Translate this page into:
Preparation of a novel biodegradable film by co-fermentation of straw and shrimp shell with Aureobasidium pullulans and Photobacterium sp. LYM-1
⁎Corresponding author. lyu.yongmei@ycit.edu.cn (Yongmei Lyu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The green and high-value recycling of shrimp shell and straw remains a worldwide problem. This study aimed to investigate the potential utilization of a fermentation broth (FB) which contains shrimp shell and straw as a new source for preparation of biodegradable films. Aureobasidium pullulans and Photobacterium sp. LYM-1 were used in the fermentation. The cellulase activity was 115.92 U/mL and chitinase activity was 17.89 U/mL in FB. The polysaccharides concentration in FB was 1.05 mg/mL after 7 days of fermentation. An eco-friendly PVA-reinforced FB biodegradable film (FBBF) was successfully prepared and the effect of different plasticizers and surfactants on the mechanical, structural, and impermeability properties of the film was determined. The formation of new bonds between PVA and FB was proved by FTIR spectroscopy. The FBBF containing 0.25 % (w/v) glycerol and 0.01 % (v/v) tween-20 showed better strength properties. Elongation and water-swelling properties were highly improved by adding 0.2 % (m/v) citric acid. According to FE-SEM images, the smooth and tight surface of citric acid added FBBF was observed. Interestingly, the FBBF film showed good heat/moisture capacity, antifungal, and degradation properties. This report reveals a new green, and high-value recycling of straw and shrimp shell by the co-fermentation with A. pullulans and Photobacterium sp. LYM-1. It is also a novel way for the preparation of biodegradable film.
Keywords
Shrimp shell
Straw
Fermentation
Biodegradable film
High-value
- FB
-
fermentation broth
- FBBF
-
FB biodegradable film
- LB
-
Luria–Bertani medium
- YPD
-
Yeast Extract Peptone Dextrose Medium
- PVA
-
polyvinyl alcohol
- P
-
the film only contains PVA
- PF
-
the film contains PVA and Fermented Broth (FB)
- PFGlT
-
the film contains PVA, FB, Glycerol, and Tween-20
- PFBGl
-
the film contains PVA, FB, boric acid, and glycerol
- PFCGl
-
the film contains PVA, FB, citric acid, and glycerol
- PFGaGl
-
the film contains PVA, FB, Glutaraldehyde, and glycerol
- PFC
-
the film contains PVA, FB, and citric acid
- PFGa
-
the film contains PVA, FB, and Glutaraldehyde
- PFGl
-
the film contains PVA, FB, and Glycerol
- Rs
-
Rotation speed
- WBF/WAF
-
the weight before/after fermentation of substrates
- Rsc
-
the rate of substrates consumption
- FE-SEM
-
field emission scanning electron microscopy
- ATR-FTIR
-
Attenuated total reflectance-Fourier transform infrared
- Sr
-
Swelling ratio
- FWS
-
Film water solubility
- RH
-
the relative humidity
Abbreviations
1 Introduction
Non-renewable petro-based synthetic polymer plastic films have been used in a broad range of applications, including houseware, agriculture, transportation, and construction (Cherubini and Ulgiati, 2010; Koller et al., 2017; Kourmentza et al., 2017). The amount of plastic films used in mulching and greenhouse over the world reached approximately 7.4 million tons in 2019, which implied a huge increase compared with the 4.4 million tons produced in 2012 (Li et al., 2021). The increase in environmental impact from plastic films, along with the market demand for materials, has stimulated a major change to sustainable film systems based on natural, renewable, degradable and recyclable polymeric materials (Călinoiu and Vodnar, 2018).
In the last decades, the novel biodegradable films from biomass and biomass-derived polymers have been postulated as an alternative strategy for lowering the environmental impact of fossil-based plastic waste (Assis et al., 2018; Salari et al., 2018). Biopolymers are usually classified by source: 1. Polymers extracted/removed directly from biomass such as polysaccharides (e.g., chitosan, starch, cellulose and galactomannan) and proteins (e.g., gluten, zein, and casein) (Liu et al., 2020; Hemapriya et al., 2020; Mostafa et al., 2018). 2. Polymers produced by chemical synthesis from renewable bioderived monomers, such as polyvinyl alcohol (PVA), which is a polymer with good film-forming capacity, fully biodegradability, wide-ranging crystallinity (Wong et al., 2020), and polylactic acid (PLA) (Özge Erdohan et al., 2013), and 3. Polymers produced by microorganisms, such as some polysaccharides (e.g., gellan gum and amylopectin) and polyhydroxyalkanoates (PHA) (Ferreira et al., 2016; Mensitieri et al., 2011). Natural biopolymer degradable films are commonly produced by polysaccharides, lipids, proteins, and their combinations (Sun et al., 2018). Among these categories, polysaccharides are the most common material due to their abundant renewable sources, good film-forming ability, and gas barrier property (Gasti et al., 2021). Nevertheless, the limitations of natural polymer materials, such as their poor mechanical properties and the difficulties encountered in their large-scale production, have restricted their application in many areas including agricultural mulches (Sikder et al., 2021). The modification of natural polymer-based films by biodegradable plasticizers (glycerol) and cross-linkers (PVA) have been thoroughly studied (Stachowiak et al., 2020).
The agri-food wastes have been sustainably recycled in many fields, such as the production of high-value bioactive compounds (Fernando et al., 2021), biogas (Tawfik et al., 2021), films (Freitas et al., 2021), and heavy metals removal (Talebi et al., 2021). The biodegradable films derived from seeding mucilages have been explored based on their functional hydrophilic properties (Beikzadeh et al., 2020; Ghadiri Alamdari et al., 2021). However, the materials from agri-food waste are commonly modified by chemical ways before being used for the synthesis of biodegradable films (Behera et al., 2021; de Campos et al., 2011). Approximately 6–8 million tons of crab and shrimp shells waste are produced annually and mostly discarded (Yan and Chen, 2015) although, these shells are rich in protein and chitin. Chitin is chemically produced from prawn and crab waste in industry. Recent studies have reported the chitin extraction by enzymatic hydrolysis and fermentation methods (Babu et al., 2013; Battampara et al., 2020; Freitas et al., 2014; Hajji et al., 2015). However, only a few studies were reported on the preparation of biodegradable films from the fermentation of crab and shrimp shells waste. As a type of agricultural waste, straw is rich in cellulose, the most abundant agricultural polymer in nature. The regular structure and hydroxyl arrangement of cellulose make this polymer commonly used in packaging materials, such as paper, cardboard, and corrugated cardboard (Babu et al., 2013; Freitas et al., 2014). The preparation of organic macromolecules by fermentation is a common strategy, but the utilization of straw fermentation for the preparation of biodegradable films is still rare (Pakizeh, 2021).
In the present report, the degradable films were prepared using a fermentation broth (FB) containing shrimp shell and straw. The compatibility between two agri-food waste-degrading strains and their hydrolysis properties was explored. The fermentation broth biodegradable film (FBBF) was prepared and optimized by applying different plasticizers and surfactants. At last, the potential application and degradability of the obtained film were explored. Our results showed that the developed method for the preparation of the biodegradable film could be a promising approach for the eco‐friendly bioconversion of chitin- and cellulose-rich wastes into high-value products.
2 Material and method
2.1 Material and strains
Yeast extract, tryptone, and glucose were purchased from Sangon Biotech (Shanghai, China). Boric acid (99 % purity), citric acid (99 % purity) and glutaraldehyde (99 % purity) were of analytic-grade and were purchased from Aladdin (Shanghai, China). Polyvinyl alcohol (Mw 89,000–98,000, 99 % hydrolyzed), tween-20 (99 % purity), and glycerol (99 % purity) were purchased from Macklin (Shanghai, China). Rice straw and shrimp shells were purchased from Yancheng Farmer’s market, Jiangsu province, China. Photobacterium sp. LYM-1 (GDMCC No. 1.3009) was isolated by our team and preserved in the Microbial Culture Collection Center of Guang-dong Institute of Microbiology. Aureobasidium pullulan 2012 was obtained from the College of Food Science and Technology, Nanjing Agricultural University, China.
LB (Luria–Bertani) medium, containing 5 g/L yeast extract, 10 g/L tryptone, 10 g/L sodium chloride, pH 7.0, was used in the experiments. LB medium was sterilized at 121 °C for 20 min. LB agar plates contained 15 g/L agar.
YPD (Yeast Extract Peptone Dextrose Medium) medium, containing 20 g/L tryptone, 10 g/L yeast extract, and 20 g/L glucose, was used in the experiments. This medium was sterilized at 121 °C sterilization for 20 min. YPD agar plates contained 15 g/L agar.
Fermentation medium, containing 15 g/L straw, and 30 g/L shrimp shell, was sterilized at 121 °C for 20 min and used in the experiments.
