Translate this page into:
Profile characterization and biological activities of cold pressed Garden Cress (Lepidium sativum) seed oil
⁎Corresponding author at: University of Carthage, National Institute of Applied Sciences and Technology, LR11ES26, LIP-MB ‘Laboratory of Protein Engineering and Bioactive Molecules’, Tunis, Tunisia. l_rezig@yahoo.fr (Leila Rezig)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Lepidium sativum is cultivated mainly for the edible oil from its seeds, and considered as an unutilized and neglected crop despite its important properties. Its oil fraction is used to produce soap and stabilize linseed oil when it is mixed with wild mustard seed oil. Once converted into fatty acid methyl esters, it represents a good substitute for imported petroleum diesel after alkaline transesterification reaction. In the current study, Lepidium sativum seeds cultivated in Tunisia and the physicochemical properties and nutrient profile of its cold pressed seed oil were investigated. The antioxidant, antibacterial, and anti-inflammatory activities of the above oil were also assessed. Lepidium sativum seed oil was abundant in both linolenic (35.59 ± 1.9%) and oleic (21.14 ± 0.63%) acids, and high amounts of β-sitosterol (42.57 ± 2.96 mg/100 g), campesterol (20.04 ± 1.4 mg/100 g) and Δ 5,24 stigmastadienol (11.82 ± 0.45 mg/100 g) were detected. The total tocopherol content of Lepidium sativum seed oil reached 136.83 ± 7.6 mg/100 g with a predominance of γ-tocopherol (86.23%). Its seed oil exhibited an IC50 of 10.33 ± 0.05 mg/mL and a radical scavenging activity of 415.6 ± 40 Trolox Equivalent Antioxidant Capacity (TEAC) for the DPPH and the ABTS assays, respectively. While the thermal analysis proved a high thermal stability of Lepidium sativum seed oil, that of eight bacteria and one fungal strain showed no noticeable bacterial or antifungal effects. It was also revealed that Lepidium sativum seed oil held a remarkable anti-inflammatory activity. Hence, the obtained results evidenced remarkable chemical, antioxidant and anti-inflammatory properties of Lepidium sativum seed oil, which might potentially be promising for enhancing human health and preventing age-related diseases.
Keywords
Lepidium sativum seeds
Cold Pressed Lepidium sativum Seed Oil
Bioactive compounds
Antioxidant activity
Anti-inflammatory activity
Antimicrobial activity
- TC
-
Total Carbohydrates
- FAMEs
-
Fatty Acid Methyl Esters
- FID
-
Flame Ionization Detector
- SFA
-
Saturated Fatty Acid
- UFA
-
Unsaturated Fatty Acid
- FFAs
-
Free Fatty Acids
- ST
-
Sterols
- TMS
-
Trimethylsilyl ether sterols
- TPC
-
Total Polyphenol Content
- GAE
-
Gallic Acid Equivalents
- TFC
-
Total Flavonoïd Content
- CE
-
Catechin Equivalents
- LPS
-
Lipopolysaccharide
- NO
-
Nitric Oxide
- RSA
-
Radical Scavenging Activity
- TEAC
-
Trolox Equivalent Antioxidant Capacity
- OIT
-
Oxidative Induction Time
- MIC
-
Minimum Inhibitory Concentration
- HRBC
-
Human Red Blood Cell
- TGA
-
Thermogravimetric analyses
- FTIR
-
Fourier-transform infrared
- SEM
-
Scanning Electron Microscope
Abbreviations
1 Introduction
Garden Cress (Lepidium sativum), commonly known as Hab-alrashed in Arab-speaking countries, is a comestible plant belonging to the Cruciferae (Brassicaceae) or mustard family (Diwakar et al., 2010). Lepidium sativum is also a fast growing, glabrous, and erect annual herb that is widely cultivated in temperate countries. It is widely and diversely applied for culinary and medicinal purposes (Nehdi et al., 2012). Although the origin of Lepidium sativum is unknown till now according to Rahimi et al. (2019), it is believed to be native of Egypt and Western Asia (Diwakar et al., 2010; Nehdi et al., 2012), and to have ultimately spread to North Africa and Europe. The seeds of Lepidium sativum are basically brownish-red, oval-shaped, triangular at one end, smooth, about 3–4 mm in length and 1–2 mm in width. Lepidium sativum seeds are proven to contain 25% of protein, 14–24% of lipids, 33–54% of carbohydrates and 8% of crude fiber (Datta et al., 2011).
Although the odor of the seed oil is pungent, the whole fruits or seeds of Lepidium sativum are used, fresh or dried, as a seasoning and a condiment due to their peppery flavor, and in baking (Jansen, 2004). According to recent studies, namely Abdel-Aty et al. (2019) and Abdou Mohamed et al., (2019), Lepidium sativum seeds are intrinsically bestowed with interesting anti-carcinogenic, anti-inflammatory, hepatoprotective and antioxidant activities, and cardiovascular diseases inhibition, respectively. This inhibitory characteristics emanates from the remarkable hypolipidemic effect of the seed oil. Egyptians were reported to use Lepidium sativum seed oil mixed with wild mustard seed oil to stabilize unstable linseed oil (Diwakar et al., 2010). It is also used in soap manufacture (Zia-Ul-Haq et al., 2012). Furthermore, Nehdi et al. (2012) have demonstrated that after converting Lepidium sativum seed oil chemically via an alkaline transesterification reaction to its fatty acid methyl esters (FAMEs), their physicochemical properties were within the ASTM D6571 limits for biodiesel. Therefore, it has been proven to be a good substitute for imported petroleum diesel.
Although Lepidium sativum seed and seed oil have gained much interest in recent years, the composition, the physico-chemical properties and the biological activities of its seed oil originating from North Africa still remain fairly unexplored.
The objective of the current study is to assess the chemical composition and physical properties of cold pressed Lepidium sativum seed oil. Specifically, the fatty acid (FA) composition and phytosterol and tocopherol contents, together with the biological activities and oxidative stability were determined.
2 Material and methods
It is to be noted that the present investigation adopted either analytical reagent (AR) grade or HPLC grade by Merck KGaA, Darmstadt, Germany.
2.1 Raw material
Approximately 6 kgs of Lepidium sativum seeds were brought from a private plant nursery in the town of Menzel-Bouzelfa in the north-eastern coast of Tunisia with the following coordinates: latitude 36°40'5.3“; longitude 10°40'35.8”; elevation 50 m. Initially, the seeds were sorted out (only unbruised seeds were taken), washed to remove impurities, and eventually air-dried in a forced circulation oven at 40 ± 0.5 °C for 24 h and stored in glass jars.
2.2 Chemical composition of Lepidium sativum seeds
2.2.1 Moisture and protein content
While the percentage of moisture was calculated using (AOAC, 1990) analytical methods, that of protein was computed from the nitrogen content using the Kjeldahl conversion factor of N (%) × 5.3.
2.2.2 Ash, fat, fibre, and total carbohydrates contents
The total ash content was determined by the process of mineralising the sample seeds at 600 °C for 8 h. Soxhlet extraction with hexane as a solvent was used to calculate fat content with an extraction time of 8 h. Acid detergent fibre (ADL) were determined according to the methods of Van Soest et al. (1991). The total carbohydrates (TC) were measured as follows: TC = 100 minus moisture, protein, ash, fibre and fat percentages altogether.
2.3 Oil extraction
Lepidium sativum seeds were cold pressed in a Komet screw oil expeller DD 85 G (IBG Monforts Oekotec GmbH & Co. KG, Mönchengladbach, Germany). The seeds (2 kg) were subsequently ground and squeezed by conical screw rotation. The obtained oil was driven through a perforated tube and the meal was evacuated through a calibrated orifice. The oil was then separated from plant remains through a centrifuge (15 min). The seed oil was filtered and frozen stored at a temperature of −20 °C.
2.4 Chemical analysis
Lepidium sativum seed oil’s chemical analysis was carried out according to AOCS Official methods, 1997: Cd 3d-63 for the acid value, Cd 8-53 for the peroxide value, Cd 1-25 for the iodine value, Cd 3-25 for the saponification value, Ca 6a-40 for the unsaponifiable matter. A 10 mL pycnometer at 25 °C was used for the determination of the specific gravity and an Abbé refractometer at 40 °C for the calculation of the refractive index.
As regards the K232 and K270 values, they were measured by a Shimadzu UV-1800 spectrophotometer (Kyoto, 604-8511 Japan) as the specific extinction of a 1% solution of Lepidium sativum seed oil in cyclohexane in 1 cm cell path at wavelengths of 232 and 270 nm.
2.5 Fatty acid composition
Fatty acid composition was calculated by the analytical methods described by the 2568/91 EEC regulation (European Economic Community, 1991). Regarding the fatty acid methyl esters (FAME) analysis, it was carried out by means of agitating a solution of 0.2 g of oil and 3 mL of hexane with 0.4 mL of 2 N methanolic potassium hydroxide. A 4890D Hewlett-Packard Gas Chromatograph furnished with an HP-INNOWax fused silica capillary column (Cross-Linked PEG), 30 m × 0.25 mm × 0.25 m and a flame ionization detector (FID) was used for FAME analysis. Besides, the inlet and detector temperatures were set to 230 °C and to 250 °C. As for the oven temperature, it was first set to 120 °C for 1 min and then elevated to 240 °C at a frequency of 4.0 °C/min for 4 min. An amount of 1 µL of FAME was injected, and nitrogen was used as carrier gas at 1 mL/min. Inlet split ratio was 1:100. Peaks were detected by reference to standards.
2.6 Bioactive compounds analysis
2.6.1 Tocopherol composition
The NF EN ISO 9936:2006 method was used to determine tocopherol composition. An amount of 0.5 g of the seed oil was diluted with 5 mL of hexane, and a 5 μL sample was injected. The sample was analysed using Agilent 1100 HPLC (CA, USA) with a G 1354 quaternary pump, a G 1313 A standard autosampler, a G 13211 fluorescence detector set to λ excitation = 295 nm, and λ emission = 330 nm and a Chemstation software. A normal-phase column (hypersil silica, Hewlett Packard), with dimensions 250 mm × 4.6 mm × 5 μm, was used with hexane / isopropanol (99.5: 0.5, v/v) as a mobile phase. The system was isocratically operated at a flow of 0.5 mL /min. Separations were done at 30 °C and the quantification was based on an external standard method. Tocopherol standards mixed in hexane solution (2 mg/mL) were prepared based on α-, β-, γ-, and the δ- tocopherols.
