Translate this page into:
Preventive and management approach of triptonide, a diterpenoid compound against streptozotocin-induced diabetic retinopathy in Wistar rat model
⁎Corresponding author at: Department of Ophthalmology, The First People ' s Hospital of Lanzhou City, Lanzhou, Gansu Province 730050, China. wgq13919897373@hotmail.com (Guoqiang Wu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Diabetic retinopathy (DR) is a serious complication in the patients with diabetes, which develops due to the increased blood glucose status. DR is a severe condition, which may causes permanent vision loss in many diabetic patients worldwide. The current experimentation was dedicated to disclose the healing property of triptonide against streptozotocin (STZ)-induced DR in Wistar rats via regulation of inflammatory markers and crucial metabolic factors. DR was induced to the animals via injecting the one-time dosage of STZ (60 mg/kg) through intraperitoneal route. The DR rats was then orally administered with 25 mg/kg of triptonide for 60 days. The animal bodyweight and level of blood glucose was assessed. The triglycerides, LDL, cholesterol, HDL, inflammatory, and oxidative stress markers were measured using corresponding available standard kits. The status of MCP-1, MMP-9 and VEGF was enumerated by available commercial kits. The morphometric investigation of animal retina tissues was also measured. The relative protein expressions of apoptotic markers like Bax, Bcl-2 and its combined ratio as well measured. Overall triptonide administration effectively reduced the food consumption and glucose level and enhanced the body mass of STZ induced rats. The TNF-α, IL- 1β & 6, TG, LDL, cholesterol and HDL, MCP-1, MMP-9 and VEGF status was considerably down regulated by the triptonide treatment. Our compound triptonide also enhanced the levels of antioxidants; with improved morphometric measures like retinal thickness, cell numbers and reduced alterations in islets ultrastructure morphology by histopathological examination. Ultimately, our outcomes recognized that the triptonide ameliorated the STZ-induced diabetic retinopathy in wistar rats.
Keywords
Diabetic retinopathy
Triptonide
Streptozotocin
Inflammation
Apoptosis
1 Introduction
Diabetes mellitus (DM) is an enduring global metabolic disorder and is a fourth major factor of global mortality because of the expansion of numerous related microvascular or blood vessel complications (Roden 2016). The incidence of DM rapidly increasing worldwide and distressing more young aged teenagers and even children in both developing and developed nations, exhibiting as an authoritative health complication to the peoples around the world with numerous other related problems (Park and Roh 2016). Recent studies stated that, in 2019 the global occurrence of DM was 9.3%, in upcoming 2030 and 2045 this percentage is estimated to increase to the percentage of 10.2% and 10.9% respectively (Saeedi et al. 2019). Furthermore, the extended impairment of DM fallout in accelerated secondary problems like diabetic retinopathy (DR), nephropathy and myopathy by modifying the functions of micro and macro-vascular blood vessels (Harding et al. 2019). DR is a problematic disease condition, which causes permanent vision loss in patients with uncontrolled up regulation of glucose status (Klein, 2007).
Several factors are contributed to the expansion of DR, for instance oxidative stress, microvascular damage, and pro-inflammatory intermediates (Guzman et al., 2016). DR is considered as a critical condition and a pivotal cause of decreased life quality of patients. The disease condition of DR is existing in reply to the endured damage of retina and altered blood-retinal barrier functions. The early indication of DR includes the presence of dark spots, hang in visualization, unbalanced and blurred vision, impairments in color vision, dark areas, and ultimately loss of vision in advanced condition (Cheloni et al. 2019). These conditions arises due to the upregulation in glucose level, hypertension, increased triglycerides and elevated urinary proteins that subsequently fallout in the development of hemorrhage and detachment of retinal region and fibrous tissues development (Kusuhara et al., 2018). Furthermore, DR as well upraises the opportunities to damage retinal blood vessel which again leads to seepage or hemorrhage (Wang and Lo, 2018). Inflammation in ocular tissues was characterized as a crucial participants of several ocular disorders like uvea inflammation, optic nerve damage and finally retinopathy (Perez and Caspi 2015).
The inflammatory mediators such as TNF-α and interleukins (IL)-1β was stated to the unintentional communication with insulin signaling and confrontation (Zhu et al. 2015). Furthermore, diabetic retinopathy is correlated to variable factors, like new blood vessel developments, in which VEGF plays a crucial role in each stage (Kant et al. 2009; Eichler W, Yafai et al. 2004). VEGF stimulate the movement, partition and propagation of endothelial cells which consequently enhance the action of plasminogen activators in plasma that will endothelial cells, which leads to neovascularization (Jorge et al. 2006; Zhang et al. 2006). Previous findings show that MMP-9 (matrix metalloproteinase-9) similarly plays a critical function in neovascularization. Besides, MCP-1 serves crucial functions in the progression of various impediments of DM (Bin Chen et al. 2017; Kim et al. 2009). Numerous drugs have been established, such as synthetic drugs, antiplatelet drugs, aldose reductase inhibitors and inhibiting end products; nevertheless, these drugs are confronted by side effects (Joussen et al. 2002).
