Translate this page into:
Pyrazoles containing organic extracts of Litsea glutinosa (Lour.) C. B. Rob enervate chemical-induced diarrhea in animal models evident in ligand-receptor interaction
⁎Corresponding author at: International Professor (Distinguished Scholar Category), Walailak University, Nakhon Si Thammarat 80160, Thailand, Department of Biochemistry & Molecular Biology, University of Chittagong, Chittagong 4331, Bangladesh. atiar@cu.ac.bd (Md. Atiar Rahman),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Litsea glutinosa bark extract displayed an excellent anti-diarrheal role in an animal model. Methanol extract showed the best in anti-diarrheal effect. Gastrointestinal motility was profoundly reduced by methanol extract. Restoration of electrolytes and immunoglobulin G were consistent. Impacts were evident in the computational study of strong ligand-receptor interaction. Heterocyclic 1-(2-Fluorophenyl)pyrazole-4-carboxylic acid remarkably supported the anti-diarrheal effect.
Abstract
This research investigated the effect of organic extracts from Litsea glutinosa (Lour.) C. B. Rob bark and its five heterocyclic compounds on induced diarrheal models. The bark of L. glutinosa was extracted with chloroform, ethyl acetate, and methanol. The resultant extracts were examined for disc-diffusion-guided activity against diarrhea-causing bacteria and chemical-induced anti-diarrheal properties in castor oil- and magnesium sulfate-induced diarrheal models. The effect of the extracts on gastrointestinal motility was tested in activated charcoal meal and barium sulfate milk models. The effects of the extracts on electrolytes (Na+, K+, Cl- and HCO3–), creatinine, triglycerides (TG), C-reactive protein (CRP), and immunoglobulin E (IgE) were assessed in the blood serum of treated animals. From the GC–MS analysis of the L. glutinosa methanol extract, five heterocyclic compounds were selected, and their interactions with target receptors were investigated using molecular docking techniques. The methanol extract (MExLG) showed the highest zone of inhibition for Shigella dysentriae (ZOI, 24 ± 0.9 mm) and E. coli (ZOI, 16.00 ± 1.14 mm), Salmonella paratyphi (18.23 ± 3.06 mm) and Vibrio cholerae (22.10 ± 2.62 mm). For both castor oil- and barium sulfate-induced diarrhea, MExLG achieved the highest levels of diarrheal inhibition 82.5% and 77.33%, respectively. MExLG showed the best in Na+, K+, Cl-, and HCO3– equivalence. Serum creatinine, TG, CRP and IgE levels were significantly (P < 0.05) restored by both MExLG and ethyl acetate extract (EAxLG). Out of five compounds, 1-(2-Fluorophenyl)pyrazole-4-carboxylic acid had the closest ligand-receptor interaction to that of the standard anti-diarrheal drug loperamide. The results demonstrate that the 1-(2-Fluorophenyl)pyrazole-4-carboxylic acid of MExLG could be positioned as a potential anti-diarrheal target through further affirmation in a dose–response cell-line study.
Keywords
Litsea glutinosa
Diarrhea-causing bacteria
C-reactive protein
IgE
1-(2-Fluorophenyl)pyrazole-4-carboxylic acid
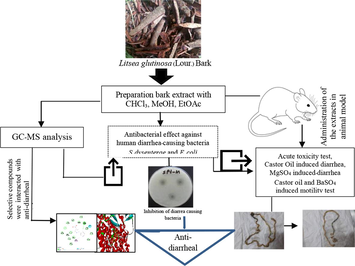
1 Introduction
Diarrhea is characterized by an increase in the frequency of bowel movements, unformed stools associated with a growing tendency of defecation, and abdominal pain (Guerrant et al., 2001). It is the world's third-highest killer disease, contributing substantially to pediatric morbidity and mortality, especially in malnourished children (Thielman and Guerrant 2004, Mehmood et al., 2011). The diarrheal disease accounts for an estimated 17.5–21 % of all deaths in children under the age of 5 years, equivalent to 1.5 million deaths per year, despite the efforts of international organizations to control this disease (Boschi 2013). Africa and South-East Asia account for 78% of all diarrheal fatalities in children, which puts a great financial load on healthcare expenses (Boschi 2013). Additionally, antibiotics used to treat diarrhea sometimes cause adverse effects, and microbes often become resistant to them (Gilani 2005). Therefore, the search for safe and more effective agents from plant origin has continued to be an essential area of active research.
Medicinal plants are usually recommended to treat gastrointestinal disorders including constipation and diarrhea because they contain a variety of ingredients that have effect-enhancing and side effect-neutralizing potential (Gilani 2005) and are considered to be quite safe when used for a long time. In recent years, several technological and scientific developments — including improved analytical tools, genome mining and engineering strategies, and microbial culturing advances — are addressing such challenges and opening up new opportunities for plant-based drug discovery. Consequently, interest in plant products as drug leads is being revitalized (Gilani 2005). Due to society's reliance of resource-limited areas on herbal medicine for their health care needs, the WHO recommended integrating folk and modern medicine to control health problems (Azaizeh et al., 2010). Accordingly, the ethnobotanical survey reported that there are several plants that have claimed anti-diarrheal role, but therapeutic and safety measures on some of these herbs including Litsea glutinosa have not been reported (Snyder and Merson 1982, Giday et al., 2010, Kidane et al., 2014).
The well-known evergreen species Litsea glutinosa, which is primarily found in tropical Asia Australia, North and Central America, India, Southern China, Malaysia, and Thailand, is a member of the Lauraceae family and locally known as meda pata in Bangladesh (van der Werff 2001, Ngearnsaengsaruay et al., 2011, Anjum et al., 2023). Its bark and leaves are used as a demulcent and mild astringent for diarrhea and dysentery due to its balsamic and mucilaginous nature, and the paste of its roots is used as a poultice in sprain and bruise (Haque et al., 2014, Anjum et al., 2023). Mucilaginous polysaccharides found in L. glutinosa leaves are thought to have antispasmodic and emollient properties, making them a useful component of poultices (Leong et al., 2016). The methanol extract of bark showed antibacterial activity against sixteen tested microorganisms, both gram-negative and gram-positive bacteria (Mandal et al., 2000). The mucilage, isolated from the leaves of L. glutinosa, exhibit anti-diabetic (type II) and antioxidant property (Palanuvej et al., 2009). Although a relatively recent report revealed that L. glutinosa leaves have antidiarrheal properties, scientific proof of the same for the bark has not yet been discovered. In this study, three distinct solvent extracts of L. glutinosa bark were examined for their anti-diarrheal properties in animal models of chemically induced diarrhea. The results were then confirmed by interfacing the most prevalent heterocyclic compounds from the most effective extract with anti-diarrheal receptor proteins.
2 Materials and methods
2.1 Chemicals and reagents
All the chemicals and reagents used in this research were of analytical grade unless specified otherwise. Loperamide hydrochloride was collected from the local pharmaceutical manufacturer “Square Pharmaceuticals Pvt. Ltd., Bangladesh”. NaCl, Barium sulfate, Gum acacia, and Magnesium sulfate were procured from Merck KGaA, Darmstadt, Germany. Charcoal was purchased from Qualikems Fine Chem Pvt. Ltd, India. Chloroform (99/8%), ethyl acetate (ACS reagent, ≥99.5%), and methanol (99.8%) were purchased from Sigma-Aldrich, St Louis, USA.
2.2 Collection of plant material
The bark of Litsea glutinosa (Lour.) C.B.Rob. was collected from Chittagong University hilly areas (GPS Coordinate: 22.46918,91.79492) and was identified by a taxonomist Prof. Dr. Sheikh Bokhtear Uddin. A sample specimen (Accession Number LAMLG-A121) of L. glutinosa has been preserved in the institutional herbarium of the University of Chittagong.
2.3 Preparation of the extracts
Shade-dried bark powder (1.5 kg) of L. glutinosa was soaked into n-hexane in an Erlenmeyer flask to exclude the fatty substances from the plant material. The residue was then successively macerated into chloroform, ethyl acetate, and methanol for three days in each solvent with mild stirring, and supernatants were filtered by eight-layered Muslin cloth followed by Whatman filter paper # 1. The filtrates were evaporated using a rotatory vacuum evaporator (RE200, BIBBY Sterilin Ltd. Staffordshire, UK) under reduced pressure at 40 °C. The concentrated reddish-brown crude extracts were collected in a Petri dish and allowed to air dry for complete evaporation of solvents. The crude yield was calculated to be 3.5%. For upcoming studies, the crude extracts of ethyl acetate, methanol, and chloroform are further abbreviated as CExLG, EaxLG, and MexLG, respectively.
2.4 Phytochemical analysis of L.glutinosa methanol extract
The bioactive compounds extracted from the methanol extract of L. glutinosa bark were analyzed by gas chromatography (GC-2010 plus, Shimadzu Corporation, Kyoto, Japan), coupled with a mass spectrometer (GCMS- TQ 8040, Shimadzu Corporation, Kyoto, Japan). A fused silica capillary column (Rxi-5 ms; 30 m, 0.25 mm ID, and 0.25 μM) was used for GC maintaining sample inlet temperature at 250◦C. A 1.0 µL sample was injected in splitless mode. The oven temperature was programmed as 75 °C (1 min); 25 °C, 125 °C (1 min); 10 °C, and 300 °C (15 min). The aux (GC to MS interface) temperature was set to 250 °C. The total run time was 36.50 min, and the column flow rate was 1.5 mL/min He gas. An electron ionization (EI) type mass spectroscopy (MS) was used in Q3 scan mode at 200 °C ion source temperature, 250 °C interface temperature, 1.17 kV detector voltage, and 50–1000 m/z mass range were set for MS. Individual compounds with m/z ratio was searched in “NIST-MS Library 2014. Total Ionic Chromatogram (TIC) was used to determine the peak area and percentage amounts of each compound.