2.6.2 Sterol analysis (ST)
NF EN ISO 12228 method (1999) was used for sterol separation. Lepidium sativum seed oil (250 mg) was refluxed during 15 min with a 5 mL ethanolic KOH solution (3%, w/v). A quantity of 1 mg of FLUKA cholesterol was added as an internal standard with a few anti-bumping granules. The compound was diluted at once using ethanol (5 mL). The unsaponifiable part was eluted through a glass column filled with an aluminium oxide slurry in ethanol (1:2, w/v) with 5 mL of ethanol and 30 mL of diethyl ether at a flow of 2 mL/min. A rotary evaporator was used to evaporate the solvent at 40 °C under reduced pressure. Ether was then completely evaporated under a nitrogen stream. Sterols were determined through a FLUKA silica gel F 254 plate which was developed in the solvent system n-hexane/diethyl ether (1:1, v/v). Similarly, for the detection of sterols, methanol was sprinkled over the thin-layer plate. Sterol bands were scratched from the plate and extracted with diethyl ether. Trimethylsilyl ether sterol derivatives (TMS) were prepared in a glass vial screw cap by adding up 100 µL of a silylating reagent, namely N-methyl-N-(trimethylsilyl) trifluoroacetamide/pyridine (1:10, v/v). The TMS were subsequently heated to 105 °C during 15 min.
Mixtures of sterol standard solutions were derivatized. The eluted compounds were cholesterol, sitosterol, stigmasterol, ergosterol and campesterol. The TMS sterol derivatives analysis was carried out using an Agilent 6890 N GC (CA, USA) equipped with an FID and the GC Chemstation software. An HP-5 ms GC column was used (0.32 mm i.d. × 30 m in length and 0.25 µm in film thickness, CA, USA). Helium (He) was used as a carrier gas with a flow of 1.99 mL/min in the split/splitless injector with a split ratio of 1:200. With respect to the detection and injection temperatures, they were set to 320 °C with an injection volume of 1 µL. Aiming to guarantee a maximal elution of all STs, all analyses were set to 71 min. It is trusty to note that the used parameters are as follows: Injection temperature was at 320 °C; Column temperature: a gradient of 4 °C/min from 240 °C to 255 °C; Sterol peaks were determined following the NF EN ISO 12228 (1999) method.
2.6.3 Total polyphenol content determination (TPC)
The identification of phenolic compounds was conducted through a 2-time extraction of an oil solution in hexane, whereby 5 g of oil was added to 10 mL of hexane along with a 10 mL methanol/water mixture (80:20, v/v). A rotary evaporator was used to remove the methanolic content at 45 °C. The obtained extract was then reduced to a concentration of 20 mg/mL in methanol. An amount of 100 µL of the 2 N Folin-Ciocalteu phenol reagent (Merck Schuchardt OHG, Hohenbrunn, Germany) was mixed with a 20 µL of the resultant extract and 80 µL of a saturated sodium carbonate solution (16%, w/v) was further added to the reaction compound in 5 min, precisely. Wavelength was set to 760 nm and assessed against a blank solution with a Multiskan GO microplate reader for 2 h. Each value was determined as mg of gallic acid equivalents (GAE) per g oil (Vazquez-Roncero et al., 1973; Gutfinger, 1981).
2.6.4 Total flavonoid content determination (TFC)
The whole flavonoid content was calculated in conformity with the Dewanto et al. method (2002). Twenty microliters of the methanolic extract (obtained as previously described in 2.6.3) was added to 16% of NaNO2 in 20 µL. Six minutes later, 40 µL of 10% AlCl3 and 100 µL of NaOH (5 M) were added. The resultant volume was set to 200 µL and mixed with distilled water. A wavelength of 510 nm was used for the absorbance reading against a blank reading with a Multiskan GO microplate reader. The total flavonoid content of the Lepidium sativum seed oil was measured as mg catechin equivalents per gram of oil (mg CE/g of oil).
2.7 Biological activities determination
2.7.1 Antimicrobial activity
The antibacterial activity of Lepidium sativum seed oil at 200 mg/mL, 500 mg/mL, and 1000 mg/mL in pure Dimethylsulfoxide (DMSO) was assessed towards four Gram-positive strains: Staphylococcus aureus ATCC 25923, Bacillus Cereus ATCC 11778, Staphylococcus aureus ATCC 6538 and Enterococcus feacium 19434 and four Gram-negative strains: Listeria monocytogenes ATCC 15313, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853 and Klebsiella pneumonia ATCC 35657. The antifungal activity was determined against one fungal species: Candida albicans ATCC 10231.
The agar diffusion disc technique was used according to Rίos and Recio (2005). A suspension of the tested microorganisms was spread on the relevant solid media plates (Luria-Bertani) and incubated at 37 °C overnight. A day later, 4–5 loops of pure colonies were transferred to sterile saline solutions in a test tube for each bacterial strain and adjusted according to the 0.5 McFarland turbidity standard (∼108 cells/mL). Sterile cotton was dipped into the bacterial suspension and the agar plates were streaked three times, each time turning the plate at a 60° angle, and finally rubbing the swab through the edge of the plate. Sterile paper discs (Glass Microfibre filters, Whatman; 6 mm in diameter) were placed onto inoculated plates and impregnated with the diluted solutions (10 µL). While Ampicillin (10 μg/disc) was used as positive control for all strains except Candida albicans for which Nystatin (100 μg/disc) was employed, the same solvent (pure DMSO) applied for the dilution of the Lepidium sativum seed oil was used as a negative control in similar conditions. Inoculated plates with discs were placed in a 37 °C incubator. Within 24 h of incubation, the results were assessed by measuring the growth inhibition zones surrounding the discs. The antimicrobial activity was then detected by the occurrence of clear inhibition zones around the discs. It should be noted that the test was conducted in triplicate.
2.7.2 Anti-inflammatory activity measurement
The anti-inflammatory effect of Lepidium sativum seed oil was assessed in-vitro through the RAW 264.7 murine macrophage cell line (ATCC) by nitrite accumulation. Cell growth was performed through 24-well plates at a density of 2 105 cells/mL for 24 h at 37 °C before being treated with various concentrations of Lepidium sativum seed oil dissolved in the DMSO or the positive control (L-NAME) for 1 h. The final concentration of solvent in the culture medium was maintained at 0.1% (v/v) to avoid solvent toxicity. Lipopolysaccharide (LPS) (100 µg/mL) was added to the treatment group of plates, while medium or LPS alone was added to the control group. Following a 24 h LPS stimulation at 37 °C under 5% CO2, the cell free supernatants were collected and Griess's reagent (1% sulfanilamide, 5% phosphoric acid and 0.1 % N-(1-naphthyl)-ethylene diamine dihydrochloride) was used to assess nitric oxide (NO) levels (Green et al., 1990). The resazurin assay protocol was used for checking the cytotoxicity of samples on cells. For the assessed concentrations (0–300 µg/mL) of Lepidium sativum sample, we found no significant cytotoxic effect. It is to worthy to note that the nitrite concentration in the samples was calculated using a sodium nitrite standard curve, and absorbance was measured at 540 nm.
2.7.3 Antioxidant activity
The antioxidant activity of Lepidium sativum seed oil was evaluated through DPPH and ABTS assays. For the DPPH assay, a Lepidium sativum seed oil sample was tested for its scavenging ability against the stable 1,1- diphenyl-2-picrylhydrazyl (DPPH) radicals (Kalantzakis et al., 2006). One millilitre of a compound of 0.5 g oil in a 5 mL of ethyl acetate was mixed with 4 mL of a 10−4 M ethyl acetate DPPH solution within a 10 mL screw cap test tube. The reaction mixture was then vortexed intensely for about 10 s. Steady-state stability was reached by keeping the tube in total darkness for exactly 30 min. Absorbance was subsequently measured at 515 nm against a blank solution. In the meantime, 1 mL of ethyl acetate was mixed with 4 mL DPPH solution for the sake of preparing and evenly measuring a control sample solution.
DPPH Radical Scavenging Activity (RSA) was determined as the DPPH concentration reduction% of the Lepidium sativum components and was calculated according to the following equation:
% [DPPH]red = 100 × (1 − [DPPH]30 /[DPPH]0), where [DPPH]0 and [DPPH]30, using DPPH concentrations in the control sample (t = 0) and in the test mixture after the 30 min reaction, respectively.
The ABTS decolorization assay was applied to measure the free-radical scavenging capacity (Re et al., 1999) with some minor adjustments. ABTS was dissolved in water to obtain a concentration of 7 mM. The ABTS•+ radical was generated by reacting a 140 mM K2S2O8 solution with this stock solution, leaving the mixture in the dark at room temperature for 12–16 h before use. ABTS•+ solution was diluted with methanol to reach an absorbance of 0.7 ± 0.02 at 730 nm. After a 2-min incubation in a glass cuvette at 30 °C, absorbance readings were taken after adding an adequate volume of the Lepidium sativum seed oil sample, with methanol as a blank, or Trolox standard, to 1 mL of diluted ABTS•+ solution. A decrease in absorbance was obtained at 734 nm. All the measurements were carried out in triplicate. The free radical scavenging capacity was calculated as ABTS•+ inhibition percentage of the Lepidium sativum seed oil sample against a Trolox standard curve (prepared at different concentrations in the range of 40–500 mM of Trolox). The results were described as mM of Trolox equivalent antioxidant capacity (TEAC, mM).
2.8 Oxidative stability
Oxidative stability was determined by the 743 Rancimat apparatus from Metrohm Co., Basel, Switzerland). Such an instrument is used to automatically detect oxidation stability of both oils and fats. We resorted to oxidative-induction time (OIT) using 3.5 g of Lepidium sativum seed oil to determine the stabilisation level. The purified airflow rate was of 10 L/h and temperature was adjusted to 100 °C. It is to be noted that volatile acids condensed in the distilled water and were conductometrically measured during the oxidation process. The induction period was described as the time required to attain the conductivity curve inflection point (Halbault et al., 1997).
2.9 Physical properties of Lepidium sativum seed oil
2.9.1 Fourier transform infrared spectroscopy (FTIR) analysis
FTIR analytical technique was used to identify the functional groups found in the Lepidium sativum seed oil sample using a Thermo Scientific Nicolet 380 FTIR spectrophotometer. The FTIR spectra recorded in the absorption band mode ranged from 500 to 4000 cm−1 with a spectral resolution of 4 cm−1 at 32 scans. It should be noted that Lepidium sativum seed oil was analysed without any prior treatment. To test repeatability, analyses were conducted in triplicate and average spectra were used.
