Translate this page into:
Preparation and properties of lignin-based polyurethane materials
⁎Corresponding author. gcj@tust.edu.cn (Guichang Jiang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this paper, lignin-based polyurethane (LPU) films were successfully prepared by tape casting using modified alkaline lignin instead of partial polycarbonate diol (PCDL) in reaction with isophorone diisocyanate (IPDI), with diethylene glycol (DEG) as chain extender and dibutyltin dilaurate (DBTDL) as catalyst. The factors influencing the hydroxylation modification of alkali lignin were discussed, and the optimal reaction conditions were determined: reaction temperature of 50 °C, reaction time of 60 min, hydrogen peroxide to lignin mass ratio of 1.2:1, and iron hydroxide to alkali lignin mass ratio of 2 %. The lignin-based polyurethane films with different R values were also prepared by changing the ratio of isocyanate groups to hydroxyl groups (R-value), and the effects of different R values on the polyurethane properties were investigated. It was characterized by Fourier transform infrared spectroscopy (FTIR), mechanical property tests, thermogravimetric analysis (TGA), scanning electron microscopy (SEM), moisture and oxygen permeability property tests, and UV spectroscopy tests. The results show that changing the R-value can improve the mechanical properties, thermal stability, barrier properties, and UV shielding properties of polyurethane materials. When the R-value is 1.8, the lignin-based polyurethane film material has the best performance.
Keywords
Alkali lignin
Polyurethane film
Hydroxylation modification
R-value
1 Introduction
With the continuous development of society, ordinary polyurethane has been unable to meet the needs of modern society, and the polyols used in synthetic polyurethane mainly come from petroleum resources, in the increasingly serious environmental resource problems, it is urgent to choose an environmentally friendly and renewable material to replace petroleum-based polyols (Bontaş et al., 2023; Głowińska et al., 2023). Some renewable materials, including lignin, cellulose, starch, rosin, vegetable oils, etc., which contain hydroxyl groups or are modified to obtain hydroxyl groups, have attracted a lot of attention as potential polyurethane raw materials. Lignin is a complex aromatic macromolecule that is the second most abundant natural polymer in nature as the main component of the lignocellulose matrix (Wang et al., 2023). Compared to hemicellulose and cellulose, which are easily converted and valuable, lignin has a very complex structure and low exploitation rate and has long been regarded as a by-product waste of the pulp and paper industry (Ossanna et al., 2023), which can rarely be used in production.
Therefore, it is particularly important to improve the utilization rate of lignin. One of the ways through which lignin is used is to synthesize lignin polymers, and many researchers have synthesized lignin polyurethanes (Saito et al., 2013). Ma et al., in recent years, have focused on the synthesis of polyurethane elastomers and polyurethane foams using lignin and modified lignin. It also illustrates some problems in the utilization process of lignin and its application prospects in various fields (Ma et al., 2020). Mona Alinejad et al. detail the structure of lignin and its extraction and modification methods in the article. The current situation and application potential of lignin replacing polyol in polyurethane were discussed, and lignin could be used as a substitute for polyol (Alinejad et al., 2019). Wang et al. synthesized lignin-based polyurethanes using three unmodified acerolia sulfate lignin as the main hydroxyl donors. The study showed that the synergistic effect of the molecular weight of lignin and secondary polyols could regulate the mechanical properties of lignin-based polyurethanes (Wang et al., 2019). Li et al. derived kraft paper pine lignin into liquid polyols by oxypropylation method, and compared them with commercial polyols from the perspective of mechanical properties. A series of lignin-based polyurethanes were synthesized with sucrose polyols and glycerol polyols with different weight percentages. It is also pointed out that the best compressibility of rigid polyurethane foam can be obtained by using lignin polyols (Li and Ragauskas, 2012). Therefore, due to the advantages of cheapness, abundance, and renewables, it is crucial to develop effective strategies for converting lignin into high added value products.
