Translate this page into:
Preparation and properties evaluation of novel silica gel-based fracturing fluid with temperature tolerance and salt resistance for geoenergy development
⁎Corresponding authors. zhoufj@cup.edu.cn (Fujian Zhou), liyuan2022@petrochina.com.cn (Yuan Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Various polymers are the most widely used product to provide rheology for water-based fracturing fluid, however, they have weaknesses in terms of temperature resistance and salt resistance. The change from organic-based to inorganic thickeners may be a meaningful attempt. Inorganic silica gel is a potentially alternative viscosifier with high temperature resistance, excellent proppant carrying capacity, and can even be used to prepare fracturing fluids with high salinity of produced water. In this paper, the silica gel viscosifier was firstly prepared using sol–gel method. Then, the gelation time under different influencing factors and rheological properties of the silica gel viscosifier were studied in detail. Subsequently, the silica gel-based fracturing fluid was prepared by adding the desired amount of drag reducer to the silica sol solution, and the properties of novel fracturing fluid were systematically evaluated with regard to the temperature and shearing resistance, drag reduction, and static proppant suspension. The potential mechanism of gelation process by syneresis of silica gel was revealed at last. Results showed that the microstructure of silica gel is synthetic, amorphous and consists of a three-dimensional network of SiO2 particles. The effect of SiO2 concentration on the gelation time is more pronounced than other factors such as temperature and pH level. In addition, the silica gel viscosifier exhibits strong salt resistance, whether monovalent ions (Na+, K+) or divalent ions (Ca2+, Mg2+), and the gelation time decreased significantly with the increase of salt concentration. The shear viscosity of the silica gel viscosifier increased with an increase of SiO2 concentration, showing shear thinning behavior as well. Meanwhile, the silica gel-based fracturing fluid prepared by adding drag reducer into silica sol solution also presented excellent thermal stability and shear resistance, drag reduction and proppant suspension performances. The retained viscosity can be maintained above 50 mPa·s after shearing at 180 °C for 60 mins; the drag reduction rate shows a declining trend at high displacement due to the gelation process; the settling rate of 40/70 mesh sand proppant with 35 % sand ratio is less than 30 % after standing at 90 °C for 4 h. In addition, the gelation process is essentially the formation of Si-O-Si linkage by dehydration between SiO2 particles, which is gradually extended at both ends and sides of the chain, and eventually forms a rigid, highly porous, entangled network. Findings found in this study provide a research basis for the popularization and application of silica gel-based fracturing fluid.
Keywords
Silica gel
Gelation time
Salt resistance
Rheological properties
Gelation mechanism
1 Introduction
Hydraulic fracturing is an effective stimulation treatment for unconventional reservoirs development, such as shale and tight reservoirs, natural gas hydrate, and geothermal energy (Li et al., 2022; Xu et al., 2022; Wei et al., 2022). Fracturing fluid performance is one of the vital technologies determining the effectiveness of fracturing stimulations. In hydraulic fracture operation, the fracturing fluid is essential for transmitting hydraulic pressure to fracture, propagating the fracture, transporting and suspending proppant until closure of the fracture (Zhang et al., 2023; Xu et al., 2023; Bai et al., 2021; Liu et al., 2021). In recent decades, various polymers are the most widely used product to provide rheology for water-based fracturing fluid, whether it is guar gum bio-based polymer or synthetic polymer such as modified polyacrylamide (Du et al., 2022). The performances of these fracturing fluids are well known, as are their drawbacks. For example, bio-based polymer fracturing fluids fail to tolerate high temperatures, and the process for synthetic polymers is complex and costly, making it difficult to apply on a large scale (Yao et al., 2021). Besides, the polymer-based fracture fluids are difficult to break completely, always causing great damage to reservoirs (Zhang et al., 2023).
On the other hand, with the development of horizontal drilling and sequential multistage fracturing technology, water consumption and produced water volume of fracturing have been increasing (Liu et al., 2020; McMahon et al., 2015; Li et al., 2016). Both the use of freshwater or near freshwater for fracturing operations and the treatment of produced water can significantly improve production costs (Boschee, 2014; Lebas et al., 2013). Formulating a fracturing fluid with treated produced water is an apparent solution for reducing cost and minimize the use of freshwater. Produced water generally consists of surface water, formation water, and flowback water from previous well treatments. It usually contains hydrocarbons, various levels of salinity and hardness, suspended solids, residual production chemicals, and bacteria (McMahon et al., 2015; Lebas et al., 2013). Hence, a series of negative problems may be encountered when directly using produced water to prepare water-based fracturing fluid. One problem is the massive mental cations in the solution could greatly affect the hydration and dissolution properties of conventional polymer viscosifier (Banerjee et al., 2009; Ruyle and Fragachan, 2015). The other problem is that the high-valent cations would consume the hydroxide ions in the solution, thus reducing the number of hydroxy-boron produced and affecting the continuation of the cross-linking reaction (Zhang et al., 2022). Moreover, the presence of abundant saprophytic bacteria in the produced water may cause the bio-based polymer viscosifier susceptible to microbial corrosion (Yao et al., 2021). These tricky issues undoubtedly pose a huge challenge for using produced water to prepare fracturing fluid.
There have been extensive research works in recent years focused on the cost-effective use of produced water for fracturing fluid preparation. Several scholars have engaged in removing salts/ions from produced water to mitigate the negative effects of produced water. Generally speaking, it is too costly to treat high-salinity produced water to the extent like freshwater used direct to prepare fracturing fluid (Zhang et al., 2022). Using chelating agent to stabilize the harness ions in the produced water is another method reported to minimize the adverse effect. Elsarawy and co-workers (Elsarawy et al., 2016) evaluated the effects of five different chelating agents on the tolerance of high-TDS fracturing fluids, and finally concluded that the use of HEDTA and GLDA increased the fluid system's tolerance to Ca2+ and Mg2+, while the other three chelating agents (di-ammonium EDTA, di-sodium EDTA, and sodium gluconate) showed a breaking effect on the viscosity of the fracturing fluid. The other method is to develop functional fracturing fluid systems that are tolerant to the high-salinity conditions. New polymers and crosslinkers, which have high tolerance to mono-and divalent cations has enabled the use of high-salinity produced water to formulate different fracturing fluid systems (Oseh et al., 2023; Liang et al., 2015). For synthetic polymers, the most effective solution to improve salt resistance is to introduce different functional groups, such as the sulfonate group, hydrophobic monomer, and annular material, etc. (Zhang et al., 2022). At present, international and domestic related research mainly focuses on the development of salt-resistant polymers based on acrylamide (AM) monomers, and the groups containing strong electrolyte groups or cyclic structure groups are the main monomers that can be introduced into polymers in polymerization reactions. For guar gum-based fluid systems, the functional modification of guar gum and the exploration of suitable crosslinker are potential measures to realize the preparation of fracturing fluid with produced water (Liang et al., 2015; Kakadjian et al., 2013). Although polymer-based viscosifier have shown notable improvements in salt resistance, there are still limitations and challenges to using produced water.
Laboratory and field data indicate that silica gel is a potential viscosifier for high salinity, high hardness produced water. Due to its highly functional applications, silica gel has excellent practicability in oil, gas and geothermal well operations (Liu and Ott, 2020). These measures include drilling, water shutoff, enhanced oil recovery conformance control, reservoir stimulation and so on (Soric et al., 2004; Pham and Hatzignatiou, 2016; Hatzignatiou et al., 2016; Lakatos et al., 2018; Elphingstone et al., 1980). Silica gel was first proposed as a water-based fracturing fluid viscosifier in the 1970 s. Early research found that this form of silica could provide a high-viscosity, thixotropic fluid with the ability to maintain viscosity at very high temperatures (McDonald et al., 2016; McDonald et al., 2015). In 1980, the silica gel-based fracturing fluid was first used to successfully fracture seven geothermal wells in the Nigorikawa field in Southwest Hokkaido, Japan, as an economical alternative to crosslinked bio-based polymer fluids (Katagiri and Ott, 1983). In addition, the maximum temperature of the fractured geothermal wells up to 507 °F. The latest application case was in 2019, during a single-stage fracturing process of a San Andres formation well drilled in western Texas, 500 barrels of silica gel-based fracturing fluid were prepared using on-site produced water (the salinity is 45380 mg/L) and mixed with 3500 lb of 40/70 mesh quartz sand, and then injected into the formation at a displacement of 15 bbl/min (Harman et al., 2021). The application results show that the cost of silica gel-based fracturing fluid prepared using untreated produced water is comparable to a standard 20 lb/gal crosslinked guar fluid. Field applications of silica gel-based fracturing fluids have proven that the new viscosifier not only be a technical success, but also has the same or lower cost than current technology. Although silica gel fracturing fluid has achieved good results in field application, there are few reports on the indoor research and evaluation of this fluid system, and the key performance parameters are not clear. Many mechanism studies are still in the blank stage, and the technology is not fully mature, so it is necessary to conduct an in-depth study.
