Translate this page into:
Preparation, characterization of some transition metal complexes of hydrazone derivatives and their antibacterial and antioxidant activities
⁎Corresponding author. samar.mostafa@gebri.usc.edu.eg (S.A. Aly)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A new series of metal complexes of Pd(II), Cd(II) and Cu(II, I) of polydentate Schiff base ligand (H2L), namely ((Z)-2-(phenylamino)-N'-(thiophen-2-ylmethylene) acetohydrazide) have been prepared. The ligand and its metal complexes have been characterized based on various physicochemical studies as elemental analyses, molar conductance, spectral (UV–Vis, MS, IR, 1H NMR, 13C NMR and XRD), magnetic moment measurements and thermal studies (TG, DTG). In the view of previous studies, the ligand (H2L) acts as polydentate one and coordinates with metal ions to form all metal complexes. The kinetic and thermodynamic parameters of decomposition process (ΔG, ΔH, ΔS) were calculated. The possible structures of the metal complexes have been computed using the molecular mechanic calculations using the hyper chem. 8.03 molecular modeling program. The calculations are performed to obtain the optimized molecular geometry. The antibacterial study of the selected compounds was assayed against two pathogenic bacteria. Moreover, the complexes (Cu II, I), Cd(II), Pd(II)) and the ligand revealed excellent antioxidant properties and could be useful in fighting the free radicals which occur in close connection with cancerous cells. It was remarkable that the two complexes (Cu II, I) demonstrated stronger antioxidant effects than their parent compounds. It is clear that the new complexes are good active compounds for use in a variety of applications.
Keywords
Metal complexes
Thermal behavior
Powder X-ray diffraction
Molecular modeling
Biological activity
1 Introduction
The development of new derivatives of hydrazone have revealed that they own a wide variety of biological activities such as antimicrobial, anticonvulsant, antidepressant, antiinflammatory, analgesic, antiplatelet, antimalarial, anticancer, antifungal, antitubercular, antiviral and cardio protective (kaan et al., 2019). Hydrazones/azomethines imines possess —NHN⚌CH— and constitute an important class of compounds for novel drug development. A number of researchers have prepared and evaluated the biological activities of hydrazones (Rathinam and Sankaran, 2015; El-Sharief et al., 2013; Loncle et al., 2004; Abdel-Aal et al., 2006; Chavarria et al., 2012; Savini et al., 2004; Bijev, 2006; Imramovsky et al., 2007; Daniela et al., 2019).
Some derivatives of hydrazone such as 2-thaizoylare found to be connected with various biological activities such as anti-inflammatory (Mohamed et al., 2013; Bakr et al., 2012), antiviral (Kesel, 2011), antioxidant (Hasnah et al., 2012; Jaishree et al., 2012), antitumor (Adriana et al., 2015), anticancer (Naidu et al., 2011). Metal complexes of hydrazones are also recognized for their pharmacological activity. Some of them for instance Cu(II) and Ga(III) complexes of hydrazones derivatives are found to be highly cytotoxic to human tumor cell lines (Ferreira et al., 2016; Angel et al., 2014; Recio Despaigne et al., 2012; Dominik et al., 2017; Jiayang et al., 2019).
Diketohydrazides of hydrazine own (C—N) and two (C—O) groups that are considered as electron donors causing the chelating ability of these ligands to different transition metal ions (Shah, 2016; Yousef et al., 2012, 2013, 2014, 2015).
Hydrazones are effective in inhibiting corrosion because their construction contains two nitrogen atoms and an imine bond which act as active adsorption centres (Turuvekere et al., 2016). Previous studies reported the anti-corrosion potential of some new hydrazone derivatives on copper corrosion in NaCl medium and found the maximum percentage inhibition effectiveness (Sherif and Ahmed, 2010). Many combinations of bisisatins were obtained to possess biological activities towards different kinds of targets inside cancer cells (Ibrahim et al., 2016). Addition of certain heterocyclic rings on site 3 of indolin-2- as pyrrole moiety was useful in synthesis of numerous anticancer compounds (Sun and McMahon, 2000; Recio-Despaigne et al., 2012).
We have reported the effect of gamma irradiation on some Ru(II), Pd(II), VO(II) and Hg(II) complexes of (Z)-N-(furan-2-yl)methylene)-2-(phenylamino)acetohydrazide (Samar and Hanaa, 2019). Investigation of synthesized metal complexes was determined by using elemental analyses, magnetic moment, molar conductance, FT-IR, 1H NMR, UV–Vis., spectroscopy and thermal techniques. Spectral, thermal, powder X-ray diffraction patterns (XRD) and surface morphological studies were performed for unirradiated and irradiated metal complexes. Molecular modeling structures, the energies of the highest occupied molecular orbital, energies of the lowest unoccupied molecular orbital, energy gap, the absolute electronegativity, χ, the chemical potential, Pi, the absolute hardness, η and the softness (σ) were calculated for the same compounds. The antibacterial activities of the synthesized compounds were investigated (Samar and Hanaa, 2019).
In the present work, we report the syntheses, spectroscopic characterization and thermal studies of novel Pd(II), Cd(II), Cu(II,I) complexes with 2-phenylamino-N-(thiophene-2-yl) methylene) acetohydrazide ligand. These complexes have been characterized by various physicochemical techniques. The antibacterial and antioxidant activities of the ligand and some selected metal complexes were investigated.
2 Experimental
2.1 Materials and methods
All the chemicals used in preparation of ((Z)-2-phenylamino-N-(thiophene-2-yl) methylene)acetohydrazide ligand (H2L) and Pd(II), Cd(II), Cu(II,I) complexes were of Analar grade and procured from Sigma, Aldrich and Fluka. All reactions were carried out under normal atmospheric conditions.
2.2 Synthesis of Schiff base ligand (H2L)
((Z)-2-(phenylamino)-N'-(thiophen-2-ylmethylene)acetohydrazide ligand has been prepared as shown in Scheme 1. The anilinohydrazide (1.0 mmol, 1 g) in ethanol (20 mL) was added to an ethanolic solution of 2-thiophene carbaldehyde (1.0 mmol, 0.37 g) (Xavier1 and Srividhya, 2014). The resulting mixture was stirred at room temperature for about 6 h. The buff solid product was collected by filtration and washed several times with ethanol and dried over P4O10.
Synthesis of ligand (H2L).
2.2.1 [(H2L)], (M.F.: C13H13N3OS and M.W.: 259)
Elemental analysis of (H2L) gave, C = 60.0% (60.2), H = 4.70% (5.0) and N = 16.0% (16.2), (where the numbers in parenthesis represent the calculated value from the chemical formula). Electronic absorption spectra of (H2L) in DMF solution, λmax/nm: 250, 325. Selected IR frequencies (KBr disk, cm−1): 3381, 3244, 1628 and 690.
2.3 Synthesis of the metal complexes
Metal complexes were prepared by adding stoichiometric amount of the appropriate metal salt, Na2PdCl4, CdCl2, Cu(ClO4)2, CuCl and CuI in EtOH to (Z)-2-(phenylamino)-N'-(thiophen-2-ylmethylene) acetohydrazide (20 mL) in the same solvent. The reaction mixture was heated with stirring at ca. 60 °C for 6 h. The resulting solids were filtered off, washed several times with EtOH, and dried under vacuum over P4O10.
2.3.1 [Pd(H2L)(OH)Cl]·1/2H2O] C1, (M.F.: C13H15N3O1½S Pd and M.W.: 426.5)
Elemental analysis of C1 gave; C = 36.5% (36.57), H = 3.20% (3.50), N = 9.80% (9.85), Cl = 8.30% (8.30) and Pd = 24.58% (24.85) (where the numbers in parenthesis represent the calculated value from the chemical formula). Electronic absorption spectra of C1 in DMF solution, λmax/nm: 323, 399, 520. Selected IR frequencies (KBr disk, cm−1): 3431, 3194, 3135, 1602, 438 and 512.
2.3.2 [Cd(H2L)Cl2], C2(M.F.: C13H13N3OSCl2Cd and M.W.: 442.5)
Elemental analysis of C2 gave; C = 35.37% (35.25), H = 3.08% (2.94), N = 9.6% (9.5), Cl = 16.20% (16.00) and Cd = 25.4% (24.5) (where the numbers in parenthesis represent the calculated value from the chemical formula). Electronic absorption spectra of C2 in DMF solution, λmax/nm: 316. Selected IR frequencies (KBr disk, cm−1):3413, 3171, 3069, 1707, 427 and 503.
2.3.3 [Cu(H2L)(HL)·OH] C3 (M.F.: C26H26N6O3S2Cu and M.W.: 597.5]
Elemental analysis of C3 gave; C = 52.60% (52.21), H = 4.50% (4.35), N = 14.30% (14.06) and Cu = 10.56% (10.63) (where the numbers in parenthesis represent the calculated value from the chemical formula). Electronic absorption spectra of C3 in DMF solution, λmax/nm: 325, 379, 420 and 615. Selected IR frequencies (KBr disk, cm−1): 3336, 3042, 3019, 1608, 1534, 452, and 575.
2.3.4 [Cu(H2L)2Cl]·1/2H2O] C4 (M.F.: C26H27N6 O2½ S2 ClCu and M.W.: 626.0]
Elemental analysis of C4 gave; C = 49.7% (49.84), H = 4.20% (4.31), N = 13.40% (13.42), Cl = 5.46% (5.67) and Cu = 10.05% (10.14) (where the numbers in parenthesis represent the calculated value from the chemical formula). Electronic absorption spectra of C4 in DMF solution, λmax/nm: 338, 389, 440 and 607. Selected IR frequencies (KBr disk, cm−1): 3432, 2923, 1674, 1604, 455, and 511.
