Translate this page into:
Recent advances in dyes uptake by microplastics in aquatic environments: Influencing factors and ecotoxicological behaviors
⁎Corresponding author. b.ramavandi@bpums.ac.ir (Bahman Ramavandi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Understanding the adsorption mechanisms and interaction of dye pollutants and microplastics in natural water is vital to evaluating potential risks. This review article discusses the bibliometric analysis and the adsorption behavior of dyes to microplastics. The review also examined the impact of environmental (salinity, pH, and temperature) and physicochemical (particle size and active area) factors on dye uptake by microplastics. The maximum amount of Cyan dye adsorbed onto polyethylene microplastics was reported to be 2874.4 mg/g. Polystyrene (PS) microplastics exhibited the highest adsorption capacity for aniline (0.060 mg/g) due to its surface area of 0.7214 m2/g. In 50 % of dye uptake studies on microplastics, the equilibrium condition was reached within 24 h. However, in a few cases, equilibrium was achieved in 8 days. The desorption efficiency of malachite green in the simulated gastric fluid at high temperatures was 81.4 %. The concentration of dyes in the isotherm studies of their adsorption by microplastics varied widely (5–160 mg/L). According to the criterion of R2 > 0.95, the Langmuir isotherm demonstrates a better fit with the data in most of the studies. The lowest uptake of dyes was observed at a pH of 1.5 under the same conditions. Studies have shown that higher temperatures can increase the ability of microplastics to attract and release organic and inorganic pollutants. The potential ecological effects of ‘microplastic-dye’ on organisms and the methods for removing microplastics were investigated. This paper has provided data for the assessment of the potential risks of ‘microplastic-dye’ to aquatic organisms.
Keywords
Microplastic
Dye
Organic pollutant
Adsorption
Aquatic environment
1 Introduction
Environmental severe problems such as water and soil pollution are due to the development of chemical, agricultural, pharmaceutical, and textile industries (Sharma et al., 2021). Several pollutants, including anionic/cationic dyes, are discharged from the textile, paint, tanneries, paper and pulp, and dye manufacturing industry (Lim et al., 2022) (Mansor et al., 2020; Liang et al., 2021). Among these industries, textile produces the most dye-containing wastewater (Mansor et al., 2020); (Liang et al., 2021) . Releasing toxic and hazardous chemicals from various activities has led to an alarming increase in water resource pollution (Balarak, 2015). In the textile industry's dyeing process, different dye molecules, salts, and additives are commonly used (Gnanasekaran et al., 2021), and most commercial textile dyes are highly water-soluble (Carneiro et al., 2010). During the dyeing process, approximately 10–15 % of dye contaminants enter the aqueous environment (Du et al., 2022). Previous studies show that water sources receive about 14,000 tons of dyes annually (Liu, 2021). Dyes are highly toxic and can severely damage aquatic ecosystems (Mansor et al., 2020). Various dyes can be easily detected in water even at concentrations as low as 1 mg/L (Sharma et al., 2021). Dye pollutants can reduce the amount of light entering the water, lower oxygen levels (Lim et al., 2022; Tkaczyk et al., 2020), and negatively affect the survival of phytoplankton and the food chain (Moorthy et al., 2021). Also, other consequences of dyes in the ecosystems are metabolic stress, neurosensorial damage, flora necrosis, and decreased growth of fauna (Pinheiro et al., 2022). Dyes that float on the surface water undergo degradation by sunlight (Dev et al., 2021). Ecological effects of azo dyes have been reported in freshwater fish species (Abe et al., 2019). After acute exposure of Daphnia magna to azo dyes, toxic effects have been observed (Lach et al., 2022). Pollutants can remain in sediments and fish for long periods even though the water may appear clear (Ben Slama, 2021).
Table 1 shows the characteristics of synthetic dyes. Dyes are complex molecules that bind to various surfaces, such as fabrics and leather (Maheshwari et al., 2021; Yu et al., 2024). Zhou et al. (2020) found that textile printing and dyeing procedures can lead to significantly high levels of microfibers in wastewater, as high as 54,100 microfibers per liter in China (Zhou et al., 2020). These microfibers are a type of microplastic (MP) that have a strong adsorption capacity of pollutants. Heavy metals, pathogens, polychlorinated biphenyls (PCBs), and polycyclic aromatic hydrocarbons (PAHs) can be adsorbed by MPs (Zhang, 2022). MP pollution and its impact on water bodies such as freshwater, deep sea, and polar regions is a global problem (Zhou et al., 2020; Ma, 2022).
Synthetic dyes
Uses
Solubility in fresh water
Solubility in seawater
Class, Examples
Acid dye (anionic)
Primarily for wool and silk, nylon, and acrylic
Soluble
Fair
Azo, Acid red 27, anthraquinone, Acid blue 25, xanthenes, Acid brown13, diphenylamine, Acid blue 9
Basic dye (cationic)
Primarily for wool, silk, nylon, and cotton
Soluble
Very poor
Diphenylmethane or ketone imine, Basic yellow, Triarylmethane, Basic green 4, and Basic violets (Oxazine, Basic blue 12), Azine (Basic red 5), Xanthene (Basic violet 10)
Reactive dye (anionic)
Primarily for cotton apparel
Depends on the types of dyes
Good
Azo, Reactive red 3, Anthraquinones, Reactive blue 19
Direct dye (anionic)
Primarily for cellulosic fabrics
Depends on the types of dyes
Poor to good
Azo, Direct blue 1, Phthalocyanine, Direct blue 86, Triphenodioxazine, Direct blue 106
Disperse dye (nonionic)
Primarily for acetate, polyester, nylon, and cellulose fibers
Slightly soluble
Good
Azo, Disperse orange 30, Anthraquinones, Disperse violet 1, Nitrodiphenylamine, Disperse yellow 26
Sulfur dye (nonionic)
Used for linen, cotton, and jute
Insoluble
Good
Sulfur blue 15, Leuco sulfur black1, Sulfur green 3
Vat dye (nonionic)
Used for cotton and wool
Insoluble, soluble (Leuco salts)
Good
Indigo class, Vat blue 1, Anthraquinones, Vat black 25, Violanthrone, Vat green 1
The findings of various studies indicate that the amount of plastic waste in our oceans exceeds 5 × 1012 pieces. In addition, it is predicted that by 2050, there will be about 12 × 109 tons of plastic waste in landfill sites or the natural environment (You, 2021; Wang, 2023). Coastal cities, ports, shipping operations, and landfills along the coast are the primary contributors to plastic pollution in oceanic environments (Alfaro-Núñez, 2021). However, there are reports that plastics have been recycled in concrete asphalt production for road construction (Boom, 2023; Noor and Rehman, 2022).
Microplastics are typically produced from decomposing larger macroplastic pieces through chemical, physical, and biological processes (Du et al., 2022). MPs are tiny pieces which aquatic organisms can ingest. Once MPs enter an organism's gastrointestinal tract (GIT), any toxic substances may be released in MPs and disrupt the organism's normal biological functions (Wang, 2023). MPs play a role in spreading environmental pollutants, particularly synthetic dyes (Tubić, 2023). MPs’ complex structure and large surface make them prone to absorbing and releasing organic contaminants in the ocean (Du et al., 2022). It is worth noting that the desorption process tends to be more significant in the GIT environment compared to natural water (Wang, 2023). For example, Alfaro-Núñez et al. (2021) analyzed 16 different marine species to determine the level of MPs in their bodies based on their feeding habits. The results showed that Carnivorous species had the highest levels of MPs, while Detritivore species that feed on dead organic matter had the lowest levels of MPs (Alfaro-Núñez, 2021).
Despite their high concentrations and widespread environmental occurrence, the adsorption of dye contaminants onto MPs has received limited research focus (You, 2021). Few studies have examined how MPs interact with hydrophilic pollutants (Anastopoulos et al., 2022). Dye adsorption experiments on MPs contribute to understanding their role as carriers of co-existing contaminants and their potential environmental risks. When dyes are absorbed into MPs, they can have a more toxic impact on aquatic organisms due to their synergy (You, 2021). The primary source of toxicity in MPs originates from the pollutants they have adsorbed (Chen, 2023). So, by understanding these interactions, the researchers can evaluate the environmental effects of dyes and the potential of MPs as absorbents for removing these contaminants from water (Tubić, 2023). The use of plastic products has caused severe challenges to the environment. Thus, efficient technologies for the simultaneous removal of multiple pollutants from wastewater require more attention (Li et al., 2023; Liu, 2023). Yang et al. (2024) used the catalyst-mediated sono-degradation to remove dyes and MPs (Yang, 2024). Other researchers have introduced novel and green techniques to eliminate a mix of MPs and dyes (Li et al., 2023); (Mayorga-Burrezo et al., 2023; Khalid et al., 2023; Peng, 2024) .
