Translate this page into:
Recent advancement and development of chitin and chitosan-based nanocomposite for drug delivery: Critical approach to clinical research
⁎Corresponding authors at: Department of Environmental Science and Engineering, Government College University, Faisalabad 38000, Pakistan (S. Ali). Laboratory of Quality of Vegetables and Medicinal Plants Department of Vegetable Crops and Medicinal Plants, University of Life Sciences in Lublin, 15 Akademicka Street, 20-950 Lublin, Poland (A. Najda). Pharmacology Department, Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt (Abdel-Daim M.). agnieszka.najda@up.lublin.pl (Agnieszka Najda), shafaqat@mail.cmuh.org.tw (Shafaqat Ali), shahid@ujs.edu.cn (Shahid Hussain)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This review depicts the exposure of chitin and chitosan base multifunctional nanomaterial composites for promising applications in field of biomedical science structure, synthesis as well as potential application from a colossal angle. We elaborated critically each of the chitin and chitosan base nanomaterial with its potential application toward biomedical science. For different biomedical applications it use in form of hydrogels, microsphere, nanoparticles, aerogels, microsphere and in form of scaffold. Due to this it had been blended with different polymer such as starch, cellulose, alginate, lipid, hyaluronic acid, polyvinyl alcohol and caboxymethyl cellulose. In this review article, a comprehensive overview of combination of chitin and chitosan base nanomaterial with natural as well as synthetic polymers and their biomedical applications in biomedical field involving drug delivery system all the technical scientific issues have been addressed; highlighting the recent advancements.
Keywords
Chitin
Chitosan
Natural polymer
Nanomaterials
Drug delivery
Cancer
- Cur
-
Curcumin
- UV
-
Ultraviolet
- PVA
-
Poly vinyl Alcohol
- LWMG
-
low molecular weight gelator
- HPMC
-
Hydroxypropyl methyl cellulose
- CS
-
Chitosan
- IBM
-
Imatinib mesylate
- CML
-
chronic myeloid leukemia
- DDS
-
Drug delivery system
- BC
-
Bacterial Cellulose
- RA
-
Retinoic acid
- PEG
-
Polyethylene glycol
- VEGF
-
vascular endothelial growth factor
- VPF
-
vascular permeability factor
- CD
-
Cyclodextrin
- LCST
-
lower critical solution temperature
- HA
-
Hydroxyapatite
- IBD
-
Inflammatory bowel disease
- UC
-
ulcerative colitis
- MT
-
Metoprolol Tartrate
- CD
-
Crohn's disease
- NSC
-
N-succinyl chitosan
- FDDS
-
Floating drug delivery system
- PEO
-
Polyethylene Oxide
- OG
-
Okra gum
- GO
-
graphene oxide
- 6-TG
-
6-thioguanine
- PAA
-
Poly(acrylic acid)
- FS
-
free-standing
- BMSC
-
bone marrow stromal cells
- QPM
-
Quaternary polymethacrylate
- MIPs
-
Molecularly imprinted polymers
- SAPs
-
Superabsorbent polymers
- Pas
-
Peptide amphiphiles
- UTI
-
urinary tract infections
- CSC
-
Cardiac Stem Cell
- SPIONs
-
Superparamagnetic iron oxide nanoparticles
- SSD
-
Silver sulfadiazine
- PEI
-
Polyethyleneimine
- QS
-
Quinapyramine sulfate
- PPy
-
Polypyrole
- DOX
-
Doxorubicin
- SLN
-
solid liquid nanoparticles
- CD
-
Bacterial cytosine deaminase
- 5-FC
-
5-fluorocytosine
- HER
-
human epidermal growth factor receptor
- Col
-
collagen
- BTE
-
Bone tissue engineering
- FDA
-
Food and Drug Administration
- PCL
-
Polycaprolactone
- TACE
-
transcatheter arterial chemoembolization
- DNA
-
deoxyribonucleic acid
- MRI
-
magnatic resonance imaging
- PEI
-
polyethylenimine
- SEM
-
scanning electron microscope
- DLS
-
dynamic light scattering
- SAXS
-
small angle X-ray scattering
- FT-IR
-
Fourier-transform infrared spectroscopy
- TEM
-
transmission electron microscope
- XRD
-
X-ray diffraction
- GCS
-
glycol chitosanstearate
- NMR
-
nuclear magnetic resonance
- CLSM
-
Confocal microscopy
- GA
-
galvanometric analysis
- HPLC
-
high performance liquid charomatography
- PLAG
-
poly(lactic-co-glycolic acid)
- CMC
-
carboxymethyl cellulose TC, tocopheryl succinate
- PAMAM
-
polyamidoamine
- ZP
-
Zeta-potential
Abbreviations
1 Introduction
Nanotechnology offers a leading-edge technology as a new therapeutic approach since very long (Ahmad et al., 2020; Salata, 2004). Discovery of potential polymeric nanomaterials has advanced the spectrum of translational research in Biomedicine (Pervaiz et al., 2019). Considering the status of Biology and Medicine, nanotechnology involves the polymeric and metallic nanomaterials, nanodevices and also those structures that have small length scales (Coutinho et al., 2020). One of the most important, abundant, bridgeable, biocompatible and polymeric nanomaterials being used as a successfully investigated and reported polymer is chitin and its derivative chitosan (Sultankulov et al., 2019). Chitin (C8H13O5N) is extended long-chain polymer of N-acetylglucosamine and is made from modified glucose. It is the main component of cell walls in fungi, the exoskeletons of arthropods, for example, crustaceans and insects, the radulae of molluscs, cephalopod beaks, and the scales of fish and Lissamphibia’s (Muzzarelli, 2013). The structure of chitin is comparable to another polysaccharide which is cellulose, forming crystalline nanofibrils or whiskers. In terms of function, it may be compared to the protein keratin (Huang et al., 2020). Chitin as a polymer is one of the most abundant element of marine environment and comes after cellulose on Earth (Rudall and Kenchington, 1973). Many techniques have been reported regarding chitosan and chitosan extraction and synthesis from different sources. One of the most commonly employed method includes grinding of shells and demineralization using the dilute acidic medium. Following the deproteinization with aqueous solution of sodium hydro-oxide and potassium hydro-oxide from residual material. But the biggest problems such processes and methods offer to environment is pollution due to toxic waste material. Hence, to account for the environmental control during all this makes it a costly and laborious method. More eco-friendly extraction and synthesis and deproteination have gained attention such as bacterial fermentation and use of proteolytic enzymes (Yang et al., 2020). Chitosan due to its potential physiochemical properties and biological properties it offers wide range of application in the cosmetic industry, textile industry, food industry, biotechnological applications and in pharmaceutical industry, medicine, agricultural application (Huang et al., 2020). In terms of its intrinsic properties (purity, molar mass, viscosity, acetylation degree, quality, physical form chitosan can be characterized. Both molar mass and acetylation properties are very important for its characterization which influence it’s the chitosan performance, its synthesis, characterization and application (Abdou et al., 2008). Usage of polymer-based nanomaterials have been intensively investigated for their varied application and most important of these is the use of chitosan based polymeric nano-material in tissue engineering and regenerative medicine field (Thakur et al., 2014).
1.1 Chitin
The word chitin is derived from a Greek word meaning ‘tunic’ referring to the protective shell (Poornima and Korrapati, 2017). Chitin is composed of N-acetylglucosamine units linked in the orientation of (1–4) as a linear array with a molecular weight of 2–3 million Daltons. It’s properties includes insolubility in water (Barbosa et al., 2019). It is a versatile structural component made from monomers and it can form a solid structure in the insect’s wings as well as can form stronger component by combining it with component calcium carbonate in the shell of a clam (Muzzarelli, 2013), undermining the structure of chitin is the glycosidic bonds form between modified monomeric glucose molecule. Series of these glycosidic bonds in substituted glucose molecules form a long chain of chitin. Chitin distribution in different organisms shown in Table 1. Chitin has different categories based on its structural properties. Alpha Chitin due to its crystalline structure is tightly packed with antiparallel chains offering more stronger hydrogen bonding (Pillai et al., 2009). B-isoforms existed in Squid undermining higher solubility, affinity and reactivity towards solvents. B-form properties are due to lose hydrogen bonding between chains. Third chitin isoform is Gama-chitin which is reported to a variant of Alpha-Chitin (Tao et al., 2020) (see Fig. 1).
Sr.#
Specie
Structural component
Significance
1
Arthropod
Hard E.S made of Chitin and other protein
Protective body plan
2
Mollusks
Chitin is a part of radula
Aids in preying
3
Fungi
Chitin makes up the call wall of fungi
Rigid cell wall, retain shape
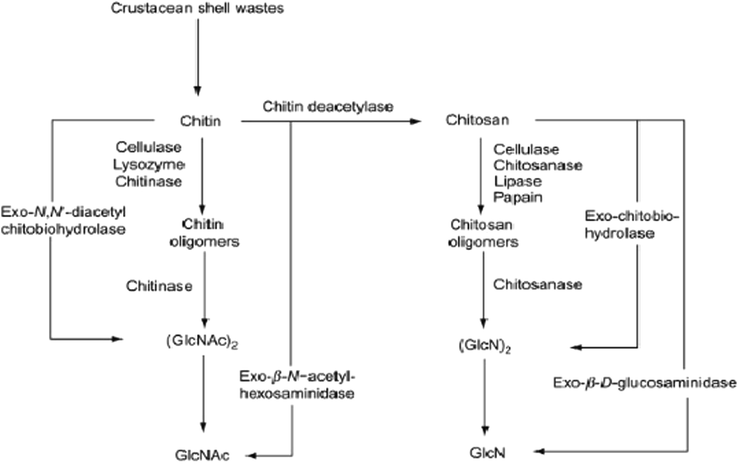
Enzymatic hydrolysis of chitin and chitosan to their monomers.
1.2 Source
Mushrooms are reported to be a very first source of chitin. Whereas Mycelia and fungal spores of chytridiaceae, Blastocladiaceae, Ascomydes, penicillium (20% chitin), Aspergilliun niger (sizable part) are the potential commercial sources found in literature (Roncero, 2002). Over 100 gigatons are synthesized in the biosphere per annum (Ahmad et al., 2020). In the United States, waste material consisting of shells and heads of lobster, crabs and shrimps are processed for the extraction of chitin (Zhang et al., 2020).
1.3 Historical view
In the beginning of 19th century, chitin marks its existence in France by the work of a chemist known as Henri Braconnot. The term “fongine” had been given to a residue remained after the extraction of fungi (Ahmad et al., 2020) and named “chitine” Odier (Odier, 1823). Apparently, in 1799 Charles Hatchett was a person to discovered chitin from the shells of marine animal (Kashif et al., 2020). Charles Rouget proposed a new term “chitine modifiee” after treating it with caustic potash solution in 1859, which make it soluble in organic solvent (Rouget, 1859). In 1894, an experiment was undertaken for the acid soluble derivative of chitin from the shells of Scorpio, spiders and crabs referring it to chitosan for the very first time by Felix Hoppe-Seyler (Rouget, 1859). Researchers spend the next six years (1894–1930) in studying the chitin determination in animals and its chemistry. Nothing yet is reported about their structure and nomenclature to validate it structural and functional differences from a closely related polymer cellulose. X-Ray diffraction had been reported to be the most reliable method for their validation reported since between 1930 and 1950. Identification of Natural polymers and Natural fiber gain attention in the scientific community afterwards. George W. Rigby patented the first chitosan films and fibers in mid-1930 (Rigby, 1936) and used for the very first time in papermaking industry(Lubs et al., 1937); as adhesive (Maxwell, 1939); photography (Martin and Middleton, 1938); textile (Heckert, 1937). Intensive elucidation of chitin and chitosan properties have been employed between 1970 and 1980, today there are more than 2500 applications f chitin, chitosan and their other reported derivatives in literature (Lee and Huang, 2019). Historical land marks are summarized in a Table 2.
Landmarks
Era
Name
Significance
Reference
Discovery
1799–1894
Charles Hatchett (English Chemist)
Decalcified the shells with mineral acid of crab, prawns, lobsters, crayfish.
(Kashif et al., 2020)
Henri Braconnot (French Chemist)
Discovered alkaline insoluble fraction from fungi, identified nitrogen content from a distillation fraction. Obsereved different consistencies in his fongine
(Braconnot, 1813)
Auguste Odier
Isolate insoluble alkaline fraction from cockchafer by hot caustic potash treatment. And findout that chitine is nitrogen free.
Presented a mémoire
at the Société d’Histoire Naturelle de Paris on a new sub-
stance found in the elytra of insects(Odier, 1823)
(Ahmad et al., 2020)
John George children (British Chemist, mineralogist and zoologist)
He used elemental analysis to analyzed the remaining residues after repeated extraction. Given the
empirical formula C11H17O7N2(Chen et al., 2018)
Charles Rouget (French physiologist)
Observed the boiling chitine in conc. Potassium Hydroxide to dilute sol of organic acid.
He given the name chitine mofifiee(Rouget, 1859)
Georg Ledderhose
Study the hydrolysis of chitine with Conc. HCL
(Araki, 1895)
(Ledderhose, 1876)
Flex Hoppie-Seyler (Germen physiologist and chemist)
Discovered Chitosan, demonstrate relation between chitin and chitosan.
(Tsai et al., 2019a)
1.4 Chitosan
Chitosan is a derivative of chitin and also referred to as amino-polysaccharides. Along the chain axis it has turn for every 10.1–10.5A°. There are three categories of chitosan namely alpha-chitosan, beta-chitosan and gamma-chitosan. Most common among them is the Alpha-chitosan. Reactive functional group of chitosan are also of three types sat C2, C3 and C6 position (Chandy and Sharma, 1990). Factor influencing physiochemical properties of chitosan include its molecular weight, chain length, charge densities, charge distribution, degree of deacetylation and chain length etc. High viscosity, insolubility in water, the tendency at high PH to coagulate with protein and property of being chemically inert makes chitosan to be a limiting factor in synthesis and characterization (Yi et al., 2005). Basically, chitosan is a copolymer of N acetylglucosamine and D-glucosamine unit. Chitosan has an effective absorption enhancing a property as an inherent mucoadhesive property aided due to conformational flexibility of linear chains (Huang et al., 2020). Chitosan is obtained by partial deacetylation of chitin and the degree of deacetylation is proportional to the transformational degree of chitosan from chitin.
2 Blends and composite of Na-Alg
Chitin and chitosan had been reported to blend with different type of synthetic and natural polymer to increase the mechanical as well as improve its adhesion properties. It is blended with the different types of cheaper materials to give them mechanical strength. It makes blends, hydrogels, micelles, Nano-carrier, Nano-gels, Nano-composites, Nanoparticles, tubes, cationic system, aerogels etc. to exhibit its excellent properties in drug delivery as well as in gene delivery, bone and tissue engineering and in food. This polymer has excellent blends as well as composites for the wastewater treatment by making hydrogels, composites and blends to remove different type of pollutants especially organic and inorganic as well as dyes. Na-Alg coating also becomes more impressive by adding different sort of antimicrobial agents. The different sorts of chitin and chitosan composites exhibit much functionality in different type of cancer and diabetic treatment. This polymer exhibits a lot of application in drug carrying and in the chemotherapeutic and stem cell treatment. For the purpose of food, this polysaccharide helps in the properties of gelling and food packing. This polymer blended as well as form composites with different type of materials to remove the organic pollutant as well as to remove metallic as well as dyes from aqueous solution (Table 3).
Sr#
Composite
Type of nanomaterial
Characterization Techniques
Applications
Ref.
Chitin for Drug Delivery
1.
Chitin/ PVA
Hydrogel
FTIR, AFM, SEM
Use for drug carriers for medical therapies
(Peng et al., 2019)
2
Hexadecyl-quaternized chitin
Micelles
H NMR, TEM, CLSM
Use for DOX delivery
(Zeng et al., 2020)
3
Chitin/Cadmium chloride
Nanogels
XRD, PL, DLS, SEM, AFM, FTIR
Use for loading of BSA protein drug.
(Rejinold et al., 2011)
4
Chitin/ Hallosytes naotubes
Nanocrytsals
SEM, TEM, AFM
Use for biomedical applications.
(Ahmad et al., 2020)
5
Chitin/ Fibrin
Nanocomposites
SEM
They have great potential in controlling bleeding and preventing mediastinitis after cardiac surgery.
(Sundaram et al., 2018)
6
Chitin/ Rifampicin
Nanoparticles
SEM
used to treat S. aureus and a variety of other bacteria that can persist inside PMNs.
(Smitha et al., 2015)
7
Chitin/SCH
Nanoparticles
FTIR, XRD
Use for otoneurological pathology
(Petrova et al., 2018)
8
Chitin/Ag/Copper
Nanocomposites
FTIR, XRD, SEM, TEM, EDS, XRD, 13C NMR
Use for human breast cancer MCF-7 cell line
(Solairaj et al., 2017)
9
Chitin/ Starch
Nanoparticles
FTIR, XRD, AFM
Use as cell substrate
(Rodríguez et al., 2018)
10
Chitin/Hematite
Nanostructured particles
XRD, SEM, HRTEM
Use for hematite base biomaterials
(Wysokowski et al., 2014)
11
Chitin/Methotrexate
Nanogels
HPLC
Use for drug delivery application
(Panonnummal et al., 2018)
Chitosan For Drug Delivery
12
Chitosan/PVA
Nanoparticles
TEM, UV, SEM
Use for delivery of anticancer drugs
(Khdair et al., 2016)
13
Chitosan/Sodium Nitrate
Nanoparticles
FTIR, DLS, TGA,
Use for delivery of DOX
(Soares, 2016)
14
Chitosan/HA
Nanoparticles
FTIR, DLS, TEM
Use to encapsulate a chemotherapeutic drug (5-Fu)
(Wang et al., 2017)
15
Chitosan
Nanoparticles
TEM, DLS, UV, FTIR
Use as as potential nanocarriers for combined drug delivery and hyperthermia application
(Zamora-Mora et al., 2017)
16
Chitosan/Paromomycin
Nanoparticles
SEM, FTIR, GC
Use for for the treatment of leishmaniasis, especially when the current drugs are impaired by resistance.
(Esfandiari et al., 2019)
17
Chitosan/Lipid
Hybrid nanoparticles
TEM, DSC, TGA
Use for controlled delivery of cisplatin
(Khan et al., 2019)
18
Chitosan/ API
Nanoparticles
SEM, TEM
Have great potentials as efficient nano-carriers in tumor treatment
(Cheng et al., 2019)
19
Chitosan/ Human serum albumin
Nanoparticles
TEM, Zeta Potential
Use for nose-to-brain drug delivery.
