Translate this page into:
Pyroelectric field drived photocatalysis by ZnFe2O4/NaNbO3 heterojunction for dye degradation through integration of solar and thermal energy
⁎Corresponding authors. huzhenglong@hbust.edu.cn (Zhenglong Hu), juanxiong@hubu.edu.cn (Juan Xiong)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A novel composite of ZnFe2O4/NaNbO3 (ZFO/NNO) nanorods with a p-n heterojunction structure was successfully synthesized via the hydrothermal method. The NNO nanorods were heated in situ using the photothermal effect of ZFO and subsequently cooled to room temperature, creating a cyclic heating and cooling process for ZFO/NNO. The catalytic activity of the resulting nanorods was assessed through the degradation of Rhodamine B, reaching 98% degradation efficiency after 180 min, attributed to the synergistic effect of pyrocatalysis and photocatalysis. Compared with NNO and ZFO individually, the enhanced performance of ZFO/NNO can be attributed to several synergistic factors, including the expanded spectral range of light absorption for ZFO, the establishment of the built-in electric field of the p-n junction between ZFO and NNO, and the NNO pyroelectric effect that further improved the charge transfer efficiency. Superoxide radicals and holes generated from photo-induced electrons were identified as the active species. Hydroxyl radicals generated from pyroelectrically-induced charge also participated in catalytic reaction. The combined effect of pyroelectric and photocatalytic processes could significantly improve the coupled pyro-photocatalytic reaction for pyroelectric semiconductor heterostructure, enabling the utilization of multiple energy sources, including solar and thermal energy.
Keywords
Sodium niobate (NaNbO3)
Zinc ferrite (ZnFe2O4)
Heterojunction
Pyroelectric effect
Photocatalysis
1 Introduction
Photocatalytic technology is a widely recognized and significant approach for utilizing solar energy and has the potential to alleviate global environmental pollution and energy crises (Tu et al., 2020; Dai et al., 2023; Li et al., 2021). Significant advances have been made in the field of photocatalysis since the seminal paper reported by Fujishima and Honda in 1972. However, the practical application of photocatalytic degradation of contaminants still faces major challenges due to the high recombination rate of electron-hole pairs and low sunlight utilization efficiency. Natural diurnal temperature fluctuations, in addition to solar energy, also offer an inexhaustible energy resource (Ou et al., 2016; Wu et al., 2020; Jiang et al., 2021). Developing a new and environmentally friendly catalytic technology that utilizes diurnal temperature variations to treat dye wastewater is crucial.
Certain materials exhibit a property known as the pyroelectric effect. This effect describes the variation in spontaneous polarization that these materials undergo in response to temperature changes between hot and cold states (Jiang et al., 2021; Chen et al., 2019; Zhang et al., 2021). Temperature fluctuations and mechanical vibrations induce ion displacement as the dipole moment changes within pyroelectric and piezoelectric materials. This creates a net electric charge and an electric potential on the material's surface. The intrinsic electric field can effectively address rapid carrier recombination in the bulk phase (Liu et al., 2022; Li et al., 2014). Additionally, pyroelectric-induced surface charge generate reactive oxygen species (ROS), including hydroxyl radicals (·OH), superoxide radicals (·O2−), singlet oxygen (1O2), and hydrogen peroxide (H2O2) (Benke et al., 2015; Wu et al., 2016; Huang et al., 2022). Pyro-catalytically generated ROS are widely thought to have huge potential for disinfection and treatment of dyes (Raufeisen et al., 2020). Qian et al. combined the pyroelectric effect with electrochemical oxidation using ZnO nanorods and reported a thermocatalytic process with a decomposition rate of approximately 98.15 % for Rhodamine B (RhB) solution under 22–62 °C heating–cooling cycles (Qian et al., 2017). Jia et al. reported the pyro-catalytic activity of pyroelectric BiFeO3 nanoparticles for dye degradation under thermal cycles of 27–38 °C (Wu et al., 2016). Liu et al. reported on the enhanced pyro-photo-electric performance of Ba1−xSrxTiO3 catalyst attributed to the acceleration of carrier separation and transfer efficiency due to the built-in polarization electric field present in pyroelectric materials (Liu et al., 2022).
Sodium niobate (NaNbO3, NNO), a perovskite material with semiconductor properties, has attracted considerable research interest due to its excellent nonlinear optics, ferroelectricity, ionic conductivity, and photo-refractive properties. Moreover, NNO exhibits excellent pyroelectric properties, including a large pyroelectric coefficient of about 100 μC∙m−2∙K−1 and a high Curie temperature (370 °C) (Jung et al., 2011). These properties make it a promising choice for room-temperature pyroelectric catalytic processes (Guo et al., 2023; Zhang et al., 2019). Zhang et al. demonstrated the effectiveness of combining pyroelectric catalysis and photoelectrochemical catalysis in an NNO-based system, proving the feasibility of pyroelectric-assisted photoelectrochemical performance (Zhang et al., 2021; Li et al., 2020; Zhang et al., 2021). You et al. documented the pyrocatalytic decomposition performance of NNO with different morphologies under a heating–cooling cycle of 23–50 °C (You et al., 2018). Liu et al. reported on an optimized NNO nanostructure and noted that the synergy between the optimized pyroelectric effect and surface reaction dynamics can enhance the dynamics of photo- and pyro-generated carrier transfer (Li et al., 2022). Liu et al. substantiated the synergistic effect of piezoelectric/pyroelectric-driven mechano/pyro bi-catalysis of NNO nanofibers (You et al., 2018). However, the wide band gap of NNO (Eg ∼ 3.3 eV) significantly limited its photoreaction in the solar spectral region, indicating that most solar energy was not utilized by the original NNO photocatalyst. Additionally, the currently available pyroelectric materials, whose pyrocatalytic capability depends on the variation of ambient temperature, exhibit low pyrocatalytic efficiencies. Under steady-state conditions, the short-circuit pyrocurrent (I) available for pyrocatalytic reactions can be calculated as (Wang et al., 2022; You et al., 2022):
Photothermal agent nanostructures, which absorb light and convert it into heat, are one such ideal candidate (Mateo et al., 2021; Yang et al., 2021). ZnFe2O4 (ZFO), a type of ferrite with a spinel structure, exhibits various functionalities, including soft magnetism, antibacterial properties, and photocatalysis, and is inexpensive, non-toxic, environmentally friendly, and highly chemically stable (Sun et al., 2024). Importantly, ZFO possesses a narrow band gap of about 1.9 eV, enabling efficient utilization of solar energy (Zhang et al., 2022). Recent studies have highlighted ZFO's potential as a superior photothermal agent, demonstrating a superior and stable ability to convert light into heat. For example, Yang et al. reported ZFO nanoparticles that exhibited a temperature increase of 36.8 °C and a photothermal conversion efficiency of 37.7 % under near-infrared light irradiation of 808 nm (Wang et al., 2019). Wang et al. investigated a composite structure of ZnO/ZFO and proposed that the photothermal effect of ZFO accelerated the migration of reactive oxygen species (ROS) and, consequently, the catalytic reaction rate (Zhang et al., 2023). Additionally, Han et al. documented the synthesis of ZFO with oxygen vacancies via a soft-chemistry method, demonstrating its high performance in the photothermal catalytic degradation of gaseous organic pollutants (Sun et al., 2021).
In addition to the utilization of solar and thermal energy, one-dimensional (1D) materials, such as nanowires, nanorods, and nanofibers, have attracted significant research interest for nanostructure design due to their high aspect ratio, quantum confinement effects, and a significantly higher density of active sites, which are expected to lead to substantially enhanced photocatalytic activities (Qian et al., 2017; You et al., 2018; Xiao et al., 2015; Antonin et al., 2024). Motivated by these considerations, this work investigates a photocatalyst synthesized through the hydrothermal method comprised of ZFO-decorated NNO nanorods. In this composite structure combining pyroelectric and photothermal materials, light irradiation heats the catalyst, while the surrounding environment cools it. This cyclic heating and cooling process removes the requirement for additional energy input. The study demonstrates that the photocatalyst temperature can be modulated from room temperature to 45 °C under simulated sunlight irradiation. The degradation efficiency of RhB reached 98 % under temperature variation and light irradiation, achieving a degradation rate that was two times higher than that of its photocatalysis-only counterpart and 2.8 times higher than that of pristine ZFO, respectively. This improvement in dye degradation was attributed to the synergistic combination of pyroelectric catalysis with photocatalysis. This research has the potential to introduce a new approach for synergistic pyroelectric catalysis and elucidate the joint enhancement mechanism of the pyroelectric effect and photocatalysis, thus offering promising applications for pollutant treatment.
2 Experimental section
2.1 Synthesis of materials
All chemicals used in this experiment were analytical grade reagents, purchased from Sinopharm Chemical Reagent Co. Ltd. (China), and employed without any further purification steps.
2.1.1 Synthesis of NNO
NNO nanorods (NNO NRs) were synthesized via hydrothermal reaction and subsequent annealing treatment, following a previously reported method (Li et al., 2020; Li et al., 2022). To prepare the solution, 0.57 g Nb2O5 powder was added to 40 mL of 10 M NaOH aqueous solution and stirred for 2 h. The resulting suspension was transferred into Teflon-lined stainless-steel autoclaves, and a hydrothermal reaction was conducted at 160 °C for 2 h. After the reaction, the autoclaves were removed and allowed to cool down under ambient conditions. The precipitate obtained after filtration was washed with deionized water and ethanol to achieve a neutral pH in the supernatant. Then, the precipitate was dried at 80 °C for 12 h. The resulting NNO NRs were obtained after being treated at 500 °C for 2 h. For comparison purposes, NNO microcubes (NNO MCs) were prepared by extending the hydrothermal reaction time to 8 h while keeping all other conditions unchanged (Yu et al., 2012).