Management of diabetes has extended traditional events in Chinese medicine. Researches have revealed that traditional Chinese medicine can suggestively recover diabetes and its correlated problems (Zhang and Jiang 2012). Triptonide (TPN) is a significant diterpene compound that demonstrates anti-inflammation, anticancer, immunosuppressive, anti-inflammatory, and other biological properties (Xiang et al. 2019; Zhang M et al. 2019; Zhang B et al. 2019; Wang et al. 2019; Yue-Juan Ling et al., 2020). Many previous investigations have emphasized that the triptonide has outstanding biological activities like antioxidant and anti-inflammatory properties, further previous studies states that triptonide does not tempt serious liver toxicity in experimental animals. (Dong et al. 2019; Hu et al. 2018; Yang et al. 2017).
The beneficial effects of triptonide against the DR were not explored yet. Henceforth, the present study was dedicated to decrypt the salutary activities of triptolide against the STZ (streptozotocin)-induced DR in rats via altered biochemical parameters and inhibiting inflammation signals.
2 Materials and methods
2.1 Chemicals and reagents
Streptozotocin (STZ), Triponide, were obtained from Sigma-Aldrich, USA. All the corresponding assay kits to estimate the biochemical markers were obtained from Abcam, Beijing, China. Thermofisher, Massachusetts, United States of America, Cayman Chemical, Michigan, USA. and R&D Systems, Inc., Minneapolis, Canada, respectively.
2.2 Investigational animals
Three months matured male Wistar rats with weight around 230 ± 50gm were selected for present study. Animals were acquired from Shanghai Laboratory Animal Centre and rats were adapted to animal house condition formerly before the experiment start. All animals were maintained in excellent conditions that is with 22–24 °C temperature, 50% moisture; 12-hr light and 12-hr dark sequences. Animals permissible to get pure water and pellet diet in animal cage during the entire experimentation period. The present investigation was ethically permitted by the approved animal ethical committee directed by subsequent guidelines of National Institutions of Animal Health Care and Use.
2.3 Experimental design
Randomly, experimental rats were split into four groups (n = 6). Group I rats are control. Group II remained only with diabetic retinopathy induction. In which, rats were treated intraperitonially (i.p) with 60 mg/kg of STZ, which was freshly equipped with citrate buffer (0.1 M). Meanwhile, group I control rats established only with citrate buffer along with regular pellet diet. Later with STZ encounter for 48hr, the glucose level was estimated and the rats with 200 mg/dl of glucose level and above was categorized as diabetic. Group III rats was induced with STZ and oral administration of 25 mg/kg of triptonide for 60 days. Group IV rats were induced with diabetic as well as with metformin, a standard drug (300 mg/kg) for 60 days. Subsequently with completion of experiments, the blood sample was collected using retro-orbital plexus. The retina tissues were collected from the experimental rats and homogenated using triphosphate buffer (50 mM of) at 7.4 pH and samples centrifuged at 5000 rpm for 10 min at 4 °C. The final homogenate was used for further investigations.
2.4 Analysis bodyweight, food uptake, blood glucose, and HbA1c levels
The bodyweight and food uptake of experimental rats were sensibly assessed during the experimental period. The blood glucose level was noticed by means of glucometer (HUIDA instruments, Jiangsu, China). The HbA1c status was measured with the help of commercially available kit in accordance with manufacturer guidelines (Thermo Fisher Scientific, Massachusetts, United States of America).
2.5 Lipid profile and atherogenic index (AI) measurement
The crucial biochemical like lipid levels and associated lipids like triglyceride (TG), cholesterol (Ch), LDL and HDL were studied using corresponding kits according to the procedure specified by manufacturer (Cayman Chemical Company, Michigan, United States of America). The AI (atherogenic index) was studied according to the following equation which means: (Ch - HDL-Ch)/HDL-cholesterol.
2.6 Antioxidants measurement
The very important and vital biochemical status such as SOD, GSH, relative SOD/CAT, and GSSG (GSH/oxidized glutathione) proportion in the experimental rats were measured with the corresponding kits as in accordance with the manufacturer protocol (Abcam, Tokyo, Japan).
2.7 Pro-inflammatory marker levels
The Interleukins (IL-1b, IL-6) and TNF-α in the rat retina of the experimental rats was assessed using corresponding analyzing kits according to the recommended manufacturer instruction (R&D Systems, Beijing, China).
2.8 Analysis of MCP-1, MMP-9 and VEGF levels
The status of MCP-1, MMP-9 and VEGF were measured according to commercially available kits in accordance with the manufacturer recommendation (My BioSource, Incorporation, San Diego, USA).
2.9 Morphometric measurement
The morphometric measurement of retina of the experimental rats were achieved by using computer attached image examination method to notice the retinal thickness and ganglion cell (GCL) numbers. To analyze this, Image J software was employed as defined in previous studies (Jiang et al. 2010).
2.10 Measurement of anti-apoptotic marker levels
The apoptotic markers like Bax, Bcl-2, and Bax/Bcl-2 proportions was measured in the present study. The levels of apoptotic markers were studied using respective assay kits. Each assay was done in triplicate as per the guidelines specified by the manufacturer (Elabsciences, Texas, USA).
2.11 Histopathological analysis
To examine the alterations in ultrastructure levels, the paraffin-fixed pancreas was segmented at 4 μm by means of a semi-automated microtome (RM2255; Leica Micro-systems, Mumbai, India). The tissue segments were then kept on glass slides by means of hot plate. Subsequently, the tissue sections were deparaffinized by xylene solution and the moisture content was absorbed by dissimilar ethanol dilution (100% and 90%). The segments were stained with H&E. Finally, the sections were assessed by using simple light microscope (ZEISS microscopes, Oberkochen, Germany) beneath a magnification of X200.
