Propofol-loaded nanomicelle with improved anesthetic, pharmacokinetic, hemocompatibility, safety, and permeation profiles
⁎Corresponding author at: Department of Anesthesiology, the First Affiliated Hospital, Zhengzhou University, NO.1, Jianshe East Road, Zhengzhou 450000, China. yanqiu.ai_2021@yahoo.com (Yanqiu Ai)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Propofol is known as one of the most potential anesthetics which suffer from limited duration of anesthesia water insolubility. Therefore, in the present study, a formulation of propofol- carboxylic acid-poly [ethylene glycol (COO-PEG)]-b-poly[ D,L lactide (PDLA)]-nanomicelle was developed and its characterization was done by transmission electron microscopy (TEM) and dynamic light scattering analysis (DLS) studies. Afterwards, drug release assay was carried out to determine the efficacy of COO-PEG-PLDA nanomicelle in drug delivery systems. Cytotoxic, hemolytic, pharmacokinetic, and permeation assays were also followed by anesthetic efficacy of propofol- COO-PEG-PLDA nanomicelle in the rat hind paw model. The results indicated that the diameter, hydrodynamic radius and zeta potential of fabricated drug-loaded nanomicelles were 30 nm, 73.24 nm and –23.59 mV, respectively and the nanomicelles were stable for a period of 50 min. Also, COO-PEG-PLDA nanomicelle showed a sustained propofol release at physiological pH. Moreover, it was indicated that COO-PEG-PLDA nanomicelle reduced the cytotoxic and hemolytic effects of propofol and increased the steady-state flux (Jss) by 6.64 times (***P < 0.001) and the cumulative amount of propofol permeated per cm2 of esophageal epithelium after 5 h (Q5h) by 6.01 times (***P < 0.001). Furthermore, pharmacokinetic data showed that COO-PEG-PLDA nanomicelles provided several fold increases in plasma concentration of propofol compared to free propofol at different time intervals. Finally, it was shown that COO-PEG-PLDA nanomicelles improved the induction time of anesthesia, anesthesia period and walking time induced by propofol. In conclusion, it may be suggested that encapsulation of propofol into diblock copolymer nanomicelles can be developed as a new novel therapeutic strategy, facilitating future research as a topical anesthetic.
Keywords
Propofol
Diblock copolymer
Nanomicelle
Pharmacokinetic
Hemolytic activity
Permeation
Anesthetic efficacy
1 Introduction
Propofol is a drug with sedative and hypnotic effects that is widely prescribed to induce and maintain general anesthesia (Barr, 1995). Rapid onset of action, rapid recovery even after a long period of time and anti-nausea effect are some of the benefits of this drug (Barr, 1995). Almost all propofol-like drugs have irritating and irritating effects on the skin and mucous membranes (Marik, 2004; Tan and Onsiong, 1998). Propofol is insoluble in water and oily at room temperature, which it is formulated as an oil emulsion in water (Baker et al., 2005).
Controlled drug delivery in the body is one of the most important categories in the pharmaceutical industry (Ramasamy et al., 2017). In the usual methods of taking the drug, the drug will be distributed throughout the body and all parts of the body will be affected and side effects of the drug will occur. Therefore, in some cases, larger amounts of the drug should be prescribed (Ramasamy et al., 2017). With nanotechnology, targeted drug delivery can be achieved and the time, place and speed of drug release can be controlled, and higher efficiency can be achieved while reducing side effects (Patra et al., 2018). According to the definition of ISO TS 18110, any product related to the pharmaceutical industry containing nanomaterials whose function or properties are enhanced with nanotechnology is considered a nano-product (Zhang and Ge, 2018). These drugs show different effects such as anticancer, analgesic, cholesterol and lipid control, anti-inflammatory, antiarrhythmic, nervous system stimulant, anticoagulant and anesthetic (Zhang and Ge, 2018). There are several active companies that have used nanotechnology in various ways to create or improve various properties of drugs (Shapira and Wang, 2009). Liposomes, micelles, lipids, proteins, polymers and nanocrystals are among the nanomaterials used in patented drugs. Polymeric-based formulations can be used as potential strategies to reduce the side effects of propofol (Kwon, 2003; Wakaskar, 2018). However, the current formulation, in addition to painful injections, has other disadvantages such as microbial contamination, hyperlipidemia, anaphylaxis, which occurs in long-term prescriptions (Baker et al., 2005; Feng et al., 2017). Therefore, the effort to introduce a better emulsifier has been conducted so far.
