Translate this page into:
Qualitative assessment on cisplatin loaded CeO2/Au/GO hybrid as theranostics platform in HeLa cell lines
⁎Corresponding authors. saranya.j@rajalakshmi.edu.in (J. Saranya), mrshaik@ksu.edu.sa (Mohammed Rafi Shaik)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Nanomaterials have been increasingly popular in bioimaging and cancer therapy due to their unique characteristics to reachtarget-specific tumours. Due to this uniqueness, a drug delivery platform made of nanomaterials has been developed to deliver theanti-cancer drug to target sites. As the number of incidences of cancer increases, it is critical to provide a medication delivery platform for treating cancer as soon as possible. Also, nanosystems based on carbon have been widely used as a possible biomarker for cancer imaging and therapeutics. This research work primarily focuses on the development of a spherical-shaped porous CeO2/Au/GO hybrid nanocomposite to serve as a nanoplatform to treatcervical cancer. The stacked layer of graphene oxide (GO) was loaded with porous aminated cerium oxide nanoparticles (CeO2 NPs) and gold nanoparticles (Au NPs). X-ray Diffraction (XRD),Field Emission Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) were used to investigate their physio-chemical properties and morphology. Furthermore, HeLa cells were interacted with the suggested porous Au/GO hybrid, CeO2/Au/GO hybrid nanosystem and cisplatin-loaded CeO2/Au/GO hybrid system (CeO2/Cis-Au/GO hybrid), under in-vitro conditions to assess the anti-cancer efficacy of the proposed nanoplatforms. In this study, the minimum concentration of Au/GO at which nearly 50% of the cells remain dead (IC50 concentration)is considered to be 62.5 µg/mL and 31.2 µg/mL for CeO2/Au/GO. Further, the cisplatin anticancer drug was chemically bonded with CeO2/Au/GO hybrid nanosystem for testing the apoptotic efficacy of cancer cells under in-vitro conditions. The IC50 value was 62.5 µg/mL which affirmed the anticancer property of the CeO2/Cis-Au/GO system with HeLa cells. According to the findings from the antiproliferative assay, CeO2/Au/GO nanoplatforms resulted in superior cytotoxicity effects on cervical cancer in comparison with CeO2/Cis-Au/GO and Au/GO nanoplatform. Lastly, the proposed CeO2/Au/GO hybrid nanosystem was subject to dual staining investigation using Acridine Orange/Ethidium Bromide (AO/EB) dyes for recording the morphological changes incurred and also to visualize live and dead cells using fluorescence spectroscopy. Based on these findings, the developed CeO2/Au/GO hybrid nanosystem can be taken to in vivo studies for the validation to act as a theranostic platform for cervical cancer.
Keywords
Cisplatin
Graphene oxide
CeO2 NPs
Au NPs
HeLa cell line
Theranostic platform
1 Introduction
The wellness of women is very important for society and cancer is a major threat to their lives. Cervical cancer is the fourth most common cause of death in women worldwide, and it remains a major issue in all developing nations. Cervical cancer develops in women who have been infected with the Human Papilloma Virus (HPV) and spreads throughout their reproductive system through a process known as carcinogenesis. In India, a quadrivalent vaccination (GardasilTM, promoted by Merck) and a bivalent vaccine (CervarixTM, marketed by Glaxo Smith Kline) are available to prevent HPV infections. Besides, many people are not aware of and interested to get vaccinated due to the huge cost involved in it. Nanomaterials, which can help target these specific malignant cells, can assist solve these problems (Chen et al., 2015; Zaman et al., 2016). Metal oxide-based systems have recently become popular in a variety of biomedical applications (Kwon et al., 2018; Singh et al., 2018; Zhang et al., 2008). For many decades, hybrid nanoplatforms using metal-oxide and metals for the treatment of various ailments in human beings have been popular. Metal oxide Nanosystems such as Titanium di-Oxide (TiO2), Zirconium di-oxide (ZrO2), and copper di-oxide, have a variety of morphologies and outstanding physio-chemical properties that aid in the treatment of a variety of biological problems (Manne et al., 2020; Sharma et al., 2015).