2.12 Statistics analysis
All values are depicted as mean ± SD of triplicates. GraphPad Prism Software version 7.0 was employed for the statistical investigations. Values were scrutinized using one-way ANOVA consecutively Tukey’s multiple assessments test. P < 0.05 was viewed as substantial or significant.
3 Results
3.1 Efficacy of triptonide on the body weight, food consumption, blood glucose, and HbA1c levels in the STZ-induced DR rats
As demonstrated in Fig. 1, the regular food intake, glucose, and HbA1c levels was upregulated in the STZ induced group II DR rats. Coming to body weight, STZ induction decreased the animal bodyweight. The management with 25 mg/kg of triptonide along with STZ induction noticeably reduced the food intake, glucose level, and HbA1c level in group III Wistar rats. Triptonide as well increased the animal bodyweight of the STZ induced group III animals. The group IV metformin administered animals as well exhibited improved bodyweight and reduced the food uptake, glucose, and HbA1c levels in STZ induced DR rats, which was comparable with the triptonide treatment.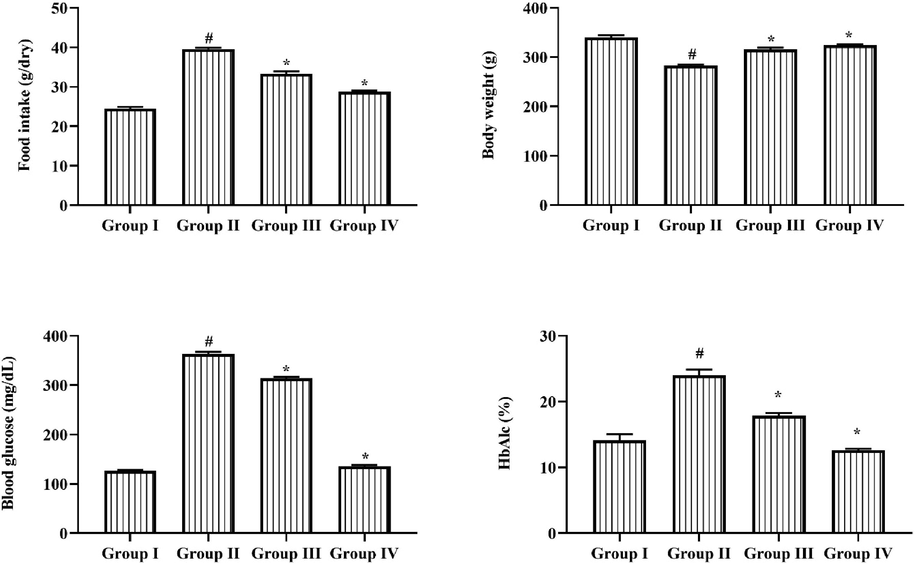
Effect of Triptonide on the food intake, bodyweight, blood glucose, and HbA1c levels in the STZ- inducedDR rats. Efficacy of triptonide on the food consumption, body mass, blood glucose, and HbA1c status in the STZ induced DR rats. Outcomes were characterized as mean ± SD of triplicates. Statistics were reviewed by one-way ANOVA successively Tukey’s post hoc test. Note: ‘*’ p < 0.05 associated with control ‘#’ p < 0.01 related with DR induced animals. Group I: normal rats, Group II: DR induced rats by STZ (60 mg/kg). Group III: DR rats administered with the 25 mg/kg of triptonide. Group IV: DR rats treated with standard drug metformin (300 mg/kg).
3.2 Outcome of triptonide on the lipid levels and atherogenic index
The DR rats exhibits upregulated the triglycerides, LDL, Chol, and AI levels in animal serum when compared with normal group I rats. While high density lipoprotein level was downregulated in the STZ provoked rats (Fig. 2). But the same was effectually controlled by the triptonide management. The management with 25 mg/kg of triptonide to STZ induced rats showed the substantial decrease in TG, LDL, Chol, and AI and also enhanced the HDL in the DR rats. The group IV metformin treated animals also modified the alterations in lipid contents in the DR rats.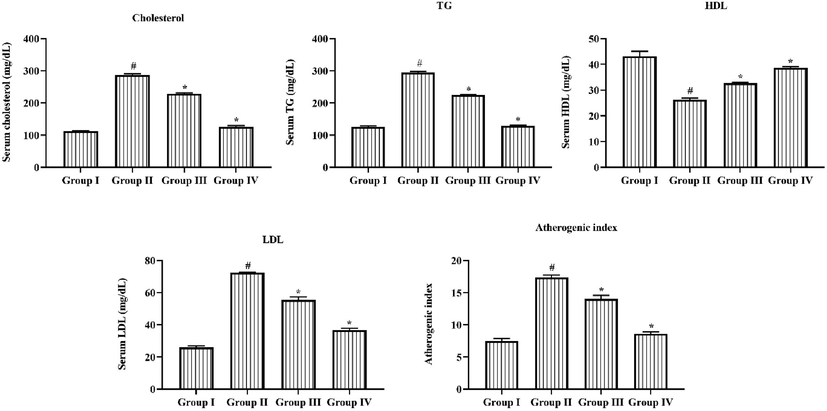
Efficacy of Triptonide on the Lipid profiles and Atherogenic Index of STZ induced retinopathy rats. Effect of triptonide on lipid status and atherogenic index of STZ tempted retinopathy rats. Grades were signified as mean ± SD of triplicates. Statistics were scrutinized by one-way ANOVA sequentially Tukey’s post hoc test. Note: ‘*’ p < 0.05 equaled with control ‘#’ p < 0.01 equaled with retinopathy rats. Group I: normal rats, Group II: DR induced rats by STZ (60 mg/kg). Group III: DR rats administered with the 25 mg/kg of triptonide. Group IV: DR rats treated with standard drug metformin (300 mg/kg).
