Translate this page into:
Reactive melt blending of low-density polyethylene with poly (acrylic acid)
*Tel.: +964 947811923127 tahseen12_saki@yahoo.com (Tahseen A. Saki)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 17 June 2011
Abstract
Reactive melt blending of low-density polyethylene LDPE with a low molecular weight poly (acrylic acid) PAA in the presence of benzoyl peroxide (BPO) free radical initiator was studied by means of a HAAKE torque rheometer and a capillary rheometer FTIR spectroscopy and was used to characterize the structure of LDPE/PAA blends. The rheological behavior of blends has been investigated at different temperatures (170, 190, 210 and 230 °C) and shear rates (5.43, 18.1, 54.3, 181 and 543 S−1). With a rise of temperature and increasing shear rates the shear stress of blends increases nonlinearly. The melt viscosity increases with the increase in the PAA proportion but decreases with the increasing shear rate and shear stress. The melts of all the blends are pseudoplastic in nature. The flow behavior index (n) of the blends decreases with the increase in the PAA proportion and with the increase in the temperature. The temperature sensitivity of the flow behavior of the blends is studied using Arrhenius plots. The system exhibits relatively low activation energy which means less sensitivity to temperature with an increase in the PAA ratio.
Keywords
Low-density polyethylene
Poly(acrylic acid)
Reactive blend
FTIR
Rheological behavior
Flow index
Activation energy
1 Introduction
Low-density polyethylene LDPE is the one of the most important thermoplastics today, it has gained an important position among other polymers due to its wide range of mechanical, physical and chemical properties.
Low-density polyethylene is a partially crystalline solid with a degree of crystallinity in the 50–60% range that leads to several properties such as opacity, rigidity (stiffness), tensile strength, tear strength, and chemical resistance (Billmeyer, 1971).
However, its use in blending with other polymers has been limited due to the absence of polar functional groups and lack of reactive sites on LDPE back bone. Chemical modification through functionalization or grafting leads to the introduction of polar group onto polymer main chain as pendent unites or short chain branching via free radical reactions initiated by organic peroxides to generate reactive sites on polymer chain for grafting (Ghosh et al., 1998). Another way of increasing the adhesion, compatibilization, wettability properties of LDPE is its modification in the bulk by the addition of small amounts of polar polymer or low molecular weight additives. Different monomers were grafted onto LDPE such as maleic anhydride, unsaturated carboxylic derivatives and vinyl or acrylic compounds containing more than one functional group (Ghosh et al., 1998; Ghosh and Dev, 1998). LDPE modified with polar monomer has found wide application as reactive compatibilizer in LDPE blends in which the second component of the mixture is a polar polymer.
Compatibility of the immiscible blend components can be greatly improved using peroxides, taking advantage of high reactivity of polyolefins to free radical that good starting point for promoting compatibilization between polypropylene PP/polyethylene PE blends, although the individual component responds differently to peroxide (PP degraded &PE crosslink), on the other hand, low molecular weight compounds can be used by adding in the melt to initiate grafting – coupling reactions forming graft or block co-polymers during processing.
Compatibility between polyethylene with other polyolefins by addition of peroxide having been obtained in several researches, Vogel and Heinze studied effects of processing conditions on crosslinking LDPE/EVAc ethylene vinyl acetate copolymer by DCP when mixed in internal mixer at 150–210 °C (Vogel and Heinze, 1993), Abraham et al., prepared LDPE/LLDPE blend in internal mixer at 160 °C by DCP dicumyl peroxide (Abraham et al., 1992), Yu et al., mixed LDPE/PP blend by internal mixer at 180 °C uses 2,5-dimethyl-2,5-di-t-butylperoxy hexane as radical coupling agent (Yu et al., 1990, 1992, 1994).
The main aim of chemical modification of LDPE molecules is to enhance blend compatibilization and to improve some useful properties such as compatibility polyethylene with polystyrene (Kim et al., 1997), polyamide, polyester (Karl Fink, 2005), starch (Huang et al., 2005) poly (butylenes terephthalate) (Yang et al., 2003). Functionalization of polyolfines for improving adhesion is usually carried out through carboxylation with unsaturated monomers (eig. Maleic anhydride, acrylic acid) used at low concentration (Ghosh and Dev, 1998) as well as the population and a proximity of the propagation poly(acrylic acid) chains increase (acrylic acid suffered homopolymerization when grafted onto polyolfines in the melt thus bringing in a reduction in the graft yield) (Ghosh and Dev, 1998; Kim et al., 1997; Bhattacharya and Inamdar, 2007) which lead to the higher rate of termination and consequently lower grafted degree on polymer backbone. On the other hand, if the amount of the functional group is too small, few graft copolymers are formed and thus cannot play efficient roles as compatibilizers; the final physical properties of the polymer blend become poor (Kim et al., 1999). In this work, preparation blend of low-density polyethylene LDPE with a much higher concentration of carboxylate (low molecular weight poly(acrylic acid))by reactive blending in the batch mixers is made in the presence of an organic peroxide. The react blends were examined by Fourier transform infrared (FTIR) spectroscopy in attempts to confirm the “in situ” formation of compatibility copolymers and study rheological characterization of the grafted copolymers.
