Quercetin ameliorates liver fibrosis in Wilson disease and EMT involving suppression of the Hedgehog signaling pathway
⁎Corresponding author. tanglulu618@126.com (Lulu Tang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Liver fibrosis is a fundamental pathological alteration observed in individuals with Wilson disease. Quercetin (Que) has shown potential in inhibiting fibrosis in various diseases. However, the precise mechanism by which Que alleviates liver fibrosis in Wilson disease remains unclear. The present study aimed to investigate the potential role of Que in liver fibrosis of Wilson disease using male Toxic Milk (TX) mice and Cu2+-induced hepatoblastoma cells (HepG2 cells). In TX mice, Analyses using Histopathology, Immunohistochemical staining, Enzyme-linked immunosorbent assay (ELISA), and Western blot, revealed that Que treatment facilitated the efflux of Cu2+ from the liver, improvement of liver function indicators and pathological damage of liver tissue, and significantly inhibited Hedghog (Hh) signaling pathway, leading to significant downregulation of key proteins and genes involved in this pathway, including Gli1, Ptch1, Smo, and Shh. Moreover, Que can reverse epithelial-mesenchymal transition (EMT), and we have also investigated key proteins and genes of EMT and the specific transcription factors controlling E-cadherin expression at the transcriptional level (Slug, Snail, ZEB-1, and Twist) and found that Que can regulatory their expression levels. In Cu2+-induced HepG2 cells, we using Hh Signaling pathway Inhibitor: GDC-0449, found blocking Hh Signaling pathway can reverse the EMT and consistent with in vivo results. Therefore, Que may have the potential to ameliorate liver fibrosis in Wilson disease and EMT by suppressing the Hh signaling pathway.
Keywords
Wilson disease
Liver fibrosis
Quercetin
EMT
Hedgehog signaling pathway
1 Introduction
Wilson disease, also known as hepatolenticular degeneration, is an autosomal recessive disorder of Cu2+ metabolism caused by mutations in the ATP7B gene. The liver plays a central role in maintaining Cu2+ homeostasis in the body. Dysfunction of ATP7B results in reduced Cu2+ excretion, leading to the accumulation of Cu2+ in the liver (Członkowska et al., 2018). Consequently, liver injury emerges as the earliest and most prevalent manifestation of Wilson disease. In response to chronic liver injury, epithelial cells undergo epithelial-mesenchymal transition (EMT) and invade the interhepatic stroma, progressively advancing to liver fibrosis. Liver fibrosis, a fundamental pathological alteration observed in nearly all Wilson disease patients, acts as a pivotal stage in the progression to cirrhosis. Cirrhosis and its associated comorbidities stand as the primary cause of mortality in individuals afflicted with Wilson disease. However, liver fibrosis is reversible, and antifibrotic therapy can arrest or even reverse its progression (Gerosa et al., 2019). Recent studies have unveiled the transformation of intrahepatic hepatocytes, hepatic stellate cells, and bile duct cells into myofibroblasts through EMT, contributing to the development of liver fibrosis (Chen et al., 2020). Some experimental studies have shown that Hedgehog (Hh) signaling pathway activity in non-alcoholic fatty liver disease (NAFLD) patients is positively correlated with the degree of liver injury and plays an important role in the EMT process(Guy et al., 2012; Pratap et al., 2012).
Hh signaling pathway is critical in organogenesis and tissue remodeling and is involved in various acute and chronic liver diseases (Choi et al., 2011; Varjosalo et al., 2006). There is growing evidence based on extensive studies on hepatic stellate cells (HSC) that the Hh pathway may also govern the EMT transformation of certain types of hepatocytes during liver injury (Choi et al., 2009; Michelotti et al., 2013; Xie et al., 2013). Comparable findings have been observed in cholangiocytes and malignant hepatocytes (Choi et al., 2011). Notably, Hh signaling assumes a critical role in liver pathophysiology, and numerous studies have confirmed its activation during EMT (Choi et al., 2009). In the healthy adult liver, Hh signaling remains dormant. However, upon activation during liver injury, it orchestrates the capillarization of hepatic sinusoidal cells, stimulates the activation and proliferation of hematopoietic stem cells, and promotes biliary tract dilation, thereby contributing to liver fibrosis. Aberrant activation of Hh signaling in liver fibrosis further fosters the development of cirrhosis and hepatocellular carcinoma (Omenetti et al., 2011b). Consequently, the inhibition of Hh signaling pathway activation emerges as a promising avenue for the treatment of liver fibrosis.
Liver fibrosis is a fundamental pathological alteration observed in individuals with Wilson disease, and in recent years, reports have confirmed that liver fibrosis is dynamic and reversible (Gerosa et al., 2019). Heavy metal chelators, represented by penicillamine, are the first line of treatment for Wilson disease. Because of their many adverse effects and poor drug safety, their clinical application has been greatly restricted (Weiss et al., 2013). This pressing clinical issue remains a focus of global research. From the perspective of overall diagnosis and treatment, traditional Chinese medicine (TCM) in the treatment of liver fibrosis in Wilson disease can reconstruct the biliary copper excretion pathway in a multi-target, multi-link, and multi-pathway manner, and the composition is natural, with almost no adverse reactions, and is widely used in clinical practice (Tang et al., 2023; Yang et al., 2012). It is worth mentioning that quercetin (Que) is a well-known flavonoid and has strong pharmacological activity and low side effects (Tang et al., 2020). And we found that Que exists in the TCM compounds that is commonly used in the treatment of liver fibrosis in Wilson disease in our hospital, and is an important active ingredient in the TCM compounds (Wei et al., 2021; Yang et al., 2023; Zhao et al., 2022). Interestingly, several studies have proposed that Que exerts its anti-fibrotic effects by inhibiting the Hh signaling pathway in various fibrotic conditions, such as renal fibrosis, pulmonary fibrosis, and myelofibrosis (Liu et al., 2019; Tang et al., 2012; Tibes and Mesa, 2014). Studies have confirmed that the Hh signaling pathway is closely related to EMT in tissue fibrosis (Omenetti et al., 2011a). Based on these findings, we propose the hypothesis that Que may exert antifibrotic effects and reverse EMT in Wilson disease by inhibiting the activation of the Hh signaling pathway.
2 Materials and methods
2.1 Animal models
Male Toxic Milk (TX) mice (strain: C3HeB/FeJAtp7btx-J/J) were obtained from the Jackson Laboratory and housed in a specific pathogen-free (SPF) animal facility at the College of Life Sciences, Anhui Agricultural University. The mice were kept under controlled conditions, including a temperature range of 18–20 °C, alternating light and dark cycles, and 50 %-60 % humidity. They had ad libitum access to food and water. The mice weighed between 20 and 30 g. Que (Lot Number: N1841; purity [HPLC]: 95.00 %) was purchased from APE x BIO (USA). In this study, we randomly assigned 30 six-month-old pure TX mice to five groups: the Wilson group, Que low-dose group (30 mg/kg/day, Que-L), Que medium-dose group (60 mg/kg/day, Que-M), Que high-dose group (90 mg/kg/day, Que-H), and the penicillamine group (0.1 g/kg/day, Pen). The doses of Que were selected based on previous studies (Yang et al., 2019) that reported no adverse effects associated with Que administration. Additionally, six wild-type mice with the same genetic background were included as the Control group. The Control group mice received an equivalent volume of 1 % sodium Carboxymethyl cellulose (CMC-Na) solution daily. Ethical approval for the experiment was obtained from the Ethics Committee of Anhui University of Traditional Chinese Medicine (approval number: AHUCM-mouse-2021011). After 6 weeks of treatment and following a 12-hour fasting period with no access to food or water, all mice were anesthetized, with blood samples collected from the eyes. Their livers were immediately excised, with a portion fixed in 4 % paraformaldehyde for further analysis, while the remaining tissue was stored at −80 °C for subsequent biochemical analysis.
