Translate this page into:
Preparation and evaluation of transdermal hydrogel of chitosan coated nanocurcumin for enhanced stability and skin permeability
⁎Corresponding authors at: Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, Qassim University, Qassim 51452, Saudi Arabia (H.A. Mohammed). adelpharma2004@svu.edu.eg (Adel M. Ahmad), ham.mohammed@qu.edu.sa (Hamdoon A. Mohammed), naaltwaijry@pnu.edu.sa (Najla Altwaijry)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Curcumin (CR) is a natural product that has great interest in biopharmaceutical applications. It has several biological effects that antagonizes inflammation, oxidative stress, and cancer. On the other hand, low aqueous solubility, stability, and dissolution rate are the main challenges to using curcumin. In the present investigation, curcumin was formulated as a nanosystem known as nanocurcumin using a combination of polyvinyl pyrrolidone (PVP) with different polymers as stabilizing agents. The nanocurcumin formulations were prepared and evaluated by measuring the particle size, surface charge, drug content, and stability. The selected nanocurcumin formulation prepared using PVPK30-Poloxamer 188 demonstrated a significantly smaller particle size (272.1 ± 15.32 nm) and a higher settlement volume ration as compared with other formulations. Also, the selected formulation showed higher solubility and dissolution rates as compared with other stabilizer combinations. Chitosan was added to the selected nanocurcumin formulation as a coating polymer to enhance short-term storage colloidal stability. The result of stability proved that chitosan (0.05%)-coated nanocurcumin dispersion showed good stability during storage as compared with non-coated nanocurcumin. Non-coated nanocurcumin and chitosan-coated curcumin were loaded into the Carbopol934 hydrogel matrix to investigate the effect on curcumin permeability across the skin. The results revealed that nanocurcumin hydrogel formulations showed a significantly higher amount of curcumin permeated across the skin.
Keywords
Curcumin
Nanotechnology
Hydrogel
Skin permeability
HPLC
Chitosan Coated Nanocurcumin
1 Introduction
Solubility is an important parameter to evaluate drug performance, regardless of the different routes of administration. However, dealing with poorly water-soluble compounds, which represent at least 40% of all new products, is one of the major obstacles facing pharmaceutical development (Merisko-Liversidge et al., 2003; Merisko-Liversidge and Liversidge, 2008). Subtherapeutic drug solubility may produce several undesirable effects, such as precipitation upon administration, inconsistent dosage response, and inappropriate pharmacokinetics. Low water solubility of active pharmaceutical ingredients (API) results in a limited rate of dissolution, decreased absorption, and low oral bioavailability. In addition, using drug solutions containing other types of solvents might produce toxicity issues and in-vivo precipitation. Recently, pharmaceutical preparations have faced various issues related to solubility challenges because 60% of synthetic drugs have solubility problems (Jabeen et al., 2022). Nanotechnology is a valuable approach that has a great interest in solubility enhancement issues via particle size reduction and increasing surface area that lead to enhanced dissolution rate (Abdellatif et al., 2021; Mohammed et al., 2023b). The utilization of nanoparticulate systems offers variable benefits compared to conventional approaches in terms of efficient absorption, limited degradation, enhanced stability, and extended release (Aboubakr et al., 2022; Mohammed et al., 2023a; Quan et al., 2021).
Curcumin (CR), the natural phenolic substance isolated from Curcuma longa L., has shown several biological performances, e.g., anti-inflammatory, hepatoprotection, anti-bacterial, antitumor, antifungal, and antioxidant activities without toxicity even at high doses (Mohandas and Rangasamy, 2021). Despite its excellent suitability for transdermal and tropical applications, CR's limited water solubility, poor stability, and low bioavailability limit its medicinal efficacy and administration. Furthermore, the absence of water solubility reduces flexibility for CR formulation and administration (El-Refaie et al., 2015).
In recent years, drug nanocrystals (nanodrug form) have attracted great interest and have been shown to be one of the most effective nanoplatform systems for formulating poorly soluble products (Gao et al., 2008; Shegokar and Müller, 2010). Nanocrystals can be defined as very finely dispersed drug particles in an aqueous medium for different routes of administration. It is a colloid drug delivery system without a carrier and has particles with a mean size of 10 to 1000 nm (Gao et al., 2008), consisting of free active drug and a low concentration of surfactant to maintain stabilization condition (Gigliobianco et al., 2018). These nanocarriers provide drug delivery systems that offer several benefits, including high drug loading, limited utilization of excipients, improved chemical stability, lower toxicity, improved solubility, and dissolution of poorly soluble drugs (El-Badry et al., 2013). Reducing particle size to the nanometer range results in increased surface area, which leads to an improved dissolution rate (Abdelbary et al., 2015). Colloidal nanoparticles can be stabilized using minimum amounts of ionic stabilizers (sodium lauryl sulfate or lecithin) or nonionic surfactants (Poloxamer-407 or 188, and Tweens). The stabilizer acts as a wetting agent for the drug particles to thoroughly wet them and to overcome Ostwald’s ripening and aggregation, thus resulting in a physically stable nanoplatform system (El-Refaie et al., 2015). Mucoadhesive delivery systems are considered a hopeful approach for drug administration due to the localization of the drug product for an extended period at the absorption site. In recent years, the use of nanosystems coated with mucoadhesive polymers has established itself as a promising approach, allowing extended residence time, enhancing formulation stability, and improving the absorption of drug products through different mucosal tissues (Al-Jubori et al., 2021; Ibraheem et al., 2022; Liu et al., 2008). In that context, Zou, Peng, et al. (Zou et al., 2013) compared the pharmacokinetics of polymeric nanocurcumin and solvent-solubilized curcumin formulations in rats for cancer therapy. They found that nanocurcumin showed an increase in the plasma Cmax of curcumin by 1749 fold compared to solvent-solubilized curcumin. Additionally, the nanocurcumin fraction following burst release is available for tumor delivery via the enhanced permeation and retention effects commonly observed for nanoparticle formulations. Furthermore, curcumin nanoparticles have been prepared by Bhawana, et al., using the wet milling method for improved aqueous solubility and antimicrobial effects. They found that the prepared curcumin nanoparticles have a narrow particle size distribution in the range of 2–40 nm. Unlike curcumin, nanocurcumin was found to be freely dispersible in water without any surfactants. Also, they discovered that the enhanced solubility of curcumin nanoparticles resulted in more effective activity against fungal and bacterial strains (Bhawana et al., 2011). Stable curcumin PLGA nanoparticles were developed by Shen, Hao, et al. (Shen et al., 2013) to overcome the low bioavailability and rapid clearance of curcumin. Nanoparticles were generated using flash nanoprecipitation integrated with spray drying for alleviation of morphine tolerance in mice when orally administered after subcutaneous injections of morphine. Using hydrogel as a carrier for curcumin delivery offers the application via different routes of administration, such as oral, topical, and nasal routes. Since then, different pharmacological actions have been known for curcumin, such as antitumor, anti-inflammatory, and antimicrobial effects (Madamsetty et al., 2023). The pharmacokinetic parameters of curcumin can be improved by using different nanocarriers loaded into hydrogels. Such a study used curcumin nanomicelles loaded into dextran hydrogel to enhance its release during inflammation and promote fibroblast proliferation and collagen synthesis during the wound healing process (Alibolandi et al., 2017). Further, chitosan hydrogel with nanocurcumin was utilized previously to enhance transdermal permeability and wound healing activity (Nair et al., 2019).
Chitosan is a polysaccharide made of chitin-derived N-acetyl-d-glucosamine residue and linear 1–4-linked d-glucosamine. Chitosan has been widely used among mucoadhesive polymers because of its ability to improve medication absorption by interacting with the negatively charged mucosal barrier (Garcia et al., 2017). Studies have shown that coating nanoparticles with this polysaccharide for medication delivery is a promising application. Consequently, chitosan-coated nanoparticles have demonstrated that they are stable and ideal for drug incorporation (Hu and Luo, 2021; Tai et al., 2020).
