Translate this page into:
Recent advances in β-cyclodextrin-based catalyst systems for the synthesis of heterocyclic compounds via multicomponent reactions (MCRs)
⁎Corresponding author. a.poursattar@urmia.ac.ir (Ahmad Poursattar Marjani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cyclodextrins (CDs) are essential compounds because of their wide applications in many fields. They are cyclic oligosaccharides with lipophilic internal cavities and hydrophilic external surfaces that link the α-D-glucopyranose portion. β-Cyclodextrin (β-CD) is a non-toxicity, cheap, green, and renewability macrocycle with excellent performance in organic transformation. β-CD is a green catalyst with satisfactory catalytic activity in diverse organic changes. Regarding green chemistry’s goals, β-CD opens the way to effective catalysts for various reactions. Heterocyclic compounds are among the most prominent organic compounds in numerous organic materials, pharmaceuticals, and natural products. Heterocyclic compounds are essential in pharmaceutical products and show different biological activities in multiple illnesses. Thus, there is a tendency to develop novel, green, and helpful syntheses of heterocyclic systems, which has been a big challenge in synthetic organic chemistry. Using β-CD as catalysts for synthesizing heterocyclic compounds makes the procedure milder, easier, and less toxic, making it an eco-friendly substitute compared to the reported approaches. This review underlines the applications of β-CD-based catalysts for synthesizing heterocyclic compounds covering 2019–2023. This study will be helpful to researchers in the investigation areas of organic synthesis, medicinal chemistry, synthetic materials, and pharmacological medicine..
Keywords
β-Cyclodextrin (β-CD)
Heterocyclic compounds
Multicomponent reactions
Supramolecular catalysis
One-pot
- CD
-
Cyclodextrin
- β-CD
-
β-Cyclodextrin
- NPs
-
Nanoparticles
- MCRs
-
Multicomponent reactions
- TCR
-
Three-component response
- BA
-
Barbituric acid
- [β-CD/Im](OTs)2
-
β-Cyclodextrin/imidazolium-based dicationic ionic liquid
- Ils
-
Ionic liquids
- SPIONs
-
Iron oxide nanoparticles
- M-Starch
-
Magnetic starch
- ChCl
-
Choline chloride
- p-TSA
-
p-Toluenesulfonic acid
- Er(OTF)3
-
Erbium(III) trifluoromethanesulfonate
- DABCO
-
1,4-Diazabicyclo[2,2,2]octane
- SDS
-
Sodium dodecyl sulfate
- NWs
-
Nanowires
- OMWCNTs
-
Oxidized multi-walled carbon nanotubes
- SB-DBU, SB-DBU Cl
-
Silica-bonded 5-n-propyl-octahydro-pyrimido[1,2-a]azepinium chloride
- SSA‐MNPs
-
Silica sulfuric acid magnetic nanoparticle
- PEI@Si–MNPs
-
Polyethyleneimine-modified superparamagnetic Fe3O4 NPs
- CAN
-
Ceric ammonium nitrate
- SO3H
-
Sulfonic acid
- MNPTC
-
Magnetic nano phase transfer catalyst
- OPD
-
o-Phenylenediamine
- CIT
-
Citric acid
- β-CD.NS
-
β-Cyclodextrin-based nanosponges
- MCM-41
-
Mobil Composition of Matter No. 41
- GA
-
Guanidine
- Met
-
Metformin
- GO
-
Graphene oxide
- β-CDH
-
β-CD hydrate
- MNPs
-
Magnetic nanoparticles
- VSM
-
Vibrating-sample magnetometer
- TEM
-
Transmission electron microscopy
- SEM
-
Scanning electron microscopy
- TGA
-
Thermogravimetric Analysis
- EDX
-
Electron-dispersive X-ray analysis
- XRD
-
X-ray diffraction analysis
- FT-IR
-
Fourier Transform Infrared
- 13C NMR
-
Carbon Nuclear Magnetic Resonance
- 1H NMR
-
Proton Nuclear Magnetic Resonance
Abbreviations
1 Introduction
The expansion of green and sustainable paths for the fabrication of chemical production is a fundamental target in modern organic synthesis. The striking plan for organic synthesis is to extend methods by exerting only green materials without creating chemical wastage. In this regard, reducing organic pollutants and developing new green catalysts are essential goals of green chemistry. One of the fast-developing fields of research related to expanding green catalysts for chemical conversions) Abdussalam-Mohammed et al., 2020; Anastas et al., 2002; Nozad et al., 2022; Mohammadi Dehcheshmeh et al., 2021; Zamani et al., 2019; Gozali Balkanloo et al., 2023).
β-CDs have drawn much notable among the various supramolecular catalysts due to their green biomimetic materials and unique nature. They are a well-known class of polysaccharides obtained from starch degradation using enzymatic conversion. These cyclic oligosaccharides have a hydrophilic outer surface and hydrophobic central cavity (Scheme 1) (Crini, 2014; Szejtli, 1998). They can make inclusion complexes with a wide range of guest compounds by including them in the cavity without forming covalent bonds (Fig. 1). The size of the central cavity plays a significant role in creating inclusion complexes (Khan et al.,1998; Engeldinger et al., 2003; Kfoury et al., 2018). CDs are somewhat low-price, non-toxic, biodegradable, and available, so developing catalysts based on these supramolecular oligosaccharides is highly advantageous. Modified CDs present many perfect opportunities and challenges for the chemistry community (Kfoury and Fourmentin, 2023, R Rao et al., 2010). Many reports have been published on using CDs in fields like pharmaceutical chemistry (Puskás et al., 2023), analytical chemistries (Cid-Samamed et al., 2022), and catalysis (Noël et al.,2021; Kanchana et al., 2020; Payamifar and Poursattar Marjani, 2023a, 2023b; Hapiot et al., 2017; Payamifar and Poursattar Marjani, 2024; Hapiot et al., 2017; Payamifar et al., 2021) during the past years indicate the interest of scientists in this field because it has outstanding properties. Developing a new and proper process for preparing β-CD-based catalysts with high catalytic performance, reusability, and simple workup is desirable. Recently, β-CDs and their derivatives as practical catalysts have drawn attention to synthesizing heterocycle compounds owing to their economical and straightforward accessibility (Mishra et al., 2018; Abbasi, 2017).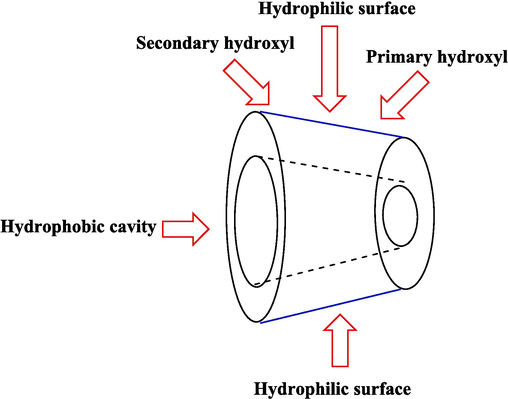
Structure representation of native β-CD.
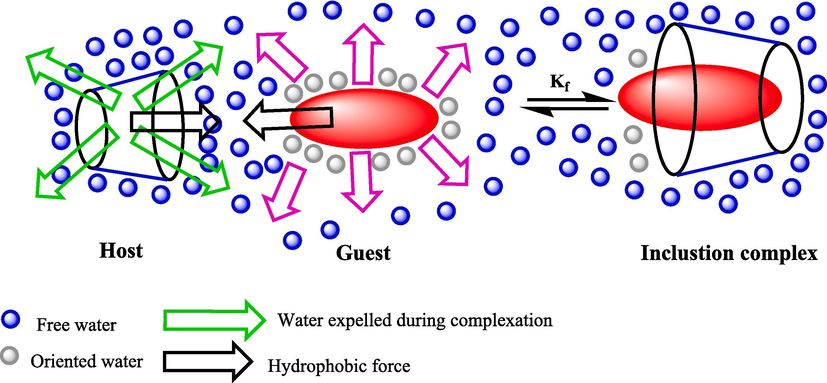
The construction of an inclusion complex between β-CD and a guest molecular.
The progress of various chemicals of fascinating drug-like molecules utilizing multicomponent reactions (MCRs) permits high combinatorial usefulness in making diversity. The conventional route for preparing complicated molecules was contrary to MCRs, allowing the complex molecules to lead to products in one pot. The role of MCRs is extensive in all areas of the chemistry world because they offer many products with the lowest effort in price- and time-effective methods. MCRs have been employed to prepare an extensive scope of various categories of heterocyclic compounds presenting various biologically active and medical applications. Some organic compounds that transform more than two components into their products via one-pot reactions are created by MCRs. This approach has advantages such as simplicity, economics, efficiency, and eco-friendliness compared to conventional chemical reactions (Cioc et al., 2014; Ugi, 2001; Meng et al., 2014; Younus et al., 2021; Zhi et al., 2019). The history of heterocyclic chemistry began in the 1800s, coinciding with the development of organic chemistry. The formation of heterocycle compounds is the most important reaction and is crucial to organic chemistry. They have one or more hetero atoms in their structure. They can be cyclic or noncyclic, vastly expanded in nature, and essential to our daily lives with an expansive scope of applications (Joule, 2020; Cabrele and Reiser, 2016; Jacobi, 2018; Bibak and Poursattar Marjani, 2023; Bikas et al., 2023; Payamifar et al., 2024a; 2024b; Khashaei et al., 2022; Bibak et al., 2024). They are found in pharmaceuticals (Kabir and Uzzaman, 2022), agrochemicals (Sanemitsu and Kawamura, 2008), and veterinary products. Also, they have applications such as sensitizers (AL-Adilee et al., 2016), anti-oxidants (Tsolaki et al., 2014), and corrosion inhibitors (Goni et al., 2021). They are employed as tools in the synthesis of other organic compounds.
These excellent biological properties make heterocyclic compounds a suitable candidate for developing the pharmaceuticals field. They display an expansive range of biological activities, namely anti-cancer (Ali et al., 2015), anti-viral (De et al., 2021), anti-bacterial (Azab et al., 2013), anti-inflammatory (Sharma et al., 2020), anti-diabetic (Singh, 2022), and anti-tumor (El-Hag et al., 2019). Several heterocyclic compounds have been shown to have biological activity (Scheme 2).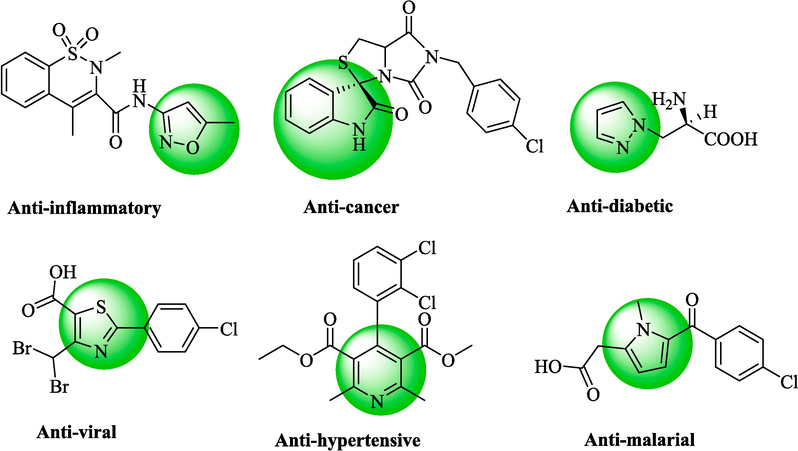
Some structures of the biologically active fused heterocycles.
The preparation of myriad heterocycles was published via MCR. Still, these works do not deliver an acceptable result regarding reaction factors like pricey transition metal catalysts, long reaction times, and halogenated solvents. Given these drawbacks, diverse new procedures were regarded about environmental safety. Developing green and improved ways of synthesizing heterocycles remains a challenging objective. Expanding a profoundly efficient catalytic system is highly preferred with the rising need for practical organic conversion based on green sustainability in chemistry (Daştan et al., 2012; Safari et al., 2023; Nishanth Rao et al., 2021; Adhikari et al., 2022; Ameta and Dandia, 2014; Chaudhuri et al., 2021). Recently, β-CD, a powerful catalytic system, received significant attention for synthesizing heterocycles. β-CDs have been broadly employed as a valuable catalyst for synthesizing heterocycles that make many products (Abbasi, 2017; Cioc et al., 2014; Mishra et al., 2018). As mentioned earlier, the critical characteristic of CD compounds is that they can organize the inclusion of complex metal ions for hosting in their cavity with non-covalent bonding. This feature makes them water-soluble catalysts with high stability in water. Along this line, CD catalysts effectively conduct chemical transformations in aqueous media. CDs are gaining attention in greener synthetic routes due to their reactions being performed in neat, aqueous organic media, and it will inspire chemists to design and develop new heterocyclic compounds by using CDs as a perfect catalyst. This review highlights the advances in synthesizing heterocyclic compounds based on β-CD catalysts. We hope this discussion will be necessary and draw more interest in various research fields.
2 β-CD catalysts for synthesizing heterocycles
2.1 Synthesis of five-membered heterocycles
In 2019, Bahadorikhalili et al. reported a green procedure for the fabrication of imidazo[2,1-b][1,3,4]thiadiazol-5-amines 4 and imidazo[1,2-a]pyridines 5 from the corresponding benzaldehyde (1), semicarbazide (2), and isocyanides (3) using βCD-IL@MStarch in ethanol as an environmentally friendly solvent at 50 °C (Scheme 4) (Bahadorikhalili et al., 2019). Iron oxide nanoparticles (SPIONs) were obtained using co-precipitation of Fe3+ and Fe2+ ions for the preparation catalyst. Then, they were modified by starch (M−Starch). Separately, β-CD-IL was synthesized in two stages. Initially, a tosylation is the reaction of the tosylate β-CD by methyl imidazole. The polymerization of β-CD-IL with hexamethylene diisocyanate on M−Starch was obtained from the final β-CD-IL@M−Starch catalyst (Scheme 3). This new catalyst was identified using TGA, TEM, VSM, and FTIR techniques. In the TEM photo of this magnetic nanocatalyst, the dark spots represent the existence of Fe3O4 nanoparticles. The brighter parts correspond to the organic groups, including starch, ionic liquid β-CD, and the polymer linker. The magnetic behavior of the nanocatalyst was examined using the VSM analysis so that the superparamagnetic behavior of the catalyst could be confirmed. This catalyst was recycled successfully for ten consecutive runs. The imidazothiadiazolamine derivatives were attained in good isolated yields (80–91 %). Gadad et al. reported the synthesis of imidazothiadiazolamine derivatives by using MCRs. Reactions of 5-substituted-1,3,4-thiadiazol-2-amine with aldehydes and isocyanides in the presence of trimethylsilyl chloride or perchloric acid in dry ethanol at reflux for 14 h. are reported (Gadad et al., 2008). Compared with this method, employing β-CD-IL@M starch catalyst has some advantages like mild reaction conditions, easy reaction procedure, and high isolated yields.
The structure of β-CD-IL@M starch.
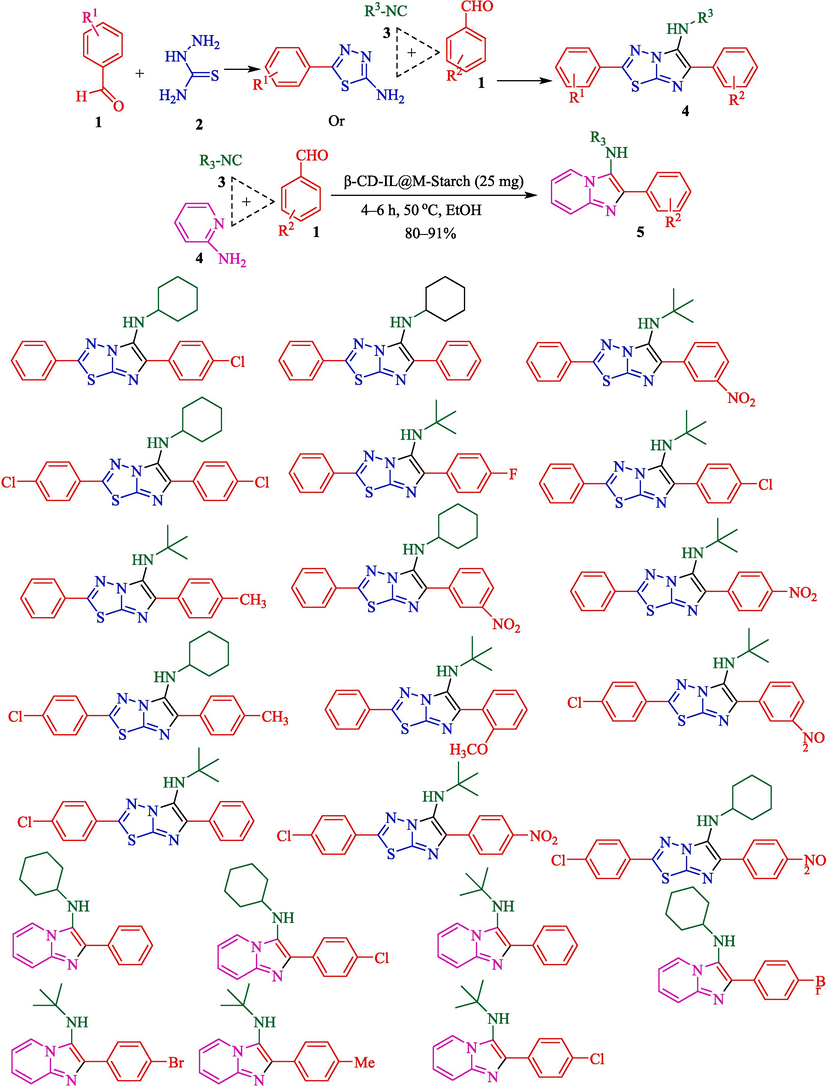
Synthesis of imidazothiadiazolamine and imidazopyridinamine derivatives.
Jain et al. declared a helpful route for the formation of 1,4-disubstituted 1,2,3-triazoles 8 from coumarin azides (6), terminal alkyl 7 via click reaction by using 2.5 mol% of Fe3O4@CD-CIT as a phase-transfer catalyst by lower Cu loading in H2O under ultrasonic irradiation conditions at 40 °C (Scheme 6) (Jain et al., 2019). The steps of catalyst preparation are shown in Scheme 5. Diverse techniques, including FTIR, XRD, 1H NMR, TEM, and VSM, identified the new magnetic compound Fe3O4@CD-CIT. TEM image indicated that particles were nearly spherical with a range of 5–10 nm. This reaction performed well, providing 1,4-disubstituted-1,2,3-triazoles were good to superior yields (91–97 %). Fe3O4@CD-CIT could be recycled by magnetic separation for 6 consecutive runs with a tiny reduction in performance. The SEM result of the recycled catalyst after the 7 times indicated no notable difference in the SEM photos of the fresh and the recovered catalyst, which furnished proof for the satisfactory structural stability of the catalyst under the utilized reaction conditions. In the same ultrasound irradiation method, Jiang et al. reported the synthesis of 1,4-disubstituted-1,2,3-triazoles derivatives by using 1,3-dipolar cycloaddition reaction employing CuSO4·5H2O (10 mol%) and sodium ascorbate (20 mol%) as the catalyst in t-BuOH/H2O as reaction solvents and at room temperature (Jiang et al., 2011). Compared to the mentioned report, this method has some benefits like short-time reaction, recyclability, Gram scale synthesis, and high yield. The probable reaction mechanism for click reaction has been proposed in Scheme 7. At first, Cu(I) coordinated acetylide complexes created through the interaction of terminal alkyne and Cu(I) species (Complex 1″). The host–guest complex is constructed by β-CD of MNPTC (Complex 3″) in the next step. The formed complex reacts with coumarin azide, creating complex 4″, which converts into an intermediate 5″. Then, complex E intermediate provides heterocyclic complex 6″, which undergoes protonolysis and gets the related 1,2,3-triazole derivatives.
The provision of Fe3O4@CD-CIT.
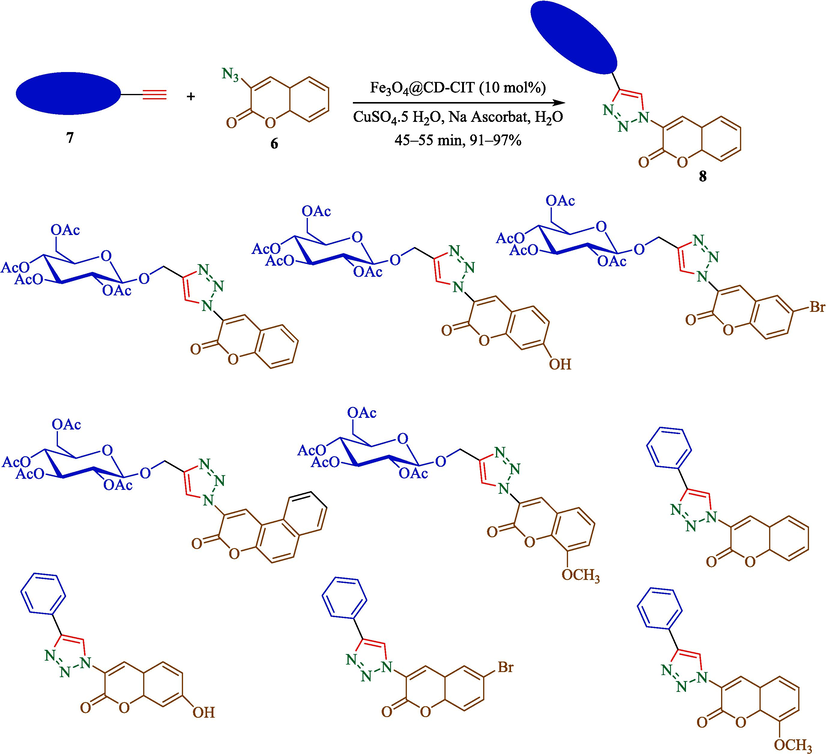
The preparation of 1,2,3- triazoles derivatives by Fe3O4@CD-CIT.
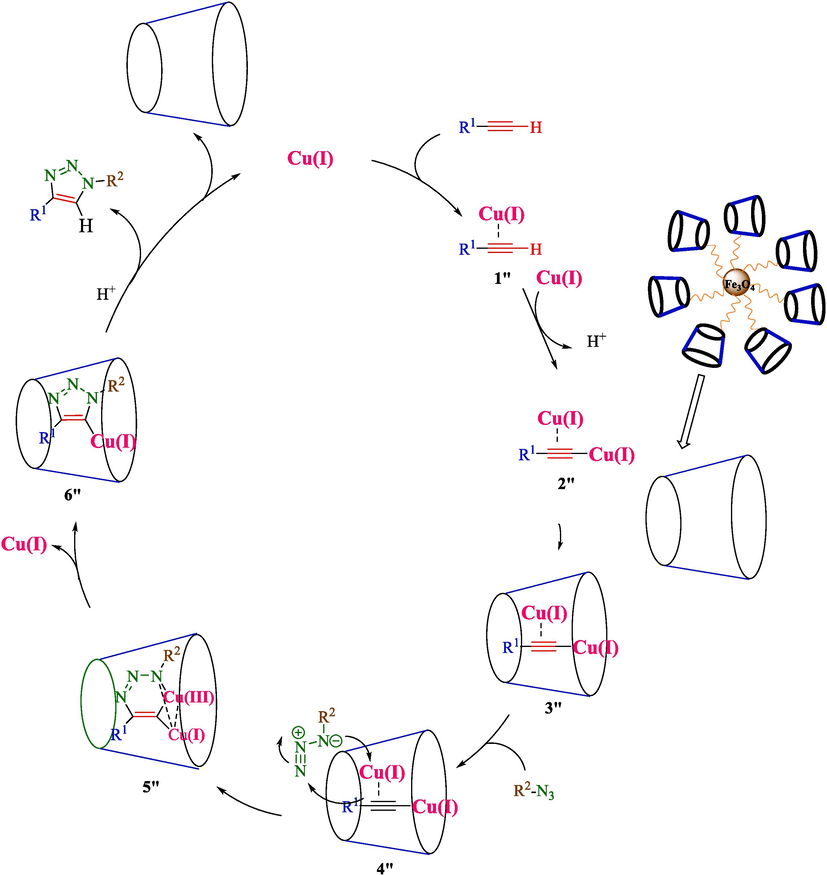
Mechanism for the preparation of 1,2,3- triazoles derivatives by Fe3O4@CD-CIT.