Motivated by the apparent knowledge gaps from the existing studies, this paper mainly developed a new silica-based fracturing fluid system, and evaluated its comprehensive performance through laboratory experiments. The silica gel viscosifier was first prepared using sol–gel method, and then the microscopic morphology of self-prepared silica gel was characterized. Afterwards, the bottle test method was employed to visually observe the gelation process of silica gel and roughly estimate the gelation time under static conditions, and then the effect of SiO2 concentration, temperature, pH, and salt ions on the gelation time was further explored. The rheological properties of silica gel viscosifier were also reflected by apparent viscosity and viscoelastic modulus. Subsequently, the silica gel-based fracturing fluid was prepared by adding the desired amount of drag reducer to the silica sol solution, and three important performance including temperature and shear resistance, drag reduction and static proppant suspension of the novel fracturing fluid system were evaluated in succession. At last, the potential mechanism of gelation process by polymerization and dehydration of silica gel was revealed. The findings of this study contribute to provide an efficient fracturing fluid system for the fracture stimulation operation of geoenergy.
2 Materials and methods
2.1 Materials
Hydrochloric acid (HCl) and sodium silicate are the raw materials required for the preparation of silica gel. In order to facilitate dissolution, sodium metasilicate nonahydrate (Na2SiO3·9H2O) with a modulus of 1 was used in this study. Magnesium chloride (MgCl2), calcium chloride (CaCl2), potassium chloride (KCl), and sodium chloride (NaCl) were used to investigate the effect of salt ions on the gelation process. All these inorganic materials are of analytical purity grade, supplied by Shanghai Macklin Biochemical Co., Ltd. Besides, a high-viscosity drag reducer (DR-900), which was provided by Beijing Kemax Oilfield Chemicals Co., Ltd. DR-900 is a kind of cationic polyacrylamide copolymer with molecular weight of 8 × 106, white emulsion in appearance, avirulent and odorless. All the used chemical materials are non-toxic.
2.2 Experiment methods
2.2.1 Preparation of the silica gel
Among numerous silica gel synthesis methods, sol–gel is the most flexible and suitable route for oil field preparation (Su et al., 2023; Zhou et al., 2021). According to relevant literature (Baskaran et al., 2022; Singh et al., 2014); there are two different pathways to prepare silica gel, one is to add acid to sodium silicate solution and the pH reduced to induce gelation; the other, in contrast, is to add sodium silicate to the acid solution and the pH raised to induce gelation (Iler, 1979). The difference between the two preparation methods is reflected in the surface area, pore volume and specificity of the formed silica gel (McDonald et al., 2016). The method of increasing pH by adding alkali in acid solution is beneficial to the formation of silica gel with larger internal and external surface area, thus having stronger suspension and thixotropic properties (Liu and Ott, 2020; McDonald et al., 2015).
As a starting example for a simple 80 g silica gel fluid sample, 2.1 g concentrated HCl was added to 47.9 mL of deionized water with constant agitation, to prepare 0.49 mol/L HCl solution. 3.8 g Na2SiO3·9H2O was prediluted with 30 mL of deionized water, to prepared 0.44 mol/L sodium silicate solution. The diluted Na2SiO3 solution was slowly added into the diluted HCl solution under constant agitation, and the increasing pH value was constantly measured with a pH meter. The pH value of the mixed solution is about 7, and the desired gelation pH range of 5.0 to less than 10.5. The option exists to make minor adjustments to pH with the addition of less or more alkali, which has slightly influence on the final SiO2 concentration, but we ignored this little difference in this study. The above example would prepare a silica gel that is about 1.0 % SiO2 by weight. Analogously, if silica gel with different SiO2 concentration is desired, one can keep the molar concentration of HCl and Na2SiO3 solution increase or decrease simultaneously while keeping the acid and alkali volume unchanged.
2.2.2 Characterization
The micromorphology and structure of the self-prepared silica gel were characterized by environmental scanning electron microscopy (E-SEM) and transmission electron microscopy (TEM), respectively. During the E-SEM experiments, the crosslinked block silica gel can be directly observed under electron microscope after gold spraying treatment. For TEM experiments, it is necessary to dilute the silica gel with deionized water to prepare a low-concentration standard solution, which is dropped on the carbon support membrane and then dried for observation.
2.2.3 Determination of the gelation time
The gelation time of silica gel is similar to the crosslinking time of conventional polymer-based fracturing fluids, which is an important parameter to assess the delayed crosslinking property of the novel fracturing fluid. In this study, the bottle test method was employed to visually observe the gelation process and roughly estimate the gelation time under static conditions, which is also considered as a convenient and inexpensive experimental technique. Gelation time is defined as the case where the bottle can be turned over without any movement of the silica gel, which means the flowability of the fluid is completely lost. Firstly, filling a specified volume of fluid sample in a transparent glass bottle, and then, the bottle was placed at room temperature or in an oven preheated at the test temperature and taken out periodically for observation. This method was applied to investigate the effects of SiO2 concentration, temperature, pH, and the type and addition of salt ions on the gelation time.
2.2.4 Rheological properties test
Rheological characteristic is one of the vital properties of silica gel as viscosifier. The stability, drag reduction performance and proppant suspension capacity of fracturing fluid are closely related to rheological properties (Luo et al., 2015; Li et al., 2017; Liu et al., 2020). The apparent viscosity and viscoelastic properties of silica gel are the two parameters that were focused on in this study. Apparent viscosity can be tailored to suit field equipment, reservoir environments and fluid design (Liu et al., 2023; Liu et al., 2023b), while the viscoelastic modulus is the key parameter to evaluate the proppant suspension capacity.
The apparent viscosity measurements of the silica gel viscosifier was performed by using Fann® 35 six-speed rotary viscometer. Unlike conventional polymer-based fracturing fluids, the apparent viscosity readings of silica gel viscosifier were taken at 600 rpm, 300 rpm, 200 rpm, 100 rpm, 6 rpm, and 3 rpm, because silica gel is a highly viscous, thixotropic fluid. In addition to focusing on the apparent viscosity at 300 rpm, there are other two parameters used to evaluate the rheological properties, one is the plastic viscosity (PV), which can be calculated by the viscosity reading at 600 rpm minus the viscosity reading at 300 rpm; the other is the yield point (YP), which can be calculated by the viscosity reading at 300 rpm minus the PV (Guria et al., 2013). Moreover, the shear viscosity was measured at ambient temperature by using the HAAKE MARS Ⅲ rotational rheometer, and the shear rate was set between 0.1 ∼ 1000 s−1.
The viscoelasticity modulus of the prepared silica gel viscosifier with different concentrations were also determined using the HAAKE RS6000 rheometer under certain tension from low to high frequency shear conditions. The main experimental procedures are as follows: stress sweeping of the prepared fluid sample was firstly performed, and then the stress values near the inflection point were scanned using the frequency approach to acquire the viscous modulus (G’’) and the elastic modulus (G’) of the test sample. Based on the obtained intersection value of G’’ and G’ and the range of elastic region, the viscoelastic strength of the silica gel can be determined.
2.2.5 Temperature and shearing resistance test
Fracturing fluids are constantly sheared when flowing in the wellbore, while the wellbore temperature increases with depth, and the fracturing fluid reaching the bottom of the well also needs to have sufficient viscosity to transport the proppants farther inside the fracture network. Therefore, it is necessary to evaluate the thermal stability and shearing resistance of the fracturing fluid. In this study, HAAKE MARS III rheometer was used to conduct the temperature and shearing resistance experiments of the silica gel-based fracturing fluid. The silica gel-based fracturing fluid was prepared by adding 0.1 % DR900 drag reducer to the silica sol solution under shearing. (It is worth noting that the addition of drag reducer is mainly to play the role of drag reduction, while the main viscosity-increasing effect relies on the silica gel, therefore, the amount of DR900 drag reducer in the fluid system is generally not more than 0.2 %.) After gelation, the temperature and shearing resistance tests were carried out under 170.3 s−1 shear rate at 180 °C. 170.3 s−1 usually indicates the shear rate in the wellbore during operation process. Ramping up the test sample to target temperature at a constant rate of 3.0 ± 0.5 °C/min, and then sheared it under 170.3 s−1 for 60 mins. The terminal apparent viscosity retained able to reflects the thermal stability and shearing resistance of the silica gel-based fracturing fluids.
2.2.6 Drag reduction test
The drag reduction capacity of a fracturing fluid can be evaluated by testing the drag pressure difference generated as the test sample flows through a pipe. The above preparation process shows that the silica gel is a mixture of acid and alkali, and the drag reduction efficiency of the inorganic brine is usually similar to that of the water, which does not have a significant drag reduction effect. Based on this, the drag reduction experiment of silica gel-based fracturing fluid requires the addition of a small amount of DR900 drag reducer before the gelation, and the concentration of which does not exceed 0.2 %.
