Translate this page into:
Promoting effects of thoria on the nickel-manganese mixed oxide catalysts for the aerobic oxidation of benzyl alcohol
⁎Corresponding authors. krsprasad_fed@kluniversity.in (K.R.S. Prasad), sfadil@ksu.edu.sa (Syed F. Adil)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Due to the recent advancement in the development of various characterization techniques, mixed metal oxide (MMO) based catalysts have gained tremendous attention in the field of catalysis. In this study, we demonstrated the synthesis of a series of novel MMO based catalysts by a facile co-precipitation method. The detailed structure and composition of thoria promoted NiMnO catalysts was investigated using various microscopic and spectroscopic techniques such as, SEM, EDAX, XRD, TGA, BET and TPR. In order to study the effect of the content of thorium oxide on the catalytic activity of the as-prepared material various samples were prepared by the addition of low quantities of thorium oxide with 1%, 3% and 5% on NiMnO. The catalytic performances of the as-prepared catalysts were evaluated towards the aerobic oxidation of benzyl alcohol using molecular oxygen as oxidant. Furthermore, in order to investigate the effect of the calcination temperatures on the catalytic activities of the as-prepared materials, the samples were calcined at three different temperatures at 300 °C, 400 °C and 500 °C. The catalysts displayed significant enhancement in catalytic activity towards the catalytic conversion of benzyl alcohol (C6H5CH2OH) to benzaldehyde (C6H5CHO). Detailed kinetic studies of the reactions using gas chromatography have revealed that the variation of calcination temperature and the percentage of thoria had significant effect on the catalytic performances of the materials. Among all synthesized catalysts ThO2-(5%)-NiMnO catalyst calcined at 400 °C exhibited the highest catalytic performance and stability for the selective oxidation of alcohols.
Keywords
Oxidation catalyst
Mixed metal oxides
Nanocatalyst
Thoria
NiMnO
Catalysis
1 Introduction
Mixed-metal oxides have attracted considerable attention as efficient catalysts in a variety of industrially significant applications (Gawande et al., 2013). They represent one of the most significant categories of solid state catalysts which are commonly employed either as active phases or as support materials (Cargnello et al., 2013; Sattler et al., 2014). Amongst all types of metal oxide based catalysts, those which comprises of more than one kind of metal are generally referred to as mixed metal oxides (MMO’s) (Dabbagh et al., 2005; McFarland and Metiu, 2013; Villa et al., 2009; Villa et al., 2010). MMO’s display a variety of applications such as, memory alloys (Druker et al., 2014), semiconductors (Vignesh et al., 2014), oxygen sensors, cathodes for Solid Oxide Fuel Cells [SOFC] (Thursfield et al., 2012) and anodes for electrochemical oxidations (Moradi and Dehghanian, 2014), photoconductive thin films (Godines et al., 2014), electrode materials for lithium batteries (Aragon et al., 2011) and various other environmental applications (Yang and He, 2012). Besides, their excellent stability MMO’s exhibit enhanced catalytic activities when compared to their respective single-component metal oxides, and thus have also been significantly exploited in the field of catalysis (Xie et al., 2015). The enhanced catalytic properties of the MMO’s can be attributed to the significant increase in the surface area of the material and the change in the chemical states of the metal ions (Wu et al., 2014). There are so many literature reports have been reported for various catalytic transformations by using MMO’s (Qwabe et al., 2015; Xie et al., 2015). Among these MMO’s, Ni-Mn based MMO’s exhibit superior stability. The catalytic activity of a mixed Ni-Mn-oxide system has been carried out in liquid-phase oxidation of phenol. The MMO’s catalysts for numerous catalytic transformations have been widely reported as promising catalysts (Stoyanova and Georgieva, 2003; Tang et al., 2011).
Additionally, in several studies the catalytic activities of MMO’s have been further enhanced by incorporating various lanthanides as dopants (Primus et al., 2014). For instance, in a recent report the combination of cerium–tungsten−titanium in the form of WO3/CeOx-TiO2 nanoparticles were used as catalysts for selective catalytic NOx reduction (Michalow-Mauke et al., 2015). In this doped MMO’s catalyst, cerium is existing as a dopant in TiO2 and is well dispersed on the surface of the nano catalysts. The addition of cerium considerably enhanced the interactions between different metal oxides involved (TiO2 and WO3) and increased the surface area of the material, due to which the NOx reduction efficiency of the nano catalyst increased many folds.
Among different actinide based metal oxides, thoria has gained prominent significance due to their excellent properties that are considerably valuable for a variety of applications (Lundeen and VanHoozer, 1967). Especially in the field of catalysis, thoria based MMO’s were successfully applied for various industrially important transformations. For instance, thorium-doped meso structured zirconia exhibited excellent potential in the non–aqueous catalytic reactions, particularly in the form of precursor to the practical catalysts to obtain mesoporous super acid SO42−/ZrO2 catalysts (Yu et al., 2008). Furthermore, thorium dioxide has also been extensively used for various other applications (Yousefi et al., 2015).
Additionally, thoria is also recognized as an excellent promoter in the field of catalysis due to its high catalytic performance and thermal stability towards metal oxides. Several cases are reported in the literature, where ThO2-based catalysts prepared by different methods, including, calcination, air decomposition etc., have demonstrated enhanced performance due to the presence of thoria. For example, in a recent study thoria excellently promotes the catalytic activity and selectivity of thoria based CaO2 (Th0.8Ca0.2O2−δ) catalysts towards the oxidative coupling of methane (Baidya et al., 2011). In these catalysts, the conductivity of pure ThO2, which is an elevated temperature p-type conductor, which is enhanced by doping with Ca2+ cations, that is a lower valent cation, which generates more oxide ion vacancies. It was revealed that active oxygen species formed at the oxide ion vacancy sites in Ca-doped ThO2 catalysts were mainly responsible for the enhanced catalytic performance and selectivity toward Ca2+ products. In another study, thoria not only enhanced the performance but also improved the selectivity of olefins and long-chain hydrocarbons during the hydrogenation of carbon monooxide. The thoria-based catalysts displayed extremely high catalytic activity at low temperatures. During this study, it was observed that thoria demonstrated a tendency to spread onto and to act together intimately with SiO2 and Al2O3 which were used as support materials, due to which the catalytic activity of material is promoted several times when compared to non-supported catalysts (Chen and Wang, 1993). Apart from this, thoria is also used as a blanket material in nuclear industry (Bianconi, 2014), as ceramic container for molten metals and alloys, and is also applied as a super conductor material for the preparation of various electrodes (Abou-Sekkina and Elsabawy, 2002).
