Production of polyhydroxyalkanoate from sesame seed wastewater by sequencing batch reactor cultivation process of Haloferax mediterranei
⁎Corresponding author. diya.safadi@rss.jo (Diya Alsafadi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Due to the high cost of bioplastic production, sesame wastewater, generated from the sesame seed hulling process, was investigated to be used as inexpensive and renewable carbon source for the production of biodegradable polyhydroxyalkanoate (PHA) by extreme Haloferax mediterranei. The sesame wastewater (SWW) was hydrolyzed using different concentrations of hydrochloric acid (0.4. 1.00 and 2.00 M) at different period of times (15, 60 and 90 min). The concentration of salt (NaCl) and nitrogen source (NH4Cl and yeast) required for H. mediterranei cells growth and the accumulation of PHA biopolymer was optimized. A maximum 0.53 g/L concentration of PHA was achieved when the SWW extract media was supplemented with 100 g/L NaCl and 6.0 g/L yeast extract. The cultivation was scaled-up using sequencing batch reactor (SBR) fermentation under non-sterile conditions. The SBR results showed that SWW needs an auxiliary carbon source to obtain high PHA production. Consequently, the system fed with SWW and glucose produced higher PHA (20.9 g/L) than the system fed with SWW.
Keywords
Biodegradable plastics
Polyhydroxyalkanoate
Haloferax mediterranei
Sesame wastewater
Acid pretreatment
Sequencing batch reactor
1 Introduction
To solve the depletion of global resources and to reduce water and energy consumption, there is a new trend for making wastewater treatment plants (WWTPs) more sustainable and developed. The main function of WWTPs is to remove organics, nitrogen, phosphorus, and other pollutants from wastewater. With the widespread toward the sustainability of WWTPs, a shift on its operation from nutrients removal to simultaneous pollutants removal as well as resource recovery (Xu et al., 2019). The resources that can be recovered from WWTPs as valuable byproducts such as hydrogen as source of energy (Patel et al., 2021), fertilizers, enzymes, heavy precious or radioactive metals, and emerging pollutants like pharmaceuticals and hormones (Puyol et al., 2016). Among the various nutrients recovered from WWTPs, carbon can be also recovered as bioplastic such as polyhydroxyalkanoates (PHAs) (Patel et al., 2021). This approach has attracted a lot of attention in recent years, due to overproduction and accumulation of non-degradable petrochemical plastics in the environment and increased the concern of possible depletion of fossil fuels which are the main source of petrochemical plastics.
Polyhydroxyalkanoates (PHAs) are renewable bio-polyesters of hydroxyalkanoic acid which are synthesized by several bacteria and archaea. Recently, PHAs production has attracted a lot of attention in recent years as “green plastic” (Sohn et al., 2022). PHAs showed same chemical and physical features to those of non-biodegradable polymers, but they are also biodegradable (Shrivastav et al., 2010). Accordingly, PHAs had replaced the fossil fuels-based plastics and there is an increasing demands on using them in a variety of disposable packaging goods; such as antimicrobial food containers (Ibrahim et al., 2022a) and low-cost bags for growing and transporting seedlings and mulching in the agriculture sector (Kalia et al., 2021). Furthermore, PHAs high biocompatibility and non-toxicity make them attractive candidates for biomedical applications such as tissue engineering, sutures, scaffolds, biocontrol agents, and drug delivery carriers (Ray et al., 2017, Kalia et al., 2021, Ibrahim et al., 2022b).
Sesame paste (tahini) is a popular foodstuff in Middle Eastern and Eastern Mediterranean countries which is produced from milling of roasted sesame (Sesamum indicum) seeds (Torlak et al., 2013). The effluent of the tahini industry, sesame seed wastewater (SWW) is characterized by its high chemical oxygen demand (COD), total solids (TS), carbohydrates, oil and greases, and total phenols (Nweke et al., 2014). This indicates that the discharge of SWW into the environment without any treatment causes damage to the aquatic life. Alternatively, SWW can be considered as a potential low-cost feedstock for PHAs production and their application for biodegradable plastic production could represent solution for their harmful disposal processes.
Several types of bacteria were tested to produce PHAs form wastewater. For example, Bacillus strains were utilized to produce safe and compatible PHAs from coconut wastewater as carbon source (Akhter et al., 2022). Mozejko-Ciesielska et al. 2022 used Paracoccus homiensis in the production of PHAs from diary wastewater, and the result indicated that both homo and hetero structures of PHAs biopolymers were obtained.
Haloferax mediterranei is an extremely halophilic organism (salt-loving microorganism), at which it can only grow in highly saline medium between 150 and 200 g/L. The advantage of high salinity of H. mediterrane media reduced the need for sterilization and facilitating cultivation process to be operated continuously in open vessel in large scale (Parroquin Gonzalez and Winterburn 2022). Compared to others PHAs producing organisms (Cupriavidus necator, Azotobacter vinelandii, Alcaligenes Spp., Pseudomonas oleovorans and Bacillus megaterium), H. mediterranei has unique advantages for PHAs production due to the ability to use low cost renewable carbon sources and the use of simple and convenient PHAs polymer extraction techniques (Koller and Muhr 2014, Koller 2017, Priya et al., 2022).