In this study, the self-developed loop friction test apparatus was used for drag reduction experiments, which mainly consists of a screw pump, the piping system, a fluid supply system, and an integrated control computer. Wherein, a stainless-steel pipeline with an inner diameter of 8 mm and a length of 3 m was preferably selected for this experiment. During the test, the frictional pressure difference generated by fresh water (ΔPw) under different pump rates was measured first, and then frictional pressure difference generated by fracturing fluid (ΔPf) was measured as well. The drag reduction rate (DR) of the fracturing fluid can be figured out by following equation:
There is a fact that with the progress of silica gel gelation, the viscosity of the formed silica gel-based fracturing fluid will increase. According to the Reynolds formula, the flow pattern of the fracturing fluid will change, which will seriously affect the efficiency of drag reduction. Consequently, this research only explored the decline rule of drag reduction rate under the highest flow rate (the upper limit of the device is 2500 kg/h). The drag reduction rate was recorded every 30 s during this experiment, and the total test time was determined to be 12 min by fully considering the field operation displacement and the well depth.
2.2.7 Proppant suspension test
The proppant suspension and carrying capacity of the fracturing fluid determines the settlement pattern and transport distance of the proppant in the fracture, which directly affects the fracture conductivity. In this work, static sand settling experiments of silica gel-based fracturing fluid were carried out to study the settling behaviors of quartz sand proppant with different sand ratios (10 %, 15 %, 20 %, 25 %, 30 %, and 35 %, all represent volume ratio) under various temperatures (30 °C, 90 °C). The mesh size of quartz sand proppant used in these experiments is 40/70. Firstly, mix the proppant and silica gel-based fracturing fluid evenly in a beaker, then pour it into the graduated glass cylinder, and record the falling height of the sand column within 4 h at 30 °C. Subsequently, the glass cylinders were placed in a constant temperature oven at 90 °C for 4 h to test the height of the sand column further reduced. Additionally, mono-mesh carbon steel shot with different diameters were used to conduct single-particle proppant settling experiments to measure the settling velocity. The reason for using carbon steel shots with a specific gravity of 7.8 for the experiments is because conventional industrial proppants (such as 20/40 mesh quartz sand or ceramic) settle extremely slow in the silica gel-based fracturing fluid systems, and the settlement height is difficult to measure accurately even after 24 h of standing. At first, place the steel shots on the surface of crosslinked silica gel-based fracturing fluid and record the time consumed by the steel shot falling to a certain height. Then, the terminal settling velocity of the steel shot inside the silica gel-based fracturing fluid can be calculated according to the settling height and time.
3 Results and discussion
3.1 Structural characterization
Fig. 1 displays the SEM images of the self-prepared silica gel with 1.0 % SiO2 by weight at different resolutions. Unlike conventional crosslinked polymer-based fracturing fluids, the microstructure of silica gel exhibits highly porous characteristics, and the interconnections between pores create a complex pore network. As can be seen from the enlarged image, the silica gel shows an irregular agglomerated morphology. This is mainly because the gel network formed during the gelation process was fragmented continuously by a stirrer, resulting in the showing discrete gel fragments (Elphingstone et al., 1980). However, due to the continuing polymerization, these gel fragments will restructure, directly causing the gel fragments shrink and the internal fluid is expulsed. This is the “syneresis” process of silica gel. It is worth noting that no obvious SiO2 spherical particles were observed in the images, and the surface of silica gel is amorphous. This is closely related to the morphology of gel fragments, the degree of polymerization of SiO2 and the extent of syneresis. The experimental results of TEM in Fig. 2 further confirm the microstructure characteristics of silica gel is synthetic, amorphous and consists of a three-dimensional network of silica particles. The sample drying process resulted in the precipitation of little sodium chloride crystals, which adhered to the surface of SiO2 particles, so that small amounts of chloride and sodium signals were observed in the energy spectrum analysis.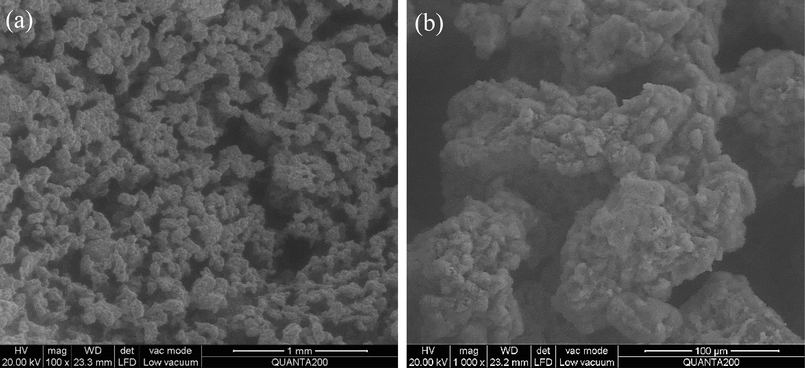
The SEM images of silica gel under different resolutions, (a) 1 mm, (b) 100 μm.
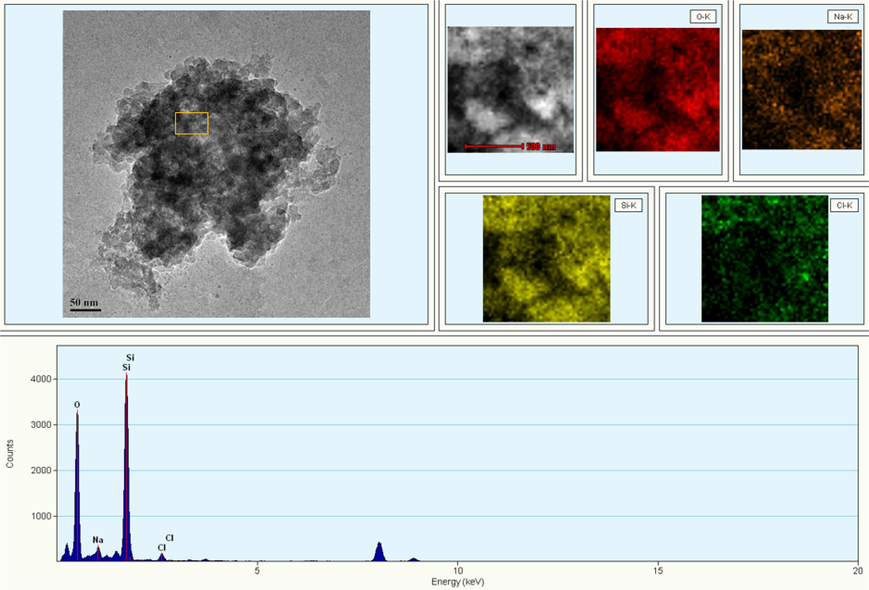
The TEM scanning results of silica gel.
3.2 Influencing factors of gelation time
The gelation time of silica gel is similar to the delayed crosslinking time of conventional polymer-based fracturing fluid, which is one of the key parameters to evaluate whether the novel fluid system can be applied in the field operation. The gelation time should be controlled within a reasonable range to ensure that the injected fluid meets the performance requirements of low friction during flow in the wellbore and strong proppant-carrying capacity inside the fractures (Zhou et al., 2022; L et al., 2022). If the gelation time is too short, the viscosity gradually increases as the fracturing fluid flow in the wellbore, and the flowing friction will increase rapidly; if the gelation time is too long, it is not conducive to the long-distance migration of proppant-carrying fracturing fluid within the fractures, which will easily cause sand plugging in the near-wellbore area, thus affecting the production of fracturing wells.