2.3.5 [Cu2(H2L)2(HL)(OH)] C5 (M.F.: [C39H41N9O4S3Cu2] and M.W.: 938.1]
Elemental analysis of C5 gave; C = 50.1% (49.9), H = 4.50% (4.3), N = 13.5% (13.4) and Cu = 14.32% (13.5) (where the numbers in parenthesis represent the calculated value from the chemical formula). Electronic absorption spectra of C5 in DMF solution, λmax/nm: 340, 379 and 600. Selected IR frequencies (KBr disk, cm−1): 3444, 3336, 3091, 1606, 1534, 485, and 571.
2.4 Physical measurements
The elemental analyses (C, H, N) were performed at Cairo University, Giza, Egypt using CHNS-932 (LECO) Vario Elemental Analyzer. Metal and halide content were estimated by complexometry using EDTA following standard literature methods (Bassett et al., 1978). The metal content was also confirmed from the thermal results. The Fourier Transform Infrared (FTIR) measurements were performed (4000– 400 cm−1) in KBr discs using Nenexeus-Nicolidite-640-MSA FT-IR, Thermo-Electronics Co. The UV–visible absorption spectra were measured in DMF solution using 4802 UV/vis double beam spectrophotometer. The 1H NMR and 13C NMR spectra have been recorded in DMSO-d6 as a solvent using Varian Gemini 200 NMR spectrophotometer and Varian-Oxford Mercury at 300 MHz, respectively.
Molar conductivities of complexes were measured at 30 °C in DMF solution (10−3M) using a CON 6000 conductivity meter. Magnetic susceptibilities of the complexes were measured at room temperature by the modified Gouy method using magnetic susceptibility Johnson Matthey Balance. The effective magnetic moments were calculated using the relation µeff = 2.828 (XmT)1/2B.M., where Xm is the molar susceptibility corrected for diamagnetism of all atoms in the compounds. Thermal analysis (TG/DTG) was obtained out by using a Shimadzu DTA/TG-50 Thermal Analyzer with heating rate of 10 °C/min in nitrogen atmosphere with a following rate 20 mL/min in the range of ambient temperature up to 800 °C using platinum crucibles. Electro spray mass spectra (ESI–MS) for the complexes were performed at the National Research Center, Egypt by the Thermo Electron Corporation. The electron impact (EI) mass spectrum for the ligand was run on Shimadzu-QP 2010 plus Mass Spectrometer, Micro analytical Laboratory, Faculty of Science, Cairo University, Egypt. X-ray powder diffraction analyses of solid samples were measured using APD 2000 PROModel GNR-X-ray Diffractometerat (NRC, Tanta University, Egypt). X-ray diffractograms gives computer control formally finished by PHILIPS®MPDX́PERT X-ray diffratometer ready with Cu radiation CuKα (λ = 1.540 56 Å). The x́pertdiffractometer has the Bragg- Brentano geometry. The x-ray tube used was a copper tube operating at 40 KV and 30 mA. The scanning range (2θ) was 5–90° with step size of 0.050° and counting time of 2 s/step. Quartz was used as the standard material to accurate for the instrumental expansion. This identification of the complexes was done by a known method (Harikumaran and Appukuttan, 2012). From the fitidentified Scherrer formula, the average crystallite size (D) is where λ is the X- ray wavelength in the nanometer, K is factor related to crystallite shape, with a value about 0.9 and ß is the peak width at half maximum height. The value of ß in the 2θ axis of diffraction shape must be in radians. The θ is the Bragg angle and be able to in radians since the Cos θ compatible with the same number.
2.5 Theoretical calculation (molecular modeling)
Molecular modeling was applied to investigate the three dimensional arrangements of atoms in the complexes by employing semi-emperical molecular orbital calculations at the PM3 level provided by the HyperChem program (HyperChem, 2007).
2.6 Biological applications
2.6.1 Antibacterial activity
The in vitro antibacterial activity studies were carried out in the department of Microbial Biotechnology, Genetic Engineering and Biotechnology Research Institute, Sadat City University, Egypt, by using Broth Dilution Method with some alterations (Wikler et al., 2009) to investigate the inhibitory effect of ligand and synthesized complexes (C1-C5) on the sensitive organisms Streptococcus pyogenes as Gram- positive bacterium and Escherichia coli as Gram–negative bacterium. Nutrient broth medium was prepared by using Brain Heart Infusion (BHI) broth and distilled water. The test compounds in measured quantities were dissolved in DMSO which has no inhibition activity to get two different concentrations (1 mg/mL, 5 mg/mL) of compounds. The strains selected for the study were prepared in (BHI) broth medium with shaking and autoclaved for 20 min 15 lb of pressure and at 121 °C before inoculation. The bacteria were then cultured for 24 h at 37 °C in an incubator. One ml of the standard bacterial culture was used as inoculation in a nutrient broth. For growth studies, culture of microbial cells were inoculated and grown aerobically in BHI broth for control and along with various concentrations of the test compounds in individual flasks. Growth was calculated turbidometrically at 650 nm using conventional Spectrophotometer, in which turbidity produced is measured by taking absorbance and compared with turbidity produced by control. The growth rate of different bacteria in absence as well as in presence of test compounds was performed for each concentration. Absorption measurements were accomplished by spectrophotometer after 24 and 48 h of incubation to determine the number of viable organisms per milliliter of sample and were used to the calculated the % inhibition (Umit et al., 2018; Abdülmelik et al., 2019; Nuraniye et al., 2019).
2.6.2 Assay for DPPH free-radical scavenging potential
The nitrogen centered stable free radical 2,2′-diphenyl-1-picrylhydrazyl (DPPH) has often been used to characterize antioxidants (Gülçin et al., 2010; Ercan and Ilhami, 2011; Leyla et al., 2015). It is reversibly reduced and the odd electron in the DPPH free radical gives a strong absorption maximum at λ 517 nm, which is purple in colour (Blois, 1958). Each sample at different concentrations (100, 200 and 300 µg) in HPLC methanol was added to the solution [3.9 mL, 0.004% (w/v)] of DPPH0 in methanol. The tubes were kept at an ambient temperature for 30 min and the absorbance was measured at λ 517 nm. All tests were performed in triplicate and mean were calculated. The scavenging activity (SCA) was expressed as a percentage of scavenging activity on DPPH: where A control is the absorbance of the control (DPPH solution without test sample) and A test is the absorbance of the test sample (DPPH solution plus scavenger).
Butylated hydroxyl anisole (BHA) and tert-Butylated hydroxyl qunione (TBHQ) were used as positive controls. All tests were run in triplicate and an average was used (Gülçin, 2006a,b; Ak and Gülçin, 2008).
3 Results and discussion
3.1 Physical properties
The purity of the ligand and Pd(II), Cd(II), Cu(II, I) complexes have been checked by TLC. All of the metal complexes are coloured solids except complex C2, stable towards air, slightly soluble in alcohols, insoluble in most common organic solvents, but soluble in DMF and/or DMSO. The elemental analyses display that, complexes (C1, C2) are 1L:1M, while (C3,C4) and (C5) are 2L:1M and 3L:2M stoichiometry, respectively (Table 1). The measured values of the molar conductance of complexes in 10−3 M DMF solution at room temperature were in the range of 12–20 (ohm−1 cm2 mol−1). These values reveal that all complexes are non-electrolyte (Bayoumi et al., 2013). The analytical and physical data are compatible with the suggested structures. Ʌm = molar conductivity ohm−1 cm2 mol−1 in 10−3 M DMF.
No.
Compounds
Emperical formula
Color
Mol. wt.
Found (Calc.)%
Ʌm
C
H
N
M
Cl
H2L
C13H13N3OS
Buff
259
60.0
(60.23)4.7
(5.0)16.00
(16.20)–
–
–
C1
[Pd(H2L)(OH)Cl]·1/2H2O
C13H15N3O1½SPd
Yellow
426.5
36.50
(36.57)3.20
(3.5)9.80
(9.85)24.58
(24.85)8.30
(8.30)12
C2
[Cd(H2L)Cl2]
C13H13N3OSCl2Cd
white
442.5
35.37
(35.26)3.08
(2.94)9.60
(9.50)24.50
(25.40)16.20
(16.00)20
C3
[Cu(H2L)(HL).OH]
C13H13N3OS2Cl2Cu
Brown
597.5
52.60
(52.21)4.50
(4.35)14.30
(14.06)10.56
(10.63)−
20
C4
[Cu(H2L)2Cl]·1/2H2O
C26H27N6O2½S2ClCu
Brownish green
626.0
49.70
(49.84)4.20
(4.31)13.40
(13.42)10.05
(10.15)5.46
(5.67)14
C5
[Cu2(H2L)3OH)2]
C39H41N9O5S3Cu2
Green
938.1
50.10
(49.90)4.50
(4.30)13.50
(13.40)14.32
(13.50)–
18
3.1.1 1H NMR spectrum of the ligand
The 1H NMR spectrum of the ligand was verified in DMSO-d6 solution (Fig. 1). The spectrum shows that the different non-equivalent protons resonate at different values of applied field. The 1H NMR spectrum exhibits one signal at δ 4.14 ppm assigned to —NH of amine protons and the multiple peaks observed in the region of (NH—CH2) and (OC—NH—CH2) signals indicate chemically non-equivalent methylene protons. Furthermore, the spectrum displays a multiple signal at δ (6.58–7.63 ppm) assigned to aromatic ring protons. Also, the spectrum depicts signals at δ (8.48, 11.46 and 7.63 ppm) to (2H, —CH⚌N—), (2H, —CO—NH—) and thiophene ring protons, respectively. Moreover, the spectrum exhibits signals at δ 3.77 ppm due to nonequivalent methylene protons. Thus, the 1H NMR result supports the assigned of geometry.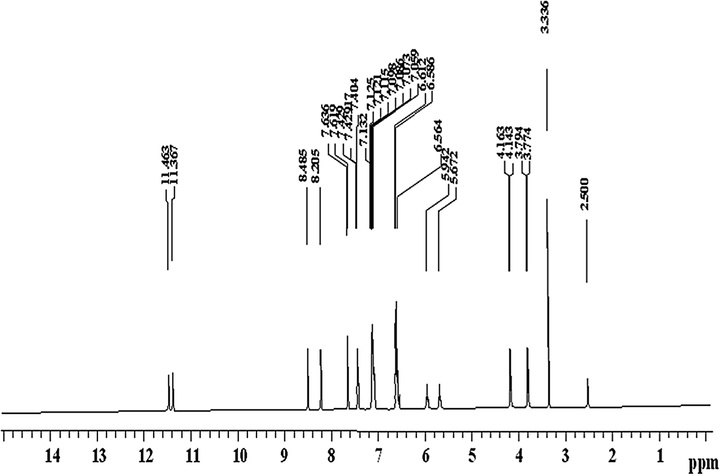
Proton nuclear magnetic resonance spectrum of (Z)-2-(phenylamino)-N′-(thiophen-2-ylmethylene) acetohydrazide (H2L).
