Translate this page into:
Pulmonary protective effects of ultrasonic green synthesis of gold nanoparticles mediated by pectin on Methotrexate-induced acute lung injury in lung BEAS-2B, WI-38, CCD-19Lu, IMR-90, MRC-5, and HEL 299 cell lines
⁎Corresponding author. hugen0517@sina.com (Gen Hu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Regarding applicative, facile, green chemical research, a bio-inspired approach is being reported for the synthesis of Au nanoparticles by pectin (PEC) as a natural reducing and stabilizing agent without using any toxic and harmful reagent under ultrasonic condition. The biosynthesized Au NPs@PEC were characterized by advanced physicochemical techniques like ultraviolet–visible (UV–Vis), Fourier Transformed Infrared spectroscopy (FT-IR), Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Energy Dispersive X-ray spectroscopy (EDX), and X-ray Diffraction (XRD) study. It has been established that pectin-stabilized Au nanoparticles have a spherical shape with a mean diameter from 5 to 10 nm. In the medicinal part of the present research, the lung BEAS-2B, WI-38, CCD-19Lu, IMR-90, MRC-5, and HEL 299 cell viability was determined by trypan blue assay. The caspase activity colorimetric assay kit and Rhodamine123 fluorescence dye were used to determine the caspase-3 activity and mitochondrial membrane potential, respectively. Apoptosis and DNA fragmentation were determined by the TUNEL test. Also, the inflammatory cytokines concentrations were evaluated by the Rat inflammatory cytokine assay kit. Au NPs@PEC-treated cell cutlers decreased significantly (p ≤ 0.01) the caspase-3 activity, inflammatory cytokines concentrations, and DNA fragmentation, and enhanced the mitochondrial membrane potential and cell viability in the high concentration of Methotrexate-treated lung BEAS-2B, WI-38, CCD-19Lu, IMR-90, MRC-5, and HEL 299 cells. In the antioxidant test, the IC50 of Au NPs@PEC nanocomposite and BHT against DPPH free radicals were 203 and 181 µg/mL, respectively. Finally, Au NPs@PEC may be used as a pulmonary protective supplement to treat acute lung injury.
Keywords
Pectin
Gold nanoparticles
DNA fragmentation
Apoptosis
Antioxidant
Acute lung injury
1 Introduction
Nanotechnology is the field to yield modern systems, tools, and materials by taking control at the atomic and molecular levels using the features that appear on those surfaces (Cheng et al., 2003; Li et al., 2005; Fukuda et al., 2006; Wang et al., 2010; You et al., 2012). Applications for nanotechnology in medical diagnostics, food, medicine, biotechnology, environment, energy, chemistry, physics, etc, introduce this technology as an interdisciplinary and cross-sectoral context (Staroverov et al., 2009; Gholamian et al., 2013). The interdisciplinary nature of nanoscience and nanotechnology as the field to yield modern systems, tools, and materials with precision atoms and molecules, will sooner or later affect the health and medical sector (Liao et al., 2015; Fazaeli et al., 2010; Konda et al., 2014). Drug use is currently volumetric, so most cells in the body need medication. In the new method, the drug is directed directly to specific cells with new injection devices and delivered to the required location. By this mechanism, small and large diseases can be diagnosed and treated at the beginning of their formation (Staroverov et al., 2009; Gholamian et al., 2013). The National Nanotechnology Project is being implemented in European countries, the United States and Japan with high priority in various fields. Nanotechnology and nanoscience emerging fields can move materials very accurately, to understand and control unprecedented fundamental components of physical objects. It seems that these developments will change the way we design and build everything from vaccines to computers. The plan would increase investment in nanotechnology about twice as much each year as last year. A branch of nanotechnology is the formulation of new drugs with metallic nanoparticles (Fazaeli et al., 2010; Konda et al., 2014; Mazaahir et al., 2012; Kiasat et al., 2013; Celardo et al., 2011; De Jong and Borm, 2008).
Nanoparticles are widely used in tissue engineering scaffolds, targeted drug delivery and disease diagnosis (Borm et al., 2006; Stapleton and Nurkiewicz, 2014; Patra et al., 2018). At present, many drug delivery systems are made of nanoparticles and different materials have been used as drug stimulants or enhancers to ameliorate the effectiveness of treatment and the durability and stability as well as the safety of pulmonary protective drugs (Stapleton and Nurkiewicz, 2014; Patra et al., 2018; Itani and Al Faraj, 2019). The substances used to release pulmonary protective drugs are divided into different polymers, magnetic, and biomolecules. These materials can also provide surface modifications such as binding to target antibodies and ligands to make the nanoparticles act purposefully to increase the effectiveness of the treatment (Itani and Al Faraj, 2019; Trojer et al., 2013; Liu et al., 2014).
In this work we have proposed the ‘green’ approach for synthesis of stable pectin capped Au nanoparticles (Au NPs@PEC) with desirable physico-chemical properties (Scheme 1). Due to the non-toxic effects of pectin to human, it is an interesting and valuable compounds in pharmaceuticals and biomedical usages. So, we used pectin in research work as an ecofriendly natural polymer with minimum side effects and importantly as a natural reducing/stabilizing and solid support agent for fabrication Au nanoparticles without using any toxic and harmful reagent under ultrasonic condition. Then, the prepared Au NPs@PEC were characterized by advanced physicochemical techniques. Also, the in vitro pulmonary protective effects of ultrasonic green synthesis of Au NPs@PEC on Methotrexate-induced acute lung injury in lung BEAS-2B, WI-38, CCD-19Lu, IMR-90, MRC-5, and HEL 299 cell lines were evaluated.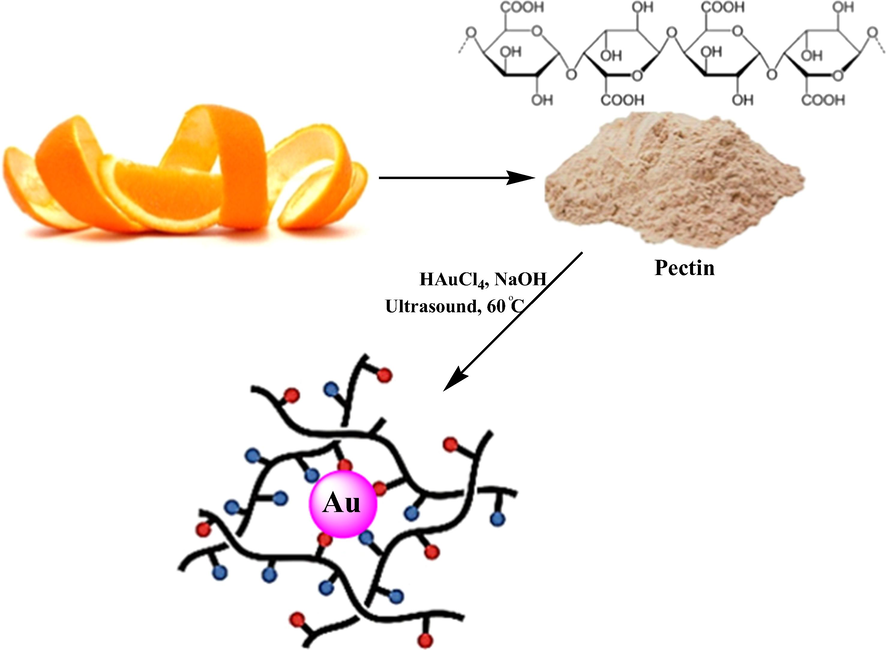
Synthesis of Au NPs@PEC under ultrasound conditions.