In this work, the experimental study into the gelation time covered a broad range of SiO2 concentration, temperature, pH levels, and salt ions (Na+, K+, Ca2+, Mg2+) concentration. Fig. 3 shows the graphical representation of gelation time versus pH levels, temperature, and SiO2 concentration. Table 1 provides the test matrix and the original data of the bottle tests. All the tests were performed with deionized water. Longitudinal comparative experimental results, one can see that the SiO2 concentration has the most significant effect on gelation time. Under certain pH level and temperature conditions, the gelation time decreased significantly as the SiO2 concentration rose. For example, at 60 °C and pH = 8.5, the gelation time decreased from 67.4 mins to 0.6 min with increasing SiO2 contact from 0.75 % to 1.50 %. Horizontal comparison, under the condition of a certain SiO2 concentration, the influence of pH level on the gelation time is greater than the temperature. Although an increase in temperature also decreases the gelation time to some extent, the change amplitude is slightly less than that of pH level. For instance, at the 1.0 % SiO2 condition, the gelation time decreased from 27.0 mins to 16.0 mins with rising temperature from 30 °C to 90 °C, under the pH = 8.5. However, the gelation time dropped from 27.0 mins to 6.2 mins with decreasing pH level from 8.5 to 7.0, likewise at 30 °C and 1.0 % SiO2 conditions. In addition, the influence of pH level on gelation time shows a parabolic-like characteristic, with the fastest gelation time occurred at pH = 7, while increasing or decreasing pH level would increase gelation time.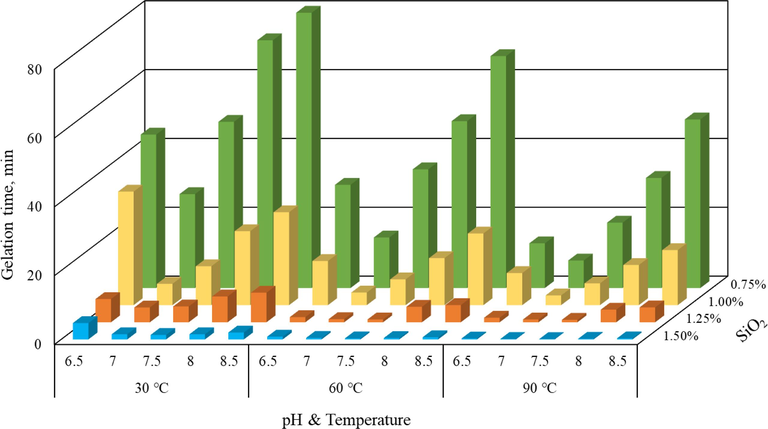
Gelation time test results under different influencing factors.
Temperature, ℃
pH
Gelation time with different SiO2 conc., min
0.75 %
1.0 %
1.25 %
1.5 %
30
6.5
44.6
33.1
6.8
4.8
7.0
27.3
6.2
4.3
1.5
7.5
48.3
11.3
4.6
1.3
8.0
72.1
21.5
7.5
1.5
8.5
84.1
27.0
8.6
2.0
60
6.5
30.2
12.8
1.5
0.8
7.0
14.7
3.6
0.9
0.4
7.5
34.5
7.50
0.8
0.3
8.0
48.5
13.6
4.5
0.4
8.5
67.4
20.8
5.1
0.6
90
6.5
13.0
9.3
1.3
0.3
7.0
8.1
2.8
0.8
0.1
7.5
19.1
6.3
0.7
0.1
8.0
32.1
11.7
3.7
0.2
8.5
49.0
16.0
4.3
0.3
Except for the above three factors, salt ions also have a significant influence on gelation time. In fact, fracturing fluids always inevitably interact with salt ions during field application. On the one hand, the injected fracturing fluid will interaction with formation water, which is a mixture of original and injected water, containing various of inorganic salt ions. On the other hand, reservoir rock is composed of multiple minerals, and the interactions of fracturing fluids with reservoir rock takes place throughout the whole fracturing process. These ions will affect the gelation time, and they might even lead to precipitation of calcium and/or magnesium silicate at high concentrations. This negative impact needs to be considered in order to determine the tolerance of silica gel-based fracturing fluid systems to salt ions.
Four different inorganic chlorides were applied to analyze different cation effects on the gelation time of silica gel. These experiments were performed by adding a specific concentration of salt ion to the hydrochloric acid solution, fully dissolved and then slowly add the sodium silicate solution, adjust the pH level of the mixed sample to an interesting value, and record the gelation time. Note that the experimental temperature is 30 °C, and the SiO2 content in the final prepared silica gel is about 1.75 %. Besides, the original Na+ in sodium silicate is maintained in roughly the same range, and the content is lower than that of additional salt ions, so it was not considered as the focus of this study. Fig. 4 shows the influence of four different cations on the gelation time. Because the mixed solution of a specific pH value cannot be accurately obtained during the experiment, a large number of data points were randomly collected. From the Fig. 4(a) and (b), one can see that the effect of pH value on the gelation time showed a parabolic-like distribution, while the addition of Na+ and K+ significantly accelerated the gelation process. Results also indicated that increasing salt ion concentration decreases the gelation time at different pH levels. The shortest gelation time occurred in the pH range of 7 ∼ 8, and with the increase of salt addition, the gelation time at different pH levels showed a downward trend, which was more obvious when the salt concentration reached 10.0 %. Moreover, K+ demonstrates the stronger capability to shorten the gelation time compared to Na+. Fig. 4(c) and (d) show the influence of Ca2+ and Mg2+ on gelation time respectively. In contrast to Na+ and K+, there is an obvious precipitation area when the divalent cations at high concentrations. This is mainly because the precipitation of high-valence Ca2+ and Mg2+ with the surface SiO2- 3 (Hatami et al., 2021). Also, high valent cations could react with hydroxyl ion in the solution, forming insoluble spices. Nevertheless, at the same addition dosage (such as 0.1 %), divalent cations have a more visible effect on the gelation time than the monovalent cations. It is clear that the introduction of Ca2+ and Mg2+ can indeed reduce the gelation time to a certain extent, even in the case of 0.01 % addition. The higher the ion concentration, the shorter gelation time. Furthermore, the effect of Ca2+ on the gelation process was slightly stronger than Mg2+ — consistent with other studies in the previous literature (Hatami et al., 2021; Hatzignatiou et al., 2018).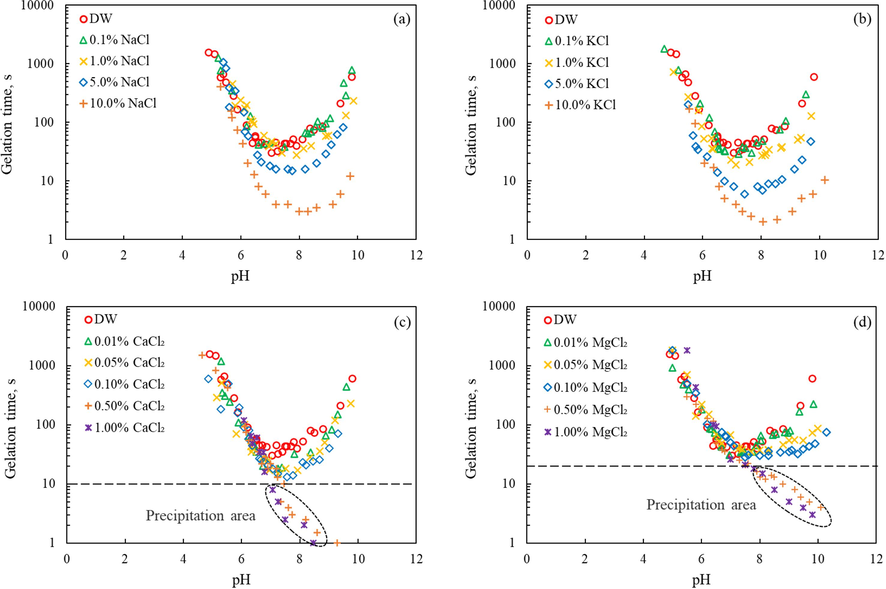
Effect of salt ion type and concentration on gelation time, (a) Na+, (b) K+, (c) Ca2+, (d) Mg2+.
The SiO2 nanoparticles in silica gel carry a negative charges surface, and the added cations act as counterions, which will enter the electrical double layer of the nanoparticles. This will lower the zeta potential of the nanoparticles, thereby destabilizing the silica gel system and leading to gelation. This effect becomes more pronounced as the salt concentration increases. Due to their higher valence and larger size, the divalent salt ions make it easier to reduce zeta potential and promote gelation (Huang et al., 2017).
3.3 Rheological characteristics
The rheological characteristics of silica gel viscosifier with different concentrations tested by the Fann® 35 six-speed rotational viscometer are shown in Table 2. During the experiment, deionized water, 10 % NaCl solution and 0.5 % CaCl2 solution were used to prepare silica gel to evaluate the effects of salt ions on rheological properties. Since silica gel is a highly viscous and thixotropic fluid, the rotational speed is set from high to low during the test, and the continuous shear inevitably leads to a downward trend in the viscosity value. The viscosity value measured at 300 speed is considered to be the apparent viscosity of silica gel. As the table lists, the apparent viscosity measured in different solutions gradually increases with the rising concentration of SiO2. The addition of 10 % NaCl had a negligible effect on the apparent viscosity of the silica gel, while the addition of 0.5 % CaCl2 caused a significant decrease in the apparent viscosity at various concentrations. The variation curves of PV and YP with SiO2 concentration were plotted and illustrated in Fig. 5. The comparation between the three PV curves revelated that with the presence of salts, the PV drops sharply, indicating the flocculation caused by salt ions reduces the number of dispersed gel fragments, resulting in a decrease in internal friction inside the silica gel. On the contrary, the YP shows an increasing trend with the addition of salt ions, mainly because the introduction of inorganic salt ions will increase the flocculation inside the silica gel to a certain extent, making its internal structure more disordered and amorphous, reflecting the increase of the yield point.