The 1H NMR spectrum of the ligand was verified in DMSO-d6 solution δ ppm: (Fig. 1). The 1H NMR spectrum exhibits signal at 2.36 (s, 2H, CH2), 4.14 (br, 1H, NH), 6.58–7.63 (m, 8H, aromatic system), 5.94 (s,1H, CH), 11.36 (s,1H, NH) (amide α to hydrazone linkage).
3.1.2 13C NMR spectrum of the ligand
The 13C NMR spectrum of the ligand (DMSO-d6) features a signal at 57.5 ppm corresponding to the methylene group. The aromatic carbons of the phenyl ring are observed at 113.5, 120.8, 129.5 and 147.6 ppm (El-Saied et al., 2017) whereas the thiophene ring carbons appear at 127.2, 128.5 and 130 ppm (Nursen and Perihan, 2004; Chandrasekaran et al., 2014). The signal of the azomethine carbon is observed at 125 ppm (Juvansinh et al., 2015). The chemical shift appeared at 171 ppm can be assigned to the carbonyl group (see Fig. 2).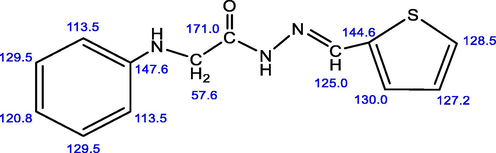
13C NMR of (Z)-2-(phenylamino)-N′-(thiophen-2-ylmethylene) acetohydrazide.
3.1.3 Mass spectrum of the ligand
The electron impact (EI) mass spectrum of the ligand (Fig. 3) confirms the proposed formula, the observed peak at m/z = 259amu (M) corresponding to the ligand moiety (C13H13N3OS, atomic mass m/z = 259 molecular ion peak). The series of peaks in the range m/z = 51 [C4H4-H]+, 77 [C6H5]+, 106 [C7H8N]+ base peak, 132 [C8H8NO-2H]+, 149 [C8H9N2O]+, may be assigned to different fragments.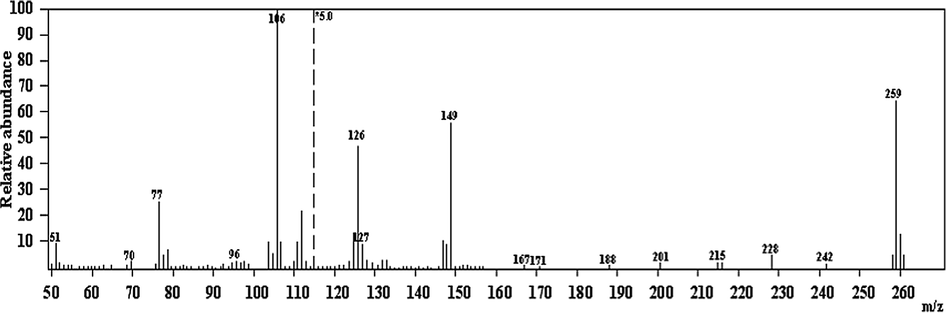
Mass spectrum of (Z)-2-(phenylamino)-N′-(thiophen-2-ylmethylene)acetohydrazide(H2L).
3.1.4 Infrared spectra of the ligand and its metal complexes
The IR spectrum of free ligand is compared with IR spectra of its metal complexes, to investigate the mode of bonding Schiff base ligand to metal ion. The characteristic bands of the IR spectrum of the Schiff base ligand are listed in Table 2 and Fig. 4. The broad band at 1628 cm−1 is assigned to the (—C⚌O, —CH⚌N) stretching frequency. The IR spectrum of the ligand additionally shows a broad band at 3381, 3244 cm−1 due to the stretching vibration of υ(NH) group (Table 2). On complexation, the IR spectra of all complexes display the bands of the imine groups has shifted to lower wave number as compared of free Schiff base ligand. Also the bands of carbonyl group was shifted to lower wavenumber accompanied by a decrease in their intensity, indicating that they are involved in complex formation (Morsy et al., 2011; Samar, 2017). Complexes (C3, C5) showed that the ligand in keto-enolform and enolic oxygen is added to the coordination of complexes (Cescon, 1962; Kumar and Ramesh, 2005; Balasubramanian et al., 2006; Yin and Chen, 2006). The appearance of two new bands at 427–485 and 503–575 cm−1 ranges corresponds to ν(M−N) and ν(M−O), respectively (Fernandez et al., 2006; Majumder et al., 2006). Complexes (C1 and C4) under investigation included water molecules. The broad bands in the 3336–3444 cm−1 region are due to water of crystallization or coordinated of hydroxo group (Fathy and Samar, 2004). From the IR spectral data and the results mentioned above, it is concluded that, the ligand behaved as a neutral bidentate ligand coordinating through the imine nitrogens and the carbonyl groups oxygen in complexes (C1, C2). While in complex C5 the ligand produces binuclear complex.
NO.
Compound
ν H2O/OH
ν (N—H)
ν (C⚌O)
ν (C⚌N)
ν (C⚌S)
ν(N—N)
ν (M—N)
ν (M—O)
H2L
–
3381,3244
1628
1628
690
1226
–
–
C1
Pd(H2L)(OH)Cl]·1/2H2O
3431
3194,3135
1602
1522
653
1220
438
512
C2
[Cd(H2L)Cl2]
3413
3171,3069
1607
1595
641
1223
427
503
C3
[Cu(H2L)(HL).OH]
3336
3042,3019
1608
1534
690
1220
452
575
C4
[Cu(H2L)2Cl]·1/2H2O
3432
2923
1614
1604
691
1225
455
511
C5
[Cu2(H2L)3OH)2]
3444
3336,3091
1606
1534
642
1245
485
571
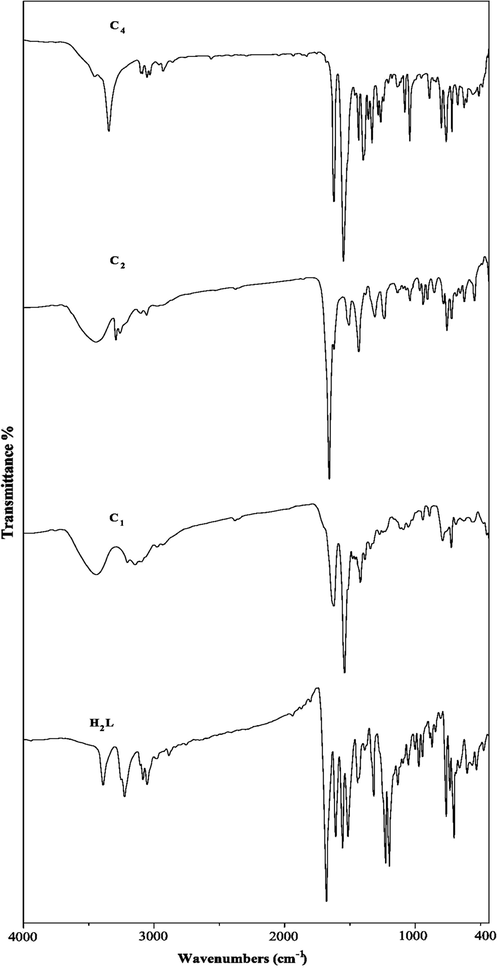
Infrared spectra of ligand and its metal complexes (C1, C2, C4).
3.1.5 Electronic spectra and magnetic moments
The electronic spectra of hydrazone ligand and M2+, M1+ complexes [where M = Pd(II), Cd (II) and Cu(I, II)] were recorded in 10−3 DMF solution in the range 190–800 nm. Within the UV spectrum of the ligand (Table 3) was observed the existence of two absorption bands assigned to the transition π–π* at 250, 325 nm (Table 3). While the electronic spectrum of Pd(II) complex (C1) displayed bands at 323, 399 and 520 nm, this spin-allowed transition may correspond to 1A1g → 1B1g transition indicating a square planar geometry (Shankarwar et al., 2015; Hanaa and Ocala, 2015). The electronic spectrum of Cd(II) complex exhibited non-ligand band at 316 nm, referring to L → M charge transfer transition in tetrahedral geometry (Mohamed et al., 2018). Pd(II) and Cd(II) complexes exhibit diamagnetic character (Azam et al., 2012).