Nanoparticles are good contrast agents due to their high sensitivity, small size, and composition. On the surface of nanoparticles, appropriate targeted ligands could be conjugated. Nanoparticles and nanocomposites can be made for a variety of applications by combining different functional materials and subjecting them to variety of applications (Al Harby et al., 2022; El Batouti and Fetouh, 2021; Kim et al., 2017; Xu and Qu, 2014). Because of their ability to maintain redox equilibrium in pathological conditions, cerium dioxide nanoparticles (CeO2 NPs) are widely used for the development of theranostic platforms (Casals et al., 2020). Nanoceria has a greater potential to shift oxidation states (Corsi et al., 2018). CeO2 Nanoparticles with fluorite crystal structure have been chosen as catalysts, promoters for various chemical reactions, and specific indicators that contribute to antioxidant, anti-inflammatory, and anti-proliferative action against cancer cells (Das et al., 2017; Liying et al., 2015; Mittal and Pandey, 2014; Nourmohammadi et al., 2019). Toxicity assessments of cerium oxide nanoparticles on mouse fibrosarcoma reveal that nanoceria increases ROS levels and induces apoptosis in cancer cells (WEHI164) in a dose-dependent manner, whereas normal cells (L929) only exhibit minimal levels of toxicity even at concentrations exceeding 250 µg/mL in the MTT experiment (Nourmohammadi et al., 2019). CeO2NPs-induced apoptosis in A549 cells is largely mediated by ROS-driven DNA damage and cell cycle arrest (Mittal and Pandey, 2014). The toxicity effects and apoptotic behavior of CeO2NPs functionalized with graphene oxide nanoparticles (GO NPs) on HeLa cell lines were reported which affirms to act as a therapeutic platform (Saranya et al., 2020). CeO2/ZnO/GO nanoplatform demonstrated superior anti-cancer activity with HeLa cell line at an IC50 concentration of 62.5 µg/mL after 72 h of incubation (Saranya et al., 2022).
Gold nanoparticles (AuNPs) have been used as nanocarriers in the drug delivery platform due to their high surface to volume ratio, high specificity and low toxicity. AuNPs have unique physico-chemical properties and are easy to surface functionalize (Giljohann et al., 2010; Mittal and Pandey, 2014). The potential of silica-coated gold (Au@SiO2) nanoparticles combined to antibodies against the scavenger receptor class B type I (SR-BI) were investigated for visual tracking and cervical cancer treatment. Fluorescein isothiocyanate (FITC)-labeled Au@SiO2-SR-BI antibody was synthesized and subjected to western blot and immunofluorescence assays. As a result, photothermal ablation of solid tumors was observed when FITC-Au@SiO2-SR-BI was activated using 808 nm wave (Yu et al., 2022).
GO NPs, have exhibited their significant role in delivering anti-cancer drugs (Croitoru et al., 2019; Mahanta et al., 2019). High drug loading and release abilities are considered to be important properties of graphene-based materials (Zhou et al., 2019). Doxorubicin (DOX) and camptothecin (CPT) are two anticancer medicines that can be loaded into GO NPs(Zhang et al., 2010). As a result, they can administer multiple drugs at the same time. The medication can be covalently loaded onto graphene. These studies have revealed that GO Nanocarriers are very promising for biomedical applications (Abdelhamid and Hussein, 2021). CeO2/GO hybrid exhibited excellent anti-cancer activity on MCF-7 cell line at an IC50 concentration of 62.5 µg/mL after 72 h of incubation (Saranya et al., 2023). Because of the distinct cytotoxicity and apoptotic behavior of CeO2, Au, and GO NPs as standalone and as hybrids, we decided to investigate the CeO2/Au/GO platform as a theranostic platform for improved anticancer efficacy and drug delivery applications. As per current knowledge, no one has tested the cytotoxicity of the CeO2/Au/GO nanocomposite. The main objective of this work is to study the cytotoxicity effects of Au/ GO, CeO2/Au/GO, and CeO2/cis-Au/GO nanoplatforms that cause apoptosis in the HeLa cell line.CeO2/Au/GO nanocomposite is combined with cisplatin drug and is subjected to a toxicity study for understanding the synergetic effects of both which can give better %cell viability in HeLa cell lines under in-vitro conditions.
2 Materials and methods
2.1 Chemicals and equipments
All the chemicals and solvents used for this work were pure and of good analytical grade. Cerous nitrate hexahydrate (CeN3O9·6H2O) and sodium hydroxide (NaOH) were procured from Fischer Scientific, India. Gold Nanoparticles were obtained from Sisco Research Laboratories Pvt. Ltd (SRL), India. The HeLa cell lines for this study were procured from the International Vaccination Centre, The King Institute of Preventive Medicine and Research, Guindy.
The XRD spectra of the developed nanosystem were obtained using a RIGAKU Miniflux 2C model. Surface morphology was obtained using SUPRA 55. TEM analysis and Selected Area Electron Diffraction (SAED) was obtained using TECNAI G2 TF20-ST. A spectrophotometer was used to collect the absorption spectra (Labman scientific instruments, India). Metzer Inverted Confocal Microscope, India, was used to obtain the confocal pictures of HeLa cells.The steps given below were performed while culturing theHeLa cell line under invitro conditions.