2.2 Cell culture and grouping
The international hepatocyte models for Wilson disease in vitro have been constructed using HepG2 cells (Chandhok et al., 2016; Strand et al., 1998). The HepG2 cells used in this study were obtained from Wuhan Pronosai (Wuhan, China, item no. CL-0103). The cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10 % fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were maintained at 37 °C with 5 % CO2. CuCl2·2H2O (Macklin, Shanghai, China) was used for the in vitro experiments. The experiments were divided into five groups: Control group, Model group (treated with 150 μmol/L CuCl2, Cu2+), Que low-dose group (50 μmol/L Que + final concentration of 150 μmol/L CuCl2, Cu2++Que-50), Que medium dose group (75 μmol/L Que + final concentration of 150 μmol/L CuCl2, Cu2++Que-75), and Que high dose group (100 μmol/L Que + final concentration of 150 μmol/L CuCl2, Cu2++Que-100). Hh Signaling Inhibitor: GDC-0449 (Vismodegib, APE x BIO, A3021) was used in this study.
2.3 Enzyme-linked immunosorbent assay (ELISA)
ELISA kits were used to measure the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), total bilirubin (TBIL), total protein (TP), and hydroxyproline (HYP). A copper ion detection kit (Nanjing Jiancheng Institute of Biological Engineering, China) was used to detect the levels of copper ions.
2.4 Reverse-transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from liver tissue using a tissue homogenizer and Trizol reagent (R0016, Beyotime Biotechnology, China). The RNA-Quick Purification Kit (ES Science, Shanghai, China) was used for RNA purification. Reverse transcription was performed using a reverse transcription kit (Enzy Artisan, Shanghai, China) to generate cDNA. Quantification of mRNA levels was carried out using a PerfectStart Green qPCR SuperMix (EnzyArtisan, Shanghai, China). RT-qPCR amplification was performed in a LightCycler 480 real-time PCR system. Glyceraldehyde phosphate dehydrogenase (GAPDH) was used as an internal reference gene. The relative expression levels of the target genes were calculated using the 2-ΔΔCt method.
2.5 Histopathology
Fresh liver tissue was fixed in 4 % paraformaldehyde for 48 h. After fixation, the samples underwent a dehydration process and were then embedded in wax blocks. Sections were stained with Hematoxylin-eosin staining (HE), Masson staining, and Sirius scarlet staining to observe the degree of inflammation and liver fibrosis. Photographs of the stained liver tissue sections were captured using a microscope. Masson stain, and Sirius scarlet staining were quantified using by Image J 1.8.0 software.
2.6 Immunofluorescence
HepG2 cells were seeded in well plates containing cell crawls, grouped accordingly, and subsequently treated with Cu2+ and Que. Following the treatment, immunofluorescence staining was performed. The cells were first fixed and permeabilized, and then a primary antibody targeting E-cadherin / Vimentin was applied and left to incubate overnight at 4 °C in a wet box. The cells were then exposed to a secondary antibody at room temperature for 2 h, while being protected from light. To ensure proper visualization, the cell crawls were rinsed three times with phosphate-buffered saline (PBS) and subsequently restained with 4′,6-diamidino-2-phenylindole (DAPI). The resulting fluorescence signal was visualized using a Nikon Eclipse C1 fluorescence microscope (Tokyo, Japan), equipped to detect and capture the DAPI fluorescence signal.
2.7 Immunohistochemical staining
Liver tissue sections, approximately 4 μm thick, were deparaffinized in xylene and then rehydrated using graded ethanol. The sections were stained with specific antibodies against Alpha-smooth muscle actin (α-SMA), Collagen I, E-cadherin, and Vimentin, following a standardized staining protocol. To enhance the staining process, antigen retrieval was performed using high-pH ethylenediamine tetraacetic acid (EDTA) (pH 9.0), followed by air injection. Subsequently, the sections were subjected to a 10-minute treatment with 3 % hydrogen peroxide (H2O2) at 37 °C. The α-SMA, Collagen I, E-cadherin, and Vimentin antibodies were then applied to the sections and incubated for 60 min at 37 °C. Afterward, the sections were incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody for 30 min at 37 °C. To visualize the staining, an Enhanced DAB Chromogenic Kit was utilized, followed by a 10-minute incubation at 37 °C for tablet refixation and sealing. Throughout the procedure, each step was followed by three 5-minute washes with PBS solution containing Tween 20. The integrated optical density (IOD) values of immunoreactive positive cells were analyzed using image J 1.8.0 software.
2.8 Western blot analysis
Samples of liver tissue were collected and stored at −80 °C. For HepG2 cells, treat the grouping with Cu2+ and Que in a six-well plate and continue to culture for 12 h in a 37 °C, 5 % CO2 incubator. Total protein was extracted from the liver tissue or HepG2 cells using a protein lysate, and the protein concentration was determined using the bicinchoninic acid (BCA) Protein Analysis Kit (P0010S, Beyoncé Institute of Biotechnology). The extracted proteins were separated by 8 % sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) / 10 % SDS-PAGE gel electrophoresis for 90 min. Subsequently, the proteins were transferred to polyvinylidene fluoride (PVDF) membranes using a wet transfer method. The membranes were then blocked with 5 % milk for 90 min at room temperature. Primary antibodies against E-cadherin (1447S, Cell Signaling, USA), N-cadherin (13116 T, Cell Signaling, USA), Vimentin (5741 T, Cell Signaling, USA), Desmin (5332 T, Cell Signaling, USA), Collagen I (72026 T, Cell Signaling, USA), α-SMA (19245 T. Cell Signaling, USA), Smoothened (SMO) (92981S, Cell Signaling, USA), Patched1 (Ptch1) (2468 T, Cell Signaling, USA), GLI1 (2643S, Cell Signaling, USA), Sonic hedgehog (Shh) (2207 T. Cell Signaling, USA), Snail (bs-1371R, Bioss), Slug (bs-1382R, Bioss), ZEB1 (ab203829, Abcam), Twist (bs-2441R, Bioss), and β-actin (AF7018, Affinity, USA) were added to the membranes. After overnight incubation, the membranes were triply washed. HRP-labeled goat anti-rabbit IgG (H + L) antibody (A0423, Bain Marie Biotech) or HRP-labeled goat anti-mouse IgG (H + L) antibody (A1050, Boster) was added and incubated for 2 h at room temperature. Finally, the membranes were treated with an ultra-sensitive electrochemiluminescence (ECL) solution (D046-1, Bridgen, China), placed in a chemiluminescent analyzer, and the resulting bands were analyzed using Chem Studio 515 (Analytik Jena AG, Germany). The grayscale values of the bands were obtained and further analyzed using Image J 1.8.0 software for statistical analysis.
2.9 Statistical analysis
All data were presented as mean ± standard deviation (SD), and statistical analysis was performed using GraphPad Prism 9.4.0 statistical software. Group comparisons were assessed using one-way analysis of variance (ANOVA). A p-value < 0.05 was considered statistically significant.
3 Results
3.1 Effect of Que on liver damage in TX mice
The chemical structure of Que is shown in Fig. 1A. TX mice were randomly assigned to five groups (Wilson group, Que-L, Que-M, Que-H, and Pen group), with wild-type mice of the same genetic background serving as controls. After six weeks of treatment and a 12-hour fasting period, the animals were anesthetized, and blood samples were collected from the eyes. The livers were immediately excised for further analysis (Fig. 1B). The Cu2+ content in liver tissues was measured, and the results showed that all three doses of Que significantly facilitated the excretion of Cu2+ from the liver in TX mice compared to the Wilson group (Fig. 1C). Serum biochemical index results demonstrated that the Wilson group exhibited significantly increased levels of AST, ALT and TBIL, and significantly decreased levels of ALB and TP compared to the Control group (Fig. 1D). In contrast, the Que-M and Que-H groups showed significantly lower levels of AST, ALT and TBIL, and significantly higher levels of ALB and TP compared to the Wilson group. Histopathological examination of liver tissues using HE staining (Fig. 2A) revealed that the Control group exhibited a normal morphological structure of liver lobules, while the Wilson group displayed extensive hepatocellular edema, disorganized hepatic cords, and narrowed and occluded sinuses. However, after intervention with Que, the degree of liver pathology significantly improved compared to the Wilson group. Among them, the Que-H group had the most obvious improvement in hepatocellular edema, and the structure of hepatic cord and hepatic sinusoids was clear. These findings suggest that Que has the potential to improve liver injury in TX mice with Wilson disease.