No.
Compound
λmax(nm)
µeff(B.M.)
H2L
250, 325
–
C1
Pd(H2L)(OH)Cl]·1/2H2O
323, 399, 520
Dia
C2
[Cd(H2L)Cl2]
316
Dia
C3
[Cu(H2L)(HL).OH]
325, 379, 420, 615
1.75
C4
[Cu(H2L)2Cl]·1/2H2O]
338, 389, 440, 607
Dia
C5
[Cu2(H2L)3 (OH)2]
340, 379, 600
Dia
Also, The electronic spectra of the copper complexes (C3-C5), showed strong absorption bands in the spectra at 325–340 nm attributed to the transition of the ligand between different energy levels. The other band at 379–440 nm is probably assigned to ligand to metal charge transfer (LMCT) transition. One more spectral band appeared at 600–615 for Cu complexes which is due to d-d transitions, which are consistent with the distorted square-pyramidal structure (Li and Xu, 1999; Hathaway et al., 1987). The room temperature magnetic moment value of the Cu complex (C3) is found to be 1.75B.M. which are close to the spin only value of one unpaired electron. However, the complexes (C4 and C5) showed diamagnetic character. The proposed structures are shown in Scheme 2.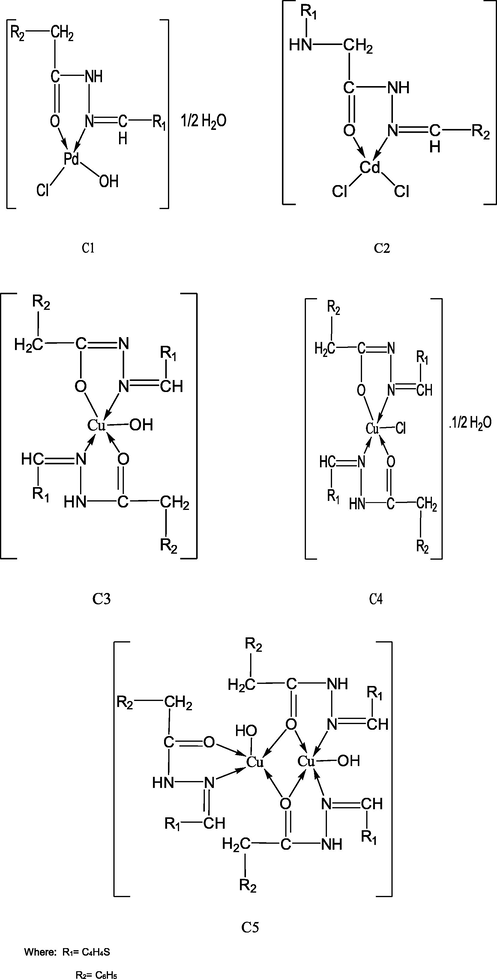
Suggested chemical structure of Pd(ll), Cd(ll), Cu(l, ll) complexes.
3.1.6 Thermal studies of the ligand and its complexes
The thermogravimetric (TG/DTG) analysis is very important technique used to assess the stability of compounds and support their geometry structure. TG/DTG results of ligand and its complexes (C1-C5) are listed in Table 4 and Figs. 5a–5c. The thermal studies of the reported compounds show good agreement with the suggested theoretical formulae obtained from analytical and spectral data (Ahamed et al., 2003). where ia = Dehydration, ib = Decompostion, ic = Finial residue.
No
Compound
Temp. range/oC
DTGTemp. range/°C
TGAMass loss%
Calc. (F)Reaction
Leaving species
H2L
293
30–320
100(99.89)
iib
Decomp.
C1
Pd(H2L)(OH)Cl]·1/2H2O
46
243, 334, 41429–103
103–799
At7992.11(3.0)
69.22 (68.47)
28.60(28.28)ia
iib
ic
−1/2H2O
Decomp.
PdO
C2
[Cd(H2L)Cl2]
249, 341, 627
192–799
At79974.69(75.50)
25.40 (24.50)iib
ic
Decomp.
Cd
C3
[Cu(H2L)(HL).OH]
197, 247, 506
697, 776155–776
At 77672.64 (72.20)
27.36 (27.80)iib
ic
Decomp.
CuO + 7C
C4
[Cu(H2L)2Cl]·1/2H2O
78
236, 638,
70633–100
100–799
At 7991.44(1.47)
76.28(76.45)
22.29(22.08)ia
iib
ic
−1/2H2O
Decomp.
CuO + 5C
C5
[Cu2(H2L)3OH)2]
197, 697, 776
135–799
At 79973.61(73.50)
25.91(26.50)iib
ic
Decomp.
2CuO + 7C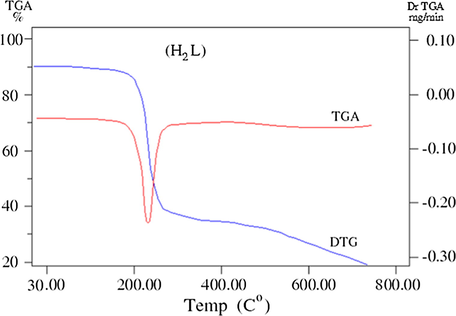
TG/ DTG curves of the ligand.
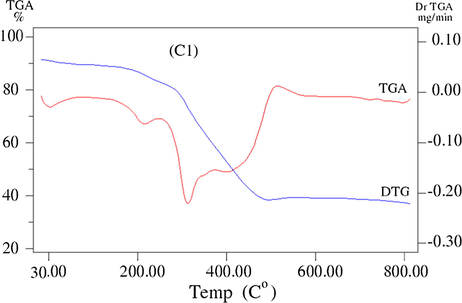
TG/ DTG curve of the Pd(ll) complex (C1).
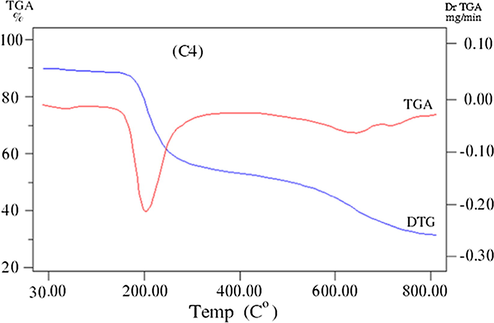
TG/ DTG curve of the Cu(l) complex (C4).
3.1.6.1 Ligand
The TGA curve of the ligand shows one successive decomposition step within the temperature range 30–320 °C with endothermic DTG peak at 293 °C which assigned to total mass loss parts of ligand (Calc./Found%:100/99.89%).
3.1.6.2 Palladium(II) chelate
The TGA thermoanalytical curves of Pd(II) complex (C1) shows mass loss (Calc./Found%: 2.11/3.0) with DTG peak at 46 °C, assigned to liberation of half molecule of lattice water within the temperature at 29–103 °C. The thermoanalytical curves also shows one step of decomposition within temperature range 103–799 °C with mass loss of (Calc/Found%:69.22/68.47) associated with DTG peaks at 243, 334, 414 °C leaving metal oxide PdO (Calc./Found%: 28.60/28.28) as final residue (Mishra et al., 2009).
3.1.6.3 Cadmium(II) chelate
The TGA curve of Cd(II) complex (C2) displays steady part till 192 °C indicating the absence of any outside lattice water or solvent within the temperature range 30–192 °C followed by decomposition step within temperature range 192–799 °C with mass loss (Calc./Found%: 74.69/ 75.50) associated with DTG peaks at 249, 341, 627 °C ended with Cd metal (Calc./Found%: 25.40/24.50) as final thermo product.
3.1.6.4 Copper (II, I) chelates
The TGA thermoanalytical curves of Cu complexes (C3–C5) were discussed as follows. The TGA thermogram of Cu(II) complex (C3) shows steady part till 155 °C indicating the absence of any hydrated molecules. The complex decomposes in one step in the temperature range 155–776 °C with mass loss (Calc./Found% 72.64/72.20) associated with DTG peaks at 247, 506, 697, 776 with the production of CuO and carbon residue.
The TGA curve of (C4) complex displays mass loss (Calc./Found%: 1.44/1.47) within the temperature ranges 33–100 °C with DTG peak at 78 °C which corresponds to loss of half molecule of lattice water. The thermogram also shows more stage of decomposition within temperature range 100–799 °C associated with DTG peaks at 236, 638, 706 °C, according to mass loss (Calc./Found%: 76.28/76.45), respectively, leaving metal oxide contaminated with carbon atoms as residue.
Moreover, the TGA thermoanalytical curves of (C5) complex exhibits a steady part till 135 °C, indicating the absence of any outside residual solvent. This step was followed by gradual decomposition within temperature range 135–799 °C associated with DTG peaks at 197, 697, 776 °C, respectively. The decomposition stage ended with the formation of CuO contaminated with carbon atoms as final residue (Calc./Found%: 25.9/26.5), respectively (Harinath et al., 2013). The slight higher value found for the percentage of the residue when compared with the theoretical value for pure metal oxides shows that the residue is contaminated with carbon (Hanaa, 2005).
3.1.7 Kinetic and thermodynamic parameters
The kinetic parameters of activation energy (E), reaction order (n) and the pre-exponential factor (A) were calculated using the Coats–Redfern equation (Coats and Redfern, 1964). As well as the thermodynamic activation parameters for the thermal decomposition steps in complexes; enthalpy (ΔH*), entropy (ΔS*) and free energy of decomposition (ΔG*) were evaluated using the following relationships:
where k is Boltzmann constant and h is Plank’s constant and R is the gas constant. The kinetic parameters obtained from the previously mentioned methods are listed in Table 5 and Figs. 6a–6d. Where: E: the activation energy (KJ.mol−1), ΔH*: the activation enthalpy (KJ.mol−1), ΔS*: the entropy (J.K−1.mol−1), A: the pre-exponential factor (s−1), ΔG*: the free energy (KJ.mol−1).