Despite the good aqueous solubility and dissolution properties of nanocrystal formulations (nanodrug forms), they are not widely used for topical and transdermal applications. Therefore, it is required to load a nanoform of drug into an appropriate gelling system (hydrogel) to overcome these challenges (Jabeen et al., 2022; Yao et al., 2022). Hydrogel is considered an acceptable medium for nanodrug suspensions that prolong the duration of topical administration.
The present study aimed to develop nanocurcumin formulations using polyvinylpyrrolidone K30 (PVPK30) combined with different stabilizers to enhance the aqueous solubility and dissolution rate of CR. The antisolvent nanoprecipitation method was used, and the formulation was tested for particle size, polydispersity index (PDI), zeta potential, solubility, and dissolution rate characteristics. Then the formulation was coated with chitosan to enhance the short-term stability of nanocurcumin and characterized for particle size, zetapotential, in-vitro drug release, and physical and chemical stability. Finally, chitosan-coated nanocurcumin (CS-NCR) was loaded into Carbopol934 hydrogel to investigate skin permeation. The developed hydrogel formulations of chitosan-coated nanocurcumin were investigated by measuring the viscosity, rheological properties, pH, drug release (in vitro), and ex-vivo permeability performance in the rats' skin model to assess transdermal delivery of chitosan-coated nanocurcumin in comparison with noncoated nanocurcumin. It’s a new trial to investigate the effect of combining two stabilizers (PVP and Polxamer) and a coating agent (chitosan) on the short-term (<3 months) storage colloidal stability of nanocurcumin dispersion. As part of the novelty of the work, the skin permeability of coated and uncoated nanocurcumin was also evaluated by measuring the permeated curcumin concentration using a newly validated HPLC method. The results showed that transdermal permeability was improved by using nanocurcumin.
2 Materials and methods
2.1 Materials
Curcumin (purity > 95%) was obtained from SD Fine-Chem Limited, Mumbai, India. KH2PO4, PEG200, Tween80, Na2HPO4, and NaOH were purchased from United Company for Chem. and Med. Prep., Egypt. Acetonitrile and methanol were purchased from Fisher Scientific, A Millipore® purification system (Bedford, MA, USA) was used to obtain the Milli-Q water used to prepare buffer solutions, Chitosan (CS), low molecular weight (98% degree of deacetylation), was obtained from Industrial Manufacturing Co., Tokyo, Japan. Brij ® 35 was obtained from Merk Co., Germany.
2.2 Preparation of curcumin nanocrystals (CR-NC)
In this study, nanocurcumin formulations were prepared utilizing a modified antisolvent nanoprecipitation technique as previously reported (Abdelbary et al., 2015). Briefly, CR was dissolved in ethanol (organic phase). The antisolvent phase was prepared by dissolving the stabilizer combination of PVPK30 with (Poloxamer 188, Tween 80, HPβ-CD and Brij®35) at a ratio of 1:2 (CR: stabilizer combination, w/w) with PEG200 (costabilizer) (1 ml) in distilled water (Table 1). At a temperature of 25 °C, the organic phase was added slowly at a rate of 1 ml/min into the antisolvent phase and stirred on a magnetic stirrer for 20 min. Instantly, nanodrug particles precipitated from the antisolvent. Then, nanocurcumin dispersions were placed in a rotary evaporator at 40 °C for 15 min for complete evaporation. The developed nanocurcumin formulations were stored at −80 ± 1 °C in the refrigerator before freeze-drying. The composition of the developed formulations is listed in Table 1. NCR: Nanocurcumin.
Ingredients
NCR1
NCR2
NCR3
NCR4
Curcumin (mg)
50
50
50
50
PVPK30-Poloxamer188 (mg)
100
–
–
–
PVPK30-Tween80 (mg)
–
100
–
–
PVPK30-HPβ-CD (mg)
–
–
100
PVPK30-Brij®35 (mg)
–
–
–
100
PEG200 (ml)
1
1
1
1
Ethanol (ml)
2
2
2
2
Water (ml)
8
8
8
8
2.3 Characterization of nanocurcumin dispersions
2.3.1 Settlement volume ratio assessment
The developed formulations of nanocurcumin dispersions were kept away from light at room temperature for one month. The physical stability of the produced nanoformulations was monitored via visual detection and a calculated settlement volume ratio (F). The settlement volume ratio is the ratio of volume or height between before and after sedimentation for a definite time to select an appropriate stabilizer combination (Allouni et al., 2009; Sun et al., 2010a). It was estimated according to the following Eq. (1):
2.3.2 Measurement of particle size and zeta potential
Particle size and polydispersity index (PDI) were measured by dynamic light scattering using a Zetasizer Nano ZS® instrument (Malvern Instruments, Malvern, UK) equipped with a backscattered light detector operating at 173° (Aboubakr et al., 2022). The zeta-potential values were estimated by laser Doppler anemometry using a Malvern Zetasizer Nanoseries ZS® instrument. The measurements were carried out in triplicate.
2.3.3 HPLC evaluation of CR
A Symmetry C18 column with a length of 150 mm, a width of 4.6 mm, and a silica particle size of 5 µm was used for chromatographic separation. The mobile phase was composed of a CH3OH and H2O mixture, whereas K2HPO4 (15 mM at a ratio of 54/46 v/v) was used to adjust the pH to 3.0. The column temperature was 35 °C, and the flow rate was adjusted at 1 ml per minute. The detection wavelength was 385 nm, and the injection volume was 20 µL In accordance with ICH recommendations, many chromatographic parameters were tested to optimize and verify the HPLC method (Borman and Elder, 2017).
2.3.4 Characterization of Freeze-Dried powder of nanocurcumin
2.3.4.1 Drug content
Nanocurcumin (specific weight) was dissolved in methanol. The samples were filtered by a Millipore filter with a pore size of 0.45 µm. Then, the amount of CR in the filtrate was determined using the chromatographic method.
2.3.4.2 Saturation solubility
In pH 7.4 phosphate buffered saline, the saturation solubility of free CR and nanocurcumin containing different stabilizers was determined. An excess of sample (equivalent to 5 mg) was kept in medium in a tightly closed glass vial and shaken continuously for 48 h in a water bath (DAIHAN Scientific Co., Seoul, South Korea) at 50 rpm and 37 °C (Yadav et al., 2009). The supersaturated suspension was clarified using a membrane disc filter (0.45 µm) and analyzed using the proposed HPLC method.
2.3.4.3 Dissolution study
By using the USP XXIV type II dissolution apparatus, the dissolution test was assayed in PBS (pH 7.4 and 0.5 % ethanol). Free CR and nanocurcumin (freeze-dried) were incorporated into the 500 ml of dissolution vehicle and stirred at 50 rpm and a temperature of 37 ± 0.5 °C. 5 ml samples were taken at the following times: 0, 5, 15, 30, 60, 90, and 120 min, and substituted directly by newly prepared dissolution medium. The samples were analyzed using the chromatographic method for dissolved CR.
2.3.4.4 Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) (Jeol, JSM-5200, Tokyo, Japan) was displayed to detect the surface morphology of nanocurcumin. A droplet of the tested sample was put onto an aluminum specimen stub, dried overnight, and sputter-coated with gold prior to imaging.
2.4 Preparation of selected nanocurcumin formulation coated with chitosan
The selected nanocurcumin dispersion was added dropwise into chitosan solution (CS, 1% acetic acid solution) using a syringe at a volume ratio of 1:1 on a magnetic stirrer for 60 min. The drop rate was 2.5 ml/min. The chitosan final concentrations were 0.01%, 0.03%, 0.05%, and 0.07% (w/v) after mixing with the nanocurcumin dispersion (Table 2). Then particle size and zetapotential were measured using the same procedure as in Section 2.3.2. Coated NCR: chitosan coated nanocurcumin.
