Translate this page into:
Recent advances in the piperazine based antiviral agents: A remarkable heterocycle for antiviral research
⁎Corresponding authors. matloob.ahmad@gcuf.edu.pk (Matloob Ahmad), mezaki@imamu.edu.sa (Magdi E.A. Zaki)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
A growing interest in pharmacology has made heterocyclic chemistry as one of the emerging branches of organic chemistry. Piperazine is an excellent heterocycle that possesses a large span of pharmaceutical applications. Piperazine derived compounds have shown multiple therapeutic activities such as antioxidant, antibacterial, analgesic, anticancer, antihypertensive, antiallergic, anti-inflammatory, antimalarial, antipsychotic, cardioprotective, antifungal, antidepressant and antiviral. FDA has approved many piperazine scaffold-based drugs for the treatment of various viral infections, therefore establishing the pharmacological importance of piperazine derivatives. Only a few reviews on antiviral activities of piperazine containing compounds are available in the literature, despite of its great medicinal significance. This review deals with piperazine derived compounds as antiviral agents covering the literature reports from 2010 to 2023.
Keywords
Antiviral piperazine derivatives
Anti-SARS-CoV-2 agents
Anti-HIV agents
Anti-HCV agents
Anti-TMV agents
Anti-influenza agents
1 Introduction
Viral infections affect millions of people worldwide and present significant health challenges. Viral diseases including emerging, re-emerging and chronic infections are increasing health concerns throughout the world. Ebola virus, Marburg virus, human immunodeficiency virus (HIV), Chikungunya virus, dengue virus, severe acute respiratory syndrome (SARS), hepatitis C virus, Middle East respiratory syndrome (MERS), coronavirus (CoV), and human-avian influenza viruses render immense trouble for human beings. From 1940 to 2004, 5.3 viruses have emerged per year and among them, 60–70% viruses are human pathogens (Stramer, 2014). Several viral epidemics have been observed in previous two decades such as severe acute respiratory syndrome coronavirus (SARS-CoV) epidemic in 2002; influenza A H1N1 pandemic in 2009; Middle East Respiratory Syndrome (MERS) in 2012; and acute respiratory syndrome coronavirus-2 (SARS-CoV-2) epidemic in 2019, the newly COVID-19 pandemic. Therefore, the prohibition and cure of viral diseases is a world-wide health challenge. The discovery of novel antivirals have assumed more urgency today because of the arising and re-appearing of viruses like severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which causes COVID-19 (Brown and McCullough, 2020). During the previous ten years, the development and use of antiviral agents has achieved significant success for treatment of some viral infections (De Rycker et al., 2018). Investigations in molecular virology has opened new corridors in the knowledge and awareness of virus properties, the nature of the obligate intracellular parasitism of viruses and to some extent, the mechanisms of viral diseases (Moradpour et al., 2016; Guo et al., 2017). Previously, a single agent was used for the treatment of a specific viral infection but now the concept of broad-spectrum antiviral agents (BSAAs) is becoming popular. The hypothesis of BSAAs has recently gained stimulus due to the epidemics of ebola, influenza, zika, dengue and other viral infections. BSAAs obstruct a wide variety of human viruses (Bekerman and Einay, 2015; Debing et al., 2015).
Although some highly potent drugs have been developed against these viruses but the problem of drug resistance is also associated with this advancement. The development of drug resistance is a big hindrance in the alleviation of viral infections (Little et al., 2002; Shafer et al., 2007). Heterocyclic compounds form the core skeleton of several beneficial medicinal agents (Rizvi et al., 2012; Ahmad et al., 2013; Ahmad et al., 2014; Khalid et al., 2015; Akhtar et al., 2019; Rafiq et al., 2023a) and hence are essential in the new drug development. Nitrogen-containing heterocycles also displayed interesting biological activities in various pharmacological agents like antimicrobial (Ahmad et al., 2012; Naz et al., 2017) and anticancer (Rasool et al., 2017; Kanwal et al., 2018; Tandon et al., 2019; Walayat et al., 2019). Among nitrogen containing heterocycles, piperazine has acquired consideration due to its wide pharmacological activities (Rathi et al., 2016). In this regard, piperazine can also be considered as the most ubiquitous and functional heterocyclic moiety for the discovery of novel biological active analogues (Brito et al., 2019). In this review, we have elaborated the most potent piperazine derivatives as antiviral agents. We have summarized and highlighted the antiviral activities of piperazine-based compounds predominantly after the year 2009.
2 Piperazine-based FDA approved antiviral drugs and piperazine derivatives in clinical development
Piperazine is a privileged scaffold which has been considerably probed for various pharmacological activities. Piperazine can bind to multiple moieties to result in several marketed drugs. For example, atevirdine (1 Fig. 1), a non-nucleoside reverse transcriptase inhibitor contains a piperazine ring in its structure (Morse et al., 2000). Bictegravir sold under the trade name Biktarvy (2) has been approved by the FDA and is available as a single tablet regimen for the cure of HIV-1 and simian immunodeficiency viruses (SIV) (Mandal et al., 2019). Vicriviroc (3) has been successfully developed to phase II/III clinical studies and accredited for clinical applications in the last five years as HIV-1 co-receptor antagonist (Lou et al., 2014). Delavirdine (4) received FDA approval as a remedy for HIV infection (Chaudhuri et al., 2018). Dolutegravir (5) is also FDA accepted drug which is used with other medicines to treat HIV/AIDS infection as an integrase strand transfer inhibitor (INSTI) (Nilavar et al., 2020). Indinavir (6) was certified by the FDA in 1996 for treating HIV disorder and its docking studies predicted it as capable of SARS-CoV-2 inhibition (Mamidala et al., 2020). Letermovir (7), developed by Merck & Co. and sold by the trade name prevymis, is given for the treatment and prophylaxis of cytomegalovirus infection (Kim, 2018). Nucleozin (8) is an antiviral agent which is identified to target the viral nucleoprotein. It also inhibits the nuclear accumulation of nucleoprotein. Nucleozin unites to influenza A nucleoprotein which is a versatile RNA-binding protein vital for viral replication (Amorim et al., 2013). Vesatolimod (9) is pharmacodynamically active agent for HIV infected individuals and is in clinical trials. In phase II study, vesatolimod has been identified as an immune-inducing medicine for chronic HBV patients (Janssen et al., 2018).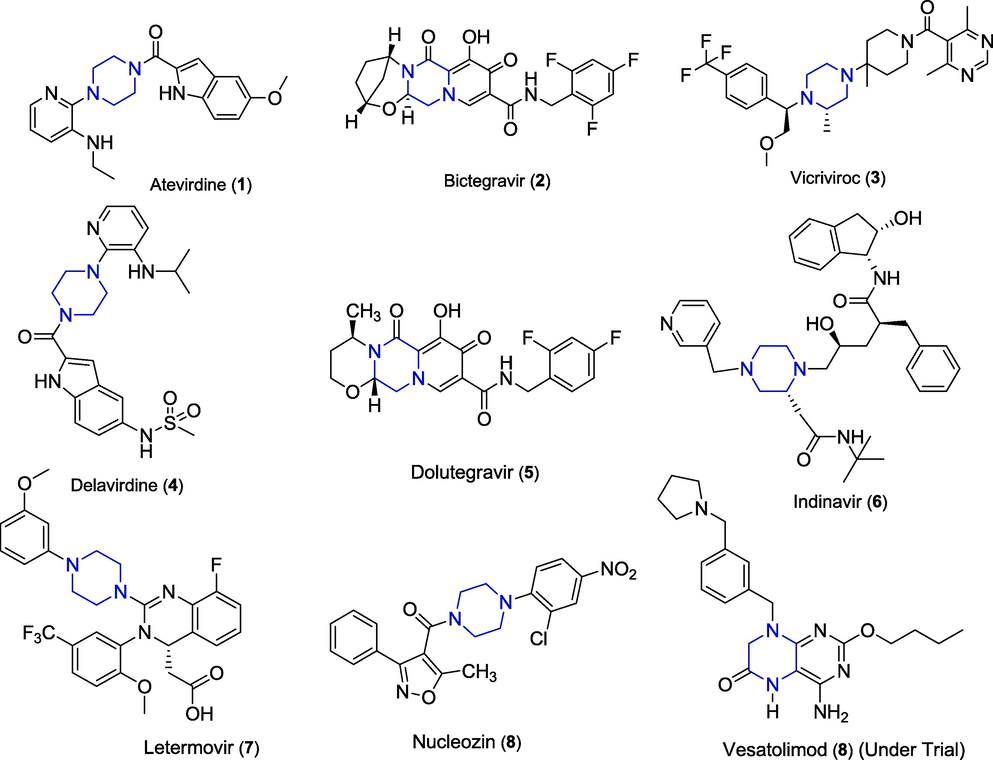
Piperazine-based FDA approved antiviral drugs and compounds in clinical development.
