Translate this page into:
Preparation and characterization of dummy template molecularly imprinted polymers coupled with HPLC for selective extraction of spiked cloprostenol from milk samples
⁎Corresponding author at: School of Pharmacy, Department of Pharmaceutical Analysis, Xi’an Jiao-tong University, Xi’an 710061, China. Department of Pharmaceutical Analysis, College of Pharmacy, Shenzhen Technology University, Shenzhen 518118, China. fuqiang@mail.xjtu.edu.cn (Qiang Fu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study was conducted to develop a particular sorbent for the selective extraction of the luteolytic drug cloprostenol from milk samples. Cloprostenol's luteolytic activity has been proved in animals, whereas the production of prostaglandins is discussed extensively in the literature, while extraction of cloprostenol is rarely discussed. The major objective was to get sufficient molecularly imprinted polymers (MIPs) for selective extraction and detection of cloprostenol. After a series of experiment, MIPs and their analogues NIPs, were synthesized. In these experiments, radical polymerization with non-covalent interactions has been used to synthesize MIPs and NIPs. Different parameters to determine the optimization conditions such as solvent volume, cross-linker, and template ratio were studied in various sets to get better possible characteristics of MIPs. The HPLC was used to evaluate the retention capacity which ranges from 68.5% to 94.1%. The mechanism of adsorption was studied by the isothermal assay and kinetic studies. The kinetic studies exhibited a high retention capacity within 20 min of MIPs contact. The percentage of polymer yield ranged from 53.5% to 92.3%. HPLC coupled with a UV detector showed the limit of detection and limit of quantification of 30.5 ng/mL and 86.7 ng/mL, respectively, for the determination of analytes from milk samples. The recovery of cloprostenol for all spiked samples of milk was higher than 91.54%. Furthermore, the studies showed that MIPs are specific in the adsorption of cloprostenol compared to its other structural analogues. The outcomes also showed the significance of cross-linker concentration and the amount of solvent during the process of polymerization. These parameters greatly affect the preorganization of complementary functional groups, meanwhile, it creates specific cavities for the targeted drugs.
Keywords
Prostaglandins
Luteolytic drugs
Selective extraction
Molecularly imprinted polymers
Milk samples
Cloprostenol
- PGF2
-
Prostaglandin F2
- SEM
-
Scanning electron microscope
- RSD
-
Relative standard deviation
- SPE
-
Solid-phase extraction
Abbreviations
1 Introduction
Cloprostenol is a prostaglandin F2 (PGF2) analogue made synthetically (Asher et al., 1996) which works as an agonist for the FP receptor and can cause luteolysis (Cao et al., 2002; Shahzad et al., 2020; Walton et al., 2002). The drug stimulates uterine contractions to induce parturition in pregnant animals (Minela et al., 2021). Cloprostenol's luteolytic activity has also been proved in animals (Brito et al., 2002; Hirsbrunner et al., 2003; Ribeiro et al., 2012), whereas the production of prostaglandins is discussed extensively in the literature(Zhu et al., 2021) (Corey et al., 1970; Corey et al., 1969; Das et al., 2007), while extraction of cloprostenol is rarely discussed. The production of cloprostenol from numerous lactone-derived particles might be a well-recognized technique as a synthetic analogue of PGF2 (Chen et al., 2015; Lust et al., 2011; Mudduluru et al., 2011; Sato et al., 2000; Vostrikov et al., 2020). Cloprostenol causes the contraction of uterine and bronchial muscles and also causes vasoconstriction of blood vessels (Cavanagh). Cloprostenol is readily absorbed through the skin. Exposure to cloprostenol is a great risk to human health for pregnant women, childbearing age and asthmatics. The contraction of bronchial muscles may result in severe bronchospasm and the contraction of uterine muscles result in abortion. There is no antidote available against the exposure of cloprostenol, there are only supportive measures such as immediately decontaminating of exposed area with water and soup. In case of exposure to cloprostenol in the eyes, it must be washed with water for 15–20 min. Cloprostenol may affect the fertility of the unborn child and damage the respiratory system(Lust et al., 2011) Because of the hazards of cloprostenol in milk and water, general treatment processes including physical adsorption, oxidation, or bioremediation were required. Cloprostenol, on the other hand, can be found in traces and is difficult to extract and remove from the mixtures.
Cloprostenol is usually analyzed or detected using HPLC, GC, and LC-MS, among other techniques (Kalikova et al., 2008; Martins et al., 2011; Whang et al., 2008; Zagitov et al., 2023). Before using these instruments, however, a reliable sample pretreatment is needed. Generally, solid phase extraction (SPE) is used to pretreat samples. Furthermore traditional SPE, such as charcoal, silica, C8, and C18, however, have really no selectivity for the compounds being studied (Huang et al., 2015). In contemporary times, MIPs have certainly been considered as a promising adsorbent for crafting artificial receptors that are compatible with the template molecules in size, shape, and functional moieties (Dong et al., 2021; Martín-Esteban, 2016). The molecularly imprinted polymers synthesized employing the surface imprinting technique gains additional active binding sites on the material surface, allowing for faster mass exchange, recognition, and recovery(Bashir et al., 2020). The molecules have similar structure and characteristics are used as dummy template in preparation of molecularly imprinted polymer (Basak et al., 2022; Marc et al., 2018). Molecularly imprinted polymers exhibit high strength at various pH levels, exceptional mechanical consistency at various temperatures, and show robustness in a variety of solvents (Luo et al., 2014). Molecularly imprinted technique and its subcategories including dispersive SPE (DSPE), single drop micro extraction technique, stir-bar sportive extraction method, solid phase microextraction (SPME) are regarded as selective and environment-friendly techniques (Aguilera-Herrador et al., 2008; Xu et al., 2007; Zielinska et al., 2012). Dispersion is done in the DSPE to enhance the surface range between the sorbents and the sample solution. It also lessens the time for extraction and the quantity of sorbent (Pastor-Belda et al., 2016). The Conventional method is also successfully used for preparing MIPs via solution polymerization followed by mechanical grinding of the resulting bulk polymers generated to give small particles and sieve the particles into the desired size ranges, which diameter usually in the micrometer range. This method, by far the most popular, presents many attractive properties, especially to newcomers. In fact, it is fast and simple in its practical execution and it does not require sophisticated instrumentation (Yan and Row, 2006). The conventional method could be used for preparation of molecularly imprinted polymer due to the desirable adsorption capacity, stability, reuse-ability, cost-effectiveness, simplicity of method and available facilities in the laboratory.
The scope of this study was to develop and employ MIPs as sorbents that may be specific for selective extraction of cloprostenol from complex media, to prepare MIPs coupling with HPLC. Here we also noticed the aspects that influence on the adsorption efficiency and tweak experiments settings. This is the first time cloprostenol has been selectively adsorbed and extracted from samples of milk.