Complex
n
Step
r
T (K)
E*
ΔH*
A
−ΔS*
Δ G*
C1
1
01st
2nd0.9926
0.9672330.75
669.6224.476
18.69421.730
13.132643661.330
548491248.30.1429
0.092668.910
75.109
C2
0.66
1st
0.9865
599.36
8.273
3.293
2,753,329,374
0.0783
50.200
C3
1
2nd
0.9938
511.16
95.953
91.705
18.7038612
0.2333
210.948
C4
1
0.51st
2nd0.9989
0.9993344.57
606.3040.332
10.49837.468
5.4599819.302904
1,029,166,7190.1780
0.086698.789
57.931
C5
1
1st
0.9825
482.88
47.452
43.437
550951.946
0.1473
114.596
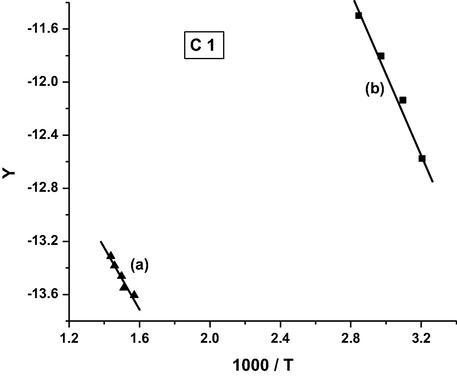
Coats-Redfern plot for complex C1.
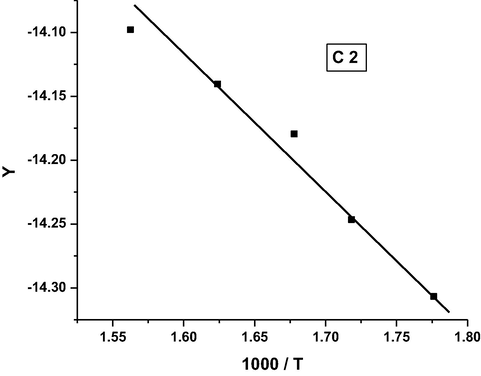
Coats-Redfern plot for complex C2.
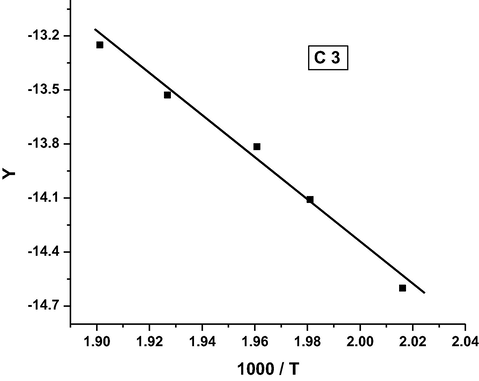
Coats-Redfern plot for complex C3.
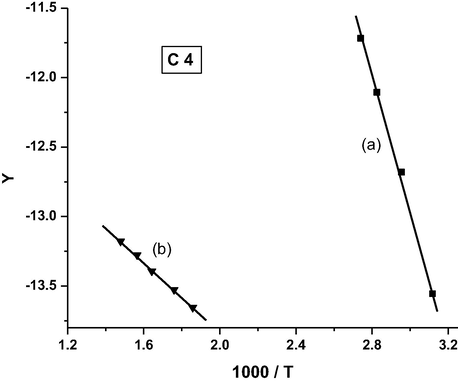
Coats-Redfern plot for complex C4.
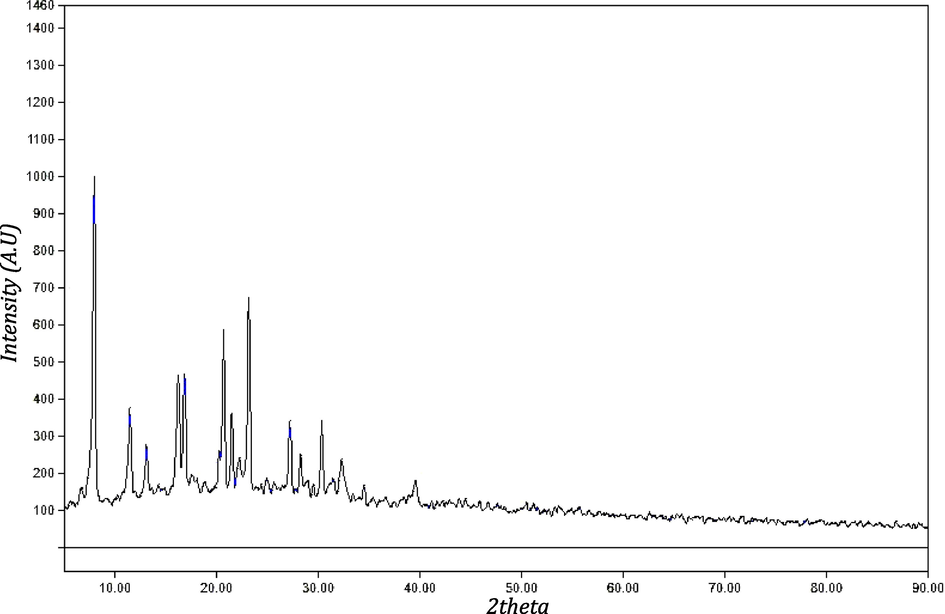
X-ray diffraction pattern of C4 complex.
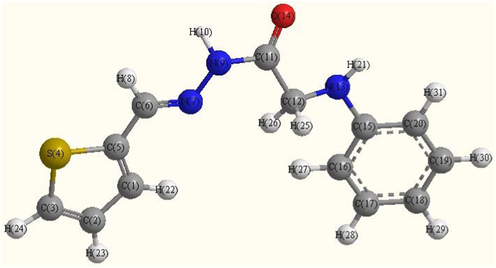
Optimized molecular structure of ligand.
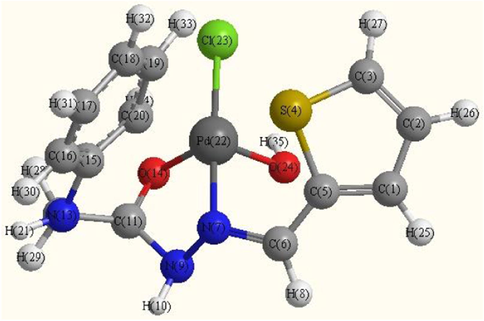
Optimized molecular structure of Pd(II) complex (C1).
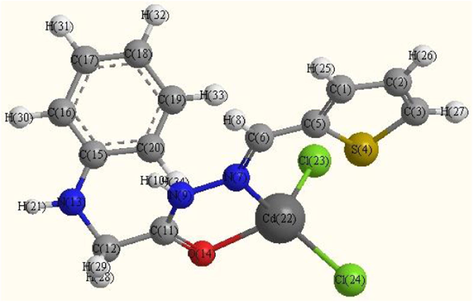
Optimized molecular structure of Cd(II)complex (C2).
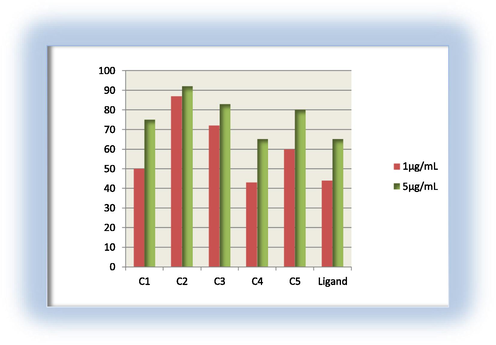
Antibacterial activity for the ligand and its metal complexes against E. coli.
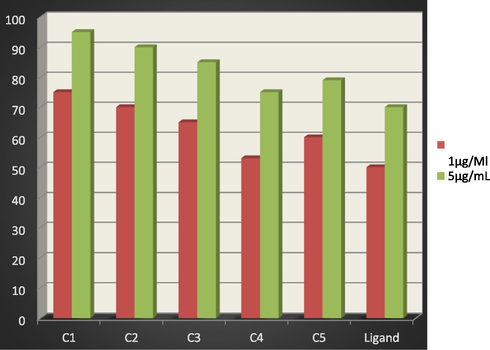
Antibacterial activity for the ligand and its metal complexes against S. pyogenes.
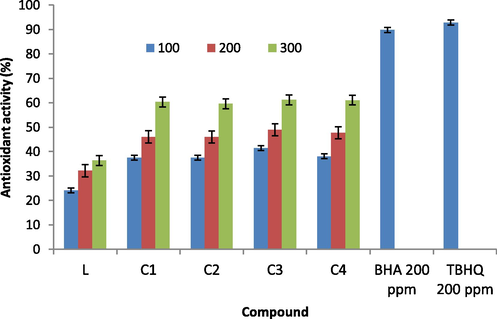
Antioxidant activity of ligand and its complexes (C1-C4) as determined by DPPH.
From the results, the following remarks can be pointed out:
-
–
The positive sign of ΔG* for the complexes under investigation revealed that the free energy of the final residue is higher than that of the initial compound and all the decomposition steps are non-spontaneous processes.
-
–
The negative values of ΔS*for the degradation process indicated a more order activated complex than reactant and/or the reaction was slow (Frost and Pearson, 1961).