2 Materials
Low-density polyethylene (LDPE grade SCPILEN 22004) of density 0.921 gm/cm3 and melt flow index 0.32 g/10 min was procured from state company for petrochemical industry, Basrah, Iraq. Benzoyl peroxide BPO has been used as graft and cross-linking agent supplied from Fluka. Acrylic acid (obtained from BDH) polymerized to low molecular weight poly (acrylic acid) PAA in the store. Molecular weight (Mv) of this poly (acrylic acid) was determined with viscosity testing method in this case, Mark-Houwink Eq. (1) as used
By substituting these data in Eq. (1) the molecular weight was found to be 11,370–11,376 dalton.
About 2 g of gross LDPE (95 wt.%)/PAA (5 wt.%) product prepared in this laboratory was dissolved in 100 mL boiling xylene and precipitated in 100 mL acetone to remove unreacted PAA during melt mixing. The precipitated LDPE/PAA was filtered, washed, and then dried in an air oven.
Approximately 0.5 g of the purified LDPE/PAA sample mentioned above was dissolved in 50mL of hot xylene, and then titrated with 0.05N KOH in methanol using phenolphthalein as an indicator. The equation to calculate degree of grafting of PAA can be expressed as Eq. (2):
3 Blend preparation
All blends were prepared by melt-mixing of the components in an internal mixer (Haake Rheometer 90) with rotor speed of 50 rpm and at 160 °C. Benzoyl peroxide was added in four portions at 2 min, mixing was continued for 2 min after the last addition. The total mixing time was 10 min in all cases.
The blends having different compositions from PAA wt.% = 1–5 at the fixed peroxide concentration (0.2 g). In all cases, the blends were compression molded into sheets immediately after mixing was finished for FTIR analysis.
4 FTIR spectroscopy
Fourier transforms infrared ray FTIR measurement by Shimadzu FTIR – 8400S for neat LDPE and PAA besides LDPE/PAA/BPO blends.
Unmodified LDPE film had absorption bands at the expected frequencies for saturated aliphatic groups, including strong intensities at 2936–2916, 2863–2843 and 720–750 cm−1 for methylene vibration, as well as, less induce absorption at 1380–1375 cm−1indicating methyl groups as shown in Fig. 1. FTIR spectrum of thin film of PAA observed a weak absorption peak at 1640 cm−1 in PAA molecules indicates the presence of trace quantities of the —C⚌C— double bond stretching in the PAA molecules, Fig. 2. This confirms the availability of sites for reacting (cross-linking) with free radicals on LDPE chains. This band at 1640 cm−1 disappears in the reactive LDPE/PAA blends spectra may be due to cross-linking reactions. Fig. 3 shows reactive blends spectra, strong band 3100–2900 cm−1 and sharp absorption stretching carboxylic acid at 1725–1700 cm−1, lower intensity absorption bending at 1440–1395 cm−1, 3400–3200 cm−1 broad band for hydroxyl group stretching vibration. All these bands indicate the presence of carboxylic acid in the LDPE/PAA blends.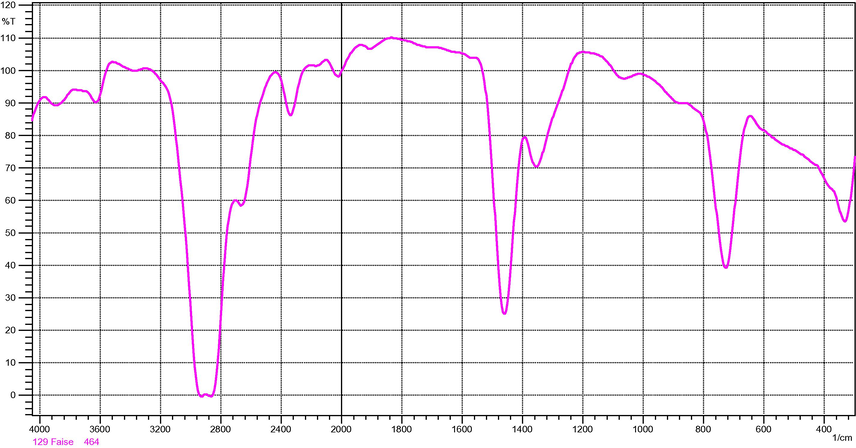
FT-IR low density polyethylene.