This review stands out due to its innovative approach to exploring the intricate relationship between two significant environmental pollutants (MPs and dye), offering a comprehensive analysis of how various factors, such as size, shape, and surface properties of MPs, as well as dye characteristics, can influence their interaction and subsequent ecotoxicological impacts. The study offers insights into the environmental behavior, ecological toxicity, sources, distribution, consequences, and fate of microplastics in aquatic ecosystems, providing a foundation for discussing the unique contributions to environmental pollution research. In addition, in this research, the complex aspects of ‘MPs–dye’ simultaneous pollution, one of the ecological challenges, become apparent.
Accordingly, this paper reviewed the uptake of dyes into microplastics, the influence of the characteristics of microplastic particles and dyes on the amount of adsorption–desorption, the ecotoxicological effects of ‘MPs-dye’, and the decontamination methods of ‘MPs-dye’.
2 Methodology
Google Scholar was used to search papers and review the topic. Many keywords like ‘microplastic + dye + adsorption + sorption + uptake + water + distilled water + removal + aquatic environment’ were used to find related articles. Web of Science, Scopus, and Science Direct databases used the exact search keywords to avoid an incomplete search. To understand more about the current research on MPs and dyes, a bibliometric analysis was conducted in the field of MPs and dyes. The original data were gathered from Scopus by searching for “microplastic and dye and adsorption or phthalocyanine or azo dye or malachite green or Rhodamine B or crystal violet or aniline or methylene blue or methyl orange”. Software VOSviewer 1.6.17 was used to co-occurrence keyword network visualization, overlay visualization map, and map density visualization. The output of the Scopus database was imported into the software. The size and label of the bubbles and the thickness of the lines in the network are essential for the interpretation and analysis of maps. Fig. 1a is a network visualization. It shows three bubbles (MPs, dye, and adsorption) are big. Thus, conducting research in this field is essential. A stronger connection is seen between adsorption and MPs, indicated by thicker lines. Fig. 1a–c shows keywords detection results between 2018 and 2024. Fig. 1b shows an overlay visualization map, and the color of the lines and bubbles suggest that this topic is gaining novel attention. Most of the research in this case was conducted from 2022 until 2024. Based on Fig. 1c, a map of density visualization, the color is beside the hot spot. According to this figure, polyethylene terephthalate (PET), polypropylenes (PP), and plastic waste have been used in most studies. The keyword ‘particle size’ is one of the most popular keywords among all the keywords. So, ‘particle size’ has an essential effect on adsorption. Fig. 2 shows the number of studies of MPs and dye. This figure also emphasizes that the most articles related to the topic were published in 2022 and 2023.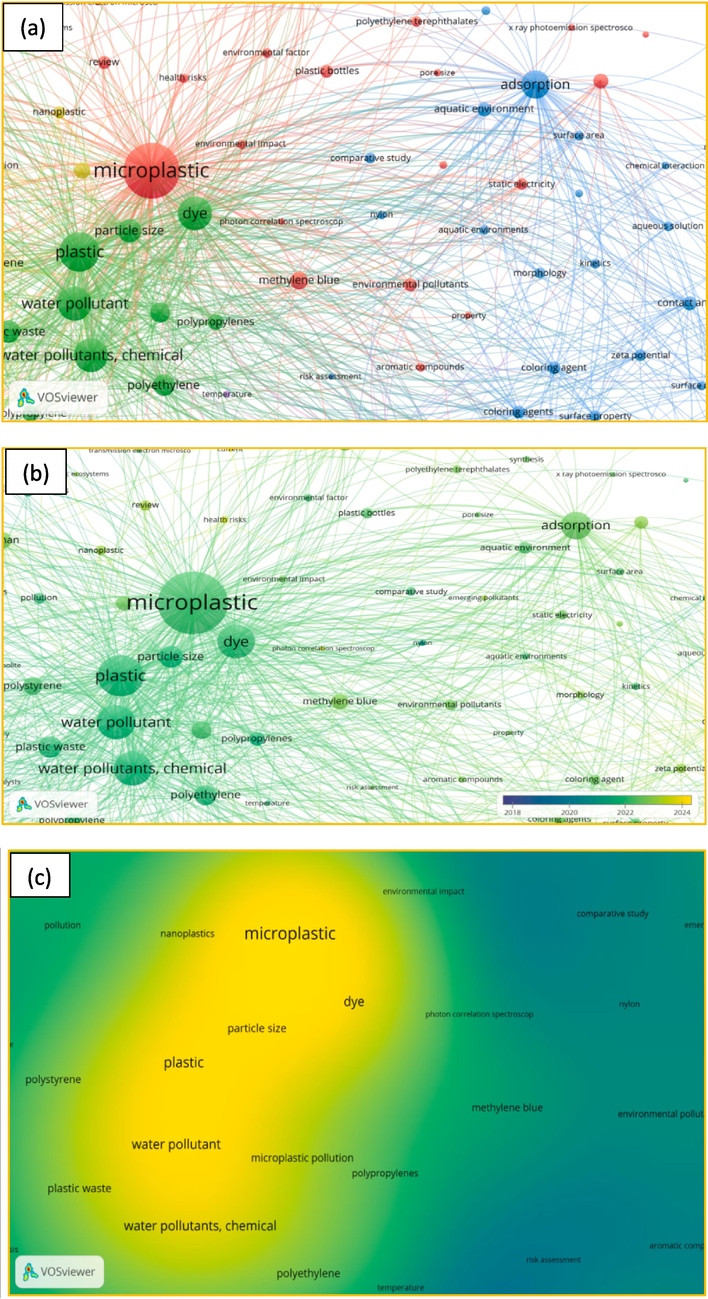
(a) Network visualization, (b) overlay visualization, and (c) density visualization.
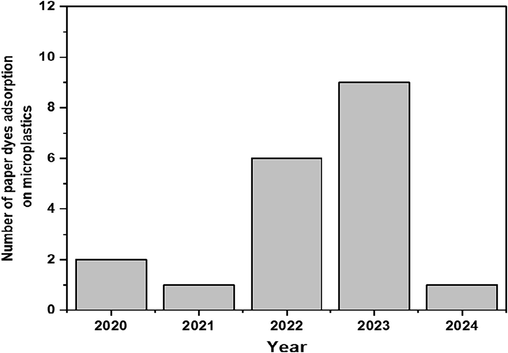
The number of papers on dye uptake by MPs published from 2020 to 2024.
3 Review and discussion
3.1 Mps-dyes interaction in the aquatic media
Numerous studies have extensively explored the uptake behaviors of organic contaminants such as dyestuffs, antibiotics, and PAHs onto MPs (Du et al., 2022; Ding, 2023). The adsorption affinity of different pollutants to the surface of MPs is different (Guo and Wang, 2019) and vastly depends on the plastic’s and contaminants’ specific properties. The adsorption behavior of one pollutant type compared to others is not always similar. Therefore, focusing on the unique characteristics of the contaminant and the plastic material is critical in studying adsorption processes (Tubić, 2023). To evaluate the environmental effects of MPs, it is necessary to understand the interactions between organic compounds and MPs (Hüffer et al., 2018).
3.2 Adsorption and desorption behavior of dyes onto MPs
3.2.1 Adsorption kinetics
Valuable information about the rate and mechanisms of adsorption is provided by kinetic experiments (Gao et al., 2021). Table 2 shows the data of the adsorption kinetics of dyes onto MPs. You et al. (2021) found that 90.3 % of methylene blue (MB) can be removed from aged PE in aqueous media (You, 2021). Research has been done to investigate the kinetics of reaching equilibrium on polyethylene (PE), polyvinyl chloride (PVC), PET, and PP (Tubić, 2023). For instance, in 50 % of dye uptake studies on MPs, equilibrium was reached within 24 h. However, in a few cases, equilibrium was achieved in 8 days (Du et al., 2022; Wang, 2023; Alfaro-Núñez, 2021; Boom, 2023; Noor and Rehman, 2022). Lower molecular weight can limit their ability to enter active sites, and it causes an extended state of equilibrium; however, accessibility is not exclusively influenced by molecular weight (Tubić, 2023). Table 2 demonstrates the uptake of various dyes onto MPs following the pseudo-second-order kinetic model. This finding suggests that chemical is the primary mechanism governing the interaction between dyes and MPs (Du et al., 2022; Lach et al., 2022). It was also found that the Elovich model describes the rate control of chemical uptake on an energetically heterogeneous adsorbent as a diffusion process (Tubić, 2023). There were three steps in the uptake of MB on the aged PE MPs: a fast adsorption process, a transition portion, and a quasi-equilibrium stage (You, 2021). Furthermore, Chakraborty et al. (2023) show the application of interaparticle diffusion to examine the mechanisms involved in the adsorption of methyl orange (MO) and MB dye onto carbonized MP particles (Chakraborty, 2023).