Formulation
Coated NCR-F1
Coated NCR-F2
Coated NCR-F3
Coated NCR-F4
NCR dispersing (ml)
10
10
10
10
Chitosan (%)
0.01
0.03
0.05
0.07
Final volume
20
20
20
20
2.5 Stability studies
Non-coated nanocurcumin (NCR) and chitosan-coated nanocurcumin (CS-cNCR) dispersions were kept in closed amber glass bottles and stored at two different storage conditions (4 °C and 25 °C), room temperature, and refrigerated conditions for one month. At definite time intervals, the examined formulations were withdrawn and evaluated in terms of particle size and entrapment efficiency.
2.6 Preparation of free curcumin (CR), non-coated nanocurcumin (NCR), and chitosan coated nanocurcumin (CS-cNCR) hydrogel formulations
Carbopol hydrogel was developed simply by adding carbopol934 (1%, w/v) gradually to distilled water on a magnetic stirrer at 50 °C until a homogenous plain gel is produced. Little drops of triethanolamine were added to neutralize the Carbopol, and the bubbles produced were removed by sonication for 10 min. To prepare free CR, NCR, and CS-cNCR hydrogel formulations, the calculated amount of CR in different formulations equivalent to 0.5% w/v was dissolved in minimal amounts of water before being gradually added to the performed Carbopol gel with continuous stirring overnight (24 h at 25.0–2.0 °C) until homogenous hydrogel formulations were obtained. The prepared hydrogel formulations were kept in the refrigerator for other studies.
2.7 Evaluation of the developed hydrogel formulations
2.7.1 pH and drug content determination
The clarity of hydrogel preparations was examined visually under adequate lighting for the presence of particles or turbidity. The CR content in the prepared hydrogel formulations was determined by dissolving 0.5 g in methanol, and the drug concentration was measured by HPLC. The pH of free CR, NCR, and CS-cNCR hydrogel formulations was measured using a pH meter (3500 pH meter, Jenway, UK). All the determinations were conducted in triplicate (n = 3).
2.7.2 Viscosity
The CR, NCR, and CS-cNCR hydrogel preparation viscosities were measured on a Brookfield Digital Viscometer (Model DV-II; Brookfield Engineering Laboratories, Inc., Stoughton, MA) at room temperature. Viscosity was detected using spindle no. S94 at 15 rpm.
2.8 In-Vitro drug release
The release manner of curcumin from the prepared formulations (CR, NCR, and CS-cNCR hydrogel formulations) was performed employing a diffusion dialysis approach across a semi-permeable cellophane membrane (molecular weight cutoff of 12,000–14,000, Sigma Chemicals, St. Louis, MO, USA) according to the previous reported method with slight changes (El-Mahdy et al., 2020). In brief, the examined preparation (0.5 g) was kept over a cellophane membrane (previously soaked) that was fixed at the lower end of the glass tube. The glass tube was dipped in a beaker containing 100 ml of phosphate buffer saline (PBS) (pH 7.4, 0.25 % ethanol) at 37 ± 0.5 °C and agitated at 50 rpm for 24 h using a shaking bath, (DAIHAN Scientific Co., Seoul, South Korea). Aliquots of 5 ml were removed at predetermined points of 0.5, 1, 2, 4, 6, 12, and 24 h. Sink conditions were maintained by replacing each withdrawn sample with an equal volume of fresh release medium. The drug content was estimated by the validated chromatographic method. Furthermore, the release profile of free curcumin suspension was performed at the same time as the above procedure. In vitro release studies were performed in triplicate.
2.9 Kinetic studies
Kinetic studies were performed by analyzing the in vitro release data by the linear regression method (R2) using different kinetic models (zero order, first order, Higuchi diffusion model, and Korsmeyer-Peppas) (Mircioiu et al., 2019).
2.10 Ex-vivo permeation analysis
The permeation across rat skin of CR from noncoated NCR, CS-cNCR (chitosan coated nanocurcumin) hydrogel formulations, and free CR suspension was conducted displaying abdominal skin rat as reported in previous work (Pillai and Panchagnula, 2003). This research was designed and performed according to ethical standards approved by the ethical committee at the Faculty of Pharmacy, South Valley University (Approval # P.S.V.U215). Animals were sacrificed, and then the dorsal side hairs of skin from the abdominal section were removed.
The skin was dipped into phosphate buffered saline (PBS) at pH 7.4 for 4 h to stabilize the membrane before beginning the experiment. The donor (sample fitted at the fixed skin at the end of the glass tube) and the receptor (beaker) were placed at 37 ± 0.5 °C in a thermostatic shaker water bath that rotated at 50 rpm. Examined samples (0.5 g of hydrogels, 0.5 ml of drug suspension) were put on the skin. At definite time points (up to 24 h), aliquots of 5.0 ml were removed and replaced by an equal amount of fresh buffer medium, and then the drug content was determined using the validated HPLC method. The analysis was done in triplicate, and the average value was calculated. The cumulative amount (µg) across the skin was plotted versus time. The apparent permeability coefficient (Papp, cm/s) of curcumin through skin was estimated using Fick’s first law of diffusion based on the following equation (2):
(2)where J is the flux across the skin membrane and C0 is the initial concentration of curcumin (µg /ml) in the donor compartment.
2.11 Skin irritation study
A skin irritation test was carried out to prove that the hydrogel formulations of nanocurcumin and the ingredients used for nanocurcumin fabrication were safe. Approval of the Ethical Committee“ of the Faculty of Pharmacy, South Valley University, Egypt, has been achieved prior to the study's conduct. The skin irritancy test was conducted to assess any visual reaction of the nanocurcumin hydrogel formulations on the skin of male rats (180–200 g) regarding the reported method mentioned by Sara M. Soliman et al. (Soliman et al., 2010). Animals were divided into three groups: the first group was a control (no treatment), the second group received free NCR hydrogel, and the third group received the selected CS-cNCR. A dose of 1 g of hydrogel preparations was applied daily to a specific area of the shaved dorsal side of the rats for three days (Kaur et al., 2007). The skin of rats was observed for the development of erythema and edema periodically for 3 days.
2.12 Statistical analysis
All experiments were conducted three times, and the findings were expressed as mean and SD. GraphPad Prism version 8.4.3 (GraphPad, San Diego, CA) was utilized to evaluate the obtained data. A one-way analysis of variance (ANOVA) with a Tukey test was used. *p < 0.05 indicated significantly different results.
3 Results and discussion
3.1 Chromatographic method
3.1.1 Development of HPLC method
CR is composed of three polyphenolic compounds named curcumin, demethoxycurcumin, and bisdemethoxycurcumin. For the development of the HPLC method, several attempts were made to select optimum chromatographic conditions. Methanol was selected as an organic modifier because it gave better resolution and the best baseline separation for the peaks. The experiment demonstrated that the high percentage of methanol as a mobile phase resulted in the elution of curcuminoids as a single peak. However, decreasing the ratio of methanol to 55 % induced good separation and better resolution of the curcuminoids. CR showed a high degree of stability at the acidic pH, so pH 3.0 was selected. After many trials, the best resolution for the peaks was obtained when the temperature of the column was 35 °C.