2.5 Evaluation of antibacterial activity
2.5.1 Microorganisms
The antibacterial activity of the L. glutinosa bark extracts was tested on gram-negative bacterial strains of Shigella dysenteriae (ATCC 13313), Escherichia coli (ATCC 25922), Salmonella paratyphi (ATCC 9150) and Vibrio cholerae (ATC C 14033). The gram-negative bacterial strains were collected from the Bangladesh Institute of Tropical and Infectious Diseases (BITID), Bhatiary, Sitakundu, Chittagong, Bangladesh. Bacterial strains were held on nutrient agar at 4 °C and subcultured once a month in the laboratory.
2.5.2 Agar disc diffusion assay
The disc diffusion method was used to assess the extract’s antibacterial activity (Rios et al., 1988). To obtain 108 CFU/mL bacterial suspension, overnight bacterial cultures were diluted in Mueller-Hinton broth (OD 600 = 0.08). The spread plate technique was used to inoculate 20 mL of Mueller-Hinton agar media with 200 µL of diluted cultures, which were then allowed to dry in a sterile chamber. Five filter paper discs (Whatman® antibiotic assay discs; diam. 6 mm) were mounted on the inoculated agar surface. The extracts were loaded onto the filter paper discs in a volume of 50 µL (1–3 mg/mL) and allowed to dry entirely. Kanamycin (30 μg/disc) was used as standard antibacterial discs. The plates were incubated for 24 h at 37 °C. The zone of inhibition (ZOI) was used to determine the antibacterial activity. Each test was carried out three times.
2.6 Acute toxicity study of the crude L. glutinosa extracts
The OECD-423 Guidelines were used to conduct the acute toxicity test. Wistar albino rats were randomly assigned to three groups, each with two rats. Experimental animals were given a single dosage of 500–2500 mg/kg body weight of CExLG, EAxLG, and MExLG for three successive days. After dosing, individual animals were observed for the first 30 min, then special attention for the next 24 h paying especial attention for any unusual reactions, such as changes in the eyes, skin, fur, mucous membranes, autonomic system, and central nervous systems, respiratory system, and allergic syndromes. Any unusual changes are recorded for the next seven days. Finally, the median lethal dose (LD50 > 2000 mg/kg bw) was selected as an effective dose for animal intervention (Zaoui et al., 2002).
2.7 Animal care and maintenance
Six-seven weeks aged Wistar Albino rats (both sex, body weight 150–180 g) were procured from the animal house of the Bangladesh Council of Scientific and Industrial Research (BCSIR), Chittagong. The animals were acclimatized under standard laboratory conditions (relative humidity 55.0 ± 5.0%, room temperature 23.0 ± 0.50° C, and 12 h light: dark cycle) for 7 days. The animals were caged individually during the experiments and supplied with a standard pellet diet and water. All animal experimentations were maintained and carried out with the guidelines of the Institutional Animal Ethics Committee (Reference no EACUBS2018-6).
2.7.1 Animal grouping and dosing
The three bark samples-CExLG, EAxLG, and MExLG were individually dissolved in olive oil and fed to the rats at a concentration of 200 mg/kg body weight. Wistar albino rats were separated into five groups, each with six individuals:
Normal Control (NC): Saline 2 mL/kg BW was administered orally.
Reference Control (RC): Loperamide 5 mg/kg BW was administered orally.
Chloroform extract-treated group (CExLG200): 200 mg/kg BW CExLG was administered orally.
Ethyl acetate extract-treated group (EAxLG200): 200 mg/kg BW EAxLG was administered orally.
Methanol extract-treated group (MExLG200): 200 mg/kg BW MExLG was administered orally.
2.7.2 Induction of diarrhea by castor oil
The Shoba (Shoba and Thomas 2001) and Franca (Franca et al., 2008) procedures for the castor oil-induced diarrheal tests were adopted, with a minor modification. Animals that developed diarrhea after receiving 0.5 mL of castor oil were included in the experiment. The grouped rats were fasted for 24 h to induce diarrhea with the administration of 1 mL of castor oil 30 min before the treatment. Each animal was then placed in an individual cage, the floor lined with transparent paper, and the floor lining was changed every hour. The total number of fecal outputs was noted in the 4 h following the onset of diarrhea. And even total fluid content of the faces was determined by using the weight difference of the fresh and dry stool (dried for 24 h at room temperature in a shaded area). Evacuation classification based on stool consistency was assigned as follows: normal stool = 1, semi-solid stool = 2, and watery stool = 3, and the mean evacuation index (EI) was calculated for each group. The activity of each group was expressed as percent inhibition (%) of diarrhea which was calculated for all groups compared with the negative controls. The percent inhibition of defecation was calculated as follows:
Percent (%) inhibition of defecation = [(A-B)/A] × 100, where A indicates the mean number of defecations caused by castor oil; B indicates the mean number of defecations caused by drugs or extract.
2.8 Induction of diarrhea by magnesium sulfate
Diarrhea was induced in Wistar Albino rats by magnesium sulfate using Doherty's modified protocol (Doherty 1981). Experimental rats fasted for 18–24 h before the test with free access to water. Normal control and reference control groups were given 1 mL of olive oil to get the same stress condition as others. LGxCH, LGxEA, and LGxME groups were given the chloroform, ethyl acetate, and methanolic bark extracts at 200 mg/kg BW, respectively. After 1 h, all groups received 1 mL (5 g/kg) of MgSO4 orally. Then animals were placed in cages lined with adsorbent papers and observed for 4 h for diarrhea defined as watery (wet), unformed stool. The result for the control group was considered 100%. The activity of each group was expressed as percent inhibition (%) of diarrhea calculated as follows:
Percent (%) inhibition of defecation = [(A − B)/A] × 100, where A indicates the mean number of defecations caused by MgSO4; B indicates the mean number of defecations caused by drug or extract.
2.9 Gastrointestinal motility test
2.9.1 Castor oil-induced gastrointestinal transit in rats
This experiment was carried out by the method described by Inayathulla (Inayathulla et al., 2010). Briefly, the adult rats selected without sex discrimination were fasted for 18 h and divided into five groups of six animals each. Castor oil (1 mL) was administered orally to the animals. One hour later, the NC group was administered 1.0 mL/100 g of 0.9% NaCl in distilled water (normal saline); the RC group received the standard drug, atropine sulfate, at a 5 mg/kg dose through the oral route. Rats of groups CExLG, EAxLG, and MExLG received 200 mg/kg of respective extracts p.o. After 30 min of the administration, 1 mL of the charcoal meal (10% suspension in 5% gum acacia) as a marker diet was given orally to rats in each group. The rats were sacrificed by ether (20% v/v) anesthesia, the small intestine was carefully separated without stretching the mesentery, and blood was collected by the heart puncture method. The collected blood was immediately centrifuged at 3000 rpm for 20 min at room temperature to prepare serum for biochemical analyses. For each animal, gastrointestinal transit was calculated as the percentage distance traveled by charcoal meal to the total length of the intestine and the distance traveled by charcoal meal from the pylorus to the caecum. The inhibitory effect of the extracts on gastrointestinal transit was calculated relative to the control group:
2.9.2 Gastrointestinal motility test with barium sulfate milk
This experiment is carried out by the method described by (Chatterjee 1993). Wistar albino rats were fasted for 18–24 h with free access to water and divided into five groups of five animals each. NC and RC groups were given 1 mL of olive oil to get the same stress condition as others. After 30 min, 2 mL of 10% barium sulfate solution was administrated in all groups. Rats were sacrificed after 30 min and blood was collected by heart puncture method. The collected blood was immediately centrifuged at 3000 rpm for 20 min at room temperature to prepare serum for biochemical analyses. The distance traversed by barium sulfate milk was measured and expressed as a percentage of the total length of the small intestine (from the pylorus to the ileocecal junction). The percentage of inhibition compared with the control group was determined by using the following equation:
2.9.3 Assay of serum electrolytes and biochemical parameters
Serum sodium, potassium, chloride, bicarbonate, creatinine, triglycerides (TG), C-reactive protein (CRP) and immunoglobulin E (IgE) were measured by using reaction kits on a semi-autoanalyzer (Humalyzer 3000, Human).
2.10 Molecular docking
2.10.1 Molecular docking analysis
Based on a literature study to unveil anti-diarrheal activity, the receptors/enzymes were selected for molecular docking (Bulbul et al., 2020). The crystal structure of receptors Cystic fibrosis transmembrane receptor, Calcium-activated chloride channel, Guanylate cyclase receptor, and A2B receptor were imported from the RCSB Protein Data Bank (PDB), an online database (https://www.rcsb.org/) and best binding sites were selected by using an online tool PockDrug (Hussein et al., 2015). The chemical structure of major identified compounds Levoglucosenone; 1-Ethyl-2-hydroxymethyl imidazole, 5-(Hydroxymethyl)-2-(dimethoxymethyl)furan, 3-O-Methyl-d-glucose, and 1-(2-Fluorophenyl)pyrazole-4-carboxylic acid from L. glutinosa methanol extract by GC–MS was extracted from the PubChem repository (https://pubchem.ncbi.nlm.nih.gov/). The molecular docking study followed Hossen et al. and briefly described the methodology (Hossen et al., 2021).