(Piazzini et al., 2019)
20
Chitosan/ Chlorin e6
Nanoparticles
SEM, AFM, UV
Use for controlled release of DOX upon NIR irradiation
(Bhatta et al., 2019)
21
Chitosan/CMC
Nanoparticles
Zeta Potential, TEM, EDX, FTIR
Use for moderate and persistent DOX released
(Li et al., 2019)
22
Chitosan/ Peptide
Nanoparticles
1H NMR, FTIR, TGA, CLSM
Use for targeted cancer therapy
(Qian et al., 2019)
23
Chitosan/Folic acid
Nanoparticles
UV, TEM
Use for delivery of tetracycline, doxorubicin and tamoxifen
(Chanphai et al., 2019)
24
Chitosan/Glutaraldehyde
Nanoparticles
FTIR, SEM, TEM, UV
Use for pulmonary Drug Delivery
(Islam et al., 2019)
25
Chitosan/Fucoidan
Nanoparticles
FTIR, Zeta Potential, TEM
Use for for enhanced oral delivery of insulin
(Tsai et al., 2019a)
26
Chitosan/Polylactide
Nanoparticles
FTIR, FCA
Use for Delivery of Therapeutics for Triple-Negative Breast Cancer Treatment
(Gomillion, 2019)
27
Chitosan/Cadmium
Quantum Dots
XRD, FTIR, UV, TEM
Potential candidate for the drug delivery of Sesamol
(Abdelhamid et al., 2019)
28
Chitosan/Silica Nanoparticles
Thin Film
FTIR, TGA, XRD, UV, SEM
Use for DOX delivery
(Chen et al., 2019)
30
Chitosan/Polycaprolactone
Nanofibers
SEM, FTIR, DSC, TGA
Use for the alternative therapeutic modality for cancer treatment with combinatorial efficacy of naturally occurring phytochemicals
(Balan et al., 2019)
31
Chitosan/PVA
Nanoparticles
SEM, DSC, FTIR
Use for for oral delivery and sustained release of the immunosuppressant drug mycophenolate mofetil
(Mohammed et al., 2019)
32
Chitosan/BSA
Nanoparticles
TEM, DLS
Use for safe drug carriers in further in vivo investigation
(Montero et al., 2019)
33
Chitosan/PEG-PLGA
Hydrogels
TEM, EM
Use for ocular drug delivery
(Rong et al., 2019)
34
Chitosan/Curcumin
Nanoparticles
FESEM, EDX, XRD, FTIR, DSC
Use for for transdermal delivery.
(Nair et al., 2019)
35
Chitosan/Albumin
Nanoparticles
DLS, TEM
Use as a hydrophobic drug nanocarrier in pharmaceutical and medical applications
(Razi et al., 2019)
2.1 Drug delivery
2.1.1 Chitin base nanomaterials for drug delivery
2.1.1.1 Chitin/PVA composite nanomaterials
Poly (vinyl alcohol) (PVA) has gained much attention in the field of hydrogel formation because of its unique characteristics (biodegradable, biocompatible and hydrophilic in nature). In last few years, a lot of efforts have done on the formation of PVA based hydrogels. Choi et al., reported the synthesis of eggshell membrane-based PVA hydrogels by means of electron beam irradiation and characterize these materials (Choi et al., 2017). Sun et al., used repeating freeze–thaw process that results in the formation of a high tough PVA hydrogel. This was done via immersing NaCl solution (Sun et al., 2017). Gao et al. has been worked on PVA by using trimethylol melamine as chemical crosslinking material and carboxymethyl cellulose as reinforcement material (Gao et al., 2017). PVA hydrogels have been successfully applied in drug delivery systems due to its unique features.
Peng et al. 2019, (Peng et al., 2019) synthesized the hydrogels of ChWs/PVA hydrogels for the purpose of drug delivery [Fig. 2]. Crab shell consisted of fibrous and porous conformation, having microfibers that possessed the structure of hierarchical nature [Fig. 2 (a)]. They blended the solution of polyvinyl alcohol (PVA) in minor acidic medium with suspended nanowhiskers of α-chitin, that when deacetylated to some extent by the surface carbonization of fibrils, showed conformation of jelly-like nature [Fig. 2(b)]. There took place cross-linking reaction between every constituent [Fig. 2(c)]. The technique of freezes thawing was used to further develop the “monolith PVA/ChWs” [Fig. 2(d)]. A condensed three dimensional conformation was obtained by the crosslinking of linear polymer, offering a large surface area for drug loading [Fig. 2(e)].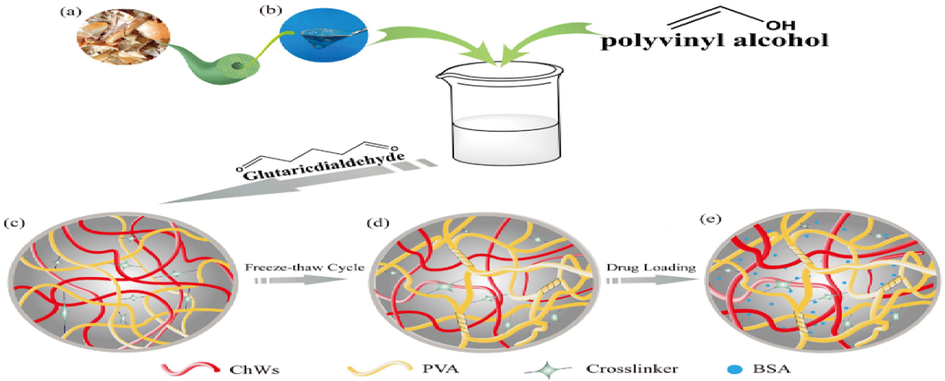
Schematic process illustrating the formation of PVA/ChWs hydrogels Images of 1 (a) shrimp and crab shell flakes (b) Image of colloidal partially deacetylated α-chitin nanowhiskers (c) Schematic of PVA/ChWs (30% ChWs) hydrogels after chemical cross-linking (d) Schematic of PVA/ChWs (30% ChWs) hydrogels after physical cross-linking. (e) Schematic of drug-loaded hydrogel (Peng et al., 2019).
2.1.1.2 Chitin-based nanomaterials for drug delivery
The controlled release of antitumor drugs can be achieved by the use of polymeric micelles (Chang et al., 2018). The self-assembly of amphiphilic co-polymers in water results in the fabrication of polymeric micelles only when the concentration of copolymer is reached to a point is known as critical micelle concentration (CMC) (Rapoport, 2007). In amphiphillic co-polymers, the inner part is hydrophobic portion. Hydrophobic portion provides environment for the encapsulation of various drugs with improved bioavailability as well as solubility (Upponi et al., 2018). However, the outer shell (hydrophobic part) acts as stabilizer between aqueous environment and hydrophobic part. The incorporation of micelles with drug have advantageous of particular size, enhanced circulation time and drug loading ability (Bi et al., 2016). The enhanced permeability is the observation that the vasculature of cancerous part is leaky as compared to normal tissue due to nanosized of the micelles (Cho et al., 2016). Various factors like chain length of polymer, molecular weight as well as its chemical composition have significant role on drug loading capacity by decreasing its toxicity along with its enhanced therapeutic effect (Mohamed et al., 2017). However, polymeric micelles have disadvantages such as non-biodegradable, high cost, drug leakage and cytotoxicity (Moshe et al., 2017).
Peng et al. 2019, (Zeng et al., 2020) fabricated derivates of a series of amphiphilic chitin [Fig. 3]. In order to manufacture the novel micelles, he introduced “hydrophobic hexadecyl groups” as well as “hydrophilic quaternary ammonium” for the delivery of DOX. Through the reaction of one-pot, “quaternized chitins” were fabricated equivalently. First, at low temperature, chitin was dissolved in the aqueous system of urea that was eco friendly to fabricate chitin solution. Then, “(3-chloro-2-hydroxypropyl) trimethylammonium chloride (CHPTAC)” was dropped into the solution of chitin gradually. Under alkaline conditions, CHPTAC formed epoxide. Then, it was reacted with the chitin’s hydroxyl group. By the formation of ether bonds, the quaternary ammonium moieties were conjugated. In the meantime, the hexadecyl bromide reacted with quaternary chitin’s hydroxyl group that remains behinds in basic condition. In this way, through substitution reaction, the amphiphilic chitin was obtained. White powder of amphiphilic chitin was obtained after precipitation as well as washing of crude product as well as dried it in vacuum at 50 °C.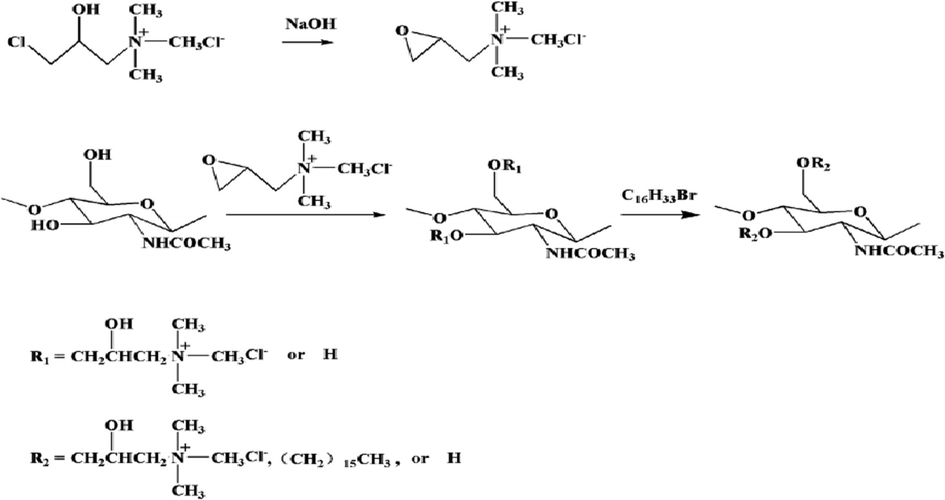
The pathway for the synthesis of amphiphilic chitin (Zeng et al., 2020).
2.1.1.3 Chitin/Cadmium chloride composite nanomaterials
Rejinold et al., 2011 established a chitin nanogels (CNGs) class i.e. Rh was less than 100 nm. As a model protein, its fundamental ability of multiple functionalities with “Bovine Serum Albumin (BSA)” as well as QDs was also established. As shown in [Fig. 4].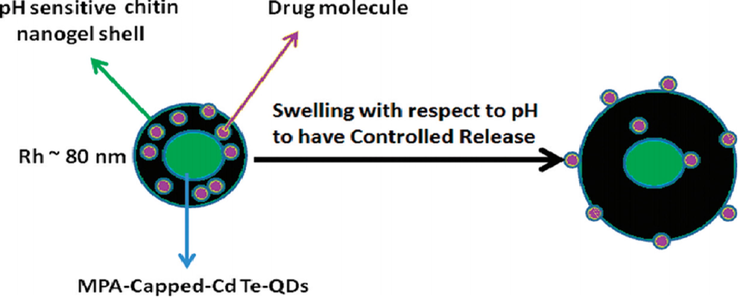
Schematic representation for the concept for designing multifunctional BSA loaded-CdTe QDs-chitin hybrid nanogel (BSA-QD-CNGs) and its potential extending applications in the biomedical field (Zeng et al., 2020).
In the network of chitin nanogels, through in situ immobilization, chitin nanogels were conjugated with CdTe QDs. It might be demonstrated the functions as well as properties from each building blocks. Under 25 °C with controlled stirring, the solution of chitin was stirred for an hour as well as added the methanol during stirring to get the clear turbid solution. Then washed the above solution with distilled water several times until the methanol was completely eliminated. This synthesis route is illustrated in [Fig. 5].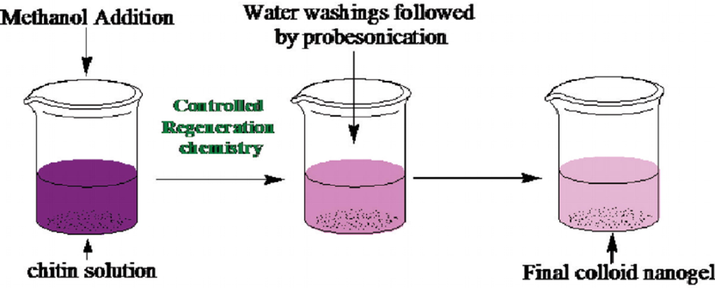
Synthesis route for the chitin nanogels (CNGs) by controlled regeneration chemistry (Rejinold et al., 2011).
2.1.1.4 Chitin/Hallosytes composite nanomaterials
Halloysite nanotubes are tubular in shape having empirical formula Al2Si2O5(OH)4·nH2O6. The diameter of HNTs ranges from 40 to 70 nm. Its length ranges from 200 to 2000 nm (Liu et al., 2014). The outer surface of HNTs is composed of SiO2 (negatively charged) while the inner part is Al2O3 (positively charged) (Zhao et al., 2016). But, the overall charge is negative. The cylindrical nature of HNTs is responsible for enhanced mechanical reinforcement, thermal stability and sustained release of drugs (Liu et al., 2014). HNTs find its application in drug delivery approach, healing of wounds, biosensors and tissue engineering (Massaro et al., 2018). The biological applications of HNTs were explored by Massaro, Lazzara (Massaro et al., 2017). The modification of HNTS by carbon dots result in the formation of fluorescent label. HNts-CDs are one of the promising candidates for oral gene therapy that manipulate the release of ct-DNA. Their cylindrical nature help in the controlled release of drugs as well as tracking of delivered molecules (Massaro et al., 2019). The toxicity of HNTs-CDs has been checked on the model of mice, zebrafish and C.elegans model18-20. The biological applications of HNTs rely upon the connection between HNTs and cells. Techniques like atomic force microscopy and laser confocal visualization have revealed that HNTs are present within the cell in nuclear vicinity (Dzamukova et al., 2015).
Zhao et al. 2019, (Ahmad et al., 2020) estimated the features of physical chemistry of two one-dimensional nanoparticles. To calculate the in vitro nanoparticles cytotoxicity, the “rat osteosarcoma cells (UMR-106)” as well as “mouse bone marrow mesenchymal cells (mBMSCs)” were utilized. In this way, C. elegans, as well as cell toxic evaluations, were carried out [Fig. 6]. For one day, rat “osteosarcoma cells” as well as mouse “bone marrow mesenchymal cells” were seeded with different concentration. With different concentration, the suspension of nanoparticles was fabricated and then sterilized through utilizing microwave. With final concentrations, the sterilized solution was added into the culture plates and incubated the solution for one day. To evaluate the one-dimensional nanoparticles cytotoxicity, the CCK-8 assay was utilized. To estimate the living as well as dead cells, AO/EB dual-fluorescent dyes were utilized.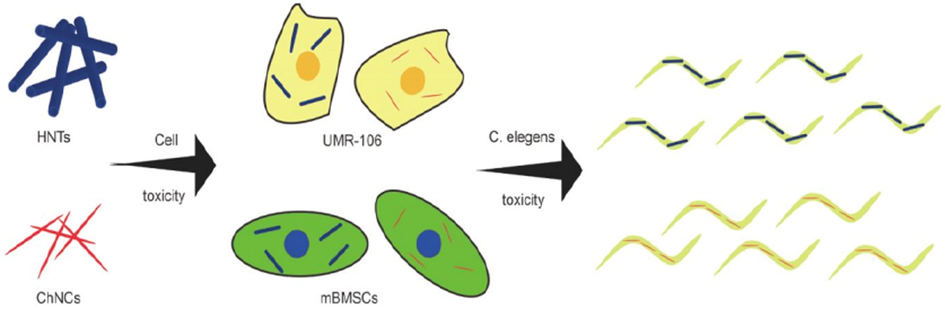
Toxicological evaluation of HNTs and ChNCs (Ahmad et al., 2020).
2.1.1.5 Chitin/Fibrin composite nanomaterials
Medianstrernotomy cause mediastinitis which is deep sternal wound infection (DSWI) and involves long period of hospitalization, increases morbidity and mortality rate upto10-40% (Risnes et al., 2010). DSWI occur with an incidence rate of about 1–5% (Singh et al., 2011). The complications associated with coronary artery bypass graft surgery are mediastinitis as well as bleeding after surgery. To overcome these limitations, the commonly applied antihemorrhagic agent is bone wax. Although, several studies concluded at the site of surgery bone wax plays critical role in increasing infection (Vestergaard et al., 2010). At the site of sternal wound infection, both types of pathogens are present. But, it was reported that concentration of gram-positive bacterium is much more as compared to gram-negative bacterium 8. Sternal closure with muscle flap, vacuum-assisted closure therapy and duration of antibacterial therapy were utilized in DSWI cure (Cotogni et al., 2015). However, vancomycin has been successfully employed toward gram-positive bacterium (Methicillin resistant S. aureu) and also effective in treatment of mediastinitis. In spite of this, deafness and impaired renal function along with resistance toward pathogens are resulting with high dose vancomycin (Engleman et al., 2007).
Sundaram et al. 2018, (Sundaram et al., 2018) fabricated a tGNPsCH-FB gel i.e. a bio-adhesive. Utilizing a dual syringe applicator, this gel was prepared with antibacterial as well as hemostatic property. As shown [Fig. 7] in both the solutions of thrombin as well as fibrinogen, tGNPs was dispersed separately. These solutions contained chitin gel i.e. 400 mg. for the formation of in situ tGNPsCH-FB gel. An equal volume of these solutions was injected simultaneously.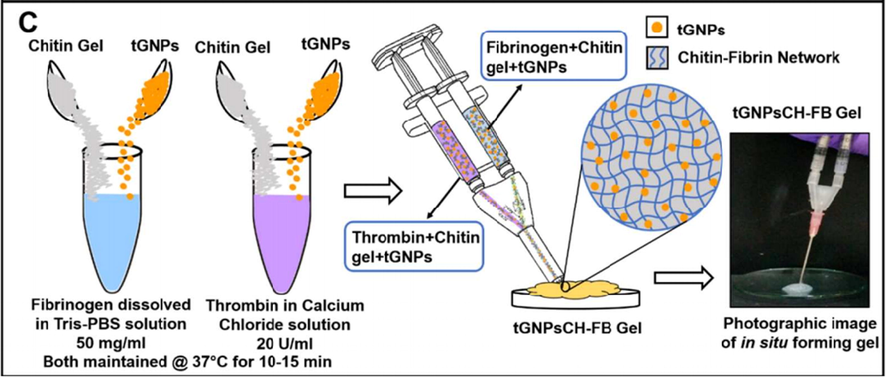
Schematic diagram illustrates the synthesis of in situ forming tGNPsCH-FB gel (Sundaram et al., 2018).
