Translate this page into:
Preparation of nano-Co3O4-coated Albizia procera-derived carbon by direct thermal decomposition method for electrochemical water oxidation
⁎Corresponding author. maziz@kfupm.edu.sa (Md. Abdul Aziz)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
It is obtained that nano-Co3O4-coated carbon prepared by thermal decomposition of Co(NO3)2·6H2O at 300 °C on home-made Albizia procera (Roxb.) leaves derived carbon is an efficient electrocatalyst for electrochemical water oxidation in 0.1 M NaOH (aq.) solution. The loading of nano-Co3O4 on the carbon was changed by varying the amount of precursor of cobalt (100–1000 mg) and using a constant amount of the carbon (200 mg) during thermal decomposition. The prepared samples were characterized by physical techniques, including X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), energy dispersive spectroscopy (EDS), thermo-gravimetric analysis (TGA), fourier transform infrared spectroscopy (FTIR), high-resolution transmission electron microscopy (HRTEM), diffuse reflectance spectroscopy (DRS), Brunauer-Emmett-Teller (BET) and X-ray photoelectron spectroscopy (XPS). XRD, TEM, FESEM, and EDS confirmed the formation of uniformly distributed nanoparticles of single-phase Co3O4 on the surface of carbon. The XRD data reveals formation of nano-Co3O4 with average particle sizes in the range of 9–17 nm. The FESEM micrographs demonstrate that Co3O4 nanoparticles, having irregular morphology, are uniformly and densely covered on the surface of supporting carbon.. The prepared samples were immobilized on the filter paper derived carbon electrode (FPCE) to study their electrocatalytic properties toward water oxidation. The cyclic voltammetric studies showed that the nano-Co3O4-C prepared using 400 mg of Co(NO3)2·6H2O (nano-Co3O4-C-400), which possesses meso- and macropores with BET surface area of 192.4 m2/g, reaches a current density of 28 mAcm−2 at 1.5 V and electrochemical water oxidation starting potential of 0.7 V. In this work, it is also shown that the current densities, at 1.5 V, increase by increasing the amount of cobalt oxide in the prepared samples though. The nano-Co3O4-C-400 catalyst shows optimum performance for electrochemical water oxidation in terms of starting water oxidation potential, reasonable amount of Co3O4 and moderate level of current density at 1.5 V.
Keywords
Albizia procera derived carbon
Cobalt (II) nitrate hexahydrate
Thermal decomposition
Nano-Co3O4-carbon
Electrochemical water oxidation
1 Introduction
The current resources of energy are insufficient to fulfill the future demands of global energy (Asif and Muneer, 2007). Therefore, novel technological developments to utilize sustainable, clean, and viable energy resources are highly needed (Chu and Majumdar, 2012; Dragunov et al., 2018; Turner et al., 1999; Zotov et al., 2017). Among the available resources of energy, hydrogen gas (H2) is one of the emerging energy carriers, but generally nature exist hydrogen cannot generate energy due to its low concentration. To generate enough amount of hydrogen to produce energy, electrochemical water (H2O) splitting could be an option. Here, H2O could undergo electrochemical splitting to produce green fuel in the form of hydrogen gas (Jiao et al., 2015; Züttel et al., 2010). However, the efficiency of such hydrogen generation significantly depends on favorable catalysts to promote the hydrogen evolution reaction (HER) and achieve fast kinetics in practical applications. During the past few decades, several heterogeneous and molecular catalysts (Blakemore et al., 2015; McCrory et al., 2013; Zhang et al., 2016) have been produced to accomplish water oxidation at lower overpotential. In particular, earth-abundant metal catalysts (Hunter et al., 2016; Roger et al., 2017) could possibly substitute the Ru, Pt, and Ir based benchmark oxygen evolution reaction (OER) catalysts (Reier et al., 2012). Amongst the abundantly available transition metals, much attention has been concerted on cobalt, since the pioneer work of Nocera (Kanan and Nocera, 2008). Indeed, several catalysts based on chalcogenides, phosphides, phosphates, and oxides of cobalt have been developed for water splitting (Deng and Tüysüz, 2014; Gerken et al., 2011; J. Wang et al., 2016).
The nano-sized transition metal oxides, including Co3O4, TiO2, Fe2O3, CuO and NiO, have attracted much research interest due to their unique electrochemical, electronics, chemical and physical properties (Fernández-García et al., 2004; Khomane et al., 2008). Specifically, research has been conducted using nano-Co3O4 for various applications such as electrochemical water splitting (Chen et al., 2015), electrochemical gas sensing (Guo et al., 2013; Jia et al., 2009; Xu and Cheng, 2016), fuel cells (Babar et al., 2018), solar cells (Kabre et al., 2011; Sharma et al., 2015), supercapacitors (X. Wang et al., 2016), catalysis (Ma, 2014; Sharma et al., 2015), and batteries (Su et al., 2014).
Due to its indispensable wide range of applications, several approaches have been developed to synthesize nano-Co3O4 materials with various morphologies, sizes, and shapes (Ahmed Qasem et al., 2017; Fernández-García et al., 2004; Harish et al., 2016; Luisetto et al., 2008; Qasem et al., 2019; Xu and Zeng, 2003; Zhou et al., 2013). Among the numerous approaches developed to prepare nano-Co3O4, thermal decomposition method has been reported as one of the simple, time effective, and promising methods (Ahmed Qasem et al., 2017).
For preparing nano-Co3O4 material using thermal decomposition method, a suitable cobalt precursor is required. Among the reported cobalt precursors are cobalt hydroxide decomposed at 500 °C (Mahmoud and Al-Agel, 2011), cobalt malonate (Bhattacharjee et al., 2013a), cobalt oxalate rods (Ahmed et al., 2008), cobalt in plant extracts (Aspalathus linearis) (Xu and Zeng, 2003), cobalt-citrate (Raman et al., 2016), cobalt (II)-tartrate complex (Bhattacharjee et al., 2013b), and cobalt hydroxyl carbonates (Diallo et al., 2015). Most of these precursors require pre-reaction, making it tedious and time consuming. So, it would be better to employ a simple and straightforward method to prepare pure nano-Co3O4 through a thermal decomposition of an abundant cobalt inorganic precursor such as Co(NO3)2·6H2O. Qasem et al. (2019) reported a simple and direct thermal decomposition of Co(NO3)2.6H2O at 520 °C to prepare pure nano-Co3O4 in the air atmosphere without any pre-reaction.