2.9.2 Thermal analysis
Thermogravimetric (TG) curves were acquired with a TGA-50 Shimadzu analyzer. Thermogravimetric analyses (TGA) were carried out in the temperature range from 25 to 600 °C using a high-purity synthetic air (0 gas) atmosphere (100 mL/min). Alumina crucibles were used with 10 °C/min as a heating rate, and 5 mg as sample mass. For data analysis, the Shimadzu TA-60WS (2.20) software was applied to simultaneously measure the thermogravimetric (TG) and first derivative thermogravimetric (DTG) curves.
2.9.3 Rheological measurement
AR 2000ex rheometer (TA Instruments, Ltd., Crawley, UK) was used to determine oil viscosity. According to the cone-plate geometry, shear rate ranged from 0.01 to 500 s−1 with a temperature range of 20 up to 120 °C. What is trustworthy to mention is that temperature stability was attained using a Peltier-type connected water bath (±0.1 °C). The diameter, cone angle and the gap between the cone and plate were 40 mm, 1° and 200 µm, respectively (Chouaibi et al., 2012). Shear stress was calculated as a shear rate function and data were provided according to the Newtonian law model:
Furthermore, data related to viscosity dependence toward temperature were fitted to the following exponential models:
2.10 CIE L* a* b* coordinates
CieLab colour coordinates (L*, a* and b*) were immediately measured with a Lovibond Tintometer PFX-195 (UK) spectrophotometric colorimeter. The system comprises the L* value which stands for brightness, ranging from L* = 0 at the bottom of the axis (black) to L* = 100 at the top (white). While a* denotes the red and green value and varies from -a* = a shift towards green to + a* = a shift towards red, b* denotes the yellow and blue value and ranges from -b* = a change to blue to + b* = 100 a change to yellow.
2.11 Microscopic observations
Seed structural morphology, seed flour, and defatted flour of Lepidium sativum were all observed using a German Philips XL-30 ESEM scanning electron microscope (SEM). This technique allows researchers to obtain surface-sensitive images of any solid material at scales ranging from the magnifying glass (×10) to the electron microscope in transmission (×500,000 or more).
2.12 Data assessment
All the conducted experiments in the present study were carried out in triplicate. The findings were described as mean values ± standard deviations (SD).
3 Results and discussion
3.1 Chemical composition of Lepidium sativum seeds
Table 1 shows the chemical composition of Lepidium sativum seeds. Lepidium sativum L. seeds are rich in lipids (23.2 ± 0.6%), proteins (24.55 ± 0.8%), and carbohydrates (35.75 ± 0.07%). The high oil content makes the seeds suitable for its application in the oil industry. Meanwhile, higher lipid and protein contents indicate that Lepidium sativum seeds are a good source of energy (Zia-Ul-Haq et al., 2012). The high lipid content of the seeds agrees well with those previously cited by Moser et al. (2009) (22.7 ± 0.4%), Al-Jasass and Al-Jasser (2012) (23.19 ± 0.12%) and Jain et al. (2016) (23.4 ± 0.05%). The oil content of Lepidium sativum seeds was lower than those previously cited by Gokavi et al. (2004) (27.48 ± 0.14%), Nehdi et al. (2012) (26.7%), and Zia-Ul-Haq et al. (2012) (28.03 ± 1.05%). However, when compared to other edible oil seeds belonging to the same botanical family (Cruciferae), Lepidium sativum seeds are relatively poor in oil content. Values are means ± Standard Deviations (SD) of three determinations. % (w/w).
Component (%)
Moisture
2.63 ± 0.75
Crude oil
23.2 ± 0.60
Crude protein
24.55 ± 0.8
Crude fibre
8.5 ± 0.04
Total ash
5.37 ± 0.05
Total carbohydrates
35.75 ± 0.07
While protein content of Lepidium sativum seeds is in a similar range to those reported by Al-Jasass and Al-Jasser (2012) and Zia-Ul-Haq et al. (2012) with contents of 24.19 ± 0.5% and 24.18 ± 1.54%, respectively, it is higher than those cited by Jain et al. (2016) (22.81 ± 0.09%) and Gokavi et al. (2004) (22.5%).
It is worth noting that Lepidium sativum seeds are rich in total carbohydrates. In fact, the 90% of non-starch polysaccharides of this fraction was proven to greatly decrease p-cresol level, in blood, urine, and feces of individuals with autism compared to neurotypical children, which is recognized as a deleterious compound (El-Ansary et al., 2019).
Ash content indicates that the seeds are a remarkable source of minerals. The low moisture content in Lepidium sativum seeds may increase their shelf life during packaging and storage and limit fungal and bacterial infection.
Moreover, the variation in proximate composition could be attributed to differences in agronomic practices, cultivation climate, ripening phases and the eco-geological conditions of the area from which the seeds were collected.
All the above chemical qualities certainly account for the efficiency of Lepidium sativum seeds, and hence for their unrealized exploitation.
3.2 Chemical analysis
Lepidium sativum seed oil physicochemical features are elucidated in Table 2. The specific gravity value of Lepidium sativum seed oil is comparable to that found by Nehdi et al. (2012) (0.879). However, it is lower than that previously cited by Moser et al. (2009) (0.912) and Diwakar et al. (2010) in Lepidium sativum seed oils extracted by Soxhlet (0.9 ± 0.001), by cold pressing (0.91 ± 0.001) and by supercritical CO2 extractions (0.91 ± 0.001). Nonetheless, the specific gravity value is higher than that reported by Zia-Ul-Haq et al. (2012) in Lepidium sativum seed oil extracted by Soxhlet with a mixture of n-hexane/2-propanol (3:1, v/v). The iodine value of Lepidium sativum seed oil (165 ± 0.21 g I2/100g) is higher than that reported in the literature in Lepidium sativum seed oil extracted by supercritical CO2, by a hydraulic press, by solvent, and by Soxhlet (Moser et al., 2009; Diwakar et al., 2010; Yeng et al., 2017). The elevated iodine value is ascribed to an elevated amount of unsaturated fatty acids (Table 3). Hence, it is fair to say that the higher iodine value of the Lepidium sativum seed oil is clear evidence of its higher qualities as both an edible and a drying oil (Rezig et al., 2012). The high value of the refractive index (1.47 ± 0.00) affirms a significant oil unsaturation. The refractive index also provides valuable information about the purity of oils, as each oil has certain range for this parameter and any data deviation from the set specification may indicate oil adulteration (Zia-Ul-Haq et al., 2012). Values are means ± Standard Deviations (SD) of three determinations. SAFA: saturated fatty acids; MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids. Values are means ± Standard Deviations (SD) of three determinations.
Parameter
Refractive index (40 °C)
1.47 ± 0.00
Specific gravity (25 °C)
0.898 ± 0.08
Acid value (mg KOH/g oil)
0.56 ± 0.01
Saponification value (mg KOH/g oil)
179 ± 0.45
Iodine value (g I2/100 g)
165 ± 0.21
Peroxide value (meq O2/kg oil)
2.4 ± 0.03
Unsaponifiable matter (%)
1.7 ± 0.01
K232
1.92
K270
0.71
IC50 (mg/mL)
10.03 ± 0.05
Oil Stability index (h)
26.7 ± 0.67
Fatty acids
Composition
Palmitic C 16:0
9.45 ± 0.39
Stearic C 18:0
2.38 ± 0.56
Oleic C 18:1
21.14 ± 0.63
Linoleic C 18:2
10.89 ± 0.95
Linolenic C 18:3
35.59 ± 1.90
Arachidic C 20:0
3.1 ± 0.40
Eicosenoic C 20:1
10.3 ± 0.45
Erucic C 22:1
3.7 ± 0.02
Nervonic C 24:1
3.45 ± 0.10
SAFA
14.93 ± 0.07
MUFA
38.59 ± 1.08
PUFA
46.48 ± 0.09
It is to be noted that the acid and peroxide values of Lepidium sativum seed oil were low, and even lower than those determined by the Codex Alimentarius (1982). This postulates permitted maximum acid and peroxide values of 10 mg KOH/g oil and 10 meq of O2/kg oil, respectively for vegetable oils. The lower acidity of the Tunisian Lepidium sativum seed oil indicates that it is edible and might have a long shelf life. In fact, a higher acidity is related to the presence of Free Fatty Acids (FFAs), which are much more susceptible to oxidation than the fatty acids present in the triacylglycerol molecules (Rezig et al., 2019). The peroxide value suggests that the oil started to degrade since it was identified as a highly unsaturated oil. The specific extinction coefficients taken at 232 and 270 nm were 1.92 and 0.71. These values reveal that Lepidium sativum seed oil contains primary (hydroperoxides) and secondary oxidation products.
For the unsaponifiable matter, low content was observed in the examined Lepidium sativum seed oil. The saponification value was calculated as 179 mg KOH/g of oil, which is accordance with those cited by Zia-Ul-Haq et al. (2012) and Diwakar et al. (2010) on Lepidium sativum seed oil extracted by Soxhlet (179 ± 0.3 mg KOH/g) and by a hydraulic press (178.85 ± 0.46 mg KOH/g), respectively. Saponification value was, however, higher than that of Lepidium sativum seed oil extracted by supercritical carbon dioxide (174 ± 0.82 mg KOH/g) and lower than that extracted by Soxhlet (182.23 ± 0.73 mg KOH/g) (Diwakar et al., 2010). Lepidium sativum seed oil showed an unsaponifiable matter content within the range of that reported by Diwakar et al. (2010) in cold pressed Lepidium sativum seed oil (1.69 ± 0.24%), but lower than that reported by Diwakar et al. (2010) in Lepidium sativum seed oil extracted by Soxhlet (1.39 ± 0.1%) and by supercritical carbon dioxide extraction (1.16 ± 0.3%). The unsaponifiable matter contains many olefinic compounds including pigments, such as chlorophylls and carotenoids, and other heterocyclic compounds present in Lepidium sativum seed oil. When compared to other vegetable oils, the unsaponifiable matter of Lepidium sativum seed oil is higher than those of sesame (1.2%), white melon (1.1%), corn (0.92%), cotton (0.52%), palm (0.34%), peanut (0.33%), palm kernel (0.22%), coco kernel (0.09%), Cucurbita pepo var. ‘Essahli’ (1.32%), Citrullus lanatus var. ‘Crimson’ (1.04%), and Cucumis melo var. ‘Ananas’ (1.14%) (Rezig et al., 2019; Musara et al., 2020).