Polyurethane (PU), short for urethane, is a polymer containing a basic repeating unit carbamate group (–NHCOO-) on the backbone. It is synthesized by reactions between isocyanates (–CNO), polyols (–OH), and other additives (Fang et al., 2019). The isocyanates used in the synthesis of polyurethanes include diphenylmethane diisocyanate (MDI), toluene diisocyanate (TDI), dicyclohexylmethylmethane-diisocyanate(HMDI), isophorone diisocyanate (IPDI) and so on. Polyols used in the synthesis of polyurethanes are mainly classified as polyethers (Gu et al., 2022) and polyester polyols. Polyether polyols are oligomers containing ether bonds (-R-O-R-) in the main chain and more than two hydroxyl groups in the side or end groups, commonly used are polypropylene glycol (PPG), polytetrahydrofuran diol (PTHF), etc. Polyurethanes synthesized with polyether polyols have better flexibility. Polyester polyols are generally obtained by condensation of organic dicarboxylic acids with polyols or polymerization of lactones with polyols, and polyurethanes synthesized with polyester polyols have greater mechanical strength (Ng et al., 2017). There are many types of chain extenders used in the synthesis of polyurethane, mostly small molecule alcohols, amines, or alcohol amine compounds (Kalajahi et al., 2017; Kwak et al., 2003; Kwon et al., 2022), such as propylene glycol, 1,4-butanediol, diethyltoluenediamine, 3,5-dimethylthiotoluenediamine, diethanolamine, triethanolamine, etc. In the polyurethane reaction system, due to the high activity of the chain extender, the reaction system can be quickly crosslinked and expanded, and the performance of polyurethane can be adjusted by changing the amount of chain extender (Sun et al., 2021; Zhao et al., 2023). Alkali lignin is a common biodegradable biomass material and its hydroxylation modification and co-synthesis of polyurethane with polyester polyol has not been reported. Modification of alkali lignin with hydrogen peroxide and sodium hydroxide, mixing it with isophorone diisocyanate (IPDI and polycarbonate diol (PCDL), and preparing lignin-based polyurethane films in the presence of catalyst dibutyltin dilaurate (DBTDL) are innovative explorations. The overall idea flow and LPU synthesis route of the experiment are shown in Fig. 1.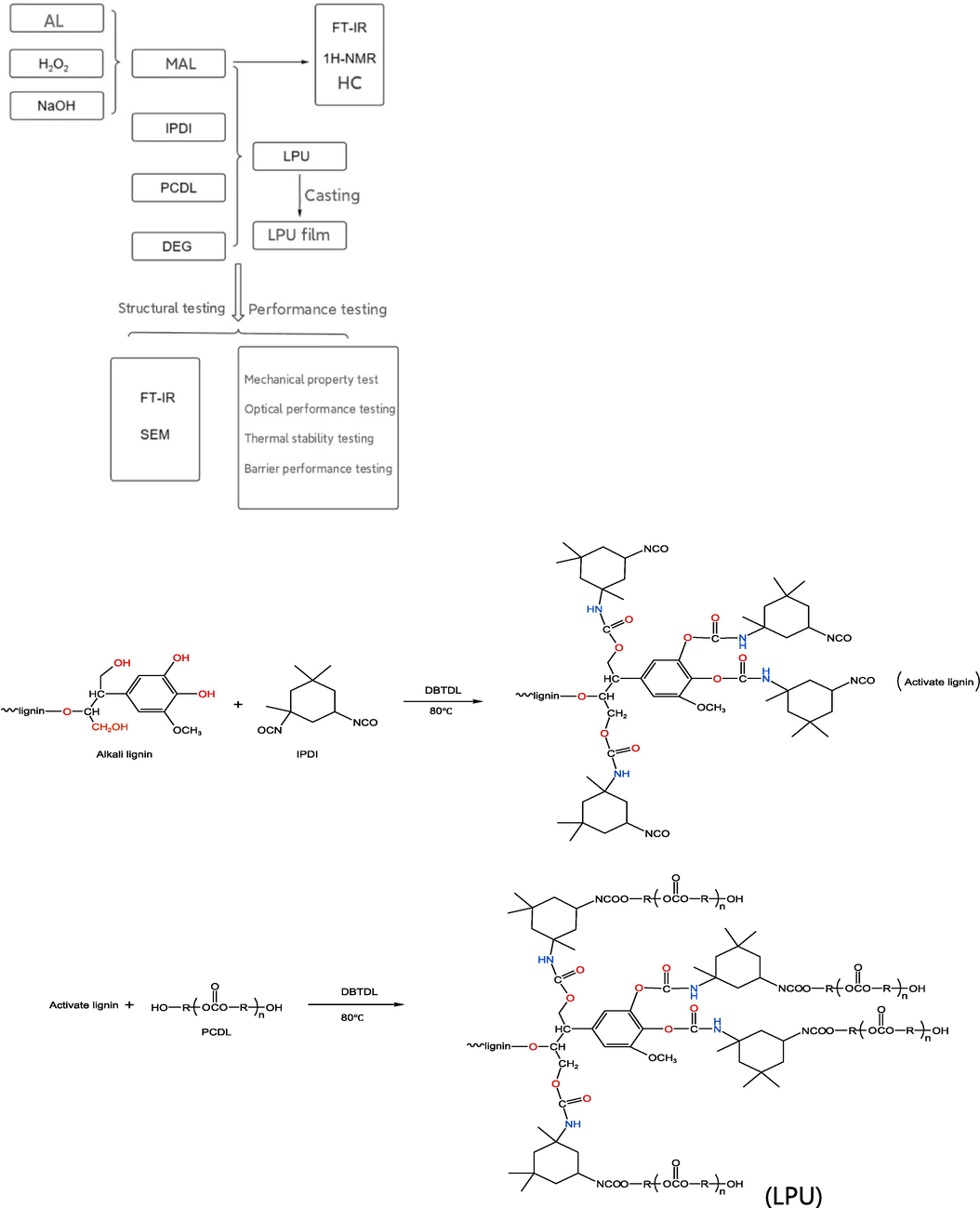
Experimental flowchart and LPU synthesis roadmap.
2 Experimental methods
2.1 Materials
Alkali lignin (chemicals, Anhui, China) was purified by alkali-soluble acid precipitation and dried at 50 °C for 12 h. Isophorone diisocyanate (IPDI) (Chemicals, Shandong, China) is frozen at 0 °C for 12 h. Polycarbonate diol (PCDL) (Chemicals, Shandong, China) and Diethylene glycol (DEG) (Chemicals, BASF, Germany) were dried at 80 °C for 12 h. Dibutyltin (DBTDL) (Chemicals, Tianjin, China) and N, N-Dimethylformamide (DMF) (Chemicals, Tianjin, China), Sodium hydroxide (Chemicals, Tianjin, China), hydrogen peroxide (Chemicals, Tianjin, China), ferric hydroxide (Chemicals, Tianjin, China) and hydrochloric acid (Chemicals, Tianjin, China) are stored at room temperature.
2.2 Hydroxylation modification of alkaline lignin
5 g of alkaline lignin was dissolved with 50 mL of 1 % sodium hydroxide solution and then added to the reaction vessel equipped with magnetic stirring, condensation reflux, and constant temperature water bath device, and then a certain mass of catalyst iron hydroxide, 30 % mass fraction of hydrogen peroxide solution was added in turn, and the reaction was carried out at 50 °C for one hour. The catalyst was removed by repeated centrifugal washing at the end of the reaction. After that, the pH was adjusted to 2 with 10 % hydrochloric acid, and the lignin was precipitated and then centrifuged and washed repeatedly with deionized water to neutralize. Finally, it was put into an oven and dried at 60 °C for 24 h to obtain hydroxylated modified alkali lignin.
2.3 Preparation of alkaline lignin polyurethane
The alkali lignin and isophorone diisocyanate (IPDI) were added to a three-necked flask and heated in an oil bath at 80 °C under a nitrogen atmosphere, and the catalyst dibutyl dilaurate (DBTDL) was added after 1 h of reaction, polycarbonate diol (PCDL) was added after 2 h of reaction, and diethylene glycol (DEG) was added after 4 h of reaction for the chain expansion reaction, and the whole reaction lasted for 8 h.
2.4 Preparation of alkaline lignin polyurethane films
According to the ratio of 50 mL N, N-dimethylformamide (DMF) dissolved 5 g of alkali lignin polyurethane (LPU), the ingredients were poured into a beaker and placed on a magnetic stirrer and stirred for 1 h to fully dissolve alkali lignin polyurethane (LPU) in N, N-dimethylformamide (DMF). After the reaction, the magnetic rotor was removed and the solution was allowed to stand for 30 min and then poured onto a polytetrafluoroethylene (PTFE) plate and then dried in an oven at 60 °C for 24 h, and finally the film was removed with forceps to obtain a lignin-based polyurethane film.