-
–
All the complexes have negative entropy, which indicates that the complexes are formed spontaneously.
-
–
The positive values of ΔH* means that the decomposition processes were endothermic (El-Ayaan et al., 2007).
-
–
Kinetics of the thermal decomposition stages of the complexes under study obeys, in most cases, the first order kinetics.
-
–
The values of ΔG* increased for the subsequent decomposition stage of complex C1. This increase in the values of ΔG* reflected that the rate of removal of the subsequent ligand was lower than that of the precedent ligand, this is due to increasing in the values of TΔS* from one step to another which overrides the values of ΔH* (Maravalli and Goudar, 1978; Yusuff and Sreekala, 1990; Vinodkumar et al., 2000).
-
–
It was found that the order of the activation energy values of the first decomposition step for the complexes is: C3 > C5 > C4 > C1 > C2.
3.1.8 X-ray diffraction
XRD pattern of copper complex (C4) is revealed in Table 6 and Fig. 7. The X-ray powder diffraction analysis of (C4) complex was carried out to give information about atomic or molecular arrangement as shown in Fig. 7. The spectra showed sharp peaks indicating their crystalline nature. The X-ray powder diffraction patterns were attained in the range of 5–90° and in steps of 0.050°. The full width at the half- maximum (FWHM) of diffraction peaks observed from the refinement was used to evaluate the particle size. Also, to investigate the crystalline information about complex, the average crystallite sizes (D) were calculated from the most sharp peaks using Sherrer’s formula (Harikumaran and Appukuttan, 2012).
Compounds
Peak
02Th
d-sp
β-FWHM
D(nm)
C4[Cu(H2L)2Cl]·1/2H2O]
1
7.97
11.0838
0.2887
4.81
2
11.49
7.6963
0.3286.6
4.24
3
13.13
6.7385
0.3059
4.56
4
16.23
5.4561
0.3577
3.90
5
16.87
5.2519
0.2602
5.38
6
20.71
4.2849
0.2827
4.98
7
21.51
4.1270
0.2126
6.64
8
22.28
3.9872
0.4925
2.86
9
27.24
3.2714
0.2814
5.07
10
3235
2.7653
0.5139
2.74
Mean of D
4.107
The diffraction peaks indicate that synthesized of copper complex (C4) is in the nanometer range. Also, the average crystallite size of C4 complex is 4.107 nm. From the value of (D), we can obtain the dislocation density (δ) value of complex which indicates the number of dislocation lines per unit area of the crystal. The value of (δ) is related to the average crystallite size (D) from the equation:
The calculated value of (δ) found to be 0.0593.
3.1.9 Molecular modeling
Many attempts to prepare crystals for X-ray crystallography were unsuccessful. The computational strategy in this manuscript is to determine the geometry of the ligand and its complex. Thus, molecular modeling calculations were considered. Thus, the geometries of the complexes were optimized by hyper chem. 8.03 Molecular Modeling and Analysis Program using the molecular mechanics calculations (PM3) (HyperChem, 2007).
The optimized geometries of the ligand and its complexes are shown in Figs. 8a–8c. The optimized lengths of bonds in addition to bond angles (Supplementary materials) are listed in Tables (S1-S3). The energetic properties of these compounds are listed in Table 8.
Compound
Bond length
C5—C6
C6⚌N7
N7—N9
N9—C11
C11—C12
C11—O14
C12—N13
N13—C15
M—N
M—O
Ligand
1.4443
1.3093
1.3857
1.4450
1.5130
1.2188
1.4840
1.4380
---
---
C2
1.4428
1.3135
1.3731
1.4564
1.5271
1.2144
1.4822
1.4461
2.2087
2.1872
C1
1.3996
1.4041
1.4872
1.4570
1.5034
1.3338
1.4868
1.4366
1.9674
2.0006
1.9784
The assignment of the theoretical parameters
Ligand
C2
C1
Total energy*
−2980.14
−3108.34
−3755.85
Kinetic Energy*
145.37
198.75
13.70
Binding energy*
−3125.511
−3307.10
−3769.552
Potential energy*
−62198.038
−77434.766
−100987.210
Isolated Atomic Energy*
−59072.527
−74127.670
−97217.658
Electronic Energy*
−385803.42
−450035.21
−679902.63
Core-Core Interaction*
323605.38
372600.45
578915.42
Heat of Formation
238.344
141.46
−175.046
Dipole moment (Debye)
2.195
6.121
6.221
HOMO (eV)
−8.976991
−8.948892
−7.934258
LUMO (eV)
−0.732292
−0.975450
−0.741812
From selected bond lengths collected in Table 7 one can conclude the following remarks:
-
In all complexes the metal ions are coordinated to the ligand via N9 and O14 atoms.
-
A large variation in C6⚌N7 and N7—N9 bonds length on coordination via the N7 atom.
-
The C6⚌N7 bond length increases in all complexes than ligand.
-
In Pd complex the bond distance N7—N9 is enlarged due to their participation in coordination which becomes weaker due to the formation of Pd—N bonds (El-Gammal et al., 2014) M—N bond of Cd complex is larger than Pd complex.
-
In general, the M—Cl bond length is longer than that of M—N and M—O bonds.
-
The bond angles of the ligand moiety are altered somewhat upon coordination (Mohamed et al., 2014).
-
The other bond lengths and bond angles also suffer some changes, but not significantly.
-
The bond angles in Pd(II) complex are quite near to a square planer, and tetrahedral geometry in Cd(II) predicting dsp2 hybridization. The bond angle values show a large distortion around the metal centers.
-
The HOMO and LUMO namely the Frontier Molecular Orbitals (FMOs) are very popular quantum chemical parameters. The chemical reactivity is a function of interaction between HOMO and LUMO levels of the reacting species (Kenichi, 1982). The higher EHOMO implies that the molecule is a good electron donor and has great ability to complex formation. LUMO energy presents the ability of a molecule receiving electron (Geerlings et al., 2003; Seda et al., 2009). The lower ELUMO value, the more ability of the molecule is to accept electrons (Guo and Chengha, 2007).
3.2 Biological applications
3.2.1 In vitro antibacterial activity
The antibacterial activities of ligand H2L as well as its metal complexes (C1- C5) were tested against S. pyogenes as Gram- positive bacteria and E. coli as Gram–negative bacteria. According to the data depicted in Figs. 9 and 10, the following results are summarize as follow:
1-All of the tested compounds are found to have remarkable antibacterial activity. The active sequence of the ligand and its tested complexes against S. pyogenes in the concentration of 1 μg/mL, 5 μg/mL follows the trend: C1 > C2 > C3 > C5 > C4 > H2L.
2- Also all the tested complexes except C4, have higher activity than their metal free ligand against E. coli for concentration of 1 μg/mL as the follow trend: C2 > C3 > C5 > C1 > H2L > C4, and for the concentration of 5 μg/mL, the activity follows the trend: C2 > C3 > C5 > C1 > H2L, C4.
3- It is found that, C1 complex has the highest toxicity against Gram-positive bacteria in both two concentrations; C2 has the highest toxicity against gram-negative bacteria in both concentrations. Moreover, it is found that all tested complexes are more toxic against both Gram-positive and Gram-negative bacteria than that of the free ligand in the both concentrations, except C4 in gram-negative bacteria, having higher antibacterial activities than that of the free ligand. This would suggest that, chelation/complexation could enhance the lipophilic character of the central metal atom, which subsequently favors its permeation through the lipid layers of the cell membrane and blocking the metal binding sites on enzymes of microorganism which as explained by Tweedy’s chelation theory (Tweedy, 1964). It is suspected that other factors such as liposolubility, dipole moment, conductivity influenced by metal ion, geometry, number and type of metal ions and counter ions, and moieties as azomethine linkage present in these compounds may be possible reasons for their remarkable antibacterial activities (Mehmet et al., 2010; Dharmpal et al., 2017; Saeed et al., 2009; Najma et al., 2010).
3.2.2 Antioxidant activity
The process of scavenging DPPH-free radicals has been used to assess the antioxidant activity of the present compounds (Bilgicli et al., 2015). The data of the suppression ratio for DPPH are shown in Fig. 11. The results were compared with those of standard antioxidants. The percentage radical scavenging activity of the synthesized compounds has been carried out at different concentrations (100, 200 and 300 µg mL−1). The DPPH scavenging activity was expressed as IC50, the effective concentration at which 50% of the radicals were scavenged, have been calculated to assess the antioxidant activities. A lower IC50 value indicates greater antioxidant activity. IC50 values lower than 10 mg/mL usually imply effective activities in antioxidant properties. Fig. 11 shows that, all of the tested compounds exhibited very good radical scavenging activities at different concentrations. From the obtained results, C3,C4 complexes (IC50 = 0.019, 0.211 µg mL−1) showed good activity better all tested compounds and the positive standard. The ligand (IC50 = 0.514 µg mL−1) possesses the lowest scavenging ability as compared to the positive standard and all tested complexes. The order of IC50 values of ligand and its metal(II,I) complexes was as follows: C3 > C4 > C1 > C2 > Ligand. The free radical scavenging activity of the compounds depends on the structural factors and other structural features as type and geometry of metal ions.
4 Conclusions
In this summary, Pd(II), Cd(II) and Cu(I, II) complexes of 2-phenylamino-N-(thiophene-2yl) methylene)acetohydrazide ligand(H2L) have been studied. The analytical and physical techniques recommended the chemical structure of the new complexes. The biological applications such as antibacterial activity and antioxidant of ligand and its complexes are reported. The results found can be summarized as follows:
-
The ligand behaves as neutral bidentate or tetradentate
-
Pd(II) complex (C1) is square planar and Cu(II, I) complexes (C3-C4) are square pyramidal in geometry. While Cd(II) complex (C2) has tetrahedral geometry.