Among various catalytic conversions the oxidation of alcohols to their respective aldehydes or ketones, while maintaining selectivity is one of the most crucial and demanding functional group conversions in organic synthesis (Farnesi Camellone and Marx, 2014). These reactions usually serve many purposes from both industrial and synthetic points of view, since the resulting compounds obtained from these reactions are versatile intermediates of valuable compounds, such as agricultural, pharmaceuticals, and fine chemicals (Karimi et al., 2014). In our previous study, we have successfully demonstrated efficient catalytic activity of MMO towards the selective oxidation of alcohols (Adil et al., 2015). For this purpose, mixed oxide of nickel manganese doped with vanadia nanoparticles were synthesized by co-precipitation method, which displayed excellent catalytic activity towards the oxidation of benzyl alcohol.
With encouraging results obtained previously by the use of ceria (Sultana et al., 2015), which is a lanthanide oxide, in this study thorium oxide of actinide series as a promoter was selected, in order to study if similar pattern of catalytic performance could be obtained. We herein, report the synthesis of thoria promoted nickel manganese MMO’s as oxidation catalyst for the selective oxidation of benzyl alcohol to benzaldehyde. The effect of the amount of dopant material on the catalytic activity of NiMnO was studied by the preparation of catalysts with varying percentage of thoria, such as 1%, 3%, 5%, which were later calcined at temperatures such as at 300 °C, 400 °C, 500 °C. The as-prepared catalysts were characterized by various techniques including scanning electron microscopy (SEM), thermo gravimetric analysis (TGA), X-ray diffraction (XRD), Brunauer–Emmett–Teller (BET), and temperature-programmed reduction (TPR). Furthermore, the catalytic activities of as-synthesized catalysts were evaluated and the conversions monitored by gas chromatography.
2 Experimental
2.1 Materials
Ni(NO3)2·6H2O (nickel nitrate), Mn(NO3)2·4H2O (manganese nitrate), Th(NO3)4 (thorium nitrate), NaHCO3 (sodium bicarbonate), C6H5CH2OH (benzyl alcohol), C6H5CHO (benzaldehyde), C6H5CH3 (toluene) [AR, Merck] were purchased from sigma aldrich pure chemical industries co., ltd. All the chemicals were used as received without further purification. Deionized water was used to prepare the required solutions.
2.2 Catalyst preparation
Thoria promoted NiMnO oxidative catalysts were synthesized by facile co-precipitation technique with addition of low quantities of thorium oxide in 1%, 3%, and 5% molar rates. In a typical procedure stoichiometric volume of 0.2 M solution of nickel nitrate and manganese nitrate were mixed in a round bottomed flask, to this, desired volume of 0.2 M thorium nitrate solution was added. The resulting solution was heated to 90 °C, while stirring using a mechanical stirrer. Subsequently, 1 M solution of NaHCO3 was added drop wise until the solution attained a pH 9. The resulting solution was continued to stir at the same temperature for about 3 hours, and then left on stirring over night at room temperature. Finally, the solution was filtered using a Buchner funnel under vacuum and dried at 70 °C overnight. The dried product was then calcined at temperatures such as 300 °C, 400 °C, 500 °C.
2.3 Catalyst characterization
The morphology of mixed metal oxides and elemental analysis was determined using scanning electron microscopy (SEM) and energy dispersive X-ray analysis (EDAX) which is carried out using Jeol SEM model JSM 6360A (Japan). Powder X-ray diffraction studies were carried out using a D2 phaser (Bruker) X-ray diffractometer, the wavelength of X-ray (1.542 Å). Thermogravimetric Analysis (TGA) was studied employing Perkin-Elmer Thermogravimetric Analyzer 7. The surface area of the material was measured using BET, on a NOVA 4200e surface area and pore size analyzer.
2.4 Catalyst testing
The catalytic performance of the synthesized products was evaluated by carrying out oxidation reaction using benzyl alcohol as a model reaction and the kinetics of the reaction was studied using gas chromatography analyses.
In a typical reaction, catalyst weighing 150 mg was taken in a glass flask with 0.2 mL (2 mmol) benzyl alcohol with 8 mL toluene as solvent; the mixture was then refluxed at 100 °C while vigorous stirring. Oxygen was bubbled at a flow rate of 20 mL min−1 into the mixture once the reaction temperature was attained. After the completion of reaction, the solid catalyst was separated by centrifugation and the liquid samples were analyzed by gas chromatography to evaluate the conversion of the product by (GC, 7890A) Agilent Technologies Inc., equipped with a flame ionization detector (FID) and a 19019S-001 HP-PONA capillary column.
2.5 Catalyst reusability
The catalytic performance of the synthesized mixed metal oxides revealed that ThO2-(5%)-NiMnO catalyst calcined at 400 °C yields a 100% conversion product and hence in order to understand the reusability of the catalyst, the same catalyst was subjected to oxidation reaction several times and the conversion product obtained was studied using gas chromatography analyses.