2.5 Characterization
IR spectra were recorded with a BRUKER-VECTOR22 FT-IR spectrophotometer using KBr disks. Weigh a small amount of alkali lignin powder to be tested about 1–2 mg, and potassium bromide powder 150–170 mg, add the two to the mortar and fully grind in one direction, and then press into thin sheets with a tablet press, and then put them into an infrared spectrometer for testing. The wavenumber range is set to 4000–500 cm−1, the resolution is 4 cm−1, and the number of scans is 32 times. The microstructure of polyurethane film sections was observed by the SU-1510 scanning electron microscope. The polyurethane film samples were glued on conductive adhesive, then gold sprayed and observed under an accelerating voltage of 10.0 kV. The mechanical properties of the PU films were measured on a universal testing machine (GT-TS-2000, Taiwan) according to GB/T 1040–2006 with a tensile speed of 200 mm/min at 23 ± 2 °C to obtain the tensile strength and the breaking elongation. The samples were cast from DMF solutions and shaped to strip products. A TGA Q500 thermogravimetric analyzer was used to measure the temperature as a function of the mass of 5–7 mg of LPU sample, which was heated from room temperature to 600 °C in the air at a rate of 20 °C/min. The oxygen permeability of LPU film is tested according to GB/T 1038–2000 “Test Method for Gas Permeability of Plastic Films and Sheets by Differential Pressure Method”, and the gas permeability coefficient Pg of the film is calculated according to the following formula. Where Pg is the gas transmission rate of the film in 10–14 cm3∙cm (cm2⁄∙s∙ Pa), Qg is the gas transmission amount in cm3/m2∙d∙Pa, and D is the thickness of the film sample in cm. The moisture permeability of LPU films is tested according to GB1037-1988 “Test Method for Water Vapor Permeability of Plastic Films and Sheets by Cup Method”, and the moisture permeability coefficient is calculated according to the following formula ere Pv is the water vapor transmission coefficient in g∙ cm/(cm2∙s∙Pa), WVT is the amount of water vapor transmission in g/m2∙24 h, D is the thickness of the specimen in cm, ΔP is the difference in water vapor pressure between the two sides of the specimen in Pa. The film samples were cut into rectangular slices of 4 cm × 8 cm and the UV absorption spectra of the films were tested in the range of 200–800 nm using a UV −2700 spectrometer. The thickness of the specimen is 3 mm. The length of the sample between the two pneumatic grips of the Testing Machine was 50 mm. Five measurements were conducted for each sample, and the results were averaged to obtain a mean value. The hydrogen spectra were analyzed by NMR spectrometer AV III 400 M, and the acetylated alkaline lignin and modified lignin were fully dissolved in deuterated chloroform, respectively, with sample masses of 5–10 mg, and the instrument frequency was set to 400 MHz, and the tests were performed at room temperature. The hydroxyl content in alkali lignin was determined according to HG/T 2709–1955 Determination of hydroxyl group of polyester polyol. The acetylation method was used, using the acetic anhydride in the acetylation reagent and the specimen for the acylation reaction, hydrolyzing the remaining acetic anhydride with water, titrating the resulting acetic acid with potassium hydroxide ethanol standard solution, and doing a blank experiment to calculate the hydroxyl value by their difference. Where X1 is the hydroxyl value of the specimen in mg KOH/g, X2 is the acid value of the specimen in mg KOH/g, V1 is the volume of potassium hydroxide ethanol standard titration solution consumed in mL for the blank experiment, V2 is the volume of potassium hydroxide ethanol standard titration solution consumed in mL for the titration of the specimen, c is the concentration of potassium hydroxide ethanol standard titration solution in mol/L, and m is the mass of the specimen in g. The sample molecular weight distribution was obtained by GPC using a PL-GPC50 gel permeation chromatograph with a mobile phase of chloroform (CDCL3) at a flow rate of 1 mL/min and sample concentrations in the range of 2–5 mg/ml.
3 Results and discussion
3.1 Reaction mechanism and structural analysis of alkaline lignin
3.1.1 Reaction mechanism of alkali lignin modification
The reaction mechanism of alkali lignin modification is shown in Fig. 2. The reaction of lignin hydroxylation modification can be carried out according to the free radical mechanism, iron hydroxide will generate trivalent iron ions, trivalent iron ions and hydrogen peroxide will react to generate hydroxyl radicals and divalent iron ions, divalent iron ions and hydrogen peroxide will react to generate hydroxyl radicals and trivalent iron ions, hydroxyl radicals and alkali lignin will react to generate an intermediate, and then this intermediate and trivalent iron ions will generate hydroxylated lignin and divalent iron ions. In the whole reaction, there are two reactions: hydroxylation and hydrogen peroxide decomposition, so the decomposition of hydrogen peroxide into oxygen is inhibited as much as possible, thus promoting the production of hydroxyl radicals from hydrogen peroxide.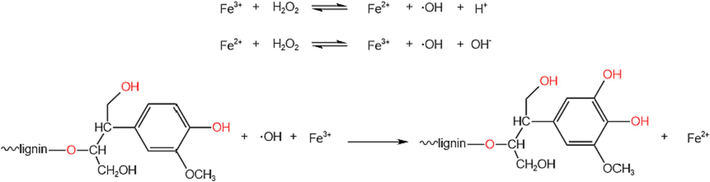
Reaction mechanism of alkali lignin modification.
3.1.2 Infrared spectrum comparison of lignin
In this study, the infrared spectra of alkali lignin and modified alkali lignin were examined. As shown in Fig. 3. the peak at 3433 cm−1 is the infrared absorption peak of the hydroxyl group in alkali lignin, 2932 cm−1 and 2929 cm−1 are the infrared absorption peaks of the sub-methyl group in alkali lignin, and 1606 cm−1 and 1609 cm−1 are the infrared absorption peaks of the benzene ring. It can be seen from Fig. 3 that no new absorption peaks appeared in the IR spectra after the hydrogen peroxide treatment, no new functional groups appeared in the lignin, and the complete structure of the lignin was retained. The IR spectra of lignin before and after the modification are consistent with the previous inference of the reaction mechanism, which also indicates the successful modification of lignin.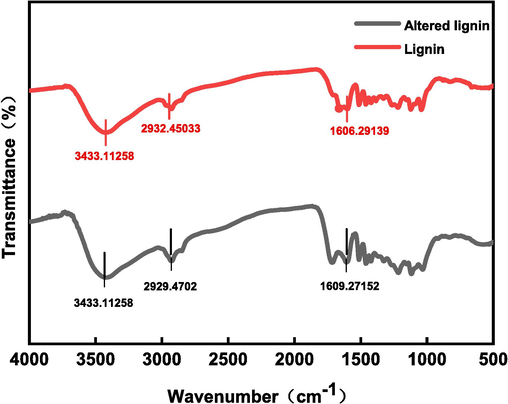
Comparison of infrared spectra before and after alkali lignin modification.