2.2 Compatibility test of A. pullulans and Photobacterium sp. LYM-1 strains
The plate confrontation method was conducted to test the compatibility of two strains based on the previous method (Shi et al., 2017). A. pullulans was smeared on the surface of the YPD agar plate and the Photobacterium sp. LYM-1 was inoculated in the middle. Then Photobacterium sp. LYM-1 was smeared on the surface of the LB agar plate and A. pullulans was inoculated on it. Plates were incubated at 28 °C for 3 d. The growth and bacteriostatic circle of the two strains were observed.
2.3 Seeding liquid preparation and liquid fermentation
To prepare inoculum cultures, a single cryopreserving bead was incubated in 10 mL of sterile LB medium for Photobacterium sp. LYM-1 and YPD medium for A. pullulans, at 28 °C and 180 rpm for 24 h.
In the experimental group, 2.5 mL inoculum culture of Photobacterium sp. LYM-1 and 2.5 mL inoculum culture of A. pullulans (1:1, v/v) was conducted in 100 mL of fermentation medium and co-cultured at 28 °C and 180 rpm for 8 d. In the control group, only Photobacterium sp. LYM-1 or A. pullulans was applied into the medium and fermented under the same conditions. The experimental group and control groups were set at 3 repeats. Every 24 h, 1 mL of fermentation broth was collected from each flask and the specimens were filtered and centrifuged at 10,000 rpm for 10 min. The extracts were filtered through filter paper and stored at −20 °C for further analysis (Dias et al., 2018).
2.4 Determination of enzyme activity and sugar content in the fermentation process
To measure the chitinase activity, colloidal chitin was used as the substrate and prepared based on the method previously described by Joshi et al. (Joshi et al., 1989). The DNS colorimetric method (3,5-dinitrosalicylic acid) was used to measure the content of reducing sugar. The reaction system containing 100 μL of 1 % colloidal chitin and 50 μL of the fermentation broth (FB) was incubated at 37 °C for 30 min and quenched in a boiling water bath for 5 min. The samples were added 300 μL of DNS reagent and boiled in a water bath for 10 min. The samples were quantified to 1 mL by distilled water after cooling. Two hundred microliters of the supernatant were used to measure the absorbance at 520 nm (Multiskan Sky Plate-reader, Thermo Scientific). Inactivated FB (the inactivation was carried out by heating) was used as a negative control. One unit of chitinase activity (U/mL) was defined as the amount of enzyme for conversion of colloidal chitin into 1 μg of reducing sugar per min at 37 °C.
ΔOD is the change value of absorbance OD;
V is always the total volume of reaction liquid (mL);
N is the dilution ratio of enzyme solution;
ΔT is reaction time;
Venzyme is enzyme liquid volume (mL).
To measure the cellulase activity, the method reported by Garcia et al. was used (Garcia et al., 2018) with minor modifications. DNS colorimetric method was used to measure the content of reducing sugar. The reaction system was composed of 100 μL of 1 % carboxyl cellulose (dissolved in acetic acid-sodium acetate buffer, pH4.8) and 50 μL of FB. The reaction mixture was incubated at 50 °C for 30 min. The reaction was stopped by heating the reaction mixture in a boiling water bath for 5 min. The other steps were the same as those in the measurement of chitinase activity. Cellulase activity (U/mL) was calculated according to the amount of enzyme for conversion of carboxymethyl cellulose into 1 μg of reducing sugar per min at 50 °C.
The content of reducing sugars was measured using the method previously reported by Lam et al. (Lam et al., 2021). The standard curve was prepared as follows. Briefly, 1 mg/ mL glucose solution was prepared and diluted to 0.02, 0.04, 0.06, 0.08, 0.1, 0.12, 0.14, 0.16, 0.18, and 0.2 mg/mL, respectively. One hundred fifty of standard glucose and 300 μL of DNS reagent were mixed and boiled for 5 min The absorbance at 520 nm was tested. The standard curve of glucose was constructed using glucose concentration (abscissa) versus absorbance (ordinate).
FB was directly reacted with DNS reagent to calculate the concentration of reducing sugar in the medium. The measured absorbance was used to calculate the concentration of reducing sugar in the FB. The standard curve was as follows (Eq. (2)):
X is the reducing sugar concentration in fermentation broth;
Y is the absorbance of the specimen;
A is the slope of the regression equation;
B is the y-intercept of the regression equation;
C is the dilution ratio of the specimen.
The total amount of sugars was measured by the phenol–sulfuric acid method (Lam et al., 2021). The standard curve was prepared as follows: 1 mg/mL glucose solution was prepared. Then, the standard solution was diluted to 0.02, 0.04, 0.06, 0.08, 0.1, 0.12, 0.14, 0.16, 0.18, and 0.2 mg/mL, respectively. Two hundred microliters of glucose solutions and 0.2 mL of phenol (mass fraction: 5 %) were mixed. Then, 1 mL of concentrated sulfuric acid was added. The absorbance of the samples was measured at 490 nm. The standard curve of the glucose solution was obtained.
The absorbance of the FB was measured at 490 nm. The measured absorbance was used (Eq. (3)) to calculate the total concentration of sugars in the FB.
X is the total sugar concentration in fermentation broth;
Y is the absorbance of the specimen;
A is the slope of the regression equation;
B is the y-intercept of the regression equation;
C is the dilution ratio of the specimen.
2.5 Extraction and purification of polysaccharides from the FB
The fermentation broth was centrifuged at 8000 rpm for 10 min and the supernatant was collected. Then, the polysaccharides were concentrated to 2 mg/mL by using a rotary evaporator (Shanghai Lu Xiangyi TGL-16M) at 55 °C. The concentrated FB was dialyzed at 4 °C for 2 days to remove the small molecules, e.g., salts, monosaccharides, and amino acids. Polysaccharides were precipitated by 95 % ethanol (1:4, FB/ethanol) at 4 °C for 12 h. The polysaccharides were collected by centrifugation at 4000 rpm for 10 min. Distilled water was used to dissolve the polysaccharides. Redissolved solution was mixed with Sevage reagent (trichloromethane:n-butanol, 4:1, v/v) and spun for 30 min. After centrifugation, the water phase was collected and this step was repeated for several times to remove the proteins in the solution as much as possible. The resulting solution was diluted three times with anhydrous ethanol and held at 4 °C for 24 h. The precipitated polysaccharides were then collected by centrifugation and dissolved in distilled water.
2.6 Preparation of biodegradable film using FB as the substrate
An appropriate amount of polyvinyl alcohol 17–99 (PVA) was dissolved in distilled water by stirring to obtain a 2 % (w/v) homogeneous solution. 10 mL of polysaccharides solution (75 %, w/v) and 10 mL of PVA solution (2 %, w/v) were mixed and stirred. The solution was then cast on a petri-dish and dried at 50 °C for 24 h. The FB biodegradable film (FBBF) was obtained.
2.7 Optimization of FBBF film formulation
A set of experimental designs were carried out for the optimization of FBBF film by adding different additives. The additives included glycerol, citric acid, glutaraldehyde, boric acid and tween-20. Through a series of preliminary experiments, the optimized concentrations for each additive are shown in Table 1.
Code of samples
FB
(v/v)PVA
(w/v)CA
(w/v)BA
(w/v)GL
(w/v)TW
(v/v)GA
(v/v)
P
/
1 %
/
/
/
/
/
PF
75 %
1 %
/
/
/
/
/
PFC
75 %
1 %
0.2 %
/
/
/
/
PFB
75 %
1 %
/
0.05 %
/
/
/
PFGa
75 %
1 %
/
/
/
/
0.2 %
PFGl
75 %
1 %
/
/
0.25 %
/
/
PFGlT
75 %
1 %
/
/
0.25 %
0.01 %
/
PFCGl
75 %
1 %
0.2 %
/
0.25 %
/
/
PFBGl
75 %
1 %
/
0.2 %
0.25 %
/
/
PFGaGl
75 %
1 %
/
/
0.25 %
/
0.2 %
2.8 Hardness property test
The mechanical properties in terms of hardness of the FBBFs were determined at room temperature by using a texture tester (Shanghai Baosheng Technology). The method described by Cao et al. was used with some modifications (Cao et al., 2020). The biodegradable film samples were cut into square slices (10 cm × 10 cm), clamped with a clamp and put into the instrument. For this purpose, a 2 mm diameter rod probe (TA39) was used to analyze the compression test at a target distance of 5.0 mm and applied at a test speed of 0.5 mm/s. Measurements were made in triplicate and the results were shown as mean value ± standard deviation.
The hardness (MPa) was calculated according to the following Eq. (4):
F is the force on the biodegradable film specimen (N);
R is the radius of rod probe (mm).
2.9 Elongation at break (Eb)
Elongation at break (Eb) was carried out following the method by Chen et al. (2019) using a texture tester (Shanghai Baosheng Technology). The crosshead’s maximum strength capacity was 500n. Rectangular samples (10 cm × 10 cm) were used as the specimens. A fixed crosshead rate of 5 mm/min was used in all cases and results were taken as the mean (±standard deviation) of the three trials. SPSS software was used to test the significance of the mean values of all parameters through analysis of variance.