2 Experimental
2.1 Synthesis of Au NPs@PEC
To initiate the reaction 20 mL of a freshly prepared aqueous solution of HAuCl4 (10 mM) was added to 100 mL of aqueous pectin (0.1 g) solution under ultrasonic conditions and left for 10 min at 60 °C. Then the pH of the solution was adjusted to 11.0 (NaOH, 3 wt%) and the reaction mixture was kept with sonication at 60 °C for 60 min. The progress of the reaction is monitored by the change of light yellow color solution to wine red, indicating the formation of Au NPs. The prepared Au NPs@PEC were collected by centrifugation and washed several times with DI-water.
2.2 Pulmonary protective analyses of Au NPs@PEC
2.2.1 Cell culture
In the recent study, lung BEAS-2B, WI-38, CCD-19Lu, IMR-90, MRC-5, and HEL 299 cells were used. In the beginning, 100 µg/mL streptomycin (Sigma), 100 IU/mL penicillin (Sigma), and 10 %fetal bovine serum (FBS, Gibco) supported in cell cultures and standard case in T-25 cm2 tissue culture bottles (5% incubated at 37 °C in CO2). It made changes in the Methotrexate cells morphology, so apoptotic cells made the cell being treated with diluted Methotrexate with 100 mM water. For HAuCl4·H2O and Au NPs@PEC were dissolved first in DMSO and transferred to the cultural environment with a 0.1% final volume. 18 h after the washing and plating, they were divided into various groups including I) Methotrexate: Cell culture with 100 μM Methotrexate; II) Control: Cell culture medium without Methotrexate, HAuCl4·H2O and Au NPs@PEC; III) T1: Cell culture with 100 μM Methotrexate and 2 μg of HAuCl4·H2O; IV) T2: Cell culture with 100 μM Methotrexate and 4 μg of HAuCl4·H2O; V) T3: Cell culture with 100 μM Methotrexate and 2 μg of Au NPs@PEC; VI) T4: Cell culture with 100 μM Methotrexate and 4 μg of Au NPs@PEC (Han et al., 2020).
2.2.2 Cell viability
Trypan blue was administrated to evaluate the cell viability. Vital Dye, is observed by penetrating dead cells in the Blue lobes in the Neubauer Lamela. 96 wells with a 6 × 104 cell/mL density were coated on the cultural plate for 12 h, next, they were cultured with different I-VI treatments and incubated at 5% CO2 at 37 °C for 48 h. After cell trypsinization, 200 μL cell suspension was suspended by Neubauer Lamela 120–180 s after mixing with 0.5% 50 μL of Trippan blue. The cell viability of samples was determined by following the formula (Han et al., 2020).
Cell viability: Non-colored cells number/Total cells number
2.2.3 Cell death index
To determine the cell death index in several treatments of I-VI, the staining of TUNEL was used Eight random wells were chosen to gain TUNEL positive cells with an Olympus AX-70 fluorescence microscope. The cell death index ratio to apoptotic cells to total cells is equal (Han et al., 2020).
2.2.4 Inflammatory cytokines secretion
The pro-inflammatory cytokines concentrations including IL-1β, IL-6, and TNFα were determined using Rat V-Plex Kit.
2.2.5 Mitochondrial membrane potential (MMP)
For half an hour various groups were exposed to 20 mg / mL rhodamine-123. Then, the cells were washed by PBS. Consequently, 1000 μL triton X-100 was transferred to wells and held for 2 h at 4 °C. The solutions were taken into microtubes to be centrifuged for 20 min at 16000 rpm. Finally, cells fluorescent absorbing was done by a fluorescent microplate reader (490 nm excitation and 518 nm emissions) (Han et al., 2020).
2.2.6 Caspase-3 activity
To plate the cells, the well cell culture plate with the PRMI1640 medium was applied. After 18 h, PBS was used to wash the plates. Then, several groups of I-VI were transferred to the cells. Trypsin was applied to separate the cells from the flask. To remove the supernatant, various samples were centrifuged for 20 min. Then the centrifuging was performed by adding lysate buffer and consequently, they were transferred to the well cell culture plate. Later, 5 μL N-acetyl-Asp-Glu-Val-Asp-p-nitroanilineDEVD-pNA was transferred to the well cell culture plate and incubated for 2 h in 37 °C. Biotek (USA) Spectrophotometer was used to record the release of pNA related to the caspase-3 effect (Han et al., 2020).
2.3 Analysis of antioxidant properties of Au NPs@PEC by DPPH
The free radical scavenging test was first performed by Blois in 1958, and after some modification by numerous studies in its current form. DPPH method is one of the most widely used methods for estimating antioxidant content. DPPH is a stable radical that reacts with hydrogen atom compounds. This test is based on the inhibition of DPPH, which causes the decolorization of DPPH solution by adding radical species or antioxidants. DPPH changes color from purple to yellow by taking an electron from the antioxidant compound. The free radicals in DPPH are adsorbed at 517 nm, which follows Beer Lambert's law, and decreased absorption is linearly related to the amount of antioxidants; the higher the amount of antioxidants, the more DPPH is consumed and the more purple turns yellow (Han et al., 2020).
In a recent study, the degree of inhibition of DPPH radicals was evaluated by, Cronstein (2005). For this purpose, solutions with different samples of the Au NPs@PEC nanocomposite of variable concentrations (0–1000 µg/mL) as well as synthetic antioxidant BHT in methanol solvent were prepared. The test method was that one ml of DPPH methanolic solution (at a concentration of 1 mM) was added to 4 mL of the extract and the resulting mixture was stirred vigorously. The test tubes were placed in a dark place for 60 min. After this period, the absorbance was read at 517 nm. Finally, the DPPH radicals’ inhibition percentage of the Au NPs@PEC nanocomposite was calculated by the below formula (Han et al., 2020):
IC50 factor was used to evaluate better the antioxidant activity, which indicates the concentration of the Au NPs@PEC nanocomposite that can reduce the concentration of free radical DPPH. The initial is 50% of the initial value, and the lower the amount, the greater the antioxidant activity (Han et al., 2020).
3 Results and discussion
3.1 Structural characterization of Au NPs@PEC
AuNPs capped by pectin macromolecules were fabricated by a ‘green’ approach via chemical reduction of Au3+ cations under ultrasonic conditions (Scheme 1). The polysaccharide pectin is acted as a reducing and stabilizing agent in this work. The hemiacetal hydroxy groups in the pectin macromolecule therein not only facilitate the bio-reduction of the Au3+ ions, but also forms a protective shell over the tiny NPs to prevent their agglomeration, corrosion and auto-oxidation in air. Physicochemical characteristics of the as-synthesized Au NPs@PEC nanocomposite were determined via UV–Vis, FT-IR spectroscopy, FE-SEM, TEM, EDX and XRD analysis. In addition to the visible detection by color change, the formation of Au NPs could also be monitored spectroscopically by the UV–Vis technique. Fig. 1 displays the typical surface plasmon resonance (SPR) excitation peak of Au NPs, being observed at around 545 nm. This is also a representation of the bathochromic shift derived from the reduction of Au3+ to Au0.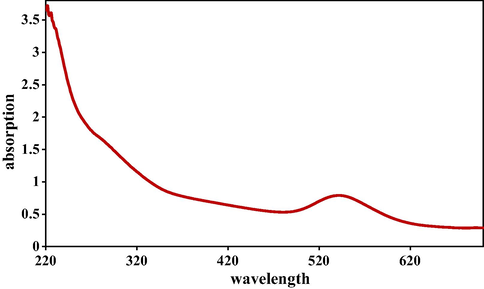
UV–visible absorption spectra of Au NPs@PEC.