In 2020, Patil et al. represented an eco-friendly procedure for the synthesis of benzyl pyrazolyl naphthoquinones 15 (Scheme 9) and pyrazolopyranopyrimidines 12 (Scheme 8) by β-CD-SO3H as a heterogeneous reusable catalyst in H2O at 25–30 °C (Patil et al., 2020). β-CD-SO3H was synthesized by a simple one-step process and then characterized by several analyses like TGA, FT-IR, XRD, 13C NMR, 1H NMR, EDAX, BET, and acid-base titration. The EDAX analysis of β-CD-SO3H showed carbon and oxygen as the main elements related to the β-CD scaffolding. In contrast, the sulfur peak in its respective energy position at 2.2–2.4 keV also proved the construction of the expected catalyst. The loading of the SO3H group was noticed to be 0.8625 mmol of the functional group per gram of catalyst. The results indicated the proper usage of β-CD-SO3H and H2O to prepare pyrazolo pyranopyrimidines (86–94 %). Compared to reported methods, this approach showed better results (Table 1). Simplicity, mild and fast way, a green solvent, an easy work-up, and an inexpensive catalyst cost are the main advantages of this procedure. They also suggested the mechanism of the reaction (Scheme 10). Initially, aryl hydrazine/hydrazine hydrate 10 is reacted with ethyl acetoacetate (11) to generate the pyrazolone ring 13, an isomeric form. Simultaneously, the electrophilicity of carbonyl carbon of aldehyde 1 increases owing to the hydrogen bonding of β-CD-SO3H. Nucleophilic attack of 2-hydroxy-1,4-naphthoquinone 14 to activated aldehyde leads to the creation of intermediate 7″, followed by Knoevenagel condensation to construct intermediate 8″. Then, Michael added intermediate 13 and unsaturated Knoevenagel product 8 to form intermediate 9″, which undergoes a tautomeric proton shift to generate the desired product 15.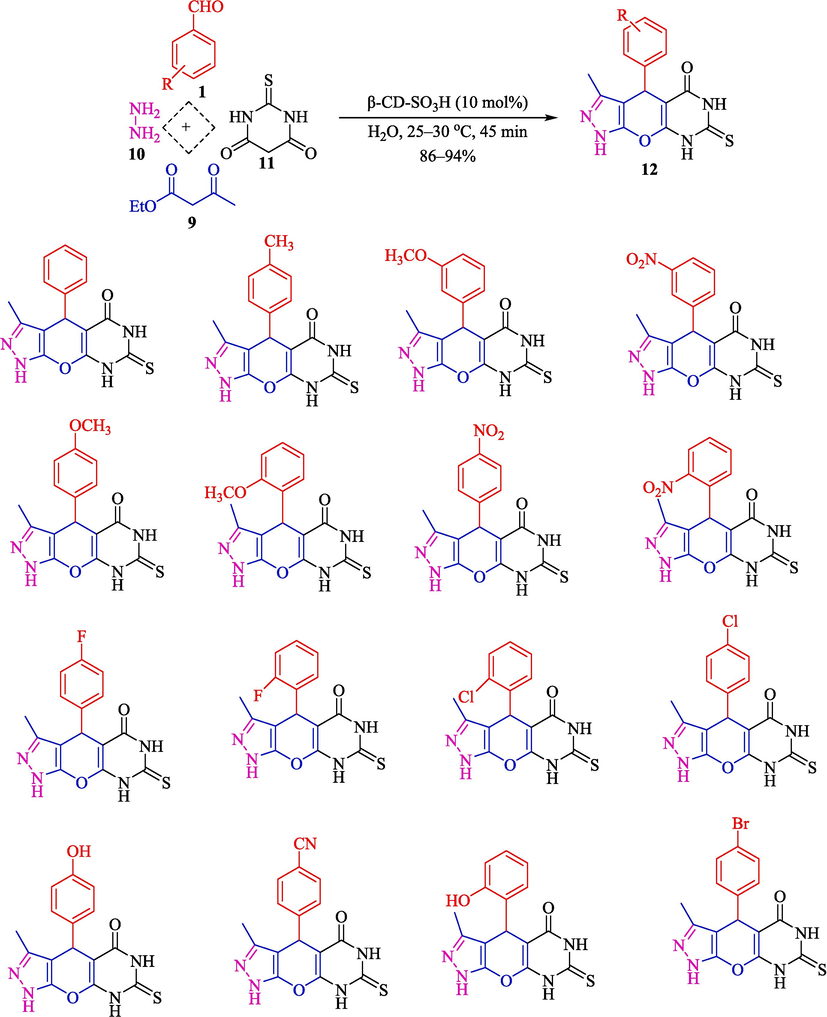
Synthesis of pyrazolopyranopyrimidines 12.
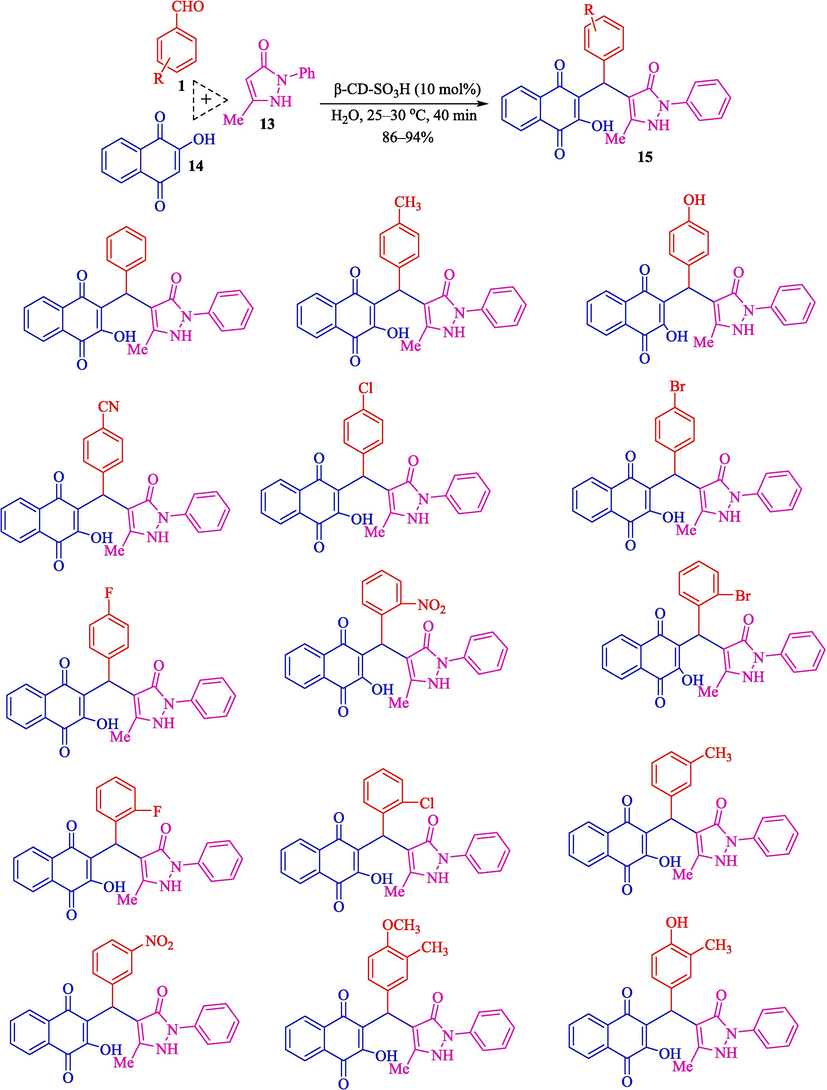
Synthesis of benzylpyrazolyl naphthoquinones.
Entry
Catalyst
Solvent
Condition
Time (min)
Yield (%)
Reference
1
Er(OTF)3
EtOH
Reflux
2 h
88–96
Kumar et al., 2017
2
p-TSA
H2O
Reflux
24
75–84
Lakshmanan and Ramalakshmi, 2016
3
MgCl2
EtOH
100 ℃
20–60
80–90
Fu et al., 2016
4
–
H2O
MW, 120 ℃
7–9
70–90
Wang et al., 2012
5
β-CD-SO3H
H2O
Rt
40
88–94
Current discussed report
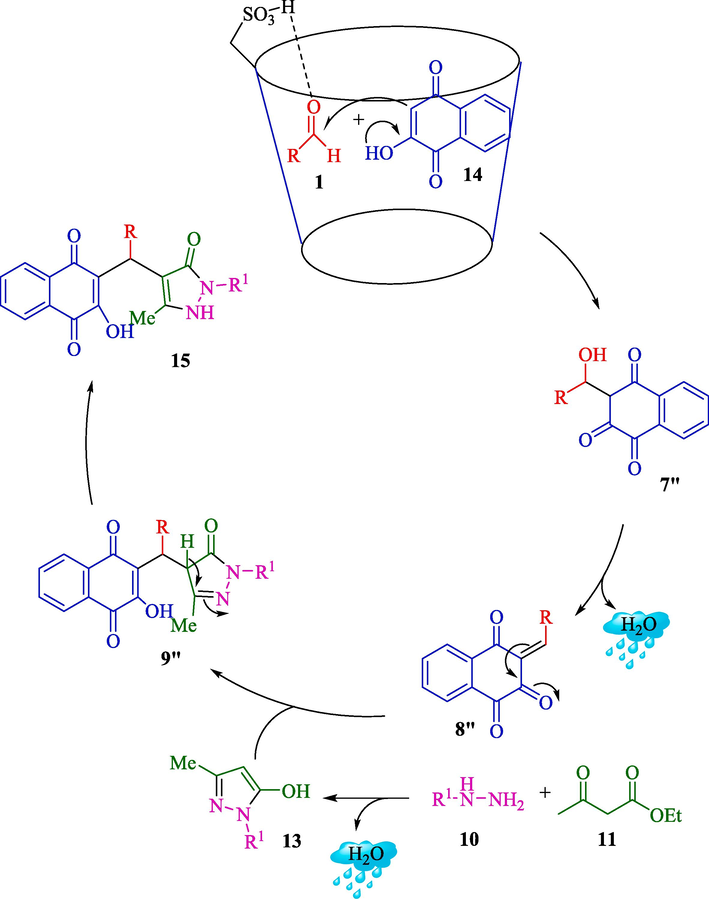
Plausible reaction mechanism.
The one-pot reaction of aromatic aldehydes 1, ethyl acetoacetate (9), hydrazine hydrate (10), and barbituric acid (BA, 16) has been represented for the synthesis of pyrazolopyranopyrimidines 17 by Akolkar and et al. (Scheme 11) (Akolkar et al., 2020). This method is green, rapid, and ultrasound-assisted. They used β-CD as a recyclable catalyst in H2O for synthesizing pyrazolopyranopyrimidines with superb yield (84–93 %). The result showed that β-CD was recycled for five runs. The comparative study with a former reported method for synthesizing pyrazolopyranopyrimidines is exhibited in Table 2. The authors proposed a possible mechanism demonstrated in Scheme 12. The first condensation of hydrazine hydrate and ethyl acetoacetate creates an intermediate equilibrium with its enolate 10″. Then, intermediate 11″ is made by Knoevenagel condensation of BA and aryl aldehyde. The intermediates 10″ and 11″ in the hollow of β-CD undergo Michael's addition to generate 12″. Intramolecular cyclization by the nucleophilic addition of oxyanion to the C⚌O group provides intermediate 13″. Eventually, the dehydrating intermediate D will convert to the expected product.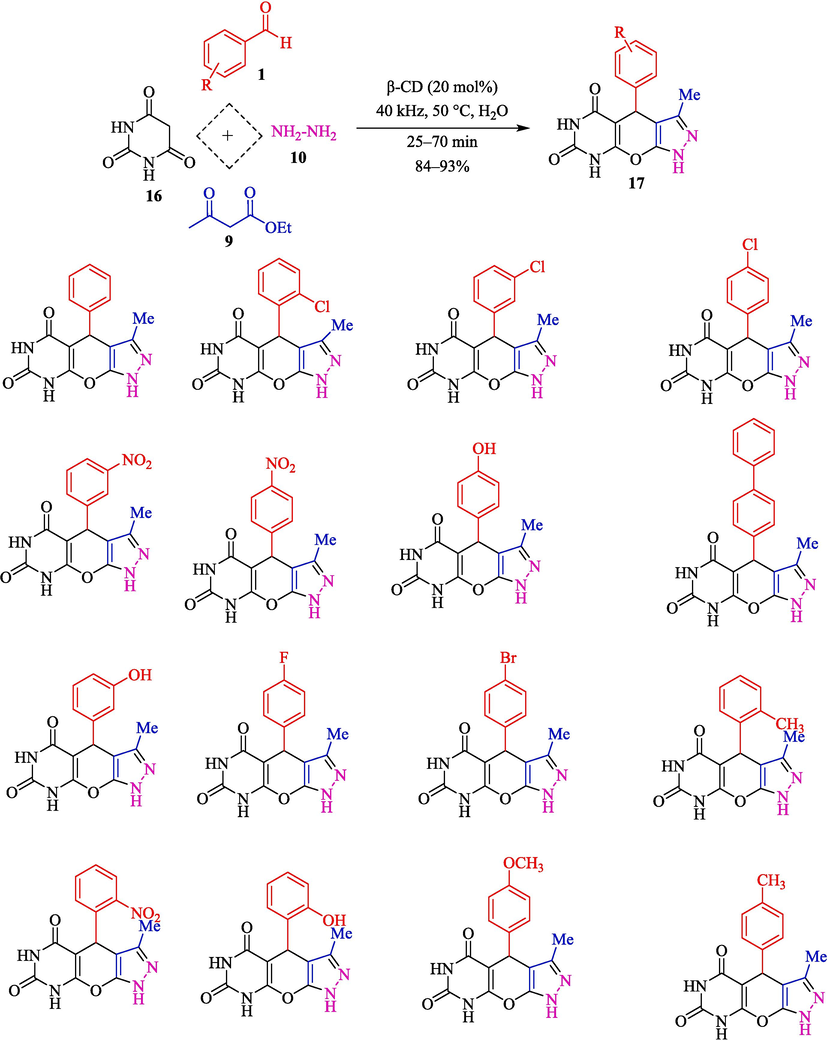
The preparation of pyrazolopyranopyrimidines by β-CD as a catalyst under ultrasound conditions.
Entry
Catalyst and solvent
Temperature
Time (min)
Yield (%)
References
1
DABCO, H2O
Reflux
20–45
84–99
Heravi et al., 2014
2
SDS, H2O
Reflux
40
87–96
Ahanthem et al., 2018
3
TiO2 NWs, EtOH/H2O
Reflux
45–100
83–95
Dastkhoon et al., 2015
4
OMWCNTs, EtOH/H2O
Reflux
65–100
85–94
Khodabakhshi et al., 2016
5
Meglumine, H2O
rt
15–360
83–95
Li et al., 2014
6
Cu2+@MSNs-(CO2−)2, H2O
rt
60–120
75–92
Nasresfahani and Kassaee, 2017
7
ChCl:Urea, EtOH
80 ℃
60
75–92
Tipale et al., 2018
8
β-CD, H2O
50 ℃
25–70
84–93
Current discussed report
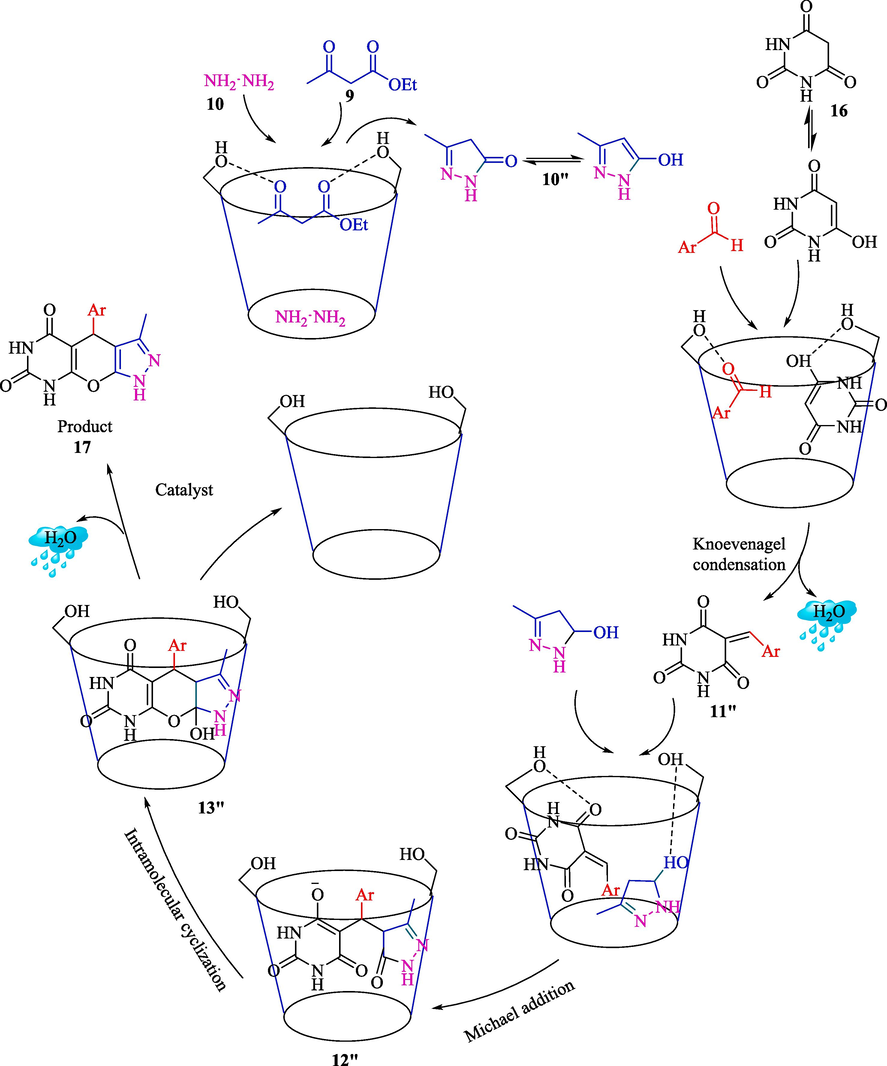
The possible mechanism for one‑pot synthesis of pyrazolopyranopyrimidines.
Dhananjaya et al. represented a preparation of 4-oxo-4,5,6,7-tetrahydroindoles 21 by β-CD (10 % w/w) in water (Dhananjaya et al.,2020). This reaction was conducted in a three-component response (TCR) of phenacyl bromide (18), primary amine (19), and dimedone (20). A spectrum of products was obtained using this green procedure with a high yield (82–92 %) (Scheme 13). The catalyst plays a critical role in raising the efficiency of the reaction, mainly by activating the bromo group of the phenacyl bromide and assisting the water solubility of all the reactants. β-CD could be recycled up to the third cycle with no notable product yield loss. This method has some advantages compared to previous approaches (Zhang et al., 2013; Caliskan et al., 2020), such as metal-free conditions, non-toxic catalysts, the ability to recycle, and active biological products. A reasonable reaction mechanism for this MCR reaction by β-CD is illustrated in Scheme 14. The nucleophilic attack by the dimedone 20 through its enol form on the bromo group having carbon the phenacyl bromide 18 was also facilitated by β-CD afforded the tri-keto intermediate 14″. On further reaction with amine 19, the 14″ afforded an enamine intermediate 15″ that on intramolecular cyclization furnished the desired product 21.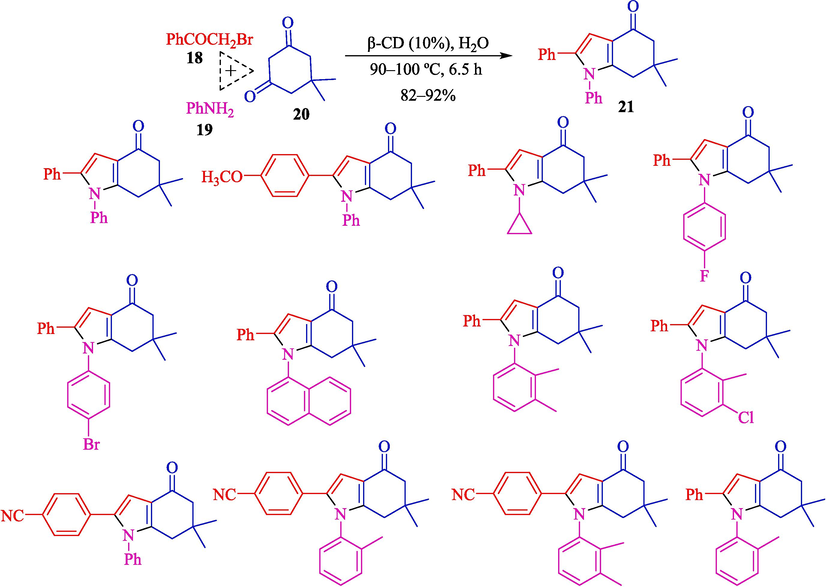
The preparation of 4-oxo-4,5,6,7-tetrahydroindoles by β-CD catalyst.
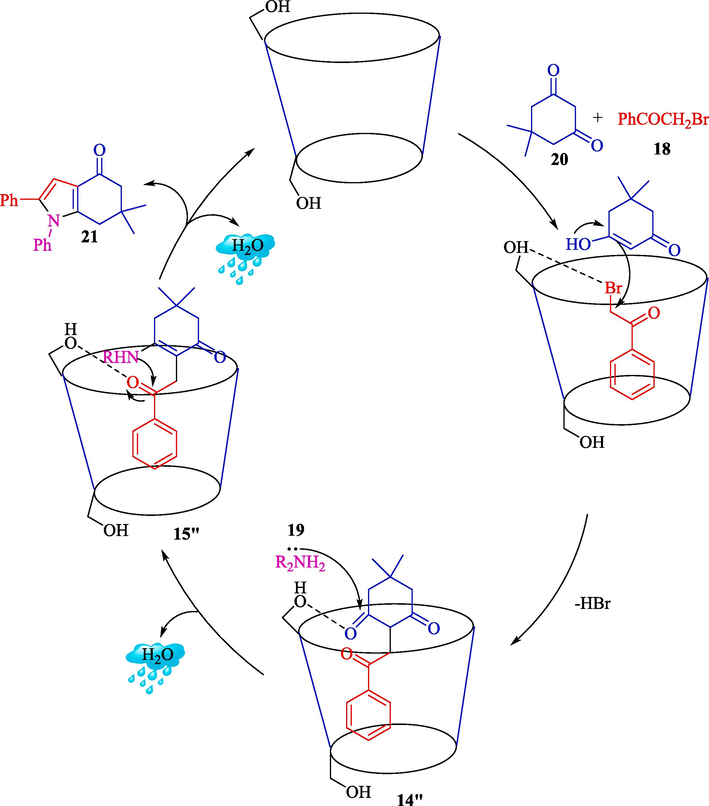
Offered path for the construction of 4-oxo-4,5,6,7-tetrahydroindoles.
Bahadorikhalili et al. prepared Cu@β-CD@MGO as a nanocatalyst for the preparation of N-(alkyl)-2-phenylimidazo[1,2-a]pyridin-3-amines 5 via an efficient and atom-economical method (Bahadorikhalili et al.,2020). The TCR obtained aldehydes (from 22), pyridin-2-amine (4), and isocyanides 3 were developed, and N-(alkyl)-2-phenylimidazo[1,2-a]pyridin-3-amine produced provided in high-yield (65–75 %) (Scheme 15). Techniques like XRD, TGA, TEM, SEM, VSM, FT-IR, and ICP analyzed this new nanocatalyst. SEM and TEM results showed that iron oxide nanoparticles (20–25 nm) were seen as dark spots within the graphene oxide nanosheets. The FTIR spectrum of βCD@MGO indicated the attachment of β-CD to the MGO. The bands at 3430 cm−1 are owing to the O—H groups of β-CD connected to the surface, and the band at 1691 cm−1 was allocated to the C⚌O. This nanocatalyst exhibited high reusability with no noticeable leaching detected after ten times. At the start of the reaction, aerobic oxidation of benzyl alcohol to the relevant aldehyde occurs in Cu@βCD@MGO catalyst. The fantastic benefit of this stage is that the oxidation reaction happens in the presence of oxygen in the air and without the requirement for other oxidizing reagents. A comparison between the present approach and formerly reported strategies for synthesizing phenylimidazo[1,2-a]pyridine derivatives demonstrated the excellent activity and high performance of Cu@βCD@MGO catalyst for synthesizing the cited compounds. In most cases, using non-reusable catalysts restricts these compounds' industrial production. Additionally, utilizing toxic or high-temperature conditions is one of the other disadvantages of some of the previous reports (Bharate et al., 2013, Bode et al., 2011). The possible mechanism was offered by the authors and displayed in Scheme 16. At the start of the reaction, aerobic oxidation of benzyl alcohol 22 to the corresponding aldehyde 1 occurs in the presence of Cu@βCD@MGO catalyst. The created aldehyde reacts to pyridine-2-amine 4, forming 16″ intermediated. The reaction of 16″ intermediated with isocyanide derivative 3 leads to forming 17″ intermediated. An intramolecular cyclization reaction occurred in 17″ intermediated, which gives 18″ intermediated. The desired phenylimidazo[1,2-a]pyridines 5 will be formed by a 1,3-H-shift in the compound 18″.![Synthesis of phenylimidazo[1,2-a]pyridines by Cu@β-CD@MGO.](/content/184/2024/17/10/img/10.1016_j.arabjc.2024.105967-fig16.png)
Synthesis of phenylimidazo[1,2-a]pyridines by Cu@β-CD@MGO.
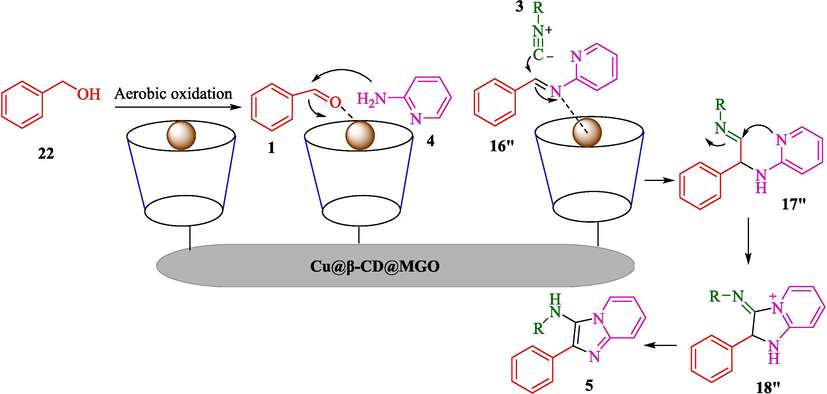
The suggested way for this reaction is using Cu@β-CD@MGO.
