Translate this page into:
Quercetin-albumin nano-complex as an antioxidant agent against hydrogen peroxide-induced death of spinal cord neurons as a model of preventive care study
⁎Corresponding authors at: No. 747, West Section of Zhonghua Road, Zhumadian 463600, Henan Province, China. Pshi.medicine@gmail.com (Pin Shi), lulucheng.neurosurgery@yahoo.com (Lulu Cheng)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Herein, the quercetin-human serum albumin (HSA) nano-complex was formulated and its capability to decrease H2O2-stimulated cytotoxicity against spinal cord neurons as a model of preventive care study was investigated by cellular assays. Quercetin molecules were first encapsulated into HSA as a shell and the loading efficiency along with drug release and different properties of formulated nano-complex were determined using TEM, SEM, and DLS approaches. Afterwards using CD spectroscopic method and theoritical analysis, the structural changes and binding redisues of HSA after interaction with quercetin were investigated, respectively. Finally, the antioxidant activity of quercetin-HSA nano-complex against H2O2 (200 µM)-stimulated cytotoxicity in spinal cord neurons was explored by MTT, SOD and CAT activity, and morphological changes assays. It was shown that the fabrication of quercetin-HSA nano-complex resulted in the improved bioavailability of quercetin with an entrapment efficiency of 68.99% and percent yield of 53.19%. Also, molecular docking study showed that quercetin molecules spontaneously bind to HSA through hydrogen bonds and van der Waals interactions. Furthermore, CD data indicated that the quercetin did not change the secondary structure of HSA. Finally, cellular assays displayed that the pre-treatments of spinal cord neurons with quercetin-HSA nano-complex attenuated the H2O2-triggerd neuron mortality through a significant increase in the activity of SOD and CAT and prevention of morphological changes. In conclusion, it may be suggested that the formulation of quercetin into nano-complex can improve its medical properties and this new developed drug formulation may hold a great promise in the advancement of antioxidant compounds in preventive care services.
Keywords
Quercetin
HSA
Nano-complex
Spinal cord neurons
Antioxidant
Preventive care
1 Introduction
Neurons are damaged by physical, chemical, and pathological factors, and degeneration is a permanent injury (Ohno and Ikenaka, 2019; Pei et al., 2017). Each neuron is a functional and structural unit that is specialized in sending and receiving signals (Hahn et al., 2019). When a nerve is damaged, the connection of the axon to the cell body is disrupted, and its distal part begins to degenerate simultaneously in a retrograde manner (Pathak et al., 2018). However, neurons generally lack the power to be reproduced after birth. But they can resist certain degrees of damage and start to be recovered to some extent (Andreone et al., 2020; Takeoka and Arber, 2019; Wu et al., 2020). If a nerve fiber is damaged, changes can occur in both the peripheral nerves and the central nerves, which can lead to cell death if these changes are severe (Andreone et al., 2020). Some chemicals and nerve damage trigger a number of free radicals, followed by intracellular action and subsequent reaction that eventually lead to large-scale changes (Ballance et al., 2019; Martinelli et al., 2020). Studies in the mammalian nervous system show that after the pressure and impact on the nerve cell during the developmental stages and after the formation of the relevant nerve cells, they suffer from cell death to varying degrees, depending on the severity of the pressure, the location of the injury, the type of nerve cell, its location in the nervous system, and age of the patient (Andreone et al., 2020; Schimmel et al., 2017). The spinal cord is highly sensitive to oxidative stress due to physiological and biochemical reasons (Ren et al., 2019; Zhang et al., 2016). Therefore, maintaining homeostasis-essential oxidation status is essential for spinal cord neuron cells (Andrabi et al., 2020). In addition, the antioxidant levels of the spinal cord are limited compared to other tissues (Danková et al., 2019; Jia et al., 2019). Therefore, the various mechanisms involved in the production of endogenous oxygen and exogenous reactive oxygen species (ROS) and the role of oxidative stress in neurodegenerative disease have been well-discussed (Coyoy-Salgado et al., 2019; Xu et al., 2020). Oxidative imbalance occurs when antioxidant capacity does not overcome free radicals, which can lead to tissue damage, cell death, or disease onset (Valko et al., 2007). Antioxidants are compounds that significantly delay or prevent substrate oxidation (Asadi-Samani et al., 2019; Martinelli et al., 2020). Antioxidants are biologically active compounds that protect the body against damage caused by ROS, which lead to disease (Suleman et al., 2019; Sumran and Aggarwal, 2019).
Due to the increasing use of herbal and natural remedies instead of chemical substances, neuroscientists are also paying attention to this issue and are presenting a large part of their studies with a new approach to using these natural substances (Singh et al., 2019; Sumran and Aggarwal, 2019). Although phenolic compounds and some of their derivatives are highly effective in preventing autooxidation, only a small number of them are allowed to be used in food or medical sciences as antioxidants (Rahaiee et al., 2020; Rojas and Buitrago, 2019). The most important natural antioxidants in food are phenolic and polyphenolic compounds (Jagadeesan et al., 2019; Vella et al., 2019).
Quercetin is a flavonoid plant pigment and has a potential antioxidant (Caddeo et al., 2019) and anticancer activity (Ezzati et al., 2020). Several studies have shown that phenolic compounds like quercetin could be effective in stabilizing double-layered phospholipids against ROS-induced peroxidation in different cells (Jin et al., 2019; Li et al., 2019; Song et al., 2020).
Although it is soluble in organic solvents such as dimethyl sulfoxide (DMSO), ethanol, and acetone, it is insoluble in water and acidic conditions and it decomposes rapidly in neutral and alkaline conditions (López et al., 2020; Tang et al., 2019). Today, various carriers of drug delivery have been used to increase the solubility of this valuable substance in body fluids and, consequently, to increase the antioxidant effects of quercetin (Birinci et al., 2020; Ersoz et al., 2020; Ghayour et al., 2019).