2.10.2 Evaluation of pharmacokinetic parameters
The absorption, distribution, metabolism, excretion, and toxicity (ADME/T) properties analysis of five bioactive compounds (Levoglucosenone; 1-Ethyl-2-hydroxymethyl imidazole, 5-(Hydroxymethyl)-2-(dimethoxymethyl)furan, 3-O-Methyl-d-glucose, and 1-((2-Fluorophenyl)pyrazole-4-carboxylic acid) from MExLG was evaluated by Lipinski's rule of fives (Lipinski et al., 2012) and Veber's rules (number of rotatable bonds; topological polar surface area)(Veber et al., 2002). The ADME/T properties analyses were analyzed through QikProp (Schrödinger Release 2017–1: QikProp, Schrödinger, LLC, New York, NY, USA). QikProp is an efficient ADME/T prediction tool that forecasts whether the selected compound would exhibit satisfactory ADME/T performances.
2.10.3 Determination of toxicological properties
AdmetSAR online tool was used to determine the toxicological properties of the selected compounds, while a prime concern during the development of new drugs is toxicity (Yang et al., 2019). In this study, Ames toxicity, carcinogenic properties, acute oral toxicity, and acute rat toxicity were predicted.
2.11 Statistical analysis
All the data are presented as a mean ± SD. The data were analyzed by One-Way-ANOVA (Analysis of Variance) using SPSS (statistical package for social science) software (Version 20.0, IBM Corporation, NY) followed by Tukey's post hoc tests. The values at P < 0.05 were considered statistically significant.
3 Results
3.1 Natural compounds of L.glutinosa methanol extract
Methanol extract of L. glutinosa, as the most soluble fraction, has been characterized by GC–MS analysis, and forty-five volatile compounds were recorded (Table 1 and Fig. 1). Among the compounds, Levoglucosenone; 1-Ethyl-2-hydroxymethyl imidazole, 5-(Hydroxymethyl)-2-(dimethoxymethyl)furan, 3-O-Methyl-d-glucose, and 1-(2-Fluorophenyl)pyrazole-4-carboxylic acid have been found to be the most prevalent compounds (Fig. 2).
Peak#
R.Time
Area%
Name
1
3.631
0.36
N-Glycylglycine
2
3.763
0.87
2,5,5-Trimethyl-3-hexyn-2-ol
3
3.850
0.26
3-Furancarboxylic acid
4
4.001
1.46
Cyclohexanamine, N-3-butenyl-N-methyl-
5
4.087
1.91
Methyl 2-furoate
6
4.208
0.47
2-Butenedioic acid (E)-, monomethyl ester
7
4.393
0.40
Levoglucosenone
8
4.477
0.59
Heptane, 4-ethyl-
9
4.635
0.49
Glutaric acid, 3-heptyl propyl ester
10
4.783
2.31
1-(2-Thienyl)-1-propanone
11
4.983
0.01
2-Furanethanol,.beta.-methoxy-(S)-
12
5.058
0.03
2(3H)-Furanone, dihydro-4-hydroxy-
13
5.167
0.06
4H-Pyran-4-one, 3,5-dihydroxy-2-methyl-
14
5.225
0.40
1,3-Propanediol, 2-(hydroxymethyl)-2-nitro-
15
5.284
0.24
2-Propyl-1-pentanol
16
5.733
15.22
1-Ethyl-2-hydroxymethylimidazole
17
6.005
1.80
Fumaric acid, butyl 3-methylbut-3-enyl ester
18
6.208
0.58
7-Dimethyl(prop-2-enyl)silyloxytridecane
19
6.325
0.21
cis-13-Octadecenoic acid
20
6.449
2.26
5-(Hydroxymethyl)-2-(dimethoxymethyl)furan
21
6.640
1.30
Decanoic acid, 3-methyl-
22
6.811
0.86
2,4-Dimethyl-3-pentanol acetate
23
7.213
0.54
Silane, dimethyldi(but-3-enyloxy)-
24
7.292
0.22
.alpha.-Methyl mannofuranoside
25
7.538
2.36
Glutamine
26
7.636
0.43
3-Chloro-6-methoxy-2-methylbenzoic acid, m
27
7.825
0.13
Carbonic acid, butyl ethyl ester
28
7.958
0.07
(S)-(-)-1,2,4-Butanetriol, 4-acetate
29
8.036
0.19
1-Methyl-1-n-pentyloxy-1-silacyclobutane
30
8.367
0.25
D-Mannoheptulose
31
8.642
0.10
2-t-Butyl-4-oxooxazolidine-3-carboxylic acid
32
9.150
5.40
D-Allose
33
9.281
3.89
.beta.-D-Glucopyranose, 1,6-anhydro-
34
9.795
0.20
1,2-O-Isopropylidene-D-xylofuranose, TBD
35
10.147
0.42
Acetic acid, 2-ethylbutyl ester
36
10.317
0.13
3-Methylmannoside
37
10.400
0.32
4-Hydroxy-3-[3-(2-hydroxy-5-methoxy-pheny
38
10.863
3.97
1,6-Anhydro-.beta.-D-glucofuranose
39
11.226
0.50
Hydrazinecarboxamide, 2-(2-methylcyclohexy
40
12.565
37.25
3-O-Methyl-d-glucose
41
13.908
0.30
1-(2-Fluorophenyl)pyrazole-4-carboxylic acid
42
14.908
0.49
4-Hydroxy-2-hydroxymethyl-6-methylpyrimid
43
16.040
1.72
9-Octadecenoic acid, (E)-
44
17.958
0.29
R-(+)-Methyl-3-isopropyl-6-oxoheptanoate
45
18.690
0.32
E-8-Methyl-7-dodecane-1-ol acetate
Gas Chromatography-Mass spectroscopy (GC–MS) analysis spectra of MExLG. GC–MS was conducted by electron impact ionization (EI) method on a gas chromatograph coupled to a mass spectrometer. A fused silica capillary column with 0.25 m film thickness (Rxi-5 ms) is coated with DB-1 (J&W).
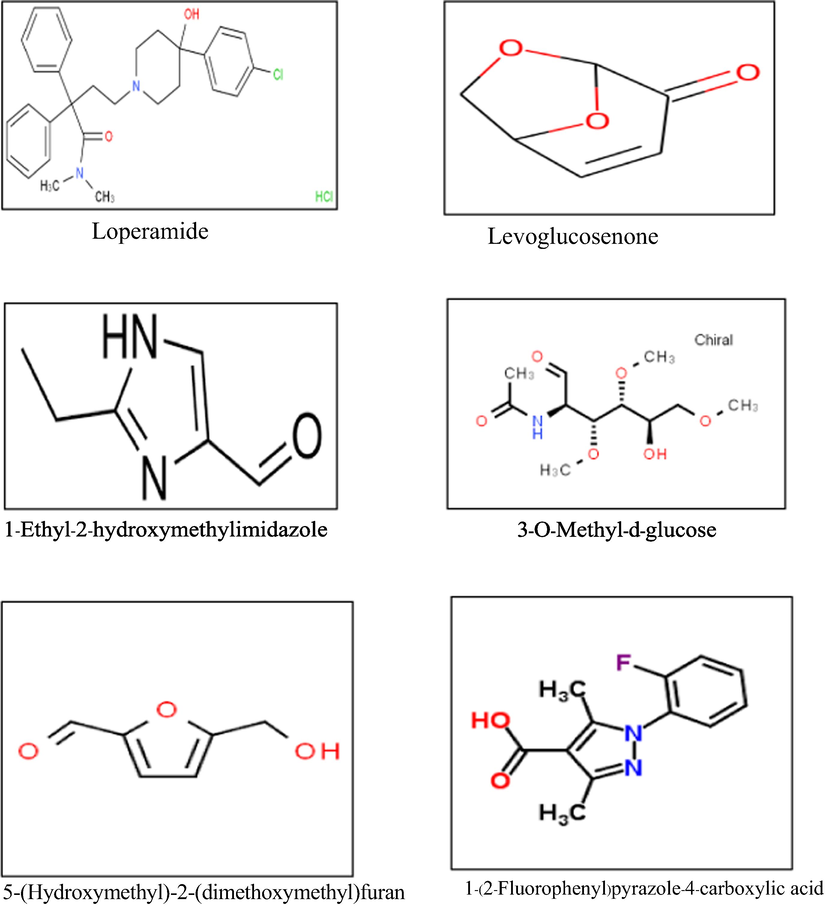
The five selected compounds were characterized from the GC–Ms spectra of MExLG.
3.2 Antibacterial effects and toxicity of the extracts
The antibacterial effect of CExLG, EAxLG, and MExLG of L. glutinosa bark against the human-pathogenic gram-negative bacteria, Shigella dysenteriae, Escherichia coli, Salmonella paratyphii and Vibrio cholerae in disc diffusion method is presented in Table 2. At the treatment dose of 3 mg/disc, MExLG was found to maximally inhibit the four bacteria S. dysenteriae, E. coli, S. paratyphii, and V. cholerae achieving the zone of inhibitions 24 ± 0.9 and 16 ± 1.14 mm, respectively. Two other extracts were deemed ineffective for bacterial inhibition.
Extract/drug
Dose
Zone of inhibition(mm)
Shigella dysentriae
E. coli
Salmonella paratyphi
Vibrio cholerae
CEXLG
(3 mg/disc)
6.50 ± 1.12
7.00 ± 2.30
8.95 ± 1.63
9.66 ± 1.10
EAxLG
(3 mg/disc)
7.50 ± 1.30
8.00 ± 1.20
10.00 ± 2.01
15.23 ± 2.00
MExLG
(3 mg/disc)
24.00 ± 0.90
16.00 ± 1.14
18.23 ± 3.06
22.10 ± 2.62
Kanamycin
30 µg/disc
35.00 ± 4.21
9.67 ± 0.75
15.00 ± 1.45
52.00 ± 3.75
In acute toxicity studies on animals, none of the organic extracts were found to be harmful. No adverse effects or behavioral responses were recorded for the oral dose of 1000, 2000, and 3000 mg/kg bw in a 7-day experimental period. In rats, no signs of physical changes (weakness, diarrhea, noisy breathing, clonic convulsion, lethargy, etc.) were observed. Additionally, there was absolutely no mortality or weight loss seen.
