Translate this page into:
Quantification of multi-class pesticides in stomach contents and milk by gas chromatography–mass spectrometry with liquid extraction method
⁎Corresponding author. shahidgcs10@yahoo.com (Shahid Iqbal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Stomach content is the best specimen in acute pesticide poisoning. Direct detection of pesticides in milk. Salt-assisted liquid extraction method. Quantification method of nine multi-class pesticides by GCMS.
Abstract
Pesticides belonging to carbamates, pyrethroids and organophosphate groups are being mostly used worldwide. These are toxic and their minute amount leads to severe illness or death when ingested through various means. In case of suicidal or homicidal incidents, trace levels of pesticides may lead to acute death. In this scenario, stomach content is the best specimen for the detection of pesticide poison. Conversely, trace levels of pesticides may reach the mammary glands of milking animals when they eat grassy feed exposed to pesticides spray. Trace levels of those pesticide residues present in milk remain stable even after pasteurization. Eventually, milk consumers are affected chronically by these pesticide residues. The current study includes the development and validation of nine multi-class pesticide residues analyses in stomach content and milk. Nine-multiclass pesticides were extracted from stomach content and milk by acetonitrile with the addition of extraction salt. Quantitative analysis of permethrin, lambda-cyhalothrin, pyriproxyfen, triazophos, profenophos, chlorpyriphos, carbofuran, phorate, and step, GC–MS was used as an analytical technique equipped with DB-5 ms capillary open tubular column (15 m × 0.25 mm × 0.25 µm) and 0.08 ml/L flow rate of helium mobile phase gas with constant pressure. LLOQ and ULOQ for all target analytes were 0.05 mg/L and 3 mg/L respectively.
Keywords
Pesticides
Salt-assisted liquid extraction
GC–MS
Stomach contents
Milk
1 Introduction
For the last few decades, humans had been applying pesticides to achieve benefits of their wish like better crops yield by killing pest species i.e., insects, molds, rodents, and weeds (Kislev et al., 2004). Pesticides are also being used in a variety of fields including agriculture, forestry, environment, and domestic residence (Kislev et al., 2004, Liu et al., 2019). The prominent use of these synthetic chemicals has affected not only the health of the human population but also other living species of this planet (Deng et al., 2019, He et al., 2019, Ujan et al., 2021). The potential effect of pesticides causes illness in multiple systems of the human body for example, dermatological, neurological, and gastrointestinal functioning (Jabbar and Mallick 1994, Alavanja 2009, Shoaib et al., 2017a). Continuous exposure to pesticides may give rise to carcinogenic, toxic, and mutagenic problems in the victim. Unfortunately, pesticides are being used in homicides and suicides in human society (Dey et al., 2010, Bahadur et al., 2018, Fu et al., 2019). The unchecked utilization of pesticides in crops is creating toxicity in natural food items including fruits, vegetables, grains, milk, and drinking water (Marchis et al., 2012, Abbaspour et al., 2019). Pesticide residues may retain in exposed food and eventually reach the body of the consumer (Özcan 2016, Ozcan and Balkan 2017, Abbaspour et al., 2019). Early studies have proved that these synthetic chemicals may destroy the immune system, urinary system, cardiac system, skin, muscular system, gastric system, and even respiratory system (Bahadur et al., 2019, Kim et al., 2019, Saha et al., 2020).
On a global scale, studies related to the toxic behavior of pesticides have proved that cardiovascular and hypertension are caused by DDT (dichlorodiphenyltrichloroethane), carbofuran, and endosulfan. Permethrin and chlorpyrifos may cause death even at trace levels when ingested (Hayat et al., 2010). Mostly a brand of pesticide contains multiple pesticides in it to enhance its effect on applied fields (Jabbar and Mallick 1994, Ozcan and Balkan 2017). Commonly, pesticides should be biodegradable, but their residues may be detected in exposed crops (Costa et al., 2008, Ozcan and Balkan 2017, Iqbal et al., 2020). It has been found that unprocessed milk of herbivores may contain pesticide residues (Mogaddam et al., 2019, Weng et al., 2020). In case of suicidal or homicidal incidents where any pesticide was used, then stomach content is the best sample for analysis if properly preserved (Bahadur et al., 2017, Shoaib et al., 2017b, Song et al., 2019). Traces of pesticides can also be detected in other biological samples like blood, urine, or even the liver (Kim et al., 2018). Traces of pesticides in milk can cause chronic health problems in consumers (Costa et al., 2018, Manav et al., 2019). Selection of the best analytical tool for the detection of pesticides where sensitivity would not be compromised and it should be cost-effective has always been a challenge for scientists all over the world (Jouyban et al., 2018, Seebunrueng et al., 2020). Multiple techniques are available for the detection of pesticides, but the hyphenated instrument Gas chromatography–Mass Spectrometer (GC–MS) is one of the best tools in this sense (Özcan 2016, Ozcan and Balkan 2017, Manav et al., 2019, Sun and Wu 2020).