2.2 Preparation of CeO2 / Au /GO nanocomposite
CeO2 NPs were prepared according to the methodology described in (Saranya et al., 2020).Graphene oxide nanoparticles (GO NPs) were developed using the hummer method (Yu et al., 2016). 1 g CeO2 NPs and Au NPs were dissolved in 100 mL distilled water and the same were subject to ultrasonication for 30 min. 0.25 g of GO was mixed with 50 mLdistilled water and the same was subject to ultrasonication for 2 h. Finally, the two solutions are blended to form a hybrid nanocomposite solution. Lastly, the mixtures were stirred for 2 h and were cleaned twice with distilled water. The resultant was further allowed to settle down for a few hours.
2.3 Sample preparation (CeO2/ Cis -Au/GO) and dilution
Initially, 1000 µg/mL of cisplatin was added to a well containing the HeLa cell line. Then, for each of the eight concentrations (7.8, 15.6, 31.2, 62.5, 125, 250, 500, and 1000 µg/mL), half of the concentration will be the sample (CeO2/Au/GO) and the other half will be cisplatin. (For example, a 1000 µg/mL stock solution contains 500 µg/mL cisplatin and 500 µg/mL CeO2/Au/GO). The same procedure is repeated for other concentrations as well. The above procedure was performed to develop the CeO2/ Cis -Au/GO hybrid and the same was subjected to the MTT assay protocol for the estimation of % cell Viability.
2.4 Serial dilution
In Eppendorf tube 1, which contains 500 µl of medium without serum, 500 µl of the stock solution is introduced. The solution is the end result of this mixing. This is repeated in Eppendorf tube 2 by adding 500 µl of the newly generated solution. This process of aliquoting and resuspension continues until the last tube is reached, diluting the stock concentration.
2.5 Steps involved in culture media
Stock is prepared using Dulbecco's Modified Eagle Medium (DMEM) after checking its sterile condition. Certain supplements are required for cell cultivation. Serums were typically derived from bovine sources (FBS) and the same is used as a common supplement in cell culture media. Serum-free medium was also prepared. To inhibit the growth of opportunistic bacteria and avoid light-induced deterioration of the culture media components, commercial and produced culture media in liquid form are normally stored at 4 °C in the dark. Prior to inoculation with cultures, the media should be warmed to an appropriate temperature for cell growth. 1 mg of CeO2/Au/GO was mixed with 1 mL of Dimethyl Sulfoxide (DMSO) and was kept in an Eppendorf tube as the initial stock solution. To prevent cell death, the serum is added to cell culture media. As the next step, 500 µl of DMEM without serum is added to another 5 Eppendorf tube, which is used for various concentrations.
2.6 In vitro examination of anticancer efficacy using Au/GO, CeO2/Au/GO, and CeO2/Cis- Au/GO system
To estimate the efficacy of Au/GO and CeO2/Au/GO nano-systems for a variety of biomedical applications, the cytotoxicity effects of drug-free and cisplatin-loaded Nanosystems on HeLa (Human Epitheloid Cervix Cancer) cell lines must be studied. After treatment with Au/GO, CeO2/Au/GO, and CeO2/Cis-Au/GO hybrid Nanosystem, the number of live cells was measured using a standard Methyl Thiazolyl Diphenyl Tetrazolium Bromide (MTT) assay (Mosmann, 1983). The purpose of the MTT assay is to evaluate the %cell viability by increasing tetrazolium salt and through monitoring the metabolization (Das et al., 2017). In vitro examination is initiated once the volume of HeLa cells per well in 96-well microtiter plates reaches one hundred thousand. HeLa cells were grown in 96 well microtiter plates at a concentration of 1x106 cells/mL. Later, the cells were pre-treated at 37 °C for 24 h in the presence of 5% CO2 and 95% humidity with varying concentrations of Au/GO, (CeO2/Au/GO), and CeO2/Cis-Au/GO hybrid nanosystem (7.8, 15.6, 31.2, 62.5, 125, 250, 500, and 1000 µg/mL). The developed cells were further treated with MTT (10 mL) and were incubated for another 4 h. Once the crystals were dissolved in 200 µl of DMSO, the absorbance was recorded at 570 nm. This procedure was replicated thrice on the same day, and the concentration required for a 50% cell inhibition was estimated using Eq (1).
2.7 Apoptosis study using direct fluorescence microscopic analysis
This study detects the apoptotic behaviour of the developed nanosystem on HeLa cell lines through morphological induction. The cells were grown in a 3:1 mixture of ethanol and phosphate buffer solution (PBS) for an incubation period of 1 h at room temperature. After an hour, AO and EB at a 1:1 ratio were combined with three sets of concentrations of hybrid nanocomposite (1000, 62.5, and 7.8 µg/mL). Excess unbonded dye was removed and the stained cells were studied under a fluorescence microscope (Mittal and Pandey, 2014).