3.3 Effectiveness of triptonide on antioxidants level in experimental rats
Fig. 3 illustrates that the levels of crucial antioxidants such as SOD, Glutathione, ratio of SOD/CAT and GSH/GSSG proportion was radically reduced in the retina of STZ-induced DR rats in comparison with group I animals. Significantly, the 25 mg/kg of triptonide noticeably augmented the level of SOD, Glutathione transferase, GSH/GSSG ratio and SOD/CAT ratio in retina tissues of STZ challenged group III rats. The same result was observed in treatment with standard drug metformin in STZ induced group IV animals, where the antioxidants level exposed improved level in DR animals.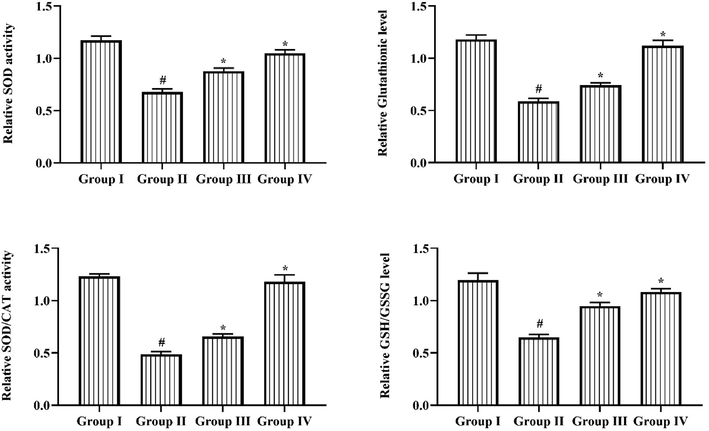
Outcome of Triptonide on antioxidants status in STZ induced DR rats. Outcome of triptonide on antioxidants status in STZ-induced DR rats. Outcomes were denoted as mean ± SD of triplicates. Statistics were reviewed by one-way ANOVA successively Tukey’s post hoc test. Note: ‘*’ p < 0.05 associated with control ‘#’ p < 0.01 associated with retinopathy rats. Group I: normal rats, Group II: DR induced rats by STZ (60 mg/kg). Group III: DR rats administered with the 25 mg/kg of triptonide. Group IV: DR rats treated with standard drug metformin (300 mg/kg).
3.4 Triptonide effect on the pro-inflammatory cytokines
Fig. 4 represents the TNF-α and IL-1β, and IL-6 status in the retina of untreated normal and induced experimental animals. STZ triggered diabetic retinopathy rats illustrates the upsurge in the TNF-α, IL-1β, and IL-6 in the retina. However, the oral administration of 25 mg/kg of triptonide to the DR rats showed the considerable diminution in these cytokine levels. Likewise, metformin treatment too reduced these cytokine levels in retina of DR rats.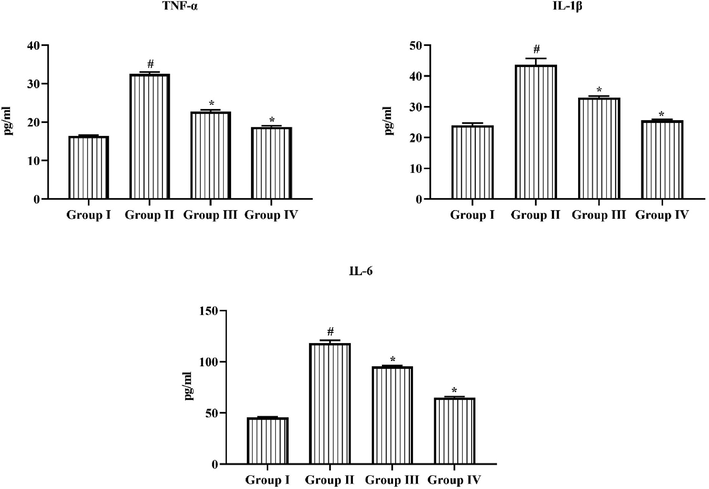
Triptonide on the pro-inflammatory markers in the STZ induced retinopathy rats. Triptonide on the pro-inflammatory markers in STZ treated rats. Outcomes were symbolized as mean ± SD of triplicates. Statistics were studied by one-way ANOVA sequentially Tukey’s post hoc test. Note: ‘*’ p < 0.05 linked with control ‘#’ p < 0.01 linked with retinopathy animals. Group I: normal rats, Group II: DR induced rats by STZ (60 mg/kg). Group III: DR rats administered with the 25 mg/kg of triptonide. Group IV: DR rats treated with standard drug metformin (300 mg/kg).