Selective and efficient extraction of target analytes from the matrix is another challenge for scientists. Liquid phase extraction and solid-phase extraction are the most common methods (Barci et al., 2020, Durak et al., 2020, Sun and Wu 2020). An extraction method known to be AOAC was introduced in 2007 that was developed to extract pesticides from fruits and vegetables (Westland and Dorman 2013). Acetonitrile as extraction solvent along with the salt of magnesium is added to the sample. For the removal of interfering matrices from the sample, solid-phase extraction (SPE) sorbent and lipid Enhanced Matrix Removal (EMR) sorbent is applied in addition (Lehmann et al., 2018, Ajibola et al., 2020). GC–MS has dual options as it can be used for qualitative and quantitative measures as well (Ozcan et al., 2017, Kusano et al., 2019).
Remember, a complex matrix of whole milk and stomach content contains a large number of interfering compounds like lipids and proteins which can mask the recovery and sensitivity of target pesticides (Costa et al., 2018, Manav et al., 2019). The pH of both types of samples vary from each other as the pH of whole milk is 6–7 while the pH of the stomach is 1–2 approximately (Costa et al., 2018). In this scenario, a unique extraction method is needed to achieve the expected results of better recovery of pesticides and their sensitive detection by GC–MS.
The present work is unique and efficient in the sense that it provides better cleaning of samples from the interfering matrices with minimum solvent consumption. Acetonitrile along with sodium acetate buffer is selected for extraction. Moreover, salts including anhydrous NaCl and MgSO4 are added to the sample for phase separation and better recovery. Simultaneous quantitation of nine pesticides of different groups is made possible on GC–MS operated on SIM mode with less run-time. Simple, cheap, effective, robust, green, reproducible, and sensitive are essential characteristics of this novel method.
2 Materials and methods
2.1 Chemicals and reagents
Pesticide standards for permethrin, lambda-cyhalothrin, pyriproxyfen, triazophos, profenophos, chlorpyriphos, carbofuran, phorate, and step (Fluka), internal standard Brucine (Fluka), extraction solvent Acetonitrile (ThermoScientific), Sodium Chloride, QuEChERS Extraction salt-Magnesium sulfate: Sodium acetate = 4:1 (Agilent Technologies USA), Lipid EMR powder (Agilent Technologies USA), Deionized Water (ACS grade, Acros Organics), Ammonium Chloride (ACS Grade, Acros Organics), Ammonium Hydroxide 25% (ACS Grade, Acros Organics).
2.2 Preparation of standard solutions
A stock solution of all nine pesticides was prepared in acetonitrile with a concentration of 100 ppm (µg/L). Then the working solution of each pesticide was prepared from the stock solution to make the concentration of 10 ppm (µg/L) in the same solvent. A working solution of internal standard with a concentration of 40 ppm (µg/L) was prepared in acetonitrile solvent. All standard solutions were stored in the refrigerator at 3 ⁰C temperature. To make a buffer solution of 9.5 pH, 0.045 Kg NH4Cl was added in 1 L of deionized water and mixed well with a magnetic stirrer. Then NH4OH was added dropwise to adjust the final pH of 9.5.
2.3 Salts and sorbents
Ready to use QuEChERS extraction salt (Sodium acetate: Magnesium sulfate = 1:4) and Enhanced Matric Removal (EMR) sorbent were purchased from Agilent Technologies.