3.3 Effect of L.glutinosa extracts on castor oil-induced and magnesium sulfate-induced diarrhea
Three distinct organic extracts of L. glutinosa bark produced a marked anti-diarrheal effect in the rats. In the castor oil-induced model, MExLG decreased the average number of unformed feces (1.75 ± 0.5) and achieved the maximum diarrheal inhibition of 82.50%; the inhibition was statistically significant (P < 0.05) in comparison to that of Loperamide, the standard anti-diarrheal drug (Fig. 3).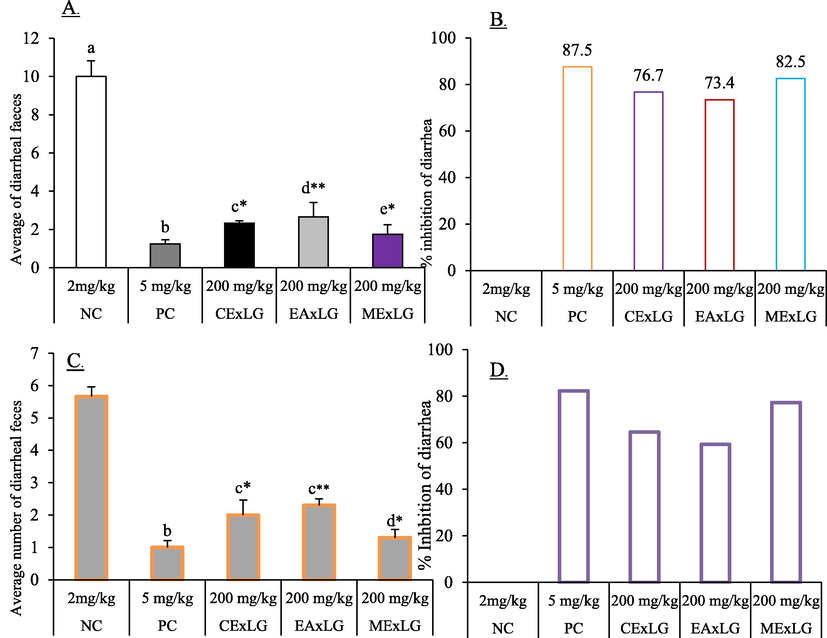
Effect of CExLG, EAxLG, MExLG on controlling different events of A. Attenuation of average diarrheal feces B. % inhibition of diarrhea in castor oil-induced diarrhea; C. Attenuation of average diarrheal feces D. % inhibition of diarrhea in BaSO4. Data are presented as Mean ± SD. The superscript values (a-e) denote the significant (P < 0.05) differences between and among the treatments. Data were analyzed by One-Way-Analysis of Variance (ANOVA) using the statistical software SPSS (Version 22.0, IBM Corporation, NY) followed by a post hoc test for multiple comparisons.
The effect of L. glutinosa extract was similarly reflected in the magnesium sulfate-induced diarrheal model. The most remarkable anti-diarrheal effect was noted for MExLG, which showed the maximum diarrheal inhibition of 77.33% and the minimum number of average diarrheal feces. In a 4 h observation, the CExLG and EAExLG showed 64.6% and 59.3% diarrheal inhibition, respectively. Loperamide, a reference anti-diarrhea medication, inhibited diarrheal activity by 82.33%. The effects of the extracts in the magnesium sulfate-induced diarrheal model are summarized in Table 3. Data are presented as Mean ± SEM for six animals. Data were analyzed by One-Way-Analysis of Variance (ANOVA) using the software “Statistical Package for Social Sciences” (SPSS, Version 22.0, IBM Corporation, NY). The superscript alphabetical letters (a-e) denote the statistical significance among and between the groups at least in the experimental condition. Values were considered significant at P < 0.05.
Group of
treatmentDose
Castor oil-induced diarrhea
Magnesium sulfate-induced diarrhea
The total average number of diarrheal feces
% inhibition of diarrhea
The total average number of diarrheal feces
% of inhibition of diarrhea
NC
2 mg/kg
10.00 ± 0.81a
–
5.66 ± 0.3a
_
RC
5 mg/kg
1.25 ± 0.21b
87.50
1.00 ± 0.21b
82.33
CExLG
200 mg/kg
2.33 ± 0.12c
76.70
2.00 ± 0.46c
64.60
EAExLG
200 mg/kg
2.66 ± 0.75d
73.40
2.30 ± 0.20c
59.30
MExLG
200 mg/kg
1.75 ± 0.50e
82.50
1.30 ± 0.26d
77.33
3.4 Effect of L.glutinosa extracts on castor oil-induced and barium sulfate-induced gastrointestinal motility assay
The effect of the extracts on the castor oil-induced intestinal motility is displayed in Table 4, and the photographs of the intestinal changes are placed in Fig. 4A. The intestinal length of the normal control animal was 93 ± 0.03 cm which has been extended due to the increased motility by castor oil. The MExLG was found to minimize the length most effectively (101 ± 0.05 cm), while the distance traveled by the marker was measured at 60 cm, and the peristalsis index attained by MExLG was 39.79. The shortest intestinal traveling made by the marker was 51 ± 0.03 cm for CExLG, which was ultimately reflected by its maximum diarrheal inhibition of 40.00%. The effect of L. glutinosa extract on barium sulfate-induced gastrointestinal motility is summarized in Table 4 and Fig. 4B. The total length of the intestine in the BaSO4-induced model was found to be 99 cm which was significantly indifferent from that (98 cm) displayed by both CExLG and MExLG; however, the distance traveled by the marker in MExLG was lower than in CExLG. In addition, MExLG showed the maximum diarrheal inhibition of 59.40%, which consistently reflected the anti-diarrheal potential of MExLG through its lowest peristalsis index, 39.79. Data are shown as Mean ± SEM of six animals in each group. Data were analyzed by One-Way-Analysis of Variance (ANOVA) using the software “Statistical Package for Social Sciences” (SPPSS, Version 22. IBM Corporation, NY), followed by Tukey's Post Hoc test for multiple analysis. Values are significantly (P < 0.05) different from each other and differences are denoted by the superscript letters (a-e).
Group
Dose
The total length the of intestine (cm)
Distance traveled by marker (cm)
% of inhibition
Peristalsis index
Cast-oil
BaSO4
Cast-oil
BaSO4
Cast-oil
BaSO4
Cast-oil
BaSO4
NC
2 mg/kg
93 ± 0.03a
99 ± 0.09a
85 ± 0.11a
88 ± 0.21a
–
–
91.39
88.88
RC
5 mg/kg
112 ± 0.1b
102 ± 0.13b
40 ± 0.03b
42 ± 0.1b
52.94
52.27
35.71
41.17
CExLG
200 mg/kg
121 ± 0.12c
98 ± 0.2c
51 ± 0.1c
70 ± 0.13c
40.00
20.45
42.14
71.42
EAExLG
200 mg/kg
113 ± 0.07d
103 ± 0.11d
75 ± 0.03d
54 ± 0.05d
11.76
38.63
66.37
52.42
MExLG
200 mg/kg
101 ± 0.05e
98 ± 0.15c
60 ± 0.2e
39 ± 0.02e
29.41
55.68
59.40
39.79
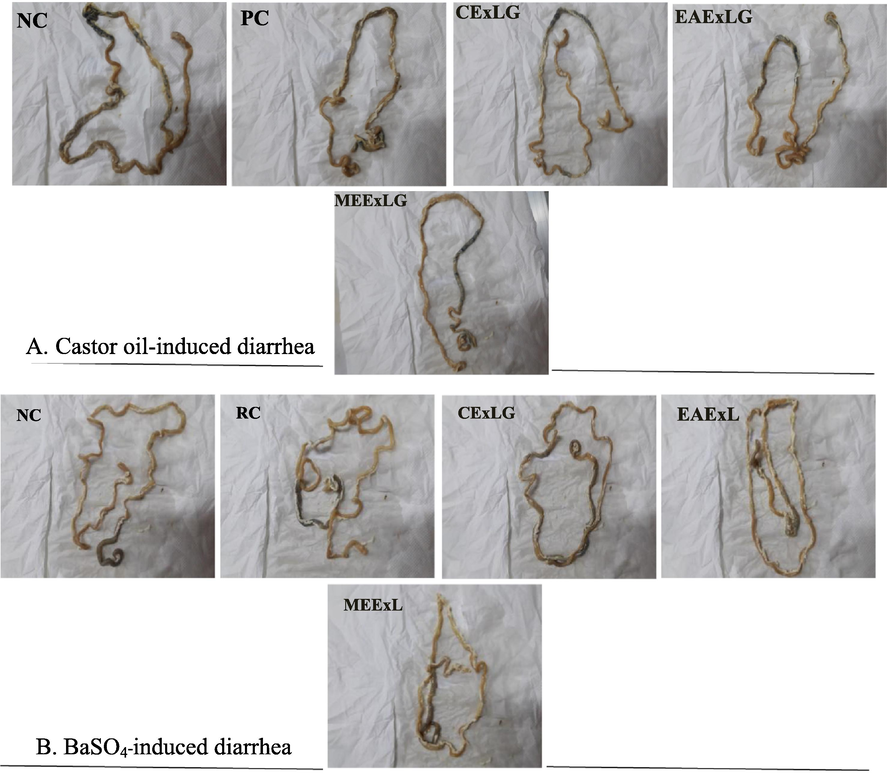
Photographs of isolated rat small intestinal tracts showing the distance traveled by activated charcoal in A. Castor oil-induced motility test; B. BaSO4-induced motility test after administrating CEXLG; EAxLG and MExLG.