After optimization of the chromatographic conditions, the curcuminoids were separated with retention times of 4.82, 5.07, and 5.54 min for demethoxycurcumin, bisdemethoxycurcumin, and curcumin, respectively (Fig. 1).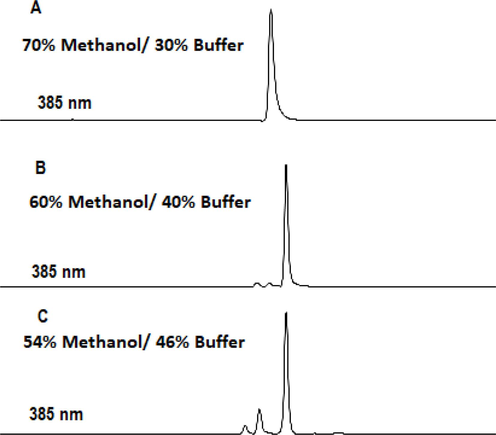
The optimization of chromatographic conditions. (A) Curcuminoids (5 ug/mL) were eluted with 70% methanol/30% buffer (B) Three curcuminoids well separated by 54% methanol /46% buffer at pH 3.0 (C) HPLC detection of curcuminoids in CR-NCs at 385 nm.
3.1.2 Validation of HPLC method
In accordance with the recommendations of the International Conference on Harmonization (ICH), the chromatographic technique was verified. The considered validation parameters were accuracy, precision, LOD, and LOQ. The HPLC method showed good linearity over CR concentrations ranging from 3.0 to 75 ug/mL with an r value nearly equal to 1. The LOD and LOQ values (0.931 and 2.82 ug/mL respectively) indicated good linearity of the method (Table 3). Table 4 shows the results of the accuracy test of the HPLC method; the RSD % is lower than 2 and the overall recovery (%) is within 101.1 ± 0.38. Three operational standard solutions were created in order to assess the intra-day accuracy, covering the complete defined calibration curve in concentrations of 5, 30, and 60 μgmL-1 and then three injections of each solution into the chromatographic apparatus. The intra-day and inter-day RSD values were found to be well under the 2% limit, demonstrating the accuracy of the existing approach (Table 5). The 5 g/mL peaks had tailing and asymmetry factors of 1.25 and 0.63, respectively. The HETP was 0.00538 cm, and the theoretical plate number was found to be higher than 2x103. These numbers show that the system is suitable for the suggested test and is within acceptable bounds (Table 6). The chromatograms' well-shaped peaks provided evidence of the method's adequate specificity. N.B. AF = Asymmetry factor, TF = Tailing factor, NTP = Number of theoretical plates, HETP = height equivalent to a theoretical plate.
Parameter
Value
ʎmax
385 nm
RT
5.54 min
Linearity range (µgmL−1)
3.0–75
LOD (µgmL−1)
0.931
LOQ (µgmL−1)
2.82
Regression equation
Y = a + bx
Slope
2218.8
Intercept
292.08
(R2)
0.999
Standard solution (ug/mL) (n = 3)
Found
% Recovery
Mean ± SD
RSD (%)
5
4.969
99.38
4.96 ± 0.047
0.94
4.918
98.36
5.012
100.24
30
30.006
100.02
30.03 ± 0.25
0.85
30.306
101.02
29.796
99.32
60
61.218
102.03
60.21 ± 0.87
1.44
59.616
99.36
59.821
99.71
Standard solution (ug/mL)
Intra-day precision
Mean ± RSD
Inter-day precision
Mean ± RSD
Found
% Recovery
Found
% Recovery
5
4.9595
99.19
4.97 ± 0.30
4.9645
99.29
4.98 ± 0.57
4.9675
99.35
5.0205
100.41
4.989
99.78
4.9835
99.67
30
29.487
98.29
29.77 ± 0.82
29.916
99.72
29.81 ± 0.71
29.895
99.65
29.577
98.59
29.931
99.77
29.964
99.88
60
60.186
100.31
60.37 ± 0.41
59.382
98.97
59.93 ± 1.41
60.27
100.45
59.508
99.18
60.66
101.1
60.906
101.51
Parameters
CR
Acceptable limits
AF
0.636
<1.5
TF
1.25
<2
NTP
2783
<2000
HETP (cm)
0.00538
3.2 Characterization of freeze dried non-coated nanocurcumin
3.2.1 Drug content
The drug content of the fabricated nanodrug particles was measured by the HPLC-developed method. The results shown in Table 7 revealed that total drug content was in the range of 90.94 to 99.6%, which indicates that drug loss was lower during the preparation process. According to the results, the formulation, NCR1, was selected for further analysis as it showed the highest total drug content (containing PVPK30-Poloxamer188) at 99.67%. Coated NCR: chitosan coated nanocurcumin. *p < 0.05 Significant difference of NCR1 compared with other formulations.
Formulation
Particle size (nm)
PDI
Zeta potential (mV)
Drug content %
NCR1
272.1 ± 15.32*
0.315* ± 0.053
−25.5 ± 0.149
99.67
NCR2
282.1 ± 12.27
0.514 ± 0.023
–
99
NCR3
360.1 ± 18.27
0.562 ± 0.09
–
98.3
NCR4
482.7 ± 20.17
0.532 ± 0.07
–
90.94
Coated NCR-F1
288.0 ± 20.32
0.414 ± 0.034
–
99.4
Coated NCR-F2
294.66 ± 18.34
0.234 ± 0.023
–
98.9
Coated NCR-F3
312.1 ± 15.32
0.489 ± 0.098
+30.5 ± 0.35
99.61
Coated NCR-F4
527.1 ± 17.22
0.634 ± 0.063
–
99.2
3.2.2 Saturation solubility of lyophilized CR-NC
Fig. 1 shows the aqueous solubility of free CR and nanocurcumin formulations. Curcumin is a hydrophobic biologically active flavonoid with a very poor aqueous solubility of about (2 μg/ mL) (Homayouni et al., 2019). Also, the solubility of CR in water has been reported between 11 ng/mL and 10 μg/mL, which matches our observations as shown in Fig. 2. The solubility of curcumin after formulation in nano-curcumin forms was observed to be more than 150-fold higher as compared with free curcumin. The enhanced solubility of curcumin is attributed to the nanosized form in which the drug exists. In addition, the enhanced solubility of CR might be largely attributed to the reduction of its particle size in the nanoformulations, which have been decreased further by freeze drying. The current results of CR solubility are consistent with the known theory that decreased particle size increases the surface and consequently improves solubility (Allotey-Babington et al., 2018). The nanocurcumin formulation containing PVPK30-Poloxamer 188 showed a marked increase (p < 0.05) in the solubility of CR as compared with the formulations containing other stabilizers. Therefore, PVPK30-Poloxamer is considered as an ideal stabilizer combination that could spread the CR particles at the interface with water to prevent re-aggregation in aqueous solutions.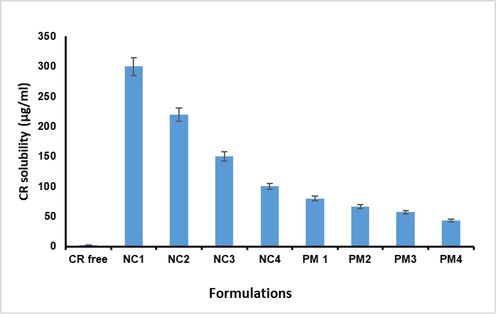
Saturation solubility of CR, physical mixtures, and nanocrystals with different stabilizers in water. Each value expressed as the mean ± SD (n = 3).