2.1.1.6 Chitin-based nanomaterials for drug delivery
The regular/ repeated use of antibiotics in order to overcome intracellular bacterial infections for longer period of time is essential. In host cells, concentrations below minimum inhibitory concentration (MIC) exist in the intracellular fluid which holds the life of pathogens that develop resistance against antibiotic (Armstead and Li, 2011). The successful removal of pathogen can be done by increasing the concentration of antibiotic in intracellular fluid for extended period of time. However, the main difficulty is the transfer of the drug to the intracellular fluid (Gnanadhas et al., 2013). The main approach toward this problem is the encapsulation of drugs with the help of microparticles or nanoparticles which increases the efficiency of drug delivery systems. The intracellular chlamydial infection can be treated by the encapsulation of Azithromycin and Rifampin with the help of PLGA nanoparticles as reported by Toti, Guru (Toti et al., 2011). The better efficiency of Ciprofloxacin/carboxymethyl chitosan NPs in the treatment of E. coli pathogens as compared to pure ciprofloxacin was observed. This is due to easily absorption in the cells (Zhao et al., 2013). Like other pathogens, Salmonella sp was better treated with ciprofloxacin loaded chitosan dextran sulphate (CD) nanocapsules as studied by Gnanadhas, Ben Thomas (Gnanadhas et al., 2013). It was observed that the Brucellamelitensis and Brucella abortus destroyed by using PLA/PLGA microspheres. The pulmonary tuberculosis can be medicated by using PLGA microspheres using rifampicin (O'Hara and Hickey, 2000).
Smitha et al. 2014, (Smitha et al., 2015) fabricated RIF-ACNPs for RIF intracellular delivery that was utilized for the intracellular S. aureus infection treatment. In ethanol, RIF was dissolved and then added the solution of AC. Through the addition of TPP dropwise, the solution was stirred for about 3 h to get a turbid suspension. Through the centrifugation, the formation of nanoparticles was recovered for about half an hour. Then obtained pellets were washed with distilled water and resuspended in the “phosphate buffered saline (PBS)”.
2.1.1.7 Succinyl chitin-based nanomaterials
The otoneurological disorders resulting from hypoxic and ischemic pathogenesis can be cured by using succinate (natural endrogeneous metabolite) produced during Krebs cycle (Benit et al., 2014). Under anaerobic conditions, the use of succinate as exogeneous administration is associated with generation of ATP but it limits or lows the concentration of citrate, lactate and pyruvate (Benit et al., 2014). The antihypoxic and cytoprotective effects of succinate in case of unbalanced consumption of endogenous metabolic substrates have been exploited successfully in several pharmacological drugs based on succinate solutions (e.g., Reamberin, Cytoflavin, Remaxole, Mexidol, etc.) (Volchegorskii et al., 2017). Substrate replacing drug has certain drawbacks i.e., small aggregation in targeted cells. This happens due to the small cycling time in blood. As succinate (an endrogeneous metabolite), has also used by those organs (skeletal and liver muscles) even that are not associated in pathological process. Because of the high metabolic activity as well as small size they play an important role in distribution (Volchegorskiĭ et al., 2014). Low molecular weight drug have also be restricted due to existence of histohematic barrier which are ineffective for ligand receptors interaction (McCall et al., 2010). So, medicine using succinate as substrate can be improved by following two main routes; 1) by enhancing the cycling time into blood 2) to establish some ways that are more useful in the penetration of medicine to capillary to tissue in targeted organs in the presence of histohematic barriers.
Petrova et al. 2018, (Petrova et al., 2018) fabricated the nanoparticles of succinyl-chitin (SCH) as well as estimated their pharmacological action. Followed through free amino groups succinylation, the α-chitin modification included the partial deacetylation as shown in [Fig. 8]. At 20 °C, utilizing succinic anhydride at equal molar ratio as acylating agent, the deacetylated chitin was N -cylated. Through the addition of 3% NaHCO3 solution into the reaction mixture, the product was converted into a Na-salt that further followed through dialysis against freeze drying as well as deionized water. For 5 days, the freeze drying solution of SCH was stirred as well as added to water. In the ultrasound dispergator, the solution was sonicated for 10 min to manufacture a SCH nanoparticles dispersions.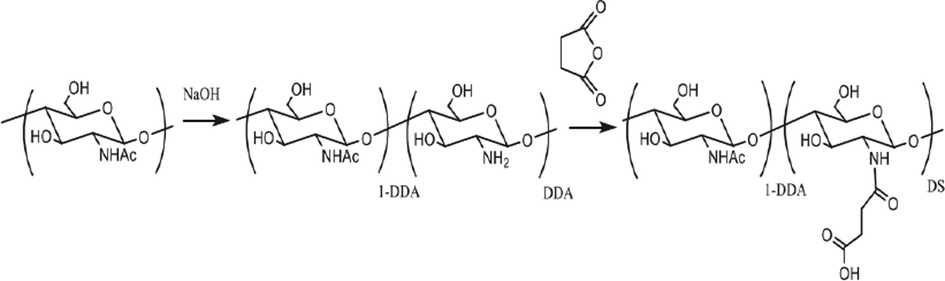
Synthesis of N-succinyl-chitin (Petrova et al., 2018).
2.1.1.8 Chitin/Ag composite nanomaterials
In polymer-metal nanocomposites, the metallic nanoparticles are distributed in polymeric matrix. These nanocomposites have extensive applications in biosensors, treatment of tumors, labeling of cells, pharmaceutical industry, drug delivery systems and molecular imaging (Wise and Brasuel, 2011). Metallic nanoparticles like Ag, Ni, Cu, Pt, Co, Pd, Au possess unusual physical as well as chemical properties that entirely different from their corresponding bulk or individual metals (Duan and Wang, 2013). Among the above mentioned nanoparticles, Cu and Ag are drawing much attention as antimycotic medication, antiparasitic drugs, antineoplastic drugs, insecticide agents and antibiotic (Agnihotri et al., 2014). Currently, Ag nanoparticles are very interesting because of their applications in purification of water, production of crops, textile industry, biomedical engineering and food processing (Venugopal et al., 2017). Cu is a fundamental element for aerobes as well as human beings. It plays an important role in the synthesis of DNA, metabolism of energy and respiration (Santini et al., 2013). Moreover, Cu based nonmaterial are more toxic for cancerous tissues as compared to normal tissues (Acilan et al., 2017). The fabrication of chitin based nanocomposites has gained much interest due to their mechanical, optical, catalytic and electronic characteristics.
Solairaj et al., 2017, (Solairaj et al., 2017) estimated the chitin cytotoxicity against the cells of “human breast cancer” (MCF-7), that was incorporated with Cu as well as Ag nanocomposite. Through deproteination as well as demineralization, CNP was fabricated from the Penaeus monodon Fabricius utilizing basic as well as acid treatment. By mixing 10 mg CNP as well as 1 mL of AgNP that was chemically prepared, CNP/AgNP was fabricated. Through reducing the Cu(II) sulphate utilizing a reducing agent i.e. NaBH4 in the presence of CNP, the CNP/CuNP was synthesized. I this work different metallic nanoparticle toxicity have been checked for MCF-7 cancer cell line [Fig. 9].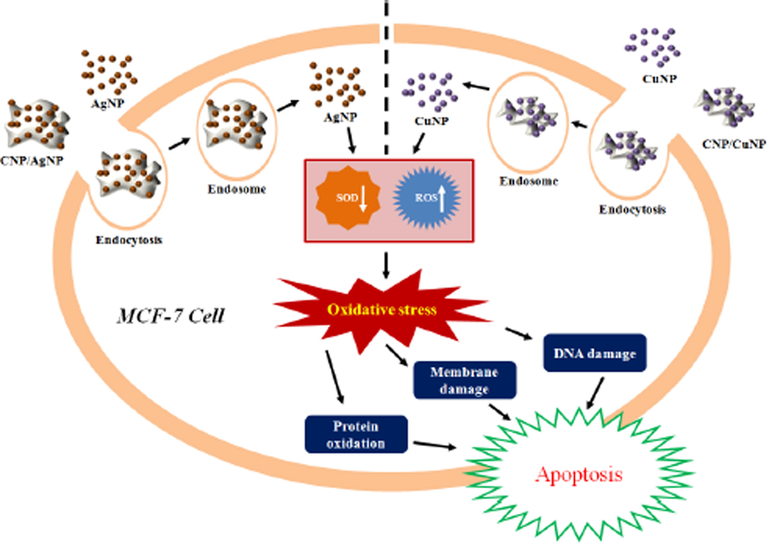
Hypothetical mechanism of cytotoxic activity of AgNP, CuNP, CNP/AgNP and CNP/CuNP against MCF-7 cells SOD↓ denotes decreased SOD activity; ROS↑ denotes increased ROS generation (Solairaj et al., 2017).
2.1.1.9 Chitin/Starch composite nanomaterials
Exopolysaccharides (EPS) are produced by certain bacteria which are embedded in accumulation of biopolymers (Flemming and Wingender, 2010). It is responsible for the protection of bacteria against UV radiations, heavy metal ions and dehydration (Ehling-Schulz et al., 1997; Scherer et al., 1988). In cyanobacterium, EPS are designed of hetropolysaccharides. The combination of ten different monosaccharides (xylose, glucose, uronic acid and galactose) form hetropolysaccharides. The anionic nature of monosaccharides can be decided by the existence of acidic sugars such as C6H10O7 accompanied by anionic organic (such as acetyl group) and inorganic substituents (phosphate or sulphate) (De Philippis and Vincenzini, 1998).
Rodríguez et al. 2018, (Rodríguez et al., 2018) examined the interaction of cell-substrate polysaccharides films by utilizing as low adhesion cell-substrates. Starch NPs, as well as Chitin whiskers, were accurately weighed by mixing into distilled water as well as stirred for one day. At different concentration, the suspension of starch NPs, as well as chitin whiskers, was added to EPS. By utilizing sonication, the solution was homogenized for 10 min. The obtained product was poured as well as dried at 40 °C for approximately one day.
2.1.1.10 Chitin/Hematite composite nanomaterials
The effective and well-established way to fabricate inorganic/organic-based nonmaterial possessing polymorphism, complex morphology and hierarchical organization is extreme Bionimetics (Ehrlich et al., 2013). The combination of these materials leads to the formation of functional materials that have used in biosensors, drug delivery systems, catalysis, electrochemistry and photonics (Yan et al., 2012). The basic principle of extreme biomimetics is mineralizing the biological molecules over circumstances that imitate aquatic niches such as hot springs and hydrothermal vents. Accordingly, essentially that biological molecules must be stable in both thermally as well as chemically under in-vitro conditions. Thermodynamics, nucleation and kinetic crystal growth has been strongly influenced by the choice of appropriate biomolecule (Xu et al., 2007). A large number of examples related to biomineralization phenomenon in hydrothermal vents and hot springs have been reported. At higher or very low temperature, the proteins as well as peptides undergo denaturation. Due to this, niche under extreme conditions supported polysaccharides “as template” in the process of biominerilazation. In living organisms, the use of polysaccharides as templating agent and nucleating agent is due to their structure, properties, chemical composition and a variety of reactive groups (Hedrich et al., 2013). The formation of exopolysaccharides in case of both gram-positive as well as gram-negative microbes having the ability to obtain Fe3+ which result in inducing precipitation of haematite externally to cell. Microorganisms are protected through this process (Poorni and Natarajan, 2014).
Wysokowski et al. 2014, (Wysokowski et al., 2014) synthesized a hybrid material that contains Fe2O3 utilizing “3D tube-like fibrous a-chitin scaffolds” [Fig. 10]. In ultra-pure water, anhydrous FeCl3 was dissolved. Then, it was added into the mixture of HCl as well as ultra-pure water. In a further step, the addition of sponge chitin fragment to solution as well as it was converted into the vessel of Teflon-based of the hydrothermal reactor. Then, for 2 days, it was heated at 90 °C. Then, the template of chitin was covered with the nanoparticles of Fe2O3 and carefully isolated. Then, in the ultrasound bath for about 20 min, washed it with distilled water. Then, for 2 days, dried at 90 °C and brought the pH up to 6.8. By utilizing liquid N2 as well as an agate mortar, the chitin-Fe2O3 fragments were disrupted mechanically to get nanosized powder.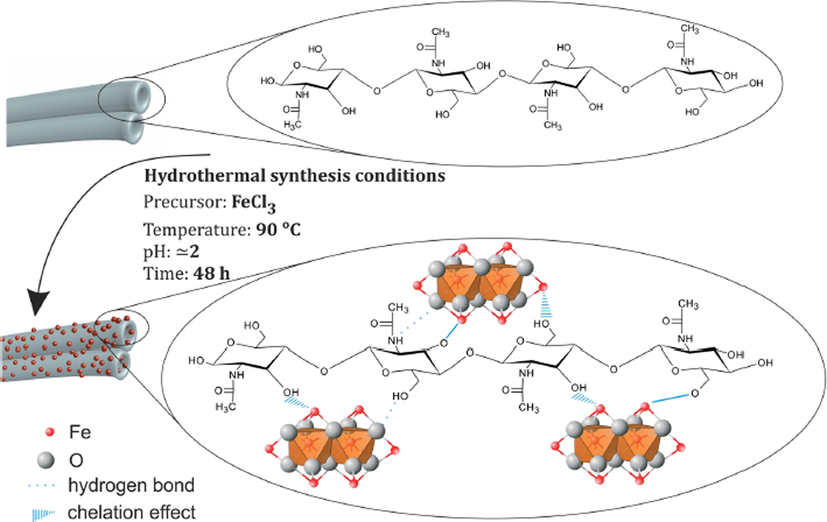
A schematic view on possible mechanism of chitin–hematite interactions under hydrothermal conditions (Wysokowski et al., 2014).
2.1.1.11 Chitin-based nanomaterials for drug delivery
Hyperproliferation as well as incomplete differentiation of keratinocytes are associated with psoriasis disease occur with occurrence rate about 2–3% in 0.7% Indians (Raza et al., 2013). The existence of unknown antigen at spinous layer resulting in stimulation of antigen presenting cells which causes psoriasis. Then the antigen reached towards regional lymph node that is associated with differentiation along with proliferation of T-cells. The activation of T-cells is responsible in disturbance of cell division as well as it also liberate chemo-kinesis and cytokinesis that effect the differentiation of keratinocytes (Rioux and Abbas, 2005). So, psoriasis is auto-immunogenic in nature. In moderate to severe cases, this disease requires systemic therapy (Collamer and Battafarano, 2010; Laws and Young, 2012).
Panonnummal, 2018, (Panonnummal et al., 2018) carried out the analysis of the “anti-psoriatic efficacy”, toxic nature of “methotrexate loaded chitin nanogel (MCNG)” being delivered orally and “biodistribution”, comparing to “methotrexate tablet (MTX)”. “Methotrexate tablet” was made to mix in “Phosphate Buffered Saline (PBS)” to make solution of tablet. The animals were given specified dose by adjusting the volume of administered MCNG and solution of tablet. “Oral sample administration tubes” were used to administer the sample to the animals.
2.1.1.12 Chitosan base nanomaterials for drug delivery
2.1.1.12.1 Chitosan/PVA composite nanomaterials
The use of anticancer drugs in the treatment of cancer is restricted due to resistive nature of these drugs (Tomasetti, 2014). Tumor drug resistance occurs through different mechanisms like entrapment of anticancer drugs in intracellular acidic compartment, p-gp glycoprotein efflux transport and unfavorable acidic cancerous environment (Tomasetti, 2014; Tcherniuk and Oleinikov, 2015). These mechanisms make the anticancer drug to be less effective, cure rate decreases and limits its applications in clinical field. Moreover, the drug delivery systems are of considerable interest by the scientific community (Pérez-Herrero and Fernández-Medarde, 2015). For past few years, NPs have gained much attention in drug delivery system for the treatment of cancer (Ediriwickrema and Saltzman, 2015). The better performance of anticancer drug can be achieved through enhancing accumulation of therapeutic agents through “enhance and retention effect”.
Khdair 2016, (Khdair et al., 2016) established novel drug delivery system that based on nanoparticles by utilizing modified surfactant of “aerosol-OT (AOT)” as well as “polymer of chitosan”. They also investigate their utilization in a number of drugs. By using the “double-emulsion solvent evaporation-crosslinking” technique, they articulated the “chitosan diacetate” as well as “chitosan triacetate” into nanoparticles. To stabilize the primary polymer-drug w/o emulsion, “AOT (Aerosol-OT)” was utilized. Because of their unique properties, for example, anionic surfactant, forming reverse micelles ability in the solvent of non-polar, strong emulsifying as well as double tails. For the formation of secondary emulsion, polyvinyl chloride was utilized because it considered as o/w emulsifier that stabilized the primary emulsion. In the polymer, calcium chloride was utilized to crosslink the acetate groups, this stabilized the “polymeric AOT” matrix.
2.1.1.13 Chitosan base sodium nitrate
A chemotherapeutic drug “Doxorubicin (DOX)” belongs to family anthracyclines played a significant role as medication in leukemia, breast cancer, lymphoma and lung cancer. It is one of the promising candidate in drug delivery systems (Swain et al., 2003; Soares, 2012). The formulation of liposomal doxorubicin over other anticancer drugs is accepted but it also causes the damage to heart muscles (Davies et al., 2004). Different methods like nanocarrier based platforms have been utilized that increases the effectiveness of drug delivery systems. The tailoring of nanoparticles permitted with selection of polymeric matrices in order to meet all requirements (Baptista et al., 2013).
Paula et al. 2016, (Soares, 2016) fabricated the delivery system of “doxorubicin (DOX)” based on chitosan. By ionotropic gelation, the nanoparticles of O-HTCC, as well as chitosan, were fabricated through adding a standard concentration of tripolyphosphate as well as the polymeric solution that was dissolved in CH3COOH. Then, the product was stored in a dry place after the freeze-drying process.
2.1.1.14 Chitosan/HA composite nanomaterials
A member of mucopolysaccharide “Hyaluronic acid (HA)” also has been approved to treat cancer cells as drug carrier because it particularly targeted CD44 receptors. HA is one of the basic constituents in extracellular matrix and chemically composed of alternative glucuronic disaccharides well as N-acetylglucosamine (Auvinen, 2000; Eliaz and Szoka, 2001; Amirghofran, 2008; Choi, 2010; Cho, 2011).
Wang 2017, (Wang et al., 2017) through the interactions between “CD44” as well as “HA (hyaluronic acid)”, designed “HA-CS (hyaluronic acid-coated chitosan) nanoparticles” to increase the efficiency of antitumor. By utilizing the ion gelation process, they fabricated “5-Fu-loaded-coated chitosan nanoparticles”, then, the obtained precipitate was resuspended in distilled water after the addition of hyaluronic acid and the solution of Na-salt. For the synthesis of “5-Fu-loaded hyaluronic acid-coated chitosan nanoparticles”, at the surface of chitosan nanoparticles through charge adsorption process, hyaluronic acid was conjugated. This is due to strong interaction between the anionic group of hyaluronic acid carboxyl group and the cationic group of chitosan. Through centrifugation, the product was precipitated and then, these nanoparticles were separated. Then, to eliminate the chitosan as well as hyaluronic acid, washed the final product with distilled water.