2.4 Gastric contents and milk
A sample of gastric contents was collected from victims of pesticide poisoning. The whole milk of buffalos and cows was taken from Dairy Farms in the village of Punjab, Pakistan.
2.5 Gas chromatography–mass spectrometer
A GC system (7890B), auto-injector (G4513A), autosampler (7694) coupled with Inert MS system XL MSD (5975C) of Agilent Technologies were used as an analytical tool. Enhanced Mass Hunter Software was used to operate GC–MS. Injection volume was 2 µL set in the method using 5 µL micro-syringe. The split-less mode of the inlet was operated at 250 °C. Wall coated open tubular DB-35 ms capillary column (film thickness 0.25 µm × internal diameter 0.25 mm × column length 15 m) of Agilent Technologies was used in the GC system. Helium was used as carrier gas at constant pressure mode with a flow rate of 0.08 ml/min. Temperature programming of GC system includes 100 °C initially with 0.5 min hold-time. Then the temperature was raised to 300 °C at the rate of 20 °C/min, hold time 3.5 min, the total run time of 14 min. The temperature of the MS transfer line was 280 °C. Electron Impact (EI) at the voltage of 70 eV was applied for ionization purposes. The temperatures of the ionization chamber and mass analyzer were set to 300 °C and 150 °C respectively. For quantification of analytes, the SIM model was applied. Table 1 includes selected ions for internal standard and all analytes.
Pesticides and their Retention time with quantifier and qualifier ions
Analyte
RT (min)
Target ion
Q1
Q2
1-Sufotep
4.94
322.1
97.5
202.1
2-Phorate
5.06
75.2
121.2
260.2
3-Carbofurane
5.33
164.1
149.2
131.1
4-Chlorpyrifos
6.62
197.3
199.1
314.3
5-Profenofos
7.62
337.1
339.2
208.4
6-Triazophos
8.23
161.4
162.3
172.1
7-Pyriproxyfen
9.36
136.2
226.2
137.2
8-Lambda-Cyhalothrin
9.45
181.1
141.1
180.3
9-Permethrin
9.94
183.2
163.2
165.2
10-Brucine (IS)
11.02
394.5
379.1
395.5
2.6 Extraction scheme
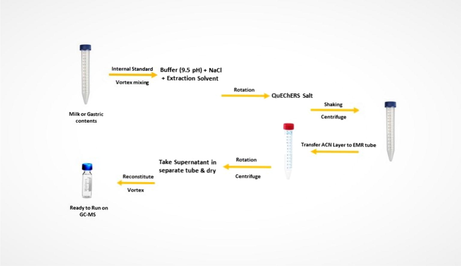
For efficient recovery of analytes from the sample, salt-assisted liquid extraction was used. First, 2 ml of each gastric content and milk was collected in a 15 ml plastic tube using the pipette. Then 50 µL IS (internal standard) was spiked in each tube and vortexed for 30 sec on a digital vortex mixer. Then 300 mg NaCl, 2 ml of buffer solution (9.5 pH), and 5 ml acetonitrile were added in each tube and then by auto-rotator tube were shaken for 15 min. After that extraction salt of QuEChERS (2 g) was added to each plastic tube, shaken for 2 min, and centrifuged for 5 min at 4000 rpm in a digital centrifuge machine. The supernatant of acetonitrile was put into a new 15 ml plastic tube having 1 g of EMR powder already in it. After rotation and centrifugation, the upper layer of solvent was transferred into a new 15 ml plastic tube, and the solvent was evaporated in a turbo-vaporizer at 40 °C. Then 50 µL of acetonitrile was added to each tube for reconstitution and then transferred to GC–MS auto-sampler vial. Fig. 1 shows the overall extraction procedure.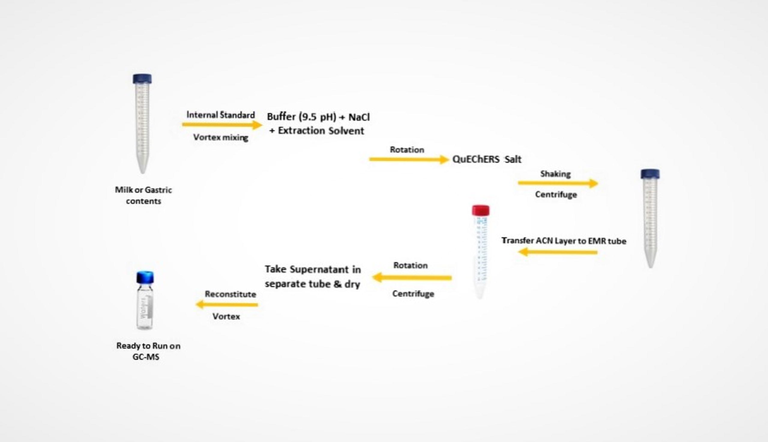
Schematic extraction procedure.