As reported by the scientific community (Bajdich et al., 2013; Deng and Tüysüz, 2014; Gerken et al., 2011), cobalt oxide is one of the suitable catalysts for electrochemical water oxidation. Particularly, the cobalt oxide can electrooxidize water to O2 at basic pH selectively (Gerken et al., 2011). But as there are limited resources of metals (especially cobalt) in the world, which restrict their practical applications in O2 evolution reactions. Therefore, to preserve metals (cobalt), it would be better if we can coat cobalt oxide on some cheap scaffolder, like biomass derived carbon and to achieve similar performance for electrochemical water oxidation. Though, several carbon materials such as carbon nanotube (SWCNT or MWCNT), mesoporous carbon and graphene have been extensively reported as conductive substrates, due to their high conductivity and specific surface area (Liang et al., 2012; Wu et al., 2012), yet they are all relatively expensive.
Herein, we employ the direct thermal decomposition of Co(NO3)2·6H2O at 300 °C without any pre-reaction, not only to prepare pure nano-Co3O4, but also coated it on home-made carbon (Albizia procera derived carbon). The idea of combining nano-Co3O4 with carbon substrate comes to save cobalt and to improve the catalytic properties, including activity and stability. The home-made carbon coated with nano-Co3O4 demonstrates a decent activity and has gained the author attention as a catalyst for electrochemical water oxidation.
2 Experimental
2.1 Materials and method
All chemical reagents including cobalt (II) nitrate hexahydrate Co(NO3)2·6H2O (ACS reagent, ≥98%) and sodium hydroxide NaOH (ACS reagent, ≥97.0%) were used directly as purchased from Sigma-Aldrich (http://www.sigmaaldrich.com) without any further purification. Filter papers were purchased from Whatman. Copper tape (one-sided adhesive) and scotch tape-898 (one-sided adhesive) premium grade were both obtained from 3 M, United states.
Initially, the carbon was prepared by pyrolysis of dried powder (≤100 µm) of Albizia procera (Roxb.) leaves at 800 °C. The details of the preparation will be summarized in somewhere else. The preparation of nano-Co3O4 on this home-made carbon (nano-Co3O4-C) is illustrated in Fig. 1 and tabulated in Table 1. Initially, 100 mg of Co(NO3)2·6H2O and 200 mg of Albizia procera (Roxb.) leaves derived carbon were added directly into 100 mL of de-ionized (DI) water in two separate beakers and ultrasonicated using sonicator (Bransonic Ultrasonic cleanser: 5510E-DTH) for one hour to prevent particles from aggregation. The two separate solutions were mixed together and sonicated at room temperature for 40 min. The resultant mixture was heat treated at 110 °C for one hour under rigorous stirring (at 300 rpm) to remove the water. Afterward, the mixture was dried overnight in an electric oven at 60 °C. The final dried mixture was transferred to a boat type alumina crucible and thermally decomposed at 300 °C for 180 min in an electric tube furnace (OTF-1200X-S/www.mtixtl.com (USA)) to obtain nano-Co3O4-C. Initially, the temperature was increased at heating rate of 10 °C/min from the room temperature, to a maximum temperature of 300 °C, which was later maintained for 180 min. At the elevated temperature, Co(NO3)2·6H2O-C was decomposed to nano-Co3O4-C through the formation of Co(NO3)2·4H2O-C, Co(NO3)2·2H2O-C, Co(NO3)2·H2O-C, Co(NO3)2-C, CoOOH-C and Co2O3-C intermediate compounds (Aslan et al., 2019). Next, the temperature was decreased at cooling rate of 5 °C/min from 300 °C to the room temperature. The nano-Co3O4-C obtained by using 100 mg Co(NO3)2·6H2O is denoted as nano-Co3O4-C-100 in rest of this manuscript. The procedure was repeated for 200, 400, 600, 800 and 1000 mg of precursor to obtain nano-Co3O4-C-200, nano-Co3O4-C-400, nano-Co3O4-C-600, nano-Co3O4-C-800 and nano-Co3O4-C-1000, respectively. The idea here is to study the effect of increasing amount of precursor on water oxidation catalytic properties including activity and stability.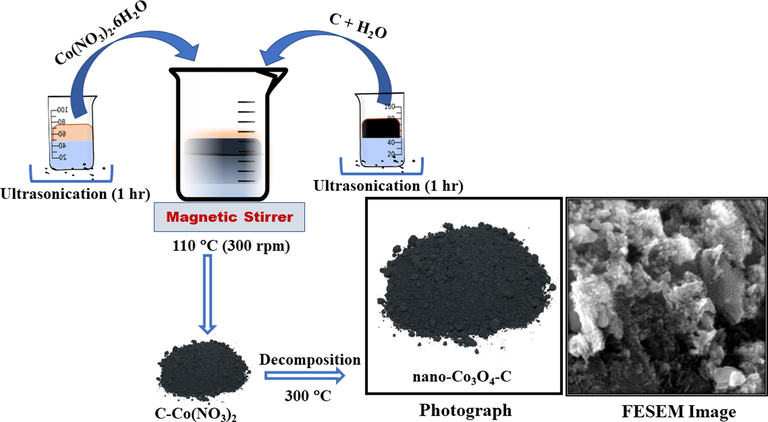
Schematic representation for the preparation of nano-Co3O4-C.
S. No.
Carbon mass (mg)
Co(NO3)2·6H2O mass (mg)
Name of the samples
1
200
100
Nano-Co3O4-C-100
2
200
200
Nano-Co3O4-C-200
3
200
400
Nano-Co3O4-C-400
4
200
600
Nano-Co3O4-C-600
5
200
800
Nano-Co3O4-C-800
6
200
1000
Nano-Co3O4-C-1000
2.2 Materials characterization
The X-ray diffraction (XRD) patterns of nano-Co3O4-C-100, 200, 400, 600, 800, and 1000 samples were recorded at a scan rate of 1.00° per min in the 2θ-range from 5° to 80° by a Rigaku Miniflex II diffractometer equipped with Cu K-alpha radiation. The morphological properties of the prepared samples were obtained using a scanning electron microscope (Tescan Lyra-3). The energy dispersive X-ray spectroscopy (EDS) spectra were recorded on a Lyra 3 attachment to the FESEM using LINK INCA program system. Further morphological and diffraction properties of the synthesized nano-Co3O4-C-400 sample was obtained using a transmission electron microscope (JEOL JEM 2100F). Thermogravimetric Analysis (TGA) was carried out by heating 16 mg of nano-Co3O4-C-400 from 30 °C to 1000 °C at a heating rate of 10 °C/min in the nitrogen atmosphere. An X-ray photoelectron spectrometer (XPS) (ESCALAB 250Xi XPS Microprobe, Thermo Scientific, USA) was applied for chemical analysis of the prepared samples. The band gap of nano-Co3O4-C-400 was calculated using a Cary 5000 high performance UV–Vis-NIR spectrophotometer with photometric performance in the range of 175–3300 nm, equipped with diffuse reflectance spectrometer. Fourier transform infrared spectroscopy (FTIR) was carried out using Thermo Scientific NICOLET 6700 spectrometer, and the spectra were obtained in the range of 4000–500 cm−1 with 32 scans per spectrum and 4 cm−1 resolution.