Dye
MP type
Dye content (mg/L)
MP dose (g/L)
Equilibrium time (h)
Kinetic model and parameters
Other fitting models
Ref.
Pseudo –first order
Pseudo-second order
k1
(1/h)qe
(mg/g)k2
(g/mg.h)qe
(mg/g)
1MB
Aged PE
40
1
72
0.010
7.711
0.006
12.987
Elovich and intra-layer diffusion
(You, 2021)
Cyan
Powdered PE
100
1
0.11
399.1
3.1044
436.9
Elovich
(Tubić, 2023)
Granulated PE
100
–
192
2.75
448.1
0.0052
478.5
PVC
100
–
192
0.22
497.2
0.0006
521
PET
100
–
192
1.33
409.7
0.0036
437.7
PP
100
–
192
11.3
389.2
0.0315
400.8
Magenta
Powdered PE
100
–
72
1.35
268.4
0.0056
286.2
Elovich
(Tubić, 2023)
Granulated PE
100
1
72
5.17
229.2
0.0277
237.5
–
PVC
100
–
72
0.22
491.0
0.0006
515.3
–
PET
100
–
72
0.69
462.3
0.0024
482.4
PP
100
–
72
12.3
385.9
0.0231
401.9
–
1 MG
PVC-UV/KMnO4
20
5 mg/ 50 mL
60
0.1528 ± 0.0183 g/mg.h
133.199 ± 3.991
0.0013 ± 0.0001
148.589 ± 2.609
–
Wang et al, 2023
MG
Aged PA
57
1.66
36
0.225
33.41
0.007
37.65
–
(Lin et al., 2020)
Virgin PA
57
1.66
12
2.165
2.528
1.310
2.645
–
1 MO
PET waste
20
10
30 min
0.066 1/min
0.361
1.062 g/mg. min
2.000
Intraparticle diffusion
(Chakraborty, 2023)
MB
PET waste
20
10
30 min
0.071 1/min
0.247
2.284 g/mg. min
1.967
1RR120
Polyamide nylon 6
35
0.4 g/ 0.05 L
24
0.416 1/min
1.49
0.690 g/mg. min
3.40
–
(Afmataj, 2023)
1RhB
PVC
10
20 mg/15 mL
24
0.034 ± 0.003
0.960 ± 0.012
0.412 ± 0.007
2.483 ± 0.011
–
(Du et al., 2022)
PS
10
20 mg/15 mL
24
0.023 ± 0.001
0.574 ± 0.010
1.807 ± 0.086
1.530 ± 0.091
PET
10
20 mg/15 mL
24
0.023 ± 0.001
0.473 ± 0.008
2.841 ± 0.016
1.283 ± 0.089
MG
Polyurethane plastic waste
PUPW-AC-C-A600
0.02 g/50 mL
200 min
0.015 1/min
699
3.77 g/mg. min
1211
Intraparticle diffusion
(Li, 2021)
MB
1BY28Plastic wastes
160
1600.4
0.830 min
45 min71.75 × 103
1/min
85.64 × 103
1/min9.88
123.7316.19 × 103
g/mg.min
1.22 × 103
g/mg.min
99
226.24
–(Dahdouh et al., 2020)
Aniline
PS
5
1
24
0.9423 1/min
0.0437 mg/g
4.2223 g/mg. min
0.0600
Webber-Morris model
(Chen, 2023)
1CV
Aged PE
10
1
48
–
–
–
1.681
Elovich
(Du et al., 2022)
Aged PP
10
1
48
–
–
–
4.201
MB
Cyanobacteria-plastic (pp) porous carbon
500
20 mg/25 mL
–
0.06243
686
0.8944 × 10-4
862
–
(Li, 2022)
MO
MG
Polyamide 6
10
10
1
148
48-
-
-–
-
–
-–
-Elovich, Intraparticle diffusion
(Wang, 2023)
MO
MGPolyamide 66
10
101
1
48
–
–
–
–
Intraparticle diffusion
MG
PE
10
25
5 d
−0.0508
69.8 μg/g
−0.0169
μg/g·h153.9 μg/g
–
(Çiftçi et al., 2023)
MB
Aged PP
20
1
48
1.8973
Intraparticle diffusion-
Weber-Morris(Lin et al., 2020)
3.2.2 Adsorption isotherms
Several isotherm studies have focused on dye adsorption onto MPs, as shown in Table 3. The concentration of dyes used in the isotherm studies varied widely, ranging from 5 mg/L to 160 mg/L. Studies have primarily examined the relationship between initial concentration and adsorption capacity of various dye compounds such as phthalocyanine)Cyan), Magenta, MG, RhB, basic yellow 28)BY28(, reactive red 120) RR120(, MO, CV, aniline, and MB. These studies have reported MPs with high uptake capacities. For example, using the Langmuir model, the powdered polyethylene (PEp) sorption capacity for Cyan was 2874.4 mg/g (Tubić, 2023). According to the value of R2 > 0.95 that has been done in most of the studies, the Langmuir isotherm has shown a better fit with data than other models. The Langmuir adsorption model explains the monolayer adsorption process, where a single layer of adsorbate molecules is formed on a uniform MP surface. This model assumes that the MP surface is homogeneous and there is no significant interaction between the adsorbed molecules and the uptake sites with uniform energies (Behnamfard and Salarirad, 2009). DOM: dissolved organic matter; HA: Humic acid; FA: fulvic acid
Contaminant
MPs type
Initial dye content, pH, salinity, DOM condition
Identification techniques
Sorption capacity and coefficient derived from isotherm models
Ref.
Langmuir Qmax (mg/g)
Langmuir Kd (L/mg)
Freundlich Kf (mg/g) (L/mg)1/n
MB
PE
Dye: 5–40 mg/L, pH 3–11, NaCl: 0.0–0.6 M
SEM–EDS, BET, FTIR, XRD, TGA, DSC
–
0.036–0.096
(You, 2021)
Cyan
PEp
SEM–EDS, FTIR
2874.4
0.020
82.7
(Tubić, 2023)
PEg
Dye: 1–200 mg/L, pH 7.3 ± 0.1
SEM–EDS, FTIR
1535.9
0.030
95.2
PVC
SEM–EDS, FTIR
184.6
0.030
16.9
PET
–
SEM–EDS, FTIR
123.4
0.025
11.1
PP
–
SEM–EDS, FTIR
104.6
0.023
9.16
Magenta
PEp
Dye: 1–200 mg/L, pH 7.3 ± 0.1
SEM–EDS, FTIR
184.4
0.006
2.4
PEg
–
SEM–EDS, FTIR
210.5
0.003
1.27
PVC
–
SEM–EDS, FTIR
142.1
0.009
3.43
PET
–
SEM–EDS, FTIR
101.5
0.006
1.47
PP
–
SEM–EDS, FTIR
123.2
0.009
3.24
MG
PVC- UV/KMnO4 composite oxidation
Dye: 5–50 mg/L, pH 7
FTIR, XRD, XPS, SEM, AFM
215.352 ± 24.325
0.0362 ± 0.0083 L/g
8.4055 ± 0.408 L/g
(Wang, 2023)
MO
CMPs PET waste
Dye: 5–70 mg/L, pH 3
SEM-EDX, FTIR, X-ray (EDX)
5.678
1.282
2.851
(Chakraborty, 2023)
MB
PET waste
Dye: 5–70 mg/L, pH 11
SEM-EDX, FTIR, X-ray (EDX)
6.561
0.785
2.343
RR120
Polyamide nylon 6 (PN6) powder
Dye: 10–60 mg/L, pH 2
–
3.96
2.148
2.329
(Afmataj, 2023)
RhB
PVC
Dye: 1–25 mg/L, pH 7.36, NaCl: 0.05 %, 0.5 %, 1 %, 2 %, 3.5 %, pH 3–9, HA: 2–20 mg/L
SEM, BET, XPS FTIR, XRD
4.598 ± 0.185
0.392 ± 0.009
1.211 ± 0.144
(Du et al., 2022)
PS
Dye: 1–25 mg/L, pH 7.36,
SEM, BET, XPS FTIR, XRD
2.482 ± 0.145
0.250 ± 0.012
0.544 ± 0.072
PET
Dye: 1–25 mg/L, pH 7.36,
SEM, BET, XPS FTIR, XRD
1.788 ± 0.089
0.480 ± 0.028
0.568 ± 0.043
MG
Polyurethane plastic waste
PUPW-AC-C-ADye: 5–150 mg/L, pH 2–7
BET, XPS FTIR, XRD, Raman spectroscopy analysis (RS)
1428
0.14
6.37
(Li, 2021)
MG
Aged PA
Dye: 5–100 mg/L, pH 7
SEM-EDAX, FTIR
63.48
0.177 L/g
12.69
(Lin et al., 2020)
Virgin PA
Dye: 5–100 mg/L, pH 7
SEM-EDAX, FTIR
5.40
0.114 L/g
1.24
BY28
MBSulphonated Waste Poly Methyl
Dye: 40–160 mg/L, pH 7
SEM-EDX, FTIR
222.222