-
X-ray diffraction pattern prove that the crystallite size of the copper complex (C4).
-
The computational calculations showed that metal complexes C1 exhibit square planer and C2 is tetrahedral geometrical arrangement which agrees well with the experimental observation.
-
In vitro biological applications showed the effective antibacterial and antioxidant activities of the tested compounds. The antibacterial and antioxidant activities of these compounds increase with their coordination.
-
In addition to the complex of copper (C3) is more greater antioxidant than all tested compounds.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis and antiviral evaluation of some sugar arylglycinoylhydrazones and their oxadiazoline derivatives. Arch. Pharm. Chem. Life Sci.. 2006;339, 656–663
- [CrossRef] [Google Scholar]
- Phytochemical content, antidiabetic, anticholinergic, and antioxidant activities of endemic lecokia cretica extracts. Chem. Biodiversity. 2019;16 e1900341
- [CrossRef] [Google Scholar]
- Synthesis, anticancer activity, and genome profiling of thiazolo arene ruthenium complexes. J. Med. Chem.. 2015;58:8475-8490.
- [CrossRef] [Google Scholar]
- Thermal investigation of iron(III) and manganese(II, III) complexes of dianils derived from 6-formylkhellin. J. Therm. Anal Calorim.. 2003;73:987-1000.
- [CrossRef] [Google Scholar]
- Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact.. 2008;174:27-37.
- [CrossRef] [Google Scholar]
- ROS-mediated cytotoxic effect of copper(II) hydrazone complexes against human glioma cells. Molecules. 2014;19:17202-17220.
- [CrossRef] [Google Scholar]
- Synthesis and photoluminescent properties of a Schiff-base ligand and its mononuclear Zn(II), Cd(II), Cu(II), Ni(II) and Pd(II) metal complexes. Arabian J. Chem.. 2012;5(1):45-53.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, electro chemistry, catalytic and biological activities of ruthenium(III) complexes with bidentate N, O/S donor ligands. Spectrochim. Acta, Part A. 2006;65:678-683.
- [CrossRef] [Google Scholar]
- Synthesis, antimicrobial, antioxidant, anti-inflammatory, and analgesic activities of some new 3-(20 -thienyl)pyrazole-based heterocycles. Med. Chem. Res.. 2012;21:1418-1426.
- [CrossRef] [Google Scholar]
- Vogel’s Textbook of Quantitative Inorganic Analysis Including Elementary Instrumental Analysis (4th edition,). London: Longman Group; 1978. p. :316.
- Cu(II), Ni(II), Co(II) and Cr(III) complexes with N2O2-chelating Schiff's base ligand incorporating Azo and sulfonamide moieties: spectroscopic, electrochemical behavior and thermal decomposition studies. Int. J. Electrochem. Sci.. 2013;8:9399-9413. www.electrochemsci.org
- [Google Scholar]
- New heterocyclic hydrazones in the search for antitubercular agents: synthesis and in vitro evaluations. Lett. Drug Des. Discov.. 2006;3:506-512.
- [CrossRef] [Google Scholar]
- J. Coord. Chem.. 2015;68:4102.
- [CrossRef]
- Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199-1200.
- [Google Scholar]
- Day, A.R., Preparation of some benzimidazolylamino acids. Reactions of amino acids with o-phenylenediamines. J. Org. Chem.. 1962;27(2):581-586.
- [CrossRef] [Google Scholar]
- Initial evaluation of the antitumour activity of KGP94, a functionalized benzophenone thiosemicarbazone inhibitor of cathepsin L. Eur. J. Med. Chem.. 2012;58:568-572. 10.1016/j.ejmech.2012.10.039
- [Google Scholar]
- Synthesis, characterization and antimicrobial studies of novel compound and its metal complexes derived from nicotinic acid. Der Chemica Sinica. 2014;5(5):81-90. www.pelagiaresearchlibrary.com
- [Google Scholar]
- 4-(3-Nitrophenyl)thiazol-2-ylhydrazone derivatives as antioxidants and selective hMAO-B inhibitors:synthesis, biological activity and computational analysis. J. Enzyme Inhib. Med. Chem.. 2019;34:597-612.
- [CrossRef] [Google Scholar]
- Trivalent transition metal complexes derived from carbohydrazide and dimedone. Arab. J. Chem.. 2017;10:S1795-S1801.
- [CrossRef] [Google Scholar]
- Structurally related hydrazone-based metal complexes with different antitumor activities variably induce apoptotic cell death. Dalton Trans.. 2017;46:4759-4767.
- [CrossRef] [Google Scholar]
- Preparation, characterization and antimicrobial activities of N'-((3-(hydroxyimino) butan-2-ylidene)-2 (phenylamino) acetohydrazide and its metal complexes. Egypt. J. Chem.. 2017;60(1):1-24.
- [CrossRef] [Google Scholar]
- Synthesis of thiosemicarbazones derived from N-(4-hippuric acid)thiosemicarbazide and different carbonyl compounds as antimicrobial agents. Eur. J. Med. Chem.. 2013;67:263-268.
- [CrossRef] [Google Scholar]
- Synthesis, thermal and spectral. Studies of first-row transition metal complexes with Girard-T reagent-based ligand. Spectrochim. Acta, Part A. 2007;68:211-219.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, DFT and biological studies of isatinpicolinohydrazone and its Zn(II), Cd(II) and Hg(II) complexes. Spectrochim. Acta, Part A. 2014;127:144-156.
- [CrossRef] [Google Scholar]
- Polyphenol contents and in vitro antioxidant activities of lyophilised aqueous extract of kiwifruit (Actinidia deliciosa) Food Res. Int.. 2011;44:1482-1489.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of UO2(VI), Sn (IV), and Th (IV) complexes of 4-formylazohydrazoaniline antipyrine. Afinidad. 2004;61(514):516-520.
- [Google Scholar]
- Bismuth(III) complexes with 2-acetylpyridine- and 2-benzoylpyridine-derived hydrazones: Antimicrobial and cytotoxic activities and effects on the clonogenic survival of human solid tumor cells. Bioorg. & Med. Chem.. 2016;24:2988-2998.
- [CrossRef] [Google Scholar]
- The crystal structures of some -o-hydroxy Schiff base copper(II) complexes derived from (R) and (S) secbutylamines. J. Mol. Struct.. 2006;787:1-7.
- [CrossRef] [Google Scholar]
- Kinetics and Mechanisms. New-York: Wiley; 1961. https://doi.org/10.1021/ed038p535.1
- Electrochemical and DFT studies of β-amino-alcohols as corrosion inhibitors for brass. Electro Chim. Acta. 2007;52:4554-4559.
- [CrossRef] [Google Scholar]
- Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem. Toxicol.. 2010;48:2227-2238.
- [CrossRef] [Google Scholar]
- Antioxidant and antiradical activities of L-carnitine. Life Sci.. 2006;78:803-811.
- [CrossRef] [Google Scholar]
- Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid) Toxicol.. 2006;217:213-220.
- [CrossRef] [Google Scholar]
- Structural and thermal studies of some aroylhydrazone Schiff’s bases-transition metal complexes. J. Therm. Anal. Calorim.. 2005;81:339-346.
- [CrossRef] [Google Scholar]
- New 15-membered tetraaza (N4) macrocyclic ligand and its transition metal complexes: spectral, magnetic, thermal and anticancer activity. Spectrochim. Acta A. 2015;138:553-562.
- [CrossRef] [Google Scholar]
- Synthesis, spectral characterization and antioxidant activity studies of a bidentate Schiff base, 5-methyl thiophene-2-carboxaldehyde-carbohydrazone and its Cd(II), Cu(II), Ni(II) and Zn(II) complexes. Spectrochim. Acta A. 2013;101:264-272.
- [CrossRef] [Google Scholar]
- Microwave-assisted synthesis and antioxidant properties of hydrazinyl thiazolyl coumarin derivatives. Chem. Cent. J.. 2012;6(32):1-10.
- [CrossRef] [Google Scholar]
- Hathaway, B.J., Wilkinson, G., Gillard, R.D., McCleverty, J.A., (Eds.) 1987. New York: Pergamon, 6, pp. 317–410.
- Syntheses, spectral, surface morphological and gamma ray irradiation studies of some oxomolybdenum(V) and dioxomolybdenum(VI) complexes of an azo dye derived from 4-aminoantipyrine. J. Korean Chem. Soc.. 2012;56:217-227.
- [CrossRef] [Google Scholar]
- HyperChem, 2007. Release 8.03 for Windows, Molecular Modeling System, Hypercube Inc.
- Bis-isatin hydrazones with novel linkers: Synthesis and biological evaluation as cytotoxic agents. Eur. J. Med. Chem.. 2016;108:415-422.
- [CrossRef] [Google Scholar]
- A new modification of anti-tubercular active molecules. Bioorg. Med. Chem. Lett.. 2007;15:2551-2559.
- [CrossRef] [Google Scholar]
- In vitro antioxidant properties of new thiazole derivatives. J. Saudi Chem. Soc.. 2012;16:371-376.
- [CrossRef] [Google Scholar]
- Enhancing singlet oxygen generation in semiconducting polymer nanoparticles through fluorescence resonance energy transfer for tumor treatment. Chem. Sci.. 2019;10:5085-5094.