3.1.3 Comparison of NMR hydrogen spectra of lignin
Fig. 4(a) shows the hydrogen spectrum of alkali lignin, and Fig. 4(b) shows the hydrogen spectrum of modified alkali lignin. To improve the solubility of lignin in a deuterated reagent and exclude the effect of hydroxyl groups in water, acetylation was done on lignin. The peak of the alcohol hydroxyl group after acetylation of alkali lignin is at 1.9 ppm to 2.2 ppm, the peak of phenolic hydroxyl group after acetylation of alkali lignin is at 2.2 ppm to 2.5 ppm, and the absorption peak of benzene ring is at 6.5 ppm. It can also be seen from the hydrogen spectra that the modified alkali lignin retains the complete structure of lignin.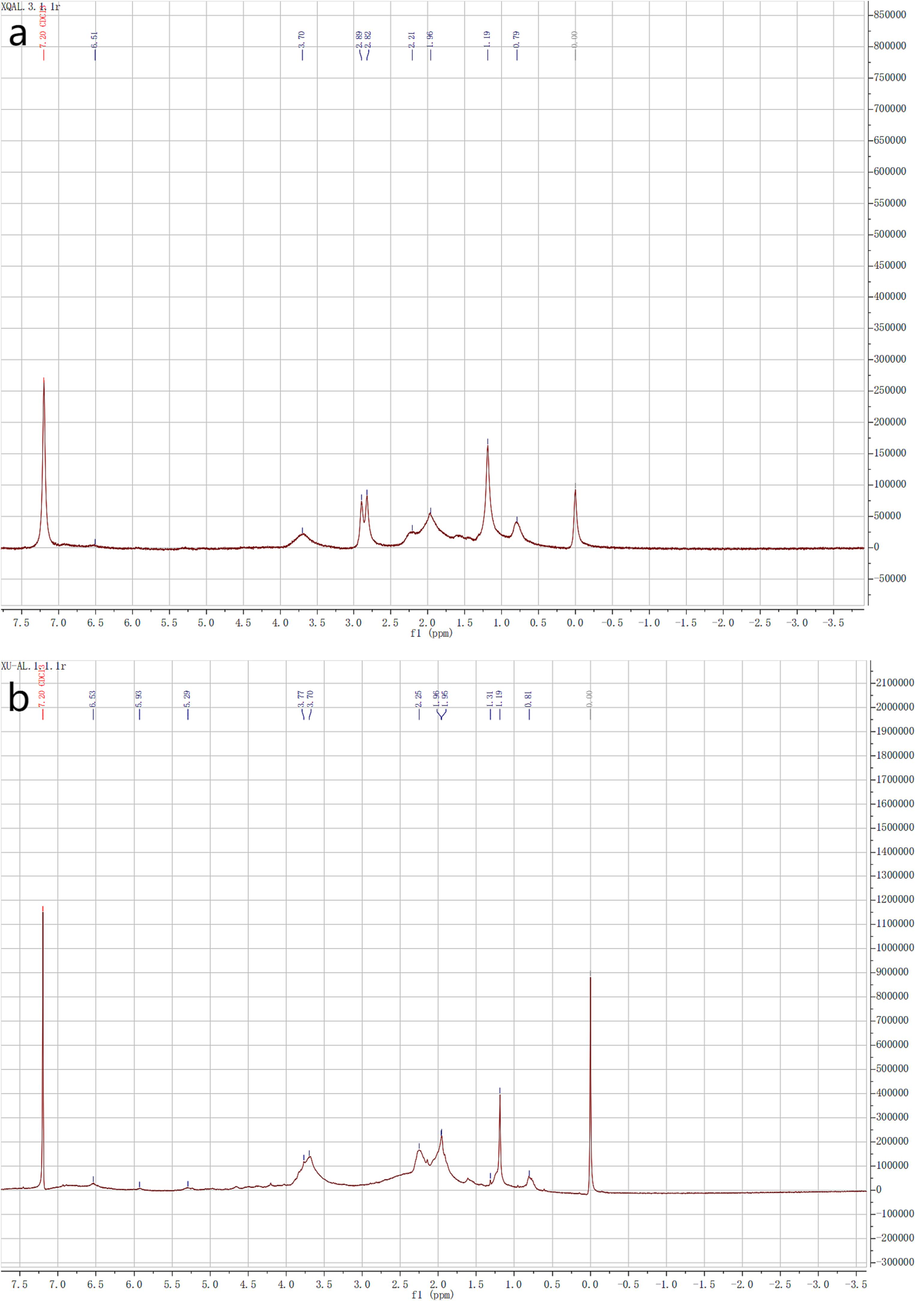
(a) nuclear magnetic resonance hydrogen of alkali lignin, and (b) nuclear magnetic resonance hydrogen spectrum of modified alkali lignin.
3.1.4 GPC analysis of alkaline lignin
The heavy average molecular weight (Mw), number average molecular weight (Mn), and polydispersity (PDI) of alkali lignin before and after modification were determined by GPC, as shown in Table 1. Table 1 shows that the Mn of the hydroxylation-modified treated alkali lignin was 1330 g/mol, which was much lower than that of the unmodified alkali lignin 1796 g/mol, which might be caused by the cleavage of lignin during the modification of treatment. In addition, the polydispersity of the modified lignin sample was 1.19, and this low polydispersity phenomenon may be because the lignin underwent another acid precipitation treatment after the treatment with hydrogen peroxide and sodium hydroxide, which made the precipitated lignin fraction more homogeneous, which is beneficial for the utilization of lignin.
sample
Mn(g/Mol)
Mw(g/Mol)
PDI
Alkaline lignin
1796
2361
1.31
Altered lignin
1330
1579
1.19
3.2 Effect of different reaction conditions on the hydroxyl content of alkaline lignin
The reactivity of lignin determines how much it reacts with isocyanate groups. Due to the problem of low lignin activity, it is difficult to meet the experimental requirements by simply adding lignin to the reaction system to synthesize polyurethane, because the group in lignin that reacts with isocyanate is hydroxyl group (–OH), so increasing the content of hydroxyl group can improve its reactivity. Therefore, in this experiment, alkali lignin was modified by hydroxylation and the effect of different reaction conditions on the hydroxyl content of alkali lignin was investigated.
3.2.1 Effect of reaction time on the hydroxyl content of alkali lignin
The effect of reaction time on the hydroxyl content of lignin is shown in Fig. 5. From the figure, it can be seen that the hydroxyl content increased greatly from 0 to 60 min of the reaction, and after 60 min, it was difficult to increase the hydroxyl content. The reason may be that after 60 min the hydroxyl group on lignin reacts completely, resulting in a large reduction of its active site, which is difficult to participate in the subsequent reaction. Therefore, 60 min is the best reaction time for lignin modification.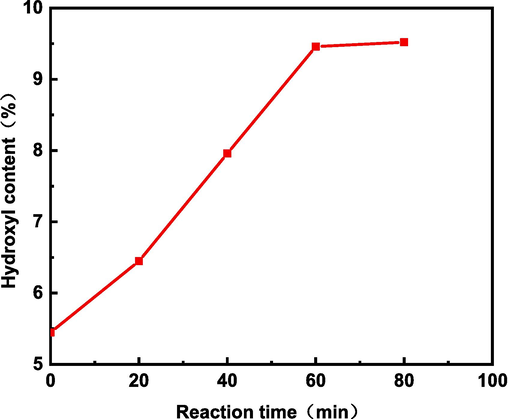
Effect of reaction time on hydroxyl content of modified alkali lignin.