3.3 In-vitro dissolution study
Fig. 2 demonstrates the dissolution profiles of the curcumin in the different formulations. As shown, the dissolution behavior of CR and its nanoform particles was presented as a percentage of drug dissolved over 120 min, with the percentage of drug dissolved after 30 min serving as a comparison parameter between the pure drug and nanocurcumin. The dissolution profiles of lyophilized nanocurcumin containing different stabilizers combined with PVP are illustrated in Fig. 3. The dissolution rate of CR was markedly enhanced in the nanocurcumin suspension, a significant amount p < 0.05, as more than 80% of the drug dissolved in 30 min from all the formulations when compared to less than 20% of pure CR. This might be because of the increased surface area of the drug and thus better wettability, as well as the dispersion of nanocurcumin in the dissolution medium.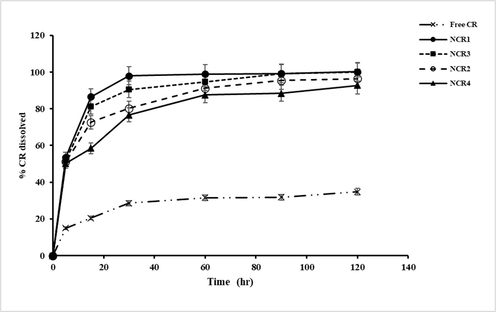
Dissolution profiles of free CR, and CR nanocrystals with different stabilizers in phosphate buffer Saline (pH 7.4 and 0.5 % ethanol) at 37 ± 0.5 °C. Each value expressed as the mean ± SD (n = 3).
The findings demonstrated in Fig. 2 indicated that the NCR4 formulation containing Brij35 exhibited a significant p < 0.05 lower percentage of dissolved curcumin. It was reported that saturation solubility increases with decreasing particle size. The nanocurcumin stabilized by PVP and curcumin with a particle size of 272.1 ± 15.32 nm showed the highest drug dissolution rate at 97% of drug dissolved in 30 min, whereas, Brij35 containing nanocrystals with a particle size of 482.7 ± 20.17 nm showed about 70.9% of drug dissolved within 30 min of the dissolution test. The increased dissolution rate might be attributed to the highest solubilizing and wetting effects of Poloxamers. Furthermore, it was found that nanocurcumin containing HPβCD showed a higher percent of drug dissolved (90%) as compared with Tween80 and Brij35. It may be noticed that the critical parameters determining drug dissolution are particle size as well as the mechanism by which the stabilizer enhances drug dissolution (Sundar et al., 2019). The obtained results are consistent with the Noyes-Whitney equation, which indicates that the increase in saturation solubility and the decrease in particle size result in an enhancement of drug dissolution rate (Liu et al., 2018). Since nanocrystals prepared by using PVP-Poloxamer as a stabilizer combination exhibited promising results regarding particle size, saturation solubility, and dissolution rate, they were selected for further studies.
3.4 Scanning electron microscopy (SEM) analysis
Fig. 4 illustrates the SEM image. It reveals that the prepared NCR1 selected formulation (containing PVP-Poloxamer combined stabilizer) had a spherical shape without any aggregations and confirmed the uniform distribution of nanodrug particles. Also, the preparation was observed as smooth and had a better surface character.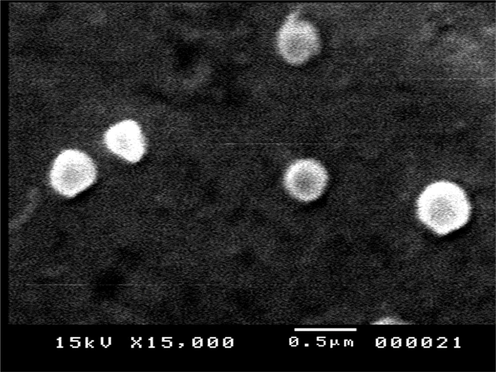
SEM image of nanocurcumin (NCR1).
3.5 Characterization of noncoated nanocurcumin and chitosan coated nanocurcumin dispersions; determination of particle size and zetapotential
Fabrication of nanocrystals involves the generation of many small particles with different surface areas, leading to marked increases in the Gibbs free energy of the system. Particle aggregation is affected by the activation energy, which is adjusted by the incorporation of surfactant stabilizers into the system. The incorporation of these stabilizers leads to a reduction in the interfacial tension between the particles and the dispersion medium and increases the wettability of the drug particles. Furthermore, the addition of nonionic surfactants can provide steric stabilization and prevent particle aggregation. It has been reported that the selection of an adequate stabilizer is a crucial factor that controls particle growth during the development of uniform nanocrystals. Numerous studies have demonstrated that the selection of efficient and appropriate stabilizers leads to the maintenance of the stability of nanocrystal systems for a longer time (Gao et al., 2011; Sundar et al., 2019).
Based on the above consideration, different polymers were utilized in this study, such as Poloxamer-188, Tween 80, HPβ-CD, and Brij35 used in combination with PVPK30 (1:1). The selected stabilizers were examined in terms of their stabilization actions and particle sizes in the developed formulations. It was found that nanocurcumin prepared using a drug to stabilizer weight ratio of 2:1 produced a large particle size and rapid precipitation was observed. Increasing the amount of stabilizer content in the stabilizer-drug combination to 1: 2 (drug: stabilizer) resulted in a reduction in particle size without precipitation of nanodrug particles.
Table 8 demonstrates settlement volume ratios for different nanocurcumin formulations. It is clear that the fabricated NCR using PVPK30-Poloxamer 188 combined stabilizers (NCR1) showed significantly higher settlement volume ratio values (F) (p < 0.05) during time intervals up to one month compared with those of other nanocurcumin formulations. That NCR1 exhibited an F value of 0.97 ± 0.10 after one month (Table 8). The stability of this formulation, NCR1, might be attributed to the presence of the pyrrolidinone group in PVP, which is important for nanodrug particle stabilization. The pyrrolidinone group is considered an acceptable hydrogen acceptor, especially for the —OH and —NH groups of nanoformulations, and thus, stabilizes the formulation (Kumar et al., 2022). In addition, due to balancing the hydrophobic and hydrophilic properties in poloxamer and PVP combinations as obtained in various studies (Emam and Mohamed, 2021; Rask et al., 2016; Sun et al., 2010b). Therefore, the PVPK30-Poloxamer combination could be considered a promising combination to produce table nanocurcumin without any precipitation. *p < 0.05 Significant difference of NCR1 when comparing with other formulations.
Time intervals
NCR1
NCR2
NCR3
NCR4
1 Week
0.99 ± 0.093*
0.90 ± 0.023
0.980 ± 0.012
0.966 ± 0.032
2 Weeks
0.98 ± 0.05*
0.87 ± 0.01
0.970 ± 0.03
0.940 ± 0.03
3 Weeks
0.972 ± 0.10*
0.76 ± 0.09
0.85 ± 0.05
0.8 ± 0.12
4 Weeks
0.97 ± 0.10*
0.70 ± 0.05
0.80 ± 0.15
0.75 ± 0.15
The results of the preliminary study revealed that the size of the formulated nanocurcumin without stabilizer was found 620 ± 35.14 nm with a PDI of 0.34 ± 0.3, whereas the same formulation in which the size was measured after 24 h was found to be 2000 ± 43.6 nm with a PDI of 0.64 ± 0.1. This agrees with an earlier study that showed a time-dependent increase in the size of particles due to the aggregation of formed nanodrug particles (Bose et al., 2021).
Table 7 shows the evaluation parameters of the fabricated nanodrug formulations. It was noticed that NCR that was stabilized using Poloxamer188-PVPK30 exhibited a significantly (p < 0.05) smaller particle size (272.1 ± 15.32) as compared with that of other stabilizers. Poloxamer (a nonionic surfactant) boosts the steric stabilization of the developed dispersions at their interfaces. The external hydrophilic polyethylene oxide blocks solvate and extend into the aqueous medium, generating a steric barrier, while the triblock copolymer chains are linked to the nanoparticle surface by hydrophobic interactions with PPO blocks. Also, it was observed that this formulation tends to be stable for longer without any precipitation (Sundar et al., 2019).
According to previous experimental observation of settlement volume ratios (F) and particle size, (NCR1) non-coated nanocurcumin was selected for further characterization and for coating with chitosan. We suggested that the short-term stability of nanocurcumin dispersion might be improved by using chitosan as a coating agent, that the calculated settlement volume ratio (F) significantly reduced for the selected nanocurcumin formulation after more than one month.