3 Piperazine-based antiviral agents
3.1 Anti-HIV activity of piperazine derivatives
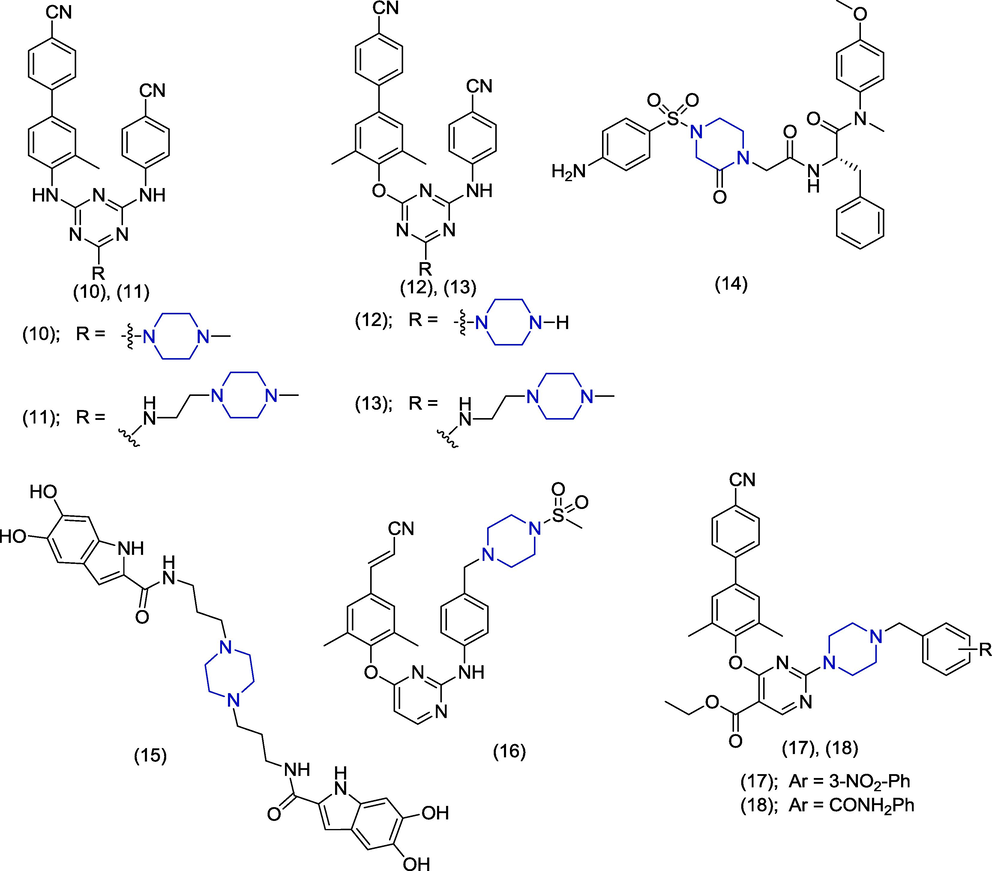
Piperazine derivatives demonstrate a variety of antiviral activities (Fig. 2). Idoxuridine was the first antiviral drug licensed in 1963 and after that ninety (90) antiviral drugs have been approved which comprise 13 different functional groups (De Clercq and Li, 2016). HIV is a retrovirus which persists all over the map and is communicated by tainted blood transfusion, unprotected coupling and hypodermic syringes. In 2021, there were 38.4 million victims of HIV with 1.5 million new patients https://www.unaids.org/en/resources/fact-sheet. Presently, there is no licensed vaccine for HIV prevention. Anti-HIV agents reduce the dissemination of HIV infection and improve the health of HIV infected individuals (Cohen et al., 2011). Current HIV treatments comprise a combination of therapeutics that target viral reverse transcriptase, protease enzymes and viral entry. However, the existing therapeutic agents are associated with major side effects and HIV can develop resistance against this agent. Therefore, there is an immediate demand for the addition of novel antiviral agents with a distinct mode of action. In 2020, Jin et al. designed novel series of non-nucleoside HIV-1 reverse transcriptase inhibitors comprising biphenyl-substituted diaryltriazine core. The 4-cyanobiphenyl and 4-cyano aniline group are present in all derivatives (Jin et al., 2020). The synthesized compounds exhibited significant activity against both the wild type (WT) HIV-1 and a panel of HIV-1 mutant strains. However, the piperazine-based derivatives (10) and (11) (Fig. 3) were found to have excellent anti-HIV-1 IIIB wild-type activities with EC50 values of 0.0033 ± 0.6 µM and 0.005 ± 1.0 µM, respectively with reference to nevirapine (EC50 = 0.0045 ± 2 µM), efavirenz (EC50 = 0.001 ± 0.3 µM) as standard drugs. Compounds (12) and (13) also presented prominent activity against WT HIV-1 reverse transcriptase with IC50 values of 0.242 ± 0.020 µM and 0.368 ± 0.021 µM, respectively. In derivatives 10 and 11, the 4-cyanobiphenyl group is attached to triazine ring through the nitrogen bridge. In the case of derivatives 12 and 13, the 4-cyanobiphenyl group is connected to the triazine ring through the oxygen bridge. Sun et al. designed, synthesized and studied the HIV-1 capsid inhibition activity of novel benzenesulfonamide-containing phenylalanine derivatives. Derivatives having 2-piperazinone scaffold exhibited prominent anti-HIV activity. The phenylalanine derivative (14) having 2-piperazinone moiety was found to have 5.78-fold better anti-HIV activity (EC50 = 0.09 ± 0.03 μM) in comparison to the reported standard PF-74 (EC50 = 0.52 ± 0.18 μM). Derivative 14 presented a selectivity index>383.36. Derivative 14 carries a p-amino-substituted benzenesulfonyl group. Compound 14 also showed beneficial pharmacokinetic properties such as oral bioavailability and were found non-toxic till a dose of 1000 mg/kg (Sun et al., 2020). Esposito and co-researchers explored dihydroxyindole-2-carboxylic acid derivatives as blockers of reverse transcriptase associated ribonuclease (RNase) and HIV-1 integrase. Among all synthesized compounds, the bis-propyl-piperazine linker containing symmetric compound (15) exhibited significant inhibition (IC50 = 7.0 ± 0.1 μM) of HIV-1 integrase (IN) activity. The dioxole analogue of derivative 15 was found to be inactive (IC50 > 100 μM) (Esposito et al., 2020). Huang and his team discovered potent diarylpyrimidine-type derivatives in which heterocyclic rings such as piperazine, piperidine and morpholine were introduced. The HIV-1 inhibitory activity as a non-nucleoside reverse transcriptase inhibitor (NNRTI) against wild-type and E138K mutant virus was carried out. These derivatives presented prominent potency towards WT HIV-1 strain upon evaluation by anti-HIV assay. However, the piperazine containing compound (16) was found as the most agent (EC50 = 0.0035 µM) with prominent selectivity index (SI < 48774). Most importantly the compound (16) also demonstrated excellent activity against resistant virus strain E138K with an EC50 value of 0.0075 µM. Compound 16 also exhibited better water solubility (30.92 ± 0.02 µM) which makes it a strong candidate for the future drug (Huang et al., 2019). In 2018, Jin et al. synthesized a series of substituted piperazine-1-yl-pyrimidine derivatives as HIV-1 non-nucleoside inhibitor. The antiviral activity was evaluated as reverse transcriptase inhibitors in MT-4 cells. The results revealed inhibitory activities of 17 (EC50 = 31.5 µM) and 18 (EC50 = 3.36 µM) analogs against wild-type strain. In molecular docking studies of compound 17 with binding pocket of non-nucleoside reverse transcriptase (NNIBP), the biphenyl group interacted with aromatic rich pocket. The pyrimidine ring was involved in hydrogen bond formation with NH group of Lys10. The nitro group is also involved in hydrogen bond interactions. The piperazine ring increased the flexibility and lipophilicity of these derivatives (Jin et al., 2018).
Antiviral activities of piperazine derivatives.
Anti-HIV agents having piperazine core.
In 2017, Zhou et al. carried out the structural modification of a novel viral infectivity factor (Vif) antagonist and reported that piperazine derivative 19 (Fig. 4) has good anti-HIV activity (EC50 = 8.24 µM) by inhibiting Vifin non-permissive H9 cells. But derivative 19 was found more cytotoxic (CC50 = 30.6 µM) in this series and showed less selectivity index (3.71) (Zhou et al., 2017). In 2015, Rivero-Buceta studied the anti-HIV activity of different compounds and also the mechanism of anti-viral activity. These derivatives contain 2,4,6-triethylbenzene as a central scaffold. Studies reported that piperazine containing compound (20) demonstrated the HIV-1 integrase inhibitory and gp120 binding capacity in the moderate range having EC50 value>10 µM. The piperazine ring served as a linker and 2,3,4-trihydroxybenzoyl group is attached at position 1,3,5 of the triethylbenzene through the piperazine linker group (Rivero-Buceta et al., 2015). Zhao et al. discovered the novel N-aryl piperazine tetrahydroquinoline derivatives as chemokine receptor (CXCR4) inhibitors. Compounds (21) and (22) emerged as the most potent derivatives against HIV1 with the IC50 values of 0.02 µM in HIV-1 repression assay. These derivatives contain benzoyl group and phenyl sulfonyl group attached with piperazine ring (Zhao et al., 2015). Ashok and co-workers synthesized and evaluated the β-carboline derivatives against HIV-1 viral strain. These compounds consist of central pyridoindole scaffold and different derivatives were synthesized by altering the phenyl substituent of piperazine ring. Among the reported analogues, the compound (23) demonstrated significant activity (EC50 = 0.53 µM) as an inhibitor of syncytia formation and decent selectivity index (SI = 483). Compounds (24), (25) and (26) exhibited moderate activities presenting EC50 values of 3.8 µM, 3.8 µM and 2.8 µM and selectivity index values > 105, >105 and 3.85 respectively. Compound (23) also showed inhibition of p24 antigen expression in acute HIV-1IIIB infected cell line presenting an EC50 value of 1.1 µM (Ashok et al., 2015). Derivatives in this series followed the Lipinski’s rule of five with minor violations (Lipinski, 2004).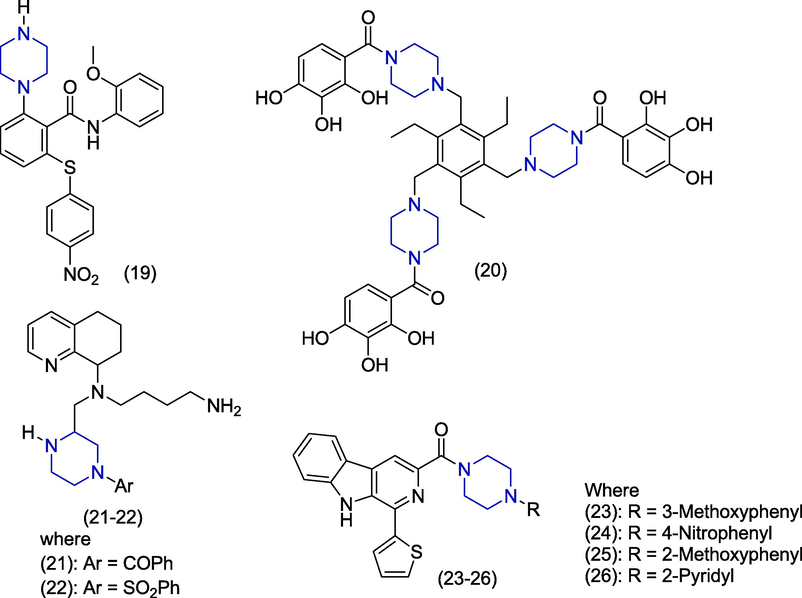
Piperazine derivatives as anti-HIV agents.
