Translate this page into:
Preparation and tribology properties of water-soluble fullerene derivative nanoball
⁎Corresponding author. Tel./fax: +86 2260274489. wangziqing1978@163.com (Guichang Jiang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Abstract
Water-soluble fullerene derivatives were synthesized via radical polymerization. They are completely soluble in water, yielding a clear brown solution. The products were characterized by FTIR, UV–Vis, 1H-NMR, 13CNMR, GPC, TGA, and SEM. Four-ball tests show that the addition of a certain concentration of the fullerene derivatives to base stock (2 wt.% triethanolamine aqueous solution) can effectively increase both the load-carrying capacity (PB value), and the resistance to wear. SEM observations confirm the additive results in a reduced diameter of the wear scar and decreased wear.
Keywords
Lubricant additive
Chemical
Coefficient
Fullerene (C60), which has a spherical π-electron system, indicates interesting magnetic (Allemand et al., 1991; Makarova et al., 2001), superconductivity(Hebard et al., 1991; Grant, 2001), electrical (Haddon et al., 1995), and biochemical properties (Tokuyama et al., 1993; An et al., 1996). Not surprisingly, fullerenes and their derivatives have attracted a lot of attention in the recent years and have been successfully applied to materials science and biological technology. However, the application of fullerenes and their derivatives has now been limited because of their low solubility in water and frequently used solvents.
Since C60 shows a poor solubility in common solvents (it is only soluble in few solvents), many efforts have been made to modify this molecule in complex pathways. One access to modified C60 through covalent and noncovalent binding describes a mechanochemical technique (Braun et al., 1994). Also studies for the fullerene–cyclodextrin complex by mechanochemical synthesis have been reported (Braun et al., 1994). Although the functionalization of C60 could bring about numerous potential applications in improving the properties of composites, there are still two major obstacles to overcome, i.e. the wettability of C60 and interfacial interaction with composite materials. So, further development in the modification of C60 is still required. Herein, water-soluble fullerene–vinylpyrrolidone random copolymers with different fullerene contents have been synthesized by the radical polymerization of vinylpyrrolidone in the presence of fullerene.
The lubricating properties of C60 have been speculated since the time of its discovery because of its unique spherical shape with cage diameter of 0.71 nm, low surface energy, high load-bearing capacity, high chemical stability, weak intermolecular and strong intramolecular bonding (Kratschmer et al., 1990; Kroto et al., 1985; Feng, 1997; Bhushan et al., 1993), and it was expected to act as tiny ball bearing (Blau and Haberlin, 1992). Theoretical simulations indicated that fullerene molecule would be able to roll between graphite sheets, as well as between hydrogen-terminated surfaces of diamond under relatively low load (White et al., 1993). The lubricating properties of C60 particles as additives to liquid lubricants were investigated by a few groups (Yan et al., 1993). The results found that the presence of C60 was able to increase the load-carrying ability and decrease the friction coefficient and wear. However, in these experiments C60 particles were dispersed in liquid lubricants by physical or mechanical means (such as solid grinding, solvent evaporation, ultrasound). Since they are soluble in only few non-polar solvents (such as benzene, toluene, and carbon disulfide), C60 particles were present in the form of molecular clusters or super-corpuscles (Yan et al., 1993). Up to this date, the lubricating properties of soluble C60 and its derivatives have seldom been studied. In this paper, water-soluble fullerene derivatives were prepared, in which the fullerene was reacted with the polymer by a chemical reaction. The frictional behavior of the water-soluble C60–vinylpyrrolidone copolymer was investigated for the first time. Then, the assembly behavior of the water-soluble fullerene derivatives was investigated by SEM. The results show that the fullerene derivatives create morphology that is sphere-like. The purpose of synthesis of this fullerene derivative nanoball was its use as a lubricant additive. This fullerene derivative has the possibility to cause a microcosmic rolling effect between two rubbing surfaces as nanometer tiny ball.