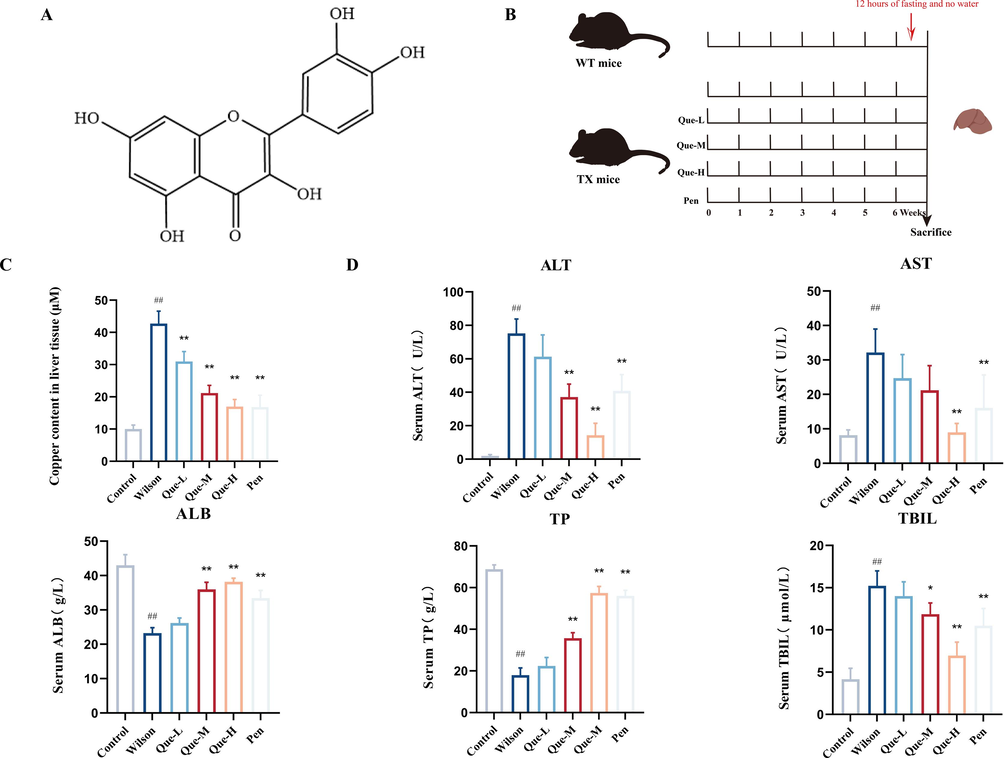
- Effect of Que on the liver of TX mice. (A) Chemical structure of Que (B) Que treatment for TX mice method (C) Determination of copper concentration in liver tissue, n = 6. (D) Serum levels of ALT, AST, ALB, TP, and TBIL, n = 6. #P < 0.05, ##P < 0.01, vs. Control, *P < 0.05, **P < 0.01, vs. Wilson. Data are expressed as mean ± SD.
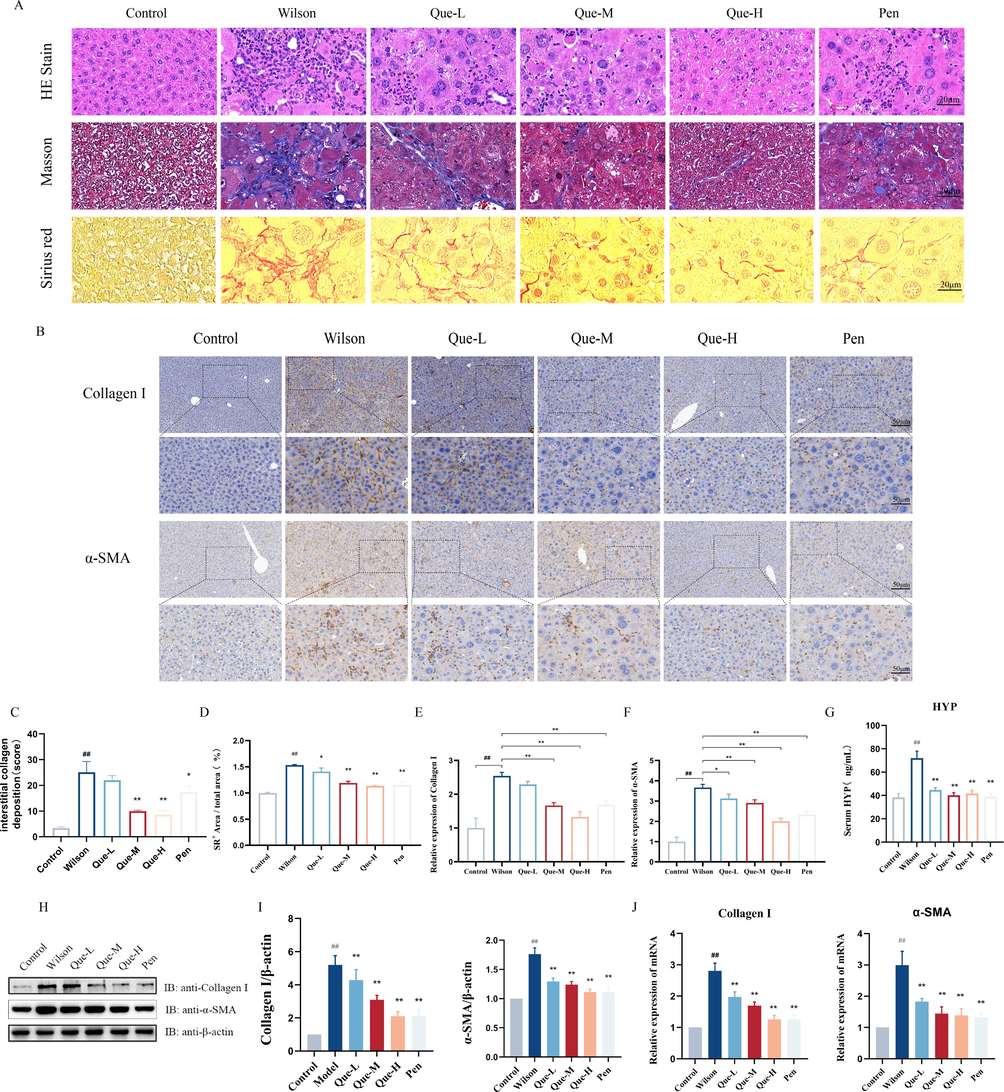
- Effect of Que on liver fibrosis in TX mice. (A) Liver HE staining (magnification × 400, scale bar = 20 μm); using Masson staining (magnification × 400, scale bar = 20 μm); using Sirius scarlet staining (magnification × 400, scale bar = 20 μm). (B) Immunohistochemical staining of liver tissue for Collagen I and α-SMA (magnification × 200, scale bar = 50 μm). (C-D) Statistical analysis of the extent of collagen deposition and morphometric quantification of the SR-positive area in the liver tissues of each group. n = 3. (E-F) The ratio of Collagen I, α-SMA positive cells / total cells in liver tissues was determined, n = 3. (G) Serum HYP level, n = 6. (H-I) Quantification of Collagen I and α-SMA in liver tissues. The protein expression of Collagen I and α-SMA. Relative protein levels were normalized by β-actin, n = 3. (J) The mRNA expression of Collagen I and α-SMA in liver tissue. n = 6. #P < 0.05, ##P < 0.01, vs. Control, *P < 0.05, **P < 0.01, vs. Wilson. Data are expressed as mean ± SD.