Test condition
Six-speed rotary viscometer taken at 25℃, shear rate, r/min; viscosity, mPa·s
Deionized water
10.0 % NaCl brine
0.5 % CaCl2 brine
SiO2 conc.
600
300
200
100
6
3
600
300
200
100
6
3
600
300
200
100
6
3
0.75 %
25
14
9.5
5
1
1
20
11
8.5
3.5
1
0.5
15
8
5
2.5
1
0.5
1.00 %
38
20
13
6
1.5
1
25
15
10
3
1.5
1
23
13
7.5
4.5
1
0.5
1.25 %
48
25.5
15
8
2
1.5
32
19
14
7
2
1
28
15
9.5
5
1
0.5
1.50 %
54
28
15
9
2.5
1.5
37
22.5
18
8.5
4
3
30
19
10
6
1
0.5
1.75 %
62
33
17
10
3
2
44
27
16.5
10
3
2
41
25
16
9
2
1.5
2.00 %
71
38
26
17.5
6
5
53
33
24
13.5
11.5
9
52
31
20
11.5
4
2
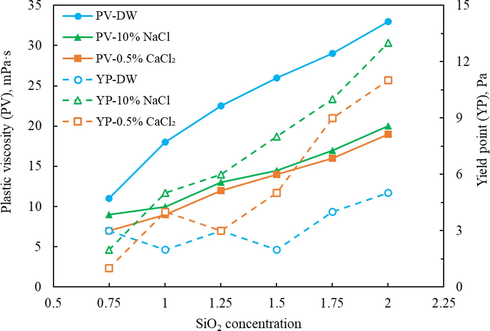
The variation curves of PV and YP with SiO2 concentration.
In addition, the shear viscosity was also measured at ambient temperature by using the rotational rheometer, and the shear rate range was between 0.1 ∼ 1000 s−1. Rheological measurements were performed multiple times to ensure the repeatability of the final results, and the test results of silica gel with different SiO2 concentrations is shown in Fig. 6. It was found that the shear viscosity of the silica gel increased as the SiO2 concentration increases. A shear thinning characteristic was also observed for the silica gel within the test shear rate interval. Silica gel builds viscosity via the smaller fragments that mechanically entangle and interlock to form a much larger, three-dimensional microstructure. This entangled structure is easily damaged under high-speed shear, resulting in a decrease in viscosity (McDonald et al., 2015; Iler, 1979; Liu et al., 2022). However, after a long period of resting, these small fragments after shearing will be entangled again, so that the viscosity of the silica gel system will be restored, which is an important feature of thixotropic fluid.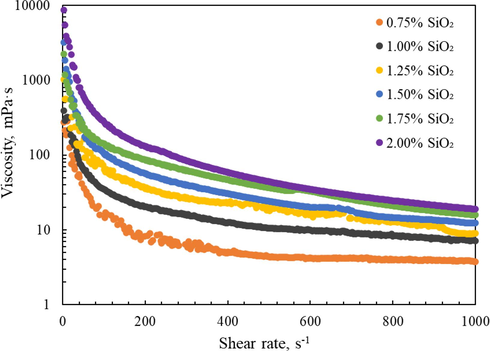
Relationship between viscosity and shear rate for samples.
The viscoelastic behavior of prepared silica gel with different SiO2 concentrations was measured at ambient temperature. Modulus of silica gel samples varies with angular frequency, as shown in Fig. 7. Unlike the conventional polymer-based fracturing fluid system, the silica gel system with different SiO2 concentrations exhibited the characteristic that the G’ was always greater than the G’’ in the entire tested angular frequency range, and there are no crossover points between the two curves, indicating that the silica gel system has a dominant elastic response behavior, which may be attributed to the higher entanglement-structural strength. As the figure depicts, both G’ and G’’ of silica gel samples showing an increasing trend with the SiO2 concentration increasing, especially for the G’ value. This is mainly because silica gel forms a denser three-dimensional structure under high concentration of SiO2, and the gel strength is further enhanced as hydrogen bonds formed between silica gels. The microstructural strengthening is manifested by a simultaneous increase in G’ and G’’. The large G’ reflects the rigidity and strength of the gel, and the large G’’ indicates that the silica gel consumes less energy in the flow process. Furthermore, the enhanced viscoelastic characteristics may relate to proppant transportation (Gomaa et al., 2015; Ba Geri et al., 2019; Yao et al., 2022), but this property must be correlated with additional data from another source.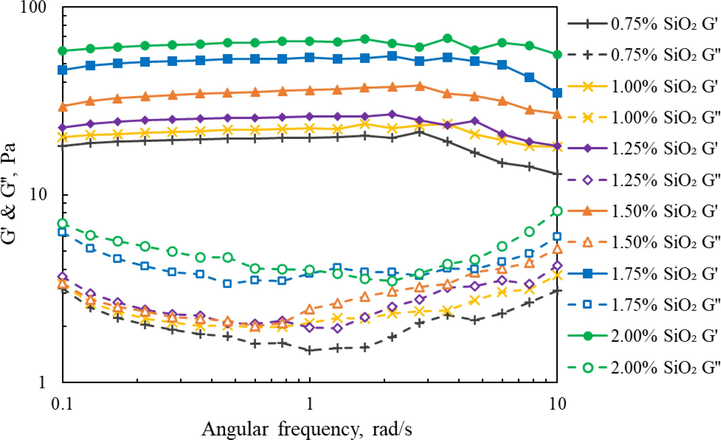
Viscoelastic modulus curves of silica gel under various SiO2 concentrations.
3.4 Performance of temperature and shearing resistance
The temperature and shearing resistance performance is a key parameter to evaluate whether the silica gel-based fracturing fluid could be used in high-temperature deep well fracturing. The HAAKE MARS Ⅲ rheometer was applied to measure the terminal apparent viscosity of silica gel fracturing fluid with different SiO2 concentrations after shearing at 180 °C and 170.3 s−1 for 60 mins. The obtained rheological curves are presented in Fig. 8. As shown in the figure, the apparent viscosity of the novel fracturing fluid with 1.0 % SiO2 increases first and then decreases, and the moment when the maximum viscosity occurs is basically similar to the gelation time of this system. The terminal apparent viscosity after shearing is basically maintained at about 50 mPa·s, indicating that the thermal and shear stability of the prepared silica gel-based fracturing fluid meets the requirements of field applications. By contrast, the apparent viscosity variation curve of silica gel-based fracturing fluid with 1.5 % SiO2 content showed a decreasing trend followed by a stable trend. This is due to the short gelation time of silica gel with 1.5 % SiO2 content, which gelated rapidly after pouring the mixed solution into the rheometer rotor, resulting in a high initial apparent viscosity in the rheological curve. Moreover, the increase of SiO2 content in the system leads to a significant increase in the retained viscosity after shearing. It is also worth noting that after adding a little drag reducer to the silica sol solution, the rheological properties of the formed fracturing fluid system are further improved (compared with the silica gel thickener data listed in Table 2), probably due to the drag reducer was fully dissolved in the free water, and there might be existence of a synergistic mechanism between the network structure of silica gel and the flexible molecular chain of polyacrylamide polymer (Sun et al., 2023; Sun et al., 2023; Wang et al., 2022), thus forming a denser microstructure and enhancing the rheology properties.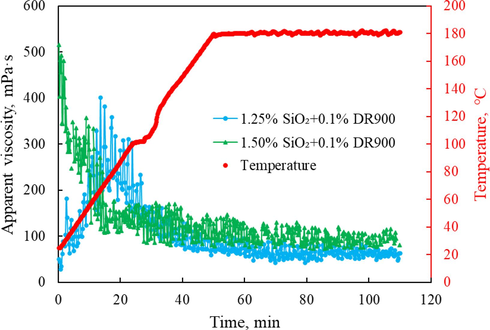
Variation of apparent viscosity with temperature for silica gel-based fracturing fluid with different SiO2 concentrations.