One of the most important biological functions of human serum albumin (HSA) is its ability to bind to natural and synthetic bioactive compounds (Esfandfar et al., 2016; Nosrati et al., 2019). Therefore, HSA, as a carrier, seems to be a good option for interaction with substances such as herbal compounds with therapeutic and medicinal properties (Nosrati et al., 2019; Pan et al., 2019). On the other hand, recent advances in nanotechnology and drug delivery require extensive research in the preparation of the nano-complex derived from bioactive materials. HSA in the blood is the most abundant protein in plasma, which plays a key role in regulating blood osmotic pressure (Al-Harthi et al., 2019). The interaction of small molecules/drugs with HSA can provide useful information about the complex properties of these substances, which indicate the behavior of their distribution, the active concentration and their metabolite in the bloodstream (Yamasaki et al., 2013). Therefore, the study of the interaction between quercetin and HSA can provide useful findings in the field of formulation and the applied dose of drugs.
2 Materials and methods
2.1 Materials
HSA, quercetin, Dulbecco’s Modified Eagle’s Medium with a 1:1 mixture of Ham's F12 (DMEM: F12), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromidefor (MTT), and fetal bovine serum (FBS) were obtained from Sigma-Aldrich Co. (USA). All other materials were of analytical grade. Quercetin in medium containing co-solvents or ethanol was assessed to develop a dissolution system which provides sink condition for studying curcumin loading and release assays.
2.2 Methods
2.2.1 Fabrication of quercetin-HSA nano-complex
Quercetin solution (5 mg/ml, dissolved in 1% ethanol) was added dropwise to the HSA solution (20 mg/ml, dissolved in phosphate buffer, pH 7.4) with a stirring rate of 200 rpm for 2 hr to dissolve the quercetin in the solution. The obtained dispersion was rotary evaporated to remove ethanol at 30 °C for 60 min under reduced pressure. The resulting sample was centrifuged at 10000*g for 20 min and the pellet (nano-complex powder) was resuspended in a phosphate buffer and maintained at 4 °C.
2.2.2 Characterization of quercetin-HSA nano-complex
The morphology and size of quercetin-HSA nano-complex were investigated using scanning electron microscopy (SEM, zeiss, Germany) and transmission electron microscopy (TEM, Zeiss, Germany), respectively. The hydrodynamic radius and zeta potential values were determined using a Zetasizer (ZetaPlus zeta potential analyzer, Brookhaven, USA).
2.2.3 The percent yield and drug entrapment
The percent yield and drug entrapment were determined using Eq. (1) and Eq. (2), respectively as following (Jithan et al., 2011):
2.2.4 Drug release assay
The release solution was assessed in phosphate buffer medium (pH 7.4, 10 mM) added by 1% ethanol, where this assay can be usually employed to explore the release of the small molecules from a variety of nano-formulations (Namasivayam and Robin, 2018; Salehiabar et al., 2018). This medium with a pH 7.4 as the physiological pH was used to determine the quercetin release from the nano-complex based on the previous studies (Cheng et al., 2018; Islam et al., 2018). For this assay, 50 mg of prepared quercetin-HSA nano-complex was solubilized in 200 ml of the phosphate buffer at room temperature under constant stirring at 150 rpm. As, the final quercetin concentration was 5 mg/ml, after determining the concentration of supernatant, it was found that around 4.5 mg/ml quercetin is entrapped in 50 mg of nanocomplex. At defined time intervals (1 till 24 hr), 10 ml of the solution was removed and followed by filtration. Finally, the amount of released drug was determined by UV-spectrophotometer at 390 nm. A standard calibration curve (concentration vs. absorbance) was plotted for this assay (Fig. S1).
2.2.5 Molecular docking study
It is necessary to use fast and computational tools such as molecular docking to predict the design of drugs. Since this method is based on the interaction of atoms with each other and in which the desired ligand or chemical compound is attached randomly or with a special algorithm at the level of specific macromolecules, in this study, the ability of the desired plant compound, namely, quercetin in creating interaction with HSA was evaluated by molecular docking method.
In order to model the interaction between quercetin and HSA as a receptor, the X-ray structure of HSA with a PDB ID: 1AO6 was extracted from the online Protein Data Bank RCSB PDB (http://www.pdb.org). The geometry of the quercetin was optimized using Avogadro software (Libavogadro Library, Pittsburgh, PA, USA). The visualization of the complexes was done using CHIMERA (www.cgl.ucsf.edu/chimera) and PyMOL (http://pymol.sourceforge.net/) tools. The iGemdock v2.1 software (Hsu et al., 2011) was used as the molecular docking tool. This software generates profiles of hydrogen-bonding (H), Van der Waals (V) and electrostatic (E) interactions. The empirical scoring function of iGemdock was estimated using the following Eq. (3) (Hsu et al., 2011):
2.2.6 Circular dichroism (CD) study
The CD study was done to explore the secondary structural changes of HSA (5 µM, dissolved in phosphate buffer, pH 7.4, 10 mM) in the presence of varying concentrations of quercetin (2–20 µM, dissolved in 1% (v/v) ethanol) using a spectropolarimeter (Aviv, NJ, USA) in the λ range of 190–260 nm. The relevant software (CDNN) was then used to quantify the secondary structural changes of HSA.
2.2.7 Spinal cord neurons culture
First, the body of the two-day-old rat was sterilized and after anesthetizing, the spinal cord was removed (All procedures were done based on the ethics committee protocols approved by Luoyang Central Hospital and Zhengzhou University). The spinal cord was then stored in PBS on ice and washed in the culture medium solution. Then, the meningeal layers around the spinal cord were slowly removed, separating the spinal cord, and trying to prevent any fibroblastic cells from transferring to the cell culture medium. After removing the spinal cord parts and washing them with a PBS buffer, two or three pieces with a length of 1 mm were placed in the middle of the petri dish. Finally, the cell culture medium including DMEM/F12, FBS 10%, penicillin and streptomycin 1% was added to the cells at 37˚C, 5% CO2, and 95% humidity.
2.2.8 MTT assay
To explore the safety of quercetin against the spinal cord neurons, the cells were treated with different concentrations (1–50 µM) of quercetin for 48 hr and the MTT assay was run to investigate the cell viability. In the next step, the cells were pretreated with a fixed safe concentration of quercetin or quercetin-HSA nano-complex for 24 hr followed by addition of H2O2 (200 µM) for another 24 hr. Finally, MTT solution (0.5 mg/ml) was added for 4 hr and after the preparation of samples the absorbance was monitored at 570 nm using an ELISA plate reader. The cells without any treatment were considered as negative control cells.