3.2 Effect of Que on liver fibrosis in TX mice
The liver tissues of the Wilson group exhibited a significant increase in fibrous hyperplasia compared to the control group, as revealed by Masson staining and Sirius scarlet staining (Fig. 2A). However, after Que intervention, both the Que-M and Que-H groups showed significant improvements in collagen deposition and the Sirius Red (SR)-positive area in the liver tissues. Among them, the Que-H group exhibited the most effective reduction in liver fibrosis (Fig. 2C-D). Immunohistochemical analysis (Fig. 2B) demonstrated that the expression of α-SMA and Collagen I, which are markers of activated fibroblasts involved in collagen synthesis and liver fibrosis development, was minimal in the control group. In contrast, the liver tissues of the Wilson group exhibited a substantial increase in α-SMA and Collagen I-positive cells. However, Que treatment significantly reduced the number of α-SMA and Collagen I-positive cells in the liver tissues (Fig. 2E-F). Additionally, Que treatment led to a significant decrease in the protein and mRNA levels of Collagen I and α-SMA in the liver tissues of TX mice (Fig. 2H-J). HYP is a major component of collagen tissue that indicates the degree of fibrosis. The ELISA results (Fig. 2G) demonstrated that Que treatment significantly decreased the HYP content in the serum of TX mice, further confirming its inhibitory effect on α-SMA and Collagen I activation, and ability to reduce liver fibrosis in TX mice.
3.3 Effects of Que on the EMT in the liver tissues of TX mice
EMT plays a vital role in the generation of myofibroblasts during liver fibrosis. Our study observed that the livers of TX mice exhibited an increase in mesenchymal markers N-cadherin and Vimentin, and a decrease in epithelial markers E-cadherin and Desmin. However, following treatment with Que-M and Que -H, there was a significant decrease in the protein and mRNA expression levels of N-cadherin and Vimentin, as well as a significant increase in the protein and mRNA expression levels of E-cadherin and Desmin (Fig. 3C-E). Immunohistochemistry results further confirmed that Que treatment significantly upregulated E-cadherin but downregulated the protein expression of Vimentin (Fig. 3A-B). We also investigated the protein and mRNA expression levels of four transcription factors (including Slug, Snail, ZEB-1, and Twist) regulating E-cadherin expression at the transcriptional level. Results demonstrated that Que-M and Que-H treatment groups significantly suppressed the high expression of these four transcription factors in the liver specimens of TX mice (Fig. 3C-E). The Que-H treatment group showed outcomes comparable to those of the positive drug control group (Pen). These findings suggest that Que attenuates EMT in the liver tissue of TX mice by inhibiting these four transcription factors.
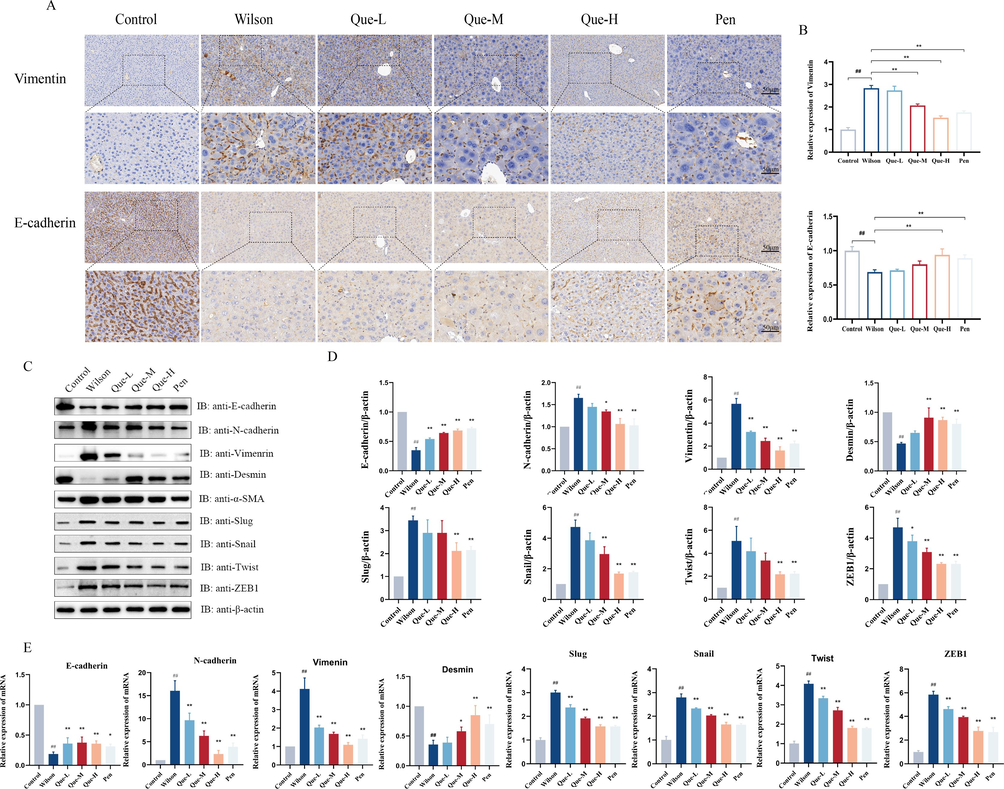
- Effects of Que on the EMT in the liver tissues of TX mice. (A) Immunohistochemical staining for Vimentin, E-cadherin expression, (magnification × 200, Scale bar = 50 μm). (B) the ratio of Vimentin, E-cadherin positive cells / total cells was determined in the liver tissue, n = 3. (C- D) Protein expression of EMT signaling pathway, E-cadherin, N-cadherin, Vimentin, Desmin, Slug, Snail, Twist, ZEB1, and relative protein levels were normalized by β-actin, n = 3 (E) The mRNA expression of E-cadherin, N-cadherin, Vimentin, Desmin, Slug, Twist, ZEB1, Snail, Twist, ZEB1, n = 6. #P < 0.05, ##P < 0.01, vs. Control, *P < 0.05, **P < 0.01, vs. Wilson. Data are expressed as mean ± SD.
3.4 Que suppressed the activation of Hh signaling pathway in TX mice
In Wilson disease, Cu2+ deposition elicits proliferation of hepatic tissue cells through paracrine signaling loops involving the Hh signaling pathway and other proliferation-related signaling pathways. Accordingly, our investigation evaluated the protein and mRNA expression levels of key molecules within the Hh signaling pathway in the liver tissue of TX mice. As depicted in Fig. 4A-C, Gli1, Ptch1, Shh, and Smo expression exhibited an upregulation in the liver tissue of TX mice. Following treatment with Que, a notable reduction in the expression of Gli1, Ptch1, Shh, and Smo was observed compared to the Wilson group. The Que-H group demonstrated outcomes similar to those of the positive drug control group (Pen). These results provided evidence that Hh signaling pathway activation may occur due to Cu2+ deposition within the liver and foster liver fibrosis. Previous investigations have also shown that inhibition of Hh signaling pathway can mitigate fibrosis progression. Our study revealed that Que treatment effectively inhibited Hh signaling pathway activation, suggesting its anti-fibrotic effect arises from its ability to impede excessive Hh signaling pathway activation.