Polymeric micelles resulting from self-assembly of copolymers are potential platforms in drug delivery (Kwon and Okano, 1996; Deshmukh et al., 2017). Variety of synthesis and ease of joining functional groups are two extremely attractive features of these systems (Kwon and Okano, 1996; Deshmukh et al., 2017). Key features of copolymeric micelles include a hydrophobic site to dissolve hydrophobic drugs and a hydrophilic cover to stabilize the loaded drug from external stressors (Kwon and Okano, 1996; Deshmukh et al., 2017). In addition to the ability of polymeric micelles to act as a carrier of hydrophobic drugs, the nanometer diameter of drug-carrying micelles provides potential advantageous relative to free drugs (Kwon and Okano, 1996; Deshmukh et al., 2017).
Therefore, in this paper, the propofol-loaded diblock copolymer micelles were fabricated, characterized and their anesthetic effects were evaluated.
2 Materials and methods
2.1 Materials
Propofol, carboxylic acid-poly [ethylene glycol (COO-PEG)]-b-poly [ D, L lactide (PDLA)], PEG average Mn 5000, PDLA average Mn 16,000 were purchased from Sigma Company (St Louis, USA). All chemicals were analytical grade and distilled water was employed in all assays.
2.2 Preparation of propofol-COO-PEG-PLDA nanomicelle
Propofol (5 mg) and COO-PEG-PLDA (30 mg) were hydrated in methanol (10 mL), followed by addition of distilled water dropwise to the solution, incubation at 50 °C for 10 min, vortex for 10 s, filtered (0.22 µm) and finally freeze-dried. Afterward, 5 mg of propofol-COO-PEG-PLDA nanomicelle was rehydrated in distilled water and the propofol loading capacity and nanoparticle yield was calculated based on the reported equations in the previous reports (Wang et al., 2017; Li et al., 2017) using UV/VIS absorbance of at 285 nm (Shimadzu UV-1900i, Japan).
2.3 Characterization of propofol-COO-PEG-PLDA nanomicelle
The characterization of propofol-COO-PEG-PLDA nanomicelle was done by transmission electron microscope (TEM) (Zeiss-EM10C-100 KV, Germany) and dynamic light scattering (DLS) (ZEN314, England) analysis. Following lyophilization, propofol-COO-PEG-PLDA nanomicelle was rehydrated at 0.1 mg/mL distilled water, centrifuged (2500 rpm) and analyzed.
2.4 Drug release assay
The propofol release assay was done by a UV/VIS absorbance of at 285 nm (Shimadzu UV-1900i, Japan) using two different pH: 7.4 and 4.5, which represent the pH of blood and lysosomes and endosomes (Kievit et al., 2011), respectively. 2 mL of propofol-COO-PEG-PLDA nanomicelle solution poured into dialysis bags (MWCO 3.5 kDa) and placed in 48 mL of release medium at 37 °C for 120 min. At different time intervals, 0.5 mL of release medium was withdrawn and propofol concentration was detected at 285 nm.
2.5 Cytotoxic effect against human peripheral blood mononuclear cells (PBMCs)
This assay was done to assess the effect of the propofol in the form if free or nano-formulated state on normal human cells. To isolate the PBMCs, fresh blood samples (10 mL) were collected under medical and ethical committee control from healthy volunteer donors and the cells were isolated through Ficoll-hypaque density centrifugation method. The cytotoxic effect was then evaluated by the MTT assay.
2.6 MTT assay
After incubation of cells with serial concentrations of propofol or propofol-COO-PEG-PLDA nanomicelle (1, 2, 5, 10, 20, 50, 100, and 200 μg/mL) for 24 h at 37 °C and 5% CO2, a fixed volume of medium was gently removed and replaced by 20 μL of MTT solution (5 mg/mL of PBS) and incubated for 4 h. The plates were then treated with 200 μL of DMSO for 5 min at 37°Cfolowed by reading the absorbance of samples at 540 nm using a Multiskan MK3 microplate reader (Labsystems, Helsinki, Finland).
2.7 Hemolytic assay
Fresh human red blood cells (RBCs) were centrifuged (3000 rpm, 10 min) at 4 °C, washed, diluted 12-fold with PBS, added to a 200 μL of propofol or propofol-COO-PEG-PLDA nanomicelle in PBS (pH 7.4) at varying concentrations of 1, 2, 5, 10, 20, 50, 100, and 200 μg/mL. PBS and distilled water were used as negative and positive controls, respectively. The solutions were vortexed, incubated at 37 °C for 2 h, and centrifuged (3000 rpm, 5 min). A total of 100 μL of supernatant was then removed and the absorbance values of the supernatants were read at 540 nm employing a Multiskan MK3 microplate reader (Labsystems, Helsinki, Finland). The zero hemolysis (0%) and the total hemolysis (100%) was assigned when the samples were in the presence of PBS and distilled water, respectively.