2.2.9 Superoxide dismutase (SOD) assay
SOD activity was assessed by SOD Activity Assay Kit (ab65354)/Abcam (UK) based on the manufacturer's protocol. Briefly, after treatment and harvesting, the cells were lysed in a lysis buffer (0.1 M Tris/HCl, pH 7.4 containing 0.5% Triton X-100, 5 mM β-ME, 0.1 mg/ml PMSF) in ice cold. The cells were then centrifuged at 14,000 × g for 5 min at 4 °C. Finally, the supernatant was collected and transferred to a clean tube. The cell extract was then added by reaction mix and incubated at 37 °C for 20 min and the absorbance was read at 450 nm using an ELISA plate reader.
2.2.10 Catalase (CAT) assay
CAT activity was assessed by CAT Activity Assay Kit (ab118184)/Abcam (UK) based on the manufacturer's protocol. Briefly, after sample preparation, the cells were added by H2O2 and incubated 30 min at 25 °C flowed by adding a stop solution to samples. Afterwards, development mix solution was added and the samples were incubated at 25 °C for 10 min. Finally, the absorbance was read at OD570 using an ELISA plate reader.
2.2.11 Morphological assay
Qualitative analysis of normal and damaged cells was explored using morphology assay. Subsequently, cells were exposed to different treatments and their underlying morphological changes were observed by optical images in an inverted microscope (Zeiss, Germany).
2.2.12 Statistical analysis
Statistical analyses were done using SPSS software and the resultant data were expressed as mean ± standard deviation (SD) of three independent experiments. Statistical difference was reported using Student’s t-test or ANOVA, considering P < 0.05 as statistically significant.
3 Results:
3.1 The characterization of prepared quercetin-HSA nano-complex
The nano-complex formation through desolvation method was confirmed for quercetin with HSA employing SEM (Fig. 1A), TEM (Fig. 1B) and zeta sizer [Fig. 1C] techniques. SEM image (Fig. 1A) showed that quercetin-HSA nano-complex was potentially fabricated and were typically in the 99–505 nm size range and had a spherical-shaped morphology. TEM image (Fig. 1B) also indicated that the size of the prepared nano-complex is around 100 to 500 nm and quercetin molecules have been entrapped by HSA biopolymers. Indeed, due the sample preparation for SEM and TEM analysis, i.e. fixation and dehydration, drying and coating, then the observation of different size prepared nano-complex with a large size range can be expected. DLS study was then run to further support this explanation. The Zetasizer measurement showed that the hydrodynamic radius of quercetin-HSA nano-complex was about 210.37 nm (PDI = 0.218) (Fig. 1C) with a charge distribution of around −31.98 mV. This data indicated that the prepared nano-complex had a good colloidal stability. The entrapment efficiency was also indicated to be 68.99%, determined by UV-spectrophotometer and percent yield was 53.19%.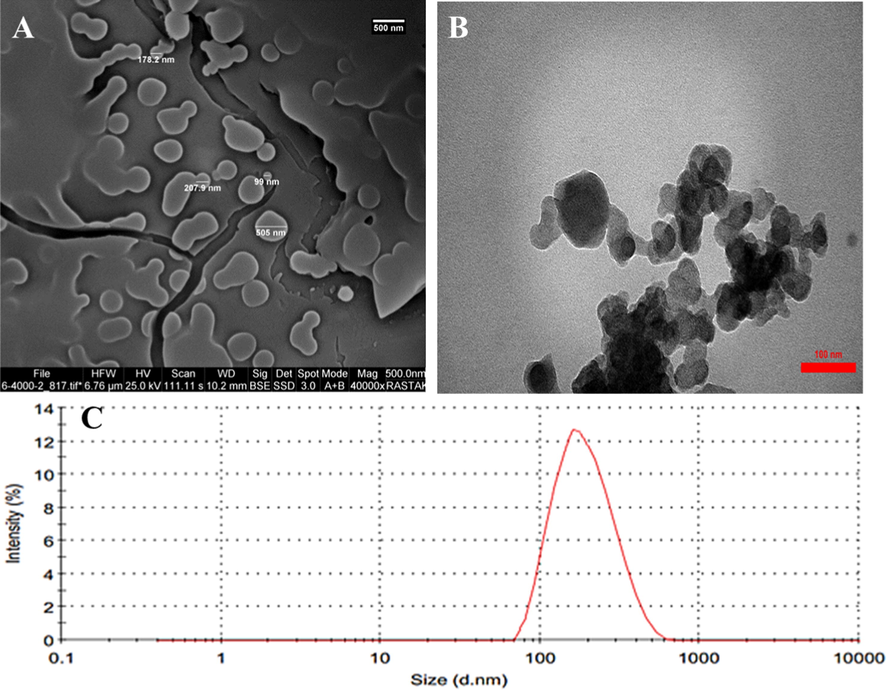
SEM image (A), TEM image (B), and DLS plot (C) of prepared quercetin-HSA nano-complex prepared through desolvation method. SEM and TEM images reveal that the dimension of quercetin-HSA nano-complex is in the range of 100 to 500 nm. The DLS analysis indicates that the hydrodynamic radius of quercetin-HSA nano-complex is about 210.37 nm.
3.2 Drug release assay
The quercetin release from HSA nano-complex solubilized in phosphate buffer pH 7.4, 10 mM was approximately 3.2-fold higher than that of free quercetin (Fig. 2) after 24 hr. This data indicated that the solubility of quercetin in the quercetin-HSA nano-complex may be increased in vitro and in vivo, thereby increasing higher plasma quantity. Indeed, the low plasma concentration of quercetin is almost due to its low level of dispersion in aqueous medium and remarkable reduction in the blood circulation time. Therefore, this data indicated that nano-formulation of quercetin by means of HSA can increase the dissolution rate of the encapsulated drug.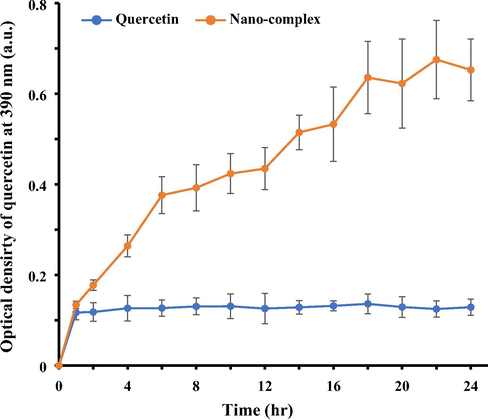
Drug release of quercetin over time after nano-formulation into the quercetin-HSA nano-complex as determined by UV-spectrophotometer at 390 nm. The quercetin release from nano-complex in phosphate buffer (pH 7.4, 10 mM) was higher than that of free quercetin.