1 Experimental methods
1.1 Materials
Fullerene (C60) was obtained from Wuhan University (purity 99%). Vinylpyrrolidone and benzoyl peroxide were purchased as commercial products from Acros. Vinylpyrrolidone was distilled under vacuum at 90 °C. Toluene was distilled at 110 °C. All other reagents and solvents such as dimethyl sulfoxide (DMSO) were used as received without further purification.
1.2 Preparation of water-soluble fullerene derivatives
A solution of 30–100 mg of C60 in 30–100 mL of toluene or 1, 2-dichlorobenzene (or pyridine, dioxane) was mixed with 0.1–4.0 g of vinylpyrrolidone. The mixture was deoxygenated by bubbling dry nitrogen gas for ∼30 min, followed by the addition of 150 mg of BPO (benzoyl peroxide). The polymerization reaction was carried out in a glass bottle at 70–80 °C for 30 h. A change in color from violet (characteristic of C60 in toluene) to red–brown was observed during the reaction. After solvent removal under reduced pressure, the residue was dissolved in THF, and unreacted C60 was removed by centrifugation (5000 rpm, 30 min) and filtration through 0.45 μm membrane filters. The THF solution was concentrated under reduced pressure and subsequently poured into an excess of heptane for precipitating the copolymer (a solid sample with almost brown color). The copolymer was dissolved in water and was further purified by column chromatography (Sephadex G-25, distilled deionized water as eluent). After drying under vacuum, the final copolymer sample (water-soluble fullerene derivative) was obtained (purity 95%). A polymer of neat vinylpyrrolidone was prepared under the same conditions to be used as a reference. FTIR (KBr): ν 2959, 1660, 1458, 1422, 1295, 1120, 658, 530 (C60) cm-1; 1H NMR (300 MHz, CDCl3, TMS): δ = 1.50–1.75 (m, 2H, CH2CH), 1.86–2.01 (m, 2H, CH2), 2.10–2.32 (m, 2H, CH2CO), 3.01–3.30 (m, 2H, CH2 N), 3.60–3.82 (m, 1H, CH) ppm; 13C NMR (75 MHz, CDCl3, TMS): δ = 17.2, 18.1, 31.8, 45.0, 94.2, 140–150 (C60), 174(f) ppm.
1.3 Characterization
The molecular weights and their distributions of the copolymers were determined by gel permeation chromatography (GPC) utilizing a Waters model 515 pump and a model 2410 differential refractometer with three Styragel columns HT2, HT3, and HT4 connected in a serial fashion. THF was used as the eluent at a flow rate of 1.0 mL/min. Polystyrene standards with dispersity of 1.08–1.12 obtained from Waters were employed to calibrate the instrument. IR spectra were recorded with a Perkin–Elmer FT-IR spectrophotometer using KBr disks. 1H NMR spectra were recorded in CDCl3 at 20 °C on a Bruker AM 300 MHz apparatus. 13C NMR spectra were recorded in CDCl3 at 20 °C on a Bruker AM 75 MHz apparatus. UV–vis absorption spectra were recorded on a Hitachi U-3300 spectrophotometer.
Thermogravimetric analyses (TGA) were carried out under nitrogen with a TA Instruments Q500 thermogravimetric analyzer in the 25–1000 °C range, at a 10 °C/min rate (HiRes method).
1.4 Self-assembly of water-soluble fullerene derivatives
The solvent-induced assembly of water-soluble fullerene derivatives was investigated by dissolving the fullerene derivatives in THF:water (80:20, v/v), filtering and dropping on a glass patch. After evaporation for 48 h under ambient conditions, the samples were observed by SEM.