3 Result and discussion
3.1 Scanning electron microscopy (SEM)
The Scanning Electron Micrograph images of ThO2 promoted NiMnO shows that the catalysts have a combination of spheres and needles morphology. The structural visualization of the sample confirm that the rugged structure appears to be more prominent in the catalyst calcined at higher temperatures such as 400 °C, 500 °C, while the catalyst calcined at 300 °C appear to be a mixture of spheres and needles. The SEM images of the catalyst calcined at temperatures such as 300 °C, 400 °C and 500 °C is given in Fig. 1. Moreover, to understand the morphological changes of the reused catalyst, a comparison was made between the SEM images of the fresh catalyst and the reused one. From Fig. 2 it can be observed that the needles like structure on the surface changes and the morphology appears to be porous, which can be easily be noticed in the insets given in Fig. 2. The elemental composition of the of ThO2-(5%)-NiMnO calcined at 400 °C temperatures was investigated by energy-dispersive X-ray spectroscopy (EDX), which discloses the clear elemental composition summary of the catalyst prepared as displayed in Fig. 3.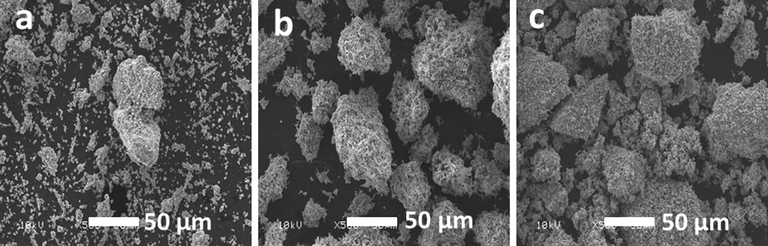
SEM micro graphs of ThO2-(5%)-NiMnO calcined at temperatures such as (a) 300 °C; (b) 400 °C; (c) 500 °C.
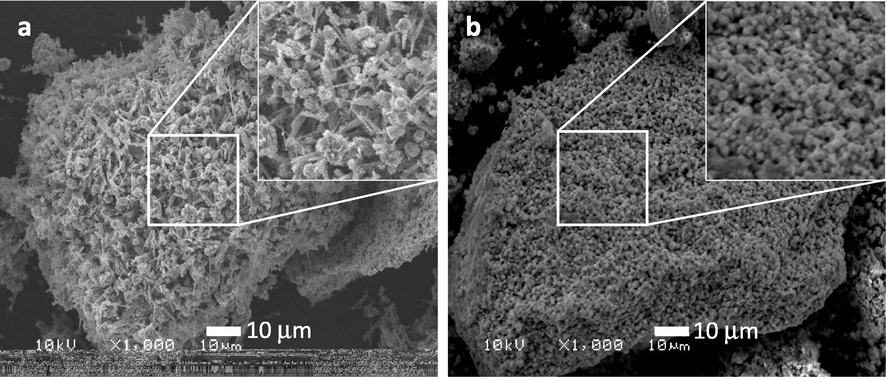
SEM micro graphs of ThO2-(5%)-NiMnO calcined at 400 °C temperatures (a) Fresh; (b) Re-used.
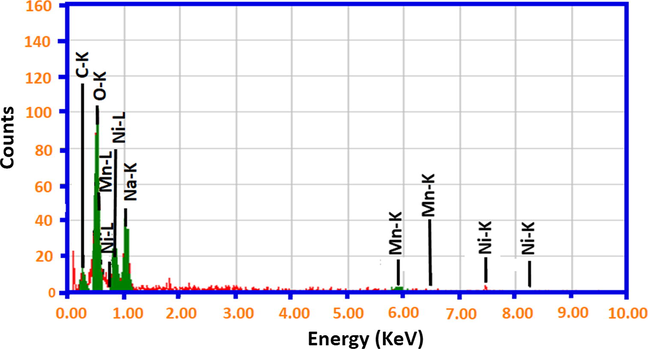
Elemental composition from the EDX analysis of the as-synthesized catalysts of ThO2-(5%)-NiMnO calcined at 400 °C temperature.
3.2 X-ray diffraction (XRD)
The phase identification and crystalline structures of the ThO2 promoted NiMnO catalyst was analyzed using XRD and the diffraction patterns obtained are illustrated in Fig. 4A. The diffraction peaks confirmed that the mixed metal oxides formed were crystalline in nature. The diffractograms obtained indicate the formation of different phases of the mixed metal oxides. Moreover, comparing with the XRD patterns of various types of NiMnO, it was observed that the obtained patterns are in good agreement with the various NiMnO salts such as nickel manganese (IV) oxide NiMnO3 (ICSD#31853), Hexanickel manganese(IV) oxide, Ni6MnO8 (ICSD# 80301), NiMn2O4 (ICSD# 181111). The diffraction pattern of the catalyst calcined at 300 °C shows that nickel manganese (IV) oxide NiMnO3 (ICSD#31853) along with nickel manganese oxide (1/2/4), NiMn2O4 (ICSD# 181111) is present. The other prominent signals found correspond to the manganese carbonate MnCO3 (ICSD# 100677). However, as the calcination temperature was increased to 400 °C, the diffraction signals indicate a considerable decrease in concentration of manganese carbonate MnCO3 (ICSD# 100677) in the catalyst. While a slight decrease in the NiMn2O4 (ICSD# 181111) was also observed along with the formation of a new phase of nickel manganese mixed oxide. Hexa nickel manganese(IV) oxide, Ni6MnO8 (ICSD# 80301) appears along with nickel manganese (IV) oxide NiMnO3 (ICSD#31853). In the catalyst calcined at 500 °C it was observed that the nickel manganese oxide phase (1/2/4), NiMn2O4 (ICSD# 181111) disappears. Hence, it can be concluded that changes in calcination temperature, induce phase changes in the catalyst and the 400 °C calcination temperature the phase changes appear to be in transition. Reflection from thorium oxide were not observed,and the low percentage present in the catalyst could be possible reason for its absence. The XRD pattern of the fresh catalyst and used catalyst was compared to understand the phase changes in the catalyst after it is used, it was found that the used catalyst undergoes phase transition as can be seen in Fig. 4B.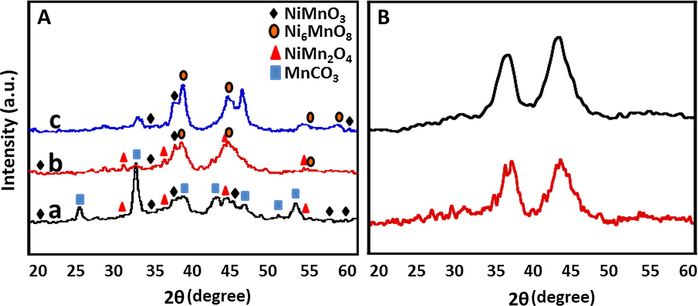
XRD diffractograms of (A) ThO2-(5%)-NiMnO calcined at temperatures such as (a) 300 °C; (b) 400 °C; (c) 500 °C: (B) ThO2-(5%)-NiMnO calcined at 400 °C; (a) Fresh (b) Used.