3.3 Molecular docking approach
The molecular docking simulation between the HSA and small molecules can be performed to achieve the correlation between drug and receptor for biomedical applications (Eskandari et al., 2018). In this molecular docking, the docking energy was calculated and the binding energy for quercetin and HSA was shown to be −101.23 in the unit of the software score. The contributions of hydrogen bond and van der Waals forces are −75.72 and −25.52 respectively.
Fig. 3A shows the geometry of quercetin's most stable conformer and Fig. 3B displays the quercetin-HSA complex. The ligand with surrounding active site residues within 4 Å is shown in Fig. 3C. Also, the contributions of each residue in binding energy are tabulated in Table 1.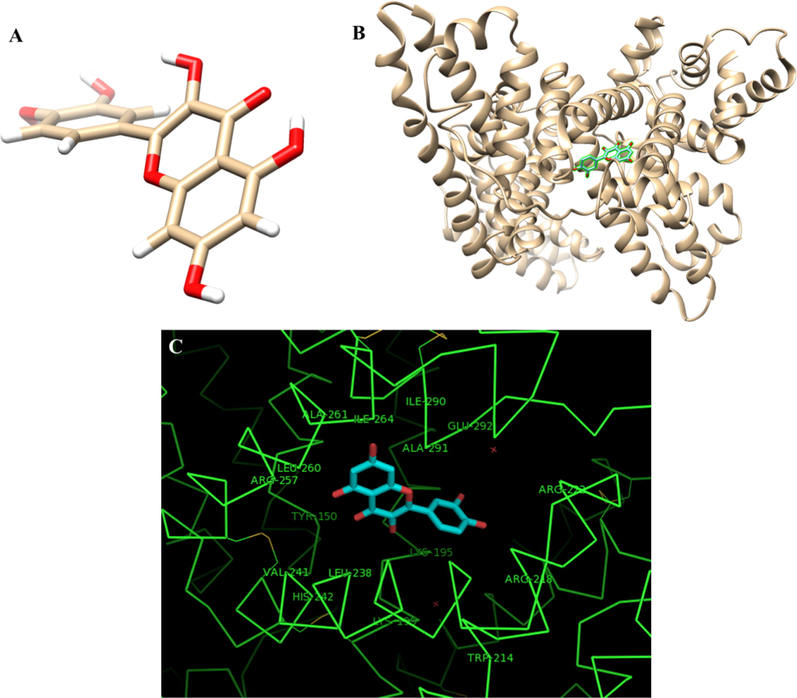
The geometry of quercetin molecule (A), The best docked models of the quercetin with HSA (B), the interacting residues of the HSA with quercetin (C). The docking score (binding energy) was calculated to be −101.23 in the unit of the software score.
Residue interacted
Energy score
H-S Tyr-150
−3.6
H-S Lys-195
−3.5
H-S Lys-199
−3.4
H-S Arg-222
−3.5
H-S His-242
−6.7
H-S Arg-257
−3.5
V-S Tyr-150
−5.7
V-S Lys-199
−5.3
V-S Trp-214
−4.4
V-S Arg-222
−5.4
V-S Leu-238
−7.2
V-S Arg-257
−5.9
V-S Leu-260
−5.1
V-M Ala-291
−7.8
The results showed that the quercetin compound showed a potential interaction with the HSA. In addition, the study of amino acids involved in interactions showed that amino acids such as Ala-29, Leu-238, and His-242 play a key role in the interaction of quercetin with HSA. Examination of the status of the amino acids involved in the interaction with the HSA also showed that the amino acids located in the regions of 150–199 and 222–291 of the HSA contain key residues to establish a potential interaction with quercetin (Table 1).
3.4 CD study
The structural symmetry of biological macromolecules such as a protein, results in a difference in the absorption of rotating pulsed light (Pishkar et al., 2017; Mehrabi et al., 2020). The CD technique helps to study the second structure of the protein by determining the CD spectra in the far UV region (260–190 nm), which is due to the absorption of peptide bonds (Zeinabad et al., 2016). In other words, the percentage of the second structure of protein, including α-helix, β-sheets, β-turns, and random coil structures, is quantified by CD approach.
Far UV-CD spectrum of HSA protein solutions in the absence and presence of different concentrations of quercetin (2–20 µM), are shown in Fig. 4A. The spectrum includes a negative band with two minimum peaks at 208 and 222 nm, representing the α-helix structure of HSA protein. As shown in Fig. 4A, after addition of different concentrations of quercetin, the typical ellipticity changes [θ] of CD signals almost remained unchanged, indicating that the typical secondary structure of protein was preserved. Indeed, the decrease in [θ] indicates the destruction of the α-helix structure, which may be due to the complex formation of the small molecules with HSA polypeptide residues, destroying the hydrogen bonding network and the second protein structure. As, in the case of quercetin interaction with HSA, the [θ] values of HSA molecules were not significantly reduced, it may be indicated that the interaction of quercetin did not induce substantial changes in the secondary structure of HSA. The changes in the content of the secondary structure of the HSA in the presence of different concentrations of quercetin were calculated using CDNN software and were displayed in Table 2 and Fig. 4B. In the CD ranges before and after the addition of the different concentrations of quercetin, the α-helix content is seen to be 62.1% and 58.2%, in the absence and presence of the highest concentration of quercetin (20 µM) respectively, indicating that the quercetin compound has stimulated a slight HSA destabilization.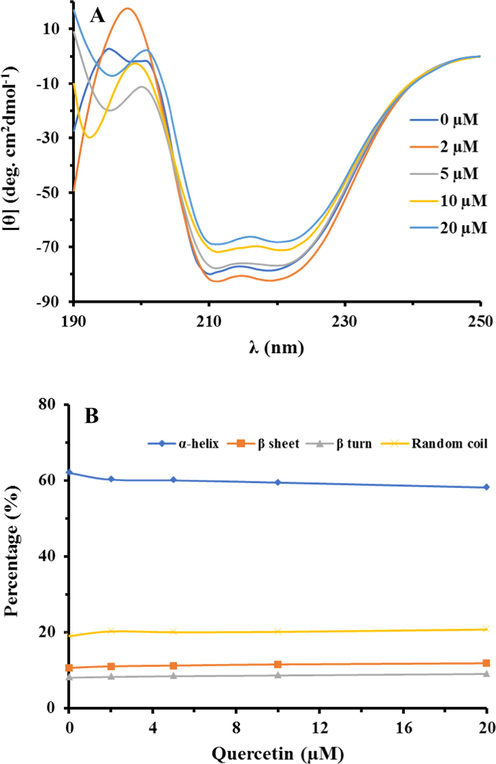
The CD spectra of HSA (A) and the content of the secondary structure of the HSA (B) in the presence of different concentrations of quercetin. This fata indicates that the quercetin stimulates a slight destabilization effect on the HSA structure.