So far, the most reported fermentation processes for PHAs production using H. mediterranei were based on batch or fed-batch fermentation processes (Cui et al., 2017a, Cui et al., 2017b, Ferre-Guell and Winterburn 2019; Alsafadi et al., 2020b). The results had indicated that these processes have the disadvantage to give products with variable qualities. In addition, the use of these processes yielded low quantities of PHAs, especially when toxic carbon sources were used in the process (Koller and Muhr 2014). Consequently, continuous PHA fermentation strategy using sequencing batch reactors (SBRs) has been considered as a potential alternative to increase the PHA productivity with desired composition and minimal variations in product properties (Atlić et al., 2011, Tan et al., 2011, Koller and Muhr 2014).
This study investigates the use of SWW as renewable carbon sources for PHA production by H. mediterranei. Continuous cultivation process using SBRs under non-sterile conditions has been developed to achieve high PHAs productivity. The structure and the molecular weight of the produced polymer was also determined. To the best of our knowledge, this work represents the first attempt to produce PHA from SWW using continuous cultivation mode.
2 Material and methods
2.1 Chemical reagents and standards
All chemical reagents, unless stated otherwise, were analytical grade. Standard solutions used in volatile fatty acids (VFAs) analysis were prepared from concentrated formic acid (≥98 %, Sigma), acetic acid (glacial) (100 %, Merck), propionic acid (≥99 %, Merck), butyric acid (≥99 %, Merck). NaCl was purchased from Merck. Chloroform (HPLC grade) was sourced from Scharlab. Ammonium chloride was obtained from Chemlab. Glucose and yeast extract were purchased from Oxoid. Monodisperse polystyrene standards (PSt Quick C) for Gel permeation chromatography analysis were purchased from TOSOH Corporation.
2.2 Microorganisms and growth conditions
Haloferax mediterranei was obtained from German Collection of Microorganisms and Cell Cultures DSMZ (Germany), as lyophilized sample of strain DSM1411. H. mediterranei was initially grown in nutrient-rich medium (Alsafadi and Al-Mashaqbeh 2017). The culture was incubated for 4 days in a rotary shaker (230 rpm) and at a temperature of 37 °C. The strain was maintained in vials containing 20 % glycerol and 80 % medium at 80 °C.The stored strain was streaked on salt agar medium and incubated at 37 °C for 48 h.
2.3 Characterization of sesame wastewater and pre-treatment process
Fresh sesame seed wastewater (SWW) samples were collected from Kasih Canned Food Production Co (Amman – Jordan) and representative samples were prepared for further analysis. The samples were stored in plastic containers at 4 °C. The sample preparation process has significant importance to obtain repeatable results and increase measurements reliability (Sazali et al., 2019). Chemical oxygen demand (COD), ammonical nitrogen (NH4-N), chloride (Cl) and total suspended solid (TSS), density and Electrical conductivity (EC) were determined according to Standard Methods (Eaton et al., 2005). The SWW samples were pre-treated with different concentrations of hydrochloric acid (HCl). In each experiment, 300 mL SWW was hydrolyzed using 300 mL of 0.4, 1.0 and 2.0 M HCl concentrations at different reaction times (15, 60, 90 min), while keeping the temperature of the solution at 100 °C. After cooling to room temperature, the hydrolysate was neutralized to pH 7.2 using 1 M NaOH. The volume of solution was completed to 500 mL and then filtered by Whatman grade 41.
2.4 Production of polyhydroxyalkanoate (PHA)
A single H. mediterranei colony grown on solid media was inoculated into 100 mL of the liquid media (Alsafadi et al., 2020a). The culture was incubated with constant shaking (230 rpm) at a temperature of 37 °C. After three days, 3.0 mL of a selected pre-culture was transferred into 100 mL of hydrolyzed SWW with final carbohydrates concentration of 10.0 g/L. The media was supplemented by different concentrations of NaCl (50, 100, 150 and 200 g/L), yeast extract (6 and 12 g/L) or ammonium chloride (0.04 and 1.00 g/L).
2.5 Determination of cell growth and cell dry mass
The cell growth was analyzed by measuring the optical density at 520 nm (OD520nm), using a Biochrom Libra S50 UV–visible spectrophotometer. To determine the cell dry mass (CDM), 3.0 mL of the broth containing H. mediterranei cells was centrifuged at 9000 rpm for 15 min, and then the supernatant was discarded. The remaining pellet was washed twice with 3.0 M NaCl solution. The pellet was then dried in an oven at 105 °C to a constant mass.
