Translate this page into:
Protective effects and structure-activity relationship of oligopeptide AYAPE from Isochrysis Zhanjiangensis on oxidative stress in HUVECs and SH-SY5Y cells
⁎Corresponding author. zjqian@gdou.edu.cn (Zhong-Ji Qian)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Oligopeptide (AYAPE, AYP) has been isolated from Isochrysis Zhanjiangensis and it has been proven to have good biological activity potential, but its structural characteristics and structure-activity are not yet very clear. As an oligopeptide of five amino acids, the structural characteristics of AYP are the key factors of its activity. Therefore, through evaluating the antioxidant mechanism and stress degree of AYP in different cells, the active action sites were analyzed to explain the antioxidant structure activity relationship of AYP. The results indicate that under oxidative stress, 50 μM AYP can restore 78.53 % of cell viability. AYP through NF-κB and MAPK signaling pathways inhibit inflammation and cell apoptosis. And then, it activates Nrf2/Keap1 pathway, promotes Nrf2 nuclear transfer, and increases the expression of antioxidant enzymes, thus exerting antioxidant effects. In addition, in order to further explore the structure activity relationship of AYP, quantum mechanics and molecular docking were combined for analysis. The results showed that tyrosine, alanine, proline, and glutamic acid contained in AYP can all contribute to antioxidant activity, and the key amino acids that mainly play an antioxidant role are tyrosine and alanine. Therefore, this study provides a new idea for the rational design of marine oligopeptides based on the activity mechanism in the application of food and health products.
Keywords
Oligopeptide
Isochrysis Zhanjiangensis
Antioxidant
Anti-apoptotic
Active site
1 Introduction
Pathological condition known as oxidative stress is brought on by an imbalance between the oxidative and antioxidant systems, which leads to an overproduction of reactive polymers such as reactive oxygen species (ROS) (Li et al., 2021). Lipids, proteins, organelles, and nucleic acids will be harmed by high quantities of ROS in cells, triggering the cell death process (Redza-Dutordoir and Averill-Bates, 2016). Because cells that fail to repair damage or adapt to oxidative stress may enter permanent growth stagnation or die due to apoptosis, it will cause various pathological changes. Thus, therapeutic intervention options for neurological and cardiovascular disorders may be provided by pharmaceutical methods to deal with oxidative stress (Pan et al., 2014). For example, velvet antler polypeptide VAP II has a protective effect on HUVECs damage, which demonstrates that it has an anti-apoptosis impact brought on by oxidative stress and may take part in the mitochondrial pathway's function (Zhu et al., 2017). In addition, studies have shown that the polypeptide PCF of Chlamys farreri evaluated the effect of H2O2 induced neuronal cell death, which can promote endogenous antioxidant defense components, suppress the phosphorylation of MAPK family members and prevent the production of reactive oxygen species (Zhu et al., 2017). However, these studies are to analyze the antioxidant effect of polypeptides at the cellular level. If theoretical studies at the molecular level are combined, the antioxidant mechanism of polypeptides will be more perfect. marine antioxidant peptides and their amino acid modified peptides have potential to alternative antioxidants (Fan et al., 2022). In comparison to enzymatic antioxidants, antioxidant peptides from food offer a few advantages. Due to their having a simpler and more stable structure, and besides antioxidant activity, they also have some other nutritional and functional characteristics (Sarmadi and Ismail, 2010).
Marine biological resources are not only an important component of the marine ecological environment, but also an important source of food and nutrition for humans. For decades, marine natural products such as mollusca, sponges, sea squirts, and marine, microalgae have been developed for their excellent biological activity in the nutrition, pharmaceutical, and cosmetic industries (Oh et al., 2019). Among them, marine microalgae is a single celled algae with photosynthetic and simple reproductive characteristics (Pina-Pérez et al., 2017). They can often be applied in different fields such as biofuels, feed, natural products, food, and pharmaceuticals. (Maeda et al., 2018). In particular, microalgae Isochrysis has potential development prospects for the food industry because of its high fat and protein contents (Bonfanti et al., 2018). Isochrysis Zhanjiangensis is a marine microalgae distributed in the South China Sea. It was first found in Zhanjiang Bay, China, and is often used as bait and feed for aquaculture industry (Chen et al., 2020). In previous studies, the oligopeptide AYAPE (AYP), isolated from Isochrysis Zhanjiangensis, has multiple biological activities and has a significant effect on preventing inflammation and blocking tumor angiogenesis (Tang et al., 2022). It has also been shown to be resistant to ultraviolet radiation and skin aging (He et al., 2022). However, the structural characteristics of peptides that must be considered in the structure–activity relationship have not yet been elucidated.
Studies have shown that peptides with molecular weights between 500 and 1,500 Da have stronger biological activity than other peptide sequences, and their mechanism of action depends on the sequence, composition, and molecular weight of amino acids (Ngo et al., 2013; Qian et al., 2008). Compared with biological activity and mechanism research, comprehensive research on the structural characteristics of polypeptides is relatively lagging behind. Therefore, the analysis of the structural characteristics of bioactive peptides can provide a theoretical basis for the development of their biological activities.
Due to the complex spatial structure of peptides, it is difficult to accurately obtain their active sites and electron transfer pathways through experimental methods. In this case, quantum chemical methods demonstrate greater advantages. Quantum chemical calculations have been widely used in structure–activity relationship studies of active peptides for their ability to describe the physicochemical properties of peptides and to elucidate their electron transfer mechanisms (Fu et al., 2022). Molecular docking can also be used to elucidate the structure–activity relationship and antioxidant Mechanism of peptides (Wen et al., 2021; Vakser. 2014). Therefore, quantum mechanics and molecular docking can be combined to evaluate the action mechanism of peptides.