2.8 Permeation assay
In vitro permeation of 1% propofol solution or 1% propofol-COO-PEG-PLDA nanomicelle across rat esophagus epithelium was caried out in Franz type vertical diffusion cells with permeation area of 1.98 cm2 and receptor compartment of 10 mL in volume under constant magnetic stirring (300 rpm) at 37 °C for 5 h. The epithelium was placed between chambers over a cellulose filter without any damage to the barrier. The compartments were filled with filtered/degassed PBS (pH 7.4) and the donor chamber buffer was replaced by 1 mL of either % propofol solution or 1% propofol-COO-PEG-PLDA nanomicelle. The samples were analyzed by HPLC and the steady-state flux (Jss) and lag time (tlag) were obtained by the linear portion of the cumulative amount of propofol permeated per cm2 of esophagus epithelium (Q) over time.
2.9 Pharmacokinetic assay in vivo
Female Sprague-Dawley rats (250 g) were randomly divided into four groups and were given a single injection of free propofol (150 mg/kg, over 5 min, 0.4 mL) or propofol-COO-PEG-PLDA nanomicelle (150 mg/kg propofol-equivalent dosing dissolved in 150 mM NaCl (n = 10). Two control groups received 150 mM NaCl and equivalent dose of COO-PEG-PLDA nanomicelle (n = 8). About 0.25 mL of blood was collected through the catheter after different time intervals (0,2, 5, 10, 20, 30, 50, 100, and 120 min), centrifuged (2000 rpm, 10 min), diluted plasma 1: 4 in cold 0.1% phosphoric acid in methanol, vortexed, centrifuged (13,000 rpm,10 min), and analyzed by HPLC. Animals were handled in accordance with ethical rules approved by Department of Anesthesiology, Zhengzhou University, China.
2.10 Hind paw model
After pacing the rats in wired mesh floor cage 45 min, enhancing forces were applied to the wound hyperalgesic based on previous study (Grant et al., 1997). Hyperalgesic response was defined based on the paw withdrawal with a force 20% of baseline. Afterwards, wounds were infiltrated with 0.3 mL propofol 1%, propofol-COO-PEG-PLDA nanomicelle (n = 10) 1% or controls (NaCl or nanomicelle, n = 8). The rats were then exposed to the forces and the absence of reflex (paw withdrawal) was considered as anesthesia (Grant et al., 1997). In this study, the time interval between drug injection and the balance reflex dilution was recorded as the induction time of anesthesia. The interval between the loss of the balance reflex and its return was the length of the anesthesia period. The interval between the disappearance of the balance reflex and the animal's ability to walk again after anesthesia was defined as the walking time.
2.11 Statistical analysis
All data are expressed as mean ± standard deviation (SD) of three independent assays. Statistical analysis of significance was reported based on Student’s t test. P < 0.05 is considered as statistically significant.
3 Results and discussion
3.1 Characterization of propofol-COO-PEG-PLDA nanomicelle
The TEM photo of propofol-COO-PEG-PLDA nanomicelle is depicted in Fig. 1A. They show a smooth spherical morphology with a diameter of about 20–30 nm. To explore the colloidal stability, DLS study was run to determine the hydrodynamic radius, polydispersity index (PDI) and zeta potential values of fabricated propofol-COO-PEG-PLDA nanomicelle. Results shown in Fig. 1B indicated that the particles of COO-PEG-PLDA nanomicelle and propofol-COO-PEG-PLDA nanomicelle are 63.29 nm (PDI: 0.193) and 73.24 nm (PDI: 0.201), respectively. Also, zeta potential values indicated that the charge distribution of COO-PEG-PLDA nanomicelle and propofol-COO-PEG-PLDA nanomicelle samples are −19.67 mV and –23.59 mV, respectively (Fig. 1C). The data indicated that loading of nanomicelles by propofol slightly increases the hydrodynamics radius and zeta potential values of complex, pointing out the potential loading of drug and colloidal stability of the nano-based system.