2.1.2 Preparation of ZFO nanoparticles
ZFO nanoparticles were synthesized using a solvothermal method following a previously documented procedure (Xu et al., 2022). Briefly, 0.06 mmol of Fe(NO3)3·9H2O was dissolved in a 30 mL solution of ethylene glycol/isopropyl alcohol (volume ratio: 5/3) under stirring at room temperature. Subsequently, 0.03 mmol of Zn(CH3COO)2·2H2O was added and stirred until complete dissolution. The mixture was then transferred to a 50 mL Teflon-lined autoclave and heated at 180℃ for 12 h. After cooling naturally to room temperature, the product was isolated by centrifugation, washed three times with purified water and once with anhydrous ethanol, and finally dried at 80℃ for 10 h.
2.1.3 Preparation of ZFO/NNO composites
NNO/ZFO composites were prepared by a sequential hydrothermal-solvothermal method. The NNO NRs/MCs prepared via the aforementioned hydrothermal method were added to a reactor containing ZFO, and the reaction was maintained at 180℃ for 12 h. After natural cooling, the products were washed and then heat-treated at 500℃ for 30 min, yielding the composite products of ZFO/NNO nanorods (abbreviated as ZFO/NNO NRs) and ZFO/NNO microcubes (abbreviated as ZFO/NNO MCs). Scheme 1 depicts the formation routes of the catalysts, providing a brief procedural overview of the synthesis of NNO NRs/MCs, ZFO nanoparticles (ZFO NPs), and NNO/ZFO heterojunctions.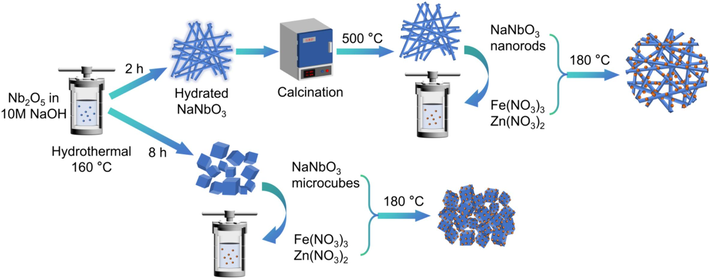
A schematic illustration of the synthetic process of the ZFO/NNO composites.
2.2 Characterization of the catalysts
The morphology and microstructure of the samples were investigated using a scanning electron microscope (SEM, S4800, Hitachi Co., Japan) and a transmission electron microscope (TEM, 2100F, JEOL Co., Japan). X-ray diffraction (XRD, AXSSM-D8, Bruker, Germany) with Cu Kα radiation was used to determine the crystal structure and phase purity of the samples. X-ray photoelectron spectroscopy (XPS) was recorded using Escalab 250Xi (Thermo Scientific Instruments, USA). UV–vis absorbance spectra were recorded using a UV-2600 instrument (Shimadzu Co., Japan) with BaSO4 as the reference. Electron spin resonance (ESR) signals of the radical spin were recorded using an E500 spectrometer (Bruker Co., Germany). The 5,5-dimethyl-1-pyrroline N-oxide (DMPO) was used as a spin trap to detect hydroxyl (∙OH) and superoxide (·O2−) radicals. The Brunauer-Emmett-Teller (BET) surface area was obtained using the nitrogen (N2) adsorption–desorption technique at 77 K on an ASAP 2460 (Micromeritics Co., USA) instrument.
The temperature variation of different samples under simulated solar light illumination (AM 1.5 G standards, 100 mW/cm2, 300 W Xe lamp, PLS-SXE300D, Perfectlight Technology Co., Beijing, China) was measured with a DT-1000 infrared thermometer (Shenzhen Weirdpower Technology Co., China).
The photoelectrochemical performance of catalysts was obtained using an electrochemical station (CHI660E, Chenhua Instruments, China). A three-electrode system was used, with a saturated calomel electrode and Pt serving as the reference and counter electrodes, respectively. The electrolyte used was a 0.5 M Na2SO4 solution. To achieve a total dispersion, 5 mg of the sample powder was added to 2 mL of ethyl alcohol and ultrasonicated for proper mixing. The resulting dispersion was then uniformly applied onto a pre-cleaned FTO glass measuring 1 cm × 1 cm. The coated glass was used as the working electrode after being dried in air at 393 K for 48 h. Electrochemical impedance spectroscopy (EIS) was conducted in the 0.1 Hz − 1 MHz range using a 5 mV AC voltage amplitude. Mott–Schottky plots (M-S plots) were recorded at a frequency of 1000 Hz. The Nernst equation was employed to convert the measured potential to that of a normal hydrogen electrode (NHE), as follows (Qian et al., 2020):
2.3 Evaluation of pyro-photodegradation activity
The photocatalytic degradation performance of the as-prepared samples (NNO NRs, ZFO/NNO NRs, and ZFO/NNO MCs) was evaluated using the degradation of organic dye RhB. To establish adsorption–desorption equilibrium between RhB and the catalysts, a 50 mL degradation solution of RhB (10 mg·L−1) was poured into a quartz beaker containing 50 mg of the as-prepared photocatalyst and stirred in darkness for 1 h. The whole system was then irradiated under simulated solar light for 180 min. A 3 mL aliquot of the suspension was collected every 30 min. The extracted solution was centrifuged at 8000 rpm and analyzed for RhB concentration using a UV–vis spectrophotometer in the range of 450–650 nm. The degradation performance of the catalysts is usually expressed by the relative concentration of the dye and is calculated using the following formula:
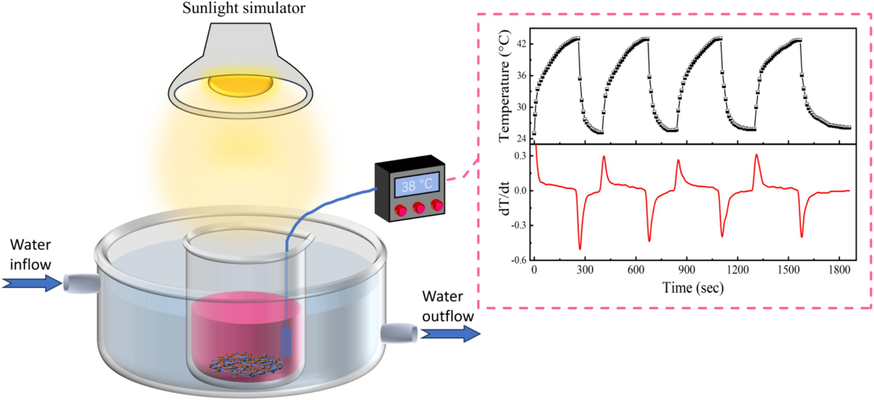
The schematic diagram of pyro-photocatalysis and temperature profile of the RhB solution under simulated solar light.
2.4 Theoretical calculation
Density functional theory (DFT) calculations base on the first-principles approach were performed by CASTEP program (Cambridge Sequential Total Energy Package). A Perdew–Burke–Ernzerhof (PBE) function based on the Generalized Gradient Approximation (GGA) was used for this computational procedure. Projected augmented wave (PAW) potentials were employed to describe the ionic cores and represent valence electrons using a plane wave basis set with a kinetic energy cutoff of 520 eV (Modak et al., 2016). Partial occupancies of the Kohn-Sham orbitals were allowed with a Gaussian smearing method and a width of 0.05 eV. Electronic convergence was achieved when the energy change was less than 10−5 eV. Geometry optimization was considered converged when the energy change was smaller than 0.05 eV∙Å−1. A vacuum spacing of 18 Å was applied in a direction perpendicular to the plane of the structure. Brillouin zone integration was performed using a 2 × 2 × 1 Monkhorst-Pack k-point mesh for the structure.
3 Results and discussion
3.1 Material characterization
Scheme 1 shows the schematic illustration of the hierarchical ZFO/NNO heterojunction synthesis process. Perovskite NNO NRs were synthesized via a two-step hydrothermal and calcination process, using Nb2O5 and NaOH as precursors. NNO materials with two different morphologies (nanorods and microcubes) could be obtained by adjusting the hydrothermal reaction times. After being mixed with a zinc and iron source for 2 h under magnetic stirring, both zinc and iron ions were deposited onto the surface of the NNO. Due to the good chemical stability of NNO NRs, ZFO NPs were grown in situ on them. The crystal phase and morphologies of pure NNO and ZFO, as well as ZFO/NNO composites, could be directly observed via XRD, SEM, and TEM images.
Fig. 1(a) shows the XRD patterns of the prepared samples (NNO, ZFO, ZFO/NNO NRs, and ZFO/NNO MCs). The NNO NRs diffraction pattern exhibited strong peaks at 2θ values of 22.9°, 32.6°, 46.5°, 52.6°, 58.1°, and 68.1°, which perfectly matched the reference pattern of orthorhombic NNO (JCPDS 33-1270). This result confirmed the successful synthesis of NNO using low-temperature hydrothermal followed by calcination (Yamazoe et al., 2013). The pattern of the ZFO sample (after being heat-treated at 500℃ for 30 min) showed main diffraction peaks at 2θ values of 29.9°, 35.3°, 42.8°, 53.1°, 56.6°, and 62.2°, which corresponded to cubic ZFO (JCPDS 22-1012). The corresponding crystal planes for the diffraction angles were labelled in Fig. 1(a). Both ZFO/NNO NRs and MCs exhibited XRD patterns consistent with the superposition of the two-component phases of ZFO and NNO. No diffraction peaks from impurities were observed, indicating the absence of a chemical reaction between NNO and ZFO during the solvothermal process. Furthermore, the results of the FWHM (Full Width at Half Maximum) of the primary diffraction peaks of NNO and ZFO, the grain size, and the mass percentage of the two phases in the composite were presented in Figure S1 of the Supplementary Materials.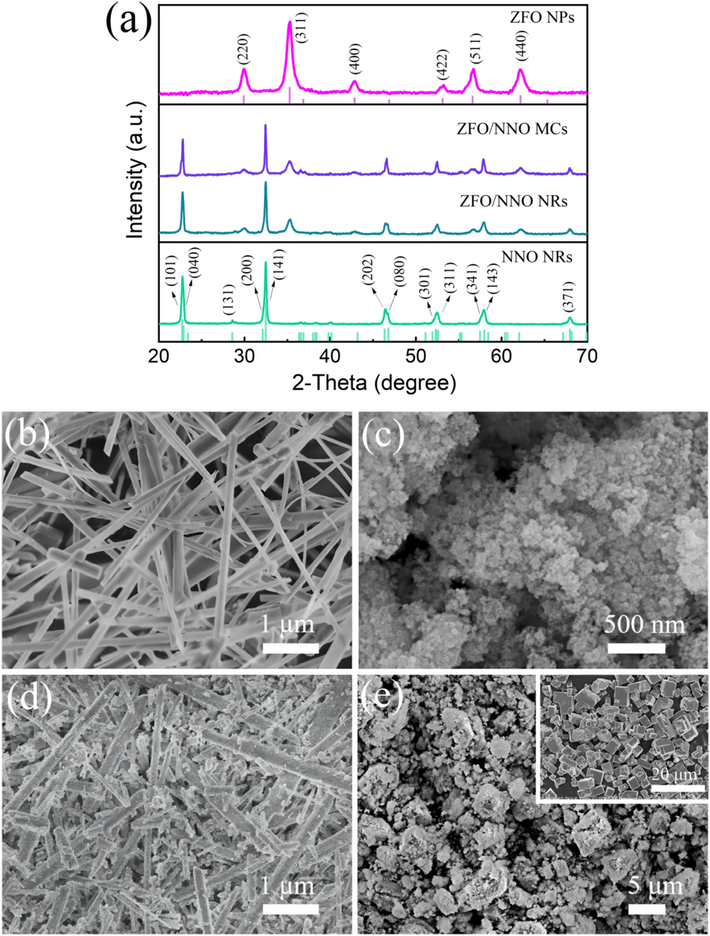
XRD patterns (a) and morphologies of NNO NRs (b), ZFO NPs (c), ZFO/NNO NRs (d), and ZFO/NNO MCs (e). The inset of (e) is the morphology picture of NNO MCs.