3.5 Effect of triptonide on the MCP-1, MMP-9 and VEGF levels
Fig. 5 shows the MCP-1, MMP-9 and VEGF status in normal and treated experimental rats. These marker levels were extremely augmented in STZ induced DR rats when related to control. Remarkably, the oral treatment of 25 mg/kg of triptonide was significantly repressed these above-mentioned biomarkers in the DR rats. The same result observed in standard drug metformin administered group IV animals in which MCP-1, MMP-9 and VEGF status in the DR rats.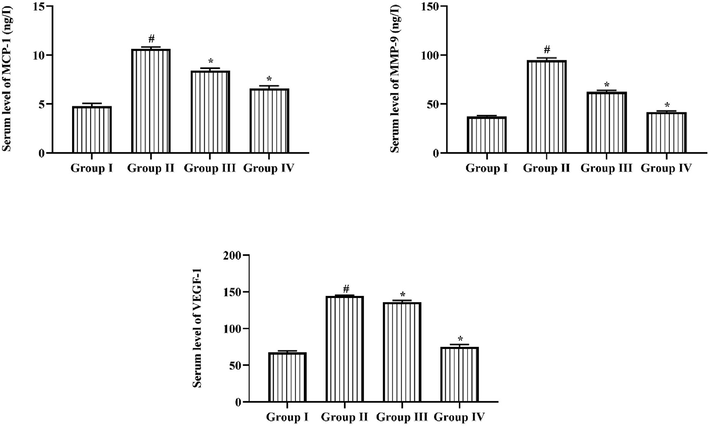
Result of Triptonide on the MCP-1, MMP-9, and VEGF levels in the STZ induced retinopathy rats. Result of Triptonide on MCP-1, MMP-9 and VEGF in STZ nduced retinopathy animals. Consequences were signified as mean ± SD of triplicates. Statistics were examined by one-way ANOVA repeatedly Tukey’s post hoc test. Note: ‘*’ p < 0.05 related with control ‘#’ p < 0.01 related with retinopathy rats. Group I: normal rats, Group II: DR induced rats by STZ (60 mg/kg). Group III: DR rats administered with the 25 mg/kg of triptonide. Group IV: DR rats treated with standard drug metformin (300 mg/kg).
3.6 Efficacy of triptonide on morphometric analysis of retina
The consequences of morphometric examination of retina was described in Fig. 6. It exposed that the retinal width and the CGL numbers were reduced in group II rats when related to normal group I rats. Conversely, the 25 mg/kg of triptonide administration to STZ treated group III animals displayed the noteworthy enhancement in the thickness and the CGL numbers in the, which is different to the STZ alone treated group II rats. Similar, outcome were found in metformin treated group IV animals, where enhanced total cells and thickness.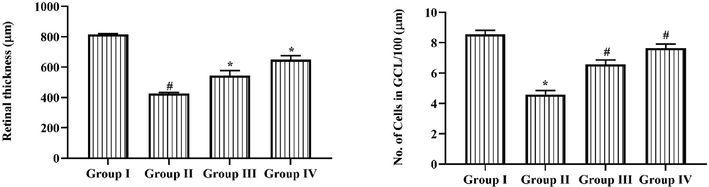
Efficacy of Triptonide on the morphometric examination of retina in the STZ induced DR rats. Efficacy of triptonide on the morphometric examination of retina in STZ treated animals Outcomes were characterized as mean ± SD of triplicates. Statistics were examined by one-way ANOVA successively Tukey’s post hoc test. Note: ‘*’ p < 0.05 associated with control ‘#’ p < 0.01 associated with retinopathy rats. Group I: normal rats, Group II: DR induced rats by STZ (60 mg/kg). Group III: DR rats administered with the 25 mg/kg of triptonide. Group IV: DR rats treated with standard drug metformin (300 mg/kg).
3.7 Consequence of triptonide on the Bax and Bcl-2 expression
Fig. 7 representing the apoptotic protein expressions in retina tissues of the experimental rats. The relative expressions of Bax and Bax/Bcl-2 ratio in group II animals were found to be augmented, while the relative expression of Bcl-2 was repressed in the STZ induced DR rats in comparison with normal group I rats. Remarkably, the triptonide (25 mg/kg) oral administration displayed notable downregulation in apoptotic protein expressions and enhanced the Bcl-2 expression in retina of STZ tempted group III rats. Metformin also substantially controlled these expressions in DR rats.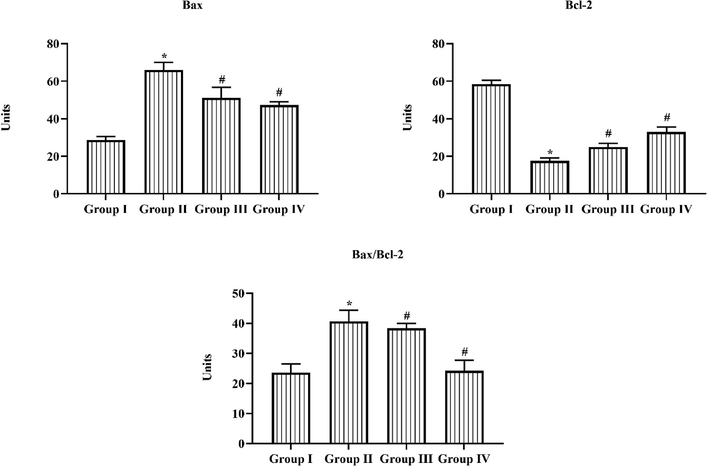
Effect of Triptonide on the Bax and Bcl-2 expression in the STZ induced retinopathy rats. Triptonide on apoptotic inducing factors like Bax, Bcl-2 and its combined ratio of bcl-2 and bax, in the STZ (60 mg/kg) induced and triptonide managed animals was restrained by ELIZA kit. The relative intensity was signified as percentage in all groups of experimental rats. Statistics were examined by one-way ANOVA successively Tukey’s post hoc test. Note: ‘*’ p < 0.05 associated with control ‘#’ p < 0.01 associated with retinopathy rats. Group I: normal rats, Group II: DR induced rats by STZ (60 mg/kg). Group III: DR rats administered with the 25 mg/kg of triptonide. Group IV: DR rats treated with standard drug metformin (300 mg/kg).