2 Material and method
2.1 Materials
2-Vinyl pyridine (2-VP) Sigma Aldrich, AIBN (2,20-azobisisobutyronitrile) Sigma Aldrich 98%, EGDMA (ethylene glycol dimethacrylate)Sigma Aldrich > 95%, cloprostenol (99.5%) Sigma Aldrich, Bimatoprost (99.5%) Sigma Aldrich, dinoprostone (99.9%) Sigma Aldrich, progesterone (99.72%) Sigma Aldrich, absolute ethanol from Sigma Aldrich, dimethyl sulfoxide from Sigma Aldrich, glacial acetic acid 99.8% from Sigma Aldrich, methanol from Fischer Scientific and acetonitrile from Fischer Scientific used was HPLC grade.
3 Method
3.1 Optimization for MIPs and NIPs
Firstly, the method for preparation of molecularly imprinted polymer was optimized by investigating various types of functional monomers (AA, MAA,2-VP,4-VP), solvent (methanol, ethanol, acetonitrile) for polymerization, dummy templates and other reaction conditions e.g. volume of solvent and amount of cross linker. The optimization showed the remarkable results for 2-VP as functional monomer in preparation of molecularly imprinted polymer for target molecules, furthermore volume of solvent and amount of cross linker for polymerization was optimized (Table 1).
Serial number
Experiments
(Types of MIPs)Cross linker mole % of monomer
Solvent (Ethanol) mL
1
MIP-A
40
2
2
MIP-B
40
4
3
MIP-C
40
6
4
MIP-D
80
2
5
MIP-E
80
4
6
MIP-F
80
6
7
MIP-G
160
2
8
MIP-H
160
4
9
MIP-I
160
6
3.2 Preparation of MIPs and NIPS
The amount 0.68 g (6.5 mmol) of functional monomer (2-VP), weighed and put in tubes (50-mL), then ethanol was used as solvent and the dummy template molecules (Bimatoprost) were added. To achieve a homogeneous solution, the mixture was stirred for 10 min before being left undisturbed for a few minutes. After that, each tube was filled with the required amount of cross-linker EGDMA and stirred for 5 min. In the polymerization mixtures, the initiator AIBN (2,20-azobisisobutyronitrile) was dissolved in ethanol trace and added to mixture. The solutions were passed with nitrogen dioxide for 10 min for removal of the dissolved O2. In addition, each tube was deoxygenated with N2 to maintain an inert and O2-free environment. Subsequently both tubing of MIPs and NIPs were placed in a 60 °C thermostatic bath for 24 h. The MIPs were collected from the tubes after synthesis; the material sieved, grounded, and washed out with ethanol and then with ultrapure water. Finally, this material was dried by using an oven (at 40 °C). The NIPs were synthesized in the same manner as MIPs, with the same conditions and procedure, but without dummy template molecules.
3.3 Removal of dummy template and recovery of target molecules from MIPs
The removal of dummy template was carried out by placing the dried MIPs in 250-mL beakers. The various types of solvents such as methanol, ethanol, DMSO and combination of solvents were investigated for the removal of dummy template. Similarly, various solvents such as methanol, ethanol, ACN and the combination of these solvents were investigated for recovery of cloprostenol from MIPs. Single solvent and mixture of solvents were used in each beaker to investigate the removal of dummy template (Bimatoprost) and recovery of target molecules (cloprostenol). Then placed in a mechanical shaker for 24 h at ambient temperature. Complete removal of dummy template was checked by using HPLC (Elkady et al., 2018) and recovery of target molecules was investigated by using validated HPLC method of British Pharmacopeia-2019, Volume VI. Decantation was used to get the MIPs. An additional solvent of ethanol was used for the final cleaning by using filtration. After that, the polymers were vacuum filtered and dried in an oven by using Petri dishes.
3.4 Size reduction of MIPs and NIPs
MIPs were sieved after size reduction by grinding. The fraction of particles was used in all subsequent tests.
3.5 Characterization of MIPs and NIPs
3.5.1 Infrared spectroscopy
With a FTIR instrument ALPAA-P, Bruker, the FTIR spectrum of the MIPs in the form of potassium bromide pellets was obtained. The scan range of 400-–4000 cm−1 was used for infrared spectral analysis. According to the position and strength of the absorption peak in the resulting infrared spectroscopy, the relevant structural information of the imprinted polymers and non-imprinted polymer was determined.
3.5.2 Electron scanning microscope
The samples were also examined using a SEM these images were taken by a Scanning Electron Microscope of TM-1000 under vacuum. The gold was sprayed on the samples and dried in oven then observed under the scanning electron microscope. The physical appearance and morphological characteristics of MIPs and NIPs were noted by scanning electron microscopic analysis.
3.6 Identification and analysis of cloprostenol by HPLC
Monogram of British Pharmacopeia-2019, Volume VI for identification and assay of cloprostenol was used for further studies. Calibration curves of cloprostenol were determined by using 100 mL volumetric flasks in acetonitrile and methanol (75/25). The stock solution has a concentration of 100 µg/L. Appropriate dilutions were made from the stock solution and later used for standard curves. The calibration curve of cloprostenol was obtained between the concentration of 0.05 µg/mL and 1.6 µg/mL, the ethanol, methanol, water, and acetonitrile were chosen as solvents for the separation processes. The solutions were sonicated and filtered before being stored for future use. Cloprostenol was detected and analyzed using HPLC, waters system Alliance with empower 3.0 software, PDA Detector, auto sampler, and integration system, USA.
3.7 Retention studies of MIPs and NIPs
The retention studies were carried out by using 15 mL falcon tubes, each tube contained 50 mg of polymers such as MIPs or NIPs, while the standard solution of 5 mL of cloprostenol (50 µg/L) was added. Then the tubes containing the mixture were stirred for 1 h on a reciprocal shaker and afterward centrifuged for 5 min. Then the supernatant was obtained and filtered by a filter of polyvinyldene fluoride (PVDF) having a size of (0.22 µm) and collected into the Eppendorf tubes. The supernatant was analyzed by using HPLC-UV to get the concentration of cloprostenol by HPLC.
3.8 Adsorption studies
50 mg of MIPs and NIPs were taken into 20 mL falcon tubes and then subjected to 5 mL of cloprostenol (25 µg/ml) solution. This mixture was put into a mechanical shaker for 1 h and then centrifuged. The supernatant was then filtered with 0.22µ membrane filter before being transferred to HPLC analysis.
3.9 Kinetics studies
In 10 falcon tubes, MIPs, NIPs, and cloprostenol solutions were added to investigate the retention potential at various contact times. The tubes were shaken for 0, 5, 10, 20, 40, 60, 80, 100, and 120 min on a mechanical shaker. They were then easily moved and centrifuged at 4000 rpm for 5 min. The obtained supernatant was then filtered with 0.22µ membrane filter before being transferred to HPLC vials for analysis.