3.5 Performance of drag reduction
Silica gel-based fracturing fluid will inevitably gelation when pumped in ultra-deep wells, and the increase of apparent viscosity will lead to the decrease of drag reduction effect. Therefore, this paper focuses on exploring the variation trend of drag reduction efficiency of the silica gel-based fracturing fluid with time at the maximum flow rate (2500 kg/h). The drag reduction performance of the 0.1 % DR-900 slickwater and the silica gel-based fracturing fluid with different SiO2 concentrations were evaluated with the loop friction test apparatus in the lab. Fig. 9 shows the test result of three samples. As the figure depicted, the drag reduction rate of DR900 slickwater system changed very little within 12 min, just from 76.28 % to 75.39 %. However, the drag reduction rate decreased significantly within 12 min of the silica gel-based fracturing fluids. For silica gel fracturing fluid with 1.0 % SiO2 content, the drag reduction rate decreased from 76.23 % to 41.68 %; for silica gel fracturing fluid with 1.5 % SiO2 content, the drag reduction rate decreased from 75.64 % to 37.05 %.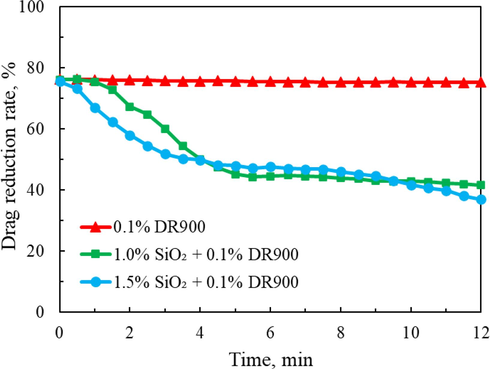
Drag reduction rate as a function of test time.
Compared with slickwater fluid system, the drag reduction effect of silica gel-based fracturing fluid is not satisfactory. This is mainly because silica gel is a mixture of silicate and hydrochloric acid, and the inorganic salt solution itself does not have the ability to reduce drag. By adding a little amount of DR900 drag reducer to the solution, the silica gel-based fracturing fluid has a pronounced drag reducing ability, and the maximum drag reduction rate can reach more than 70 % at the initial moment. However, the apparent viscosity gradually increases with the constant gelation of the silica sol during the circulation flow, and the polymer molecular chain is randomly doped with the formed silica gel, resulting in the disordered extension of the molecular chain and the decrease of drag reduction rate. It should be noted that due to the limitation of equipment, the maximum pump rate is only 2500 kg/h in the laboratory, and the corresponding operation displacement (take 3.5 in. tubing as an example) is about 3.7 m3/min calculated by the linear velocity. For higher operation displacement on site, the flow time of fracturing fluid in wellbore can be further reduced, and the drag reduction effect in a short time hopefully meet the needs of on-site fracturing operation.
3.6 Performance of proppant suspension
Static proppant settling experiment is the simplest and most effective method to evaluate the proppant suspension capacity of fracturing fluid. According to the different experimental methods, it is usually divided into multi-particle proppant settlement test and single-particle proppant test experiment (Xu et al., 2022). Generally speaking, slower settling velocity represents stronger suspension capacity. In this work, the static proppant settling tests were carried out using specified graduated glasses in the laboratory. Fig. 10 shows the static proppant suspension experimental graph of silica gel fracturing fluid with 1.0 % SiO2 concentration under different sand ratio, time, and temperature conditions. As the figure shown, the silica gel suspension column dropped significantly after standing for a period of time, and a part of water was generated inside the graduated glass, which was mainly due to the syneresis process of silica gel. Syneresis is the process, whereby the silica gel shrinks and the entrained water/brine is extruded from the gel structure. The process of syneresis is closely related to time and temperature. It can be seen form the Fig. 11 that the sand settling rate also increased with the time and temperature. The term “settling rate” is defined as the ratio of the height of the suspended sand slurry at a specific moment to its initial height. When the suspended sand fracturing fluid, which had been standing for 4 h at 30 °C, was then placed in a constant temperature oven at 90 °C, the sand settling rate was further improved after standing for 4 h again. In addition, the sand settling rate basically increases gradually with the increase of sand ratio, but the experimental group with 30 % sand ratio exhibits an unexpected result, there may be an operation error. From the experimental data, it can be observed that the sand settling rate is around 15 % when standing for 4 h at 30 °C, while the temperature increases by 90 °C, the settling rate increases by less than 5 %, about 20 %.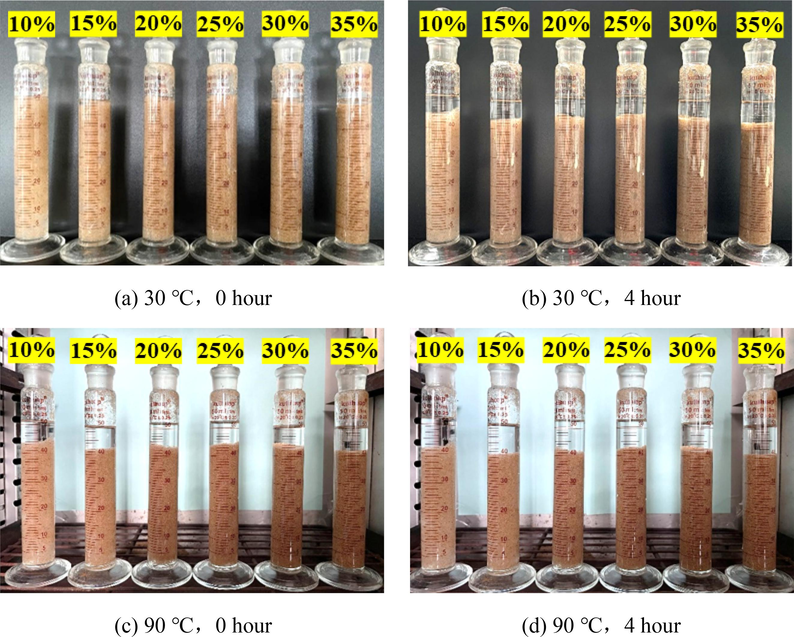
Sand settling at different conditions using 40/70 mesh size.
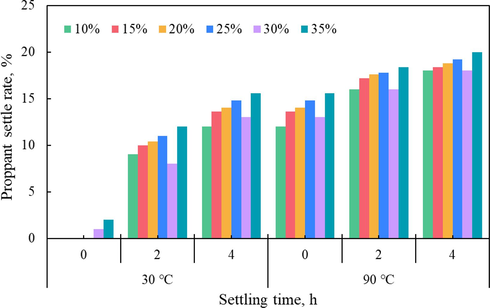
Proppant settling rate varies with time at different conditions.
Static settling experiments of single-particle proppant for silica gel-based fracturing fluid were carried out using steel shots with different particle sizes. The reason for choosing steel shots with large particle size is that regular proppant settles extremely slowly in silica gel-based fracturing fluid and the experimental test error is large. Also, the silica gel-based fracturing fluid will continue to undergo syneresis reaction during the long-term standing process, causing a denser gel structure and even the formation of gel blocks, which negatively affects the proppant settling. Fig. 12 shows the terminal settling velocity of single steel shot with various diameters in the two silica gel-based fracturing fluids with different SiO2 contents at 30 °C. As the particle diameter increases, the calculated settling velocity of steel shots shows an exponential increase trend, and the silica gel-based fracturing fluid with 1.0 % SiO2 content is more obvious. Compared with conventional aqueous hydraulic fracturing fluids, such as guar gum-based and polymer-based, the settling velocity of single-particle proppant in silica gel-based fracturing fluid is extremely slow (Ba Geri et al., 2019; Biheri and Imqam, 2022; Zhang et al., 2016; Hu et al., 2015), indicating the excellent proppant-carrying capacity. This is mainly because the elasticity of the silica gel system is strong, and the syneresis process promotes the formation of a denser and more stable three-dimensional structure of silica gel, leading to the proppant has a higher resistance when settling inside it.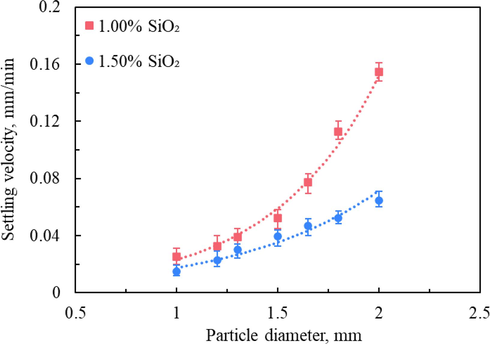
The settling velocity of single steel shot with different diameters measured at 30 °C.