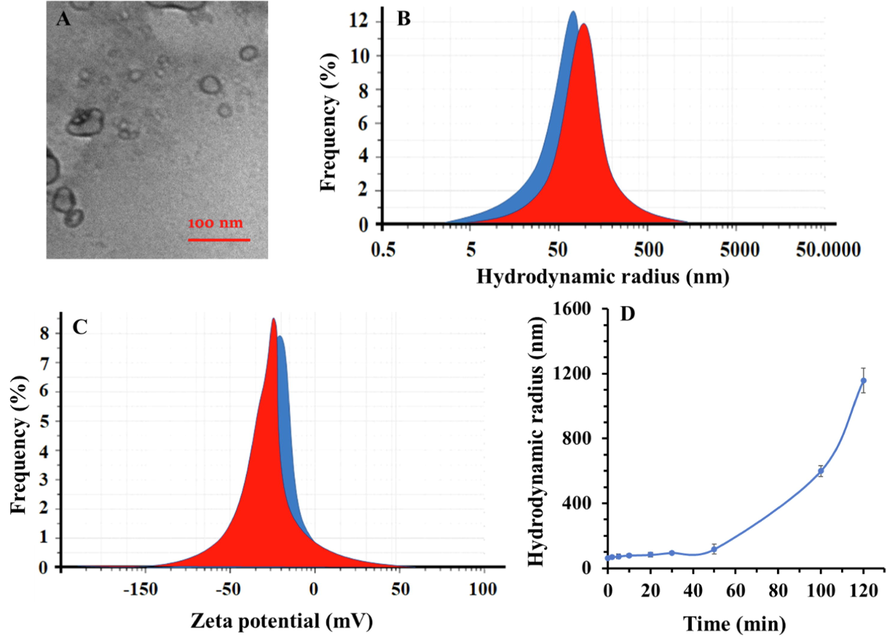
- (A) TEM image of propofol-COO-PEG-PLDA nanomicelle, (B) hydrodynamic radius analysis of COO-PEG-PLDA nanomicelle (blue) and propofol-COO-PEG-PLDA nanomicelle (red), (C) zeta potential value of COO-PEG-PLDA nanomicelle (blue) and propofol-COO-PEG-PLDA nanomicelle (red), (D) hydrodynamic radius of propofol-COO-PEG-PLDA nanomicelle over time (120 min). Data are reported as mean ± SD (n = 3).
Also, to explore the stability of propofol-COO-PEG-PLDA nanomicelle, DLS study was run for 120 min and during different time intervals, hydrodynamic radius was determined. As shown in Fig. 1D, the hydrodynamic radius of propofol-COO-PEG-PLDA nanomicelle is almost constant for 50 min, afterward it sharply increases, implying that the structure integrity of nanocomplex is stable during 50 min.
3.2 Drug release assay
It was found that the encapsulation efficiency and the drug loading capacity of propofol-COO-PEG-PLDA nanomicelle were 49.83 ± 7.65% and 57.79 ± 8.94%, respectively with a nanoparticle yield of 86.91 ± 3.63%.
Drug release assay was performed at two different buffer solutions (pH 7.4 and pH 4.5) individually. The cumulative propofol release from COO-PEG-PLDA nanomicelles was shown to reach 100% at pH 4.5 after 30 min, whereas at pH 7.4 the drug release was about 97% after 120 min (Fig. 2). Obviously, the nanomicelles showed a higher drug release ratio in the acidic microenvironment while a lower propofol release in the physiological pH. Indeed, the propofol-COO-PEG-PLDA nanomicelles are stable in physiological condition, limiting the release of propofol. However, the nanomicelles can dissolve into the acidic solution, which destabilizes different bonds cleavage, therefore the nanomicelles are destabilized and increase the release rate of propofol.
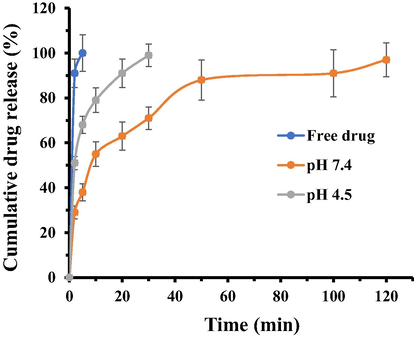
- The in vitro cumulative propofol released from the COO-PEG-PLDA nanomicelles in different pH media over a time period of 120 min. Data are reported as mean ± SD (n = 3).
3.3 MTT assay
The in vitro cytotoxic activity of propofol and propofol- COO-PEG-PLDA nanomicelles against PBMCs was measured by the MTT assay at serial concentrations. It is displayed in Fig. 3 that the cytotoxic activity of the both propofol and propofol- COO-PEG-PLDA nanomicelles was dose dependent. In fact, as long as the concentration enhanced, the percentage of cell viability reduced. However, the cytotoxic effect was different for propofol and propofol- COO-PEG-PLDA nanomicelles; the percentage of viable PBMCs incubated with free propofol has reduced to 50% at a concentration of 200 μg/mL. However, the propofol- COO-PEG-PLDA nanomicelles was less cytotoxic, with the concentration causing 50% of viable population equal to >200 μg/mL.