The results revealed that nanocurcumin formulations coated with chitosan exhibited a significantly higher (F) value of 0.92 ± 0.050 after two months (p < 0.05) as compared with noncoated nanocurcumin (0.77 ± 0.10) at the same time. In addition, it was observed that the optimum concentration of chitosan to develop highly stable coated nanocurcumin was 0.05% w/v and no precipitation or sedimentation was detected.
Furthermore, as shown in Table 7, the mean nanocrystal size was definitely increased by the chitosan coating. By electrostatic contact between the positively charged amine (NH3+) groups of chitosan and the negatively charged polar head groups of poloxamer, it effectively proved the successful coating of chitosan Further, the results proved that the mean size of the nanocrystals increased with increasing chitosan concentration (Table7). As chitosan concentration increased from 0.01% to 0.07% (w/v), the particle size of the nanosystem increased from 288.0 ± 20.32 to 527.1 ± 17.22 nm.
The zeta potential of the selected formulation (NCR1) was found to be −25.5 ± 0.149 mV. Poloxamer, a non-ionic surfactant, is used as a stabilizer, which provides steric stabilization. Therefore, the presence of a negative zeta potential might be attributed to the hydroxyl group in nanocurcumin. Generally, a zeta potential value of ± 20 mV is appropriate for providing stable nanosystems. This result indicates that the prepared formulation might be free from instability problems. Noncoated nanocurcumin showed a negative surface charge and became positive with the coating of chitosan. The zeta potential value of uncoated nanocurcumin is significantly smaller than that of nanocurcumin coated with chitosan, this may be due to the layer effect of the nonionic surfactant poloxamer. The increase in the surface charge of chitosan-coated nanocurcumin is attributed to the increase in amino groups positively charged in chitosan molecules, indicating that the developed nanocrystals were successfully coated.
3.6 Stability studies
Stability studies were performed on the selected formulation (NCR1) and chitosan coated NC (CS-cNCR, F3). The results of the stability studies are presented in Table 9. When nanocurcumin was kept at room temperature, the particle size significantly increased. (p < 0.005) form 272.1 ± 15.32 to 510.9 ± 12.12 nm in 30 days. Although there was a nonsignificant increase from 272.1 ± 15.32 to 289.7 ± 20.25 under refrigerated storage settings, this indicates higher stability in these conditions. The findings demonstrated that temperature has an impact on the aggregation of nanoparticles and that for liquid nanosuspension, aggregation was stronger at ambient temperature than in a refrigerator. The increase in particle size at room temperature is believed to be due to the aggregation of the particles (Han et al., 2021). When compared with chitosan-coated nanocurcumin, aggregation was less for both conditions (room temperature and refrigerator). Refrigerated conditions have no significant impact on particle size, whereas room temperature conditions have a more unfavorable effect. Accordingly, chitosan coating results in increased nanocurcumin dispersion stability. The chemical stability of the formulation was monitored under different storage conditions, and the results are shown in Table 9. As noticed, the change in the drug content of the examined formulation is non-significant at room temperature or refrigerated conditions. Thus, both noncoated and chitosan-coated nanocurcumin are chemically stable at both storage conditions. However, chitosan coating and storage in the refrigerator are advocated for better physical stability. NCR1: nanocurcumin prepared using PVPK30-Poloxamer stabilizer combination (1:2, drug:stailizer combination). Coated NCR-F3 (CS-cNCR1): Chitosan coated nanocurcumin at concentration of 0.05%.
Storage Temperature
Zero time
1 month
4 °C
At room temperature 25 °C
Formulation
NCR1
Coated NCR-F3
NCR1
Coated NCR-F3
NCR1
Coated NCR-F3
Size (nm)
272.1 ± 15.32
312.1 ± 15.32
289.7 ± 20.25
345.8 ± 15.0
510.9*±12. 12
350.65 ± 10.5
PDI
0.315 ± 0.06
0.489 ± 0.098
0.504 ± 0.03
0.512 ± 0.08
0.620 ± 0.02
0.521 ± 0.01
Drug content
91.5 ± 5.34
96.66 ± 2.5
89.80 ± 1.0
95.88 ± 2.3
85.43 ± 1.3
93.80 ± 1.3
3.7 Evaluation of the developed hydrogel formulations
Curcumin hydrogel in different formulations was elegant, clear, while the hydrogel of free CR was found to be opaque. No lumps or air bubbles were observed. The content of CR in the different prepared hydrogel formulations ranged from 97.5 ± 1.5 to 99.0 ± 1.8%. Hence, these matches with theoretical limits (100 ± 5) indicate high homogeneity and uniformity of the fabricated hydrogel formulation. Furthermore, pH determination results showed that all the developed hydrogel formulations exhibited pH values ranging from 6.9 to 7.2 that were acceptable for skin application, which confirmed the compatibility of these formulations. (Kumar et al., 2022). The higher pH values might be related to the addition of triethanolamine during hydrogel preparation. Viscosity is a crucial parameter for hydrogel characterization that influences the release of drugs. Table 10 shows the viscosity measurements of the prepared hydrogel formulation; they showed an acceptable range for topical administration. It is clear that the viscosity value was significantly increased by using nanocurcumin and chitosan-coated nanocurcumin, this might be attributed to the incorporation of polymer stabilizer (PVP-Poloxamer) and chitosan in nanocurcumin and coated nanocurcumin, respectively. These findings were consistent with previous reports (Salem et al., 2020). The skin irritation study exhibited that the prepared hydrogel formulations did not demonstrate any sign of edema or erythema (null irritation). This result indicated the compatibility of the developed formulations with the skin.
Formulation
Drug content (%)
pH
Viscosity (mPas)
Skin irritation
Appearance
Free CR hydrogel
97.7 ± 0.12
6.9 ± 0.02
3150 ± 75
Null
Opaque yellow
NCR hydrogel
98.1 ± 0.15
7.3 ± 0.05
4950 ± 80
Null
Clear yellow
CS-cNCR
hydrogel
99 ± 0.19
7.1 ± 0.06
4150 ± 55
Null
Clear yellow
3.8 In-vitro release study
The release profiles of CR from the developed hydrogel formulations containing free CR, nanocurcumin, and chitosan-coated nanocurcumin are depicted in Fig. 5 and compared with the free CR solution. It is clear that free CR showed a significantly (p < 0.05) slower release pattern in comparison with other hydrogel formulations, with only 14.50 ± 0.05 % release in 24 h, which is related to the poor aqueous solubility of CR as reported in different studies. The hydrogel formulation of nanocurcumin exhibited an accelerated release rate with a higher cumulative amount of CR release (95.43 ± 0.2% in 24 h); this result may be due to the smaller particle size and high surface area of the nanocurcumin hydrogel when compared with pure curcumin. Similar observations were obtained in the previous investigation (Kumar et al., 2022). In the case of hydrogel containing chitosan-coated nanocurcumin, the result revealed that CR was released in a significant (p < 0.05) slow pattern when compared with noncoated nanocurcumin (83.4 ± 0.2% in 24 h). This could be attributed to repulsion forces between negatively charged nanocurcumin and Carbopol gel matrix, while a possible attraction force between Carbopol and cationic chitosan in the hydrogel of coated nanocurcumin, hence decreasing the release rate of CR (Salem et al., 2020).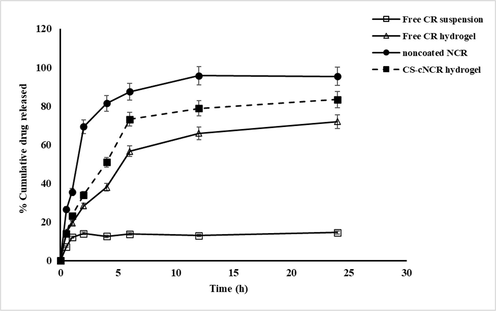
in-vitro release patterns of CR from different formulations in PBS (pH 7.4 at 37 °C) n = 3, error bars prove ± S.D.