Fig. 2 displays the FT-IR spectra of Pectin and Au NPs@PEC. In the FT-IR spectra of pectin (Fig. 2a) the broad peak appeared at 3444 cm−1 corresponds to the overlapped stretching vibrations of OH groups. The alcoholic C—O stretching and O—H bending peaks are observed at 1077 cm−1 and 1658 cm−1 respectively. Fig. 2b represents the corresponding peaks of Au NPs@PEC. The broad peak that appeared at 3445 cm−1 corresponds to the overlapped stretching vibrations of OH groups. The alcoholic C—O stretching and O—H bending peaks are observed at 1066 cm−1 and 1635 cm−1, respectively. This is attributed to the strong complexation of Au NPs with the pectin and OH functions.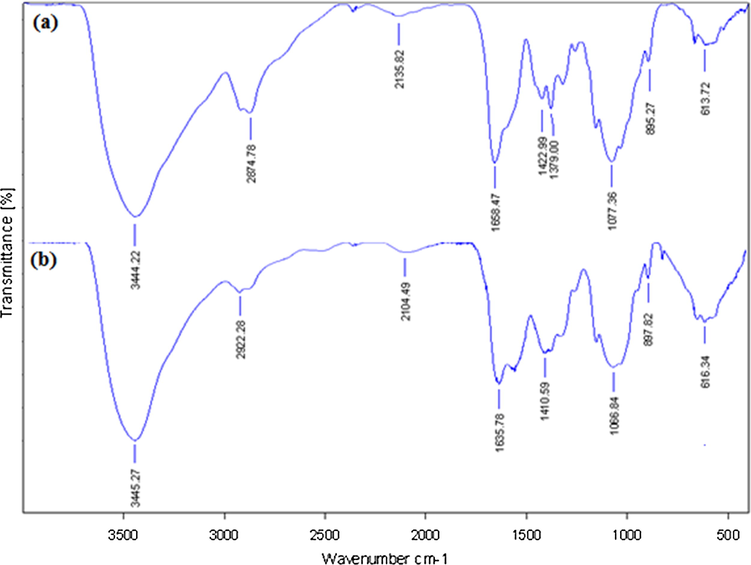
FT-IR analysis of pectin (a) and Au NPs@PEC (b).
Shape, size and structural morphology of the as-synthesized Au NPs@PEC nanocomposite were investigated by FE-SEM and TEM analysis. Fig. 3 displays the FE-SEM image with pseudo-spherical shaped particles. A homogeneous layer of the pectin polymers over the surface can be seen on close observation. This makes the particles fluffy. Due to high surface energy, the particles have high tendency to agglomerate forming lump like appearances. This is somewhat can be noticed in Fig. 3. High concentration during sample preparation in manual may be another reason. Some more detailed structural features are obtained from TEM images (Fig. 4). They are absolutely spherical and well separated from each other. The particle sizes are homogeneously distributed with an average particle diameter of 10–15 nm. The bio-inspired synthesis is greatly responsible for the size and shape the selective generation of NPs.
FE-SEM image of Au NPs@PEC nanocomposite.
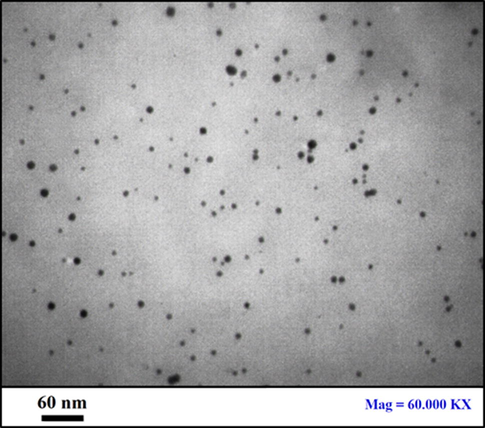
TEM image of Au NPs@PEC nanocomposite.
The synthesis of Au NPs mediated by pectin and its corresponding composition was validated from EDX analysis, as depicted in Fig. 5. The profile shows a major and distinct signal of Au appeared at 2.2 keV. However, some small and weak peaks of C and O as constitutional elements are also observed. They can be corresponded to the pectin, being associated with the Au NPs surfaces.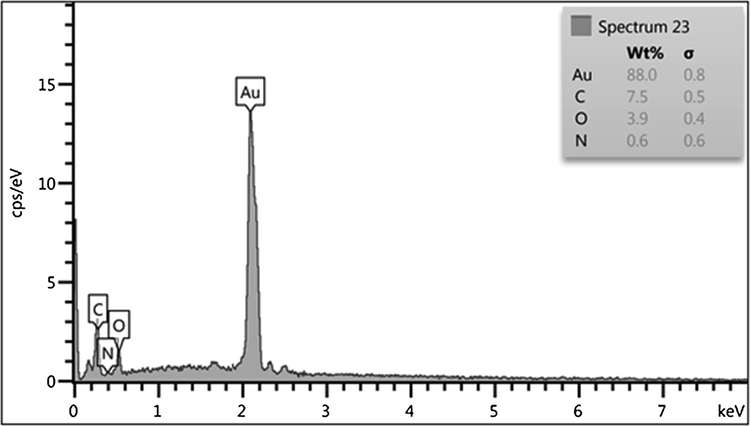
EDX spectrum of Au NPs@PEC nanocomposite.
Finally, the phase structure and crystallinity of the Au NPs@PEC nanocomposite were ascertained from XRD analysis. The profile exhibits a single phase and highly crystalline material. It also indicates the sample of great purity with insignificant contaminations. Fig. 6 represents the well-distinguished four sharp diffraction peaks at 2θ values of 38.2, 44.4, 64.6 and 77.6 respectively which correspond to the (1 1 1), (2 0 0), (2 2 0) and (3 1 1) planes of the face centered cubic (fcc) Au crystal (JCPDS No.65-2870).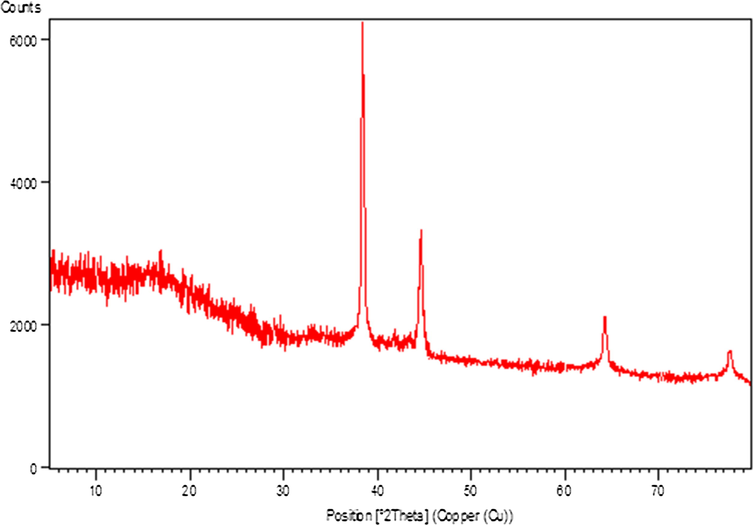
X-ray diffraction study of Au NPs@PEC.