FESEM images in Fig. 1(b) confirm the successful synthesis of bare NNO with a nanorod-like morphology and relatively smooth surface, consistent with previous literature (Zhu et al., 2006). Fig. 1(c) shows that pure ZFO products consisted of slightly aggregated nanoparticles, requiring further analysis using TEM for more accurate size determination. Fig. 1(d) and (e) show ZFO/NNO composites where ZFO NPs are well-distributed on the surface of NNO NRs and MCs, respectively. The inset of Fig. 1(e) shows that the bare NNO products have a cube-like morphology with smooth surfaces. Notably, the absence of morphological alterations in the products suggests the successful preservation of their original form despite the incorporation of ZFO. This finding further confirms the successful synthesis of ZFO/NNO composites.
The microstructure and chemical composition of the ZFO/NNO nanorod composites were analyzed by TEM. Fig. 2(a) shows abundant ZFO NPs loaded onto the surface of NNO NRs. The NNO NRs exhibited an average diameter of approximately 100 nm, as illustrated in Fig. S2(a) of the supplementary materials. The inset reveals the ZFO NPs slightly aggregated structure with a mean size of approximately 10–20 nm (see Fig. S2 (b)). Fig. 2(b) shows the high resolution transmission electron microscopy (HRTEM) image of the ZFO/NNO NRs. Two distinct lattice fringes were observed, with lattice spacings of 0.271 nm and 0.255 nm, which can be assigned to the (141) plane of NNO and the (311) plane of ZFO, respectively. Furthermore, the high-magnification annular dark-field image and corresponding scanning The TEM-EDS images (Fig. 2(c)-(e)) demonstrated that the product is composed of the elements sodium (Na), niobium (Nb), zinc (Zn), iron (Fe), and oxygen (O). These findings collectively suggest that well-formed ZFO/NNO composites were created between ZFO NPs and NNO NRs. The mass and atomic fraction percentages of the elements were determined by means of TEM-EDS (TEM energy dispersive spectrometer). The results are presented in Figure S3 of the Supplementary Materials.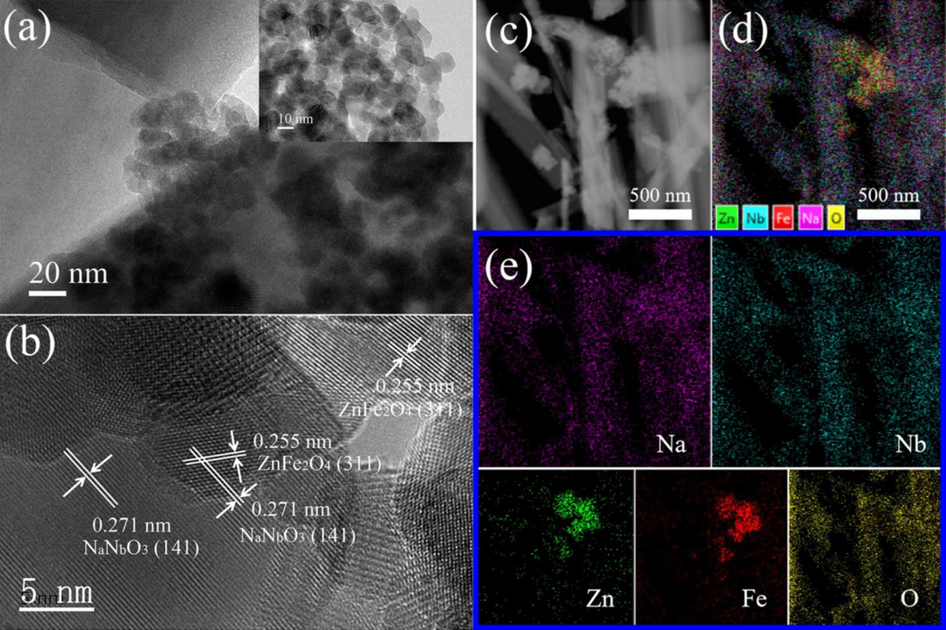
TEM images of ZFO/NNO NRs (a) and ZFO NPs (inset); HRTEM (b) and scanning TEM-EDS images (c, d, e) of ZFO/NNO NRs.
Due to the presence of surface-reactive sites, specific surface area and pore size are known to significantly influence the photocatalytic activity of materials. The variety of surface areas was determined by measuring nitrogen adsorption–desorption isotherms. As shown in Fig. 3(a), all samples exhibited type IV isotherms with H3 hysteresis loops (Xiao et al., 2015), characteristic of mesoporous materials with gas (liquid) adsorption capability. Based on the results, the ZFO/NNO NRs composite showed a higher specific surface area (SBET) compared to pristine NNO MCs, ZFO/NNO NRs and ZFO/NNO MCs. This finding suggests that the composite structure consisting of nanorods and nanoparticles can provide a larger surface area, offering more active sites for the reaction resulting in a stronger photocatalytic performance at the interface (Zhao et al., 2019; Zhu et al., 2020). Fig. 3(b) shows the pore size distribution of the synthesized catalysts. NNO NRs exhibited a wide distribution, indicating the presence of irregularly stacked nanorods. The incorporation of ZFO NPs led to a narrower distribution in ZFO/NNO NRs, possibly due to partial blockage of inter-rod spaces by ZFO. The values of BET surface area and pore volume of different samples are listed in Table 1.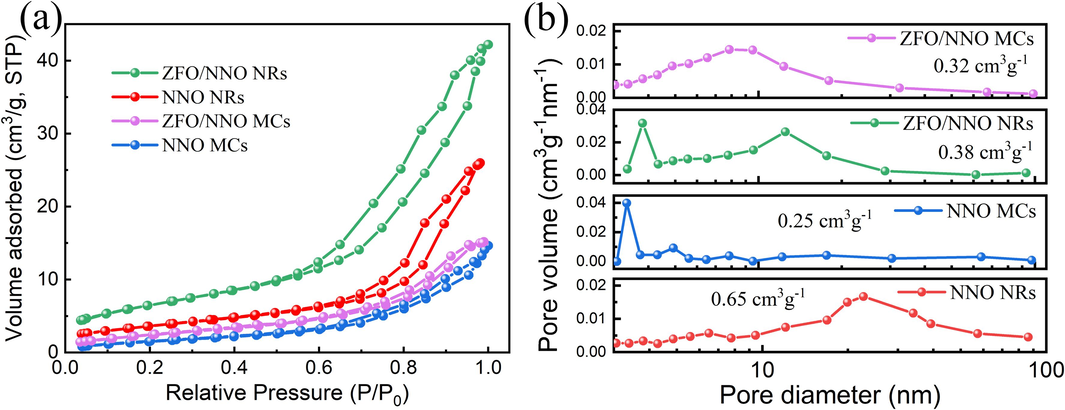
N2 adsorption–desorption isotherms (a) and pore size distribution plots (b) of the as-prepared catalysts.