3.7 Analysis of gelation mechanism
The essence of gelation is the process in which SiO2 particles form a hydrous, amorphous gel structure through self-polymerization and condensation. The initial step toward formation of silica gel is the aggregation of some of the particles into short chains as shown in Fig. 13. At first, initially formed monomeric silicic acid in the sodium silicate solution condenses to form colloidal particles, as shown in the Fig. 13(a). The SiO2 is also present as a multiplicity of anionic species derived from silicic acid. When sodium silicate solution is mixed with acid, neutralization of the stabilizing alkali occurs, and silicate anions form polymeric silica connected by the formation of new siloxane bonds (Si-O-Si) with the expulsion of water molecules (McDonald et al., 2016; Wilhelm and Kind, 2014). This reaction is generally controlled by pH level, temperature, and SiO2 concentration; also by order of addition. Fig. 13(b) shows the result of the reaction is that many polymerized short chains are formed in the solution. As the reaction continues, the chain length is increased, and the free particles begin to be added to the sides as well as the ends of the chains, thus a lot of branched chains is created (McDonald and Li, 2017), as depicted in Fig. 13(c). Free particles within this region are continuously added to the network which soon contains all the particles within its domain, forming a “microgel region” of what has been called “gel phase”. Each region of the gel phase increases with the increase of the sol particles in the surrounding liquid region until the gel phase occupies the majority of the total volume (Iler, 1979). At this point, the gel region grows together into a continuous coherent gel network, as Fig. 13(d) shown. Meanwhile, there is an increase in viscosity as the gel phase develops. Once the silica sol is fully converted to silica gel, there is a period of time when secondary reactions occur and the gel consolidates or shrinks with the expulsion of water and salts. This shrinkage process is known as syneresis, which can last for several hours, depending on the overall size of the silica gel.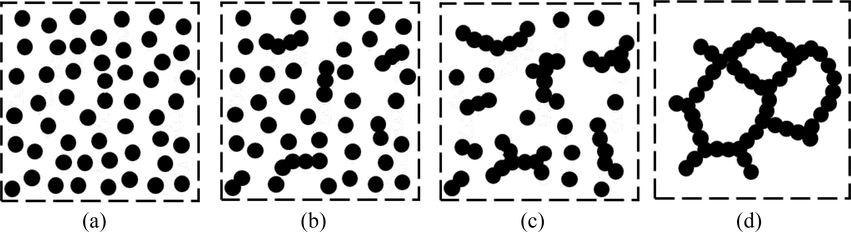
Silica sol aggregate to become silica gel (the three-dimensional process is represented only in two dimensions).
In fact, the mechanism of each stage of the gelation process is the same, that is, two silicate anions react to form a Si-O-Si linkage with expulsion of one water molecule (McDonald et al., 2016; Iler, 1979), as shown in Fig. 14. However, in the first stage, the polymerization of monomeric silicic acid leads to particles of massive silica; while in the subsequent stage, since it is impossible to accurately fit two particles together on a common surface, the number of Si-O-Si bonds between particles is fewer in number than those within the particles themselves (Bergna, 1994). When the two silica particles contacted in water, they will grow together because the solubility of silica in the crevice at the point of contact is less than the solubility of silica on the surface of the particles. As a result, the neck diameter of the joined particles increases until the solubility difference becomes smaller (Iler, 1979). The two particles connected together are in a fixed position relative to each other, forming an oval structure, and the linkage effect reinforced at the same time (Fig. 14). As more and more particles are connected together, a rigid, highly porous, entangled network of branching chains is final generated.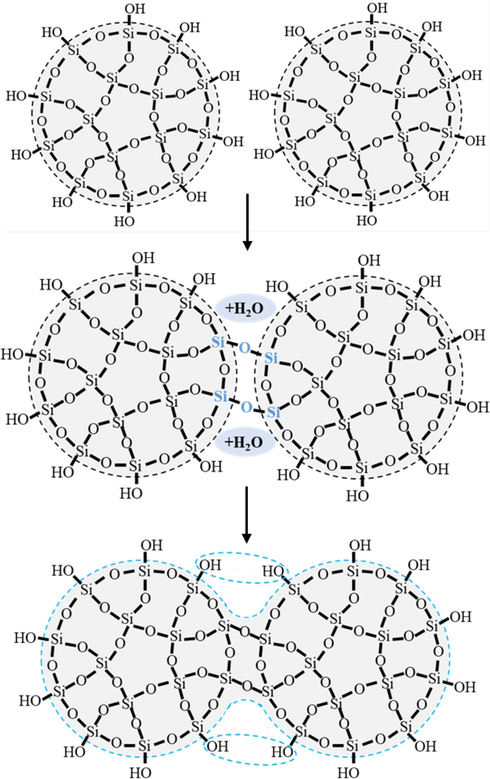
Schematic illustration of the polymerization process.
4 Conclusions
In this paper, we firstly prepared the silica gel viscosifier using sol–gel method, and the micromorphology was characterized by E-SEM and TEM. Then, the gelation time under different influencing factors and rheological properties of the silica gel viscosifier were studied by a series of laboratory experiments. Afterwards, the silica gel-based fracturing fluid was prepared by adding 0.1 % DR900 drag reducer to the silica sol solution, and the properties of novel fracturing fluid were systematically evaluated with regard to the temperature and shearing resistance, drag reduction efficiency, and static proppant suspension. At last, the potential mechanism of gelation process by polymerization and dehydration of silica gel was revealed. Based on the experimental results of this research, we draw the following conclusions:
-
1)
The micromorphology characteristics of silica gel is synthetic, amorphous and consists of a three-dimensional network of silica particles.
-
2)
Gelation time is positively correlated with temperature and SiO2 concentration, while the effect of pH level on gelation time shows a parabolic-like characteristic, with the fastest gelation time occurred at about pH = 7. Compared with temperature and pH level, the effect of SiO2 concentration on the gelation time is more pronounced. Besides, the gelation time decreased significantly with the salt ion concentration increasing, and the effect of divalent ions on the gelation time is more obvious.
-
3)
The rheological properties of silica gel viscosifier demonstrated that the shear viscosity increased as the SiO2 concentration rose, exhibiting shear thinning behavior as well. Viscoelastic modulus test results show that the silica gel exhibits G’ was always greater than G’’, indicating that the silica gel has a dominant elastic response behavior.
-
4)
Silica gel-based fracturing fluid presents excellent temperature and shearing resistance, drag reduction and static proppant suspension performances. The retained apparent viscosity can be maintained above 50 mPa·s after shearing at 180 °C for 60 mins; the drag reduction rate can reach more than 70 % at the initial moment, but it shows a downward trend with the gelation process; the settling rate of 40/70 mesh sand proppant with 35 % sand ratio is less than 30 % after standing at 90 °C for 4 h, and the settling velocity of single steel shot proppant with large diameter in silica gel is extremely slow.
-
5)
The essence of silica sol polymerization to form silica gel is the formation of Si-O-Si linkage by dehydration between SiO2 particles, which is gradually extended at both ends and sides of the chain, and eventually forms a rigid, highly porous, entangled network. Meanwhile, there is an increase in viscosity as the gel phase develops.
Although the novel silica gel-based fracturing fluid shows excellent performance in temperature tolerance and salt resistance, it is still at a disadvantage in shear resistance and drag reduction. Further validation study is recommended to research on how to improve the shear resistance and drag reduction performances of the silica-based fracturing fluid as well as investigate the conductivity of proppant-filled fractures.
Acknowledgement
This research was financially supported by National Natural Science Foundation of China (Grant No. 52004306 and 52174045).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Investigate the rheological behavior of high viscosity friction reducer fracture fluid and its impact on proppant static settling velocity. In: In SPE Oklahoma City Oil and Gas Symposium. 2019.
- [Google Scholar]
- Viscoelastic characterization effect of high-viscosity friction reducers and proppant transport performance in high-TDS environment. In: In SPE Annual Technical Conference and Exhibition. 2019.
- [Google Scholar]
- Optimization and friction reduction study of a new type of viscoelastic slickwater system. J. Mol. Liq.. 2021;344:117876
- [Google Scholar]
- Sorption of water vapor, hydration, and viscosity of carboxymethyl hydroxypropyl guar, diutan, and xanthan gums, and their molecular association with and without salts (NaCl, CaCl2, HCOOK, CH3COONa, (NH4)2SO4 and MgSO4) in aqueous solution. Langmuir. 2009;25(19):11647-11656.
- [Google Scholar]
- Sol-gel derived silica: A review of polymer-tailored properties for energy and environmental applications. Microporous Mesoporous Mater. 2022111874
- [Google Scholar]
- Bergna, H.E. 1994. The Colloid Chemistry of Silica: An Overview; American Chemical Society: Washington, DC, USA.
- Proppant transport using high-viscosity friction reducer fracture fluids at high-temperature environment. SPE J.. 2022;27(01):60-76.
- [Google Scholar]
- Boschee, P. 2014. Produced and flowback water recycling and reuse: economics, limitations, and technology. Oil and Gas Facilities, 3(01), 16-21.
- Water-soluble polymers for high-temperature resistant hydraulic fracturing: A review. J. Nat. Gas Sci. Eng. 2022104673
- [Google Scholar]
- Elphingstone, E.A., Misak, M.D., Briscoe, J.E., 1980. Methods of Treating Subterranean Well Formations, p. 4215001. US.
- The effect of chelating agents on the use of produced water in crosslinked-gel-based hydraulic fracturing. In: In SPE Low Perm Symposium. 2016.
- [Google Scholar]
- Proppant transport? Viscosity is not all it's cracked up to be. In: In SPE Hydraulic Fracturing Technology Conference. 2015.
- [Google Scholar]
- Rheological analysis of drilling fluid using Marsh Funnel. J. Pet. Sci. Eng.. 2013;105:62-69.