2.1.1.15 Chitosan-based nanomaterials for drug delivery
Hyperthermia increases the effectiveness of chemotherapy that help to kill cancer cells (Huang et al., 2012). Hyperthermia is a thermal procedure in which temperature raises up-to 40–46 °C that kills tumour cells (Jordan et al., 1999; Laurent et al., 2011). In magnetic hyperthermia (MH), magnetite (Fe3O4) nanoparticles have been employed as heating agents. This process is based on altering magnetic field (AMF) by irradiation of nanoparticles with low frequency in the range of 100–900 kHz. By utilizing various mechanisms, nanoparticles transform the energy absorbed by magnetic field into heat. This transformation is strongly dependent upon various factors like viscosity, agglomeration state (Gupta and Gupta, 2005) and size of nanoparticles. However, the use of polymer-drug carrier as heating agents increase the drug release rate as reported carboxymethyl dextran-coated magneto-liposomes (Guo et al., 2015) as well as carrageenan beads in addition to carboxymethyl chitosan (Mahdavinia et al., 2015). The results declared that the utilization of altering magnetic field causes the motion of nanoparticles which is associated with the relaxation of polymer chains.
Mora et al. 2016, (Zamora-Mora et al., 2017) synthesized the ionotropic-based responsive material that was coupled with CS NPs. These chitosan NPs were loaded with “magnetic iron oxide NPs” as well as “5-Fu”. For the production of final ferrofluid concentrations, a standard concentration of ferrofluid was dispersed. In the atmosphere of nitrogen, each ferrofluid solution with a volumetric ratio of chitosan was blended under continuous stirring. In the sodium tripolyphosphate aqueous solution, “5-fluorouracil” was mixed to get final product. Then, this solution was added dropwise into the solution of “chitosan” as well as “ferrofluid”. Then, the final product was stored after freeze drying process. The obtained product was named as “5-FU-CS-MNPx” where x represented the concentration of “Fe3O4-MNPs”.
2.1.1.16 Chitosan base paromomycin
A member of family Trypanosomatidae belonging to genus Leishmania (L.) is obligateintramacrophage protozoan that causes Leishmaniasis. Leishmaniasis is one of the leading cause of various vector borne infection diseases (Alvar et al., 2012), and ultimately threats to global health. It comes out in three ways such as mucocutaneous, visceral and cutaneous (Henry et al., 2007). Among all catagories, cutaneous is commonly caused in new world via L. amazonensisin, L. mexicana. However, L. aethiopica, L. tropica nad L. major are main causes of cutaneous in ancient times (Akhoundi et al., 2016). L. major which produces cutaneous leishmanias occurs with an incidence rate of about 0.7–1.2 million is a global health issue. The disease may be in the forms of mole, rashes or acne is widespread throughout the world. Although, Chemotherapy have been employed in the treatment of this disease (Silva-Jardim et al., 2014). But, with implementation of drugs some problems like toxic and resistive nature of drugs, expensive and treatment period is too long have been studied (Kedzierski et al., 2009). Drugs like Glucantim® and Pentostam® are included in the category of first-line leishmaniasis drugs and now replaced by second line drugs which show more effectivness and less harmful. Yet, amphotericin B and miltefosine (included in second line drug) also have some problems such as abnormalities of physiological development, life threatening, need to spend time in hospital and high-priced (Wiwanitkit, 2012). Recently, a second line drug paromomycin (PM) also called as aminosidine showed remarkable activity in the treatment of leishmanial (Wiwanitkit, 2012) due to low-priced and protection over a variety protozoa and bacteria (Sundar and Chakravarty, 2008). This drug is neither mutagenic nor teratogenic in nature. In the treatment of cutaneous leishmanias, based on parasitic specie and geographical area different drugs like gentamicin, urea and methylbenzothonium have been used (Sundar and Chakravarty, 2008). Different methods have been utilized that increases the effective life of drug by decreasing the time-period of treatment and reduces toxic nature of drugs. For this purpose, nanomaterials (Sattarahmady et al., 2016); nano-emulsions, and nanonisomes (Nazari-Vanani et al., 2018) have been used as drug carrier that hit only target cell and do not harm normal or healthy tissues. As nanoparticles possess quantum phenomenon and have high surface aspects. For this reason, NPs increases particle activity and hence biological function also increases (Sattarahmady et al., 2018).
Esfandiari et al. 2019, (Esfandiari et al., 2019) prepared the nanoparticles of “PM-loaded mannosylated CS (MCS)”. These nanoparticles were fabricated by adding the solution of dextran into the solution of “mannosylated CS”. The solution of triphosphate was synthesized in distilled water and then added this into previous solution dropwise to obtain the gelation solution through insulin syringe. The final product was stirred after sonication process. Then, washed with phosphate buffer saline when the product was precipitated by ultracentrifugation. At last, the product was stored after freeze-drying process. For the formation of nanoparticles of “PM-loaded MCS” without dextran followed the same procedure.
2.1.1.17 Chitosan/Lipid composite nanomaterials
In order to overcome the drawbacks of liposomal and polymeric nanoparticles system, a novel lipid based polymer drug delivery system has been introduced (Mandal et al., 2013). Hydrophobic lipid moieties linked with biocompatible hydrophilic polymers can self assemble into nanoparticles. Phospholipids (liposomes) exhibt remarkable property in biocompatible drug delivery systems and are non-immunogenic in nature. But, it was observed that lipid has degraded at high temperature (Robinson, 1996). A more effective drug delivery approach has been introduced by the combination of lipid and polymer (Elsabahy and Wooley, 2012). Lipid-based polymer hybrid nanoparticles have been introduced to overcome the limitation of uncontrolled as well as non-specific drug delivery approach (Tahir et al., 2017). Ovarian, testicular, lung and esophageal cancer can be cured with the aid of Cisplatin (a chemotherapy medication) (Comis, 1994). Moreover, Cisplatin shows poor solubility in oil and water phases that restricted the development of nanoparticles having more drug loading ability and encapsulation (Hamelers and De Kroon, 2007). However, Cisplatin as intravenous medication have adverse effect on healthy liver and kidney tissues. If the cisplatin is encapsulated with lipid polymer systems then it is safely transported to the targeted cell (tumors) (Kim et al., 2008) and in this way, the effectiveness of antitumor drug also increases.
Khan 2019, (Khan et al., 2019) articulated the nanoparticles of lipid-chitosan hybrid that was loaded with cisplatin through ionic gelation process. In the solution of acetic acid, chitosan was dissolved. Lipid was mixed in pure ethanol. Cisplatin was mixed with continuous stirring in acetic acid. Then, for the formation of nanoparticles through ionic gelation process, the ethanolic solution was added dropwise into the solution of drug. By lyophilization, the chitosan as well as lipid nanoparticles were centrifuged.
2.1.1.18 API- chitosan nanomaterials
The effectiveness of chemotherapeutic agents and their toxicity can be decreased by the fabrication of nano-scale drug delivery systems (nDDS) (Sun, 2017). However, many efforts on nanomedicine as clinical trials have done but the use of them still ask some questions related to lower serum stability, interaction of tumor cell with respondence to host cells, circulation in blood, allowing the passage of drugs through cytoplasmic vesicles, captured by the reticular endothelial system (RES) and short term circulation (Batrakova and Kabanov, 2008). However, amount of drug at tumor site has been reduced by means of these biological barriers. Different techniques have been utilized in order to increase the efficiency of therapeutic drugs (Danhier et al., 2010). PEGylation is the most commonly used process that increases circulation time of nDDS through surface modification of polyethylene glycol (PEG). The presence of hydrophilic shell inhibit opsonin proteins to be adsorbed which, in turn, increases stability of serum as well as circulation time along with preventing from destroying via RES (Shen et al., 2015). Recent research showed that PEG coated nanocarriers have low bioavailability in cancerous part because their drug release rate from endosomes as well as cellular uptake is slow (Stewart et al., 2016). The advances in cellular uptake can be done by the surface modification of nanocarriers depending upon cellular receptors and targeting groups with the help of ligands like peptides and antibodies (Zhong et al., 2017). Circulation time as well as stability can be enhanced by modifying the surface of nanoparticles with the introduction of ligands. This happens as a result of charge on ligand. So, there is an urgent need to demonstrate a multifunctional nano-carrier at cancerous part with enhanced rate of blood circulation, higher cellular uptake along with higher stability (Sun, 2017). It was reported that fluorinated functionalized material (due to its characteristics like stability, improvement in cell membrane permeation and resisting protein permeation) have played a significant role in drug delivery approach (Dong et al., 2010). An efficient protein delivery approach was developed (Zhang et al., 2018); in order to avoid protein denaturation and improvement in the process of endocytosis by the co-assembly of proteins along with fluroamphiphiles into NPs (Zhang et al., 2018). Yan, Wang (Yan et al., 2017) reported the synthesis of fluorinated poly (orthoester)-based nanospheres that are better for cellular uptake both in-vivo and in-vitro as well as efficient intracellular drug release (Yan et al., 2017). For the successful treatment of tumors, it is necessary to develop an efficient intracellular drug release (Fu et al., 2017). Keeping in view of this, different stimulus-sensitive drug delivery approaches had been proposed (Thambi et al., 2014). The widely employed approach toward drug release control in either lysosomes or endosomes having pH in the range of 4–5.5 trailored by endocytosis is acid sensitive drug delivery approach (Du et al., 2014).
Cheng, 2019, (Cheng et al., 2019) fabricated modified “carboxymethyl chitosan-based composite NPs”. This type of nanoparticles was developed to realize the effective intracellular drug release as well as greater permeability of cell membrane. In this process, “N-(3-Aminopropyl)-imidazole” was added gradually after the formation of carboxymethyl chitosan solution in phosphate buffer. The pH was maintained by HCl solution. Then, NHS as well as EDC.HCl was mixed into above solution. The reaction was performed for 12 h [Fig. 11]. For the elimination of smaller particles, the product was kept into dialysis bag. Through lyophilization, the “N-(3-Aminopropyl)-imidazole carboxymethyl chitosan (API-CMCS)” was fabricated.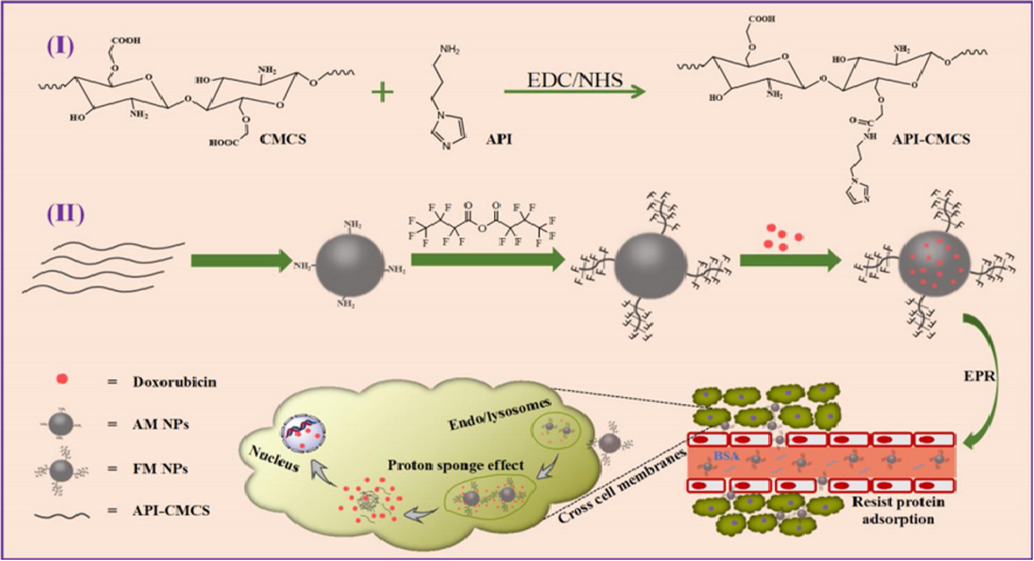
The preparation and drug delivery of AM and FM NPs (Cheng et al., 2019).
2.1.1.19 Chitosan/HSA composite nanomaterials
Due to existence of blood cerebrospinal fluid as well as blood brain barrier (BBB), mostly the drugs are not sufficiently absorbed by the brain which is responsible for allowing passage of specific molecules (Mistry et al., 2009; Sant et al., 2012). The novel approach “Nose-to-brain (NTB) delivery” has been implemented that directly targets the drug into brain. For this purpose, different products have been introduced such as hormones, anti-tumor drugs, vaccines, antimigraine and pain killers (Pires et al., 2009; Sonvico et al., 2018). The passage of drugs systematically as well as locally through intranasal route nullifies the effect of hepatic metabolism of drugs along with gastrointestinal degradation of oral administration. Nasal mucosa is highly vascularized that helps in increasing absorption rate of therapeutic agent, patient compliance, speed of therapeutic drug and safety. In addition, both large as well as small sized molecule easily enters through nasal cavity. Drugs entry through nasal cavity to the brain occurs via various routes. The first route is olfactory nerves that constitute the main path in nose to brain drug delivery approach. Second route is trigeminal nerves that end in respiratory epithelia. The third and last route is the passage of drug from respiratory epithelium to circulation and reach the BBB (Bonaccorso et al., 2017; Mistry et al., 2009). Moreover, this delivery route also have some drawbacks; degradation with the aid of enzymes, reduced volume of nasal passage, drug delivery system, removal of drug, an appropriate equipment for delivery, and potential nasomucosal toxicity (Md, 2018; Sonvico, 2018). HSA is an endogenous protein, biocompatible, biodegradable, safe to use and non-immunogenic. Also HSA exhibit good properties for the adhesion at mucosal surface that allows it to stay long time in nasal cavity. Due to its targeting properties, the cellular uptake can also be enhanced by HSA (Elzoghby et al., 2012). To enhance certain features such as pharmacological effects of synthetic as well as plant-derived molecules, bioavailability and stability albumin also has been utilized as nanocarrier. In nose to b rain delivery of anti-Alzheimer drugs as well as R-flurbiprofen HAS nanoparticles played a significant role (Bilati et al., 2005).
Piazzini 2019, (Piazzini et al., 2019) formulated the “human serum albumin nanoparticles (HAS NPs)” that was considered as nose-to-brain carrier. For nasal application, the effect of chitosan that was coated on the performance of “HSA nanoparticles” was examined. By the process of desolvation, HSA nanoparticles were prepared. Under magnetic stirring, the HSA was mixed in the solution of sodium chloride. By utilizing syringe, the ethanol was added to get turbid solution. Through the addition of glutaraldehyde solution, the obtained product was hardened. The pellet was redispersed in water after two hours.
2.1.1.20 Chitosan base chlorin e6 composite nanomaterials
The emission as well as excitation of photo-responsive nanocarriers by means of disruption and disassembly results in external stimuli photo controlled drug release system (Peng et al., 2015). Nanocarriers that have played a significant role in controlled drug delivery approach, bioimaging and biosensors, are nanotubes, nanoclusters, nanoparticles, nanogel, nanorod, micelles and liposome (Yang et al., 2015). Photo-triggered drug release approach is based on the absorption of NIR light and the conversion of this light into photo-thermal heat (Wang, 2014; Peng, 2015). The drug release approach involve the usage of drugs such as gene therapeutics, growth factors, chemo and chemokines (Costa et al., 2015). The study of interaction of these systems with NIR region is under consideration and also used in-vivo as well as in-vitro tracking of delivery. When nano-carriers interact with tissue or cell, various factors like surface chemistry and physical characteristics are of great interest (Cole et al., 2009). However, at cellular level, the interaction of drug with cell as well as biodegradability of nano-carrier is still a question.
Bhatta et al. 2019, (Bhatta et al., 2019) carried out the synthesis of nanoparticles loaded with “doxorubicin (DOX)” and decorated with Ce6 by the use of chitosan and “tripolyphosphate (TPP)”, DOX and chlorin e6 (Ce6), by the technique of “ionic-gelation. Under constant ultrasonic stirring, by the addition of known concentration of triphosphate with chitosan solution, the chitosan nanoparticles were fabricated. The product was stored overnight after the stirring for 4 h and then, the solution was filtered after chlorine e6 was mixed in water. Under continuous stirring for 2 h, the Ce6 solution was added dropwise into the chitosan nanoparticles dispersion. Nanoparticles were resuspended in deionized water after centrifugation. The “doxorubicin (DOX)” was mixed into “triphosphate (TPP)” solution before the formation of “Ce6-CSNPs” for DOX encapsulation.
2.1.1.21 Carboxymethyl chitosan-based nanomaterials
The extracellular pH of cancerous cell played a significant role in degradation of cancer which is more acidic than normal or healthy tissue (Min et al., 2010). In this way, pH based drug delivery approach is highly useful for the treatment of cancer (Lee et al., 2008; Shang et al., 2014). Under acidic gastric conditions, pH based carrier method cause a burst release and under alkaline intestinal microenvironment controlled drug release is observed (Feng et al., 2013). For the sustainable release of drug, only few researchers observed the weakly acidic environment as compared to cancerous part (Lee et al., 2010; Risbud et al., 2000). So, there is an increasing demand of developing carriers which are pH sensitive through less acidic cancerous environment.
Li et al. 2019, (Li et al., 2019) formulated nanoparticles of carboxymethyl chitosan. Through adding the different concentration of rectorite (REC) into carboxymethyl chitosan solution, CMC/REC composites were synthesized. To maintain the pH of solution, sodium hydroxide as well as hydrochloric acid was applied. When the solution converted into light blue emulsions, the solution of nanoparticles colloidal was obtained.
2.1.1.22 Chitosan base peptide nanomaterials for drug delivery
Breast cancer is one of the leading cause of death in women and treated with chemotherapy (Ferlay et al., 2010); but it has certain adverse effects on human health due to systematically distribution of active ingredients. The chemotherapeutic agents are harmful for both type of cells such as normal cells and cancerous tissues. They also cause hepatorenal failure as well as alopecia (DeSantis et al., 2014). A chemotherapeutic drug “Paclitaxel (PTX)” also utilized but it has poor targeting ability and adverse effects. Nanoscale formulations have been introduced that increases the effectiveness of clinical approaches toward breast cancer (Nam et al., 2013) as well as overcome the poor targeting ability of PTX (Lammers et al., 2012). Targeted carriers have been gained much attention because they don’t destroy normal cells and are highly specific in their action (Talluri et al., 2016). Based on EPR effect, nanoparticles are absorbed at tumor site employing passive targeting functionality (Allen and Cullis, 2013). The tumor cell as well as normal cell have different environment. The blood vessels of tumor cell are bent, dilated and lack of lymphatic flow. These are responsible for pressure in cancer cell which is much more than normal cells. Also, the temperature of cancerous part in body is more as compared to normal tissue due to uncontrollable division of cancer cells as well acidic pH of cancer cell (Van der Zee, 2002). These factors help the drug delivery system to selectively target the cancer tissue.