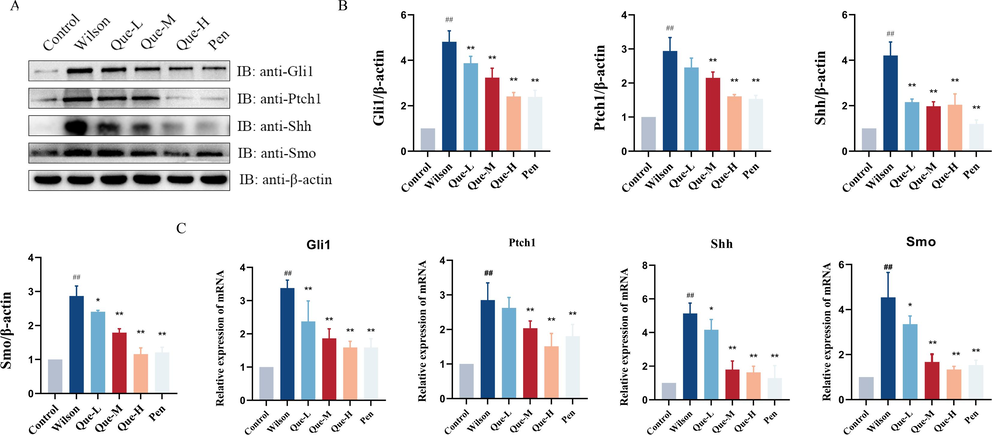
- (A-B) Western blot analysis showing the expression levels of Gli1, Ptch1, Shh and Smo proteins in different groups. Relative protein levels were normalized by β-actin, n = 3. (C) The mRNA expression levels of Gli1, Ptch1, Shh, and Smo in liver tissues, n = 6. #P < 0.05, ##P < 0.01, vs. Control, *P < 0.05, **P < 0.01, vs. Wilson. Data are expressed as mean ± SD.
3.5 Effects of Que on cellular copper in Cu2+-induced HepG2 Cell models
HepG2 cells, which are commonly used as a cellular model for Wilson disease, were utilized in this study. The cells were seeded in 96-well plates at a density of 5000 cells per well during the logarithmic growth stage. They were then divided into six groups, with the CuCl2 concentrations ranging from 50 μmol/L to 300 μmol/L, and incubated for 8, 12, and 24 h. The incubation was carried out in a 37 °C environment with 5 % CO2. Based on the IC50 value (Fig. 5A), it can be seen that when CuCl2 was applied to the HepG2 cells for 12 h, its IC50 was 149.9 μmol/L, and the cell wall adhered well and the state was relatively stable. Therefore, HepG2 cells were applied to a concentration of 150 μmol/L CuCl2 for 12 h to replicate the Wilson disease model. For the Que treatment groups, concentrations of 25 μmol/L to 150 μmol/L were added to the cells 2 h prior to the addition of Cu2+, and the final concentration of Cu2+ was 150 μmol/L. The subsequent steps followed the aforementioned protocol. The IC50 value was calculated, and the optimal concentration of Que was determined to be 75 μmol/L (Fig. 5B). The results demonstrated (Fig. 5C) that Que significantly reduced the Cu2+ content in Cu2+-induced HepG2 cells. Additionally, Que exhibited a dose-dependent ability to release intracellularly accumulated Cu2+ and promote its excretion from the cells.
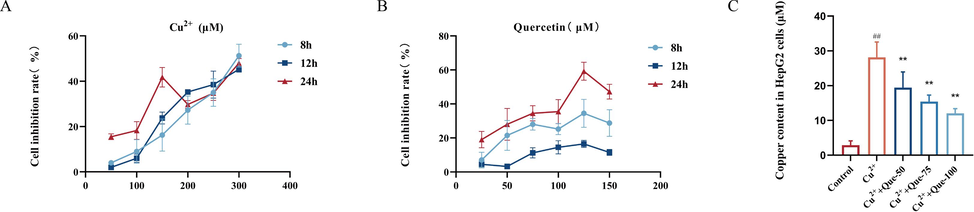
- Effects of Que on cellular copper in Cu2+-induced HepG2 cells model. (A) CCK8 assay screening for optimal Cu2+-induced concentration. (B) CCK8 assay screening for optimal Que concentration. (C) Effect of Que on Cu2+ content in Cu2+-induced HepG2 cells. #P < 0.05, ##P < 0.01, vs. Control, *P < 0.05, **P < 0.01, vs. Model. Data are expressed as mean ± SD.
3.6 Effects of Que on the EMT in the Cu2+-induced HepG2 Cell models
To further validate the impact of Que on liver fibrosis, we investigated its effect on Cu2+-induced EMT in HepG2 cells. As shown in Fig. 6A-C, Cu2+ induction resulted in elevated expression levels of α-SMA, Vimentin, and N-cadherin, while causing a decrease in the expression levels of E-cadherin and Desmin. Consistent with our in vivo findings, the expression levels of four transcription factors (Slug, ZEB-1, Snail, and Twist) were significantly upregulated. Importantly, compared to the model group, treatment with Que led to a dose-dependent decrease in the expression of α-SMA, Vimentin, N-cadherin, Slug, ZEB-1, Snail, and Twist at both the mRNA and protein levels, while concurrently enhancing the expression of E-cadherin and Desmin at the protein and mRNA levels. Immunofluorescence analysis further confirmed that Que significantly upregulated E-cadherin (Fig. 6D-E) and downregulated Vimentin (Fig. 6F-G) protein expression. Additionally, Que dose-dependently attenuated or even reversed the Cu2+-induced alterations in EMT in HepG2 cells. Notably, a high dose of Que exhibited a more potent inhibitory effect on EMT and the associated transcription factors in a dose-dependent manner.
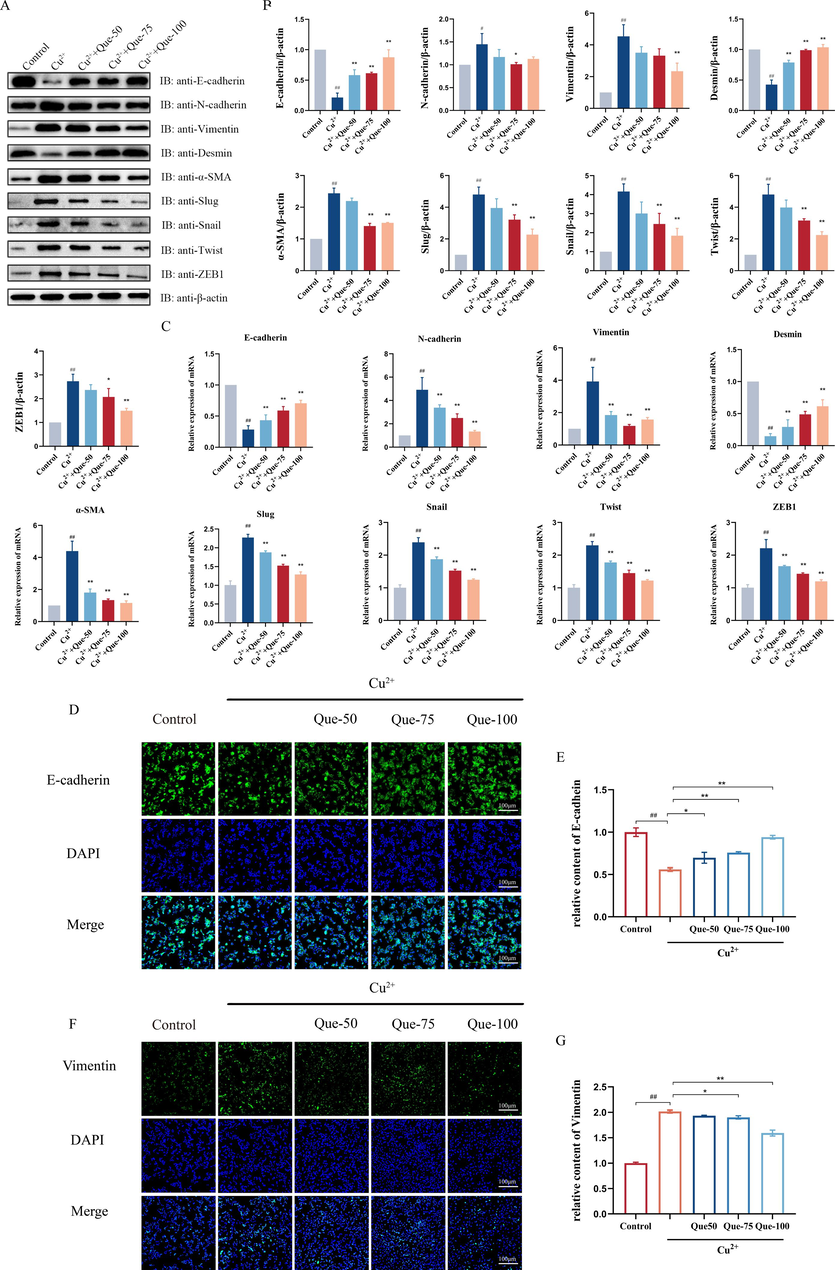
- Effects of Que on the EMT in the Cu2+-induced HepG2 Cells model. (A-B) Protein expressions of E- cadherin, N-cadherin, Desmin, Vimentin, α-SMA, Slug, Snail, Twist, and ZEB1 in Cu2+-induced HepG2 cells. Relative protein levels were normalized by β-actin, n = 3. (C) The mRNA expression of E-cadherin, N-cadherin, Desmin, Vimentin, α-SMA, Slug, Snail, Twist, and ZEB1 in Cu2+-induced HepG2 cells, n = 6. (D) Immunofluorescence of E-cadherin in Cu2+-induced HepG2 cells (magnification × 100, scale bar = 100 μm). (E) Relative fluorescence intensity of E-cadherin. n = 3. (F) Immunofluorescence of Vimentin in Cu2+-induced HepG2 cells (magnification × 100, scale bar = 100 μm). (G) Relative immunofluorescence intensity of Vimentin, n = 3. #P < 0.05, ##P < 0.01, vs. Control, *P < 0.05, **P < 0.01, vs. Model. Data are expressed as mean ± SD.