3.2.2 Effect of reaction temperature on the hydroxyl content of alkali lignin
As the reaction temperature rises, the more energy the reactants can get, the more violent the reaction becomes. As shown in Fig. 6, the hydroxyl content reaches the maximum at 50 °C. This is because as the temperature increases, the number of activated molecules increases and the hydroxyl radicals increase, thus increasing the degree of lignin reaction. However, as the temperature continued to increase, that is, after 50 °C, the hydroxyl content decreased instead, probably because a side reaction occurred at this time when oxygen was produced by the decomposition of hydrogen peroxide. This was confirmed by the large number of bubbles produced in the reaction vessel at 60 °C and 70 °C. Therefore, 50 °C is the best reaction temperature for lignin.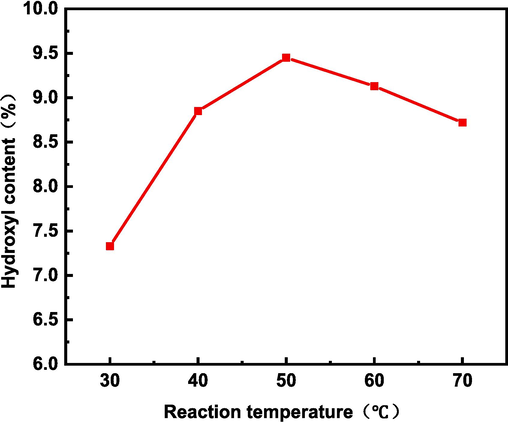
Effect of reaction temperature on hydroxyl content of modified alkali lignin.
3.2.3 Effect of mass ratio of iron hydroxide and alkaline lignin on hydroxyl content
The relationship between the mass ratio of iron hydroxide and alkali lignin and the hydroxyl content is shown in Fig. 7. When their mass ratio is 0.02, the hydroxyl content reaches the highest. This is because in the reaction system, the trivalent and divalent iron ions can be recycled and used, and a small amount of iron hydroxide is sufficient for the hydroxylation reaction. During the hydroxylation reaction of alkali lignin, iron hydroxide produces iron ions, which generate hydroxyl radicals with hydrogen peroxide, but iron ions can also catalyze the decomposition of hydrogen peroxide into oxygen. Therefore, when the mass ratio is above 0.02, the lignin hydroxyl content starts to decrease. This is because the increase of iron hydroxide leads to the faster decomposition of hydrogen peroxide into oxygen, which leads to the decrease of hydroxyl radical content and affects the hydroxylation modification of lignin, thus leading to the decrease of hydroxyl content. Therefore, it is not better to add more iron hydroxide to the catalyst, and the best ratio of iron hydroxide to lignin mass of 0.02 was selected.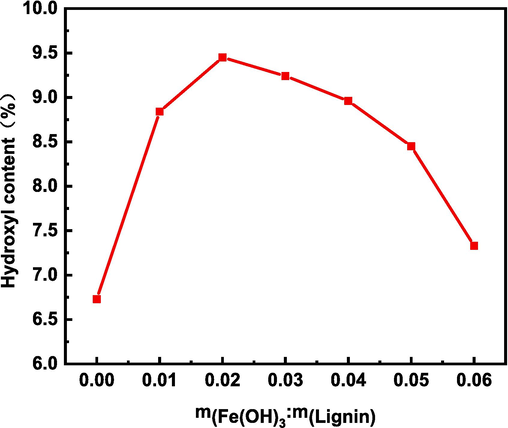
Effect of mass ratio of iron hydroxide and alkaline lignin on hydroxyl content.
3.2.4 Effect of hydrogen peroxide and alkaline lignin mass ratio on hydroxyl content
The relationship between the mass ratio of hydrogen peroxide and alkaline lignin and the hydroxyl content is shown in Fig. 8. The amount of hydrogen peroxide determines the amount of hydroxyl content, and when the mass ratio is less than 1.2, as the mass ratio keeps increasing, the free radicals produced also keep increasing, and therefore the hydroxyl content keeps increasing. When the mass ratio is greater than 1.2, the active sites on the lignin that can react are greatly reduced, and it is difficult for the free radicals to react with them, and the hydroxyl content cannot continue to increase. Therefore, to save cost, 1.2 is the best mass ratio of hydrogen peroxide and alkaline lignin.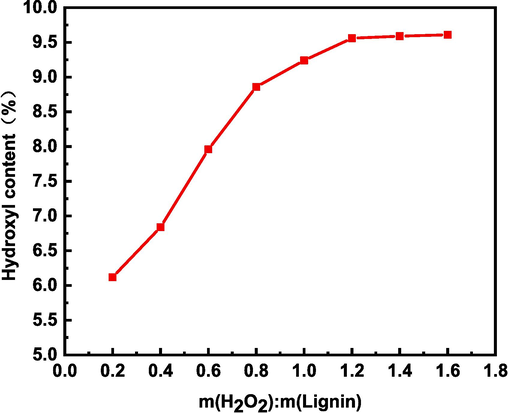
Effect of mass ratio of hydrogen peroxide to alkaline lignin on hydroxyl content.
3.3 Analysis of the effect of R-value on the structure and performance of LPU membranes
3.3.1 Distribution ratio of LPU groups with different R values
Table 2 shows the distribution ratios of LPU groups with different R values.
Samples
IPDI(g)
PCDL2000(g)
DEG(g)
Lignin(g)
R = 1.6
10
17.03
1.24
2.72
R = 1.7
10
16.00
1.19
2.56
R = 1.8
10
14.97
1.14
2.40
R = 1.9
10
13.93
1.09
2.23
R = 2.0
10
12.28
1.01
1.96
3.3.2 LPU infrared spectra with different R values
Fig. 9 shows the infrared spectra of LPU membranes with different R values, and each sample was successfully prepared. The benzene ring characteristic absorption peaks belonging to lignin were observed around 1516 cm−1, 1425 cm−1 and 1635 cm−1 for all LPUs, which not only showed that the lignin structure was not destroyed during the reaction but also indicated that the LPU structure obtained under different R values was the same. It can be seen from the figure that with the increase of R-value, the carbonyl (C = O) expansion vibration peak of the aminoester group at 1739 cm−1 gradually increases, because when the R-value increases, the content of isocyanate groups increases, increasing carbonyl content.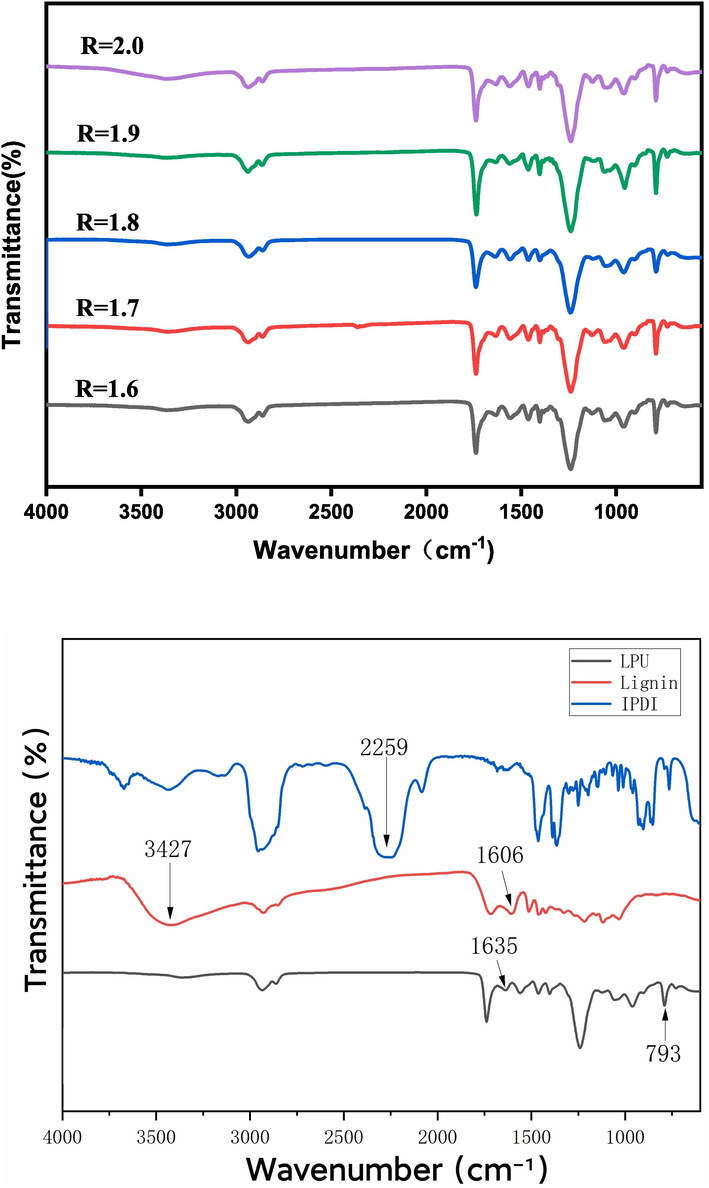
LPU infrared spectra with different r values.