[Quercetin] (µM)
α-helix (%)
β-sheets (%)
β-turns (%)
Random coil (%)
0
62.1
10.7
8.1
19.1
2
60.3
11.1
8.3
20.3
5
60.1
11.3
8.5
20.1
10
59.5
11.6
8.7
20.2
20
58.2
11.9
9.1
20.8
3.5 MTT assay
MTT assay was done to measure the viability of spinal cord neurons in the presence of varying concentrations of quercetin compound. As shown in Fig. 5A, the viability of spinal cord neurons was determined to be 92.87%, 87.24%, 71.21% (*P < 0.05), 39.86% (**P < 0.01), and 29.17% (***P < 0.001) in the presence of 1 µM, 10 µM, 20 µM, 40 µM, and 50 µM of quercetin after 48 hr. Therefore, it was indicated that quercetin can induce a significant cytotoxicity against spinal cord neurons in the concentrations of above 10 µM. Therefore, based on this study, it was determined that 10 µM of quercetin or quercetin-HSA nano-complex can be used as the highest concentration of quercetin which does not trigger any significant cytotoxicity effects against spinal cord neurons. Hence, in the next step, the spinal cord neurons were pretreated for 24 h with 10 µM of quercetin or quercetin-HSA nano-complex, followed by exposure to 200 µM of H2O2 for 24 h, and the cell viability was explored by MTT assay. As shown in Fig. 5B, the cell viability was dramatically (**P < 0.01) decreased in the case of cells exposed to 200 µM of H2O2. However, pretreatment of the spinal cord neurons with 10 µM of quercetin or quercetin-HSA nano-complex resulted in the recovery of cell viability to 61.68% (#P < 0.05) and 79.23% (##P < 0.01), respectively. Also, it was indicated the quercetin-mediated cell recovery was more pronounced ($P < 0. 05) in the case of quercetin-HSA nano-complex than free quercetin. This data indicated that nano-formulation of quercetin has resulted in the enhancement of its potential protective effects against H2O2-induced cell mortality.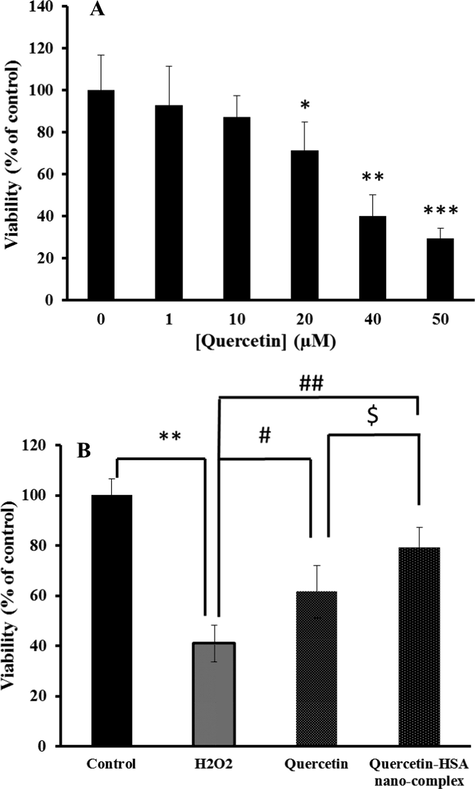
The MTT assay of spinal cord neurons in the presence of varying concentrations of quercetin for 48 hr (A) and the protective effect of 10 µM of quercetin or quercetin-HSA nano-complex on the H2O2-induced mortality in spinal cord neurons (B). *P < 0.05, relative to control cells, #P < 0.05, relative to H2O2-treated cells, $P < 0.05, relative to quercetin-treated cells.