3.2 Pulmonary protective activities of Au NPs@PEC
Methotrexate is an anti-metabolite and structurally analog of folic acid. It is believed that its effect is based on changes in the use of folic acid in the body. It has been used successfully in treating blood malignancies since 1948, in treating psoriasis since about 1960, then in treating rheumatoid arthritis, and finally in treating chronic juvenile arthritis about ten years ago (Bannwarth et al., 1994; Herfarth et al., 2012; Cronstein, 2005; American Rheumatoid Arthritis Guidelines, 2002; Weinblatt, 2013). The mechanism of action of the methotrexate is not fully understood. It was initially thought that this drug; as a cytotoxic element with a mechanism similar to the potential effect on purine and pyrimidine synthesis, could affect malignant immune responses (Herfarth et al., 2012; Marks and Edwards, 2012). In 1980; the effect of the methotrexate as an inhibitor of the proliferation of activated lymphocytes and other cells that cause tissue damage in rheumatoid arthritis, that is, it was mentioned with a similar role in cancers (Cronstein, 2005; American Rheumatoid Arthritis Guidelines, 2002; Weinblatt, 2013; Marks and Edwards, 2012; Donahue et al., 2019). Due to serum antibodies as a rheumatoid factor in these patients; various studies have been performed on the effect of the methotrexate on IgM-RF in the serum of patients with different results (Marks and Edwards, 2012; Donahue et al., 2019; Herfarth, 2016; Tsang-A-Sjoe and Bultink, 2015). Although none of the studies have been able to determine how methotrexate works to reduce secretion or non-response to interleukin; however, various studies suggest that at least one of the mechanisms by which methotrexate prevents inflammation in rheumatoid arthritis is modulating the production of cytokines in the joint membrane (Herfarth, 2016; Mol et al., 2008). Low-dose methotrexate increases adenosine release; a potent inhibitor of neutrophil accumulation at the site of inflammation (Tsang-A-Sjoe and Bultink, 2015; Mol et al., 2008; Gromnica-Ihle and Krüger, 2010).
Methotrexate is used orally, intravenously, intramuscularly, and subcutaneously. Sixty percent of oral methotrexate is probably absorbed from the proximal part of the jejunum in direct proportion to dose and plasma concentration (Herfarth, 2016; Tsang-A-Sjoe and Bultink, 2015; Mol et al., 2008; Gromnica-Ihle and Krüger, 2010). The half-life of the methotrexate is approximately two hours, with half of the drug bound to the plasma protein being replaced by salicylates, and an increase in plasma free methotrexate is seen following the use of drugs that have a strong tendency to bind to protein, Methotrexate is metabolized to 7-hydroxymethotrexate (Donahue et al., 2019; Herfarth, 2016; Tsang-A-Sjoe and Bultink, 2015; Mol et al., 2008; Gromnica-Ihle and Krüger, 2010). 80% of methotrexate is excreted unchanged in the urine within 4–28 h and the dose should be reduced in patients with kidney problems. The effect of the methotrexate appears 3–6 weeks after treatment, its importance in treating juvenile rheumatoid arthritis is the clear effect of the drug at completely lower doses, which, especially if consumed for a long time, can cause poisoning and side effects (Mol et al., 2008; Gromnica-Ihle and Krüger, 2010; Scheinfeld, 2006).
However, the study on treating rheumatic diseases in children with methotrexate is promising. But the toxic side effects of the drug are potentially significant and should be considered (Mol et al., 2008; Scheinfeld, 2006). Complications such as mouth ulcers are relieved by taking one milligram a day of folic acid. Gastrointestinal intolerance is not common in children and in case of severe nausea, the injectable type can be used (Scheinfeld, 2006; Rajagopalan et al., 2002). Clinically, bone marrow suppression is more common in people who have had folic acid deficiency since the beginning of treatment, and of course in these cases the warning is to reduce or stop the drug. Potentially important side effects of the drug are liver complications, which are less common in children than adults due to lower risk factors such as alcohol consumption, and can be prevented by using the appropriate dose and if the patient is constantly monitored (Rajagopalan et al., 2002; Goodsell, 1999; Wessels et al., 2008; Böhm, 2004). Because methotrexate is an immunosuppressive element, the risk of developing infections in children should be considered. Although the possibility of viral and bacterial infections has been reported, there is no documented evidence of even pneumocystis carinii seen in adults in children (Goodsell, 1999; Wessels et al., 2008; Böhm, 2004; Brody et al., 1993). Previous studies have shown that the risk of developing blood disorders after taking drugs that are effective in rheumatoid arthritis, including methotrexate, is negligible (Böhm, 2004; Brody et al., 1993; Meyer et al., 1950). Complications such as pneumonitis, which has been reported in adults as 0.6–0.3% in children, have recently been reported. Complications such as oligospermia and alopecia are rare. There is a possibility of kidney complications that are increased with salicylates. Central nervous system complications do not appear to be problematic with anti-rheumatic doses (Böhm, 2004; Brody et al., 1993; Meyer et al., 1950; Matera et al., 2018).
Methotrexate is consumed as a chemical drug and has many side effects (Bannwarth et al., 1994; Herfarth et al., 2012; Cronstein, 2005; American Rheumatoid Arthritis Guidelines, 2002; Weinblatt, 2013). This drug may influence the lower respiratory tract (Including lung, secondary bronchi, primary bronchi, and trachea) and upper respiratory system (including larynx, pharynx, and nasal cavity); its metabolites may be adverse to the excretion and digestive system (Cronstein, 2005; American Rheumatoid Arthritis Guidelines, 2002; Weinblatt, 2013; Marks and Edwards, 2012; Donahue et al., 2019; Herfarth, 2016). The breakdown pathway of Methotrexate passes by the kidneys and liver, and subsequently, the side effects potential is high in the recent organs (Cronstein, 2005; American Rheumatoid Arthritis Guidelines, 2002; Weinblatt, 2013; Marks and Edwards, 2012; Donahue et al., 2019; Herfarth, 2016; Tsang-A-Sjoe and Bultink, 2015). In a study, the Methotrexate effects on the brain (hippocampus and cerebral cortex) and lung are indicated. They mentioned the high consumption of Methotrexate caused pulmonary and neuronal damages (Weinblatt, 2013; Marks and Edwards, 2012; Donahue et al., 2019; Herfarth, 2016; Tsang-A-Sjoe and Bultink, 2015; Mol et al., 2008). Methotrexate directly affects lower and upper respiratory systems and raises the reactive oxygen species (ROS) by decreasing the antioxidant activity level in plasma. So, it needs to assess the Methotrexate toxic effects in lower and upper respiratory system cells and investigate the compounds which may reduce these toxic effects (Gromnica-Ihle and Krüger, 2010; Scheinfeld, 2006; Rajagopalan et al., 2002; Goodsell, 1999).
Various reports have revealed which Methotrexate declines the anti-inflammatory cytokines such as IL10, IL13, IL4, IL5, and IL3 and raises the pro-inflammatory cytokines such as IL1β, IL1α, TNFα, and IL6.