Chen et al. synthesized piperazine-based triazine derivatives (27), (28), (29) and (30) (Fig. 5) and also evaluated them against wild-type and resistant strains of HIV-1 (K103N/Y181C). Derivative (29) presented EC50 values of 0.030 ± 0.0035 µM and ≥ 1.2 µM against wild-type and K103N/Y181C strains of HIV-1 in the MT-4 cell line (Chen et al., 2015). Hu et al. used fragment linking technique to design and synthesize novel 2-methylpiperazine analogues as CCR5 antagonist. Promising antiviral activities were observed for piperazine containing derivatives (31–34) having IC50 values of 0.0472 µM, 0.146 µM, 0.0314 µM and 0.0751 µM, respectively with reference to maraviroc (IC50 = 0.0055 µM). Derivative 31 carries 4-cyanobenzyl group while 32–34 carry 4-fluorobenzyl group attached at the position 4 of the piperazine group. Substitution of 4-fluorobenzyl by methyl benzyl or simple benzyl group produced less active derivatives. These derivatives in the concentration of 10 μM exhibited no cytotoxicity upon evaluation by CCK-8 assay (Hu et al., 2015). Tuyishime and coworkers used virtual blaze screening procedure to analyze million available compounds. Fifty derivatives were scrutinized for anti-HIV potential by using viral inhibition assays. The compound (35) appeared as the most potent against HIV-1YU-2 displaying an IC50 value of 13.1 ± 1.7 µM. The compound (35) contains a piperazine ring connected to acenaphthene group. The replacement of piperazine with di-pyrrolidine produced weakly active derivatives. Then, the acenaphthene scaffold was modified to produce more active derivatives (Tuyishime et al., 2014). In 2013, T. Liu et al. designed and synthesized new piperazine derivatives by using fragment linking and bioisosteric principles. Among different evaluated compounds, compound (36) was found the most potent anti-HIV-1 inhibitor (IC50 = 0.44 µM) while other piperazine containing derivatives showed inferior activities. Derivative 36 also showed CC5 antagonistic activity having an IC50 value of 6.29 µM (T. Liu et al., 2013).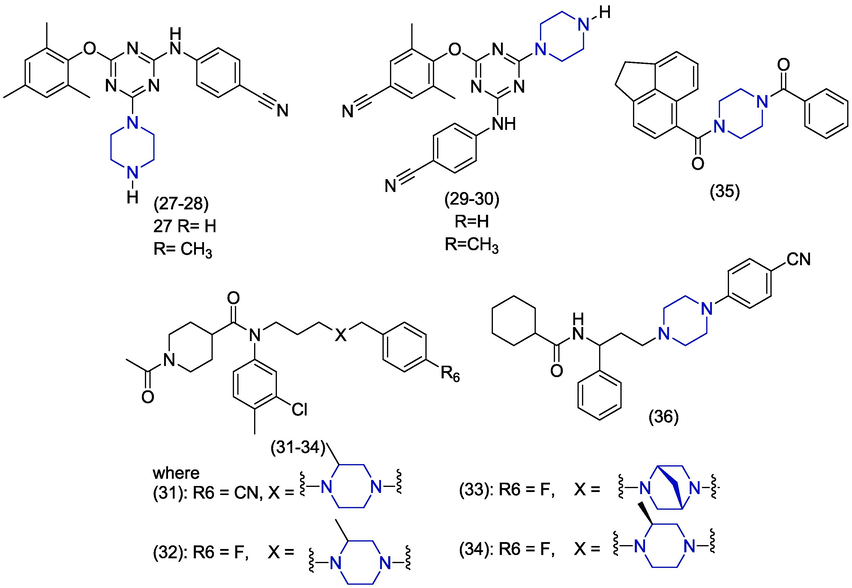
Piperazine derived compounds as anti-HIV agents.
Serrao et al. discovered the new 5-carbonyl-1H-imidazole-4-carboxamide analogues as HIV-1 inhibitors. Various aromatic rings and amines were introduced at positions 4 and 5. Piperazine containing derivatives (37 and 38) (Fig. 6) showed excellent activities towards the inhibition of integrase lens epithelium-derived growth factor (IN-LEDGF/p75) and HIV-1 with IC50 values>20 µM (Serrao et al., 2013). Yeung et al. synthesized of indole-7-carboxamides and evaluated their HIV-1 inhibition capabilities. The piperazine containing indole derivatives were found as potent antiviral agents. The analogues (39–43) displayed promising in vitro antiviral activities with the EC50 values of 5.2 × 10-4 µM, 2.9 × 10-4 µM, 2 × 10-5 µM, 5.8 × 10-6 µM, and 4 × 10-5 µM, respectively. Derivatives 39–41 emerged as most promising because they were less toxic with CC50 values>300 µM. While derivatives (42) and (43) are more toxic presenting CC50 values of 29 µM and 9.2 µM, respectively. Derivative 39 exhibited better oral bioavailability and human liver microsomal stability (Yeung et al., 2013). Zentner et al. reported the piperazine derivatives as HIV-1 matrix (MA) protein inhibitors. The compound (44) came out as the most active inhibitor of HIV-1 MA with IC50 values in the range of 7.5 to 15.6 µM against different subtypes. This compound competes with phosphatidylinosityl 4,5-biphosphate (PI[4,5]P2) for MA protein binding. The compound 44 interacted with HIV-I matrix protein presenting a Kd value of 22.6 ± 3.1 μM (Zentner et al., 2013). In 2012, Ali et al. reported the synthesis of benzamide analogues and also evaluated HIV-1 inhibition studies. Upon evaluation of antiviral activities, the piperazine containing analogue (45) showed prominent antiviral potential against H9 and MT4 cell lines having IC50 values of 3.9 µM and 3.7 µM, respectively and presented CC50 value of 70 µM. Compound 45 carries a piperazine ring attached with ring B through a carbonyl group. Derivatives having thioether linkage between ring A and B showed better activity. Similarly the amide or sulfonamide group was found effective between ring B and C (Ali et al., 2012).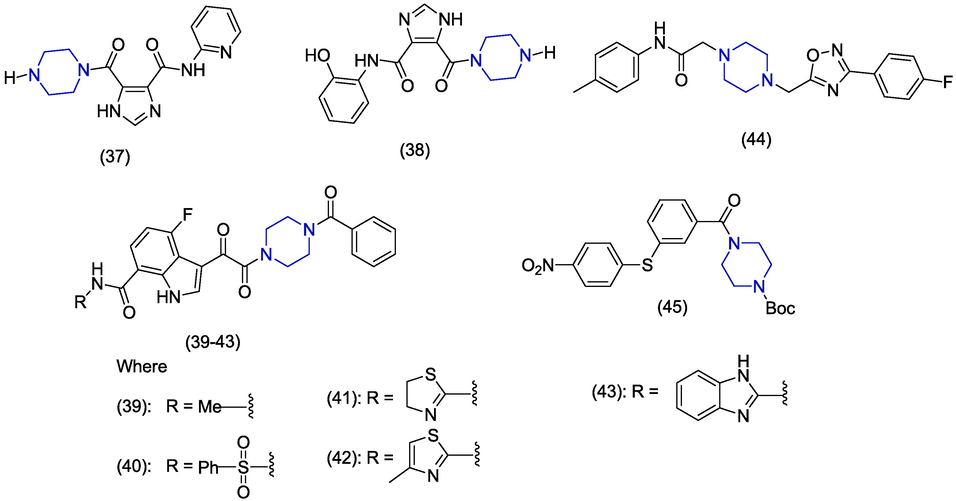
Piperazine derivatives as anti-HIV agents.
Dong et al. designed and synthesized novel 1,4-disubstituted piperidine/piperazine analogues and also evaluated antiviral activities against HIV-1 Bal (R5) in the CEMX174 5.25 M7 cells. Most of the synthesized derivatives demonstrated potent anti-HIV-1 activities showing IC50 values. The piperazine hydrochloride analog (46) (Fig. 7) exhibited promising anti-HIV-1 activity (IC50 = 0.00617 µM) and lower cytotoxicity (CC50 < 100 µM). The compound (46) has also much higher solubility (25 mg/mL at 25 °C) and shorter plasma half-life (0.76 h) than the reference drug TAK-220 hydrochloride (2 mg/mL at 25 °C). The derivative (47) also showed excellent (IC50 = 0.00185 ± 0.17 µM) anti-HIV-1 activity than the reference TAK-220 but showed higher cytotoxicity (CC50 = 30.47 ± 7.99 µM) which obstruct it as a potential drug candidate. Derivatives 46 and 47 carry 4-fluorobenzyl and 4-trifluoromethyl benzyl fragments attached with piperazine. Replacement of benzyl group by benzoyl produced less active derivatives (Dong et al., 2012). Solyev et al. synthesized novel 5′-carbamate prodrugs of 3′-azido-3′-deoxythymidine (AZT) and also evaluated their anti-HIV properties. The piperazine-based analogue (48) of AZT showed moderate viral activity in MT-4 cell culture with an EC50 value of 20 µM. This prodrug was stable and showed>24 h half-life (Solyev et al., 2012). Venkatraj et al. synthesized triazine dimers as anti-HIV agents. Alkylidenediamine, arylendiamine and piperazine act as linkers in compounds (49) and (50). Piperazine containing triazine dimers (49) and (50) have shown moderate antiviral activities against HIV-1 in TZM-b1 cells with EC50 values of 6.07 µM and 3.19 µM respectively in comparison to standard compound TMC120 (EC50 = 0.002 μM). Derivative 49 is a heterodimer in which triazine monomers are carrying different group (Venkatraj et al., 2012). Tabarrini et al. synthesized a series of naphthyridone derivatives as anti-HIV agents. The piperazine containing analogue (51) showed a prominent anti-HIV (EC50 > 0.08 µM) activity and good selectivity (SI ≥ 3707). Compound 51 carries a benzothiazole piperazine scaffold attached at position #7 of 1,6-naphthyridone scaffold. Therefore, shifting the position of naphthyridone nitrogen from 8 to 6 produced highly active derivatives (Tabarrini et al., 2011). Tabarrini et al. presented the synthesis of quinolones derivatives having vinyl and amino group at position #1. The compound (52) emerged as the most potent derivative against HIV-1 (EC50 = 2.30 ± 0.30 µM, SI 〈1 2 9) and HIV-2 (EC50 = 2.84 ± 1.82 µM, SI 〈1 0 4) and exhibited excellent selectivity. Derivative 52 carries a benzothiazole piperazine scaffold attached at position #7 of quinolone ring. The compound 52 bears an amino group at position #1 and the substitution of amino group by vinyl group produced active derivatives. But derivatives having the vinyl group showed more toxicities (Tabarrini et al., 2010). Xu et al. discovered the anti-HIV piperazinone phenylalanine derivatives. The compound 53 was found to be the most promising antiviral small molecule acting against HIV CA protein through in vitro approach. The compound 53 showed EC50 value of 5.89 ± 2.03 µM, CC50 value of 16.36 ± 3.38 µM and selectivity index ratio of 2.78. Molecular docking simulations acknowledged that the compound 53 had 20% probability of showing H-bond interactions with key residue Glu71 (Xu et al., 2022).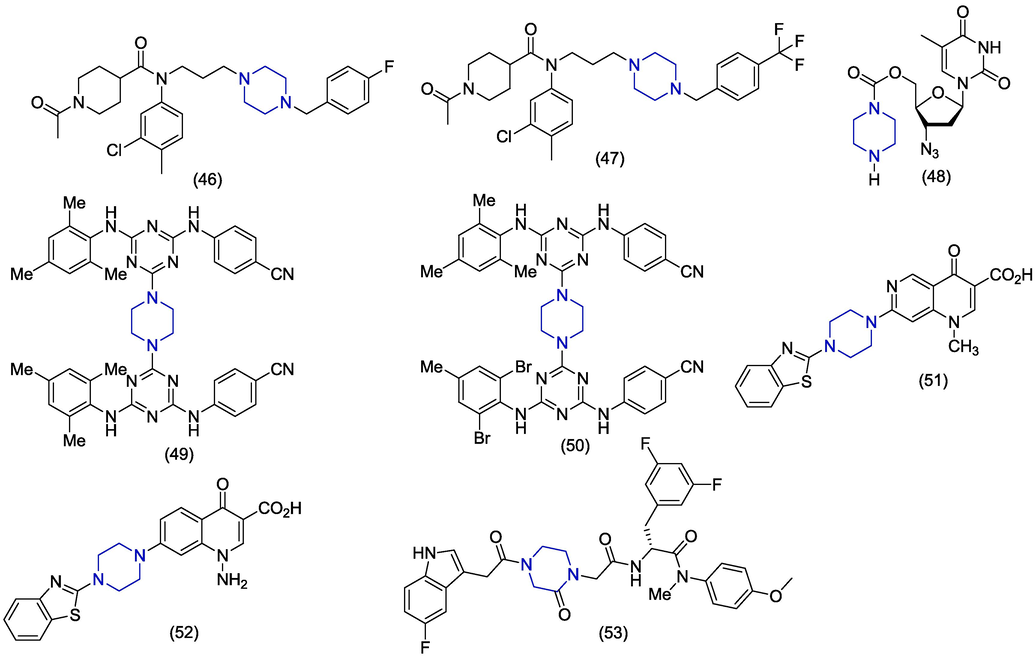
Anti-HIV agents possessing basic skeleton of piperazine.