2.7 Method validation
For validation of this newly developed, guidelines of SWAGTOX were adopted. The major parameters that were observed and measured are percent recovery, standard deviation (RSD), precision, accuracy, correlation factor (r2), the limit of detection (LOD), the limit of quantification (LOD), specificity and interference. Six calibrators (0.01, 0.05, 0.10, 0.30, 0.50, 2.0 µg/L respectively), positive controls (0.1 µg/L) and negative controls were made in pesticide-free gastric contents and milk. After extraction, the calibrators and controls were run on GC–MS in triplicated for five days.
3 Results and discussion
3.1 Optimization of the extraction procedure
Some useful additions were made in AOAC 2007.1 to get the desired results. The extraction was optimized for gastric contents and milk samples for efficient recovery of target pesticides. A buffer with 9.5 pH (ammonium hydroxide/ammonium chloride) was used with the addition of table salt. A mixture of anhydrous magnesium sulfate and sodium acetate was mixed followed by the addition of polar organic solvent acetonitrile. For the enhanced removal of interfering compounds like lipids and cholesterol from the samples, the EMR sorbent was mixed in acetonitrile supernatant. The final separated layer of acetonitrile was dried under heat and air pressure in a turbo-vaporizer. Finally, the reconstituted sample was made ready in the auto-sampler vial to run on the instrument.
3.2 Optimization of instrumental parameters
After optimization of the extraction procedure, a new analytical method was designed and validated on GCMS with GC system (7890B), auto-injector (G4513A), autosampler (7694) coupled with Inert MS system XL MSD (5975C) of Agilent Technologies were applied as an analytical instrument. For this purpose, standards of all pesticides were run at scan mode on GCMS to obtain retention times and mass spectrums of respective analytes. Wall coated open tubular DB-35 ms capillary open tubular column (0.25 µm film thickness × 0.25 mm × column length 15 m) of Agilent Technologies was used in the GC system. Helium was used as carrier gas at constant pressure mode with a flow rate of 0.08 ml/min. The Electron Impact (EI) ionization source was set on 70 eV voltage. After obtaining the mass spectrum and retention time of each analyte, the total runtime was set to 14 min that is very short. For quantitative analysis, scan mode changed to selected ion monitoring (SIM) mode.
After running the standards of pesticides on GC–MS at scan mode with 50–500 m/z range, the result was compared with the NIST library database. After this, an un-extracted quality control check (QC) was run at preset instrumental conditions. Then, three ions for each analyte were selected and the test mix was run again but this time on SIM mode. Calibrators and controls prepared in gastric contents and milk with known concentrations were also run on the newly developed and optimized method after the extraction procedure.