Compared with the infrared images, it can be seen that the infrared absorption peak of the hydroxyl group in lignin is at 3427 cm−1, but there is no such absorption peak in the LPU, indicating that the hydroxyl group is involved in the reaction. At 2259 cm−1 is the infrared absorption peak of the isocyanate group, but it is not present in the LPU, indicating that the isocyanate is also fully involved in the reaction. The absorption peak at 2929 cm−1 was the tensile vibration of C–H, which was significant in LPU, indicating that the preparation of LPU did not affect the absorption peak, the infrared absorption peaks of the benzene ring at 1606 cm−1 and 1635 cm−1 indicated that lignin was successfully involved in the reaction, and the infrared absorption peak of carbamate at 793 cm−1 indicated that lignin-based polyurethane was successfully synthesized (Wu et al., 2023; Lai et al., 2021).
3.3.3 Scanning electron microscope with different R values
Fig. 10 shows the microscopic cross-sectional structures of LPU membranes with different R values. From the figure, it can be seen that there are black and white areas in the cross sections of LPU films with different R values, and there are fewer white areas in the cross sections with low R values, which indicates that LPU is more compatible and has smooth cross sections but a small amount of particles, which may be due to the buildup of lignin that fails to integrate into the polymer network structure. This also indicates that the hydroxyl groups in the lignin cannot be fully activated and are less active in the case of a low R-value. From the figure, it is concluded that as the R-value increases, the white area becomes more and more white, the compatibility becomes worse, and the cross-section becomes coarser and coarser.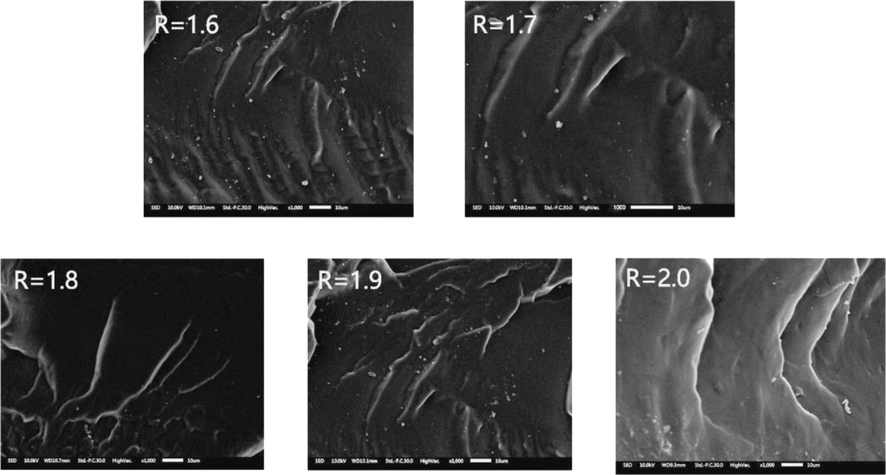
Scanning electron microscope with different r values.
3.3.4 Effect of different R values on mechanical properties of LPU
Fig. 11 shows the effects of different R values on the mechanical properties of LPU films. When the R values were 1.6, 1.7, 1.8, 1.9 and 2.0, the corresponding average tensile strengths were 32.56 MPa, 34.26 MPa, 37.24 MPa, 40.69 MPa and 48.32 MPa, respectively, and the corresponding average elongations at break were 899.66 %, 840.65 %, 824.69 %, 801.23 % and 753.65 %. As the R-value increases, the tensile strength of LPU becomes greater and the elongation at break becomes lower. the tensile strength of LPU increases steadily at R values from 1.6 to 1.9, while the growth rate of tensile strength accelerates at R values from 1.9 to 2.0. this is because thermosetting plastics depend on their network structure, and increasing the R-value increases the content of hard segments in polyurethane, while the degree of cross-linking of the material and the hardness of the material will also increase. When the R-value exceeds 1. 9, as the degree of cross-linking continues to increase, it leads to stronger intermolecular forces and more difficult movement of the chain segments, so the tensile strength will be greater and the trend of elongation at break is also affected by this.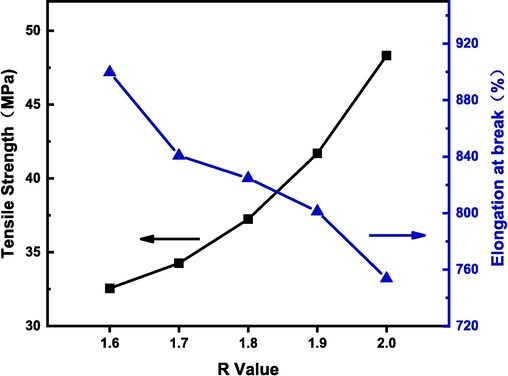
Effect of different R values on mechanical properties of LPU.