2.3 Electrode fabrication
The filter paper derived carbon electrode (FPCE) was prepared using a pyrolysis process by cutting filter papers into pieces of 2 cm by 2 cm area. These pieces of filter papers were placed in an alumina flat bottom boat shaped crucible, which was later inserted into a cylindrical alumina tube with both ends of the tube sealed. Nitrogen gas was passed through one end for about 5 min to flush out oxygen and the moisture from the tubular furnace. The sized filter papers were heated at 850 °C for 5 h in the presence of nitrogen atmosphere. The resulting FPCE were fixed with scotch and copper tapes, as shown in Fig. 2(a). The entire surface area of FPCE was covered except a 0.2 cm2 area which serves as a substrate for the working electrode. To prepare the working electrode nano-Co3O4-C/FPCE, a solution of 10 mg of the prepared nano-Co3O4-C was made in 5 mL of DI water which was sonicated for 5 min. 30 µL volume of the dispersed solution was immobilized on the exposed 0.2 cm2 area using a drop and drying method, at room temperature. To assess the water oxidation catalytic activity of the nanostructured composites in alkaline condition (2OH− → O2 + 2H+ + 4e−), the prepared working electrodes were tested as a selective oxygen evolving anode shown in Fig. 2(b).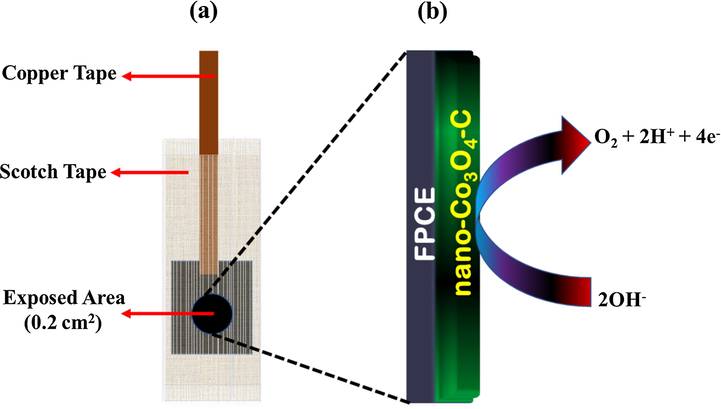
Schematic (a) of the FPCE and (b) representation for a water-splitting electrocatalytic activity on an integrated nano-Co3O4-C/FPCE oxygen-evolving anode.
2.4 Electrochemical measurement
The electrochemical measurements were performed using a CHI (760E) electrochemical workstation. The prepared nano-Co3O4-C/FPCE was used as the working electrode for electrochemical water oxidation in 0.1 M of NaOH (aq.) at room temperature. Ag/AgCl and platinum wire were used as the reference electrode and the counter electrode respectively, to complete the three-electrode cell.
3 Results and discussion
3.1 XRD analysis of nano-Co3O4-C
The X-ray diffraction (XRD) patterns of all the prepared samples were obtained to study the effect of different amount of Co(NO3)2·6H2O precursor on the crystalline and phase structures of nano-Co3O4-C. The XRD patterns obtained for nano-Co3O4-C-100, nano-Co3O4-C-200, nano-Co3O4-C-400, nano-Co3O4-C-600, nano-Co3O4-C-800, and nano-Co3O4-C-1000 are shown in Fig. 3. The diffraction peaks for all the samples are identical which shows that all the prepared samples exhibit crystalline states and display the same phase. The major peaks in each pattern occurred at 2θ values of 19.1°, 25.2°, 31.4°, 36.9°, 44.8°, 59.4° and 65.2° which correspond to 111, 220, 311, 400, 422, 511, and 440 planes of face centered cubic nano-Co3O4 crystal according to JCPDS database (JCPDS 42-1467) (Kang and Zhou, 2015). The unidentified peak in between 22° to 30° is related to carbon. We calculated crystallite sizes of the prepared nano-Co3O4 on C using Debye-Scherrer formula (Zsigmondy and Scherrer, 1912) (Eq. (1)):
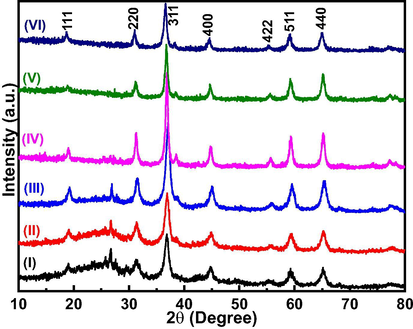
XRD patterns of (I) nano-Co3O4-C-100, (II) nano-Co3O4-C-200, (III) nano-Co3O4-C-400, (IV) nano-Co3O4-C-600, (V) nano-Co3O4-C-800, and (VI) nano-Co3O4-C-1000.
3.2 Morphological and compositional characterization of nano-Co3O4-C
The morphological and compositional details of the as-prepared nano-Co3O4-C samples were characterized by FESEM and EDS measurements. In Fig. 4(A–F), the high magnification FESEM images of nano-Co3O4-C-100, nano-Co3O4-C-200, nano-Co3O4-C-400, nano-Co3O4-C-600, nano-Co3O4-C-800 and nano-Co3O4-C-1000 are shown. A careful inspection of these micrographs demonstrates that nano-Co3O4 nanoparticles are uniformly distributed on the surface of the supporting carbon in all the prepared samples (Fig. 4(A–F)). It can be seen that the particles adopt irregular morphology with different sized particles, but it is obvious that nano-Co3O4 were uniformly and densely covered on the surface of carbon after the thermal decomposition process. The SEM micrograph of nano-Co3O4-C-100 is presented in Fig. 4(A). It is noted that the surface of the pure carbon is relatively smooth, which is reported in our earlier paper (Shah et al., 2019). The SEM images (Fig. 4(A)) shows that the surface of nano-Co3O4 modified carbon is relatively rough, which indicates the presence of nano-Co3O4 on the carbon surface. It is also observed that the nano-Co3O4 is uniformly distributed all over the carbon surface (Fig. 4(A–C)). While the other FESEM micrographs suggest that higher concentrations of the cobalt precursor resulted in the formation of more nanoparticles, leading to agglomeration. There is some agglomeration of particles in nano-Co3O4-C-600, nano-Co3O4-C-800, and nano-Co3O4-C-1000, which can decrease the electrochemical activity for water oxidation. However, in Fig. 4(A–C), the uniform dispersion of nano-Co3O4-C-100, nano-Co3O4-C-200 and nano-Co3O4-C-400 is evident, which provide an effective path for electrochemical water oxidation.