3.8 Effect of triptonide on histological examination
In group I control rats, many circular and extended islets were observed and consistently dispersed all over the cytoplasm, in which the nucleus is stained lightly than the neighboring pancreatic acinar cells, this was depicted in Fig. 8. Group-I animal exhibits that histology of islets was regular and undamaged. Whereas in group II STZ alone induced rats exhibits that altered morphology of islet, in which small islets were demolished and only few islets are viewed in the segments. Unfilled gaps are observed by the islets among the acinar cells, where intact ultrastructure is somewhat disturbed. Further vital portion of the islets was necrotic. At the same time STZ induced along with triptonide (25 mg/kg) group-III rats showed condensed gaps among the pancreatic acinar cells with moderate recover of ultrastructure. The similar outcomes were noted in metformin treated group IV rats, where the histology of islets was normal and unchanged.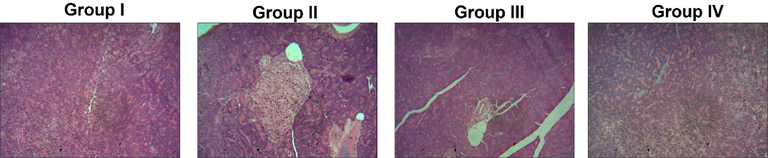
Outcome of Triptonide on the pancreas histopathology of the STZ induced DR rats. Triptonide on histopathology observation of normal and experimental rats. Group I: normal untreated demonstrates normal and regular ultrastructure, Group II: DR rats with STZ (60 mg/kg) exhibits disturbed islets with much gap in acinar cells in pancreas. Group III: Induced with STZ as well as with the 25 mg/kg of triptonide, showed reduced gaps between pancreatic acinar cells with excellent recovery of structure with intact of islet along with nucleus. Group IV: STZ induction along with treatment of metformin (300 mg/kg) in retinopathy animals also exposed the same intact nucleus and regular ultrastructure which was comparable with group I animals.
4 Discussion
The retina in the eye is one of the important metabolically dynamic tissue, which primarily affected by diabetes mellitus (Qiao et al. 2020). Chronic hyperglycemia condition leads to hemodynamic, microvascular and alterations in neurons which lead to diabetic retinopathy (Kang and Yang 2020). The mechanisms undergo DR are with multifaceted approach but hold the changes in inflammation, oxidative pressure, ischemia, neural apoptosis and angiogenesis (Li et al. 2020; Al-Kharashi 2018). DR is the prime factor of diabetic mellitus associated ocular damage among young and aged individuals globally (Wong et al. 2016). The microvascular injury in the retina such as collapsed blood barrier, drop in capillary, and retinal microaneurysms or out pouch of blood are crucial features of diabetic retinopathy (Chawla et al. 2016). The inflammatory intermediaries are the crucial players in the development of both initial and late phase of diabetic retinopathy.
Augmentation of TNF-α and other interleukins ultimately worsen the DR development (Yang et al. 2006). The over accretion of TNF-α and IL-6 in the vitreous fluid of the diabetic retinopathy individuals were highlighted earlier (Sato et al. 2009). TNF-α is a well-known marker that vigorously contributes to the augmentation of inflammatory responses (Gao et al. 2007; Huang et al. 2011). The upregulated inflammatory molecules were related with the diabetes mellitus. And further these features could communicate with resistance of insulin (Jager et al. 2007). In present study, we recognized that the STZ induced DR rats established the serious upregulation in TNF-α, IL-1β and IL-6 status in the retina. Remarkably, the triptonide administration to the STZ induced rats considerably repressed these cytokine statuses. VEGF plays a vital role in diabetic initiation and grounds for early-stage expansion of diabetic retinopathy disease condition.
Consistent diabetes leads to a condition tissue hypoxia and subsequently results in increased VEGF. It upraises the penetrability and expansion of blood vessels that contributing in the development of diabetic retinopathy (Kota et al. 2012). Previous studies states that the management of VEGF in non-diabetic animals formed serious irregularities in the animal retina that resembles diabetic retinopathy, which confirms the VEGF role in the DR progression (Bressler et al., 2020). Oxidative pressure initiates the inflammatory controllers that ultimately contributes to retina cell damage and sequentially results in DR development. Furthermore, oxidative stress can correspondingly alter numerous growth features like VEGF and trigger other metabolic pathways concerned in the diabetes progression (Keshari et al. 2013; Son et al. 2013). In this examination, we revealed that the VEGF status was extremely increased in the STZ alone induced group III rats, which was successfully abridged by the triptonide management. The MMP-9 is a proteinase family that might degrade the extracellular matrix components and executes a serious function in both normal and disease conditions (Griselda et al. 2020).
