Translate this page into:
Rapid and sustainable process with low toxicity for cyanation of silver nitrate by DC arc-discharge in presence of acetonitrile
⁎Corresponding author. mollaebrahimilo@yahoo.com (Ali Reza Molla Ebrahimlo)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Use of Direct current (DC) arc-discharge in the chemistry reactions is very novel method. In this technique, the DC arc-discharge as a high-energy source conducts reaction to cleaving of the C-CN bond in acetonitrile and causes the cyanation of silver nitrate. This process due to non-use of cyanide ion can be proposed as a green and sustainable method. The obtained Silver cyanide from this procedure was characterized by its spectroscopic data (FT-IR and XRD).
Keywords
DC arc-discharge
Cyanation
Silver nitrate
Acetonitrile
Sustainable process
1 Introduction
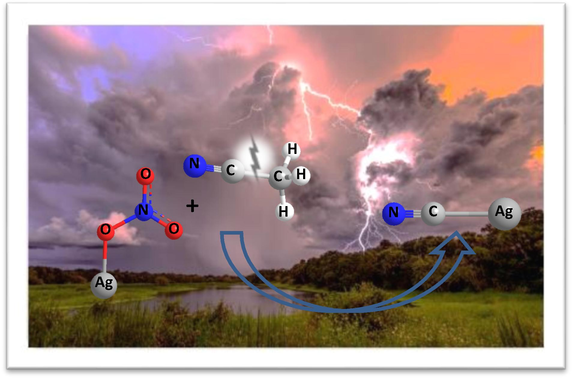
Green chemistry is very important in human health and environmental protection. Therefore, chemists are always trying to use methods and materials that reduce environmental pollution. Metal cyanides are of considerable interest owing to their commercial importance. For example, AuCN and AgCN are used in mining, electroplating, and photography, dyeing, pharmaceuticals and rubber (Gail et al., 2000). Among the different types of cyanides, transition metal cyanides have a remarkable history that spans nearly three centuries, dating back to 1704 when the Berlin artist Diesbach accidentally discovered Prussian Blue (Dunbar and Heintz, 2007). In the traditional method for the synthesis of silver cyanide are used as cyanating agents to react with silver salt solution or silver oxide solid (Gail et al., 2000; Dunbar and Heintz, 2007). However, Cyanide ion is known as a very toxic compound. In addition, the waste from the traditional method is always a threat to humanity and the environment. Based on this, it is very important to find a convenient and sustainable method for the synthesis of silver cyanide. In this regard, Zou et al. have reported (Zou et al., 2013) a mild photo-assisted route to conduct the cyanation of transition metal nitrates (Ag+, Zn2+, Yb2+, and Ni2+) using acetonitrile as the cyanating agent coupled with room-temperature C—CN bond cleavage. Compared with cyanating agents traditional (KCN, NaCN and HCN) which release free toxic cyanide ions in solution, acetonitrile is much less toxic due to its stable C—CN bond and has been widely used as a polar aprotic solvent in organic synthesis and purification. However, it is a challenge to use acetonitrile as a cyanating agent because of the difficulty in cleaving its C—CN bond. In fact, metal-mediated splitting of the C—CN bond of acetonitrile has only been shown to occur with the aid of complicated and expensive metal complexes such as those containing a Pt (Muetterties et al., 1971), Mo (Churchill et al., 1999), Rh (Taw et al., 2002, 2003), Ag (Guo et al., 2009), Cu (Marlin et al., 2001; Lu et al., 2004; Li et al., 2009), Ni (García et al., 2004) metal center. Here, we report a facile and fast cyanation of silver nitrate by arc discharge method in presence acetonitrile, which is very fast compared to the previous method.
2 Experimental
All substrates were obtained from Merck of the highest quality and were used without further purification. Graphite electrodes were from Aldrich with a purity of 99.999% having a diameter of 6 mm and length of 150 mm. The DC power supply was from STAR model 200D (0–3 V). Ruhmkorff coil was used for induction coil. The product was characterized by a comparison with authentic samples XRD and IR spectra. The X-ray diffractometer (XRD, Siemens D500) and Fourier transform-infrared spectrometer (FT-IR, Thermonicolet Nexus 670) was used to ascertain the synthesis of AgCN. The Ultra violet-visible Spectrophotometer (UV–Vis, HACH DR/4000 U) and proton nuclear magnetic resonance (1H NMR, BRUKER DRX300 AVANCE) was used to confirm the mechanism of reaction.