FESEM images of (A) nano-Co3O4-C-100, (B) nano-Co3O4-C-200, (C) nano-Co3O4-C-400, (D) nano-Co3O4-C-600, (E) nano-Co3O4-C-800, and (F) nano-Co3O4-C-1000.
The elemental composition and purity of the prepared nano-Co3O4-C were analyzed by energy dispersive X-ray analyses. Several small and large areas of the nano-Co3O4-C-100, nano-Co3O4-C-200, nano-Co3O4-C-400, nano-Co3O4-C-600, nano-Co3O4-C-800, and nano-Co3O4-C-1000 were examined by EDS. As expected, the entire samples show the presence of cobalt Co, oxygen O, and carbon C. For example, the EDS spectra of nano-Co3O4-C-400 are displayed in Fig. 5(A). The presence of some extra elements like Ca and Si in the EDS spectra might be due to the impurities of the carbon which was prepared from natural plants. EDS was also used to map the distribution of carbon, cobalt, and oxygen in the prepared nano-Co3O4-C-400. A visualization of the uniform distribution of cobalt, oxygen and carbon over the surface of the sample is given in Fig. 5(B). The other samples show similarly uniform elemental distributions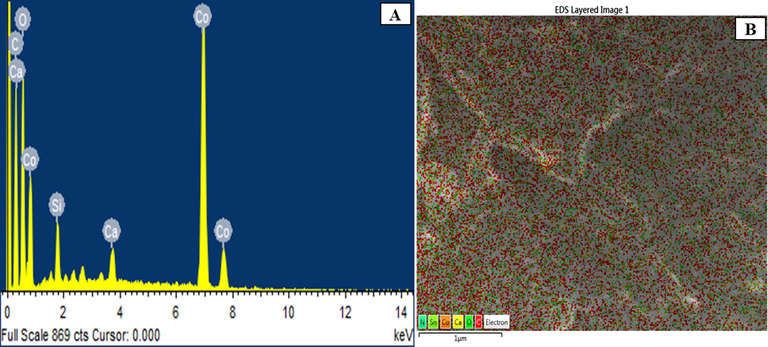
(A) EDS spectrum and (B) Elemental mapping of nano-Co3O4-C-400.
3.3 Transmission electron microscopy (TEM) of nano-Co3O4-C
Further analysis of nano-Co3O4-C-400 sample (optimum electrocatalyst, which has been described later of this manuscript) was performed using TEM. It is shown in Fig. 6 (a and b) that Co3O4 sample is composed of well dispersed and uniformed nano-structured particles with average particle size of 11.0 nm, which is closed to diameter obtained by XRD analysis (9.7 nm) . The related high-resolution transmission electron microscopy (HRTEM) image (Fig. 6(c)) shows the aligned lattice fringes with d spacing of 0.28 nm correspond to the (2 2 0) plane of cubic Co3O4 (Yu et al., 2013) which is one of the identified crystal plane of our prepared Co3O4 -C by XRD analysis (Fig. 3). The selected area electron diffraction (SAED) rings, shown in Fig. 6(d), confirms the polycrystalline nature of Co3O4 and agrees with the crystal planes presented in the XRD data (Bergmann et al., 2015).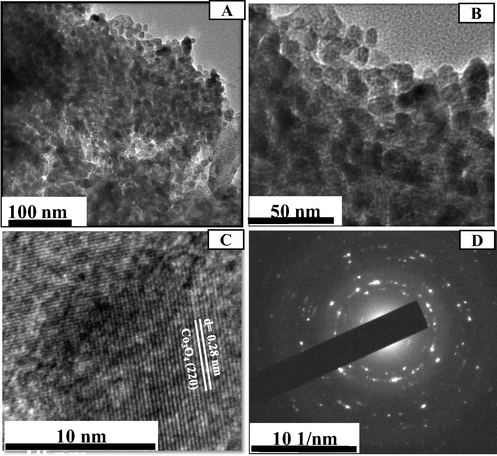
(a, b) TEM, (c) HRTEM images and (d) SAED pattern of typical nano-Co3O4-C-400.
3.4 Thermogravimetric analysis of nano-Co3O4-C
The thermogravimetric analysis (TGA) of nano-Co3O4-C-400 was carried out at a heating rate of 10 °C/minutes from 30 °C to 1000 °C in ambient atmosphere. The TGA curve in Fig. 7 revealed that the first mass loss occurred upon heating from 40 °C to 100 °C, at this point ∼10% of the original weight of nano-Co3O4-C-400 was lost. This mass loss relates to removal of moisture from nano-Co3O4-C-400. The second observation of a sharp mass loss occurred when temperature is increased from 320 °C to 450 °C. This mass loss is due to the burning of carbon though the used carbon is stable up ≤400 °C (Shah et al., 2019). Perhaps, the presence of Co3O4 decreased burning temperature of the carbon. Besides, Co(NO3)2·6H2O could be decomposed to cobalt oxide at ≤270 °C as per our earlier report (Ahmed Qasem et al., 2017). This discussion also proves that the selection of 300 °C for preparation of nano-Co3O4-C by thermal decomposition of Co(NO3)2·6H2O in the presence of home-made carbon was optimal for preserving the carbon.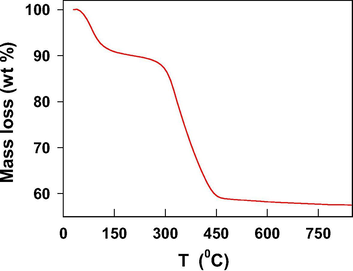
TGA spectrum of nano-Co3O4-C-400 in air.