2.6 Total carbohydrate
The total carbohydrate concentration was determined by the Anthrone-sulfuric acid method using glucose as standard. The volatile fatty acids (VFAs) were determined using Agilent 1100 HPLC equipped with a diode array detector and 8 mm Rezex ROA-organic acid H column (Phenomenex).
2.7 Fermentation process from sesame wastewater using sequencing batch reactor system (SBR)
The cultivation experiments were carried out using a lab scale BioStat A bioreactor (Sartorius, Germany) with total volume of 5.0 L. The temperature was controlled at 37 °C and the pH value was adjusted at 7.2 using either 1.0 M NaOH or 1.0 M HCl solutions. A constant air flow rate of 0.75 vvm (volume of air per volume of reactor per minute) was controlled during the cultivation process. The dissolved oxygen concentration was controlled at 20 % by the automatic variation of the stirring speed (200–800 rpm). Foaming was suppressed by the automatic addition of 1 % (w/v) antifoam A (Sigma). The cultivation was designed as continuous sequencing batch reactor containing four cycles, each one consists of filling phase, reaction phase, settling phase and withdrawal phase. The SBR system had been stopped after 583 h. During the cultivation process, samples were taken for quantification of CDM, PHA concentration and carbohydrate consumption.
2.8 PHA extraction and characterization
PHA extraction was performed as the following: after PHA production, the biomass-containing H. mediterrane cells were harvested by centrifugation (9000 rpm) for 15 min. The cell pellets were suspended in 0.10 % sodium dodecyl sulphate. After that, the cells were disrupted by vortex to produce clear lysate. The suspension was then centrifuged (9000 rpm) for 15 min. The crude formed PHA was washed twice by ethanol and water respectively. The resulting material was dissolved in 10 mL chloroform at 40 °C while the undissolved material was removed. The solvent was evaporated, yielding a thin film of PHA (Alsafadi and Al-Mashaqbeh 2017). The chemical composition of the formed PHA was determined using 500 MHz NMR spectrometer (Bruker Advance III).
2.9 Molar mass determination of PHA biopolymer
The molar mass data for PHA biopolymer were calculated from gel permeation chromatography (GPC) measurements on TOSHO HLC-8320 GPC (EcoSEC) equipped with a refractive index (RI) detector and TSKgel GMHHR-M column (7.8 mm I.D × 30 cm length × 5 μm particle size). The pump and column ovens were set at 35 ˚C and chloroform was used at a flow rate of 1.0 mL/min as eluent. Monodisperse polystyrene standards (PSt QuickC) were used to prepare a calibration curve with a working range of 500 Da–2110 kDa. The PHA biopolymer (12.0 mg) was dissolved in 2.0 mL chloroform and the molar mass data such as number average molecular weight (MW), weight average molecular weight (Mn) and polydispersity (Mw/Mn) were calculated using standard analysis software (EcoSECLogonManagerVersion1.02).
3 Results and discussion
3.1 Characterization of sesame wastewater and pre-treatment process
The physiochemical characterization of SWW was described in Table 1. As expected the SWW was characterized by high chemical oxygen demand (COD) (3750 mg/L) and total solids (TS) (10245 mg/L). These values were higher than the reported COD value for municipal wastewater (260–900 mg/L) and TS for untreated domestic wastewater (120–400 mg/L) (Metcalf et al., 2003). Furthermore, the SSW was rich in high amounts of carbon source such as carbohydrates and volatile fatty acids which can be utilized as substrates for PHAs production.
| Parameter | Result |
|---|---|
| pH-value | 5.4 |
| EC (ms/cm) | 2.5 |
| COD (mg/L) | 3750 |
| TSS (mg/L) | 10,245 |
| Cl (mg/L) | 280 |
| NH4 (mg/L) | 70 |
| Volatile fatty acids (mg/L) | 112 |
| Carbohydrates (mg/L) | 966 |
| Density (g/cm3) | 1.02 |
For PHAs production the H. mediterranei was initially cultivated in SWW and the cell growth was monitored by measuring optical density at 520 nm (material and method part). The results showed that H. mediterranei was unable to grow on SWW medium (data not shown), therefore; SWW was hydrolyzed into simpler constituents such as monosaccharides and fatty acids to feed H. mediterranei cells. Among the hydrolysis methods reported in the literature, chemical hydrolysis of SWW using HCl was selected because this methodology is less expensive than other hydrolysis methods such as enzymatic method. In order to identify the best conditions for SWW acid hydrolysis, different concentrations of HCl (0.4, 1.00 and 2.00 M) with different times (15, 60 and 90 min) were investigated. The total carbohydrates for the hydrolyzed SWW were measured at different experimental conditions (Fig. 1A). The carbohydrates yield from SWW acid hydrolysis was significantly increased with increasing HCl concentration and time. This trend was also observed in previous study when the rice straw (agricultural waste) was hydrolyzed prior the PHA production process (Ahn et al., 2016).