3.3 Fatty acid composition
Table 3 illustrates the Lepidium sativum seed oil fatty acid composition analyzed by gas chromatography. We analyzed nine fatty acids, six of which proved to be unsaturated. In terms of content, the most significant acids were linolenic (35.59%) and oleic acids (21.14%) followed by linoleic acid (10.89%), eicosenoic acid (10.3%) and palmitic acid (9.45%). The high contents of linolenic and linoleic acids and the distribution of saturated (14.93%), unsaturated (38.59%), and polyunsaturated fatty acids (46.48%) are proven to accord well with those found in previous studies conducted on Lepidium sativum seed oil (Diwakar et al., 2010; Al-Jasass and Al-Jasser, 2012; Zia-Ul-Haq et al., 2012).
It is worth noting that the oleic acid content of the Lepidium sativum seed oil is in the same range of that cited by Zarrouk et al. (2019) on Milk thistle seed oil extracted from Tunisian seeds collected from Sousse (21.39 ± 0.02%) and Bizerte (19.03 ± 0.01%) regions but lower than those reported on olive (48.22 ± 0.03% to 76.13 ± 0.2%) and argan (45.05 ± 0.19% to 45.75 ± 0.28%) oils. Oleic acid is a bioactive nutrient that has been shown to cross the blood–brain barrier. Therefore, it helps to prevent and/or treat neurological disorders, such as Alzheimer's and Parkinson's disease, which become more common as people get older (Vatassery et al., 2004; Mitchell et al., 2009). This finding is in good agreement with that obtained by Debbabi et al. (2016) on the protective effect of oleic acid on 7-ketocholesterol (7KC)-induced mitochondrial and peroxisomal dysfunction in murine microglial BV2 cells. Such a lipid peroxidation product (7KC) may be increased in the body fluids and tissues of patients with age related diseases, including neurodegenerative diseases, and trigger microglial dysfunction involved in neurodegeneration (Anderson et al., 2020; Nury et al., 2021).
In terms of erucic acid content, which is common among the members of the Brassicaceae family, the latter was less than 5% and conforms to the World Health Organization norms for erucic acid content in edible oils (Diwakar et al., 2010).
The fatty acid composition of Lepidium sativum seed oil is interesting from the nutritional point of view for its higher contents of unsaturated fatty acids, especially in ω-3 fatty acid which is beneficial in protecting against neurodegenerative disease.
3.4 Bioactive compounds analysis
3.4.1 Tocopherols
Seed oils are a potential source of tocopherols that are known to be highly effective in preventing oxidative deterioration of polyunsaturated fatty acids in most plants thanks to their natural lipophilic and antioxidant properties. The tocopherol composition of Lepidium sativum seed oil obtained by HPLC is shown in Table 4. The major components were γ-tocopherol accounting for about 86.23% of the total tocopherols, followed by δ-tocopherol, β-tocopherol, and α-tocopherol. These results agree well with those found by Diwakar et al. (2010) in Lepidium sativum seed oils extracted with a hydraulic press, by Soxhlet using petroleum ether as solvent and by supercritical carbon dioxide in which γ-tocopherol represented 80.4%, 80.9%, and 80.3% of total tocopherols, respectively. In our study, γ-tocopherol content was lower than those found by Moser et al. (2009) (79.04%) in Lepidium sativum seed oil extracted by Soxhlet with hexane. With respect to other Mediterranean vegetable oils, the Lepidium sativum seed oil exhibited a higher content of γ-tocopherol. In fact, according to Zarrouk et al. (2009), the γ-tocopherol content ranges from 5 ± 1 mg/kg to 22 ± 1 mg/kg for olive oil, from 84 ± 4 mg/kg to 92 ± 7 mg/kg for Milk thistle seed oil, and from 12 ± 1 to 434 ± 7 mg/kg for argan oil. Given its ability to cross the blood brain barrier, γ-tocopherol, which is found at elevated levels in cold pressed Lepidium sativum seed oil, could be useful to attenuate the toxic effects of 7KC. Such hypothesis is confirmed by Debbabi et al. (2016) who have proven that γ-tocopherol is able to impair 7KC-induced loss mitochondrial transmembrane potential associated with increased permeability to propidium iodide. The latter is an indicator of plasma membrane damages and murine microglial BV-2 cell death. This isomer is also able to prevent the decrease in ABCD3 protein levels, which allows the evaluation of peroxisomal mass, and in mRNA levels of ABCD1 and ABCD2, encoding two peroxisomal transporters involved in peroxisomal β-oxidation.
Tocopherol
Composition
α-Tocopherol
0.43 ± 0.1
β-Tocopherol
6.8 ± 1.2
γ-Tocopherol
118 ± 3.4
δ-Tocopherol
11.6 ± 2.9
Total
136.83 ± 7.6
Sterol
Composition
Cholesterol
2.09 ± 0.06
24-methylenecholesterol
0.21 ± 0.01
Stigmasterol
2.14 ± 0.13
Sitosterol
42.57 ± 2.96
Sitostanol
0.81 ± 0.1
Campesterol
20.04 ± 1.4
Campestanol
0.34 ± 0.01
Clerosterol
0.4 ± 0.02
Δ 7-Campesterol
0.45 ± 0.15
Δ 5,24 Stigmastadienol
11.82 ± 0.45
Δ 5-Avenasterol
1.03 ± 0.11
Δ 7-Avenasterol
0.48 ± 0.02
Δ 7-Stigmastenol
0.12 ± 0.05
Total
82.5 ± 2.97
The low content of α-tocopherol (0.31%) is in compliance with that obtained in a previous study conducted by Nehdi et al. (2019) on Lepidium sativum seed oil, in which this isomer represented 0.45% of total tocopherols. Furthermore, α-tocopherol was found in higher amounts in Lepidium sativum seed oil extracted by Soxhlet using a mixture of n-hexane/2-propanol (3:1, v/v) (12.3%) (Zia-Ul-Haq et al., 2012). It is worth mentioning that β-tocopherol was not detected in Lepidium sativum seed oil extracted by a hydraulic press, by Soxhlet and by supercritical carbon dioxide (Moser et al., 2009; Diwakar et al., 2010). According to Fatnassi et al. (2009), when compared to other tocopherols, the α-tocopherol is highly approved for both humans and animals thanks to its higher biological activity, yet γ-tocopherol shows a higher antioxidant capacity than α-tocopherol. Total tocopherol content in Lepidium sativum seed oil (136.83 ± 7.6 mg/100 g) is higher than those reported by Diwakar et al. (2010) but lower than that found by Moser et al. (2009) (179.9 ± 0.9 mg/100 g).
3.4.2 Sterols
Table 4 illustrates sterol composition of Lepidium sativum seed oil obtained by GC-FID. Thirteen phytosterols were identified: cholesterol, 24-methylenecholesterol, stigmasterol, sitosterol, sitostanol, campesterol, campestanol, clerosterol, Δ7-campesterol, Δ 5,24-stigmastadienol, Δ5-avenasterol, Δ 7-avenasterol and Δ7-stigmastenol. The β-sitosterol accounted for 42.57 mg/100 g and had the highest sterol content, followed by the campesterol (20.04 mg/100 g), and the Δ 5,24- stigmastadienol (11.82 mg/100 g). Thus, β-sitosterol, campesterol, and Δ 5,24- stigmastadienol were the major sterols, and together they made up 90.21% of Lepidium sativum seed oil. It is noteworthy to say that β-sitosterol was also the sterol marker in Lepidium sativum seed oil extracted by Soxhlet with hexane and represented only 40.38% of total sterols. This phytosterol was followed in descending order by campesterol, avenasterol, cholesterol, stigmasterol, dihydrolanosterol, and β-amyrin. Cholesterol, sitosterol, and stigmasterol contents were higher than those found in the present study (Moser et al., 2009).
The Lepidium sativum seed oil overall phytosterol content (0.825 mg/g) was lower than those cited by Zarrouk et al. (2019) in olive oil (1726 ± 45 mg/kg to 2936 ± 59 mg/kg), Milk thistle seed oil (5088 ± 71 mg/kg to 5891 ± 118 mg/kg), and argan oil (1881 ± 25 mg/kg to 2330 ± 79 mg/kg). Along with the effect of the intake of phytosterols on the development of neurodegenerative diseases, these bioactive nutrients have been recently reported to possess anti-neurodegenerative effects (Cheng et al., 2014; Dierckx et al., 2019). This has been confirmed by treating neuroinflammation which is a common pathological feature of many neurodegenerative diseases, namely Alzheimer and Parkinson (Ransohoff, 2016; Russo and McGavern, 2016; Subhramanyam et al., 2019).
It is interesting to note that brassicasterol is absent in Lepidium sativum seed oil, which typically comprises 5–20% of phytosterols in oils from other plants in the Brassicaceae family, such as rapeseed, canola, and mustard oils (Kochhar, 1983; Phillips et al., 2002).
3.4.3 Total polyphenol and flavonoid contents
Thanks to their diverse chemical structures, phenolic phytochemicals have the potential to protect cellular components against oxidation (Bahloul et al., 2014). In the same way, polyphenols are known for not only their potential health virtues, but also their capacity to prevent several chronic diseases, such as cancer and high blood pressure (Liara et al., 2013). It has also been reported that naturally synthesized polyphenols contribute to the effective fighting of neurodegenerative disorders such as brain aging (Sarubbo et al., 2018). So far, no previous studies have investigated the total polyphenol and flavonoid contents of Lepidium sativum seed oil. The total polyphenol content (TPC) of Lepidium sativum seed oil obtained by the Folin-Ciocalteu method was of 0.23 ± 0.00 mg GAE/g. Zia-Ul-Haq et al. (2012) and Kasabe et al. (2012) have reported TPCs of 120.26 ± 1.52 mg CE/g and 21 mg GAE/g, respectively, in the methanolic extract of Lepidium sativum seeds. Ait-yahia et al. (2018) have also reported a TPC of 1.629 ± 0.13 mg GAE/g in n-butanol Lepidium sativum seed extract.
According to Ismail et al. (2010), flavonoids are the most common and most widely distributed group of plant phenolic compounds, which are usually considered as very effective antioxidants. The total flavonoid content of Lepidium sativum seed oil obtained according to Dewanto et al. method (2002) was of 0.78 ± 0.02 mg CE/g. This value was higher than that found by Różańska et al. (2018) when investigating extra virgin olive oil (26.6 ± 13.5 mg/kg).