The Friedel-Crafts alkylation reaction of indoles 23 with aryl, heteroaryl as well, as alkyl aldehydes 1 by β-cyclodextrin hydrate (β-CDH) for the synthesis of bis-(indol-3-yl)-methanes 24 reported by Das et al. (Scheme 17) (Das et al., 2020). This reaction indicates good chemoselectivity under mild reaction conditions. The reactions were completed at 60 °C in H2O as a safe solvent, providing products with good to high yields (87–94 %). The other supramolecular catalysts, like α- and γ- CD, were less helpful in this reaction. The β-CDH catalyst could be recycled six times without an appreciable drop in performance. Some of the previous studies listed in Table 3 involved the usage of pricey catalysts (Sun et al., 2015; Fu et al., 2020; Bahuguna et al., 2018; Huo et al., 2013), multistep synthesis of catalysts (Bahuguna et al., 2016) long reaction time (Sun et al., 2015; Fu et al., 2020; Bahuguna et al., 2018), and lack of chemoselectivity (Gao et al., 2017; Huo et al., 2013). The current β-cyclodextrin hydrate-catalyzed metal-free protocol in aqueous medium mainly does away with these weaknesses (entry 7). The possible mechanism was demonstrated in Scheme 18. The nucleophilic attack from 2-methylindole 23 to benzaldehyde 1 with the water molecules inside the cavity of β-CD hydrate) the intermediate 19″ is enhanced and rapidly created in the first stage. Subsequent dehydration of the intermediate 19″ makes the corresponding 20″, which, on further nucleophilic attack by another 2-methylindole 23, affords the product 24.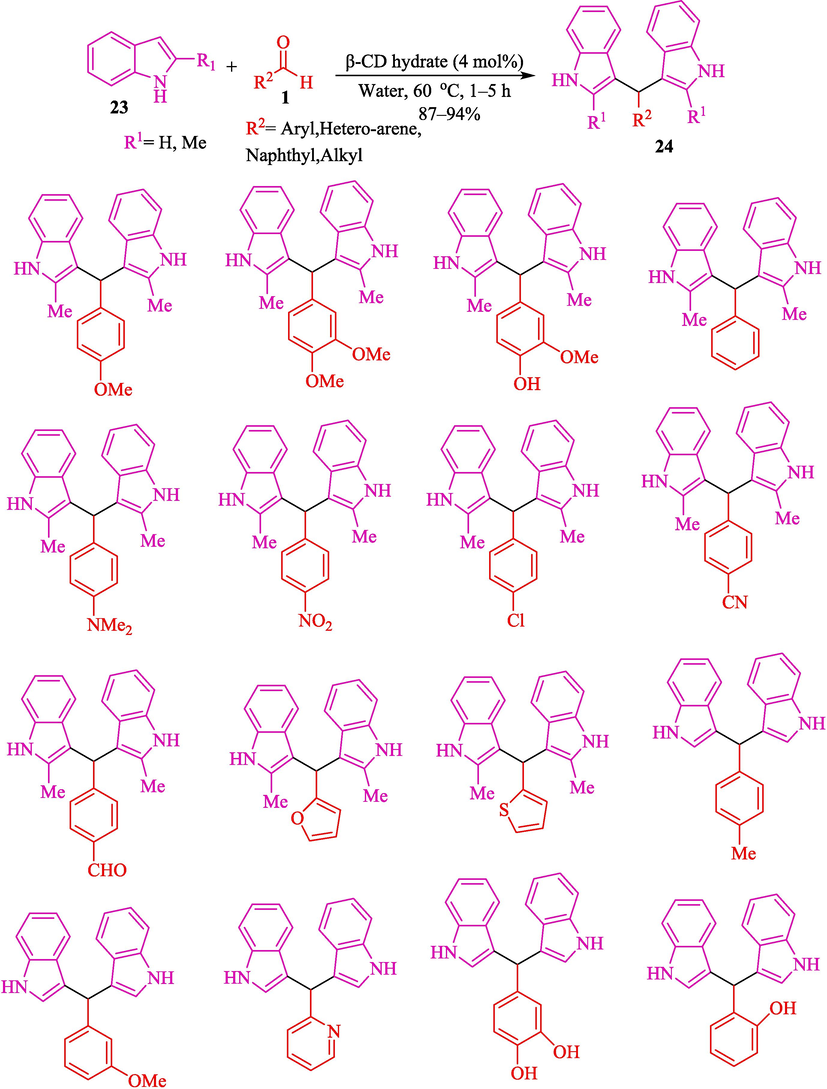
Synthesis of bis-(indolyl)methanes by β-CD hydrate catalyzed.
Entry
Catalyst
Solvent
Time (h)
Temp (℃)
Yield
(%)Reference
1
–
Ethyl lactate: H2O
0.5
rt
91
Gao et al., 2017
2
[DABCOH][HSO4]
–
2
90
79
Tong et al., 2016
3
α-Chymotrypsin
H2O
24
70
90
Sun et al., 2015
4
Lipase enzyme
H2O
36
55
95
Fu et al., 2020
5
ZnO-RGO
EtOH: H2O
12
rt
86
Bahuguna et al., 2018
6
TPPMS/CBr4
CH3CN
4
rt
72
Huo et al., 2013
7
β-CD hydrate
H2O
3
60
89
Current discussed report
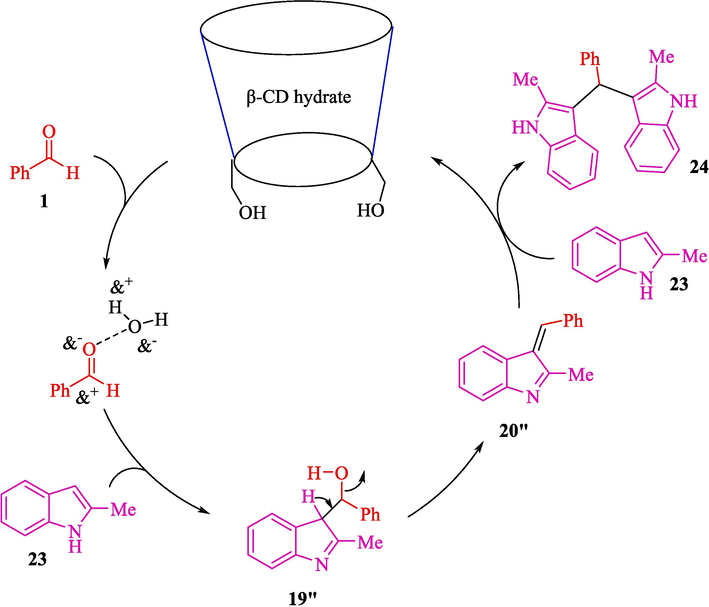
The suggested mechanism for reactions of indoles with aldehydes catalyzed by β-CD hydrate.
In 2022, Bhadke at el. illustrated a green protocol for the synthesis of pyrazolones 26 from ethyl acetoacetate (9) and phenylhydrazine (25) via the β-CD (12 mol%) catalyzed in H2O at 80 °C for 20–30 min. β-CD acts as an effective host and powerful catalyst (Scheme 19) (Bhadke et al., 2022). This procedure reduced the restrictions of former traditional strategies, some of which needed transition metal catalysts, long reaction times, harsh reaction conditions, or costly reagents. β-CD furnishes such innate advantages as eco-friendly conditions, low prices, simplification of protection-deprotection stages, and decreased waste.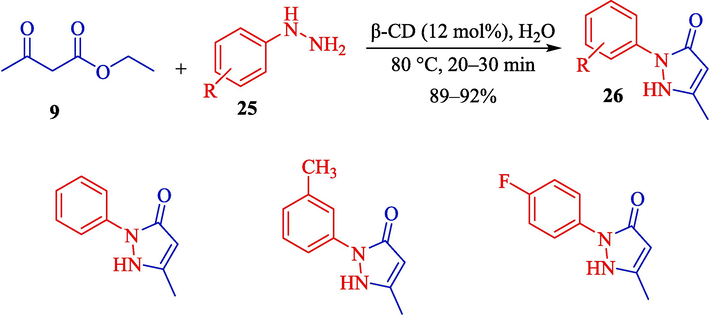
Synthesis of pyrazolones derivates 26.
Among the various procedures famous in the literature for the synthesis of triazoles utilizing click chemistry, one that meets the goal of the green strategy is the usage of CDs as a phase transfer catalyst. In 2022, for the first time, Madhuri and at el. illustrated the synthesis of spirochromanone attached 1,2,3-triazoles 29 and spirochromanone conjugates, including bis-1,2,3-triazoles (Scheme 20) (Madhuri et al., 2022). Spirochromanone linked 1,2,3-triazoles and spirochromanone conjugates comprising bis-1,2,3-triazoles were prepared for the first time and are assessed for probable anti-bacterial activity. The process applies easy, practical click chemistry, a low-cost phase transfer catalyst, and water as an eco-friendly solvent.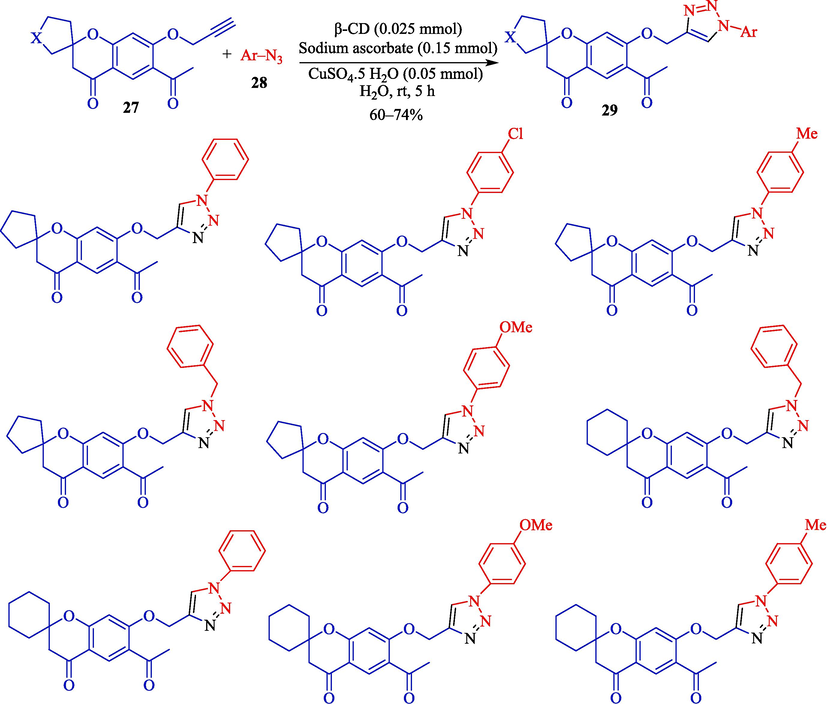
The preparation of triazole moieties by β-CD catalyst.
In 2022, Tajbakhsh et al. described a facile method for synthesizing isoxazole derivatives 31 through a TCR from aryl aldehydes 1, ethyl acetoacetate (9), and hydroxylamine hydrochloride (30) using Cu@Met-β‐CD as a biodegradable, and reusable catalyst (Scheme 22) (Tajbakhsh et al., 2022). The steps of this catalyst preparation are provided in Scheme 21. This catalyst was analyzed using EDX, SEM, FT-IR, TGA, and XRD analyses. In the FT‑IR analysis, the strong absorption bands at 3380 cm−1 and 1640 cm−1 correspond to OH groups’ stretching and bending vibrations, respectively, were observed. The aliphatic CH absorption bands of β-CD can be seen at 2925 cm−1. The peak at 1370 cm−1 corresponds to the characteristic bands of the S⚌O tosyl group. The peak of 1624 cm−1 corresponds to stretching bonds C⚌N of metformin, which shifted to 1650 cm− 1 and varied the form of the peak in Cu@Met-β‐CD upon complexation with copper. This work has advantages like clean workup, short reaction times, excellent yields, and an environmentally friendly process. The recovery of the Cu@Met-β‐CD was tested, and the results showed that catalysts were successfully recycled seven consecutive times with little activity reduction. The SEM images of the catalyst indicated round shape morphology in average size, mainly in the range of < 50 nm. Table 4, compares Cu@Met-β‐CD and various catalysts in synthesizing 4-(4-hydroxybenzylidene)-3-methylisoxazol-5(4H)-one. The Authors also suggested the mechanism for the reaction (Scheme 23). Firstly, the Cu immobilized in functionalized β-CD works as a Lewis acid and enhances the electrophilic properties of the carbonyl groups in ethyl acetate. Then, the nucleophilic attack of the NH2 group 30 occurs at the activated carbonyl carbon of 9, resulting in an oxime intermediate 21″. The condensation provides isoxazol-5-ones as the heterocyclic compound 22″. The obtained carbanions can also be utilized in condensation reactions with aldehydes to produce electrophilic arylidene isoxazole-5-ones 23″.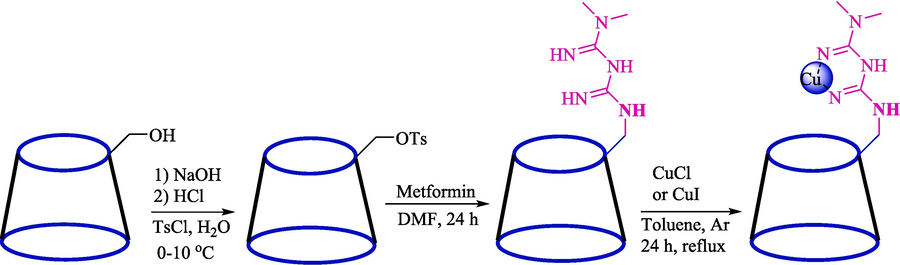
Steps catalyst preparation.
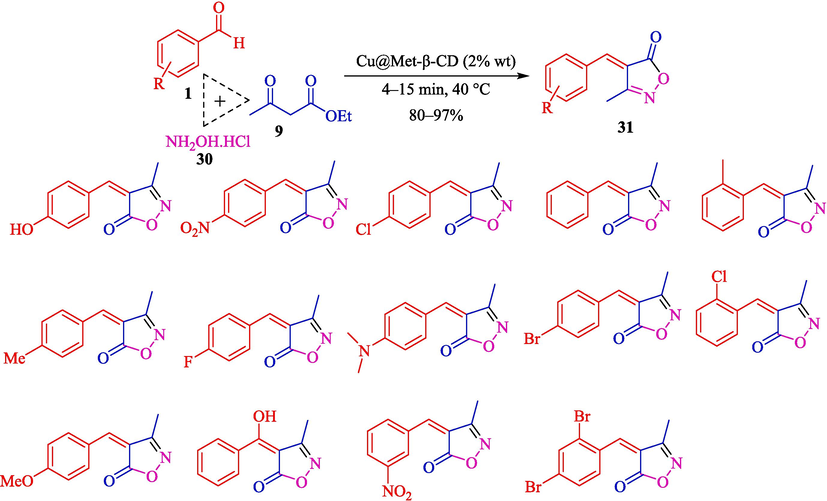
The synthesis of isoxazole derivatives using Cu@Met-β‐CD.
Entry
Catalyst
Condition
Time (min)
Yield (%)
Reference
1
Sodium acetate
EtOH/H2O, rt
30
90
Aslam et al., 2020
2
DABCO
EtOH, rt
30–180
87
Kim et al., 2018
3
Modified-MMT
H2O, 70 °C
7–70
92
Mashhadinezhad et al., 2018
4
Cu/TCH-pr@SBA-15
Solvent-free, 80 °C
8
95
Kalhor et al.,2020
5
L-Valine
EtOH, reflux
1–240
95
Kour et al., 2020
7
Cu@Met-β‐CD
H2O, rt. up to 50 °C
2–5
97
Current discussed report
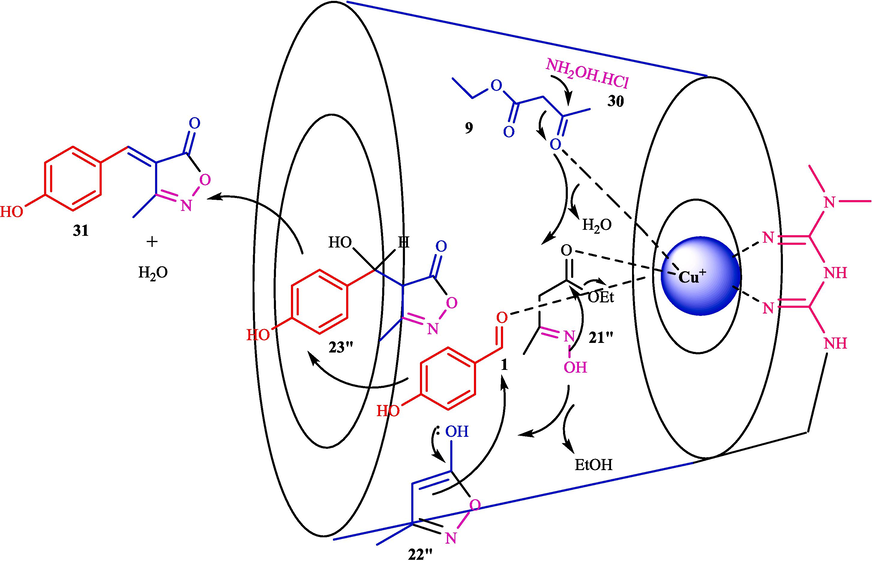
Suggested path for the reaction by Cu@Met-β‐CD.
In 2023, Morita et al. designed a green procedure for synthesizing 2,3-dihydrobenzofurans 34 (Scheme 25) and 1,2,3-trisubstituted indanes 37 (Scheme 26) stereoselectively using permethylated β-CD-tagged NHC–gold(I) (Morita et al., 2023). The structure of β-CD-NHC-AuCl is displayed in Scheme 24. The gold(I) catalyst performs perfectly in these reactions, which could be recovered in five runs. The suggested reaction mechanism for preparing 1,2,3-trisubstituted indanes is shown in Scheme 27. At first, the OH group of benzylic alcohol 36 is activated by coordinating a gold center, possibly inner the hydrophobic cavity of the β-CD, creating an active intermediate 24″. The addition of trans-anethole 35 to the intermediate 24″ provides the intermediate 25″ with an electron-rich aromatic ring and an electron-deficient ring. After cyclization between the aromatic ring and the benzylic part in the intermediate 25″, product 37 was afforded.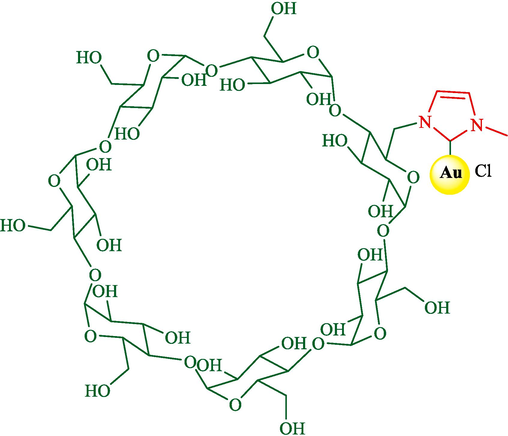
The structure of β-CD-NHC-AuCl catalyst.
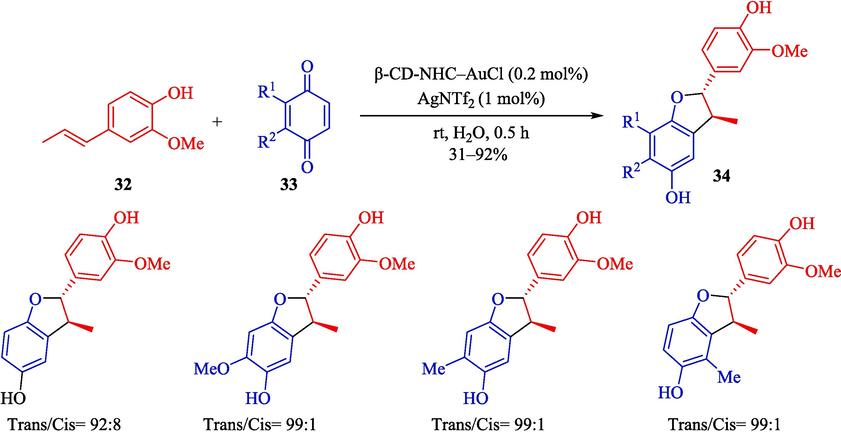
Preparation of 2,3-dihydrobenzofurans derivates by β-CD-NHC-AuCl.
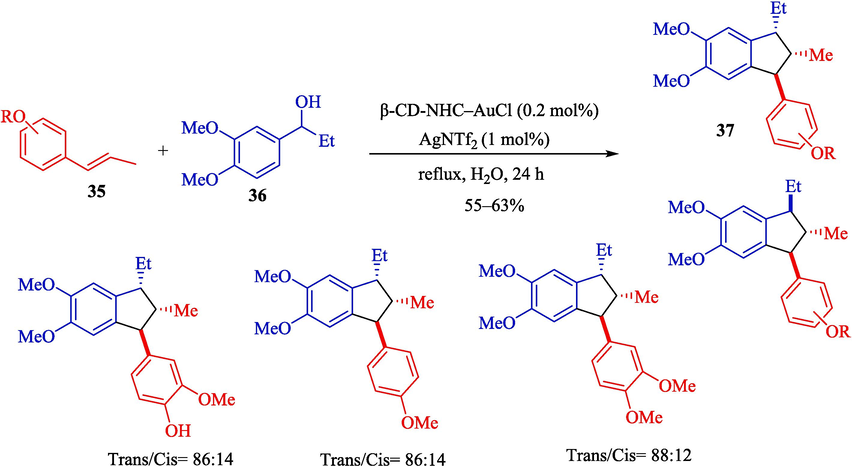
Preparation of 1,2,3-trisubstituted indanes products by β-CD-NHC-AuCl.
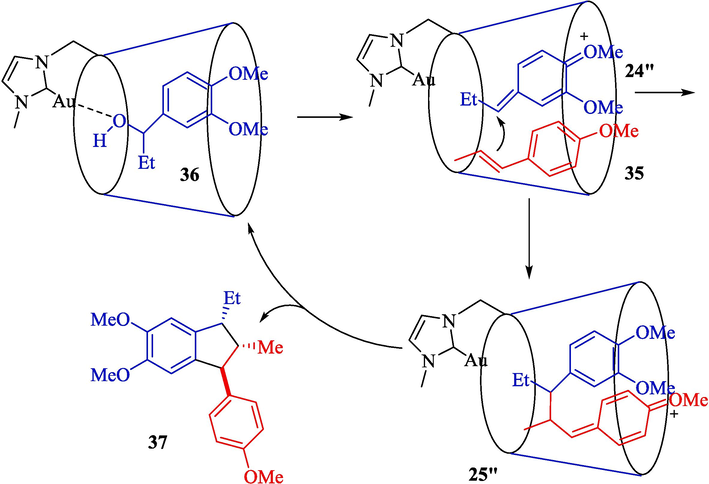
Suggested reaction mechanism for preparation of 1,2,3- trisubstituted indanes.
Paul et al. reported a green method for the preparation of 1,2,3-functionalized 4-hydroxy pyrrolidine-5-ones 40 from aldehydes 1, amines 38, and dimethylacetylenedicarboxylate (39) employing a supramolecular catalyst β-CD (Scheme 28) (Paul et al., 2023). This reaction was performed without metal salt in a water/ethanol medium. The reactions were completed in a time (8 h), and the product was established with satisfactory yields (64–90 %). The offered mechanism for synthesizing pyrrolidine-2-one derivates is indicated in Scheme 29. β-CD activates both aryl aldehyde 1 and di(methyl/ethyl) acetylene dicarboxylate 39 compounds simultaneously as the active electrophilic species, facilitating the reaction. The condensation of aryl amines 38 with these activated electrophilic aldehydes with the loss of H2O, along with the reaction of H2O with activated electrophilic di(methyl/ethyl) acetylene dicarboxylate compound, may then produce the corresponding intermediates 26″ and 27″, respectively. After that, the reaction between these two intermediates 26″ and 27″ will yield the corresponding intermediate 28″. Eventually, a series of tautomerization, cyclization, and aromatization reactions create the intermediates 29″, and 30″, and finally, the product 40.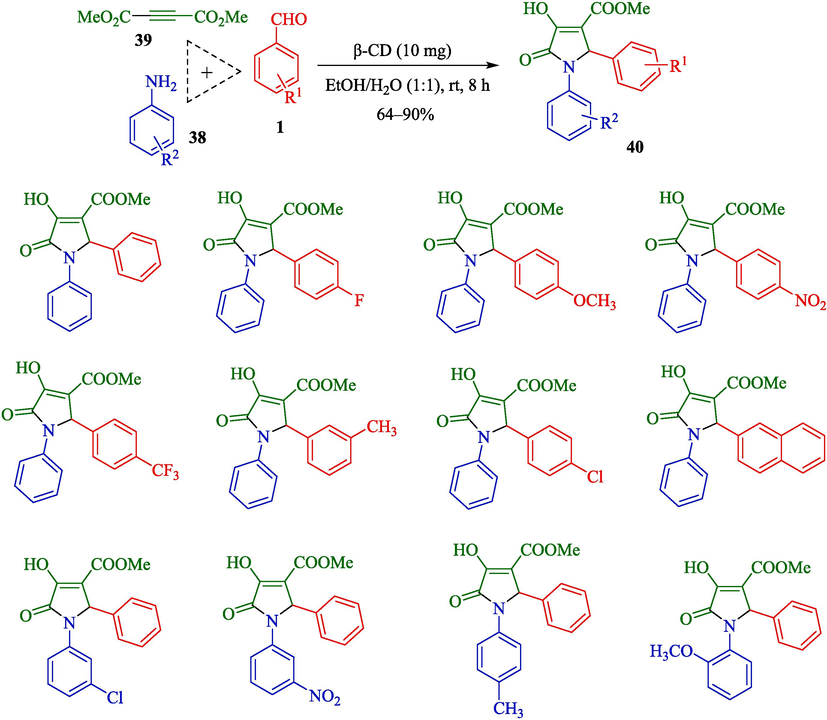
Synthesis of functionalized pyrrolidine-2-ones using β-CD.