3.3.5 TGA analysis of different R values
The effect of different R values on the thermal stability of polyurethane was studied by the TGA method, and the TGA curve of LPU film is shown in Fig. 12. LPU chain is divided into rigid chain segments and soft segments, and the thermal stability of soft segments is higher than that of rigid chain segments. Therefore, the whole weight loss process is divided into two stages, the first stage starts at about 230 °C, which is related to the breakage of carbamate bond and degradation of lignin in LPU, and the breakage of carbamate bond will produce primary amine, olefin and secondary amine. The second weight-loss stage occurs at 365 °C, which is related to the decomposition of soft segments in LPU, and the final thermal decomposition process stops roughly at 600 °C.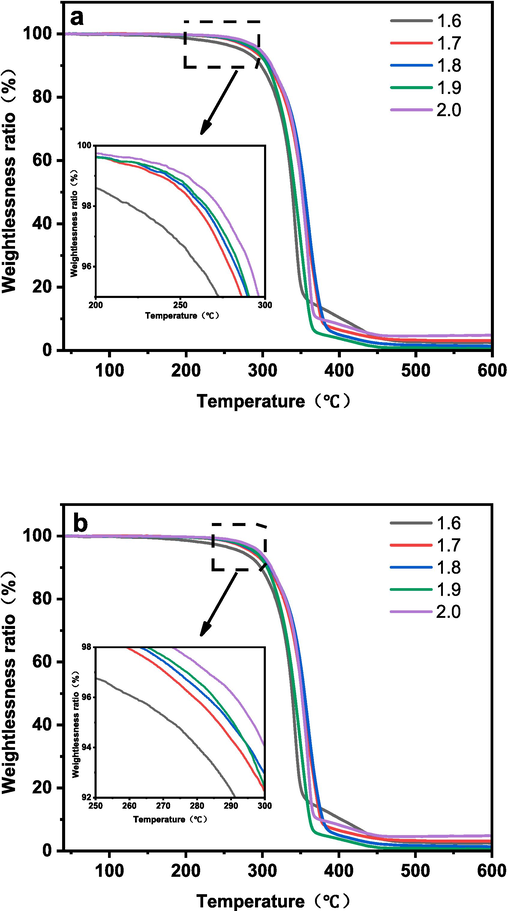
TGA analysis of different R values, (a) TGA amplification curve when weight loss is 2.5%, and (b) TGA amplification curve when weight loss is 5%.
As can be seen from the Fig. 12, the temperatures of LPU membranes with different R values at 2.5 % weight loss were 235.0 °C, 265.4 °C, 269.3 °C, 271.8 °C, and 278.4 °C, respectively. The temperatures at 5 % weight loss were classified as 272.7 °C, 286.2 °C, 289.8 °C, 290.6 °C, and 296.4 °C. With the increase of the R-value, the thermal decomposition temperature of LPU is higher, which is because with the increase of the R-value, the number of polar groups in the molecular chain increases, the hydrogen bond becomes stronger, the intramolecular strength increases, and the degree of cross-linking of LPU increases, the heat resistance increases, and the thermal stability of LPU is improved.
3.3.6 Effect of different R values on barrier properties of LPU membrane
The arrangement density and structural state of polymer molecular chains affect the barrier properties of film materials. The effects of different R values on the barrier properties of LPU films were analyzed by the variation of oxygen permeability coefficient and moisture permeability coefficient. Fig. 13 shows the effect of different R values on the change of oxygen permeability coefficient and moisture permeability coefficient. As shown in Fig. 13, with the increase of R-value, the oxygen permeability coefficient and moisture permeability coefficient of LPU film are constantly decreasing. The oxygen permeability coefficient decreased from 13.57x10-14cm3·cm/(cm2∙s∙pa) to 6.12x10-14cm3∙cm/(cm2∙s∙pa), and the moisture permeability coefficient decreased from 8.23 × 10-14 g∙ cm/(cm2∙s∙Pa) to 3.64 × 10-14 g∙ cm/(cm2∙s∙Pa). This is because with the increase of R-value, the content of the hard segment increases, the content of the polar group isocyanate group increases, the crystallinity of LPU molecular chains is improved, the molecular chains are arranged more closely, and the path of small molecule gas passes through becomes complicated, so that the oxygen permeability and moisture permeability of LPU are reduced, and the barrier is improved.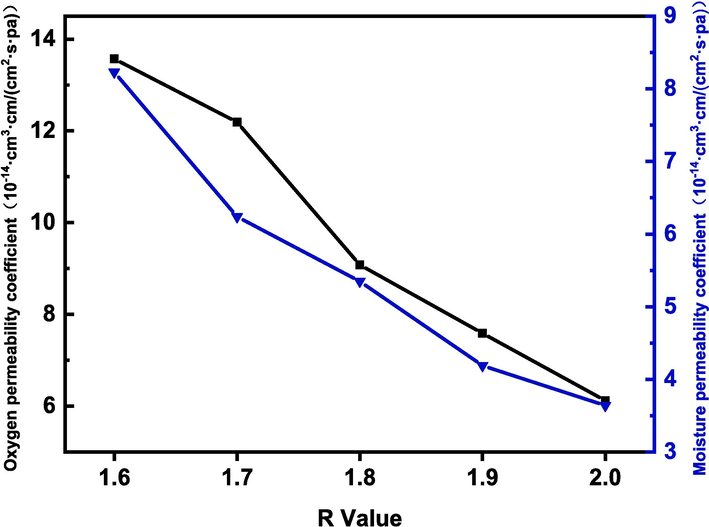
Effect of different R values on barrier properties of LPU membrane.
3.3.7 Effect of different R values on optical properties of LPU films
The optical properties of LPU films with different R values were analyzed by UV–visible spectroscopy. It can be seen from Fig. 14 that the transparency of LPU films decreases with the increase of R-value, and the transmittance is reduced to 0 at wavelengths less than 400 nm. Material transparency was also modified by decreasing the amorphous nature of the materials with the increase in the R values. The main reason is that the R-value increases, the order of the hard segment of the LPU membrane increases, the molecular bond hydrogen bond of the hard segment increases, the degree of microphase separation of the soft and hard segment increases, and the crystallization behavior and crystallinity of the hard segment increase (Xu et al., 2018; Gomez et al., 2016). The crystallinity is not conducive to the transmission of light, resulting in a decrease in the transparency of the LPU film. In addition, if the surface of the film is not smooth and uniform, it may cause light scattering, deviating from the direction of incident light and thus reducing the light transmittance. The sudden jump in the R-value from 1.9 to 2.0 may be due to some error in the sample itself, or it is possible that an R-value of 2.0 does not meet the requirements of the test.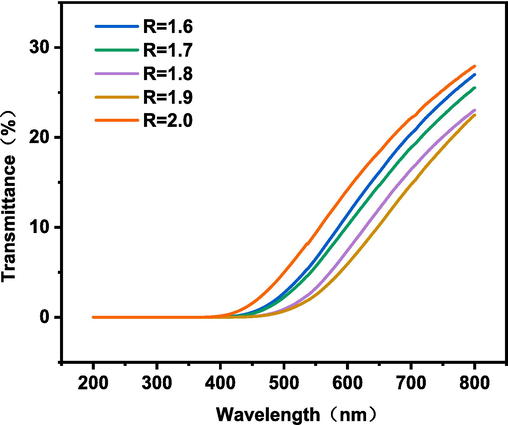
Effect of different R values on optical properties of LPU films.