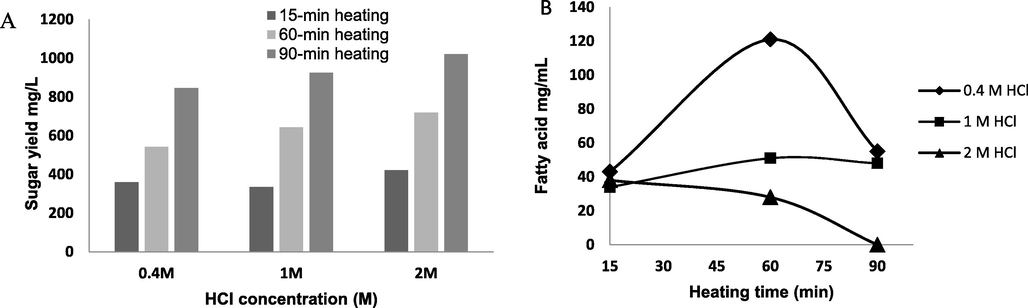
- Hydrolysis of SWW at different HCl concentrations and incubation time. A: total carbohydrates for the hydrolyzed SWW. B: Total volatile fatty acids for the hydrolyzed SWW.
As illustrated in Fig. 1A, the treatment of SWW with 2.0 M HCl would be optimal for maximum carbohydrates yields; however, the high acid concentration has the effect to cause a precipitation of organic compounds in the SWW. This was confirmed by measuring volatile fatty acids (VFAs) concentration (Fig. 1B). This figure shows that the treatment of SWW with 2.00 M HCl caused a significant decrease in VFAs content. Therefore, 1.00 M HCl was chosen for hydrolysis of SWW.
3.2 PHA production at different nitrogen sources and NaCl concentrations
In order to optimize PHA production in hydrolyzed SWW media, the influence of cultivation conditions (nitrogen source and NaCl concentrations) on the growth of H. mediterranei (Fig. 2) and the consumption of total carbohydrates in the media (Fig. 3) were studied.
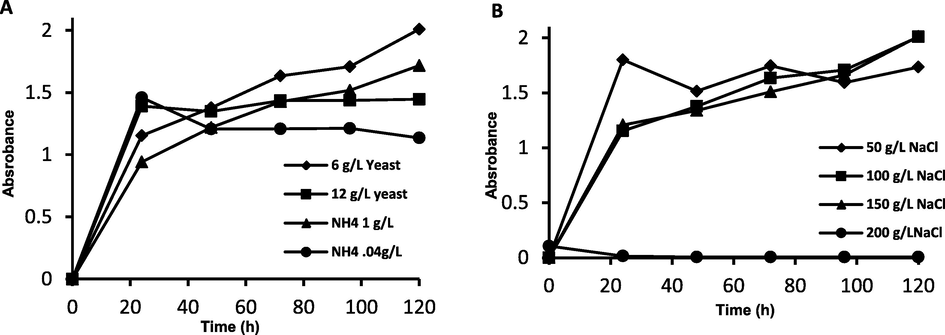
- Growth time profile of H.mediterranei A: different nitrogen sources of different concentrations and B: different NaCl concentrations.
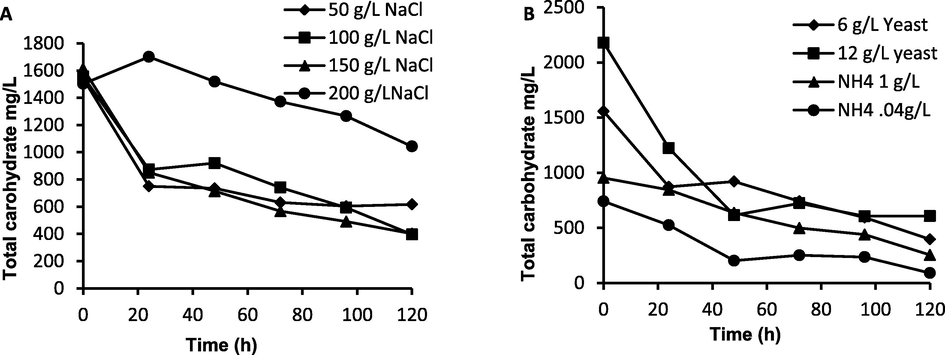
- Total carbohydrate consumed during growth time A: at different NaCl concentrations and B: at different nitrogen sources with different concentrations.
As illustrated in Fig. 2A, the media containing yeast extract (organic nitrogen source) produced a higher growth rate of H. mediterranei than ammonium chloride (inorganic nitrogen source) at concentration of 6.0 g/L. This result was supported by the high consumption of total carbohydrates when 6.0 g/L of yeast was used (∼500 mg/L) (Fig. 3B). Fig. 2B showed that the added NaCl to the H. mediterranei culture media should be maintained at 100 g/L, which is much less than the required salt concentration (22 %) for optimum extremely halophilic H. mediterranei cell growth and PHA production (Alsafadi and Al-Mashaqbeh 2017). This indicates that there was another amount of salt resulted from the neutralization of the HCl-catalyzed hydrolysis of SWW process which also contributed to the salinity of the fermentation medium. Increasing the added salt to 200 g/L caused as significant inhibition in the cell growth. This confirmed by the low carbohydrates consumption at 200 g/L NaCl concentration (Fig. 3B).