Samples
NNO NRs
NNO MCs
ZFO/NNO NRs
ZFO/NNO MCs
SBET (m2/g)
12.3
4.6
24.9
5.4
Pore volume (cm3/g)
0.65
0.25
0.38
0.32
XPS analysis was performed to determine the elemental composition and chemical state of NNO, ZFO, and ZFO/NNO NRs. The C 1s peak at 284.8 eV was used for calibration. Fig. 4(a) depicts the complete survey XPS scans of NNO, ZFO and ZFO/NNO NRs, verifying the presence of Na, Nb, Zn, Fe, and O elements. Fig. 4(b) shows high-resolution XPS spectra of Na 1s in NNO and ZFO/NNO composites. The binding energies were 1071.6 eV and 1072.1 eV for NNO and ZFO/NNO, respectively, corresponding to Na+. Fig. 4(c) displays the Nb 3d XPS spectra, which exhibited a spin–orbit doublet with peaks at 206.6 eV and 209.3 eV for Nb 3d5/2 and Nb 3d3/2, respectively. These peaks confirmed the Nb5+ oxidation state in NNO. As shown in Fig. 4(d), the peaks observed at 1021.6 and 1044.6 eV corresponded to Zn 2p3/2 and Zn 2p1/2, respectively, indicating the existence of Zn2+ in ZnFe2O4. In Fig. 4(e), the XPS peaks located at 711.8 and 725.6 eV for Fe 2p were attributed to the spectra of Fe 2p3/2 and Fe 2p1/2, indicating the presence of Fe3+ ion. The two shake-up satellite signals located at ∼ 719.7 and 733.6 eV confirm the presence of the element Fe in the synthesized materials as Fe3+. Furthermore, the binding energies of Na 1 s and Nb 3d in ZFO/NNO NRs composite exhibited a mild shift towards the positive side as compared to those in pure NNO. Conversely, in the ZFO/NNO NRs composite, the peaks of Zn 2p and Fe 2p shifted to lower binding energies compared to those of the pristine ZFO. These binding energy shifts for the heterojunction components might be explained by the strong interaction between ZFO and NNO. The enhancement (upward shift) of binding energy theoretically indicated a weakened electron screening effect caused by the decreased electron concentration. Conversely, an increase in electron concentration led to a decrease (downward shift) in binding energy due to the promoted electron screening effect (Xu et al., 2021; Li et al., 2023). Consequently, in this case, it is highly conceivable that the observed upward and downward shifts of binding energy result from the increased electron concentration of ZFO and decreased electron concentration of NNO caused by the strong interaction based on the interfacial charge transfer from NNO to ZFO. In addition, high-resolution XPS O 1s spectra of the pristine NNO, ZFO and heterojunction samples were shown in Figure S4 of the Supplementary Materials.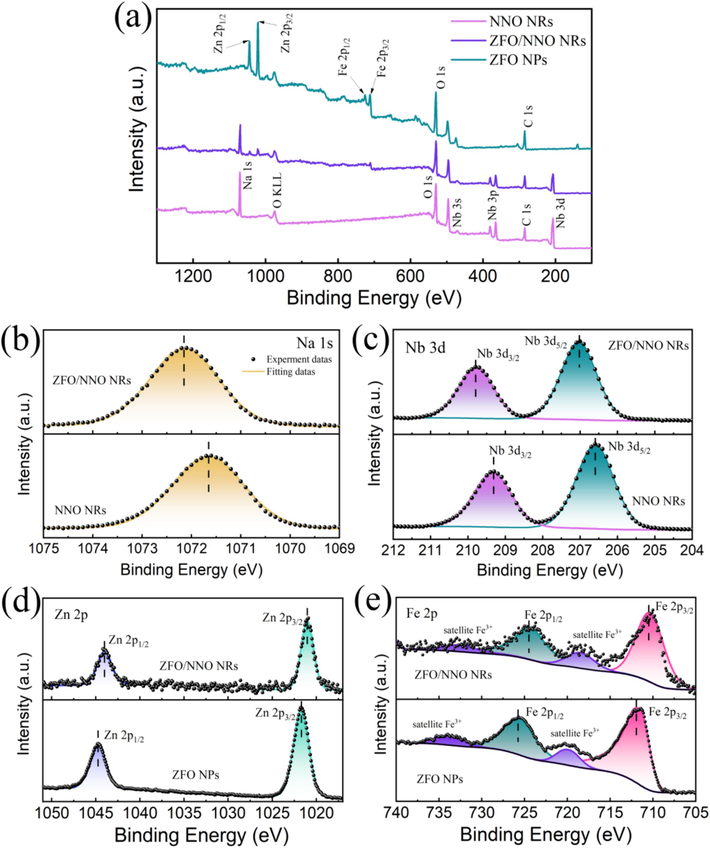
XPS fully scanned spectra (a) and high-resolution XPS spectra of Na 1s (b), Nb 3d (c), Zn 2p (d). and Fe 2p (e) in NNO, ZFO and ZFO/NNO NRs.
Light absorption is crucial for semiconductor photocatalysts. Fig. 5(a) shows the UV–Vis absorbance spectra of the prepared NNO, ZFO, and ZFO/NNO composites. The unmodified NNO exhibited a strong UV absorption edge at around 375 nm, as shown in its UV–Vis absorbance spectrum. Pure ZFO NPs exhibited broad light absorption from UV to visible light (below 650 nm), potentially beneficial for a wider range of light-activated reactions. In the case of ZFO/NNO composites, the UV–Vis absorbance spectra showed a combined absorption feature of ZFO and NNO. Additionally, ZFO/NNO NRs showed a stronger absorption compared to the ZFO/NNO MCs composite. This could be attributed to the agglomerated ZFO NPs covering the surface of NNO MCs, which reduced scattering of visible light, leading to a decrease in the absorption intensity of the composites (Zhao et al., 2019). The results of UV–Vis DRS indicated that the heterojunction broadened the photo-absorption range and improved the limited light absorption ability of NNO, which is highly advantageous in the activation of more charge carriers.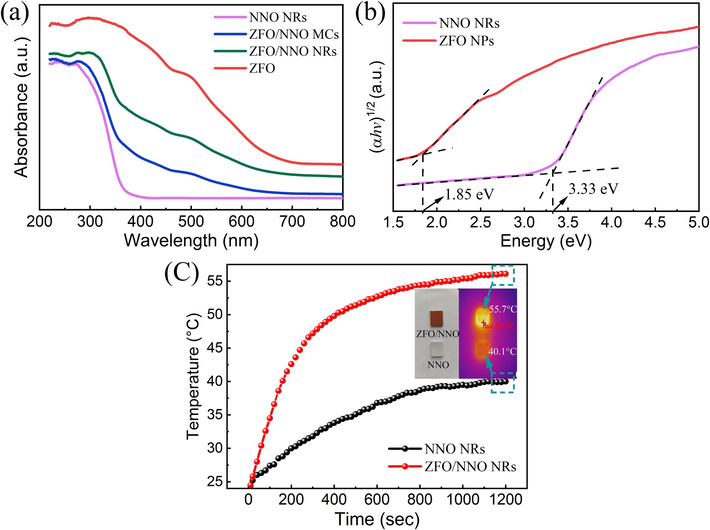
(a) UV–vis absorbance spectra, (b) plots of (αhv)1/2 vs. hv of the prepared NNO, ZFO, and ZFO/NNO composites, and (c) temperature–time profile of the surface of the sample under simulated solar light irradiation. The inset shows thermal imaging photos corresponding to the hottest temperatures.
Furthermore, the optical band gap of the samples could be estimated from plots of (αhv)1/2 vs. photon energy (hv)(Fig. 5(b)) based on the Kubelka-Munk method and Tauc’s equation as follows (Khan et al., 2020):
Building on its light absorption properties, ZFO is a typical photothermal agent that absorbs light energy and converts it into heat energy (Yang et al., 2021). The temperature changes of the samples' surfaces were recorded with an infrared thermometer to quantify the photothermal effect, as shown in Fig. 5(c). The surface temperature of pristine NNO NRs steadily increased from the ambient temperature of 24°C to reach ∼ 40 °C within 20 min under simulated solar light irradiation. In contrast, ZFO/NNO NRs composite exhibited a rapid temperature rise within 200 s, followed by a gradual increase to a maximum temperature exceeding 55 °C within 20 min. These results demonstrate that the addition of ZFO could enhance both visible light absorption and thermal conversion (Li et al., 2023).
3.2 Pyro-photocatalytic degradation performance
To examine the critical role of the photocatalyst, a control experiment was conducted. As shown in Fig. 6(a), negligible degradation of the RhB solution occurred under simultaneous light irradiation and temperature fluctuations in the absence of catalysts. It is well-known that RhB dye is highly stable, and the pyrolysis of organic molecules typically occurs at elevated temperatures (200–700 °C) (Inyang and Dickenson, 2015). Moreover, no decomposition of the RhB solution was observed, even with the NNO or ZFO/NNO pyrocatalysts under dark conditions. This is attributed to the limited intrinsic excitation in semiconductors, resulting in insufficient excitons to generate active free radicals (Luo et al., 2022). Then, the photo-pyrocatalytic performance of the ZFO/NNO heterojunctions under light irradiation and temperature fluctuations was investigated for their combined effects on RhB degradation. The experimental conditions consisted of simultaneous light irradiation and temperature fluctuations, as well as light irradiation alone. Fig. 6(b) demonstrated the degradation performance of RhB using NNO, ZFO, and ZFO/NNO heterojunctions as photo-pyrocatalysts, respectively. Evidently, the RhB exhibited some degradation under light conditions regardless of the catalyst sample used. In particular, the degradation of RhB was notably improved under conditions of temperature fluctuation, which implied that utilizing temperature fluctuations to promote the degradation of dyes for the pyroelectric catalysts is an effective approach. Fig. 6(c) and (d) display the kinetic plots of the degradation reactions corresponding to Fig. 6(a) and (d), respectively. These reactions were simulated using the Langmuir-Hinshelwood kinetic method. The equation for the Langmuir-Hinshelwood model is as follows (Goutham et al., 2022):
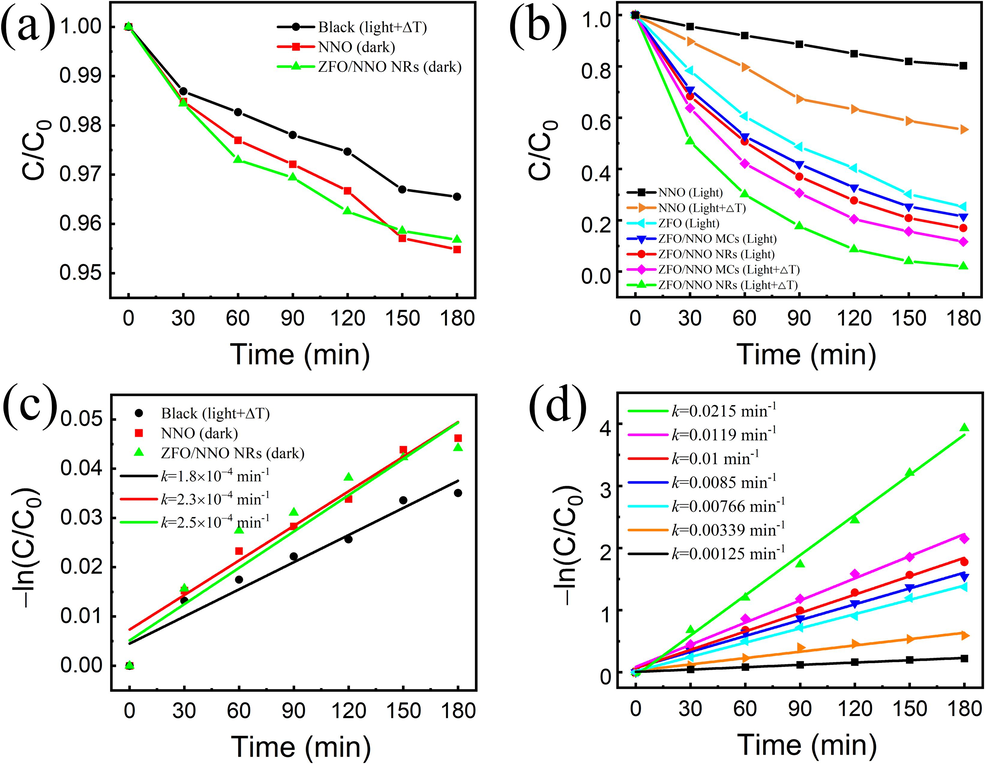
Photocatalytic performance: the concentration–time plots of RhB solution under dark (a) and light irradiation and temperature variation together (b), the linear fitting curves of kinetic rate constants (c) and (d) calculated from (a) and (b), respectively.