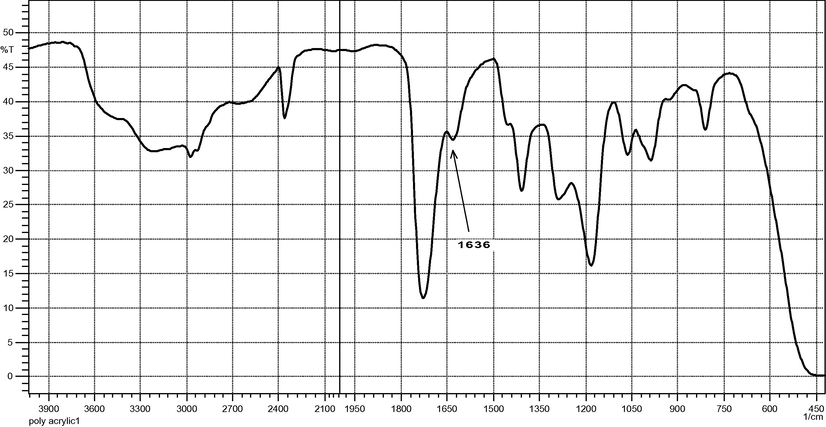
FT-IR of low molecular weight poly(acrylic acid).
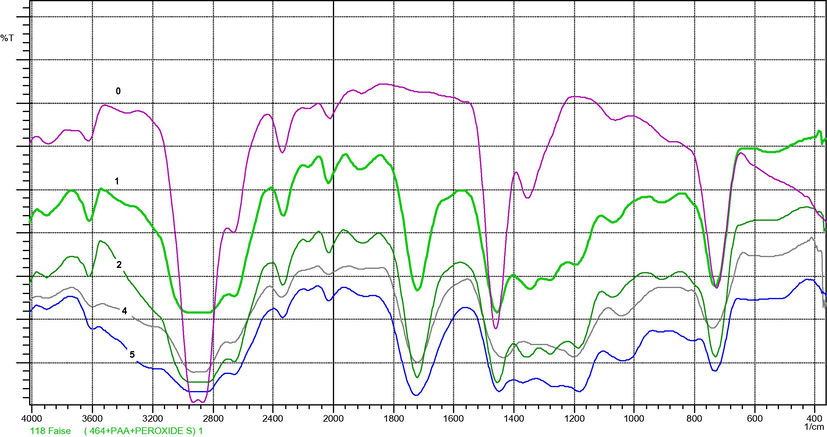
FT-IR spectra of LDPE/PAA blend, 0 = neat LDPE, 1 = 1% PAA, 2 = 2% PAA, 4 = 4% PAA, 5 = 5% PAA. Note: spectrum of the ratio 3% PAA do not show in FT-IR spectra Fig. 3 because of interface with other spectrum.
5 Reaction mechanism
Covalent crosslinking reactions mediated by a third, added reagent might give the same type of copolymer structure as that which results from direct crosslinking reactions. In this case, the added reagent is a radical initiator or other type of activating agent such as a condensing agent. Such activating agents are usually not incorporated into the final copolymer.
Radical initiators may promote radical formation on each of two immiscible polymers. A crosslinked copolymer results through radical–radical coupling between the two polymers at a melt phase interface. Self-coupling of each polymer may compete with cross-coupling as is the case with most 3-body reactions in which an added reagent is capable of reacting with each of the two immiscible polymers.
Immiscible blends of polyolefins have been compatibilized by copolymer formation brought about by radical coupling across a melt phase boundary Polyolefin/LDPE blends have been compatibilized by addition of a radical initiator (Utracki, 2002).
Low density polyethylene and poly (acrylic acid) are totally immiscible because of their different molecular structures. Both have reactivity to free radical reactions but different responses to peroxide. Several researches refer to the ability of grafting functional group according to the reactive blend on LDPE which is above that of other polyolefins and this was the reason for choosing LDPE for blend. It is well established that incorporation of peroxide in the molten LDPE induces a cross-linking reaction of the LDPE molecules. Double bonds of the incorporated PAA seemed to act as a new site for the grafting initiation, leading to the promotion of grafting. On the other hand, it is plausible that the double bonds in the PAA also act as a cross-linked point to result in grafted chains with a cross-linked structure.