Following PHA production, H. mediterranei were harvested and PHA was extracted as described in the method part. The data for PHA yields at different NaCl concentration and different nitrogen sources are presented in Table 2.
| Nitrogen Source | NaCl (g/L) | PHA (g/L) |
|---|---|---|
| Yeast extract (6.0 g/L) | 100 | 0.53 |
| Yeast extract (12.0 g/L) | 100 | 0.52 |
| NH4Cl (1.0 g/L) | 100 | 0.02 |
| NH4Cl (0.04 g/L) | 100 | No PHA |
| Yeast extract (6.0 g/L) | 50 | No PHA |
| Yeast extract (6.0 g/L) | 150 | 0.41 |
| Yeast extract (6.0 g/L) | 200 | No PHA |
Table 2 showed that 100 g/L NaCl was the best concentration required for PHA production (0.53 g/L) when the yeast at 6 g/L was fixed during the experiments. As the NaCl concentration increased, lower PHA production obtained until no PHAs were formed when the concentration of NaCl reached 200 g/L. The results in Table 2 revealed that the highest polymer yield (0.53 g/L) at 100 g/L NaCl and 6.0 g/L yeast. These batch experimental conditions were used in the fermentation process from sesame wastewater using sequencing batch reactor system (SBR).
3.3 PHA production from sesame wastewater using sequencing batch reactor (SBR) system
The SBR system was designed in four steps including filling, reaction, settling and discharging. The aim of this experiment is to evaluate the performance of SBR system for producing PHA by H. mediterranei using SSW as carbon source. During the cultivation process samples were periodically taken for quantification of total carbohydrate, cell dry mass (CDM) and polymer concentration (PHA). Fig. 4 showed the results of SBR experiment at different feeding cycles (glucose, mix of glucose and SSW, pure SSW and glucose). Firstly, the startup phase of the SBR was initiated by adding two liters medium contained glucose as carbon source (10 g/L) in order to determine the optimal time of reaction cycle. After 167 h, another one liter glucose medium was added to the reactor to maintain the working volume three liters for the rest of SBR experiment. When the carbohydrate depleted at 213 h, the experiment was stopped for 24 h (settling step). In fact, the settling step was not completed as the settling of H. mediterranei cells was very poor. Therefore, the SBR mode has been modified to three steps (filling, reaction and discharging step). The first cycle of SSW was started at 213 hr by adding 300 mL (10 %SSW and 90 % glucose) as shown in the Fig. 3. The results showed that CDM was decreased while the PHA was highly increased to around 19 g/ L containing around 54 % of PHA. Then, 300 mL media containing 20 % SSW and 80 % glucose was treated from 319 h to 487 h. The results showed that there was a significant increase in H. mediterranei growth and the CDM increased from 32 g/L to 50 g/L, this has led to an increase in the PHA productivity from 19 g/L to 24 g/L. The highest PHA content recorded in this cycle around 75 % PHA/CDM. Then, 450 mL of SSW (100 %) was added to the rector two times at 487 h and at 518 h. This addition showed a significant decrease in the CDM (6.5 g/L) and PHA concentration (20.5 g/L) at the end of this cycle. This is suggesting that it is very difficult for H. mediterranei to treat the pure waste (SSW) without adding glucose. The inhibitory effect of SSW could be attributed to the present of high concentration toxic compounds for H. mediterranei growth, such as phenolic compounds (248 mg/L) (Isik et al. 2021). Finally, 300 mL glucose medium was added to the reactor at 559 h and treated until 583 h. In this cycle the SBR system return back to the startup stage and the CDM increased from 20 to 40 g/L and the PHA decreased to 2 g/L. This indicate that the H. mediterranei cells are not completely inhabited by SSW. The maximum PHA concentration (20.5 g/L) produced from the SBR system was higher than the reported PHA concentration from several studies that used glucose as feedstock and employed fed-batch fermentation (13.0 g/L) (Koller et al., 2015) and shake flask fermentation (5.1 g/L) (Han et al., 2015) for the production of PHA using H. mediterranei. In previous study, Phasakanona et al. 2014 had investigated the production of PHA and the treatment efficiency of the SBR system when it was used to treat synthetic domestic wastewater. The results indicated that Bacillus aryabhattai MSU 504 gave high performance of a sequencing batch reactor system and the PHA production increased when the synthetic domestic wastewater was mixed with glycerol waste. The highest PHA concentration and PHA yield were recorded at 1,086.87 mg/L and 61.42 % as dry sludge weight, respectively (Phasakanon et al., 2014). The continuous cultivation processes obtained from different strains had been introduced by previous studies. Halomonas campaniensis strain LS21 was used to produce PHAs from the sea water using open and continuous cultivation process. This process showed the advantages to decrease the cost of producing PHA due to the use of cheap substrate and the implementation of non– sterilization conditions that causes a reduction in energy consumptions (Yue et al., 2014). Tan et al. 2011 had introduced an unsterile-continuous fermentation process based on halophilic Halomonas TD01 for the production PHA. The CDM was 40 g/L containing 60 % poly(3-hydroxybutyrate) in the first fermentor with glucose salt medium (Tan et al., 2011). Another study had introduced a multistage system, constructed from five stirred tank, sorted in continuous series of reactors. The Cupriavidus necator bacteria were grown in the first stage and the fermentation liquor formed continuously fed the other reactors. This continuous multistage system had increased the volume productively of the PHAs (1.8 g L-1h−1) when it was compared with the data obtained from the continuous two-stage and in fed-batch mode (Atlić et al., 2011).