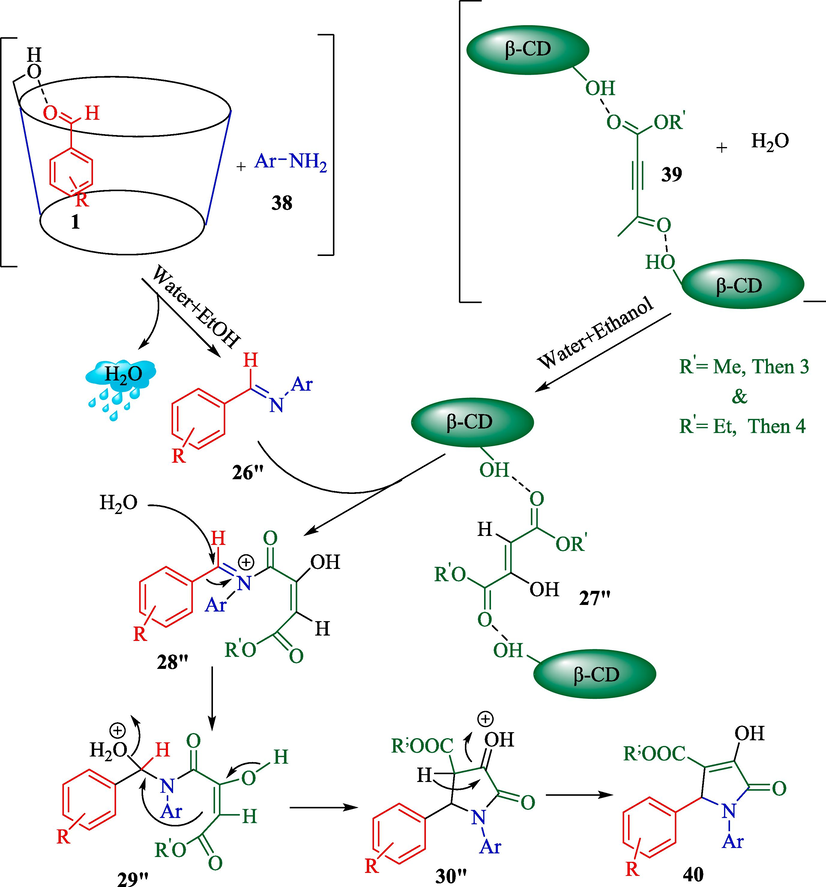
Recommend mechanism for this reaction by β-CD.
2.2 Synthesis of six-membered heterocycles
Ahadi et al. introduced MNPs@β‐CD@Cu(OAc)2 as an impressive catalyst for the preparation of spiropyrans 43 from isatin (41), dimedone (20), and 2-substituent-acetonitrile 42 (Scheme 31) (Ahadi et al., 2019). This nanocatalyst was prepared by surface‐modified magnetic support by the Cu(II)‐β‐CD complex. The preparation step of MNPs@β‐CD@Cu(OAc)2 is shown in Scheme 30. TGA, FT-IR, XRD, SEM, and VSM characterized the structure of the nanocatalyst. The result obtained from the SEM analyst showed that the average diameter of the nanocatalyst was about 33 nm. The reusability of the nanocatalyst was examined, and it turned out that the catalyst recycled six runs with no significant loss in performance. The chief properties in this work are green condition, short reaction period, facile workup, and reusability catalytic by using an external magnet. Table 5, compares MNPs@β‐CD@Cu(OAc)2 catalyst with other catalysts reported for the preparation of spiropyrans. The suggested mechanism for the reaction is exhibited in Scheme 32. The carbonyl group at isatin 41 could be activated by MNPs‐β‐CD@Cu (OAc)2 in the β‐CD cavity, so the nucleophilic attack by the CH acidic group of 2-substituent-acetonitrile 42 (Knoevenagel condensation) could be carried out to attained the intermediate 31″. Then, Michael's addition is done by enolized dimedone 20 to create the intermediate 32″. Then, nucleophilic attack through oxygen onto the CN group, and next, tautomerization provides the 43 product.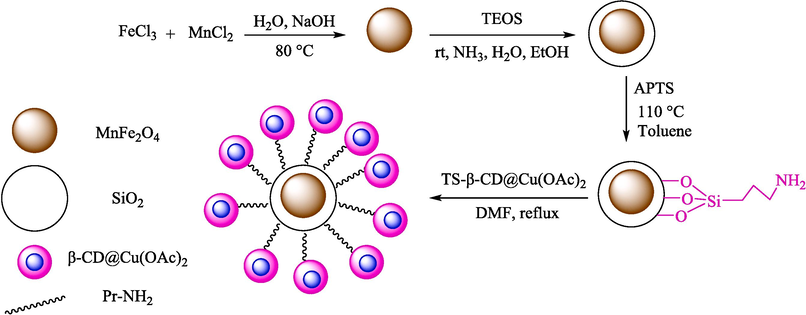
Synthesis of this new nanocatalyst.
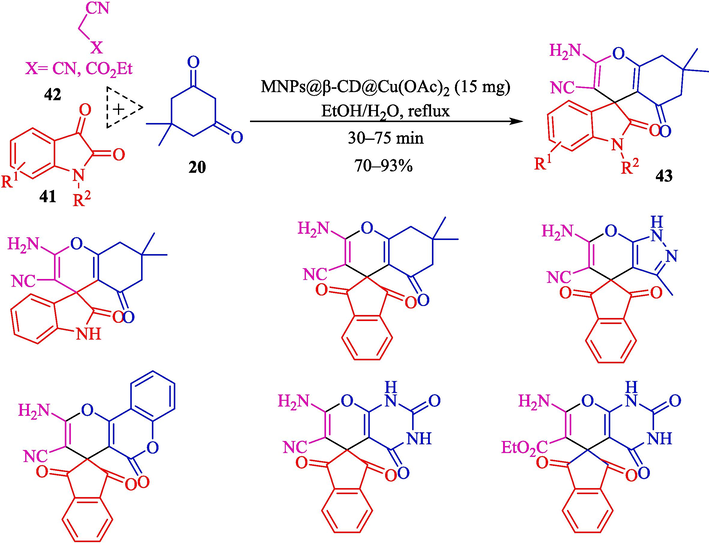
Preparation of the spiropyrans by MNPs@β‐CD@Cu(OAc)2.
Entry
Catalyst
Condition
Time (min)
Yield (%)
Reference
1
SB‐DBU
EtOH:H2O, 50 °C
150
97
Hasaninejad et al., 2013
2
SSA‐MNPs
EtOH:H2O, 60 °C
80
95
Karimi et al., 2015
3
PEI@Si–MNPs
H2O, 40 °C
40
92
Khoobi et al., 2015
4
Sodium stearate
H2O, 60 °C
3 h
95
Wang et al., 2010
5
MNPs@β‐CD@Cu(OAc)2
EtOH:H2O, 80 °C
35
93
Current discussed report
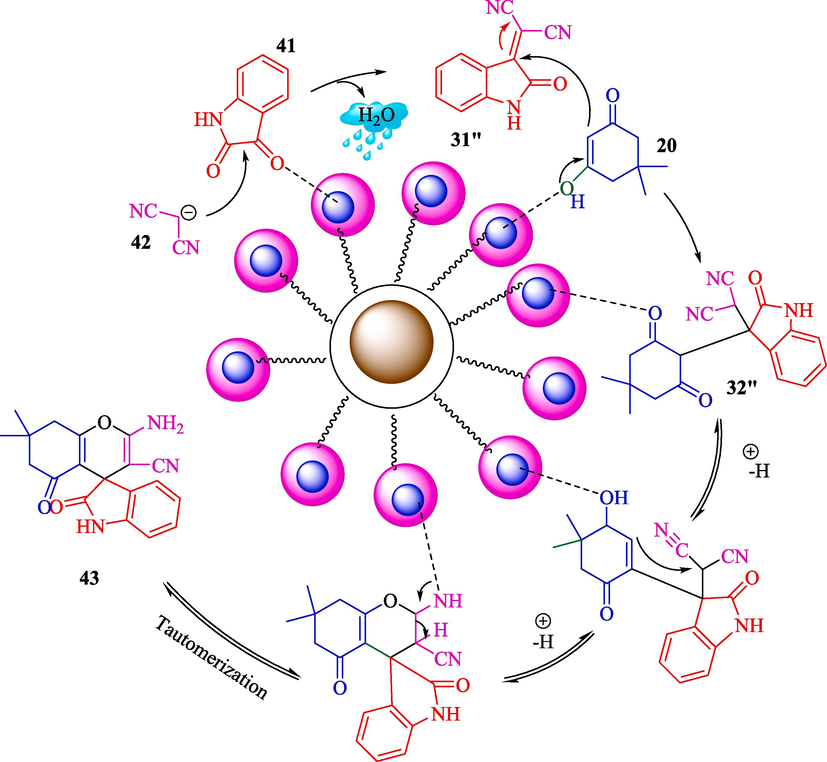
The proposed way to synthesize spirogyra is by using a nanomagnetic catalyst.
An efficient method of the preparation of pyrano[2,3-d]pyrimidine-2,4,7-triones 45 from aryl aldehydes 1, BA (16), and Meldrum acid (44) has been developed by β-CD catalyst by Bhosle et al. (Bhosle et al., 2019). This approach obtained various pyrano[2,3-d] pyrimidine-2,4,7-triones in superb yields (Scheme 33). This reaction was conducted under mild water at 65 °C via a one-pot MCR. Compared to α-and γ-CD, β-CD gives excellent results as a catalyst. α- and γ- CD yield 45 and 39, respectively![The preparation of pyrano[2,3-d]pyrimidine-2,4,7-triones by β-CD.](/content/184/2024/17/10/img/10.1016_j.arabjc.2024.105967-fig34.png)
The preparation of pyrano[2,3-d]pyrimidine-2,4,7-triones by β-CD.
Mohammadian and Akhlaghinia introduced Fe3O4/COS@β-CD-SO3H NPs as highly efficient and recoverable magnetic nanocatalysts for the preparation of spirooxindoles 43 via one-pot, MCR from isatin (41), dimedone (20), and malononitrile (42) in water (Scheme 35) (Mohammadian and Akhlaghinia, 2019). The structure of magnetic nanocatalysts is indicated in Scheme 34. This new magnetic nanocatalyst was characterized by different techniques comprising XRD, FT-IR, EDX, SEM, VSM, TGA, and TEM analyses. The TEM image of magnetic nanocatalysts showed the presence of sphere particles with a size of 16–25 nm. Spirooxindole products were synthesized by reacting various isatins, ethyl cyanoacetate or malononitrile, and 1,3-dicarbonyl compounds. The recoverability of the nanocatalyst was executed eight consecutive times with no noticeable drop in activity. The significant prominent benefits of this approach are easy workup, short-time reaction, catalyst reusability, and high product yields. Table 6 compares the performance of Fe3O4/COS@β-CD-SO3H NPs and other catalysts to prepare spirooxindoles 43. The presented mechanism is shown in Scheme 36. The condensation of isatin 41 with nitrilo-active methylene components 42 to afford 33″. In the next stage, the electron-deficient adduct 33″ is attacked via acid-promoted Michael addition of activated 20 (1,3-dicarbonyl compound) to give intermediate 34″. Further, intramolecular cyclization provides the product 43.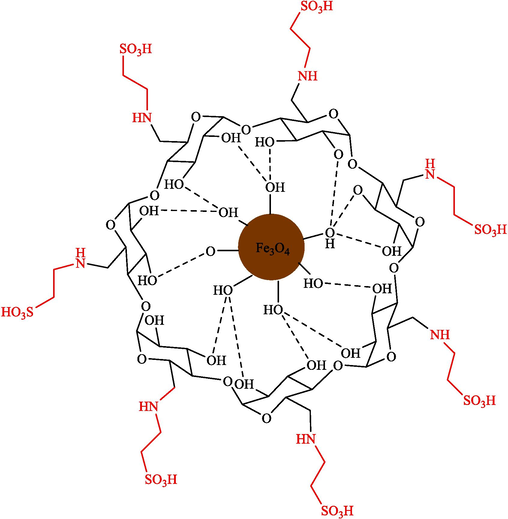
Structure of Fe3O4/COS@β-CD-SO3H NPs catalyst.
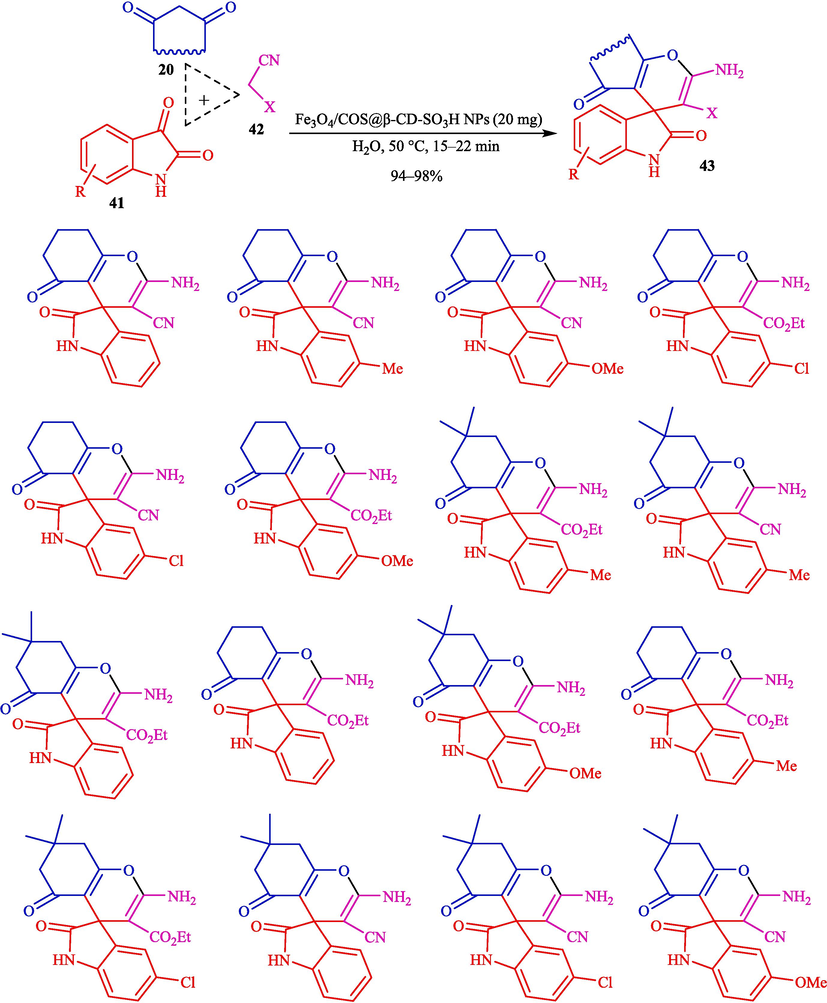
Synthesis of different structurally spirooxindoles using Fe3O4/COS@β-CD-SO3H NPs.
Entry
Catalyst
Time (min)
Solvent
Temperature (°C)
Yield
(%)Reference
1
I2
60
H2O
50
80
Kidwai et al., 2013
2
Cu(OAc)2·H2O
240
–
80
86
Mohamadpour et al., 2016
3
Sodium stearate
180
H2O
60
95
Wang et al., 2010
4
Fe3O4/COS@β-CD-SO3H NPs
20
H2O
50
98
Current discussed report
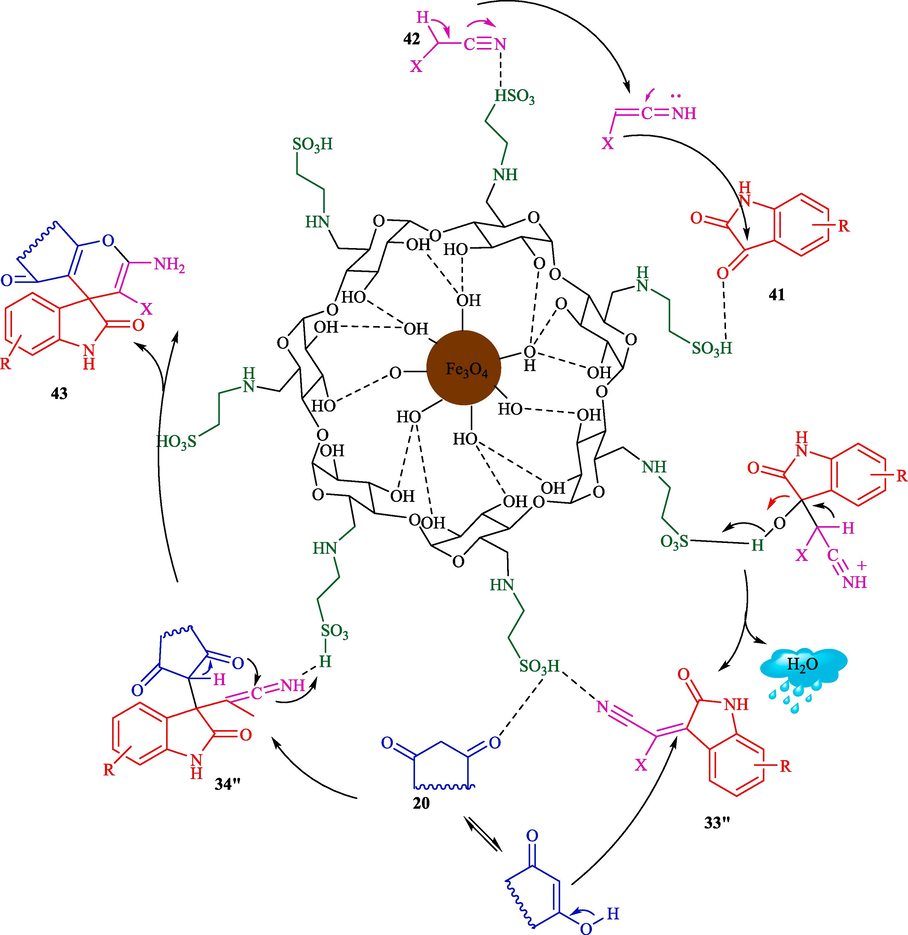
Proposed mechanism for synthesis of spirooxindoles.
Bhosle et al. explained a procedure for the preparation of novel isoniazid fused chromeno[4,3-b]quinolins 48 from isoniazid (46), dimedone (20), 4-hydroxycoumarin (47), and aryl aldehydes 1 using β-CD in the H2O at 60–65 °C (Scheme 37) (Bhosle et al., 2020). This approach provided high yields of the products (71–94 %) via one-pot conditions. The catalyst recovered four runs without a substantial performance drop. The probable reaction mechanism is displayed in Scheme 38. The reaction is possibly performed by forming 35″ and 36″ in the inner cavity of β-CD. Michael's addition happens with the in situ-created intermediate 37″, followed by intramolecular cyclization and dehydration to provide the final product 48.![The preparation of chromeno[4,3‑b]quinolin‑isonicotinamides by β‑CD catalyst.](/content/184/2024/17/10/img/10.1016_j.arabjc.2024.105967-fig38.png)
The preparation of chromeno[4,3‑b]quinolin‑isonicotinamides by β‑CD catalyst.
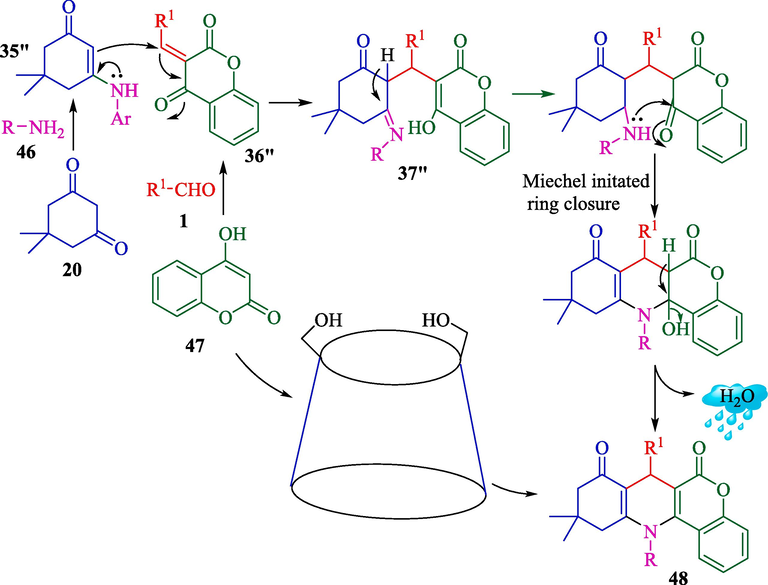
Plausible reaction mechanism for this one-pot reaction.
In 2020, Nipate et al. described for the first time an eco‐friendly strategy for synthesizing 2‐phenyl‐3,4‐dihydroimidazo[4,5‐b] indoles 50 via a one-pot, TCR of aldehyde 1, isatin (41), and NH4OAc (49) by β-CD as a reusable and biodegradable catalyst in EtOH/H2O at 80 °C within 90 min (Scheme 39) (Nipate et al., 2020). This catalyst recycled for four consecutive runs with a slight decrease in activity. This protocol has some advantages, such as a green catalyst, clean reaction, easy recovery of catalyst, and shorter reaction time. The corresponding compounds were gained in 71–95 % yields under mild conditions. The probable reaction mechanism is presented in Scheme 40. In the first stage, the aldehyde that attaches to the OH group of the β‐CD cavity raises the electrophilicity of the carbonyl group reaction, letting it react with ammonia, providing an intermediate 38″. This intermediate 38″ could again react with ammonia, undergoing dehydration and quickly creating a key intermediate 39″. Then cyclocondensation, cyclization, dehydration, and tautomerization generate produce 50.![Synthesis of imidazo[2,3‐b]indoles.](/content/184/2024/17/10/img/10.1016_j.arabjc.2024.105967-fig40.png)
Synthesis of imidazo[2,3‐b]indoles.
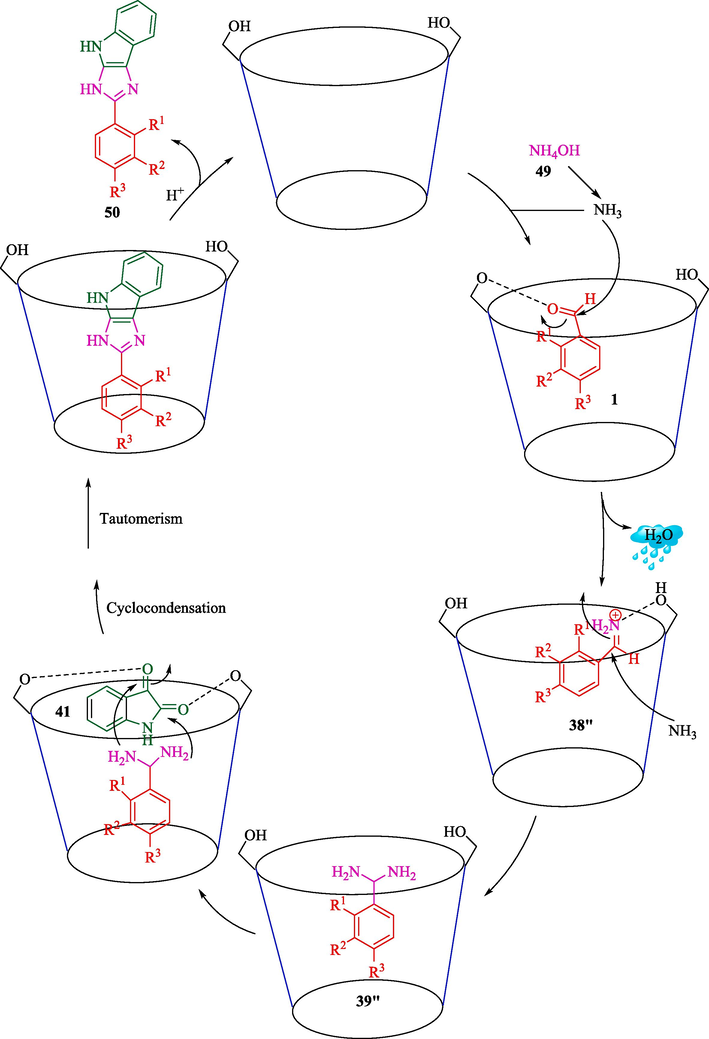
Possible reaction mechanism.