3.10 Adsorption isotherms
To obtain adsorption isotherms, 8 different concentrations of cloprostenol ranging from 25 µg/L to 100 µg/L and 4 temperatures (298 K, 303 K, 308 K, and 313 K) were used in the experiments which have shown significant variation in adsorption studies, and these obtained samples were then put on a mechanical shaker for 24 h at specified temperature, then centrifuged for 2 min at 4000 rpm to get clear sample to analyze by HPLC. The concentrations of cloprostenol were determined by HPLC after the supernatants were shifted into separate Eppendorf tubes.
3.11 Selectivity of MIPs
The selectivity of MIPs in potable water samples was investigated by two extensively used prostaglandin analogues (Dinoprostone, Progesterone) at different quantities and this depends on the recognition process.
3.12 Validation of SPE-HPLC method
The material developed for extraction of cloprostenol was used for extraction of cloprostenol and then target molecules were analyzed with the HPLC-UV. The process was validated by various validation parameters i.e. linearity, accuracy, precision, limit of detection, and limit of quantification. A calibration plot was constructed by range of 0.05–1.6 µg/mL of cloprostenol spiked samples.
3.13 Reuse capacity of MIPs and cost effectiveness
For this, MIP (50 mg) were mixed with cloprostenol solution (5 mL). The analyte was being adsorbed on the MIPs and the percentage of the obtained was investigated. The amount of the analyte to retain in the MIPs was calculated. In addition, the amount of analyte separation was calculated using previous information of the volume added into the MIPs and the elution mixture using eluent. The cost effectiveness of the extraction method was studied along with reusability of MIPs.
3.14 Preparation of samples and analysis
An amount of 25 µg (25 µg/ml) of cloprostenol was spiked in 1 mL of cow milk, and then 50 mg of MIPs were added to the tubes of 15 mL. The 3 mL of acetonitrile was added, then kept the mixture on a shaker for 1 h, afterwards, centrifuged for five minutes at 12000 rpm. The supernatant was then obtained and subjected to evaporation, this leads to drying under gentle nitrogen. In 1 mL of methanol, the residues were redissolved. The eluents were collected for examination; the residues were analyzed by using HPLC.
4 Results and discussion
4.1 Synthesis of MIPs and NIPs
According to the experimental design method, MIP formulations of cloprostenol were developed in the optimized conditions for polymerization in all further experiments. After the first optimization of MIPs, two of them showing the finest results were nominated for each analyte and developed in larger amounts. Consequently, these are nominated as MIPs/NIPs. Fig. 1 illustrated the structure of each type of constituents used to manufacture the molecularly imprinted polymer and its analogues in a reaction. The Fig. 2 showed the schematic diagram of the experiment for synthesis of molecularly imprinted polymer for cloprostenol.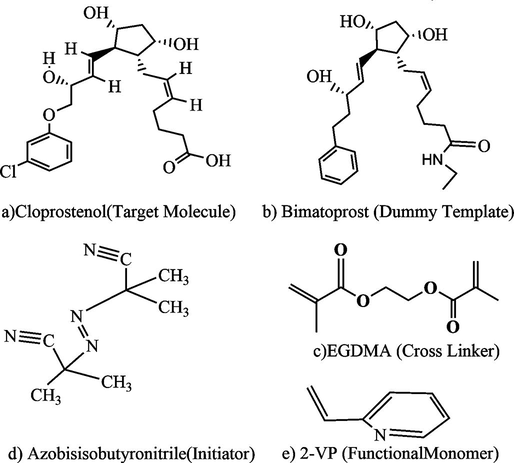
Structures of a) Cloprostenol b) Bimatoprost c) EGDMA d) Azobisisobutyronitrile e) 2-VP.
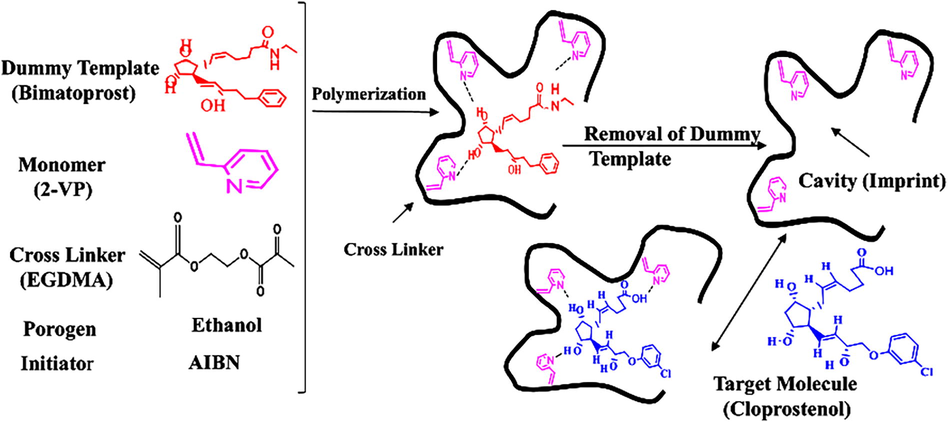
Schematic illustration of the preparation of molecularly imprinting polymer.
This bar diagram, Fig. 3a, demonstrated that the MIP-D and MIP-G showed the maximum yield, 92.3% and 89.1%, respectively. This can also be observed that MIP-A gives the lowermost yield. This relationship between the % yield of MIP-D, MIP-G, and MIP-A can be attributed to the experiment settings (Table 1). MIP-D and MIP-G yield an extreme measure of cross-linker and the smallest measure of the solvent volume.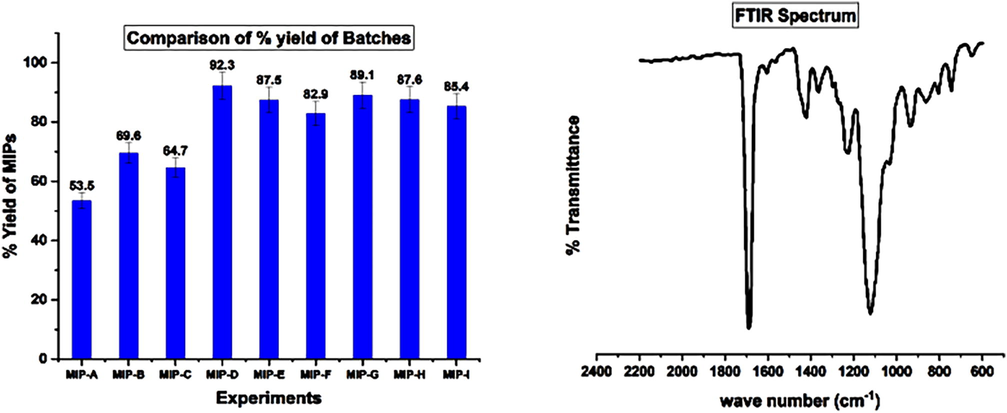
a: Yield of the MIPs in various conditions. 3b: FT-IR spectrum of MIP.
The conditions for this may lead to the existence of a gelling influence and so not a fine homogenous blend; this increased the viscosity of the MIP samples and improved the polymerization rate. As a result, this reaction may be radical and chaotic and results in a slowing the termination rate. The template, monomer and solvent connections under such conditions might represent a highly cross-linked copolymer dimension.