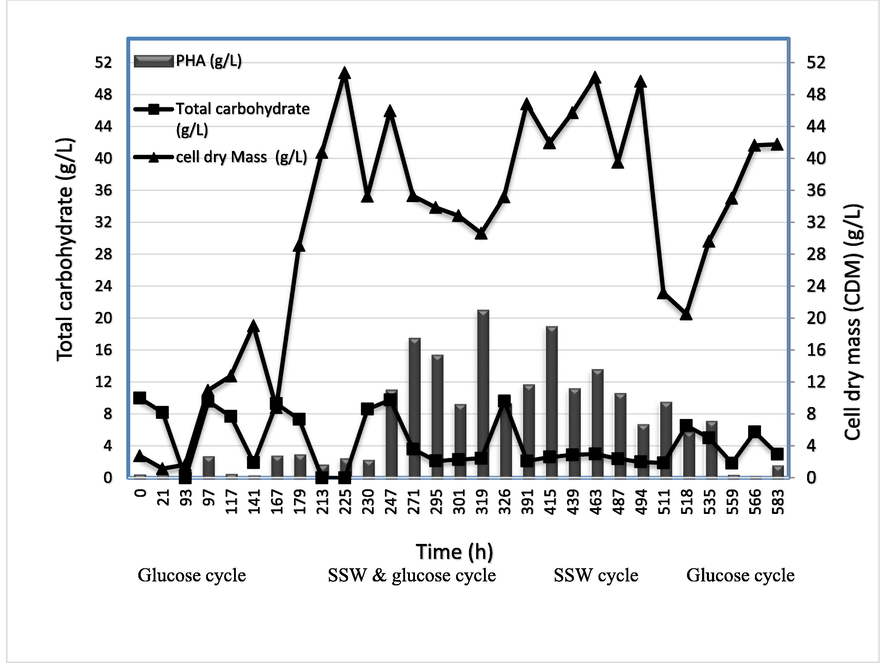
- Cell dry mass of H.mediterranei, PHA amount, and carbohydrate concentrations in sequencing batch reactor (SBR).
3.4 Characterization of PHA
3.4.1 1H NMR spectrometry
1H NMR spectroscopy was used to determine the chemical structure of the biopolymer produced from SSW by H. mediterranei. The biopolymer was extracted and purified as described in the materials and methods part. Then, the sample was dissolved in deuterated chloroform and tetramethyl silane (TMS) was used as internal reference compound for 1H NMR chemical shift (δ) assignments (δ = 0 ppm). The 1H NMR spectrum showed that the chemical structure of the polymer is 3-hydroxybutyrate-co-3-hydroxyvalerate (PHBV) (Fig. 5). The percentage of the 3 HV unit in the PHBV co-polymer was calculated as reported previously (Alsafadi et al., 2020b), and found to be 10 mol%.
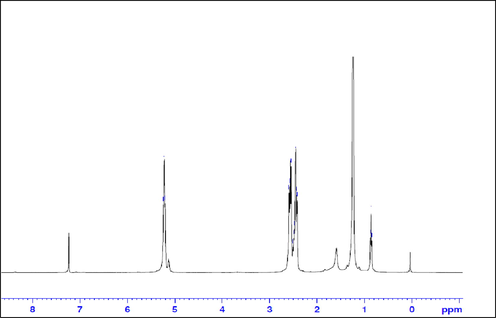
- The 1H NMR spectrum of the PHBV polymer.