Therefore, in the melt mixing and peroxide, the grafting and cross-linking reaction of LDPE and PAA took place, Scheme 1.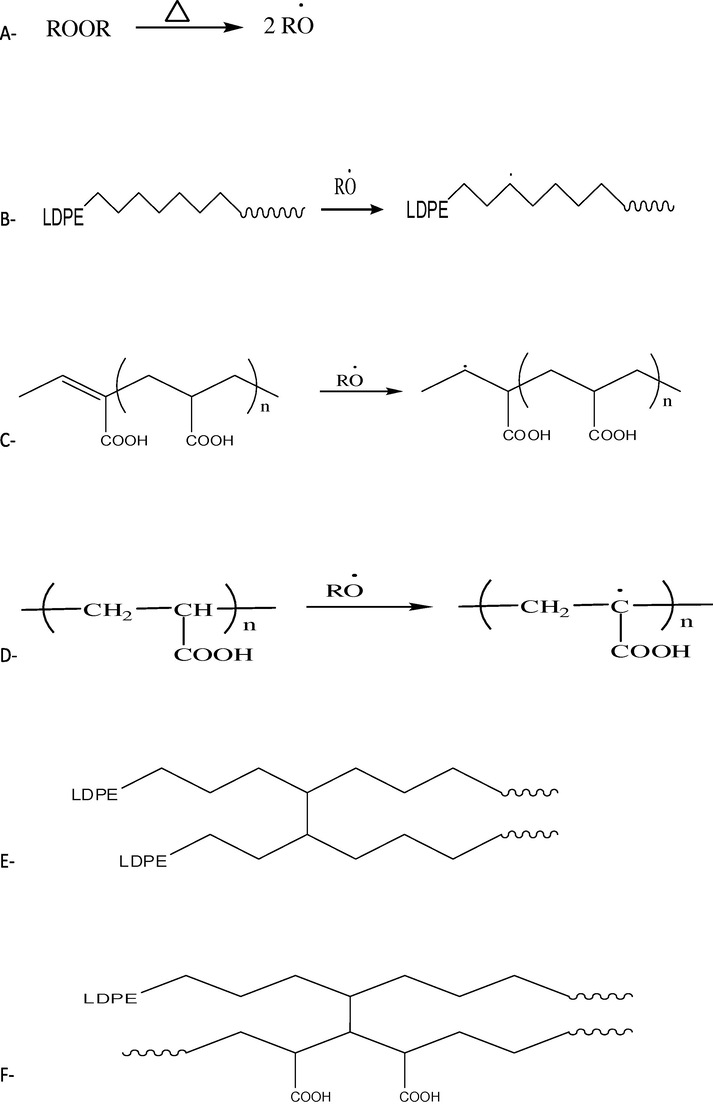
Possible reactions of the free radical in the LDPE/PAA/BPO system, (A) peroxide decomposition, (B) free radical generation on LDPE, (C and D) free radical generation on PAA, (E)crosslinking LDPE, (F) crosslinking or grafting reaction.
From FTIR results we can conclude that the grafting reaction of PAA on LDPE chains occurs by abstraction of hydrogen atom from tertiary carbon atom at branches of the LDPE backbone when reacting with free radical source (benzoyl peroxide decomposition). The peroxide cross-linking may occur at the tertiary carbon on both polymers (Scheme 1D) this obtained when grafted maleic anhydride onto ethylene vinyl acetate copolymer (Soares and Colombaretti, 1999) or unsaturated —C⚌C— of the PAA chains or both sites with LDPE macro radical chains that lead to. This result is in agreement with the FTIR observation in the previous study.
This result suggests that, from the point of view of the thermal response, the branched molecular structure obtained in this case may be similar to the conventional branched structure of PEs.
6 Rheological properties
The rheological properties of polymeric materials vary with their chemical structures. Therefore, it is highly desirable to be able to relate the rheological properties of polymeric materials to their chemical structures.
Rheological behavior of polymeric melts is an important aspect to understand the flow behavior of the materials during processing. Capillary rheometry is the most common technique used to determine deformation of polymeric melt under shear flow (Sombatsompop and Dangtungee, 2002).
A capillary rheometer (Instron model 3211) was used to study the shear flow properties of the low-density polyethylene blends. The experiment was carried out using a wide range of cross head speed (0.06, 0.2, 0.6, 2.0 and 6.0 cm min−1) at different temperatures 170, 190, 210 and 230 °C. Dimensions of the capillary die used were 1.257 mm, the length to diameter (L/D) ratio 80/9 and 90° entry angle. The apparent values of shear stress, shear rate and shear viscosity were calculated using Eqs. (3)–(14) (Manual, xxxx):
The shear rate (γ) for Newtonian fluid was calculated from the equations:
From Eqs. (7) and (9), substitution in Eq. (10) we get:
The apparent viscosity (ηa) was calculated from the equation:
7 Shear stress of the blends
It is known that the properties of grafted LDPE changes from nonpolar to polar due to the orientation of acrylic groups (Oh et al., 2001).