3.5 Effect of L.glutinosaextracts on the serum electrolytes and biochemical markers
Table 5 summarizes the effects of L. glutinosa extracts on controlling sodium (Na+), potassium (K+), chloride (Cl-), and bicarbonate (HCO3–). The MExLG significantly (P < 0.05) maximized the Na+, Cl- and HCO3– in Castor oil-induced diarrhea compared to the reference control. The increment of Na+, K+, and Cl- by EAxLG in BaSO4-induced model was found to be slightly higher than that by MExLG while impacts of both of them were statistically significant compared to the reference control. However, the effect EAxLG on HCO3– was not insignificant in comparison to the reference control. Data are shown as Mean ± SEM of six animals in each group. Data were analyzed by One Way of Analysis of Variance (ANOVA) using the software “Statistical Package for Social Sciences” (SPPSS, Version 22. IBM Corporation, NY), followed by Tukey's Post Hoc test for multiple analyses. Values are significantly (P < 0.05) different from each other and differences are denoted by the superscript letters (a-e).
Group
Dose
Conc. of serum Na + mEq/L
Conc. of serum K + mEq/L
Serum Cl- level (mEq/L)
Serum HCO3– level (mEq/L)
Cast- oil
BaSO4
Cast- oil
BaSO4
Cast- oil
BaSO4
Cast- oil
BaSO4
Control
2 mg/kg
141 ± 1.10a
138 ± 1.00a
5.80 ± 1.30a
5.70 ± 0.10a
88 ± 1.10a
98 ± 1.12a
24.81 ± 0.23a
24.50 ± 1.0a
RC
5 mg/kg
146 ± 0.4b
144 ± 1.12b
9.10 ± 0.20b
7.4 ± 0.09b
107 ± 0.9b
103 ± 2.1b
25.69 ± 0.66b
25.34 ± 0.09a
CExLG
200 mg/kg
132 ± 2.10c
140.5 ± 1.88c
7.10 ± 1.00b
6.70 ± 0.23c
97 ± 0.15c
99 ± 0.8c
23.25 ± 0.5c
24.22 ± 1.2a
EAExLG
200 mg/kg
149 ± 1.20d
149 ± 2.00d
7.50 ± 0.90bc
5.0 ± 0.21d
96.50 ± 0.17c
101 ± 0.25d
25.87 ± 0.45b
26.87 ± 0.03b
MExLG
200 mg/kg
155 ± 1.53e
142.6 ± 1.10c
6.0 ± 0.41a
5.3 ± 0.07d
107 ± 0.85b
97.66 ± 0.08e
26.75 ± 0.1d
25.53 ± 0.81c
The effect of the L. glutinosa extracts on the serum creatinine, triglyceride (TG), c-reactive protein (CRP) and immunoglobulin E (IgE) is presented in Table 6. The creatinine level was maximally minimized by the EAxLG in castor oil-induced model although effect of MExLG administration was also significant (P < 0.05) compared to reference control. The creatinine concentration in MExLG group was statistically significant compared to RC group in BaSO4-induced model. The MExLG impacted the highest reduction of TG both in castor oil-induced (133 ± 0.9) and BaSO4-induced model (81 ± 1.06). Interestingly, the C-reactive protein attenuating capacity of EAxLG and MExLG was not significantly dissented. The IgE levels for EAxLG and MExLG were 7.72 ± 0.22 and 8.36 ± 0.55 in castor oil-induced model; and 9.71 ± 0.1 and 8.88 ± 0.09 in BaSO4-induced model. The MExLG was noticed as more impactful than the EAxLG in reducing the IgE concentration in both the models. Data are shown as Mean ± SEM of six animals in each group. Data were analyzed by One Way of Analysis of Variance (ANOVA) using the software “Statistical Package for Social Sciences” (SPPSS, Version 22. IBM Corporation, NY), followed by Tukey's Post Hoc test for multiple analyses. Values are significantly (P < 0.05) different from each other and differences are denoted by the superscript letters (a-e).
Group
Dose
Creatinine level mg/dL
TG level mg/dL
C reactive protein(mg/dl)
IgE level KIU/mL
Cast- oil
BaSO4
Cast- oil
BaSO4
Cast- oil
BaSO4
Cast- oil
BaSO4
NC
2 mg/kg
0.53 ± 0.03a
0.49 ± 0.01a
193 ± 0.21a
143 ± 1.00a
0.57 ± 0.03a
1.02 ± 0.01a
9.81 ± 0.23a
11.91 ± 0.11a
RC
5 mg/kg
0.32 ± 0.11b
0.22 ± 0.09b
133 ± 0.76b
66 ± 0.87b
0.41 ± 0.11b
0.96 ± 0.1a
8.02 ± 0.05b
9.49 ± 0.02b
CExLG
200 mg/kg
0.38 ± 0.02b
0.36 ± 0.01c
178 ± 0.19c
118 ± 0.37c
1.21 ± 0.90c
0.79 ± 0.05b
11.8 ± 0.30c
8.40 ± 0.21c
EAExLG
200 mg/kg
0.27 ± 0.1bc
0.45 ± 0.1a
138 ± 0.45d
127 ± 0.24d
0.35 ± 0.45ab
0.62 ± 0.2ab
7.72 ± 0.22b
9.71 ± 0.1d
MExLG
200 mg/kg
0.44 ± 0.13d
0.41 ± 0.13a
133 ± 0.9b
81 ± 1.60e
0.47 ± 0.21ab
0.72 ± 0.13ab
8.36 ± 0.55d
8.88 ± 0.09d
3.6 Effect of MExLG compounds in in silico molecular docking study
Table 7 displays the results of the docking analysis for the anti-diarrheal activity of the chosen MExLG compounds in terms of their drug-likeliness characteristics. This study showed that four major receptors, Cystic fibrosis transmembrane receptor, Calcium-activated chloride channel, Guanylate cyclase receptor, and A2B receptor, were involved in intestinal motility and are related to exploring anti-diarrheal activity. The binding affinity and docking scores for all the ligand molecules are summarized in Table 8. In the case of Cystic fibrosis, transmembrane receptor CFTR (PDB ID:5UAK), 1-(2-Fluorophenyl)pyrazole-4-carboxylic acid showed the docking score −8.9, where 1-(2-Fluorophenyl)pyrazole-4-carboxylic acid interacts with the amino acid TRP A:277 and ALA A:274 of the Cystic fibrosis transmembrane receptor (Fig. 5). The docking score was −9.3 for the interaction of 1-(2-Fluorophenyl)pyrazole-4-carboxylic acid with a calcium-activated chloride channel (PDB ID: 5NL2). The interaction of the same compound with guanylate cyclase receptor (NCBI accession number P25092) had the docking score −8.2 while 1-(2-Fluorophenyl)pyrazole-4-carboxylic acid interacted with the amino acid TYR A:563, CYS A:564, VAL A:621 of and ILE A:514. A2B receptor (A2B receptor, NP_000667) was found to bind with 1-(2-Fluorophenyl)pyrazole-4-carboxylic acid through the amino acid SER A:68, HIS A:280, and VAL A:250 showing a docking score −8.7. MW, molecular weight; HBA, hydrogen bond acceptor; HBD, hydrogen bond donor, Log P, lipophilicity; QPlogPo/w, Predicted octanol/water partition coefficient; QOlogBB, Predicted blood–brain partition coefficient; QplogS, Predicted aqueous solubility, S in mol/dm − 3. None of the selected compounds, except Levoglucosenone was found to show toxicity in the Ames test which is widely used to check the mutagenic effects of any chemical, however; none of them is identified to show carcinogenic effects. Their oral toxicity level was III defying that their LD50 is between 50 mg/kg < LD50 < 300 mg/kg except for 3-O-Methyl-d-glucose which had an LD50 between 300 mg/kg < LD50 < 2000 mg/kg.