4.2 Removal of dummy template and recovery of target molecules from MIPs
The results indicated that % removal of dummy template from molecularly imprinted polymers in Fig. 4a, while Fig. 4b showed the number of elutions to remove the dummy template. The results indicated that the combination of DMSO and ethanol showed maximum % removal of dummy template with four elutions of solvents mixture. In Fig. 4c, % recovery of target molecules with different solvents and combination of solvents has been shown, Fig. 4d showed, the recovery of target molecules with number of elution.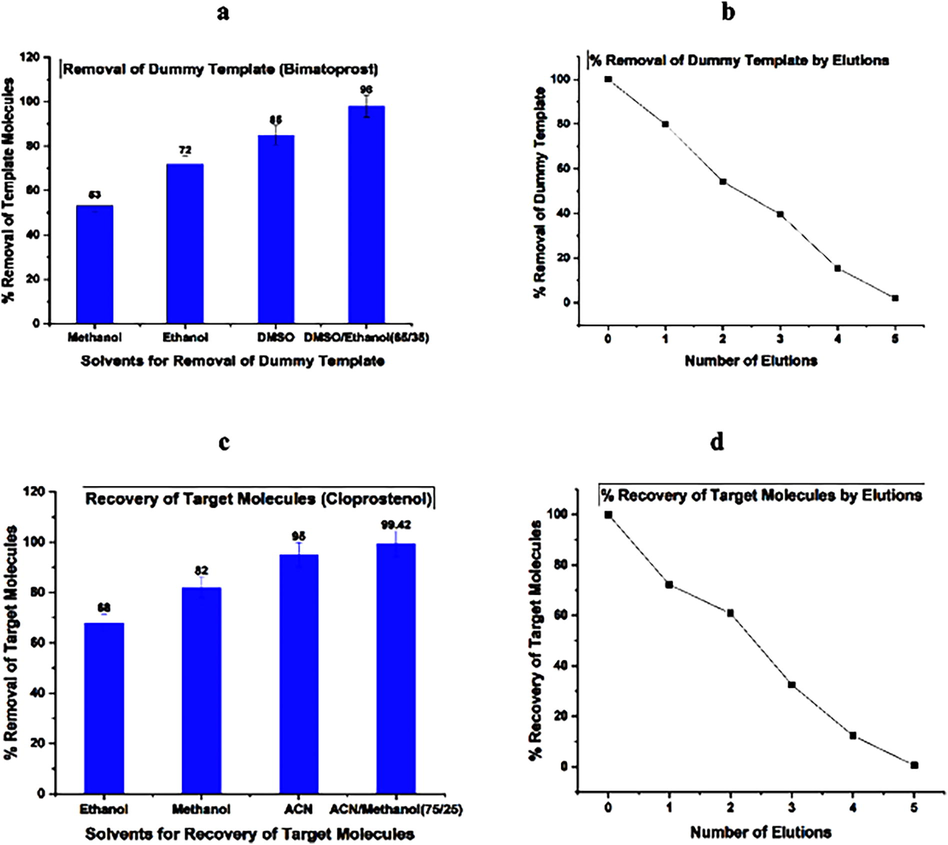
a: Removal of Dummy Template 4b: % Removal of dummy template by Elution 4c: Recovery of Target Molecules 4d: % Recovery of Target Molecules by Elution.
4.3 Characterization of MIPs
4.3.1 Fourier transfer spectroscopy
Fig. 3b demonstrated the FTIR spectrum of MIP-D and MIP-G for cloprostenol. This spectrum showed characteristic peaks related to the functional moieties of the monomers in the MIP. The characteristic peaks placed in spectrum at 1720 cm−1 and 1140 cm−1 represent C⚌O and C—O corresponds to the O—C⚌O of EGDMA. The bands can be observed between 1600 cm−1 and 1445 cm−1 and this was qualified to the stretching vibration C⚌N of pyridine functional monomer. There were multiple vinyl groups in cross linker and functional monomer. The characteristic peaks at 1630 cm−1, 990 cm−1 and 910 cm−1 in FTIR spectrum evident of polymerization reaction.
4.3.2 Scanning electron microscopy:
The morphological characterizations of MIP-D and MIP-G were investigated by scanning electron microscopy. The outcomes were shown in Fig. 5a for MIP-D and Fig. 5b MIP-G, respectively. Here we could observe that there were marked variations in the generalized morphology of the MIPs. While these modifications were correlated to the prior observation and the changes in the amount of the cross-linker and amount of the solvent during the reaction.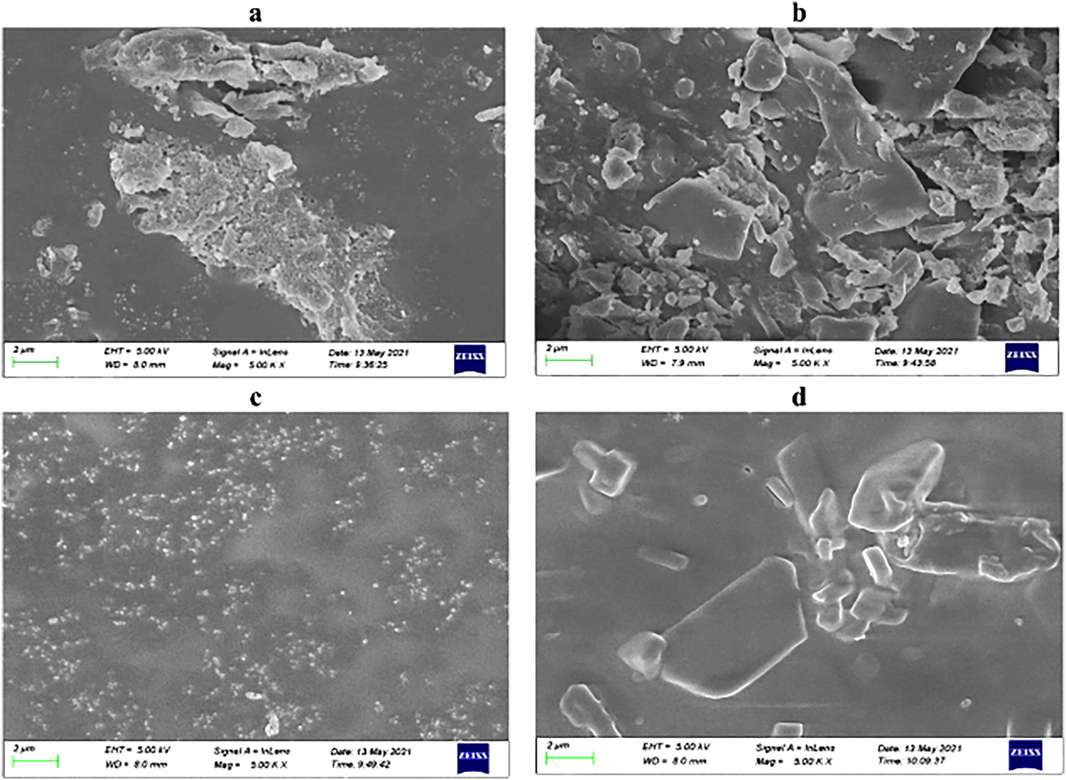
SEM microscopies of 5a: MIP-D; 5b: MIP-G.