This study first demonstrates that AYP protects cells from damage by inhibiting oxidative stress. Using VC as a positive control, the cell models of HUVECs and SH-SY5Y cells induced by H2O2 indicate that AYP can reduce ROS levels, inhibit mitochondrial damage, and also inhibit the phosphorylation of the NF-κB/MAPK pathway, protecting cells from apoptosis and inflammation. In addition, it exerts antioxidant effects by activating the Nrf2/Keap1 pathway. Finally, utilizing quantum chemistry and molecular docking to analyze the active sites of peptides provides a theoretical basis for the activity of AYP and links its structure and mechanism of action. This may provide some new ideas for discovering the mode of action and activity basis of marine bioactive oligopeptides (Fig. 1).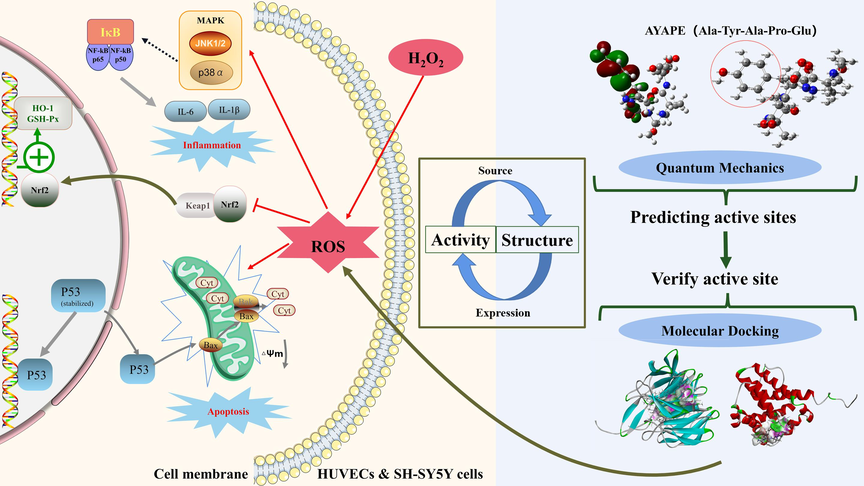
Experimental design of antioxidant mechanism of AYAPE. The left side represents the cellular pathway through which AYP exerts protective effects in two cells, while the right side represents the key site where AYP exerts antioxidant activity. Analyze the antioxidant function of AYP through two levels: cell model and molecular structure.
2 Material and methods
2.1 Materials and chemicals
AYP (Ala-Tyr-Ala-Pro-Glu) came from previous studies (Chen et al., 2020), with a molecular weight of 549.57 Da and a purity of 98.88 %. Human Umbilical Vein Endothelial Cell (HUVEC) and SH-SY5Y were purchased from Suzhou Chuanglian Biotechnology. DCFH-DA fluorescent probe and RIPA buffer were purchased from Shanghai Bioyotime Biotechnology. Mouse antibody JNK (sc-7345), P-JNK (sc-6254), p38 (sc-7972), P-p38 (sc-166182), p65 (sc-8008), P-p65 (sc-1365408), p50 (sc-8414), P-p50 (sc-271908), IκB-α (sc-1643), P-IκB-α (sc-8404), p53 (sc-126), cytochrome c (sc-13156), Nrf2 (sc-365949), Keap 1 (sc-365626), Heme Oxygenase-1 (sc-136960), and secondary antibodies: goat anti-mouse IgG-HRP (sc-2005) and goat anti-rabbit IgG HRP (sc-2004) were products of Santa Cruz Biotechnology (Shanghai, China). Rabbit antibodies Bax (D3R2M) and GPX1 (C8C4) were purchased from Cell Signaling Technology (Danvers, MA). Human IL-6 and IL-1β ELISA kits were obtained from NeoBioscience Technology. Vitamin C, JC-1 kit, and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Solarbio Life Science (Beijing, China). Other chemical reagents are analytical grade.
2.2 Cell culture and viability assay
The HUVECs and SH-SY5Y cells were cultured in 90 % DMEM, 10 % FBS, and 1 % penicillin/streptomycin complete medium and grown in 37 °C, 5 % CO2 incubator. After the cells grew completely, different concentrations of AYP (20, 50, and 100 μM) were added. After 24 h, added 100 μL of 10 % CCK-8 solution. After 30 min incubation at 37 °C without light, measure absorbance at 450 nm with a microplate reader (BioTek, Winooski, VT, USA).
2.3 Enzyme-linked immunosorbent assay
AYP (20, 50, and 100 μM) and VC (200 μM) were cultured with the cells for 2 h, then H2O2 was added. The supernatant was collected after 24 h, and the supernatant was centrifuged (12,000 rpm, 4 °C, 10 min). The concentrations of IL-6 and IL-1β in the supernatant of HUVECs were then determined as prescribed by the manufacturer.
2.4 Detection of reactive oxygen species
The cells were seeded in 24-well plates for growth and cultured with different concentrations of AYP (20, 50, and 100 μM). After 2 h, add H2O2 to achieve the final concentration of 600 μM. Positive control at 50 μg/mL Rosup was added, and after 20 min, cleaned with PBS. DCFH-DA (10 μM) was added to each well, and photos were taken under a Fluorescence microscope after 30 min incubation in dark (Olympus, Tokyo, Japan).
2.5 Detection of mitochondrial membrane potential
The positive control group was given CCCP (10 μM) to pre-treat for 30 min. Incubate the cells in JC-1 working solution for 20 min in the dark at 37 °C, and then add serum-free culture medium. Finally, cells were observed using a fluorescence microscope (Olympus, Tokyo, Japan).
2.6 Annexin-V and PI double staining method
Cells grow on a 24-well plate, and after 24 h of treatment, add 100 μL binding buffer solution. Treat cells with Annexin-V and PI dyes in a dark environment for 10 min, and complete the shooting under a fluorescence microscope within one hour (Olympus, Tokyo, Japan).
2.7 Immunofluorescence
After 24 h incubation, cells were washed with PBS, added 4 % paraformaldehyde, and fixed at 4 °C for 30 min. 0.2 % TritoX-100 was added to infiltrate on ice for 10 min and was sealed in 5 % bovine serum albumin for 1 h. P53 antibody or Nrf2 antibody (1:500) was added overnight at 4 °C. Incubate goat anti-mouse IgG Dylight 488 (1:500) in dark for 2 h. After PBS washing, add DAPI dye to stain the nucleus. Observing cells under a fluorescence microscope (Olympus, Tokyo, Japan).
2.8 Western blot assay
The cells were cultured with AYP (20, 50, and 100 μM) and VC (200 μM) for 2 h, then H2O2 was added to make the final concentration of 600 μM. Cleaned with PBS after 24 h, and cell lysate was added (RIPA: PMSF = 100:1). After obtaining the sample by BCA quantitative method, the protein was separated by SDS-page and transferred to nitrocellulose (NC) membrane. Blocking with 7 % skim milk for 2 h and incubated overnight with primary antibodies (mouse antibody 1:500, rabbit antibody 1:1000). Incubate in secondary antibody (1:2000) for 2 h after washing. At last, the strip was analyzed by the ECL analysis system (Syngene, Cambridge, UK).