3.3 Applications
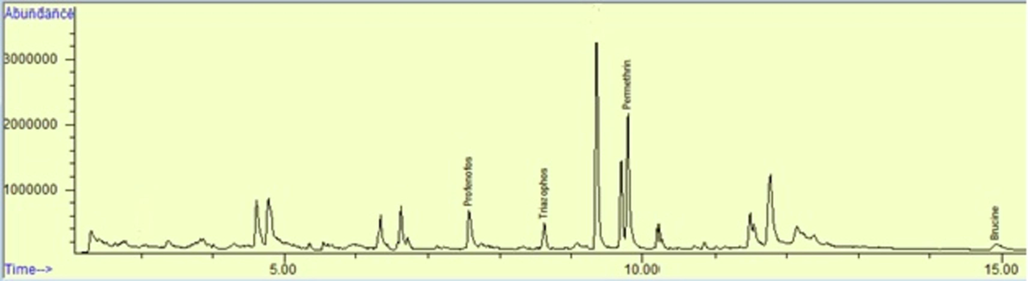
The newly developed, optimized and validated method was applied on multiple milk samples of buffalos and cows collected from different dairy farms of Punjab, Pakistan (Fig. 2, Tables 1, 2, 3, and 4). It was also applied on gastric contents sample of a person who died due to pesticide intake in a suicidal attempt. Results revealed that the cause of death was permethrin and the stomach contents of the victim contained 0.17 mg/L of this pesticide (Fig. 3). One of the results of a milk sample of a cow shows that it contained traces of profenofos, triazophos, and permethrin with amounts of 0.029 mg/L, 0.022 mg/L, and 0.84 mg/L respectively (Fig. 4).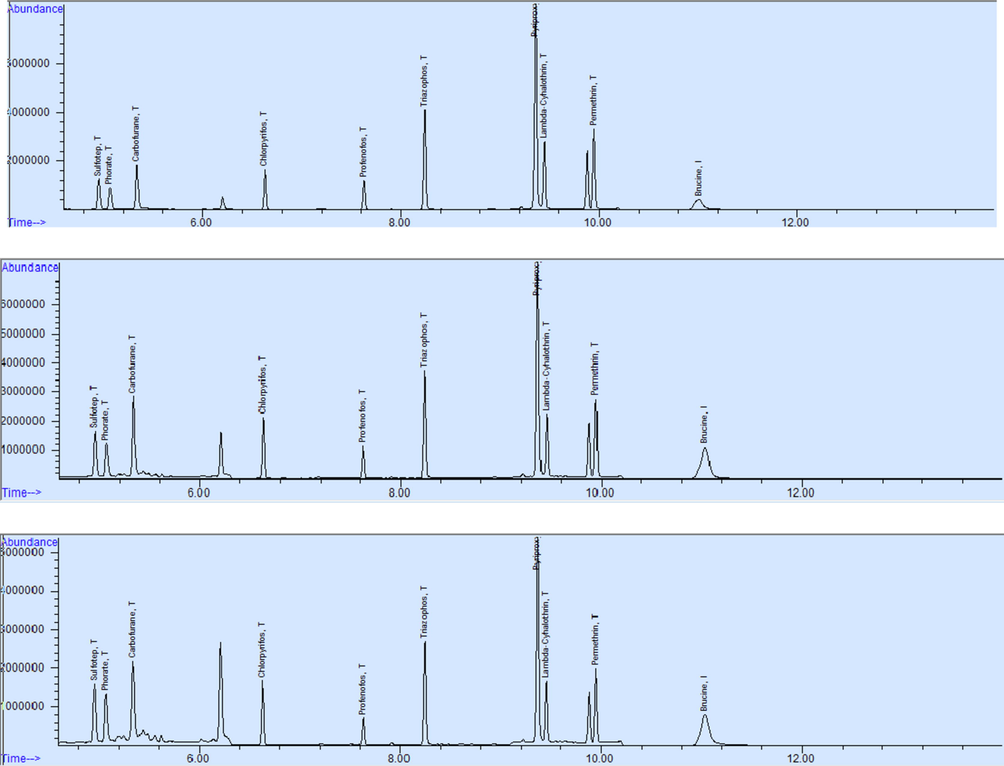
(a) Positive QC run at SIM mode on GCMS, (b) positive QC spiked in stomach contents run at SIM mode on GCMS and (c) positive QC spiked in milk run at SIM mode on GC–MS.
0.01 mg/L
0.05 mg/L
Sr#
Av Conc.
Av. recovered conc.
Bias
%Recovery
Av Conc.
Av. recovered conc.