Naeimi and Rahmatinejad reported a convenient approach for the fabrication of 3,4-dihydropyrano[3,2-c]chromenes 52 from aryl aldehyde 1, 4-hydroxycoumarin (47) and ethyl cyanoacetate (51) using Fe3O4 nanoparticles supported on β-CD-guanidine as a reusable, and heterogeneous catalyst (Scheme 42) (Rahmatinejad and Naeimi, 2020). The step of preparation of Fe3O4-β-CD-GA is shown in Scheme 41. FTIR, X-ray diffraction, SEM, TGA, and VSM methods were used to analyze this heterogeneous catalyst. The SEM photo of the catalyst exhibits semi-spherical particles with a moderate size of almost 49 nm. The three-component coupling ethyl cyanoacetate, aryl aldehyde, and 4-hydroxycoumarin were conducted under clean and mild status. The recycling of Fe3O4-β-CD-GA nanoparticles under optimized reaction conditions is assessed. The result indicated that the nanocatalyst was recovered for five successive runs with a minor loss of catalyst activity. This procedure has benefits like easy workup, high yields, low catalyst loading, mild conditions, and catalyst reusability. The various amounts of catalyst were examined for the synthesis 52, and the results are summarized in Table 7. The suggested reaction by the authors is exhibited in Scheme 43. Knoevenagel condensation of activated ethyl cyanoacetate 51 and aldehyde 1 followed by Michel's addition of 4-hydroxycoumarin 47 with the Knoevenagel product 40″ gives intermediate 41″. Then, cyclization of the intermediate 41″ in the existence of the catalyst produces the intermediate 42″, which, through tautomerization, forms the final product 52.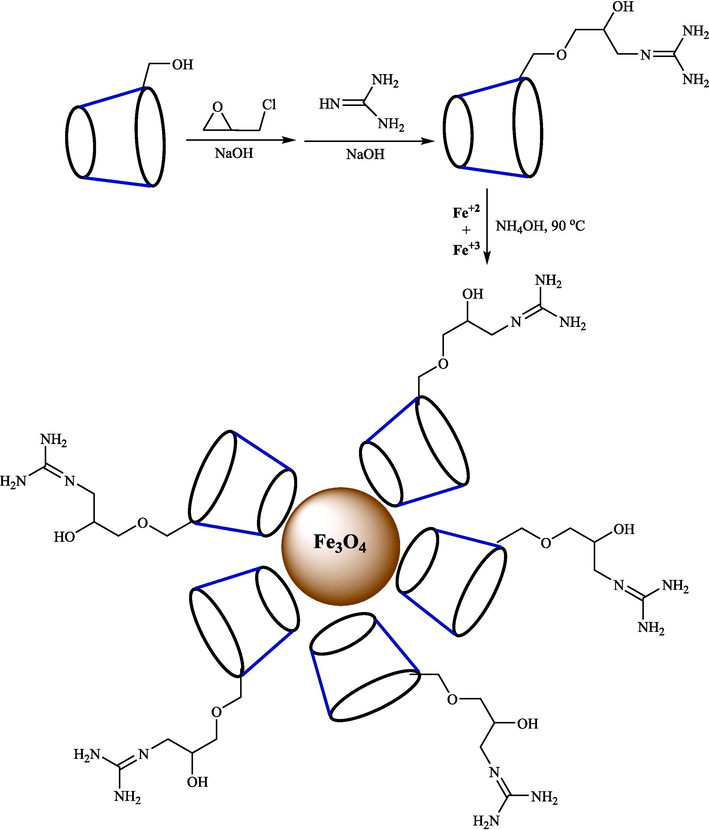
Schematic representation of the synthesis of magnetic nanocatalyst.
![Fabrication of dihydropyrano[3,2-c]chromenes using Fe3O4-β-CD-GA.](/content/184/2024/17/10/img/10.1016_j.arabjc.2024.105967-fig43.png)
Fabrication of dihydropyrano[3,2-c]chromenes using Fe3O4-β-CD-GA.
Entry
Catalyst (mg)
Time (min)
Yield (%)
1
Fe3O4-β-CD-GA (50)
80
89
2
Nano Fe3O4 (40)
180
30
3
β-CD (190)
180
42
4
Guanidine (10)
180
58
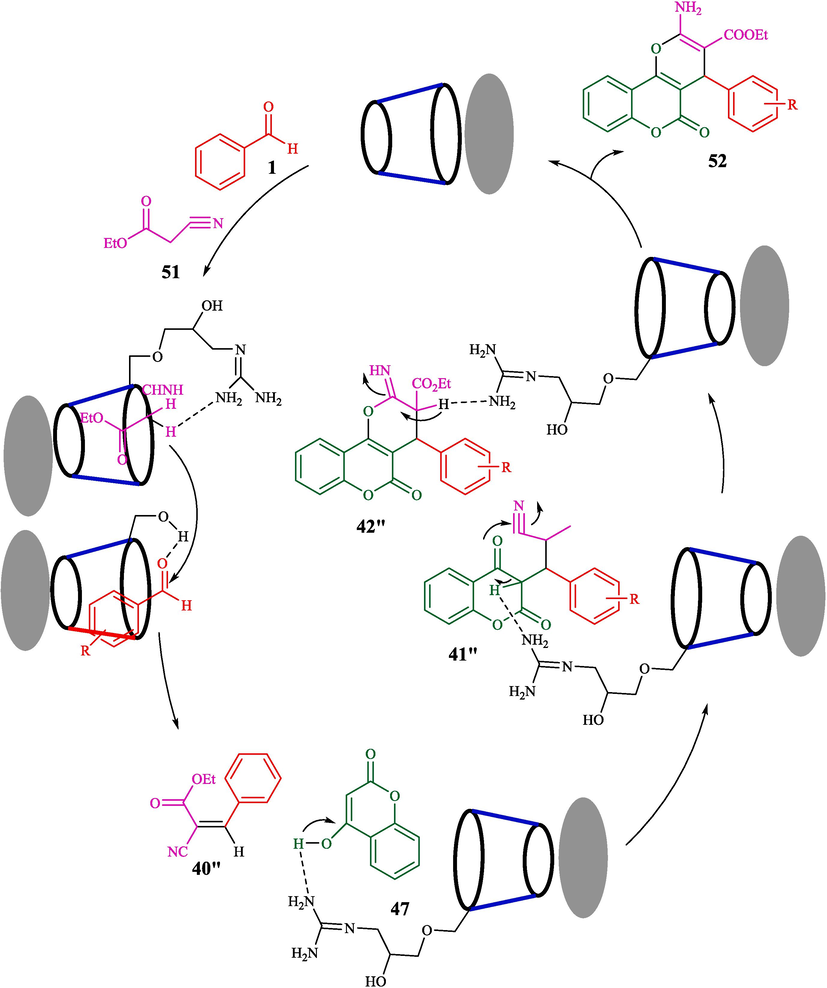
Proposed reaction pathway.
An efficient route to the synthesis of hexahydro-4H-indazol-4-ones 53 was developed by Singh et al. through the MCR of aryl aldehydes 1, hydrazine/phenylhydrazine (25), and 1,3-diketone 20 using β-CD (113 mg, 10 mol%) as a supramolecular catalyst in H2O at 60 °C for 20 min (Scheme 44) (Tiwari et al., 2020). The recycling of β-CD as a catalyst is evaluated. The result displayed that the catalyst was reused five consecutive times with little decrease in its activity. The plausible mechanism for synthesizing hexahydro-4H-indazol-4-ones 53 is presented in Scheme 45. The reaction is performed by activating the C⚌O bond of benzaldehyde 1 through β-CD-assisted construction of the anion 43″. Hydrazine 25 attacks the anion 43″ via the Knoevenagel condensation to create an intermediate 44″, which undergoes Michael's addition of dimedone 20, resulting in cyclization, leading to the building of the product 53.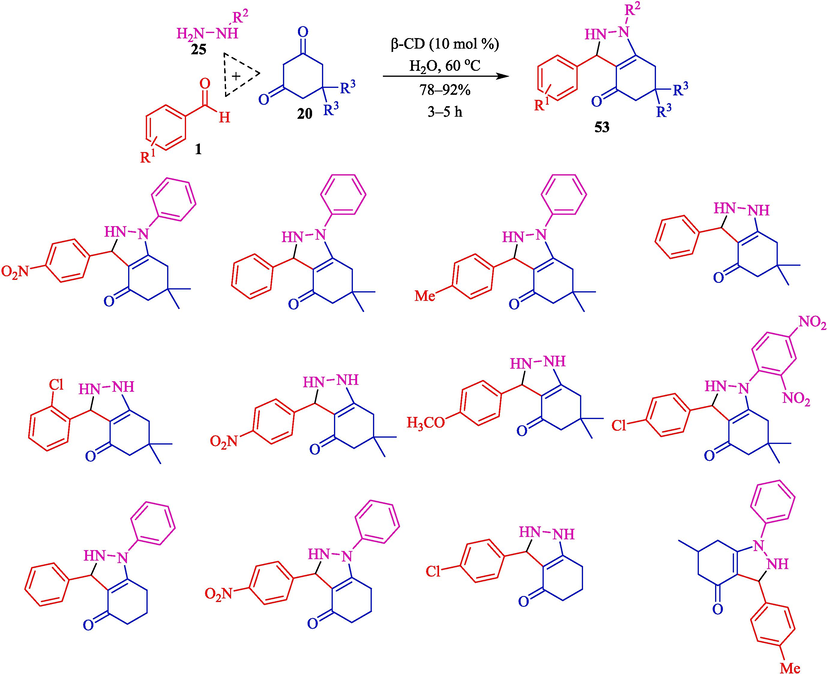
Synthesis of hexahydro-4H-indazole-4-ones 53.
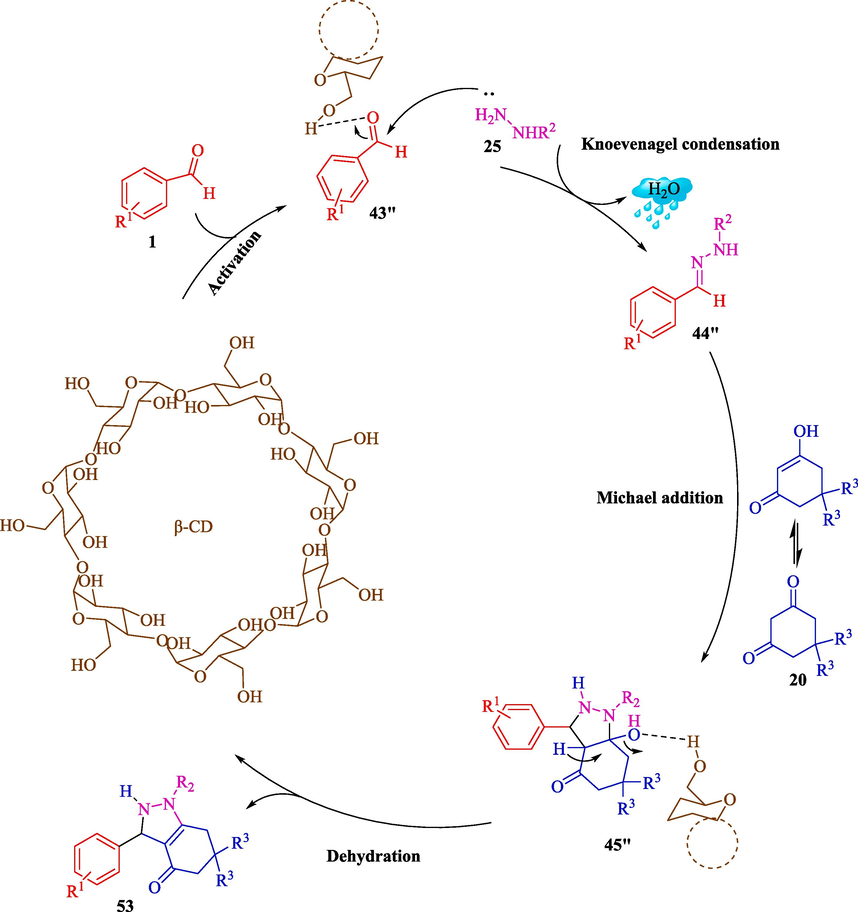
A convenient mechanism for synthesizing hexahydro-4H-indazol-4-ones.
Kardooni et al. discussed a one‑pot synthesis of Kojic acid‑based heterocycles 57 from kojic acid (56), aryl aldehydes 54, and aryl amines 55 using β-CD-based nanosponges as an efficient and biodegradable nanocatalyst via Mannich-type condensation reaction in ethanol (Scheme 47) (Kardooni et al., 2020). This catalyst was synthesized by a reaction of β-CD and dicarbonyl imidazole (as a crosslinker agent) in DMF and was stirred for four hours at 100 °C (Scheme 46). The SEM image showed that β-CDNS has an almost smooth and regular surface with several hollows and cavities. The authors found that the usage of ethanol in the presence of β-CD.NS (0.01 mol%) afforded the Kojic acid‑based heterocycles an excellent yield (80–95 %) within 25–50 min. This procedure has advantages: superior results, a short reaction period, catalyst reusability, and green reaction conditions. The catalyst recyclability was tested, and the performance of the β-CD-based nanosponge was without considerable shifts after three times. The authors suggested the mechanism of Mannich-type tri-compound condensation as displayed in Scheme 48. Here, CD units of nanosponges work as a nanocontainer, which could create the inclusion complex with the starting compound. The reaction selectivity was controlled by intermolecular hydrogen bonding between CD units of nanosponges with the guest molecules, which promoted the condensation reaction.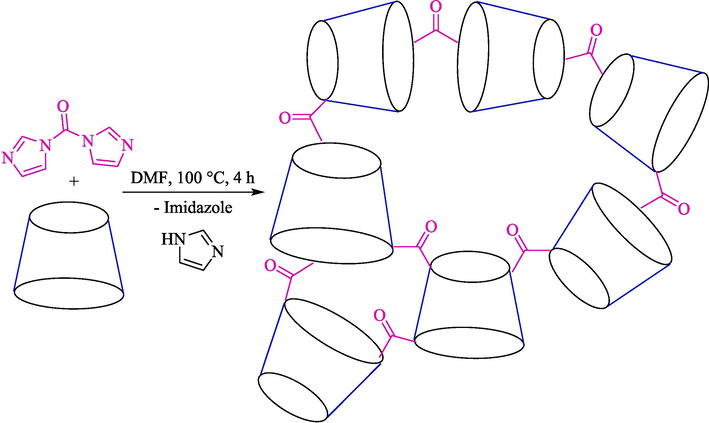
Synthesis of β-CD-based nanosponges.
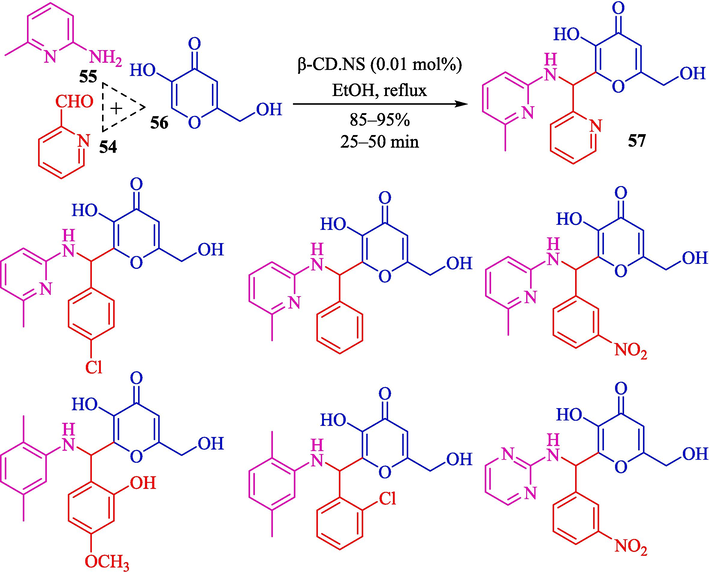
Synthesis of aryl amino kojic acid by β-CD.NS as a catalyst.
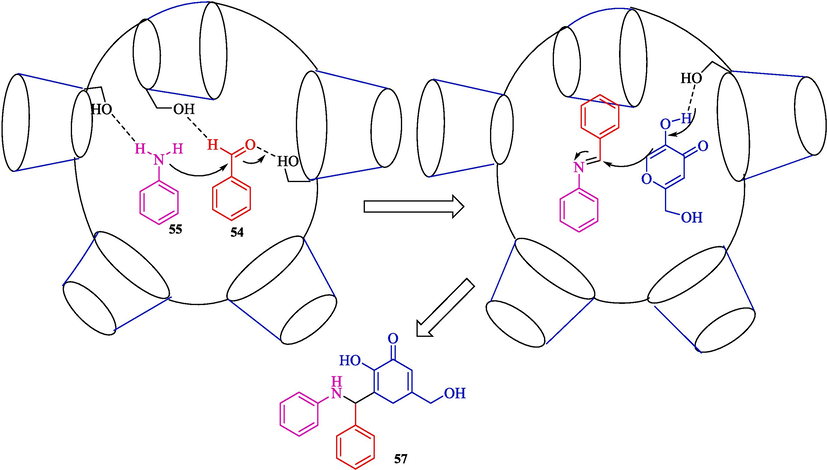
The mechanistic pathway represents the synthesizing of amino Kojic acid derivatives.
Chate et al. disclosed a straightforward preparation of 5-phenyl-5,6-dihydropyrido[2,3-d]pyrimidine-2,4,7(1H,3H,8H)-triones 59 from aryl aldehyde 1, 6-amino uracil (58), and Meldrum acid (44) through host–guest β-CD complex in water at 100 °C (Scheme 49) (Chate et al., 2020). Other CDs were examined, like α-CDs and γ-CD showed 45 and 65 % yield, respectively. This poorer yield is owing to the small or large cavity, which couldn't make inclusion complexes with the substrates. The synthesis of dihydropyrido[2,3-d]pyrimidine-2,4,7(1H,3H,8H)-triones was done in excellent yield (79–97 %) via efficient and environmental protocol. β-CD catalyst was reused several times without a noteworthy drop in catalytic performance. The probable pathway of MCRs depicted in Scheme 50 comprises condensation, Michael addition, cyclization, and elimination steps. A proton from Meldrum acid 44 active by β-CD catalyzes a Knoevenagel condensation with the C⚌O group to provide the arylidene 46″ intermediate. Then adding arylidene intermediate 46″ with 58 cyclized presents the intermediate product 47″. The release of CO2 and acetone generates the product 59.![The formation of 5-phenyl-5,6-dihydropyrido[2,3-d]pyrimidine-2,4,7(1H,3H,8H)-triones 59 using β-CD.](/content/184/2024/17/10/img/10.1016_j.arabjc.2024.105967-fig50.png)
The formation of 5-phenyl-5,6-dihydropyrido[2,3-d]pyrimidine-2,4,7(1H,3H,8H)-triones 59 using β-CD.
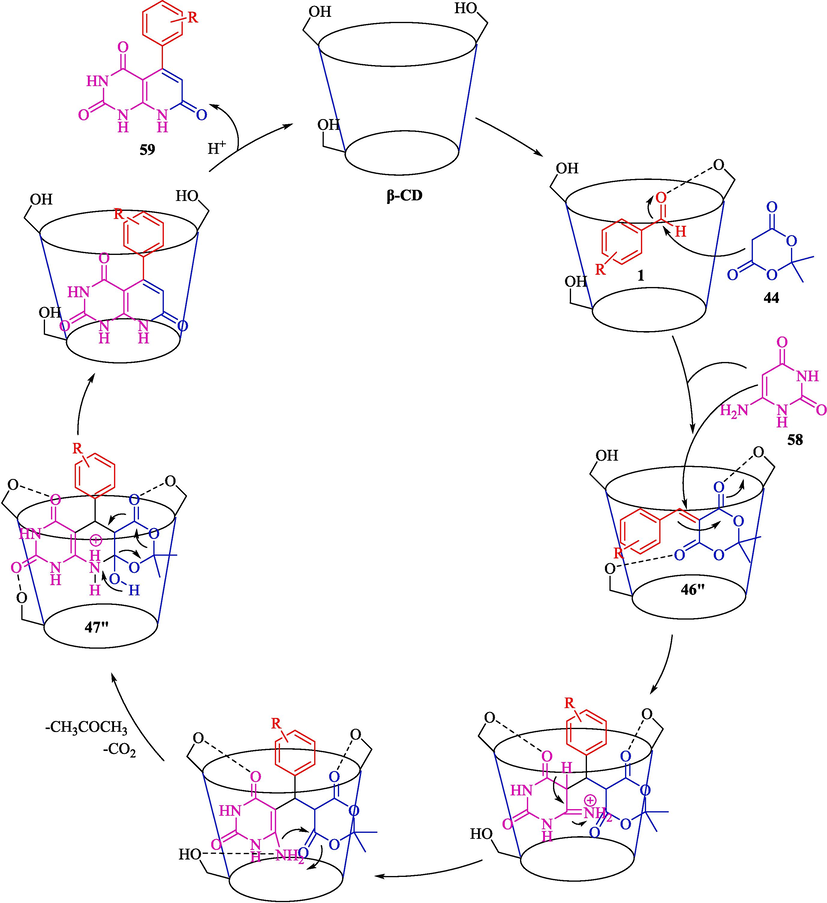
The possible way for the compounds 59 using β-CD.
Avvadukkam et al. reported a solvent-free method for synthesizing pyrano[2,3-d:6,5-d′]dipyrimidines 61 catalyzed by β-CD as efficient catalysts with assist the microwave irradiations (Avvadukkam et al., 2021). The TCR of aryl aldehyde 1, Meldrum's acid (44), and 6-amino-1,3-dimethyluracil (60) were developed, and pyrano[2,3-d:6,5-d′]dipyrimidine products afford good to excellent yields (85–96 %) (Scheme 51). The catalyst was recyclable for up to three runs without a notable change in performance. Some valuable features of this method are short reaction time, good to high yields, no tedious workup, eco-friendly strategy, no need for separation of products, and mild conditions.![The provision of pyrano[2,3-d:6,5-d′]pyrimidines.](/content/184/2024/17/10/img/10.1016_j.arabjc.2024.105967-fig52.png)
The provision of pyrano[2,3-d:6,5-d′]pyrimidines.
In 2021, a simple, eco-friendly, and solvent-free approach for the Hantzsch reaction from aryl aldehyde 1, dicarbonyl compounds (20 and 9), and ammonium acetate (49) was developed by Moheiseni et al. using [β-CD/Im](OTs)2-silica as a catalyst (Moheiseni et al., 2021). This catalyst was prepared in the structure of a dicationic ionic liquid ([β-CD/Im](OTs)2) and supported on the silica gel that the structure of [β-CD/Im](OTs)2-Silica is displayed in Scheme 52. This new catalyst was characterized in various ways. SEM images of this nanocomposite catalyst revealed a layered structure with an enormous surface area. Three-component Hantzsch condensation of aryl aldehydes, ammonium acetate, and dimedone or ethyl acetoacetate was performed. Polyhydroquinolines 62 and 4-dihydropyridines 63 were obtained with excellent yield (84–98 %) (Scheme 53).2-Silica.](/content/184/2024/17/10/img/10.1016_j.arabjc.2024.105967-fig53.png)
The structure of [β-CD/Im](OTs)2-Silica.
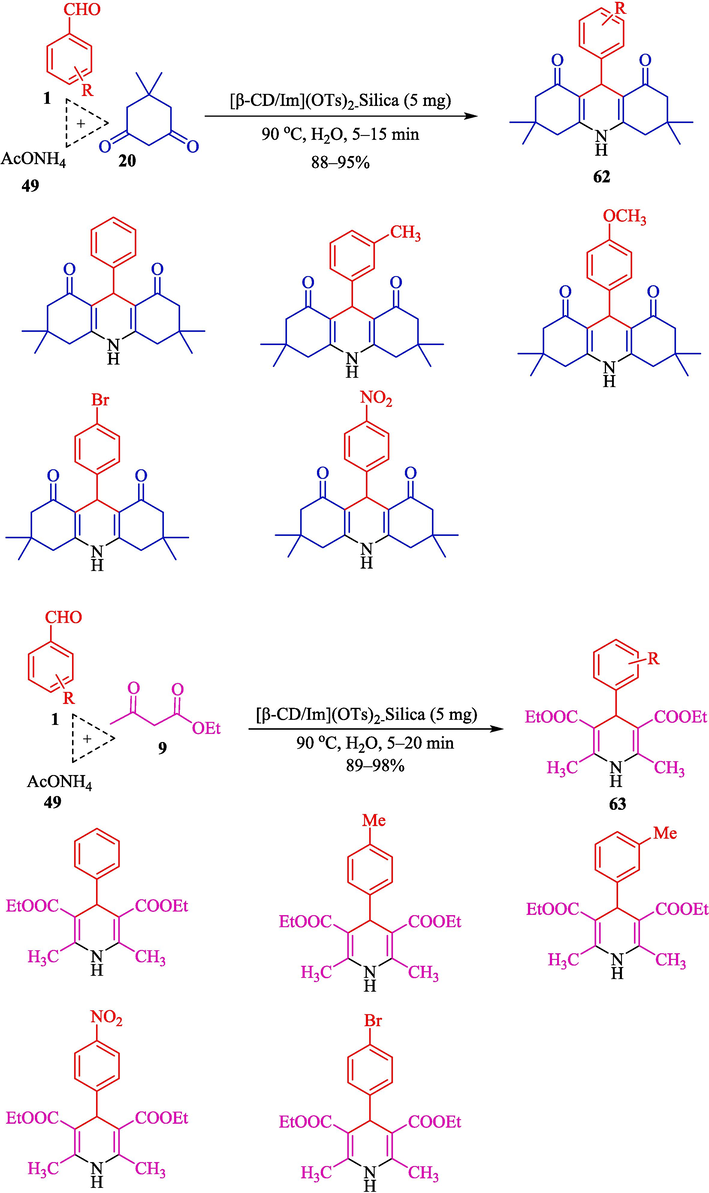
Synthesis of 1,4-dihydropyridines 62 and 63.