Orally bioavailable, CCR5 antagonists as HIV-1 inhibitors were discovered by Tagat et al. (2001). The compound 54 exhibited modest binding data for CCR5-RANTES with Ki value of 0.007 µM, and CCR5 binding towards muscarinic receptor with Ki value of 0.25 µM. The compound 54 inhibited the entry and replication of HIV-1 with IC50 value of 0.001 µM. McCombie et al. (2003) reported the pharmacokinetic profile of piperazine-based CCR5 antagonists as HIV-1 inhibitors by inserting some modifications in the compound 54 reported by Tagat et al. The unsymmetrical nicotinamide-N-oxide moiety in compound 54 was replaced with symmetrical isonicotinamides as well as 4,6-dimethyl pyrimidine-5-carboxamides. The newly made compound 55 showed the CCR5 binding data with Ki value of 0.003 µM, and binding affinity of CCR5 towards muscurainic receptor was observed to be 0.456 µM. IC50 value noted while entrying into the cell was 0.005 µM. Sriram et al. (2005) described the anti-HIV effect of some biologically active lamivudine prodrugs, The study reported that among the series of compounds, compound 56 displayed the better anti-HIV potential with EC50 value; 1.11 ± 0.52 µM, CC50 value; 123 ± 14.3 µM with selectivity index of 110. In 2009, Wang et al. reported a drug candidate that demonstrated the antiviral activity in HIV-1- infected subjects. The compound 57 containing azaindole moiety displayed the better results than others. The inhibitory studies carried out to evaluate the anti-HIV-1 activity for compound 57 were EC50, CC50 and IC50 with 0.88 ± 0.46 µM, > 300 µM, and > 40 µM values, respectively (Wang et al., 2009). Jiang et al. (2023) discovered diarylpyrimidine derivatives bearining piperazine as potent HIV-1 non-nucleoside reverese transcriptase inhibitors. The compound 58 showed the most potent antiviral activity against HIV-1 by showing EC50 value of 0.0014 ± 0.00019 µM and CC50 value 10.15 ± 1.82 µM. (In silico studies are depited in Table 1).
Serial No.
Compound
Binding Potential
Interactions at Enzyme Active Site
Ref.
Docking Score (kcal/mol)
Binding Free Energy (kcal/mol)
H-bond interactions
Hydrophobic interactions
SARS-CoV-2
1
Hydroxyethylamine analogs 138
–
−52.14 kcal/mol
GLN189, GLU166
HIS41, MET49, MET165, GLU166
(Gupta et al. 2021)
2
(2S,3S)-3-amino-1-(4-(4-(tert-butyl)benzyl)piperazin-1-yl)-4-phenylbutan-2-ol 137
–
55.62 kcal/mol
HIS234, THR340
HIS234, LYS289
(Kumar et al. 2021)
3
(N'1E,N'4E)-N'1,N'4-bis(4-nitrobenzylidene)piperazine-1,4-bis(carbothiohydrazide) 135
−6.87 kcal/mol
–
THR26, GLN189, ASN142, GLY143
–
(Omar et al., 2021)
4
1,1′-(piperazine-1,4-diylbis(methylene))bis(indoline-2,3-dione) 136
−6.81 kcal/mol
–
HIS163
HIS41, THR25
(Omar et al., 2021)
5
7-(4-(N-substituted-carbamoyl-methyl)piperazin-1yl)-chalcone 134
−8.9 kcal/mol
–
CYS145, HIS41, GLN189, MET49, MET165, ASN142, THR190, GLY143
(Alaaeldin et al., 2022)
HIV
6
(E)-3-(3,5-dimethyl-4-((2-((4-((4-(methylsulfonyl)piperazin-1-yl)methyl)phenyl)amino)pyrimidin-4-yl)oxy)phenyl)acrylonitrile 16
−12 kcal/mol
–
LYS101
TYR181, TYR188, TRP229
(Huang et al., 2019)
7
2-(4-((3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl)methyl)piperazin-1-yl)-N-(p-tolyl)acetamide 44
–
–
TRP36, THR81, ARG76, LEU21, ARG22, LYS98
(Zentner et al., 2013)
8
diarylpyrimidine derivatives 58
–
−17 kcal/mol
TYR181, LEU100A,
VAL179A, LYS103A, PHE227A, HIS235A
(Jiang et al., 2023)
Respiratory Syncytial Virus (RSV)
9
benzo[d]thiazol-2-ylmethyl 4-benzylpiperazine-1-carbodithioate 103
–
–
GLY18, LYS19, HIS22, SER24, HIS25, GLU10, HIS14
CYS15(Cancellieri et al., 2015)
10
benzo[d]thiazol-2-ylmethyl 4-phenylpiperazine-1-carbodithioate 102
–
−47.40 ± 6.39 kJ/mol
GLY18, HIS25
GLU10, HIS14, CYS15, LEU16, LYS19, ARG45
(Ferla et al., 2020)
TMV
11
4-(4-chlorophenyl)-N-(2-nitrophenyl)piperazine-1-carboxamide
124
−2.632 kcal/mol
−41.436 kcal/mol
GLN39, GLN38
VAL114,THR111,ASN91, ILE94,GLU95,GLN36,THR37,GLN38,GLN39
(Nagalakshmamma et al., 2020)
Influenza Virus
12
1-(4-(4-((7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)oxy)phenyl)piperazin-1-yl)ethanone 94
–
–
LYS643, TRP706, GLN408, ARG673
(Pagano et al., 2014)
13
(3Z,6E)-3-benzylidene-6-(2-methylpropylidene)-1-(2-(pyrrolidin-1-yl)ethyl)piperazine-2,5-dione 96
–
−102.25 kcal/mol
ARG371
–
(Winyakul et al., 2022)
Niphah Virus
14
(6-fluoro-3-hydroxypyrazin-2-yl)(4-(5-(4-hydroxyphenyl)-1,3,4-oxadiazol-2-yl)piperazin-1-yl)methanone 142
−8.909 kcal/mol
−55.229 kcal/mol
GLN530, ALA532, ILE580
VAL507, PRO488, ALA532, CYS240, TYR581, ALA558, ILE580, PRO590, ILE588, ILE217, CYS216
(Lipin et al., 2021)
Hepatitis C Virus
15
N,N’-(3,3′-(Piperazine-1,4-diyl)bis(propane-3,1-diyl))bis(4-chlorobenzene-sulfonamide) 74
–
–
TRP501
ARG393, THR411
(Bassetto et al., 2017)
The structure–activity relationship (SAR) of the studies reported here in context of piperazine-based anti-HIV derivatives, piperazine containing indole derivatives (39–43) showed the best anti-HIV activity among the other groups attached with the piperazine-moiety displaying EC50 in the range of 5.8 × 10-6 µM to 5.2 × 10-4 µM. Other moieties which exhibited the excellent anti-HIV activity (EC50) includes; diarylpyrimidine derivatives (16, 58), biphenyl-substituted diaryltriazine (10), benzenesulfonamide-containing phenylalanine (14), β-carboline derivatives (23), and azaindole moiety (57) in the range of 0.0014 µM to 0.88 µM.
3.2 Anti-hepatitis B virus activity of piperazine derivatives
Hepatitis B virus (HBV) is a double-stranded DNA enveloped virus which infects>250 million people worldwide. About 900,000 deaths are reported annually due to hepatitis B virus infection (Razavi-Shearer et al., 2018). The higher death rate is due to the complications of cirrhosis, hepatocellular carcinoma and end-stage liver disease associated with HBV (Trépo et al., 2014). Interferon (IFN)-α,adefovir dipivoxil [bis(POM)PMEA] and lamivudine (3TC) are formally approved as the treatment of hepatitis B infection. However, the treatment does not completely cure the virus and virus reappears in patients after the withdrawal of drug (Ghany, 2017). Side effects and development of resistance necessitate the discovery of novel anti-HBV agents (De Clercq, 2004). In 2020, Li et al. synthesized a series of amide-containing α-hydroxytropolones as potential compounds for HBV drug development. The compound 59 (Fig. 8) was the most active (EC50 = 0.33 ± 0.13 µM) against HBV replication among the piperazine derivatives. The compound 59 showed higher cytotoxicity (CC50 = 4.5 ± 1.4 µM) and lower selectivity index (SI = 13.7) which makes it a poor candidate towards drug development. However, the piperazine containing compound 60 was also a potent compound (EC50 = 0.46 ± 0.22 µM) against hepatitis B virus replication based on cytotoxicity value (CC50 < 30 µM) and selectivity index (SI < 80) (Li et al., 2020). Gerasi et al. designed and synthesized novel substituted imidazo[4,5-b]pyridines and evaluated their anti-HBV activities. HBV infectious system was used to determine the anti-HBV activity of the synthesized derivatives at the level of HBV rcDNA secretion. Monochlorosubstituted imidazo[4,5-b]pyridine derivatives presented better activity as compared to dichlorosubstituted derivatives. The piperazine containing derivative 61 (EC50 = 25.31 ± 1.45 μM) expressed excellent anti-HBV activity but presented low selectivity (SI < 7.9) (Gerasi et al., 2020). In 2018, Ren et al. reported the morpholine and piperazine-based novel analogues as HBV capsid inhibitor. The piperazine containing compound 62 was found to have low anti-HBV activity (EC50 < 16.4 µM) as compared to morpholine analogues which showed better anti-HBV activities (Ren et al., 2018). In 2014, Xu et al. screened benzimidazole derivatives and identified novel piperazine containing benzimidazole 63 as a potent antiviral agent. Derivative 63 demonstrated prominent inhibition of hepatitis B virus (EC50 = 0.6 µM) and HBsAg secretion (EC50 = 1.5 µM) accompanied by less toxicity (CC50 = 24.5 µM). Furthermore, it does not activate cellular stress reaction or host protein secretion and therefore it might be hypothesized that it is an excellent compound for drug development (Xu et al., 2014). In 2011, P. Wang et al. synthesized substituted aryl propenamide analogues and also separated the Z and E isomers. The Z isomer was found to have potent antiviral activity against HBV. The piperazine containing derivative 64 has less anti-HBV activity with an EC90 value of ˃10 µM as compared to the piperidine and pyrrolidine derivatives (P. Wang et al., 2011). Kiruthika et al. reported a novel piperazine-based derivative 65 against tenofovir resistant hepatitis B virus that targets the hepatitis B surface antigen (HBsAg) using computational and in vitro approaches. The compound 65 was screened virtually and predicted binding free energy against HBsAg using MMGBSA analysis, and the score found was −50.01 kcal/mol. The compound 65 shows hydrogen bond interaction with Cys76 and Ala45. The in vitro analysis was performed on the Huh7 cells and the IC50 value was found to be 11.39 µM against tenofovir-resistant hepatitis B virus (Kiruthika et al., 2021). Tan et al. (2006) presented 2-benzenesulfonylalkyl-5-substituted-sulfanyl-[1,3,4]-oxadiazoles as potential anti-hepatitis B virus agents. Among the reported series of anti-HBV agents, compound 66 was seen to inhibit the expression of HIV antigens HBsAg and HBeAg. The compound 66 displayed the EC50 value of 1.63 µM which showed the greater reduction in virion production compared to that of nucleoside analog 3TC showing EC50 value of 2.96 µM.