Chain entanglements and branch entanglements provide non-Newtonian rheology for higher molar mass PE. Entanglements of branches increase the melt strength of PE with long branches, especially LDPE (Nwabunma, 2008).
Figs. 4 and 5 show the shear stress vs. shear rate of LDPE/PAA blends. It can be seen that the shear stress increases with increase in the shear rate and at a low shear rate, the LDPE/PAA blends exhibit slightly higher shear stress than the neat LDPE and increase with increasing shear rate. These results again indicate that the graft copolymer was formed in situ at the interface of LDPE/PAA blends. (See Fig. 6.)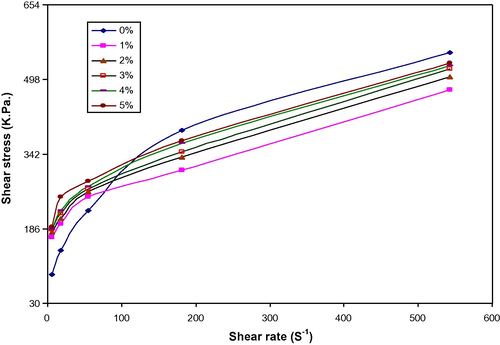
Shear stress vs. shear rate for LDPE/PAA blends at different PAA (wt.%).
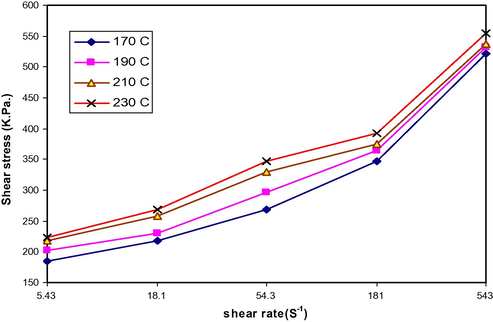
Shear stress vs. shear rate for LDPE/PAA blends at PAA (3 wt.%) and various temperature
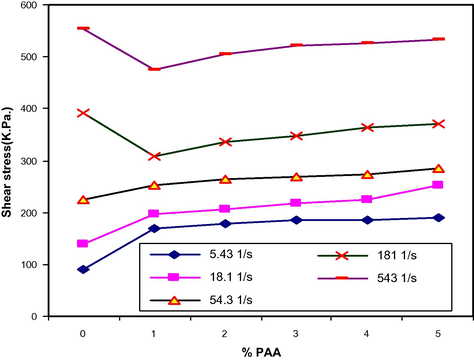
Shear stress vs. PAA (wt.%) for LDPE/PAA blends at different shear rate.
As it starts to move, the carbon-carbon chains of the molecule will tend to orient in the direction of flow. If the force is applied very slowly, so that the Brownian motion overcomes the orienting caused by the flow, the mass of the polymer will move with a rate proportional in the applied stress. This is Newtonian flow and corresponds straight to the section at the beginning of the curve on Fig. 4.
As we increase the flow rate, two things happen. The chain moves so rapidly that it is not sufficient for the Brownian motion to have an appreciable effect. Secondly, the molecules being oriented in one direction separate because of their side chains. This separation reduces the intermolecular forces, permitting them to slide over each other much more easily (Frados, 1976).
As the polarity of the copolymer increases with the increasing content of acrylic acid, the bonding with ionic groups on blend increases. The shear stress therefore increases correspondingly.
8 Viscosity of the blend
The viscosity was determined by dividing the apparent shear stress and apparent shear rate and the plot of viscosity vs. shear rate is shown in Fig. 7. For all blends, the pseudoplastic characteristics can be observed from the decrease of viscosity with increasing shear rate.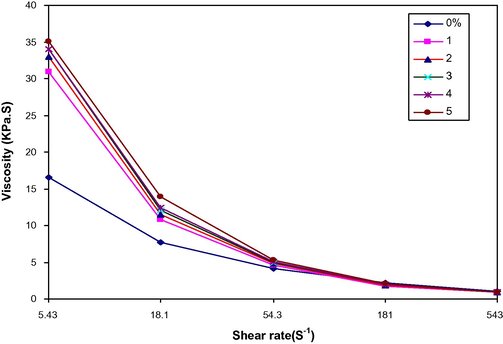
Viscosity vs. shear rate for LDPE/PAA blends at different PAA (wt.%).
Introduction of the polar acrylic acid unit into the polymer structure increases its polarity while at the same time disrupting the interchain bonding and crystalline nature of PE. Initially, the disruption of the interchain interaction of PE predominates. Consequently, flow is enhanced and melts index increases. Further increase in acrylic acid content results in the predominance of chain polarity and hence results in intermolecular attraction. This leads to a decrease in the flow properties.