3.3 TGA
The samples of the synthesized catalyst were subjected to the thermal analysis in order to ascertain their thermal stability and to understand their degradation pattern shown in Fig. 5. The values in percentage of mass lost during the thermogravimetric analysis of the synthesized catalyst are present in Table 1. From the results obtained the following conclusions can be drawn. The total mass loss of the catalysts (1%, 3% and 5% Thoria) calcined at temperatures such as (300 °C, 400 °C and 500 °C) observed was found to be different for each sample. However, the mass loss for 1%, 3% and 5% catalysts were similar. The mass loss of the 1%, 3% and 5% catalysts at 300 °C was 26%, 26% and 24% respectively. For catalysts calcined at 400 °C a mass loss of the 19%, 17% and 13% was observed and the catalysts calcined at 500 °C displayed the best thermal stability with 18%, 11% and 9% mass loss. The high percentage of mass loss of the catalyst calcined at lower temperatures can be attributed to the degradation of remnants of carbonates that was detected in the XRD diffractogram. Hence, from the TGA investigation of these catalysts, it can be concluded that the mass loss of the catalyst decreased slightly with increasing the percentage of doping and decrease markedly with increase in calcination temperature. Therefore, the catalyst with ThO2-(5%)-NiMnO calcined at 500 °C is thermally most stable among the other synthesized catalysts.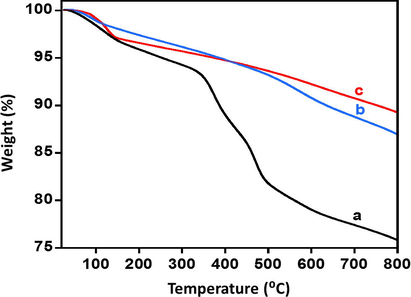
Thermal stability pattern of the ThO2-(5%)-NiMnO calcined at (a) 300 °C; (b) 400 °C; (c) 500 °C.
Catalyst
Calcination temperature
300 °C
400 °C
500 °C
ThO2 -(1%)- NiMnO
26%
19%
18%
ThO2 -(3%)- NiMnO
26%
17%
11%
ThO2 -(5%)- NiMnO
24%
13%
9%
3.4 BET
Surface areas and pore size information was obtained by subjecting the prepared samples to BET analysis. The results of BET surface areas are summarized in Table 2. It was observed that upon an increase in the % of ThO2 in the catalyst, there was a decrease in the surface area. The calcination temperature of the material also showed effect on the surface area, while there was a meager change in pore size, no change was observed in the pore diameter. The probable reason in the decline in the specific surface with the increasing % of ThO2 could be due to thoria occupying the internal walls resulting in decrease of, total pore volume and average pore size (Daza et al., 2010; Liao et al., 2013).
Catalystsa/calcination temp.
Surface area (m2/g)b
V (Pore) (cm3/g)c
D (Pore) (Å)d
(1%)
300 °C
53.9
0.021
18.6
400 °C
46.7
0.018
18.6
500 °C
29.8
0.012
18.6
(3%)
300 °C
37.0
0.014
18.5
400 °C
44.5
0.018
18.5
500 °C
31.4
0.012
18.5
(5%)
300 °C
56.5
0.022
18.6
400 °C
39.4
0.015
18.5
500 °C
22.7
0.009
18.6
3.5 Temperature programmed reduction of catalysts (H2-TPR)
It is supposed that the active species in supported catalysts for catalytic reactions are the reduced metallic particles present at the surface of the catalyst. Temperature programmed reduction is a very accessible technique to find number of reducible metallic species present in catalysts and interaction between active metal and support (Molina and Poncelet, 1998). TPR profiles of the prepared samples after calcination at 300 °C, 400 °C and 500 °C are shown in Fig. 6 and data are summarized in Table 3. It is observed that TPR profiles of thoria promoted Ni-Mn mixed metal oxide catalysts are different, both in broadness and H2 consumptions, which indicates that the H2 consumption increases with the calcination temperature. The difference in TPR pattern revealed that calcination temperature has a definite effect on reduction behavior of catalysts. As the temperature is increased, the main peak grows larger in height and width, which is probably due to existence of more reducible species in catalyst. In fact, the positive shift in temperature favors the strong metal support interaction. In all cases, the main reduction peak being obtained at high temperature in range 275–400 °C and it increase with increasing calcination temperature. The profile for ThO2-(5%)-NiMnO calcined at 300 °C catalyst shows two reduction peaks, a smaller one at 200 °C and a larger one at 320 °C. Both can be attributed to the reduction of catalyst as Ni6MnO8 and Ni2O3, indicating that there are two kinds of oxide species present on both inner and outer surface of support, respectively. The profile for ThO2-(5%)-NiMnO calcined at 400 °C catalyst shows two peaks at 275 °C and 325 °C which are identified as NiMn2O4 and MnO2. The small peak at 275 °C shows a weak or no interaction of Ni2+ with the lattice oxygen of support and bigger peak shows strong interaction due to some ion exchange between active species and support.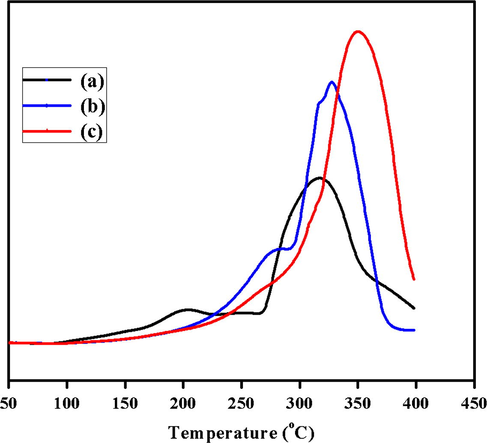
TPR of ThO2-(5%)-NiMnO catalyst calcined at temperatures such as (a) 300 °C; (b) 400 °C; (c) 500 °C.