2.9 Quantum chemical calculation
Using chembiodraw ultra 14.0, the two-dimensional structure of the polypeptide was drawn. Two dimensional structures were converted to three-dimensional structures using chembio3d ultra 14.0. The molecular force field mm2 was used for preliminary optimization, and the preliminary optimized structure was used for Gaussian calculation. In Gauss 09, the peptide was geometrically optimized using the b3lyp-d3bj level, and dispersion correction was performed to obtain electronic structure information.
2.10 Molecular docking
After obtaining the three-dimensional structure of the polypeptide, mmff94 force field was used to optimize it. From the protein database( https://www.rcsb.org/pages/jobs )download the 3D structures of Keap1 (PDB ID: 2FLU) and Bax (PDB ID: 1F16). Molecular docking and analysis were performed using Discovery Studio software. According to the score and binding energy value, the optimal docking pose of AYP in the two key proteins was obtained.
2.11 Statistical analysis
Perform data analysis using Image J (version 1.46R, NIH) and GraphPad Prism 8.4 (GraphPad Software, San Diego, CA). Compare all data between groups using one-way analysis of variance and Dunnett's multiple comparison test. Data are presented as mean ± standard deviation (n = 3). Gaussian 09 and GaussView 5.0 were used to analyze the electronic properties of AYP.
3 Results and discussion
3.1 Protective effect of AYP on oxidative damage of HUVECs and SH-SY5Y cells
As previously reported (Tang et al., 2022), AYP has a sequence of amino acids and a chemical structure (Fig. 2A). The results of the effects of AYP on HUVECs and SH-SY5Y cells showed that, after the treatment of AYP below 200 μM (10, 20, 50, and 100 μM), the cell survival rate of HUVECs and SH-SY5Y cells did not change significantly, indicating that AYP (20, 50, and 100 μM) acts on cells with non-toxic effects (Fig. 2B). Therefore, follow-up experiments used concentrations of 20, 50, and 100 μM. With the increase of H2O2 concentration, the cell vitality also gradually decreases, the cell survival rate is approximately 50 % when acting on 600 μM (Fig. 2C). But pretreatment with AYP can significantly protect cells from oxidative stress damage under H2O2 stimulation (Fig. 2D). As shown in Fig. 2E, ROS content of cells increased due to oxidative stress, but ROS decreased significantly after pretreatment with AYP (Rosup as a positive control), indicating that AYP has excellent inhibitory effect on oxidative stress.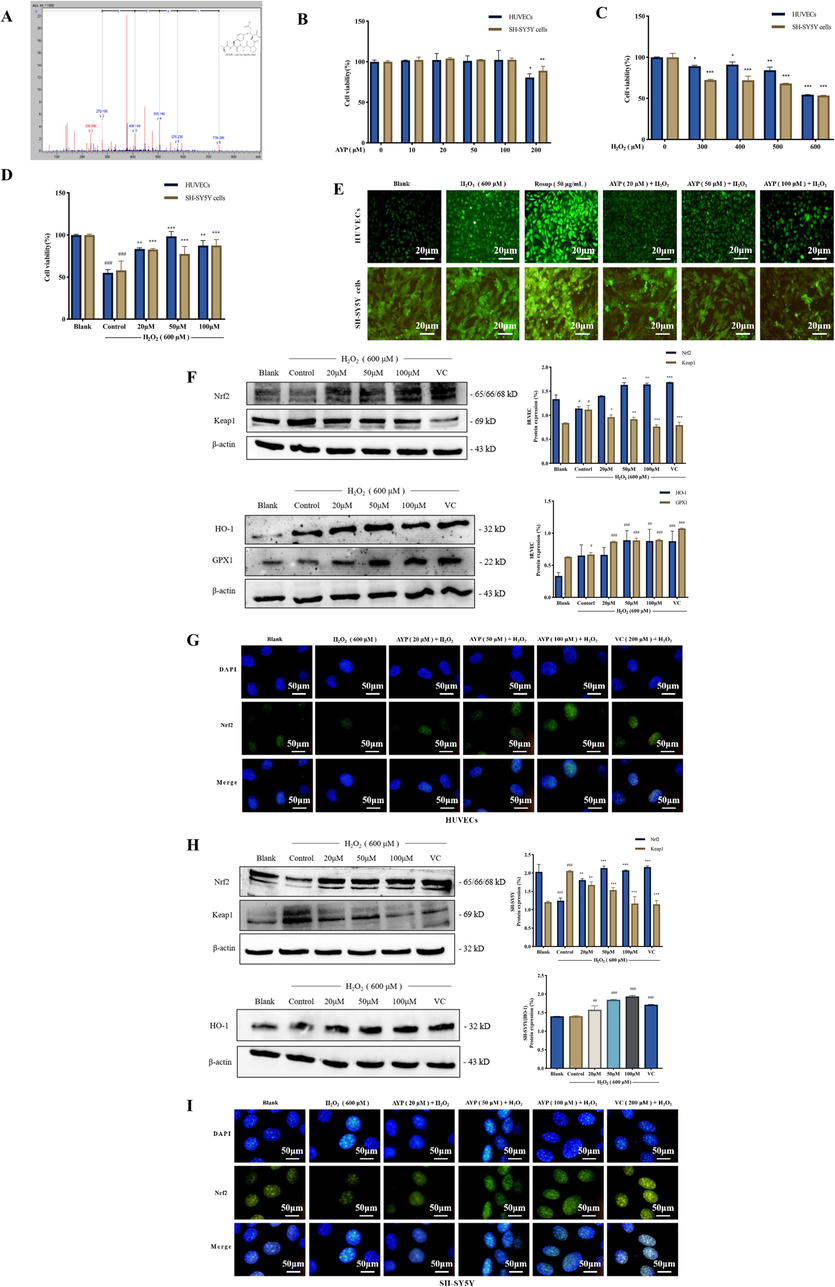
AYP inhibits H2O2-induced oxidative stress in cells. (A) The amino acid sequence and chemical structure of AYP; (B) The effect of AYP on the activity of HUVECs and SH-SY5Y cells; (C) The effect of H2O2 stimulation on the activity of HUVECs and SH-SY5Y cells; (D) The protective effect of AYP preconditioning on HUVECs and SH-SY5Y cells under H2O2 stimulation; (E) The expression of ROS in HUVECs was measured by DCFH-DA fluorescence probe to determine the total level of oxidative stress; (F) The expression of the antioxidant protein in HUVECs was measured by western blotting, and the protein expression was evaluated by Image J; (G) The expression of Nrf2 protein in HUVECs nucleus was observed by staining Nrf2 protein green and nucleus blue with immunocytochemistry; (H) The expression of the antioxidant protein in SH-SY5Y cells was measured by western blotting, and the protein expression was evaluated by Image J; (I) The expression of Nrf2 protein in SH-SY5Y cells was observed by staining Nrf2 protein green and nucleus blue with immunocytochemistry. The data is displayed as mean ± SD (n = 3). #p < 0.05; ##p < 0.01; ###p < 0.001, # compared with the blank group. *p < 0.05; **p < 0.01; ***p < 0.001, *compared with the control group (H2O2 induction group).