The kinetic analysis of release data demonstrated that the drug release from the hydrogel of pure curcumin and nanocurcumin was best fitted to first-order kinetics according to the highest R2 (Table 11), which confirmed that the release of the drug almost depended on its concentration. While the hydrogel formulation of chitosan coated nanocurcumin was compatible with the Higuchi diffusion model as shown in Table 11, proving a diffusion determinant mechanism. Further, the Korsmeyer–Peppas model was utilized to analyze the release result of CR and identify the mechanisms of drug release. Based on the Korsmeyer-Peppas equation, the n values for Fickian (diffusional) and zero-order release kinetics are between 0.5 and 1, whereas 0.5 < n < 1 for non-Fickian (anomalous) release. In the current work, the n values for CR hydrogel formulations ranged from 0.525 to 0.695, indicating a non-Fickian drug diffusion and drug release manner in which drug release is governed by gel diffusion and erosion. These observations were consistent with previous investigations (Kumar et al., 2022).
Formulation
Correlation coefficient (R2)
(n)
Korsmeyer–Peppas equation
Zero-order model
First- order model
Higuchi diffusion model
Korsmeyer–Peppas model
Pure CR hydrogel
0.869 ± 0.011
0.917 ± 0.014
0.951 ± 0.015
0.990 ± 0.011
0.525 ± 0.014
Nanocurcumin hydrogel
0.701 ± 0.021
0.910 ± 0.012
0.839 ± 0.014
0.975 ± 0.012
0.695 ± 0.019
Chitosan coated hydrogel
0.814 ± 0.024
0.886 ± 0.027
0.928 ± 0.021
0.998 ± 0.011
0.612 ± 0.012
3.9 Ex-vivo permeation analysis
CR permeated across the skin from free CR, nanocurcumin, and chitosan coated nanocurcumin Carbopol hydrogels is depicted in Fig. 6, and permeability parameters are listed in Table 12. ER: Enhancement ratio.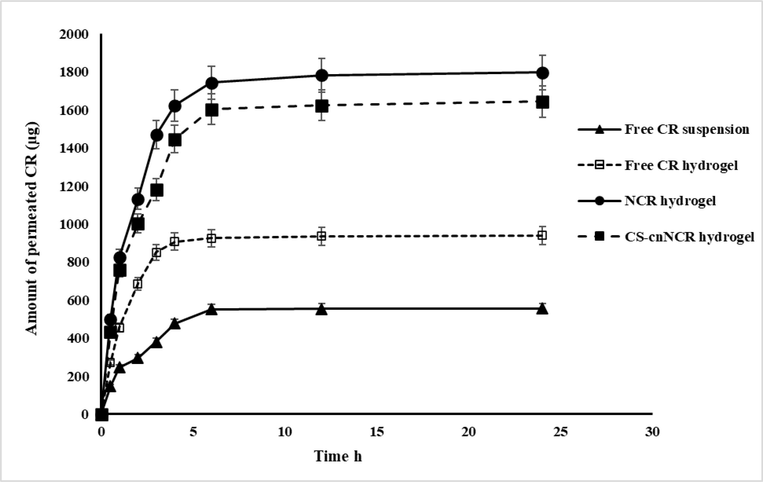
Skin permeation of cr from different formulations in pbs (pH 7.4 at 37 °C) n = 3, error bars prove ± S.D.
Sample
Papp(cm/sec)x10-6
Amount permeated (μg)
ER
Free CR suspension
2.783 ± 1.92
555.9 ± 41.92
–
NCR hydrogel
8.688 ± 1.20**
1797.53 ± 28.02*
2
Coated NCR hydrogel
7.794 ± 1.50**
1644.79 ± 31.90*
1.75
Free CR hydrogel
4.501 ± 1.30
940.018 ± 36.54
–
The developed nanocurcumin and chitosan coated nanocurcumin hydrogel demonstrated a significantly (p < 0.05) higher skin permeation compared with free CR suspension and hydrogel containing an equivalent amount of CR. As shown in Fig. 6, it is noticed that only 940 μg of CR of pure hydrogel was permeated via rat skin over 24 h; meanwhile, the cumulative amount of CR permeated from nanocurcumin and chitosan coated nanocurcumin hydrogels was 1797.53 and 1644.79 μg, respectively. The permeability coefficient values were 1.7 and 2.0 times higher for chitosan-coated nanocurcumin and nanocurcumin hydrogel compared with the pure drug hydrogel. The higher permeability of CR from NCR and CS-cNCR might be explained according to the small particle size, high surface area, and enhanced solubility of CR from both coated and noncoated nanocurcumin hydrogel formulations. Further, it is reported that Carbopol934 might demonstrate permeation enhancer properties and prolong the retention time at the site of application. (Majithiya et al., 2006). Swelling of Carbopol 943 because of the water absorption from the epithelial tissue results in extensive penetration into the skin and hence localizes the formulation in the skin, providing a higher drug concentration gradient across the epithelium (Singh et al., 2013). So, in the current study, Carbopol was selected as a gelling agent to provide a highly permeable hydrogel system. Further, the non-significantly (p > 0.05) higher permeability of non-coated nanocurcumin hydrogel (NCR1 hydrogel) compared to CS-coated nanocurcumin might be due to its smaller size and the swelling and prolonged residence time effects of Carbopol.
4 Conclusion
In this current work, we prepared nanocrystals based on the stabilizer combination PVP-Poloxamer188 and then coated them with chitosan to accommodate curcumin at a high theoretical yield. Aqueous solubility and the rate of dissolution of CR in nanocurcumin formulations were significantly increased as compared with pure curcumin. Chitosan was utilized for coating the developed nanodrug particles to evaluate its effects on both, physical and chemical stability of CR. After chitosan coating, the zeta potential changed from negative to positive, proving that nanocurcumin particles were coated by chitosan. The stability studies revealed that the chitosan-coated NCR formulation was stable in terms of settlement volume ratio, particle size, and aggregation at different two temperatures when compared with the noncoated nanocurcumin. In addition, the permeability of curcumin through the rat skin was significantly enhanced by nanocurcumin formulations as compared with free curcumin, which was attributed to the small particle size. The hydrogel formulations of nanocurcumin were homogenous, and no sign of irritation was observed. Consequently, the use of a drug-stable nanosystem (curcumin) results in an improvement of the skin permeability of free curcumin, and the utilization of chitosan as a coating polymer for nanocurcumin is considered a promising stabilizer coating polymer for enhancement of nanosystem colloidal stability. This study offers an initial point of view to investigate different coating polymers and examine their enhanced effects on nanocurcumin stability and skin permeability in topical and transdermal preparations.
Acknowledgment
The authors extend their appreciation to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R89), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia for funding this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Preparation, optimization, and in vitro simulated inhalation delivery of carvedilol nanoparticles loaded on a coarse carrier intended for pulmonary administration. Int. J. Nanomed.. 2015;10:6339.
- [Google Scholar]
- Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity. Nanotechnol. Rev.. 2021;10:1493-1559.
- [Google Scholar]
- Glutathione-loaded non-ionic surfactant niosomes: a new approach to improve oral bioavailability and hepatoprotective efficacy of glutathione. Nanotechnol. Rev.. 2022;11:117-137.
- [Google Scholar]
- Synthesis and preparation of biodegradable hybrid dextran hydrogel incorporated with biodegradable curcumin nanomicelles for full thickness wound healing. Int. J. Pharm.. 2017;532:466-477.
- [Google Scholar]
- Layer-by-Layer nanoparticles of tamoxifen and resveratrol for dual drug delivery system and potential triple-negative breast cancer treatment. Pharmaceutics. 2021;13:1098.
