Translate this page into:
Rapid assessment of the impact of microwave heating coupled with UV-C radiation on the degradation of PAHs from contaminated soil using FTIR and multivariate analysis
⁎Corresponding author. mohammad.alghouti@qu.edu.qa (Mohammad A. Al-Ghouti)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The presence and fate of polyaromatic hydrocarbons (PAHs) in the environment are receiving a great concern. In this study, three oil-contaminated soils (industrial area, Dukhan city, and artificial soils) were utilized to examine the effect of microwave (MW) heating and UV-C irradiation on the PAHs degradation. A rapid assessment of the impact was evaluated using Fourier transform infrared (FTIR) and multivariate analysis. The total organic matter values for the maximum PAHs reduction were evaluated based on the FTIR spectra of the contaminated soils followed with the principal component analysis (PCA). The results showed that the highest total organic carbon reduction was achieved for the industrial soil sample that required a high MW power and long MW exposure time. On the other hand, the Dukhan city soil sample, which has the lowest total organic carbon, required a high MW power and short MW exposure time followed by UV-C treatment for 20 min to reach the maximal FTIR transmittance reduction. The cluster analysis was also used to evaluate the impact of MW heating, and MW heating followed by UV-C irradiation on the degradation of PAHs. The PCA results of the industrial city sample showed that neither MW treatment (100% MW, 15 min exposure time) followed by UV-C treatment for 20 min nor 10 min is significantly different from the MW treatment (100% MW, 15 min exposure time). However, for the Dukhan sample, the UV-C treatment at 10 min after high MW power and long exposure time (100% MW, 15 min exposure time) was the most efficient treatment.
Keywords
Polyaromatic hydrocarbons
Microwave heating
UV-C irradiation
Soil contamination
Fourier transform infrared
Multivariate analysis
1 Introduction
Polycyclic aromatic hydrocarbons (PAHs) are organic compounds classified as widely spread toxic environmentally persistent contaminants found in different structures with varied biological toxicity (Abdel-Shafy and Mansour, 2016). In general, PAHs exist in the environment as a mixture of two or more compounds. Sources of PAHs in the environment can be classified into natural or anthropogenic sources. Anthropogenic sources have been contributed largely to the amount of PAHs in the environment which include domestic sources, industrial sources, and agricultural sources (Mojiriai et al., 2019). Consequently, PAHs can exist in different compartments in the environment such as air, water, soil, and biota (Lee, 2010). The number of aromatic rings determines the categorization of the PAHs into (i) low molecular weight (LMW), which represents a group with less than four aromatic rings such as naphthalene, acenaphthene, fluorene, phenanthrene, and (ii) high molecular weight (HMW), which represents a group with four or more aromatic rings such as pyrene (Adeniji et al., 2017). Because PAHs tend to cause mutations, cancer, and cell toxicity, they have been described as hazardous and toxic compounds (Hamoudi-Belarbi et al., 2018). The solid nature of PAHs is due to their high boiling and melting points. Moreover, they are characterized by their aqueous solubility, which decreases with increasing molecular weight and low vapor pressure. On the other hand, increasing their molecular weights makes them more susceptible to oxidation and reduction (Masih et al., 2012). Because of the high lipophilic nature of PAHs, they are very soluble in organic solvents.
Nowadays, PAHs are dealt with great concern as they are the most frequently detected pollutants in the environment. The concentrations of PAHs and their patterns of generation vary based on the contamination source (Abdel-Shafy and Mansour, 2016). The degradation difficulty of PAHs depends on the physicochemical properties of individual PAHs (Al-Kaabi et al., 2018). The LMW-PAHs have high water solubility, while HMW-PAHs are very hydrophobic as demonstrated in log Kow above five (Jonsson et al., 2007). Thus, several degradation techniques and mechanisms were investigated, which can be categorized into physical, chemical, biological, and thermal techniques as shown in Table 1. Various studies showed that among different thermal techniques, MW heating has been considered a successful technique for the treatment of PAHs-contaminated soil. MW treatment has been shown quick and effective pollutant removal with high efficiency and low costs (Falciglia et al., 2018a, 2018b). Treatment using MW heating does not depend on heat transfer, and accordingly heating times can be much lower than traditional thermal treatment (Sivagami et al., 2019). Hence, microwave treatment reduces the treatment periods significantly, cost, and the risk of contamination. Microwaves are bands of electromagnetic radiation with different frequencies range 300 MHz to 300 GHz. Frequency describes the number of waves that pass in a fixed place through a specific time (seconds) (Kostas et al., 2017). The principle of the microwave irradiation treatment is based on the dissipation of the electromagnetic field energy and transferring this energy into heat required for the thermal desorption of the pollutants (Buttress et al. 2016). Thermal treatments for PAHs contaminated soils are based on using heat to destroy or volatilize PAHs from soils (Krouzek et al., 2018). Therefore, these pollutants will change into a gaseous phase causing their mobility to increase. Pollutants at the gaseous phase can be collected through other treatments (Falciglia et al., 2018a, 2018b). As reported by Li et al. (2009), MW energy is transformed into thermal energy to degrade pollutants over volatilization by heating. Hence, compared to other thermal techniques, MW heating is more cost-effective and time-efficient, and PAHs degradation increases with increasing the microwave heating power in a short time (Sivagami et al., 2019; Falciglia et al., 2016; Shanker et al., 2017; Kamil and Talib, 2016; Rabodonirina et al., 2019; Liao et al., 2018). Xiao et al. studied the potentiality to prepare coal-based activated carbon using MW radiation and evaluate the possible application of the produced AC (activated carbon) for removal of PAHs based on the adsorption capacity of the produced AC.
Classification
Technology
Advantages
Disadvantages
Reference
Physical
Solvent extraction, supercritical/subcritical fluid extraction, and electrokinetic remediation.
Easily applied, use non-toxic and biodegradable extraction agents
Secondary treatment is required.
(Hamoudi-Belarbi et al., 2018; Benamar et al., 2019)
Chemical
Chemical oxidization, photocatalytic degradation, and electro-kinetic remediation
Degradation of PAHs and prevention of transfer to another medium
Formation of toxic by-products
(Gan et al., 2009; Dong et al., 2019; Li et al., 2020; So et al., 2019)
Biological
Bioremediation and phytoremediation
Environmentally friendly, no waste treatment
Long treatment duration
(Gan et al., 2009; Rabodonirina et al., 2019; Lu et al., 2019; Li et al., 2020)
Thermal
Thermal desorption, incineration, microwave heating, and soil vapor extraction
Can degrade HMW hydrocarbons e.g. PAHs
It requires secondary treatment for gaseous by-products and requires high temperature.
(Koshlaf and Ball, 2017; Sivagami et al. 2019; Falciglia et al., 2018a, 2018b)
Various factors should be considered before choosing the appropriate treatment technique such as pollutant type and concentration, contamination site, properties of the contaminant sink (air, water, or soil), the time consumed by a treatment to have effective results, the application of treated effluent, soil grain size, pH and others (Ram et al., 1993; Kuppusamy et al., 2017).
Soil is considered as a sink for several environmental contaminants including PAHs in where soil properties change based on soil type, contaminant type, and concentration of the contaminant, as well as the contamination period (Riccardi et al., 2013). PAHs are toxic to the indigenous microbes in the soil and they affect their enzymatic activity leading to a decrease in their microbial activity (Sakshi et al., 2019).
UV irradiation has been considered an efficient method to eliminate organic pollutants like PAHs. Organic pollutants in different matrixes such as water or soil can absorb the UV light. Later the molecules are electronically excited forming series of reactions that lead to photodegradation of pollutants (Xu et al., 2013; Bai et al., 2019). It worth mentioning that the photodegradation process in the environment is a naturally occurring major pathway for the transformation of most PAHs. PAHs are capable of absorbing photons within 280–400 nm and 400–760 nm by their conjugated π-orbital electron systems, and they are categorized as photoactive pollutants (Gbeddy et al., 2020). However, Gbeddy et al., (2020) have been studied the role of 254 nm UV photons which is in the range of UV-C on photochemistry of PAHs and other transformed products due to PAHs absorption of UV-C irradiation. The results showed that UV-C irradiation had different effects on the fate of different analytes.
In this study, UV treatment has been used as a second treatment after microwave treatment to enhance the remediation process. Therefore, the photochemical processes of PAHs can be enhanced and accelerated in the presence of a heating process such as microwave heating.
For the soil environment, the photodegradation of PAHs significantly depends on the soil depth and the depth of the contaminant in the soil, temperature, soil particle size, soil moisture, and organic matter (Zhang et al., 2010). Also, the photodegradation of each compound of different PAHs depends on the light absorption spectrum. In the presence of radicals, UV remediation can be more efficient and results in a higher degradation rate than matrix without radicals (Gan et al., 2009). For example, high soil moisture where the water content is high, the presence of UV light can break down water molecules and produce (⋅OH) hydroxyl radicals. Hydroxyl radicals can enhance the photodegradation of PAHs, this process is called an advanced oxidation process. Wlodarczyk-Makula (2011) investigated the impact of UV irradiation on treating wastewater regarding PAHs removal. The study deals with two wastewater sample types from a municipal treatment plant with different concentrations of PAHs 0.8 μg/L and 1.2 μg/L; a standard mixture of the 16 priority compounds of PAHs was added to both samples. All samples were exposed to UV-C irradiation of 264 nm wavelength for 10, 20, 30, and 60 s. After the UV treatment, the PAHs concentration in all samples and the control sample (without adding the standard mixture of PAHs) were measured using gas chromatography-mass spectrometry (GC–MS). It was found that the exposure of UV irradiation to the control and the sample reduced the PAHs concertation to 65% and 84% respectively. Besides, the results showed that the efficiency of the removal was based on the molecular weight and the number of rings in different compounds of PAHs. A prolonged exposure from 10 to 20 s to the UV irradiation significantly increased the removal efficiency of PAHs, while it was not significant when the exposure time increased from 30 to 60 s. Eker and Sengul, 2018 did a study to compare the efficiencies of PAHs degradation from soil by solar irradiation (UV-A) and UV-C treatments. Soil samples from the industrial area were exposed to three conditions, namely ambient air, UVA, and UVC irradiation. The PAH removal obtained from ambient air condition, UVA, and UVC condition was 35%, 90%, 86%, respectively. As well, the effects of adding catalysts (TiO2 and H2O2) at different concentrations (1%, 10%, and 20% of dry soil weight) on PAHs removal under ambient air condition (mimic real conditions) was studied. The PAHs removal was the highest for a dose of 20% TiO2 (89%), while was the highest for a dose of 1% of H2O2 (88%). In terms of different molecular weight of different PAHs, the removal efficiencies were increased for both LMW-PAHs and HMW-PAHs with the adding of catalysts. However, without adding the catalysts, the removal efficiency was higher for the LMW-PAHs; 3-ring and 4-ring compared to 5-ring and 6-ring.