Catalyst reusability was conducted in the reaction of ammonium acetate, dimedone, and benzaldehyde, and the catalyst recovered four runs with a slight decline in performance. This protocol has advantages: short-time reaction, clean reactions, perfect yields, and green and reusable catalysts. The comparison of diverse catalysts for the Hantzsch coupling is shown in Table 8.
Entry
Catalyst/condition
Catalyst loading (mg)
Time (min)
Yield (%)
Reference
1
Silica gel/NaHSO4
60
6 h
85
Igder et al., 2015
2
HClO4-SiO2/80 ℃
50
20
95
Maheswara et al., 2006
3
CAN
28
60
92
Ko and Yao, 2006
4
L-Proline
115
30
95
Kumar and Maurya, 2007
5
[β-CD/Im](OTs)2-Silica
5
15
98
Current discussed report
Ghalambaz et al. introduced MCM-41-β-CD/NH2 (Scheme 54) as a heterogeneous catalyst for the high-efficiency synthesis of pyrans 64 through a one-pot, multicomponent process. The TCR of malononitrile (42), aryl aldehyde 1, and 4-hydroxycoumarin (47) lead to the formation of pyrans in 76–88 % yield (Scheme 55) (Ghalambaz et al., 2021). This new catalyst has β-CD and amino basic units with pore channels that preparation through a surfactant-templated sol–gel approach. SEM, TEM, XRD, TGA, TGA, BET, and FT-IR analyzed this heterogeneous catalyst. The TEM image of MCM-41-β-CD/NH2 displayed a particle size of around 200 nm. This nanocatalyst was recycled for five runs without loss of activity. The method has several advantages, including being environmentally friendly, reusability of catalyst, good yields of pyran heterocycle, and short reaction times.
The structure of MCM-41-β-CD.NH2.
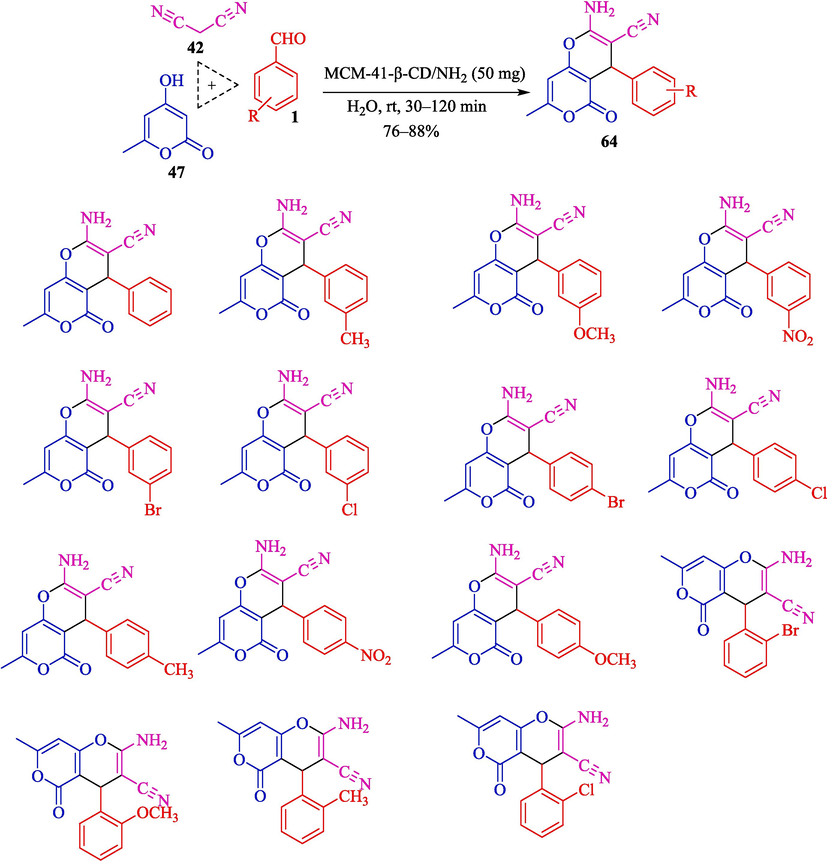
Synthesis of various pyran derivatives by MCM-41-β-CD/NH2.
Mitra et al. explained a metal-free and green protocol for the preparation of 2-amino-4,6- diphenylnicotinonitriles 66 from aryl aldehydes 1, acetophenone (65), malononitrile (42), and ammonium acetate (49) (Scheme 56) (Mitra et al., 2021) and 2,3-dihydroquinazolin-4(1H)-ones 68 from aryl aldehydes 1, isatoic anhydride (67), and ammonium acetate (49) (Scheme 57) via one-pot, MCRs using β-CD as a recoverable catalyst in water and solvent-free conditions. The 2-amino-4,6-diphenylnicotinonitriles and 2,3-dihydroquinazolin-4(1H)-ones were attained in good to high yield (75–94 %). The critical features of this method are that it is an inexpensive, greener protocol without metal catalysts or toxic acid. The authors offered probable mechanisms of both reactions that are shown in Scheme 58 (formation of the pyridine motif) and Scheme 59 (construction of the 2,3-dihydroquinazolin-4(1H)-ones). β-CD as a catalyst activates the aryl aldehyde 1 and acetophenone compound 65 as the active electrophile species. The reaction of malononitrile 42 and ammonium acetate 49 with these two activated electrophiles provides the corresponding intermediates 48″ and 49″, respectively. Then, the reaction between these 48″ and 49″ intermediates will create the corresponding intermediate 50″. After the sequence of tautomerization, cyclization, and again tautomerization generates the product 66 (Scheme 58). In the first step, isotonic anhydride 67 coordinates with the β-CD cavity; the reaction of ammonium acetate (49) forms a 51″ intermediate, which is then isolated. The following step facilitates the nucleophilic attack by the electron-rich nitrogen of the NH2 group to the electrophilic carbonyl carbon center of aldehydes 1, which β-CD activates. Then, the elimination of H2O, followed by another nucleophilic attack by the NH2 group of the amide to the carbon center on the substituted imine 52″ leads to the expected product 68 (Scheme 59).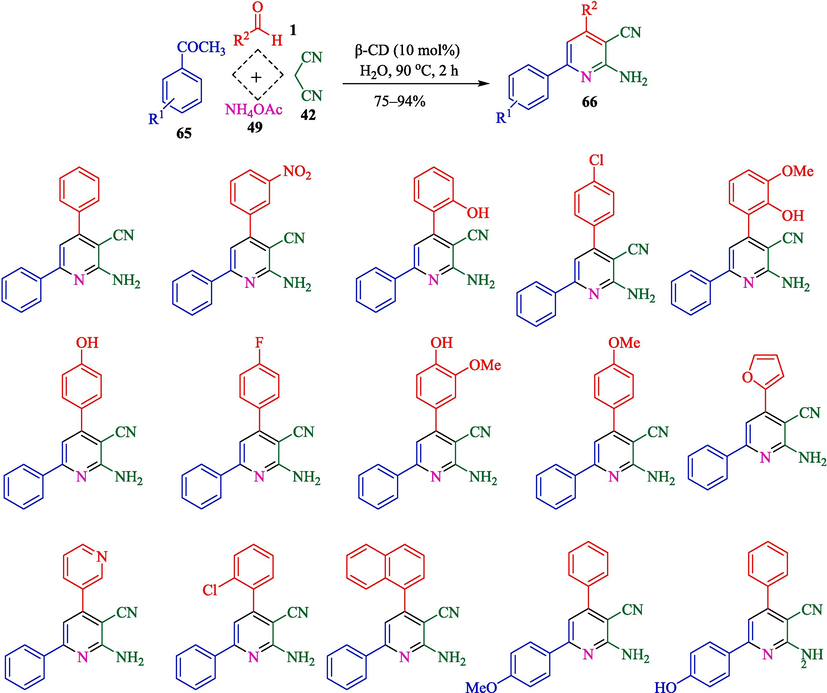
Preparation of diversified 2-amino-4,6-diphenylnicotinonitriles.
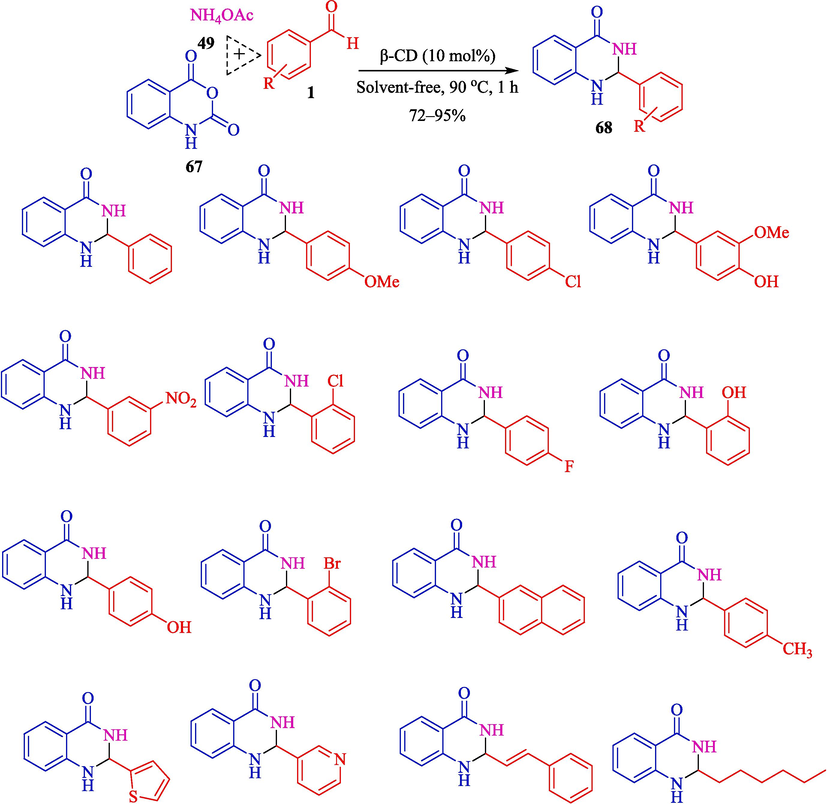
Fabrication of the 2,3-dihydroquinazolin-4(1H)-ones.
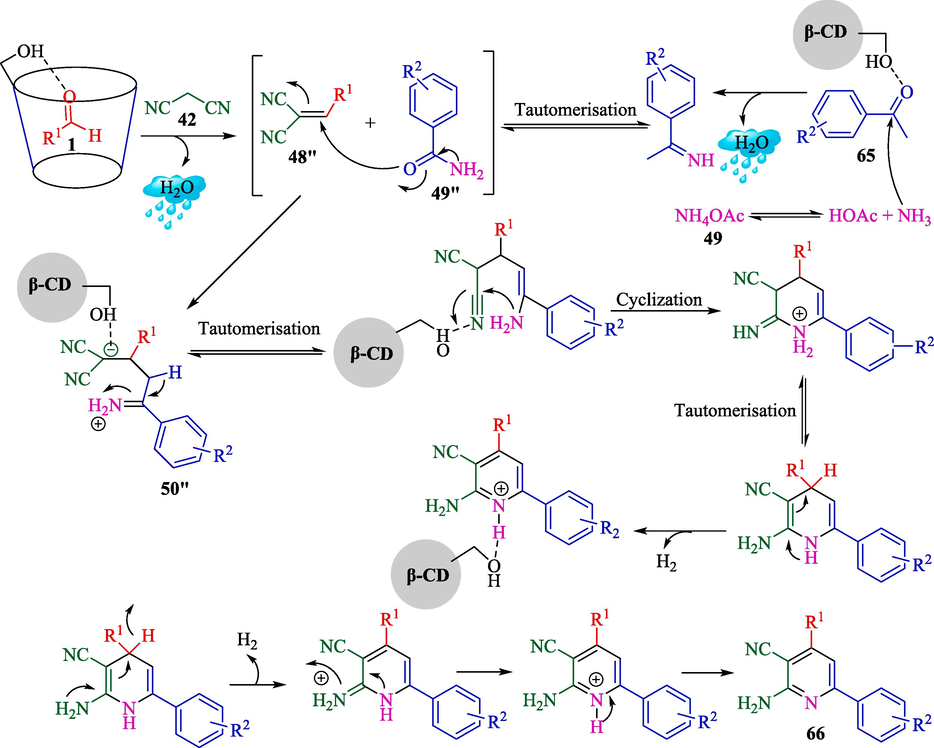
The suitable pathway for synthesizing pyridine motif using β-CD catalyst.
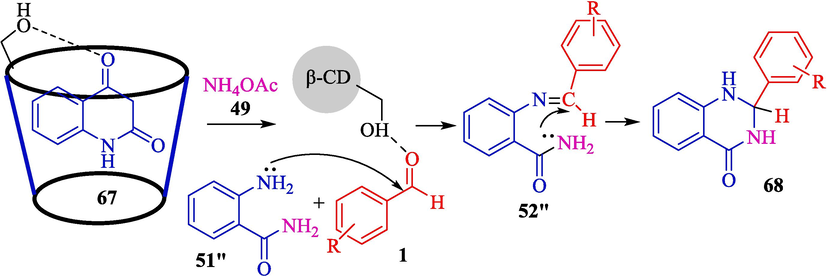
The stepwise synthesis pathway of 2,3-dihydroquinazolin-4(1H)-ones.
In 2022, Jadhav and co-workers designed an efficient approach for synthesizing 2-amino-4H-pyranoquinolines 70 from TCR of aryl aldehydes 1, 8-hydroxyquinoline (69) and malononitrile (42), or ethyl cyanoacetate by β-CD as a robust catalyst in water under ultrasound irradiation (Jadhav et al., 2022). The 2-amino-4H-pyranoquinolines were provided in 84–93 % yield (Scheme 60). They presented a gram-scale synthetic method for synthesizing 2-amino-4H-pyranoquinolines with perfect results. β-CD was recovered in four cycles with no notable loss in activity. Using water (a greener solvent), recycling the catalyst, and synthesizing pyranoquinolines, essential in pharmaceutical chemistry, are the main advantages of this work. The suggested pathway for the reaction is displayed in Scheme 61. Firstly, the Knoevenagel condensation of the aryl aldehyde 1 with the active methylene compound 42 generates the arylidene adduct 53″. Then, Michael's addition of 69 to the Knoevenagel adduct 53″ yields an intermediate 54″, which undergoes aromatization followed by intramolecular cyclization facilitated by β-CD to the C–N triple bond to provide the cyclic intermediate 55″ via intermediate 54″. Ultimately, 70 products are created in situ by tautomerization of the imino group to an amino group.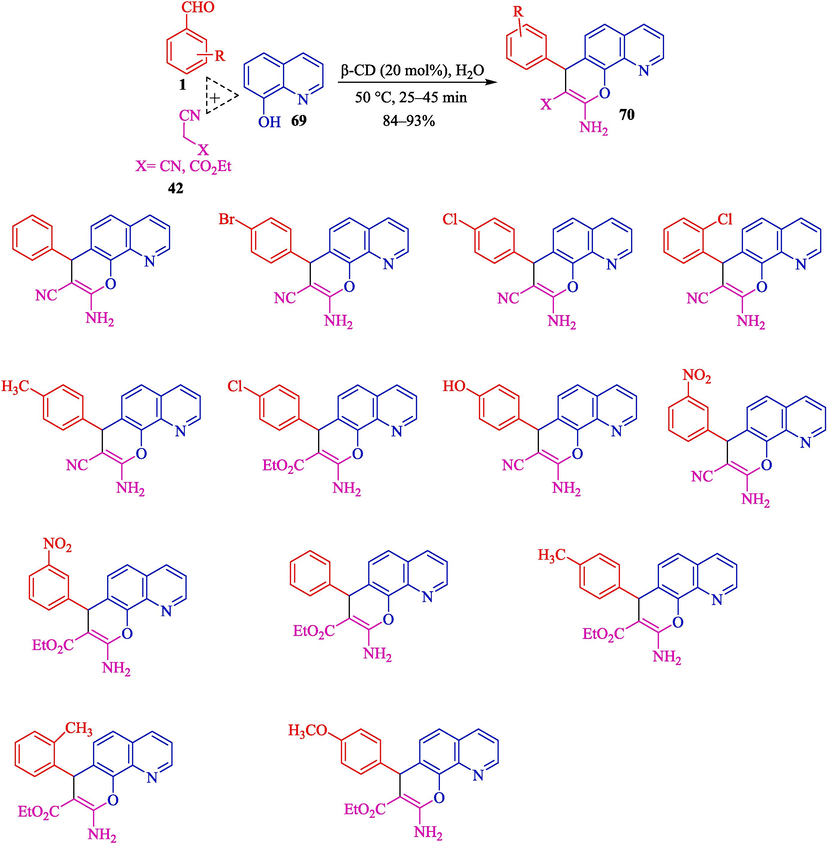
The synthesis of pyranoquinolines by β-CD catalyst.
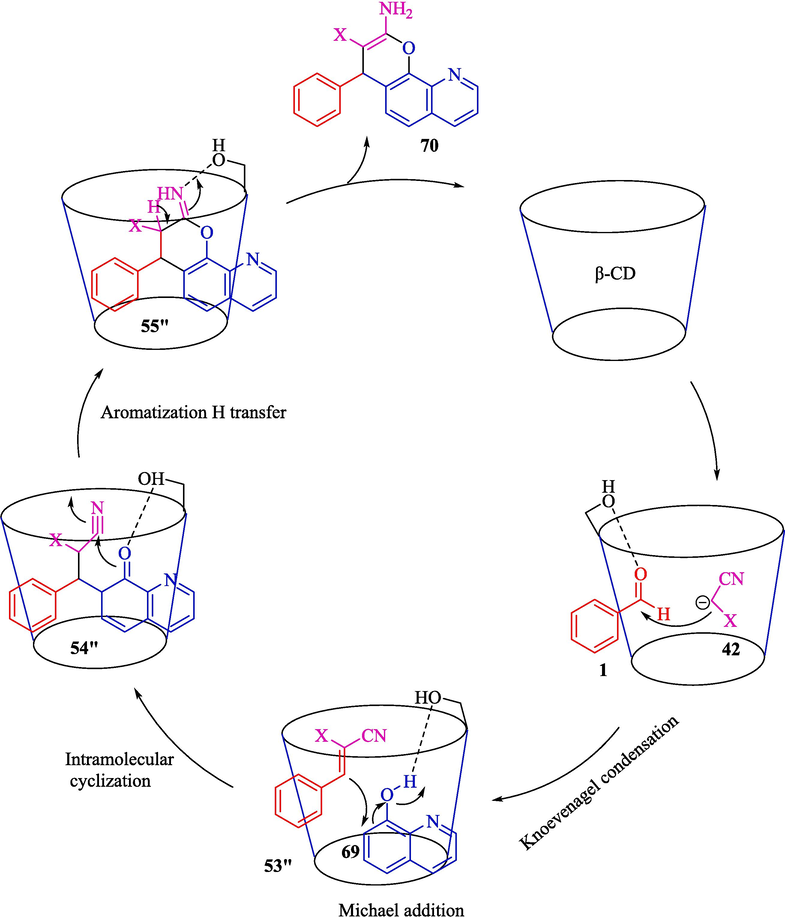
The suitable mechanism for the reaction.
During the same years, Wang et al. used β-CD-SO3H as a reusable catalyst for synthesizing tetrahydrobenzo [4,5] imidazo[2,1-b] quinazolin-1(2H)-ones in water (Wang et al., 2022). This approach is efficient and green for the preparation of tetrahydrobenzo[4,5]imidazo[2,1-b]quinazolin1(2H)-ones series in high yields through a MCR using β-CD-SO3H under mild status. The best procedure for synthesizing these compounds is the high product yields, higher atom efficiency, and reusability of β-CD-SO3H.
Liao et al. explained the preparation of indeno[1,2-b]quinoxalines 73 from o-phenylenediamine (OPD, 71) and 2-indanones 72 using β-CD as an excellent catalytic (Scheme 62) (Liao et al., 2022). Optimization of the reaction status was studied, and the optimal catalytic consisted of β-CD (15 mol%) in H2O at room temperature within 12 h. This approach is milder, easier, and slightly toxic than previous methods, leading to an eco-friendly option. The β-CD can be recycled and reused for four consecutive runs with a slight drop in activity. The superior advantages of the current method are excellent yields, environmental friendliness, reusable catalyst, low cost, and a broad substrate scope, making it a strong strategy for synthesizing indeno[1,2-b]quinoxalines. The mechanism proposed for the reaction is displayed in Scheme 63. First, OPD is included in the cavity of β-CD to create the OPD-β-CD inclusion complex. Then, the first Schiff base reaction happens, releasing a mole of H2O to form an intermediate 56″. The generated intermediate 56″ is immediately oxidized by oxygen in the O2 to form the intermediate 57″, leading to the second Schiff base reaction. After cyclization and removal, a mole of H2O product 73 was generated.![Synthesis of indeno[1,2-b]quinoxalines by β-CD catalyst.](/content/184/2024/17/10/img/10.1016_j.arabjc.2024.105967-fig63.png)
Synthesis of indeno[1,2-b]quinoxalines by β-CD catalyst.
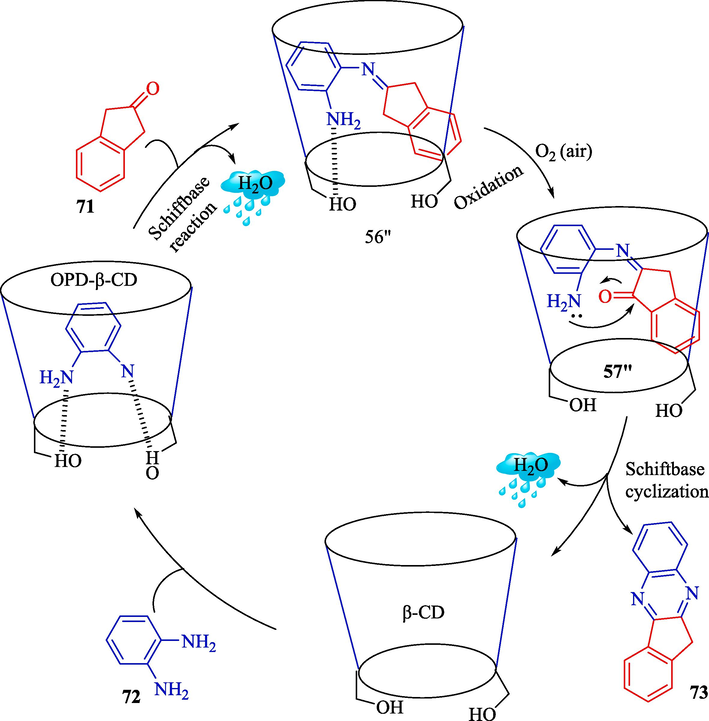
A possible mechanism for the reaction.
Xu's group illustrated a suitable procedure for forming spiroindolines by β-CD-SO3H in water at 50 °C. Under mild conditions, a one-pot, TCR of various isatin 41, diketones 74, and malononitrile (42) was carried, and spiroindoline products 75 were obtained in 38–99 % yields (Scheme 64) (Xu et al., 2022). β-CD-SO3H displayed high catalytic activity that was successfully recycled five times with no notable effect on the product outcomes. A comparison of catalytic performance β-CD–SO3H with the formerly reported catalysts for the synthesis of spiroindoline 75 is shown in Table 9.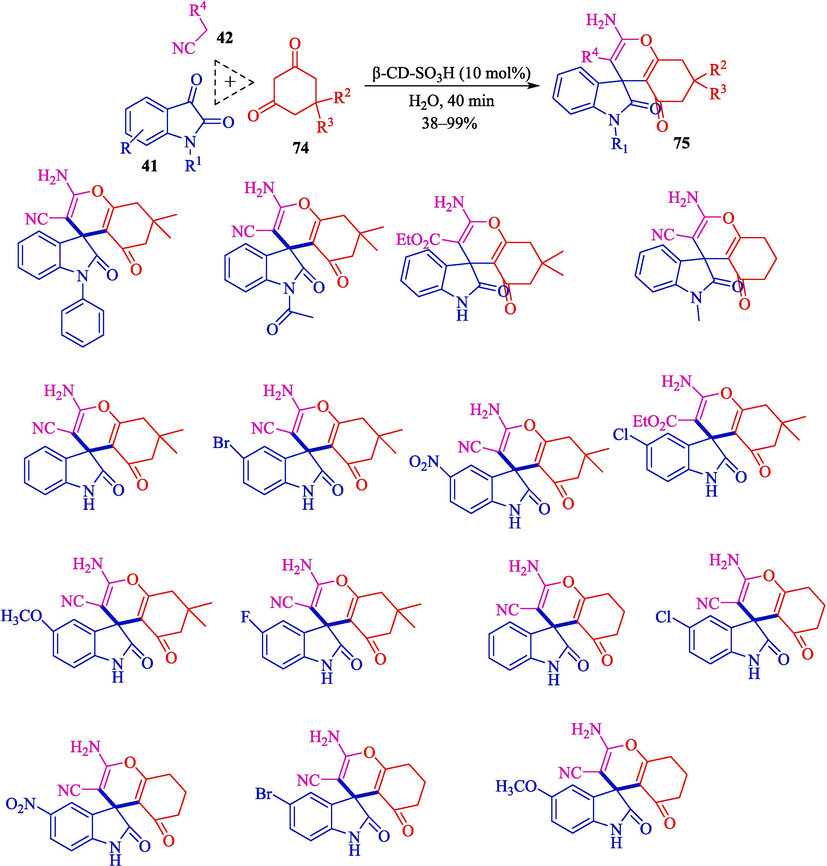
Synthesis of drivers spiro indole derivatives by β-CD-SO3H catalyst.