Qian et al. 2018, (Qian et al., 2019) carried out the formation of “paclitaxel (PTX) loaded K237-peptide functionalized hybrid chitosan/poly(N-isopropylacrylamide) nanoparticles (NPs)” and analyzed their effectivity for “KDR/Flk-1-overexpressing human breast cancer” [Fig. 12].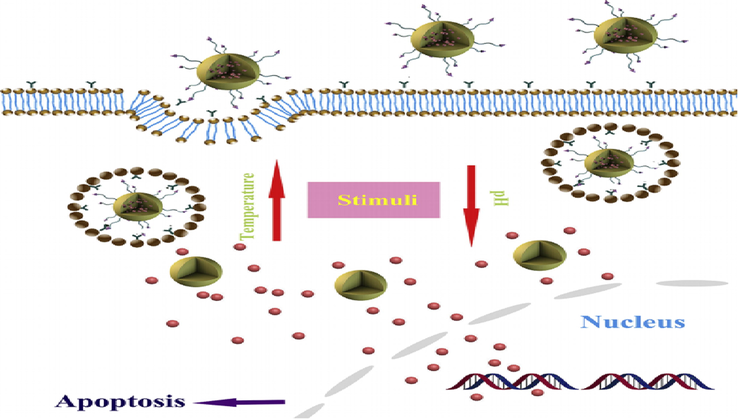
Schematic Illustration of Peptide Functionalized Dual-responsive Chitosan Nanoparticles for Controlled Drug Delivery to Breast Cancer Cells (Qian et al., 2019).
In this process, the obtained functional nanoparticles were RAFT-based method [Fig. 13]. Under the atmosphere of nitrogen, the mixture of “anhydrous DMF” as well as “CS-RAFT agent” was magnetically stirred. “N-isopropylacrylamide (NIPAM)” as well as “azobisisobutyronitrile (AIBN)” were added after the completion of dissolved process of “CS-RAFT agent”. To get “N-acetyl CS-g-PNIPAM”, the product was precipitated in “10-fold diethyl ether” and then filtered. By agitation the product in aqueous sodium hydroxide solution, acetyl group were removed. In the solution of DMSO, “K237 peptide” solution was added in “CS-g-PNIPAM”. To avoid the denaturation of “K237 peptide”, “N-hydroxysuccinimide (NHS)” as well as “N- (3-dimethyl-aminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC)” were mixed in the solution. Against sodium hydroxide solution, the mixture was dialyzed for 72 h and then lyophilized to obtained “K237-CS-g-PNIPAM”.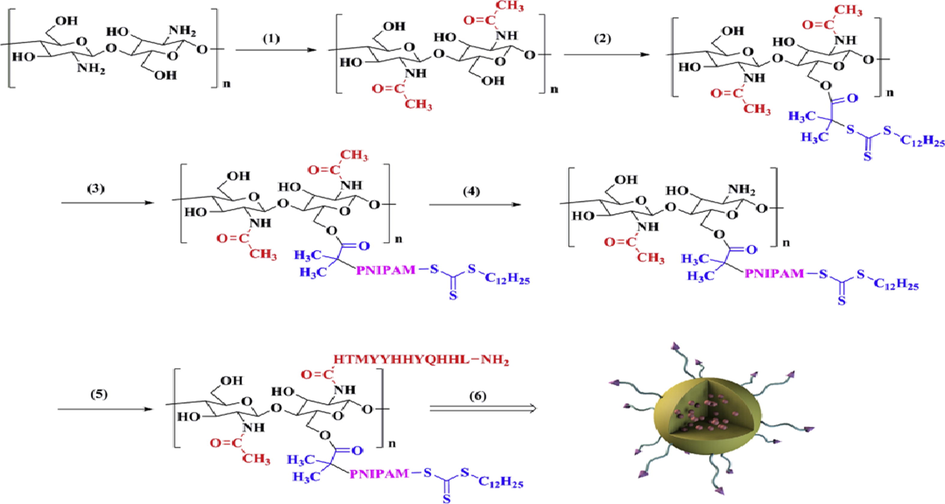
The synthesis of K237-CS (PTX)-g-PNIPAM NPs. (1) Acetic anhydride, rt, 4 h; (2) DDACT, DCC, DMAP, rt, 48 h; (3) NIPAM, AIBN, 60 °C, 48 h; (4) Hydrolysis, rt; (5) K237 peptide, EDC, NHS; (6) PTX, self-assemblyFig. 1. The synthesis of K237-CS (PTX)-g-PNIPAM NPs. (1) Acetic anhydride, rt, 4 h; (2) DDACT, DCC, DMAP, rt, 48 h; (3) NIPAM, AIBN, 60 °C, 48 h; (4) Hydrolysis, rt; (5) K237 peptide, EDC, NHS; (6) PTX, self-assembly (Qian et al., 2019).
2.1.1.23 Chitosan-folic acid conjugate nanomaterials for drug delivery
Infectious diseases are treated with the help of tetracycline antibiotics (Chopra and Roberts, 2001). Doxorubicin has the ability to fight against cancer and show remarkable activity as chemotherapy agent (Ma and Mumper, 2013). Throughout the world, estrogen receptor-positive breast cancer have been cured with tamoxifen drug (Errico, 2015). The use of natural as well as synthetic nanocarriers along drugs like tamoxifen and doxorubicin is now under consideration (Bourassa et al., 2014). Folic acid-polymer conjugates have tremendous effect on drug delivery system and there is an important task to encapsulate folic acid-Cs nanoparticles that show remarkable drug loading efficacy.
Chanphai et al. 2018, (Chanphai et al., 2019) used the nanocapsules of chitosan-folic acid for encapsulating the tamoxifen, doxorubicin and tetracycline [Fig. 14]. They added the solution of folic acid into chitosan with constant stirring. They prepared solutions in water and then made various dilutions of doxorubicin and tetracycline in Tris-HCl (pH 7.2). Tamoxifen similarly made in water/ethanol 50% and after this, made its serial dilutions in Tris-HCl (pH 7.2). The formulation of the conjugates of Drug-chitosan-folic acid was achieved by adding the solution of drug to the nanocapsules of chitosan-folic acid in Tris–HCl by stirring when required for the confirmation of the synthesis of solution with homogeneous nature.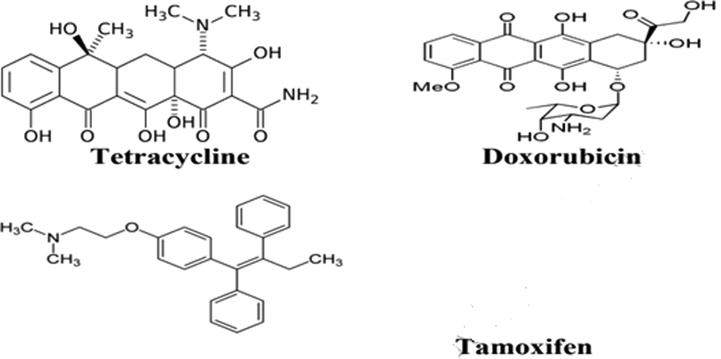
Chemical structures of tetracycline, doxorubicin and tamoxifen (Chanphai et al., 2019).
2.1.1.24 Glutaraldehyde cross-linked chitosan nanomaterials
Glutaraldehyde belongs to one of the lethal chemical but no significant information related to digestion is available. Exposure to glutaraldehyde may cause respiratory tract infection. Zissu, Bonnet (Zissu et al., 1998) found that when mice are repeatedly exposed to glutaraldehyde vapor at 0.1 ppm for 78 weeks, the histopathology report does not show any evidence related to lesions in pulmonary tract of mice. However, Halatek, Opalska (Halatek et al., 2003) published that when the rat model is exposed to 0.1 ppm glutaraldehyde vapor even only for 4 weeks, rat got infection in lungs.
Islam et al. 2019, (Islam et al., 2019) carried out analysis for degrading the chitosan with low molecular weight, having 92% “degree of deacetylation (DD)”, and prepared their nanoparticles in the solution of lysozyme at temperature of 37 °C. The synthesis of nanoparticles was done by crosslinking the chitosan with glutaraldehyde in the medium of water-in-oil emulsion. The blank particles of chitosan were synthesized by dissolving the powder of chitosan in the solution of acetic acid. The formation of particles was done by the addition of the solution of glutaraldehyde, then done its washing by using diethyl ether and hexane, then carried out centrifugation and at last freeze-drying at temperature of −80 °C.
2.1.1.25 Chitosan/fucoidan composite nanomaterials
Tsai et al. 2018, (Tsai et al., 2019a) carried out the synthesis of nanoparticles of trimethyl chitosan (TMC)-fucoidan (FD) and analyzed their use in oral delivery of insulin. They applied simple technique of polyelectrolyte complex to fabricate the nanoparticles by developing electrostatic interaction between fucoidan and trimethyl chitosan. The mixing of fucoidan and trimethyl chitosan (or chitosan) was done in deionized H2O. The dissolution of insulin and magnesium sulfate was done in the solution of fucoidan and then dropped the solution in premixed state into the freshly made solution of TMC (or chitosan) and then homogenized thoroughly by stirring. The same above mentioned method was opted to fabricate the fucoidan and TMC (or chitosan) based “insulin-loaded nanoparticles”.
2.1.1.26 Chitosan/polylactide composite nanomaterials
After the use of insulin in the treatment of diabetes, the medical uses of proteins and peptides in the cure of various diseases have gained much interest. Type-1 diabetes requires daily injection of insulin twice a day that is painful for a patient. The convenient method for the intake of drug (proteins and peptides) is oral administration but it has also some problems like rapid degradation by enzymes as well as poor intestinal permeability (Chen et al., 2013). There is an urgent need to develop Oral administration of proteins as well peptides in order to limit the disadvantages of drug delivery systems. Brown algae yields Fucoidan (a polysaccharide) along with Undariapinnatifida, Eckloniakurome and Fucusvesiculosus. The main composition of Fucoidan includes sulphates and L-fucose with small proportions of mannose, xylose, uronic acid and galactose. These components help in various anti cancer, anti viral, anti inflammation, anti coagulant and antioxidant properties. Further, investigation revealed that Fucoidan also has the ability to maintain the blood-glucose level (Zhao et al., 2018). This can be done by the inhibition of α glucosidase and α amylase (Kim et al., 2014); that contribute protection to pancreases and stimulate secretion of insulin. It was also observed that the use of fucoidan also provide protection to pulmonary tract (Kolsi et al., 2018); in both human and animal patient. However, TMC also played a significant role in anticancer potential of fucoidan through oral route as reported by Hayashi, Sasatsu (Hayashi et al., 2011). In this way, both Fucoidan (FD) and TMC combine together that have significant role in oral administration of insulin and also have the benefit to overcome the complications faced by only FD as well as amelioration of diabetes.
DeVeaux et al. 2019, (Gomillion, 2019) achieved the synthesis of “chitosan (CS)/polylactide (PLA) nanoparticles” by using the technique of “solvent evaporation”, in order to obtain therapeutics delivery. For this purpose, they pipetted the solution of tamoxifen into the solution of polylactic acid by continuously stirring in order encapsulate the tamoxifen. Then, poured the solution in the solution of chitosan. The oil in water emulsion was obtained by sonicating the solution at 40 °C and after this, removed the emulsion from sonicator and put on hot plate at same temperature and stirring continuously for removing the excessive dichloromethane (DCM). Then pipetted the “CS/PLA/tamoxifen emulsion” in the microtubes that were sterilized, and the pH was neutralized and residual polyethylene oxide and other contaminations from emulsion were washed by pipetting 200 μL “phosphate-buffered saline (PBS)” and 300 μL C2H5OH ethanol, respectively. Then centrifugation of emulsion was carried out, aeration of supernatant was done and the pellets of the nanoparticles of tamoxifen were washed by using “phosphate-buffered saline (PBS)” and prior to using it, was resuspended in “Dulbecco’s modified Eagle’s medium (DMEM) [Fig. 15].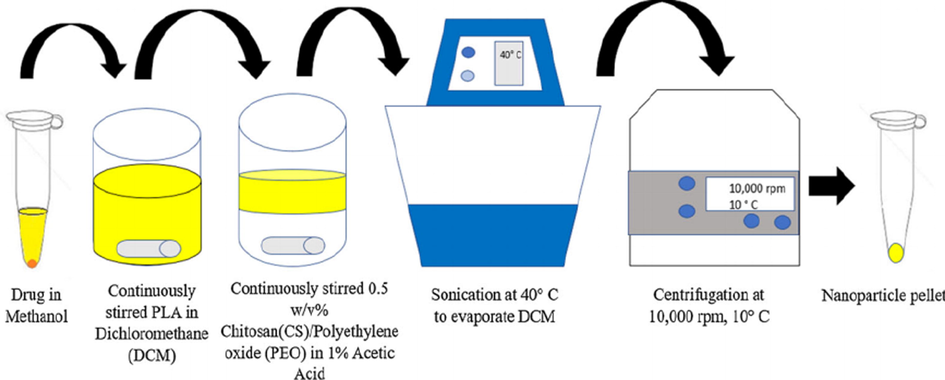
Fabrication of drug-loaded nanoparticles via solvent evaporation method (Gomillion, 2019).
2.1.1.27 Chitosan/Cadmium chloride composite nanomaterials
Triple negative breast cancer (TNBC) is one of the threatening type in breast cancer. The treatment of TNBC is highly difficult because TNBC cells do not have any type of human epidermal growth factor receptor type 2, estrogen receptor and progesterone receptors (Foulkes et al., 2010). Family history, age, life style and use of oral contraceptives are the primary reasons that are concerned with development of TNBC. Researchers declared that changes or mutation in tumor suppressor genes (such as BRCA1 and BRCA2) have contribution in growth of TNBC but the exact cause is still unknown (Severson et al., 2015). Studies have shown that there is the greater chance of developing metastasis breast cancer in African-American women in contrast to Caucasian women because the former have more genes like BRCA1 as well as BRCA2 (Haffty et al., 2006). It was also observed that TNBC mostly found in African women suggested it is transmitted from their descendants (Newman and Kaljee, 2017). Further, epidemiological studies evaluated that there are lesser chances for the European women to diagnose TNBC than American and African women (Boyle, 2012). The prediction of hormone positive breast cancer is easy as compared to TNBC. Meanwhile, the diagnosis of hormone positive breast cancer in Africa and America women is lower than other groups and the detection of TNBC is even much lower in Africa America women and hence, mortality rate also increases. PLA has been successfully applied in dialysis, dialysis media and surgical sutures. PLA exhibit excellent biocompatibility as well as poor cell toxicity. The soft tissue compatibility of PLA is related to strong hydrophobicity in addition to higher crystalline nature (Dev et al., 2010).
Abdelhamid et al. 2019, (Abdelhamid et al., 2019) fabricated “CdS quantum dots modified chitosan (CdS@CTS)” by hydrothermal method [Fig. 16]. They formed solution of chitosan in acetic acid and purged it with N2 gas under “magnetic stirring”. Then mixed the solution with Cd(NO3)2 under constant stirring for 24 h. Then slowly added a freshly formed Na2S·9H2O solution in it. In the last step, they added ammonium hydroxide while stirring constantly. Then washed the particles and centrifuged them for separation. The dispersion of obtained quantum dots of cadmium sulfide (CdS) was then carried out in the solution of chitosan by the use of ultrasonication. The ultrasonication was done with chitosan (CTS) to get cadmium sulfide solution. The “Sesamol-CdS@CTS” was obtained by using this solution. The addition of Sesamol was done to the suspension of CdS@CTS under varied stirring rate as well as temperatures. Then the prepared colloid was purified by the help of dialysis against water at room temperature.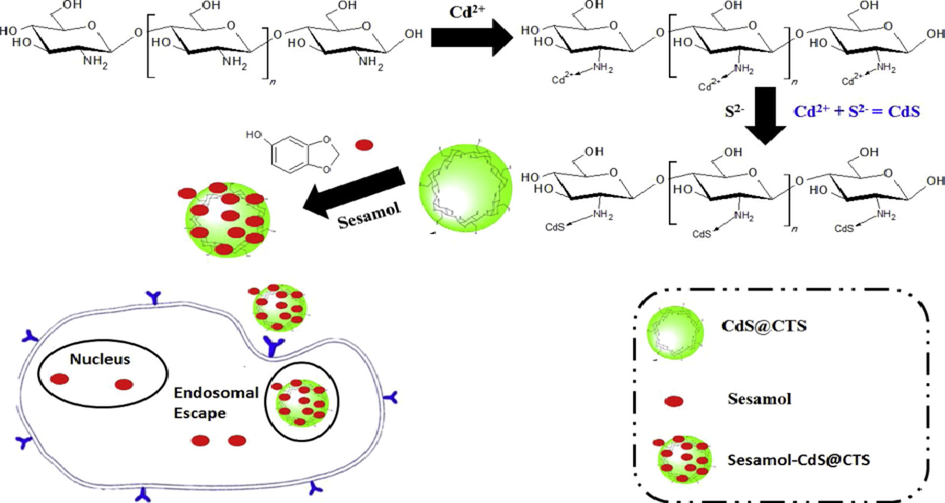
Schematic representation of the synthesis of CdS@CTS and Sesamol-CdS@CTS and their applications for drug delivery (Abdelhamid et al., 2019).
2.1.1.28 Chitosan/Silica nanoparticles composite nanomaterials
On the basis of remarkable properties of quantumS dots (QDs) such as Cadmium sulphide (CdS) have gained much attention in the field of research (Chand et al., 2017). Conventional organic dyes do not show tunable optical properties but QDs have the ability to possess optical features in the range of UV to near-infrared region (>650 nm) emission (Abdelhamid, 2019). In addition, QDs also possess size tunable light emission, good photo-chemical stability along with high quantum field (Matea et al., 2017). QDs find its application in lithium sulphur battery and in biological field such as biomedical sensing and imaging technology (Abdelhamid and Wu, 2015); in-vitro tumor cell diagnosis biomarkers and study of proteins (Abdelhamid and Wu, 2015). However, it was reported that in random forest mode, the toxic nature of CdS NPs is due to surface aspects (Oh et al., 2016). The surface modified conjugates can be produced by using some biomolecules like chitosan and derivatives of CS for several biological field that have no toxic effects in mice (de Carvalho et al., 2017). When the surface of inorganic QDs coated, it is not only responsible in the stability of colloidal NPs but it also give protection from leakage of Cd ions from particle surface and deterioration (Hezinger et al., 2008).