2.1 General procedure
In a pyrex twonecked round-bottomed flask, AgNO3 (1 mmol) was dissolved in 8 mL of acetonitrile and 2 mL of water. Two graphite electrodes were connected at the poles of the induction coil that was connected to the DC power supply; one of the electrodes (positive pole) immersed in solution and the other electrode (negative pole) is placed half a centimeter above from the solution surface (Scheme 1). The solution was stirred under arc discharge with output voltage 2.5 for 2 min at room temperature (The carbon arc appears between the hanging carbon electrode and the solution surface). After 2 min AgCN was collected and washed with water (20 mL) to give white solid (0.1 g, 74%).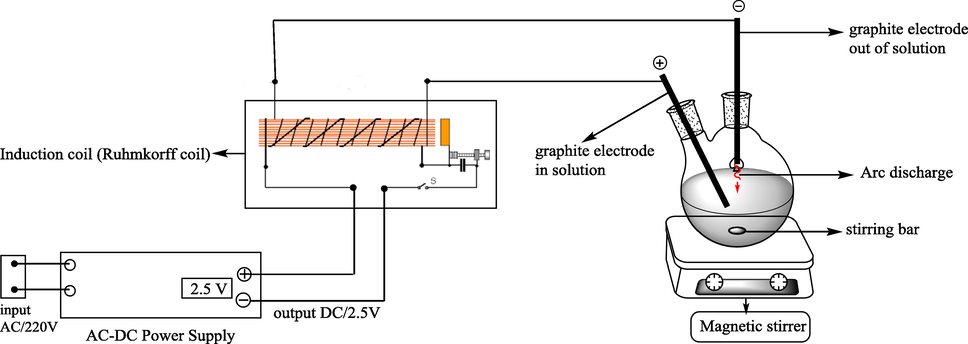
DC arc-discharge system.
3 Results and discussion
Arc discharge interactions with aqueous solutions have a lot of value in chemistry. In 1784, Henry Cavendish (Cavendish, 1784) discovered that an electrical arc in atmospheric air would make a nearby solution of water acidic. In the DC arc-discharge system, it is generally assumed that the aqueous environment forms a dielectric barrier that does not allow charge to be transferred from the arc discharge into the bulk of the liquid, and electrolytic reactions are typically neglected in these systems as well, because no current is passed through the solution. In our system, two graphite electrodes were used as cathode and anode that one of the electrodes (positive pole) immersed in solution and the other electrode (negative pole) is placed half a centimeter above from the solution surface then the electric discharge is applied to the solution (Scheme 1),
We recently reported (Molla Ebrahimlo, 2017) a green oxidation of aromatic aldehydes by arc discharge method in presence CH3CN-H2O2 with high yields and short reaction times. Herein, we use DC arc-discharge for cyanation of silver nitrate at room temperature. For synthesis of AgCN, 1 mmol of AgNO3 was dissolved in 8 mL acetonitrile, for avoiding ignition of acetonitrile during arc discharge was added 2 mL water, product as white solid precipitates with applying arc discharge during 2 min. The white solid product was confirmed to be AgCN by XRD and IR analysis. By comparing XRD of the synthetic and commercial AgCN, it is observed that both are the same at seven picks. Seven XRD peaks at 2θ = 24.0, 29.7, 38.4, 49.5, 52.8, 58.6 and 61.8 can be exactly indexed as the (1 0 1), (1 1 0), (0 1 2), (2 1 1), (3 0 0), (1 2 2) and (2 2 0) plane reflections of AgCN (Fig. 1).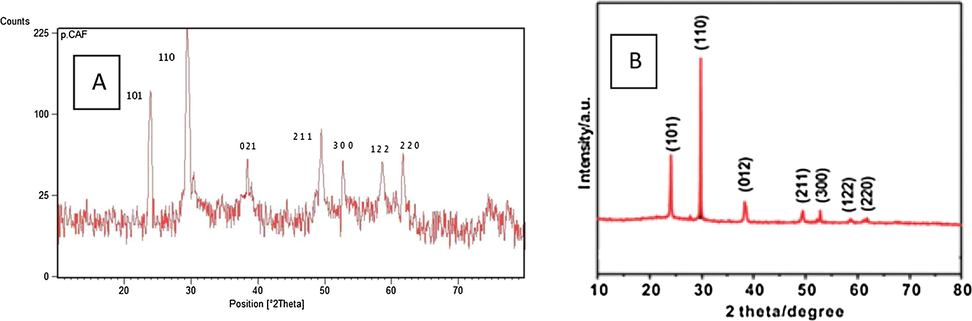
XRD patterns; (A) synthesized AgCN, (B) commercial AgCN.