Compounds
Lipinski Rules
Lipinski'sViolations(≤1)
Veber Rules
QPlogPo/w(-2 to 6.5)
QPlogBB (−3 to 1.2)
QPlogS (−6.5 to 0.5)
% of human oral absorption (<25% is poor and > 80% is high)
MW(〈5 0 0)
HBA(<10)
HBD(<5)
Log P(≤5)
Nrb(≤10)
TPSA(≤140 Å2)
Loperamide
477.04
3
1
5.09
1
0
43.78
4.67
0.8500
−3.519
78.57
Levoglucosenone
126.11
3
0
−0.13
0
0
35.53
0.24
0.6500
1.319
76.736
1-Ethyl-2-hydroxymethyl imidazole
126.16
3
1
0.40
0
0
38.05
0.21
0.8820
−1.43
65.71
5-(Hydroxymethyl)-2-(dimethoxy methyl)furan
172.18
4
1
1.06
0
0
51.83
0.60
0.8250
−0.605
51.43
3-O-Methyl-d-glucose
194.18
6
4
−2.72
0
0
107.22
−1.70
0.7250
1.039
71.43
1-(2-Fluorophenyl)pyrazole-4-carboxylic acid
206.18
3
1
1.71
0
0
55.12
1.60
0.8250
−3.444
82.86
Compound Name
Binding affinity
Cystic fibrosis transmembrane receptor: CFTR
(PDB ID:5UAK)Calcium-activated chloride channel (PDB ID: 5NL2)
Guanylate cyclase receptor: (NCBI accession number P25092)
A2B receptor
(A2B receptor, NP_000667)
Loperamide
−9.6
−9.6
−9.9
−9.3
Levoglucosenone
−7.3
−7.2
−6.5
−6.5
1-Ethyl-2-hydroxymethyl imidazole
−6.4
−8.2
−6.6
−6.2
5-(Hydroxymethyl)-2-(dimethoxy methyl)furan
−7.4
−7.6
−6.7
−7.0
3-O-Methyl-d-glucose
−6.8
−7.0
−7.0
−5.9
1-(2-Fluorophenyl)pyrazole-4-carboxylic acid
−8.9
−9.3
−8.2
−8.7
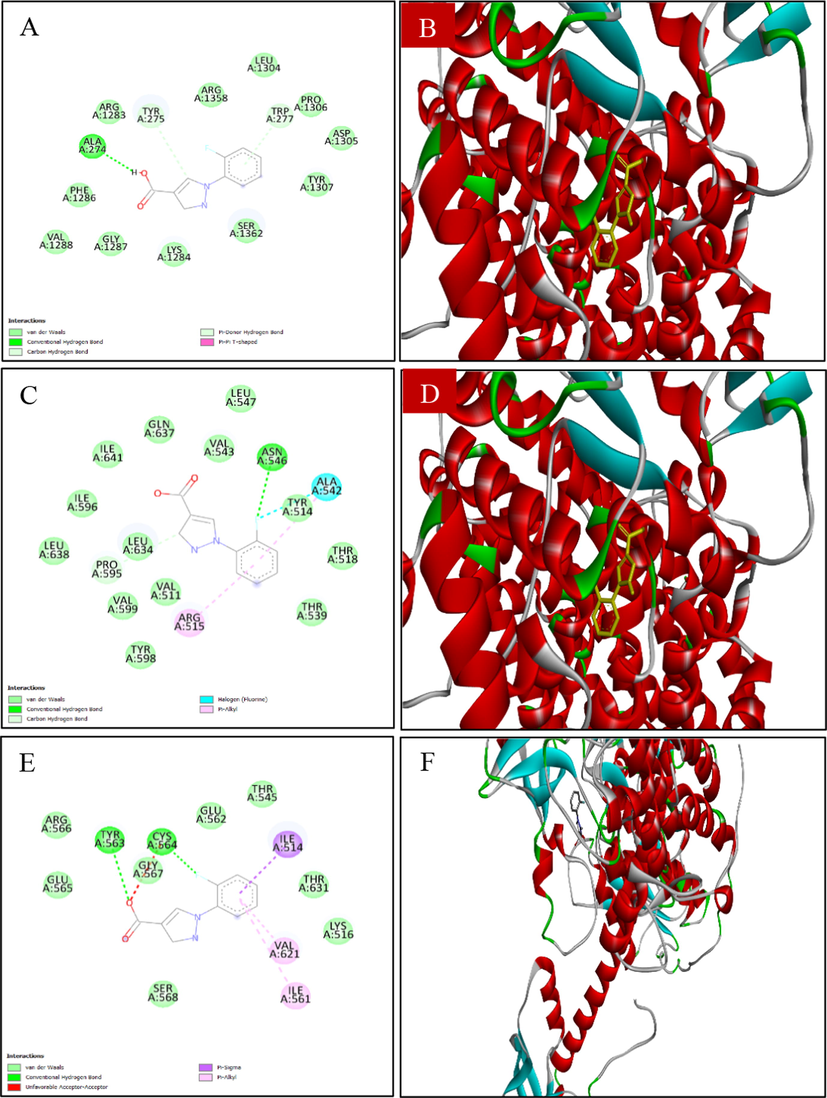
Docking analysis of (A, B) 2D and 3D view of 1-(2-fluorophenyl)-1H-pyrazole-4-carboxylic acid: Best binding affinity with CFTR; (C, D) 2D and 3D view of 1-(2-fluorophenyl)-1H-pyrazole-4-carboxylic acid: Best binding affinity with Calcium-activated chloride channel (E, F) 2D and 3D view of 1-(2-fluorophenyl)-1H-pyrazole-4-carboxylic acid: Best binding affinity with GC-C receptor; (G, F) 2D and 3D view of 1-(2-fluorophenyl)-1H-pyrazole-4-carboxylic acid: Best binding affinity with A2B receptor and (I, J) 2D and 3D view of Loperamide: Best binding affinity with GC-C receptor.
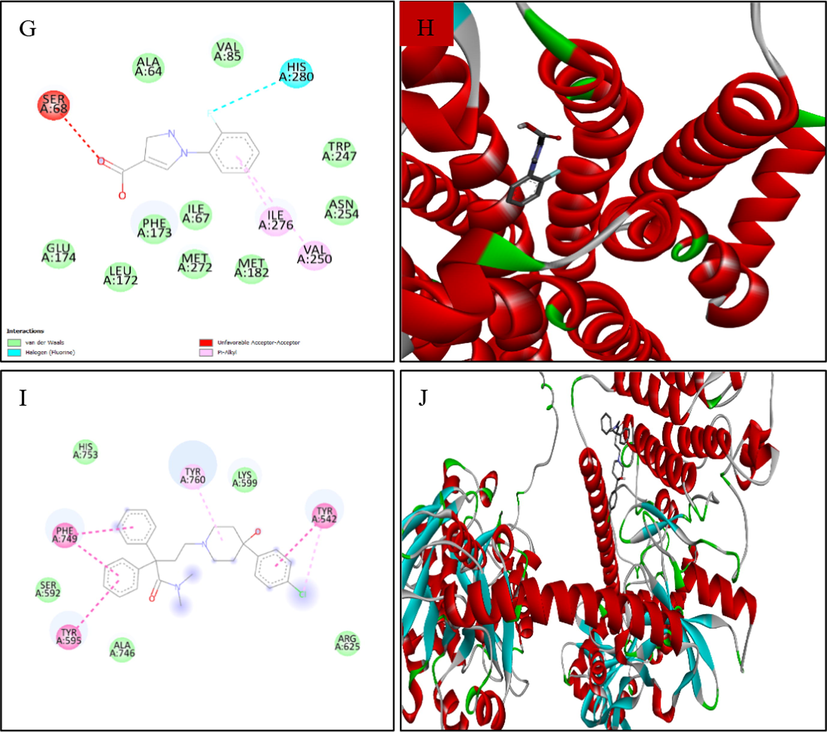
Docking analysis of (A, B) 2D and 3D view of 1-(2-fluorophenyl)-1H-pyrazole-4-carboxylic acid: Best binding affinity with CFTR; (C, D) 2D and 3D view of 1-(2-fluorophenyl)-1H-pyrazole-4-carboxylic acid: Best binding affinity with Calcium-activated chloride channel (E, F) 2D and 3D view of 1-(2-fluorophenyl)-1H-pyrazole-4-carboxylic acid: Best binding affinity with GC-C receptor; (G, F) 2D and 3D view of 1-(2-fluorophenyl)-1H-pyrazole-4-carboxylic acid: Best binding affinity with A2B receptor and (I, J) 2D and 3D view of Loperamide: Best binding affinity with GC-C receptor.
3.7 Pharmacokinetic and toxicological impacts
The pharmacokinetic properties of different compounds are investigated using QikProp ADME/T (absorption, distribution, metabolism, and excretion/transport) prediction tool. The study revealed that Levoglucosenone; 1-Ethyl-2-hydroxymethyl imidazole, 5-(Hydroxymethyl)-2-(dimethoxy methyl)furan, 3-O-Methyl-d-glucose, and 1-(2-Fluorophenyl)pyrazole-4-carboxylic acid did not disobey the Lipinski's and rule Veber's rule. Hence, these five compounds exhibited drug-like attributes (Table 8) and were more likely to be orally available as they maximally obeyed Lipinski's and Veber's rules. Furthermore, toxicological properties were also predicted using the admetSAR online server, where the study demonstrated that compounds are non-carcinogenic (Table 9). Therefore, four bioactive constituents could be considered promising drug candidates with good oral bioavailability through further extensive studies that are still necessary, like a clinical trial on animal models. However, 1-(2-Fluorophenyl)pyrazole-4-carboxylic acid has been shown to have the best binding effects as a future therapeutic target (Fig. 5). NAT, Non Ames toxic; AT, Ames toxic; NC, Non-carcinogenic; Category-III (50 mg/kg < LD50 < 300 mg/kg); Category-IV (300 mg/kg < LD50 < 2000 mg/kg).
Compounds
Structure
Parameters
Ames toxicity
Carcinogens
Acute oral toxicity
Rat Acute Toxicity
Loperamide
NAT
NC
II
3.221
Levoglucosenone
AT
NC
III
1.657
1-Ethyl-2-hydroxymethylimidazole
NAT
NC
III
1.564
5-(Hydroxymethyl)-2-(dimethoxymethyl)furan
NAT
NC
III
1.047
3-O-Methyl-d-glucose
NAT
NC
IV
2.344
1-(2-Fluorophenyl)pyrazole-4-carboxylic acid
NAT
NC
III
1.962
4 Discussion
This research has investigated the antidiarrheal effects of Litsea glutinosa bark extracts (CExLG, EAxLG, MExLG). The work was primed to evaluate the effects of extracts against four diarrhea-causing gram-negative bacteria. MExLG manifested the best antibacterial effects which may be mechanistically supported by the higher solubility of methanol and its greater potential to extract a wide range of polyphenolics effective against bacteria (Birru et al., 2016).