The applied force disturbed the long chain polymer from its equilibrium position and the molecules got disentangled in the direction of the force and this caused a reduction in viscosity (Xu et al., 1999; George et al., 1999).
Fig. 7 shows the proposed interaction between the LDPE/PAA with the presence of BPO. The increase in viscosity of polymer blends upon the addition of peroxide and this has been reported by Ghosh et al. (1997). The increase in viscosity indicates that there is less slippage at the interface of LDPE/PAA blends as a result of the addition of BPO.
Another way, the decreasing trend of viscosity (η) with increasing shear rate (γ), commonly referred to as “shear-thinning behavior,” is believed to arise from the stretching of an “entangled” state of polymer chains to an “oriented” state when the applied shear rate is higher than a certain critical value (Han, 2007).
Fig. 8 shows a plot of viscosity vs. shear stress at temperature 170 °C of LDPE/PAA blends with peroxide. For all blends, it can be seen that the viscosity decreased with increasing shear stress, and the difference of the viscosity of LDPE/PAA blends higher than that of LDPE at low shear stress than that at high shear stress, however, when the PAA increased from 1 % to 5 wt.%, the viscosity did not obviously change, Fig. 8.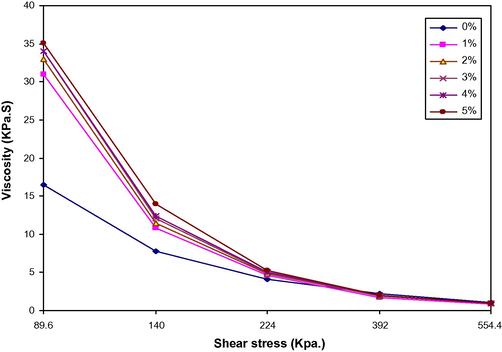
Viscosity vs. shear stress for LDPE/PAA blends at different PAA (wt.%).
Again at a similar temperature, the LDPE/PAA blends show higher viscosity than the LDPE due to the better interaction between LDPE and PAA with the presence of BPO, Figs. 7 and 8.
At a similar shear rate 5.43 S−1, LDPE/PAA blends exhibit higher viscosity than the LDPE alone and increases viscosity with increasing temperature (Fig. 9). The addition of PAA increases the interaction between LDPE/PAA blend chains becoming stronger. This result can be explained; an increase in the temperature would be expected to make the polymer more viscous due to more interaction.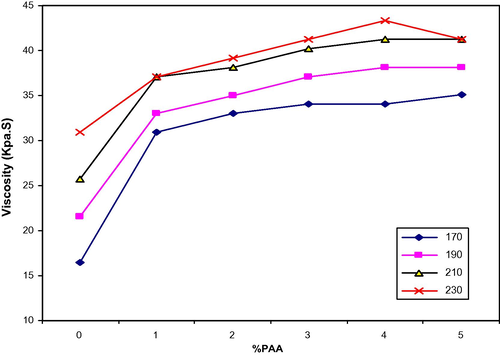
Viscosity vs. PAA (wt.%) for LDPE/PAA blends at different temperature and shear rate = 5.43 S−1.
As discussed before, the viscosity of the blends depends on the strength of the intermolecular structure of the blend components. PAA branch chains grafted onto LDPE imparted hindrance to the movement of LDPE chains at high temperature because these branch chains are readily tangled with each other at high temperature. These results that are similar to grafted copolymerization of oleic acid onto LDPE using DCP as an initiator in the molten state (Liu et al., 2003) have different results and structures from those of LLDPE-g-AA (Huang et al., 2001).
9 Power law index and activation energy
Power-law is behavior characterized by a power (n) relationship between shear stress and shear rate. The power-law equation was applied to describe the rheological behavior of the system.
The melt flow behavior can be described by power law, which is expressed by Ostwald and de Waele model. The equation for this model is given as follows:
The power-law index n basically represents the slope of the shear stress (τ) vs. shear rate (γ) curve and n − 1 is the negative slope of the η vs. γ curve in the medium- to high-shear-rate range (Shenoy and Saini, 1996).
The variation of power law index, n of LDPE/PAA blends with BPO is presented in Table 1. It can be seen that the value of n decreases with the addition of PAA. This might be due to the formation of grafted PAA onto LDPE with BPO besides the lower value of n indicates the pseudoplastic nature of the LDPE/PAA with BPO. The increased interaction between LDPE/PAA chains will obviously increase the rigidity of the polymer chain and consequently prevent the chains from slippage.