2.10 Water absorption and solubility test
Water absorption of the FBBF film samples was established in terms of swelling (Behera et al., 2021; Orsuwan et al., 2016; Yadav et al., 2020). The dried film specimens were sectioned into 5 cm × 5 cm pieces and pre-weighed W0. Each sample was placed in a Petri-dish containing 200 mL of distilled water and held at 25 °C for 24 h until equilibrium swelling was reached. The swollen samples were removed from the water. The moisture on the surface of the film was sucked up with filter paper, weighed and recorded with W1. Sucked films were dried at 50 °C and recorded the weight W2.
The swelling ratio (Sr) and the solubility were calculated according to the following Eqs. (5) and (6):
2.11 Attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy analysis
ATR-FTIR analysis was carried out by the IR Affinity-1S, SHIMADZU equipment (Kyoto, Japan). For each sample, a total of 45 scans with a resolution of 4 cm−1 were obtained in transmittance mode using a diamond ATR crystal cell. The measurements were recorded in the range of 600–4000 cm−1. All specimens were examined using the KBr pellet technique.
2.12 Field emission scanning electron microscopy (FE-SEM)
The surface morphology and differences of the FBBF films were visualized and evaluated using a field emission electron microscope (Nova NanoSEM450) at 10 kV acceleration voltage and 6 × 10−5 Pa vacuum. The film specimens were coated with a thin gold layer under the vacuum state before the analysis.
2.13 Film opacity
The films were cut to 1 × 4 cm2 strips and placed in a UV–vis spectrophotometer (JASCO, V-650). The opacity values of the films were calculated according to the following Eq. (7).
2.14 Heat/moisture-preservation properties of FBBF film
Potentilla supina L. Sp. Pl. was selected as a model plant to evaluate the heat-preservation property of the FBBF film. Optimized FBBF film (PFCGl) was chosen as the standard film in this study. Five seeds were planted into each pot (diameter of 10 cm) with nutrient soil and grown in the humidity-controlled chamber at 10 °C/70 % RH in dark and 24 °C/70 % RH in light, respectively. was covered on the pot. The pot without film and covered by the commercial plastic film were negative and positive controls, respectively. The surface temperature of the soil in the pots covered by different films was measured by a waterproof digital thermometer (SK-250WP II-N, Suzhou, China). The soil temperature was recorded at 8 a.m. every day. All specimens were done with three replicates and the average value was calculated.
2.15 Degradation property
The biodegradability property was carried out based on the previous method (Behera et al., 2021; de Campos et al., 2011). Rectangular-shaped FBBF films (10 cm × 10 cm) were dried to constant weight W0, then wrapped with the gauze to keep it from the soil adhesion. The prepared specimens were buried in garden soil and covered to a depth of 5.0 cm, and then placed in a humidity-controlled chamber at 25 °C and 30 % RH. After a certain interval of time (10 d, 20 d, and 30 d). specimens were carefully cleaned using tissue paper and dried to constant weight Wn. The weight loss (Wl) was determined at different time points and the results were calculated according to Eq. (8):
Wl is the degradation rate of FBBF film (%);
Wn is the residue weight of the FBBF film after a certain time point (g);
W0 is the initial weight of FBBF specimen (g).
2.16 Statistical analysis
All the studies were done three times to indicate repeatability. All the quantitative data were analyzed using SPSS 15.0 software. All data were presented as means of a homogenous set of three replications ± standard deviations. A one-way analysis of variance (ANOVA) was used to determine the significant differences between groups. Differences were considered statistically significant at p ≤ 0.05 by Duncan’s test.
3 Results and discussion
3.1 Compatibility test of the strains
Straw is one of the abundant lignocellulosic waste materials with the least recycling in the world. Shrimp and crab shells contain a lot of organic compounds without effective exploitation. A. pullulans, which is able to efficiently degrade cellulose in straw, and Photobacterium sp. LYM-1, which can degrade shrimp and crab shells, were used for the fermentation of straw and shrimp shells. The compatibility of these two strains was explored for the co-fermentation of straw and shell. Fig. 1 showed that there was no obvious antagonism between A. pullulans and Photobacterium sp. LYM-1. Two strains were amplified at a similar rate and no bacteriostatic zone was observed between them after 24 h. After 48 h, the two strains could fuse without obvious boundaries. This result demonstrated that these two strains could grow together with compatibility.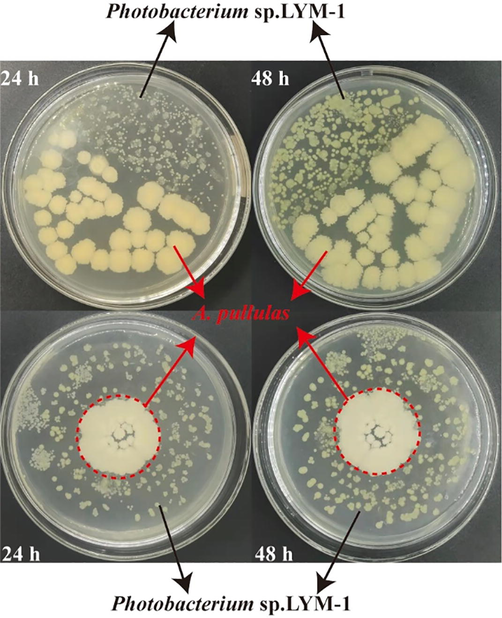
The compatibility assay between A. pullulans and Photobacterium sp. LYM-1.
3.2 Determination of chitinase activity and cellulase activity
In this report, cellulase activity and chitinase activity were measured to indicate the efficiency for digesting straw and shrimp shell in the broth within the two strains. Fig. 2a showed that cellulase activity increased in both A. pullulans monoculture and co-culture group, while, Photobacterium sp. LYM-1 had no cellulase activity. The highest activity was 115.92 U/ mL in A. pullulans monoculture after 7 d. In agreement with this result, a previous report indicated that Aspergillus sp. showed a good ability to degrade cellulose (Dias et al., 2018). For chitinase activity, the maximum production of chitinase was observed in the monoculture fermentation employing Photobacterium sp. LYM-1 in the consortium, with 19.67 U/mL, after 5 d (Fig. 2b). On the other hand, co-culture broth showed a similar activity after 5 d. Photobacterium sp. was normally isolated from marine seawater with chitin hydrolysis properties (Wang et al., 2019). The transparent ring experiment confirmed the high efficiency of Photobacterium sp. LYM-1 to degrade chitin (the data not shown). Considering the efficiency and economy, the co-culture of A. pullulans and Photobacterium sp. LYM-1 was the promising combination for preparation of degradable film material.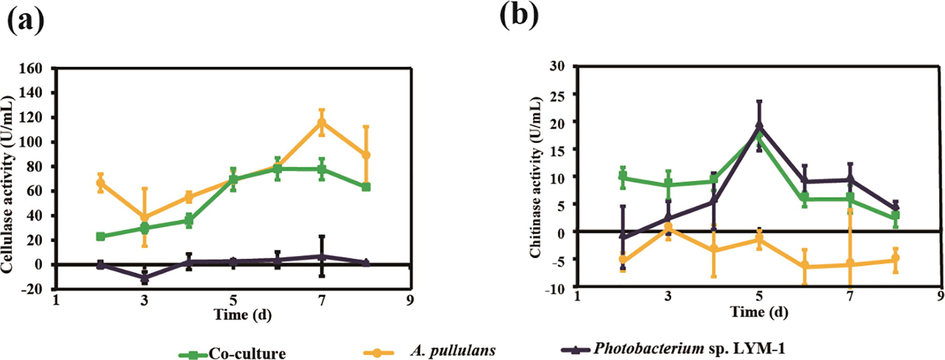
Activity of chitinase and cellulase during fermentation in the FB.