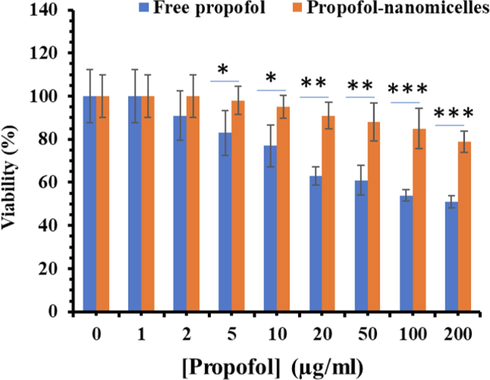
- Cytotoxic effects of propofol and propofol-COO-PEG-PLDA nanomicelles at different propofol concentrations ranging from 1 to 200 μg/mL, which were incubated with PBMCs at 37 °C for 24 h (n = 3). *P < 0.05, ** P < 0.01, ***P < 0.001.
3.4 Hemolytic assay
The free propofol revealed a dose-dependent hemolytic activity which enhanced rapidly with the increase of propofol concentration. The hemolysis percentage of propofol reached 44.1% at the propofol concentration of 200 μg/mL, whereas the hemolysis percentage of propofol-COO-PEG-PLDA nanomicelles was around 21% at similar concentration of propofol (Fig. 4). Thus, it can be deduced that COO-PEG-PLDA nanomicelles had superiority in hemocompatibility and could be used as a potential candidate for intravenous administration.
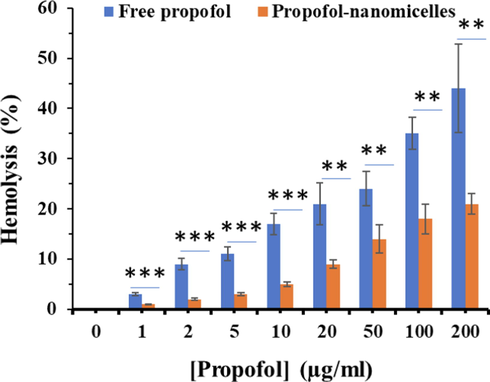
- Hemolysis percentages of propofol and propofol-COO-PEG-PLDA nanomicelles at different propofol concentrations ranging from 1 to 200 μg/mL, which were incubated with human RBCs at 37 °C for 2 h (n = 3). ** P < 0.01, ***P < 0.001.
3.5 In vitro permeation assay across rat esophageal epithelium
In order to improve the buccal permeation of topical formulations, in the present study, we demonstrated the efficacy of COO-PEG-PLDA nanomicelles to increase propofol permeation across the rat esophagus epithelium. It was calculated that the COO-PEG-PLDA nanomicelles increased the Jss by 6.64 times (***P < 0.001) and the Q5h by 6.01 times (***P < 0.001) (Table 1). Besides the improved permeation, a lack of lag time was also observed, characterizing an immediate transport of the drug.
| Formulation | Jss (μg·cm·h) | ERa | Q5h (μg·cm) | ERb | R2 |
|---|---|---|---|---|---|
| Propofol | 60.08 ± 10.08 | 1.0 | 151.20 ± 12.91 | 1.0 | 0.94 |
| Propofol- nanomicelle | 419.81 ± 70.37*** | 6.33 | 921.02 ± 128.57*** | 6.88 | 0.97 |
a Enhancement ratio between the steady-state flux of propofol-COO-PEG-PLDA nanomicelle in comparison with free drug.
b Enhancement ratio between the cumulative amount of propofol permeated per cm2 of oesophageal epithelium after 5 h of propofol-COO-PEG-PLDA nanomicelle in comparison with free drug.
*** P < 0.0001.
3.6 Pharmacokinetic assay in vivo
In order to assess the integrity of the COO-PEG-PLDA nanomicelles in vivo, the concentration of propofol in plasma was estimated over the course of 120 min (Fig. 5). The data indicated that the COO-PEG-PLDA nanomicelles provided 2.01-, 1.91-, 2.20-, 2.43- and 1.74-fold increase in plasma concentration of propofol compared to free propofol at different time intervals of 2, 5, 10, 20, and 30 min, respectively. The data also indicated that after 50 min, the concentration of free propofol was zero, whereas COO-PEG-PLDA nanomicelles provided 3.5 mg/mL propofol even after 120 min. These data showed that the pharmacokinetic properties of propofol upon loading into COO-PEG-PLDA nanomicelles greatly increased in comparison with free propofol.