Chen et al., 2018, 2019 carried out the fabrication of a “pH/GSH dually responsive delivery system” and a “folic acid (FA) target” on the basis of the nanoparticles of “mesoporous silica” by coating it with the shell of chitosan by various chemical variations on the surface of “mesoporous silica nanoparticles (MSN)” [Fig. 17]. Then the self-assembly of “low molecular weight chitosan (LMW chitosan)” was done on MSN surface by the help of “electrostatic interactions”. They increased the activity of drug delivery and release by further crosslinking the shell of chitosan molecules by “N,N’-bis(acryloyl) cystamine (BAC)” by the help of “GSH-sensitive disulfide bond”. Then they carried out conjugation of chitosan nanoparticles with folate (“self-targeting agent”), for minimizing the side effects as well as identifying the cancer cells. They finally synthesized the system of drug delivery based on “pH/GSH dual-responsive MSN” by some processes of chemical changes on the surface, that possessed the capability of controlling as well as targeting.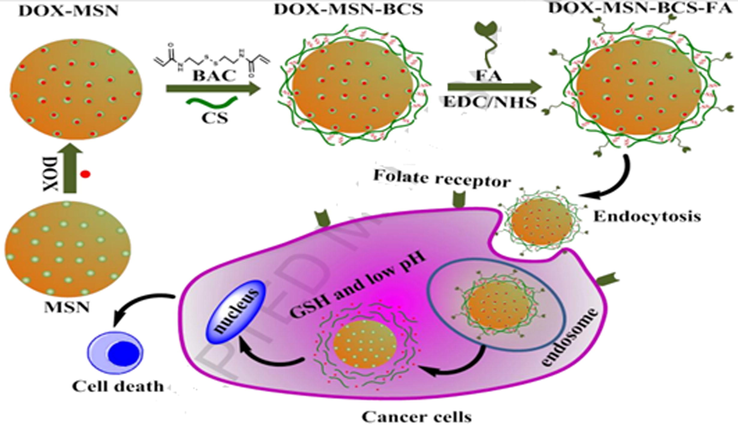
Schematic diagram showing the synthesis procedure of DOX-MSN-BCS-FA and the extracellular and intracellular trafficking for DOX-MSN-BCS-FA to cancer cells (Chen et al., 2019).
2.1.1.29 Chitosan/Polycaprolactone composite nanomaterials
The magnetic properties as well as dimensions of iron oxide nanoparticles make it a suitable candidate in the development of functional nanostructures (Mahmoudi et al., 2011; Zhao et al., 2009). These feature allow the application of NPs in environmental and biological field (Zhao et al., 2009). Among all iron oxides, Magnetite (Fe3O4) nanoparticles have been extensively studied due to its chemical stability, harmless nature, ease of fabrication and biocompatibility (Hong et al., 2008; Zhao et al., 2009). Because of this reason, magnetite NPs have been used in magnetically guided drug delivery, MRI contrast agent and bimolecular separation (Hong et al., 2008; Zhao et al., 2009). In addition to these, Fe3O4 NPs have also found its application in catalysis along with purification of water from heavy metals and organic dyes (Tran et al., 2010).
Balan et al. 2019, (Balan et al., 2019) carried out analysis for therapy of anticancer, on the combined influence of the nanocomposites made by combining ferulic acid (FA) and resveratrol (RS). For this, they dissolved “polycaprolactone (PCL)” into “dichloromethane”: C2H5OH with equal volume ratio. Then added chitosan nanoparticles and resveratrol loaded chitosan nanoparticles that were obtained freshly, into the polycaprolactone and ferulic acid blended solution of polycaprolactone and stirred continuously for 2–3 h. Then loaded this blended homogenized solution in a syringe having “vertically fixed stainless steel needle” on “syringe pump” (used for controlling the rate of flow). Gave a voltage of 20 kV to the needle and fixed the distance between collector and needle to about 12–15 cm. Then finally collected the as-spun nanofibers.
2.1.1.30 Chitosan composite nanomaterials
Skin is one of the largest sense organ comprises of 8% of the body mass having approximate 1.8 m2 surface area. Skin protects us from UV radiation coming from the sun, microorganisms and other harmful agents. However, the exposure of these factors causes skin carcinogenesis (Kvam and Tyrrell, 1997). There are more chances in light-skinned population to detect with skin cancer. Major categories of skin cancer are; 1) melanoma, 2) non-melanoma skin cancer, 3) basel cell carcinoma and 4) squamous cell carcinoma. Non-melanoma skin cancer found with morbidity rate around 2–3 million people each year. However, this type of cancer is harmless and rarely, it is associated with death. But, causes diseases which has harmful influence on human health (Brandt et al., 2019; Narayanan et al., 2010). Treatment options in skin cancer are excisional surgery, radiation therapy and biological therapy. But, when cancer is treated through surgery, it leads to incomplete removal of cancerous part. Then, treatment with radiation or through chemotherapy but these also have adverse effect on normal healthy tissues. After that, it was found that plant derived phenolic compounds have also been used in treatment of tumor (Huang et al., 2009). Yet, Ferulic acid (FA) is a hydroxycinnamic acid possess antitumor as well as antioxidant properties. It is mostly found in plant cell wall (Kumar and Pruthi, 2014).
Mohammed et al. 2018, (Mohammed et al., 2019) orally achieved the release development of “mycophenolate mofetil (MMF)” by the use of the nanoparticles of “poly(lactic-co-glycolic) acid (PLGA)” or “polylactic acid (PLA)” coated with chitosan, for daily single time dosing. For obtaining the polymeric nanoparticles (PNPs), the dissolution of PLGA and MMF was done in chloroform and poured it by syringe pump to polyvinyl alcohol (PVA) in H2O onto a “vortex mixer”. Then decreased its size by ultrasonication. Then poured this emulsion in beaker to 0.5% PVA, stirred constantly to harden it. Then done its ultracentrifugation and washed with deionized H2O for removing extra surfactant. Then suspended the pellet of PNP in deionized H2O. Then addition of sucrose was done for sake of cryoprotection. The frozen of obtained suspension of PNP was done at −80 °C and lyophilization was done for 3 days. For preparing “chitosan coated PNPs (CS-PNPs)”, the synthesis of “MMF-loaded nanoparticles (PLGA or PLA)” was done similar to above but the addition of chitosan solution in acetic acid wad carried out into the PVA during the step of hardening, and stirred for about 6 h at 23 °C.
2.1.1.31 Chitosan base human serum albumin
End-stage organ failure is treated with solid organ transplantation. In 2015, the total number of solid organs transplanted is 126,670 in more than 102 countries that exceeds 5.7% ratio than 2014 (Carmona et al., 2015). This can be achieved by the advancement in immune suppressive pharmacology therapy. When an organ is transplanted, our immune system recognizes the transplanted organ like foreign object that generates a complicated response resulting in the damage of the organ. Immunosuppressive medication must be used in order to prevent the transplant rejection. However, missed doses of the medicine results in increased health care expenditure, graft impairment and increased rate of mortality (Dew et al., 2007). Yet, one in four patients fail to take medicine as prescribed by physician (Butler et al., 2004). The type of drugs that are commonly utilized for the immunosuppression of transplant organs are mycophenolic acid (MPA) like mycophenolatemofetil (MMF), a corticosteroid and calcineurin inhibitor (CNI). Non-adherence to prescribed medication is due to the difficulty of remembering multiple doses of immunosuppressive drugs (Muduma et al., 2016). A corticosteroid and CNI are advised to take once a day. But MMF is prescribed to take twice-a-day. The adverse effects of digestive diseases can be lessened through the further division of medicine into four times. The one-daily dose of MMF is helpful for patients that results in better clinical findings. Owing to various dosage uptakes, there should be a flexible dosing platform. The formulation of nanoparticles can help in this regard. MMF which shows sufficient solubility in water frequently transformed into MPA within body and plays a critical role in proliferation of T and B- lymphocytes. MMF is anti-proliferative and immunosuppressant drug (He et al., 2011).
Montero et al. 2018, (Montero et al., 2019) used the technique of ionic crosslinking with the solution of “tripolyphosphate (TPP)” being “crosslinking agent”, for the fabrication of “bovine serum albumin (BSA)” and chitosan based nanoparticles [pFig. 18]. They prepared various amounts of nanoparticles by changing BSA (A) and chitosan (C) proportions in primary mixture. So, when BSA and chitosan were dissolved fully in acetic acid, then they mixed various concentrations of the solutions of polymer and stirred constantly for 30 min. After this, they sonicated continuously while dropping 5 mL of the obtained solution into TPP (100 mL), in a water–ice bath, maintained the suspension by stirring continuously and then done its ultracentrifugation and after this, washed by using H2O, and finally freeze-dried the as-prepared nanoparticles at −110 °C for 24 h.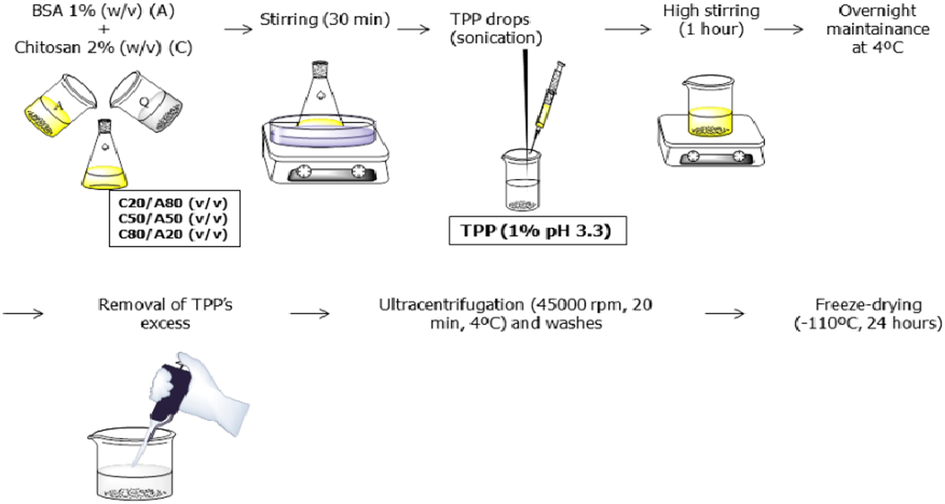
Schematic representation of the synthesis of chitosan-BSA based nanoparticles (Montero et al., 2019).
2.1.1.32 Chitosan base polylactide composite nanomaterials
Although, many treatment advances in the spectrum of cancer has been developed. But, these methods have certain drawbacks such as adverse toxic effects on human health. So, there is an urgent need to develop a method that is more reliable. Many efforts have been done to establish a drug delivery approach by the use of NPs (Huang, 2017). In drug delivery approach, Bovine serum albumin (BSA) employed in encapsulation of genes, paclitaxel betulinic acid and tamoxifen (Martínez et al., 2011). BSA have also integrated in many nano-systems that increases its efficacy (Mattu et al., 2013).
Rong et al. 2018, (Rong et al., 2019) developed a system for “double-controlled” release of drug (insulin) for delivery in ocular way by “subconjunctival injection” and also analyzed the safety and biocompatibility of this system. They prepared the solution of chitosan initially and after this added the solution of insulin in this chitosan solution with magnetic stirring. Then addition of the solution of “Tripolyphosphate (TPP)” was carried out in the mixture formed above with stirring for fabrication of nanoparticles. The freeze-drying of the obtained insulin-loaded chitosan nanoparticles (ICN) was done and was stored finally. Then dissolution of “PLGA-PEG-PLGA” in deionized H2O was done and in the obtained solution, the ICN was added with stirring. The obtained composite hydrogel was in liquid form at 25 °C while in solid form at 37 °C.
2.1.1.33 Chitosan base curcumin nanomaterials for drug delivery
Transdermal drug delivery (TDD) is preferred route as compared to oral drug administration because it prevents digestive disorders and pre-systemic metabolism that decreases the dosage frequency along with controlled levels of plasma (Ren et al., 2009). But, TDD also affects the outermost layer of epidermis (stratum corneum) that act as barrier. Several techniques like physical, nano and chemical carrier has been employed to increase the permeation through stratum corneum. Among these carriers, nanocarriers present a number of advantages (improved stability and prolonged release of drug) as compared to conventional transdermal formulation. But the main limitation in the use of nanocarriers is poorly penetrated in the skin (Zhao et al., 2010). It was believed that healthy skin is impermeable for the penetration of nanoparticles. Although, recent research has been revealed that NPs rely upon size, type of material and surface charge has the ability to deeply penetrate in the skin (Palmer and DeLouise, 2016). However, nanocarriers system (nano vesicles as well as polymeric NPs) does not affect the structure of skin and help in transdermal penetration (William and Barry, 2004). NPs permeation through the skin takes place through these pathways; 1) intercellular lipid spaces 2) trans-appendageal (hair follicles and sweat glands) 3) transcellular route. Transcellular route involves the passage of drug by lipid structures of intracellular regime and corneocytes (Pegoraro et al., 2012).
Nair et al. 2019, (Nair et al., 2019) carried out the fabrication of nanoparticles of chitosan loaded with curcumin and also analyzed for its effectivity in “transdermal delivery”. For this, they prepared the solution of chitosan by applying magnetic stirrer and then added the solution of curcumin (in C2H5OH) in it drop by drop. Then added the TPP crosslinker in it with different amounts dropwise, for obtaining chitosan and TPP in mass ratio between 3:1 and 5:1. Carried out stirring of the obtained suspension and centrifugation for getting the pellets of nanoparticles. Then cryoprotectant was used and redispersion was done for pellets and were finally faced to freeze-drying.
2.1.1.34 Chitosan base albumin nanomaterials for drug delivery
Albumin is a globular and the most abundant protein in nature. Its concentration in human serum ranges between 35 and 50 g/L. It is synthesized in liver and responsible for the transportation of many bio-molecules such as nutrients, hormones and fatty acids (Larsen et al., 2016). Due to its properties like biocompatibility, low-immunogenicity, high solubility in water, non-toxic behavior and bio-degradability have been extensively utilized in drug delivery systems (Elzoghby et al., 2012; Kudarha and Sawant, 2017). Human serum albumin (HSA)/NPs named as Abraxane applied in tumor therapy. BSA is relatively cheap, similar to HSA and has been used as drug nanocarriers in pharma industry (Elzoghby et al., 2012). The number of amino acid residues in BSA is 583. Its molecular weight is 69.3 kDa. It consists of 17 disulphide bonds with one free thiol group. The iso-electric point of BSA is 4.7. Two tryptophan residues are present at position 212 in hydrophobic pocket at subdomain II A along at position 134 corresponds to subdomain IB that is exposed to solvent (Elzoghby et al., 2012; Kudarha and Sawant, 2017; Larsen et al., 2016). BSA attracts cationic polymers because in solution it is negatively charged. CS is non-toxic, biodegradable, cationic and biodegradable in nature and has been used in drug delivery approach (Zhao et al., 2018). The composition of chitin is 1,4-linked 2-acetamido-2-deoxy- β-D-glucan and 1,4-linked 2-amino-2-deoxy-β-D-glucan and is produced during deacetylation of chitin (Birch and Schiffman, 2014). By the encapsulation of CS and albumin, the formation of NPs is associated as gene carrier along with the delivery of antitumor drugs like alprazolam, ibuprofen and paclitaxel (Esfandyari-Manesh et al., 2016). Aggregation of NPs under physiological conditions and its poor solubility are some drawbacks in using CS as drug carrier (Birch and Schiffman, 2014; Zhao et al., 2018).
Razi et al. 2019, (Razi et al., 2019) fabricated the nanoparticles of “reduced bovine serum albumin (rBSA)” and “glycol chitosan (GC)” (rBG-NPs), that were made stable by the availability of “disulfide bonds” and “hydrophobic interactions” for the delivery of paclitaxel (PTX). They mixed the solutions of GC and rBSA for fabricating rBG-NP. For this purpose, they synthesized first the solution of GC in acetate buffer by stirring for complete dissolution and hydrated overnight. Similarly the solution of rBSA was obtained and diluted with HCl. The synthesis of rBG-NPs was achieved when the solution of rBSA was added in the solution of GC while stirring at 25 °C, and finally the adjustment of pH was done by addition of NaOH or HCl [Fig. 19].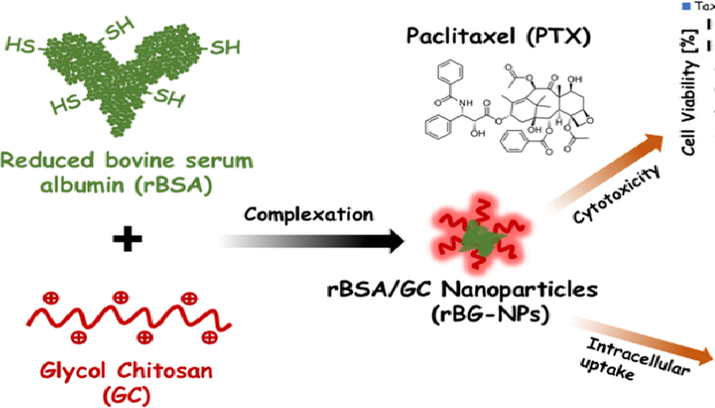
Formation of stable nanoparticles with biological components for encapsulation and cellular delivery of PTX (Razi et al., 2019).
3 Conclusion
This potential polymer have novel properties such as non-toxicity, low cost transparency, non-immunogenic, biocompatibility as well as biodegradability. Hydrogels, microsphere, blends, microparticles, nanogels, electrospun fibers, films, nanoparticles, nanocomposites, scaffold as well as prodrugs based on chitin and chitosan natural or synthetic polymers are used as promising candidate for biomedical application such as drug delivery. Due to its vast use in different novel applications it produce interest in mind of researchers for making its potential blend with inorganic/organic, natural as well as synthetic polymer for different promising application in different fields in future.
Funding
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Acknowledgement
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Nanoparticles assisted laser desorption/ionization mass spectrometry. Handb. Smart Mater. Anal. Chem. 2019:729-755.
- [Google Scholar]
- Synthesis of CdS-modified chitosan quantum dots for the drug delivery of Sesamol. Carbohydr. Polym.. 2019;214:90-99.
- [CrossRef] [Google Scholar]
- Proteomics analysis of the mode of antibacterial action of nanoparticles and their interactions with proteins. TrAC, Trends Anal. Chem.. 2015;65:30-46.
- [Google Scholar]
- Improved antimicrobial activity of polypropylene and cotton nonwoven fabrics by surface treatment and modification with chitosan. J. Appl. Polym. Sci.. 2008;108(4):2290-2296.
- [Google Scholar]
- Synthesis, biological characterization and evaluation of molecular mechanisms of novel copper complexes as anticancer agents. Biochimica et Biophysica Acta (BBA)-Gen. Sub.. 2017;1861(2):218-234.
- [CrossRef] [Google Scholar]
- Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv.. 2014;4(8):3974-3983.
- [Google Scholar]
- Role of nanoparticle in cosmetics industries. Biol. Synth. Nanopart. Appl. 2020:173.
- [CrossRef] [Google Scholar]
- Relief role of lysine chelated zinc (Zn) on 6-week-old maize plants under tannery wastewater irrigation stress. Int. J. Environ. Res. Public Health. 2020;17(14):5161.
- [Google Scholar]
- Hydrogel scaffold-based fiber composites for engineering applications. Hybrid Fiber Compos.: Mater. Manuf. Process Eng. 2020:307-350.
- [Google Scholar]
- A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl.Trop. Dis.. 2016;10(3):e0004349.
- [CrossRef] [Google Scholar]
- Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev.. 2013;65(1):36-48.
- [Google Scholar]
- Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7(5):e35671 22693548
- [CrossRef] [Google Scholar]
- Evaluation of CD44 and CD44v6 in colorectal carcinoma patients: soluble forms in relation to tumor tissue expression and metastasis. J. Gastrointestinal Cancer. 2008;39(1–4):73. 19333790
- [CrossRef] [Google Scholar]
- Nanomedicine as an emerging approach against intracellular pathogens. Int. J. Nanomed.. 2011;6:3281.