Bias
%Recovery
1
0.011
0.009
10
90%
0.02
0.0206
3
103%
2
0.001
0.02
3
0.021
0.02
4
0.011
0.02
5
0.001
0.02
0.10 mg/L
0.30 mg/L
1
0.003
0.0419
4.8
104.75%
0.02
0.077
3.8
96.25%
2
0.002
0.02
3
0.001
0.01
4
0.101
0.21
5
0.102
0.12
0.50 mg/L
2.0 mg/L
1
0.201
0.1605
0.3
100%
0.2
0.2918
8.8
91.90%
2
0.201
0.12
3
0.0001
0.1
4
0.2
0.01
5
0.2
1.02
Analyte
0.01 mg/L
0.3 mg/L
2.0 mg/L
Av Response
RSD
Av Response
RSD
Av Response
RSD
Sulfotep
0.132
17.55
1.05455
7.487
4.3112
19.42
0.1338
1.15376
6.3212
0.1853
1.26558
4.2322
0.1359
1.14479
5.1322
Phorate
0.3995
16.27
6.1022
19.07
21.649
13.52
0.4976
5.1012
22.878
0.4638
4.101
17.948
0.5899
4.2112
24.997
Carbofurane
0.0007
15.71
0.0047
17.37
0.019
10.1
0.0009
0.0047
0.02
0.001
0.0057
0.016
0.001
0.0037
0.02
Chlorpyrifos
1.2022
4.421
13.241
16.06
38.816
17.11
1.1012
9.1041
58.904
1.1051
11.1231
46.824
1.1014
10.211
50.716
Profenophos
6.563
12.55
40.62
12.53
157.56
19.32
5.4972
50.88
140.75
5.7161
50.54
197.65
4.8441
40.96
214.64
Triazophos
3.1087
19.33
18.9502
18.27
80.15
18.23
2.7095
18.97
78.394
2.7097
18.9701
85.261
1.9069
26.5901
113.283
Pyriproxyfen
2.922
1.667
22.9022
11.81
63.29
16.61
2.993
20.9346
95.492
2.902
18.9133
78.291
2.999
24.9435
80.091
Lambda-cyhalothrin
0.0043
4.057
0.023
7.607
0.0732
12.42
0.0047
0.025
0.0994
0.0044
0.022
0.0871
0.0046
0.026
0.0855
Permethrin
0.503
13.66
4.9903
19.28
23.56
12.81
0.705
4.9902
25.73
0.606
6.9905
28.39
0.602
6.9906
20.96
Parameter
Acceptance criteria
Outcomes
Matrix interference
No interference from matrix or diluent must be observed.
No matric interference was observed both in extraction and instrumental procedure.
Precision
It should not be more than 20%
It was less than 20% for all analytes.
Accuracy (% recovery)
It should be 85–115%
It was 90–105% for all analytes.
Linearity (r2)
r2 value must be ≥ 0.985
It was 0.9988–0.9999
LOD
3 Standard deviation
It was 0.01 ppm
LOQ
10 Standard deviation
It was 0.01 ppm
Reproducibility and repeatability
Both must be meted
The assay was both reproducible and repeatable.
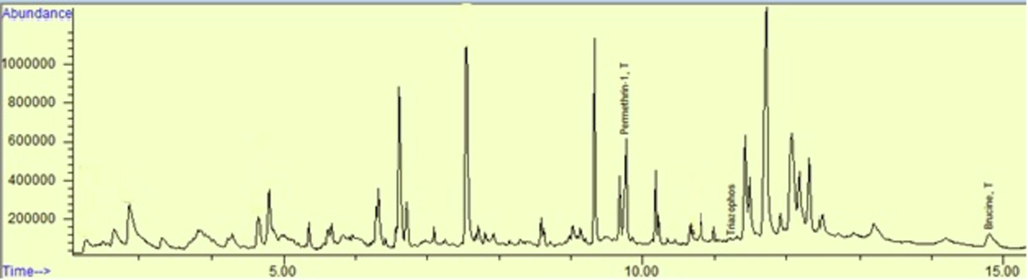
Result of gastric contents sample of a victim who died due to pesticide intake.
Result of milk sample of a cow.