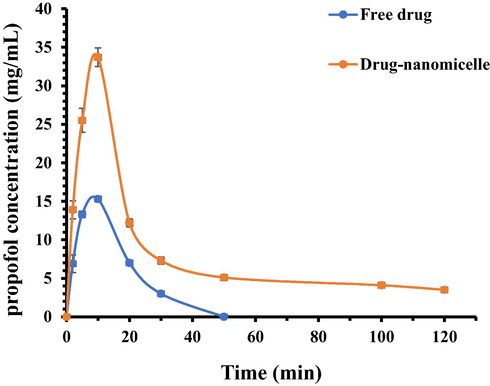
- Pharmacokinetics of free propofol or propofol- COO-PEG-PLDA nanomicelles in rats. Data are reported as mean ± SD (n = 3).
3.7 Anesthetic efficacy
In the present study, induction time of anesthesia (Fig. 6A), anesthesia period (Fig. 6b) and walking time (Fig. 6C) was determined. As shown in Fig. 6, after the injection, induction time of anesthesia (Fig. 6A), anesthesia period (Fig. 6b) and walking time (Fig. 6C) were significantly (**P < 0.01) different from those of free drug, indicating the improvement of the anesthetic efficacy of propofol in the form of nanomicelle. Indeed, the encapsulation of propofol in COO-PEG-PLDA nanomicelles increased anesthetic success (**P < 0.01) of propofol.
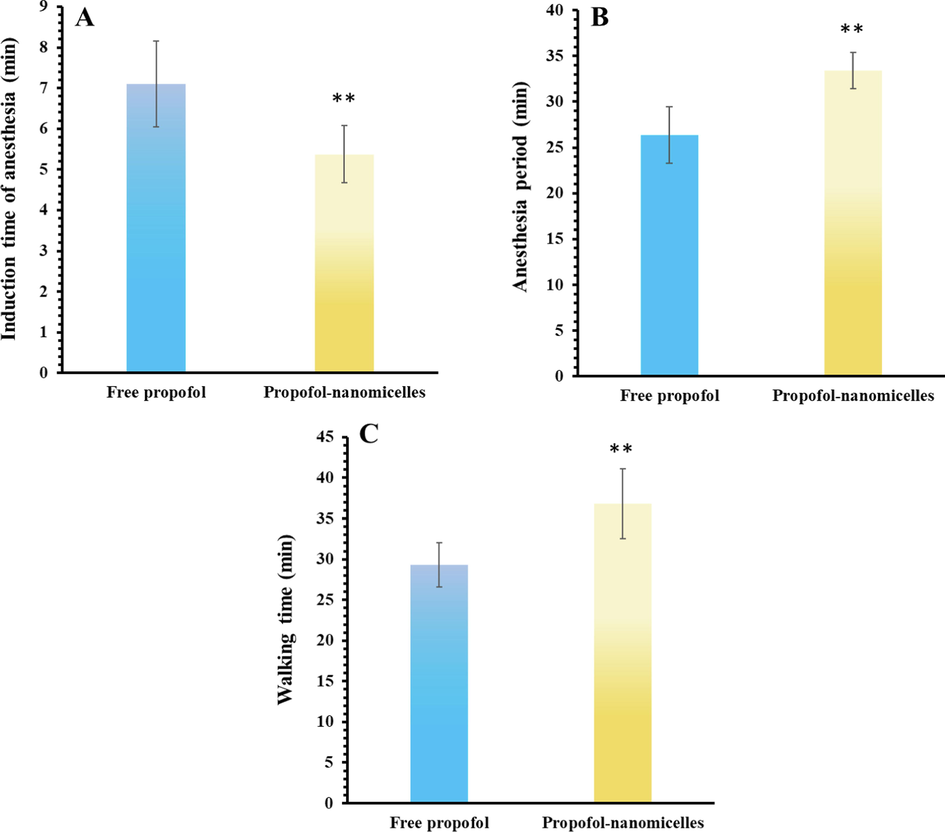
- (A) Induction time of anesthesia, (B) anesthesia period, (C) walking time after infiltration of free propofol or propofol- COO-PEG-PLDA nanomicelles in rat paws. Data are reported as mean ± SD (n = 3), ** P < 0.01.