3 Results and discussion
3.1 X-ray Diffraction analysis
Fig. 1 depicts the XRD patterns of the developed CeO2/Au/GO Nanocomposites. The peaks at (1 1 1), (2 0 0), (2 2 0), (3 1 1), and (2 2 2) planes affirm the formation of CeO2 NPs with cubic fluorite structure with reference to JCPDS 81–0792. The obtained pattern was found to be identical to that of pure CeO2 NPs, indicating that the crystal structure of CeO2 NPs is not altered and affected by GO. The degree of crystallinity and the intensity of the peaks decrease due to the absorption of CeO2 NPs on the surface of the GO sheets. The existence of CeO2 NPs and Au NPs on the GO sheets is confirmed through three distinctive peaks found at 2 theta = 27.64°,38.25°and 60.5° respectively. The average grain size of the CeO2/Au/GO nanocomposite was found to be 75 nm, as estimated using Scherrer’s relation.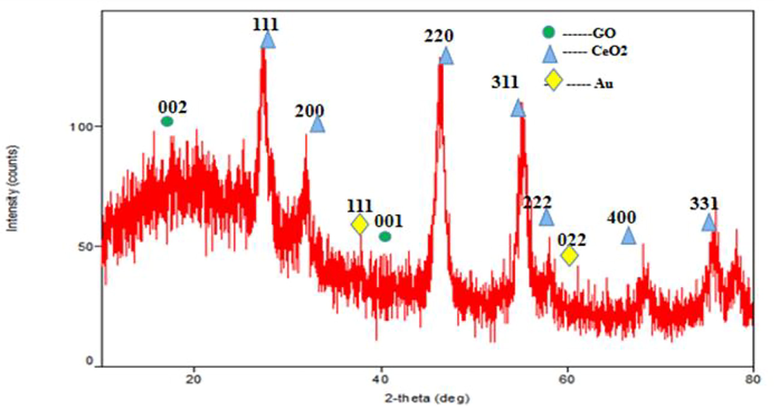
XRD Analysis of CeO2/Au/GO Nanocomposites.
3.2 Surface morphology analysis
The surface morphology of the developed Au/GO nanocomposite and CeO2/Au/GO nanocomposite was examined using SEM, as shown in Fig. 2(a-d). Fig. 2(a-b) represents the SEM images of the CeO2/Au/GO nanocomposite and Fig. 2(c-d)represents the SEM images of the Au/GO nanocomposite respectively.CeO2 NPs were identified as clusters with severe agglomeration and CeO2/Au/GO nanocomposites were identified with a nano-rectangular shape of 100 nm in size. This has occurred due to the incorporation of GO sheets, which resulted in the nucleation and development of CeO2 NPs with more active sites on them(Duan et al., 2016; Wang et al., 2019). Previous research has shown that nanomaterials with no agglomeration have a surface charge, indicating that they are stable (Mahendran and Ponnuchamy, 2018). This hierarchical structure is also advantageous for improving anticancer activity and hence, strengthening the cytotoxicity effects against HeLa cells when combined with CeO2 NPs and Au/GO NPs.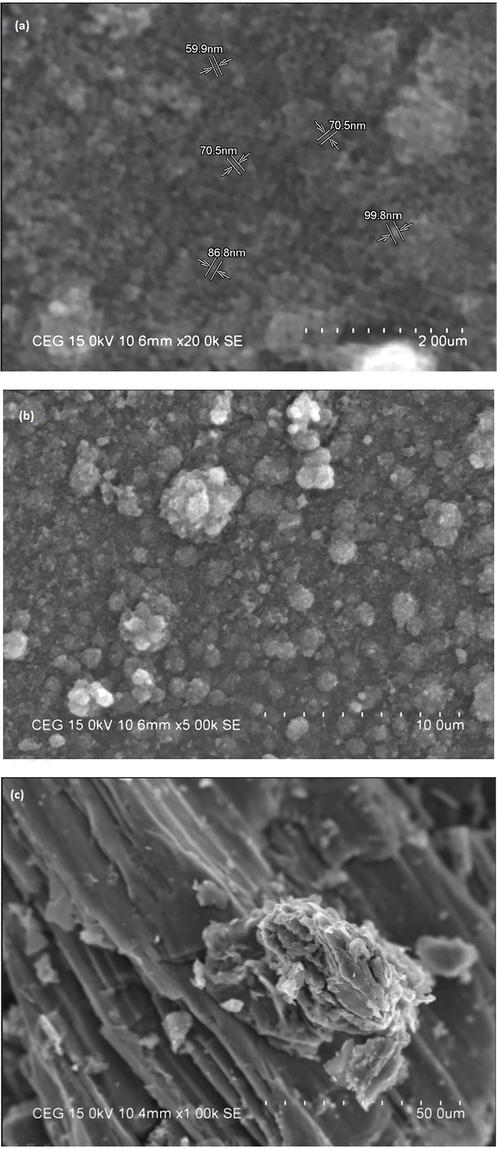
SEM images of(a, b) CeO2/Au/GO nanocomposite and (c, d) Au/GO nanocomposite.