The reusability of photocatalysts is essential for practical applications, especially considering their separation and stability. To assess the reusability of ZFO/NNO NRs composite for practical applications, their photocatalytic stability under cyclic degradation with light irradiation and temperature variation was investigated. The ZFO/NNO NRs heterojunction exhibited good stability, as shown by the slight (only 6 %) decrease in degradation efficiency observed after five consecutive cycles (Fig. 7(a)). XRD patterns of the samples before and after the reaction cycle (Fig. 7(b)) showed almost identical peaks, indicating that the crystal structure remained largely unchanged. In summary, these results suggest that ZFO/NNO heterojunctions exhibit stability in the photocatalytic degradation of RhB dye. Additionally, the magnetic properties of the ZFO/NNO catalyst were evaluated. When a magnet was placed near the bottle containing the catalyst dispersion for one hour, the ZFO/NNO catalyst responded to the magnet by moving towards the inner wall of the bottle (Fig. 7(c)), suggesting that the introduction of ZFO made the catalyst magnetic. The complexation of catalysts with magnetic particles offers a solution to the challenge of separating photocatalysts from degradation solutions.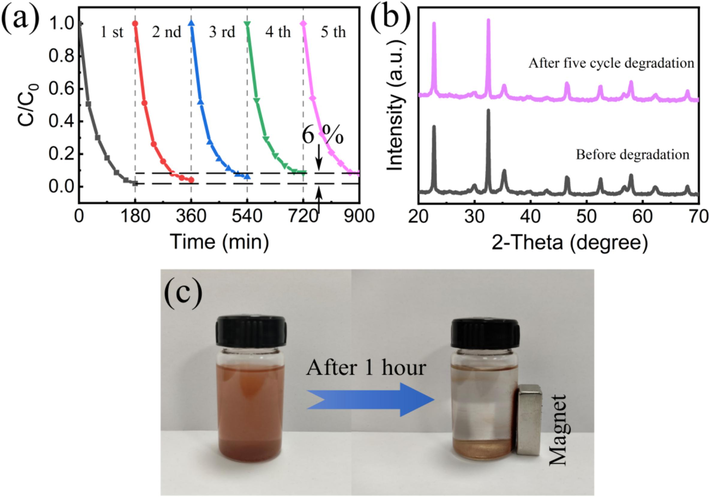
(a) The degradation cyclic performance; (b) the XRD patterns of the ZFO/NNO catalyst before and after the degradation reaction; (c) a photograph of the catalyst held by a magnet.
3.3 Radical and hole-trapping experiments
Identifying the primary active species generated by photocatalysts is crucial for understanding the degradation mechanism. To identify the primary reactive species involved, different radical scavengers were added to the RhB solution: tert-butanol (TBA) for hydroxyl radicals (∙OH), p-benzoquinone (BQ) for superoxide radicals (·O2−), and ethylenediaminetetraacetate (EDTA) for holes (h+) (Yang et al., 2021; Zhang et al., 2023; Sun et al., 2021; Xiao et al., 2015). As shown in Fig. 8(a), the percentage degradation of RhB after 3 h of photocatalysis varied in their suppressive effect, implying that the active species include hydroxyl radicals, superoxide radicals, and holes. With the addition of BQ, the degradation ratio was only 23 %, indicating that the superoxide radical was the primary reactive species during the degradation process. We found that 27 % of RhB was degraded after adding EDTA to capture h+, suggesting that h+ also played an important role in the degradation of RhB. The degradation ratio of RhB showed slight suppression after the addition of TBA, implying that the influence of hydroxyl radicals was moderate for photo-pyro-catalytic degradation.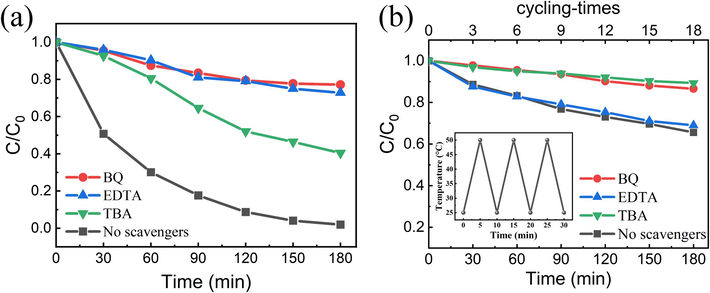
Radicals-trapping experiments of (a) ZFO/NNO NRs under light irradiation and (b) NNO NRs under temperature cycling with and without radical scavengers. The inset of (b) is the setup temperature variation for cold-hot cycles.
To understand the role of temperature fluctuations, an RhB degradation experiment with NNO NRs and radical scavengers was conducted in the dark to avoid the production of photogenerated carriers. The temperature cycling was achieved with a temperature-controllable water bath device (HH-S1A, Guangzhou Instrument Manufacturing Co., LTD, China), set in the temperature variation range of 25–50°C at 5 °C/min. Fig. 8(b) shows the influence of different scavengers on the degradation rate of RhB in the NNO pyro-catalytic degradation process. After the addition of these scavengers, the degradation efficiency was inhibited to some extent. Among them, the degradation efficiency decreased significantly after adding BQ and TBA, indicating that ·O2− and ∙OH were responsible for the main degradation reaction. The reaction process can be inferred as follows.
3.4 Pyro-Photocatalytic mechanism
To understand the impact of the heterojunction on charge separation, photoelectrochemical measurements were performed to analyze the charge transfer mechanism of the samples. Fig. 9(a) displays the Nyquist plots of electrochemical impedance spectroscopy (EIS) for NNO and ZFO/NNO NRs, measured using an electrochemical workstation. The arc radius of the analyzed Nyquist curve is generally considered proportional to the charge transfer impedance at the interface (Zhang et al., 2022; Xu et al., 2021). As shown in Fig. 9(a), the arc radius of ZFO/NNO NRs was smaller than that of NNO, with the smallest radius observed under light irradiation and temperature fluctuations. This indicated the lowest charge transfer resistance, which translated to the highest separation and transfer efficiency of charge carriers at this time. Therefore, the combination of ZFO with NNO and the pyroelectric effect stimulated by photothermal excitation reduced the interfacial charge transfer resistance and enhanced the separation and utilization efficiency of the charge carriers, ultimately improving photocatalytic performance.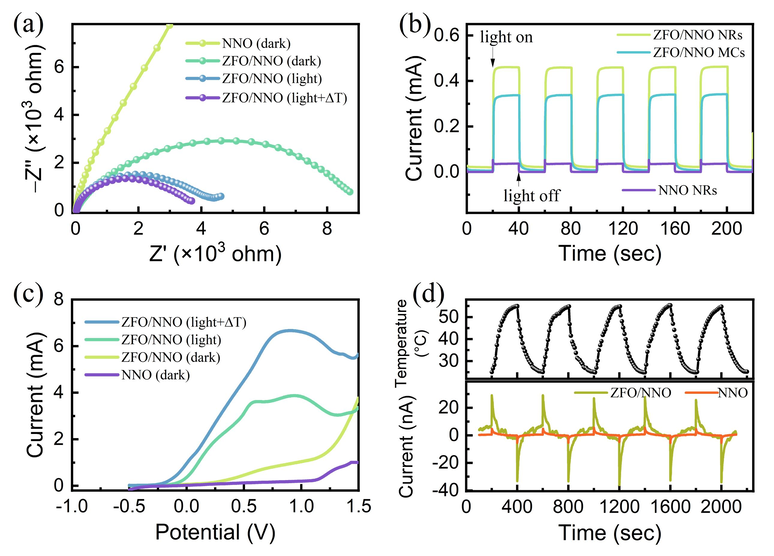
(a) Nyquist plots of EIS, (b) transient photocurrent response curves, (c) LSV curves, and (d) pyroelectric current–time curves of NNO and ZFO/NNO NRs composite.
To further investigate the reproducibility and stability of photocatalysts in response to light, a transient photocurrent response experiment was conducted under chopped-light irradiation (light switched on and off every 20 s). Fig. 9(b) shows the current–time (I-t) responses of the photoelectrodes under chopped-light irradiation. The responses were stable, reproducible, and showed a clear response to the light on–off cycles. Compared to the other samples, the ZFO/NNO NRs heterostructure showed the highest photocurrent intensity. The photocurrent response curve of the ZFO NPs electrode was presented in the Supplementary Materials, see Figure S6. Fig. 9(c) displays the linear sweep voltammetry (LSV) curves of NNO and ZFO/NNO NRs. The ZFO/NNO electrode exhibited a higher current response under light irradiation compared to darkness. Additionally, an increase in temperature during the process resulted in the maximum photocurrent being achieved at the same bias voltage. The experimental results of the photocurrent response discussed above suggest that the ZFO/NNO catalyst generated a significant number of electrons and holes, as well as compensating charge on the catalyst surface that could be excited and transferred, under the combined effect of light and temperature change (Goutham et al., 2022). This allowed for greater participation of charge carriers generated by photoexcitation and the pyroelectric effect in the photocatalytic reaction.