3.6 SOD and CAT activity assay
The activity of SOD and CAT in spinal cord neurons was determined to explore the protective capability of quercetin or quercetin-HSA nano-complex on the H2O2-induced oxidative stress in spinal cord neurons. As shown in Fig. 6A, the SOD activity in control group, H2O2-treatd group, H2O2-induced group pretreated with quercetin, and H2O2-treated group pretreated with quercetin-HSA nano-complex were 39.71 unit/mg protein, 18.87 unit/mg protein, 48.23 unit/mg protein, and 75.19 unit/mg protein, respectively. The statistical analysis showed that both quercetin (##P < 0.01) and quercetin-HSA nano-complex (###P < 0.001) increased the SOD activity relative to H2O2-tread group, however the nano-formulation of quercetin had a more significant ($P < 0.05) effect on CAT activity than free quercetin.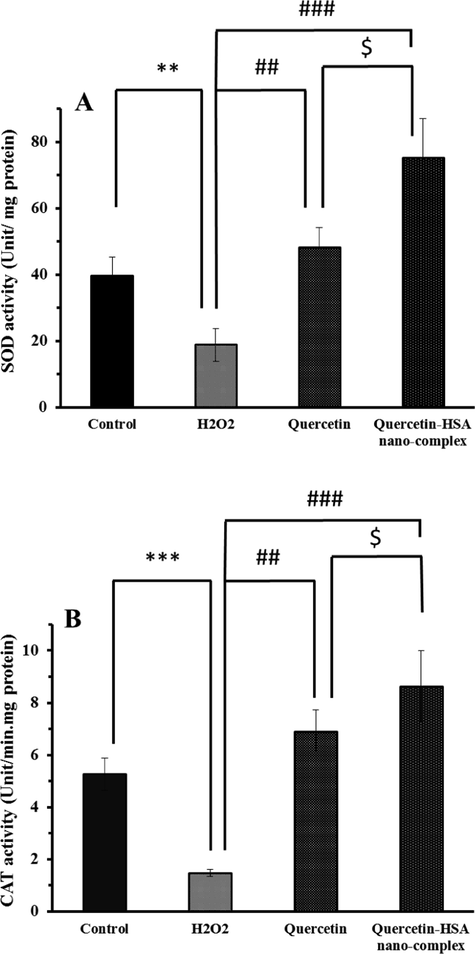
The SOD activity (A) and CAT activity (B) of spinal cord neurons in the presence of different treatments. *P < 0.05, relative to control cells, #P < 0.05, relative to H2O2-treated cells, $P < 0.05, relative to quercetin-treated cells.
The CAT activity in different groups also showed that the quercetin (##P < 0.01) and quercetin-HSA nano-complex (###P < 0.001) had a profound effect on enhancement of CAT activity relative to H2O2-treated group (Fig. 6B). Furthermore, it was observed that quercetin-HSA nano-complex enhanced the CAT activity more significantly (&P-value < 0.05) than free quercetin against H2O2-induced oxidative stress.
3.7 Morphological assay
The morphological assay of spinal cord neurons was carried out by optical microscope to evaluate the protective effect of quercetin or quercetin-HSA nano-complex on the H2O2-induced oxidative stress in spinal cord neurons. Fig. 7 shows the morphological changes of spinal cord neurons as control (Fig. 7A), H2O2-treatd group (Fig. 7B), H2O2-induced group pretreated with quercetin (Fig. 7C), and H2O2-treated group pretreated with quercetin-HSA nano-complex (Fig. 7D). As shown in Fig. 7A, the spinal cord neurons show a typical form of neurons in the cell culture medium, however, the exposure of the cells to 200 µM of H2O2 has led to the deformation of the neurons and segmentation of axons (Fig. 7B). But, Fig. 7C and Fig. 7D showed that after pretreatment of the cells with quercetin or quercetin-HSA nano-complex, respectively, these compounds have induced a protective effect against oxidative stress-stimulated morphological changes and this protective effect was more pronounced in the case of quercetin-HSA nano-complex than free quercetin.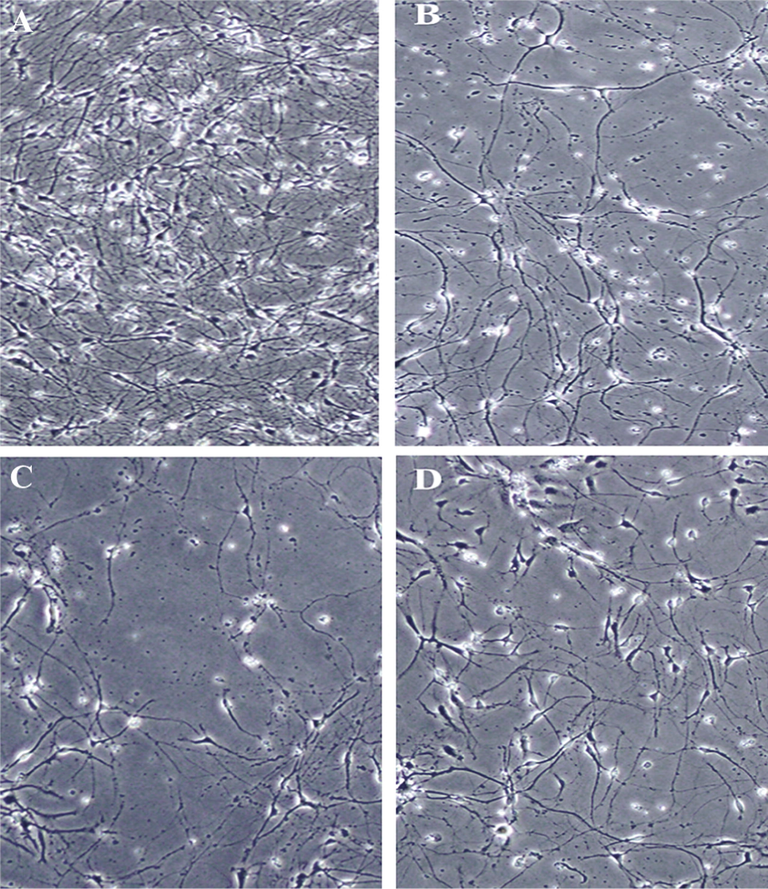
The morphological changes of spinal cord neurons as control (A), H2O2-treatd group (B), H2O2-induced group pretreated with quercetin (C), and H2O2-treated group pretreated with quercetin-HSA nano-complex (D). Quercetin-HSA nano-complex triggers a protective effect against H2O2-induced morphological changes on spinal cord neurons.