1.5 Measurement of tribology properties
Since pure water has poor lubricity and is corrosive, a 2 wt.% of triethanolamine aqueous solution was chosen as base stock. Triethanolamine is widely used in the metal-working fluids as a multifunctional additive. The fullerene-containing polymer prepared above was used as a lubricant additive in the base stock. The tribological measurements were carried out by using an MQ-800 four-ball tribotester at a rotational speed of 1450 rpm and at a temperature of 25 °C. The maximum nonseizure load was obtained by GB3142–82, similar to ASTMD2783; the wear scar diameter was measured under a load of 200 N and test duration of 30 min; friction coefficient was measured under a load of 200 N and test duration of 10 s. The steel balls used in the tests were made of GCr15 (AISIE52100) bearing steel with the 64–66 surface HRC hardness and 0.012 μm of surface roughness Ra. In order to study the effect of C60 in the C60−vinylpyrrolidone copolymer, the same concentration of the polyvinylpyrrolidone was used as a reference additive and measured under the same conditions.
2 Results and discussion
2.1 Synthesis of water-soluble fullerene derivatives
Water-soluble fullerene derivatives were prepared according to the procedure shown in Scheme 1. They are soluble in polar solvents such as water, acetone, tetrahydrofuran and dimethyl sulfoxide. The solubility of derivatives in water is approximately 5 mg/ml (calculated as C60 residue), which is larger than that of the inclusion complex of cucurbit (Braun et al., 1994) uril and C60. One reasonable explanation for the increase of solubility is that the intrinsic solubility of PVP is larger than that of cucurbit(Braun et al., 1994)uril. The covalent attachment of C60 to the polyvinylpyrrolidone backbone was confirmed by a variety of techniques. FTIR spectra were measured in KBr matrices, and the results were compared with that of neat polyvinylpyrrolidone samples prepared under the same experimental conditions (Fig. 1). As shown in Fig. 1, observed FTIR spectrums of the copolymers with C60 contents are different from that of neat polyvinylpyrrolidone. For the copolymers with C60 contents, the FTIR spectrum shows extra absorption in the 520–530 cm−1 region, which is typical with respect to substituted C60 (Bunker et al., 1995). UV absorption spectra were measured in CH3OH, and the results were compared with that of polyvinylpyrrolidone (Fig. 2). As shown in Fig. 2, the observed UV absorption spectra of copolymers with C60 contents are very different from that of polyvinylpyrrolidone. The somewhat structured absorption band of free C60 is replaced by a steadily decreasing curve, typical for substituted C60 (Bunker et al., 1995). UV absorption wavelength maximum of copolymers with C60 contents is 202 nm, different from that of free C60 and polyvinylpyrrolidone, which is typical with respect to substituted C60. The emission wavelength can be attributed to the C60-containing sites in the polyvinylpyrrolidone structure. In the 1H NMR spectrum of the copolymers with C60 contents (Fig. 3), peaks corresponding to the CH2 aliphatic protons adjacent to the ketone group of the vinylpyrrolidone and the other CH2 aliphatic protons adjacent to the CH group of vinylpyrrolidone, were observed at 2.10–2.32 and 1.50–1.75 ppm, respectively. The peak related to the CH aliphatic protons from vinylpyrrolidone also appeared between 3.60 and 3.82 ppm. In the 13C NMR spectrum of the copolymers with C60 contents (Fig. 4), peak corresponding to the C60 was observed at 140–150 ppm (Guhr et al., 1994). The C60−vinylpyrrolidone copolymers proved to be extremely soluble in a variety of organic solvents such as H2O, CHCl3, etc. This solubility behavior is similar to poly-vinylpyrrolidone itself and totally unlike C60, which demonstrates the dramatic effect the polymer attachment has on the processability of fullerene derivatives. The weight percent of C60 incorporated into the copolymers could be determined by TGA since the polyvinylpyrrolidone backbone decomposes at 230–280 °C to leave C60 which undergoes only minor weight loss up to 600 °C. Comparison with starting materials and physical mixtures confirmed the TGA results. Interestingly the weight percent of C60 was found to be ca. 80% of the theoretical value. The above results are all consistent, with monoaddition being the dominant process, and demonstrate the viability of this procedure for the preparation of copolymers with high loadings of C60.
Synthesis of water-soluble C60−vinylpyrrolidone copolymers.

FTIR spectra of C60−vinylpyrrolidone copolymer (a) and polyvinylpyrrolidone (b).