The Methotrexate cytotoxicity effects significantly decrease cell proliferation and raise cell death in lower and upper respiratory system cells (Rajagopalan et al., 2002; Goodsell, 1999; Wessels et al., 2008; Böhm, 2004; Brody et al., 1993; Meyer et al., 1950). A research demonstrated that Methotrexate by cell proliferation inhibition, caspase-3 and caspase-9 activation, and apoptosis increasing, induced cell death (Goodsell, 1999; Böhm, 2004). The results of the recent study agreed with the above findings and indicated that Methotrexate at high concentrations (100 μM) significantly (p ≤ 0.01) decreased the cell viability and raised the caspase-3 activity and inflammatory cytokines concentrations. Treating the cells with both doses of Au NPs@PEC raised the cell viability and cell proliferation levels due to the cell cytotoxicity lowering (Figs. 7–10).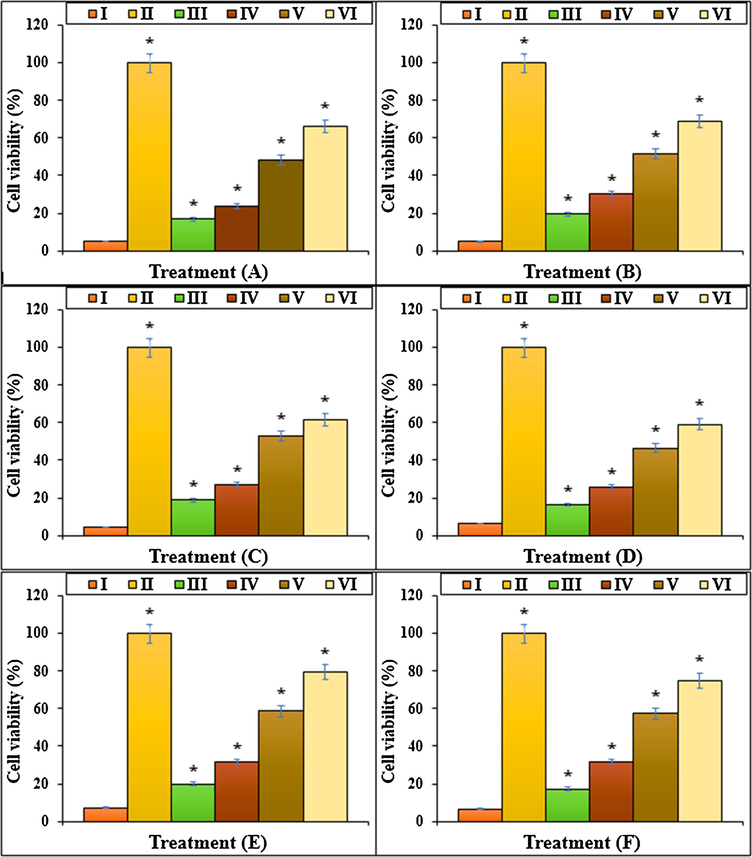
The cell viability of different treatments after 48 h. I: Methotrexate, II: Control, III: Methotrexate and 2 μg HAuCl4, IV: Methotrexate and 4 μg HAuCl4, V: Methotrexate and 2 μg Au NPs@PEC, VI: Methotrexate and 4 μg Au NPs@PEC. A: BEAS-2B, B: WI-38, C: CCD-19Lu, D: IMR-90, E: MRC-5, F: HEL 299.
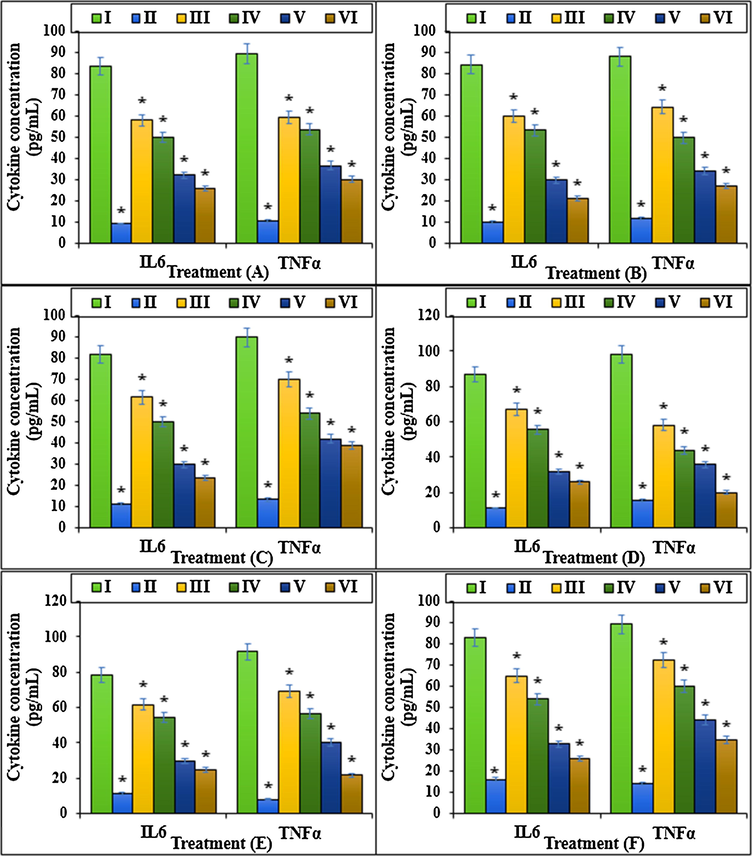
The cytokine concentration (IL6 and TNFα) of different treatments after 48 h.I: Methotrexate, II: Control, III: Methotrexate and 2 μg HAuCl4, IV: Methotrexate and 4 μg HAuCl4, V: Methotrexate and 2 μg Au NPs@PEC, VI: Methotrexate and 4 μg Au NPs@PEC. A: BEAS-2B, B: WI-38, C: CCD-19Lu, D: IMR-90, E: MRC-5, F: HEL 299.
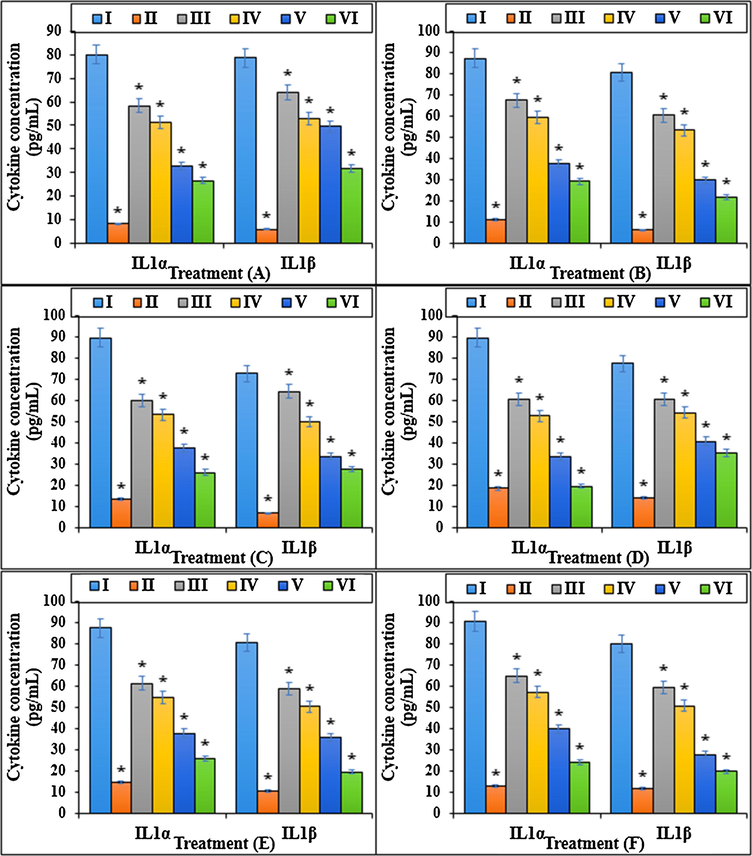
The cytokine concentration (IL1α and IL1β) of different treatments after 48 h.I: Methotrexate, II: Control, III: Methotrexate and 2 μg HAuCl4, IV: Methotrexate and 4 μg HAuCl4, V: Methotrexate and 2 μg Au NPs@PEC, VI: Methotrexate and 4 μg Au NPs@PEC. A: BEAS-2B, B: WI-38, C: CCD-19Lu, D: IMR-90, E: MRC-5, F: HEL 299.