3.3 Determination of reducing sugar, total sugar and polysaccharide contents
Polysaccharide is an ideal polymer for biodegradable film preparation. The concentration of polysaccharides in the FB was investigated in this report. The reducing sugar in A. pullulans monoculture fermentation broth was decreased at the first 4 d (Fig. 3a), indicating that A. pullulans grew fast. Subsequently, a slight increase in the concentration of reducing sugar was detected on day 5, indicating the high production of cellulase, which is consistent with the cellulase activity result. After 6 d, the concentration of reducing sugar tended to be stable, indicating that the amount of consumed and synthetized reducing sugar by the cells reached a balance. The concentration of reducing sugar retained stably in the monoculture of Photobacterium sp. LYM-1. The total sugar production was analyzed and shown in Fig. 3b. There was no big difference between the monocultures and the co-culture. Interestingly, the total sugars slowly decreased and then increased, which demonstrated that the cells use them as nutrients during the first stages of the fermentation, and then the cells synthetize extracellular saccharides.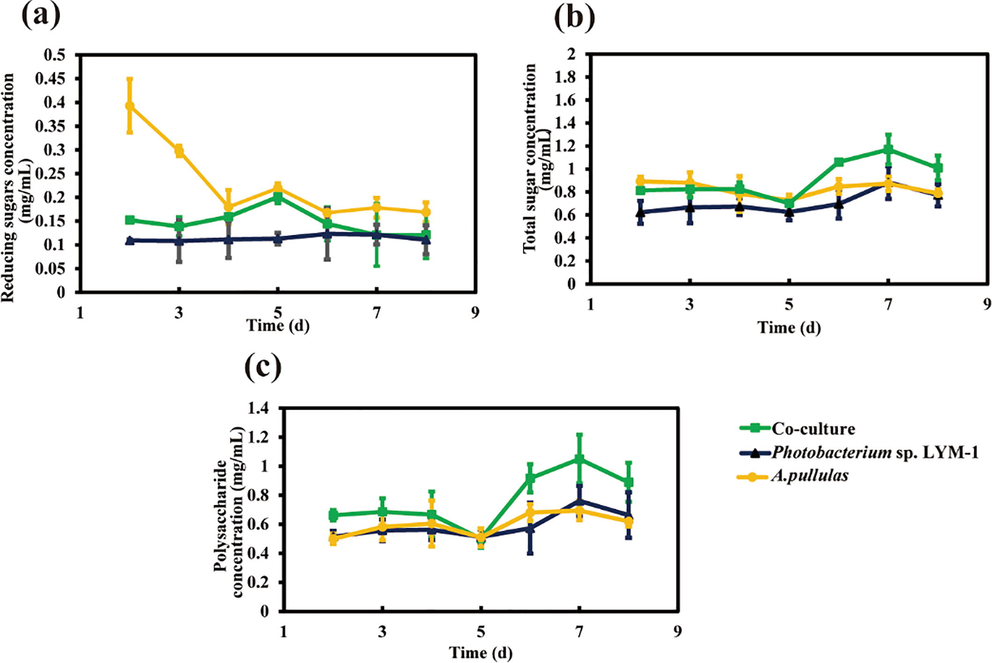
Reducing sugar, total sugar and polysaccharide production during the fermentation. (a) Reducing sugar production in the FB, (b) Total sugar production in the FB, and (c) Polysaccharide concentration in the FB.
The concentration of polysaccharides was measured to examine the difference between the amount of reducing sugars and total sugars. The polysaccharide concentration in the FB of the co-culture decreased during the first 5 d. Subsequently, the concentration of polysaccharides increased and the highest concentration of 1.17 mg/mL was observed after 7 d. The content of polysaccharides in the FB of the monocultures showed a similar trend. The result proved that Aureobasidium sp. is a high-quality bacterium for producing extracellular polysaccharides (Ravella et al., 2010).
The consumption of straw and shrimp shell by A. pullulans and Photobacterium sp. LYM-1 in the FB is shown in Table 2. The co-culture of the two strains displayed a higher metabolic rate compared to the monocultures. The rate of substrates consumption (Rsc) was 63.39 % in the co-culture. However, the metabolic rate of substrates in the A. pullulans monoculture was only 46.57 %, and the metabolic rate in Photobacterium sp. LYM-1 monoculture was 53.11 %. Collectively, A. pullulans and Photobacterium sp. LYM-1 showed compatibility, and the co-culture of both strains allowed high extracellular enzymatic activities, metabolic rate, and economy. For these reasons, the co-culture of A. pullulans and Photobacterium sp. LYM-1 was chosen for the preparation of the biodegradable film. Note: Values with the different superscript letters in the same line indicate that they are statistically different (p < 0.05). The values are presented as mean ± SD (n = 3).
Fermentation type
Time (d)
Tm (°C)
Rs (rpm)
WBF (g)
WAF (g)
Rsc (%)
Photobacterium sp. LYM-1
8
28
200
4.5
2.11 ± 0.14b
53.11 ± 0.03b
A. pullulans
8
28
200
4.5
2.98 ± 0.20a
46.57 ± 0.04c
Co-culture
8
28
200
4.5
1.64 ± 0.08c
63.39 ± 0.02a
3.4 Preparation of degradable film
The fermentation broth (FB) was collected and the polysaccharides were purified. The crosslinker-PVA-reinforced fermentation broth biodegradable film (FBBF) was prepared. In most cases, the mechanical and waterproof properties of the biodegradable film prepared from biomass without plasticizers could not meet the requirements of industrial application (Das et al., 2020; Wang et al., 2021). In this study, different additives commonly used in commercial biodegradable films were explored. The experiment for the optimal ratio of each additive in FBBF films were conducted in the early stage (data not shown). The optical ratio of each additive is shown in Table 1. As can be seen in Fig. 4, the film with fermentation broth was darker than the one obtained without fermentation broth, and the film with glycerol was softer and more elastic than the one without glycerol. The properties of different films were compared (Table 3). Note: All the data are shown as means ± SD, n = 3. Means with different superscript letters within a column are significantly different (p < 0.05).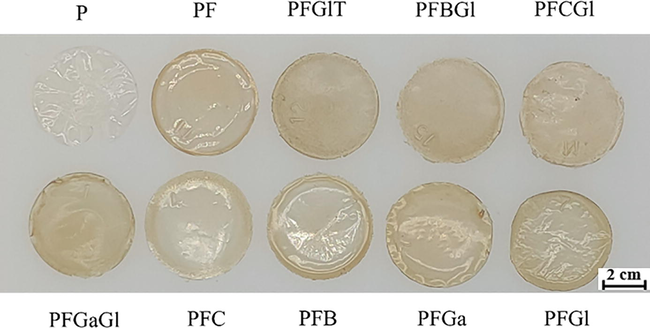
The optimizations of FBBF film with different additives. P means the film only contains PVA; PF means the film contains PVA and Fermentation Broth (FB); PFGlT means the film contains PVA, FB, glycerol, and tween-20; PFBGl means the film contains PVA, FB, boric acid, and glycerol; PFCGl means the film contains PVA, FB, citric acid, and glycerol; PFGaGl means the film contains PVA, FB, glutaraldehyde, and glycerol; PFC means the film contains PVA, FB, and citric acid. PFGa means the film contains PVA, FB, and glutaraldehyde; PFGl means the film contains PVA, FB, and glycerol.
Code of Samples
Thickness (μm)
Hardness (MPa)
Eb (%)
Sr (%)
FWS (%)
Opacity
(Abs600/d)
P
13.09 ± 4.66a
2.01 ± 0.78a
5.31 ± 2.17a
607.77 ± 23.12c
33.42 ± 3.21c
1.31 ± 0.21a
PF
18.26 ± 2.11b
2.31 ± 1.09a
4.86 ± 1.86a
659.11 ± 33.42c
22.34 ± 6.42ab
4.53 ± 0.51b
PFC
19.36 ± 3.25b
2.65 ± 0.78a
118.18 ± 36.01bc
619.21 ± 45.02c
18.12 ± 4.23a
4.75 ± 0.23b
PFB
14.58 ± 6.21a
2.12 ± 0.57a
6.21 ± 3.12a
133.91 ± 19.18a
76.60 ± 13.24e
4.53 ± 0.41b
PFGa
18.62 ± 3.26b
3.01 ± 0.82ab
91.39 ± 12.45b
712.36 ± 32.14d
50.12 ± 5.36d
4.82 ± 0.31b
PFGl
23.50 ± 4.25c
3.38 ± 1.18b
86.56 ± 21.23b
216.34 ± 22.31b
29.24 ± 7.12b
5.21 ± 0.48b
PFGlT
13.00 ± 4.62a
3.66 ± 1.32ab
67.27 + 21.24b
227.43 ± 33.12b
28.71 ± 6.54b
4.85 ± 0.21b
PFCGl
20.14 ± 7.24b
3.49 ± 0.84b
135.49 ± 12.14c
204.16 ± 11.36b
20.47 ± 3.87ab
4.93 ± 0.32b
PFBGl
19.58 ± 3.24b
3.52 ± 0.72b
62.51 ± 13.62b
233.91 ± 19.18b
56.60 + 13.24d
4.96 ± 0.42b
PFGlGa
20.54 ± 2.76b
3.11 ± 0.72ab
111.59 ± 11.54c
234.61 ± 34.44b
20.32 ± 4.46ab
5.01 ± 0.46b
3.5 Effects of different additives on the physical properties of the FBBF film
3.5.1 Hardness
The hardness, which represents the maximum load value of the compression cycle to attain the maximum deformation, is one of the most important properties of a material. The results for the hardness of the biodegradable films are shown in Table 3. Surprisingly, it was found that the hardness of the films modified with additives was higher than that observed in the film without additives. These results are in agreement with the previous reports (Dai et al., 2002; Li et al., 2017; Ma et al., 2016; Mitrea et al., 2020; Z. Zhang et al., 2020). This result can be explained by considering the formation of hydrogen bonds between glycerol, PVA and the polysaccharides in FB. In the membrane formation fluid, substitution reaction between boric acid and —OH, esterification reaction between citric acid and —OH, acetal reaction of glutaraldehyde contributes to the improvement of the film hardness.