MIP-D, Fig. 5a was shown polymerization with 2 mL solvent. The resulted finished product acquired the form of pellets and powders, and the granules were much homogenous than those of MIP-G. The less volume of the solvent and high ratio of cross-linker may result in inflexible shaped cuts that can be created in the grinding of MIP-G, Fig. 5b. The Fig. 5c and d showed the images of NIP-D and NIP-G respectively. The images of NIPs were clearly different from the MIPs in morphology which showed the adsorption properties of MIPs.
4.4 High performance liquid chromatography
An investigative scheme of British Pharmacopeia-2019, Volume VI is permissible to identify and quantify of cloprostenol. The analysis was performed by using HPLC coupled with UV-detector. The conditions for this method were summarized in the Table 2. A distinctive peak for the desired analyte cloprostenol could be observed at 3 min of the taken readings, Fig. 6.
Parameters
Analysis of cloprostenol
Mobile phase
ACN 35%: Buffer 65%
Column
C18, 250 × 4.6 cm, 5 µm
Flow rate of mobile phase
1.8 mL/min
Column oven temperature
25 °C
Detector
UV–Visible
wavelength
220 nm
Injection volume
50 µL
Time of analysis
7 min
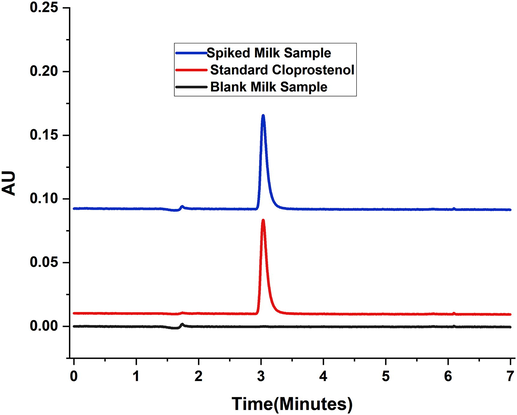
Chromatograms of Blank Milk, Standard Cloprostenol and Spiked Milk Cloprostenol.
4.5 Retention capacity of MIPs
This indicated the measured retention capabilities of each cloprostenol MIP. The retention capabilities of different categories of MIPs have demonstrated in Fig. 7a. The quantities of solvent, template, and the cross-linker exhibited the change in the retention time of MIPs. Hence, a description for the higher retention capacity could be altered forms of interactions among the template and the functional monomer produced a significant measure (the creation of hydrogen bonds amongst carboxyl groups in functional monomer and carbonyl groups present in cloprostenol).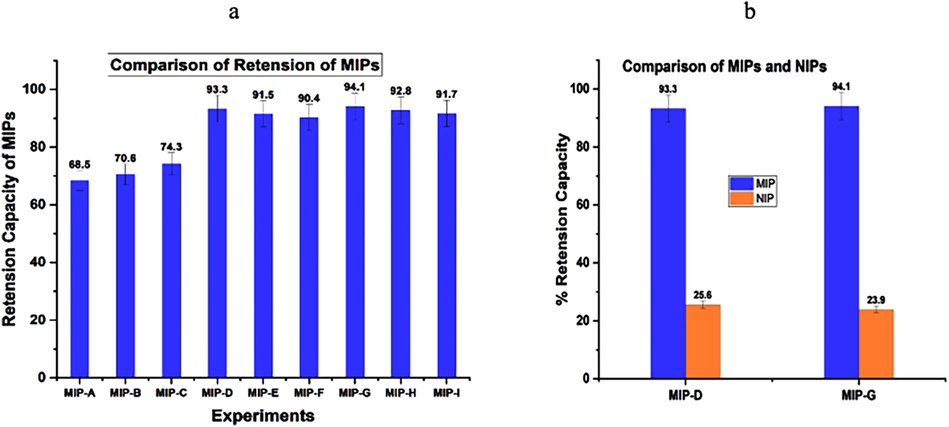
a: Retention capacities of MIPs; the initial concentration of cloprostenol was 50 µg, and MIPs of 50 mg synthesized according to the experimental setting. 7b: Comparison of MIPs and NIPs of selected batches.
In earlier studies, it was established that the one constant that might be a cross-linker was a much significant factor during the synthesis of MIPs. To check that description, the yield and retention capability of MIPs were investigated using various quantities of cross-linkers. The findings demonstrated a similar pattern to the earlier findings. The yield of synthesized MIPs showed a rise as the amount of cross-linker enhanced. The yield reduced when there was less cross-linker present. MIPs with the best performance (maximum yield & retention) might be chosen based on these findings. As a result, the most efficient polymers were chosen: MIP D and MIP G. Those MIPs and their NIPs were then synthesized in larger amounts and called batches labelled D and G, respectively, as previously described. The polymers yielded 93.3 percent and 94.1 percent cloprostenol, respectively.
4.6 Retention studies of selected MIPs and NIPs
Fig. 7b depicted the retention capabilities of the chosen batches of MIPs and NIPs. Throughout the experiment, 50 mg of MIPs or NIPs was introduced to the falcon tubes, followed by 5 mL of cloprostenol standard solution (50 µg/L).
4.7 Adsorption isotherms
In this study, MIP-D, MIP-G, NIP-D, and NIP-G were used to create adsorption isotherms to determine the maximum adsorption capacity of the polymers. The Langmuir and Freundlich models were used to analyze the experimental data for adsorption isotherms. Furthermore, fitting the adsorption data helped to correctly characterize the experimental results and determine the best model for the MIPs and NIPs that were studied. The acquired parameters for each linearized isotherm model for MIPs constructed for cloprostenol were shown in Tables 3 and 4. Freundlich and Langmuir models were used to investigate the adsorption properties(Du et al., 2013). For the adsorption isotherm, the Freundlich model demonstrates R2 > 96%. The linearity of the Langmuir model was less than R2 ˃90 percent. As a result, the findings of Freundlich were noteworthy.