Among the above-mentioned piperazine-based anti-HBV derivatives, amide containing α-hydroxytropolone derivatives (59) demonstrated the best anti-HBV activity among the other groups attached with the piperazine-moiety, with EC50 values ranging from 0.33 µM to 0.46 µM. Other molecules with excellent anti-HBV activity (EC50) include chalcone (65), oxadiazole (66), and pyrimidine derivative (62) in the range of 0.6 µM to < 16.4 µM.
3.3 Anti-hepatitis C virus activity of piperazine derivatives
Hepatitis C virus (HCV) is a single-stranded RNA virus and infection due to HCV results in chronic liver disease leading to liver fibrosis, cirrhosis and hepatocellular carcinoma. According to some estimates, there are 71 million patients of HCV all over the world (Younossi, 2017). Inspite of the availability of direct-acting antiviral agents (DAAs), the HCV can develop resistance (Li and Chung, 2019). Consequently, there is an instant demand for new anti-HCV drugs. In 2020, Hu et al. reported the mechanism of action of chlorcyclizine (CCZ) against hepatitis C virus. Chlorcyclizine 67 (Fig. 9) is an effective hepatitis C virus (HCV) entry blocker which blocks HCV E1 with EC50 values of 0.024 µM and 0.020 µM for (S)-CCZ and (R)-CCZ isomers, respectively. In molecular docking studies, chlorcyclizine formed hydrophobic interactions with HCV E1 fusion loop involving phenyl and chlorophenyl groups of chlorcyclizine. The chlorcyclizine analogue 68 was found to be more potent (EC50 = 0.002 µM) than chlorcyclizine (Hu et al., 2020). Jiang et al. synthesized benzonitrile analogues as HCV inhibitors. The activity was evaluated by measuring the HCV-RNA level in the Huh7.5 cell line by using the qRT-PCR method. The piperazine containing derivative 69 was found as the most prominent HCV inhibitor presenting an EC50 value of 0.022 µM and selectivity index of 600.The alkylation of the amino group and halogen substitution at the para-position are beneficial for the activity of these derivatives. Further evaluation of the mode of action showed that this compound acts at the point of viral entry into the host and was found specific against HCV (Jiang et al., 2020). Makino et al. discovered piperazine containing anti-HCV agent 70 while investigating the derivatives of cyclosporine. Derivative 70 presented an EC50 value of 0.22 µM but were found less active as compared to the carbamoyl derivatives in this series. But compound 70 presented less immunosuppressive activity as compared to other members in this series (Makino et al., 2020). In 2018, Rolt et al. synthesized and disclosed the in vitro anti-HCV activity of analogues of an antihistamine chlorcyclizine. The derivative 71 exhibited significant anti-HCV activity (EC50 = 0.0023 µM) with low toxicity (CC50 = 19.8 µM) and large selectivity index (SI = 8609) in Huh7.5.1 cell line. Derivative 71 carries an ethyl group at the nitrogen of piperazine and it was found more active as compared to methyl, propyl, isopropyl and cyclobutyl containing derivatives. Authors concluded that this drug can be included in some directly acting antiviral agents (Rolt et al., 2018). In 2017, Bassetto et al. synthesized series of novel piperazine-based analogues and also evaluated in vitro antiviral activity against HCV replication in Huh5-2 cell line. Derivatives were synthesized by modifications of aromatic substituents, sulfonamide groups, piperazine ring and alkyl chain. Compounds 72, 73 were found to have promising inhibition of NS3 helicase unwinding activity having EC50 values of 1.2 µM, and 1.3 ± 0.777 µM, respectively with reference to VX-950 (EC50 = 0.8 ± 0.2 μM), primuline and aurintri-carboxylic acid. The central piperazine nucleus, two sulfonamides groups and at least one propyl chain were found essential for the activity. Derivative 73 inhibited NS3 helicase enzyme activity having a Kd value of 21 ± 13. The in silico study of a single compound 74 was reported (Bassetto et al., 2017). (In silico studies are depited in Table 1).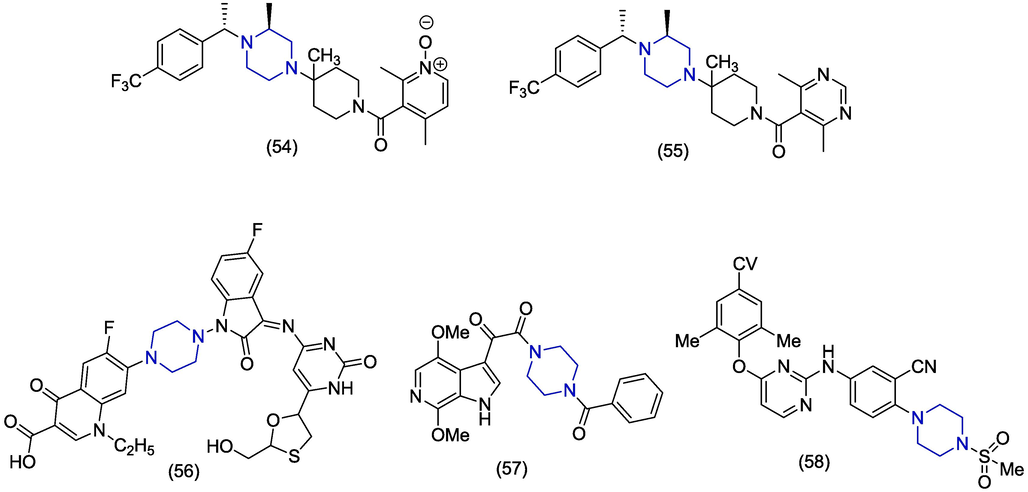
Piperazine nucleus as pharmacophoric unit to fight against HIV infections.
In 2016, Vausselin et al. identified the novel piperazine containing derivatives of 2-amino benzimidazole as antiviral agents. The compound 75 (Fig. 10) inhibited HCV infection at IC50 of 1.39 ± 0.69 µM and also showed a greater therapeutic index (TI = 18). Derivative 75 was found specific against HCV and inhibited HCV at various stages of its life cycle (Vausselin et al., 2016). In 2015, Jiang et al. reported the synthesis and antiviral activity of novel N-phenylacetophenone and N-phenyl-benzamide derivatives against HCV and EV71A strains. The piperazine was added as a substituent in one derivative but compound 76 did not display good antiviral activity (IC50 > 168.36 µM) against EV71 (strain SZ98) (Jiang et al., 2015). Magri et al. synthesized the amidino-urea derivatives of an antiviral drug moroxydine. Derivatives having aryl piperazine group exhibited prominent activity. The presence of a p-chlorophenyl group at the N1 of amidine was also beneficial for activity. The novel amidino-urea derivatives 77 and 78 exhibited excellent HCV inhibition activity having EC50 values of 1.30 µM and 1.69 µM in the fresh addition assay (FA). Derivatives 77 and 78 presented EC50 values of 11.2 µM and 10.4 µM in the standard assay with reference to ribavirin (EC50 = 30.4 µM) and telaprevir (EC50 = 0.45 µM) as standard drugs. The derivatives 77 and 78 showed better activity than the reference drug ribavirin which has poor activity (EC50 = 33.5 µM) and high cytotoxicity (CC50 = 30.5 µM). In the FA, drugs were added to the cell medium at 1/10th of the concentration taken initially after every 24 h post-infection (Magri et al., 2015). In 2014, Rynearson et al. synthesized 2-aminobenzoxazole derivatives with different substituents at position #6 and #7. Upon evaluation of their antiviral activities as inhibitors of HCV internal ribosome entry site, the piperazine derived benzoxazole 79, 80 and 81 derivatives were found inactive (Rynearson et al., 2014). In 2010, Najda-Bernatowicz et al. reported the synthesis of novel tropolone derivatives attached with cyclic amines like piperidine, piperazine and pyrrolidine. The antiviral activity was studied by specific subgenomic inhibition of HCV replication. The piperazine containing analogue 82 was found to be the most active (IC50 = 3.2 µM) in helicase inhibition assay. The compound 82 carries methyl piperazine group at positions 3, 5 and 7 of tropolone scaffold. The derivative 82 inhibited RNA amplification with EC50 value of 46.9 µM but presented minimum cytotoxicity (CC50 > 1000 μM) resulting in a better selectivity index (SI > 21.3). The 3,5,7-tri[(4′-methylpiperazin-1′-yl)methyl]tropolone 82 is the most favourable tropolone derivative which can be used as a lead to design new anti-HCV compound in future (Najda-Bernatowicz et al., 2010). (In silico studies are depited in Table 1).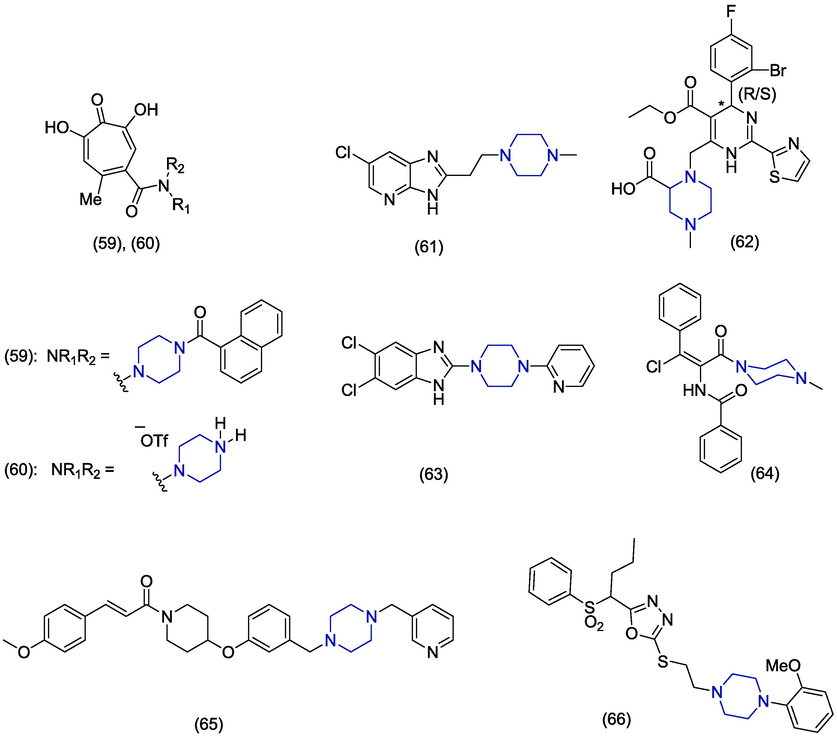
Piperazine-based anti-HBV agents.