Based on our result, it was indicated that the COO-PEG-PLDA nanomicelles improved the pharmacokinetic properties of propofol, which may result in a minimization in consecutive injections. It was also found that drug-loaded nanomicelles had lower cytotoxic and hemolytic effects and greater permeation features. There are two probable explanations for this profile. Propofol could be entirely covered by nanomicelles, which can result in the greater permeation of propofol and inhibition the interaction of RBCs with propofol (Shao et al., 2010). In addition, COO-PEG-PLDA nanomicelles could provide potential safety and hemocompatibility due to long-term sustained release and preventing the accumulation of the high concentration of propofol at target site (Liu et al., 2016).
In this study, rat esophageal mucosa was used as a potential target due to close permeability characteristics to those of human tissue. Indeed, esophageal mucosa can be separated very easily and provide a high surface area surface for permeation assays (Del Consuelo et al., 2005). However, it should be noted as the target site of propofol is CNS (Rehberg and Duch, 1999) and the presence of some histological differences between this barrier and blood-brain barrier (Del Consuelo et al., 2005), some experiments should be conducted in the future studies to compare the transport of propofol in the form of free or nano-formulated states across esophageal mucosa and blood brain barrier.
Although we did not assess the different effects of free or nano-formulated states of propofol after topical or intravenous injection, it should be noted that both application approaches can mitigate compound action potentials (CAPs) peak intensity based on nerve conduction. However, the half-maximal inhibitory concentration (IC50) as a marker of propofol’s efficacy depends on the chemical structure and how to use this medicine topically or by injection (Magori et al., 2019).
Different formulations have been developed to date including lipid-based emulsions, non-lipid formulations (such as surfactants, nanoparticle carriers and cyclodextrins), and prodrugs (Baker et al., 2005).
Formulations containing long-chain triglycerides (LCTs) are the most widely available propofol formulations, the most common of which is Diprivan (Sarkar et al., 2016). This formaldehyde has been shown to reduce the common side effects of free propofol and also increases the anesthetic efficacy (Kirkpatrick et al., 1988; Picard and Tramèr, 2000). There is another type of formulation whose lipid content is a combination of medium chain triglycerides (MCTs) and LCT oils. Propoven and propofol-®Lipuro are the most famous emulsions in this category (Kirkpatrick et al., 1988; Kam et al., 2004).
Propofol iodine-free triglyceride microemulsions (Aquafol) contain non-ionic and co-surfactants for emulsifying propofol in water (Lee, 2010). Pharmacokinetic and pharmacodynamic properties of this product are significantly similar to the common formulation (Diprivan), but have more pain during injection (Picard and Tramèr, 2000; Jung et al., 2010). Another lipid-free product is provided which contains sulfobutyl-ether-β-cyclodextrin and water, but this formulation has not been successful in reducing pain during injection (Babu and Godiwala, 2005). By adding the phosphate group to the propofol molecule, propofol can be dissolved in water. So far, two types of propofol phosphorylated prodrugs have been synthesized: propofol phosphate and phosphonooxy methyl propofol 4 (2 and 6 diisopropyl phen-oxymethyl phosphate) (Bengalorkar et al., 2011; Dinis-Oliveira, 2018). The prodrugs must first be converted to propofol in the presence of alkaline phosphatase to begin its sedative effects, which is present in the vascular and hepatic endothelium and is responsible for the conversion of pro-propofol to propofol. Therefore, activation of prodrug takes 1–4 min after injection, while the anesthetic effect of the usual formulation is much faster (Bengalorkar et al., 2011; Dinis-Oliveira, 2018). In general, as these experiments were done in vivo, some pre-clinical and clinical trials are required to further discuss about the potential biomedical application of this nano-based product.
4 Conclusion
In the present study, the development of propofol-COO-PEG-PLDA nanomicelles with great physicochemical characteristics and potential pharmacokinetic, safety, and permeation properties was reported. When compared to free propofol, this nano-based platform also improved the anesthetic effect of this drug. In general, this strategy may pave the way for future application of propofol-COO-PEG-PLDA nanomicelle as a topical nano-based formulation to promote further in vivo clinical trials.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Toward the development of an injectable dosage form of propofol: preparation and evaluation of propofol-sulfobutyl ether 7-β-cyclodextrin complex. Pharm. Dev. Technol.. 2005;9(3):265-275.
- [Google Scholar]
- Propofol: the challenges of formulation. J. Am. Soc. Anesthesiol.. 2005;103(4):860-876.
- [Google Scholar]
- Propofol: a new drug for sedation in the intensive care unit. Int. Anesthesiol. Clin.. 1995;33(1):131-154.
- [Google Scholar]
- Fospropofol: clinical pharmacology. J. Anaesthesiol. Clin. Pharmacol.. 2011;27(1):79.