3.2 AYP inhibits oxidative stress through the Nrf2/Keap1 pathway
The breakdown of intracellular redox equilibrium caused by oxidative stress-induced apoptotic signaling. This will lead to an increase in reactive oxygen species or a reduction in antioxidants, and permanent oxidative alteration of DNA, proteins, or lipids that results in a number of pathological hazards (Circu and Aw. 2010). A key pathway of cellular resistance to oxidative stress is Nrf2-Keap1 (Sajadimajd and Khazaei. 2018). Numerous antioxidant enzymes controlled by the Nrf2/Keap1 pathway can scavenge reactive oxygen substances and have detoxifying and neutralizing effects. In addition to guarding against oxidative damage from the outside environment, this also strengthens the body's antioxidant capability (Wu et al., 2022). As shown in Fig. 2F and H, it can lower Keap1 levels under condition of oxidative stress and promote Nrf2 expression. Moreover, the expression of antioxidant enzymes HO-1 and GPX1 increased after treatment with AYP, which can resist the damage caused by oxidative stress. Nrf2 is a key factor for cells to resist oxidative stress. The expression of Nrf2 in the nucleus was observed by immunofluorescence (Fig. 2G, I). The results showed that AYP could activate the expression of Nrf2 in the nucleus under H2O2 stimulation, thus increasing the expression of Nrf2 transcriptional antioxidant enzymes such as HO-1 and GPX1 in the nucleus. These results suggest that AYP can protect against oxidative stress by activating Nrf2/Keap1 pathway.
3.3 Inhibitory effect of AYP on apoptosis of HUVECs and SH-SY5Y cells
Excessive ROS under oxidative stress may damage mitochondrial structure, and start crucial apoptotic processes (Zheng et al., 2021). As shown in Fig. 3A, with the increase of the protective concentration of AYP, the number of cells entering the late stage of apoptosis is significantly reduced, and the cells can stay in the early stage of apoptosis. Therefore, it can be preliminarily determined that AYP can effectively inhibit apoptosis. In the early stages of cell apoptosis, the decline in mitochondrial membrane potential is a significant occurrence. It can be observed from Fig. 3B that the mitochondrial membrane potential decreases significantly under the stimulation of H2O2. But the AYP treatment can effectively inhibit this situation, thus reducing the number of cells entering apoptosis (CCCP as a positive control). The expression of pro-apoptosis related proteins was analyzed by western blotting. Overexpression was induced by H2O2. The expression of P53 and cytochrome c proteins decreased in a dose-dependent manner after treatment with AYP (Fig. 3C, E), indicating that AYP effectively inhibited the expression of apoptosis related proteins. P53 is a key protein in the apoptosis pathway. Through immunofluorescence, it can be observed that after pretreatment with AYP, the expression of P53 in the nucleus is significantly reduced (Fig. 3D, F). Therefore, it is possible to conclude that AYP can substantially prevent cell apoptosis following oxidative stress.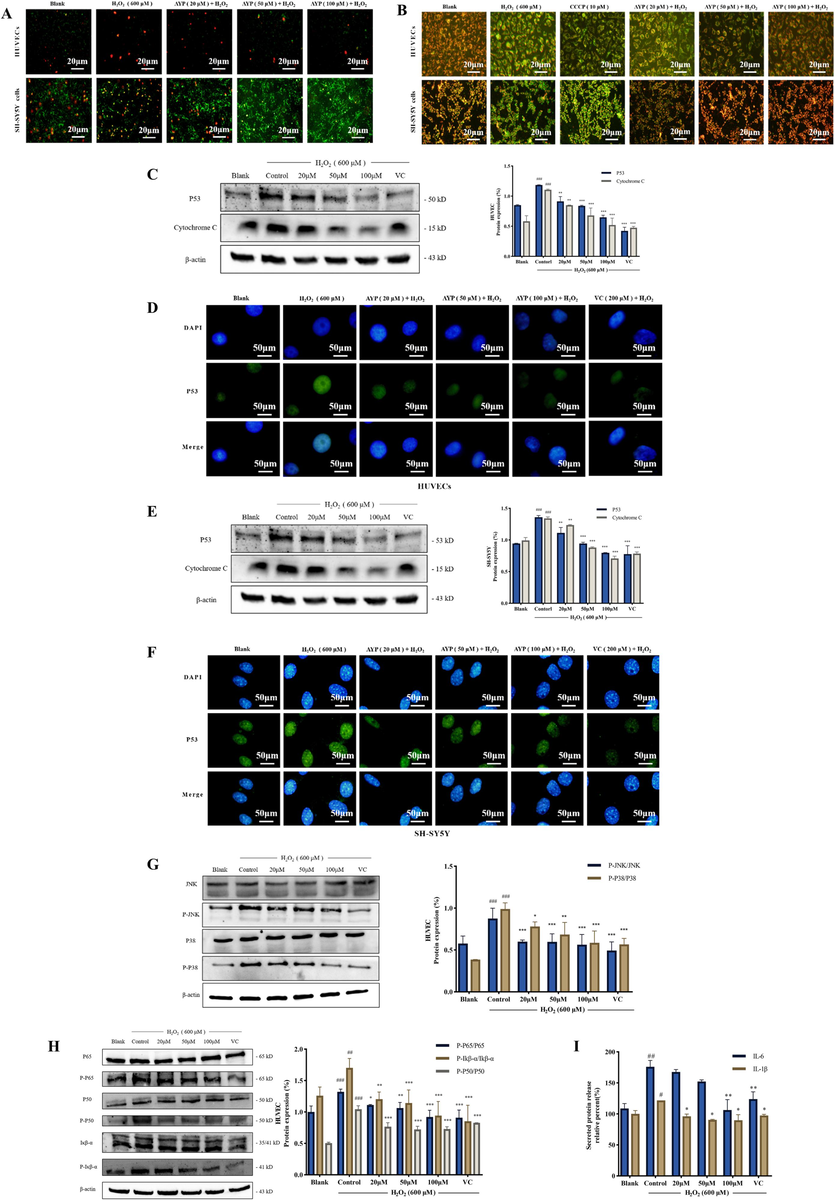
Inhibitory effect of AYP on apoptosis and inflammation pathway. (A) AnnexinV-FITC/PI double staining fluorescence image shows the apoptosis of HUVECs and SH-SY5Y cells. Red indicates late apoptosis, while green indicates early apoptosis; (B) The JC-1 experiment was used to detect the changes in mitochondrial membrane potential. Red indicates normal mitochondria; Green indicates abnormal mitochondria; (C) The expression of the pro-apoptotic protein in HUVECs was measured by western blotting, and the protein expression was evaluated by Image J; (D) Immunocytochemistry was used to observe the expression of P53 protein in HUVECs, by staining P53 protein green and nucleus blue; (E) The expression of the pro-apoptotic protein in SH-SY5Y cells was measured by western blotting, and the protein expression was evaluated by Image J; (F) Immunocytochemistry was used to observe the expression of P53 protein in SH-SY5Y cells, by staining P53 protein green and nucleus blue; (G) The expression of MAPK signal pathway related proteins was analyzed by western blotting, and the protein expression was evaluated by Image J; (H) Western blotting analysis NF-κB signal pathway related proteins was evaluated by Image J; (I) Detection of inflammatory factors IL-6 and IL-1β released by HUVECs with Elisa kit. The data is displayed as mean ± SD (n = 3). #p < 0.05; ##p < 0.01; ###p < 0.001, # compared with the blank group. *p < 0.05; **p < 0.01; ***p < 0.001, *compared with the control group (H2O2 induction group).