Numerous previous studies have revealed that the MMP-9 vigorously contributes in the instigation of diabetic retinopathy (Kowluru et al., 2012). It was previously emphasized that the MMP- 9 action was amplified in numerous stages of DR (Kowluru et al. 2016). With all these evidences in present study we observed that the MMP-9 was upregulated in the STZ induced retinopathy rats and further MMP-9 level was substantially downregulated by the treatment of triptonide.
The retina tissues frequently experience the several metabolic illnesses and changes in gene expressions. The diabetic disorder might result in necrosis of retinal capillary cells with subsequent modification in histology. That is in present study in histology observation of inducer alone group II STZ displayed much altered morphology of islet, much gaps are detected by the islets between the acinar cells, with disturbed intact ultrastructure. Whereas STZ induced along with triptonide group-III animals displayed reduced gaps between the pancreatic acinar cells with sensible recovery of ultrastructure. The activated inflammatory and other pathways in diabetic might enhance the apoptosis of capillary cells which was the authoritative histological characteristics of retinopathy (Jiang et al. 2017). In current work, we as well studied the levels of antioxidants, which is seems to be extremely downregulated in the retina. Fascinatingly, the triptonide management noticeably enhanced the antioxidants status in the DR rats.
The Bax, an apoptosis inducing feature accomplishes central functions in the retina neuron cell death through this programmed cellular death. Bcl-2 promotes the cell endurance through its capability to inhibit the cellular programmed death or apoptosis. Meantime, Bax/Bcl-2 is important for the initiation of apoptosis, the elevated Bax/Bcl-2 proportions thus takes part in the cell death of retina (Sharpe et al., 2004). With this coinciding evidence, in current study the relative expressions of Bax and Bax/Bcl-2 proportion was augmented and Bcl-2 was downregulated in STZ induced retinopathy rats. But, triptonide administration considerably regulated the apoptotic protein expressions in the animal retina, which was induced by STZ in group III animals.
5 Conclusion
Our outcomes from this present work revealed that the triptonide reduced the STZ-induced DR in wistar rats via increasing the animal body weight, and the down regulation of blood glucose level, lipid profile, oxidative pressure, inflammatory intermediaries, enhanced antioxidants, and also by inhibiting crucial factors like VEGF, MCP-1 and MMP-9. These outcomes reinforced the reference that triptonide might be an optimistic medication to manage the diabetic retinopathy. Still, the complementary research necessary in future to sort tangible confirmation about the remedial efficacy of triptonide against DR.
Funding
Model construction of regional prevention and treatment of diabetic retinopathy, Sichuan Science and Technology Support Program, 2016FZ0091. This project was supported by Researchers Supporting Project number (RSPD2023R712), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Role of oxidative stress, inflammation, hypoxia and angiogenesis in the development of diabetic retinopathy. Saudi J. Ophthalmol.. 2018;32(4):318-323.
- [Google Scholar]
- Bressler, N.M., Beaulieu, W.T., Bressler, S.B., Glassman, A.R., Melia, B.M., Jampol, L. M., Jhaveri, C.D., Salehi-Had, H., Velez, G., Sun, J.K., DRCR Retina Network., 2020. Anti-vascular endothelial growth factor therapy and risk of traction retinal detachment in eyes with proliferative diabetic retinopathy: pooled analysis of five DRCR retina network randomized clinical trials. Retina. 40(6), 1021-1028.
- The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci.. 2020 Dec;21(24):9739.
- [Google Scholar]
- Microvascular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J. Endocrinol. Metab.. 2016;20(4):546-551.
- [Google Scholar]
- Global prevalence of diabetic retinopathy: protocol for a systematic review and meta-analysis. BMJ Open. 2019;9:e022188.
- [Google Scholar]
- Effects of quercetin on the expression of MCP-1, MMP-9 and VEGF in rats with diabetic retinopathy. Experimental Therap. Med.. 2017;14:6022-6026.
- [Google Scholar]
- Triptonide acts as a novel antiprostate cancer agent mainly through inhibition of mTOR signaling pathway. Prostate. 2019;79(11):1284-1293.
- [CrossRef] [Google Scholar]
- PEDF derived from glial Müller cells: A possible regulator of retinal angiogenesis. Exp. Cell Res.. 2004;299:68-78.
- [Google Scholar]
- Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation. 2007;115(2):245-254.
- [Google Scholar]
- Mechanisms involved in the development of diabetic retinopathy induced by oxidative stress. Redox. Rep.. 2016;22(1):1-7.
- [Google Scholar]
- Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62:3-16.
- [Google Scholar]
- Comparative metabolism of tripolide and triptonide using metabolomics. Food Chem. Toxicol.. 2018;115:98-108.
- [CrossRef] [Google Scholar]
- TNF alpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest. Ophthalmol. Vis. Sci.. 2011;52(3):1336-1344.
- [Google Scholar]
- Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate- 1 expression. Endocrinology. 2007;148:241-251.
- [Google Scholar]
- MiR-874 inhibits metastasis and epithelial-mesenchymal transition in hepatocellular carcinoma by targeting SOX12. Am. J. Cancer. Res.. 2017;7(6):1310-1321.
- [Google Scholar]
- Application of isoproterenol inhibits diabetic-like changes in the rat retina. Exp. Eye. Res.. 2010;9:171-179.
- [Google Scholar]
- Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study) Retina. 2006;26:1006-1013.
- [Google Scholar]
- Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J.. 2002;16:438-440.