4 Conclusion
The novelty of the present work is proved by its ability to extract nine multiclass pesticides from complex samples like stomach content and animal milk especially. The extraction methodology is unique that involves polar organic solvent acetonitrile. The extraction is assisted with inorganic salt (Sodium acetate: Magnesium sulfate = 1:4) which is very cheap. Enhanced matrix removing sorbent is applied additionally for the cleaning of samples from interfering compounds especially fatty acids. A very sensitive analytical method is developed and validated on GC–MS for the simultaneous quantification of nine pesticides of different classes. The selected ion monitoring mode of GC–MS with a very short run time of only 14 min provides very high sensitivity. The lower limit of quantification (LLOQ) is 0.01 mg/L and the limit of detection (LOD) is 0.005 mg/L for all nine target pesticides. This novel method is validated as per SWAGTOX guidelines. No interference of problematic compounds is observed during application. It is proved as unique, simple, robust, rugged, sensitive, selective, and economical.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for supporting this work through research groups program under grant number R.G.P.2/124/43. Rami M. Alzhrani would like to acknowledge Taif University Researchers Supporting Project Number (TURSP-2020/209), Taif University, Taif, Saudi Arabia. The authors extend their appreciation to the Research Center at AlMaarefa University for funding this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Monitoring of nine pesticides in different cereal flour samples with high performance liquid chromatography-diode array detection. Anal. Methods. 2019;11:4022-4033.
- [Google Scholar]
- Simultaneous determination of multiclass antibiotics in sewage sludge based on QuEChERS extraction and liquid chromatography-tandem mass spectrometry. Anal. Methods. 2020;12:576-586.
- [Google Scholar]
- Introduction: Pesticides use and exposure, extensive worldwide. Rev. Environ. Health. 2009;24:303-310.
- [Google Scholar]
- Bahadur, A., Saeed, A., Iqbal, S., et al., 2017. Biocompatible waterborne polyurethane-urea elastomer as intelligent anticancer drug release matrix: a sustained drug release study 119, 57-63.
- Bahadur, A., Saeed, A., Shoaib, M., et al., 2019. Modulating the burst drug release effect of waterborne polyurethane matrix by modifying with polymethylmethacrylate 136, 47253.
- Bahadur, A., Shoaib, M., Iqbal, S., et al., 2018. Regulating the anticancer drug release rate by controlling the composition of waterborne polyurethane 131, 134-141.
- Modified QuEChERS method for multiresidue determination of pesticides in pecan nuts by liquid chromatography tandem mass spectrometry. Food Analy. Methods.. 2020;13:793-801.
- [Google Scholar]
- Determination of 23 organochlorine pesticides in animal feeds by GC-MS/MS after QuEChERS with EMR-lipid clean-up. Anal. Methods. 2018;10:5171-5180.
- [Google Scholar]
- Evaluation of an alternative fluorinated chitosan as a QuEChERS adsorbent for pesticide residue analysis in apple samples. Anal. Methods. 2019;11:3460-3466.
- [CrossRef] [Google Scholar]
- Comparative in vitro evaluation of anti-inflammatory effects of aerial parts and roots from Mikania scandens. J. Adv. Pharm. Educ. Res.. 2010;1
- [Google Scholar]
- Validation of ultrasonic-assisted switchable solvent liquid phase microextraction for trace determination of hormones and organochlorine pesticides by GC–MS and combination with QuEChERS. Food Chem.. 2020;305:125487.
- [CrossRef] [Google Scholar]
- A comprehensive analysis of 201 pesticides for different herbal species-ready application using gas chromatography–tandem mass spectrometry coupled with QuEChERs. J. Chromatogr. B. 2019;1125:121730.
- [Google Scholar]
- Determination of pesticide residues in blood samples of villagers involved in pesticide application at District Vehari (Punjab), Pakistan. Afr. J. Environ. Sci. Technol.. 2010;4:666-684.
- [Google Scholar]
- Monitoring of 49 pesticides and 17 mycotoxins in wine by QuEChERS and UHPLC–MS/MS analysis. J. Food Sci.. 2019;84:2688-2697.
- [Google Scholar]
- Modified QuEChERS extraction method followed by simultaneous quantitation of nine multi-class pesticides in human blood and urine by using GC-MS. J. Chromatogr. B. 2020;1152:122227.
- [Google Scholar]
- Jabbar, A., Mallick, S., 1994. Pesticides and environment situation in Pakistan, JSTOR.
- A lighter-than-water deep eutectic-solvent-based dispersive liquid-phase microextraction method in a U-shaped homemade device. New J. Chem.. 2018;42:10100-10110.
- [Google Scholar]
- Review of the QuEChERS method for the analysis of organic pollutants: Persistent organic pollutants, polycyclic aromatic hydrocarbons, and pharmaceuticals. Trends Environ. Anal. Chem.. 2019;22:e00063.