0.338
70.798
(Dahdouh et al., 2020)
Methacrylate (SW PMMA)
Dye: 40–160 mg/L, pH 7
SEM-EDX, FTIR
97.087
1.272
60.226
Aniline
PS
Dye: 1–5 mg/L, pH 7, NaCl: 0–500 mg/L, CaCl2: 0–500 mg/L
SEM, FTIR
–
–
0.0023
(Chen, 2023)
CV
Aged PE
Dye: 10 mg/L, pH 6.82, T = 25 °C, NaCl: 0–15 mg/L, HA: 0–40 mg/L
SEM–EDS, BET, FTIR
–
1/n > 1
(Du et al., 2022)
Aged PP
Dye: 10 mg/L, pH 6.82, T = 25 °C, NaCl: 0–15 mg/L, HA: 0–40 mg/L
SEM–EDS, BET, FTIR
–
–
0.5 < 1/n < 1
MB
CPCs-800–50 %
(cyanobacteria-plastic(pp) porous carbon)Dye: 500 mg/L, pH 2–10, content of PP: 0–50 %
BET, FTIR, XRD
667
0.45
–
(Li, 2022)
MO
MG
PA6
Dye: 5–40 mg/L, pH = 2–10,T = 25, 35, 45 °C, seawater: 0–3.5 %, FA: 0–30 mg/L
SEM, BET, FTIR, XRD
11.1588
5.68560.2442
0.14862.8721
1.2886(Wang, 2023)
MO
MG
PA66
Dye: 5–40 mg/L, pH = 6, T = 25, 35, 45 °C, seawater: 0–3.5 %, FA: 0–30 mg/L
SEM, BET, FTIR, XRD
8.8538
4.91580.1265
0.12961.4658
0.8757
MG
PE
T = 25 °C, pH = 4
ATR-FTIR, DSC, BET
221 μg/g
–
–
(Çiftçi et al., 2023)
MB
Aged PP (derived from disposable PP cups)
pH 3, 5, 7, 9, 11, NaCl: 0, 0.2, 0.3, 0.4, 0.6 M and MgCl2: 0.01, 0.02, 0.03, 0.04 M, pH 7, HA: 5, 10, 20, 30 mg/L, T = 25 °C
FTIR, XPS, SEM-EDX, EDS
2.2213
–
–
(Liu et al., 2024)
3.2.3 Adsorption mechanisms
Information can be obtained to discern the interaction mechanism between MPs and dyes by examining the active functional groups and their involvement in adsorption (Tubić, 2023). Fig. 3 shows the mechanisms that control or regulate the process. For example, You et al. (2021) investigated, at pH values > pHPZC (point of zero charge), negatively charged PE MPs adsorb cationic MB through electrostatic attraction (You, 2021). Cationic dyes are soluble in water with a negative charge that can chemically bind to fiber materials such as cotton and wool (Sharma et al., 2021). In an investigation by Zhong et al. (2022), they studied the contact angles of two different MPs and found that PE MPs have a more hydrophobic surface and can absorb hydrophobic dyes (Zhong, 2022). PE and PP polymers interact with organic compounds through non-specific van der Waals forces due to non-specific functional groups (Tubić, 2023). Lin et al. (2021) explored the impacts of the solution pH and Pb2+ content on MG uptake by aged nylon MPs. The uptake of MG by aged nylon MPs decreased with increasing Pb2+ concentration (Lin et al., 2021). Pb2+ and aged nylon MPs may interact through surface complexation (Tang et al., 2020). Carboxyl functional groups on the MP surface have a higher affinity for the uptake of Pb2+ ions. These functional groups prohibit the electrostatic interaction between MG and MPs (Lin et al., 2021). Tubić et al. (2023) reported that Magenta and Cyan dyes contain aromatic rings with π-electrons that can interact with PET through π-π bonding (Tubić, 2023). Magenta and MG are classified as synthetic dyes belonging to the introductory class. They are created from organic bases and can ionize in water, producing colored cations (Sharma et al., 2021). So, the hydrophobic interactions do not play a main role in Cyan and Magenta’s uptake process. New bands were associated with Cyan dye on granulated PEg (PEg) and PVC after adsorption (Tubić, 2023). Du, Ma and Xing (2022) discovered that the adsorption process depended on the electrostatic attraction between aged PE, aged PP, and ionic dyes. Furthermore, hydrophobic interactions between the alkenyl chains, silicon-based additives, and the benzene ring in the dyes, and the hydrogen bonding between heteroatom, like nitrogen atoms in CV and oxygen atoms in oxygen-containing functional groups of aged MPs, may also influence the uptake of dyes onto MPs (Du et al., 2022). The most frequently used colorants are nitrogen-containing dyes. Some examples of these dyes include MG, CV, lemon yellow, and Congo red (Fan et al., 2021).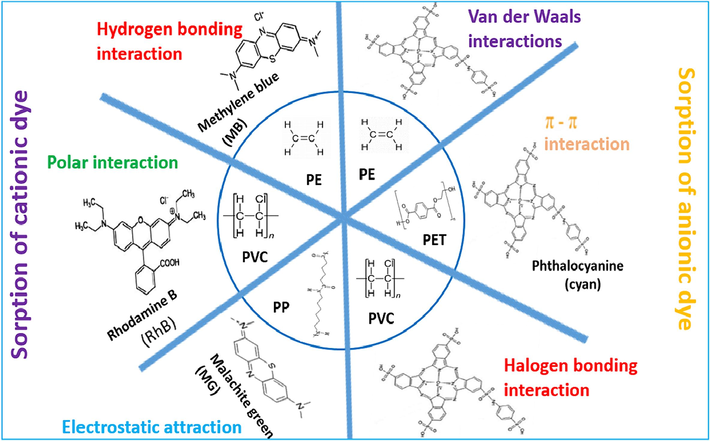
Mechanisms involved in the interactions between MPs and dyes.
Different techniques are utilized to understand adsorption mechanisms from various perspectives (see Table 3). These include the methods of FTIR, scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDX), and X-ray photoelectron spectroscopy (XPS). The EDX spectrum of nylon MPs aged with MG shows an increase in elemental C and O atoms content. This indicates MPs have absorbed MG dye (Lin et al., 2020). SEM-EDX can provide information about the material’s physical structure and the elements’ distribution (Suiyi, 2024). XPS is a surface analysis technique that provides information about the adsorbent surface’s chemical composition and oxidation states. It can help identify the presence of specific elements and chemical bonds involved in the adsorption process (Wang et al., 2024; Guo et al., 2023). The FTIR spectrum was obtained after the adsorption of MG on PVC, and it displays a reduction in the peak intensities (1427 cm−1 and 1251 cm−1). This reduction indicates an interaction between the chlorine groups in PVC and the functional groups in MG. The presence of -Cl on PVC can lead to a halogen-hydrogen bond with proton-donating functional groups (Zhong, 2022). The FTIR spectrum after the adsorption of aniline on PS showed an increase in the characteristic peaks of N–H bond formation at 1636 cm−1 and 685 cm−1. The C–N and C–H bonds on the MPs indicate the adsorption of aniline (Chen, 2023). The MB uptake mechanism onto MPs is due to hydrogen bonding between the positively charged MB molecule and the oxygen atoms in the C-O or C-O-C bonds on the surface of MPs. This bonding occurs through the sharing of electrons. It leads to the uptake of MB onto the MP’s surface (Mahabeer et al., 2023).