3.5 X-ray photoelectron spectroscopy analysis of nano-Co3O4-C
The X-ray photoelectron spectroscopy (XPS) measurements were performed to know the chemical information of the prepared nano-Co3O4-C-400. The XPS survey scan spectrum of the sample is shown in Fig. 8(A). It indicates four major peaks at around 284.6, 400, 531 and 785 eV. These peaks represent the C1s, N1s, O1s and Co2p core levels, respectively. It clearly shows the presence of Co, O, N, C, and Si. The presence of Co, O, and C is expected. While the presence of Si and N might be due to impurities in carbon that comes from the preparation from natural plant, because the presence of nitrogenous and silicate compounds in plants are very common (Ohyama, 2010; Shah et al., 2019), The high-resolution spectra of C1s, Co2p and O1s were also recorded and presented in Fig. 8(B)–(D), respectively. For a comprehensive evaluation of oxidation states and the chemical environment of each element, the peaks corresponding to C1s, Co2p and O1s were deconvoluted and fitted. Besides, the presence of characteristic XPS peaks for Co2p is related to Co3O4. As shown in Fig. 8(C), the XPS spectra for the Co2p of the composite exhibit three peaks at ∼781, ∼785 eV and ∼798 eV attributed to the Co2p3/2, Co2p3/2 (satellite) and Co2p1/2 states of Co3O4, respectively. The O1s XPS spectra, shown in Fig. 8 (D) exhibit two deconvoluted components. The component 530.2 eV is ascribed to oxygen atoms in the cobalt particles, while the other component centered at 533.5 eV is related to C—O/O⚌C—O (Babar et al., 2018; Petitto et al., 2008; Sundar et al., 2016) groups. This gives a further proof of the Co3O4-C formation by the thermal decomposition of Co(NO3)2·6H2O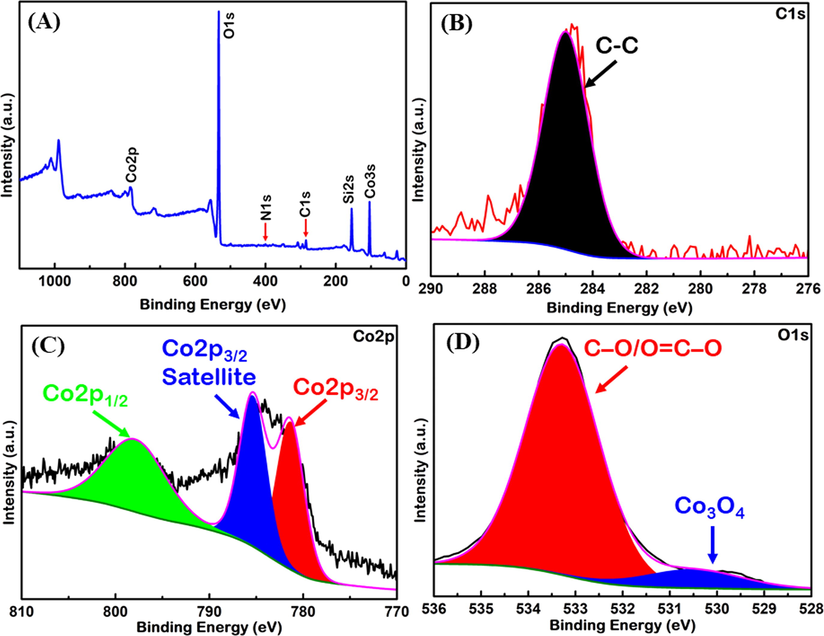
(A) XPS survey spectrum of nano-Co3O4-C-400 with four major peaks of carbon, oxygen, cobalt and nitrogen. The high resolution survey spectra of (B) C1s, (C) Co2p and (D) O1s of nano-Co3O4-C-400.
3.6 Diffuse reflectance spectroscopy of nano-Co3O4-C
The direct band gap of nano-Co3O4-C-400 was calculated using diffuse reflectance spectroscopy (DRS), that estimated by Tauc’s relation (Escobedo Morales et al., 2007) given below (equation (2));
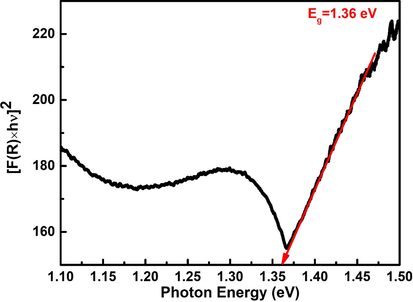
Thse band gap energy of the prepared nano-Co3O4-C-400.
3.7 Fourier Transform Infrared Spectroscopy (FTIR)
Fig. 10 represents the FTIR spectra of the prepared nano-Co3O4-C-400 and Co3O4 samples. The two sharp peaks at 572 and 661 cm−1 in the FTIR spectrum of nano-Co3O4-C-400 and Co3O4 are related to the stretching vibrations of Co—O (Xu et al., 2015). The presence of these peaks supports the formation of the Co3O4 spinel network and are associated with the OB3 (B denotes Co3+ in an octahedral hole) and the ABO (A denotes the Co2+ in a tetrahedral hole) vibrations in the spinel lattice (Xu et al., 2015). Both nano-Co3O4-C-400 and Co3O4 have two peaks at 1118. The peak at 1118 cm−1 is attributed to the stretching vibrations of C—O (Xu et al., 2015). The sharp and intense peak at 1398 cm−1 is appeared for Co3O4. This peak corresponds to the stretching vibration of NO3−, which is due to the residue of Co(NO3)2 (Xu et al., 2015). However this peak is very minor for the case of nano-Co3O4-C-400 i.e. almost all Co(NO3)2 decomposed to Co3O4 in the presence of carbon. The other minor peak at 1642 cm−1 in the spectrum of Co3O4 may be assigned for molecular water due to the absorbed moisture (Xu et al., 2015). However, the relatively intense peak of nano-Co3O4-C-400 at 1642 cm-1 could be related to the C⚌O bonds in carboxylic acid/C⚌C of olefinic group/C⚌N in addition to molecular water peak. These functional group is expected from our prepared carbon. The details related to FTIR of carbon will be summarized in our next paper with other synthesized carbonaceous materials.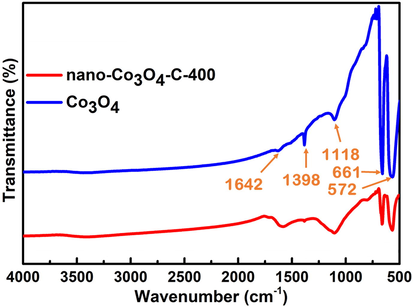
FTIR spectra of nano-Co3O4-C-400 and Co3O4.