Moreover, from the TPR pattern of the catalyst ThO2-(5%)-NiMnO calcined at 500 °C, it is observed that it yields only one reduction consumption peak between 275–400 °C which indicates a majority of reduced species has either stronger interaction/exchange with surrounding cations or with lattice oxygen, leading to higher temperature required for reduction.
3.6 Catalytic evaluation
To determine the catalytic performance of synthesized material as oxidation catalyst, the oxidation of benzyl alcohol was used as a model reaction and the reaction was carried out in the presence of the ThO2-(X%)-NiMnO catalyst. The percentage of thoria and the calcination temperature was varied, in order to confirm the optimum percentage of thoria and the calcination temperature for the best catalytic conversion of the synthesized catalyst. The reaction was carried out for 5 hours and the results obtained are discussed below and also the results are displayed in Table 4.
Catalyst
Conversion (%)
ThO2-(1%)-NiMnO
300 °C
61
400 °C
64
500 °C
44
ThO2-(3%)-NiMnO
300 °C
67
400 °C
92
500 °C
62
ThO2-(5%)-NiMnO
300 °C
64
400 °C
100
500 °C
57
3.6.1 ThO2-(1%)-NiMnO catalyst
With 1% ThO2 promoted NiMnO catalyst calcined at temperatures such as of 300 °C and 500 °C the conversion of 61% and 44% respectively was obtained. However, the 400 °C calcined catalyst yielded up to 64% conversion of benzyl alcohol to benzaldehyde.
3.6.2 ThO2-(3%)-NiMnO catalyst
The 3% ThO2 promoted NiMnO catalyst calcined at temperatures such as i.e., at 300 °C, 400 °C and 500 °C showed 67%, 92%and 62% conversion respectively.
3.6.3 ThO2-(5%)-NiMnO catalyst
With 5% ThO2 promoted NiMnO catalyst calcined at 400 °C, a 100% conversion was obtained, while the catalyst which was calcined at 300 °C and 500 °C yielded 64% and 57% conversion respectively.
Above-mentioned results clearly suggest that the calcination temperature and the percentage of the promoter play a vital role on the catalytic performance of ThO2-(5%)-NiMnO catalyst. It can be concluded that the catalysts calcined at 400 °C show better activity compared to catalysts calcined at 300 °C and 500 °C and that ThO2-(5%)-NiMnO calcined at 400 °C temperature is best combination to obtain the best catalytic activity and selectivity. In order to ascertain the effect of thoria in the catalytic performance of the catalyst, a reaction without the presences of dopant ThO2 was prepared and subjected to oxidation reaction under similar conditions. It was found that the catalyst produces oxidation product with 56% conversion after 300 min. From the above results the Turnover Number (TON) and Turnover Frequency (TOF) for the catalyst with and without the dopant (ThO2) were calculated using the formula’s mentioned (Johansson Seechurn et al., 2015). The TON for the catalyst ThO2-(5%)-NiMnO catalyst calcined at 400 °C shown in Fig. 7 was found to be 0.94, and the TOF was found to be 0.20 h−1. While the NiMnO catalyst (i.e. ThO2-(0%)-NiMnO catalyst) calcined at 400 °C the TON for the catalyst was calculated as 0.45 and the TOF was found to be 0.09 h−1. These values clearly indicate that the inclusion of ThO2 as promoter has influenced the catalytic performance positively by improving its catalytic efficiency almost 2.5 times. The specific activity for the catalyst ThO2-(5%)-NiMnO and NiMnO catalyst calcined at 400 °C was found to be 1.48 mmol g−1 h−1 and 1.33 mol g−1 h−1.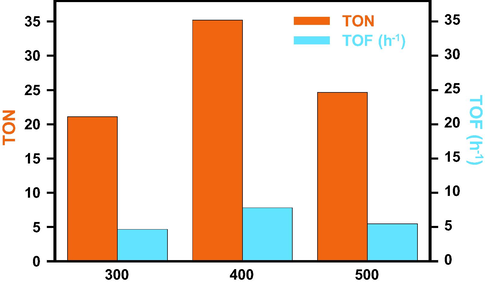
Graphical representation TOF and TON for the catalyst: ThO2-(5%)-NiMnO catalyst calcined at (a) 300 °C; (b) 400 °C; (c) 500 °C.
A graphical illustration of comparison of surface area of catalyst ThO2-(5%)-NiMnO to conversion product and the kinetics of the reaction are given in Fig. 8. Fig. 9 shows a graphical representation of time-on-line catalytic activity for the different catalysts.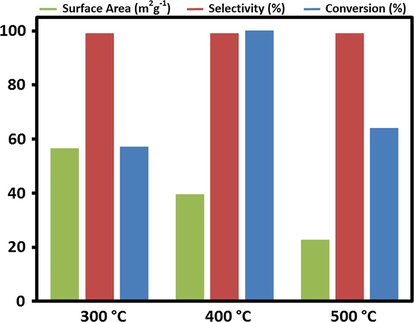
Graphical illustration of comparison of surface area of catalyst ThO2-(5%)-NiMnO to conversion product.
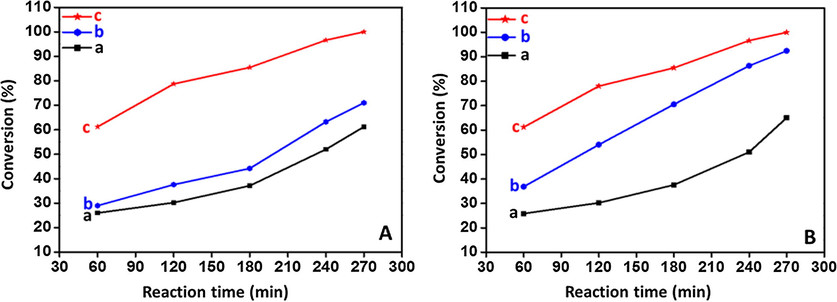
Graphical representation of time Vs Conversion (%) for the catalyst ThO2-(5%)-NiMnO catalyst (A) Calcined at temperatures such as (a) 300 °C; (b) 500 °C; (c) 400 °C; (B) ThO2-(X%)-NiMnO catalyst calcined at 400 °C where in X = (a) 1%; (b) 3%; (c) 5%.