3.2.4 Desorption efficiency
Desorption studies play a central role in understanding how adsorbates and adsorbents can be recovered (Rápó and Tonk, 2021). Desorption tests were performed to determine the stability of MPs after dye uptake (Tubić, 2023). You et al. (2021) examined the desorption efficiency of MB from aged PE MPs. They found that this process depends on the physical conditions of the environment (You, 2021). In environments such as the sea, dyes absorbed into microplastics are released into the bodies of organisms that mistakenly ingest the plastic particles. Fig. 4 represents the desorption efficiency of dyes from MPs under different conditions. Wang et al. (2023) show that the dye desorbs from MPs in the first 10 h with a faster efficiency under the simulated conditions. This phase is vital to evaluate the desorption behavior of dye in simulated gastrointestinal fluids (Wang, 2023). Du, Ma and Xing (2022) discovered that the efficiency of CV desorption from MPs (PE and PP) samples was higher in seawater than in fresh water. This might be due to NaCl in seawater, which occupies some of the adsorption sites on MPs. The competitive adsorption of NaCl with CV molecules on MPs may be responsible for the phenomenon of desorption promotion. So, the findings suggest that aquatic organisms can absorb dyes that attach to MPs through a critical exposure route depending on the organisms’ physiological conditions (Du et al., 2022). In another study, the desorption of methylene blue dye from plastic particles in distilled water was reported as 99–100 % (Arslan and Günay, 2018). In 2019, Ünlü et al. reported the 100 % desorption of two cationic dyes (RB and MG) from a fibrous plastic adsorbent (Ünlü et al., 2020). The high desorption in these studies is due to working in a distilled water. Because, like other environments (seawater and tap water), there are no ionic interferences and organic substances.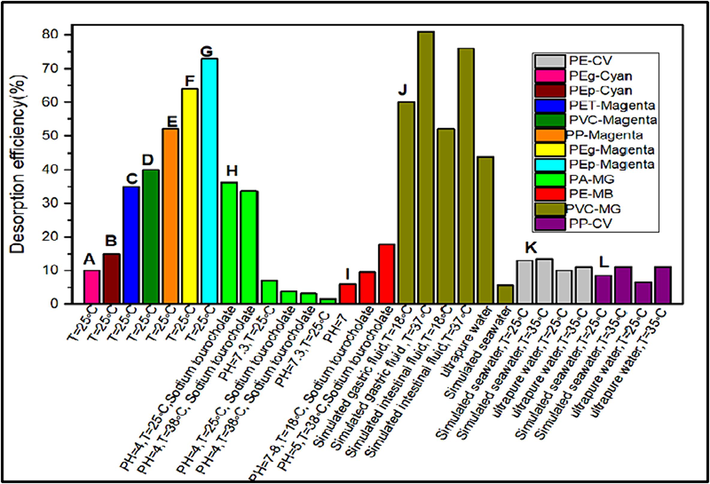
Desorption efficiency of dyes from MPs under various conditions (A, B, C, D, E, F, G: (Tubić, 2023), H: (Lin et al., 2020), I (You, 2021), J (Wang, 2023), K, L: (Du et al., 2022).
3.3 Environmental factors affecting the adsorption of dyes by MPs
Fu et al. (2021) divided the factors that play a role in the adsorption of pollutants in MPs into three categories: MPs properties, characteristics of organic pollutants, and environmental factors (Fu et al., 2021).
3.3.1 Solution pH
PH level is important in capturing dyes in adsorbents like MPs. It contributes to creating binding sites on the MP surface, which facilitates the capture of the adsorbate (Mahabeer et al., 2023). Altering a solution's pH significantly impacts a compound's properties, chemical reactivity, equilibrium conditions, and toxicity (Atugoda, 2021). You et al. (2021) show a competitive uptake between excess H+ and MB. However, when the pH level of the solution increased, the surface charge of the aged PE MPs became negative; it weakened the electrostatic repulsive force and enhanced MB uptake onto MPs (You, 2021). Mahabeer et al. (2023) reported that increasing the medium pH led to an increase in MB dye uptake on MPs derived from waste plastic. The highest uptake was observed at a pH of 12.83, with removal rates ranging from 91.5 % to 94.9 % and adsorption capacity values around 11.4 mg/g (Mahabeer et al., 2023).
On the other hand, the lowest uptake was observed at a pH of 1.5 under the same conditions. The increase in pH leads to the addition of hydroxyl groups on the MPs' surface, which makes them more electronegative (Mahabeer et al., 2023). In 2020, Dahdouh et al.'s findings showed when the pH level is < 6, the Sulphonated Waste Poly Methyl Methacrylate (SW PMMA) becomes positively charged. This decreases uptake efficiency because a high concentration of H+ ions competes with cationic dyes for the active sites on the SW PMMA surface (Dahdouh et al., 2020). The uptake capacity of aniline onto MPs increased at a pH (5–7) above the pKa value (4.6). There is an inhibitory effect in primary conditions (pH = 8) and an unfavorable adsorption process because of repulsion (Chen, 2023). The pH factor affects the adsorbent in two significant ways; firstly, it changes the ionic state of the pollutant or solubility, and secondly, it alters the surface charge of MPs (Du et al., 2022).
3.3.2 Temperature
Studies have shown that higher temperatures can increase the ability of MPs to attract and release organic and inorganic pollutants (Vieira et al., 2021). Dahdouh et al. (2020) stated that the adsorption percentage of two types of dyes, MB and BY28, on SW PMMA decreases as the temperature increases. For MB, the adsorption percentage decreases from 85.27 % to 32.49 %, and BY28 decreases from 87.86 % to 35.61 % when the temperature increases from 20 °C to 60 °C (Dahdouh et al., 2020). Chakraborty et al. (2023) studied the adsorption thermodynamics of MB and MO on CMPs at different temperatures. The results suggested that the values of ΔG, ΔH, and ΔS are negative, which indicates the dye adsorption process is favorable, spontaneous, and exothermic (Chakraborty, 2023). Chen et al. (2023) studied the uptake thermodynamics of aniline on PS at various temperatures. The results showed that the values of ΔG > 0, ΔH < 0, and ΔS < 0, indicating the dye uptake process was nonspontaneous and exothermic. The adsorption capacity of MPs to aniline in high temperatures decreased (Chen, 2023). However, Mahabeer et al. (2023) further investigated the sorption thermodynamics parameters of MB onto MPs in synthetic wastewater and drew the opposite conclusion. This phenomenon can be explained by the fact that at higher temperatures, molecules possess more kinetic energy and move around more rapidly. This thermal energy allows the MB dye molecules to overcome the activation energy for chemisorption onto the MP’s surface. As a result, the uptake capacity of the MPs increases at higher temperatures due to the more vital interaction between the dye and the adsorbent surface. The increase in temperature may have caused the pores of the MP adsorbent to become larger (Mahabeer et al., 2023). Besides, Du et al. (2022) observed a promotion of RhB adsorption on PVC with increasing temperature. One possible explanation is that as the temperature increased, the PVC material became more rigid and glass-like in its structure. This change in the material's physical properties may have made it easier for RhB to adhere and spread across the surface (Du et al., 2022). Hence, the operating temperature affects the dye uptake on the MPs surface, which is an important factor in the process (Mahabeer et al., 2023).
3.3.3 Ionic strength
A gradient solution is used to simulate the salinity of water bodies (estuaries, rivers, and oceans) (Wang, 2023). The existence and concentration of various salts in wastewater influence the salinity (Mahabeer et al., 2023). As salinity levels increase in a system, the adsorption of pollutants such as MG and RhB to negatively charged surfaces of PE and PVC decreases. Because introducing Na+ weakens the electrostatic attraction between the contaminants and the surfaces of PE and PVC. MG and RhB are stable cationic forms in neutral environments, while PE and PVC surfaces are negatively charged at pH 6 (Zhong, 2022). Similar trends were observed for RhB adsorption on PVC, PS, and PET; adsorption gradually decreased when salinity levels increased from 0 to 3.5 % (Du et al., 2022). Wang et al. (2023) discovered that the uptake of MG onto PVC MPs increases with increasing solution salinity. MG in water is cationic and ionizes to generate positively charged colored ions. This is due to dye dimerization in the solution, and high ionic strength promotes dye gathering and enhances surface uptake onto MPs (Wang, 2023). There was a reduction in aniline uptake capacity onto MPs when NaCl concentrations increased to 500 mg/L. Because of accumulation, Na+ occupies adsorption sites and inhibits dye uptake onto MPs (Anastopoulos et al., 2022). Afmataj et al. (2023) showed that phosphate or nitrate ions did not affect RR120 adsorption by PN6, meaning there was no conflict between the dye and competing anions. Aniline adsorption increased with Na+ and Ca2+ in MPs, but Na+ had a more substantial effect than Ca2+ (Afmataj, 2023). MG adsorption by aged nylon MPs decreased as Pb2+ concentration increased, but virgin MPs were unaffected by lead ions. Because aged MPs had carboxyl groups that attracted MG. Virgin nylon MPs accumulated MG mainly through hydrogen bonding, while aged nylon MPs used electrostatic attraction and hydrogen bonding (Lin et al., 2020). The ionic strength negatively affected the adsorption of CV on PE and PP, and it reduced adsorption. There are three possible reasons for this phenomenon: (1) the added cations (like Na+will compete with the cationic CV molecules for the active sites on the MPs; (2) the electrolytes increase the ionic strength in the solution, which affects the diffusion rate of CV in the solution, so the electrolyte makes the CV move slower; and (3) the presence and increase of NaCl weakens the electrostatic attraction between the anionic groups on MPs and the cationic CV. These interactions result in lower uptake capacity of MPs in higher salinity (Du et al., 2022). The researchers believed that MPs have a higher potential to cause harm in freshwater environments than seawater. The reason for this is that MPs tend to absorb certain organic pollutants more easily in freshwater environments (Du et al., 2022).