3.5.2 Elongation at break (Eb)
As shown by the Eb parameters listed in Table 3, glycerol is an effective plasticizer for improving the elongation ability of biological films. The Eb of PF film increased from 4.86 % to 86.56 % after the addition of 0.2 % (v/v) of glycerol (PFGl film). The film modified with glycerol showed better Eb properties than ones with tween 20 (PFGlT, 67.27 %) or boric acid (PFB, 6.21 %), but worse than the one with glutaraldehyde (PFGa, 91.39 %). However, the film modified with citric acid (PFC, 118.18 %) exhibited better Eb properties than PFGa. FBCGl showed the best elongation ability (135.49 %). The changes in Eb suggested that the hydroxyl in glycerol and citric acid can enter between the polymeric chains. This may result in weakening of the intermolecular force, reducing the intermolecular crystallinity, and finally increasing the plasticity of the polymer, which is consistent with the result reported by Andrade et al. (de Andrade et al., 2019).
3.5.3 Swelling ratio (Sr)
The swelling ratio is an indicator that represents the water-holding capacity of polymeric films. The swelling behavior is related to the diffusion of water molecules into the void space and macromolecular relaxation in the polymer chain network. The Sr values of different films were recorded in Table 3. The Sr of pure PVA was 607.77 %. There was no obvious difference between the addition of FB polysaccharides. For example, the Sr of PFGa was 712.35 %. The Sr of PFGl decreased almost three times compared to PF film. Furthermore, the Sr value of the PFB film was only 133.9 %. The mechanical strength of the PFB film was reduced compared to the FBC, FBGa film, and FBGlT films. These results indicated that the water-holding capacity of PF films decreased by adding boric acid, citric acid, or glutaraldehyde. As a crosslinking agent in the PF film, the additives could interact with functional groups of polysaccharides. Consequently, less free space and fewer hydroxyl groups were available for holding water molecules, which may result in a reduction of the water-holding capacity of the polymeric films. Cazón et al. reported similar observations and indicated that glycerol significantly reduced the Sr of the film (Cazón et al., 2019).
3.5.4 Film water solubility
As shown by the values listed in Table 3, the fermentation broth (FB) was an effective filler for improving the FWS of PVA. The FWS of PVA films decreased from 33.43 % to 22.34 % after the addition of 75 % FB. The changes in FWS were presumably caused by the complexity of FB, which blocked the tendency of PVA to dissolve in water. Boric acid- and glutaraldehyde-modified films showed high FWS, with 76.60 % and 50.12 %, respectively. Makishi et al. reported a similar result with the proteins blend film (Makishi et al., 2013). Although glutaraldehyde and boric acid are crosslinking agents (Kim et al., 1994), they were not considered as alternatives for further applications in this study.
3.5.5 Opacity
The opacity (or transparency) of biodegradable films is an important property to be considered for real applications. The higher the transparency the film is, the lower the opacity it has. The measured color parameters (opacity) were valued in Table 3. It can be seen in the color changes of the different FBBF films, the opacity value increased in the FBBF films compared to that in the pure PVA film. The addition of different modifiers had no significant effect on the opacity. The increase in the opacity in the FBBF films can be explained by the residual pigment in the fermentation broth. These values were similar to and in some cases even lower than those previously reported in other biodegradable films (Gasti et al., 2021; Li et al., 2019).
3.6 FTIR analysis
To compare the chemical changes in the film matrix of different FBBF films, the ATR-FTIR analysis was performed. The FTIR spectra of PVA, PF and other films modified with glycerol, tween-20, citric acid, boric acid or glutaraldehyde were recorded at room temperature in the region 600–4000 cm−1 (Fig. 5). Fig. 5a showed the differences between the P, PF, PFGl, and PFGlT films. Based on the spectrum data of previous reports (Nascimento et al., 2016), the broad absorption peak at 3266 cm−1 was assigned to —OH groups of sugars in hemicellulosic polysaccharide structure. It was found that the wave peak at 3336 cm−1, which represented —OH tensile vibration in the PVA film, shifted significantly to a low wave number in the PF film and in the films modified with glycerol and tween-20. In these films, the regions of the vibration were slightly narrower. This result suggested that there are interactions between the polysaccharides in FB and the crosslinker-PVA (Cazón et al., 2018). In addition, the peak value of —OH in the film with tween-20 decreased, indicating that the number of —OH decreased and the water absorption of the PFGlT film decreased after the addition of tween-20. The vibration at 2950–2910 cm−1 represents the asymmetric and symmetric stretching patterns of the —CH2— group (Z. Zhang et al., 2020). The band centered on 1094 cm−1 represents the vibration of the C—H stretching of PVA. The band interval between 1100 and 700 cm−1 is commonly referred to as the “fingerprint region”, which is related to the polysaccharide structure. The peaks at 1080 and 710 cm−1 were the stretching vibrations of -C—O—C from glycosidic bonds and stretching vibrations of —OH bending resulting from the pyranosidic structures of the polysaccharides in fermentation broth, respectively (Moreira et al., 2020).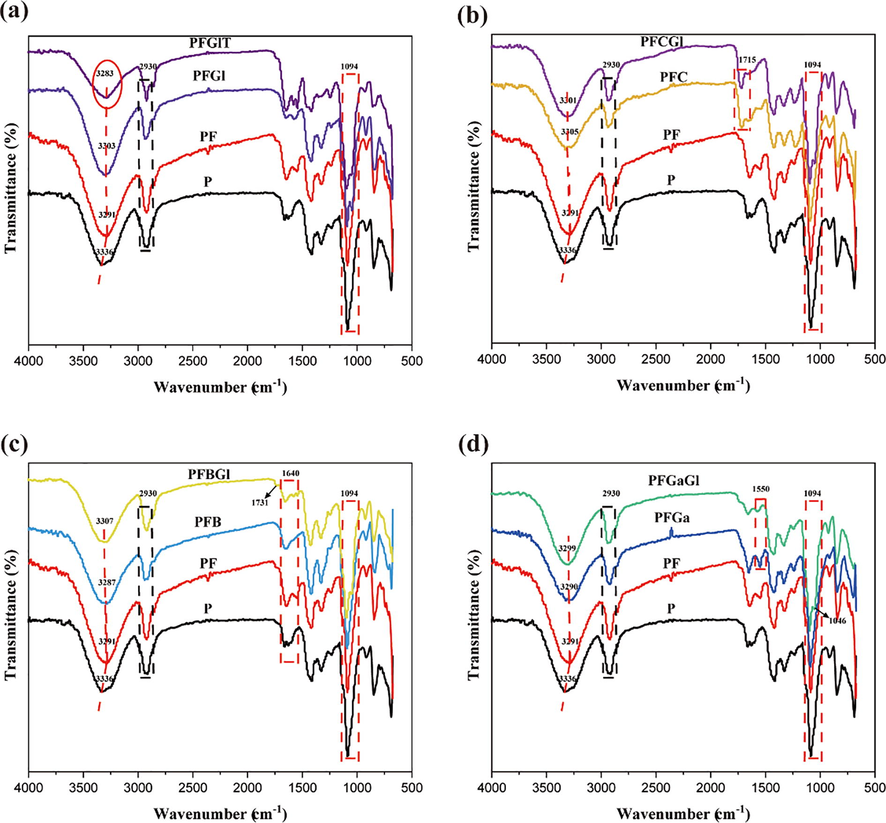
FTIR spectra comparison between different FBBF film samples. (a) The chemical differences between P, PF, PFGl, and PFGlT; (b) The chemical differences between P, PF, PFC, and PFCGl; (c) The chemical differences between P, PF, PFB, and PFBGl, (d) The chemical differences between P, PF, PFGa, and PFGaGl.
Fig. 5b represents the infrared spectrum of the modified FBBF film by citric acid, which revealed an agglomeration peak at 1715 cm−1. This was due to the ester bond formed between citric acid, PVA, and polysaccharides in FB, which played a great role in improving the waterproof performance of the FBBF film. This outcome was consistent with the waterproof performance improvement phenomenon (Abdullah and Dong, 2019). In the case of the blend of the FBBF films with boric acid (Fig. 5c), the vibration frequency at 1731 cm−1 in the infrared spectrum of PFBGl was considered to be the carbon–oxygen stretching of the borate ester group during the reaction of PVA, glycerol, and polysaccharide with boric acid (Moreira et al., 2020). The peak around 1640 cm−1 is associated with the asymmetric deformation of the —OH of water groups (Lima et al., 2018).
Fig. 5d represents the chemical changes in the film matrix of the modified film by the addition of glutaraldehyde. It is well known that aldehyde compounds can react with the hydroxyl groups of PVA through acetylation to form a dense three-dimensional network structure, which was identified in the peak at 1550 cm−1 (Kim et al., 1994; Z. Zhang et al., 2020).