- [Google Scholar]
- Q. Kang and C. Yang, “Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications,” Redox Biology, vol. 37, Article ID 101799, 2020.
- Vascular endothelial growth factor-A (VEGF-A) in vitreous fluid of patients with proliferative diabetic retinopathy. Ann. Ophthalmol. (Skokie). 2009;41:170-173.
- [Google Scholar]
- Reactive oxygen species induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. J. Cell. Biochem.. 2013;114(3):532-540.
- [Google Scholar]
- Anti-diabetic effects of new herbal formula in neonatally streptozotocin-induced diabetic rats. Biol. Pharm. Bull.. 2009;32:421-426.
- [Google Scholar]
- Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol.. 2007;14:179-183.
- [Google Scholar]
- Aberrant angiogenesis: the gateway to diabetic complications. Ind. J. Endo. Metabol.. 2012;16(6):918.
- [Google Scholar]
- Matrix metalloproteinases in diabetic retinopathy: potential role of MMP-9. Expert. Opin. Investig. Drugs.. 2012;21:797-805.
- [Google Scholar]
- Dynamic DNA methylation of matrix metalloproteinase-9 in the development of diabetic retinopathy. Lab. Invest.. 2016;96(10):1040-1049.
- [Google Scholar]
- Pathophysiology of diabetic retinopathy: the old and the new. Diabetes Metab. J.. 2018;42:364-376.
- [Google Scholar]
- Y. Li, T. Cheng, C. Wan, and Y. Cang, “circRNA_0084043 contributes to the progression of diabetic retinopathy via sponging miR-140-3p and inducing TGFA gene expression in retinal pigment epithelial cells,” Gene, vol. 747, Article ID 144653, 2020.
- Yue-Juan Ling, Ting-Yu Ding, Fu-Lu Dong, Yong-Jing Gao, Bao-Chun Jiang. Intravenous Administration of Triptonide Attenuates CFA-Induced Pain Hypersensitivity by Inhibiting DRG AKT Signaling Pathway in Mice. Journal of Pain Research 2020:13 3195-3206.
- New diagnostic and therapeutic approaches for preventing the progression of diabetic retinopathy. J. Diabetes Res.. 2016;2016:1753584.
- [Google Scholar]
- Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol.. 2015;36:354-363.
- [Google Scholar]
- Diabetic retinopathy detection using prognosis of microaneurysm and early diagnosis system for non-proliferative diabetic retinopathy based on deep learning algorithms. IEEE Access. 2020;8:104292-104302.
- [Google Scholar]
- Diabetes mellitus-definition, klassifikation und diagnose. Wiener Klinische Wochenschrift. 2016;128(2):37-40.
- [Google Scholar]
- P. Saeedi, I. Petersohn, P. Salpea et al., “Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th edition,” Diabetes Research and Clinical Practice, vol. 157, no. 594, Article ID 107843, 2019.
- Simultaneous analyses of vitreous levels of 27 cytokines in eyes with retinopathy of prematurity. Ophthalmol.. 2009;116(11):2165-2169.
- [Google Scholar]
- Control of mitochondrial permeability by Bcl-2 family members. Biochim. Biophys. Acta. 2004;1644:107-113.
- [Google Scholar]
- Reactive oxygen species in the activation of MAP kinases. Methods Enzymol.. 2013;528:27-48.
- [Google Scholar]
- Diabetic retinopathy: pathophysiology and treatments. J. Mol. Sci. Int.; 2018. p. :19.
- Triptonide inhibits human nasopharyngeal carcinoma cell growth via disrupting Lnc-RNA THOR-IGF2BP1 signaling. Cancer Lett.. 2019;443:13-24.
- [CrossRef] [Google Scholar]
- Triptonide effectively suppresses gastric tumor growth and metastasis through inhibition of the oncogenic Notch1 and NF-kappaB signaling pathways. Toxicol. Appl. Pharmacol. 2019:114870.
- [Google Scholar]
- Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappa B and posttranslational modifications of histone deacetylase in macrophages. Am. J. Physiol. Lung. Cell. Mol. Physiol.. 2006;291(1):L46-L57.
- [Google Scholar]
- Triptonide acts as a novel potent anti-lymphoma agent with low toxicity mainly through inhibition of proto-oncogene Lyn transcription and suppression of Lyn signal pathway. Toxicol. Lett.. 2017;278:9-17.
- [CrossRef] [Google Scholar]
- Zhang SX, Wang JJ, Gao G, Parke K and Ma JX: Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol 37: l-12, 2006.
- Active ingredients of traditional Chinese medicine in the treatment of diabetes and diabetic complications. Expert. Opin. Investig. Drugs. 2012;21:1625-1642.
- [Google Scholar]
- Selective activation of tumor-suppressive MAPKP signaling pathway by triptonide effectively inhibits pancreatic cancer cell tumorigenicity and tumor growth. Biochem. Pharmacol.. 2019;166:70-81.
- [CrossRef] [Google Scholar]
- Triptonide inhibits lung cancer cell tumorigenicity by selectively attenuating the Shh-Gli1 signaling pathway. Toxicol. Appl. Pharmacol.. 2019;365:1-8.
- [CrossRef] [Google Scholar]
- Association of oxidative stress biomarkers with gestational diabetes mellitus in pregnant women: a case-control study. PLoS One. 2015;10:e0126490.
- [Google Scholar]