3.5 Biological activities of Lepidium sativum seed oil
3.5.1 Antimicrobial activity
The antimicrobial activity of the Lepidium sativum seed oil was assessed by the agar diffusion disc technique according to Rίos and Recio (2005). Our results showed that the solvent (pure DMSO) employed in the dilution of the Lepidium sativum seed oil did not have any antibacterial capacity against all tested strains. Likewise, Lepidium sativum seed oil had no inhibitory effect on all tested strains. Such a result is in agreement with that found in the research work of Getahun et al. (2020) who have substantiated that the seed oil of the same plant originating from India and extracted with petroleum ether in a Soxhlet apparatus have not any activity toward Gram – (Escherichia coli ATCC 25922 and Klebsiella pneumoniae ATCC 10031) and Gram + (Saphylococcus aureus ATCC 25923 and Bacillus cereus ATCC 14579) bacteria. However, our findings are not in accordance with those cited by Alqahtani et al. (2018) on Lepidium sativum seed oil extracted by Soxhlet using petroleum ether as solvent. In fact, the results related to the Lepidium sativum seed oil antimicrobial activity against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Salmonella enterica, Klebsiella pneumonia and Candida albicans showed that the latter were susceptible to Lepidium sativum seed oil. For all mentioned bacteria and fungi, the minimum inhibitory concentration (MIC) was 47.5 mg/mL, except for Salmonella enterica that revealed a higher MIC of 90 mg/mL. The minimum bactericidal concentration of Lepidium sativum seed oil was found to be equivalent to 100 mg/mL for inhibiting the growth of all bacteria and fungi. Alqahtani et al. (2018) have confirmed the findings of Abo El-Maati et al. (2016) related to the antimicrobial activity of Lepidium sativum seed oil in terms of its spectrum against all tested microorganisms except for Staphylococcus aureus which was proven to be resistant to the used Lepidium sativum seed oil. When compared to other studies conducted on the determination of the MIC of Lepidium sativum seed oil toward bacterial and fungal strains, the variations in MIC values may be attributed to the differences in the adopted antimicrobial activity technique and the used oil extraction procedure (Alqahtani et al., 2018). The impact of the method used for the evaluation of the antimicrobial activity on the results was also consolidated by the findings of Boyanova et al. (2005).
Differences in the antimicrobial activity of Lepidium sativum seed oil is likely to emanate from the sensitivity of the strains depending on the inability of the antimicrobial agent present in the oil to diffuse uniformly into the agar. It is possible that Lepidium sativum seed oil's inefficiency is due to its low TPC. In fact, many studies have proven the positive correlation between the phenolic compounds content and the antimicrobial activity (Brahmi et al., 2020). According to Cowan (1999), polyphenols such as tannins and flavonoids, are powerful antimicrobial substances. The number and position of hydroxyl group in the aromatic ring of these compounds can also enhance their toxicity to the microorganisms. Furthermore, lipophilic flavonoids can damage microbial membranes by increasing lipid fluidity. It is worth noting that the antimicrobial activity is dependent not only on the presence of phenolic compounds, but also on the existence of numerous secondary metabolites.
3.5.2 Anti-inflammatory activity
The anti-inflammatory effect of Lepidium sativum seed oil was assessed in-vitro through the RAW 264.7 murine macrophage cell line (ATCC) by nitrite (NO) accumulation (Fig. 1). NO production was reported as a principal mediator playing an essential role in various physiological and pathophysiological processes. Meanwhile, the excess in NO may contribute to tissue injury in inflammatory diseases. Indeed, it can react with superoxide radicals to form the harmful peroxynitrite. Consequently, the prevention of NO generation may be an interesting strategy to treat various inflammatory disorders (Bourgou et al., 2010). The results illustrated in Fig. 1 are expressed as quantity of NO production in LPS-stimulated Raw 264.7 macrophages. Our results proved that LPS treatment triggered significant nitrite accumulation in control cells. Interestingly, NO production was significantly inhibited by Lepidium sativum seed oil fraction treatments. In fact, cold pressed Lepidium sativum seed oil was found to decrease NO release in a concentration-dependent manner. Such a result is in agreement with that cited by Alqahtani et al. (2018) on the anti-inflammatory activity of Lepidium sativum seed oil extracted by Soxhlet using petroleum ether as a solvent. These authors have demonstrated the presence of a concentration-dependent protection of the human red blood cell (HRBC) membrane by Lepidium sativum seed oil. The observed levels of oil protection were 21%, 11% and 7% for respective concentrations of 300, 200 and 100 µg/mL. When compared to a standard drug (diclofenac Na), the inhibition percentages of hemolysis were significantly higher, showing 65%, 63%, and 61% membrane stabilization at concentrations of 300, 200, and 100 mg/mL, respectively. When tested at 75 µg/mL, 150 µg/mL and 300 µg/mL, Lepidium sativum seed oil decreased NO production by macrophages by 56%, 66% and 69%, respectively. The IC50 value was of 60 µg/mL, indicating an interesting anti-inflammatory activity of Lepidium sativum seed oil.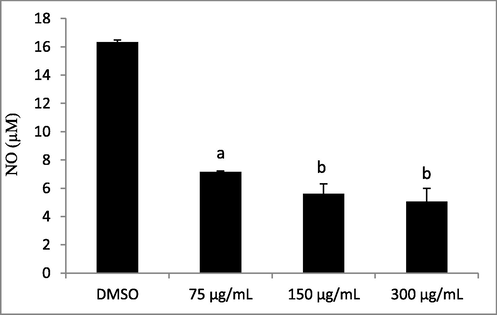
Effect of Lepidium sativum seed oil on LPS-induced NO production in RAW 264.7 macrophages. Different letters on the top of data bars indicate significant differences (p < 0.05) between mean values (±SD, n = 3).
Furthermore, Bourgou et al. (2019) have reported strong anti-inflammatory activities for carvi oils extracted with 2-methyltetrahydrofuran (MeTHF) and supercritical-carbon dioxide (Sc-CO2) with IC50 values of 27 µg/mL and 28 µg/mL, respectively. The higher anti-inflammatory activity of oil was ascribed to the effect of some active compounds, namely phenolics and sterols. It has been documented that incubating inflammatory cells with total phenolic fraction or isolated compounds from olive oil reduced anti-inflammatory enzyme activities, resulting in a reduction in NO production (Aparicio-soto et al., 2014; Cardeno et al., 2014). Besides, sterols may have a protective effect on certain mediators associated with the production of inflammatory damage. In fact, β-sitosterol has been shown to reduce NO release in activated macrophages, which has been linked to iNOS activity impairment (Moreno, 2003; Montserrat-de la Paz et al., 2012).
3.5.3 Antioxidant activities
ABTS and DPPH assays were used to assess the antioxidant activity of Lepidium sativum seed oil. The ABTS test proved that the radical scavenging activity of Lepidium sativum seed oil was of 415.6 ± 40 TEAC. To the best of our knowledge, the ABTS assay has not been used before to determine the antioxidant activity of Lepidium sativum seed oil. For the DPPH assay, the Lepidium sativum seed oil showed a considerable IC50 value of 10.03 ± 0.05 mg/mL. The Lepidium sativum seed oil antioxidant activity was higher when compared with those found in earlier literature reports (Alqahtani et al., 2018; Getahun et al., 2020) in which Lepidium sativum from Saudi Arabia and India were examined using the DPPH assay. In fact, the IC50 values of the seed oils extracted using Soxhlet apparatus with petroleum ether as solvent were of 40 mg/mL and 23.5 mg/mL for Saudi Arabian and Indian plants, respectively. Getahun et al. (2020) have described differences in the antioxidant activity to variations in seed oil compositions. It is worth noting that the IC50 value of the seed oil from the Tunisian plant is likewise lower than that found by Umesha and Naidu (2013) (23.5 mg/mL) in cold pressed Lepidium sativum seed oil. It is also well documented that the antioxidant activity is generally related to the amount and nature of phenolic compounds. In addition, an identical correlation is recorded between the flavonoid content and the DPPH assay (Brahmi et al., 2020). According to Górnaś et al. (2015), differences in the seed oils’ DPPH radical scavenging activity may also be attributed to total tocochromanol content.
3.6 Oxidative stability
Table 2 presents the Rancimat test result, indicating that the Lepidium sativum seed oil oxidation stability described as the oxidation induction time was of 26.7 h. The value was higher than that found by Nehdi et al. (2019) for Lepidium sativum seed oil extracted by the Soxhlet apparatus with hexane. Indeed, the Lepidium sativum seed oil exhibited an oxidation induction time of only 3.3 h, which is a low value that may be attributed to differences in operating conditions (temperature and air flow rate of 110 °C and 20 L/h, respectively), seed oil extraction technique and total tocopherol content (31.1 mg/100 g). Aparicio et al. (1999) followed a linear regression model for the ratio of Oleic to Linoleic Acids and the tocopherol/phenolic content in virgin olive oil. Their model revealed an adequate interrelation with the oxidative stability calculated by the Rancimat test. Phenolic and orthophenolic compounds had an impact on the olive oil oxidation stability by about 51% and 24% for fatty acids and to lesser rates on α-tocopherols, carotenoids and chlorophylls.
3.7 Physical properties of Lepidium sativum seed oil
3.7.1 FTIR analysis
The importance of IR spectroscopy in molecular structure identification is related to the rich information content and to the ability to assign certain absorption bands to functional groups. Most of the bands and shoulders of the spectrum in fats and oils may be accredited to specific functional groups (Bendini et al., 2007). Since triglycerides are the main components of fats and oils, the latter dominate the fat and oil spectra.