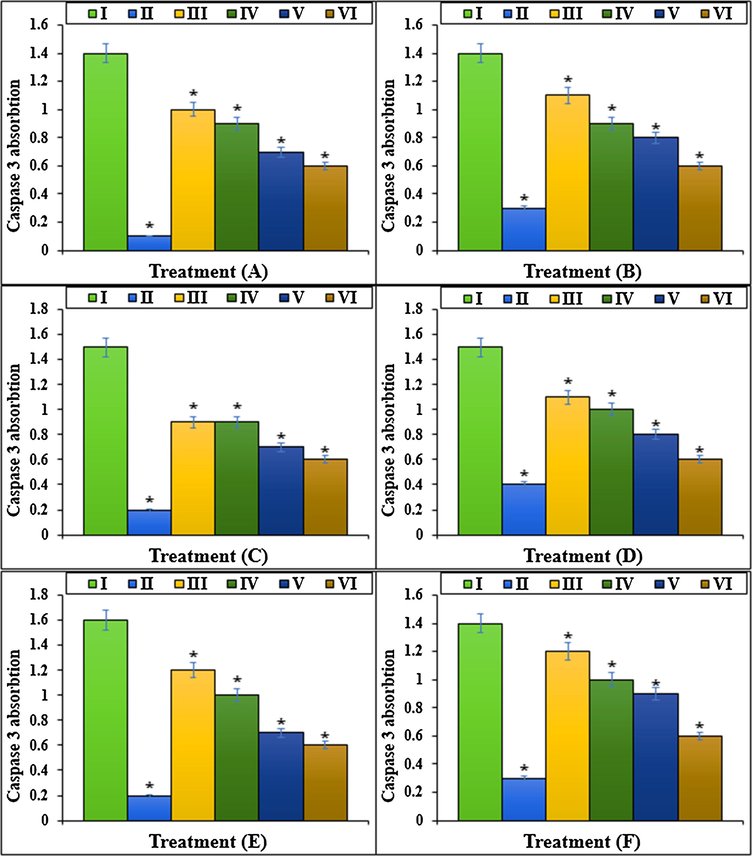
The caspase 3 absorption of different treatments after 48 h.I: Methotrexate, II: Control, III: Methotrexate and 2 μg HAuCl4, IV: Methotrexate and 4 μg HAuCl4, V: Methotrexate and 2 μg Au NPs@PEC, VI: Methotrexate and 4 μg Au NPs@PEC. A: BEAS-2B, B: WI-38, C: CCD-19Lu, D: IMR-90, E: MRC-5, F: HEL 299.
The previous studies reported that Methotrexate yielded several free radicals such as ROS in the body (Meyer et al., 1950; Matera et al., 2018). Free radicals by degrading the DNA molecules led to apoptosis and cellular degradation in the cells (Goodsell, 1999; Wessels et al., 2008; Böhm, 2004; Brody et al., 1993). ROS damages the DNA directly and enhances the apoptosis in lower and upper respiratory systems cells by producing the free radicals (Böhm, 2004; Meyer et al., 1950; Matera et al., 2018). In the recent research, the apoptosis experiment revealed that Methotrexate led to DNA fragmentation and increased the apoptosis in lung BEAS-2B, WI-38, CCD-19Lu, IMR-90, MRC-5, and HEL 299 cells. More experiments indicated that Methotrexate led to cell apoptosis by lowering the mitochondrial membrane potential. Our results revealed that Au NPs@PEC significantly (p ≤ 0.01) raised the mitochondrial membrane potential and decreased the DNA fragmentation rate in lung BEAS-2B, WI-38, CCD-19Lu, IMR-90, MRC-5, and HEL 299 cells treated with Methotrexate (Figs. 11, 12).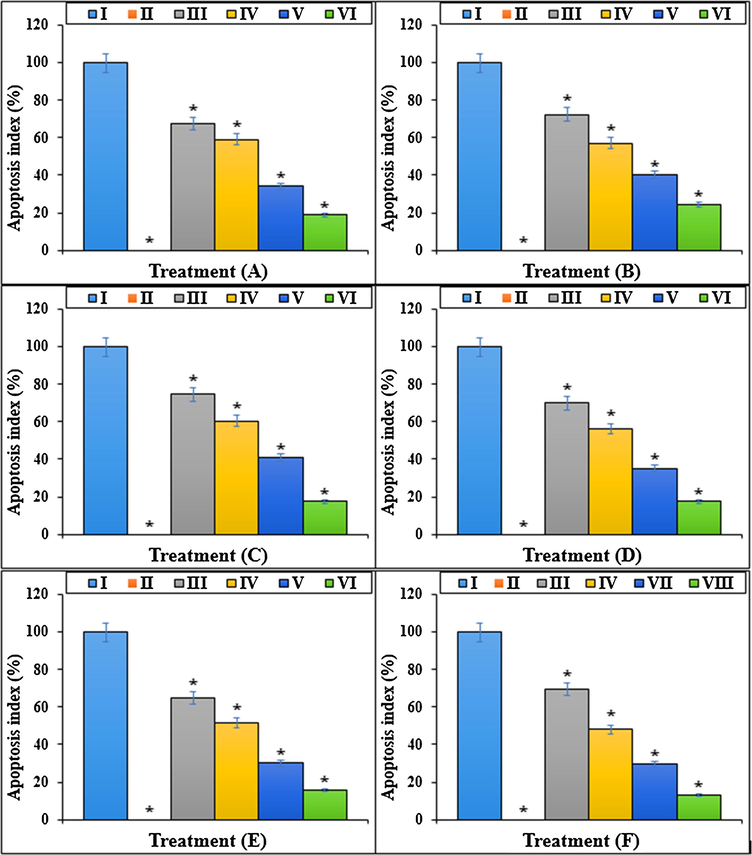
The apoptosis index of different treatments after 48 h.I: Methotrexate, II: Control, III: Methotrexate and 2 μg HAuCl4, IV: Methotrexate and 4 μg HAuCl4, V: Methotrexate and 2 μg Au NPs@PEC, VI: Methotrexate and 4 μg Au NPs@PEC. A: BEAS-2B, B: WI-38, C: CCD-19Lu, D: IMR-90, E: MRC-5, F: HEL 299.
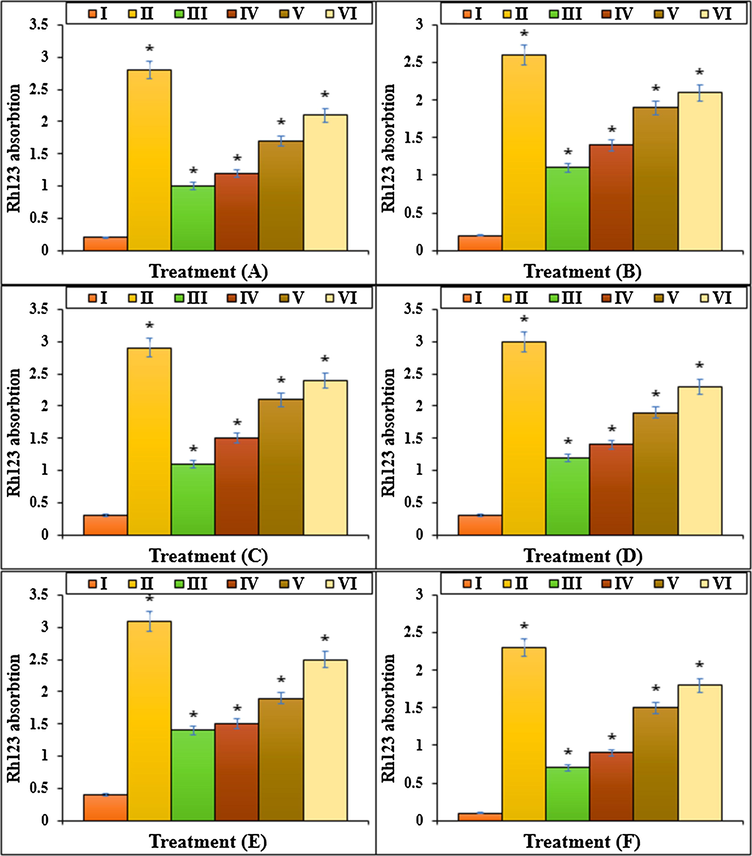
The Rh123 absorption of different treatments after 48 h.I: Methotrexate, II: Control, III: Methotrexate and 2 μg HAuCl4, IV: Methotrexate and 4 μg HAuCl4, V: Methotrexate and 2 μg Au NPs@PEC, VI: Methotrexate and 4 μg Au NPs@PEC. A: BEAS-2B, B: WI-38, C: CCD-19Lu, D: IMR-90, E: MRC-5, F: HEL 299.