Zhang et al. (2010) investigated the rates of degradation of pyrene on the surfaces of soil under UV-C light at 254 nm wavelength. The impact of some parameters which affect the photodegradation efficiency such as temperature, particle size, depth of soil, and humic acid percentage have been explored in the study. The rate of pyrene degradation was 25%, 30%, and 35% at three different temperatures, namely 20 oC, 25 oC, and 30 °C, respectively. Soil particles with different sizes represented dissimilar rates of photodegradation. The rate of photodegradation for the particle size less than 1 mm was faster in comparison to a particle size of less than 0.25 mm. Falciglia et al. (2018a, 2018b) studied the effect of MW heating coupled with UV-A treatment on PAHs removal from marine sediments. The used MW power was 650 W, 2.45 GHz in a time range of 1 to 7 min, while for the used wavelength for UV-A was 350 nm within the time range up to 36 min. Because of their dielectric properties of the sediments and the PAHs, the results demonstrated a rapid increase in the sediment temperature that occurred during the MW heating. It was noticed that more than 85% removal of PAHs within 1 min MW treatment (650 W, 2.45 GHz), and total PAHs removal was achieved with longer MW irradiation.
Fourier transform infrared spectroscopy (FTIR) is an analytical technique that is universally used in the identification, classification, and quantification of several organic compounds such as hydrocarbons (Cozzolino, 2015; Ng et al., 2017). The advantages of FTIR include ease of sample processing without requiring extraction, a different type of samples can be readily analyzed, rapid and accurate analysis (Aske et al., 2001; Schneider et al., 2016). FTIR spectroscopy is considered as one of the tools that study samples of organic components found in soil. FTIR spectroscopy provides a precise characterization of soil organic matter (SOM), mechanistic, and kinetic aspects of mineral–SOM interactions that underlie biogeochemical processes. Accordingly, the FTIR technique allows us to identify the peaks in the spectra regions linked to the functional groups (Schneider et al., 2016). Many previous studies used FTIR as an analytic method to measure the concentration of PAHs and identify different compounds of PAHs in various environmental samples (Schneider et al., 2016; Okparanma and Mouazen, 2013; Yi-Bin Qi et al., 2017). However, this paper aims to investigate the impact of MW heating and MW heating followed by UV-C irradiation on hydrocarbon degradation in the soil by means of the changes/shifting in the FTIR transmittance peaks. The redundancy in the FTIR data set was reduced by using multivariate analysis such as PCA.
To the best of our knowledge, there is no study on the assessment of the impact of MW heating followed by UV-C irradiation on the degradation of PAHs from contaminated soil using FTIR and multivariate analysis. Existing studies are principally related to evaluating the MW heating or UV irradiation on soil using long extraction times and laborious identification techniques such as chromatography. In this study, FTIR followed by PCA was promptly used for the evaluation of the PAHs degradation without the need of using a sophisticated analysis technique or solvents. In this context, this study assessed the role of MW and ultraviolet (UV-C) on the degradation of PAHs from oil-contaminated soils. The specific objectives of the study include (i) studying the physicochemical characterizations of three oil-contaminated soil samples, (ii) examining the impact of MW heating, and MW heating followed by UV-C irradiation on the degradation of PAHs from the contaminated soils by means of FTIR analysis, and (iii) the application of PCA to rapid assessment of the effect of MW heating, and microwave heating followed by UV-C irradiation on the degradation of PAHs.
2 Methods and materials
2.1 Sample collection
The contaminated weathered soil samples were obtained from two different sites in Qatar, namely Dukhan city and industrial area. Approximately 30 g were collected from each site in a clean plastic bag properly sealed and labeled. The soil samples were taken by a clean spatula from 1 to 3 cm depth. Before analysis, the samples were kept in the fridge. A sandy sample was also obtained from a clean area near the beach in Alwakra; this sample was considered as a blank sample (free from PAHs). Approximately 100 g of the sand was collected in a clean plastic bag properly sealed and labeled. The blank sample was deliberately contaminated with fresh crude oil. This was done to compare it with the weathered soil samples. The fresh oil-contaminated soil (FOCS) was prepared by mixing the sandy sample (100 g) with a mixture of 2 mL of local crude oil (liquid appearance with a density of 0.75 g/mL at 25 °C) and 5 mL of dichloromethane (DCM); DCM (greater than99.9% purity) was obtained from SIGMA-ALDRICH. The DCM was added to decrease the thickness of crude oil, so the contaminants are equally distributed between sand particles. The FOCS was homogenized and left for a minimum of 24 h under the hood to allow the volatilizing of DCM.
2.2 MW Experiment for hydrocarbon contaminated soil
Since MW heating and UV-C exposure could not be combined in one test unit, MW heating and UV-C exposure had to be carried out successively starting with microwave heating, followed by UV-C exposure. The collected soil samples were treated with high MW power (100%) which equals 1000 W, and low MW power (20%) which equals 100 W for a long MW exposure (15 min), medium MW exposure (10 min), and short MW exposure (5 min) (Panasonic Microwave NN-CD997S, 2005, with a single-mode cavity and adjustable power settings to supply microwave energy, namely 100 W, 350 W, 550 W, 700 W and 1000 W). Previous studies proved that PAHs removal from soils increases as MW power input increases (Buttress et al. 2016; Sivagami et al. 2019). This relationship has been explained by the Eq. (1), which displays a proportional relationship with power density and heating rate:
2.3 UV irradiation experiment
For studying the synergistic effect of the MW and the UV-C irradiation on the degradation of PAHs in the soils, the MW heating followed by UV-C irradiation was implemented. UV-C lamp with cabinet at Thomas Scientific was used. UVG–11 Compact UV lamp from John Morris Scientific, 254 nm wavelength and 4 W, 0.12 A. The distance between the irradiated sample and the lamp was 0.83 cm. In this study, the UV-C has been used as it composed of shorter wavelengths and higher energy required to degrade PAHs. Various studies have been used UV-C irradiation to treat either multi or specific compounds of PAHs and different patterns of generation (Gbeddy et al., 2020; Eker and Sengul, 2018; Wlodarczyk-Makula, 2011).
Two MW heating powers, namely high power (100%) and low power (20%) as well as two UV-C exposure times, namely 20 min and 10 min were chosen. The FTIR analyses were done before and after each treatment. Fig. 1 shows the graphical methods of the experiment where the MW heating and UV-C exposure had to be carried out successively starting with microwave heating, followed by UV-C exposure.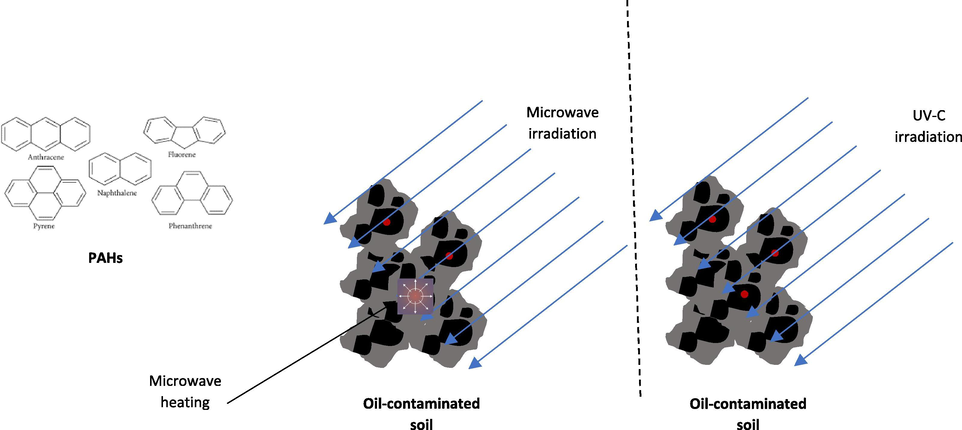
Graphical method of the experiment.
2.4 FTIR analysis
The FTIR analysis was carried out using a Perkin Elmer 400 spectrum instrument (UATR – Universal Attenuated Total Reflectance). All absorbance spectra were obtained in the 4000–400 cm−1 range by 100 scans at 1.0 cm−1 resolution, and the signal-to-noise ratio was 45000:1. The UATR-FTIR spectra displays information regarding functional groups that are present 1 μm near the surface of an internal reflection element. Moreover, it possesses the ability to obtain precise spectra of any samples in the existence of water. Fig. 2 illustrates the experimental design for each sample.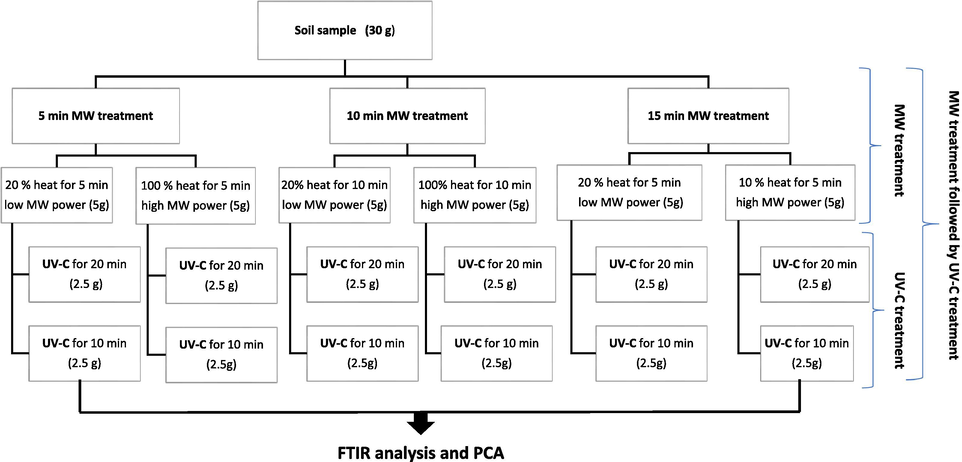
Experimental design for each sample.