4 Discussion
A large number of newly developed drugs/small molecules are insoluble in both organic and liquid intermediates, and with conventional formulation methods, such compounds will show low bioavailability. One promising approach to solving this problem is the production of drug/small molecule nano-complex (for example, quercetin-HSA nano-complex). As a matter of fact, formulation of hydrophobic small molecules into the nanoformulated species prevents them from the undesired effects. Hence, the increase in the dissolution level potentially increases the bioavailability of the small molecules and a higher dose of encapsulated drug could reach the targeted tissue. In this regard, Jithan et al., Uddin et al., Lian et al., and Sohrabi et al., reported the increased solubility of curcumin, antisense oligonucleotide, resveratrol, and silymarin respectively after interaction with HSA. Increased solubility and bioavailability of insoluble or insoluble drugs/small molecules can be investigated with the help of nanotechnology (Birinci et al., 2020; Lian et al., 2019; Sohrabi et al., 2019). In nano-suspension formulations, micron-sized drug/small molecule particles are dispersed and stabilized using an auxiliary liquid phase (Tang et al., 2019). According to studies conducted in this field and reviewing them, preparation of small molecules-HSA nano-complex have been used to re-formulate existing pharmaceutical compounds to eliminate toxicity or increase their effectiveness (Ghayour et al., 2019; Li et al., 2019; Nosrati et al., 2019; Pan et al., 2019). The main advantages of this technology are its general applicability for most drugs/small molecules and its simplicity. The main aims of this study were to prepare quercetin-HSA nano-complex and explore the special characteristics of these materials, their advantages, their potential for use as potential antioxidant agents, as well as the techniques for evaluating the stability of this formulation. Indeed, in vitro results showed that quercetin-HSA nano-complex might show potential preventive effects against oxidative stress induced by H2O2 on spinal cord neurons through the improvement of the solubility and biological activity of quercetin
5 Conclusion
HSA plays an important role in the transport of small molecules as a non-toxic and metabolizable protein in the body. HSA-based nano-complexes have been given special attention as carriers of the hydrophobic small molecules. This is due to the presence of various bonding sites and its ability to form different bonds with a variety of molecules. In this study, the HSA-quercetin nano-complex was prepared and well-characterized by different approaches. It was also indicated that the nano-formulation can increase bioavailability of quercetin as determined by drug release assay. The interaction of HSA and quercetin was assessed by CD, and molecular docking studies and the relevant data were discussed in detail. It was deduced that the hydrophilic forces play an important role in the formation of the HSA-quercetin complex and quercetin did not disturb the secondary structure of HSA. Cellular assays also indicated that both quercetin and quercetin-HSA nano-complex induced a preventive effect on the oxidative stress-induced cell mortality, enzyme deactivation, and morphological changes of spinal cord neurons and these preventive effects were more pronounced in the case of quercetin-HSA nano-complex than free quercetin. In conclusion, this study can provide a promising method for carrying bioactive substances in the appropriate form in the body.
Declaration of Competing Interest
The authors declare no conflict of interest
References
- Towards the functional high-resolution coordination chemistry of blood plasma human serum albumin. J. Inorg. Biochem.. 2019;1(198):110716.
- [Google Scholar]
- Nanoparticles with antioxidant enzymes protect injured spinal cord from neuronal cell apoptosis by attenuating mitochondrial dysfunction. J. Control. Release. 2020;10(317):300-311.
- [Google Scholar]
- Cell death and neurodegeneration. Cold Spring Harbor Perspect. Biol.. 2020;12(2):a036434.
- [Google Scholar]
- Asadi-Samani, M., Farkhad, N.K., Mahmoudian-Sani, M.R., Shirzad, H., 2019. Antioxidants as a Double-Edged Sword in the Treatment of Cancer. InAntioxidants. IntechOpen.
- Reactive oxygen species-responsive drug delivery systems for the treatment of neurodegenerative diseases. Biomaterials. 2019;21:119292.
- [Google Scholar]
- Quercetin in the form of a nano-antioxidant (QTiO2) provides stabilization of quercetin and maximizes its antioxidant capacity in the mouse fibroblast model. Enzyme Microb. Technol.. 2020;6:109559.
- [Google Scholar]
- Antioxidant activity of quercetin in Eudragit-coated liposomes for intestinal delivery. Int. J. Pharm.. 2019;30(565):64-69.
- [Google Scholar]
- Preparation, characterization, and antiproliferative activities of biotin-decorated docetaxel-loaded bovine serum albumin nanoparticles. Brazilian. J. Pharm. Sci.. 2018;54(2)
- [Google Scholar]
- The importance of natural antioxidants in the treatment of spinal cord injury in animal models: an overview. Oxid. Med. Cell. Longevity. 2019;2019
- [Google Scholar]
- Bradykinin and noradrenaline preconditioning influences level of antioxidant enzymes SOD, CuZn-SOD, Mn-SOD and catalase in the white matter of spinal cord in rabbits after ischemia/reperfusion. Eur. J. Histochem.: EJH. 2019;63(4)
- [Google Scholar]
- Quercetin-loaded nanoparticles enhance cytotoxicity and antioxidant activity on C6 glioma cells. Pharm. Dev. Technol.. 2020;19:1.
- [Google Scholar]
- Spectroscopic studies of interaction between CuO nanoparticles and bovine serum albumin. J. Biomol. Struct. Dyn.. 2016;34(9):1962-1968.
- [Google Scholar]
- Biophysical, docking, and cellular studies on the effects of cerium oxide nanoparticles on blood components: in vitro. Int. J. Nanomed.. 2018;13:4575.
- [Google Scholar]
- A review on anti-cancer properties of Quercetin in breast cancer. Life Sci.. 2020;22:117463.
- [Google Scholar]
- Nanoencapsulation of quercetin and curcumin in casein-based delivery systems. Food Hydrocolloids. 2019;1(87):394-403.
- [Google Scholar]
- Portraits of communication in neuronal networks. Nat. Rev. Neurosci.. 2019;20(2):117-127.
- [Google Scholar]
- iGEMDOCK: a graphical environment of enhancing GEMDOCK using pharmacological interactions and post-screening analysis. BMC Bioinf.. 2011;12(S1):S33.
- [Google Scholar]
- HPMA copolymer conjugate with pirarubicin: In vitro and ex vivo stability and drug release study. Int. J. Pharm.. 2018;536(1):108-115.
- [Google Scholar]
- Optimization of phenolic compounds extracting conditions from Ficus racemosa L. fruit using response surface method. J. Food Meas. Charact.. 2019;13(1):312-320.
- [Google Scholar]
- Neuroprotective role of icariin in experimental spinal cord injury via its antioxidant, anti–neuroinflammatory and anti–apoptotic properties. Mol. Med. Rep.. 2019;20(4):3433-3439.
- [Google Scholar]
- Quercetin attenuates toosendanin-induced hepatotoxicity through inducing the Nrf2/GCL/GSH antioxidant signaling pathway. Acta Pharmacol. Sin.. 2019;40(1):75-85.