- [CrossRef] [Google Scholar]
- Synthesis, spectral analysis and evolution of antimicrobial activity of Schiff base of cyanoacetohydrazide. Int. Lett. Chem., Phys. Astron.. 2015;52:100-110. http://doi.10.18052/www.scipress.com/ILCPA.52.100
- [Google Scholar]
- Investigation of inhibitory properties of some hydrazone compounds on hCA I, hCA II and AChE enzymes. Bioorg. Chem.. 2019;86:316-321.
- [CrossRef] [Google Scholar]
- Broad-spectrum antiviral activity including human immunodeficiency and hepatitis C viruses mediated by a novel retinoid thiosemicarbazone derivative. Eur. J. Med. Chem.. 2011;46:1656.-1664.
- [CrossRef] [Google Scholar]
- Role of frontier orbitals in chemical reactions. Science. 1982;218:747-754. www.jstor.org/stable/1689733
- [Google Scholar]
- Synthesis, luminescent, redox and catalytic properties of Ru(II) carbonyl complexes containing 2N2O donors. Polyhedron. 2005;24:1885-1892.
- [CrossRef] [Google Scholar]
- LC–MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind. Crops Prod.. 2015;74:712-721.
- [CrossRef] [Google Scholar]
- One-dimensional chain copper complexes [Cu(dien)(btrz)(ClO4)2] and [Cu(en)2(btrz)(ClO4)2] based on the bridging ligand 1,2-bis(1,2,4-triazole-1-yl)ethane (btrz) Trans. Met. Chem.. 1999;24:622-627.
- [CrossRef] [Google Scholar]
- Synthesis and antifungal activity of cholesterol-hydrazone derivatives. Eur. J. Med. Chem.. 2004;39:1067-1071.
- [CrossRef] [Google Scholar]
- Substituted salen and baen tetradentate Schiff-base ligands. Synthesis, characterization, and electrochemistry of cobalt(III) complexes. Inorg. Chem.. 1978;17:3389-3398.
- [CrossRef] [Google Scholar]
- Synthesis, structures and fluorescence of nickel, zinc and cadmium complexes with the N, N, O-tridentate Schiff base N-2-pyridylmethylidene-2-hydroxy-phenylamine. Polyhedron. 2006;25:1753-1762.
- [CrossRef] [Google Scholar]
- Synthesis, spectroscopic and biological studies on the new symmetric Schiff base derived from 2,6-diformyl-4-methylphenol with N-aminopyrimidine. J. Eur. Med. Chem.. 2010;45:1935-1940.
- [CrossRef] [Google Scholar]
- Structural and antimicrobial studies of coordination compounds of VO(II), Co(II), Ni(II) and Cu(II) with some Schiff bases involving 2-amino-4-chlorophenol. J. Serb. Chem. Soc.. 2009;74:523-535.
- [CrossRef] [Google Scholar]
- Synthesis, spectroscopic, thermal and molecular modeling studies of Zn2+, Cd2+ and UO22+ complexes of Schiff bases containing triazole moiety.Antimicrobial, anticancer, antioxidant and DNA binding studies. Mater. Sci. Eng., C. 2018;83:78-89.
- [CrossRef] [Google Scholar]
- Synthesis and biological evaluation of some novel thiazole compounds as potential anti-inflammatory agents. Eur. J. Med. Chem.. 2013;65:517-526.
- [CrossRef] [Google Scholar]
- Synthesis, spectroscopic characterization, DNA interaction and biological activities of Mn(II), Co(II), Ni(II) and Cu(II) complexes with [(1H–1,2,4-triazole-3-ylimino)methyl]naphthalene-2-ol. J. Mol. Struct.. 2014;1076:251-261.
- [CrossRef] [Google Scholar]
- Spectrochemical study and effect of high energatic gamma ray on copper (II) complexes. J. Solid State Sci.. 2011;13:2080-2085.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, antibacterial, antifungal, and immunomodulating activities of gatifloxacin derivatives. Med. Chem. Res.. 2010;19:1210-1221.
- [CrossRef] [Google Scholar]
- A novel method of synthesis of certain indole-3-aldehydes and their thiosemicarbazones as potential anticancer drugs. Int. J. Pharm. Biomed. Sci.. 2011;2:92-98.
- [Google Scholar]
- Some novel amino acid-schiff bases and their complexes synthesis, characterization, solid state conductivity behaviors and potentiometric studies. Z. Naturforsch.. 2004;59b:692-698.
- [Google Scholar]
- Screening the in vitro antioxidant, antimicrobial, anticholinesterase, antidiabetic activities of endemic Achillea cucullata (Asteraceae) ethanol extract. S. Afr. J. Bot.. 2019;120:141-145.
- [CrossRef] [Google Scholar]
- Design, synthesis, computational calculation and biological evaluation of some novel 2-thiazolyl hydrazones. Spectrochim. Acta A. 2015;135:984-993.
- [CrossRef] [Google Scholar]
- 2-acetylpyridine- and 2-benzoylpyridine-derived hydrazones and their gallium(III) complexes are highly cytotoxic to glioma cells. Eur. J. Med. Chem.. 2012;50:163-172.
- [CrossRef] [Google Scholar]
- Complexation of 2-acetylpyridine- and 2-benzoylpyridine-derived hydrazones to copper(II) as an effective strategy for antimicrobial activity improvement. Polyhedron. 2012;38:285-290.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, and antitumor activity of new copper(I) and mercury(II) complexes. Russ. J. General Chem.. 2017;87:1256-1263.
- [CrossRef] [Google Scholar]
- Effect of gamma irradiation on Spectral, XRD, SEM, DNA binding, molecular modling and antibacterial property of some (Z)-N-(furan-2-yl)methylene)-2-(phenylamino)acetohydrazide metal(II) complexes. J. Mol. Stru.. 2019;1185:323.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, antibacterial and anti-Inflammatory activities of enoxacin metal complexes. Bioinorg. Chem. Appl.. 2009;2009:1-6.
- [CrossRef] [Google Scholar]
- New alpha-(N)-heterocyclichydrazones: evaluation of anticancer, anti-HIV and antimicrobial activity. Eur. J. Med. Chem.. 2004;39:113-122.
- [CrossRef] [Google Scholar]
- Theoretical and spectroscopic studies of 5-fluoro-isatin-3-(N-benzylthiosemicarbazone) and its zinc(II) complex. J. Mol. Struct.. 2009;917:63-70.
- [CrossRef] [Google Scholar]
- Synthesis, spectral, thermal and antimicrobial studies of transition metal complexes of 14-membered tetraaza[N₄] macrocyclic ligand. Spectrochim. Acta A. 2015;145:188-193.
- [CrossRef] [Google Scholar]
- Synthesizing new hydrazone derivatives and studying their effects on the inhibition of copper corrosion in sodium chloride solution. Synth. React. Inorg. Met.-Org. Nano-Metal Chem.. 2010;40:365-372.
- [CrossRef] [Google Scholar]
- Inhibition of tumor angiogenesis by synthetic receptor tyrosine kinase inhibitors. Drug Discov. Today. 2000;5:344-353.
- [CrossRef] [Google Scholar]
- Ligational, potentiometric and floatation studies on Cu(II) complexes of hydrazones derived from p and o-vanillin condensed with diketo hydrazide. J. Mol. Liq.. 2016;220:939-953.
- [CrossRef] [Google Scholar]
- Inhibition activity of new thiazole hydrazones towards mild steel corrosion in acid media by thermodynamic, electrochemical and quantum chemical methods. Taiwan Inst. Chem. Eng.. 2016;67:521-531. http://dx.doi.org/10.1016%2Fj.jtice.2016.08.013
- [Google Scholar]
- Plant extracts with metal ions as potential antimicrobial agents. Phytopathology. 1964;55:910-914.
- [Google Scholar]
- Thermal studies on lanthanide nitrate complexes of 4-n-(2′-furfurylidene)aminoantipyrine. J. Therm. Anal. Cal.. 2000;61:143-149.
- [CrossRef] [Google Scholar]
- Pennsylvania. 2009;29(2):1087-.
- Xavier1, D.A., Srividhya, N., 2014. Synthesis and study of schiff base ligands. J. Appl. Chem. 7, 6–15. http://www.iosrjournals.org.
- Synthesis and characterization of di- and tri-organotin(IV) complexes with Schiff base ligand pyruvic acid 3-hydroxy-2-naphthoyl hydrazone. Inorg. Chim. Acta. 2006;359:3330-3338.
- [CrossRef] [Google Scholar]
- Thermal and spectral studies of 1-benzyl-2- phenylbenzimidazole complexes of cobalt(II) Thermochim. Acta. 1990;159:357-368.
- [CrossRef] [Google Scholar]
- Structural and biological evaluation of some metal complexes of vanillin-4N-(2-pyridyl) thiosemicarbazone. J. Mol. Struct.. 2013;1053:15-21.
- [CrossRef] [Google Scholar]
- Structural, spectral, thermal and biological studies on (E)-2-(1-(4-hydroxyphenyl)ethylidene)-N-(pyridin-2-yl)hydrazinecarbothioamide and its metal complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2014;133:568-578.
- [CrossRef] [Google Scholar]
- Synthesis, spectroscopic characterization and thermal behavior of metal complexes formed with (Z)-2-oxo-2-(2-(2-oxoindolin-3-ylidene)hydrazinyl)-N-phenylacetamide (H2OI) J. Mol. Struct.. 2012;1007:146-157.
- [CrossRef] [Google Scholar]
- Comparative ligational, optical band gap and biological studies on Cr(III) and Fe(III) complexes of hydrazones derived from 2-hydrazinyl-2-oxo-N-phenylacetamide with both vanillin and O-vanillin. Chem. Phys. Lett.. 2015;636:180-192.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.12.003.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