2.5 Data analysis
The redundancy in the data set can be reduced by using multivariate analysis such as principal component analysis (PCA) (Ashfaq et al., 2020). Through a linear combination of actual coordinates, new coordinates known as principal components will be produced, which can be helpful in assessing the obtained categorical data and draw several conclusions (Ashfaq et al., 2019; Alshuiael and Al-Ghouti, 2020). Combing FTIR and PCA can help to categorize the impact of microwave heating followed by UV-C irradiation on the degradation of PAHs from contaminated soil. In this study, the data generated from the FTIR spectra of the MW heating and UV irradiation experiments were analyzed using PCA using the Unscrambler X software (v10.4, Camo Analytics, Magnolia, TX, USA).
3 Results and discussion
3.1 Physicochemical analysis of the soil samples
In this study, three oil-contaminated soils with different organic carbon contents were used to investigate the impact of MW heating followed by UV-C irradiation on hydrocarbon degradation. Various physicochemical analyses were carried for the collected soil samples namely, soil particle size, total organic content (TOC), total organic matter (TOM), total petroleum hydrocarbon (TPH), and total nitrogen (TN) content. Physicochemical properties determine the most efficient techniques with their optimum conditions for treating contaminated soils. Table 2 shows the results of the soil analysis for the three oil-contaminated soil samples. The industrial soil has the highest concentration of TOC and TOM followed by the Dukhan soil, while the FOCS has the lowest concentration of TOC and TOM, and it has the highest water content percentage and PAHs content among the three soil types. The Dukhan soil has the highest concentration of total petroleum hydrocarbon (TPH) followed by the industrial soil and the FOCS. The three soil samples were classified as sandy soil samples based on their particle size. Furthermore, the conditions of the MW treatment can be affected by the soil moisture as it was found that the “low MW power and short exposure time” gave the high soil moisture removal. Furthermore, the results showed that the FOCS with the highest water content had the highest FTIR transmittance reduction using any treatment setups, namely the low MW power, high MW power, and short exposure time. As mentioned earlier, the TOC influences the MW treatment conditions. Vidonish et al. (2016) stated that the high TOC soil demands a high temperature of the thermal treatment to obtain the maximum contaminant removal. Additionally, Magi et al. (2002) demonstrated that soil grain size affects the PAHs binding on the soil in which clay soil particles with more surface area have more binding sites leading to the increase in PAHs adsorption. While the fine soil particles with less porosity are prone to slow migration of adsorbed pollutants leading to long-term exposure to the toxic pollutants. Saba et al. (2011) investigated certain PAHs degradation on the surface soil samples including residential soil, barren soil, industrial soil, and garden soil. The results showed that the garden soil with high organic matter had more tendency for PAHs adsorption. The method detection limits PrimacsSNC100 (less than 0.02%).
Soil Name
Total nitrogen (TN) (%)
Total organic content (TOC) (%)
Total organic matter (TOM) (%)
Water content (%)
Total petroleum hydrocarbon (TPH) (ppm)
Polyaromatic hydrocarbons (PAHs) (ppm)
Soil type
FOCS
NIL
0.02
0.036
2.14%
40.95
0.26
Sandy
Industrial soil
NIL
7.11
12.23
0.72%
254.6
0.14
Sandy
Dukhan soil
NIL
5.50
9.460
0.50%
313.3
0.05
Sandy
3.2 FTIR analysis
The FTIR spectrum of a molecule offers a unique molecular fingerprint. For the PAHs, it elucidates the binding and degradation mechanisms to/of soil. This could be done by evaluating the relative absorption or transmittance intensity for the assigned peaks. Changes/shifts in these transmittance peaks compared to the fresh soil provide information on bonding attraction with the soil matrix. Even though FTIR has been used for both qualitative and quantitative analyses, qualitative applications are of importance (Northcott and Jones, 2000). The FTIR spectra of the untreated soils from the Dukhan, industrial area, and the FOCS samples are represented in Fig. 3A, 3B, and 3C, respectively. Identification of the peaks and their corresponding functional groups before and after treatments was performed. Furthermore, the concentration of the hydrocarbons presents in the soils can be determined from the obtained absorbance/transmittance value.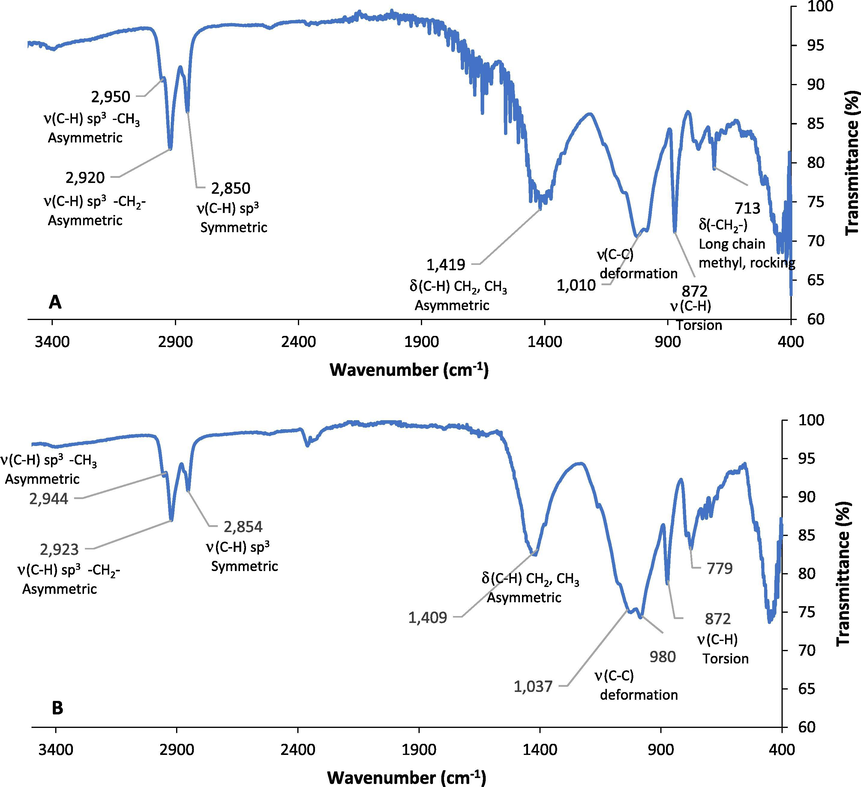
FTIR spectra of A.) Oil-contaminated soil from the Dukhan city, B.) Oil-contaminated soil from the Industrial area, C.) Artificial oil-contaminated soil with 2% crude oil (FOCS), and D. The overlay plot of the three oil-contaminated soils.
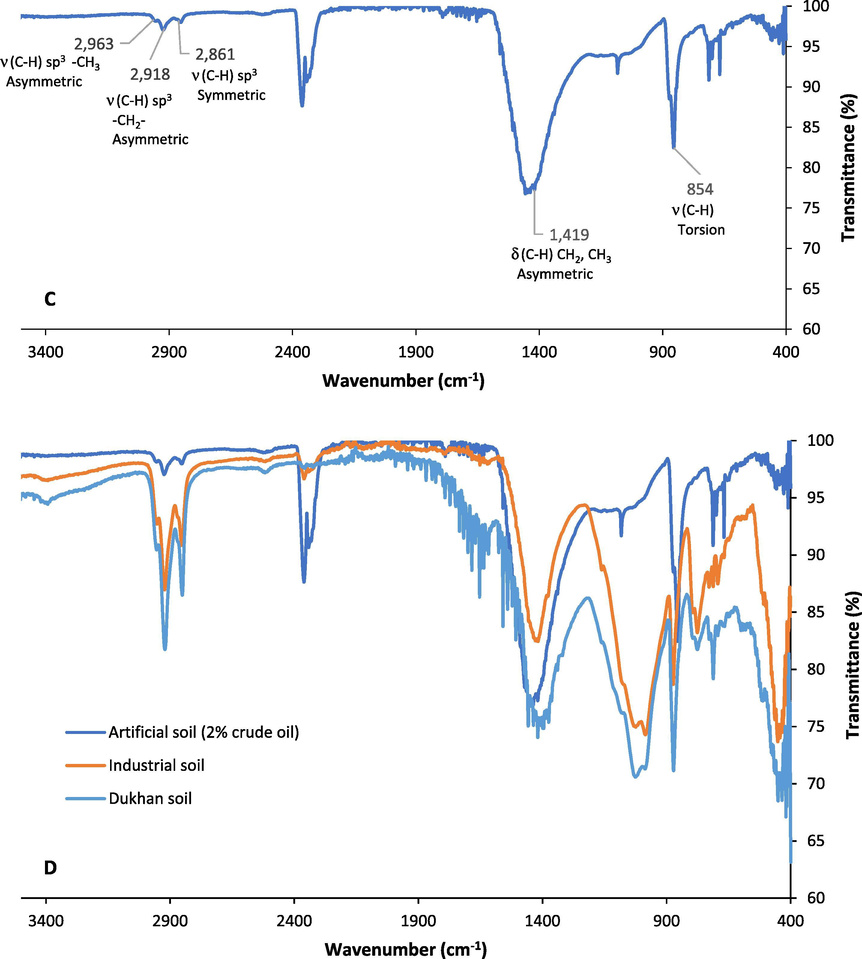
FTIR spectra of A.) Oil-contaminated soil from the Dukhan city, B.) Oil-contaminated soil from the Industrial area, C.) Artificial oil-contaminated soil with 2% crude oil (FOCS), and D. The overlay plot of the three oil-contaminated soils.