Endothelial cells are commonly used to study cardiovascular diseases, while neural cells are important tool cells for studying neural diseases. HUVECs and SH-SY5Y cells are the main research objects, which may help AYP to have greater selectivity under the effect of different diseases. In addition, it is found from the previous experimental results that the results of the two cells in the same experiment are different. Compared with SH-SY5Y cells, AYP has a more significant protective effect on HUVECs oxidative damage. Therefore, under the stimulation of H2O2, AYP can resist strong oxidative stress and ultimately reduce cell apoptosis. But in SH-SY5Y cells, AYP protects cells by enhancing their antioxidant capacity, but cannot significantly inhibit cell apoptosis under strong stimulation. Endothelial cells are upstream of nerve cells, which may induce the differentiation of nerve cells (Wurmser, 2004). In addition, the oxidative stress of endothelial cells is stronger than that of nerve cells, so the antioxidant effect of endothelial cells is stronger.
3.4 Effects of AYP on MAPK and NF-κB signaling pathways in HUVECs
AYP has a strong protective effect on the oxidative stress of HUVECs, so we will further study the anti-apoptotic effect of AYP in HUVECs through MAPK and NF-κB pathway (Fig. 3G-I). P38 and JNK are two important pathways of the MAPK pathway. These pathways have been shown to play an important role in inflammatory responses by inducing nuclear translocation of NF-κB and upregulation of proinflammatory cytokine expression (Yarmohammadi et al., 2021). As shown in Fig. 3G, H2O2 stimulation activated MAPK and increased the phosphorylation of JNK and P38, but these proteins can be affected by AYP to cause a dose-dependent decline. For inflammation-related pathways, H2O2 stimulation activated Iκβ-α phosphorylation and NF-κB activation in the nucleus, and the values of P-P65, P-P50, and p-Iκβ-α remarkably decreased after AYP treatment (Fig. 3H). Furthermore, H2O2 stimulation also induced the release of the pro-inflammatory factors IL-6 and IL-1β, which could be demonstrated by ELISA experiments that AYP inhibited the secretion of IL-6 and IL-1β in HUVECs (Fig. 3I).
3.5 The structural characteristics of AYP
AYAPE (549.57 Da) is an oligopeptide with a molecular weight of less than 1,000 Da and has good antioxidant activity. It is well known that the functional activities of polypeptides are closely related to their amino acid composition, sequence, and quantity. Amino acids in peptides such as Aspartic Acid (D), tyrosine (Y), and tryptophan (W) are thought to have high antioxidant activity (D́avalos et al., 2004). Among them, Tyr has been proposed as a chain-breaking antioxidant, largely attributable to the hydroxyl group in its aromatic structure, which can stabilize ROS through direct electron transfer (Chen et al., 2021). In addition, some scientists have found that adding Pro and Leu to the N-terminal of dipeptide (His-His) can significantly enhance the antioxidant capacity of the peptide, this is mainly due to the synergistic antioxidant effects of hydrophobic and aromatic amino acids (Wen et al., 2020). Therefore, it is speculated that the antioxidant activity of AYP may be related to the synergistic effect of Ala and Tyr. Additionally, research has demonstrated that hydrophobic residues of amino acids, including Pro and Ala, increase the solubility of peptides in the lipid phase, which facilitates the interaction of peptides with free radicals in lipids (Wen et al., 2021). As a result of hydrophobic interactions between hydrophobic amino acids, the peptide's antioxidant activity may be enhanced (Jin et al., 2016), the hydrophobic interaction between Ala-Pro may also play a role in antioxidant activity. Aside from chelating oxidation-promoting metal ions, the acidic amino acid Glu can also form complexes with metal ions through its charged side chain groups (Yang et al., 2019). In general, AYP contains tyrosine, alanine, proline, and glutamate, all of which contribute to antioxidant activity (Fig. 4A).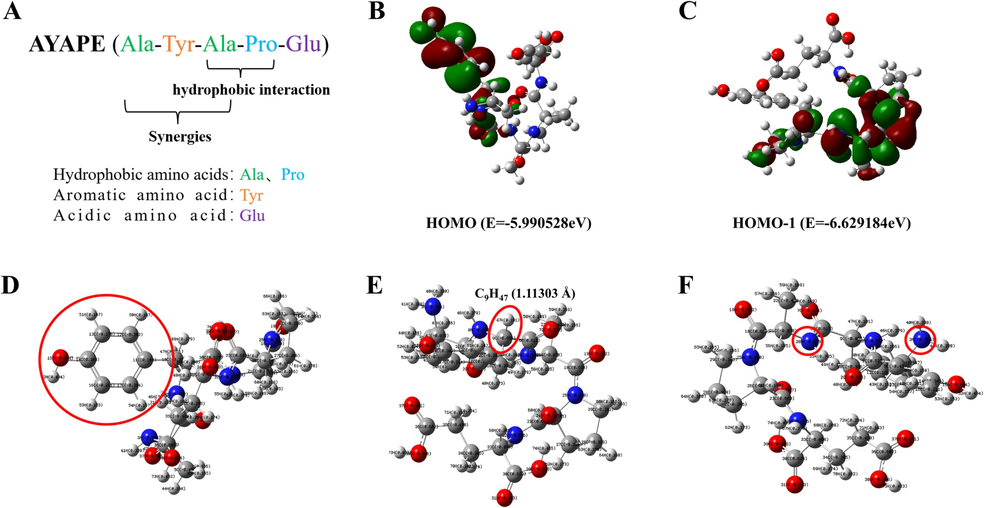
The structure of AYP. (A) The amino acid sequence and composition of AYP; (B-C) Molecular orbital energy prediction of HOMO and HOMO-1; (D-F) charge distribution (positive value is positive charge, negative value is negative charge) and bond length. (Grey: carbon, blue: nitrogen atom; red: oxygen atom; white: hydrogen atom).