- [Google Scholar]
- Preparation and characterization of albumin nanoparticles encapsulating curcumin intended for the treatment of breast cancer. Int. J. Pharmaceut. Investigat.. 2011;1(2):119.
- [Google Scholar]
- Nanoformulation s of quercetin and cellulose nanofibers as healthcare supplements with sustained antioxidant activity. Carbohydr. Polym.. 2019;1(207):160-168.
- [Google Scholar]
- Folate-conjugated human serum albumin-encapsulated resveratrol nanoparticles: preparation, characterization, bioavailability and targeting of liver tumors. Artif. Cells Nanomed. Biotechnol.. 2019;4:4.
- [Google Scholar]
- Characterization of xylitol or citric acid: choline chloride: water mixtures: Structure, thermophysical properties, and quercetin solubility. Food Chem.. 2020;15(306):125610.
- [Google Scholar]
- Antioxidants and nanotechnology: promises and limits of potentially disruptive approaches in the treatment of central nervous system diseases. Adv. Healthcare Mater.. 2020;9(3):1901589.
- [Google Scholar]
- Nanoporous iron oxide nanoparticle: hydrothermal fabrication, human serum albumin interaction and potential antibacterial effects. J. Biomol. Struct. Dyn.. 2020;13:1-2.
- [Google Scholar]
- Namasivayam, S., Robin, A.T., 2018. Preparation of nano albumin-flutamide (Nab-flu) conjugate and evaluation of its in vitro drug control release, anticancer activity and genotoxicity. 1, 1–10.
- Multifunctional nanoparticles from albumin for stimuli-responsive efficient dual drug delivery. Bioorg. Chem.. 2019;1(88):102959.
- [Google Scholar]
- Axonal and neuronal degeneration in myelin diseases. Neurosci. Res.. 2019;1(139):48-57.
- [Google Scholar]
- Albumin-modified cationic nanocarriers to potentially create a new platform for drug delivery systems. ACS Appl. Mater. Interfaces. 2019;11(18):16421-16429.
- [Google Scholar]
- Retrograde degenerative signaling mediated by the p75 neurotrophin receptor requires p150glued deacetylation by axonal HDAC1. Dev. Cell. 2018;46(3):376-387.
- [Google Scholar]
- HSYA alleviates secondary neuronal death through attenuating oxidative stress, inflammatory response, and neural apoptosis in SD rat spinal cord compression injury. J. Neuroinflammat.. 2017;14(1):97.
- [Google Scholar]
- Studies on the interaction between nanodiamond and human hemoglobin by surface tension measurement and spectroscopy methods. J. Biomol. Struct. Dyn.. 2017;35(3):603-615.
- [Google Scholar]
- Application of nano/microencapsulated phenolic compounds against cancer. Adv. Colloid Interface Sci.. 2020;4:102153.
- [Google Scholar]
- Rojas, J., Buitrago, A., 2019. Antioxidant activity of phenolic compounds biosynthesized by plants and its relationship with prevention of neurodegenerative diseases. In: Bioactive Compounds, Woodhead Publishing, pp. 3–31.
- Gal-3 is a potential biomarker for spinal cord injury and Gal-3 deficiency attenuates neuroinflammation through ROS/TXNIP/NLRP3 signaling pathway. Biosci. Rep.. 2019;39(12)
- [Google Scholar]
- Production of biological nanoparticles from bovine serum albumin as controlled release carrier for curcumin delivery. Int. J. Biol. Macromol.. 2018;1(115):83-89.
- [Google Scholar]
- Neuroinflammation in traumatic brain injury: A chronic response to an acute injury. Brain circulation.. 2017;3(3):135.
- [Google Scholar]
- Neuroprotective and antioxidant effect of Ginkgo biloba extract against AD and other neurological disorders. Neurotherapeutics.. 2019;1:1-9.
- [Google Scholar]
- Silymarin-albumin nanoplex: Preparation and its potential application as an antioxidant in nervous system in vitro and in vivo. Int. J. Pharm.. 2019;15(572):118824.
- [Google Scholar]
- Mechanism of antioxidant properties of quercetin and quercetin-DNA complex. DNA.. 2020;12(3):5.
- [Google Scholar]
- 2. Antioxidants, its role in preventing free radicals and infectious diseases in human body. Pure Appl. Biol. (PAB). 2019;8(1):380-388.
- [Google Scholar]
- Prospect of Indian herbs as sources of antioxidants in combating oxidative stress. Chem. Biol. Interf.. 2019;9(1)
- [Google Scholar]
- One pot synthesis of water-soluble quercetin derived multifunctional nanoparticles with photothermal and antioxidation capabilities. Colloids Surf., B. 2019;1(183):110429.
- [Google Scholar]
- Functional local proprioceptive feedback circuits initiate and maintain locomotor recovery after spinal cord injury. Cell Rep.. 2019;27(1):71-85.
- [Google Scholar]
- Enhanced bioavailability of orally administered antisense oligonucleotide to nuclear factor kappa B mRNA after microencapsulation with albumin. J. Drug Target.. 2013;21(5):450-457.
- [Google Scholar]
- Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol.. 2007;39(1):44-84.
- [Google Scholar]
- Characterization of polyphenolic compounds in cantaloupe melon by-products. Foods.. 2019;8(6):196.
- [Google Scholar]
- Hypoxia preconditioning protects neuronal cells against traumatic brain injury through stimulation of glucose transport mediated by HIF-1α/GLUTs signaling pathway in rat. Neurosurg. Rev.. 2020;2:1-2.
- [Google Scholar]
- Neurological recovery and antioxidant effects of resveratrol in rats with spinal cord injury: a meta-analysis. Neural Regener. Res.. 2020;15(3):482.
- [Google Scholar]
- Albumin–drug interaction and its clinical implication. Biochim. Biophys. (BBA)-General Subjects. 2013;1830(12):5435-5443.
- [Google Scholar]
- Thermodynamic and conformational changes of protein toward interaction with nanoparticles: a spectroscopic overview. RSC Adv.. 2016;6(107):105903-105919.
- [Google Scholar]
- Age increases reactive oxygen species production in macrophages and potentiates oxidative damage after spinal cord injury. Neurobiol. Aging. 2016;1(47):157-167.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.09.051.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