Among the above-mentioned piperazine-based anti-HCV derivatives, chlorocyclizine derivatives (67–68, 71) showed the best anti-HCV activity among the other groups attached with the piperazine-moiety, with EC50 values ranging from 0.0020 µM to 0.02 µM. In the EC50 range of 0.2 µM to 1.69 µM, amidino-urea containing derivatives (77), phenyl substituted derivatives (70), benzonitrile analogues (69), sulphonamides (72), and derivatives of benzonitrile (70) also exhibit excellent anti-HCV activity.
3.4 Anti-herpesvirus activity of piperazine derivatives
Herpes simplex virus (HSV) infections are endemic and extensive. Although anti-HSV therapy is efficacious, the appearance of drug-resistant species and drug toxicity disrupt the treatment (Rechenchoski et al., 2017). The development of resistance against already available nucleoside analogues has also been reported (Jiang et al., 2016). The process of finding new drugs is very necessary to compete with resistant strains. In 2010, Aytemir and Özçelik synthesized mannich bases derivatives of chlorokojic acid as antimicrobial agents. The antiviral potential of the synthesized compounds was evaluated against Herpes simplex virus Type-1 (HSV-1). Among the synthesized compounds, compound 83 (Fig. 11) showed anti-herpes virus activity with less potency (Aytemir and Özçelik, 2010).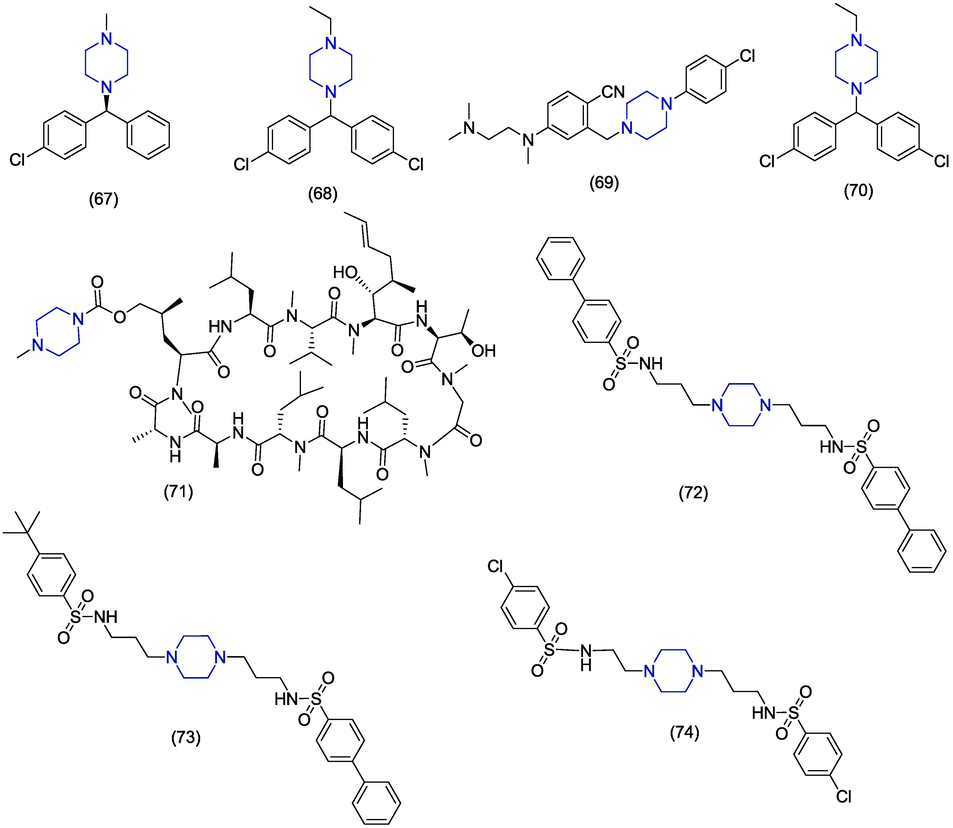
Piperazine derivatives having anti-HCV activity.
3.5 Anti-coxsackie B virus and anti-coxsackie A activity of piperazine derivatives
Coxsackie B virus (CVB) is a prevalent human enterovirus which causes frequent systemic inflammatory disease. It can cause infection of cardiac muscles and pancreas leading to type I diabetes mellitus. Currently, no vaccine or antiviral agent is accredited for the treatment or avoidance of CVB infection (Sin et al., 2017; Song et al., 2019). In 2020, Volobueva et al. synthesized novel derivatives of anti-enteroviral drug pleconaril by modifying the propyl linker group. The antiviral activity was explored against Coxsackievirus B3 (CVB3) Nancy. The piperazine containing derivatives 84 and 85 exhibited better in vitro anti-CVB3 activity presenting IC50 values of 9.1 ± 0.7 μM and 3.9 ± 0.3 μM, respectively as compared to pleconaril (IC50 = 18.4 ± 1.5 μM). Derivative 85 was found more cytotoxic (CC50 = 203.8 ± 14.7 μM, SI = 52) as compared to derivative 84 (CC50 = 1867.1 ± 90.4 μM SI = 205) as evaluated by MTT 96-well plate assay (Volobueva et al., 2020). In 2017, Hao et al. synthesized N-6 piperazine substituted adenosine analogues and also evaluated their antiviral activities against CVB3 by using MTT assay in HEp-2 cells. A number of the piperazine-based compounds exhibited better antiviral activity as compared to the reference drug. Compound 85 showed promising activity (IC50 = 5.1 ± 2.3 μM) and selectivity index (SI = 41) against CVB3 as compared to the reference drug ribavirin (IC50 = 36.8 ± 9.6 μM, SI = 29.1) (Hao et al., 2017). In 2013, Zhang et al. identified novel piperazine derivatives having antiviral potential against coxsackie virus A16 (CVA16) and human enterovirus (EV71). The piperazine containing compound 86 revealed potent activity against EV71 and CVA16 having IC50 values of 4.3 µM and 1.2 ± 0.5 µM accompanied by low cytotoxicity (TC50 = 43.1 ± 4.7 μM). Derivative 86 carries thiazole ring directly attached with piperazine. Derivative 87 shows moderate activity against EV71 and CVA16 having IC50 values of 4.9 ± 0.2 µM. In docking studies with EV71 capsid binding pocket, derivative 86 showed hydrophobic interactions with Ileu111, Val192, Met230 and Phe233. Derivative 86 also exhibited π-π interaction with Phe137 and Phe155 (Zhang et al., 2013).
According to the structure–activity relationship (SAR) of the studies discussed here regarding piperazine-based anti-coxsackie B virus hybrids, pleconaril derivatives (84, 85) and alkyl substitution on piperazine rings (86, 87) demonstrated the best anti-coxsackie B virus activity among the other groups attached with the piperazine-moiety, with IC50 values ranging from 3.9 µM to 9.1 µM.
3.6 Anti-influenza virus activity of piperazine derivatives
Influenza remains a considerable hazard to public health despite years of surveillance and treatment. The influenza vaccine is also available but mutation of the influenza virus can reduce efficacy (Petrova and Russell, 2018). In 2020, Zhang et al. designed and synthesized a novel series of 2,4-disubstituted quinazolines and checked in vitro anti-influenza A virus effectiveness. The piperazine-based analogue 88 (Fig. 12) showed medium activity against influenza A virus (IAV) presenting an IC50 value of 11.47 ± 0.54 µM and CC50 value>100 µM. However, derivative 88 showed no violation of the Lipinski’s rule of five and showed drug-likeness properties (Zhang et al., 2020). In 2016, Arns et al. reported the novel spirothiazamenthane having a unique chemical structure as an obstructer of the influenza A-M2 proton channel. The piperazine containing derivative 89 inhibited the WT (wild type) virus having an EC50 value of 2.1 µM by using plaque reduction assay in MDCK cells (Arns et al., 2016). In 2015, Bottini et al. reported the synthesis of an existing anti-influenza compound. The starting dimethoxy piperazinyl quinazolinamine compound 90 presented in vitro anti-influenza A virus activity having an IC50 value of 549 µM. The derivative 91 represents the tenfold better anti-influenza A virus profile displaying an IC50 value of 44.18 ± 11.28 µM as compared to the starting compound. The derivative 91 carries a propionyl group attached at the nitrogen of piperazine (Bottini et al., 2015). Tănase et al. synthesized novel analogues of N6-substituted adenine and pyrimidine. The antiviral activity against influenza virus was evaluated. The piperazine containing compounds 92 (EC50 = 12 ± 1 µM) and 93 (EC50 = 25 ± 2 µM) demonstrated moderate activities but better selectivity (SI = 73, 39) values. The presence of bulky substituents at position #6 of the nucleoside was necessary for optimum antiviral activity (Tănase et al., 2015). In 2014, Pagano et al. synthesized various benzofurazan derivatives and examined the activity against influenza A virus H1N1. The piperazine containing derivative 94 exhibited moderate activity (IC50 = 10 µM) towards influenza virus strain and was found to be more cytotoxic (CC50 = 20 µM) as compared to other derivatives. The ligand efficiency (LE) of compound 94 was found to be 0.22 kcal/mol (Pagano et al., 2014). Wang et al. reported antiviral piperazine containing products obtained via optimization on the JNJ4796 as novel anti-influenza A virus agents. The R-enantiomer of compound 95 exhibited excellent in vitro activity against IAV H1N1 with an IC50 value of 0.27 µM than the reference drugs Ribavirin and Oseltamivir with IC50 values 4.28 µM and 3.44 ± 0.84 µM, repectively. It is worth mentioning to report that it showed lower cytotoxicity (CC50 > 200 µM) and higher selectivity index (SI > 6666) than the reference drugs Ribavirin and Oseltamivir (Wang et al., 2021). Winyakul et al. synthesized 2,5-diketopiperazine derivatives and evaluated them as potential anti-influenza (H5N2) agents through in vitro and in silico approaches. The compound 96 displayed the best binding energy value of −102.25 kcal/mol, and hydrogen bond interactions with Arg371 when compared to the reference drug Oseltamivir carboxylate (-93.75 kcal/mol). The cytotoxicity activities were performed on the Rhesus monkey kidney epithelial cells (LL-MK2 cell lines) showing IC50 values of 287.65 µM, indicated the negligible cytotoxicity compared to the normal cells (Winyakul et al., 2022). The in silico studies are described in Table 1.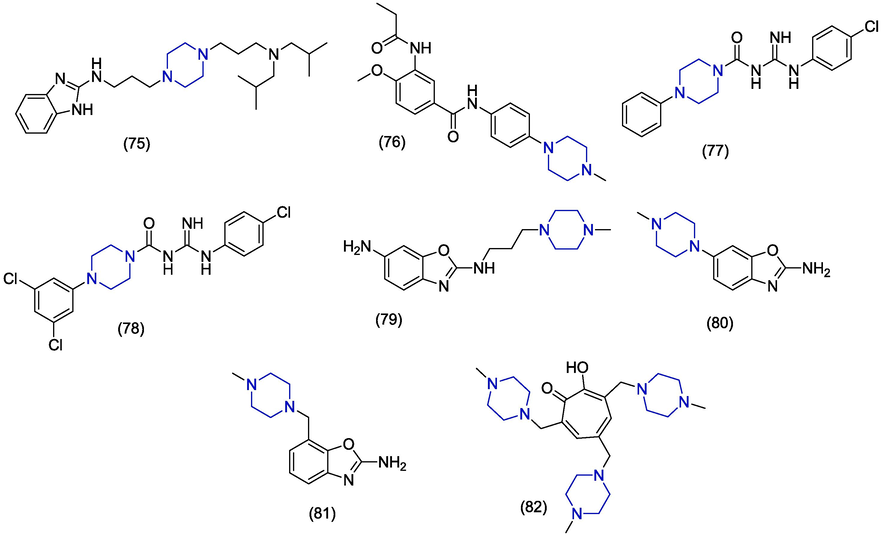
Piperazine derivatives having anti-HCV activity.