- [Google Scholar]
- Cancer chemotherapy: effect of poloxamer modified nanoparticles on cellular function. J. Drug Deliv. Sci. Technol.. 2018;47:181-192.
- [Google Scholar]
- Agglomeration and sedimentation of TiO2 nanoparticles in cell culture medium. Colloids Surf. B Biointerfaces. 2009;68:83-87.
- [Google Scholar]
- Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J. Agric. Food Chem.. 2011;59:2056-2061.
- [Google Scholar]
- Comparative in vitro evaluation of glimepiride containing nanosuspension drug delivery system developed by different techniques. J. Mol. Struct.. 2021;1231:129927
- [Google Scholar]
- Formulation and evaluation of nanosuspension of albendazole for dissolution enhancement. Nanosci. Nanotechnol. Lett.. 2013;5:1024-1029.
- [Google Scholar]
- Performance of curcumin in nanosized carriers niosomes and ethosomes as potential anti-inflammatory delivery system for topical application. Bull. Pharm. Sci. Assiut. 2020;43:105-122.
- [Google Scholar]
- Novel curcumin-loaded gel-core hyaluosomes with promising burn-wound healing potential: development, in-vitro appraisal and in-vivo studies. Int. J. Pharm.. 2015;486:88-98.
- [Google Scholar]
- Controllable release of povidone-iodine from networked Pectin@ Carboxymethyl pullulan hydrogel. Polymers (Basel).. 2021;13:3118.
- [Google Scholar]
- Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J. Nanoparticle Res.. 2008;10:845-862.
- [Google Scholar]
- Preparation of a chemically stable quercetin formulation using nanosuspension technology. Int. J. Pharm.. 2011;404:231-237.
- [Google Scholar]
- Chitosan-based mucoadhesive gel for oral mucosal toluidine blue O delivery: the influence of a non-ionic surfactant. Photodiagn. Photodyn. Ther.. 2017;20:48-54.
- [Google Scholar]
- Nanocrystals of poorly soluble drugs: drug bioavailability and physicochemical stability. Pharmaceutics. 2018;10:134.
- [Google Scholar]
- Annonaceous acetogenins nanosuspensions stabilized by poloxamer 188: Preparation, properties and in vivo evaluation. J. Drug Deliv. Sci. Technol.. 2021;66:102676
- [Google Scholar]
- Effect of high pressure homogenization on physicochemical properties of curcumin nanoparticles prepared by antisolvent crystallization using HPMC or PVP. Mater. Sci. Eng. C. 2019;98:185-196.
- [Google Scholar]
- Chitosan-based nanocarriers for encapsulation and delivery of curcumin: A review. Int. J. Biol. Macromol.. 2021;179:125-135.
- [Google Scholar]
- Ciprofloxacin-Loaded silver nanoparticles as potent nano-antibiotics against resistant pathogenic bacteria. Nanomaterials. 2022;12:2808.
- [Google Scholar]
- Silymarin nanocrystals-laden chondroitin sulphate-based thermoreversible hydrogels; A promising approach for bioavailability enhancement. Int. J. Biol. Macromol.. 2022;218:456-472.
- [Google Scholar]
- Niosomal gel for site-specific sustained delivery of anti-arthritic drug: in vitro-in vivo evaluation. Curr. Drug Deliv.. 2007;4:276-282.
- [Google Scholar]
- Development and evaluation of nanocrystals loaded hydrogel for topical application. J. Drug Deliv. Sci. Technol.. 2022;74:103503
- [Google Scholar]
- Budesonide nanocrystal-loaded hyaluronic acid microparticles for inhalation: In vitro and in vivo evaluation. Carbohydr. Polym.. 2018;181:1143-1152.
- [Google Scholar]
- Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev.. 2008;60:1650-1662.
- [Google Scholar]
- Next-Generation hydrogels as biomaterials for biomedical applications: exploring the role of curcumin. ACS Omega. 2023;8:8960-8976.
- [Google Scholar]
- Thermoreversible-mucoadhesive gel for nasal delivery of sumatriptan. AAPS PharmSciTech. 2006;7:E80-E86.
- [Google Scholar]
- Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci.. 2003;18:113-120.
- [Google Scholar]
- Drug nanoparticles: formulating poorly water-soluble compounds. Toxicol. Pathol.. 2008;36:43-48.
- [Google Scholar]
- Mathematical modeling of release kinetics from supramolecular drug delivery systems. Pharmaceutics. 2019;11:140.
- [Google Scholar]
- In vitro and in vivo synergistic wound healing and anti-methicillin-resistant Staphylococcus aureus (MRSA) evaluation of liquorice-decorated silver nanoparticles. J. Antibiot. (Tokyo) 2023:1-10.
- [Google Scholar]
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development. Nanotechnol. Rev.. 2023;12:20220517.
- [Google Scholar]
- Nanocurcumin and arginine entrapped injectable chitosan hydrogel for restoration of hypoxia induced endothelial dysfunction. Int. J. Biol. Macromol.. 2021;166:471-482.
- [Google Scholar]
- An evaluation of curcumin-encapsulated chitosan nanoparticles for transdermal delivery. AAPS PharmSciTech. 2019;20:1-13.
- [Google Scholar]
- Transdermal delivery of insulin from poloxamer gel: ex vivo and in vivo skin permeation studies in rat using iontophoresis and chemical enhancers. J. Control. Release. 2003;89:127-140.
- [Google Scholar]
- Use of 18β-glycyrrhetinic acid nanocrystals to enhance anti-inflammatory activity by improving topical delivery. Colloids Surfaces B Biointerfaces. 2021;205:111791
- [Google Scholar]
- Influence of PVP/VA copolymer composition on drug–polymer solubility. Eur. J. Pharm. Sci.. 2016;85:10-17.
- [Google Scholar]
- Novel enhanced therapeutic efficacy of dapoxetine HCl by nano-vesicle transdermal gel for treatment of carrageenan-induced rat paw edema. AAPS PharmSciTech. 2020;21:1-13.
- [Google Scholar]
- Nanocrystals: industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm.. 2010;399:129-139.
- [Google Scholar]
- Orally administered nanocurcumin to attenuate morphine tolerance: comparison between negatively charged PLGA and partially and fully PEGylated nanoparticles. Mol. Pharm.. 2013;10:4546-4551.
- [Google Scholar]
- Formulation of microemulsion gel systems for transdermal delivery of celecoxib: In vitro permeation, anti-inflammatory activity and skin irritation tests. Drug Discov. Ther.. 2010;4:459-471.
- [Google Scholar]
- Development of nanosuspension formulation for oral delivery of quercetin. J. Biomed. Nanotechnol.. 2010;6:325-332.
- [Google Scholar]
- Solubilities of crystalline drugs in polymers: an improved analytical method and comparison of solubilities of indomethacin and nifedipine in PVP, PVP/VA, and PVAc. J. Pharm. Sci.. 2010;99:4023-4031.
- [Google Scholar]
- Design, formulation and evaluation of nanosuspension for drug delivery of celecoxib. Int. J. Pharm. Res.. 2019;11
- [Google Scholar]
- The stabilization and release performances of curcumin-loaded liposomes coated by high and low molecular weight chitosan. Food Hydrocoll.. 2020;99:105355
- [Google Scholar]
- Effect of cyclodextrin complexation of curcumin on its solubility and antiangiogenic and anti-inflammatory activity in rat colitis model. AAPS PharmSciTech. 2009;10:752-762.
- [Google Scholar]
- Sprayable nanodrug-loaded hydrogels with enzyme-catalyzed semi-inter penetrating polymer network (Semi-IPN) for solar dermatitis. Nano Res. 2022:1-12.
- [Google Scholar]
- Polymeric curcumin nanoparticle pharmacokinetics and metabolism in bile duct cannulated rats. Mol. Pharm.. 2013;10:1977-1987.
- [Google Scholar]