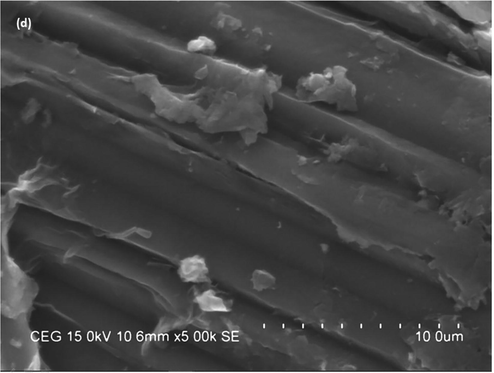
SEM images of(a, b) CeO2/Au/GO nanocomposite and (c, d) Au/GO nanocomposite.
3.3 Morphology examination using TEM analysis
As-prepared ternary nanohybrid morphology was examined using TEM, and corresponding results are presented in Fig. 3(a). According to the image, CeO2 and Au nanoparticles were decorated on the surface of the GO matrix. On the other hand, most of the CeO2 nanoparticles were formed spherically with an average size of 120–140 nm and uniformly distributed on the GO matrix. Interestingly, Au NPs were also distributed not only on the CeO2 nanoparticles but also fabricated throughout the GO matrix. However, Au NPs exhibit a size of less than 10 nm in the form of tiny nanoparticles. The inset image of Fig. 3(b) represents the selected area diffraction (SAED) of the ternary CeO2/Au/GO nanohybrid, and it is showing significant crystallinity with lattice planes of (1 1 1), (2 2 0), and (4 0 0) respectively. These lattice planes were consistent with the presence of CeO2 and Au nanoparticles.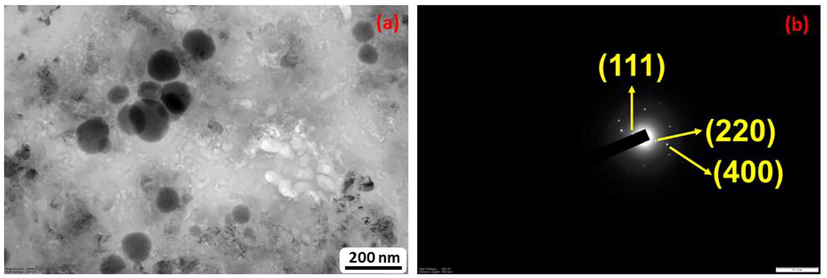
(a)TEM image of CeO2/Au/GO and (b) SAED pattern of CeO2/Au/GO.
3.4 In vitro cytotoxicity evaluation of Au/GO, CeO2/Au/GO and CeO2/Cis-Au/GO systems on HeLa cells
Fig. 4 shows the %cell viability of HeLa cells after being treated with the developed nanocomposite in various concentrations using the MTT experiment. The IC50 concentration for Au/GO hybrid, cisplatin-induced CeO2/Au/GO hybrid platform (CeO2/Cis-Au/GO) hybrid Nanoplatforms were 62.5 µg/mL and for CeO2/Au/GO hybrid it was 31.2 µg/mL. Fig. 4 showcases % live cells upon synergizing the HeLa cell line with varying concentrations of Au/GO hybrid, CeO2/Au/GO hybrid,CeO2/ Cis-Au/ GO hybrid and cisplatin drug under in-vitro conditions. The % Live cells were 10.76 for a CeO2/Au/GO hybrid with a maximum concentration of 1000 µg/mL and 69.23 for a minimum concentration of 7.8 µg/mL, respectively. Similar findings using 1000 µg/mL and 7.8 µg/mL concentrations of CeO2/Cis-Au/GO on HeLa cell Lines were recorded as 9.25% and 81.48% respectively, as shown in Table 1. The Cytotoxicity analysis reveals that at the maximum concentration of 1000 µg/mL there exists marginal improvement in the cytotoxicity effects with CeO2/Cis-Au/GO hybrid nanosystem with respect to CeO2/Au/GO hybrid platform. On the other hand, at the minimum concentration of 7.8 µg/mL and IC50 concentration of 31.2 µg/mL CeO2/Au/GO hybrid has shown superior cytotoxicity effects on the HeLa cell line. Further, the anti-cancer activity of cisplatin drug were assessed at various concentrations as shown in Fig. 4. At the maximum concentration of 1000 µg/mL, % live cells was 2.08 and at minimum concentration of 7.8 µg/mL, % live cells was 46.87. These observations affirms the inherent anti-cancer property of the cisplatin drug which was taken for in-vitro assessment of the developed Au/GO, CeO2/Au/GO and CeO2/Cis-Au/GO systems with HeLa cells. Mean value ± Standard Deviation (Three replicates).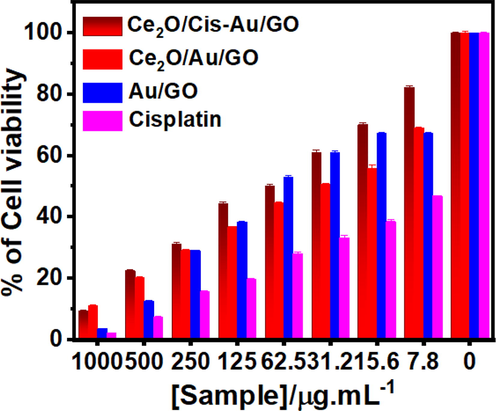
% Cell Viability study of CeO2/Cis-Au/ GO hybrid, CeO2/Au/GO hybrid, and Au/GO hybrid complexes on HeLa cells.