3.8 Brunauer-Emmett-Teller (BET) analysis of nano-Co3O4-C-400
Adsorption-desorption isotherm and BJH adsorption pore distribution for nano-Co3O4-C-400 are depicted in Fig. 11(A) and (B) respectively. The curve in Fig. 11(A) demonstrates composite Type IV + Type II isotherm i.e. nano-Co3O4-C-400 possess both meso- and macropores (Thommes et al., 2015). Fig. 11 (B) presents a wide distribution of pore diameter (Range: 1.93–170 nm) with a BJH adsorption average pore diameter of 9.76 nm. The range of pore diameter, average pore diameter (11(B)) and type of curve (11(A)) also indicate large fractions of mesopores in nano-Co3O4-C-400.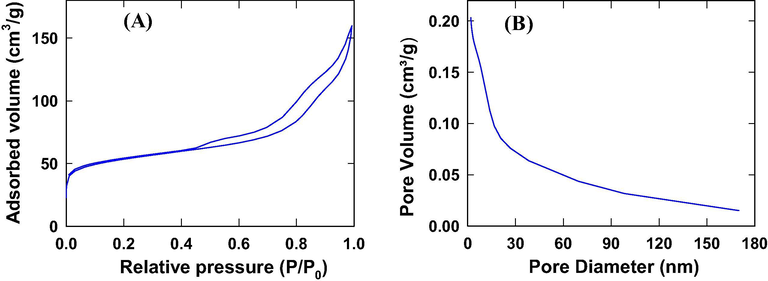
(a) Nitrogen adsorption-desorption isotherm and (b) Corresponding BJH pore size distribution of nano-Co3O4-C-400.
3.9 Electrocatalytic activities of nano-Co3O4-C
The electrocatalytic activity of nano-Co3O4-C was assessed by cyclic voltammetry (CV) in 0.1 M NaOH (aq.) solution (pH 13). In Fig. 12, the cyclic voltammograms (CVs) of the (a) bare carbon/FPCE, (b) nano-Co3O4-C-100/FPCE, (c) nano-Co3O4-C-200/FPCE, (d) nano-Co3O4-C-400/FPCE, (e) nano-Co3O4-C-600/FPCE, (f) nano-Co3O4-C-800/FPCE, and (g) nano-Co3O4-C-1000/FPCE is shown. By comparing the CVs in Fig. 12, carbon/FPCE is the least active for electrochemical water oxidation. The activity of the nano-Co3O4-C/FPCE increases with increasing the amount of precursor. The reached current densities for (a) bare carbon/FPCE, (b) nano-Co3O4-C-100/FPCE, (c) nano-Co3O4-C-200/FPCE and (d) nano-Co3O4-C-400/FPCE at 1.5 V are approximately 9.1, 12.5, 17.2 and 28.2 mA cm−2, respectively. Nano-Co3O4-C-100, nano-Co3O4-C-200, and Co3O4-C-400 started to setup oxidation of water at a low potential of 0.7 V; it is obvious that there is a significant improvement toward water oxidation by increasing the amount of cobalt oxide. The current densities for (e) nano-Co3O4-C-600/FPCE, (f) nano-Co3O4-C-800/FPCE and (g) nano-Co3O4-C-1000/FPCE at 1.5 V are 22.5, 27.1 and 35.1 mA cm−2, respectively. To further evaluate the optimum catalyst, the catalytic properties of the prepared electrodes towards electrochemical water oxidation are compared in Table 2 in terms of current densities at 1.5 V, starting potentials and overpotentials at 5 mA/cm2 determined from curves shown in Fig. 12.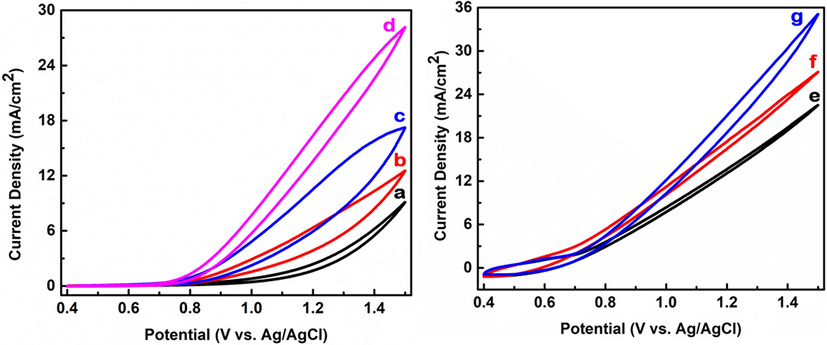
(a) Cyclic voltammograms of C/FPCE, (b) nano-Co3O4-C-100/FPCE, (c) nano-Co3O4-C-200/FPCE, (d) nano-Co3O4-C-400/FPCE, (e) nano-Co3O4-C-600/FPCE, (f) nano-Co3O4-C-800/FPCE, and (g) nano-Co3O4-C-1000/FPCE in 0.1 M NaOH (aq.). To prepare the modified electrode, 30 µL of 2 mg/ml catalyst solution (aq.) was dropped and dried.
Types of Electrodes
Current density at 1.5 V (mA/cm2)
Starting potential for water oxidation (mV vs. Ag/AgCl)
Overpotential at 5 mA/cm2 (mV vs. Ag/AgCl)
C/FPCE
9.1
700
1351
nano-Co3O4-C-100/FPCE
12.5
700
1290
nano-Co3O4-C-200/FPCE
17.2
700
1007
nano-Co3O4-C-400/FPCE
28.2
700
931
nano-Co3O4-C-600/FPCE
22.5
750
863
nano-Co3O4-C-800/FPCE
27.1
750
788
nano-Co3O4-C-1000/FPCE
35.1
780
817
This work shows that the current densities, at 1.5 V, increases by increasing the amount of cobalt oxide in the prepared samples. The nano-Co3O4-C-400 catalyst shows optimum performance for electrochemical water oxidation in terms of starting water oxidation potential, reasonable amount of Co3O4 and moderate level of current density at 1.5 V. The water oxidation current density of nano-Co3O4-C-400/FPCE is much higher than that of the electrodeposited MnOx/ITO electrode. That is, nano-Co3O4-C-400/FPCE has higher sensitivity for water oxidation than that of electrodeposited MnOx/ITO electrode (Aziz et al., 2017).