3.7 Effect of Que on Hh signaling pathway in Cu2+-induced HepG2 Cell models
Aligned with previous research, our investigation aimed to evaluate the expression levels of Cu2+-induced Gli1, Ptch1, Shh, and Smo proteins and mRNA in HepG2 cells in vitro, with a specific focus on the potential role of the Hh signaling pathway in the anti-fibrotic effect of Que. As depicted in Fig. 7, Cu2+-induced HepG2 cells demonstrated activation of the Hh signaling pathway. Importantly, treatment with Que was associated with reduced protein and mRNA expression of Gli1, Ptch1, Shh, and Smo (Fig. 7A-C). To further explore this mechanism, we added an inhibitor of the Hh signaling pathway: GDC-0449, which has been extensively studied for its efficacy in inhibiting the Hh signaling pathway and blocking Hh ligand activity. Our results revealed that compared to the model group, the addition of GDC-0449 significantly increased the expression of E-cadherin and Desmin, two proteins related to EMT, while significantly decreasing the expression of Vimentin and α-SMA. Moreover, both the Que group and the GDC-0449 group exhibited significant reductions in the expression of Hh signaling pathway-related proteins including Gli1, Ptch1, Shh, and Smo. Notably, there was no statistically significant difference between the Que group and the GDC-0449 group, and the results of the Que group closely resembled those of the GDC-0449 group (Fig. 7D-F). Based on these in vitro findings, we have reaffirmed that the inhibition of Hh activity may represent a critical molecular mechanism underlying the anti-fibrotic effect of Que in liver tissue.
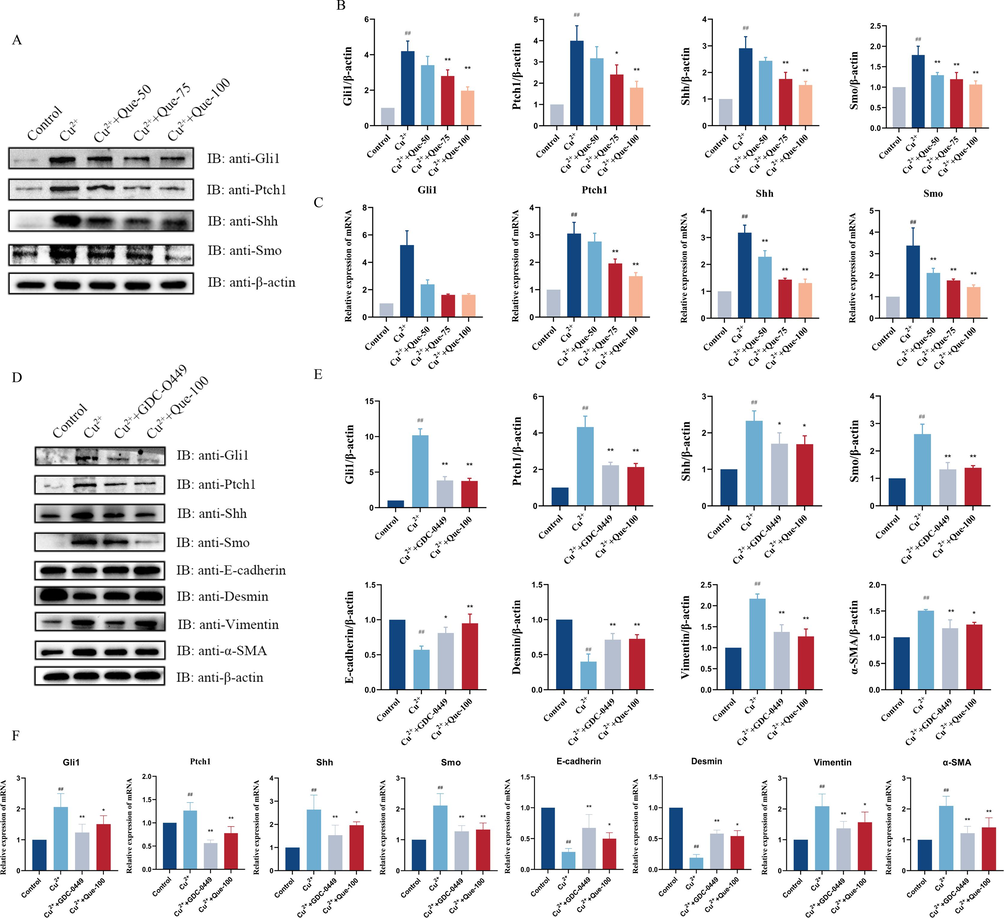
- Effect of Que on Hh Signaling in Cu2+-induced HepG2 Cell models. (A - B) WB test showing the protein levels of Gli1, Ptch1, Shh, and Smo in Cu2+-induced HepG2 cells, and relative protein levels were normalized by β-actin, n = 3. (C) RT – qPCR showing the mRNA levels of Gli1, Ptch1, Shh, and Smo in Cu2+-induced HepG2 cells, n = 6. Adding Hh inhibitor. GDC-0449. (D-E) The protein expressions of Gli1, Ptch1, Shh, Smo, E-cadherin, Vimentin, Desmin, and α-SMA. Relative protein levels were normalized by β-actin, n = 3. (F) Gli1, Ptch1, Shh, Smo, E-cadherin, Vimentin, Desmin and α-SMA mRNA expression, n = 6. #P < 0.05, ##P < 0.01, vs Control. P < 0.05, **P < 0.01, vs. Model. Data are expressed as mean ± SD.
4 Discussion
Wilson disease is an autosomal recessive monogenic condition characterized by the accumulation of Cu2+ in the body. The liver plays a central role in Cu2+ metabolism, and impaired Cu2+ metabolism leads to liver fibrosis, which is the most common hepatic pathology in Wilson disease (Chen et al., 2020; Członkowska et al., 2018). Recent studies have identified EMT as a potential mechanism underlying liver fibrosis (Giannelli et al., 2016). During EMT, epithelial cells within the liver undergo a phenotypic transition, assuming fibroblastic characteristics as they enter the hepatic mesenchyme. This process involves the transformation of various cell types, including quiescent HSCs, hepatocytes, bile duct epithelial cells, and sinusoidal endothelial cells, from an epithelial phenotype to a myofibroblast-like phenotype with mesenchymal characteristics, contributing to the progression of liver fibrosis (Choi and Diehl, 2009).