230 °C
210 °C
190 °C
170 °C
PAA (%)
K
n
K
n
K
n
K
n
2.0616
0.1533
1.9666
0.1712
1.8969
0.1762
1.7481
0.2029
0
2.1792
0.1046
2.1751
0.1
2.1226
0.1079
2.0866
0.11
1
2.2114
0.0969
2.1988
0.0979
2.1487
0.1062
2.111
0.1108
2
2.2462
0.0951
2.231
0.0946
2.1721
0.1043
2.1277
0.1101
3
2.2735
0.0903
2.2538
0.0909
2.1887
0.1022
2.1309
0.1121
4
2.2563
0.0955
2.2539
0.0934
2.1973
0.1024
2.168
0.1058
5
The n and K-values are presented in Table 1. The system shows a pseudoplastic nature (since n < 1). The flow behavior index decreases with the increasing content of the PAA and the temperature. With LDPE alone the chance of orientation increases, resulting in the observed variation of n-values.
During the addition of PAA and BPO, during melt mixing, the PAA forms LDPE-g-PAA with crosslinking, which is likely to hinder the molecular orientation. On the other hand, with an increase in temperature, enhance grafting and crosslinking reactions that led to the reduction of the chain motion or molecular vibration and the chance of orientation of molecules increase. Thus, the n-value decreases with the PAA content and the temperature.
The consistency index (K) increases with the increase in the PAA content and the temperature. The consistency index represents the viscosity at unit rate of shear (Jana and Nando, 2005). With increasing PAA content with BPO, the free volume of the system decreases, so the K-value increases. The increase in temperature and grafting or crosslinking tends to decrease the free volume of the system and thus the K-value increases.
10 Activation energy of melt flow
Controlling the processing temperature is an important means to regulate the flow ability of polymer melts. The melt viscosity of the blends as a function of temperature at the shear rate represented at 170, 190, 210, and 230 °C that increasing temperature improves the flow behavior of the melts.
It is due to the fact that when the temperature increases, the melt free volume increases, causing an enhancement of chain segments motion and a reduction of interactions between chain segments. As a result, the melt viscosity decreases with the increase in the temperature. However, the effect of temperature on melt viscosity is also dependent on the shear rate, which will be discussed in a subsequent section.
The temperature dependence of the viscosity of the blends can be expressed by the flow activation energy calculated from the Arrhenius equation as stated in Eq. (17):
Activation energy of flow, Ea is the energy that needs to be consumed for breaking up the interactions among the chain segments when the melt flows.
Ea can be evaluated from the graph of log viscosity against the reciprocal absolute temperature 1/T (calculated from the slope of straight-line; figures not shown). Table 2 shows the activation energy of the LDPE/PAA blends with BPO. It can be seen that the flow activation energy of the LDPE/PAA is lower than that of LDPE. As shown in Table 2, it is clear that the activation energy for viscous flow decreases with increase in shear rate. This behavior can be attributed to the presence of the PAA chain, which is possibly grafted onto the LDPE chains during the reaction. Thus, the LDPE/PAA blends with BPO are less sensitive to temperature.
181 S−1
18.1 S−1
5.43 S−1
PAA (%)
0.025
0.071
0.0886
0
0.027
0.0291
0.026
1
0.017
0.0323
0.028
2
0.016
0.0326
0.028
3
0.0121
0.0367
0.033
4
0.0183
0.023
0.024
5
11 Conclusion
Low-density polyethylene LDPE can be grafted or crosslinked with poly(acrylic acid) PAA via free radical generated on tertiary carbon atom and unsaturated sites on PAA.
FTIR spectra of the blends show that the band at 1640 Cm−1 for double bond on the PAA disappears and new bands appear at range 1700–1725 Cm−1 and 3200–3400 Cm−1 for carbonyl and hydroxyl in the carboxylic groups, respectively. Rheological properties of the blends refer to the grafted copolymer that was formed in situ at the interface of LDPE/PAA blends. All PAA ratio in the blends exhibited increasing shear stress and viscosity than neat LDPE. Also this system shows a pesudoplastic nature (since power law index <1). On the other hand, activation energy for viscous flow was lower than neat LDPE. Thus, the system LDPE/PAA/BPO was less sensitive to temperature.
References
- Polyacrylic acid grafting onto isotactic polypropylene fiber: methods, characterization, and properties. J. Appl. Polym. Sci.. 2007;103:1152-1165.
- [Google Scholar]
- Textbook of Polymer Science (second ed.). John Wiley & Sons, Inc.; 1971.
- Frados, Joel, 1976. Plastic Engineering Handbook, fourth ed. The Society of the Plastic Industry, p. 111.
- Rheological behaviour of thermoplastic elastomers from polypropylene/acrylonitrile–butadiene rubber blends: effect of blend ratio, reactive compatibilization and dynamic vulcanization. Polymer. 1999;40:4325.