Concentration (µg/ml)
CeO2/ cis –Au/GO (%Live cells)
CeO2/ Au/GO (%Live Cells)
Au/GO (%Live Cells)
% Live cells
Cisplatin
1000
9.35 ± 0.17
11.01 ± 0.49
3.59 ± 0.124
2.08 ± 0.23
500
22.44 ± 0.38
20.37 ± 0.32
12.65 ± 0.301
7.29 ± 0.47
250
31.30 ± 0.39
29.04 ± 0.340
29.11 ± 0.126
15.62 ± 0.34
125
44.28 ± 0.66
36.80 ± 0.11
38.47 ± 0.26
19.79 ± 0.25
62.5
50.15 ± 0.396
44.69 ± 0.25
52.92 ± 0.68
28.15 ± 0.45
31.2
61.08 ± 0.732
50.60 ± 0.26
60.93 ± 0.75
33.33 ± 0.65
15.6
70.16 ± 0.55
55.93 ± 1.177
67.25 ± 0.26
38.54 ± 0.55
7.8
82.26 ± 0.67
69.06 ± 0.36
67.25 ± 0.260
46.87 ± 0.14
Cell control
100 ± 0.35
100 ± 0.44
100 ± 0.12
100 ± 0.19
Cisplatin is a very effective anti-cancer drug which has a cross-linked DNA molecule, a positively charged super hydrophobic cell surface and is smaller in size (Avgoustakis et al., 2002; Rosenberg, 1985). Antiproliferative study was performed on cisplatin drug to affirm anticancer activity of the same as shown in Table 1.When cancer cells (MDA-MB-231 and MCF-7) were tested using various anticancer assays (MTT assay, cell morphology analysis, cell cycle analysis, comet assay, Annexin VFITC/PI staining, and DAPI staining), it was found that AuNPs derived from Mimosa pudica leaf extract were effective at killing the cancer cells [30].Based on these results, the binding of the cisplatin drug molecule with the developed CeO2/Au/GO hybrid is not effective due to its inherent super hydrophobicity property. Hence CeO2/Au/GO hybrid stands superior in the cytotoxicity analysis with the HeLa cell line under in-vitro conditions.