- [Google Scholar]
- Evaluation of pig esophageal mucosa as a permeability barrier model for buccal tissue. J. Pharm. Sci.. 2005;94(12):2777-2788.
- [Google Scholar]
- Polymeric micelles: basic research to clinical practice. Int. J. Pharm.. 2017;532(1):249-268.
- [Google Scholar]
- Metabolic profiles of propofol and fospropofol: clinical and forensic interpretative aspects. Biomed Res. Int.. 2018;1(1):1-10.
- [Google Scholar]
- Novel propofol derivatives and implications for anesthesia practice. J. Anaesthesiol. Clin. Pharmacol.. 2017;33(1):9.
- [Google Scholar]
- Wound infiltration with liposomal bupivacaine prolongs analgesia in rats. Acta Anaesthesiol. Scand.. 1997;41(2):204-207.
- [Google Scholar]
- Effectiveness, safety, and pharmacokinetic and pharmacodynamic characteristics of microemulsion propofol in patients undergoing elective surgery under total intravenous anaesthesia. Br. J. Anaesth.. 2010;104(5):563-576.
- [Google Scholar]
- Comparison of Propofol-Lipuro with propofol mixed with lidocaine 10 mg on propofol injection pain. Anaesthesia. 2004;59(12):1167-1169.
- [Google Scholar]
- Doxorubicin loaded iron oxide nanoparticles overcome multidrug resistance in cancer in vitro. J. Control. Release. 2011;152(1):76-83.
- [Google Scholar]
- Pharmacokinetics of propofol (diprivan) in elderly patients. Br. J. Anaesth.. 1988;60(2):146-150.
- [Google Scholar]
- Polymeric micelles for delivery of poorly water-soluble compounds. Crit. Reviews™ Therapeutic Drug Carrier Syst.. 2003;20(5)
- [Google Scholar]
- Polymeric micelles as new drug carriers. Adv. Drug Deliv. Rev.. 1996;21(2):107-116.
- [Google Scholar]
- Zonisamide-loaded triblock copolymer nanomicelles as a novel drug delivery system for the treatment of acute spinal cord injury. Int. J. Nanomed.. 2017;12:2443.
- [Google Scholar]
- In vitro and in vivo evaluation of puerarin-loaded PEGylated mesoporous silica nanoparticles. Drug Dev. Ind. Pharm.. 2016;42(12):2031-2037.
- [Google Scholar]
- Inhibition by general anesthetic propofol of compound action potentials in the frog sciatic nerve and its chemical structure. Naunyn-Schmiedeberg's Arch. Pharmacol.. 2019;392(3):359-369.
- [Google Scholar]
- Propofol: therapeutic indications and side-effects. Curr. Pharm. Des.. 2004;10(29):3639-3649.
- [Google Scholar]
- Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol.. 2018;16(1):71.
- [Google Scholar]
- Prevention of pain on injection with propofol: a quantitative systematic review. Anesth. Analg.. 2000;90(4):963-969.
- [Google Scholar]
- Prevention of pain on injection with propofol: a quantitative systematic review. Anesth. Analg.. 2000;90(4):963-969.
- [Google Scholar]
- Smart chemistry-based nanosized drug delivery systems for systemic applications: a comprehensive review. J. Control. Release. 2017;258:226-253.
- [Google Scholar]
- Suppression of central nervous system sodium channels by propofol. J. Am. Soc. Anesthesiol.. 1999;91(2):512-520.
- [Google Scholar]
- Propofol LCT vs propofol MCT-LCT: Randomized controlled trial. Indian J. Clin. Anaesthesia [Internet]. Diva Enterprises Private Limited. 2016;3(2):214.
- [Google Scholar]
- Angiopep-2 modified PE-PEG based polymeric micelles for amphotericin B delivery targeted to the brain. J. Control. Release. 2010;147(1):118-126.
- [Google Scholar]
- From lab to market? Strategies and issues in the commercialization of nanotechnology in China. Asian Business Manage.. 2009;8(4):461-489.
- [Google Scholar]
- General overview of lipid–polymer hybrid nanoparticles, dendrimers, micelles, liposomes, spongosomes and cubosomes. J. Drug Target.. 2018;26(4):311-318.
- [Google Scholar]
- A micelle self-assembled from doxorubicin-arabinoxylan conjugates with ph-cleavable bond for synergistic antitumor therapy. Nanoscale Res. Lett.. 2017;12(1):1-9.
- [Google Scholar]
- Nanotechnology standardization: challenges, current status and perspectives. Chin. Sci. Bull.. 2018;63(35):3697-3705.
- [Google Scholar]