3.7 Morphology of different films
The FE-SEM micrographs were employed to examine the surface structure of FBBF films (Fig. 6). Fig. 6a, 6b, and 6c showed the image of code of film P, PF, and PFCGl respectively in micrometers. The magnification times of each sample were 10,000 times, 20,000 times, 40,000 times, and 80,000 times, respectively. Only PVA-contained film showed smooth and uniform morphology without pores and cracks on its surface (Fig. 6a). Nevertheless, compared to FB added film, the surface of PF film was stronger and more compact. The surface of the blend films modified with glycerol and citric acid was much more compact and smoother than FB film (Fig. 6c). The film image color with citric acid and glycerol is fairly uniform. As a reactant, citric acid connects the reaction of polysaccharides and PVA. These results were in agreement with those reported by Jouki et al. (Jouki et al., 2013) and Dick et al (Dick et al., 2015). Such compact and smooth morphology has also been reported in seed mucilage–based films, such as gatti gum films (Zhang et al., 2016) and basil seeds (do Nascimento et al., 2021; Khazaei et al., 2014).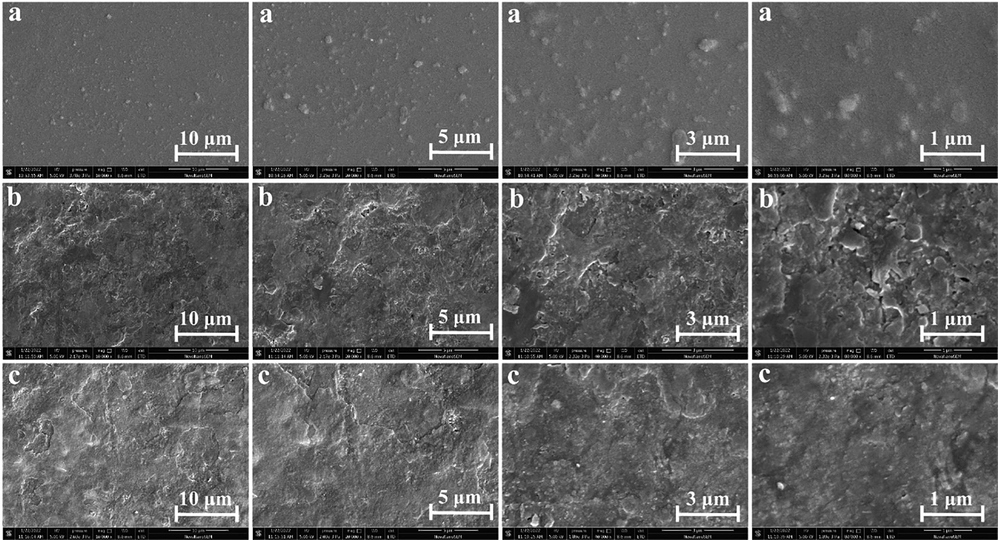
SEM micrographs of different FBBF films at different magnifications. (a) The film only contains PVA (P), (b) The FBBF film without additives (PF), (c) The optimized FBBF film (PFCGl).
3.8 Preliminary application on plant sprouting
The mechanical properties, waterproof properties, and opacity were discussed. The potential application of the optimized FBBF film (PFCGl) was conducted for plant sprouting. Potentilla supina L. Sp. Pl., a common plant planted in a greenhouse, was chosen as a model plant in this study. One group (without film, covered with PFCGl, and plastic film) grew at 10 °C in dark chamber. The plants without covering did not grow. The main reason could be the low temperature and less moisture remaining, which was not in conformity with the plant germination conditions (Rippy et al., 2004). The plants covered by PFCGl or plastic film grew well. However, only 2 plants grew in the pot covered by PFCGl, which was a lower number compared with the number of plants in the pot covered by the plastic film (5 plants). Furthermore, the height and diameter indexes of plants with the plastic film were better than ones with the biodegradable film (Table 4).
Light
Tm (°C)
Time (d)
Height (cm)
Number (strain)
Diameter (cm)
No film
No
10
/
/
0
/
FBBF film
7
5.21 ± 0.52
2
0.11 ± 0.02
Plastic film
7
6.83 ± 0.61
5
0.13 ± 0.01
No film
Yes
24
/
/
0
/
FBBF film
5
3.56
1
0.31 ± 0.02
Plastic film
5
7.13
1
0.35 ± 0.01
Another group (without film, covered with FBBF film, and plastic film) was incubated at 24 °C with light. The pots without film covering had no plants grown. The hypothesis is that the soil was dry, which is not a suitable condition to promote the sprouting. In the pots covered with FBBF film and plastic, the plants grew well. This phenomenon indicates that both FBBF film and plastic film have good moisturizing properties, which is in agreement with the swelling ratio and water-solubility properties of the FBBF films. However, the height of the plants covered with FBBF film was only 3.56 cm. By comparing the height of the plants covered by plastic and FBBF film, it can be observed that the height of the plants covered by FBBF film was half shorter than that of the plants covered with the plastic film (Table 4). The main reason may be the optical properties of the FBBF film, which were found to be worse than those of the plastic film. Interestingly, white mold appeared on the roots of plants covered with plastic film in both dark and light conditions, while no mold was observed in the plants covered by the FBBF film (Fig. 7). This result indicates that our FBBF film may have antifungal activity (Abbas et al., 2022; N. Zhang et al., 2020). In general, the developed biodegradable film showed interesting performances regarding water-retention and heat-preservation.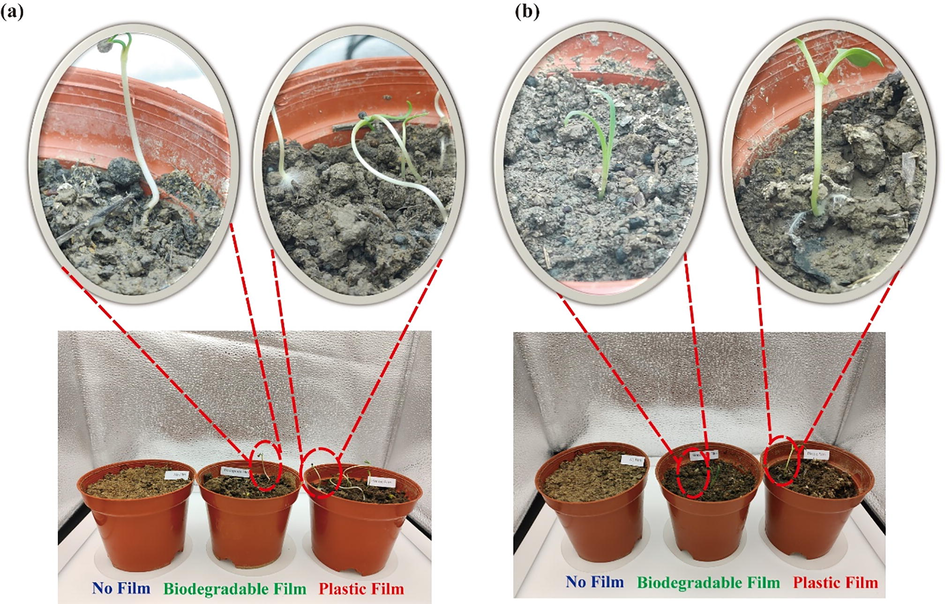
The heat/moisture-preservation and potential anti-fungi properties of the optimized FBBF film. (a) P. supina L. Sp. Pl. growing with or without film in dark conditions, (b) P. supina L. Sp. Pl. growing with or without film in light conditions.
3.9 Degradation property
Bio-based films have gained the attention of researchers in recent decades, and the degradability of biodegradable film is an essential characteristic. The biodegradability of the optimized FBBF films (PFCGl) was explored by imitating the mulch film biodegradation in soils (Sander, 2019). The biodegradation level of the films was analyzed based on the evolution of the weight loss (Wl). Fairly significant and regular levels of biodegradation were achieved in short incubation times in soil (Fig. 8). The obvious difference between the films at different time intervals were shown in Fig. 8a. After 10, 20, and 30 d, 4.4 %, 7.8 %, and 14.9 % of the weight of PFCGl film were lost, respectively. For the mulch films, the first 20 days are very important for plant sprouting. Thus, the degradation rate of PFCGl film was suitable for the plant sprouting. After 20 days, the degradation rate increased as shown in Fig. 8b.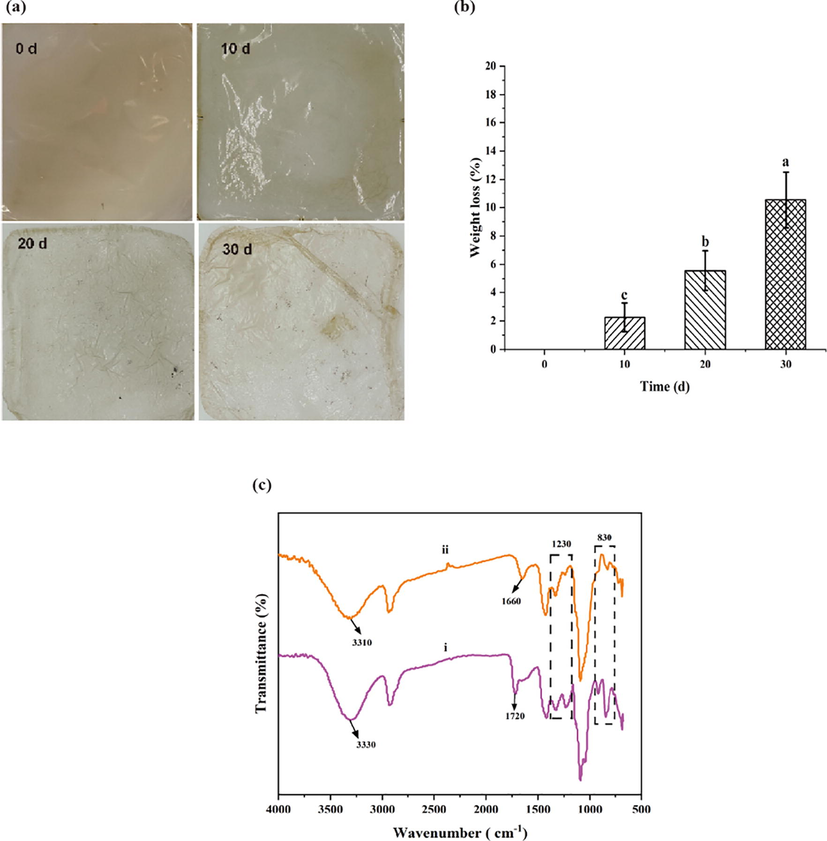
The biodegradability of PFCGl films. (a) The degradation of the PFCGl film at different time intervals. (b) The weight loss of the PFCGl film at different time intervals. (c) FTIR analysis chart of PFCGl before and after degradation, (i) The PFCGl film without burying (ii) The PFCGl film after burying for 30 d.