298 K
303 K
308 K
313 K
Polymer
n
K
n
K
n
K
n
K
MIP D
1.595
2.652
1.638
3.054
1.526
3.027
1.654
3.508
NIP D
0.583
1.464
0.634
1.210
0.697
0.775
0.649
0.946
MIP G
1.523
2.744
1.515
2.873
1.514
3.550
1.605
3.121
NIP G
0.499
2.052
0.582
1.267
0.633
0.841
0.616
1.098
298 K
303 K
308 K
313 K
Polymer
Q(max)
K
Q(max)
K
Q(max)
K
Q(max)
K
MIP D
50.910
0.039
63.891
0.035
60.270
0.041
61.012
0.046
NIP D
24.969
0.037
23.047
0.036
26.539
0.019
18.369
0.037
MIP G
62.420
0.034
50.910
0.039
134.50
0.020
108.20
0.023
NIP G
34.471
0.029
24.994
0.029
26.860
0.017
42.017
0.013
We calculated the intensity parameter “n” and the polymer's adsorption affinity “K” using the linearized Freundlich isotherm (see Table 3). At 308 K, the results revealed a significant difference in MIP and NIP affinity (K). MIP-G had a “K” value that was 4.22 and MIP-D 3.90 times greater than the control NIP. This might imply that MIP has a higher affinity for cloprostenol than NIP due to the presence of a specialized cavity for cloprostenol identification. Table 4 showed the results of the linearized Langmuir isotherms. The Q(max) (maximum adsorption capacity) of the MIPs and NIPs was calculated using the linearised Langmuir isotherm. MIP-G had the greatest Q(max) for cloprostenol at 308 K (134.50 mg/g), whereas the control (NIP-G) had 26.860 mg/g. As a result, MIP-G was able to retain 5.00 times more cloprostenol than NIP-G. The maximum value for Q(max) of MIP-D at 303 K was (60.270 mg/g), whereas the control (NIP-D) had a Q(max) of 26.539 mg/g. As a result, MIP-D kept 2.27 times more cloprostenol than NIP-D. The results of the linearized isotherm for cloprostenol were shown in Table 4.
4.8 Kinetic study of the retention of cloprostenol
Cloprostenol adsorption could be seen in Fig. 8a. It was clear that the adsorption phase attained equilibrium in a relatively short duration of time around 20 min. This could happen due to the ‘imprinted polymers’ high specific binding. Furthermore, the percentage retention capacity for MIPs and their respective NIPs was significantly higher.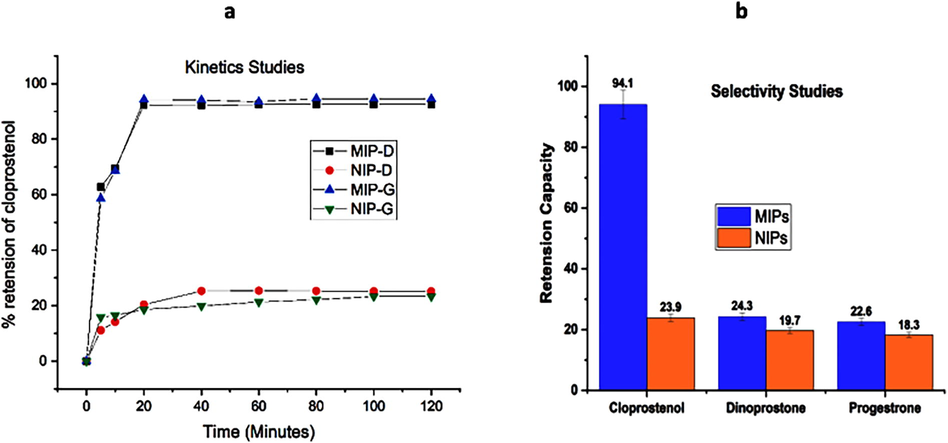
a The Kinetic study (I) MIP-D (II) MIP-G with MIPs and NIPs. 8b Selectivity Studies.
4.9 Selectivity of MIPs
The cross-reactivity of MIPs with cloprostenol was evaluated with structurally related chemicals and other prostaglandin analogues. Dinoprostone and progesterone were utilized as a structural analogue in a cross-reactivity assay to investigate cross-reactivity. All trials were carried out in three times of experiments. MIPs were tested for cross-reactivity with one analyte at a time in suitable solvent at two distinct concentrations (50 µg/L and 100 µg/L). MIPs exhibited no substantial cross-reactivity in any case, with a high recovery of the measured analytes. MIP-D had the lowest cross-reactivity when evaluated against the other prostaglandin analogues Fig. 8b. This degree of cross-reactivity could be attributed that MIPs do not always display 100% selectivity for target molecules and can interact with other structurally comparable molecules with a similar functional group and their distribution.
4.10 Validation of SPE-HPLC of method
The Linearity was determined by correlation coefficients R2 value of calibration plot of cloprostenol and spiked cloprostenol in Fig. 9a and 9b respectively. The limit of detection was determined by signal to noise ratio, the value of LOD and LOQ for the method was 30.5 ng/mL and 86.7 ng/mL, respectively (S/N = 10) Fig. 9b. The accuracy was determined by the experiments performed of concentration level at 50, 75, 100 ng/mL and each of experiment was performed (n = 3) three times shown in Table 5. The percentage recovery of the spiked samples of cloprostenol of intra-day and inter-day accuracy were calculated shown in table. The precisions were determined in terms of intra-day and inter-day experiments to determine percentage recovery and relative standard deviation at 50, 75, 100 ng/mL levels, each experiment was performed three times the % RSD of spiked cloprostenol sample by this SPE coupled with HPLC method ranges from 0.5 to 2.6 (Table 5).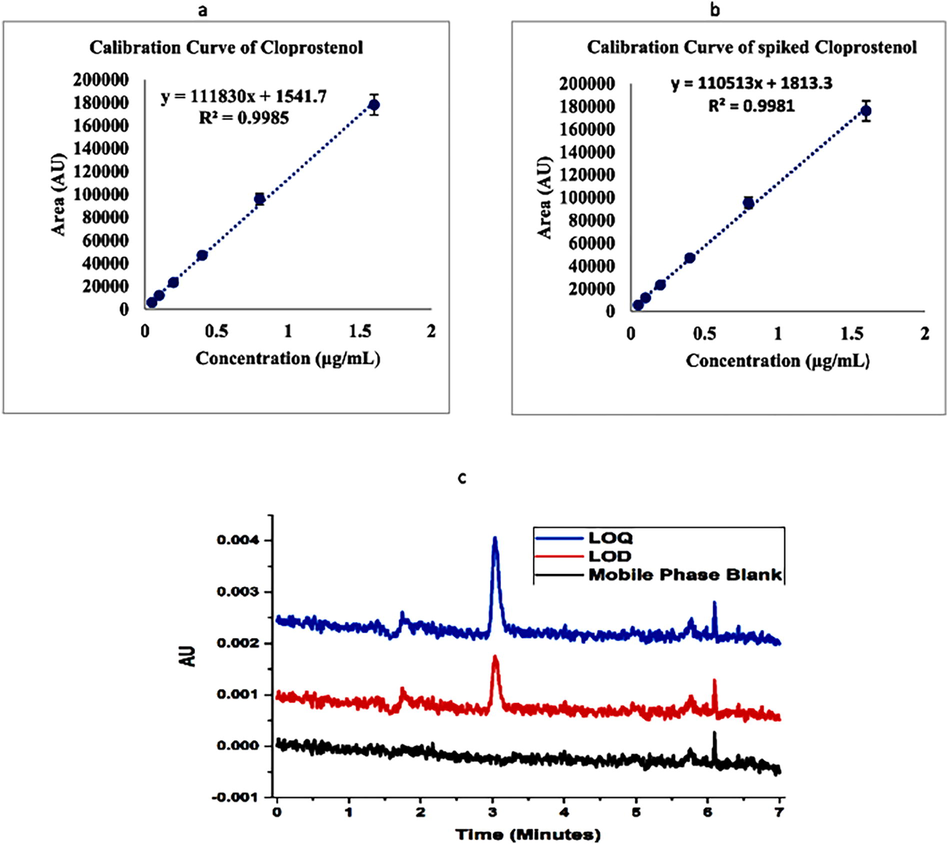
a: Calibration Curve of Cloprostenol 9b: Calibration Curve of spiked Cloprostenol 9c: Chromatograms of LOD and LOQ of Cloprostenol.