The photocatalytic performance observed in Fig. 6 was consistent with these findings. This could be attributed to the binary heterostructure and dimensional structure of ZFO/NNO NRs. The heterostructure promoted photogenerated electron-hole separation and charge transfer, leading to a higher number of charge carriers participating in the photocatalytic reaction. Additionally, the presence of NNO in the heterojunction played a crucial role due to its pyroelectric effect. Fig. 9(d) illustrates the temperature and pyroelectric current–time curves of NNO and ZFO/NNO catalysts under cold-hot cycle excitations. Temperature cycling between 25–55 °C in the dark environment was achieved using the laboratory's own hot/cold cycling apparatus, which avoids the production of photogenerated carriers. Compared to photocurrents, the pyroelectric currents of both NNO and ZFO/NNO catalysts were much less intense. They were rapidly excited when the catalyst temperature increased or decreased but quickly diminished when the temperature stabilized. The current is generated by the compensation charges in the solution, which migrates between the two poles driven by the pyroelectric field. The current intensity, according to equation (1) is directly proportional to the rate of temperature change (Yang et al., 2012; Yang et al., 2012). This pyroelectric field likely promotes the separation of charge carriers within the semiconductor photocatalyst and accelerates their migration toward the surface. This, in turn, leads to the generation of reactive radicals that participate in redox reactions with dye molecules. Furthermore, the PL spectra of the pristine NNO, ZFO and heterojunction samples were recorded using an Edinburgh FS5 fluorescence spectrophotometer, the results of which were presented in Figure S7 of the Supplementary Materials.
Density functional theory was further investigated to understand the interface charge transfer between NaNbO3 and ZFO. Fig. 10(a) and (b) show the calculated theoretical work functions of NNO (5.04 eV) and ZFO (6.06 eV). Since the Fermi level of NNO was higher than that of ZFO, electrons transferred from NNO to ZFO upon contact until their Fermi levels reached equilibrium. Subsequently, ZFO and NNO were negatively and positively charged respectively, forming an internal electric field (IEF) from NNO to ZFO. The charge transfer process derived from these findings aligned with the XPS characterization results. With the existence of IEF, the electron-hole pairs on NNO and ZFO were separated from each other, and it also caused band bending at the interface of ZFO and NNO (Tian et al., 2023).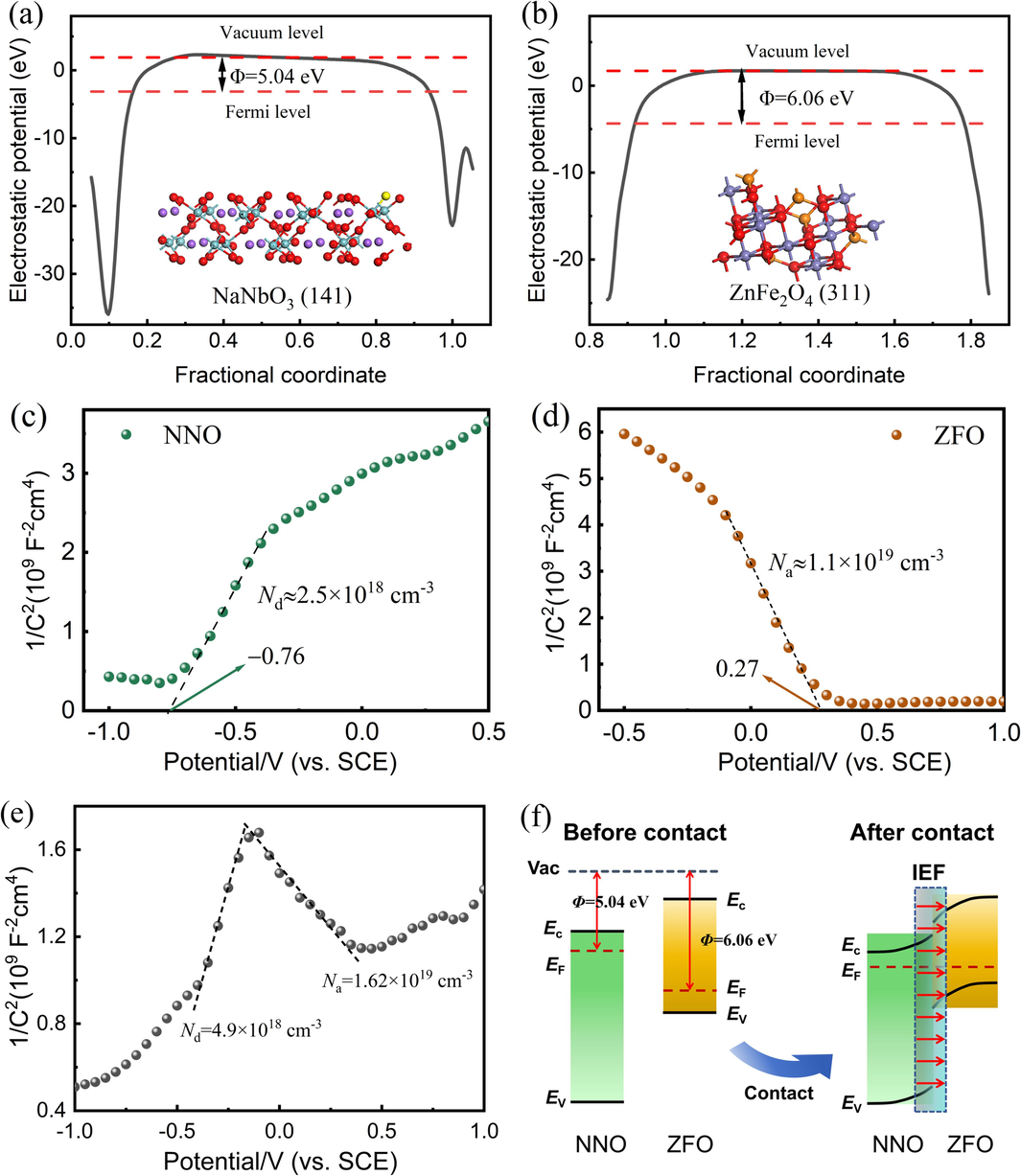
Electrostatic potentials of (a) NNO and (b) ZFO determined by DFT; Mott-Schottky plots of (c) NNO NRs, (d) ZFO and (e) ZFO/NNO NRs composite collected under light irradiation; (f) the energy level arrangement of NNO and ZFO before and after contact.
Mott-Schottky (M−S) measurements were conducted to identify semiconductor types and band structures. As shown in Fig. 10(c), the positive slope of the Mott-Schottky plot for the NNO NRs electrode indicated its n-type character. Extrapolating the linear region to the x-axis where C−2 = 0 yielded an Efb of approximately − 0.76 V (vs. SCE). Similarly, the negative slope of the curve for the ZFO NPs electrode indicated a p-type characteristic, with an Efb of around 0.27 V (vs. SCE) (see Fig. 10(d)) (Li et al., 2023). The ZFO/NNO composite exhibited an inverted V-shaped curve in Fig. 10(e), signifying the formation of a p–n heterojunction. In general, there is a 0.24 V difference between the SCE and the normal hydrogen electrode (NHE) (Qi et al., 2020). Moreover, the flat band potential was 0.1 ∼ 0.2 eV higher than its conduction band potential (ECB) for n-type semiconductors and lower than its valence band potential (EVB) for p-type semiconductors. Therefore, the EVB of ZFO was approximately 0.61 V (vs. NHE), and the ECB of NNO was approximately −0.62 V (vs. NHE). Furthermore, the EVB of NNO and the ECB of ZFO were 2.71 V and −1.24 V (vs. NHE), respectively, using the following equation (Wu et al., 2019):
As previously mentioned, Fig. 10(f) illustrates the energy level arrangement of NNO and ZFO before contact and the formation of an internal electric field after contact. After contacting, the energy levels of NNO and ZFO formed a type-II heterojunction structure, favorable for charge transfer and separation. The formation of the ZFO/NNO p-n heterojunction caused the Fermi levels of ZFO and NNO to align. Subsequently, an internal electric field was generated at the interface (Zhao et al., 2022). As a result, the band edge of NNO was bent upward due to electron loss, while that of ZFO was bent downward due to electron enrichment (Sudhir Ekande and Kumar, 2023). When exposed to simulated sunlight, electrons in both ZFO and NNO's valence band (VB) were excited to their empty conduction band (CB), leaving holes in the VB. ZFO/NNO exhibited a traditional type-II scheme, therefore electrons in ZFO's CB were further transferred to NNO's CB, through the p-n junction, whereas the holes in NNO's VB were transferred to ZFO's VB. Since the NNO's conduction band minimum (ECB) levels (−0.62 V) were more negative than the ·O2−/O2 redox potential (−0.33 eV vs. NHE), the generation of superoxide radicals was thermodynamically favorable. However, according to the theory, the valence band maximum (EVB) value of ZFO (0.61 V) was lower than the ·OH/OH− redox potential (1.99 eV vs. NHE), making the formation of hydroxyl radicals unfavorable. This contradicted the experimental results of free radical trapping, suggesting further investigation is needed to understand the pathway of hydroxyl radical production.