The obtained results of the FTIR analysis showed that the Dukhan city and the industrial area soil samples had the major peaks in the 800–1036 cm−1, 1400 cm−1, and 2800–2900 cm−1 regions corresponding to the C-H stretching, C-H (CH2, CH3) asymmetric bending, and C-H stretching (symmetric and asymmetric), respectively. These variations in the functional groups can be attributed to the fact that changes in the functional groups on the surface of the weathered samples (the Dukhan city and industrial area soil samples) occurs as a function of time. It undergoes various photooxidation reactions from the sun, unlike the non-weathered samples that did not undergo any oxidation reaction (Maletić et al., 2011; Coates, 2006; Solomon and Carangelo 1988; Munajad et al., 2017; Ţucureanu et al., 2016; Almansoory et al., 2019). Schneider et al., (2016) identified functional groups of nitro-PAHs compounds, which are 1-nitropyrene, 2-nitrofluorene, and 6-nitrochrysene in transmittance and emissivity spectra of PM1 samples (particular matter size of 1 μm) using FTIR. No sample preparation was done for obtaining the FTIR spectra. The FTIR spectral fingerprints proved that nitro-PAHs could be identified and differentiated by measuring the FTIR transmittance value. It was shown that the nitro-PAHs standard spectra could successfully classify nitro-PAHs in PM1 samples. It was observed that the transmittance and emissivity spectra in the samples presented broad bands and low intensity in comparison to the standard nitro-PAHs spectra. In this study, the peak reduction for the Dukhan city soil (the lowest water content) was achieved at high MW power and short exposure time, while the highest reduction in the FTIR peaks for the industrial area soil (low water content) was obtained at high MW power and long exposure time. The reduction of 2800–2900 cm−1 peaks, which represent the C-H stretching, in the industrial soil occurred at high MW power and long exposure time.
3.3 MW And UV treatment analysis
The UV irradiation offers an encouraging environment and plays a critical role in the transformation and degradation of PAHs on the soil matrix (Gbeddy et al., 2020). Six MW treatment variables were studied including high MW power and short exposure time, high MW power and medium exposure time, high MW power and long exposure time, low MW power and short exposure time, low MW power and medium exposure time, low MW power and long exposure time (Fig. 2), as well as twelve UV-C (in the range of 240–280 nm) treatment variables. Each MW treatment sets went through the UV-C treatment with different time exposure, 10 min, and 20 min (Fig. 2).
Fig. 4 illustrates that the high MW power causes more FTIR transmittance reduction for the industrial soil sample than the low MW power (Fig. 4A) in all identified peaks in the spectra regions in Fig. 3A and 3B which are 800–1036 cm−1, 1400 cm−1, and 2800–2900 cm−1 regions corresponding to the C-H stretching, C-H (CH2, CH3) asymmetric bending, and C-H stretching (symmetric and asymmetric), respectively. Furthermore, as shown in Fig. 4B, there is no large difference between the three spectra samples treated with MW in the same manner but differ in the UC-V treatment; MW 100 power with 15 min, MW 100 power with 15 min followed by UV-C for 10 min, MW 100 power with 15 min followed by UV-C for 20 min. It can be concluded that the industrial soil with the highest TOC and TOM values requires the use of high MW power and long exposure time to reach the maximal FTIR transmittance reduction. Zhang et al. (2010) studied the rate of degradation in soil surface for pyrene by using UV-C light (245 nm) under various parameters. The results showed as the degradation rate increased from 25% to 35%, the temperature increased from 20◦C to 30◦C. Moreover, particles with size <1 mm had faster photodegradation than particles with <0.25 mm size. This can be attributed to the fact that particles with <1 mm can scatter more light and it allows it to permeate the soil more. Eker and Sengul (2018) compared the efficiencies of PAHs degradation in soil by solar radiation UV-A and UV-C treatments. The soil samples from the industrial area were exposed to three conditions namely ambient air, UV-A, and UV-C irradiation. The PAHs removal values obtained from ambient air, UV-A and UV-C were 35%, 90%, and 86%, respectively. Furthermore, the effects of adding TiO2 and H2O2 catalysts at different concentrations (1%, 10%, and 20% of dry soil weight) for PAHs removal under ambient air conditions to mimic the real conditions were investigated. The highest PAHs removal was achieved under a concentration of 20% TiO2 (89%) while being the highest under the concentration of 1% of H2O2 (88%). Regarding the molecular weight of different PAHs, the removal efficiencies increased for both LMW and HMW PAHs by adding the catalysts. However, without the addition of catalysts, the removal efficiency was higher in LMW PAHs.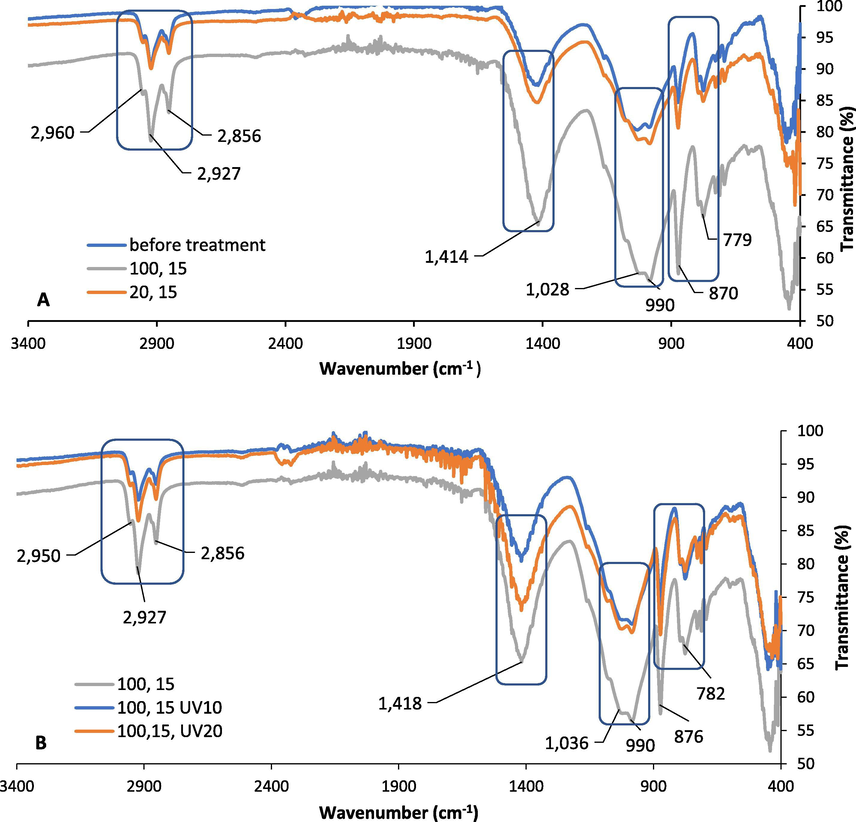
FTIR spectra of the industrial city sample A.) Before treatment and after (100% MW Power, 15 min exposure time), and (20% MW power, 15 min exposure time), B.) MW treatment (100% MW power, 15 min exposure time) and followed by UV-C treatment for 10 min and 20 min.
In order to achieve the maximum FTIR transmittance reduction, the Dukhan soil with less TOC than the industrial soil required high MW power and short exposure time followed by UV-C treatment for 20 min. Fig. 5A shows the FTIR spectra for the samples before applying any MW treatment and after two sets of MW treatment, which are high MW power (100%) and short exposure time (5 min), and high MW power (100%) and long exposure time (15 min). The FTIR results showed that there is a transmittance reduction in some peaks at 871 cm−1, 1009 cm−1, 1419 cm−1, while for the peaks 2849 cm−1 and 2919 cm−1 there was no transmittance reduction at all for the high MW power and short exposure time treatment. However, the treatment set of high MW power and high exposure time had similar FTIR spectra as the untreated sample, which indicated that the 100% high MW power treatment was efficient at short exposure time (5 min) for some peaks only. Fig. 5B shows that the FTIR spectra of the Dukhan sample of the two treatment sets (100% MW, 15 min exposure time, and 10 min UV-C) and (100% MW, 5 min exposure time, and 20 min UV-C) have a similar peak reduction compared to the untreated sample. Additionally, there was no observed transmittance reduction in peaks around 2849 cm−1 and 2919 cm−1 after treatment with UV-C and other studied treatments.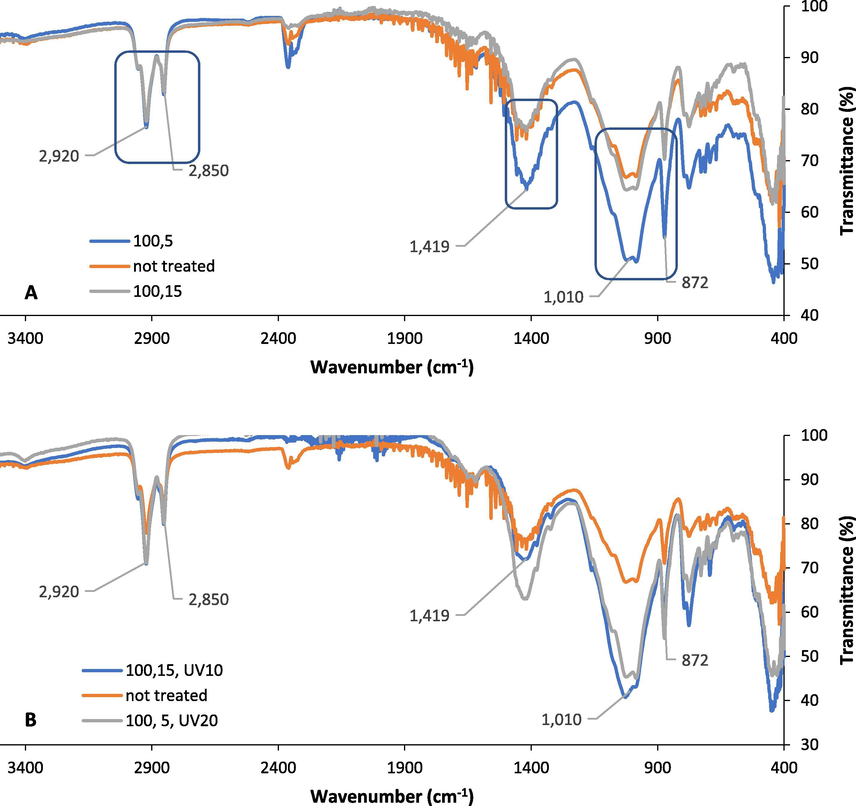
FTIR spectra for the Dukhan city sample A.) Before treatment and after (100% MW, 5 min exposure time) and (100% MW, 15 min exposure time), B.) Before treatment and after MW treatment followed by UV-C (100% MW, 5 min exposure time, and 20 min UV-C) and (100% MW, 15 min exposure time, and 20 min UV-C).