3.3.4 Presence of humic acid
Natural organic matter (NOM) comprises various components, like polysaccharides, proteins, and humic substances (HSs). HSs are formed by microbial and abiotic breakdown of plants and animal residuals, and they can be found in soil, sediments, rivers, surface water, and groundwater (Sharma et al., 2021). Humic acid is a kind of NOM that has a spiral shape with a negative charge and many oxygen-functional groups. When HSs and MPs combine, they may change their environmental behaviors and form a complex that causes water pollution and harms aquatic organisms and human health (Zhang et al., 2023). HSs have molecules of various sizes that interfere with the adsorption. The giant molecules clog the pores, and the small molecules compete for the same micropores (Liu et al., 2019). Du, Ma and Xing (2022) discovered that PE-MPs and PP-MPs had lower dye adsorption when there were HSs. Without HSs, PE-MPs and PP-MPs removed 27.67 % and 41.50 % of CV, respectively. With HSs (40 mg/L), they only removed 2.91 % and 4.91 % of CV. HSs molecules had more functional groups that could bind to CV by stronger intermolecular interactions, such as hydrogen bonding, electrostatic attraction, and hydrophobic interactions. These interactions weakened the bonds between MPs and CV (Du et al., 2022). Du et al. (2022) reported that when HSs increased, MPs adsorbed less RhB. HSs molecules had many functional groups that could affect how MPs or organics adsorbed organic pollutants. HSs might also bind to RhB through hydrophobic interaction or complexation; it reduces the amount of RhB that MPs could adsorb. Moreover, HSs are a large organic molecule, so they cover the surface of MPs and could block RhB from reaching the uptake sites (Du et al., 2022).
3.4 Effects of MPs properties on dye adsorption
3.4.1 Particle size and specific surface area
Aging processes create smaller-sized MPs (Liu, 2019). Lim et al. (2022) compared the affinity between MPs with different particle sizes and dye. They concluded that the adsorption of RR120 and BB9 onto MPs increased significantly with particle size (Lim et al., 2022). The PS particles were sieved to four size groups by Chen et al. (2023), and they showed that PS had the aniline adsorption capacity of 0.060 mg/g (P4 group) with a specific surface area (0.7214 m2/g). It was 3.3 times higher than that of the P1 group. MPs in groups were irregular in shape, and FTIR analysis showed that among the different groups, there was no difference in chemical structure. The higher adsorption is due to the larger specific surface area (Chen, 2023). There are more likely MPs in the natural environment with smaller sizes, it is due to various physical and chemical effects (Du et al., 2022).
3.4.2 Aging
Over time, microplastic particles undergo changes such as discoloration, cracks, even embrittlement and collapse (Mao et al., 2020). Cracks and pores with distorted texture surfaces attached to fragments and small grains were observed for PP by the researcher. These cracks show a weathering process, binding sites for the surrounding coexisting species, and increasing the force of interaction with dyes (Li et al., 2023). You et al. (2021) reported an increase in the adsorption capacity of the aged PE for MB from 0.63 mg/g to 8.12 mg/g. Hydrophilic with oxygen-containing functional groups increased adsorption capacity (Ben Slama, 2021). Similarly, Wang et al. (2023) demonstrated the effects of potassium permanganate (KMnO4) pre-oxidation on the physicochemical characteristics of PVC MPs and their adsorption capacity for MG in wastewater treatment plants. Pre-oxidation with KMnO4 leads to the production of MnO2 on the surface of PVC MPs and increased their hydrophilicity and MG adsorption. The presence of MnO2 particles on the surface of pre-oxidation PVC MPs was confirmed through XPS and SEM. The composite oxidation treatment intensified the adhesion of MnO2 particles. The adsorption capacity of pristine PVC for MG was 2.6 mg/g, but it increased to 7.0 mg/g for single oxidation and 140.7 mg/g for composite oxidation (Wang, 2023).
3.5 Ecotoxicological effects of ‘MPs-dye’
Studies have exhibited that there are MPs frequently in the tissues of aquatic organisms. MPs can enter organisms’ bodies, especially if less than 1 mm (Zhang, 2022). Environmental contaminants can come into contact with MPs via adsorption. This interaction can be reversed through desorption, where the pollutants are released from MPs and back into the environment (Khoshmanesh et al., 2023). Substances like MPs, whether alone or absorbed with other substances, can have harmful effects on living organisms from a toxicological perspective (Vieira et al., 2021). The presence of other toxic substances on the surface of plastic affects how easily living organisms can absorb a specific chemical; thus, it influence on the bioavailability of that particular compound (Atugoda, 2021). The toxicity of direct untreated discharges from industries using dyes affects aquaculture and humans, animals, and plants (Maheshwari et al., 2021). Most studies focused on investigating the effects of MPs and the long-lasting of persistence organic pollutants (POPs). There is a lack of research that confirms whether MPs can absorb other pollutants within organisms (De Sá et al., 2018). Moorthy et al. (2021) investigated the toxicity of a textile dye (MB) on two freshwater microalgae species (Chlorella vulgaris and Spirulina platensis). The growth rate, pigment, and protein content of both microalgae decreased with increasing concentrations of MB, and S. platensis was more susceptible to the dye than C. vulgaris (Moorthy et al., 2021). The harmful effects of RR 120 on Catla catla (a significant freshwater species) were investigated by Jagruti, 2015. The study evaluated the genotoxic and histotoxic potential of RR 120 and concluded that it has damaging effects on both blood cells and tissue in Catla catla (Jagruti, 2015).
The use of fluorescent MPs/NPs enables the observation and monitoring of their movement within biological systems, which can provide valuable insights into their interactions with molecules, cells, and tissues and their potential toxicity (Malafaia et al., 2022). MPs can play a prominent role in facilitating the transport of pollutants and acting as a source of organic compounds that promote the uptake of contaminants by organisms. This highlights the significance of understanding the impact of MPs in aquatic ecosystems (Ding et al., 2022). Malafaia et al. (2022) studied the toxicity of Nile red dye eluted from fluorescently labeling PE MPs (PE-MPs) on Physalaemus cuvieri. The study found that the Nile red dye eluted from PE-MPs can induce toxicological impacts, including changes in antioxidant activity, mechanisms regulating NO and MDA generation, and AChE activity in the animals (Malafaia et al., 2022). Various biological responses occur in organisms when they are exposed to plastic particles and chemicals (either absorbed into particles or additives) due to their joint toxicity (Bank, 2021). Common toxicity types (synergistic, additive, antagonistic, independent) depend on the physicochemical characteristics of chemical toxins, MPs, and properties of the aquatic organism (Ding et al., 2022). Xiang et al. (2022) discovered that when predators feed D. magna, they accumulate a phototoxic complex in their gut called PS/MB, which causes the accumulation of singlet oxygen. High levels of single oxygen cause significant damage and even death to individual D. magna (Xiang, 2022). To avoid harmful effects on organisms caused by synthetic dyes, it is important to treat wastewater containing dye to decrease the concentration of dye before it is released into the environment.
3.6 Removal of MP-dye
Production of wastewater containing dye has risen with the rapid development of industry and technology. Due to their negative impact on public health and organisms, it is crucial to implement strict treatment processes before discharging wastewater into natural water bodies (Jin et al., 2021). Various techniques for treating wastewater have been suggested, including membrane filtration, chemical reduction, electrochemical treatment, photocatalysis, ion exchange, and biological treatment (Xu et al., 2021). Treating wastewater containing dyes and textiles utilizes different mechanisms. These include membrane-based techniques, electric potential-based approaches, temperature-driven processes, and uptake approaches (Lee et al., 2008). In the textile industry, the dyeing process involves using different types of dyes, salts, and additives, which can harm both humans and aquatic life when present in wastewater. (Gnanasekaran et al., 2021). The paper by Mayorga-Burrezo et al. (2023) described the synthesis of Fe3O4@BiVO4 microrobots using electrostatic interactions between BiVO4 microspheres and Fe3O4 nanoparticles. These microrobots were used to remove cellulose acetate microfilaments from cigarette filters, MPs and RhB dye (Mayorga-Burrezo et al., 2023). Gnanasekaran et al., (2021) used a novel composite membrane made of metal–organic framework (MOFs) MIL-100 (Fe) nanoparticles incorporated into polysulfone (PSF) matrix for the removal of MPs adsorbing dye contaminants from textile wastewater. The membrane showed superior pure water flux, high rejection efficiency of MB (99 %), and excellent capabilities in preventing fouling for MB dye and ‘MPs + MB dye’ solutions (Gnanasekaran et al., 2021). Duan et al. (2022) showed that the elimination efficiencies of 98 % for reactive orange and 93 % for PET were obtained at optimal conditions of pH 11.75, magnesium ion content of 100 mg/L, and polyacrylamide (PAM) content of 4 mg/L (Duan, 2022). According to Khalid et al, (2023) there were excellent adsorption effectiveness, with a high removal rate of 90 % for Congo red dye at a concentration of 10 mg/L with the synthesized magnetic ionic liquid composites (MILCs). The MILCs were also successful in destroying 10-μm PS beads in about 30 min. An Artificial Neural Network (ANN) model with three layers accurately predicted the removal of Congo red dye from an aqueous solution by MILCs, with a minimal mean squared error (MSE) of 0.051 and coefficient of determination (R2) of 0.97. The ANN model showed a good capacity for generalization and prediction, confirming its capability to verify the non-linear behavior of the multicomponent adsorption system of the Congo red adsorbate (Khalid et al., 2023). Li et al. (2023) found that The developed sodium alginate/chitosan-modified graphene oxide reinforced membrane have high removal efficiencies for nanoplastics (NPs), emulsified oil, and dyes in water. It achieved removal rates exceeding 99.87 % for 500 nm NPs, 97.10 % for 50 nm NPs, over 99.17 % for six types of oil-in-water emulsions, over 99.10 % for MB, and 96.80 % for Congo red (CR) (Li et al., 2023). In research was conducted by Peng et al. (2024) the hybrid membrane demonstrated high separation efficiency for various pollutants, including dyes and pesticides, and achieved almost complete removal. It also exhibited superhydrophilicity and superoleophobicity, allowing selective penetration of water molecules and repelling non-polar pollutants. The membrane was capable of separating oil–water mixtures and removing MPs, dyes, and pesticides simultaneously (Peng, 2024). New and innovative methods must be developed to remove MPs and dyes.