The carrier concentration, which signifies the density of electron donors (Nd) in n-type semiconductors and electron acceptors (Na) in p-type semiconductors, can be determined from the slopes of the quasilinear regions near the Efb in the Mott–Schottky plots using the following equation (Sudhir Ekande and Kumar, 2023; Soltani et al., 2020):
Based on the experimental results and energy band theory, a mechanism for the pyro-photocatalytic degradation of RhB by ZFO/NNO was proposed. The pyroelectric effect of NNO plays a crucial role due to its introduction. Fig. 11(a) illustrates the pyroelectrocatalytic process for dye degradation. In the equilibrium state with constant temperature, surface-bound charges on the NNO surface were shielded by the shielding charges. When the temperature changed (ΔT≠0), the pyroelectric effect altered the orientation of the electric dipoles and the spontaneous polarization intensity of NNO. This disrupted the balance between the polarization charges and the shielding charges, leading to uncompensated surface charges. Among these charges, negative charges (q−) could rapidly combine with oxygen molecules adsorbed on the NNO surface to form superoxide radicals. Positive charges (q+) facilitated the generation of hydroxyl radicals in the dye solution (Benke et al., 2015; Zhao et al., 2022). These strong oxidizing ·O2− and ∙OH could further react with the dye molecules, leading to the decomposition of RhB dye in solution. Additionally, the imbalance of surface charge could desorb the decomposition products adsorbed on the catalyst surface (Liu et al., 2024).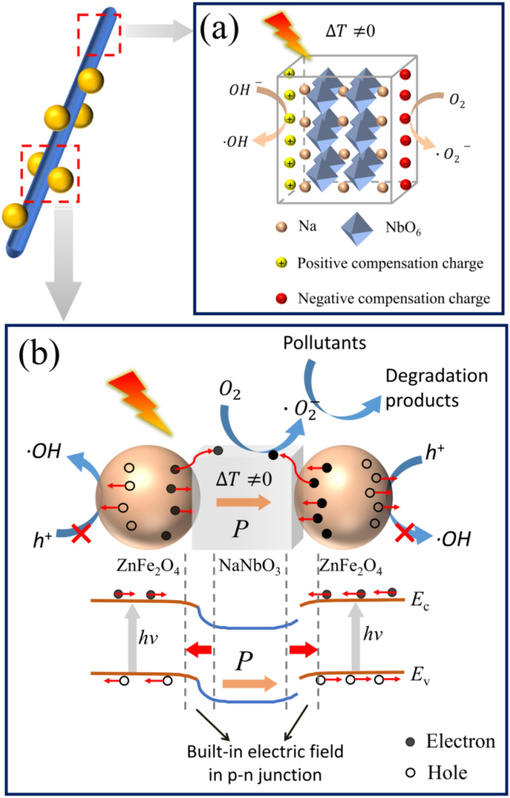
The schematic diagram for the pyro-/photocatalytic mechanism of (a) NNO and (b) ZFO/NNO composites.
When the ZFO/NNO composite was co-stimulated by light and heat, the synergy of the pyroelectric and the photoelectronic effects promoted the separation and transport of photogenerated carriers, and also generated the hydroxyl radicals (Wu et al., 2019; Naseer et al., 2022). As shown in Fig. 11(b), during temperature changes, NNO NRs generated a built-in electric field due to the pyroelectric effect. Under the influence of this pyroelectric potential, a positive polarization charge attracted electrons in its vicinity, resulting in the downward bending of the energy band edges. Conversely, a negative polarization charge repelled electrons in its vicinity, leading to an upward bending of the energy bands. In comparison to the equilibrium state with a constant temperature, this alteration in the degree of energy band bending disrupted the charge shielding effect and concurrently facilitated the separation and directional migration of the photogenerated electron-hole pairs. This ultimately led to the enhancement of the photocatalytic performance of ZFO/NNO composites. Additionally, the periodic pyroelectric field generated by the alternating hot and cold temperatures could change the polarization potential, preventing the saturation of the static built-in electric field (Wang et al., 2020). This ever-changing internal polarization electric field drove the carriers to move in opposite directions, suppressing the photogenerated electron-hole recombination and thus enhancing the photocatalytic performance.
4 Conclusions
In summary, a novel ZFO/NNO p-n junction photocatalyst with pyroelectric properties was successfully synthesized using a hydrothermal method. This work investigated a promising approach for dye decomposition that leverages the synergy of pyroelectric catalysis and photocatalysis in ZFO/NNO heterostructures. The incorporation of ZFO not only extended the photocatalyst's absorption spectrum into the visible light region, but its inherent photothermal effect also provided a heat source, contributing to the temperature fluctuations experienced by NNO. Photoelectrochemical measurements and PL spectra demonstrated a significant enhancement in the separation and migration efficiency of photogenerated charge carriers due to the combined effects of the built-in potential from the ZFO/NNO heterojunction and the spontaneous polarization potential of NNO. The N2 adsorption–desorption isotherms demonstrated that the ZFO/NNO NRs exhibited a markedly larger specific surface area (24.9 m2/g) than the ZFO/NNO MCs (5.4 m2/g). The light absorption curves demonstrated that the ZFO/NNO NRs exhibited a narrower band gap (2.37 eV of Eg) with a more extensive visible light absorption range than the ZFO/NNO MCs (2.8 eV of Eg). Under combined light irradiation and light-induced thermal cycling, the photocatalytic rate of ZFO/NNO NRs for RhB degradation (2.15 × 10−2 min−1) was more than twice its photocatalytic rate under light irradiation alone (1.0 × 10−2 min−1). Additionally, it was 2.8 and 17 times higher than the rate of ZFO (7.66 × 10−3 min−1) and NNO NRs (1.25 × 10−3 min−1) alone, respectively. While the photocatalytic rate of RhB degradation by ZFO/NNO MCs was 1.19 × 10−2 min−1. The degradation mechanism investigation suggested that the process was mainly driven by superoxide radicals and holes generated from photoexcited electrons. Radicals generated from pyroelectrically-induced charge also contributed to the degradation of dyes. The enhanced performance of ZFO/NNO under combined light and temperature changes could be attributed to several synergistic factors, including enhanced light absorption by ZFO, the establishment of the built-in electric field of the p-n junction between ZFO and NNO, and the NNO pyroelectric effect that further improved charge transfer efficiency. This “one arrow three birds” approach provided a method for rationally designing high-efficiency pyroelectric/photothermal nanostructures for photocatalysis applications, with promise for environmental remediation.
Author agreement
All authors have seen and approved the final version of the manuscript being submitted. They warrant that the article is the authors' original work, hasn't received prior publication and isn't under consideration for publication elsewhere.
CRediT authorship contribution statement
Di Zhou: Writing – original draft, Investigation, Funding acquisition, Data curation. Xiaoju Zhou: Writing – original draft, Methodology, Data curation. Zhenglong Hu: Writing – review & editing, Project administration, Funding acquisition, Conceptualization. Lili Zheng: Resources, Investigation, Data curation. Yu Tian: Methodology, Investigation, Data curation. Yafang Tu: Resources, Methodology, Investigation. Chunbo Hua: Resources, Funding acquisition, Data curation. Li Xue: Validation, Methodology, Investigation. Juan Xiong: Writing – review & editing, Project administration, Funding acquisition, Conceptualization.
Acknowledgements
This work was financially supported by the projects of National Natural Science Foundation of China (Grant No. 12304539), Natural Science Foundation of Hubei Province (Grant No. 2022CFB518), Doctoral Research Start-Up Fund of Hubei University of Science and Technology (Grant No. BK202316), Scientific Research Project of Hubei University of Science and Technology (Grant No. 2020-22GP08 and 2024-2025X08), Excellent Discipline Cultivation Project by Jianghan University (Grant No. 2023XKZ007). The authors also thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Vanessa S. Antonin, Felipe M. Souza, Victor S. Pinheiro, João P. C. Moura, Aline B. Trench, Caio Machado Fernandes, Marcos R. V. Lanza, Mauro C. Santos, Electrocatalytic hydrogen peroxide generation using WO3 nanoparticle-decorated sodium niobate microcubes, J. Electroanal. Chem. 959 (2024) 118190.
- Pyroelectrically driven ∙OH generation by barium titanate and palladium nanoparticles. J. Phys. Chem. C. 2015;119(32):18278-18286.
- [Google Scholar]
- Large Thermal–electrical response and rectifying conduction behavior in asymmetrically reduced ferroelectric ceramics. ACS Applied Electronic Materials. 2019;1(4):478-484.
- [Google Scholar]
- Recent advances in efficient photocatalysis via modulation of electric and magnetic fields and reactive phase control. Adv. Mater.. 2023;35:2370100.
- [Google Scholar]
- Synthesis and study of physicochemical properties of Fe3O4@ZnFe2O4 core/shell nanoparticles. J. Mater. Sci. Mater. Electron.. 2021;32:16786-16799.
- [Google Scholar]
- C. Goutham, K. V. Ashok Kumar, S. S. Kumar Raavi, C. Subrahmanyam, S. Asthana, Enhanced electrical and photocatalytic activities in Na0.5Bi0.5TiO3 through structural modulation by using anatase and rutile phases of TiO2, J. Materiomics, 8 (2022) 18–29.
- Piezoelectrically enhanced photocatalysis of KxNa1−xNbO3 (KNN) microstructures for efficient water purification. Nanoscale. 2023;15:6920-6933.
- [Google Scholar]
- NiFe2O4 and ZnFe2O4 nanoparticles synthesis by sol-gel auto-combustion for humidity sensor applications. J. Sol-Gel Sci. Technol.. 2023;105:416-429.
- [Google Scholar]
- Radical generation and fate control for photocatalytic biomass conversion. Nat. Rev. Chem.. 2022;6:197-214.
- [Google Scholar]
- The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: a review. Chemosphere. 2015;134:232-240.
- [Google Scholar]
- Ultrahigh piezoelectric coefficients of Li-doped (K, Na)NbO3 nanorod arrays with manipulated O-T phase boundary: towards energy harvesting and self-powered human movement monitoring. Nano Energy. 2021;86:106072
- [Google Scholar]
- Lead-free NaNbO3 nanowires for a high output piezoelectric nanogenerator. ACS Nano. 2011;5(12):10041-10046.
- [Google Scholar]
- Facile synthesis of a Z-scheme ZnIn2S4/MoO3 heterojunction with enhanced photocatalytic activity under visible light irradiation. ACS Omega. 2020;5:8188-8199.
- [Google Scholar]
- Construction of a flexible 1D core-shell Al2O3@NaNbO3 nanowire/poly(p-phenylene benzobisoxazole) nanocomposite with stable and enhanced dielectric properties in an ultra-wide temperature range. J. Mater. Chem. C. 2022;10:716-725.
- [Google Scholar]
- Synthesis of an efficient paramagnetic ZnFe2O4 agent for NIR-I/II responsive photothermal performance. J. Alloy. Compd.. 2023;936:168161
- [Google Scholar]
- Two-dimensional ultra-thin nanosheets optimize the surface reaction dynamics and photo/pyrogenerated carrier transfer of NaNbO3 for an efficient pyro-photo-electric catalytic system. Sustainable Energy Fuels. 2022;6:4227.