To check the loading effect of the catalyst on the electrode surface, we loaded different amounts of the prepared nano-Co3O4-C-400 on FPCE as described in Fig. 13(A). The data revealed that electrocatalytic properties of the nano-Co3O4-C-400/FPCE also depend on the loading amount of the nano-Co3O4-C-400 on FPCE. The water oxidation potential remains constant and the current density at 1.5 V decreases with the increase in the amount of nano-Co3O4-C-400. The stability test of nano-Co3O4-C-400/FPCE was carried out in 0.1 M NaOH for 10,000 s (Fig. 13(B)). The electrode demonstrated excellent stability since the current density remained constant throughout the measurement period. This result shows that the prepared catalyst (nano-Co3O4-C-400) is capable of sustaining water oxidation and can be utilized for practical applications such as production of H2 and O2, and the reduction of CO2 to valuable fuels.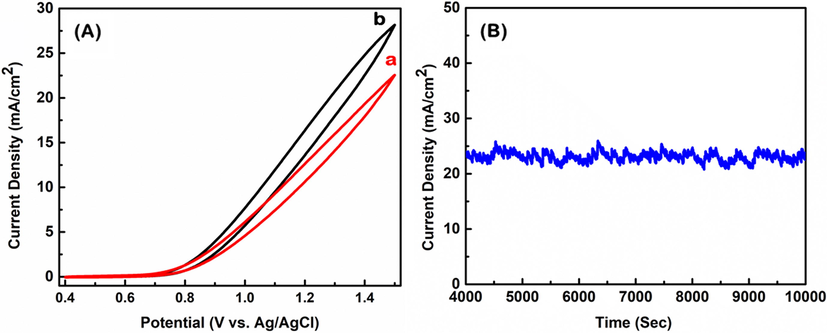
(A) Cyclic voltammograms of nano-Co3O4-C-400/FPCE in 0.1 M NaOH (aq.), to prepare the modified electrode, 30 µL of (a) 2 mg/ml and (b) 1 mg/ml catalyst solution (aq.) were dropped and dried. (B) Amperogram of nano-Co3O4-C-400/FPCE in 0.1 M NaOH at 1.0 V vs. Ag/AgCl.
4 Conclusion
Nano-Co3O4) with different morphologies were fabricated on porous carbon prepared from natural products. We demonstrated a facile method for synthesizing nano-Co3O4-C, where nano-Co3O4 was successfully decorated on home-made Albizia procera (Roxb.) based carbon using a straightforward thermal decomposition method. The prepared samples were characterized by XRD, SEM, EDS, TGA, HRTEM, FTIR, BET, DRS and XPS. The XRD data confirmed the formation of Co3O4-C nanoparticles with face centered cubic structure. EDS and XPS confirmed the presence of Co, O and C, as expected, in addition to Si and N which probably come from the natural carbon material. The TGA analysis revealed that major mass loss started at 320 °C due to carbon loss. The Co3O4-C NPs were immobilized on FPCE for electrochemical measurements. The nano-Co3O4-C/FPCE composite shows excellent electrocatalytic properties towards electrochemical water oxidation in 0.1 M NaOH (aq.). It is obtained that the nano-Co3O4-C-400/FPCE sample delivers optimum catalytic properties among all the prepared nano-Co3O4-C/FPCE catalysts. The nano-Co3O4-C-400/FPCE composite shows good stability in water oxidation. The overall electrochemical activity and stability of nano-Co3O4-C proved to be excellent and suitable agent for water oxidation. Such high-efficiency of nano-Co3O4-C/FPCE should be suitable for other electrochemical applications including production of clean H2 and O2 and conversion of CO2 to fuels.
Acknowledgement
The authors acknowledge support from the Center of Research Excellence in Nanotechnology (CENT) at the King Fahd University of Petroleum and Minerals in the Kingdom of Saudi Arabia.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
References
- Development of a microemulsion-based process for synthesis of cobalt (Co) and cobalt oxide (Co3O4) nanoparticles from submicrometer rods of cobalt oxalate. J. Colloid Interface Sci.. 2008;321:434-441.
- [CrossRef] [Google Scholar]
- Preparation of nano-Co3O4 by direct thermal decomposition of cobalt(II) nitrate hexahydrate for electrochemical water oxidation. Curr. Nanosci.. 2017;14
- [CrossRef] [Google Scholar]
- Energy supply, its demand and security issues for developed and emerging economies. Renew. Sustain. Energy Rev. 2007
- [CrossRef] [Google Scholar]
- Reaction mechanism of strontium cobaltite synthesis from equimolar mixture of Sr(NO3)2 and Co(NO3)2 ∙6H2O under air atmosphere. Thermochim. Acta. 2019;676:52-63.
- [CrossRef] [Google Scholar]
- Effect of Mn precursors on the morphology and electrocatalytic activity toward water oxidation of micro-nanostructured MnOx films prepared by voltammetric deposition. J. Mater. Sci. Mater. Electron.. 2017;28:18463-18473.
- [CrossRef] [Google Scholar]
- Electrocatalytic performance evaluation of cobalt hydroxide and cobalt oxide thin films for oxygen evolution reaction. Appl. Surf. Sci.. 2018;427:253-259.
- [CrossRef] [Google Scholar]
- Theoretical investigation of the activity of cobalt oxides for the electrochemical oxidation of water. J. Am. Chem. Soc.. 2013;135:13521-13530.
- [CrossRef] [Google Scholar]
- Reversible amorphization and the catalytically active state of crystalline Co3O4 during oxygen evolution. Nat. Commun.. 2015;6
- [CrossRef] [Google Scholar]
- Investigation of structural, optical and electrical properties of Co3O4 nanoparticles. In: AIP Conference Proceedings. American Institute of Physics Inc.; 2018.
- [CrossRef] [Google Scholar]
- Surfactant-controlled low-temperature thermal decomposition route to monodispersed phase pure tricobalt tetraoxide nanoparticles. Mater. Lett.. 2013;90:111-114.
- [CrossRef] [Google Scholar]
- Surfactant-free thermal decomposition route to phase pure tricobalt tetraoxide nanoparticles from cobalt(II)-tartrate complex. J. Sol–Gel Sci. Technol.. 2013;65:296-300.
- [CrossRef] [Google Scholar]
- Molecular catalysts for water oxidation. Chem. Rev.. 2015;115:12974-13005.
- [CrossRef] [Google Scholar]
- Facet-dependent activity and stability of Co3O4 nanocrystals towards the oxygen evolution reaction. Phys. Chem. Chem. Phys.. 2015;17:29387-29393.
- [CrossRef] [Google Scholar]
- Opportunities and challenges for a sustainable energy future. Nature 2012
- [CrossRef] [Google Scholar]
- Cobalt-oxide-based materials as water oxidation catalyst: Recent progress and challenges. ACS Catal. 2014
- [CrossRef] [Google Scholar]
- Green synthesis of Co3O4 nanoparticles via Aspalathus linearis: physical properties. Green Chem. Lett. Rev. 2015
- [CrossRef] [Google Scholar]
- A new autostabilization mechanism in the Bennet doubler circuit-based electrostatic vibrational energy harvester. Sens. Actuators, A Phys.. 2018;272:259-266.
- [CrossRef] [Google Scholar]
- Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures. RMxFS. 2007;53:18-22.