Comparison of C60−vinylpyrrolidone copolymer’s UV absorbance (a) with that of polyvinylpyrrolidone (b).

1H NMR spectrum of the copolymers with C60 (top) and vinylpyrrolidone (bottom) taken in CDCl3.

13C NMR spectrum of the copolymers with C60 taken in CDCl3.
The results presented here clearly show that C60 and vinylpyrrolidone can be copolymerized under different conditions (Table 1). With a constant benzoyl peroxide amount, C60 contents in the copolymers increase with increasing initial C60: vinylpyrrolidone reactant ratio (Table 1). There is no apparent reduction in the polymer yield at high initial C60: vinylpyrrolidone reactant ratios, which is probably due to relatively small [C60]/[initiator] values. In addition, solubility in water of the copolymers prepared with different initial C60: vinylpyrrolidone reactant ratios is significantly different (Table 1). In a classical mechanism of radical polymerization, propagation of
radicals is critical to the formation of true C60−vinylpyrrolidone copolymers. However, it has been suggested that propagation of
radicals is slow in general because of their relatively high stability. It has also been suggested that C60 competes effectively with vinylpyrrolidone (VP) for initiator radicals and, as a logical extrapolation, for VP⋅ radicals as well. In this regard, C60 may act as a radical inhibitor in the copolymerization reaction. Evidence for the inhibiting effect includes the observation that at high [C60]/[initiator] ratios polymerization yields are low in comparison with those in neat vinylpyrrolidone polymerization. Thus, the mechanism of C60−vinylpyrrolidone copolymerization may be different from the classical mechanism of radical polymerization of vinylpyrrolidone such that there is a significant population of C60 radicals and their propagation is relatively slow. However, even with the suggested inhibiting effect of C60 in the copolymerization reaction, contributions of polyvinylpyrrolidone structures are substantial even in the copolymers with high C60 contents, as shown in the observed FT-IR spectra of the copolymers. Gel permeation chromatography (GPC) results indicate that the materials do not contain oligomers, and the C60−vinylpyrrolidone copolymer and the polyvinylpyrrolidone reference have almost the same molecular weight distributions (weight- average molecular weight of 52,000–78,000 and polydispersity of 3–6). The results could be used to support a proposed copolymer mechanism as follows.
ID
C60:vinylpyrrolidone (mg:g)
Ben.peroxide (mg)
Yield (%)a
C60 wt.%
Solvent
Productb (mg/ml)
I
30:4.0
150
∼46
∼1.6
Pyridine
5
II
50:1.3
150
∼66
∼5.6
Dioxane
5
III
70:1.1
150
∼68
∼8.8
Dichlorobenzene
3
IV
100:1.0
150
∼70
∼13
Toluene
5
V
100:0.5
150
∼60
∼30
Toluene
4
VI
100:0.1
150
∼95
∼53
Toluene
2
2.2 Self-assembly of water-soluble fullerene derivatives
The assembly behavior of water-soluble fullerene derivatives was investigated by SEM. The solvent-induced assembly of water-soluble fullerene derivatives was investigated by dissolving the fullerene derivatives in THF:water (80:20, v/v), filtering and dropping on a glass patch. After evaporation for 48 h under ambient conditions, the samples were observed by SEM. SEM images showed the macrostructure of water-soluble fullerene derivatives was spheres (Fig. 5a), which is different from the irregular morphologies found for polyvinylpyrrolidone (Fig. 5b). The average sphere size was calculated from the SEM picture and the sphere sizes were homogeneous. The samples have sphere sizes ranging from 30 nm to 80 nm. The quantity of water in the incorporated solution performs an important role in the assembly and morphological shifts in the fullerene derivatives. SEM analysis shows that the average particle diameter of fullerene derivative nanoball is about 55 nm.
SEM images of C60−vinylpyrrolidone copolymers (A) and polyvinylpyrrolidone (B).