4 Conclusions
Iron hydroxide was used as a catalyst to modify lignin with hydrogen peroxide in a sodium hydroxide solution. The reaction time was exactly complete at 60 min and the reaction rate reached the fastest at a reaction temperature of 50 °C. When the reaction temperature was greater than 50 °C the reaction was violent and a large number of bubbles appeared, and the measured lignin hydroxyl content became less. When too much iron hydroxide is added, the decomposition of hydrogen peroxide is accelerated and the lignin hydroxyl content becomes smaller. When too much hydrogen peroxide is added, the hydroxyl content does not increase because the lignin reaction is already complete. Therefore, the best conditions for the reaction are: water bath heating temperature of 50 °C, sodium hydroxide solution concentration of 3 %, hydrogen peroxide to lignin mass ratio of 1.2:1, iron hydroxide to alkali lignin mass ratio of 2 %, and the lignin hydroxyl value measured under these conditions is 315.3 mgKOH/g, with a hydroxyl content of 9.56 %.
The above tests analyzed the effect of the R-value at 1.6–2.0 on LPU properties. The test results showed that the tensile strength of LPU films increased from 32.56 MPa to 48.32 MPa and the elongation at break decreased from 899.66 % to 753.65 % as the R-value increased. In terms of thermal properties, the temperatures of LPU films with different R values were 235.0 °C, 265.4 °C, 269.3 °C, 271.8 °C and 278.4 °C at 2.5 % weight loss and 272.7 °C, 286.2 °C, 289.8 °C, 290.6 °C and 296.4 °C at 5 % weight loss, respectively, indicating an improvement in thermal properties. As for the barrier properties, the oxygen permeability coefficient decreased from 13.57x10-14cm-3cm/(cm2∙s∙pa) to 6.12x10-14cm3∙cm/(cm2∙s∙pa) and the moisture permeability coefficient decreased from 8.23x10-14 g∙ cm/(cm2∙s∙Pa) to 3.64x10-14 g∙ cm/(cm2∙s∙Pa), the barrier performance is greatly improved. As for the optical properties, the transmittance kept decreasing, especially in the wavelength range of 200–400 nm when the transmittance decreased to zero, indicating that the LPU film had an obvious UV-blocking effect after the addition of lignin. Considering the mechanical properties, thermal stability, and UV barrier properties, it is most appropriate to select the R-value of 1.8.
Acknowledgments
This work was supported by the China Postdoctoral Science Foundation (No. 200902090) and Tianjin.
Enterprise Science and Technology Commissioner Project (No. 21YDTPJC00570).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Lignin-based polyurethanes: Opportunities for bio-based foams, elastomers, coatings and adhesives. Polymers. 2019;11(7):1202.
- [CrossRef] [Google Scholar]
- Lignocellulose biomass liquefaction: Process and applications development as polyurethane foams. Polymers. 2023;15(3):563.
- [CrossRef] [Google Scholar]
- Synthesis of waterborne polyurethane using snow as dispersant: Structures and properties controlled by polyols utilization. J. Mater. Sci. Technol.. 2019;35(7):1491-1498.
- [CrossRef] [Google Scholar]
- Sustainable strategy for algae biomass waste management via development of novel bio-based thermoplastic polyurethane elastomers composites. Molecules, [online]. 2023;28(1):436.
- [CrossRef] [Google Scholar]
- Transparent thermoplastic polyurethanes based on aliphatic diisocyanates and polycarbonate diol. J. Elastomers Plast.. 2016;49(1):77-95.
- [CrossRef] [Google Scholar]
- Analysis of factors influencing the efficiency of catalysts used in waste PU degradation. Polymers. 2022;14(24):5450.
- [CrossRef] [Google Scholar]
- The effect of chain extender type on the physical, mechanical, and shape memory properties of poly(ε-Caprolactone)-based polyurethane-ureas. Polym.-Plast. Technol. Eng.. 2017;56(18):1977-1985.
- [CrossRef] [Google Scholar]
- Preparation and Properties of waterborne polyurethane?urea anionomers?influences of the type of neutralizing agent and chain extender. Colloid Polym. Sci.. 2003;281(10):957-963.
- [CrossRef] [Google Scholar]
- Facile preparation and characterization of low-gloss waterborne polyurethane coatings using amine-based chain extenders. Polym. Int.. 2022;72(1)
- [CrossRef] [Google Scholar]
- Preparation and performance of lignin-based waterborne polyurethane emulsion. Indus. Crops Prod., [online]. 2021;170(2021,170):113739
- [CrossRef] [Google Scholar]
- Kraft lignin-based rigid polyurethane foam. J. Wood Chem. Technol.. 2012;32(3):210-224.
- [CrossRef] [Google Scholar]
- Lignin-based polyurethane: Recent advances and future perspectives. Macromol. Rapid Commun.. 2020;42(3):2000492.
- [CrossRef] [Google Scholar]
- Ng, W.S., Lee, C.S., Chuah, C.H. and Cheng, S.-F. (2017). Preparation and Modification of water-blown Porous Biodegradable Polyurethane Foams with Palm oil-based Polyester Polyol. Industrial Crops and Products, [online] 97(97), pp.65–78. doi:https://doi.org/10.1016/j.indcrop.2016.11.066.
- Progressive belowground soil development associated with sustainable plant establishment during copper mine waste revegetation. Appl. Soil Ecol.. 2023;186(4):104813.
- [CrossRef] [Google Scholar]
- Development of lignin-based polyurethane thermoplastics. RSC Adv.. 2013;3(44):21832.
- [CrossRef] [Google Scholar]
- Effect of chain extender on morphologies and properties of PBAT/PLA composites. J. Thermoplast. Compos. Mater.. 2021;36(3):1175-1186.
- [CrossRef] [Google Scholar]
- Fascinating polyphenol lignin extracted from sawdust via a green and recyclable solvent route. Int. J. Biol. Macromol.. 2023;234(234):123780.
- [CrossRef] [Google Scholar]
- Lignin-based polyurethanes from unmodified kraft lignin fractionated by sequential precipitation. ACS Appl. Polym. Mater.. 2019;1(7):1672-1679.
- [CrossRef] [Google Scholar]
- Wu, Z., Zhang, X., Kang, S., Liu, Y., Bushra, R., Guo, J., Zhu, W., Khan, M.R., Jin, Y. and Song, J. (2023). Preparation of biodegradable recycled fiber composite film using lignin-based polyurethane emulsion as strength agent. Industrial Crops and Products, [online] 197(2023, 197), p.116512. doi:https://doi.org/10.1016/j.indcrop.2023.116512.
- Synthesis of transparent covalently self-colored polyurethane based on anthraquinone chromophore chain extenders. Prog. Org. Coat.. 2018;123(123):1-9.
- [CrossRef] [Google Scholar]
- Synergistic effects of chain extenders and natural rubber on PLA thermal, rheological, mechanical and barrier properties. Polymer. 2023;269(269):125712.
- [CrossRef] [Google Scholar]