Fig. 2 depicts the Fourier-transform infrared (FT-IR) spectrum of cold pressed Lepidium sativum seed oil. The FTIR characteristic bands of the oil sample are detected in the 3010–2800, 1750–1000, and 1000–650 cm−1 regions. Such a finding is consistent with previous studies conducted by Nehdi et al. (2012) and Mulla et al. (2018) on Lepidium sativum seed oil extracted by hexane in a Soxhlet apparatus and petroleum ether, respectively. A band is marked at 3008.66 cm−1, indicating the C = C-H stretching (asymmetry) cis olefinic groups, while bands at 2921.68 and 2852.1 cm−1 are assigned to asymmetric and symmetric stretching vibration of the aliphatic CH2 groups, respectively. The absorption bands of Lepidium sativum seed oil at 1742.81 and 1462.9 cm−1 are associated with the ester carbonyl stretching (C = O) of fatty acids and C-H bending (scissoring) of CH2 and CH3 groups, respectively. In addition, the band at around 1377 cm−1 (1376.41 cm−1) is attributed to C-H bending of CH2 group, whereas those around 1162 cm−1 (1159.1 cm−1) and 1099 cm−1 (1097.81 cm−1) could represent the antisymmetric stretching variation of C-O. A minor band at 720.03 cm−1 represents the C-H rocking of the CH2 group.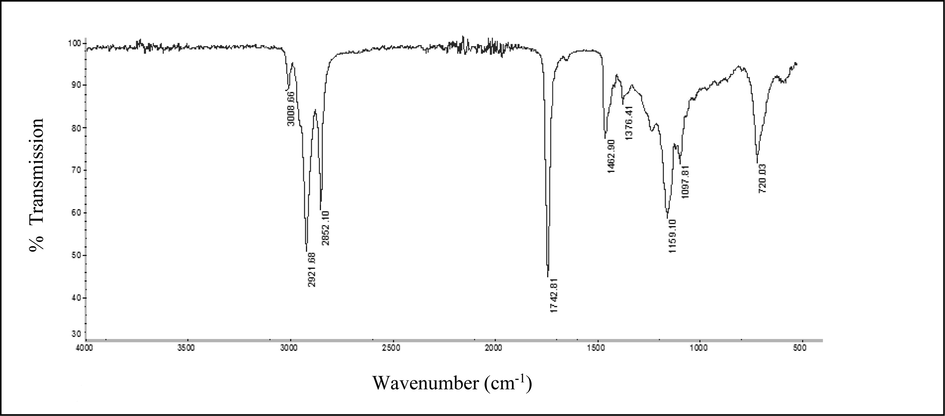
FTIR spectrum of cold pressed Lepidium sativum seed oil at frequency range of 4000–500 cm−1.
3.7.2 Thermal analysis
The Thermogravimetric analysis (TGA) is a useful tool to investigate several physico-chemical properties related to the oil behavior with respect to oxidative stability. Fig. 3 shows the TG and DTG curves of cold pressed Lepidium sativum seed oil. The thermal oxidative degradation processes for the assessed oil occurs as mainly three consecutive steps of mass loss in the temperature range between 180 °C and 600 °C. The first step of degradation begins at 180 °C and ends at 410 °C. As can be seen from the DTG curve, an exothermal peak occurs at about 360 °C (352.06 °C). The second mass-loss step occurs from 410 °C to 500 °C and describes an exothermal peak at approximately 440 °C (436.34 °C). This peak is followed by a final exothermal mass-loss step occurring between 500 °C and 600 °C and presenting an exothermal peak at about 550 °C (543 °C). According to Santos et al. (2004), the first degradation event is due to polyunsaturated fatty acid decomposition and is considered as the most important step to determine the edible oil thermal stability order. In fact, during heating, triglycerides produce volatile compounds that are constantly removed by vapor during heating. The products are mainly formed by the thermal reactions of the unsaturated fatty acids. The second thermal degradation event is reported to monounsaturated fatty acids decomposition. The last degradation event is attributed to saturated fatty acids thermal decomposition. Ciprioti (2014) reported the final exothermal mass-loss step to the combustion of the residual carbonaceous material from the previously occurring steps. The same thermal behavior was observed for Lepidium sativum seed oil extracted by Soxhlet with hexane (Nehdi et al., 2019), for rambutan (Nephelium lappaceum L.) seed fat, and for commercial olive, soybean, rapeseed, corn, and rice oils (Santos et al., 2004). It is worthwhile to note that the temperature corresponding to 5% of Lepidium sativum seed oil mass loss was recorded at around 300 °C, which accords well with the findings of Nehdi et al. (2019) for Lepidium sativum seed oil extracted by the Soxhlet apparatus (299 °C). When compared to other edible oils examined under the same operating conditions, Lepidium sativum seed oil was proven to exhibit the highest thermal stability followed in descending order by olive (285 °C) and sunflower (277 °C) oils. Nehdi et al. (2019) attributed the Lepidium sativum seed oil high thermal stability to its high antioxidant content and the presence of long-chain monounsaturated and saturated fatty acids. Such a finding is consolidated by that of Santos et al. (2004) who states that these oils doped with artificial antioxidants are more stable than non-doped oils.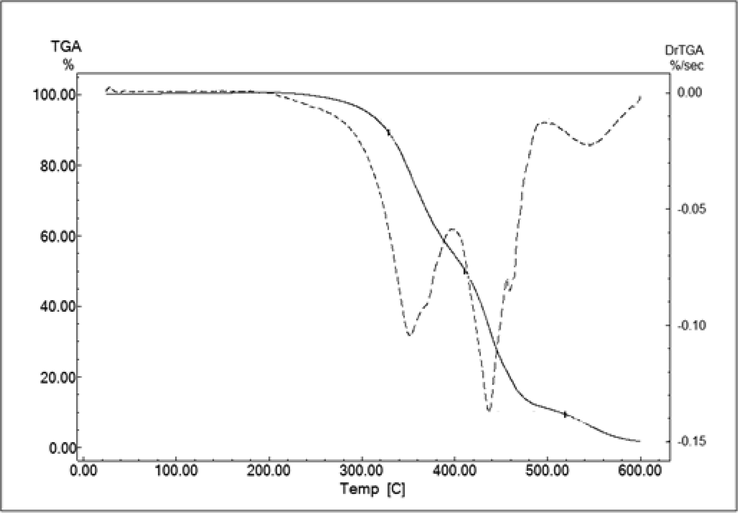
TG and DTG curve illustrations of Lepidium sativum seed oil.
3.7.3 Rheological measurement
Lepidium sativum seed oil exhibited Newtonian behavior at shear rates ranging from 0.01 to 500 s−1 at 20 °C (Fig. 4) and even at 40 °C, 60 °C, 80 °C, 100 °C, and 120 °C (data not shown). In fact, shear stress and shear rate were found to have a linear relationship. The Newtonian law model was used to describe the flow curves of the investigated Lepidium sativum seed oil sample. Based on the determination of R2 value, the Newtonian model was noted to be suitable for the rheology description of Lepidium sativum seed oil regarding flow curves (R2 > 0.9). The rheological parameters of this model at 20 °C are illustrated in Table 5. These results are in accordance with those previously obtained by Maskan (2003), Kim et al. (2010), Chouaibi et al. (2012), and Gharsallah et al. (2021). These researchers have ascertained that vegetable oils are Newtonian liquids possessing high viscosity thanks to the ability of their molecules to form long chains. Table 5 explains how Lepidium sativum seed oil viscosity value
adjusted to 20 °C (58.42 ± 0.04 mPa.s) was lower than Moringa seed oil (97.11 ± 0.02 mPa.s) (Gharsallah et al., 2021) and those of olive (76.22 ± 0.02 mPa.s), Corn (63.27 ± 0.06 mPa.s), soybean (63.23 ± 0.06 mPa.s), groundnut (69.04 ± 0.03 mPa.s), jujube seed (80.51 ± 0.02 mPa.s), and rapeseed (71.52 ± 0.02 mPa.s) oils. Yet, the value was closer to the sunflower oil (59.17 ± 0.03 mPa.s) (Chouaibi et al., 2012). Kim et al. (2010) have stated that although oil viscosity is positively influenced by the oleic acid content, it is negatively affected by linoleic acid content.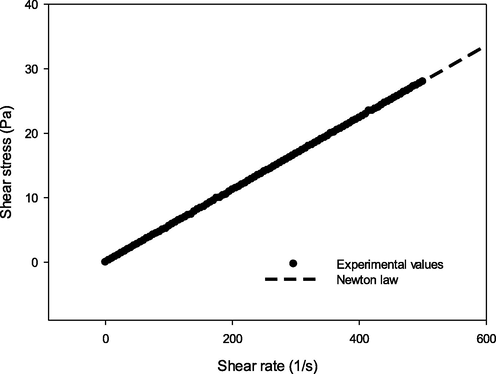
Shear stress versus shear rate of Lepidium sativum seed oil at 20 °C.
Laws
Parameters
Newton
= 58.42 ± 0.04 mPa.s
R2 = 0.99
Exponential
a = 91.37 mPa.s
b = 1.66 10−2 K−1
R2 = 0.97
Arrhenius
= 1.87 10−4 ± 0.01 mPa.s
Ea = 27.7 ± 0.03 kJ/mol
R2 = 0.98
It is fair to say that a thorough understanding of the rheological properties of natural vegetable oils is important when it comes to lubrication. Fig. 5 depicts viscosity vs temperature data pertaining to the Lepidium sativum seed oil. The findings showed that the investigated Lepidium sativum seed oil underwent an exponential decrease in viscosity (
with an increase in temperature ranging from 20 °C to 120 °C. A decrease in vegetable oil viscosity is observed when molecular distance increases and/or when intermolecular bonds decline due to a significant thermal motion of molecules (Chouaibi et al., 2012). Such phenomenon is fully consistent with previous research works conducted by Santos et al. (2005) and Kim et al. (2010). The temperature effect on Lepidium sativum seed oil viscosity was evaluated by applying the exponential and Arrhenius models. The models were found to fit apparent viscosity evolution against temperature with R2 values of 0.97 and 0.98, respectively (Table 5). Calculating activation energy (Ea) value can be carried out by plotting ln
vs 1/T in a graph (data not shown). In general, high activation energy suggests the viscosity - temperature stability of vegetable oils (Chouaibi et al., 2012). Table 5 shows the activation energy of the Lepidium sativum seed oil (27.7 ± 0.03 kJ/mol). Such a value surpasses the results found by Chouaibi et al. (2012) for corn, soybean, sunflower, and groundnut oils with respective values of 20.39 ± 0.01 kJ/mol, 20.21 ± 0.01 kJ/mol, 20.07 ± 0.03 kJ/mol, 20.78 kJ/mol. However, it remains lower than that found by Gharsallah et al. (2021) for the Moringa seed oil (30.14 kJ/mol).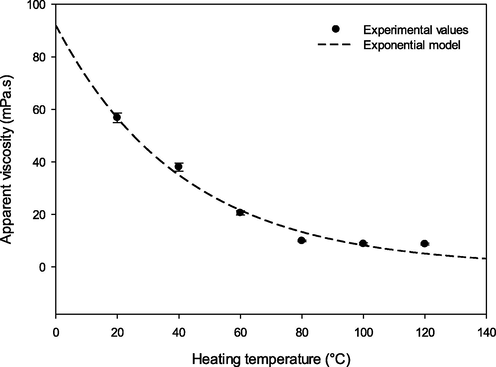
Effect of temperature on the apparent viscosity of Lepidium sativum seed oil.