Fig. 8c showed the FTIR spectra of buried PFCGl film. The —OH stretching vibration peak of PFCGl film moved to 3310 cm−1 from 3330 cm−1 after burying in the soil for 30 d, which indicated that the —OH bond between PVA molecules and polysaccharide molecules of buried PFCGl film was broken and depolymerization of the film occurred. The characteristic peak of ester bond (1660 cm−1) of the buried PFCGl film was weakened, indicating that the ester bond formed between —COOH of citric acid in the film and —OH of PVA/ polysaccharide was broken, which meant that the macromolecules in PFCGl film were degraded into the oligomers during burial. At the same time, the fingerprint area (100–700 cm−1) of polysaccharide structure was obviously weakened. The polysaccharides could be degraded by the extracellular hydrolases secreted by the microorganisms in soil (Haider et al., 2019). The FTIR spectra demonstrated that PFCGl film degraded faster in the soil than that without burying. The main reason could relate to the microorganisms in the soil. Combined with the water-retention and heat-preservation properties, the novel film PFCGl shows great potential in the field of biodegradable film mulching.
4 Conclusion
In this report, we have explored the preparation of a biodegradable film from fermented polysaccharides. The compatibility and enzymatic activities of two target strains A. pullulans and Photobacterium sp. LYM-1 were investigated. The fermentation broth biodegradable film (FBBF) was successfully established simply with purified fermented polysaccharides combining with low-cost crosslinked PVA, which was confirmed by FTIR analysis. This is the first report on the preparation of biodegradable film using a fermentation broth with straw and shrimp shell. To improve the plastic properties of the newly developed films, the effects of plasticizers and surfactants on the characteristics of the film were investigated. It was found that 0.2 % (w/v) citric acid improved the film properties. Code FBCGl was implied the optimal formulation of FBBF film considering the practicability, economy and environment friendly. The fermentation of straw and shrimp shell was demonstrated to show significant potential for the large-scale preparation of biodegradable film. The fungistasis mechanism, exact degradation time of FBBF films, and the application of fermentation residue need further investigation. The combination of FB with a low concentration of PVA, citric acid and glycerol provide a feasible method to prepare a good functional film from cheap and biodegradable waste. The potential application of the developed film for mulching was evaluated. This study lays the foundation for an environmentally benign approach that allows the preparation of biodegradable films from agri-food wastes in large scale, reducing the use of harsh chemicals.
CRediT authorship contribution statement
Xiaohong Yu: Conceptualization, Resources, Supervision, Project administration, Writing – review & editing. Mian Wang: Data curation, Writing – original draft, Funding acquisition. Yiwen Zhang: Data curation, Software. Xiaochen Liu: Software, Methodology. Xiaoyang Zhang: Data curation, Software. Jinbin Liu: Methodology, Conceptualization. Dujun Wang: Methodology, Writing – original draft. Wenbin Jin: Methodology, Writing – original draft. Yongmei Lyu: Investigation, Data curation, Writing – review & editing, Funding acquisition.
Acknowledgments
This work was supported in parts by Natural Science Research of Jiangsu Higher Education Institutions of China (Grant number 19KJA480001), Postgraduate Research & Practice Innovation Program of Yancheng Institute of Technology (Grant number KYCX21_XZ003), the Funding for School-level Research Projects of Yancheng Institute of Technology (grant numbers xjr2021027, xjr2019048).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chitosan-polyvinyl alcohol membranes with improved antibacterial properties contained Calotropis procera extract as a robust wound healing agent. Arab. J. Chem.. 2022;15:103766.
- [CrossRef] [Google Scholar]
- Biodegradable and Water Resistant Poly(vinyl) Alcohol (PVA)/Starch (ST)/Glycerol (GL)/Halloysite Nanotube (HNT) Nanocomposite Films for Sustainable Food Packaging. Front. Mater.. 2019;6:58.
- [CrossRef] [Google Scholar]
- Synthesis of biodegradable films based on cassava starch containing free and nanoencapsulated β-carotene. Packag. Technol. Sci.. 2018;31:157-166.
- [CrossRef] [Google Scholar]
- Current progress on bio-based polymers and their future trends. Prog. Biomater.. 2013;2:8.
- [CrossRef] [Google Scholar]
- Properties of chitin and chitosan extracted from silkworm pupae and egg shells. Int. J. Biol. Macromol.. 2020;161:1296-1304.
- [CrossRef] [Google Scholar]
- Chitosan/boron nitride nanobiocomposite films with improved properties for active food packaging applications. Int. J. Biol. Macromol.. 2021;186:135-144.
- [CrossRef] [Google Scholar]
- Seed mucilages as the functional ingredients for biodegradable films and edible coatings in the food industry. Adv. Colloid Interface Sci.. 2020;280:102164.
- [CrossRef] [Google Scholar]
- Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients. 2018;10:1615.
- [CrossRef] [Google Scholar]
- Effect of EGCG-gelatin biofilm on the quality and microbial composition of tilapia fillets during chilled storage. Food Chem.. 2020;305:125454.
- [CrossRef] [Google Scholar]
- Composite films of regenerate cellulose with chitosan and polyvinyl alcohol: Evaluation of water adsorption, mechanical and optical properties. Int. J. Biol. Macromol.. 2018;117:235-246.
- [CrossRef] [Google Scholar]
- Composite Films with UV-Barrier Properties of Bacterial Cellulose with Glycerol and Poly(vinyl alcohol): Puncture Properties, Solubility, and Swelling Degree. Biomacromolecules. 2019;20:3115-3125.
- [CrossRef] [Google Scholar]
- Plasticization of Cottonseed Protein/Polyvinyl Alcohol Blend Films. Polymers. 2019;11:2096.
- [CrossRef] [Google Scholar]
- Crop residues as raw materials for biorefinery systems – A LCA case study. Appl. Energy. 2010;87:47-57.
- [CrossRef] [Google Scholar]
- Effect of glycerin on structure transition of PVA/SF blends. J. Appl. Polym. Sci.. 2002;86:2342-2347.
- [CrossRef] [Google Scholar]
- Optimality of poly-vinyl alcohol/starch/glycerol/citric acid in wound dressing applicable composite films. Int. J. Biol. Macromol.. 2020;155:260-272.
- [CrossRef] [Google Scholar]
- Effect of Cellulose Nanocrystals from Different Lignocellulosic Residues to Chitosan/Glycerol Films. Polymers. 2019;11:658.
- [CrossRef] [Google Scholar]
- Biodegradation of blend films PVA/PVC, PVA/PCL in soil and soil with landfill leachate. Braz. Arch. Biol. Technol.. 2011;54:1367-1378.
- [CrossRef] [Google Scholar]
- Biomass sorghum as a novel substrate in solid-state fermentation for the production of hemicellulases and cellulases by Aspergillus niger and A. fumigatus. J. Appl. Microbiol.. 2018;124:708-718.
- [CrossRef] [Google Scholar]
- Edible film production from chia seed mucilage: Effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr. Polym.. 2015;130:198-205.
- [CrossRef] [Google Scholar]
- Formulation and characterization of crosslinked polyvinyl alcohol (PVA) membranes: effects of the crosslinking agents. Polym. Bull.. 2021;78:917-929.
- [CrossRef] [Google Scholar]
- Application of an Ultrasound-Assisted Extraction Method to Recover Betalains and Polyphenols from Red Beetroot Waste. ACS Sustain. Chem. Eng.. 2021;9:8736-8747.
- [CrossRef] [Google Scholar]
- Polysaccharide-Based Membranes in Food Packaging Applications. Membranes. 2016;6:22.