In order to understand the reusability of the catalyst, the catalyst ThO2-(5%)-NiMnO catalyst calcined at 400 °C, which yielded a 100% conversion to the desired product was selected and was subjected to oxidation reaction several times and the product conversion was studied using gas chromatography analyses. It was observed that the catalytic performance of the catalyst drops to 77% after single use and upon further reuse there is a drastic fall in the catalytic performance. The graphical representation of the product conversion results obtained from the reuse of catalyst is given in Fig. 10.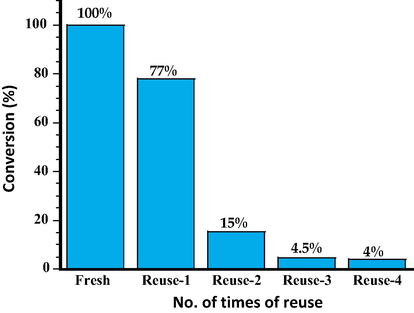
Graphical representation of conversion product (%) for the catalyst for reusability study of ThO2-(5%)-NiMnO catalyst calcined at 400 °C.
In order to understand this depreciation of catalytic performance, the reused catalyst was subjected to SEM and XRD. From the SEM image (Fig. 2) of the reused catalyst, it can be clearly observed that there is a distortion of surface morphology of the catalyst. Similarly changes in the XRD pattern obtained for the reused catalyst also point out towards certain changes in the crystal morphology, which could have led to a depreciation of catalytic performance upon single use.
In order to compare the prepared catalyst with the previously reported mixed metal oxides, the specific activities of previously reported catalysts are collected and compared. Table 5 displays the catalytic performance in terms of specific activity of the prepared catalyst compared with previous publications of the heterogeneous catalysts for oxidation of benzyl alcohol to benzaldehyde. It has found that the catalyst ThO2-(5%)-NiMnO catalyst calcined at 400 °C is the very efficient catalyst when compared to the many of the catalysts listed.
Catalyst
Conv. (%)
Sel. (%)
Temp. (°C)
Sp. activity (mmol g−1 h−1)
Ref.
NbP-S2
43
65
90
9.81
Reis et al. (2012)
Amorphous MnOx
72
99
100
9.7
Jing et al. (2007)
Au–Pd/MgO
<52
100
80
9.43
Zhan et al. (2012)
Mn6Ni4
89
99
100
8.90
Tang et al. (2011)
Mn4Ni6
73
99
100
7.4
Tang et al. (2011)
Mn8Ni2
65
97
100
6.5
Tang et al. (2011)
V2O5 (5%)-NiMnO
100
>99
100
6.67
Adil et al. (2015)
Zr (5%): NiMnO
100
>99
100
6.67
Alabbad et al. (2013)
Mn2Ni8
62
98
100
6.2
Tang et al. (2011)
Ti-SBA-15
62
95
60
6.20
Sharma et al. (2012)
OMS-2
44
99
100
5.9
Jing et al. (2007)
Au/RGO
65
93
100
5.42
Yu et al. (2013)
KMn-i/CNTs
66.8
<99
100
5.34
Yang et al. (2014)
Mn0Ni10
51
98
100
5.20
Tang et al. (2011)
ɣ-MnO2
39
98
100
5.2
Jing et al. (2007)
Ni–Cr hydrotalcite
11
98
100
4.1
Choudhary et al. (2003)
MnOOH
66
99
100
4.1
Tang et al. (2009)
CNTs-1
96.2
88.3
90
3.85
Luo et al. (2012)
1%Ag–MnO2
100
< 99
100
3.33
Adil et al. (2013)
Ni(OH)2
98
98
100
3.3
Ji et al. (2007)
Mn3O4-NiO-Ni/CNTs
32.9
< 99
100
3.29
Yang et al. (2013)
ThO2-(5%)-NiMnO
100
> 99
100
2.96
This work
Ru/Mg–LaO
75
69
120
1.84
Kantam et al. (2012)
Ni–Fe hydrotalcite
4.6
98
100
1.7
Choudhary et al. (2003)
CeO2 (5%)–NiMnO
100
> 99
100
1.48
Sultana et al. (2015)
Au(3%)-NiMnO
100
> 99
100
1.33
Siddiqui et al. (2012)
Pd/AC
48.8
45.9
110
1.30
Wu et al. (2013)
Au/CuO
85.7
< 99
80
1.29
Wang et al. (2013)
CoAl2O4
90.98
86.95
80
1.14
Ragupathi et al. (2015)
Mn10NiO
11
93
100
1.1
Tang et al. (2011)
Mg2.5Ni0.5Al HT
52
97
100
0.4
Kawabata et al. (2005)
Ni–Al hydrotalcite
31
99
100
0.1
Choudary et al. (2001)
MnOx/MCM-41
94
99
80
0.026
Wu et al. (2015)
A similar comparison of specific activity is done with other NiMnO mixed catalysts doped with various other metal oxides reported in literature. It was found that the specific activity of ThO2-(5%)-NiMnO catalyst calcined at 400 °C which is 2.96 mmolg−1h−1 is better than the specific activity of CeO2-(5%)–NiMnO (Sultana et al., 2015) and Au(3%)-NiMnO (Siddiqui et al., 2012) which was found to be 1.48 mmol g−1 h−1 and 1.33 mmol g−1 h−1 respectively. However, when similar comparisons were made with specific activity value of V2O5 (5%)-NiMnO and Zr (5%): NiMnO, it was found that the specific activity of ThO2 (5%)-NiMnO catalyst is less.