The different proton environments which are corresponding to the PHBV structure are illustrated in Table 3. Similar PHBV biopolymer was also obtained from lignocellulose biomass using alkali-halophilic Halomonas alkalicola M2 (Luo et al., 2022).
| Chemical Shift (δ) ppm | Assignment |
|---|---|
| 0.86–89 | –CH3 (hydroxyvalerate residue) |
| 1.25 | –CH3 (hydroxybutyrate monomer) |
| 1.60 | –CH2 (hydroxyvalerate monomer) |
| 2.42–2.58 | Doublets –CH2 groups adjacent to carbonyls in both hydroxyvalerate and hydroxybutyrate monomers |
| 5.25 | –CH– group connect to oxygen in both hydroxyvalerate and hydroxybutyrate monomers |
3.4.2 Molar mass of biopolymer
The molecular weight of produced PHBV was obtained by GPC (material and method). The weight average molecular weight (Mw), number average molecular weight (Mn) and polydispersity (PDI) are presented in Table 4. The Mw of the PHBV was 897.0 kDa. This value was higher than that for PHBV produced by the H. mediterranei (569.5 kDa) using glucose and yeast extract as carbon source (Don et al., 2006). The polydispersity (PDI) of the produced polymer in this study is greater than the PDI produced by H. mediterranei from glucose and yeast which indicate that the chain lengths are less homogenous.
| Mw (kDa) | Mn(KDa) | PDI [Mw/Mn] | References |
|---|---|---|---|
| 897.0 | 471.0 | 1.9 | This study |
| 569.5 | 466.4 | 1.2 | Don et al., 2006 |
4 Conclusion
In the present study, real industrial SSW was evaluated as substrate for PHAs production from H. mediterranei. The effect of pre-treatment conditions, mainly acid concentration and hydrolysis time, on the composition and yield of SSW hydrolysates was studied. Different parameters such as nitrogen source and salt concentration in the SSW feed have been optimized to maximize the production of PHAs. The results of this study indicate that the production of PHA from SSW by sequencing batch reactor cultivation process of H.mediterranei can be accomplished if the auxiliary carbon source such as glucose was added to the system.
Acknowledgments
This work was supported by the Scientific Research and Innovation Support Fund in Jordan (project No. WE/2/12/2016). We thank Dr. Othman Almashaqbeh (Royal Scientific Society) for helpful discussions during the preparation of this manuscript.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Diya Alsafadi reports financial support was provided by Industrial Research and Development Fund- The Higher Council for Science and Technology in Jordan.].
References
- Increased 3HV concentration in the bacterial production of 3-Hydroxybutyrate (3HB) and 3-Hydroxyvalerate (3HV) copolymer with acid-digested rice straw waste. J. Polym. Environ.. 2016;24:98-103.
- [CrossRef] [Google Scholar]
- Biosynthesis and characterization of bacterial nanocellulose and polyhydroxyalkanoate films using bacterial strains isolated from fermented coconut water. Process Biochem.. 2022;122:224-1223.
- [CrossRef] [Google Scholar]
- A one-stage cultivation process for the production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) from olive mill wastewater by Haloferax mediterranei. N Biotechnol.. 2017;34:47-53.
- [CrossRef] [Google Scholar]
- Optimization of nitrogen source supply for enhanced biosynthesis and quality of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by extremely halophilic archaeon Haloferax mediterranei. MicrobiologyOpen.. 2020;9(8):e1055.
- [Google Scholar]
- Utilizing the crop waste of date palm fruit to biosynthesize polyhydroxyalkanoate bioplastics with favorable properties. Sci. Total Environ.. 2020;737:139716
- [CrossRef] [Google Scholar]
- Continuous production of poly([R]-3-hydroxybutyrate) by Cupriavidus necator in a multistage bioreactor cascade. Appl. Microbiol. Biotechnol.. 2011;91(2):295-304.
- [CrossRef] [Google Scholar]
- Salinity effect on production of PHA and EPS by Haloferax mediterranei. RSC Adv.. 2017;7(84):53587-53595.
- [CrossRef] [Google Scholar]
- Effects of C/N in the substrate on the simultaneous production of polyhydroxyalkanoates and extracellular polymeric substances by Haloferax mediterranei via kinetic model analysis. RSC Adv.. 2017;7(31):18953-18961.
- [CrossRef] [Google Scholar]
- Preparation and characterization of poly(hydroxyalkanoate) from the fermentation of Haloferax mediterranei. J. Biomater. Sci. Polym. Ed.. 2006;17(12):1425-1438.
- [CrossRef] [Google Scholar]
- Eaton, A. D.,Clesceri, L. S.,Rice, E. W.,Greenberg, A. E., Franson, M. A. H., 2005. Standard Methods for the Examination of Water & Wastewater. Washington, American Water Works Association (AWWA) & Water Environment Federation (WEF). https://doi.org/10.1016/j.bej.2022.108588.
- Increased production of polyhydroxyalkanoates with controllable composition and consistent material properties by fed-batch fermentation. Biochem. Eng. J.. 2019;141:35-42.
- [CrossRef] [Google Scholar]
- Biosynthesis, characterization, and hemostasis potential of tailor-made poly(3-hydroxybutyrate-co-3-hydroxyvalerate) produced by Haloferax mediterranei. Biomacromolecules. 2015;16:578-588.
- [Google Scholar]
- A promising antimicrobial bionanocomposite based poly(3-hydroxybutyrate-co-3-hydroxyvalerate) reinforced silver doped zinc oxide nanoparticles. Sci. Rep.. 2022;12:14299.