2.3 Tribology properties of water-soluble fullerene derivatives
The maximum nonseizure load (PB value) represents the load-carrying capacity of the lubricant. The PB value was measured and the results are given in Fig. 6(A). The PB value of the base stock is 150 N, and the base stock with the C60−vinylpyrrolidone copolymer showed a higher maximum nonseizure load than that of the base stock. In other words, the fullerene containing polymer could strengthen the load-carrying capacity of the base stock. When the fullerene derivative content reaches 0.3 wt.%, the PB value is a maximum. Then, increased fullerene containing polymer resulted in a decrease in load-carrying capacity of the base stock.
Effect of the fullerene copolymer content on: (A) maximum nonseized load; (B) wear scar diameter and (C) friction coefficient.
The wear scar diameter data are given in Fig. 6(B). It is seen that the addition of the fullerene derivative can decrease the wear scar diameter of the base stock. When the fullerene containing polymer content reaches 0.3 wt.%, the wear scar diameter is minimum. But excessive fullerene copolymer content results in a larger wear scar diameter than that of the base stock. Decrease in load-carrying capacity and wear resistance at excessive fullerene-containing polymer content may be attributed to the corrosive wear, since the fullerene-containing polymer may react with the surface of the metal.
The friction coefficient measurements were carried out and the results are given in Fig. 6(C). It is indicated that with the increase of the additive content, the friction coefficient reduces rapidly from 0.233 of base stock to minimum 0.091 of that containing 0.8 wt.% fullerene copolymer.
The reduction in friction coefficient implies that the fullerene copolymer has the possibility to cause a microcosmic rolling effect between two rubbing surfaces as nanometer tiny ball. The dependence of wear scar diameter on load is shown in Fig. 7(A).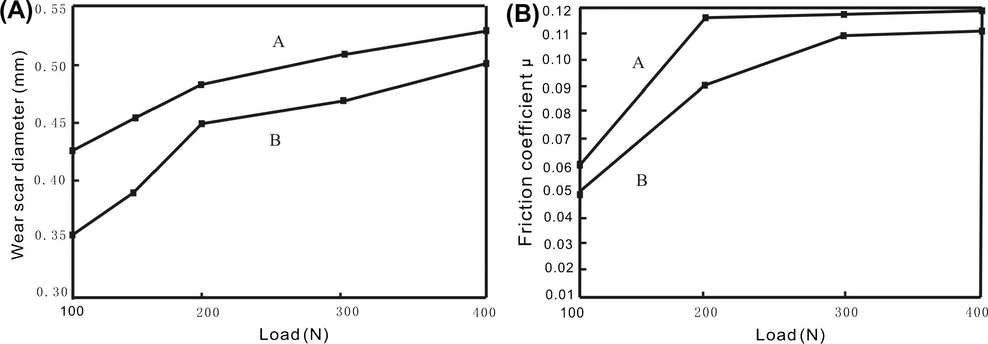
Effect of load on: (A) wear scar diameter and (B) friction coefficient. (A) Base stock with 0.3 wt.% polyvinylpyrrolidone; (B) base stock with 0.3 wt.% C60−vinylpyrrolidone copolymer.
Under testing loads, the wear scar diameter of base stock with 0.3 wt.% fullerene copolymer is smaller than that with 0.3 wt.% polyvinylpyrrolidone. It means that, the presence of fullerene-containing polymer can strengthen the wear resistance of base stock.
Fig. 7(B) shows that, during the testing loads, the friction coefficient of base stock with 0.3 wt.% fullerene copolymer is lower than that with 0.3 wt.% polyvinylpyrrolidone, especially at the load of 200 N, the difference in friction coefficient between the two additives is larger, which may be attributed to the lower load-carrying capacity of the polyvinylpyrrolidone. The direct touch between the two friction surfaces then occurred, which in turn led to a rapid rise in friction coefficient.