- [Google Scholar]
- Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am. J. Pathol.. 2000;156(2):529-536. 10666382
- [CrossRef] [Google Scholar]
- Bi-faceted delivery of phytochemicals through chitosan nanoparticles impregnated nanofibers for cancer therapeutics. Int. J. Biol. Macromol 2019 31604079
- [CrossRef] [Google Scholar]
- Nanofibers and Nanoparticles in Biomedical Applications. In: Bioengineered Nanomaterials. New York, NY, USA: CRC Press, (Taylor & Francis Group); 2013. p. :98-100.
- [Google Scholar]
- Development of methotrexate loaded fucoidan/chitosan nanoparticles with anti-inflammatory potential and enhanced skin permeation. Int. J. Biol. Macromol.. 2019;124:1115-1122.
- [Google Scholar]
- Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release. 2008;130(2):98-106.
- [Google Scholar]
- Unsuspected task for an old team: succinate, fumarate and other Krebs cycle acids in metabolic remodeling. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 2014;1837(8):1330-1337.
- [CrossRef] [Google Scholar]
- Chlorin e6 decorated doxorubicin encapsulated chitosan nanoparticles for photo-controlled cancer drug delivery. Int. J. Biol. Macromol 2019
- [CrossRef] [Google Scholar]
- T7 peptide-functionalized PEG-PLGA micelles loaded with carmustine for targeting therapy of glioma. ACS Appl. Mater. Interfaces. 2016;8(41):27465-27473.
- [CrossRef] [Google Scholar]
- Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur. J. Pharm. Sci.. 2005;24(1):67-75.
- [Google Scholar]
- Characterization of self-assembled polyelectrolyte complex nanoparticles formed from chitosan and pectin. Langmuir. 2014;30(12):3441-3447.
- [Google Scholar]
- Nose to brain delivery in rats: Effect of surface charge of rhodamine B labeled nanocarriers on brain subregion localization. Colloids Surf., B. 2017;154:297-306.
- [CrossRef] [Google Scholar]
- Locating the binding sites of antitumor drug tamoxifen and its metabolites with DNA. J. Pharm. Biomed. Anal.. 2014;95:193-199.
- [Google Scholar]
- Triple-negative breast cancer: epidemiological considerations and recommendations. Ann. Oncol.. 2012;23(suppl_6) p. vi7-vi12
- [Google Scholar]
- Braconnot, H., 1813. Nouvelles recherches analytiques sur la nature des champignons, pour servir de suite à celles qui ont été insérés dans les tomes LXXIX et LXXX des Annales de chimie. Annales de Chimie. In: Recueil de Mémoires concernant la chimie et les arts qui en dépendent et spécialement la pharmacie, 31, 237–270.
- Preliminary report on the feasibility and efficacy of the modified atkins diet for treatment of mild cognitive impairment and early Alzheimer’s disease. J. Alzheimers Dis.. 2019;68(3):969-981.
- [CrossRef] [Google Scholar]
- Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation. 2004;77(5):769-776.
- [CrossRef] [Google Scholar]
- Global organ transplant activities in 2015. Data from the Global Observatory on Donation and Transplantation (GODT) Transplantation. 2015;101:S29.
- [Google Scholar]
- Recent developments on the synthesis, structural and optical properties of chalcogenide quantum dots. Sol. Energy Mater. Sol. Cells. 2017;168:183-200.
- [CrossRef] [Google Scholar]
- Enhanced antitumor effects of epidermal growth factor receptor targetable cetuximab-conjugated polymeric micelles for photodynamic therapy. Nanomaterials. 2018;8(2):121. 2947042
- [CrossRef] [Google Scholar]
- Design of functionalized folic acid–chitosan nanoparticles for delivery of tetracycline, doxorubicin, and tamoxifen. J. Biomol. Struct. Dyn.. 2019;37(4):1000-1006.
- [Google Scholar]
- Mutlifunctional nanoparticles prepared from arginine-modified chitosan and thiolated fucoidan for oral delivery of hydrophobic and hydrophilic drugs. Carbohydr. Polym.. 2018;193:163-172.
- [Google Scholar]
- Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev.. 2013;65(6):865-879.
- [CrossRef] [Google Scholar]
- A self-targeting and controllable drug delivery system constituting mesoporous silica nanoparticles fabricated with a multi-stimuli responsive chitosan-based thin film layer. Int. J. Biol. Macromol.. 2019;122:1090-1099.
- [CrossRef] [Google Scholar]
- Surface-fluorinated and pH-sensitive carboxymethyl chitosan nanoparticles to overcome biological barriers for improved drug delivery in vivo. Carbohydr. Polym.. 2019;208:59-69.
- [CrossRef] [Google Scholar]
- PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol–gels for drug delivery. J. Control. Release. 2016;240:191-201.
- [Google Scholar]
- Self-assembled nanoparticles based on hyaluronic acid-ceramide (HA-CE) and Pluronic® for tumor-targeted delivery of docetaxel. Biomaterials. 2011;32(29):7181-7190.
- [CrossRef] [Google Scholar]
- Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials. 2010;31(1):106-114. 19783037
- [CrossRef] [Google Scholar]
- Preparation and characterization of eggshell membrane/PVA hydrogel via electron beam irradiation technique. J. Ind. Eng. Chem.. 2017;47:41-45.
- [CrossRef] [Google Scholar]
- Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev.. 2001;65(2):232-260.
- [Google Scholar]
- Stimuli-responsive interfaces and systems for the control of protein–surface and cell–surface interactions. Biomaterials. 2009;30(9):1827-1850. 19144401
- [CrossRef] [Google Scholar]
- Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum.. 2010;40(3):233-240.
- [CrossRef] [Google Scholar]
- Plasmid DNA nanogels as photoresponsive materials for multifunctional bio-applications. J. Biotechnol.. 2015;202:98-104.
- [Google Scholar]
- Deep sternal wound infection after cardiac surgery: evidences and controversies. World J. Crit. Care Med.. 2015;4(4):265.
- [Google Scholar]
- Mucoadhesive and pH responsive fucoidan-chitosan nanoparticles for the oral delivery of methotrexate. Int. J. Biol. Macromol.. 2020;158:180-188.
- [CrossRef] [Google Scholar]
- To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release. 2010;148(2):135-146.
- [Google Scholar]
- Radiation improves the distribution and uptake of liposomal doxorubicin (caelyx) in human osteosarcoma xenografts. Cancer Res.. 2004;64(2):547-553. 14744768
- [CrossRef] [Google Scholar]
- In vitro and in vivo assessment of nanotoxicity of CdS quantum dot/aminopolysaccharide bionanoconjugates. Mater. Sci. Eng., C. 2017;71:412-424.
- [CrossRef] [Google Scholar]
- Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol. Rev.. 1998;22(3):151-175.
- [Google Scholar]
- Preparation of poly (lactic acid)/chitosan nanoparticles for anti-HIV drug delivery applications. Carbohydr. Polym.. 2010;80(3):833-838.
- [CrossRef] [Google Scholar]
- Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83(7):858-873.
- [CrossRef] [Google Scholar]
- Novel fluorinated polysilsesquioxane hollow spheres: synthesis and application in drug release. Chem. Commun.. 2010;46(40):7498-7500.
- [CrossRef] [Google Scholar]
- Tumor extracellular acidity-activated nanoparticles as drug delivery systems for enhanced cancer therapy. Biotechnol. Adv.. 2014;32(4):789-803. 23933109
- [CrossRef] [Google Scholar]
- Bimetallic nanostructures with magnetic and noble metals and their physicochemical applications. Prog. Nat. Sci.: Mater. Int.. 2013;23(2):113-126.
- [Google Scholar]
- Enzyme-activated intracellular drug delivery with tubule clay nanoformulation. Sci. Rep.. 2015;5:10560.
- [CrossRef] [Google Scholar]
- Nanotherapy for cancer: targeting and multifunctionality in the future of cancer therapies. ACS Biomater. Sci. Eng.. 2015;1(2):64-78.
- [Google Scholar]
- UV-B-induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc commune. J. Bacteriol.. 1997;179(6):1940-1945.
- [Google Scholar]
- Extreme biomimetics: formation of zirconium dioxide nanophase using chitinous scaffolds under hydrothermal conditions. J. Mater. Chem. B. 2013;1(38):5092-5099. 32261100
- [CrossRef] [Google Scholar]
- Liposome-encapsulated doxorubicin targeted to CD44: a strategy to kill CD44-overexpressing tumor cells. Cancer Res.. 2001;61(6):2592-2601.
- [Google Scholar]
- Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev.. 2012;41(7):2545-2561.
- [Google Scholar]
- Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release. 2012;157(2):168-182.
- [Google Scholar]
- The Society of Thoracic Surgeons practice guidelines series: antibiotic prophylaxis in cardiac surgery, Part II: antibiotic choice. Ann. Thorac. Surg.. 2007;83(4):1569-1576.
- [CrossRef] [Google Scholar]
- Tamoxifen—Offering a long-term prevention option. Nat. Rev. Clin. Oncol.. 2015;12(2)
- [Google Scholar]
- Paromomycin-loaded mannosylated chitosan nanoparticles: synthesis, characterization and targeted drug delivery against leishmaniasis. Acta Trop. 2019:105045-105072.
- [CrossRef] [Google Scholar]
- Specific targeting delivery to MUC1 overexpressing tumors by albumin-chitosan nanoparticles conjugated to DNA aptamer. Int. J. Pharm.. 2016;515(1–2):607-615.
- [CrossRef] [Google Scholar]
- Chitosan/o-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: in vitro and in vivo evaluation. Int. J. Pharm.. 2013;457(1):158-167.
- [CrossRef] [Google Scholar]
- Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 2010;127(12):2893-2917.
- [CrossRef] [Google Scholar]
- pH-sensitive poly (ortho ester urethanes) copolymers with controlled degradation kinetic: synthesis, characterization, and in vitro evaluation as drug carriers. Eur. Polym. J.. 2017;95:275-288.
- [CrossRef] [Google Scholar]
- Carboxymethyl cellulose reinforced poly (vinyl alcohol) with trimethylol melamine as a chemical crosslinker. J. Appl. Polym. Sci.. 2017;134(11)
- [CrossRef] [Google Scholar]
- Chitosan–dextran sulphate nanocapsule drug delivery system as an effective therapeutic against intraphagosomal pathogen Salmonella. J. Antimicrob. Chemother.. 2013;68(11):2576-2586.
- [CrossRef] [Google Scholar]
- Assessing the potential of chitosan/polylactide nanoparticles for delivery of therapeutics for triple-negative breast cancer treatment. Regener. Eng. Transl. Med.. 2019;5(1):61-73.
- [Google Scholar]
- Theranostic magnetoliposomes coated by carboxymethyl dextran with controlled release by low-frequency alternating magnetic field. Carbohydr. Polym.. 2015;118:209-217.
- [CrossRef] [Google Scholar]
- Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26(18):3995-4021.
- [Google Scholar]
- Racial differences in the incidence of BRCA1 and BRCA2 mutations in a cohort of early onset breast cancer patients: African American compared to white women. J. Med. Genet.. 2006;43(2):133-137.
- [Google Scholar]
- Glutaraldehyde inhalation exposure of rats: effects on lung morphology, Clara-cell protein, and hyaluronic acid levels in BAL. Inhalation Toxicol.. 2003;15(1):85-97.
- [CrossRef] [Google Scholar]
- Nanocapsules: a novel lipid formulation platform for platinum-based anti-cancer drugs. J. Liposome Res.. 2007;17(3–4):183-189.
- [Google Scholar]
- Preparation and antitumor effect of N-trimethylchitosan/fucoidan ion-complex submicron particles. Curr. Nanosci.. 2011;7(4):497-502.
- [CrossRef] [Google Scholar]
- Heckert, W.W., 1937. Textile. Google Patents.
- Mycophenolic acid-mediated suppression of human CD4+ T cells: more than mere guanine nucleotide deprivation. Am. J. Transplant.. 2011;11(3):439-449. 21342445
- [CrossRef] [Google Scholar]
- Biomineralization in diatoms—Phosphorylated saccharides are part of Stephanopyxis turris biosilica. Carbohydr. Res.. 2013;365:52-60.
- [Google Scholar]
- Henry, J., 2007. Henry's Clinical Diagnosis and Management by Laboratory Methods. McPherson RA, Pincus MR, editors. Philadelphia: Saunders Elsevier.
- Polymer coating of quantum dots–a powerful tool toward diagnostics and sensorics. Eur. J. Pharm. Biopharm.. 2008;68(1):138-152.
- [Google Scholar]
- Synthesis, characterization and MRI application of dextran-coated Fe3O4 magnetic nanoparticles. Biochem. Eng. J.. 2008;42(3):290-300.
- [CrossRef] [Google Scholar]
- Current applications and future prospects of nanomaterials in tumor therapy. Int. J. Nanomed.. 2017;12:1815.
- [Google Scholar]
- Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr. Cancer. 2009;62(1):1-20.
- [Google Scholar]
- Enhancement of the permeability and activities of epigallocatechin gallate by quaternary ammonium chitosan/fucoidan nanoparticles. Carbohydr. Polym.. 2020;116312
- [Google Scholar]
- Polymeric nanoparticles with encapsulated superparamagnetic iron oxide and conjugated cisplatin for potential bladder cancer therapy. Biomacromolecules. 2012;13(8):2513-2520.
- [CrossRef] [Google Scholar]
- In vitro enzymatic digestibility of glutaraldehyde-crosslinked chitosan nanoparticles in lysozyme solution and their applicability in pulmonary drug delivery. Molecules. 2019;24(7):1271.
- [CrossRef] [Google Scholar]
- Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater.. 1999;201(1–3):413-419.
- [CrossRef] [Google Scholar]
- An experimental and DFT study on novel dyes incorporated with natural dyes on titanium dioxide (TiO 2) towards solar cell application. Appl. Phys. A. 2020;126(9):1-13.
- [Google Scholar]
- Leishmaniasis: current treatment and prospects for new drugs and vaccines. Curr. Med. Chem.. 2009;16(5):599-614.
- [CrossRef] [Google Scholar]
- Lipid-chitosan hybrid nanoparticles for controlled delivery of cisplatin. Drug Delivery. 2019;26(1):765-772.
- [CrossRef] [Google Scholar]
- Modified-chitosan nanoparticles: Novel drug delivery systems improve oral bioavailability of doxorubicin. Eur. J. Pharm. Sci.. 2016;93:38-44.
- [CrossRef] [Google Scholar]
- Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice. J. Control. Release. 2008;127(1):41-49.
- [CrossRef] [Google Scholar]
- Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry. 2014;98:27-33.
- [Google Scholar]
- Physico-chemical characterization and beneficial effects of seaweed sulfated polysaccharide against oxydatif and cellular damages caused by alloxan in diabetic rats. Int. J. Biol. Macromol.. 2018;117:407-417.
- [CrossRef] [Google Scholar]
- Albumin based versatile multifunctional nanocarriers for cancer therapy: fabrication, surface modification, multimodal therapeutics and imaging approaches. Mater. Sci. Eng., C. 2017;81:607-626.
- [Google Scholar]
- Potential applications of ferulic acid from natural sources. Biotechnol. Rep,. 2014;4:86-93.
- [Google Scholar]
- Induction of oxidative DNA base damage in human skin cells by UV and near visible radiation. Carcinogenesis. 1997;18(12):2379-2384.
- [Google Scholar]
- Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J. Control. Release. 2012;161(2):175-187.
- [CrossRef] [Google Scholar]
- Albumin-based drug delivery: harnessing nature to cure disease. Mol. Cell. Ther.. 2016;4(1):3.
- [CrossRef] [Google Scholar]
- Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci.. 2011;166(1–2):8-23.
- [CrossRef] [Google Scholar]
- Current and emerging systemic treatment strategies for psoriasis. Drugs. 2012;72(14):1867-1880.
- [Google Scholar]
- Intracellular pH-responsive mesoporous silica nanoparticles for the controlled release of anticancer chemotherapeutics. Angew. Chem. Int. Ed.. 2010;49(44):8214-8219.
- [CrossRef] [Google Scholar]
- Recent progress in tumor pH targeting nanotechnology. J. Control. Release. 2008;132(3):164-170.
- [Google Scholar]
- Soluble eggshell membrane protein-loaded chitosan/fucoidan nanoparticles for treatment of defective intestinal epithelial cells. Int. J. Biol. Macromol.. 2019;131:949-958.
- [Google Scholar]
- Efficient fabrication of reversible pH-induced carboxymethyl chitosan nanoparticles for antitumor drug delivery under weakly acidic microenvironment. Int. J. Biol. Macromol.. 2019;126:68-73.
- [CrossRef] [Google Scholar]
- Lubs, H.A., Roberts, J.R., Laughlin, E.R., 1937. Paper. Google Patents.
- Recent advance in research on halloysite nanotubes-polymer nanocomposite. Prog. Polym. Sci.. 2014;39(8):1498-1525.
- [CrossRef] [Google Scholar]
- Anthracycline nano-delivery systems to overcome multiple drug resistance: a comprehensive review. Nano Today. 2013;8(3):313-331.
- [Google Scholar]
- Magnetic/pH-responsive beads based on caboxymethyl chitosan and κ-carrageenan and controlled drug release. Carbohydr. Polym.. 2015;128:112-121.
- [Google Scholar]
- Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev.. 2011;63(1–2):24-46.
- [CrossRef] [Google Scholar]
- Martin, M., Middleton, E.B., 1938. Antistatic photographic film. Google Patents.
- Core–shell-type lipid–polymer hybrid nanoparticles as a drug delivery platform. Nanomed. Nanotechnol. Biol. Med.. 2013;9(4):474-491.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of thiolated alginate-albumin nanoparticles stabilized by disulfide bonds. Evaluation as drug delivery systems. Carbohydr. Polym.. 2011;83(3):1311-1321.
- [CrossRef] [Google Scholar]
- Halloysite nanotubes-carbon dots hybrids multifunctional nanocarrier with positive cell target ability as a potential non-viral vector for oral gene therapy. J. Colloid Interface Sci.. 2019;552:236-246.
- [CrossRef] [Google Scholar]
- Multifunctional carrier based on halloysite/laponite hybrid hydrogel for kartogenin delivery. ACS Med. Chem. Lett.. 2018;10(4):419-424.
- [CrossRef] [Google Scholar]
- Correction: Covalently modified halloysite clay nanotubes: synthesis, properties, biological and medical applications. J. Mater. Chem. B. 2017;5(22):4246.
- [CrossRef] [Google Scholar]
- Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomed.. 2017;12:5421.
- [CrossRef] [Google Scholar]
- Chitosan nanoparticles as therapeutic protein nanocarriers: the effect of ph on particle formation and encapsulation efficiency. Polym. Compos.. 2013;34(9):1538-1545.
- [Google Scholar]
- Maxwell, R.W., 1939. Adhesive. 1939.
- Drug delivery for treatment of inner ear disease: current state of knowledge. Ear Hear.. 2010;31(2):156.