4 Conclusions and remarks
This review focuses on the interactions and processes involved in the dyes adsorption by MPs in aquatic media. The potential environmental influences and physicochemical properties of MPs on adsorption behaviors have been emphasized. The bibliometric analysis revealed that dye adsorption by MPs has been widely studied. Keyword co-occurrence mapping analysis indicated that MPs, adsorption, and dye have high-strength links. The most investigated MPs for dye adsorption were PP, PET, and plastic waste. The dye adsorption onto MPs matched the pseudo-second-order kinetic model. This suggests that the chemistry mechanism governs the interaction between dyes and MPs. Higher temperatures increased the desorption efficiency. This research provides valuable insights for advancing wastewater treatment technologies to address dye and MP removal and conducting ecotoxicological studies. Studying the impact of MPs and dye on aquatic organisms is crucial for their health preservation. Nonetheless, there has been minimal research into the ecotoxicological effects of dyes absorbed by MPs on living organisms.
The combined contamination of MPs with organic pollutants such as dyes in natural environments is more complex, and studies on the interactions between these compounds and their risk assessment are necessary. Extended field experiments and laboratory investigations improve our comprehension of adsorption processes, the fate of pollutants, and their behavior in the environment. These experiments are crucial in developing resilient models and practical strategies to tackle environmental challenges.
CRediT authorship contribution statement
Madineh Khoshmanesh: Investigation, Methodology. Ali Mohammad Sanati: Investigation, Software, Supervision. Seyedehfatemeh Shahcheragh: Data curation, Investigation, Methodology. Sima Farjadfard: Resources, Validation, Writing – original draft. Ziaeddin Bonyadi: Conceptualization, Methodology. Bahman Ramavandi: Conceptualization, Methodology, Supervision, Writing – review & editing.
Acknowledgment
We sincerely thank Dr. Lucy Semerjian (University of Sharjah, Sharjah, United Arab Emirates) for her constructive comments that have improved the quality of the work.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Life history and behavior effects of synthetic and natural dyes on Daphnia magna. Chemosphere. 2019;236:124390
- [Google Scholar]
- Adsorption of reactive red 120 dye by polyamide nylon 6 microplastics: isotherm, kinetic, and thermodynamic analysis. Water. 2023;15(6):1137.
- [Google Scholar]
- Microplastic pollution in seawater and marine organisms across the tropical eastern Pacific and galápagos. Sci. Rep.. 2021;11(1):1-8.
- [Google Scholar]
- Microplastics as carriers of hydrophilic pollutants in an aqueous environment. J. Mol. Liq.. 2022;350:118182
- [Google Scholar]
- Synthesis and use of glycidyl methacrylate-g-poly (ethylene terephthalate) fiber containing iminodiacetate groups for the highly effective removal of basic dye. J. Mater. Sci. Eng. Adv. Technol. 2018;18:1-19.
- [Google Scholar]
- Interactions between microplastics, pharmaceuticals and personal care products: implications for vector transport. Environ. Int.. 2021;149:106367
- [Google Scholar]
- The use of low-cost adsorbent (canola residues) for the adsorption of methylene blue from aqueous solution: isotherm, kinetic and thermodynamic studies. Colloids Interface Sci. Commun.. 2015;7:16-19.
- [Google Scholar]
- Equilibrium and kinetic studies on free cyanide adsorption from aqueous solution by activated carbon. J. Hazard. Mater.. 2009;170(1):127-133.
- [Google Scholar]
- Diversity of synthetic dyes from textile industries, discharge impacts and treatment methods. Appl. Sci.. 2021;11(14):6255.
- [Google Scholar]
- Engineering properties, microplastics and emissions assessment of recycled plastic modified asphalt mixtures. Sci. Total Environ.. 2023;893:164869
- [Google Scholar]
- Assessment of water contamination caused by a mutagenic textile effluent/dyehouse effluent bearing disperse dyes. J. Hazard. Mater.. 2010;174(1–3):694-699.
- [Google Scholar]
- “Adsorption of acid and basic dye from the simulated wastewater using carbonized microplastic particles synthesized from recycled polyethylene terephthalate plastic waste bottles: an integrated approach for experimental and practical applications”, AQUA-water infrastructure. Ecosyst. Soc.. 2023;72(4):491-506.
- [Google Scholar]
- Adsorption behavior of aniline pollutant on polystyrene microplastics. Chemosphere. 2023;323:138187
- [Google Scholar]
- Microplastics and organics–a comparative study of sorption of triclosan and malachite green onto polyethylene. Water Sci. Technol.. 2023;87(5):1072-1081.
- [Google Scholar]
- Removal of methylene blue and basic yellow 28 dyes from aqueous solutions using sulphonated waste poly methyl methacrylate. J. Polym. Environ.. 2020;28:271-283.
- [Google Scholar]
- Studies of the effects of microplastics on aquatic organisms: what do we know and where should we focus our efforts in the future? Sci. Total Environ.. 2018;645:1029-1039.
- [Google Scholar]
- Response surface modeling of Orange-G adsorption onto surface tuned ragi husk. Colloid Interface Sci. Commun.. 2021;41:100363
- [Google Scholar]
- Response characteristics of indigenous microbial community in polycyclic aromatic hydrocarbons (PAHs) contaminated aquifers under polyethylene microplastics stress: a microcosmic experimental study. Sci. Total Environ.. 2023;894:164900
- [Google Scholar]
- Microplastics altered contaminant behavior and toxicity in natural waters. J. Hazard. Mater.. 2022;425:127908
- [Google Scholar]
- Adsorption of rhodamine B on polyvinyl chloride, polystyrene, and polyethylene terephthalate microplastics in aqueous environments. Environ. Technol. Innov.. 2022;27:102495
- [Google Scholar]
- Identification of naturally weathering microplastics and their interactions with ion dyes in aquatic environments. Mar. Pollut. Bull.. 2022;174:113186
- [Google Scholar]
- Coagulation performance and floc properties for synchronous removal of reactive dye and polyethylene terephthalate microplastics. Process Saf. Environ. Prot.. 2022;165:66-76.
- [Google Scholar]
- Adsorption of malachite green in aqueous solution using sugarcane bagasse-barium carbonate composite. Colloid Interface Sci. Commun.. 2021;44:100485
- [Google Scholar]
- Adsorption behavior of organic pollutants on microplastics. Ecotoxicol. Environ. Saf.. 2021;217:112207
- [Google Scholar]
- Behaviors and influencing factors of the heavy metals adsorption onto microplastics: a review. J. Clean. Prod.. 2021;319:128777
- [Google Scholar]
- A high-flux metal-organic framework membrane (PSF/MIL-100 (Fe)) for the removal of microplastics adsorbing dye contaminants from textile wastewater. Sep. Purif. Technol.. 2021;277:119655
- [Google Scholar]
- Development of pyrene-based MOFs probe for water content and investigations on their mechanochromism and acidochromism. J. Alloys Compd.. 2023;968:172004
- [Google Scholar]
- The chemical behaviors of microplastics in marine environment: a review. Mar. Pollut. Bull.. 2019;142:1-14.
- [Google Scholar]
- Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollut.. 2018;236:218-225.
- [Google Scholar]
- Evaluation of azo dye toxicity using some haematological and histopathological alterations in fish Catla catla. Int. J. Environ. Ecol. Eng.. 2015;9(5):458-461.