3.3 Antioxidant properties of Au NPs@PEC nanocomposite
In this study, we assessed the antioxidant properties of Au NPs@PEC nanocomposite by using the DPPH test as a common free radical. Free radicals are atoms, molecules, or ions with unpaired electrons and are therefore very active, unstable, and highly reactive. Free radicals are formed by breaking a bond of a stable molecule. Free radicals collide with other molecules to achieve stability and can separate electrons from them, as a result, they form a chain of more unstable molecules. A free radical can have a positive, negative or neutral charge (Mao et al., 2016; Namvar et al., 2014; Sankar et al., 2014). During the body's natural metabolism or under conditions such as smoking, pollution, the entry of unnecessary chemicals into the body in any way, radiation and stress in the body produce free radicals. The most important free radical in the human body is oxygen, damaging DNA and other molecules. Oxidative stress is the victory of free radicals over the body's antioxidant defense and biological attack on the body (Mao et al., 2016; Namvar et al., 2014; Sankar et al., 2014; Katata-Seru et al., 2018). Antioxidants are molecules that can donate an electron to a free radical without destabilizing themselves. This stabilizes the free radical and makes it less reactive. The result of oxidative stress in the body is various degeneration, eye damage, premature aging, muscle problems, brain damage, heart failure, diabetes, cancer, and overall weakness of the immune system (Sangami and Manu, 2017; Beheshtkhoo et al., 2018). Oxygen radicals are continuously produced in all living organisms and with destructive effects, lead to cell damage and death. The production of oxidant species under physiological conditions has a controlled rate, but this production increases under oxidative conditions (Radini et al., 2018). Various studies have shown that metallic nanoparticles have very significant anti-cancer effects with omitting the free radicals (Mao et al., 2016; Namvar et al., 2014; Sankar et al., 2014; Katata-Seru et al., 2018; Sangami and Manu, 2017).
The scavenging capacity of Au NPs@PEC nanocomposite and BHT at different concentrations expressed as percentage inhibition has been indicated in Fig. 13. In the antioxidant test, the IC50 of Au NPs@PEC nanocomposite and BHT against DPPH free radicals were 327 and 251 µg/mL, respectively (Table 1; Fig. 13).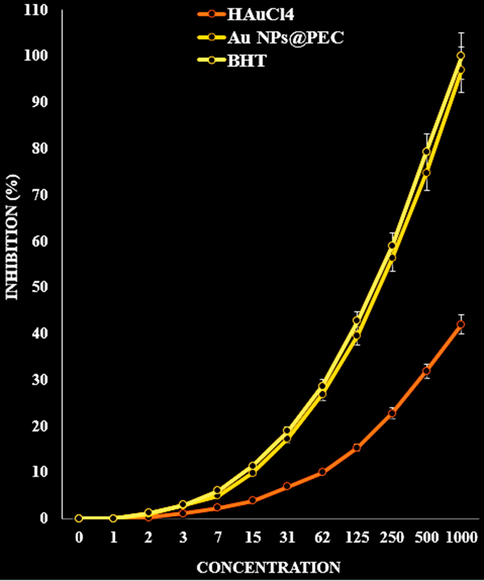
The antioxidant properties of HAuCl4, Au NPs@PEC, and BHT against DPPH.
HAuCl4 (µg/mL)
Au NPs@PEC (µg/mL)
BHT (µg/mL)
IC50 against DPPH
–
203 ± 0a
181 ± 0a
4 Conclusion
In this study, a green chemical approach is being reported for the synthesis of Au nanoparticles by using pectin (PEC) as a natural reducing and stabilizing agent without using any toxic and harmful reagent under ultrasonic condition. The prepared Au NPs@PEC was characterized by advanced physicochemical techniques like UV–Vis, FT-IR, SEM, TEM, EDX and XRD analysis. The size of obtained nanoparticles between 5 and 10 nm with suitable monodispersity and no aggregation. In the medicinal section, we understood that a high dose of Methotrexate led to cell death in lung BEAS-2B, WI-38, CCD-19Lu, IMR-90, MRC-5, and HEL 299 cell lines by the cell inflammation and apoptosis induction. Au NPs@PEC raised the cell viability in a high dose of Methotrexate-treated cells. They repressed mitochondrial membrane disruption, inflammatory cytokines (IL-1β, IL1α, IL-6, and TNF-α) yield, and caspase 3 absorption. These findings demonstrate that Au NPs@PEC suppressed Methotrexate-induced apoptosis in lung BEAS-2B, WI-38, CCD-19Lu, IMR-90, MRC-5, and HEL 299 cell lines. The Au NPs@PEC nanocomposite showed the best antioxidant activities against DPPH. Finally, Au NPs@PEC may be used as a pulmonary protective supplement to treat acute lung injury.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Guidelines for the management of rheumatoid arthritis: 2002 Update. Arthritis Rheum.. 2002;46(2):328-346.
- [Google Scholar]
- Green synthesis of iron oxide nanoparticles by aqueous leaf extract of Daphne mezereum as a novel dye removing material. Appl. Phys. A. 2018;124:363-369.
- [Google Scholar]
- Increased peripheral blood B-cells expressing the CD5 molecules in association to autoantibodies in patients with lupus erythematosus and evidence to selectively down-modulate them. Biomed. Pharmacother.. 2004;58(5):338-343.
- [Google Scholar]
- The potential risks of nanomaterials: a review carried out for ECETOC. Particle Fibre Toxicol.. 2006;3(1):11.
- [Google Scholar]
- Mechanism of action of methotrexate: experimental evidence that methotrexate blocks the binding of interleukin 1 beta to the interleukin 1 receptor on target cells. Eur. J. Clin. Chem. Clin. Biochem.. 1993;31(10):667-674.
- [Google Scholar]
- Pharmacological potential of cerium oxide nanoparticles. Nanoscale.. 2011;3(4):1411-1420.
- [Google Scholar]
- Studies on nerve cell affinity of biodegradable modified chitosan films. J. Biomater. Sci. Polym. Ed.. 2003;14:1155-1167.
- [Google Scholar]
- Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol. Rev.. 2005;57(2):163-172.
- [Google Scholar]
- Drug delivery and nanoparticles: applications and hazards. Int. J. Nanomed.. 2008;3(2):133-149.
- [Google Scholar]
- Comparative Effectiveness of Combining MTX with Biologic Drug Therapy Versus Either MTX or Biologics Alone for Early Rheumatoid Arthritis in Adults: a Systematic Review and Network Meta-analysis. J. Gen. Intern. Med.. 2019;34(10):2232-2245.
- [Google Scholar]
- Open Catal. J.. 2010;3:14-18.
- Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials. 2006;27:5259-5267.
- [Google Scholar]
- (a) Gholamian, F., Salavati-Niasari, M., Ghanbari, D., Sabet, M., 2013. J. Cluster Sci. 24, 73–84; (b) Veisi, H., Sedrpoushan, A., Faraji, A.R., Heydari, M., Hemmati, S., Fatahi, B., 2015. RSC Adv. 5, 68523–68530; (c) Veisi, H., Safarimehr, P., Hemmati, S., 2019. Mater. Sci. Eng.: C 96, 310–318; (d) Veisi, H., Mohammadi, L., Hemmati, S., Tamoradi, T., Mohammadi, P., 2019. ACS Omega 4, 13991–1400; (e) Veisi, H., Manesh, A.A., Eivazi, N., Faraji, A.R., 2015. RSC Adv. 5, 20098–20107; (f) Maleki, B., Hemmati, S., Sedrpoushan, A., Ashrafi, S.S., Veisi, H., 2014. RSC Adv. 4, 40505–40510; (g) Hemmati, S., Mehrazin, L., Pirhayati, M., Veisi, H., 2019. Polyhedron 158, 414–422; (h) Baghayeri, M., Amiri, A., Alizadeh, Z., Veisi, H., Hasheminejad, E., 2018. J. Electroanal. Chem. 810, 69–77.