- [CrossRef] [Google Scholar]
- Nano-carrier enabled drug delivery systems for nose to brain targeting for the treatment of neurodegenerative disorders. J. Drug Delivery Sci. Technol.. 2018;43:295-310.
- [CrossRef] [Google Scholar]
- Tumoral acidic pH-responsive MPEG-poly (β-amino ester) polymeric micelles for cancer targeting therapy. J. Control. Release. 2010;144(2):259-266.
- [CrossRef] [Google Scholar]
- Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pharm.. 2009;379(1):146-157.
- [Google Scholar]
- Enhanced in vitro cytotoxicity and anti-tumor activity of vorinostat-loaded pluronic micelles with prolonged release and reduced hepatic and renal toxicities. Eur. J. Pharm. Sci.. 2017;96:232-242.
- [CrossRef] [Google Scholar]
- Development and in vitro characterization of chitosan-coated polymeric nanoparticles for oral delivery and sustained release of the immunosuppressant drug mycophenolate mofetil. Drug Dev. Ind. Pharm.. 2019;45(1):76-87.
- [CrossRef] [Google Scholar]
- Biocompatibility studies of intravenously administered ionic-crosslinked chitosan-BSA nanoparticles as vehicles for antitumour drugs. Int. J. Pharm.. 2019;554:337-351.
- [CrossRef] [Google Scholar]
- Novel poly (vinyl alcohol)-based amphiphilic nanogels by non-covalent boric acid crosslinking of polymeric micelles. Biomater. Sci.. 2017;5(11):2295-2309.
- [CrossRef] [Google Scholar]
- Patient survey to identify reasons for non-adherence and elicitation of quality of life concepts associated with immunosuppressant therapy in kidney transplant recipients. Patient Pref. Adherence. 2016;10:27.
- [CrossRef] [Google Scholar]
- Chitin. Elsevier; 2013.
- An evaluation of curcumin-encapsulated chitosan nanoparticles for transdermal delivery. AAPS PharmSciTech. 2019;20(2):69.
- [CrossRef] [Google Scholar]
- Encapsulation of paclitaxel into lauric acid-O-carboxymethyl chitosan-transferrin micelles for hydrophobic drug delivery and site-specific targeted delivery. Int. J. Pharm.. 2013;457(1):124-135.
- [CrossRef] [Google Scholar]
- Investigation of anti-leishmanial efficacy of miltefosine and ketoconazole loaded on nanoniosomes. Acta Trop.. 2018;185:69-76.
- [CrossRef] [Google Scholar]
- Health disparities and triple-negative breast cancer in African American women: a review. JAMA surgery. 2017;152(5):485-493.
- [Google Scholar]
- Mémoire sur la composition chimique des parties cornées des insectes. Mem. Soc. Hist. Nat. Paris. 1823;1:29-42.
- [Google Scholar]
- Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nat. Nanotechnol.. 2016;11(5):479.
- [CrossRef] [Google Scholar]
- Respirable PLGA microspheres containing rifampicin for the treatment of tuberculosis: manufacture and characterization. Pharm. Res.. 2000;17(8):955-961.
- [Google Scholar]
- Nanoparticle-enabled transdermal drug delivery systems for enhanced dose control and tissue targeting. Molecules. 2016;21(12):1719.
- [Google Scholar]
- In vivo anti-psoriatic activity, biodistribution, sub-acute and sub-chronic toxicity studies of orally administered methotrexate loaded chitin nanogel in comparison with methotrexate tablet. Int. J. Biol. Macromol.. 2018;110:259-268.
- [CrossRef] [Google Scholar]
- Polymeric multifunctional nanomaterials for theranostics. J. Mater. Chem. B. 2015;3(34):6856-6870.
- [CrossRef] [Google Scholar]
- The preparation of α-chitin nanowhiskers-poly (vinyl alcohol) hydrogels for drug release. Int. J. Biol. Macromol.. 2019;131:336-342.
- [CrossRef] [Google Scholar]
- Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm.. 2015;93:52-79.
- [Google Scholar]
- Synthesis, spectral and antimicrobial studies of amino acid derivative Schiff base metal (Co, Mn, Cu, and Cd) complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2019;206:642-649.
- [Google Scholar]
- Preparation of N-succinyl-chitin nanoparticles and their applications in otoneurological pathology. Int. J. Biol. Macromol.. 2018;120:1023-1029.
- [CrossRef] [Google Scholar]
- Chitosan coated human serum albumin nanoparticles: a promising strategy for nose-to-brain drug delivery. Int. J. Biol. Macromol.. 2019;129:267-280.
- [CrossRef] [Google Scholar]
- Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci.. 2009;34(7):641-678.
- [Google Scholar]
- Intranasal drug delivery: how, why and what for? J. Pharm. Pharm. Sci.. 2009;12(3):288-311.
- [CrossRef] [Google Scholar]
- Flocculation behaviour of hematite–kaolinite suspensions in presence of extracellular bacterial proteins and polysaccharides. Colloids Surf., B. 2014;114:186-192.
- [Google Scholar]
- Fabrication of chitosan-polycaprolactone composite nanofibrous scaffold for simultaneous delivery of ferulic acid and resveratrol. Carbohydr. Polym.. 2017;157:1741-1749.
- [Google Scholar]
- Peptide functionalized dual-responsive chitosan nanoparticles for controlled drug delivery to breast cancer cells. Colloids Surf., A. 2019;564:122-130.
- [CrossRef] [Google Scholar]
- Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Prog. Polym. Sci.. 2007;32(8–9):962-990.
- [Google Scholar]
- Improved therapeutic performance of dithranol against psoriasis employing systematically optimized nanoemulsomes. J. Microencapsul.. 2013;30(3):225-236.
- [CrossRef] [Google Scholar]
- Self-assembled reduced albumin and glycol chitosan nanoparticles for paclitaxel delivery. Langmuir. 2019;35(7):2610-2618.
- [CrossRef] [Google Scholar]
- Multifunctional chitin nanogels for simultaneous drug delivery, bioimaging, and biosensing. ACS Appl. Mater. Interfaces. 2011;3(9):3654-3665.
- [CrossRef] [Google Scholar]
- Rigby, G.W., 1936. Process for the preparation of films and filaments and products thereof. 1936.
- Design and in vivo evaluation of an indapamide transdermal patch. Int. J. Pharm.. 2009;370(1–2):129-135.
- [CrossRef] [Google Scholar]
- Paths to understanding the genetic basis of autoimmune disease. Nature. 2005;435(7042):584.
- [Google Scholar]
- pH-sensitive freeze-dried chitosan–polyvinyl pyrrolidone hydrogels as controlled release system for antibiotic delivery. J. Control. Release. 2000;68(1):23-30.
- [CrossRef] [Google Scholar]
- Mediastinitis after coronary artery bypass grafting risk factors and long-term survival. Ann. Thoracic Surg.. 2010;89(5):1502-1509.
- [CrossRef] [Google Scholar]
- Introduction: semi-solid formulations of oral drug delivery. Bull. Technique-Gattefosse 1996:11-14.
- [Google Scholar]
- Monitoring cell substrate interactions in exopolysaccharide-based films reinforced with chitin whiskers and starch nanoparticles used as cell substrates. Int. J. Polym. Mater. Polym. Biomater.. 2018;67(6):333-339.
- [CrossRef] [Google Scholar]
- The genetic complexity of chitin synthesis in fungi. Curr. Genet.. 2002;41(6):367-378.
- [Google Scholar]
- Biocompatibility and safety of insulin-loaded chitosan nanoparticles/PLGA-PEG-PLGA hydrogel (ICNPH) delivered by subconjunctival injection in rats. J. Drug Delivery Sci. Technol.. 2019;49:556-562.
- [CrossRef] [Google Scholar]
- Des substances amylacées dans les tissus des animaux, spécialement des Articulés (chitine) Comp. Rend. 1859;48:792-795.
- [Google Scholar]
- Applications of nanoparticles in biology and medicine. J. Nanobiotechnol.. 2004;2(1):3.
- [Google Scholar]
- Microfabrication technologies for oral drug delivery. Adv. Drug Deliv. Rev.. 2012;64(6):496-507.
- [CrossRef] [Google Scholar]
- Advances in copper complexes as anticancer agents. Chem. Rev.. 2013;114(1):815-862.
- [CrossRef] [Google Scholar]
- Albumin coated arginine-capped magnetite nanoparticles as a paclitaxel vehicle: Physicochemical characterizations and in vitro evaluation. J. Drug Delivery Sci. Technol.. 2016;36:68-74.
- [CrossRef] [Google Scholar]
- Investigation of amyloid formation inhibition of chemically and biogenically from Citrus aurantium L. blossoms and Rose damascena oils of gold nanoparticles: toxicity evaluation in rat pheochromocytoma PC12 cells. Int. J. Biol. Macromol.. 2018;112:703-711.
- [CrossRef] [Google Scholar]
- A new UV-A/B protecting pigment in the terrestrial cyanobacterium Nostoc commune. Plant Physiol.. 1988;88(4):1055-1057.
- [Google Scholar]
- BRCA1-like signature in triple negative breast cancer: molecular and clinical characterization reveals subgroups with therapeutic potential. Mol. Oncol.. 2015;9(8):1528-1538.
- [CrossRef] [Google Scholar]
- Chitin-based fast responsive pH sensitive microspheres for controlled drug release. Carbohydr. Polym.. 2014;102:413-418.
- [CrossRef] [Google Scholar]
- Distribution and cellular uptake of PEGylated polymeric particles in the lung towards cell-specific targeted delivery. Pharm. Res.. 2015;32(10):3248-3260.
- [CrossRef] [Google Scholar]
- Leishmaniasis and Chagas disease chemotherapy: a critical review. J. Braz. Chem. Soc.. 2014;25(10):1810-1823.
- [Google Scholar]
- Overview and management of sternal wound infection. In: Seminars in plastic surgery. © Thieme Medical Publishers; 2011.
- [Google Scholar]
- Delivery of rifampicin-chitin nanoparticles into the intracellular compartment of polymorphonuclear leukocytes. Int. J. Biol. Macromol.. 2015;74:36-43.
- [CrossRef] [Google Scholar]
- Chitosan-based nanoparticles as drug delivery systems for doxorubicin: optimization and modelling. Carbohydr. Polym.. 2016;147:304-312.
- [Google Scholar]
- Doxorubicin vs. ladirubicin: methods for improving osteosarcoma treatment. Mini Rev. Med. Chem.. 2012;12(12):1239-1249.
- [CrossRef] [Google Scholar]
- Anticancer activity of silver and copper embedded chitin nanocomposites against human breast cancer (MCF-7) cells. Int. J. Biol. Macromol.. 2017;105:608-619.
- [Google Scholar]
- Surface-modified nanocarriers for nose-to-brain delivery: from bioadhesion to targeting. Pharmaceutics. 2018;10(1):34.
- [CrossRef] [Google Scholar]
- In vitro and ex vivo strategies for intracellular delivery. Nature. 2016;538(7624):183.
- [CrossRef] [Google Scholar]
- Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules. 2019;9(9):470.
- [CrossRef] [Google Scholar]
- Rational design of cancer nanomedicine: nanoproperty integration and synchronization. Adv. Mater.. 2017;29(14):1606628.
- [Google Scholar]
- Preparation of high tough poly (vinyl alcohol) hydrogel by soaking in NaCl aqueous solution. Mater. Lett.. 2017;194:34-37.
- [CrossRef] [Google Scholar]
- Paromomycin in the treatment of leishmaniasis. Expert Opin. Invest. Drugs. 2008;17(5):787-794.
- [Google Scholar]
- Bioadhesive, hemostatic, and antibacterial in situ chitin-fibrin nanocomposite gel for controlling bleeding and preventing infections at mediastinum. ACS Sustainable Chem. Eng.. 2018;6(6):7826-7840.
- [CrossRef] [Google Scholar]
- Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer: Interdis. Int. J. Am. Cancer Soc.. 2003;97(11):2869-2879.
- [Google Scholar]
- Development and optimization of methotrexate-loaded lipid-polymer hybrid nanoparticles for controlled drug delivery applications. Int. J. Pharm.. 2017;533(1):156-168.
- [CrossRef] [Google Scholar]
- Lipid-based nanocarriers for breast cancer treatment–comprehensive review. Drug Delivery. 2016;23(4):1291-1305.
- [CrossRef] [Google Scholar]
- Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym.. 2020;230:115658
- [Google Scholar]
- Pgp efflux pump decreases the cytostatic effect of CENP-E inhibitor GSK923295. Cancer Lett.. 2015;361(1):97-103.
- [Google Scholar]
- Progress in green polymer composites from lignin for multifunctional applications: a review. ACS Sustainable Chem. Eng.. 2014;2(5):1072-1092.
- [CrossRef] [Google Scholar]
- Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials. 2014;35(5):1735-1743.
- [CrossRef] [Google Scholar]
- Drug resistance. In: A Systems Biology Approach to Blood. Springer; 2014. p. :303-316.
- [Google Scholar]
- Targeted delivery of antibiotics to intracellular chlamydial infections using PLGA nanoparticles. Biomaterials. 2011;32(27):6606-6613.
- [CrossRef] [Google Scholar]
- Preparation of chitosan/magnetite composite beads and their application for removal of Pb (II) and Ni (II) from aqueous solution. Mater. Sci. Eng., C. 2010;30(2):304-310.
- [Google Scholar]
- Development of mutlifunctional nanoparticles self-assembled from trimethyl chitosan and fucoidan for enhanced oral delivery of insulin. Int. J. Biol. Macromol.. 2019;126:141-150.
- [CrossRef] [Google Scholar]
- Polymeric micelles: theranostic co-delivery system for poorly water-soluble drugs and contrast agents. Biomaterials. 2018;170:26-36.
- [CrossRef] [Google Scholar]
- Synthesis of silver nanoparticles (Ag NPs) for anticancer activities (MCF 7 breast and A549 lung cell lines) of the crude extract of Syzygium aromaticum. J. Photochem. Photobiol., B. 2017;167:282-289.
- [CrossRef] [Google Scholar]
- Bone healing after median sternotomy: a comparison of two hemostatic devices. J. Cardiothoracic Surg.. 2010;5(1):117.
- [CrossRef] [Google Scholar]
- The effect of 3-oxypyridine and succinic acid derivatives on the resistance to acute cerebral ischemia. Zhurnal nevrologii i psikhiatrii imeni SS Korsakova. 2014;114(12):123-127.
- [CrossRef] [Google Scholar]
- Anxiolytic and antidepressant effects of emoxipine, reamberin and mexidol in experimental diabetes mellitus. Zhurnal nevrologii i psikhiatrii imeni SS Korsakova. 2017;117(5):52-57.
- [CrossRef] [Google Scholar]
- Hyaluronic acid-coated chitosan nanoparticles induce ROS-mediated tumor cell apoptosis and enhance antitumor efficiency by targeted drug delivery via CD44. J. Nanobiotechnol.. 2017;15(1):7.
- [CrossRef] [Google Scholar]
- Magnetic/NIR-thermally responsive hybrid nanogels for optical temperature sensing, tumor cell imaging and triggered drug release. Nanoscale. 2014;6(21):13001-13011.
- [Google Scholar]
- The current state of engineered nanomaterials in consumer goods and waste streams: the need to develop nanoproperty-quantifiable sensors for monitoring engineered nanomaterials. Nanotechnol. Sci. Appl.. 2011;4:73.
- [Google Scholar]
- Interest in paromomycin for the treatment of visceral leishmaniasis (kala-azar) Ther. Clin. Risk Manag.. 2012;8:323.
- [Google Scholar]
- Synthesis of nanostructured chitin–hematite composites under extreme biomimetic conditions. RSC Adv.. 2014;4(106):61743-61752.
- [CrossRef] [Google Scholar]
- Templating assembly of multifunctional hybrid colloidal spheres. Adv. Mater.. 2012;24(20):2663-2667.
- [Google Scholar]
- Tunable dynamic fluorinated poly (orthoester)-based drug carriers for greatly enhanced chemotherapeutic efficacy. Polym. Chem.. 2017;8(13):2063-2073.
- [CrossRef] [Google Scholar]
- Development of bacterial cellulose/chitin multi-nanofibers based smart films containing natural active microspheres and nanoparticles formed in situ. Carbohydr. Polym.. 2020;228:115370
- [Google Scholar]
- Synergistic targeted delivery of payload into cancer cells using liposomes co-modified with photolabile-caged cell-penetrating peptides and targeting ligands. J. Controlled Rel.: Off. J. Controlled Rel. Soc.. 2015;213 e128 e128
- [CrossRef] [Google Scholar]
- Biofabrication with chitosan. Biomacromolecules. 2005;6(6):2881-2894. 16283704
- [CrossRef] [Google Scholar]
- Chitosan nanoparticles for combined drug delivery and magnetic hyperthermia: from preparation to in vitro studies. Carbohydr. Polym.. 2017;157:361-370.
- [CrossRef] [Google Scholar]
- Influence of metal-resistant staphylococcus aureus strain K1 on the alleviation of chromium stress in wheat. Agronomy. 2020;10(9):1354.
- [Google Scholar]
- The fluorination effect of fluoroamphiphiles in cytosolic protein delivery. Nat. Commun.. 2018;9(1):1377.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and wettability of Cu-Sn alloy on the Si-implanted 6H-SiC. Coatings. 2020;10(9):906.
- [Google Scholar]
- Pharmaceutical foams: are they the answer to the dilemma of topical nanoparticles? Nanomed.: Nanotechnol. Biol. Med.. 2010;6(2):227-236.
- [Google Scholar]
- Liquid crystal self-assembly of halloysite nanotubes in ionic liquids: a novel soft nanocomposite ionogel electrolyte with high anisotropic ionic conductivity and thermal stability. Nanoscale. 2016;8(3):1545-1554.
- [Google Scholar]
- Preparation and inductive heating property of Fe3O4–chitosan composite nanoparticles in an AC magnetic field for localized hyperthermia. J. Alloy. Compd.. 2009;477(1–2):739-743.
- [CrossRef] [Google Scholar]
- Bioactive compounds from marine macroalgae and their hypoglycemic benefits. Trends Food Sci. Technol.. 2018;72:1-12.
- [CrossRef] [Google Scholar]
- Biomedical applications of chitosan and its derivative nanoparticles. Polymers. 2018;10(4):462.
- [CrossRef] [Google Scholar]
- Preparation of biocompatible carboxymethyl chitosan nanoparticles for delivery of antibiotic drug. Biomed. Res. Int.. 2013;2013
- [CrossRef] [Google Scholar]
- Folic acid functionalized reduction-responsive magnetic chitosan nanocapsules for targeted delivery and triggered release of drugs. Carbohydr. Polym.. 2017;168:282-289.
- [CrossRef] [Google Scholar]
- Histopathological study in B6C3F1 mice chronically exposed by inhalation to glutaraldehyde. Toxicol. Lett.. 1998;95(2):131-139.
- [Google Scholar]