- [CrossRef] [Google Scholar]
- Microbial polysaccharide-based membranes: Current and future applications. J. Appl. Polym. Sci.. 2014;131
- [CrossRef] [Google Scholar]
- Valorization of Rice Straw into Cellulose Microfibers for the Reinforcement of Thermoplastic Corn Starch Films. Appl. Sci.. 2021;11:8433.
- [CrossRef] [Google Scholar]
- Catalytic properties of cellulases and hemicellulases produced by Lichtheimia ramosa: Potential for sugarcane bagasse saccharification. Ind. Crops Prod.. 2018;122:49-56.
- [CrossRef] [Google Scholar]
- Smart biodegradable films based on chitosan/methylcellulose containing Phyllanthus reticulatus anthocyanin for monitoring the freshness of fish fillet. Int. J. Biol. Macromol.. 2021;187:451-461.
- [CrossRef] [Google Scholar]
- Tomato Seed Mucilage as a New Source of Biodegradable Film-Forming Material: Effect of Glycerol and Cellulose Nanofibers on the Characteristics of Resultant Films. Food Bioprocess Technol.. 2021;14:2380-2400.
- [CrossRef] [Google Scholar]
- Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed.. 2019;58:50-62.
- [CrossRef] [Google Scholar]
- Chitin extraction from crab shells by Bacillus bacteria. Biological activities of fermented crab supernatants. Int. J. Biol. Macromol.. 2015;79:167-173.
- [CrossRef] [Google Scholar]
- Utilization of biowaste as an eco-friendly biodegradable corrosion inhibitor for mild steel in 1 mol/L HCl solution. Arab. J. Chem.. 2020;13:8684-8696.
- [CrossRef] [Google Scholar]
- Chitinase and chitobiase production during fermentation of genetically improved Serratia liquefaciens. Enzyme Microb. Technol.. 1989;11:289-296.
- [CrossRef] [Google Scholar]
- Effect of glycerol concentration on edible film production from cress seed carbohydrate gum. Carbohydr. Polym.. 2013;96:39-46.
- [CrossRef] [Google Scholar]
- Characterization of new biodegradable edible film made from basil seed (Ocimum basilicum L.) gum. Carbohydr. Polym.. 2014;102:199-206.
- [CrossRef] [Google Scholar]
- Kinetics of crosslinking reaction of PVA membrane with glutaraldehyde. Korean J. Chem. Eng.. 1994;11:41-47.
- [CrossRef] [Google Scholar]
- Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol.. 2017;37:24-38.
- [CrossRef] [Google Scholar]
- Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering. 2017;4:55.
- [CrossRef] [Google Scholar]
- Quantification of total sugars and reducing sugars of dragon fruit-derived sugar-samples by UV-Vis spectrophotometric method. IOP Conf. Ser. Earth Environ. Sci.. 2021;947:012041.
- [CrossRef] [Google Scholar]
- Environmental village regulations matter: Mulch film recycling in rural China. J. Clean. Prod.. 2021;299:126796.
- [CrossRef] [Google Scholar]
- Dual-bonding structured ternary composite film of poly(vinyl alcohol)–boric acid–nanodiamond. J. Appl. Polym. Sci.. 2017;134:45449.
- [CrossRef] [Google Scholar]
- The fabrication of a degradable film with high antimicrobial and antioxidant activities. Ind. Crops Prod.. 2019;140:111692.
- [CrossRef] [Google Scholar]
- Hydrophobization of cashew gum by acetylation mechanism and amphotericin B encapsulation. Int. J. Biol. Macromol.. 2018;108:523-530.
- [CrossRef] [Google Scholar]
- Preparation and characterization of chitosan films with three kinds of molecular weight for food packaging. Int. J. Biol. Macromol.. 2020;155:249-259.
- [CrossRef] [Google Scholar]
- Preparation and characteristics of biodegradable mulching films based on fermentation industry wastes. Int. Biodeterior. Biodegrad.. 2016;111:54-61.
- [CrossRef] [Google Scholar]
- Films based on castor bean (Ricinus communis L.) proteins crosslinked with glutaraldehyde and glyoxal. Ind. Crops Prod.. 2013;50:375-382.
- [CrossRef] [Google Scholar]
- Processing and shelf life issues of selected food packaging materials and structures from renewable resources. Trends Food Sci. Technol.. 2011;22:72-80.
- [CrossRef] [Google Scholar]
- Poly(vinyl alcohol)-Based Biofilms Plasticized with Polyols and Colored with Pigments Extracted from Tomato By-Products. Polymers. 2020;12:532.
- [CrossRef] [Google Scholar]
- An ecofriendly edible coating using cashew gum polysaccharide and polyvinyl alcohol. Food Biosci.. 2020;37:100722.
- [CrossRef] [Google Scholar]
- Production of biodegradable plastic from agricultural wastes. Arab. J. Chem.. 2018;11:546-553.
- [CrossRef] [Google Scholar]
- Arabinoxylan from Mucilage of Tomatoes (Solanum lycopersicum L.): Structure and Antinociceptive Effect in Mouse Models. J. Agric. Food Chem.. 2016;64:1239-1244.
- [CrossRef] [Google Scholar]
- Preparation of antimicrobial agar/banana powder blend films reinforced with silver nanoparticles. Food Hydrocoll.. 2016;60:476-485.
- [CrossRef] [Google Scholar]
- Characterization of antimicrobial polylactic acid based films. J. Food Eng.. 2013;119:308-315.
- [CrossRef] [Google Scholar]
- Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur. Polym. J.. 2021;17
- [Google Scholar]
- Extracellular polysaccharide (EPS) production by a novel strain of yeast-like fungus Aureobasidium pullulans. Carbohydr. Polym.. 2010;82:728-732.
- [CrossRef] [Google Scholar]
- Plant Development and Harvest Yields of Greenhouse Tomatoes in Six Organic Growing Systems. HortScience. 2004;39:223-229.
- [CrossRef] [Google Scholar]
- Development and evaluation of chitosan based active nanocomposite films containing bacterial cellulose nanocrystals and silver nanoparticles. Food Hydrocoll.. 2018;84:414-423.
- [CrossRef] [Google Scholar]
- Biodegradation of Polymeric Mulch Films in Agricultural Soils: Concepts, Knowledge Gaps, and Future Research Directions. Environ. Sci. Technol.. 2019;53:2304-2315.
- [CrossRef] [Google Scholar]
- Paenibacillus polymyxa NSY50 suppresses Fusarium wilt in cucumbers by regulating the rhizospheric microbial community. Sci. Rep.. 2017;7:41234.
- [CrossRef] [Google Scholar]
- Recent Trends in Advanced Polymer Materials in Agriculture Related Applications. ACS Appl. Polym. Mater.. 2021;3:1203-1217.
- [CrossRef] [Google Scholar]
- Effect of plasticizer and surfactant on the properties of poly(vinyl alcohol)/chitosan films. Int. J. Biol. Macromol.. 2020;164:2100-2107.
- [CrossRef] [Google Scholar]
- Synergism Effect of Surfactant and Inorganic Salt on the Properties of Starch/Poly(Vinyl Alcohol) Film. Starch - Stärke. 2018;70:1700146.
- [CrossRef] [Google Scholar]
- Agri-food wastes for heavy metals removal from water. IOP Conf. Ser. Mater. Sci. Eng.. 2021;1058:012020.
- [CrossRef] [Google Scholar]
- Strengthen “the sustainable farm” concept via efficacious conversion of farm wastes into methane. Bioresour. Technol.. 2021;341:125838.
- [CrossRef] [Google Scholar]
- Photobacterium chitinilyticum sp. nov., a marine bacterium isolated from seawater at the bottom of the East China Sea. Int. J. Syst. Evol. Microbiol.. 2019;69:1477-1483.
- [CrossRef] [Google Scholar]
- Green Production of Biodegradable Mulch Films for Effective Weed Control. ACS Omega. 2021;6:32327-32333.
- [CrossRef] [Google Scholar]
- Development of Poly(Vinyl Alcohol)-Based Polymers as Proton Exchange Membranes and Challenges in Fuel Cell Application: A Review. Polym. Rev.. 2020;60:171-202.
- [CrossRef] [Google Scholar]
- Cellulose Nanocrystal Reinforced Chitosan Based UV Barrier Composite Films for Sustainable Packaging. Polymers. 2020;12:202.
- [CrossRef] [Google Scholar]
- Structure and functional properties of active packaging films prepared by incorporating different flavonols into chitosan based matrix. Int. J. Biol. Macromol.. 2020;165:625-634.
- [CrossRef] [Google Scholar]
- Preparation and properties of glutaraldehyde crosslinked poly(vinyl alcohol) membrane with gradient structure. J. Polym. Res.. 2020;27:228.
- [CrossRef] [Google Scholar]
- Characterization of a novel edible film based on gum ghatti: Effect of plasticizer type and concentration. Carbohydr. Polym.. 2016;153:345-355.
- [CrossRef] [Google Scholar]