The worn surface in four-ball machine testing, which was obtained under a load of 200 N and a testing time of 30 min, was observed by SEM. The wear scar of base stock with 0.3 wt.% polyvinylpyrrolidone and that with 0.3 wt.% fullerene-containing polymer are shown in Figs. 8 and 9, respectively. It indicated that the wear scar obtained with the fullerene-containing polymer additive is obviously smaller and exhibits mild scratches, but in comparison, larger wear scar and sharp tracks were observed in the presence of base stock with polyvinylpyrrolidone additive. In other words, the fullerene-containing polymer can improve the microcosmic wear condition.
Morphology of the wear scar lubricated by base stock with 0.3 wt.% polyvinylpyrrolidone (100x).

Morphology of the wear scar lubricated by base stock with 0.3 wt.% C60−vinylpyrrolidone copolymer (100x).
Based on the above lubrication measurements and worn surface analyses, the lubrication mechanism of the fullerene-containing polymer additive can be deduced. The fullerene-containing polymer plays the role of a solid lubricant. Since fullerene has a very high load-carrying capacity and the fullerene copolymer is nanometer tiny balls with core–shell structure, which can penetrate into rubbing surfaces and deposit there, it is reasonable to speculate that the fullerene copolymer nanometer balls may be more effective than polyvinylpyrrolidone to support and isolate two relative motion surfaces, and therefore, the load-carrying capacity and anti-wear performance of the base stock would be improved. Moreover, the nanometer balls are expected to roll between two relative motion surfaces to reduce the friction coefficient. In addition, the shell is a polymer of neat vinylpyrrolidone, which is relatively soft but very elastic, thus improving the microcosmic condition of wear.
3 Conclusions
Water-soluble fullerene derivatives were prepared. They are soluble in water, yielding a clear brown solution. SEM analysis shows that they present an ideal spherical shape in water with a diameter ranging from 30 to 80 nm. These water-soluble fullerene derivatives exhibit good tribological properties. As a lubricant additive in base stock (2 wt.% triethanolamine aqueous solution), they can improve the wear resistance, load-carrying capacity and anti-friction ability of base stock. Excessive fullerene-containing polymer additive was disadvantageous for wear resistance and load-carrying capacity. SEM analyses indicate that the wear scars obtained with base stock exhibited a sharp grooving and serious pull-out phenomenon, while only mild scratches are observed in the presence of fullerene-containing polymer additive. The lubrication mechanism of the fullerene-containing polymer is likely attributed to its spherical shape structure, which can cause microcosmic elastic rolling as nanometer tiny balls between the two rubbing surfaces.
Acknowledgements
The Project was supported by the Tribology Science Fund of State Key Laboratory of Tribology (SKLTKF10B08). The Project was supported by the Fund of Tianjin University of Science and Technology (20100419).
References
- Electron affinities of some polycyclic aromatic hydrocarbons, obtained from electron-transfer equilibria. J. Am. Chem. Soc.. 1993;115:7918-7919.
- [Google Scholar]
- Sequence-specific modification of guanosine in DNA by a C60-linked deoxyoligonucleotide: evidence for a non-singlet oxygen mechanism. Tetrahedron. 1996;52:5179-5189.
- [Google Scholar]
- Mechanochemistry: a novel approach to the synthesis of fullerene compounds. Solid State Ionics. 1994;74:47-51.
- [Google Scholar]
- Relation between the structure of C60 and its lubricity: a review. Lubr. Sci.. 1997;9:181-183.
- [Google Scholar]
- An investigation of the microfrictional behavior of C60 particle layers on aluminum. Thin Solid Films. 1992;219:129-130.
- [Google Scholar]
- Buckminsterfullerenes (2nd ed.). New York: Longman; 1993.
- Study on tribological behavior of C60/C70 as an oil additive. Tribology. 1993;1:59-60.
- [Google Scholar]
- Fullerene–styrene random copolymers, novel optical properties. Macromolecules. 1995;28:3744-3746.
- [Google Scholar]
- Reversible covalent attachment of C60 to a polymer support. J. Am. Chem. Soc.. 1994;116:5997-5999.
- [Google Scholar]