3.6 The frontier molecular orbital and charge distributions of AYP
The frontier molecular orbital distribution of AYP is shown in Fig. 4B-C. The HOMO orbital can significantly affect the chemical reaction ability of various saturated and unsaturated compounds. As can be seen from the results, the three molecular orbitals occupying the highest energies are mainly contributed by Tyr and Ala, respectively, illustrating that these positions are most likely to lose electrons first when AYP interacts with the free radical. In addition, since the energies of these molecular orbitals are very close, peptides may rely on multiple active sites for their antioxidant activity. This provides a theoretical basis for antioxidant activity. Some scholars have shown that the smaller the difference in net charge between two atoms affects the weakening of bonds between atoms, and the more likely it is to break, prompting antioxidants to lose hydrogen atoms more readily (Mladenović et al., 2011).
As shown in the Mulliken charge distribution in Fig. 4D-F and Table 1, the charge of O15 (-0.664e) on the phenolic hydroxyl group is relatively minimal compared to other oxygen atoms. Moreover, the two atoms with the smallest difference in electrostatic charge are C9 (-0.056e) and H47 (0.181e), and the bond length of C9H47 is 1.11303 Å, which also facilitates the antioxidant activity of the polypeptide. Previous studies have also shown that the oxygen of phenolic hydroxyl can form p - π conjugation with benzene ring. The negative charge of negative ion oxygen can be dissociated to the carbon of benzene ring, dispersing the negative charge on oxygen. As a result, phenolic hydroxyl is easily able to release its hydrogen ion to terminate the chain reaction caused by free radicals (Wu et al., 2015). Besides, a previous study reported that the significant proportion of free radical scavenging sites for antioxidants are located on atoms with relatively negative charges, which can help antioxidants to provide hydrogen atoms to molecules (Nagaoka et al., 1992). The results showed that the oxygen atom (O15) on the phenol hydroxyl group has the least charge compared with other oxygen atoms, which is also beneficial to the antioxidant ability of the polypeptide. In addition, N3 in Ala1 is the least negatively charged of all atoms, and the C21H56 net charge difference (0.263e) in Ala3 is second only to C4H42 in Ala1. In conclusion, from the results of quantum mechanics, it can be found that tyrosine and alanine in AYP are the main antioxidant amino acids. Among them, the phenolic hydroxyl O–H structure and C9H47 in tyrosine are the most important antioxidant active sites, followed by C4H42 in alanine.
Amino acid
Autom
Charge
Autom
Charge
Ala1
C1
0.625
H40
0.330
O2
−0.595
H41
0.338
N3
−0.764
H42
0.201
C4
−0.072
H43
0.165
C5
−0.457
H44
0.158
H45
0.156
Tyr2
C6
0.613
H46
0.379
O7
−0.573
H47
0.181
N8
−0.592
H48
0.173
C9
−0.056
H49
0.179
C10
−0.396
H50
0.147
C11
0.160
H51
0.147
C12
−0.202
H52
0.434
C13
−0.176
H53
0.153
C14
0.343
H54
0.157
O15
−0.664
C16
−0.211
C17
−0.214
Ala3
C18
0.630
H55
0.395
O19
−0.572
H56
0.185
N20
−0.629
H57
0.156
C21
−0.078
H58
0.149
C22
−0.439
H59
0.169
3.7 Molecular docking between AYP and key proteins
Quantum mechanics can be used to calculate active sites, while molecular force fields can be used to calculate intermolecular and intramolecular interactions (Wu et al., 2021). Using molecular docking results, researchers showed that antioxidant peptides from milk can interact with Keap1, preventing Nrf2 and Keap1 from binding to one another and facilitating Nrf2 nuclear translocation (Tonolo et al., 2020). Therefore, in this study, the molecular docking of AYP with Keap1 and Bax was performed to observe the active site of AYP and verify the prediction of quantum mechanics. This study shows that hydrogen bonding is the main means of interaction between AYP and molecules. In the interaction between AYP and Keap1, a total of seven hydrogen bonds are generated, including four