3.8 CIE L* a* b* coordinates
The L*, a* and b* CieLab values of Lepidium sativum seed oil were 53.1 ± 0.33, − 0.17 ± 0.02 and 28.2 ± 0.1, respectively. It should be noted that Lepidium sativum seed oil exhibited higher L* and b* values in comparison to those cited by Diwakar et al. (2010) and Yeng et al. (2017) on Lepidium sativum seed oil extracted by petroleum ether, by a screw press, by a hydraulic press, by petroleum ether in a Soxhlet apparatus, and by supercritical carbon dioxide. This suggests that Lepidium sativum seed oil was rather pale-colored with the presence of yellow pigments such as carotenoids. Lepidium sativum seed oil was also distinguished by a negative a* value. This result is in perfect agreement with those reported by Diwakar et al. (2010). The negative a* value indicates an intense green color. The cruciferous family’s seeds are known to contain chlorophyll and its derivatives (Appelqvist, 1971) which could be extracted with the oil. Such a finding was consolidated by Sinnecker et al. (2002) who found a linear relationship between the a* value and the oil’s total chlorophyll content.
3.9 Microscopic observation
To better understand the mechanism behind the cold extraction of Lepidium sativum seed oil, the structural changes in samples were examined using the scanning electron microscope (SEM) before and after extraction. The longitudinal section of Lepidium sativum seed (Fig. 6 A) shows the seed coat tissue, or testa, which is covering the seed with thick but smooth cell walls. It is a fiber-rich outer layer that protects the embryo and guards the endosperm which is the storage tissue of the seed (Wadhwa et al., 2012). According to Prego et al. (1998), mineral nutrients, lipid and proteins reserves are principally located in both endosperm which is composed of thick-walled polygonal cells (Fig. 6 B) and embryo. Fig. 6 C shows the presence of swollen lipid cells on the surface of the cell tissues of the seed flour. After cold extraction, the latter became deflated and flattened (Fig. 6 D). This phenomenon is attributed to the depletion of lipid reserves in the seed flour. Therefore, the cold extraction technique does not affect the external morphological structure of the defatted flour’s cellular tissue. The structure remains smooth and intact.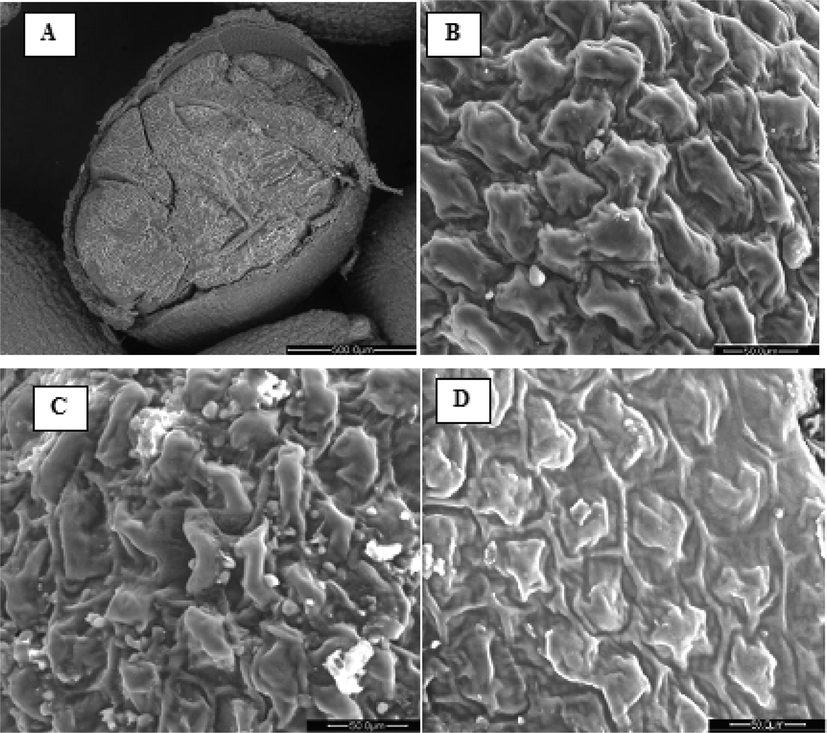
Scanning Electron micrographs of Lepidium sativum seed samples: Longitudinal section of Lepidium sativum seed (A), Endosperm (B), Lepidium sativum seed flour before extraction (C), Defatted Lepidium sativum seed flour (D).
4 Conclusion
The current research offer sufficient data about the biological and physicochemical activities of the Tunisian cold pressed Lepidium sativum seed oil. In fact, the oil was revealed to possess unique fatty acid, phytosterol and tocopherol profiles. We noted the predominance of an essential fatty acid which is the C18:2n-6 linolenic acid, followed by the C18:1n-9 oleic acid. Lepidium sativum seed oil was also proven to have high β-sitosterol and γ-tocopherol contents, and hence a rich antioxidant activity. Similarly, the cold pressed Lepidium sativum seed oil was affirmed to possess a significant anti-inflammatory activity.
Results have so far shown that the richness of Lepidium sativum in oleic acid and in bioactive compounds such as tocopherols and phytosterols could contribute in preventing and/or attenuating age-related diseases often associated with nutrients deficiency.
The results have also demonstrated that although classified by the FAO as one of the most neglected crops, garden cress seed oil nowadays remain a very potent substance that might be well-exploited in both nutritional and medical contexts.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Impact of germination on antioxidant capacity of garden cress: New calculation for determination of total antioxidant activity. Sci. Hort.. 2019;246:155-160.
- [CrossRef] [Google Scholar]
- Purslane and garden cress seeds as source of unconventional edible oils for prevention of hyperlipidemia. Pak. J. Biol. Sci.. 2019;22:537-544.
- [CrossRef] [Google Scholar]
- Antioxidant andantibacterial properties of different extracts of garden cress (Lepidium sativumL.) Zagazig J. Agric. Biochem. Appl.. 2016;43(5):1685-1697.
- [CrossRef] [Google Scholar]
- Chemical composition and biological activities of n-butanol extract of Lepidium sativum L. (Brassicaceae) seed. Trop. J. Pharm. Res.. 2018;17(5):891-896.
- [CrossRef] [Google Scholar]
- Chemical composition and fatty acid content of some spices and herbs under Saudi Arabia conditions. The Scientific World Journal.. 2012;1–5
- [CrossRef] [Google Scholar]
- Chemical composition and antimicrobial, antioxidant, and anti-inflammatory activities of Lepidium sativum seed oil. Biol. Sci. Saudi J. 2018
- [CrossRef] [Google Scholar]
- Official Methods of Analysis of the Association of the Official Analytical Chemists. USA: Association of the Official Analytical Chemists Inc, VA; 1990.
- Official Methods and Recommended Practices of the American Oil Chemist’s Society (5th ed.). Champaign, USA: AOCS Press; 1997.
- Effect of various compounds on virgin olive oil stability measured by Rancimat. J. Agr. Food Chem.. 1999;47:4150-4155.
- [CrossRef] [Google Scholar]
- Naturally occurring hydroxytyrosol derivatives: hydroxytyrosyl acetate and 3,4-dihydroxyphenylglycol modulate inflammatory response in murine peritoneal macrophages. Potential utility as new dietary supplements. J. Agric. Food Chem.. 2014;63:836-846.
- [CrossRef] [Google Scholar]
- Composition of seeds of Cruciferous oil crops. J. Am. Oil Chem. Soc.. 1971;48(12):851-859.
- [CrossRef] [Google Scholar]
- Comparative investigation of minerals, chlorophylls contents, fatty acid composition and thermal profiles of olive leaves (Olea europeae L.) as by-product. Grasas Aceites.. 2014;65(3):3-35.
- [CrossRef] [Google Scholar]
- Preliminary evaluation of the application of the FTIR spectroscopy to control the geographic origin and quality of virgin olive oils. J. Food Quality.. 2007;30:424-437.
- [CrossRef] [Google Scholar]
- Green extraction of oil from Carum carvi seeds using bio-based solvent and supercritical fluid: Evaluation of its antioxidant and anti-inflammatory activities. Phytochem. Anal... 2019;1–9
- [CrossRef] [Google Scholar]
- Bioactivities of black cumin essential oil and its main terpenes from Tunisia. S. Afr. J. Bot.. 2010;76:210-216.
- [CrossRef] [Google Scholar]
- Activity of Bulgarian propolis against 94 Helicobacter pylori strains in vitro by agar-well diffusion, agar dilution and disc diffusion methods. J. Med. Microbiol.. 2005;54:481-483.
- [CrossRef] [Google Scholar]
- Comparison of chemical composition and biological activities of Algerian seed oils of Pistacia lentiscus L., Opuntia ficus indica (L.) mill. and Argania spinosa L. Skeels. Ind. Crops Prod.. 2020;151:112456
- [CrossRef] [Google Scholar]
- Extra virgin olive oil polyphenolic extracts down regulate inflammatory responses in LPS-activated murine peritoneal macrophages suppressing NF k B and MAPK signalling pathways. Food Funct.. 2014;5(6):1270-1277.
- [CrossRef] [Google Scholar]
- Long-term cannabidiol treatment prevents the development of social recognition memory deficits in Alzheimer’s disease transgenic mice. J. Alzheimer’s. Dis.. 2014;42(4):1383-1396.
- [CrossRef] [Google Scholar]
- A comparative study on physicochemical, rheological and surface tension properties of Tunisian Jujube (Zizyphus lotus L.) seed and vegetable oils. Int. J. Food Eng.. 2012;8(2)
- [CrossRef] [Google Scholar]
- Application of Thermogravimetric Analysis in the Field of Oils and Fats. In: Chiavaro E., ed. Differential Scanning Calorimetry. Boca Raton (FL), CRC: Press; 2014. p. :123-140.
- [Google Scholar]
- Recommended Internal Standards Edible Fats and Oils (first ed.). Rome, Italy: FAO/WHO; 1982.
- Plant products as antimicrobial agents. Clin. Microbiol. Rev.. 1999;12:564-582.
- [CrossRef] [Google Scholar]
- Safety evaluation studies on garden cress (Lepidium sativum L.) seeds in Wistar rats. Int. J. Appl. Res. Nat. Prod.. 2011;4:37-43.
- [Google Scholar]
- Protective effects of α-tocopherol, γ-tocopherol and oleic acid, three compounds of olive oils, and no effect of trolox, on 7-ketocholesterol-induced mitochondrial and peroxisomal dysfunction in microglial BV-2 cells. Inter. J. Mol. Sci.. 2016;17(12):1973.
- [CrossRef] [Google Scholar]
- Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem.. 2002;50(10):3010-3014.
- [CrossRef] [Google Scholar]
- The Impact of Phytosterols on the Healthy and Diseased Brain. Curr. Med. Chem.. 2019;26(37):6750-6765.