In the analysis of the structure–activity relationship (SAR) regarding piperazine-based anti-influenza virus compounds, it was observed that the benzofuran derivative (95) demonstrated the most potent anti-influenza virus activity compared to other groups attached to the piperazine-moiety, with an impressive IC50 value of 0.27 µM. Additionally, benzofurazan derivatives (94) and 2,4-disubstituted quinazolines (88) also exhibited noteworthy anti-influenza virus activity within the IC50 range of 10 µM to 11.47 µM.
3.7 Anti-human cytomegalovirus activity of piperazine derivatives
Human cytomegalovirus is a ubiquitous DNA herpes virus that is common worldwide and is the chief cause of morbidity and death in immunocompromised patients (Davis et al., 2017). In 2013, Massari et al. synthesized piperazine containing derivatives of naphthyridone and evaluated their anti-human cytomegalovirus (HCMV) activity by using plaque reduction assay and anti-HIV activity by MTT assay. The piperazine containing compound 97 (Fig. 13) was the most significant anti-HCMV and anti-HIV agent with the EC50 values of 0.9 ± 0.2 µM and 1.3 ± 0.02 µM, respectively. The cytotoxic concentration (CC50) values of derivative 97 were 126.5 ± 27.8 µM and 10 ± 1.86 µM respectively against these viruses. The lower cytotoxicity of 97 against HCMV is responsible for its potency. However, the anti-HCMV activity of another derivative 98 was greater (EC50 = 0.19 ± 0.04 µM) than 97 but its cytotoxicity (CC50 = 15.3 ± 3.4 µM) was higher which make it less selective. The 4-(2-pyridinyl)piperazine was found as the best substituent at position #6. The replacement of the terminal pyridine ring by quinolone ring produced more cytotoxic compounds (Massari et al., 2013). In concern for structure–activity relationship, pyrimidine and benzothiazole substitution on the piperazine ring in addition to the cyclopropanyl group on the nitrogen of 1,4-dihydroquinolinoic acid in (97, 98) exhibited the best antiviral activity than the phenyl or alkyl substitution on the piperazine and quinolinoic acid’s nitrogen, respectively.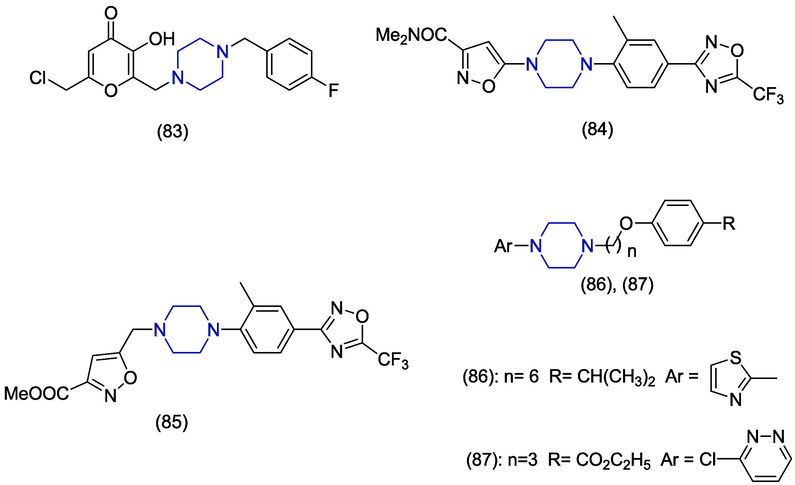
Piperazine-based anti-HSV and anti-CVB agents.
3.8 Anti-human rhinovirus (HRV3) activity of piperazine derivatives
Rhinovirus (RV) is an RNA virus and is the most endemic contributor of upper respiratory tract infections in human beings (Vandini et al.,2019). In 2017, Da Costa et al. developed a novel series of 4,5-dimethoxybenzyl analogues and studied their activity against rhinovirus (RV). The piperazine-based derivative 99 exhibited little activity against rhinovirus RV-B14 with an EC50 value of 18.10 ± 0.34 µM (Da Costa et al., 2017). Q. Zhang et al. designed, synthesized and evaluated 2,2-dimethyl-1,3-dioxolane derivatives for the development of novel anti-rhinoviral medicines. The piperazine containing compound 100 revealed excellent anti-rhinoviral activity with an IC50 value of 2.50 ± 0.7 µM versus HRV-3C protease. Docking studies of derivative 100 with HRV-3C protease crystal structure showed hydrogen bond interactions involving oxygens of 1,3-dioxolone (His40, Gly145), hydrogen and oxygen of amide (Val162, Gly164) group. The thiazole ring formed π stacking interaction with His40 (Q. Zhang et al., 2017). H. Wang et al. synthesized a novel series of chloro-pyridazine piperazines as anti-HRV3 agents. The compound 101 demonstrated meaningful anti-HRV activity with an EC50 value of < 0.0032 µM and was found to be most selective in this series. Molecular docking studies of compound 101 with HRV-3 viral protein-1 (VP1) showed that benzene ring interacts with methylene group of Tyr152. The ethoxy group formed hydrogen bonding with the amino group of Ser175. The N1 and N3 of pyridazine are involved in hydrogen bonding with the phenolic hydroxyl group of Tyr197 (H. Wang et al., 2011).
Among piperazine-based anti-human rhinovirus derivatives, it was observed that chloro-pyridazine derivatives (1 0 1) exhibited the most potent anti-human rhinovirus activity among the various groups attached to the piperazine moiety, boasting an impressive EC50 value of < 0.0032 µM. Moreover, 4,5-dimethoxybenzyl analogues (99) and 2,2-dimethyl-1,3-dioxolane derivatives (1 0 0) also demonstrated remarkable anti-human rhinovirus activity.(Fig. 14)
3.9 Anti-respiratory syncytial virus activity of piperazine derivatives
Respiratory syncytial virus (RSV) is a vital and familiar respiratory pathogen for which no vaccine is available albeit>50 years of efforts. RSV is the primary cause of acute lower respiratory infections in children (Obando-Paceco et al., 2018). In 2020, Ferla et al. discovered a family of sixteen new dithiocarbamate analogues in which benzothiazole and piperazine ring were incorporated. The antiviral activity was explored against RSV. The compound 102 (Fig. 15) was found as the best compound in the series against RSV-A (EC50 = 4.4 ± 1.1 μM) and RSV-B (EC50 = 1.3 ± 0.2 μM) in cytopathic effect inhibition assay. Compound 102 showed less toxicity to normal Hep2 cell line having CC50 value>100 μM. In derivative 102 phenyl ring is directly connected with the piperazine ring (Ferla et al., 2020). In 2015, Cancellieri et al. used structure-based drug design to synthesize novel zinc ejecting compounds in which dithiocarbamate was used as linker group. Aromatic substituent was added on one side of the carbamate group and alicyclic amine was added on the other side. Upon evaluation of their in vitro antiviral activity, compound 103 was found to be the most active against RSV having an IC50 value of 6 µM. The presence of benzyl piperazine fragment produced more active compounds in this series. Molecular docking investigations with M2-1 protein showed that thiazole ring interacted with His14 and benzyl piperazine formed hydrophobic interaction with Lys19. The dithiocarbamoyl interacted with the zinc ion of protein (Cancellieri et al., 2015).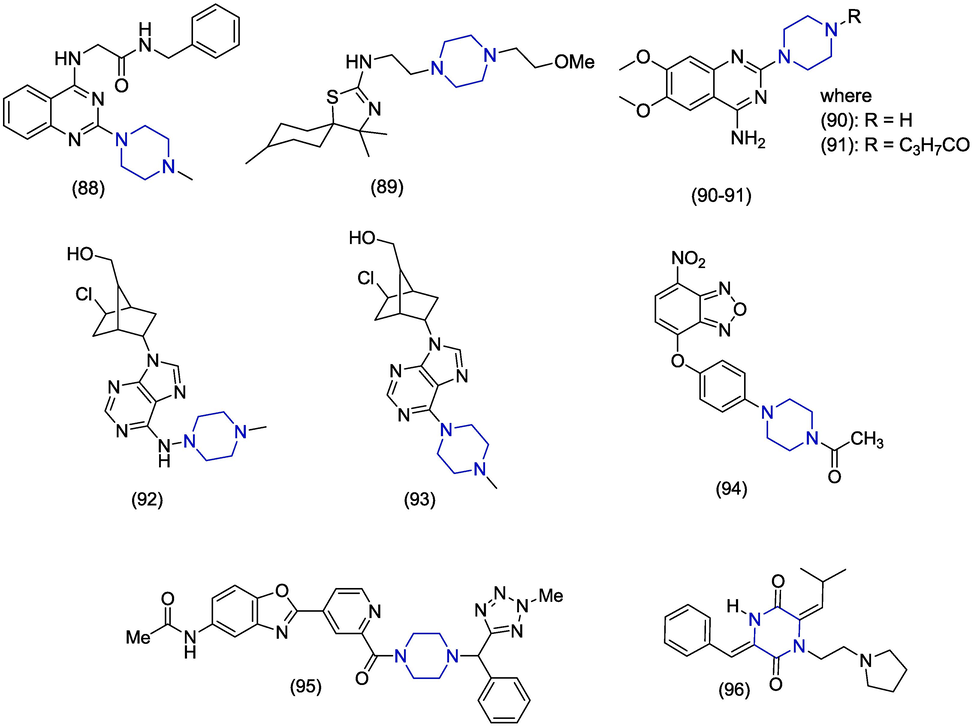
Piperazine-based anti-influenza active agents.
In the context of the studies discussed here, the structures of compounds were focused to explore the structure–activity relationship (SAR) of piperazine-based anti-respiratory syncytial virus derivatives. It was found that dithiocarbamates (102, 103) exhibited the most potent anti-HBV activity among the compounds studied.