4 Conclusions
In this study, we have successfully demonstrated the synthesis of various thoria promoted NiMnO catalysts using a facile co-precipitation method. The as-prepared catalysts were employed for the oxidation of benzyl alcohol using molecular oxygen as oxidant. During this study, it was revealed that the introduction of ThO2 as promoter to the NiMnO mixed oxide catalysts strongly influenced the catalytic performance and thermal stability of the materials. The addition of small amount of thoria on the NiMnO, a MMO catalyst, displayed a significant enhancement in the catalytic conversion of benzyl alcohol to benzaldehyde. Clearly, a synergistic effect between calcination temperatures and the chemical kinetics of the reaction was also observed. Furthermore, it was confirmed that the calcination temperature and the percentage of the promoter play an important role in the catalytic conversion. Among the various thoria promoted NiMnO catalysts prepared, ThO2-(5%)-NiMnO calcined at 400 °C has displayed best catalytic performance, providing a 100% conversion from benzyl alcohol to benzaldehyde. Moreover, from the XRD pattern of the catalyst calcined at 400 °C, it is evident that phase changes appear to be in a transition state in the catalyst at this calcination temperature, which may be responsible for better catalytic performance of the catalyst. Hence, this catalyst can be considered as most efficient among all the synthesized catalysts, including 1%, 3% calcined at (300 °C, 400 °C, and 500 °C). From above surface area analysis of the catalyst, it was concluded that all catalysts have a low surface area and the surface area does not have significant effect on their catalytic performance. From the comparison of specific activity values, it is evident that this catalyst synthesized in not the best, hence further studies will be carried out to improve the catalytic performance of the mixed metal oxides employing various other dopants etc. Moreover, the radioactive property of the element thorium can also act as a deterrent towards their extended use; hence, the radioactivity of the catalyst prepared must be evaluated before subjecting them to further studies.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the research group project No. RG-1436-032.
References
- New thorium-doped YBa2(1−x)SrxThxCu3O7− δ high-Tc superconductors. J. Supercond. Nov. Magn.. 2002;15:231-236.
- [Google Scholar]
- Vanadia supported on nickel manganese oxide nanocatalysts for the catalytic oxidation of aromatic alcohols. Nanosc. Res. Lett.. 2015;10:52.
- [Google Scholar]
- Nano silver-doped manganese oxide as catalyst for oxidation of benzyl alcohol and its derivatives: synthesis, characterisation, thermal study and evaluation of catalytic properties. Oxid. Commun.. 2013;36:778-791.
- [Google Scholar]
- Liquid phase selective oxidation of aromatic alcohols employing nanoparticles of zirconia supported on nickel manganese oxide: synthesis, characterization and catalytic evaluation. Asian J. Chem.. 2013;25:8927-8932.
- [Google Scholar]
- A new form of manganese carbonate for the negative electrode of lithium-ion batteries. J. Power Sources. 2011;196:2863-2866.
- [Google Scholar]
- Oxidative coupling of methane over Ca-and alkali metal-doped ThO2. Appl. Catal., A. 2011;391:205-214.
- [Google Scholar]
- Thorotrast and in vivo thorium dioxide: numerical simulation of 30 years of alpha radiation absorption by the tissues near a large compact source. Phys. Medica.. 2014;30:489-496.
- [Google Scholar]
- Control of metal nanocrystal size reveals metal-support interface role for ceria catalysts. Science. 2013;341:771-773.
- [Google Scholar]
- Promoting effects of thoria and molybdena on cobalt catalysts in the hydrogenation of CO. Catal. Lett.. 1993;22:165-177.
- [Google Scholar]
- The first example of activation of molecular oxygen by nickel in Ni-Al hydrotalcite: a novel protocol for the selective oxidation of alcohols. Angew. Chem. Int. Ed.. 2001;40:763-766.
- [Google Scholar]
- Solvent-free liquid phase oxidation of benzyl alcohol to benzaldehyde by molecular oxygen using non-noble transition metal containing hydrotalcite-like solid catalysts. Catal. Commun.. 2003;4:171-175.
- [Google Scholar]
- An XRD and Fourier-transformed infrared spectroscopy investigation of single and mixed gamma-alumina and thorium oxide. J. Mol. Catal. A: Chem.. 2005;238:72-77.
- [Google Scholar]
- High stability of Ce-promoted Ni/Mg-Al catalysts derived from hydrotalcites in dry reforming of methane. Fuel. 2010;89:592-603.
- [Google Scholar]
- A manufacturing process for shaft and pipe couplings of Fe-Mn-Si-Ni-Cr shape memory alloys. Mater. Des.. 2014;56:878-888.
- [Google Scholar]
- Nature and role of activated molecular oxygen species at the gold/titania interface in the selective oxidation of alcohols. J. Phys. Chem. C. 2014;118:20989-21000.
- [Google Scholar]
- Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem. Soc. Rev.. 2013;42:3371-3393.
- [Google Scholar]
- Transparent conductive thin films of Cd 2 SnO 4 obtained by the sol–gel technique and their use in a solar cell made with CdTe. Sol. Energy Mater. Sol. Cells. 2014;128:150-155.
- [Google Scholar]
- Green oxidation of alcohols by a reusable nickel catalyst in the presence of molecular oxygen. React. Kinet. Catal. Lett.. 2007;90:251-257.
- [Google Scholar]
- Amorphous manganese oxide for catalytic aerobic oxidation of benzyl alcohol. Chin. J. Catal.. 2007;28:1025-1027.
- [Google Scholar]
- Johansson Seechurn, C.C.C., DeAngelis, A., Colacot, T.J., 2015. Introduction to new trends in cross-coupling, in: Colacot, T., New trends in cross-coupling: theory and applications. The Royal Society of Chemistry, 1–19.
- Ruthenium/magnesium-lanthanum mixed oxide: an efficient reusable catalyst for oxidation of alcohols by using molecular oxygen. J. Mol. Catal. A: Chem.. 2012;359:1-7.
- [Google Scholar]
- Selective oxidation of alcohols with hydrogen peroxide catalyzed by tungstate ions (WO4=) supported on periodic mesoporous organosilica with imidazolium frameworks (PMO-IL) Tetrahedron. 2014;70:6114-6119.
- [Google Scholar]
- Nickel containing Mg-Al hydrotalcite-type anionic clay catalyst for the oxidation of alcohols with molecular oxygen. J. Mol. Catal. A: Chem.. 2005;236:206-215.
- [Google Scholar]
- Catalytic oxidation of toluene over nanorod-structured Mn-Ce mixed oxides. Catal. Today. 2013;216:220-228.
- [Google Scholar]
- Selective catalytic dehydration. Thoria-catalyzed dehydration of alcohols. J. Org. Chem.. 1967;32:3386-3389.