The FOCS sample with the lowest TOC and TOM values reached the maximum transmittance reduction at high MW power and short exposure time, and low MW power and short exposure time. From Fig. 6A, it is concluded that the highest transmittance reduction in 854 cm−1 and 1419 cm−1 peaks occurred at high MW power and short exposure time. Similarly, as shown in Fig. 6B, the low MW power was more efficient at 5 min exposure than at 10 and 15 min exposures, which indicates that longer MW exposure time can cause adverse effects on the reduction of the assigned peaks. Finally, Fig. 6C compares the two MW power treatments (100% MW, 5 min exposure time, and 20% MW, 5 min exposure time), and displayed no big difference between the two microwave power treatments. On the other hand, the UV-C treatment for the FOCS sample was not efficient compared to the MW treatment for both high and low MW power treatment as shown in Fig. 6D.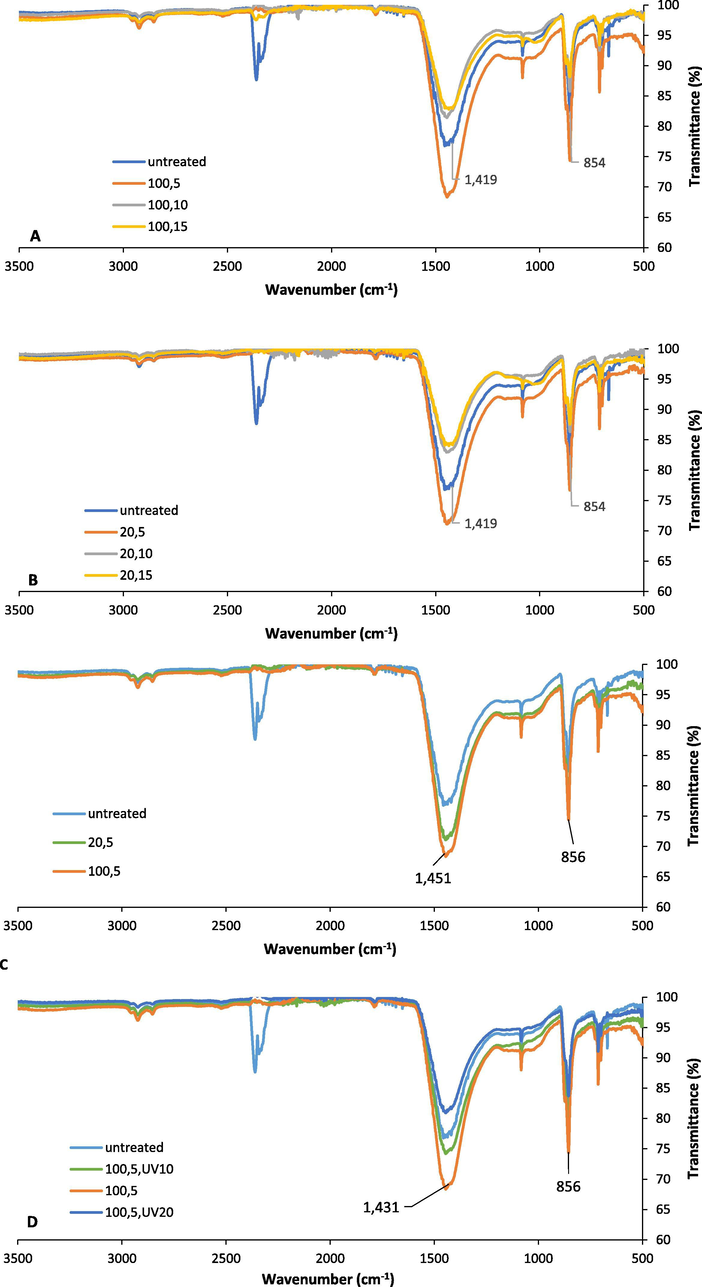
A.) FTIR spectra for the FOCS before treatment and after 100% MW power treatment for different exposure times, B.) FTIR spectra for the FOCS before treatment and after 20% MW power treatment for different exposure times, C.) FTIR spectra for FOCS sample before treatment and after (100% MW, 5 min exposure time and 20% MW, 5 min exposure time), and D.) FTIR spectra for the FOCS before treatment and after (100% MW, 5 min exposure time) followed by UV-C treatment (10 min and 20 min).
Moreover, the dielectric properties of a material
,
) plays a very crucial role in the PAHs removal by the MW heating, in which
is the irradiated materials' ability for storing electromagnetic energy or to be polarized. The dielectric loss factor is denoted by
representing the material ability to transform the electromagnetic energy to heat energy. Furthermore, the rate of energy generation within a defined volume of an irradiated material can be represented as an equation (2):
Besides, the time-dependent equation for PAHs contaminated soil remediation by microwave heating is described by Eq. (1).
Falciglia et al. (2018a, 2018b) studied the effect of photodegradation treatment on contaminated sediments with PAHs. The used MW power was 650 W, 2.45 GHz in a time range of 1 to 7 min, while for the used wavelength for UV-A was 350 nm within the time range up to 36 min. Because of their dielectric properties of the sediments and the PAHs, the results demonstrated a rapid increase in the sediment temperature that occurred during the MW heating. It was noticed that more than 85% removal of PAHs within 1 min MW treatment (650 W, 2.45 GHz), and total PAHs removal was achieved with longer MW irradiation.
The PAHs properties, namely polarity, and size, might affect the PAHs degradation and the maximum temperature reachable during the MW heating processes in addition to the PAHs removal kinetics. The degradation efficiency of hydrocarbons from the soil contaminated by hydrocarbons could be due to different mechanisms: (i) vaporization by thermal desorption, (ii) degradation by chemical bond breaking, and (iii) PAHs vapor stripping (Fig. 7) (Falciglia et al., 2018a, 2018b). Besides, the molecular structure and polarity of the PAHs are correlated with the activation energy required for the PAHs desorption from soil due to the affinity of PAHs to the soil particles. In the MW heating process, the first step would be rapid PAHs evaporation due to the effect of water stripping, while, the second one is the internal diffusion phenomena. Based on the physiochemical characterizations of the soil samples, the water content was the highest for the FOCS, and the lowest was for the Dukhan soil. This totally supports the result of the FTIR that the efficiency of degradation was the highest for the FOCS, and the lowest was for the Dukhan soil. In terms of the kinetic of hydrocarbons removal, it was shown that the Dukhan soil needed more time for PAHs degradation as the TPH was the highest (313.3 ppm).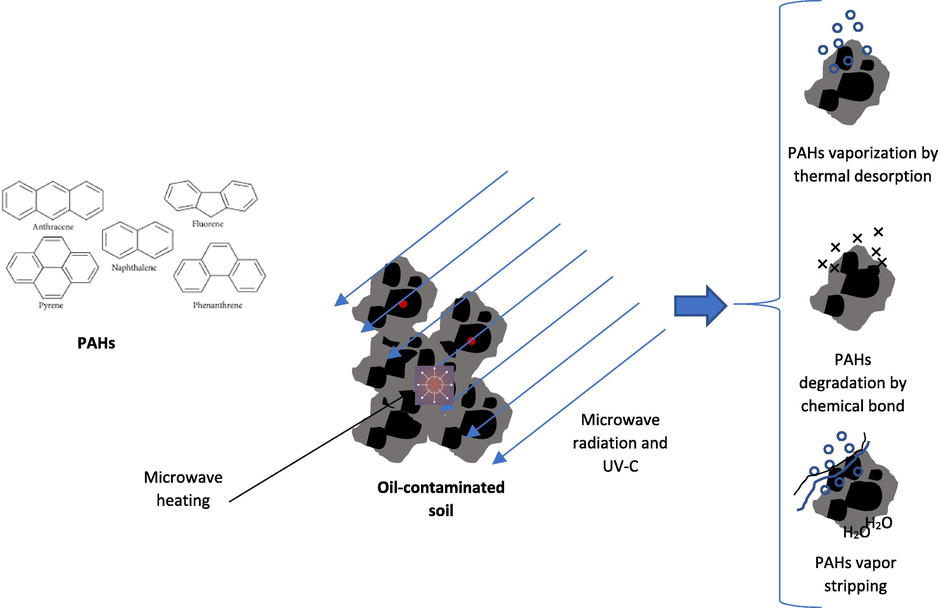
The three mechanisms of PAHs degradation during the MW heating and UV-C irradiation.
Also, the availability of the PAHs bound residues in soil, which persist in the soil matrix, may significantly reduce the accessibility and the availability for the MW radiation and UV-C irradiation. These bound residues in soil could be described as covalently bound to the soil, adsorbed residues to the soil matrix by reversible non-covalent interactions; and entrapped residues, which are retained within the soil matrix (Northcott and Jones, 2000).