In Fig. 2 is shown IR spectrum of white solid. The band at 2164 cm−1 corresponds to the stretching vibration of the C≡N bond, and 479 cm−1 is assigned to the stretching vibration of the Ag—C bond.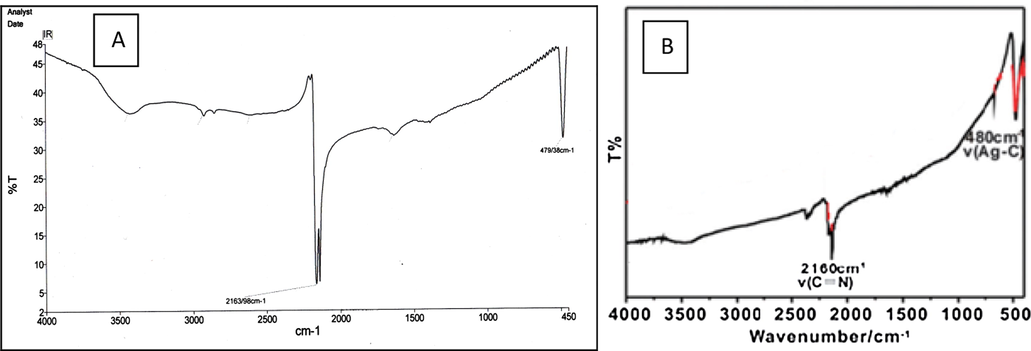
FT-IR spectrum; (A) synthesized AgCN, (B) commercial AgCN.
After applying arc discharge, the reaction solution was also examined by nuclear magnetic resonance (1H NMR) analysis and its UV–Vis spectra. In 1H NMR spectrum, the signals absorbed at about 1.95 ppm, 2.96 ppm and 5.50 ppm respectively correspond to the hydrogens in CH3CN, CH3ONO2 and H2O (Fig. 3).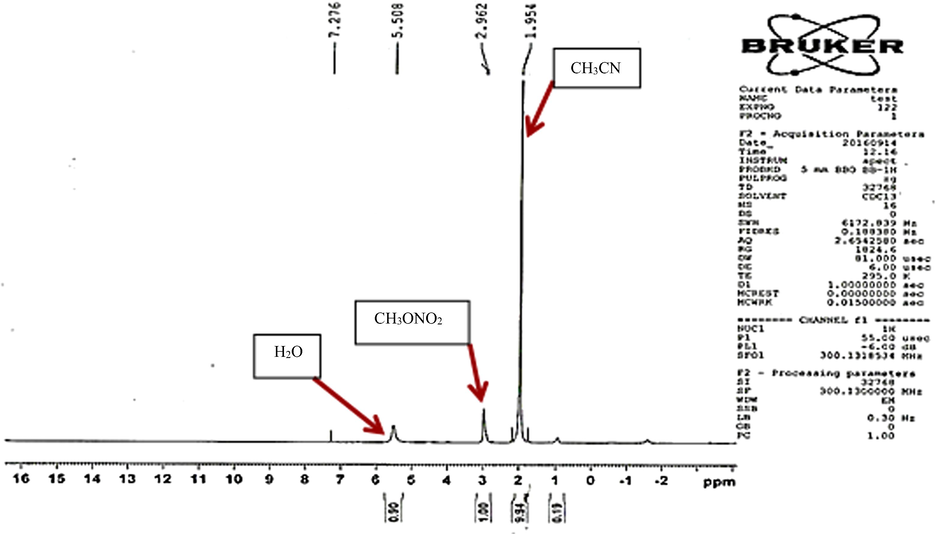
1H NMR spectrum of reaction solution.
The Matching our 1H NMR spectrum with the reported1H NMR spectrum by Zou et al. (2013) indicates that the mechanism of reaction is almost the same in both methods. Therefore, the performance of the reaction can be described as follows:
The reaction process was monitored by UV–Vis spectroscopy. As shown in Fig. 4, before arc discharge, the absorption of the 311 nm band, which is assigned to an n → π* electronic transition of NO3− in AgNO3 (Katzin, 1950; Rotlevi and Treinin, 1965; Maria et al., 1973). Interestingly, an obvious blue shift from 311 nm to 273 nm is also observed after arc discharge. This is due to the formation of CH3ONO2. The blue shift indicates CH3ONO2 has a higher energy than NO3− as reported by Maria et al. (1973).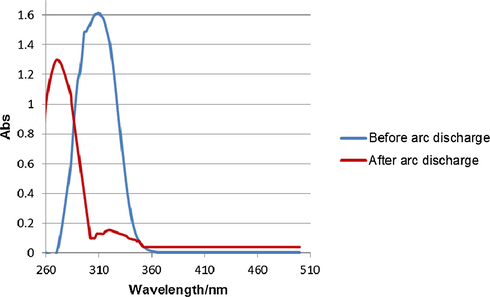
UV–Vis spectra of reaction solution before and after arc discharge.