Entry
Catalyst
Temperature
(℃)Time (min)
Yield (%)
Reference
1
Hexamethylenetetramine
60
20–150
60–93
Wang et al., 2013
2
Nanocrystalline MgO
80
90–180
80–95
Karmakar et al., 2012
3
Silica sulfuric acid magnetic NPs
60
80–120
90–95
Karimi et al., 2015
4
β-CD–SO3H
50
40
38–99
Current discussed report
Mohamadpour designed a co-friendly three-compound method for synthesizing the 2-amino-4H-chromene frameworks 77 by Knoevenagel-Michael cyclocondensation under solvent-free in perfect yield (80–96 %) (Mohamadpour, 2022a, 2022b). The reaction of aryl aldehydes 1, malononitrile (42), and resorcinol (76) was developed by per-6-amino-β-CD as a helpful catalyst at room temperature (Scheme 65). This catalyst is stable enough for six successive runs without a remarkable decline in structure and activity. This approach has the advantages of green and solvent-free reaction conditions, easy workup, superior yields, and recoverable catalysts. The suggested path for synthesizing 2-amino-4H-chromene scaffolds is indicated in Scheme 66. Knoevenagel condensation occurs between active methylene 42 compound and aryl aldehyde 1leading to intermediate 58″. Per-6-NH2-β-CD also catalyzed the resorcinol 76 attack on intermediate 58″ as Michael acceptor to provide 59″, which, after cyclizing and tautomerizing, aims the objective products 77.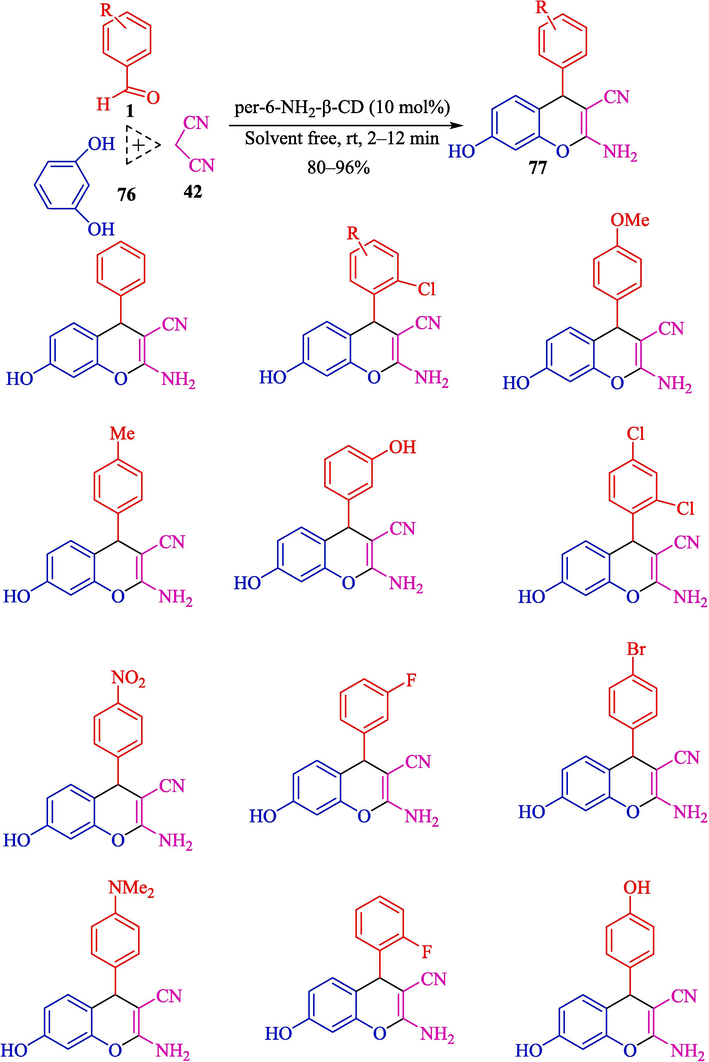
Preparation of 2-amino-4H-chromenes scaffolding by per-6-NH2-β-CD.
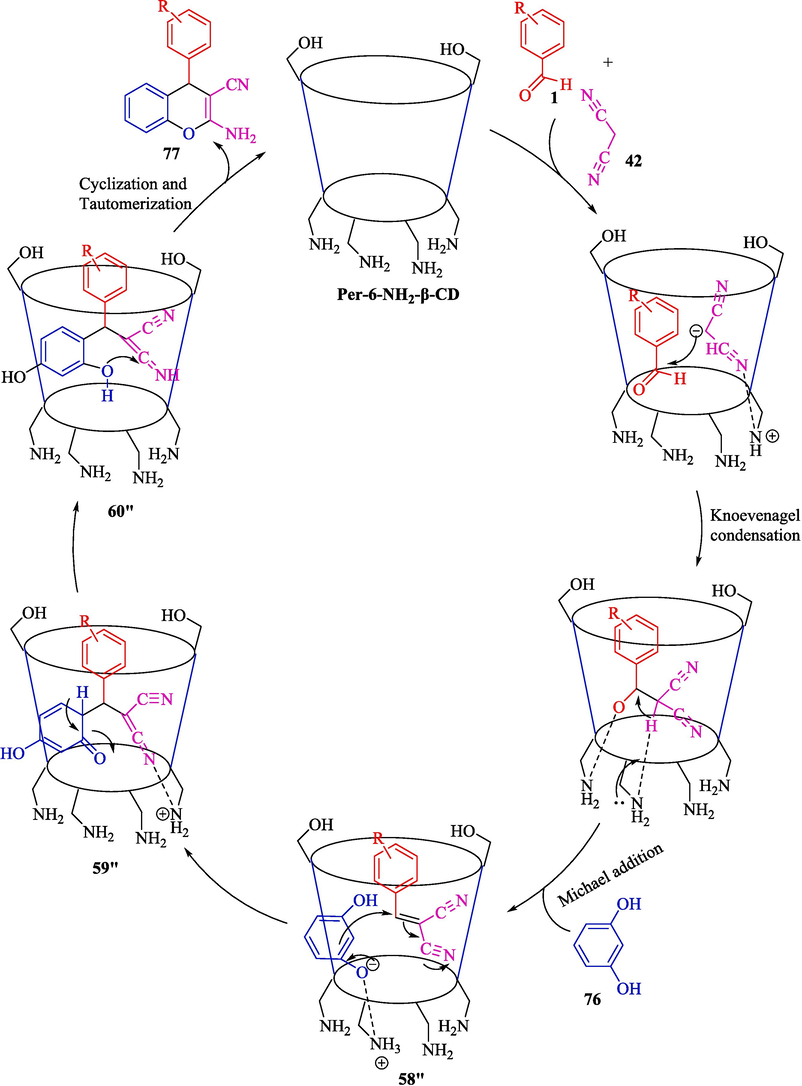
Suggested path for the reaction.
A highly effective, suitable approach has been designed for synthesizing pyrano[2,3-d]pyrimidines 78 by β-CD as a significant catalyst in water media published by the same researcher (Mohamadpour, 2022a, 2022b). Three-component Knoevenagel-Michael addition cyclo condensation reaction of malononitrile (42), aryl aldehydes 1, and BA/1,3-dimethylbarbituric acid 16 for the preparation of pyrano[2,3-d] pyrimidines were carried out and expected products obtained in 76–95 % yields (Scheme 67). The catalytic recyclability of β-CD was studied so that the catalyst could be recovered three times without a considerable drop in activity. β-CD is a highly stable catalyst that, after reaction, the structure of it doesn't change. The use of a biodegradable catalyst, high catalyst stability, easy workup, avoidance of toxic solvents, and catalyst recyclability are several of the principal features of this work. The proposed mechanism for the Knoevenagel–Michael–cyclo condensation is indicated in Scheme 68. The intermediate cyano olefin 61″ was readily created in situ from Knoevenagel condensation between β-CD solubilized aryl aldehyde 1 and active methylene compound 42 in H2O. β-CD also catalyzed the construction of the enolic form of barbituric acid 16, which could readily react with cyano olefin 61″ and provide intermediate 62″, followed by cyclization and tautomerization of 63″ and 64″ afford the products 78.![The preparation of pyrano[2,3-d]pyrimidines by β-CD catalyst.](/content/184/2024/17/10/img/10.1016_j.arabjc.2024.105967-fig68.png)
The preparation of pyrano[2,3-d]pyrimidines by β-CD catalyst.
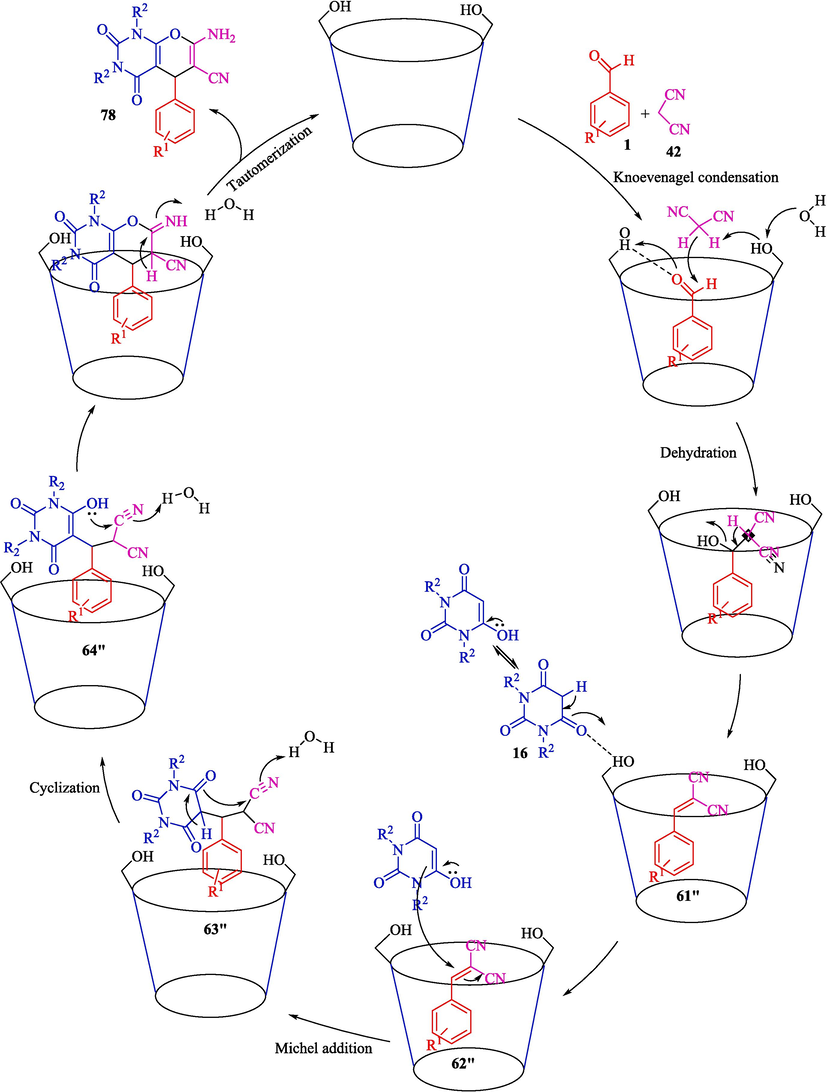
A suggested mechanistic path for the reaction.
In 2023, this researcher illustrated the preparation of xanthene derivatives 80 from β-naphthol (79), aryl aldehyde 1, and dimedone (20) by β-CD as reusable in the water media (Mohamadpour, 2023). In general, the reaction was performed by β-CD (15 mol%) in H2O solvent at 80 °C, and xanthene products were given in good yield (91–94 %) (Scheme 69). β-CD could be recovered four times without a crucial reduction in catalytic performance. This approach had excellent yields with short-period reactions. The plausible mechanism is shown in Scheme 70. The enol tautomer of β-naphthol 79 reacted with the activated aldehydes 65″. After creating intermediates (66″and 67″), it generated the products 80.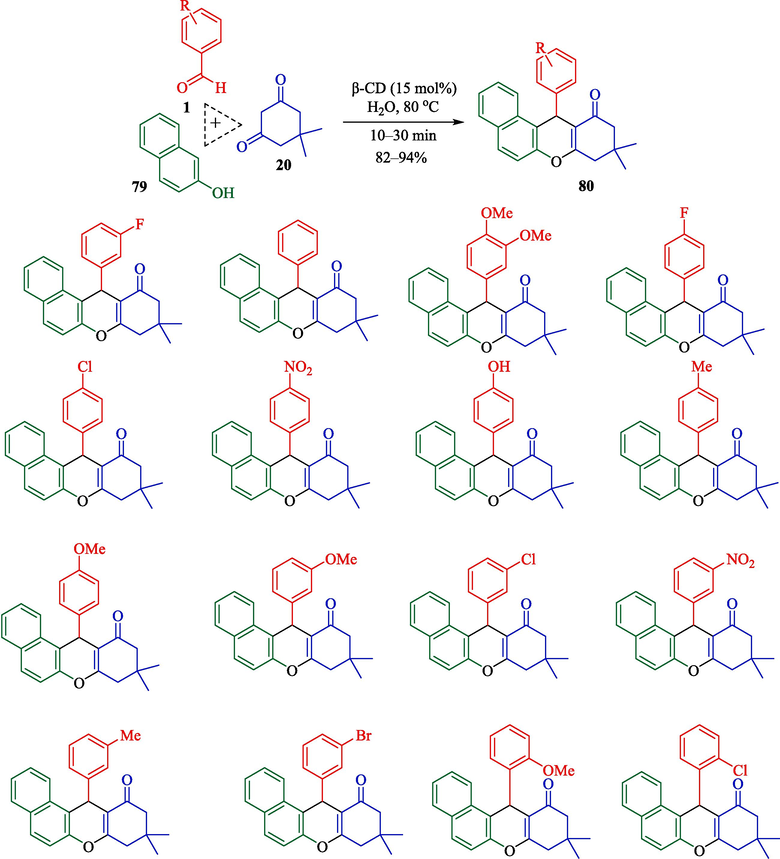
Synthesis of xanthene products using β-CD catalyst.
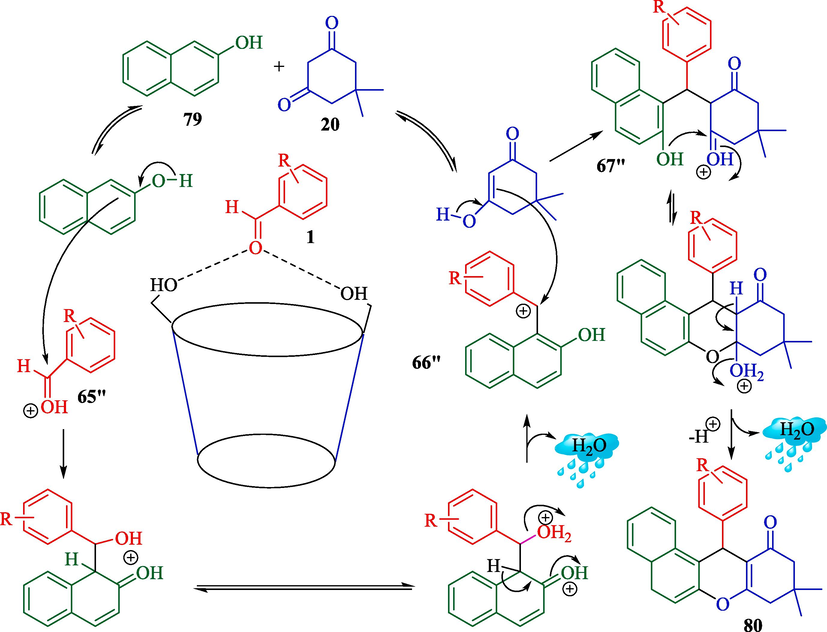
The proposed way for the preparation of xanthenes by β-CD.
2.3 Synthesis of seven-membered heterocycles
Masram et al. used a green protocol to synthesize four 1,5-benzodiazepines 81 under mild status involving β-CD as a recyclable catalyst in H2O at reflux conditions (Scheme 71) (Masram et al., 2022). The TCR of OPD (71), dimedone (20), and aryl aldehydes 1 is developed to provide good to excellent yields (93–98 %). Other CDs were also tested as catalysts. α- and γ- CD showed a lower result of the product than the β-CD. The catalyst was recovered in four runs without lowering the yield of the product. The excellent yields, easy workup, shorter reaction time, green catalyst, and lack of use of dangerous material are some advantages of this work. The plausible mechanism is displayed in Scheme 72. At first, dimedone 20 attaches to the OH group of β-CD, increasing the electrophilicity of the C⚌O group reaction. Then it reacts with 81, which subsequently provides an intermediate of imine 68″. In the next step, aldehyde carbonyl is attacked by the o-phenyl diamine amine group, which further undergoes tautomerism to create the intermediate 69″. After further cyclization, the final product 82 is generated.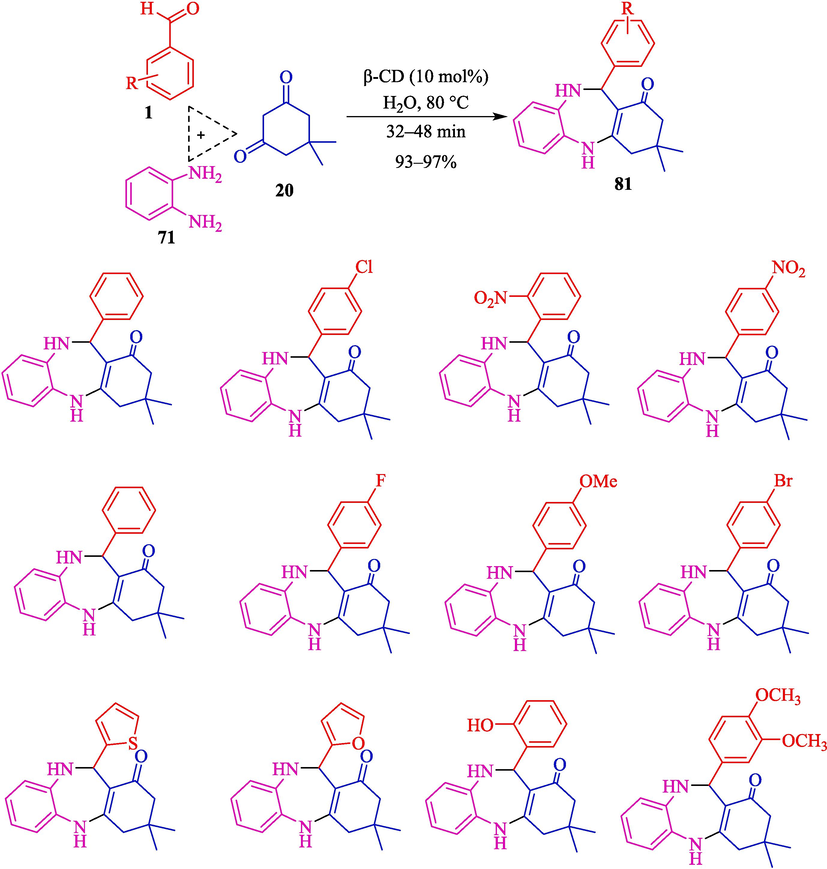
Preparation of 3,4-dihydropyrimidine-2(1H)-ones catalyzed by β-CD.
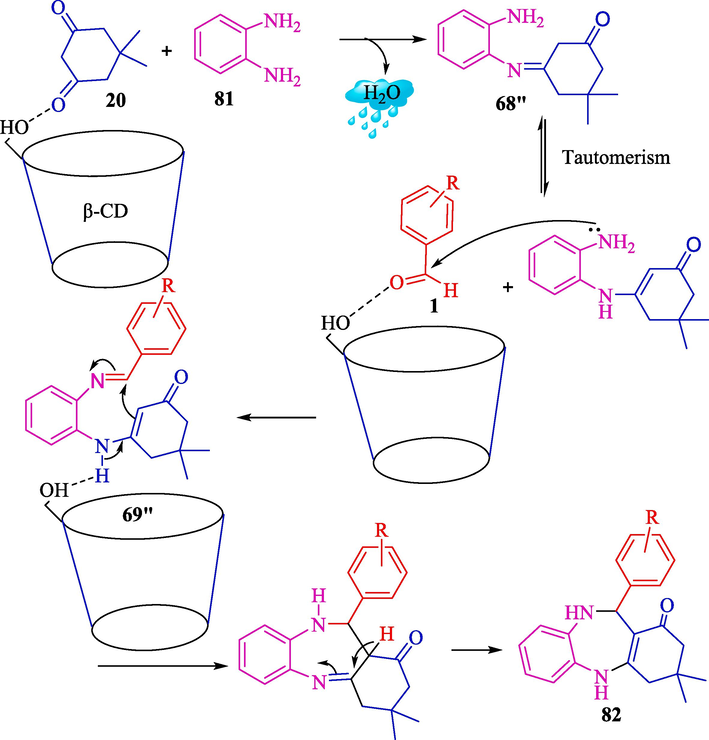
The putative mechanism for the preparation of compounds 82.
3 Conclusions
In summary, we have reported an overview of the usage of β-CD as a helpful catalyst in synthesizing diverse heterocyclic structures and printed in the last five years. It is significant to mention that a broad spectrum of the reactions catalyzed by β-CD can be performed in the H2O media, making those methods green and eco-friendly. One of the most significant advantages of β-CD catalyst is that it can be recycled and reused many times with no drop in efficiency. This kind of organic reaction has recently found more and more applications in synthesizing pharmacological and natural compounds. There is a rising attraction to using β-CD catalysts, as they play a significant role in preparing medical and organic heterocyclic material. MCR has received much interest in preparing various biological compounds for developing new drugs. Heterocyclic compounds have been a fundamental structure in medical chemistry for many years. They indicate various biological activities comprising anti-bacterial, anti-cancer activity, anti-diabetic, and anti-inflammatory. They are plentiful in many biomolecules, such as natural products, enzymes, and vitamins. Heterocyclic compound chemistry has become a critical field because of the connection between their chemistry and medical issues. This review will investigate the recent developments, catalysts based on β-CD, and their applications in heterocyclic structures. This review will benefit and be invaluable for pharmaceutical chemistry and drug design development researchers. One of the most notable attainments in this study has been helping scientists develop green and low-price catalysts based on green chemistry aims in organic reactions.
CRediT authorship contribution statement
Sara Payamifar: Writing – original draft, Investigation. Majid Abdouss: Supervision. Ahmad Poursattar Marjani: Writing – review & editing, Supervision, Investigation.
Acknowledgments
The authors would like to acknowledge the support from the Research Council of Urmia University and Amirkabir University of Technology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- β-Cyclodextrin as an efficient and recyclable supramolecular catalyst for the synthesis of heterocyclic compounds. J. Chin. Chem. Soc.. 2017;64:896-917.
- [CrossRef] [Google Scholar]
- Green chemistry: principles, applications, and disadvantages. Chem. Methodol.. 2020;4:408-423.
- [CrossRef] [Google Scholar]
- Microwave-assisted synthesis of bioactive heterocycles: an overview. Tetrahedron. 2022;126:133085
- [CrossRef] [Google Scholar]
- Cu(II)-β-cyclodextrin complex stabilized on magnetic nanoparticles: a retrievable hybrid promoter for green synthesis of spiropyrans. Appl. Organomet. Chem.. 2019;33:e4738.
- [CrossRef] [Google Scholar]
- Synthesis, antimicrobial and antioxidant activities of novel 7-thioxo-4,6,7,8-tetrahydro-pyrazolo[4',3':5,6]pyrano[2,3-d]pyrimidin-5(1H)-ones derived by SDS catalyzed multicomponent reactions in aqueous micellar media. J. Nat. Prod.. 2018;8:228-238.
- [CrossRef] [Google Scholar]
- Ultrasound-assisted β-cyclodextrin catalyzed one-pot cascade synthesis of pyrazolopyranopyrimidines in water. Catal. Lett.. 2020;150:450-460.
- [CrossRef] [Google Scholar]
- Synthesis of some transition metal complexes with new heterocyclic thiazolyl azo dye and their uses as sensitizers in photo reactions. J. Mol. Struct.. 2016;1108:378-397.
- [CrossRef] [Google Scholar]
- Heterocyclic scaffolds: centrality in anticancer drug development. Curr. Drug Targets.. 2015;16:711-734.
- [Google Scholar]
- Origins, current status, and future challenges of green chemistry. Acc. Chem. Res.. 2002;35:686-694.
- [CrossRef] [Google Scholar]
- Gravimetric, electrochemical, and morphological studies of an isoxazole derivative as corrosion inhibitor for mild steel in 1M HCl. Arab. J. Chem.. 2020;13:7744-7758.