- [Google Scholar]
- Selective liquid phase oxidation of benzyl alcohol catalyzed by carbon nanotubes. Chem. Eng. J.. 2012;204:98-106.
- [Google Scholar]
- Flame-made WO3/CeOx-TiO2 catalysts for selective catalytic reduction of NOx by NH3. ACS Catal.. 2015;5:5657-5672.
- [Google Scholar]
- Alpha-Alumina-supported nickel catalysts prepared from nickel acetylacetonate: a TPR study. J. Catal.. 1998;173:257-267.
- [Google Scholar]
- Addition of IrO 2 to RuO 2+ TiO 2 coated anodes and its effect on electrochemical performance of anodes in acid media. Prog. Nat. Sci.. 2014;24:134-141.
- [Google Scholar]
- High-resolution spectroscopy of europium-doped ceria as a tool to correlate structure and catalytic activity. J. Phys. Chem. C. 2014;118:23349-23360.
- [Google Scholar]
- Preferential oxidation of co in a hydrogen rich feed stream using Co-Fe mixed metal oxide catalysts prepared from hydrotalcite precursors. J. Mol. Catal. A: Chem.. 2015;404:167-177.
- [Google Scholar]
- Highly selective oxidation of benzyl alcohol to benzaldehyde with hydrogen peroxide by cobalt aluminate catalysis: a comparison of conventional and microwave methods. Ceram. Int.. 2015;41:2069-2080.
- [Google Scholar]
- Synthesis, characterization and catalytic activity of meso-niobium phosphate in the oxidation of benzyl alcohols. Catal. Today. 2012;192:117-122.
- [Google Scholar]
- Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev.. 2014;114:10613-10653.
- [Google Scholar]
- Preparation, characterization and application of sulfated Ti-SBA-15 catalyst for oxidation of benzyl alcohol to benzaldehyde. Catal. Commun.. 2012;29:87-91.
- [Google Scholar]
- Nano-gold supported nickel manganese oxide: synthesis, characterisation and evaluation as oxidation catalyst. Oxid. Commun.. 2012;35:476.
- [Google Scholar]
- Mixed Ni-Mn-oxide systems as catalysts for complete oxidation: Part II, Kinetic study of liquid-phase oxidation of phenol. Appl. Catal., A. 2003;249:295-302.
- [Google Scholar]
- Ceria doped mixed metal oxide nanoparticles as oxidation catalysts: synthesis and their characterization. Arab. J. Chem.. 2015;8:766-770.
- [Google Scholar]
- Insights into the nature of alumina-supported MnOOH and its catalytic performance in the aerobic oxidation of benzyl alcohol. Catal. Commun.. 2009;10:1122-1126.
- [Google Scholar]
- Catalytic performances of Mn–Ni mixed hydroxide catalysts in liquid-phase benzyl alcohol oxidation using molecular oxygen. Appl. Catal., A. 2011;403:136-141.
- [Google Scholar]
- A combinatorial approach to synthesis of the La0.8Sr0.2Co1−y MnyO3±δ family of perovskite-type mixed conducting metal oxides and characterisation of the surface oxygen mobility. Solid State Ion. 2012;225:182-185.
- [Google Scholar]
- Visible light assisted photocatalytic performance of Ni and Th Co-doped ZnO nanoparticles for the degradation of methylene blue dye. J. Ind. Eng. Chem.. 2014;20:3826-3833.
- [Google Scholar]
- Au-Pd/AC as catalysts for alcohol oxidation: Effect of reaction parameters on catalytic activity and selectivity. Appl. Catal., A. 2009;364:221-228.
- [Google Scholar]
- Pd on carbon nanotubes for liquid phase alcohol oxidation. Catal. Today. 2010;150:8-15.
- [Google Scholar]
- Selective oxidation of alcohols to aldehydes/ketones over copper oxide-supported gold catalysts. J. Catal.. 2013;299:10-19.
- [Google Scholar]
- Catalytic oxidation of benzyl alcohol over manganese oxide supported on MCM-41 zeolite. Chem. Eng. J.. 2015;271:14-22.
- [Google Scholar]
- Palladium on graphene as efficient catalyst for solvent-free aerobic oxidation of aromatic alcohols: role of graphene support. Appl. Catal., B. 2013;136:177-185.
- [Google Scholar]
- Recent development of mixed metal oxide anodes for electrochemical oxidation of organic pollutants in water. Appl. Catal., A. 2014;480:58-78.
- [Google Scholar]
- Solvent-free oxidation of ethylbenzene over hierarchical flower-like core–shell structured Co-based mixed metal oxides with significantly enhanced catalytic performance. Catal. Sci. Tech.. 2015;5:540-548.
- [Google Scholar]
- Tailoring the structure of metal oxide nanostructures towards enhanced sensing properties for environmental applications. J. Colloid Interf. Sci.. 2012;368:41-48.
- [Google Scholar]
- Mn 3 O 4-NiO-Ni/CNTs catalysts prepared by spontaneous redox at high temperature and their superior catalytic performance in selective oxidation of benzyl alcohol. J. Mol. Catal. A: Chem.. 2013;380:61-69.
- [Google Scholar]
- Enhanced catalytic activity of K-birnessite MnO 2 confined in carbon nanotubes for selective oxidation of benzyl alcohol. Catal. Commun.. 2014;46:238-241.
- [Google Scholar]
- Synthesis and characterization of ultra-fine well-dispersed thoria nano spheres through reduction reactions in nitrate bath. Prog. Nucl. Energy. 2015;85:600-604.
- [Google Scholar]
- A thermal stable thorium-doped mesoporous zirconia. Stud. Surf. Sci. Catal.. 2008;174:409-412.
- [Google Scholar]
- Reduced graphene oxide supported Au nanoparticles as an efficient catalyst for aerobic oxidation of benzyl alcohol. Appl. Surf. Sci.. 2013;280:450-455.
- [Google Scholar]
- Bimetallic Au–Pd/MgO as efficient catalysts for aerobic oxidation of benzyl alcohol: a green bio-reducing preparation method. Appl. Catal., A. 2012;439:179-186.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.arabjc.2017.01.017.
Appendix A
Supplementary material
Supplementary data 1
Supplementary data 1