The proposed mechanism is shown as Scheme 2: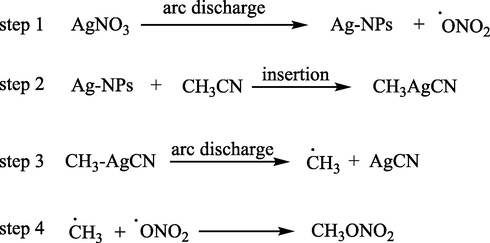
In this mechanism, the reaction between AgNO3 and CH3CN starts with the homolysis of Ag-O bond in AgNO3 by arc discharge, producing two actives species: an Silver nanoparticles (Ag-NPs), which its formation by arc discharge method in liquid reported by Ashkarran (Ashkarran, 2010), and a NO3• radical. The Ag-NPs is so active that it can insert to C—CN bond, subsequently, the C-Ag bond is broken by arc discharge and AgCN is formed. Finally, the combination of CH3• and NO3• to obtain the product CH3ONO2. However, the role of NO3− in this reaction is crucial because replacing it with other anions will not lead to reaction.
4 Conclusion
A fast and ecofriendly method for the synthesis of AgCN without using Cyanide ions was developed. We also introduced arc discharge on the solution as energy source for the reaction. Currently, we are studying the scope and limitations of this new method using other transition metal nitrates.
Acknowledgement
The authors are thankful to the research council of Islamic Azad University Khoy branch.
References
- A novel method for synthesis of colloidal silver nanoparticles by arc discharge in liquid. Curr. Appl. Phys.. 2010;10:1442-1447.
- [Google Scholar]
- The Ansa effect in permethylmolybdenocene Chemistry: A [Me2Si] Ansa bridge promotes intermolecular C−H and C−C bond activation. Organometallics. 1999;18:2403-2406.
- [Google Scholar]
- Progress in Inorganic Chemistry. John Wiley & Sons, Inc; 2007. p. :283-391.
- Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co KGaA; 2000.
- Cleavage of carbon−carbon bonds in alkyl cyanides using nickel(0) Organometallics. 2004;23:3997-4002.
- [Google Scholar]
- Ag(I)-mediated formation of pyrophosphonate coupled with C-C bond cleavage of acetonitrile. Chem. Commun. 2009:2893-2895.
- [Google Scholar]
- Variations in absorption spectrum of the nitrate group. J. Chem. Phys.. 1950;18:789-791.
- [Google Scholar]
- Solvothermal assembly of a mixed-valence Cu(I, II) cyanide coordination polymer [Cu(II)Cu(I)2(µ-Br)2(µ-CN)2(bdmpp)]n by C-C bond cleavage of acetonitrile. Cryst. Eng. Comm.. 2009;11:2751-2756.
- [Google Scholar]
- C−C bond cleavage of acetonitrile by a dinuclear copper(II) cryptate. J. Am. Chem. Soc.. 2004;126:4760-4761.
- [Google Scholar]
- Electronic absorption spectrum of nitrate ion and boron trihalides. J. Am. Chem. Soc.. 1973;95:1050-1056.
- [Google Scholar]
- Heterolytic cleavage of the C−C bond of acetonitrile with simple monomeric CuII complexes: melding old copper chemistry with new reactivity. Angew. Chem. Int. Ed.. 2001;40:4752-4754.
- [Google Scholar]
- Green oxidation of aromatic aldehydes to related acids using hydrogen peroxide in acetonitrile under arc discharge. Chim. Oggi-Chem. Today. 2017;35(6):52-54.
- [Google Scholar]
- Reactivity of trialkylphosphine complexes of platinum(0) J. Am. Chem. Soc.. 1971;93:3543-3544.
- [Google Scholar]
- Carbon−carbon bond activation of R−CN (R = Me, Ar, iPr, tBu) using a cationic Rh(III) complex. J. Am. Chem. Soc.. 2002;124:4192-4193.
- [Google Scholar]
- A Mechanistic investigation of the carbon−carbon bond cleavage of aryl and alkyl cyanides using a cationic Rh(III) silyl complex. J. Am. Chem. Soc.. 2003;125:9808-9813.
- [Google Scholar]
- Photo-assisted cyanation of transition metal nitrates coupled with room temperature C-C bond cleavage of acetonitrile. Chem. Commun.. 2013;49:1906-1913.
- [Google Scholar]