- [Google Scholar]
- Nanostructured oxides in chemistry: characterization and properties. Chem. Rev.. 2004;104:4063-4104.
- [CrossRef] [Google Scholar]
- Electrochemical water oxidation with cobalt-based electrocatalysts from pH 0–14: The thermodynamic basis for catalyst structure, stability, and activity. J. Am. Chem. Soc.. 2011;133:14431-14442.
- [CrossRef] [Google Scholar]
- Co3O4 microspheres with free-standing nanofibers for high performance non-enzymatic glucose sensor. Analyst. 2013;138:6727-6731.
- [CrossRef] [Google Scholar]
- Nanostructured porous cobalt oxide synthesis from Co3[Co(CN)6]2 and its possible applications in Lithium battery. Mater. Lett. 2016
- [CrossRef] [Google Scholar]
- Electrocatalytic oxidation and reduction of H2O2 on vertically aligned Co3O4 nanowalls electrode: toward H2O2 detection. J. Electroanal. Chem.. 2009;625:27-32.
- [CrossRef] [Google Scholar]
- Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015
- [CrossRef] [Google Scholar]
- Co3O4 Thin Films: Sol-Gel Synthesis, Electrocatalytic Properties; Photoelectrochemistry. The Ohio State University; 2011.
- In situ formation of an water containing phosphate and Co2+. Science. 2008;321:1072-1075.
- [CrossRef] [Google Scholar]
- Facile synthesis and structural characterization of Co3O4 nanocubes. AIMS Mater. Sci.. 2015;2:16-27.
- [CrossRef] [Google Scholar]
- Preparation and electrochemical characterization of lithium cobalt oxide nanoparticles by modified sol-gel method. Mater. Res. Bull.. 2008;43:2497-2503.
- [CrossRef] [Google Scholar]
- Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts. J. Am. Chem. Soc.. 2012;134:3517-3523.
- [CrossRef] [Google Scholar]
- Preparation and characterization of nano cobalt oxide. J. Nanopart. Res.. 2008;10:59-67.
- [CrossRef] [Google Scholar]
- A novel strategy to synthesize cobalt hydroxide and Co3O4 nanowires. J. Phys. Chem. Solids. 2011;72:904-907.
- [CrossRef] [Google Scholar]
- Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc.. 2013;135:16977-16987.
- [CrossRef] [Google Scholar]
- Nitrogen as a major essential element of plants. In: Nitrogen Assimilation in Plants. Trivandrum, Kerala, India: Signpost; 2010.
- [Google Scholar]
- Cobalt oxide surface chemistry: The interaction of CoO(1 0 0), Co3O4(1 1 0) and Co3O4(1 1 1) with oxygen and water. J. Mol. Catal. A Chem.. 2008;281:49-58.
- [CrossRef] [Google Scholar]
- Effect of Co(NO3)2·6H2O thermal decomposition temperature on the nano-Co3O4 product morphology and electrocatalysis of water oxidation. J. Appl. Electrochem.. 2019;49:251-259.
- [CrossRef] [Google Scholar]
- Synthesis of Co3O4 nanoparticles with block and sphere morphology, and investigation into the influence of morphology on biological toxicity. Exp. Ther. Med.. 2016;11:553-560.
- [CrossRef] [Google Scholar]
- Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and pt catalysts: a comparative study of nanoparticles and bulk materials. ACS Catal.. 2012;2:1765-1772.
- [CrossRef] [Google Scholar]
- Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017
- [CrossRef] [Google Scholar]
- Preparation and characterization of manganese oxide nanoparticles-coated Albizia procera derived carbon for electrochemical water oxidation. J. Mater. Sci. Mater. Electron. 2019
- [CrossRef] [Google Scholar]
- Green synthesis of Co3O4 nanoparticles and their applications in thermal decomposition of ammonium perchlorate and dye-sensitized solar cells. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol.. 2015;193:181-188.
- [CrossRef] [Google Scholar]
- Mesoporous hexagonal Co3O4 for high performance lithium ion batteries. Sci. Rep.. 2014;4
- [CrossRef] [Google Scholar]
- Thermal conductivity and viscosity of hybrid nanfluids prepared with magnetic nanodiamond-cobalt oxide (ND-Co3O4) nanocomposite. Case Stud. Therm. Eng.. 2016;7:66-77.
- [CrossRef] [Google Scholar]
- Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report) Pure Appl. Chem.. 2015;87:1051-1069.
- [CrossRef] [Google Scholar]
- Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater.. 2016;28:215-230.
- [CrossRef] [Google Scholar]
- Coralloid and hierarchical Co3O4 nanostructures used as supercapacitors with good cycling stability. J. Solid State Electrochem.. 2016;20:1303-1309.
- [CrossRef] [Google Scholar]
- Co3O4 nanocrystals on single-walled carbon nanotubes as a highly efficient oxygen-evolving catalyst. Nano Res.. 2012;5:521-530.
- [CrossRef] [Google Scholar]
- Synthesis and microwave absorption properties of core-shell structured Co3O4-PANI nanocomposites. J. Nanomater.. 2015;2015
- [CrossRef] [Google Scholar]
- The advances of Co3O4 as gas sensing materials: a review. J. Alloys Compd. 2016
- [CrossRef] [Google Scholar]
- Dimensional control of cobalt-hydroxide-carbonate nanorods and their thermal conversion to one-dimensional arrays of Co3O4 nanoparticles. J. Phys. Chem. B. 2003;107:12643-12649.
- [CrossRef] [Google Scholar]
- Facet-dependent electrochemical properties of Co3O4 nanocrystals toward heavy metal ions. Sci. Rep.. 2013;3
- [CrossRef] [Google Scholar]
- Homogeneously dispersed multimetal oxygen-evolving catalysts. Science (80-.). 2016;352:333-337.
- [CrossRef] [Google Scholar]
- Synthesis of carbon-coated Co3O4 composite with dendrite-like morphology and its electrochemical performance for lithium-ion batteries. J. Nanopart. Res.. 2013;15
- [CrossRef] [Google Scholar]
- Controllable electronic transformer based on the resonance structure with switching capacitor for low-rise buildings residential area power supply stabilization systems. Int. J. Electr. Power Energy Syst.. 2017;91:117-120.
- [CrossRef] [Google Scholar]
- Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen. In: Kolloidchemie Ein Lehrbuch. Springer Berlin Heidelberg; 1912. p. :387-409.
- [CrossRef] [Google Scholar]
- Hydrogen: The future energy carrier. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010
- [CrossRef] [Google Scholar]