hydrogen bonds at tyrosine, two hydrogen bonds at alanine, and one amino acid at glutamic acid (Fig. 5A). As shown in Fig. 5B, the interaction between AYP and Bax generates seven hydrogen bonds, with Ile175, Asn106, and two alanines each forming a hydrogen bond, and Asp102 forming a hydrogen bond with tyrosine. Asp102 forms a hydrogen bond with proline, while Gly108, Arg109, and Asn104 form four hydrogen bonds with glutamic acid. So, AYP mainly combines with molecules by generating hydrogen bonds. Through western blotting experiments (Fig. 5C-D), it was demonstrated that AYP pretreatment can remarkably reduce expression levels in HUVECs and SH-SY5Y cells, and also demonstrate the interaction between AYP and Bax. Tyrosine and alanine play an important role in it, which is consistent with the prediction results of quantum mechanics.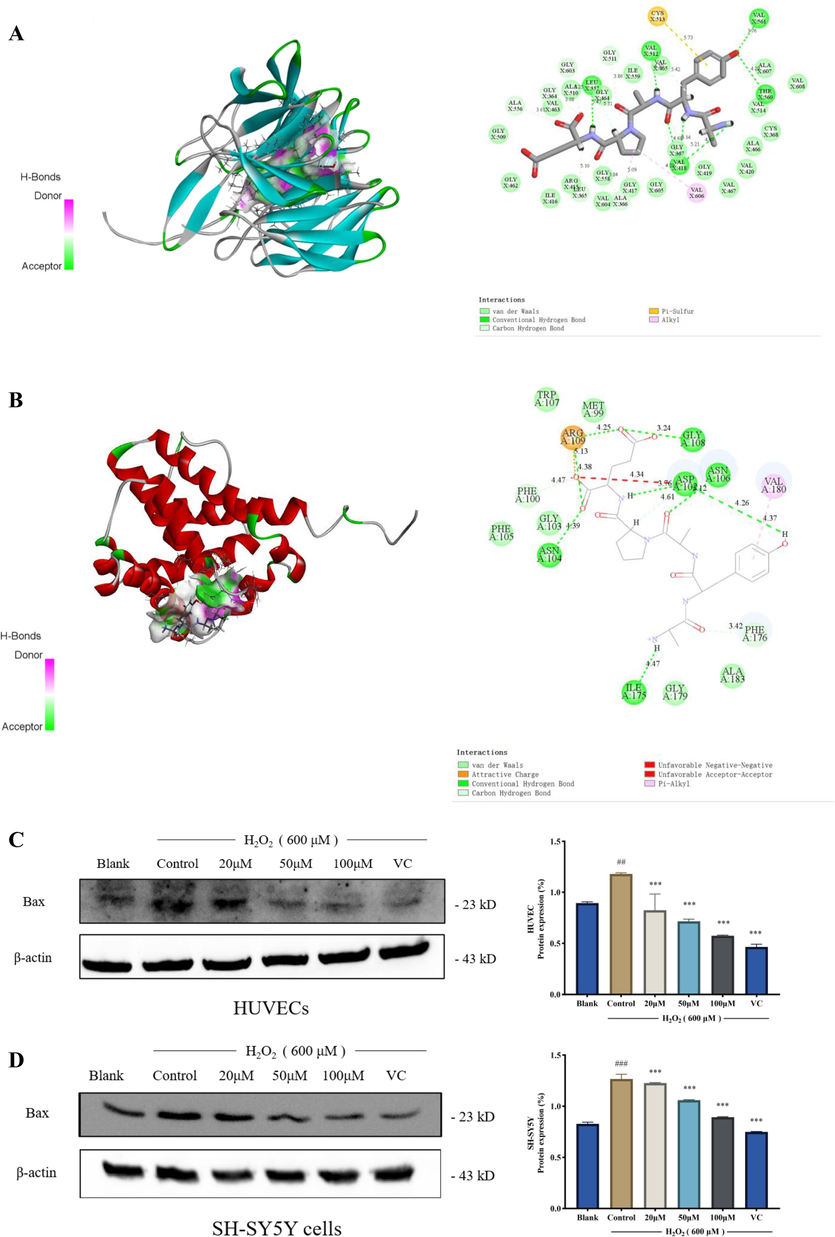
Molecular docking model of AYP with Keap1 and Bax. (A) 3D docking diagram of AYP and Keap1; (B) 3D docking diagram of AYP and Bax. (Green dotted line: hydrogen bond; pink dotted line: alkyl; red dotted line: unfavorable positive; yellow dotted line: salt bridge); (C) The expression of Bax protein in HUVECS was analyzed by western blotting, and the protein expression was evaluated by Image J; (D) The expression of Bax protein in SH-SY5Y cells was analyzed by western blotting, and the protein expression was evaluated by Image J. The data is displayed as mean ± SD (n = 3). #p < 0.05; ##p < 0.01; ###p < 0.001, # compared with the blank group. *p < 0.05; **p < 0.01; ***p < 0.001, *compared with the control group (H2O2 induction group).
4 Conclusions
This study provides new important information and further application ideas for AYP. As a marine microalgae oligopeptide with low molecular weight and high activity, AYP has a broad application prospect. In this study, a large number of cell experiments have proved that AYP has good antioxidant and anti-apoptotic activities. The cell model of oxidative stress induced by H2O2 in HUVECs and SH-SY5Y cells was used, and found that AYP has significant antioxidant effect and has different activity in the two cells, which may indicate that AYP can play different physiological roles in cells. Using the verification method of quantum mechanics and molecular docking, it further analyzed the source and mechanism of its activity from the structural level. The results found that tyrosine and alanine in AYP may be its active sites. In a word, this research could offer a fresh perspective on the origin and use of bioactive peptides. Besides the food industry, it has the potential to expand its healthful value in pharmaceuticals as well.
Acknowledgments
The research was funded by the Cultivation of Guangdong College Students’ Scientific and Technological Innovation (“Climbing Program” Special Funds, pdjh2022a0232) and Guangdong Province College Student Innovation and Entrepreneurship Training Program Project (S202210566067).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Potential of microalga Isochrysis galbana: Bioactivity and bioaccessibility. Algal Res.. 2018;29:242-248.
- [CrossRef] [Google Scholar]
- Mechanism Analysis of a Novel Angiotensin-I-Converting Enzyme Inhibitory Peptide from Isochrysis zhanjiangensis Microalgae for Suppressing Vascular Injury in Human Umbilical Vein Endothelial Cells. J Agric Food Chem.. 2020;68(15):4411-4423.
- [CrossRef] [Google Scholar]
- Purification of novel antioxidant peptides from myofibrillar protein hydrolysate of chicken breast and their antioxidant potential in chemical and H2O2-stressed cell systems. Food Funct.. 2021;12(11):48974908.
- [CrossRef] [Google Scholar]
- Reactive oxygen species, cellular redox systems, and apoptosis. Free Radical Bio Med.. 2010;48(6):749-762.
- [CrossRef] [Google Scholar]
- D́avalos, A., Miguel, M., Bartoloḿe, B., & Ĺopez-Fandĩno, R. (2004). Antioxidant activity of peptides derived from egg white proteins by enzymatic hydrolysis. J. Food Prot. 67(9), 1939–1944. 10.4315/0362-028X-67.9.1939.