3.4 Principal component analysis (PCA)
Since the study involves various variables, PCA was used to analyze all treatment sets. Thus, various experimental data groups were associated with the FTIR (i.e. reduction of relevant spectra peaks). The results were sorted out based on their similarity and variance by using PCA. PCA divides the data into clustered groups of spectra sharing the same variation characteristics and showing the differences between them (Abdel Samad et al., 2020). In this study, each sample has been gone for eighteen different sets of treatments as is illustrated in Fig. 2. Hence, the cluster analysis was also used to evaluate the impact of microwave heating followed by UV-C irradiation on the degradation of PAHs. The PCA was used in this study to compare all these 18 treatments for any variation or similarity. Various studies have been used a combination of FTIR spectroscopy and multivariate analysis such as PCA to compare or test the variations or similarities between different treatments (Oussama et al., 2020; Ashfaq et al., 2019). Fig. 8A was constructed to determine which MW treatment differs significantly from the spectra of the samples before the treatment, where the percent in x-axis and y-axis represent the variability distance, and it is shown that x-axis with 97% is more significant than the distance in y-axis with 2% (Ashfaq et al., 2019). Furthermore, the PCA showed that the high MW power and long exposure (100% MW, 15 min exposure time) treatment was the most significantly different compared to other samples before the treatment. Then, the PCA was compared with the FTIR spectra for the samples before treatment, most effective treatment samples, and the least efficient treatment based on the PCA, and the results are shown in Fig. 8A and 8B. Then, a second PCA was done to compare the most effective UV-C treatment at two different exposure times (10 min and 20 min). The PCA results showed that neither MW treatment (100% MW, 15 min exposure time) followed by UV-C treatment for 20 min nor 10 min is significantly different from the MW treatment (100% MW, 15 min exposure time). However, the three treatments described in Fig. 8B are significantly different from the untreated industrial soil. It is worth mentioning that the difference or variance in the PCA was explained based on the x-axis (97%) (Fig. 8).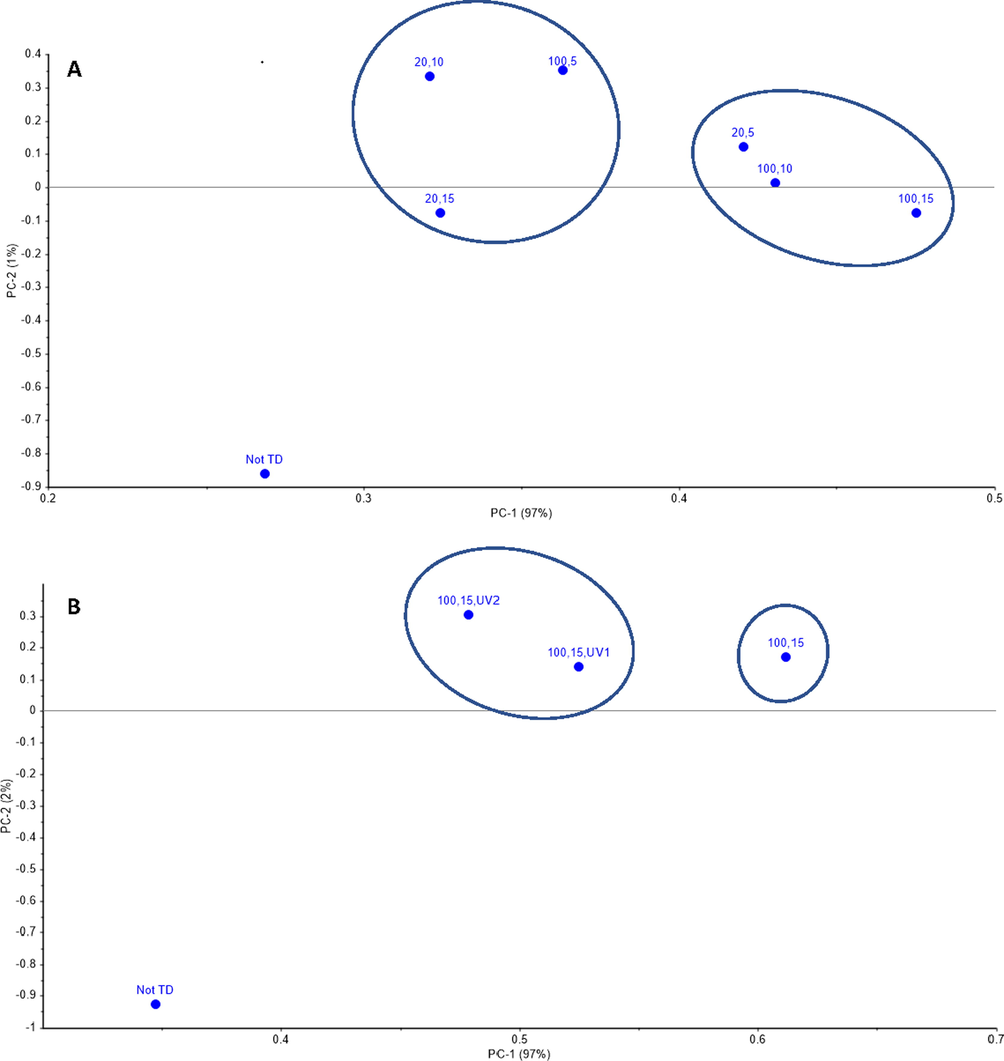
PCA results of the industrial sample after A.) MW treatments, B.) MW followed by UV-C treatments.
Fig. 9A illustrates the PCA analysis results for all MW treated Dukhan city sample to estimate the difference between MW treatments. Based on the PCA of the Dukhan city sample as shown in Fig. 9A, the variance was obtained based on the x-axis (97%), the treatment set of high MW power with short exposure time (100% MW, 5 min exposure time) was the most significantly different from all the other samples. While the treatment of low MW power with different exposure times gave similar results (central cluster in Fig. 9A). Another PCA was carried out to compare the results of samples treated with high MW power and short exposure time (100% MW, 5 min exposure time) and high MW power and long exposure time (100% MW, 15 min exposure time) with the two sets of MW followed by UV-C treated samples at 10 min and 20 min, respectively as shown in Fig. 9B and C. Fig. 9B shows that the UV-C treatment at 20 min after high MW power and short exposure time (100% MW, 5 min exposure time) is most significantly different from the three tested data sets. Furthermore, the UV-C treatment at 10 min after high MW power and long exposure time (100% MW, 15 min exposure time) is the most efficient, even better than the MW treatment alone, which was not effective (Fig. 9C).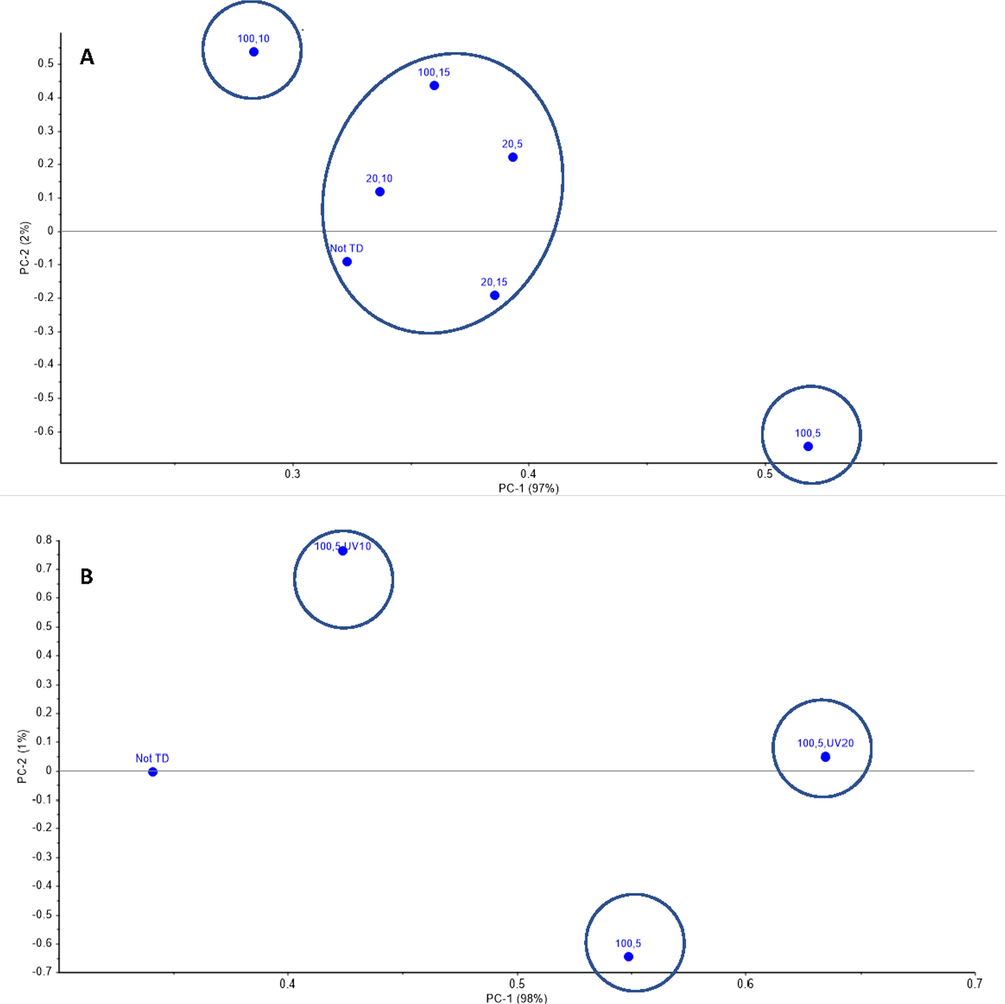
The PCA results of Dukhan city sample A.) MW treatments, B.) MW treatments followed by UV-C (100% MW, 5 min exposure time, 10 min UV-C) and (100% MW, 5 min exposure time, 20 UV-C), C.) MW treatment followed by UV-C (100% MW, 15 min exposure time, 10 min UV-C) and (100% MW, 15 min exposure time, 20 min UV-C).