3.5 Apoptotic study on CeO2/Au/GO nanocomposite using AO/EB dual staining
The biochemical interactions were recorded of the interaction of CeO2/Au/GO nanocomposite with HeLa cells. The AO dye emits green fluorescence when it enters both live and apoptotic cells, whereas the EB stain emits red fluorescence solely when it enters apoptotic cells (Das et al., 2017; Mittal and Pandey, 2014). The drastic morphological changes were recorded at 125 µg/ml of CeO2/GO nanocomposite with HeLa cells (Saranya et al., 2020). Apoptosis associated morphological changes were recorded using dual stain study through the interaction of 500 µg/ml and 250 µg/ml of CeO2/ZnO/GO nanocomposite with HeLa cells (Saranya et al., 2022). As demonstrated in Fig. 5a, the morphological changes caused by the interaction of CeO2/Au/GO combination with untreated HeLa cells emit green fluorescence due to AO absorption by live cancer cells. While 1000 µg/mL of CeO2/Au/GO interacted with HeLa cells, red fluorescence was observed, it was due to the absorption of the EB dye by the dead cancer cells (Fig. 5b). As shown in Fig. 5(c, d), the interaction of 62.5 µg/mL and 7.8 µg/mL CeO2/Au/GO nanocomposite with HeLa cells resulted in a small amount of green fluorescence among a large amount of red fluorescence. All these results demonstrated that CeO2/Au/GO hybrid nanocomposite can be used as a theranostic platform by achieving programmed cell death due to its inherent anticancer property against HeLa cells.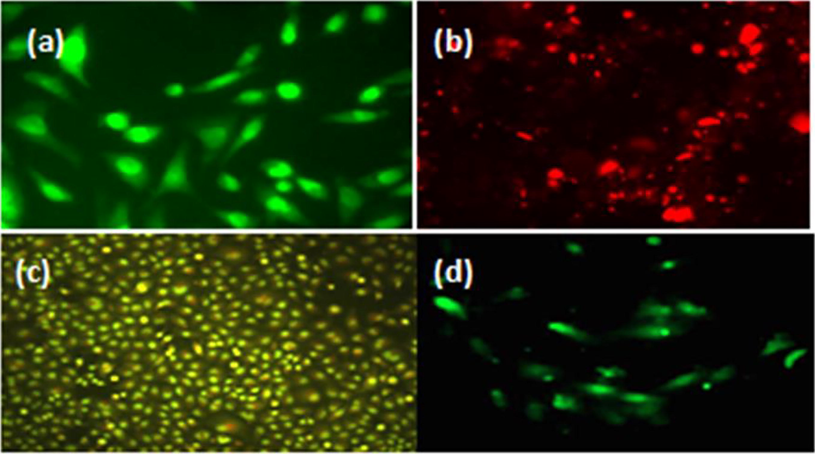
Microscopic Fluorescence images (a) untreated HeLa cells; (b), (c), (d) treated HeLa cells using 1000 µg/mL, 62.5 µg/mL, and 7.8 µg/mL CeO2/Au/GO nanocomposite.
4 Conclusions
Finally, we synthesized CeO2/Au/GO nanocomposites using an cost-effective ultrasonic method. Using XRD,SEM and TEM, we examined the crystalline properties and surface morphology of the developed nanocomposites. The anti-cancer properties of the developed Au/GO, CeO2/Au/GO, and CeO2/cis -Au/GO platforms have been confirmed and validated by cytotoxicity and apoptosis studies. Among the three Nanoplatforms, CeO2/Au/GO hybrid has attained 50% cell death at a minimum concentration of 31.2 µg/mL. The superior anticancer property revealed by CeO2/Au/GO hybrid is mainly due to the distribution of Au nanoparticles throughout the GO sheet inclusive of CeO2 NPs. Further,changes in the morphology of HeLa cells upon interaction of developed CeO2/Au/GO hybrid at various concentrations such as 1000 µg/mL, 62.5 µg/mL, and 7.8 µg/mL were recorded using dual stain study. At maximum concentration (1000 µg/mL) of CeO2/Au/GO hybrid, more cell death were seen through red flouoresence emission. On the other hand at a minimum concentration (7.8 µg/mL), more live cells were seen through green fluorescence. At 62.5 µg/mL, yellow-greenish flouoresence indicating the apoptotic behavior and mild reddish flouresence (cell death) were recorded. All these results affirm the potential to take the CeO2/Au/GO hybrid system as a theranostic platform for cervical cancer. As a future work, a comprehensive in vivo analysis can be done, in which the toxicity pattern in living organisms of the developed CeO2/Au/GO nanocomposite can be visualized and analyzed. In addition, the developed nanosystem can be subjected to Antigen-Antibody sensing for the detection of HPV 16 and 18 viruses that in turn induce E6 and E7 onco proteins.
Funding
Researchers Supporting Project number (RSPD2023R665), King Saud University, Riyadh, Saudi Arabia.
Acknowledgments
The authors acknowledge the funding from Researchers Supporting Project number (RSPD2023R665), King Saud University, Riyadh, Saudi Arabia.
Ethics Approval Statement
This study does not require ethical approval as the experimentation works were carried out under in-vitro conditions at Sasaam Biologicals Lab Services, Ashok Nagar, Chennai, Tamil Nadu, India.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Graphene oxide as a carrier for drug delivery of methotrexate. Biointerface Res. Appl. Chem. 2021;11:14726-14735.
- [Google Scholar]
- Prospects of Polymeric Nanocomposite Membranes for Water Purification and Scalability and their Health and Environmental Impacts: A Review. Nanomaterials. 2022;12:3637.
- [Google Scholar]
- PLGA–mPEG nanoparticles of cisplatin: in vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties. J. Control. Release. 2002;79:123-135.
- [Google Scholar]
- Cerium oxide nanoparticles: advances in biodistribution, toxicity, and preclinical exploration. Small. 2020;16:1907322.
- [Google Scholar]
- Nanotechnology in the management of cervical cancer. Rev. Med. Virol.. 2015;25:72-83.