Sample
Accuracy (Recovery % n = 3)
Precision (%RSD n = 3)
Spiked drug Concentration (ng/mL)
Intra-day
Inter-day
Intra-day
Inter-day
Milk
50
92.55
91.54
0.90
1.03
75
94.54
95.18
1.59
0.94
100
95.15
94.62
1.38
1.42
4.11 Reusability of MIPs
The most important feature that must be examined prior to a new material could be used in microextraction is its reusability capability. As a result, the reusability of polymers MIP-D and MIP-G was investigated to determine their reuse potential for future use in SPE.
Primarily, 50 mg of MIP-D was loaded with 5 mL solution of cloprostenol (50 µg/L) standard solution. Then the concentration of the remaining drug in the supernatant was determined after the drug were adsorbed on the MIPs, and the amount of analytes retained in the MIPs was assessed. Using four different mixtures of eluents, the percentage of analyte liberation was estimated using the amount adsorbed on the MIPs.
Four different eluents were used in the batch method, the solvents were Methanol, Acetonitrile, and different combinations of various solvents such as methanol/acetonitrile (65:35 v/v), and ethanol/methanol (30:70 v/v). The optimum elution technique was discovered to be a methanol/acetonitrile combination, which had an elution efficiency of over 93 percent, Fig. 6a, the investigation of MIP reuse was proceeded after identifying the best suited solvent mixture for the elution of analytes adsorbed in MIPs. To determine how many successive experiments may be carried out using one MIP, a series of cloprostenol adsorption and desorption assays were done, Fig. 7b. The cost effectiveness of the proposed extraction method for selective extraction of cloprostenol was studied to establish the usefulness of this extraction method. One of the significant abilities of MIPs is their reusability. This is a very important benefit in saving time and money. MIPs are used for selective extraction of drugs from complex matrix which may use less solvent as compared to other techniques of extraction. Furthermore, the advantage of this method is its low cost and very low consumption of experimental materials. This method is fast and cost-effective. The ability to recycle MIP is one of the highlights in future prospectus.
4.12 Determination of the adsorption capacity of MIPs in spiked milk samples
MIPs were used to determine the adsorption capacity for cloprostenol in milk samples. Each sample of milk was spiked with cloprostenol (50 µg/L). The conditions of the experiments and procedure of analysis were the same as those described in Section 3.4. As a result, the weight of MIPs 50 mg was added to a falcon tube of 15 mL, then 5 mL of cloprostenol spiked milk sample was added. This mixture was placed on mechanical shaker for one hour before being centrifuged for five minutes. Filtered supernatant was put in an Eppendorf tube. HPLC system coupled with UV detector was used to determine the levels of cloprostenol in the supernatant Fig. 6.
The retention capacity for all samples was higher than 93%. In the case of MIP-D, it was 93.3% (±1.9%) and 94.1% (±2.3%) for MIP-G, respectively. All measurements were carried out three times. The final results revealed good performance and possible applicability in the analysis of actual complicated samples that contain cloprostenol.
5 Conclusion
High yield of different polymers could be obtained by the process of radical polymerization which is up to 92.3%. The experimental design to optimize the conditions of polymerization for MIPs and their analogues NIPs helped to get optimum results and signify the conditions which affect the process of polymerization, so it established that the concentration of cross-linker and the amount of solvent significantly affect the process of polymerization. These parameters also effect the yield and selectivity of polymers. The kinetic studies depict significantly rapid retention of cloprostenol, the contact time of a significant retention of analyte is less than 20 min. The adsorption models of Langmuir and Freundlich were used to evaluate data. The results of adsorption model inference that cavities only allow to bind a specific analyte; these cavities are produced in the form of monolayers and multilayers on the surface of MIPs. The MIPs and NIPs showed significant differences in retention capacity inclusively. The significant difference in the value of “K” evident that MIPs showed higher affinity for cloprostenol due to the specific cavities on the surface of MIPs for the identification of selective drugs. Similarly, the value of Q(max) for MIPs of cloprostenol was higher than that of its control NIP. Furthermore, no significant cross-reactivity was reported between MIPs and other similar molecules to cloprostenol. When the MIPs were used to apply in cow milk samples in which cloprostenol was spiked, the results of analysis were practically reliable and significant. This demonstrated the great efficiency of the proposed molecularly imprinted polymers and their potential application in milk samples. This study may be useful in future prospects in which the optimization process for synthesis of molecularly imprinted polymer could be used in further research. The use of dummy template molecules could be evaluated for different method of extraction for other prostaglandin analogues.
Ethics approval and consent to participate
Not applicable.
Human and animal rights
No animals/humans were used in this research.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
The author is grateful to the Deanship of Scientific Research at King Khalid University for funding this study through the Large Research Group Project, under grant number RGP. 2/410/44.
CRediT authorship contribution statement
Aqeel Shahzad: Conceptualization, Methodology, Investigation, Formal Analysis, Writing – original draft. Abdul Majeed: Visualization, Software. Ahmed A. Lahiq: Resources. Taha Alqahtani: Resources. Ali M Alqahtani: Resources. Kamran Bashir: Data curation, Formal analysis. Musaddique Hussain: Validation, Visualization. Qiang Fu: Project administration, Resources, Writing – review & editing, Supervision.
Acknowledgements
The author acknowledges the facilities provided by Xi'an Jiaotong University, China and Bahauddin Zakariya University, Multan, Pakistan.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Direct coupling of ionic liquid based single-drop microextraction and GC/MS. Anal. Chem.. 2008;80(3):793-800.
- [CrossRef] [Google Scholar]
- Luteal support of pregnancy in red deer (Cervus elaphus): effect of cloprostenol, ovariectomy and lutectomy on the viability of the post-implantation embryo. Anim. Reprod. Sci.. 1996;41(2):141-151.
- [CrossRef] [Google Scholar]
- Recent advances in the application of molecularly imprinted polymers (MIPs) in food analysis. Food Control. 2022;109074
- [CrossRef] [Google Scholar]
- Synthesis, characterization, and application of griseofulvin surface molecularly imprinted polymers as the selective solid phase extraction sorbent in rat plasma samples. Arab. J. Chem.. 2020;13(2):4082-4091.