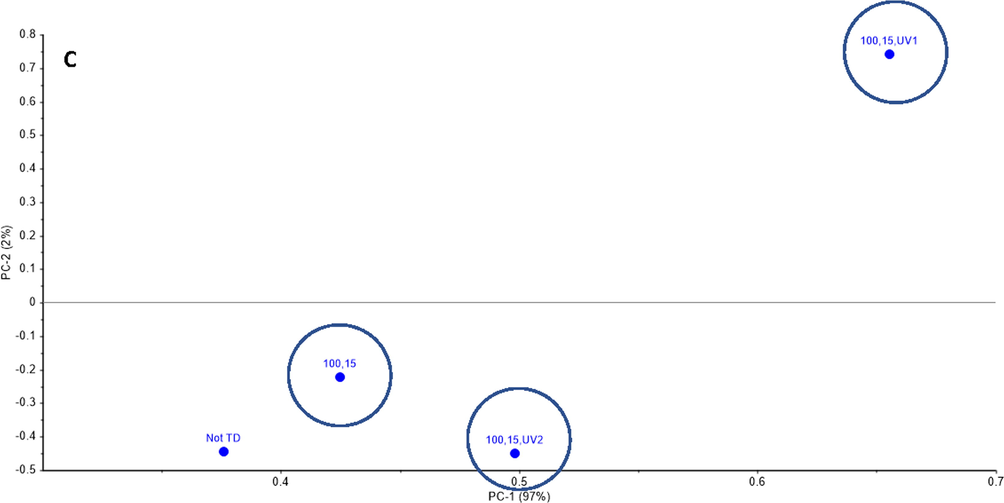
The PCA results of Dukhan city sample A.) MW treatments, B.) MW treatments followed by UV-C (100% MW, 5 min exposure time, 10 min UV-C) and (100% MW, 5 min exposure time, 20 UV-C), C.) MW treatment followed by UV-C (100% MW, 15 min exposure time, 10 min UV-C) and (100% MW, 15 min exposure time, 20 min UV-C).
Similarly, to the other samples, the PCA of the MW treatments of the FOCS sample was conducted. Fig. 10 shows the PCA results for all the MW treatment sets with an x-axis variance of 97%. It can be observed that treatments are mostly grouped in one group on the left side that is far away from the untreated samples grouped on the right side. While on the y-axis (2%), the variance was the highest for high MW power and short exposure time (100% MW, 5 min exposure time) compared to the untreated FOCS sample. It is worth mentioning that after all treatments the peaks at 2300 cm−1 corresponding effectively to CO2 disappeared (Munajad et al., 2017).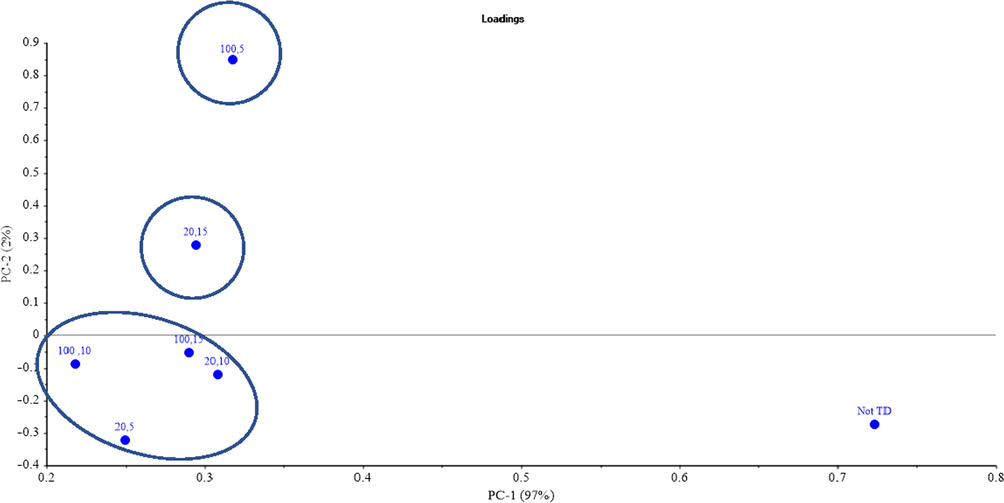
PCA results of the FOCS for all the MW treatment sets.
It could be noticed that the PCA can divulge clusters of similar behaviors. However, the cluster analysis is the recommended technique for categorizing groups of treatments. Here, the cluster analysis was used to evaluate the impact of MW heating, and MW heating followed by UV-C irradiation on the degradation of PAHs from contaminated soil. The nearest neighbor cluster method was implemented during the cluster analysis (CA). For the industrial soil sample, two clusters were obtained namely cluster 1 (100% MW and 5 min exposure time), (20% MW and 10 min exposure time), (20% MW and 15 min exposure time) and cluster 2 (100% MW and 10 min exposure time), (100% MW and 15 min exposure time), (20% MW and 5 min exposure time). While for the Dukhan city soil sample, only one cluster was obtained, namely (100% MW and 15 min exposure time), (20% MW and 5 min exposure time), (20% MW and 10 min exposure time), and (20% MW and 15 min exposure time); these have the highest similarity in behavior when the MW heating was used. For the industrial soil sample regarding MW followed by UV-C irradiation, two clusters were obtained, namely cluster 1 (100% MW, 15 min exposure time, and 10 min UV-C), (100% MW, 15 min exposure time, and 20 min UV-C), and cluster 2 (100% MW, 15 min exposure time). While three clusters were obtained for the Dukhan city soil sample, namely cluster 1 (100% MW, 15 min exposure time, and 10 min UV-C), cluster 2 (100% MW, 15 min exposure time, and 20 min UV-C), and cluster 3 (100% MW, 15 min exposure time). Ultimately, the PCA results entail significant alteration across the irradiation period.
4 Conclusion
In this study, the potentiality of using MW treatment, and MW treatment followed by UV-C treatment in treating PAHs-contaminated soil was examined. The FTIR was used to examine any difference or reduction in certain peaks and their wavenumbers. Multivariate analysis, PCA, was used to analyze for any variance in the different conditions of microwave treatment and UV treatment. This study presented the importance of the soil properties in determining the best condition with a high removal efficiency of hydrocarbon from oil-contaminated soil. The industrial soil has the highest concentration of TOC and TOM followed by the Dukhan soil, while the FOCS has the lowest concentration of TOC and TOM, and it has the highest water content percentage and PAHs content among the three soil types. The Dukhan soil has the highest concentration of total petroleum hydrocarbon (TPH) followed by the industrial soil and the FOCS.
The obtained results of the FTIR analysis showed that the Dukhan city and the industrial area soil samples had the major peaks in the 800–1036 cm−1, 1400 cm−1, and 2800–2900 cm−1 regions corresponding to the C-H stretching, C-H (CH2, CH3) asymmetric bending, and C-H stretching (symmetric and asymmetric), respectively. In this study, the peak reduction for the Dukhan city soil (the lowest water content) was achieved at high MW power and short exposure time, while the highest reduction in the FTIR peaks for the industrial area soil (low water content) was obtained at high MW power and long exposure time. The reduction of 2800–2900 cm−1 peaks, which represent the C-H stretching, in the industrial soil occurred at high MW power and long exposure time. It can be concluded that the industrial soil with the highest TOC and TOM values requires the use of high MW power and long exposure time to reach the maximal FTIR transmittance reduction. The Dukhan soil with less TOC than the industrial soil required high MW power and short exposure time followed by UV-C treatment for 20 min.
The PCA results of the industrial city sample showed that neither MW treatment (100% MW, 15 min exposure time) followed by UV-C treatment for 20 min nor 10 min is significantly different from the MW treatment (100% MW, 15 min exposure time). However, for the Dukhan sample, the UV-C treatment at 10 min after high MW power and long exposure time (100% MW, 15 min exposure time) was the most efficient treatment.
Acknowledgement
The authors would like to thank the lab technician Mr. Abdul-Ali Moghaddasi for his effort and help throughout the project.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The use of principle component analysis and MALDI-TOF MS for the differentiation of mineral forming Virgibacillus and bacillus species isolated from sabkhas. RSC Adv.. 2020;10(25):14606-14616.
- [CrossRef] [Google Scholar]
- A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet.. 2016;25(1):107-123.
- [CrossRef] [Google Scholar]
- Analytical Methods for Polycyclic Aromatic Hydrocarbons and their Global Trend of Distribution in Water and Sediment: A Review. Recent Insights in Petroleum Science and Engineering, Mansoor Zoveidavianpoor, IntechOpen, 2017 Available from:
- [CrossRef] [Google Scholar]
- A MALDI-TOF study of bio-remediation in highly weathered oil contaminated soils. J. Petrol. Sci. Eng.. 2018;168:569-576.
- [CrossRef] [Google Scholar]
- Biosurfactant produced by the hydrocarbon-degrading bacteria: Characterization, activity and applications in removing TPH from contaminated soil. Environ. Technol. Innovation. 2019;14:100347.
- [CrossRef] [Google Scholar]
- Multivariate analysis for FTIR in understanding treatment of used cooking oil using activated carbon prepared from olive stone. PLoS ONE. 2020;15(5):e0232997.
- [CrossRef] [Google Scholar]
- Investigating the effect of temperature on calcium sulfate scaling of reverse osmosis membranes using FTIR, SEM-EDX and multivariate analysis. Sci. Total Environ.. 2020;703:134726
- [CrossRef] [Google Scholar]
- Evaluating the effect of antiscalants on membrane biofouling using FTIR and multivariate analysis. Biofouling. 2019;35(1):1-14.