- Use of methotrexate in young patients with respect to the reproductive system. Clin. Exp. Rheumatol.. 2010;28(5 Suppl 61):S80-S84.
- [Google Scholar]
- Ag NPs on chitosan-alginate coated magnetite for synthesis of indazolo[2,1-b]phthalazines and human lung protective effects against α-Guttiferin. Int. J. Biol. Macromol.. 2020;164:2974-2986.
- [Google Scholar]
- Methotrexate for Inflammatory Bowel Diseases - New Developments. Digestive Diseases.. 2016;34(1–2):140-146.
- [Google Scholar]
- siRNA Conjugated Nanoparticles-A Next Generation Strategy to Treat Lung Cancer. Int. J. Mol. Sci.. 2019;20(23):6088.
- [Google Scholar]
- Green synthesis of iron nanoparticles using Moringa oleifera extracts and their applications: Removal of nitrate from water and antibacterial activity against Escherichia coli. J. Mol. Liq.. 2018;256:296-304.
- [Google Scholar]
- J. Serb. Chem. Soc.. 2013;78:469-476.
- Der Pharma Chem.. 2014;6:228-233.
- Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials. 2005;26:3919-3928.
- [Google Scholar]
- Int. J. Nanomed.. 2015;10:3315-3327.
- Formulation and characterization of hydrophilic drug diclofenac sodium-loaded solid lipid nanoparticles based on phospholipid complexes technology. J. Liposome Res.. 2014;24(1):17-26.
- [Google Scholar]
- Mechanisms of silver nanoparticle-induced toxicity and important role of autophagy. Nanotoxicol.. 2016;10:1021-1040.
- [Google Scholar]
- Protective effect of methotrexate in patients with rheumatoid arthritis and cardiovascular comorbidity. Therapeutic Adv. Musculoskeletal Disease.. 2012;4(3):149-157.
- [Google Scholar]
- Photoswitchable Antimetabolite for Targeted Photoactivated Chemotherapy. J. Am. Chem. Soc.. 2018;140(46):15764-15773.
- [Google Scholar]
- Chin. Sci. Bull.. 2012;57:2273-2279.
- Treatment of acute leukemia with amethopterin (4-amino, 10-methyl pteroyl glutamic acid) Acta Haematol.. 1950;4(3):157-167.
- [Google Scholar]
- Current evidence on surgery, systemic methotrexate and expectant management in the treatment of tubal ectopic pregnancy: a systematic review and meta-analysis. Human Reprod. Update.. 2008;14(4):309-319.
- [Google Scholar]
- Cytotoxic effect of magnetic iron oxide nanoparticles synthesized via seaweed aqueous extract. Int. J. Nanomed.. 2014;19:2479-2488.
- [Google Scholar]
- Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol.. 2018;16:71.
- [Google Scholar]
- Biosynthesis of iron nanoparticles using Trigonella foenum-graecum seed extract for photocatalytic methyl orange dye degradation and antibacterial applications. J. Photochem. Photobiol., B. 2018;183:154-163.
- [Google Scholar]
- Interaction of dihydrofolate reductase with methotrexate: ensemble and single-molecule kinetics. PNAS. 2002;99(21):13481-13486.
- [Google Scholar]
- Synthesis of Green Iron Nanoparticles using Laterite and their application as a Fenton-like catalyst for the degradation of herbicide Ametryn in water. Environ. Technol. Innov.. 2017;8:150-163.
- [Google Scholar]
- Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles. Mat. Sci. Eng. C. 2014;44:234-239.
- [Google Scholar]
- Three cases of toxic skin eruptions associated with methotrexate and a compilation of methotrexate-induced skin eruptions. Dermatol. Online J.. 2006;12(7):15.
- [Google Scholar]
- Vascular distribution of nanomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol.. 2014;6(4):338-348.
- [Google Scholar]
- (a) Staroverov, S.A., Aksinenko, N.M., Gabalov, K.P., Vasilenko, O.A., Vidyasheva I,V., Shchyogolev, S.Y.U., Dykman, L.A., 2009. Effect of gold nanoparticles on the respiratory activity of peritoneal macrophages. Gold Bulletin. 42(2), 153–156; 21. (b) Karimi Ghezeli, Z., Hekmati, M., Veisi, H., 2019. Synthesis of Imatinib‐loaded chitosan‐modified magnetic nanoparticles as an anti‐cancer agent for pH responsive targeted drug delivery. Appl. Organometallic Chem. 33, e4833; (c) Ghorbani-Vaghei, R., Hemmati, S., Hekmati, M., 2016. Pd immobilized on modified magnetic Fe3O4 nanoparticles: Magnetically recoverable and reusable Pd nanocatalyst for Suzuki-Miyaura coupling reactions and Ullmann-type N-arylation of indoles. J. Chem. Sci. 128, 1157–1162; (d) Wei Zhang, Hojat Veisi, Reyhaneh Sharifi, Delafarin Salamat, Bikash Karmakar, Malak Hekmati, Saba Hemmati, Mohammad Mahdi Zangeneh, Zhiyong Zhang, Qiang Su, 2020. Fabrication of Pd NPs on pectin-modified Fe3O4 NPs: A magnetically retrievable nanocatalyst for efficient C–C and C–N cross coupling reactions and an investigation of its cardiovascular protective effects. Int J Biological Mac. 160, 1252–1262; (e) Entezari, M., Safari, M., Hekmati, M., Hekmat, S., Azin, A., 2014. Modification of carboxylated multiwall nanotubes with benzotriazole derivatives and study of their anticancer activities. Med. Chem. Res. 23, 487–495; (f) Azizian, J., Hekmati, M., Dadras, O.G., 2014. Functionalization of carboxylated multiwall nanotubes with dapsone derivatives and study of their antibacterial activities against E. coli and S. aureus. Orient J. Chem. 30, 667–673; (g) Salehi, M.H., Yousefi, M., Hekmati, M., Balali, E., 2019. In situ biosynthesis of palladium nanoparticles on Artemisia abrotanum extract-modified graphene oxide and its catalytic activity for Suzuki coupling reactions. Polyhedron 165, 132–137; (h) Veisi, H., Karmakar, B., Tamoradi, T., Hemmati, S., Hekmati, M., Hamelian, M., 2021. Biosynthesis of CuO nanoparticles using aqueous extract of herbal tea (Stachys Lavandulifolia) flowers and evaluation of its catalytic activity. Sci. Rep. 11, 1983.
- Charged microcapsules for controlled release of hydrophobic actives Part II: surface modification by Lbl adsorption and lipid bilayer formation on properly anchored dispersant layers. J. Colloid Interface Sci.. 2013;409:8-17.
- [Google Scholar]
- Systemic lupus erythematosus: review of synthetic drugs. Expert Opin. Pharmacother.. 2015;16(18):2793-2806.
- [Google Scholar]
- Preparation of cross-linked carboxymethyl chitosan for repairing sciatic nerve injury in rats. Biotechnol. Lett.. 2010;32:59-66.
- [Google Scholar]
- Methotrexate in rheumatoid arthritis: a quarter century of development. Trans. Am. Clin. Climatol. Assoc.. 2013;124:16-25.
- [Google Scholar]
- Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology. 2008;47(3):249-255.
- [Google Scholar]
- The progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicity. Mol. Biol. Rep.. 2012;39:9193-9201.
- [CrossRef] [Google Scholar]