- [Google Scholar]
- Not only redox: the multifaceted activity of cerium oxide nanoparticles in cancer prevention and therapy. Front. Oncol.. 2018;8:309.
- [Google Scholar]
- Multifunctional platforms based on graphene oxide and natural products. Medicina. 2019;55:230.
- [Google Scholar]
- Nanoceria-mediated delivery of doxorubicin enhances the anti-tumour efficiency in ovarian cancer cells via apoptosis. Sci. Rep.. 2017;7:1-12.
- [Google Scholar]
- Morphology-controlled synthesis and microwave absorption properties of β-MnO2 microncube with rectangular pyramid. Mater Charact. 2016;112:206-212.
- [Google Scholar]
- A facile new modified method for the preparation of a new cerium-doped lanthanium cuperate perovskite energy storage system using nanotechnology. New J. Chem.. 2021;45:8506-8515.
- [Google Scholar]
- Gold nanoparticles for biology and medicine. Angew Chem Int Ed Engl. 2010;49:3280-3294.
- [Google Scholar]
- Recent development of nanoparticles for molecular imaging. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci.. 2017;375:20170022.
- [Google Scholar]
- Large-scale synthesis and medical applications of uniform-sized metal oxide nanoparticles. Adv. Mater.. 2018;30:1704290.
- [Google Scholar]
- Recent advances of cerium oxide nanoparticles in synthesis, luminescence and biomedical studies: a review. J. Rare Earths. 2015;33:791-799.
- [Google Scholar]
- Nanohybrid scaffold of chitosan and functionalized graphene oxide for controlled drug delivery and bone regeneration. ACS Biomater Sci. Eng.. 2019;5:5139-5149.
- [Google Scholar]
- Coumarin–gold nanoparticle bioconjugates: preparation, antioxidant, and cytotoxic effects against MCF-7 breast cancer cells. Appl. Nanosci.. 2018;8:447-453.
- [Google Scholar]
- Pterocarpus marsupium Roxb. heartwood extract synthesized chitosan nanoparticles and its biomedical applications. J. Genet. Eng. Biotechnol.. 2020;18:1-13.
- [Google Scholar]
- Cerium oxide nanoparticles induced toxicity in human lung cells: role of ROS mediated DNA damage and apoptosis. BioMed Research International. 2014;2014:891934.
- [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55-63.
- [Google Scholar]
- Evaluation of anticancer effects of cerium oxide nanoparticles on mouse fibrosarcoma cell line. J. Cell. Physiol.. 2019;234:4987-4996.
- [Google Scholar]
- Ultrasonic assisted cerium oxide/graphene oxide hybrid: preparation, anti-proliferative, apoptotic induction and G2/M cell cycle arrest in HeLa cell lines. J. Inorg. Organomet. Polym Mater.. 2020;30:2666-2676.
- [Google Scholar]
- Nanoarchitectonics of Cerium Oxide/Zinc Oxide/Graphene Oxide Composites for Evaluation of Cytotoxicity and Apoptotic Behavior in HeLa and VERO Cell Lines. J. Inorg. Organomet. Polym Mater. 2022:1-12.
- [Google Scholar]
- Cerium Oxide/Graphene Oxide Hybrid: Synthesis, Characterization, and Evaluation of Anticancer Activity in a Breast Cancer Cell Line (MCF-7) Biomedicines. 2023;11:531.
- [Google Scholar]
- Metal nanoparticles: a theranostic nanotool against cancer. Drug Discov. Today. 2015;20:1143-1151.
- [Google Scholar]
- ‘Green’synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J. Nanobiotechnol.. 2018;16:1-24.
- [Google Scholar]
- Interfacial synthesis of micro-cuboid Ni0.55Co0.45C2O4 solid solution with enhanced electrochemical performance for hybrid supercapacitors. Nanoscale. 2019;11:13894-13902.
- [Google Scholar]
- Cerium oxide nanoparticle: a remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater.. 2014;6:e90-e.
- [Google Scholar]
- High-efficient synthesis of graphene oxide based on improved hummers method. Sci. Rep.. 2016;6:1-7.
- [Google Scholar]
- Antibody-conjugated silica-coated gold nanoparticles in targeted therapy of cervical cancer. Am. J. Transl. Res.. 2022;14:1518.
- [Google Scholar]
- Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Ther.. 2008;83:761-769.
- [Google Scholar]
- Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small. 2010;6:537-544.
- [Google Scholar]
- Graphene oxide: A growth factor delivery carrier to enhance chondrogenic differentiation of human mesenchymal stem cells in 3D hydrogels. Acta Biomater.. 2019;96:271-280.
- [Google Scholar]