The resulting effects led to an assay of the anti-diarrheal effects of the extracts. Earlier studies using animal models have shown how plants influence gastrointestinal transit and the generation of water and electrolytes; several studies have demonstrated the effectiveness of traditionally used anti-diarrheal herbs (Palombo 2006, Tadesse et al., 2014). By employing castor oil and MgSO4-induced models in albino rats, this study sought to assess the anti-diarrheal activity of the three distinct L. glutinosa extracts. Castor oil is known to cause diarrhea through the release of its active metabolite, ricinoleic acid, by lipases in the upper section of the small intestine (Mathias et al., 1978, Gunaydin and Bilge 2018). It increases fluid retention in the intestine by lowering absorption, enhancing fluid and electrolyte output, and interacting with the smooth muscle cells' EP3 prostanoid receptors (Tunaru et al., 2012). Additionally, this metabolite also alters the motility of GI smooth muscles.
In the castor oil-induced diarrheal model, the MExLG significantly affected all measured parameters: the average number of diarrheal feces, the weight of watery stools, and the percentage of inhibition. A previous study suggested that the anti-inflammatory activities demonstrated by L. glutinosa bark were due to the inhibition of cyclooxygenase and lipoxygenase enzymes which are responsible for the biosynthesis of non-steroidal anti-inflammatory drugs (Mathias et al., 1978). Thus, the anti-diarrheal action exerted by the extracts may also be associated with the inhibition of cyclooxygenase/lipoxygenase products. The evidence supports the hypothesis that the stimulation of prostaglandin production causes castor oil-induced diarrhea (Bhowmick et al., 2014).
The gastrointestinal tract is innervated by both sympathetic and parasympathetic autonomic nervous system fibers. The peristaltic movement of the gastrointestinal system has a myogenic nature, is mostly started by local reflexes, and can happen even without neurological connections to the brain or spinal cord. Extrinsic nerves in the gut appear to play a relatively minor function in controlling the organ's peristaltic activity (Pierce et al., 1971, Phillips and Powley 2007). An earlier investigation of barium sulfate revealed that it is a traditional osmotic purgative (Chatterjee 1993). Therefore, a barium sulfate test of intestinal motility was performed to see how L. glutinosa affected peristaltic movement.
Additionally, both the central nervous system and enteric (intestinal) nervous system are in charge of controlling gut function. The autacoids (serotonin, acetylcholine, and prostaglandins) significantly influence the control of bowel motions and secretions. Our results indicate that the extract can enhance water and electrolyte absorption from the gastrointestinal tract since it slows intestinal transit in rats compared to controls, extending the time for absorption. Therefore, the anti-diarrheal activity in our experimental models can be explained by the inhibitory influence of the small intestine's propulsive movement. Prostaglandin, muscarinic, serotonin, and opioid receptors may all play a part in how the extract inhibits the gut. This is consistent with the mechanism of action of Loperamide for its anti-diarrheal effect, as presented in the literature (Ezekwesili et al., 2004). Moreover, the extract may have an anticholinergic activity and cause a reduction in intestinal motility and secretion, which agrees with the action of atropine on the intestine (Qnais et al., 2005).
Diarrhea is brought on by four pathophysiologic processes: aberrant intestinal motility, electrolyte production, elevated luminal osmolarity, reduced electrolyte absorption, and shortening of intestinal transit time (Agbor et al., 2004). In the intervention of diarrhea, ricinoleic acid stimulates gastrointestinal motility and electrolyte secretion, reducing electrolyte absorption from the intestine and colon; these are similar to the pathophysiologic processes resulting in diarrhea (Mascolo et al., 1993). Restoration of electrolytic balance by the L. glutinosa extract may be lined with its anti-diarrheal effects. Triglycerides are thought to be propulsive in aggravation of diarrhea and therefore aastor oil, a triglyceride characterized by a high content of the hydroxylated unsaturated fatty acid ricinoleic acid, is administrated to induced diarrhea. Literally, almost 90% of ricinoleate present in castor oil is mainly responsible for diarrhea production (McKeon et al., 1999). Reduced TG levels by L. glutinosa bark extract thus correlate with lessening diarrhea. In our experiment, IgE level was reduced by the treatment with L. glutinosa, which is in concordance with the strong association of chronic diarrhea and high titer of IgE with eosinophilic infiltration of gastric and antral mucosa (Estrada-Reyes et al., 2008, Sriram et al., 2010). The CRP is generally used as a monitoring or prognostic indicator in infectious diseases. It is a candidate biomarker that can differentiate between inflammatory and non-inflammatory diarrhea in patients with acute infectious diarrhea (Kim et al., 2013). The higher CRP level is related to poor prognosis. However, it is important to note that the increase in the CRP in bacterial infection is due to extracellular multiplication in the bloodstream, which induces a robust systemic inflammatory response leading to cause diarrhea (Ibrahim et al., 2011). The reduction of the elevated CRP levels in both the diarrheal models may be exerted through the common mechanism of reclaiming the restoration of CRP by treating L. glutinosa.
Molecular docking analyses were employed extensively in estimating ligand-target relationships and gaining a deeper understanding of the biological activity of natural products. It provides more insights into probable mechanisms of action and binding mode within the binding pockets of several proteins (Khan et al., 2019). Five compounds within L. glutinosa have been selected for docking tests to provide greater insight into the anti-diarrheal activity. The compounds were then docked against four targeted receptors: cystic fibrosis transmembrane receptor, Calcium-activated chloride channel, guanylate cyclase receptor, and A2B receptor.
The cystic fibrosis transmembrane conductance regulator (CFTR) is the primary chloride channel at the apical membrane of intestinal epithelial cells. It plays a significant role in intestinal fluid secretion and homeostasis. Inhibition of CFTR chloride-channel activity represents a novel approach to managing drug-induced secretory diarrhea because the CFTR channel causes excessive fluid secretion and secretory diarrhea through protein–protein interactions and cAMP/cGMP-mediated signaling. Strong binding of the selected four compounds with CFTR pondering their drug-likeliness is consistent with the proposition (Moon et al., 2015).
High-throughput screenings have yielded several chemical classes of small molecule CFTR and calcium-activated chloride channel (CaCC) inhibitors that show efficacy in animal models of diarrheas. Because, in secretory diarrhea, activation of Ca2+ signaling pathways increases the conductance of enterocyte Cl− channels, an attractive class of targets for diarrhea therapy, which include the CFTR and CaCCs. Natural-product diarrhea remedies with Cl-channel inhibition activity have also been identified that attenuated by intraperitoneal treatment with CaCC inhibitor (CaCCinh-A01) (Thiagarajah et al., 2015). The higher binding score of the selected compounds indicates their prospects to be a CaCC inhibitor.
Guanylate Cyclase-C (GC-C) is a transmembrane receptor predominantly located on intestinal epithelial cells. Receptor Guanylyl Cyclase C (GC-C) was initially characterized as an important regulator of intestinal fluid and ion homeostasis. Bacterial enterotoxin peptides are reported to bind with GC-C and stimulate cGMP secretion, which eventually causes diarrhea through a cascade of mechanisms. Therefore, searching for a new target inhibitor of GC-C is one of the most elegant ways of unfolding the anti-diarrheal drug. Our study showed a good binding interaction of four ligands out of five; their potential may be further studied to validate the computational data (Camilleri 2012).
The adenosine A2B receptors are expressed in various cell types. At the same time, its involvement in visceral hypersensitivity in animal models of IBS and regulation of intestinal secretion and motor function are cited literally. The A2B receptor activation triggers adenylate cyclase stimulation, leading to an increase in intracellular cyclic AMP (cAMP) and calcium ion levels. The recent development of pharmacological research has demonstrated the role of adenosine A2B receptor in pathophysiological processes, including intestinal inflammation (Mathias et al., 1978, Tunaru et al., 2012, Bhowmick et al., 2014), intestinal secretion, motility, and sensation (Weiglmeier et al., 2010, Aherne et al., 2011). High-affinity molecular binding of L. glutinosa bark compounds has revealed their possibilities to be a suitable inhibitor of A2B receptors to control drug-induced secretory diarrhea. According to Lipinski's law, the bioactive compound exhibited orally active drug-likeness properties. Pyrazoles among the selected compounds are reported as antibacterials and antioxidants, while high antioxidants are associated with the inhibition of diarrhea-causing pathogens (Essuman et al., 2021). Compounds with lower molecular weight, lipophilicity, and hydrogen bonding are highly permeable and have good absorption and bioavailability (Moon et al., 2015). From these results, plant-derived natural compound targets will be executed for drug discovery for various ailments, including diarrhea. We can conclude that the studied phytoconstituent is enormously responsible for the anti-diarrheal activities of L. glutionsa.
5 Conclusion
This research has revealed the anti-diarrheal effects of three distinct extracts of Litsea glutinosa while the methanol extract has remarkably attenuated the diarrheal incidence in the studied models showing no adverse effects at least in the experimental conditions. A combination of traditional use and scientific evidence affirms the future therapeutic prospects of L. glutinosa bark to control diarrhea. The effects are primarily evidenced to be attained by the major heterocyclic compounds including 1-(2-Fluorophenyl)pyrazole-4-carboxylic acid which has been found the best target for anti-diarrheal therapeutics. However, the use of a single dose of three extracts could be surpassed by administrating a multidose-response comprehensive study to affirm the clinical use of L. glutinosa methanol extract in diarrheal incidences.