- [CrossRef] [Google Scholar]
- Efficacy of PGF2α to synchronize estrus in water buffalo cows (Bubalus bubalis) is dependent upon plasma progesterone concentration, corpus luteum size and ovarian follicular status before treatment. Anim. Reprod. Sci.. 2002;73(1–2):23-35.
- [CrossRef] [Google Scholar]
- In vitro pharmacological characterization of the prostanoid receptor population in the non-pregnant porcine myometrium. Eur. J. Pharmacol.. 2002;442(1–2):115-123.
- [CrossRef] [Google Scholar]
- Plumb’s Veterinary Drug Handbook. Pocket — 7th edition. Can Vet J.. 2012;53(12):1284.
- [Google Scholar]
- An improved and efficient process for the preparation of (+)-cloprostenol. Chirality. 2015;27(6):392-396.
- [CrossRef] [Google Scholar]
- Stereo-controlled synthesis of prostaglandins F-2a and E-2 (dl) J. Am. Chem. Soc.. 1969;91(20):5675-5677.
- [CrossRef] [Google Scholar]
- Total synthesis of prostaglandins F1-alpha, E1, F2-alpha, and E2 (natural forms) from a common synthetic intermediate. J. Am. Chem. Soc.. 1970;92(8):2586-2587.
- [CrossRef] [Google Scholar]
- Recent developments in the synthesis of prostaglandins and analogues. Chem. Rev.. 2007;107(7):3286-3337.
- [CrossRef] [Google Scholar]
- Molecularly imprinted polymers by the surface imprinting technique. Eur. Polym. J.. 2021;145:110231
- [CrossRef] [Google Scholar]
- Dummy-template molecularly imprinted solid phase extraction for selective analysis of ractopamine in pork. Food Chem.. 2013;139(1–4):24-30.
- [CrossRef] [Google Scholar]
- Synchronized separation of timolol from some prostaglandin analogs (bimatoprost, latanoprost and travoprost) for determination in their combined pharmaceutical formulations using RP-HPLC. Anal. Chem. Lett.. 2018;8(1):76-87.
- [CrossRef] [Google Scholar]
- Effect of prostaglandin E2, DL-cloprostenol, and prostaglandin E2 in combination with D-cloprostenol on uterine motility during diestrus in experimental cows. Anim. Reprod. Sci.. 2003;79(1–2):17-32.
- [CrossRef] [Google Scholar]
- Application of molecularly imprinted polymers in wastewater treatment: a review. Environ. Sci. Pollut. Res. Int.. 2015;22(2):963-977.
- [CrossRef] [Google Scholar]
- HPLC method for enantioselective analysis of cloprostenol. J. Pharm. Biomed. Anal.. 2008;46(5):892-897.
- [CrossRef] [Google Scholar]
- Preparation of surface molecularly imprinted polymers as the solid-phase extraction sorbents for the specific recognition of penicilloic acid in penicillin. Anal. Methods. 2014;6(19):7865-7874.
- [CrossRef] [Google Scholar]
- Human health hazards of veterinary medications: information for emergency departments. J. Emerg. Med.. 2011;40(2):198-207.
- [CrossRef] [Google Scholar]
- Preparation and characterization of dummy-template molecularly imprinted polymers as potential sorbents for the recognition of selected polybrominated diphenyl ethers. Anal. Chim. Acta. 2018;1030:77-95.
- [CrossRef] [Google Scholar]
- Recent molecularly imprinted polymer-based sample preparation techniques in environmental analysis. Trends Environ. Anal. Chem.. 2016;9:8-14.
- [CrossRef] [Google Scholar]
- Effects of cloprostenol sodium at final prostaglandin F2alpha of Ovsynch on complete luteolysis and pregnancy per artificial insemination in lactating dairy cows. J. Dairy Sci.. 2011;94(6):2815-2824.
- [CrossRef] [Google Scholar]
- The effect of a double dose of cloprostenol sodium on luteal blood flow and pregnancy rates per artificial insemination in lactating dairy cows. J. Dairy Sci.. 2021;104(11):12105-12116.
- [CrossRef] [Google Scholar]
- Synthesis of travoprost and its analogs. Lett. Org. Chem.. 2011;8(4):234-241.
- [CrossRef] [Google Scholar]
- Determination of spirocyclic tetronic/tetramic acid derivatives and neonicotinoid insecticides in fruits and vegetables by liquid chromatography and mass spectrometry after dispersive liquid-liquid microextraction. Food Chem.. 2016;202:389-395.
- [CrossRef] [Google Scholar]
- Fertility in dairy cows following presynchronization and administering twice the luteolytic dose of prostaglandin F2alpha as one or two injections in the 5-day timed artificial insemination protocol. Theriogenology. 2012;78(2):273-284.
- [CrossRef] [Google Scholar]
- Total synthesis of prostaglandin F2alpha using nickel-catalyzed stereoselective cyclization of 1,3-diene and tethered aldehyde via transmetalation of nickelacycle with diisobutylaluminum acetylacetonate. Chem. Pharm. Bull. (Tokyo). 2000;48(11):1753-1760.
- [CrossRef] [Google Scholar]
- Identification of novel in vitro antibacterial action of cloprostenol and evaluation of other non-antibiotics against multi-drug resistant A. baumannii. J. Antibiot. (Tokyo). 2020;73(1):72-75.
- [CrossRef] [Google Scholar]
- Fluorine containing analogues of cloprostenol. J. Fluor. Chem.. 2020;235:109552
- [CrossRef] [Google Scholar]
- Prostaglandin F2alpha-induced nest-building behaviour is associated with increased hypothalamic c-fos and c-jun mRNA expression. J. Neuroendocrinol.. 2002;14(9):711-723.
- [CrossRef] [Google Scholar]
- NMR studies of the inclusion complex of cloprostenol sodium salt with beta-cyclodextrin in aqueous solution. Pharm. Res.. 2008;25(5):1142-1149.
- [CrossRef] [Google Scholar]
- Development of an improved liquid phase microextraction technique and its application in the analysis of flumetsulam and its two analogous herbicides in soil. J. Agric. Food Chem.. 2007;55(23):9351-9356.
- [CrossRef] [Google Scholar]
- Characteristic and synthetic approach of molecularly imprinted polymer. Int. J. Mol. Sci.. 2006;7(5):155-178.
- [CrossRef] [Google Scholar]
- Transformation of cloprostenol into E-type derivatives and a comparative study of their uterotonic activity. Pharm. Chem. J.. 2023;57(1):46-50.
- [CrossRef] [Google Scholar]
- A unified strategy to prostaglandins: chemoenzymatic total synthesis of cloprostenol, bimatoprost, PGF 2α, fluprostenol, and travoprost guided by biocatalytic retrosynthesis. Chem. Sci.. 2021;12(30):10362-10370.
- [CrossRef] [Google Scholar]
- Speciation analysis of aqueous nanoparticulate diclofenac complexes by solid-phase microextraction. Langmuir. 2012;28(41):14672-14680.
- [CrossRef] [Google Scholar]