- [CrossRef] [Google Scholar]
- A facile synthesis of pyrano[2,3-d:6,5-d′]dipyrimidines via microwave-assisted multicomponent reactions catalyzed by β-cyclodextrin. J. Heterocycl. Chem.. 2021;58:724-736.
- [CrossRef] [Google Scholar]
- Synthesis and antibacterial evaluation of novel heterocyclic compounds containing a sulfonamido moiety. Molecules.. 2013;18:832-844.
- [CrossRef] [Google Scholar]
- The use of magnetic starch as a support for an ionic liquid-β-cyclodextrin based catalyst for the synthesis of imidazothiadiazolamine derivatives. Int. J. Biol. Macromol.. 2019;135:453-461.
- [CrossRef] [Google Scholar]
- Efficient one-pot synthesis of phenylimidazo[1,2-a]pyridine derivatives using multifunctional copper catalyst supported on β-cyclodextrin functionalized magnetic graphene oxide. Appl. Organomet. Chem.. 2020;34:e5913.
- [CrossRef] [Google Scholar]
- Nanohybrid of ZnO-RGO as a heterogeneous green catalyst for the synthesis of medicinally significant indole alkaloids and their derivatives. ChemistrySelect. 2018;3:314-320.
- [CrossRef] [Google Scholar]
- Eco-friendly and efficient greener process for the synthesis of chalcones and pyrazolones using the supramolecular catalyst β-cyclodextrin. OPPI. 2022;54:363-369.
- [CrossRef] [Google Scholar]
- Cu–Mn spinel oxide catalyzed synthesis of imidazo[1,2-a]pyridines through domino three-component coupling and 5-exo-dig cyclization in water. RSC Adv.. 2013;3:20869-20876.
- [CrossRef] [Google Scholar]
- Straightforward multicomponent synthesis of pyrano[2,3-d]pyrimidine-2,4,7-triones in β-cyclodextrin cavity and evaluation of their anticancer activity. J. Iran. Chem. Soc.. 2019;16:1553-1561.
- [CrossRef] [Google Scholar]
- Supramolecular biomimetic catalysis by β-cyclodextrin for the synthesis of new antimicrobial chromeno[4,3-b]quinolin-isonicotinamides in water. Res. Chem. Intermed.. 2020;46:737-753.
- [CrossRef] [Google Scholar]
- Magnetically retrievable nanocatalyst Fe3O4@CPTMO@dithizone-Ni for the fabrication of 4H-benzo[H]chromenes under green medium. Sci. Rep.. 2023;13:17894.
- [CrossRef] [Google Scholar]
- MCM-41 supported 2-aminothiophenol/Cu complex as a sustainable nanocatalyst for Suzuki coupling reaction. Sci. Rep.. 2024;14:18070.
- [CrossRef] [Google Scholar]
- Synthesis of new magnetic nanocatalyst Fe3O4@CPTMO-Phenylalanine-Ni and its catalytic effect in the preparation of substituted pyrazoles. Sci. Rep.. 2023;13:2564.
- [CrossRef] [Google Scholar]
- Imidazo[1,2-a]pyridin-3-amines as potential HIV-1 non-nucleoside reverse transcriptase inhibitors. Bioorg. Med. Chem.. 2011;19:4227-4237.
- [CrossRef] [Google Scholar]
- The modern face of synthetic heterocyclic chemistry. J. Org. Chem.. 2016;81:10109-10125.
- [CrossRef] [Google Scholar]
- Synthesis of new 4-oxo-tetrahydroindol derivatives by using chemical and microbial biotransformation methods. Polycycl. Aromat. Comp.. 2020;40:1390-1396.
- [CrossRef] [Google Scholar]
- Multicomponent reactions and supramolecular catalyst: A perfect synergy for eco-compatible synthesis of pyrido[2,3-d]pyrimidines in water. J. Heterocycl. Chem.. 2020;57:2184-2193.
- [CrossRef] [Google Scholar]
- Chaudhuri, S., Ghosh, A., Chattopadhyay, S.K., 2021. Green Synthetic Approaches for Biologically Relevant Heterocycles. Elsevier, pp. 617–653. https://doi.org/10.1016/B978-0-12-820792-5.00004-4.
- Cyclodextrins inclusion complex: preparation methods, analytical techniques, and food industry applications. Food Chem.. 2022;384:132467
- [CrossRef] [Google Scholar]
- Multicomponent reactions: advanced tools for sustainable organic synthesis. Green Chem.. 2014;16:2958-2975.
- [CrossRef] [Google Scholar]
- Catalytic efficiency of β-cyclodextrin hydrate-chemoselective reaction of indoles with aldehydes in aqueous medium. Tetrahedron Lett.. 2020;61:152231
- [CrossRef] [Google Scholar]
- Environmentally benign synthesis of heterocyclic compounds by combined microwave-assisted heterogeneous catalytic approaches. Green Chem.. 2012;14:17-37.
- [CrossRef] [Google Scholar]
- Nanocatalytic one-pot, four-component synthesis of some new triheterocyclic compounds consisting of pyrazole, pyran, and pyrimidinone rings. New J Chem.. 2015;39:7268-7271.
- [CrossRef] [Google Scholar]
- Recent advances in heterocyclic compounds with antiviral properties. Chem. Heterocycl. Compd.. 2021;57:410-416.
- [CrossRef] [Google Scholar]
- In silico studies and β-cyclodextrin mediated neutral synthesis of 4-oxo-4,5,6,7-tetrahydroindoles of potential biological interest. Tetrahedron Lett.. 2020;61:151972
- [CrossRef] [Google Scholar]
- Synthesis and antitumor activity of some new fused heterocyclic compounds. Russ. J. Gen. Chem.. 2019;89:128-137.
- [CrossRef] [Google Scholar]
- Enzymatic approach to cascade synthesis of bis(indolyl)methanes in pure water. RSC Adv.. 2020;10:10848-10853.
- [CrossRef] [Google Scholar]
- MgCl2 catalyzed one-pot synthesis of 2-hydroxy-3-((5-methyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)(phenyl)methyl) naphthalene-1, 4-dione derivatives in EG. Tetrahedron Lett.. 2016;57:1104-1108.
- [CrossRef] [Google Scholar]
- Synthesis and biological evaluation of 2-trifluoromethyl/sulfonamido-5,6-diaryl substituted imidazo[2,1-b]-1,3,4-thiadiazoles: a novel class of cyclooxygenase-2 inhibitors. Bioorg. Med. Chem.. 2008;16:276-283.
- [CrossRef] [Google Scholar]
- Catalyst free synthesis of bis (indolyl) methanes and 3,3-bis (indolyl) oxindoles in aqueous ethyl lactate. ChemistrySelect.. 2017;2:11561-11564.
- [CrossRef] [Google Scholar]
- β-Cyclodextrin based mesoporous silica as eco-friendly phase transfer catalyst in the synthesis of pyran derivatives. Iran J. Org. Chem.. 2021;13:3149.
- [Google Scholar]
- Bioinspired heterocyclic compounds as corrosion inhibitors: a comprehensive review. Chem.–An Asian J.. 2021;16:1324-1364.
- [CrossRef] [Google Scholar]
- Graphene quantum dots: synthesis, characterization, and application in wastewater treatment: a review. Mater. Adv.. 2023;4:4272-4293.
- [CrossRef] [Google Scholar]
- Catalysis in cyclodextrin-based unconventional reaction media: Recent developments and future opportunities. ACS Sustain. Chem. Eng.. 2017;5:3598-3606.
- [CrossRef] [Google Scholar]
- Silica-bonded 5-n-propyl-octahydro-pyrimido[1,2-a]azepinium chloride (SB-DBU) Cl as a highly efficient, heterogeneous, and recyclable silica-supported ionic liquid catalyst for the synthesis of benzo[b]pyran, bis (benzo[b]pyran) and spiro-pyran derivatives. J. Mol. Catal. A Chem.. 2013;372:137-150.
- [CrossRef] [Google Scholar]
- A green and convenient protocol for the synthesis of novel pyrazolopyranopyrimidines via a one-pot, four-component reaction in water. Tetrahedron Lett.. 2014;55:1226-1228.
- [CrossRef] [Google Scholar]
- Triphenylphosphine-m-sulfonate/carbon tetrabromide as an efficient and easily recoverable catalyst system for Friedel-Crafts alkylation of indoles with carbonyl compounds or acetals. ACS Sustain. Chem. Eng.. 2013;1:549-553.
- [CrossRef] [Google Scholar]
- Melamine supported on hydroxyapatite-encapsulated-γ-Fe2O3: A novel superparamagnetic recyclable basic nanocatalyst for the synthesis of 1,4-dihydropyridines and polyhydroquinolines. Rev. Chem. Intermed.. 2015;41:7227-7244.
- [CrossRef] [Google Scholar]
- β-Cyclodextrin: an efficient supramolecular catalyst for the synthesis of pyranoquinolines derivatives under ultrasonic irradiation in water. Polycycl. Aromat. Comp.. 2022;42:4224-4239.
- [CrossRef] [Google Scholar]
- Robust synthesis of sugar-coumarin based fluorescent 1,4-disubstituted-1,2,3-triazoles using highly efficient recyclable citrate grafted β-cyclodextrin@magnetite nano phase transfer catalyst in aqueous media. Carbohydr. Res.. 2019;482:107736
- [CrossRef] [Google Scholar]
- Ultrasonic-assisted synthesis of chrysin derivatives linked with 1,2,3-triazoles by 1,3-dipolar cycloaddition reaction. Ultrason. Sonochem.. 2011;18:527-533.
- [CrossRef] [Google Scholar]
- Heterocyclic Chemistry. CRC Press; 2020.
- A review of the biological and medicinal impact of heterocyclic compounds. Results Chem.. 2022;4:100606
- [CrossRef] [Google Scholar]
- Cu/TCH-pr@SBA-15 nano-composite: a new organometallic catalyst for facile three-component synthesis of 4-arylidene-isoxazolidinones. RSC Adv.. 2020;10:27439-27446.
- [CrossRef] [Google Scholar]
- Cyclodextrin based palladium catalysts for Suzuki reaction: an overview. Carbohydr. Res.. 2020;489:107954
- [CrossRef] [Google Scholar]
- Hyper-cross-linked β-cyclodextrin nanosponge: a three-dimensional, porous, and biodegradable catalyst in the one-pot synthesis of kojic acid-based heterocyclic compounds. Res. Chem. Intermed.. 2020;46:1857-1868.
- [CrossRef] [Google Scholar]
- Silica sulfuric acid magnetic nanoparticle: an efficient and eco-friendly catalyst for the synthesis of spiro[2-amino-4H-pyran-oxindole] s. Can. J. Chem.. 2015;93:546-549.
- [CrossRef] [Google Scholar]
- A clean and expedient synthesis of spirooxindoles in aqueous media catalyzed over nanocrystalline MgO. Tetrahedron Lett.. 2012;53:5004-5007.
- [CrossRef] [Google Scholar]
- Cyclodextrins as building blocks for new materials. Beilstein J. Org. Chem.. 2023;19:889-891.
- [CrossRef] [Google Scholar]
- Characterization of cyclodextrin/volatile inclusion complexes: a review. Molecules.. 2018;23:1204.
- [CrossRef] [Google Scholar]
- Methods for selective modifications of cyclodextrins. Chem. Rev.. 1998;98:1977-1996.
- [CrossRef] [Google Scholar]
- A facile hydrothermal synthesis of high-efficient NiO nanocatalyst for preparation of 3, 4-dihydropyrimidin-2(1H)-ones. Sci. Rep.. 2022;12:8585.
- [CrossRef] [Google Scholar]
- The first catalytic application of oxidized carbon nanotubes in a four-component synthesis of fused heterocycles. Monatsh. Fur Chem.. 2016;147:791-795.
- [CrossRef] [Google Scholar]
- Polyethyleneimine-modified superparamagnetic Fe3O4 nanoparticles: an efficient, reusable and water tolerance nanocatalyst. J. Magn. Magn. Mater.. 2015;375:217-226.
- [CrossRef] [Google Scholar]
- Efficient entry to diversely functionalized spirooxindoles from isatin and their biological activity. Med. Chem. Res.. 2013;22:2717-2723.
- [CrossRef] [Google Scholar]
- The tyrosinase inhibitory effects of isoxazolone derivatives with a (Z)-β-phenyl-α, β-unsaturated carbonyl scaffold. Bioorg. Med. Chem.. 2018;26:3882-3889.
- [CrossRef] [Google Scholar]
- Ceric ammonium nitrate (CAN) catalyzes the one-pot synthesis of polyhydroquinoline via the Hantzsch reaction. Tetrahedron.. 2006;62:7293-7299.
- [CrossRef] [Google Scholar]
- An improved protocol for the synthesis of 3,4-disubstituted isoxazol-5(4H)-ones through L-valine-mediated domino three-component strategy. J. Chem. Sci.. 2020;132:1-10.
- [CrossRef] [Google Scholar]
- Synthesis of polyhydroquinoline derivatives through unsymmetric Hantzsch reaction using organocatalysts. Tetrahedron. 2007;63:1946-1952.
- [CrossRef] [Google Scholar]
- Er(OTf)3 assisted efficient synthesis of 3-hydroxynaphthalene-1,4-dione derivatives via pseudo four-component reactions and their biological evaluation. ChemistrySelect.. 2017;2:489-493.
- [CrossRef] [Google Scholar]
- One-pot, four-component synthesis of benzylpyrazolyl naphthoquinone derivatives and molecular docking studies. Synth. Commun.. 2016;46:2045-2052.
- [CrossRef] [Google Scholar]
- Meglumine catalyzed expeditious four-component domino protocol for the synthesis of pyrazolopyranopyrimidines in an aqueous medium. RSC Adv.. 2014;4:51580-51588.
- [CrossRef] [Google Scholar]
- Green synthesis of indeno[1,2-b]quinoxalines using β-cyclodextrin as a catalyst. Molecules. 2022;27:580.
- [CrossRef] [Google Scholar]
- β-Cyclodextrin-catalyzed synthesis, characterization, and bacterial evaluation of spirochromanone-linked 1,2,3-triazole and spirochromanone conjugates containing bis-1,2,3-triazoles. J. Heterocycl. Chem.. 2022;59:2098-2107.
- [CrossRef] [Google Scholar]
- A simple and efficient one-pot synthesis of 1, 4-dihydropyridines using heterogeneous catalysts under solvent-free conditions. J. Mol. Catal. A.. 2006;260:179-180.
- [CrossRef] [Google Scholar]
- Nanoporous Na+-montmorillonite perchloric acid as an efficient heterogeneous catalyst for the synthesis of merocyanine dyes based on isoxazolone and barbituric acid. Microporous Mesoporous Mater.. 2018;262:269-282.
- [CrossRef] [Google Scholar]
- β-Cyclodextrin: green catalyst for the efficient and expeditious synthesis of benzodiazepines under aqueous conditions. Synth. Commun.. 2022;52:2057-2066.
- [CrossRef] [Google Scholar]
- Heterogeneously copper-catalyzed oxidative synthesis of imidazo[1,2-a]pyridines using 2-aminopyridines and ketones under ligand-and additive-free conditions. RSC Adv.. 2014;4:27301-27307.
- [CrossRef] [Google Scholar]
- One-pot multicomponent approach towards the synthesis of heterocyclic compounds by using β-cyclodextrin as a catalyst. Curr. Org. Chem.. 2018;22:1959-1985.
- [CrossRef] [Google Scholar]
- β-Cyclodextrin: a supramolecular catalyst for metal-free approach towards the synthesis of 2-amino-4,6-diphenylnicotinonitriles and 2,3-dihydroquinazolin-4(1H)-one. RSC Adv.. 2021;11:1271-1281.
- [CrossRef] [Google Scholar]
- Per-6-NH2-β-CD as supramolecular host and reusable aminocyclodextrin Promoted solvent-free synthesis of 2-amino-4H-chromene scaffolds at room temperature. PAH. 2022;42:6417-6428.
- [CrossRef] [Google Scholar]
- Supramolecular β-cyclodextrin as a biodegradable and reusable catalyst promoted environmentally friendly synthesis of pyrano[2,3-d]pyrimidine scaffolds via tandem Knoevenagel–Michael–cyclocondensation reaction in aqueous media. Polycycl. Aromat. Comp.. 2022;42:2805-2814.
- [CrossRef] [Google Scholar]
- Supramolecular β-cyclodextrin as a reusable catalyst for xanthene synthesis in aqueous medium. Org Prep Proced Int.. 2023;55:317-325.
- [CrossRef] [Google Scholar]
- Copper(II) acetate monohydrate: An efficient and eco-friendly catalyst for the one-pot multi-component synthesis of biologically active spiropyrans and 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives under solvent-free conditions. Res. Chem. Intermed.. 2016;42:7841-7853.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of eco-friendly waterborne polyester-urethane-phosphorus resin for industrial coil coatings. Polym. Sci. A. 2021;63:690-704.
- [CrossRef] [Google Scholar]
- Magnetic calcined oyster shell functionalized with taurine immobilized on β-cyclodextrin (Fe3O4/COS@β-CD-SO3 H NPs) as green and magnetically reusable nanocatalyst for efficient and rapid synthesis of spirooxindoles. Res. Chem. Intermed.. 2019;45:4737-4756.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, and application of β-Cyclodextrin/imidazolium based dicationic ionic liquid supported on silica gel as a novel catalyst in Hantzsch condensation reaction. Polycycl. Aromat. Comp.. 2021;41:1094-1106.
- [CrossRef] [Google Scholar]
- Sustainable chemical synthesis of 2, 3-dihydrobenzofurans/1, 2, 3-trisubstituted indanes in water by using a permethylated β-cyclodextrin-tagged N-heterocyclic carbene–gold catalyst. Synlett. 2023;34:1425-1432.
- [CrossRef] [Google Scholar]
- Cu–immobilized mesoporous silica nanoparticles [Cu2+@MSNs-(CO2−)2] as an efficient nanocatalyst for one-pot synthesis of pyrazolopyranopyrimidines in water. ChemistrySelect.. 2017;2:9642-9646.
- [CrossRef] [Google Scholar]
- β-Cyclodextrin catalyzed access to fused 1,8-dihydroimidazo[2,3-b]indoles via one-pot multicomponent cascade in aqueous ethanol: Supramolecular approach toward sustainability. J. Heterocycl. Chem.. 2020;57:820-829.
- [CrossRef] [Google Scholar]
- Green synthesis of biologically active heterocycles of medicinal importance: a review. Environ. Chem. Lett.. 2021;19:3315-3358.
- [CrossRef] [Google Scholar]
- Cyclodextrins as multitask agents for metal nano-heterogeneous catalysis: a review. Environ. Chem. Lett.. 2021;19:4327-4348.
- [CrossRef] [Google Scholar]
- A novel and facile semi-IPN system in fabrication of solvent resistant nano-filtration membranes for effective separation of dye contamination in water and organic solvents. Sep. Purif. Technol.. 2022;282:120121
- [CrossRef] [Google Scholar]
- β-CD-SO3H: synthesis, characterization, and its application for the synthesis of benzylpyrazolyl naphthoquinone and pyrazolo pyranopyrimidine derivatives in water. Catal. Lett.. 2020;150:127-137.
- [CrossRef] [Google Scholar]
- β-Cyclodextrin: a green supramolecular catalyst assisted eco-friendly one-pot three-component synthesis of biologically active substituted pyrrolidine-2-one. RSC Adv.. 2023;13:5457-5466.
- [CrossRef] [Google Scholar]
- Nickel/β-CD-catalyzed Suzuki-Miyaura cross-coupling of aryl boronic acids with aryl halides in water. Appl. Organomet. Chem.. 2021;35:e6378.
- [CrossRef] [Google Scholar]
- Recent advances in β-cyclodextrin-based catalysts for reducing toxic nitroaromatic: an overview. Appl. Organomet. Chem.. 2023;37:e7287.
- [CrossRef] [Google Scholar]
- The electrochemical coupling reactions of organic halides compound in a valuable and practical manner for C-C and C-heteroatom formation: an overview. Arab. J. Chem.. 2024;105822
- [CrossRef] [Google Scholar]
- A new β-cyclodextrin-based nickel as green and water-soluble supramolecular catalysts for aqueous Suzuki reaction. Sci. Rep.. 2023;13:21279.
- [CrossRef] [Google Scholar]
- The recent development of β-cyclodextrin-based catalysts system in click reactions: a review. Appl. Organomet. Chem.. 2024;38:e7365.
- [CrossRef] [Google Scholar]
- The recent development of β-cyclodextrin-based catalysts system in Suzuki coupling reactions. Appl. Organomet. Chem.. 2024;38:e7458.
- [CrossRef] [Google Scholar]
- The recent list of cyclodextrin-containing drug products. Periodica Polytechnica Chem. Eng.. 2023;67:11-17.
- [CrossRef] [Google Scholar]
- Design, preparation, and characterization of Fe3O4 nanoparticles encapsulating β-cyclodextrin-bearing guanidine as a highly efficient and reusable heterogeneous base catalyst for synthesis of 3,4-dihydropyrano[3,2-c]chromenes. Appl. Organomet. Chem.. 2020;34:e5862.
- [CrossRef] [Google Scholar]
- Cyclodextrins as promoters in aqueous organic synthesis. Curr. Org. Chem. 2010;14:1308-1322.
- [CrossRef] [Google Scholar]
- Recent progress of nanocatalyst in the synthesis of heterocyclic compounds by barbituric acids. Appl. Organomet. Chem.. 2023;37:e7250.
- [CrossRef] [Google Scholar]
- Studies on the synthetic development for the discovery of novel heterocyclic agrochemicals. J. Pest. Sci.. 2008;33:175-177.
- [CrossRef] [Google Scholar]
- Recent advancements in the development of heterocyclic anti-inflammatory agents. Eur. J. Med. Chem.. 2020;200:112438
- [CrossRef] [Google Scholar]
- A Review of heterocyclic compounds for anti-diabetic activity. Syst. Rev. Pharm.. 2022;13:410.
- [Google Scholar]
- α-Chymotrypsin-catalyzed synthesis of bis (indolyl) alkanes in Water. Chin. J. Chem.. 2015;33:409-412.
- [CrossRef] [Google Scholar]
- Introduction and general overview of cyclodextrin chemistry. Chem. Rev.. 1998;98:1743-1754.
- [CrossRef] [Google Scholar]
- A green protocol for the one-pot synthesis of 3,4-disubstituted isoxazole-5(4H)-ones using modified β-cyclodextrin as a catalyst. Sci. Rep.. 2022;12:19106.
- [CrossRef] [Google Scholar]
- An efficient four component domino synthesis of pyrazolopyranopyrimidines using recyclable choline chloride: urea deep eutectic solvent. J. Heterocycl. Chem.. 2018;55:716-728.
- [CrossRef] [Google Scholar]
- Supramolecular catalysis: an efficient and sustainable multicomponent approach to the synthesis of novel hexahydro-4H-indazol-4-one derivatives. Curr. Cat.. 2020;9:92-101.
- [CrossRef] [Google Scholar]
- Ionic liquid [DABCO-H][HSO4] as a highly efficient and recyclable catalyst for Friedel-Crafts alkylation in the synthesis of bis(naphthol) methane and bis (indolyl) methane derivatives. Synthesis.. 2016;48:3559-3566.
- [CrossRef] [Google Scholar]
- Selected heterocyclic compounds as antioxidants. Synthesis and biological evaluation. Curr Top Med Chem.. 2014;14:2462-2477.
- [Google Scholar]
- Recent progress in the chemistry of multicomponent reactions. Pure Appl. Chem.. 2001;73:187-191.
- [CrossRef] [Google Scholar]
- Green synthesis of 3-hydroxynaphthalene-1,4-dione derivatives via microwave-assisted three-component reactions in neat water. J. Heterocycl. Chem.. 2012;49:521-525.
- [CrossRef] [Google Scholar]
- Sodium stearate-catalyzed multicomponent reactions for efficient synthesis of spirooxindoles in aqueous micellar media. Tetrahedron. 2010;66:339-343.
- [CrossRef] [Google Scholar]
- One-pot Synthesis of tetrahydrobenzo[4,5]imidazo[2,1-b]quinazolin-1(2H)-ones using β-cyclodextrin-SO3H as a biocompatible and recoverable catalyst in water. Org. Prep. Proced. Int.. 2022;54:40-48.
- [CrossRef] [Google Scholar]
- One-pot three-component synthesis of spirooxindoles catalyzed by hexamethylenetetramine in water. J. Heterocyclic Chem.. 2013;50:61-65.
- [CrossRef] [Google Scholar]
- Facile construction of spiroindoline derivatives as potential anti-viral agent via three-component reaction in aqueous with β-cyclodextrin-SO3H as an efficient catalyst. Green Chem. Lett. Rev.. 2022;15:139-152.
- [CrossRef] [Google Scholar]
- Multicomponent reactions (MCR) in medicinal chemistry: A patent review (2010–2020) Expert Opin. Ther.. 2021;31:267-289.
- [CrossRef] [Google Scholar]
- Agricultural waste biomass-assisted nanostructures: synthesis and application. Green Process. Synth.. 2019;8:421-429.
- [CrossRef] [Google Scholar]
- An efficient and mild synthesis of tetrahydro-4H-indol-4-one derivatives via a domino reaction in water. Synthesis. 2013;45:3007-3017.
- [CrossRef] [Google Scholar]
- Consecutive multicomponent reactions for the synthesis of complex molecules. Org. Biomol. Chem.. 2019;17:7632-7650.
- [CrossRef] [Google Scholar]