- [Google Scholar]
- Rapid removal of methylene blue and nickel ions and adsorption/desorption mechanism based on geopolymer adsorbent. Colloid Interface Sci. Commun.. 2021;45:100551
- [Google Scholar]
- Harnessing the power of iron-alumina-based ionic liquid composites for simultaneous removal of Congo red dye and microplastics. J. Clean. Prod.. 2023;429:139602
- [Google Scholar]
- Co-occurrence of microplastics and organic/inorganic contaminants in organisms living in aquatic ecosystems: a review. Mar. Pollut. Bull.. 2023;187:114563
- [Google Scholar]
- Investigating the process of electrocoagulation in the removal of azo dye from synthetic textile effluents and the effects of acute toxicity on Daphnia magna test organisms. J. Water Process Eng.. 2022;45:102485
- [Google Scholar]
- Preparation and characterization of polymer–carbon composite membranes for the removal of the dissolved salts from dye wastewater. Dye. Pigment.. 2008;76(2):372-378.
- [Google Scholar]
- Removal of malachite green dye from aqueous solution by adsorbents derived from polyurethane plastic waste. J. Environ. Chem. Eng.. 2021;9(1):104704
- [Google Scholar]
- Enhance pore structure of cyanobacteria-based porous carbon by polypropylene to improve adsorption capacity of methylene blue. Bioresour. Technol.. 2022;343:126101
- [Google Scholar]
- Multifunctional sodium alginate/chitosan-modified graphene oxide reinforced membrane for simultaneous removal of nanoplastics, emulsified oil, and dyes in water. Int. J. Biol. Macromol. 2023125524
- [Google Scholar]
- Review of organic and inorganic pollutants removal by biochar and biochar-based composites. Biochar. 2021;3:255-281.
- [Google Scholar]
- Potential of adsorption of diverse environmental contaminants onto microplastics. Water. 2022;14(24):4086.
- [Google Scholar]
- Adsorption of malachite green from aqueous solution by nylon microplastics: reaction mechanism and the optimum conditions by response surface methodology. Process Saf. Environ. Prot.. 2020;140:339-347.
- [Google Scholar]
- Hexabromocyclododecane alters malachite green and lead (II) adsorption behaviors onto polystyrene microplastics: interaction mechanism and competitive effect. Chemosphere. 2021;265:129079
- [Google Scholar]
- New insights into the aging behavior of microplastics accelerated by advanced oxidation processes. Environ. Sci. Technol.. 2019;53(7):3579-3588.
- [Google Scholar]
- Waste polystyrene foam–chitosan composite materials as high-efficient scavenger for the anionic dyes. Colloids Surfaces A Physicochem. Eng. Asp.. 2021;627:127155
- [Google Scholar]
- Calculation of carbon emissions in wastewater treatment and its neutralization measures: a review. Sci. Total Environ. 2023169356
- [Google Scholar]
- Interactions between microplastics and phthalate esters as affected by microplastics characteristics and solution chemistry. Chemosphere. 2019;214:688-694.
- [Google Scholar]
- Aging and characterization of disposable polypropylene plastic cups based microplastics and its adsorption for methylene blue. Chemosphere. 2024;349:140976
- [Google Scholar]
- Effect of agricultural organic inputs on nanoplastics transport in saturated goethite-coated porous media: particle size selectivity and role of dissolved organic matter. Environ. Sci. Technol.. 2022;56(6):3524-3534.
- [Google Scholar]
- Implementation of microplastics derived from waste plastic for uptake of MB dye: performance and LCA study. Desalination. 2023;546:116214
- [Google Scholar]
- Dye Pollution in Water and Wastewater BT - Novel Materials for Dye-Containing Wastewater Treatment. Singapore: Springer Singapore; 2021. p. :1-25.
- When toxicity of plastic particles comes from their fluorescent dye: a preliminary study involving neotropical Physalaemus cuvieri tadpoles and polyethylene microplastics. J. Hazard. Mater. Adv.. 2022;6:100054
- [Google Scholar]
- Efficient and reusable polyethylene oxide/polyaniline composite membrane for dye adsorption and filtration. Colloid Interface Sci. Commun.. 2020;39:100314
- [Google Scholar]
- Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals. J. Hazard. Mater.. 2020;393:122515
- [Google Scholar]
- Photocatalysis dramatically influences motion of magnetic microrobots: application to removal of microplastics and dyes. J. Colloid Interface Sci.. 2023;643:447-454.
- [Google Scholar]
- Acute toxicity of textile dye methylene blue on growth and metabolism of selected freshwater microalgae. Environ. Toxicol. Pharmacol.. 2021;82:103552
- [Google Scholar]
- A mini-review on the use of plastic waste as a modifier of the bituminous mix for flexible pavement. Clean. Mater.. 2022;4:100059
- [Google Scholar]
- Constructing green superhydrophilic and superoleophobic COFs-MOFs hybrid-based membrane for efficiently emulsion separation and synchronous removal of microplastics, dyes, and pesticides. Environ. Res.. 2024;243:117777
- [Google Scholar]
- Degradation of azo dyes: bacterial potential for bioremediation. Sustainability. 2022;14(3):1510.
- [Google Scholar]
- Factors affecting synthetic dye adsorption; desorption studies: a review of results from the last five years (2017–2021) Molecules. 2021;26(17):5419.
- [Google Scholar]
- Environmental factors-mediated behavior of microplastics and nanoplastics in water: a review. Chemosphere. 2021;271:129597
- [Google Scholar]
- Classification and impact of synthetic textile dyes on aquatic Flora: a review. Reg. Stud. Mar. Sci.. 2021;45:101802
- [Google Scholar]
- A novel clinoatacamite route to effectively separate Cu for recycling Ca/Zn/Mn from hazardous smelting waterwork sludge. J. Environ. Chem. Eng.. 2024;12(2):112024
- [Google Scholar]
- Pb (II) uptake onto nylon microplastics: interaction mechanism and adsorption performance. J. Hazard. Mater.. 2020;386:121960
- [Google Scholar]
- Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: a review. Sci. Total Environ.. 2020;717:137222
- [Google Scholar]
- Sorption potential of microplastics for azo-and phthalocyanine printing dyes. Dye. Pigment.. 2023;209:110884
- [Google Scholar]
- Efficient removal of cationic dyes from aqueous solutions using a modified poly (ethylene terephthalate) fibers adsorbent. Polym. Technol. Mater.. 2020;59(5):527-535.
- [Google Scholar]
- Microplastics physicochemical properties, specific adsorption modeling and their interaction with pharmaceuticals and other emerging contaminants. Sci. Total Environ.. 2021;753:141981
- [Google Scholar]
- Impact of incineration slag co-disposed with municipal solid waste on methane production and methanogens ecology in landfills. Bioresour. Technol.. 2023;377:128978
- [Google Scholar]
- Adsorption–desorption behavior of malachite green by potassium permanganate pre-oxidation polyvinyl chloride microplastics. Environ. Technol. Innov.. 2023;30:103138
- [Google Scholar]
- Comparing the adsorption of methyl orange and malachite green on similar yet distinct polyamide microplastics: uncovering hydrogen bond interactions. Chemosphere. 2023;340:139806
- [Google Scholar]
- CuCo2O4/CF cathode with bifunctional and dual reaction centers exhibits high RhB degradation in electro-Fenton systems. J. Electroanal. Chem.. 2024;956:118072
- [Google Scholar]
- Dual drive acute lethal toxicity of methylene blue to Daphnia magna by polystyrene microplastics and light. Sci. Total Environ.. 2022;840:156681
- [Google Scholar]
- Xanthated chitosan/cellulose sponges for the efficient removal of anionic and cationic dyes. React. Funct. Polym.. 2021;160:104840
- [Google Scholar]
- MX@ MIL-125 (Ti)-mediated sonocatalytic degradation for the dyes and microplastics. Sep. Purif. Technol. 2024126488
- [Google Scholar]
- Adsorption–desorption behavior of methylene blue onto aged polyethylene microplastics in aqueous environments. Mar. Pollut. Bull.. 2021;167:112287
- [Google Scholar]
- Physiological and biochemical effects of polystyrene micro/nano plastics on Arabidopsis thaliana. J. Hazard. Mater. 2024133861
- [Google Scholar]
- The contamination of microplastics in China’s aquatic environment: occurrence, detection and implications for ecological risk. Environ. Pollut.. 2022;296:118737
- [Google Scholar]
- Adsorption of typical natural organic matter on microplastics in aqueous solution: kinetics, isotherm, influence factors and mechanism. J. Hazard. Mater.. 2023;443:130130
- [Google Scholar]
- Competition adsorption of malachite green and rhodamine B on polyethylene and polyvinyl chloride microplastics in aqueous environment. Water Sci. Technol.. 2022;86(5):894-908.
- [Google Scholar]
- Microfiber from textile dyeing and printing wastewater of a typical industrial park in China: occurrence, removal and release. Sci. Total Environ.. 2020;739:140329
- [Google Scholar]