- [CrossRef] [Google Scholar]
- Biosynthesized poly (3-hydroxybutyrate-co-3-hydroxyvalerate) as biocompatible microcapsules with extended release for busulfan and montelukast. Int. J. Biol. Macromol. 2022
- [CrossRef] [Google Scholar]
- Investigation of sesame processing wastewater treatment with combined electrochemical and membrane processes. Water Sci. Technol.. 2021;84(10–11):2652-2660.
- [CrossRef] [Google Scholar]
- Polyhydroxyalkanoates: trends and advances toward biotechnological applications. Bioresource Technol.. 2021;326:124737
- [CrossRef] [Google Scholar]
- Production of polyhydroxyalkanoate (PHA) biopolyesters by extremophiles. MOJ Poly Sci.. 2017;1(2):69-85.
- [CrossRef] [Google Scholar]
- Continuous production mode as a viable process-engineering tool for efficient poly(hydroxyalkanoate) (PHA) bio-production. Chem. Biochem. Eng. Q.. 2014;28:65-77.
- [Google Scholar]
- Study on the production and re-use of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and extracellular polysaccharide by the archaeon Haloferax mediterranei strain DSM 1411.Chem. Biochem. Eng. Q.. 2015;29:87-98.
- [Google Scholar]
- Efficiently unsterile polyhydroxyalkanoate production from lignocellulose by using alkali-halophilic Halomonas alkalicola M2. Bioresour. Technol.. 2022;351
- [CrossRef] [Google Scholar]
- Metcalf,amp, Eddy, I., 2003. Wastewater engineering: treatment and reuse, fourth ed./revised by George Tchobanoglous, Franklin L. Burton, H. David Stensel. Boston : McGraw-Hill, [2003] ©2003.
- Cheese whey mother liquor as dairy waste with potential value for polyhydroxyalkanoate production by extremophilic Paracoccus homiensis. SM&T.. 2022;33:e00449.
- [Google Scholar]
- Kinetics of batch anaerobic digestion of vegetable oil wastewater. Open J. Water Pollution Treatment.. 2014;1:1-10.
- [CrossRef] [Google Scholar]
- Enhanced biosynthesis of polyhydroxyalkanoates by continuous feeding of volatile fatty acids in Haloferax mediterranei. Biochem. Eng. J.. 2022;179:108307
- [CrossRef] [Google Scholar]
- Integrating strategies for sustainable conversion of waste biomass into dark-fermentative hydrogen and value-added products. Renewable Sustain. Energy Rev.. 2021;150:111491
- [Google Scholar]
- Polyhydroxyalkanoate production from sequencing batch reactor system treating domestic wastewater mixed with glycerol waste. APCBEE Procedia.. 2014;8:161-166.
- [CrossRef] [Google Scholar]
- Effect of levulinic acid on production of polyhydroxyalkanoates from food waste by Haloferax mediterranei. Environ. Res.. 2022;214:114001
- [CrossRef] [Google Scholar]
- Resource recovery from wastewater by biological technologies: opportunities, challenges, and prospects. Front Microbiol.. 2016;7:1-23.
- [CrossRef] [Google Scholar]
- Biomedical Applications of Polyhydroxyalkanoates. Indian J. Microbiol.. 2017;57:261-269.
- [CrossRef] [Google Scholar]
- Salting-out assisted liquid–liquid extraction coupled with high-performance liquid chromatography for the determination of vitamin D3 in milk samples. R. Soc. Open Sci.. 2019;6(8):190952
- [CrossRef] [Google Scholar]
- Polyhydroxyalkanoate (PHA) synthesis by Spirulina subsalsa from Gujarat coast of India. Int. J. Biol. Macromol.. 2010;46(2):255-260.
- [CrossRef] [Google Scholar]
- Valorization of lignocellulosic biomass for polyhydroxyalkanoate production: status and perspectives. Bioresour. Technol.. 2022;360:127575
- [CrossRef] [Google Scholar]
- Unsterile and continuous production of polyhydroxybutyrate by Halomonas TD01. Bioresour. Technol.. 2011;102(17):8130-8136.
- [CrossRef] [Google Scholar]
- Fate of Salmonella during sesame seeds roasting and storage of tahini. Int. J. Food Microbiol.. 2013;163(2):214-217.
- [CrossRef] [Google Scholar]
- Free nitrous acid-based nitrifying sludge treatment in a two-sludge system obtains high polyhydroxyalkanoates accumulation and satisfied biological nutrients removal. Bioresour Technol.. 2019;284:16-24.
- [CrossRef] [Google Scholar]
- A seawater-based open and continuous process for polyhydroxyalkanoates production by recombinant Halomonas campaniensis LS21 grown in mixed substrates. Biotechnol. Biofuels.. 2014;7(1):108.
- [CrossRef] [Google Scholar]