- [Google Scholar]
- Photocatalytic conversion of methane: recent advancements and prospects. Angew. Chem. Int. Ed.. 2021;61(2):e202108069.
- [Google Scholar]
- Construction of Si nanowires/ZnFe2O4/Ag photocatalysts with enhanced photocatalytic activity under visible light and magnetic field. J. Alloy. Compd.. 2023;946:169467
- [Google Scholar]
- Lessons learned: how to report XPS data incorrectly about lead-halide perovskites. Mater. Chem. Front.. 2023;7(18):3797-3802.
- [Google Scholar]
- Recent advances of ferro-, piezo-, and pyroelectric nanomaterials for catalytic applications. ACS Appl. Nano Mater.. 2020;3:1063-1079.
- [Google Scholar]
- Pyroelectric photocatalysis: polarization mechanism insight, in situ characterizations and challenges. Chem. Eng. J.. 2024;485:149627
- [Google Scholar]
- Doping regulates pyro-photo-electric catalysis to achieve efficient water splitting in Ba1−xSrxTiO3 through solar energy and thermal resources. New J. Chem.. 2022;46:17292-17302.
- [Google Scholar]
- Bipolar charge collecting structure enables overall water splitting on ferroelectric photocatalysts. Nat. Commun.. 2022;13:4245.
- [Google Scholar]
- Unveiling the charge transfer dynamics steered by built-in electric fields in BiOBr photocatalysts. Nat. Commun.. 2022;13:2230.
- [Google Scholar]
- Fundamentals and applications of photo-thermal catalysis. Chem. Soc. Rev.. 2021;50:2173-2210.
- [Google Scholar]
- Improving visible light photocatalytic activity of NaNbO3: a DFT based investigation. RSC Adv.. 2016;6:90188-90196.
- [Google Scholar]
- Increased dielectric properties of ZnFe2O4/rGO nanohybrid via thermo-chemical route. J. Aust. Ceram. Soc.. 2022;58:1265-1274.
- [Google Scholar]
- Photothermal therapy by using titanium oxide nanoparticles. Nano Res.. 2016;9(5):1236-1243.
- [Google Scholar]
- Chromium valence change in trivalent chromium conversion coatings on aluminium deposited under applied potentials. Corros. Sci.. 2020;167:108482
- [Google Scholar]
- Hexagonal phase/cubic phase homogeneous ZnIn2S4 n-n junction photoanode for efficient photoelectrochemical water splitting. J. Alloy. Compd.. 2020;830:154639
- [Google Scholar]
- Thermo-electrochemical coupling for room temperature thermocatalysis in pyroelectric ZnO nanorods. Electrochem. Commun.. 2017;81:124-127.
- [Google Scholar]
- Thermal excitation polarized field drives photoelectric catalysis for dye degradation in a BaTiO3/CdS heterojunction through integration of solar and thermal energy. ChemPhotoChem.. 2021;5:1106-1118.
- [Google Scholar]
- Pyrocatalysis—The DCF assay as a pH-robust tool to determine the oxidation capability of thermally excited pyroelectric powders. PLoS One. 2020;15:e0228644.
- [Google Scholar]
- BiFeO3/BiVO4 p-n heterojunction for efficient and stable photocatalytic and photoelectrochemical water splitting under visible-light irradiation. Catal. Today. 2020;340:188-196.
- [Google Scholar]
- O. Sudhir Ekande, M. Kumar, Self-powered piezoelectric NaNbO3 induced band position rearrangement and electrocatalysis in MoS2/NaNbO3 heterojunction for generation of reactive oxygen species for organic pollutant removal, Chem. Eng. J. 458 (2023) 141454.
- Photothermal synergic catalytic degradation of the gaseous organic pollutant isopropanol in oxygen vacancies utilizing ZnFe2O4 and mechanism insight. J. Chem. Res.. 2021;45(7–8):773-780.
- [Google Scholar]
- Enhanced photocatalytic CO2 reduction performance via photothermal-magnetic synergistic effects for solar fuel production. ACS Sustain. Chem. Eng.. 2024;12:3298-3311.
- [Google Scholar]
- Piezo-phototronic and pyro-phototronic effects enhanced broadband photosensing. Mater. Today. 2023;68:254-274.
- [Google Scholar]
- Piezocatalysis and piezo-photocatalysis: catalysts classification and modification strategy, reaction mechanism, and practical application. Adv. Funct. Mater.. 2020;30:2005158.
- [Google Scholar]
- Enabling piezopotential in piezoelectric semiconductors for enhanced catalytic activities. Angew. Chem. Int. Ed.. 2019;58:7526-7536.
- [Google Scholar]
- Pyro-catalysis for tooth whitening via oral temperature fluctuation. Nat. Commun.. 2022;13:4419.
- [Google Scholar]
- Photothermal performance of MFe2O4 nanoparticles. Chin. Chem. Lett.. 2019;30:2013-2016.
- [Google Scholar]
- Strong pyro-catalysis of pyroelectric BiFeO3 nanoparticles under a room-temperature cold–hot alternation. Nanoscale. 2016;8:7343-7350.
- [Google Scholar]
- Effects of composition faults in ternary metal chalcogenides (ZnIn2S3+x, x = 1–5) layered crystals for visible-light-driven catalytic hydrogen generation and carbon dioxide reduction. Appl. Catal. b: Environ.. 2019;256:117810
- [Google Scholar]
- Thermal conductivity enhancement on phase change materials for thermal energy storage: a review. Energy Storage Mater.. 2020;25:251-295.
- [Google Scholar]
- One-dimensional hybrid nanostructures for heterogeneous photocatalysis and photoelectrocatalysis. Small. 2015;11:2115-2131.
- [Google Scholar]
- Interfacial chemical bond-modulated Z-scheme charge transfer for efficient photoelectrochemical water splitting. Adv. Energy Mater.. 2021;11:2003500.
- [Google Scholar]
- Hydrochloric acid-mediated synthesis of ZnFe2O4 small particle decorated one-dimensional Perylene Diimide S-scheme heterojunction with excellent photocatalytic ability. Chin. J. Catal.. 2022;43:1111-1122.
- [Google Scholar]
- Needle-like NaNbO3 synthesis via Nb6O198- cluster using Na3NbO4 precursor by dissolution precipitation method. Chem. Lett.. 2013;42:380-382.
- [Google Scholar]
- Pyroelectric nanogenerators for driving wireless sensors. Nano Lett.. 2012;12:6408-6413.
- [Google Scholar]
- Pyroelectric nanogenerators for harvesting thermoelectric energy. Nano Lett.. 2012;12:2833-2838.
- [Google Scholar]
- Photo-thermal synergy for boosting photo-Fenton activity with rGO-ZnFe2O4: Novel photo-activation process and mechanism toward environment remediation. Appl. Catal. b: Environ.. 2021;292:120198
- [Google Scholar]
- Piezoelectrically/pyroelectrically-driven vibration/cold-hot energy harvesting for mechano-/pyro-bi-catalytic dye decomposition of NaNbO3 nanofibers. Nano Energy. 2018;52:351-359.
- [Google Scholar]
- Highly efficient pyrocatalysis of pyroelectric NaNbO3 shape-controllable nanoparticles for room-temperature dye decomposition. Chemosphere. 2018;199:531-537.
- [Google Scholar]
- Accelerated pyro-catalytic hydrogen production enabled by plasmonic local heating of Au on pyroelectric BaTiO3 nanoparticles. Nat. Commun.. 2022;13:6144.
- [Google Scholar]
- Synergy of ferroelectric polarization and oxygen vacancy to promote CO2 photoreduction. Nat. Commun.. 2021;12:4594.
- [Google Scholar]
- Surface sprouting growth of Na2Nb2O6·H2O nanowires and fabrication of NaNbO3 nanostructures with controlled morphologies. Appl. Surf. Sci.. 2012;258:3490-3496.
- [Google Scholar]
- Construction of oxygen defective ZnO/ZnFe2O4 yolk-shell composite with photothermal effect for tetracycline degradation: Performance. Chin. Chem. Lett.. 2023;34:107308
- [Google Scholar]
- Facile and robust construction of a 3D-hierarchical NaNbO3-nanorod/ZnIn2S4 heterojunction towards ultra-high photocatalytic H2 production. Cat. Sci. Technol.. 2022;12:2346-2359.
- [Google Scholar]
- Pyro-electrolytic water splitting for hydrogen generation. Nano Energy. 2019;58:183-191.
- [Google Scholar]
- Enhanced photovoltaic-pyroelectric coupled effect of BiFeO3/Au/ZnO heterostructures. Nano Energy. 2021;85:105968
- [Google Scholar]
- Self-assembled LaFeO3/ZnFe2O4/La2O3 ultracompact hybrids with enhanced piezo-phototronic effect for oxygen activation in ambient conditions. Adv. Funct. Mater.. 2022;32:2205121.
- [Google Scholar]
- Recent advances in pyroelectric materials and applications. Small. 2021;17:2103960.
- [Google Scholar]
- Promising pyro-photo-electric catalysis in NaNbO3 via integrating solar and cold-hot alternation energy in pyroelectric-assisted photoelectrochemical system. Nano Energy. 2021;79:105485
- [Google Scholar]
- Designing a built-in electric field for efficient energy electrocatalysis. ACS Nano. 2022;16:19959-19979.
- [Google Scholar]
- Combined effects of octahedron NH2-UiO-66 and flowerlike ZnIn2S4 microspheres for photocatalytic dye degradation and hydrogen evolution under visible light. J. Phys. Chem. C. 2019;123:18037-18049.
- [Google Scholar]
- Photochemical deposition of amorphous MoSx on one-dimensional NaNbO3–CdS heterojunction photocatalysts for highly efficient visible-light-driven hydrogen evolution. Dalton Trans.. 2020;49(26):8891-8900.
- [Google Scholar]
- Structural evolution in a hydrothermal reaction between Nb2O5 and NaOH solution: from Nb2O5 grains to microporous Na2Nb2O6·2/3H2O fibers and NaNbO3 cubes. J. Am. Chem. Soc.. 2006;128:2373-2384.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105996.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