- [CrossRef] [Google Scholar]
- Determination of Saturate, Aromatic, Resin and Asphaltenic (SARA) Components in Crude Oils by Means of Infrared and Near-Infrared Spectroscopy. Energy Fuels. 2001;15(5):1304-1312.
- [CrossRef] [Google Scholar]
- Remediation of phenanthrene contaminated soil by coupling soil washing with Tween 80, oxidation using the UV/S2O82− process and recycling of the surfactant. Chem. Eng. J.. 2019;369:1014-1023.
- [CrossRef] [Google Scholar]
- Enhanced electrokinetic remediation of multi-contaminated dredged sediments and induced effect on their toxicity. Chemosphere. 2019;228:744-755.
- [CrossRef] [Google Scholar]
- Development and evaluation of a continuous microwave processing system for hydrocarbon removal from solids. Chem. Eng. J.. 2016;283:215-222.
- [CrossRef] [Google Scholar]
- Enhanced persulfate degradation of PAH-contaminated sediments using magnetic carbon microspheres as the catalyst substrate. Process Saf. Environ. Prot.. 2019;125:219-227.
- [CrossRef] [Google Scholar]
- Interpretation of Infrared Spectra, A Practical Approach. Encyclopedia Analyt. Chem.. 2006;10:a5606.
- [CrossRef] [Google Scholar]
- Infrared Spectroscopy as a Versatile Analytical Tool for the Quantitative Determination of Antioxidants in Agricultural Products. Foods Plants. Antioxidants (Basel). 2015;4(3):482-497.
- [CrossRef] [Google Scholar]
- Removal of Polycyclic Aromatic Hydrocarbons (PAHs) from Industrial Soil with Solar and UV Light. Polycyclic Aromat. Compd.. 2018;1–13
- [CrossRef] [Google Scholar]
- Remediation of soils contaminated with PAHs and nitro-PAHs using microwave irradiation. Chem. Eng. J.. 2016;296:162-172.
- [CrossRef] [Google Scholar]
- Microwave heating coupled with UV-A irradiation for PAH removal from highly contaminated marine sediments and subsequent photo-degradation of the generated vaporized organic compounds. Chem. Eng. J.. 2018;334:172-183.
- [CrossRef] [Google Scholar]
- A review on the microwave heating as a sustainable technique for environmental remediation/detoxification applications. Renew. Sustain. Energy Rev.. 2018;95:147-170.
- [CrossRef] [Google Scholar]
- Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs) J. Hazard. Mater.. 2009;172(2–3):532-549.
- [CrossRef] [Google Scholar]
- Application of multivariate data techniques in photochemical study of polycyclic aromatic hydrocarbons (PAHs) and transformed PAH products in road dust. Ecotoxicol. Environ. Saf.. 2020;196:110478
- [Google Scholar]
- Bioremediation of Polluted Soil Sites with Crude Oil Hydrocarbons Using Carrot Peel Waste. Environments. 2018;5(11)
- [CrossRef] [Google Scholar]
- Degradation of polycyclic aromatic hydrocarbons (PAHs) in contaminated soils by Fenton's reagent: A multivariate evaluation of the importance of soil characteristics and PAH properties. J. Hazard. Mater.. 2007;149(1):86-96.
- [CrossRef] [Google Scholar]
- Biodegradation of PAHs in Soil: Influence of Initial PAHs Concentration. IOP Conf. Ser.: Materi. Sci. Eng.. 2016;136:012052.
- [CrossRef] [Google Scholar]
- Pilot scale applications of microwave heating for soil remediation. Chem. Eng. Process. - Process Intensif.. 2018;130:53-60.
- [Google Scholar]
- Soil bioremediation approaches for petroleum hydrocarbon polluted environments. AIMS Microbiol.. 2017;3(1):25-49.
- [CrossRef] [Google Scholar]
- The application of microwave heating in bioenergy: A review on the microwave pre-treatment and upgrading technologies for biomass. Renew. Sustain. Energy Rev.. 2017;77:12-27.
- [CrossRef] [Google Scholar]
- Remediation approaches for polycyclic aromatic hydrocarbons (PAHs) contaminated soils: Technological constraints, emerging trends and future directions. Chemosphere. 2017;168:944-968.
- [CrossRef] [Google Scholar]
- Lee, B.-K. (2010). Sources, Distribution and Toxicity of Polyaromatic Hydrocarbons (PAHs) in Particulate Matter. Air Pollut. http://doi.org/10.5772/10045.
- Enhanced degradation of polycyclic aromatic hydrocarbons by indigenous microbes combined with chemical oxidation. Chemosphere. 2018;213:551-558.
- [CrossRef] [Google Scholar]
- Pilot-scale electro-bioremediation of heavily PAH-contaminated soil from an abandoned coking plant site. Chemosphere. 2020;244:125467.
- [CrossRef] [Google Scholar]
- Microwave thermal remediation of crude oil contaminated soil enhanced by carbon fiber. J. Environ. Sci.. 2009;21(9):1290-1295.
- [CrossRef] [Google Scholar]
- A PAH-degrading bacterial community enriched with contaminated agricultural soil and its utility for microbial bioremediation. Environ. Pollut.. 2019;251:773-782.
- [CrossRef] [Google Scholar]
- Distribution of polycyclic aromatic hydrocarbons in the sediments of the Adriatic Sea. Environ. Pollut.. 2002;119:91-98.
- [Google Scholar]
- Impact of hydrocarbon type, concentration and weathering on its biodegradability in soil. J. Environ. Sci. Health, Part A. 2011;46(10):1042-1049.
- [CrossRef] [Google Scholar]
- Seasonal Variation and Sources of Polycyclic Aromatic Hydrocarbons (PAHs) in Indoor and Outdoor Air in a Semi-Arid Tract of Northern India. Aerosol. Air Qual. Res.. 2012;12(4):515-525.
- [CrossRef] [Google Scholar]
- Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci. Total Environ.. 2019;696
- [CrossRef] [Google Scholar]
- Munajad, A., Subroto, C., Suwarno, 2017. Fourier transform infrared spectroscopy (FTIR) analysis of transformer insulation paper in natural ester. 2017 International Conference on High Voltage Engineering and Power Systems (ICHVEPS). http://doi.org/10.1109/ichveps.2017.8225887.
- Rapid assessment of petroleum-contaminated soils with infrared spectroscopy. Geoderma. 2017;289:150-160.
- [CrossRef] [Google Scholar]
- Experimental approaches and analytical techniques for determining organic compound bound residues in soil and sediment. Environ. Pollut.. 2000;108:19-43.
- [Google Scholar]
- Visible and near-infrared spectroscopy analysis of a polycyclic aromatic hydrocarbon in soils. Sci. World J.. 2013;2013:160360.
- [CrossRef] [Google Scholar]
- Rapid quality control of industrial flocculents using fourier transform mid-infrared spectra and multivariate analysis. Chemomet. Intell. Lab. Syst.. 2020;202:104030.
- [CrossRef] [Google Scholar]
- Removal Capacities of Polycyclic Aromatic Hydrocarbons (PAHs) by a Newly Isolated Strain from Oilfield Produced Water. Int. J. Environ. Res. Publ. Health. 2017;14(2)
- [CrossRef] [Google Scholar]
- Degradation of fluorene and phenanthrene in PAHs-contaminated soil using Pseudomonas and Bacillus strains isolated from oil spill sites. J. Environ. Manage.. 2019;232:1-7.
- [CrossRef] [Google Scholar]
- A decision framework for selecting remediation technologies at hydrocarbon-contaminated sites. J. Soil Contamination. 1993;2(2):167-189.
- [CrossRef] [Google Scholar]
- Identification of hydrocarbon sources in contaminated soils of three industrial areas. Sci. Total Environ.. 2013;450–451:13-21.
- [CrossRef] [Google Scholar]
- Adsorption kinetics of anthracene and phenanthrene in different soils of Attock Refinery Limited (ARL) Rawalpindi, Pakistan. Desalin. Water Treatment. 2011;30(1–3):333-338.
- [CrossRef] [Google Scholar]
- Sakshi, Singh, S.K., Haritash, A.K., 2019. Polycyclic aromatic hydrocarbons: soil pollution and remediation. Int. J. Environ. Sci. Technol., 16(10), 6489–6512. http://doi.org/10.1007/s13762-019-02414-3.
- FTIR analysis and evaluation of carcinogenic and mutagenic risks of nitro-polycyclic aromatic hydrocarbons in PM1.0. Sci. Total Environ.. 2016;541:1151-1160.
- [CrossRef] [Google Scholar]
- Degradation of toxic PAHs in water and soil using potassium zinc hexacyanoferrate nanocubes. J. Environ. Manage.. 2017;204:337-348.
- [CrossRef] [Google Scholar]
- Microwave (MW) remediation of hydrocarbon contaminated soil using spent graphite - An approach for waste as a resource. J. Environ. Manage.. 2019;230:151-158.
- [CrossRef] [Google Scholar]
- Naphthalene degradation by Fe(2+)/Oxone/UV - Applying an unconventional kinetics model and studying the reaction mechanism. Chemosphere. 2019;218:110-118.
- [CrossRef] [Google Scholar]
- FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem.. 2016;46(6):502-520.
- [CrossRef] [Google Scholar]
- Thermal Treatment of Hydrocarbon-Impacted Soils: A Review of Technology Innovation for Sustainable Remediation. Engineering. 2016;2(4):426-437.
- [CrossRef] [Google Scholar]
- Application of UV-rays in removal of polycyclic aromatic hydrocarbons from treated wastewater. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng.. 2011;46(3):248-257.
- [CrossRef] [Google Scholar]
- Photolysis of polycyclic aromatic hydrocarbons on soil surfaces under UV irradiation. J. Environ. Sci.. 2013;25(3):569-575.
- [CrossRef] [Google Scholar]
- Photodegradation of pyrene on soil surfaces under UV light irradiation. J. Hazard. Mater.. 2010;173(1–3):168-172.
- [CrossRef] [Google Scholar]