Despite significant progress in developing treatments for Wilson disease in recent years, medication continues to be controversial. Heavy metal chelators, such as penicillamine and sodium dimercaptopropanesulfonate (DMPS), can reduce the damage caused by Cu2+ accumulation by combining excess Cu2+ in the complex and excreting them in the urine. However, in the process of clinical treatment, copper repellents will cause irreversible aggravation of the condition of some patients, and their use is limited by significant side effects, poor drug safety and high incidence of adverse reactions (Chandhok et al., 2016; Dong and Wu, 2012). Herbal medicines have gained recognition for their unique advantages and efficacy in delaying the progression of this disease through a multi-target, multi-link, and multi-pathway approach (Chen et al., 2022). Previous evidence demonstrated that various herbal monomers and natural food derivatives derived from traditional herbal medicine may hold promise in the treatment and prevention of liver fibrosis (Li et al., 2022). Gangouling (GDL) and Gandou Fumu decoction (GDFMD) has been commended to treat liver damage in the “Guidelines for Diagnosis and Treatment of Wilson Disease with Integrated Traditional and Western Medicine” in China (2023), and are commonly used in the Department of Encephalopathy, the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, and we have also demonstrated its efficacy through a large number of clinical studies(He et al., 2020; Tang et al., 2022) and animal experiments (Cheng et al., 2023; Tang et al., 2023). Studies have shown that these TCM compounds are able to remove Cu2+ accumulated in the body through the renal metabolic pathway and reconstruct the biliary tract to remove Cu2+ from tissues (Yang et al., 2012). At the same time, some studies have used modern network pharmacology studies to confirm that GDL and GDFMD contain active ingredients such as Que and curcumin (Yang et al., 2023; Zhao et al., 2022), and RNA-sequencing (RNA-Seq) have been used to explore the mechanism of action of TCM in Wilson disease-related liver fibrosis(Wei et al., 2021). Their findings suggest that the active ingredient Que in these two TCM compounds has an anti-Wilson disease liver fibrosis effect. In addition, the previous articles and literature searches published by the research group also proved that the above-mentioned TCM compounds have anti-liver fibrotic effects on Wilson disease through signaling pathways such as Wnt/β-catenin signaling pathway (Cheng et al., 2023), JNK signaling pathway (Yang et al., 2022), PI3K/AKT/mTOR signaling pathway (Tang et al., 2023) and other signaling pathways. Que is a flavonoid with a variety of biological properties, including anti-tumor, antioxidant, anti-inflammatory and other functions, and has a good safety and bioavailability (Tang et al., 2020). Extensive data indicate the effectiveness of Que in ameliorating liver pathology (Zhao et al., 2021). Through systematic review and biological mechanism evaluation, X C Guo et al. (Guo et al., 2022) found that Que can significantly improve liver function indicators such as ALT and AST, and its therapeutic mechanism is related to multiple pathways involving anti-inflammatory and antioxidant activity and lipid accumulation, and Que may be a promising drug for the treatment of liver fibrosis. It suggests that Que holds potential as a promising drug for the treatment of liver fibrosis.
The study was aimed to investigate the effect of Que on liver fibrosis in Wilson disease using the TX mice model, which is an ideal animal model for this condition. In the in vivo experiments, elevated Cu2+ content in the liver and increased expression of α-SMA (a protein characteristic of myofibroblasts), Collagen I, and HYP (a component of collagen tissue) were observed in TX mice, indicating the presence of liver fibrosis and EMT. However, after Que treatment, an increase in Cu2+ excretion in the liver of TX mice was observed, along with significant improvement in liver function and reduction in liver fibrosis. These findings were consistent with the results of previous studies, suggesting that Que enhances Cu2+ efflux in the liver of TX mice, aligning with the mechanism of Wilson disease. In the in vitro experiments using Cu2+-induced HepG2 cells as a model for Wilson disease hepatocyte injury, elevated cellular Cu2+ content and increased α-SMA expression were observed. However, Que treatment reduced Cu2+ content and α-SMA expression in the cells. Additionally, Que suppressed the conversion of epithelial to mesenchymal cell phenotype both in vivo and in vitro, as evidenced by increased expression of E-cadherin and Desmin, as well as decreased expression of N-cadherin and Vimentin. The study also examined certain transcription factors (including slug, snail, ZEB-1, and twist) that control E-cadherin expression at the transcriptional level and found that their expression was obviously reduced both in vivo and in vitro following Que treatment (Figs. 3 and 6). Collectively, these results demonstrated that Que could effectively reverse EMT in liver fibrosis associated with Wilson disease.
Further investigation is necessary for exploring the mechanism and molecular pathway of Que's action on liver fibrosis, including its effect on the Hh signaling pathway. Previous studies have suggested that increased expression of the Hh signaling pathway may contribute to initiating the fibrosis in various tissues, and that the activation of Hh signaling pathway may cause the transformation of epithelial cells (Choi et al., 2009). Hh signaling pathway promotes myofibroblast activation as well as EMT by regulating the expression of fibrotic genes, leading to enhanced matrix accumulation and fibrotic scar formation (Grzelak et al., 2014; Guy et al., 2012). Inhibition of Hh signaling pathway has been shown to block the initiation of EMT and reverse tissue fibrosis (Gao et al., 2018). Aslam et al. (Aslam et al., 2022) found that Que could improve liver fibrosis by antagonizing the Hh signaling pathway and proposed it as a potential therapeutic target for liver fibrosis treatment. In the present study, both in vivo and in vitro experiments revealed the activation of the Hh signaling pathway. Elevated mRNA and protein expression levels of Hh ligand Shh, intranuclear transcription factor Gli1, transmembrane receptor Smo, and co-receptor Ptch1 were observed. However, treatment with Que inhibited the activation of the Hh signaling pathway, leading to significant downregulation of key proteins and genes involved in the pathway, including Gli1, Ptch1, Smo, and Shh. The study further investigated the effects of adding an inhibitor of Hh signaling pathway (GDC-0449) in Cu2+-induced HepG2 cells, and the results showed that the expression of EMT-related proteins, such as E-cadherin and Desmin, was significantly increased, while the expression of α-SMA and Vimentin was significantly decreased, and the Hh signaling pathway-related proteins Gli1, Ptch1, Smo, and Shh were significantly downregulated. These findings indicated that the activation of Hh signaling pathway might be closely associated with the induction of EMT. Our study provides evidence for the conclusion that Hh signaling is activated during liver fibrogenesis and accelerates the transition from epithelial cells to myofibroblasts via EMT. Que treatment promotes the excretion of Cu2+ in the liver, prevents overactivity of Hh signaling in vivo and in vitro, and promotes EMT-induced reversal. Therefore, the antifibrotic effect of Que on the liver of Wilson disease may be exerted by blocking the activation of Hh signaling. However, more research is needed to fully understand the specific molecular mechanisms underlying Que's action on liver fibrosis and its interaction with the Hh signaling pathway.
5 Conclusion
In summary, this study showed that in vivo experiments, Que could promote the excretion of Cu2+ in the liver, improve liver function indexes and pathological damage of liver tissue, reverse EMT, and significantly inhibit the expression of Hh signaling pathway and its key proteins and genes. In vitro experiments, Que can promote the excretion of Cu2+ in cells, and we used GDC-0449, an inhibitor of the Hh signaling pathway, and found that blocking the Hh signaling pathway can reverse EMT, consistent with the results in vivo. Therefore, Que may have the potential to ameliorate liver fibrosis and EMT in Wilson disease by inhibiting the Hh signaling pathway. The study provides valuable insights into the underlying mechanisms of liver fibrosis in Wilson disease and highlights the potential of Que as a safe and reliable option to promote Cu2+ efflux and ameliorate liver fibrosis. However, additional clinical studies are necessary to validate the application of Que in the treatment of liver fibrosis in Wilson disease.
Acknowledgments
The study was supported in part by the National Natural Science Foundation of China (No. 82205076; 81973825); National Natural Science Foundation of China [Regional Innovation and Development Joint Fund No. U22A20366]. The authors would like to express their gratitude to the Clinical Research Experimental Centre of the First Affiliated Hospital of Anhui University of Chinese Medicine for providing the experimental platform, the College of Life Sciences of Anhui Agricultural University for providing the SPF-grade animal housing, and the Biorender platform for providing the research images.