- [Google Scholar]
- Silica gel fracturing fluids an alternative to guar systems that allows the use of west Texas produced water. In: In SPE Hydraulic Fracturing Technology Conference and Exhibition. 2021.
- [Google Scholar]
- On the application of silica gel for mitigating CO2 leakage in CCS projects: Rheological properties and chemical stability. J. Pet. Sci. Eng.. 2021;207:109155
- [Google Scholar]
- Laboratory testing of environmentally friendly sodium silicate systems for water management through conformance control. SPE Prod. Oper.. 2016;31(04):337-350.
- [Google Scholar]
- Water-soluble silicate gelants: Comparison and screening for conformance control in carbonate naturally fractured reservoirs. J. Non Cryst. Solids. 2018;479:72-81.
- [Google Scholar]
- Hu, Y. T., Chung, H., Jason, M. 2015. What is more important for proppant transport, viscosity or elasticity? In SPE Hydraulic Fracturing Technology Conference.
- Systematic approach to develop a colloidal silica based gel system for water shut-off. In: In SPE Middle East Oil & Gas Show and Conference. 2017.
- [Google Scholar]
- The chemistry of silica: Solubility, polymerization. Wiley, New York: Colloid and Surface Properties and Biochemistry of Silica; 1979.
- Stable fracturing fluids from produced waste water. In: In SPE Kuwait Oil and Gas Show and Conference. 2013.
- [Google Scholar]
- L, N., Yu, J., Wang, D., et al. 2022. Development status of crosslinking agent in high-temperature and pressure fracturing fluid: A review. Journal of Natural Gas Science and Engineering, 104369.
- Revival of Green Conformance and IOR/EOR Technologies: Nanosilica Aided Silicate Systems - a Review. Lafayette, Louisiana, USA: Society of Petroleum Engineers; 2018.
- Development and use of high-TDS recycled produced water for crosslinked-gel-based hydraulic fracturing. In: In SPE Hydraulic Fracturing Technology Conference. 2013.
- [Google Scholar]
- A review of crosslinked fracturing fluids prepared with produced water. Petroleum. 2016;2(4):313-323.
- [Google Scholar]
- Rheological properties study of foam fracturing fluid using CO2 and surfactant. Chem. Eng. Sci.. 2017;170:720-730.
- [Google Scholar]
- Experimental study on plugging performance and diverted fracture geometry during different temporary plugging and diverting fracturing in Jimusar shale. J. Pet. Sci. Eng.. 2022;215:110580
- [Google Scholar]
- Reduced polymer loading, high temperature fracturing fluids using nano-crosslinkers. In: In Abu Dhabi International Petroleum Exhibition and Conference. 2015.
- [Google Scholar]
- Liu, Y., Upchurch, E. R., Ozbayoglu, E. M., et al. 2023b. Design and Calculation of Process Parameters in Bullheading and Pressurized Mud Cap Drilling. In SPE/IADC International Drilling Conference and Exhibition.
- Recycling flowback water for hydraulic fracturing in Sichuan Basin, China: Implications for gas production, water footprint, and water quality of regenerated flowback water. Fuel. 2020;272:117621
- [Google Scholar]
- Sodium silicate applications in oil, gas & geothermal well operations. J. Pet. Sci. Eng.. 2020;195:107693
- [Google Scholar]
- An improved drift-flux correlation for gas-liquid two-phase flow in horizontal and vertical upward inclined wells. J. Pet. Sci. Eng.. 2020;195:107881
- [Google Scholar]
- Experimental study of single taylor bubble rising in stagnant and downward flowing non-newtonian fluids in inclined pipes. Energies. 2021;14(3):578.
- [Google Scholar]
- Experimental and theoretical studies on Taylor Bubbles rising in stagnant non-newtonian fluids in inclined non-concentric annuli. Int. J. Multiph. Flow. 2022;147:103912
- [Google Scholar]
- Computational fluid dynamics simulations of Taylor bubbles rising in vertical and inclined concentric annuli. Int. J. Multiph. Flow. 2023;159:104333
- [Google Scholar]
- Experimental investigation on rheological properties and friction performance of thickened CO2 fracturing fluid. J. Pet. Sci. Eng.. 2015;133:410-420.
- [Google Scholar]
- McDonald, M., Li, X., 2017. The development & field results of a new, advanced form of sodium silicate as a cost effective solution for treatment for sustained casing pressure. SPE Therm. Well Integr. Des. Symp.
- McDonald, M., Ott, W. K., Elphingstone, G. 2016. Silica gel as an economical and innovative alternative to bio-based polymers in aqueous hydraulic fracturing fluids. AADE Houston: Texas. AADE-16-FTCE-18.
- Silica gel emerges as a viscosifier for aqueous hydro-frac fluids. Word Oil Shale Tech. Rep. 2015:24-26.
- [Google Scholar]
- First 100% reuse of Bakken produced water in hybrid treatments using inexpensive polysaccharide gelling agents. In: In SPE International Symposium on Oilfield Chemistry. 2015.
- [Google Scholar]
- Oseh, J. O., Mohd, N. M., Gbadamosi, A. O., et al. 2023. Polymer nanocomposites application in drilling fluids: A review. Geoenergy Science and Engineering, 211416.
- Rheological evaluation of a sodium silicate gel system for water management in mature, naturally-fractured oilfields. J. Pet. Sci. Eng.. 2016;138:218-233.
- [Google Scholar]
- Solving field produced water challenges with a novel guar-based system: A comprehensive review of actual field examples and cost savings analysis. In: In Abu Dhabi International Petroleum Exhibition and Conference. 2015.
- [Google Scholar]
- Sol-Gel processing of silica nanoparticles and their applications. Adv. Colloid Interface Sci.. 2014;214:17-37.
- [Google Scholar]
- Soric, T., Marinescu, P., Huelke, R., 2004. Silicate-based drilling fluids deliver optimum shale inhibition and wellbore stability. IADC/SPE Drilling Conference. Society of Petroleum Engineers, Dallas, Texas.
- Application of SiO2 nanocomposite ferroelectric material in preparation of trampoline net for physical exercise. Adv. Nano Res.. 2023;14(4):355-362.
- [Google Scholar]
- High-Efficiency utilization of waste shield slurry: A geopolymeric Flocculation-Filtration-Solidification method. Constr. Build. Mater.. 2023;387:131569
- [Google Scholar]
- Geopolymeric flocculation-solidification of tail slurry of shield tunnelling spoil after sand separation. Constr. Build. Mater.. 2023;374:130954
- [Google Scholar]
- Stochastic failure analysis of reinforced thermoplastic pipes under axial loading and internal pressure. China Ocean Eng.. 2022;36(4):614-628.
- [Google Scholar]
- Temperature transient analysis of naturally fractured geothermal reservoirs. SPE J. 2022:1-23.
- [Google Scholar]
- On the relation between natural and enforced syneresis of acidic precipitated silica. Polymers. 2014;6(12):2896-2911.
- [Google Scholar]
- The enhancement of performance and imbibition effect of slickwater-based fracturing fluid by using MoS2 nanosheets. Pet. Sci. 2022
- [Google Scholar]
- Adsorption characteristics, isotherm, kinetics, and diffusion of nanoemulsion in tight sandstone reservoir. Chem. Eng. J.. 2023;144070
- [Google Scholar]
- A review of experimental studies on the proppant settling in hydraulic fractures. J. Pet. Sci. Eng.. 2022;208:109211
- [Google Scholar]
- Reusing flowback and produced water with different salinity to prepare guar fracturing fluid. Energies. 2021;15(1):153.
- [Google Scholar]
- New integrated model of the settling velocity of proppants falling in viscoelastic slick-water fracturing fluids. J. Nat. Gas Sci. Eng.. 2016;33:518-526.
- [Google Scholar]
- Synthesis of a hydrophobic association polymer with an inner salt structure for fracture fluid with ultra-high-salinity water. Colloids Surf. A Physicochem. Eng. Asp.. 2022;636:128062
- [Google Scholar]
- Towards sustainable oil/gas fracking by reusing its process water: A review on fundamentals, challenges, and opportunities. J. Pet. Sci. Eng. 2022110422
- [Google Scholar]
- Application of fumed silica-enhanced polymeric fracturing fluids in highly mineralized water. J. Mol. Liq.. 2023;375:120835
- [Google Scholar]
- Preparation and crosslinking mechanism of delayed swelling double-crosslinking nano polymer gel microsphere for anti-CO2 gas channeling. J. Pet. Sci. Eng.. 2022;219:111122
- [Google Scholar]
- Preparation and characterization of high-strength geopolymer based on BH-1 lunar soil simulant with low alkali content. Engineering. 2021;7(11):1631-1645.
- [Google Scholar]