- [CrossRef] [Google Scholar]
- Method development, matrix effect, and risk assessment of 49 multiclass pesticides in kiwifruit using liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B. 2018;1076:130-138.
- [Google Scholar]
- Impetus for sowing and the beginning of agriculture: ground collecting of wild cereals. Proc. Natl. Acad. Sci.. 2004;101:2692-2695.
- [Google Scholar]
- Development of “Quick-DB forensic”: A total workflow from QuEChERS-dSPE method to GC–MS/MS quantification of forensically relevant drugs and pesticides in whole blood. Forensic Sci. Int.. 2019;300:125-135.
- [CrossRef] [Google Scholar]
- Development of a modified QuEChERS method for multi-class pesticide analysis in human hair by GC-MS and UPLC-MS/MS. Analy. Chim. Acta. 2018;999:87-98.
- [Google Scholar]
- The determination of pesticides in tea samples followed by magnetic multiwalled carbon nanotube-based magnetic solid-phase extraction and ultra-high performance liquid chromatography-tandem mass spectrometry. New J. Chem.. 2019;43:5395-5403.
- [CrossRef] [Google Scholar]
- Optimization of a modified QuEChERS method by means of experimental design for multiresidue determination of pesticides in milk and dairy products by GC–MS. Microchem. J.. 2019;144:124-129.
- [Google Scholar]
- Detection of pesticides in crops: A modified QuEChERS approach. Food Control. 2012;25:270-273.
- [Google Scholar]
- Headspace mode of liquid phase microextraction: A review. TrAC Trends Analy. Chem.. 2019;110:8-14.
- [Google Scholar]
- Determination of organochlorine pesticides in some vegetable samples using GC-MS. Polish J. Environ. Stud. 2016
- [Google Scholar]
- Multi-residue determination of organochlorine pesticides in vegetables in Kirklareli, Turkey by gas chromatography–mass spectrometry. J. Anal. Chem.. 2017;72:761-769.
- [Google Scholar]
- Residue Analysis and Determination of IMI Herbicides in Sunflower and Soil by GC–MS. Chromatographia.. 2017;80:941-950.
- [CrossRef] [Google Scholar]
- QuEChERS-based gas chromatography-electron capture/flame photometric detection method for multi-pesticide residues analysis in Andrographis paniculata: a popular Indian medicinal herb. Int. J. Environ. Anal. Chem.. 2020;100:1669-1690.
- [Google Scholar]
- A new environment-friendly supramolecular solvent-based liquid phase microextraction coupled to high performance liquid chromatography for simultaneous determination of six phenoxy acid herbicides in water and rice samples. Microchem. J.. 2020;152:104418.
- [CrossRef] [Google Scholar]
- Shoaib, M., Bahadur, A., Iqbal, S., et al., 2017. Relationship of hard segment concentration in polyurethane-urea elastomers with mechanical, thermal and drug release properties 37, 88-96.
- Shoaib, M., Bahadur, A., ur Rahman, M.S., et al., 2017. Sustained drug delivery of doxorubicin as a function of pH, releasing media, and NCO contents in polyurethane urea elastomers 39, 277-282.
- A novel extraction method for simultaneous determination of neonicotinoid insecticides and their metabolites in human urine. Anal. Methods. 2019;11:2571-2578.
- [CrossRef] [Google Scholar]
- Analysis of PAHs in oily systems using modified QuEChERS with EMR-Lipid clean-up followed by GC-QqQ-MS. Food Control. 2020;109:106950.
- [CrossRef] [Google Scholar]
- Ujan, R., Bahadur, A., Shabir, G., et al., 2021. Facile synthesis of novel fluorescent thiazole coumarinyl compounds: Electrochemical, time resolve fluorescence, and solvatochromic study 1227, 129422.
- Multi-residue analysis of 126 pesticides in chicken muscle by ultra-high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Chem.. 2020;309:125503
- [CrossRef] [Google Scholar]
- QuEChERS extraction of benzodiazepines in biological matrices. J. Pharm. Anal.. 2013;3:509-517.
- [Google Scholar]