CRediT authorship contribution statement
Md. Atiar Rahman: Conceptualization, Supervision, Project administration. Nazifa Anjum: Methodology, Data curation. Md. Khalid Juhani Rafi: Methodology, Data curation. Srabonti Saha: Methodology, Data curation. Jobaier Ibne Deen: Methodology, Data curation. Mijbah Uddin: Methodology, Data curation. Farjana Sharmen: Methodology, Data curation. Humayra Ferdousi: Methodology, Data curation. Rahni Hossain: Methodology, Data curation.
Acknowledgments
The authors thank Professor Dr. Shaikh Bokhtear Uddin for identifying the sample and offering an accession number for identification. The authors also would like to thank the Research and Publication Cell of Chittagong University for partially supporting the research project.
Funding
This research is supported by Walailak University, Thailand.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The antidiarrhoeal activity of Alchornea cordifolia leaf extract. Phytotherapy Res.: An Int. J. Devoted Pharm. Toxicol. Evaluation Natural Product Derivatives. 2004;18:873-876.
- [Google Scholar]
- The resurgence of A2B adenosine receptor signaling. Biochimica et Biophysica Acta (BBA)-Biomembranes.. 2011;1808:1329-1339.
- [Google Scholar]
- Deciphering antidiarrheal effects of Meda pata (Litsea glutinosa (Lour.) CB Rob.) leaf extract in chemical-induced models of albino rats. J. Ethnopharmacol.. 2023;308:116189
- [Google Scholar]
- Traditional Arabic and Islamic medicine, a re-emerging health aid. Evid. Based Complement. Alternat. Med.. 2010;7:419-424.
- [Google Scholar]
- In vivo analgesic, antipyretic, and anti-inflammatory potential in Swiss albino mice and in vitro thrombolytic activity of hydroalcoholic extract from Litsea glutinosa leaves. Biol. Res.. 2014;47:1-8.
- [Google Scholar]
- Antidiarrheal activity of crude methanolic root extract of Idigofera spicata Forssk. (Fabaceae) BMC Complement. Altern. Med.. 2016;16:1-7.
- [Google Scholar]
- Leea macrophylla (Roxb.) root extract reverses CCl4 induced liver injury through upregulation of antioxidative gene expression: a molecular interaction for therapeutic inception. Adv. Traditional Med.. 2020;20:35-52.
- [Google Scholar]
- Guanylate cyclase C signaling: an intestinal secretory pathway where bugs, genes and new drugs intersect. Genome Med.. 2012;4:1-3.
- [Google Scholar]
- Handbook of laboratory Mice and Rats. Department of Pharmaceutical Technology: Jadavpur University; 1993. p. :157.
- Inhibition of arachidonic acid release as the mechanism by which glucocorticoids inhibit endotoxin-induced diarrhoea. Br. J. Pharmacol.. 1981;73:549.
- [Google Scholar]
- Hypereosinophilia, hyper-IgE syndrome, and atopic dermatitis in a toddler with food hypersensitivity. J. Investigational Allergol. Clin. Immunol.. 2008;18:131.
- [Google Scholar]
- Ezekwesili, C., K. Obiora and O. Ugwu, 2004. Evaluation of Anti-Diarrhoeal Property of Crude Aqueous Extract of Ocimum gratissimum L.(Labiatae) In Rats.
- Analgesic and antidiarrheal properties of Ocimum selloi essential oil in mice. Fitoterapia. 2008;79:569-573.
- [Google Scholar]
- Evaluation of the Antidiarrheal and Antioxidant Effects of Some Chewing Sticks Commonly Used for Oral Hygiene in Ghana. Evid Based Complement Alternat Med.. 2021;7270250
- [CrossRef] [Google Scholar]
- Ethnomedicinal study of plants used by Sheko ethnic group of Ethiopia. J. Ethnopharmacol.. 2010;132:75-85.
- [Google Scholar]
- Practice guidelines for the management of infectious diarrhea. Clin. Infect. Dis.. 2001;32:331-351.
- [Google Scholar]
- Effects of nonsteroidal anti-inflammatory drugs at the molecular level. Eurasian J. Med.. 2018;50:116.
- [Google Scholar]
- Propagation, antibacterial activity and phytochemical profiles of Litsea glutinosa (Lour.) CB Robinson. Dhaka Univ. J. Biol. Sci.. 2014;23:165-171.
- [Google Scholar]
- Pretreatment of Blumea lacera leaves ameliorate acute ulcer and oxidative stress in ethanol-induced Long-Evan rat: a combined experimental and chemico-biological interaction. Biomed. Pharmacother.. 2021;135:111211
- [Google Scholar]
- PockDrug-Server: a new web server for predicting pocket druggability on holo and apo proteins. Nucleic Acids Res.. 2015;43:W436-W442.
- [Google Scholar]
- Diagnostic value of serum procalcitonin levels in children with meningitis: a comparison with blood leukocyte count and C-reactive protein. JPMA-J. Pakistan Medical Assoc.. 2011;61:346.
- [Google Scholar]
- Evaluation of anti-diarrhoeal activity of Crataeva nurvala root bark in experimental animals. Int. J. Pharm. Pharm. Sci.. 2010;2:158-161.
- [Google Scholar]
- Phytochemical profiling, in vitro biological properties and in silico studies on Caragana ambigua stocks (Fabaceae): a comprehensive approach. Ind. Crop. Prod.. 2019;131:117-124.
- [Google Scholar]
- Use and management of traditional medicinal plants by Maale and Ari ethnic communities in southern Ethiopia. J. Ethnobiol. Ethnomed.. 2014;10:1-15.
- [Google Scholar]
- Serum C-reactive protein (CRP) levels in young adults can be used to discriminate between inflammatory and non-inflammatory diarrhea. Dig. Dis. Sci.. 2013;58:504-508.
- [Google Scholar]
- 2-Benzoyl-6-benzylidenecyclohexanone analogs as potent dual inhibitors of acetylcholinesterase and butyrylcholinesterase. Bioorg. Med. Chem.. 2016;24:3742-3751.
- [Google Scholar]
- Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev.. 2012;64:4-17.
- [Google Scholar]
- Inhibitors of nitric oxide synthetase prevent castor-oil-induced diarrhoea in the rat. Br. J. Pharmacol.. 1993;108:861-864.
- [Google Scholar]
- Ricinoleic acid effect on the electrical activity of the small intestine in rabbits. J. Clin. Invest.. 1978;61:640-644.
- [Google Scholar]
- Biosynthesis of ricinoleate in castor oil. Chemicals via Higher Plant Bioeng. 1999:37-47.
- [Google Scholar]
- The antidiarrheal and spasmolytic activities of Phyllanthus emblica are mediated through dual blockade of muscarinic receptors and Ca2+ channels. J. Ethnopharmacol.. 2011;133:856-865.
- [Google Scholar]
- Drug-induced secretory diarrhea: a role for CFTR. Pharmacol. Res.. 2015;102:107-112.
- [Google Scholar]
- A revision of the genus Litsea Lam. (Lauraceae) in Thailand. Thai For. Bull. (Botany) 2011:40-119.
- [Google Scholar]
- In vitro glucose entrapment and alpha-glucosidase inhibition of mucilaginous substances from selected Thai medicinal plants. Sci. Pharm.. 2009;77:837-850.
- [Google Scholar]
- Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: modes of action and effects on intestinal function. Phytotherapy Res.: An Int. J. Devoted Pharmacol. Toxicol. Evaluation Natural Product Derivatives. 2006;20:717-724.
- [Google Scholar]
- Innervation of the gastrointestinal tract: patterns of aging. Auton. Neurosci.. 2007;136:1-19.
- [Google Scholar]
- Effects of prostaglandins, theophylline, and cholera exotoxin upon transmucosal water and electrolyte movement in the canine jejunum. Gastroenterology. 1971;60:22-32.
- [Google Scholar]
- Antidiarrheal effects of Juniperusphoenicia L. leaves extract in rats. Pak. J. Biol. Sci.. 2005;8:867-871.
- [Google Scholar]
- Screening methods for natural products with antimicrobial activity: a review of the literature. J. Ethnopharmacol.. 1988;23:127-149.
- [Google Scholar]
- Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhoea. J. Ethnopharmacol.. 2001;76:73-76.
- [Google Scholar]
- The magnitude of the global problem of acute diarrhoeal disease: a review of active surveillance data. Bull. World Health Organ.. 1982;60:605.
- [Google Scholar]
- Chronic diarrhea with Hyper Immunoglobulin E syndrome. Curr. Pediatr. Res.. 2010;14
- [Google Scholar]
- Experimental assessment of antidiarrheal and antisecretory activity of 80% methanolic leaf extract of Zehneria scabra in mice. BMC Complement. Altern. Med.. 2014;14:1-8.
- [Google Scholar]
- Secretory diarrhoea: mechanisms and emerging therapies. Nat. Rev. Gastroenterol. Hepatol.. 2015;12:446-457.
- [Google Scholar]
- Castor oil induces laxation and uterus contraction via ricinoleic acid activating prostaglandin EP3 receptors. Proc. Natl. Acad. Sci.. 2012;109:9179-9184.
- [Google Scholar]
- An annotated key to the genera of Lauraceae in the Flora Malesiana region. Blumea: Biodiversity Evol. Biogeogr. Plants. 2001;46:125-140.
- [Google Scholar]
- Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem.. 2002;45:2615-2623.
- [Google Scholar]
- Cure and curse: E. coli heat-stable enterotoxin and its receptor guanylyl cyclase C. Toxins.. 2010;2:2213-2229.
- [Google Scholar]
- admetSAR 2.0: web-service for prediction and optimization of chemical ADMET properties. Bioinformatics. 2019;35:1067-1069.
- [Google Scholar]
- Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine. 2002;9:69-74.
- [Google Scholar]