- [Google Scholar]
- Reactive processing of polyethylene: effect of peroxide-induced graft copolymerization of some acrylic monomers on polymer structure melt rheology and relaxation behavior. Eur. Polym. J.. 1998;34(10):1539-1547.
- [Google Scholar]
- Reactive melt processing of polyethylene: effect of peroxide action on polymer structure, melt rheology and relaxation behaviour. Polymer. 1997;38:6175-6180.
- [Google Scholar]
- Modification of low density polyethylene (LDPE) by graft copolymerization with some acrylic monomers. Polymer. 1998;39(1):139-201.
- [Google Scholar]
- Rheology and Processing of Polymeric Materials. Vol vol. 1. Polymer Rheology Oxford University Press, Inc.; 2007.
- Morphology, structure, and rheological property of linear low-density polyethylene grafted with acrylic acid. J. Appl. Polym. Sci.. 2001;80:2538-2544.
- [Google Scholar]
- Effect of compatibiliser on the biodegradation and mechanical properties of high-content starch/low-density polyethylene blends. Polym. Degrad. Stab.. 2005;90(1):95-105.
- [Google Scholar]
- Rheological behavior of low-density polyethylene (LDPE)–polydimethylsiloxane rubber (PDMS) blends. J. Elastomers Plast.. 2005;37:149-168.
- [Google Scholar]
- Reactive Polymers Fundamentals and Applications. William Andrew; 2005.
- Effect of molecular architecture of in situ reactive compatibilizer on the morphology and interfacial activity of an immiscible polyolefin/polystyrene blend. Polymer. 1997;38(8):1809-1815.
- [Google Scholar]
- Compatibilization mechanism of polymer blends with an in-situ compatibilizer. Polymer. 1997;38(9):2155-2164.
- [Google Scholar]
- Effect of the functional group inhomogeneity of an in situ reactive compatibilizer on the morphology and rheological properties of immiscible polymer blends. Polymer. 1999;40:2737-2743.
- [Google Scholar]
- Graft copolymerization of oleic acid onto low-density polyethylene in the molten state. J. Appl. Polm. Sci.. 2003;90:3299-3304.
- [Google Scholar]
- Manual (10-364-1A), xxxx. Capillary Rheometer for 3211 Instron Machine .
- Polyolefin Blends. John Wiley & Sons, Inc.; 2008.
- Synthesis and surface property variations polypropylene-graft-poly(ethylene glycol) J. Colloid Interface Sci.. 2001;238:43-47.
- [Google Scholar]
- Thermoplastic Melt Rheology and Processing. Marcel Dekker, Inc.; 1996.
- Melt functionalization of EVA copolymers with maleic anhydride. J. Appl. Polym. Sci.. 1999;72:1799-1806.
- [Google Scholar]
- Effects of the actual diameters and diameter ratios of barrels and dies on the elastic swell and entrance pressure drop of natural rubber in capillary die flow. J. Appl. Polym. Sci.. 2002;86(7):1762-1772.
- [Google Scholar]
- Utracki, L.A., 2002. Polymer Blends Handbook. In: Bruce Brown, S. (Eds.), Reactive Compatibilization of Polymer Blends. Kluwer Academic Publishers, p. 339 (Chapter 5).
- Reactive processing of polyethylene and ethylene-vinyl acetate copolymers. Prosecution of the reaction and produce evidence that changes. Angew. Makromol. Chem.. 1993;207(1):157-171.
- [Google Scholar]
- Melt rheology of compatibilized polystyrene/low density polyethylene blends. Polym. Int.. 1999;48(11):1113-1120.
- [Google Scholar]
- Efforts to decrease crosslinking extent of polyethylene in a reactive extrusion grafting process. J. Appl. Polym. Sci.. 2001;79(3):535-543.
- [Google Scholar]
- Rheological properties and morphology of compatibilized poly(butylenes terephthalate)/linear low-density polyethylene alloy. J. Appl. Polym. Sci.. 2003;88:206-213.
- [Google Scholar]
- Peroxide modified polyolefin blends: Part I. Effects on LDPE/PP blends with components of similar initial viscosities. Adv. Polym. Technol.. 1990;10(3):163-172.
- [Google Scholar]
- Peroxide modified polyolefin blends – Part II: effects on LDPE/PP blends with polymer components of dissimilar initial viscosities. Adv. Polym. Technol.. 1992;11(4):295-304.
- [Google Scholar]
- LDPE/PP blends modified by peroxide and radiation induced reactions. J. Appl. Polym. Sci.. 1994;52(1):99-105.
- [Google Scholar]