- Antioxidant and innate immunity of Danio rerio against Edwardsiella tarda in response to diets including three kinds of marine microalgae. Algal Res.. 2022;64:102689-102698.
- [CrossRef] [Google Scholar]
- Quantum chemical calculation for the antioxidant mechanism of two peptides from naked oat. Food Sci.. 2022;43(23):106-112.
- [CrossRef] [Google Scholar]
- Potential anti-skin aging effect of a peptide AYAPE isolated from Isochrysis zhanjiangensis on UVB-induced HaCaT cells and H2O2-induced BJ cells. J Photoch Photobio b.. 2022;233:112481-112490.
- [CrossRef] [Google Scholar]
- Preparation of antioxidative corn protein hydrolysates, purification and evaluation of three novel corn antioxidant peptides. Food Chem.. 2016;204:427-436.
- [CrossRef] [Google Scholar]
- Neuroprotective effects of aucubin on hydrogen peroxide-induced toxicity in human neuroblastoma SH-SY5Y cells via the Nrf2/HO-1 pathway. Phytomedicine. 2021;87:153577-153587.
- [CrossRef] [Google Scholar]
- Marine microalgae for production of biofuels and chemicals. Curr Opin Biotech.. 2018;50:111-120.
- [CrossRef] [Google Scholar]
- In Vitro Antioxidant Activity of Selected 4-Hydroxy-chromene-2-one Derivatives-SAR, QSAR and DFT Studies. Int J Mol Sci.. 2011;12(5):2822-2841.
- [CrossRef] [Google Scholar]
- Mechanism of antioxidant reaction of vitamin E: Charge transfer and tunneling effect in proton-transfer reaction. J Phys Chem.. 1992;96(6):2754-2761.
- [CrossRef] [Google Scholar]
- Ngo. D.H., Vo, T.S., Kim, S.K., 2013. Biological Activities of Marine Bioactive Peptides. In Kim, S. K (Eds.), Marine Proteins and Peptides (pp. 509-521). John Wiley & Sons, Ltd. 10.1002/9781118375082.ch26.
- Amino Acid Composition, Antioxidant, and Cytoprotective Effect of Blue Mussel (Mytilus edulis) Hydrolysate through the Inhibition of Caspase-3 Activation in Oxidative Stress-Mediated Endothelial Cell Injury. Mar Drugs.. 2019;17(2):135-150.
- [CrossRef] [Google Scholar]
- Asymmetric Synthesis and Evaluation of Danshensu-Cysteine Conjugates as Novel Potential Anti-Apoptotic Drug Candidates. Int J Mol Sci.. 2014;16(1):628-644.
- [CrossRef] [Google Scholar]
- Antimicrobial potential of macro and microalgae against pathogenic and spoilage microorganisms in food. Food Chem.. 2017;235:34-44.
- [CrossRef] [Google Scholar]
- Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin. Rana Catesbeiana Shaw. Bioresource Technol.. 2008;99(6):1690-1698.
- [CrossRef] [Google Scholar]
- Activation of apoptosis signalling pathways by reactive oxygen species. BBA-Mol Cell Res.. 2016;1863(12):2977-2992.
- [CrossRef] [Google Scholar]
- Oxidative Stress and Cancer: The Role of Nrf2. Curr Cancer Drug Tar.. 2018;18(6):538-557.
- [CrossRef] [Google Scholar]
- Antioxidative peptides from food proteins: A review. Peptides. 2010;31(10):1949-1956.
- [CrossRef] [Google Scholar]
- Pentapeptide AYP from Isochrysis Zhanjiangensis Exhibits Antiangiogenic Activity in HT1080 Cells and HUVECs by Suppressing Migration and Invasion In Vitro. J Agric Food Chem.. 2022;70(27):8481-8491.
- [CrossRef] [Google Scholar]
- Milk-derived bioactive peptides exhibit antioxidant activity through the Keap1-Nrf2 signaling pathway. J Func Foods.. 2020;64:103696-103703.
- [CrossRef] [Google Scholar]
- Protein-Protein Docking: From Interaction to Interactome. Biophys. J.. 2014;107(8):1785-1793.
- [CrossRef] [Google Scholar]
- Plant protein-derived antioxidant peptides: Isolation, identification, mechanism of action and application in food systems: A review. Trends Food Sci Tech.. 2020;105:308-322.
- [CrossRef] [Google Scholar]
- Study on the structure–activity relationship of watermelon seed antioxidant peptides by using molecular simulations. Food Chem.. 2021;364:130432-130438.
- [CrossRef] [Google Scholar]
- Overview of Antioxidant Peptides Derived from Marine Resources: The Sources, Characteristic, Purification, and Evaluation Methods. Appl Biochem Biotech.. 2015;176(7):1815-1833.
- [CrossRef] [Google Scholar]
- New insights into the structure-activity relationships of antioxidative peptide PMRGGGGYHY. Food Chem.. 2021;337:127678-127695.
- [CrossRef] [Google Scholar]
- Vitamin C Attenuates Oxidative Stress, Inflammation, and Apoptosis Induced by Acute Hypoxia through the Nrf2/Keap1 Signaling Pathway in Gibel Carp (Carassius gibelio) Antioxidants. 2022;11(5):935-952.
- [CrossRef] [Google Scholar]
- Cellular Interactions in the Stem Cell Niche. Science. 2004;304:1253-1254.
- [CrossRef] [Google Scholar]
- Purification and Characterization of Antioxidant Peptides Derived from Protein Hydrolysate of the Marine Bivalve Mollusk Tergillarca granosa. Mar Drugs.. 2019;17(5):251-266.
- [CrossRef] [Google Scholar]
- Inflammation suppression in doxorubicin-induced cardiotoxicity: Natural compounds as therapeutic options. N-S Arch Pharmacol.. 2021;394(10):2003-2011.
- [CrossRef] [Google Scholar]
- Heptapeptide Isolated from Isochrysis zhanjiangensis Exhibited Anti-Photoaging Potential via MAPK/AP-1/MMP Pathway and Anti-Apoptosis in UVB-Irradiated HaCaT Cells. Mar Drugs.. 2021;19(11):626-641.
- [CrossRef] [Google Scholar]
- Protective effects and plausible mechanisms of velvet polypeptide against hydrogen peroxide induced injury in human umbilical vein endothelial cells. Can J Physiol Pharm.. 2017;95(5):610-619.
- [Google Scholar]