Institutional Review Board Statement.
The animal study protocol was approved by Anhui University of Chinese Medicine Laboratory Animal Ethics Commission permission (AHUCM-mouse-2021011).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Quercetin ameliorates thioacetamide-induced hepatic fibrosis and oxidative stress by antagonizing the Hedgehog signaling pathway. J. Cell. Biochem.. 2022;123(8):1356-1365.
- [Google Scholar]
- Functional analysis and drug response to zinc and D-penicillamine in stable ATP7B mutant hepatic cell lines. World J. Gastroenterol.. 2016;22(16):4109-4119.
- [Google Scholar]
- Study on the relationship between hepatic fibrosis and epithelial-mesenchymal transition in intrahepatic cells. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2020;129:110413
- [Google Scholar]
- Perspectives of herbs and their natural compounds, and herb formulas on treating diverse diseases through regulating complicated JAK/STAT signaling. Front. Pharmacol.. 2022;13:993862
- [Google Scholar]
- Gandouling inhibits hepatic fibrosis in Wilson's disease through Wnt-1/β-catenin signaling pathway. J. Ethnopharmacol.. 2023;311:116445
- [Google Scholar]
- Epithelial-to-mesenchymal transitions in the liver. Hepatology (Baltimore, MD). 2009;50(6):2007-2013.
- [Google Scholar]
- Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol.. 2009;297(6):G1093-G1106.
- [Google Scholar]
- The role of Hedgehog signaling in fibrogenic liver repair. Int. J. Biochem. Cell Biol.. 2011;43(2):238-244.
- [Google Scholar]
- Wilson Disease. Nature Reviews. Disease Primers. 2018;4(1):21.
- Advance in the pathogenesis and treatment of Wilson disease. Translational Neurodegeneration. 2012;1(1):23.
- [Google Scholar]
- Role of canonical Hedgehog signaling pathway in liver. Int. J. Biol. Sci.. 2018;14(12):1636-1644.
- [Google Scholar]
- Liver pathology in Wilson's disease: From copper overload to cirrhosis. J. Inorg. Biochem.. 2019;193:106-111.
- [Google Scholar]
- Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J. Hepatol.. 2016;65(4):798-808.
- [Google Scholar]
- The intrahepatic signalling niche of hedgehog is defined by primary cilia positive cells during chronic liver injury. J. Hepatol.. 2014;60(1):143-151.
- [Google Scholar]
- Guo, X., Li, Y., Wang, W., Wang, L., Hu, S., Xiao, X., Hu, C., Dai, Y., Zhang, Y., Li, Z., Li, J., Ma, X., Zeng, J., 2022. The construction of preclinical evidence for the treatment of liver fibrosis with quercetin: A systematic review and meta-analysis. Phytother Res. 2022;36(10), 3774-3791.
- Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology (Baltimore, MD). 2012;55(6):1711-1721.
- [Google Scholar]
- Clinical Efficacy of Gandouling Decoction in Improving Hepatic Function with Phlegm and Blood Stasis Type Wilson's Disease. Chin. J. Exp. Tradit. Med. Formulae. 2020;26(08)
- [Google Scholar]
- Traditional Chinese medicine: An important source for discovering candidate agents against hepatic fibrosis. Front. Pharmacol.. 2022;13:962525
- [Google Scholar]
- Quercetin inhibits kidney fibrosis and the epithelial to mesenchymal transition of the renal tubular system involving suppression of the Sonic Hedgehog signaling pathway. Food Funct.. 2019;10(6):3782-3797.
- [Google Scholar]
- Smoothened is a master regulator of adult liver repair. J. Clin. Invest.. 2013;123(6):2380-2394.
- [Google Scholar]
- Hedgehog activity, epithelial-mesenchymal transitions, and biliary dysmorphogenesis in biliary atresia. Hepatology (Baltimore, MD). 2011;53(4):1246-1258.
- [Google Scholar]
- Attenuation of early liver fibrosis by pharmacological inhibition of smoothened receptor signaling. J. Drug Target.. 2012;20(9):770-782.
- [Google Scholar]
- Hepatic failure and liver cell damage in acute Wilson's disease involve CD95 (APO-1/Fas) mediated apoptosis. Nat. Med.. 1998;4(5):588-593.
- [Google Scholar]
- Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2020;121:109604
- [Google Scholar]
- Tang, L.L., Yang, W.M., Dong, T., Zhang, J., Chen, H.Z., Wang, H., Wei, T.H., JIANG, H.L., Yang, Y., 2022. Clinical Efficacy of Gandou Fumu Granules on Wilson Disease with Liver-kidney Deficiency and Phlegm-blood Stasis. Chinese Journal of Experimental Traditional Medical Formulae 28(12), 127-132.
- Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics. Int. J. Cancer. 2012;131(1):30-40.
- [Google Scholar]
- Discussion on the Mechanism of Gandoufumu Decoction Attenuates Liver Damage of Wilson's Disease by Inhibiting Autophagy through the PI3K/Akt/mTOR Pathway Based on Network Pharmacology and Experimental Verification. Mediators Inflamm.. 2023;2023:3236911.
- [Google Scholar]
- Targeting hedgehog signaling in myelofibrosis and other hematologic malignancies. J. Hematol. Oncol.. 2014;7:18.
- [Google Scholar]
- Divergence of hedgehog signal transduction mechanism between Drosophila and mammals. Dev. Cell. 2006;10(2):177-186.
- [Google Scholar]
- Comprehensive RNA-Seq Analysis of Potential Therapeutic Targets of Gan-Dou-Fu-Mu Decoction for Treatment of Wilson Disease Using a Toxic Milk Mouse Model. Front. Pharmacol.. 2021;41(08):981-990.
- [Google Scholar]
- Weiss, K.H., Thurik, F., Gotthardt, D.N., Schäfer, M., Teufel, U., Wiegand, F., Merle, U., Ferenci-Foerster, D., Maieron, A., Stauber, R., Zoller, H., Schmidt, H.H., Reuner, U., Hefter, H., Trocello, J.M., Houwen, R.H., Ferenci, P., Stremmel, W., 2013. Efficacy and safety of oral chelators in treatment of patients with Wilson disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 11(8), 1028-1035.e1021-1022.
- Cross-talk between Notch and Hedgehog regulates hepatic stellate cell fate in mice. Hepatology (Baltimore. Md.). 2013;58(5):1801-1813.
- [Google Scholar]
- Yang, H., Yang, T., Heng, C., Zhou, Y., Jiang, Z., Qian, X., Du, L., Mao, S., Yin, X., Lu, Q., 2019. Quercetin improves nonalcoholic fatty liver by ameliorating inflammation, oxidative stress, and lipid metabolism in db/db mice. Phytother Res. 2019;33(12), 3140-3152.
- TCM etiology, pathogenesis and syndrome differentiation and treatment of epatolenticular degeneration. Modern Chinese Clinical Medicine. 2012;19(04):6-9.
- [Google Scholar]
- Treatment of liver fibrosis in hepatolenticular degeneration with traditional Chinese medicine: systematic review of meta-analysis, network pharmacology and molecular dynamics simulation. Front. Med.. 2023;10:1193132.
- [Google Scholar]
- Intervention of Gandou Fumu Decoction on liver Fibrosis in Mice with Wilson's Disease Through JNK Signaling Pathway. Chin. J. Exp. Tradit. Med. Formulae. 2022;28(12):119-126.
- [Google Scholar]
- Zhao, X., Wang, J., Deng, Y., Liao, L., Zhou, M., Peng, C., Li, Y., 2021. Quercetin as a protective agent for liver diseases: A comprehensive descriptive review of the molecular mechanism. Phytother Res. 2021;35(9), 4727-4747.
- Mechanism of GanDouLing in treatment of cognitive impairment in Wilsondisease based on network pharmacology and molecular docking technology. Chinese Pharmacological Bull.. 2022;38(01):133-139.
- [Google Scholar]