- [CrossRef] [Google Scholar]
- Physicochemical properties of garden cress (Lepidium sativum L.) seed oil. J. Am. Oil. Chem. Soc.. 2010;87:539-548.
- [CrossRef] [Google Scholar]
- Characteristics of olive and olive pomace oils and their analytical methods. Regulation EEC/2568/1991. Off. J. Eur. Commun.. 1991;L.248:1-82.
- [Google Scholar]
- Lepidium Sativum Seeds as a Suggested Complex Nutritional Supplement to Treat Biomarkers Related Deficits in Autism. Nov. Tech. Nutr. Food Sci.. 2019;3(3):258-262.
- [CrossRef] [Google Scholar]
- Chemical composition and profile characteristics of Osage orange Maclura pomifera (Rafin.) Schneider seed and seed oil. Ind. Crops Prod.. 2009;29:1-8.
- [CrossRef] [Google Scholar]
- Chemical composition, antibacterial and antioxidant activities of oils obtained by different extraction methods from Lepidium sativum L. seeds. Ind. Crops Prod.. 2020;156:112876.
- [CrossRef] [Google Scholar]
- Chemical composition and profile characterization of Moringa oleifera seed oil. S. Afr. J. Bot.. 2021;137:475-482.
- [CrossRef] [Google Scholar]
- Chemical composition of garden cress (Lepidium sativum) seeds and its fractions and use of bran as a functional ingredient. Plant Foods Hum. Nutr.. 2004;59(3):105-111.
- [CrossRef] [Google Scholar]
- Seed oils recovered from industrial fruit byproducts are a rich source of tocopherols and tocotrienols: Rapid separation of α/β/γ/δ homologues by RP-HPLC/FLD. Eur. J. Lipid Sci. Tech.. 2015;117:773-777.
- [CrossRef] [Google Scholar]
- Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J. Immunol.. 1990;144:278-283.
- [Google Scholar]
- Oxidative stability of semi solid excipient mixtures with corn oil and its implication in the degradation of vitamin A. Int. J. Pharm.. 1997;147:31-40.
- [CrossRef] [Google Scholar]
- Phenolic content and antioxidant activity of cantaloupe (Cucumis melo) methanolic extracts. Food Chem.. 2010;119:643-647.
- [CrossRef] [Google Scholar]
- Effect of processing on nutrients and fatty acid composition of garden cress (Lepidium sativum) seeds. Food Chem.. 2016;213:806-812.
- [CrossRef] [Google Scholar]
- Lepidium sativum L. In: Grubben G.J.H., Denton O.A., eds. Plant resources of Tropical Africa 2: Vegetables. Netherlands: PROTA Foundation, Backhuys Publishers; 2004. p. :365-367.
- [Google Scholar]
- Stability and radical-scavenging activity of heated olive oil and other vegetable oils. Eur. J. Lipid Sci. Technol.. 2006;108:329-335.
- [CrossRef] [Google Scholar]
- Nutritional, elemental analysis and antioxidant activity of garden cress (Lepidium sativum L.) seeds. Int. J. Pharm. Pharm. Sci.. 2012;4(3):392-395.
- [Google Scholar]
- Correlation of fatty acid composition of vegetable oils with rheological behavior and oil uptake. Food Chem.. 2010;118:398-402.
- [CrossRef] [Google Scholar]
- Influence of processing on sterols of edible vegetable oils. Prog. Lipid Res.. 1983;22:161-188.
- [CrossRef] [Google Scholar]
- Physicochemical and bioactive properties of Hymenaea courbaril L pulp and seed lipid fraction. Ind. Crops Prod.. 2013;49:610-618.
- [CrossRef] [Google Scholar]
- Change in colour and rheological behavior of sunflower seed oil during frying and after adsorbent treatment of used oil. Eur. Food Res. Technol.. 2003;218:20-25.
- [CrossRef] [Google Scholar]
- On the mechanism of oleate transport across human brain microvessel endothelial cells. J. Neurochem.. 2009;110:1049-1057.
- [CrossRef] [Google Scholar]
- The sterols isolated from evening primrose oil modulate the release of proinflammatory mediators. Phytomedicine. 2012;19(12):1072-1076.
- [CrossRef] [Google Scholar]
- Effect of olive oil minor components on oxidative stress and arachidonic acid mobilization and metabolism by macrophages RAW 264.7. Free Radic. Biol. Med.. 2003;35(9):1073-1081.
- [CrossRef] [Google Scholar]
- Composition and physical properties of cress (Lepidium sativum L.) and field pennycress (Thlaspi Arvense L.) oils. Ind. Crop Prod.. 2009;30:199-205.
- [CrossRef] [Google Scholar]
- Effect of hot oven and microwave roasting on garden cress (Lepidium sativum) seed flour quality and fatty acid composition, thermal and dielectric properties of extracted oil. Int. J. Food Sci. Technol. 2018
- [CrossRef] [Google Scholar]
- Cold pressed garden cress (Lepidium sativum L.) seed oil. In: Ramadan M.F., ed. Cold pressed oils: Green technology, Bioactive compounds, Functionality, and Applications. United Kingdom: Academic press; 2020. p. :477-487.
- [Google Scholar]
- Garden cress (Lepidium sativum Linn.) seed oil as a potential feedstock for biodiesel production. Bioresour. Technol.. 2012;126:193-197.
- [CrossRef] [Google Scholar]
- Characterization of Ternary Blends of Vegetable Oils with Optimal ω-6/ω-3 Fatty Acid Ratios. J. Oleo Sci.. 2019;68(11):1041-1049.
- [CrossRef] [Google Scholar]
- NF EN ISO 12228, 1999. Association Française de Normalisation, European Norm, NF EN ISO 12228 May 1999; French norm T60-258: animal and vegetable fats and oils- determination of individual and total sterols contents-gas chromatographic method. AFNOR. Paris. 18 pp.
- NF EN ISO 9936, 2006. Association Française de Normalisation, European Norm, NF EN ISO 9936 Octobre 2006; French norm T60-239: animal and vegetable fats and oils - determination of tocopherols and tocotrienols contents-method using high-performance liquid chromatography. ISO.Genève.17 pp.
- Attenuation of 7-ketocholesterol- and 7β-hydroxycholesterol-induced oxiapoptophagy by nutrients, synthetic molecules and oils: Potential for the prevention of age-related diseases. Ageing Res. Rev.. 2021;68:101324.
- [CrossRef] [Google Scholar]
- Free and esterified sterol composition of edible oils and fats. J. Food Compos. Anal.. 2002;15:123-142.
- [CrossRef] [Google Scholar]
- Seed structure and localization of reserves in Chenopodium quinoa. Ann. Bot.. 1998;82:481-488.
- [CrossRef] [Google Scholar]
- The relationship between morphological traits and seed yield of Iranian Garden Cress accessions. Acta Sci. Pol. Hortorum Cultus.. 2019;18(3):137-145.
- [CrossRef] [Google Scholar]
- How neuroinflammation contributes to neurodegeneration. Science. 2016;353(6301):777-783.
- [CrossRef] [Google Scholar]
- Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Bio. Med.. 1999;26:1231-1237.
- [CrossRef] [Google Scholar]
- Chemical composition and bioactive compounds of Cucurbitaceae seeds: Potential sources for new trends of plant oils. Process Saf. Environ. 2019
- [CrossRef] [Google Scholar]
- Chemical composition and profile characterisation of pumpkin (Cucurbita maxima) seed oil. Ind. Crops Prod.. 2012;37:82-87.
- [CrossRef] [Google Scholar]
- Medicinal plants and antimicrobial activity. J. Ethnopharmacol.. 2005;100:80-84.
- [CrossRef] [Google Scholar]
- Inflammatory neuroprotection following traumatic brain injury. Science. 2016;353(6301):783-785.
- [CrossRef] [Google Scholar]
- Thermoanalytical, kinetic and rheological parameters of commercial edible oils. J Therm Anal. Calorim.. 2004;75:419-428.
- [CrossRef] [Google Scholar]
- Effect of heating and cooling on rheological parameters of vegetable edible oils. J. Food Eng.. 2005;67:401-405.
- [CrossRef] [Google Scholar]
- Effects of Resveratrol and other polyphenols on Sirt 1: Relevance to brain function during aging. Curr. Neuromarphacol.. 2018;16(2):126-136.
- [CrossRef] [Google Scholar]
- Relationship between color (instrumental and visual) and chlorophyll contents in soybean seeds during ripening. J Agric. Food Chem.. 2002;50:3961-3966.
- [CrossRef] [Google Scholar]
- Microgliamediated neuroinflammation in neurodegenerative diseases. Sem. Cell Dev. Biol.. 2019;94:112-120.
- [CrossRef] [Google Scholar]
- Antioxidants and antioxidant enzymes status of rats fed on n-3 PUFA rich garden cress (Lepidium sativum L.) seed oil and its blended oils. Int. J. Food Sci. Technol.. 2013;52(4):1993-2002.
- [CrossRef] [Google Scholar]
- Methods for dietary fibre, neutral detergent fiber, and non starch carbohydrates in relation to animal nutrition. J. Dairy Sci.. 1991;74:3583-3597.
- [CrossRef] [Google Scholar]
- Alpha and gamma tocopherols in cerebrospinal fluid and serum from older, male, human subjects. J. Am. Coll. Nutr.. 2004;23(3):233-238.
- [CrossRef] [Google Scholar]
- Determinación de los polifenoles totales de la ceite de oliva. Grasas Aceites. 1973;24:350-357.
- [Google Scholar]
- A review on pharmacognostical study of Lepidium sativum. Adv. Res. n Pharm. Bio Sci.. 2012;2(4):316-323.
- [Google Scholar]
- Effect of different extraction methods on yield and physico-chemical properties of garden cress (Lepidium sativum L.) oil. J. Oilseed Brasscica. 2017;8(2):138-142.
- [Google Scholar]
- Profile of Fatty Acids, Tocopherols, Phytosterols and Polyphenols in Mediterranean Oils (Argan Oils, Olive Oils, Milk Thistle Seed Oils and Nigella Seed Oil) and Evaluation of their Antioxidant and Cytoprotective Activities. Curr. Pharm. Des.. 2019;25:1791-1805.
- [CrossRef] [Google Scholar]
- Compositional study and antioxidant potential of Ipomoea hederacea Jacq. and Lepidium sativum L. seeds. Molecules. 2012;17:10306-10321.
- [CrossRef] [Google Scholar]