3.10 Anti-bovine viral diarrhea activity of piperazine derivatives
Bovine viral diarrhoea virus (BVDV) is notably infective pathogen from Flaviviridae family and is globally distributed. BVDV is responsible for serious economic damages in the cattle industry (Riitho et al., 2020). In 2018, Loddo et al. synthesized novel 9-aminoacridine-based derivatives and evaluated their anti-BVDV activity against both RNA and DNA-based viruses. The 4-(2′-hydroxyethyl) piperazine containing compounds 104 was the most potent derivative against BVDV presenting an EC50 value of 0.8 µM. Compound 104 also showed prominent in vitro inhibition (IC50 = 0.62 µM) of viral RNA dependent RNA polymerase (RdRp) enzyme. The presence of the amino group between piperazine and acridine nucleus was important for the activity of these derivatives (Loddo et al., 2018). Shiryaev et al. designed and synthesized cage compounds as inhibitors of BVDV. The piperazine-based cage compound 105 was found to have moderate antiviral activity against BVDV (IC50 = 1.69 µM) but showed low toxicity with CC50 values above 358.17 µM. In this compound, piperazine ring is condensed with adamantane fragment (Shiryaev et al., 2018). In 2016, Ibrahim et al. reported the preparation of piperazine containing Mannich bases of 7-hydroxycoumarins and studied the antiviral activities against HIV-1, BVDV, yellow fever virus (YFV), Reovirus (Reo-1), CVB-5, poliovirus (Sb-1), RSV and vesicular stomatitis virus (VSV). The piperazine containing compounds 106 and 107 endowed notable activities against BVDV with EC50 values of 0.3 ± 0.01 µM and 1.0 µM as compared to EFV and other standard compounds. These compounds were specific for the BVDV because they showed no activity against other viruses. These derivatives demonstrated significant selectivity indices and less toxicity which makes them strong candidates towards the drug development. Derivatives 106 and 107 contain p-nitrobenzoyl group and hydroxyl group at position #7 respectively. In these derivatives, piperazine was found to be most suitable linker because the replacement of piperazine by piperidine and other alkyl amines produced less active derivatives (Ibrahim et al., 2016).
Among the various groups attached to the piperazine moiety, piperazine-containing 7-hydroxycoumarin derivatives (1 0 6) exhibited the most potent anti-bovine viral diarrhoea virus activity, with an EC50 value of 0.3 µM. Additionally, other moieties that showed excellent anti-bovine viral diarrhoea virus activity include the adamantine fragment (1 0 5) and 9-aminoacridine-based derivatives (1 0 4), displaying IC50 value ranging from 0.62 µM to 1.69 µM.(Fig. 15)
3.11 Anti-human norovirus activity of piperazine derivatives
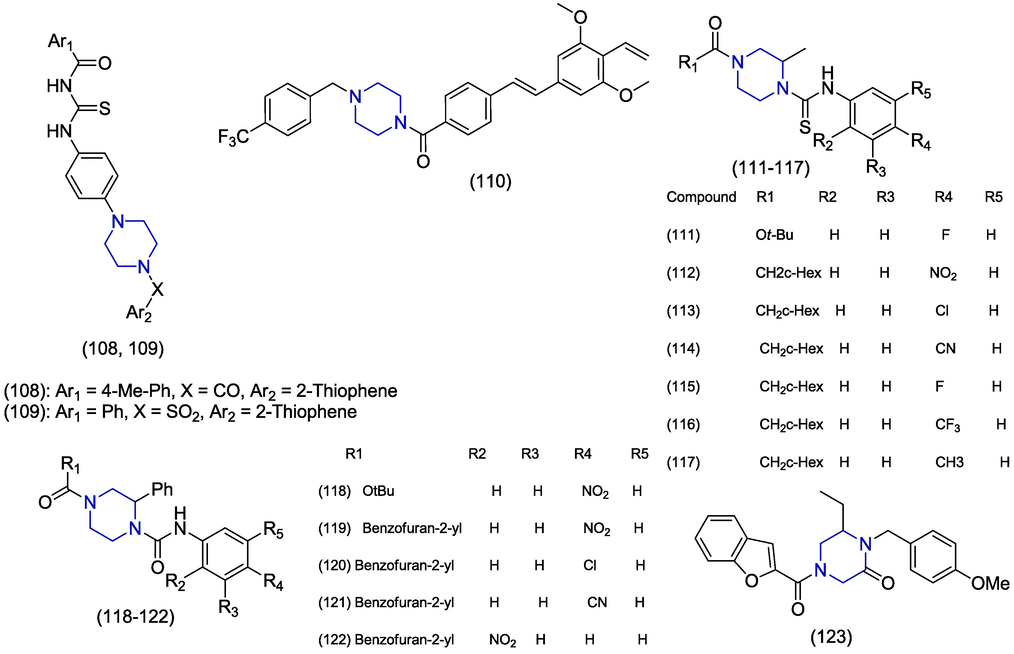
Human norovirus (HNV) is one of the most leading etiological agents which causes acute gastroenteritis worldwide and therefore poses a substantial risk to human health. The development of a vaccine for norovirus faces challenges of short-lived immunity and antigenic unpredictability (Lucero et al., 2018; Nordgren and Svensson, 2019). In 2019, Giancotti and co-workers identified new antiviral scaffolds for HNV by using computer-assisted techniques on the viral polymerase. The piperazine containing hybrid analogues 108 and 109 (Fig. 16) produced the most amazing antiviral activity with EC50 values of 6.1 ± 3.9 µM and 10.9 ± 11.1 µM respectively in HNV G1 assay (Giancotti et al., 2019). Harmalkar et al. carried out the design and synthesis of novel non-nucleoside vinyl-stilbene derivatives as potent inhibitors of norovirus. Amide derivatives of vinyl stilbene presented prominent activities and piperazine-derived amide 110 emerged as the most effective with an EC50 value of 2.43 µM. Compound 110 also exhibited better selectivity index (>41.2) and less toxicity (CC50 > 100 μM). The presence of the vinyl group was necessary for the activity because the removal of the vinyl group or replacement by ethyl produced less active derivatives. The promising anti-HNV activity of 110 may lead it towards the development of novel noroviral inhibitor (Harmalkar et al., 2019).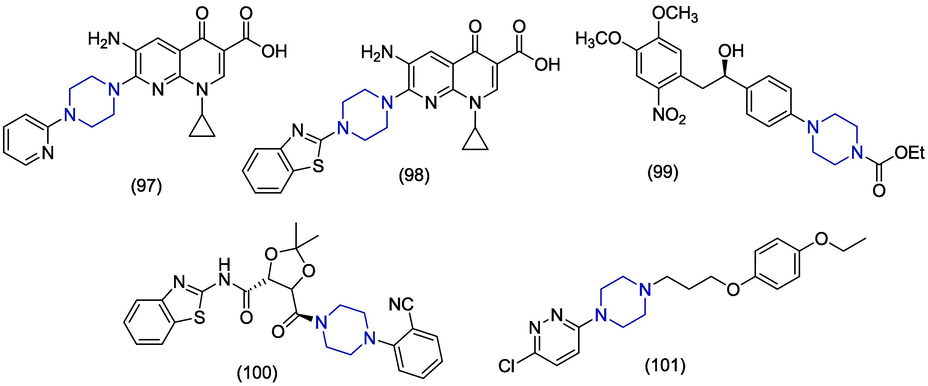
Piperazine-based anti-human cytomegalovirus and anti-human rhinovirus agents.
The studies presented here demonstrate a structure–activity relationship (SAR) in the context of derivatives of piperazine that are directed against the human norovirus. The piperazine-containing non-nucleoside vinyl-stilbene derivatives (1 1 0) with an EC50 value of 2.43 µM showed the strongest anti-human norovirus activity among the various groups attached to the piperazine moiety. The carbamothioyl benzamides (108, 109) with EC50 values ranging from 1.6 µM to 10.9 µM also demonstrated excellent anti-human norovirus activity.
3.12 Anti-adenoviral activity of piperazine derivatives
Several human diseases are associated with adenovirus which includes conjunctivitis, gastroenteritis, myocarditis, pneumonia and hepatitis and to-date no antiviral agent is licensed to treat adenovirus. Adenovirus infections are considered as self-limiting in healthy individuals but individuals with impaired immune functions have encountered severe infections (Echavarría, 2008; Lenaerts et al., 2008; Ali et al., 2016). Here, we have reported potential compounds to control adenovirus replication. In 2020, Mazzotta et al. optimized piperazine-derived urea analogues for anti-adenoviral activity and reported a library of sixty-seven compounds by the refining procedure of piperazine-based privileged scaffolds. Structural modifications were carried out by replacement of urea by thiourea, the acyl group was substituted by substituted acetyl group and the piperazine ring was modified by 2,6-dimethyl piperazine. The biological evaluations of antiviral activity and cytotoxicity identified twelve derivatives that showed>80% inhibition of human adenovirus (HAdV) infections at low micromolar (µM) and nanomolar (nM) quantities accompanied by low cytotoxicity. Furthermore, eight out of twelve studied derivatives also blocked DNA replication of human cytomegalovirus (HCMV) at low micromolar concentrations. Finally, a total of seven compounds 111–117 were identified for their>90% HAdV inhibition in the plaque reduction analysis and with CC50>100 µM. As the compound 114 significantly inhibited Phi29 DNA polymerase activity, therefore, it is suggesting that preferential targets are HAdV and CMV DNA polymerases (Mazzotta et al., 2020). In 2016 Sánchez-Céspedes et al. disclosed the synthesis, biological evaluation and SAR studies of novel phenylpiperazine derivatives. The compounds 118, 119, 120, 121 and 122 were found to have significant inhibition of DNA replication against human adenovirus (HAdV5) presenting IC50 values of 3.4 ± 1.0 µM, 2.1 ± 0.1 µM, 2.5 ± 1.2 µM, 4.7 ± 0.1 µM, and 2.5 µM respectively. In these derivatives, piperazine is linked to benzofuran scaffold and substituted phenyl ring at both nitrogens (Sáanchez-Céspedes et al., 2016). In 2014, Sáanchez-Céspedes et al. prepared piperazine-2-one derivatives and also investigated the inhibition of adenosine replication. The trisubstituted piperazine-2-one analogue presented significant antiviral activity with very little cytotoxicity. The compound 123 selectively inhibited DNA replication of Ad5 and Ad16 adenovirus displaying IC50 values of 1.3 ± 0.18 µM and 0.8 ± 0.74 µM, respectively. The studies on the mode of action showed that compound 123 did not interfere with the entry of the virus inside the host but inhibited the approach of attacking viral genome at the exact point in the nucleus. The compound 123 emerges as a potential drug towards the discovery of new antiviral agents (Sáanchez-Céspedes et al., 2014).
Among the various groups attached to the piperazine moiety, the derivative known as piperazin-2-one (1 2 3) displayed the most potent anti-adenovirus activity, with an EC50 value of 1.3 µM. Furthermore, other derivatives, namely piperazin-2-ones (118–122), demonstrated remarkable anti-adenovirus activity, with EC50 values ranging from 2.1 µM to 4.7 µM.(Fig. 16)
3.13 Anti-tobacco mosaic virus activity of piperazine derivatives
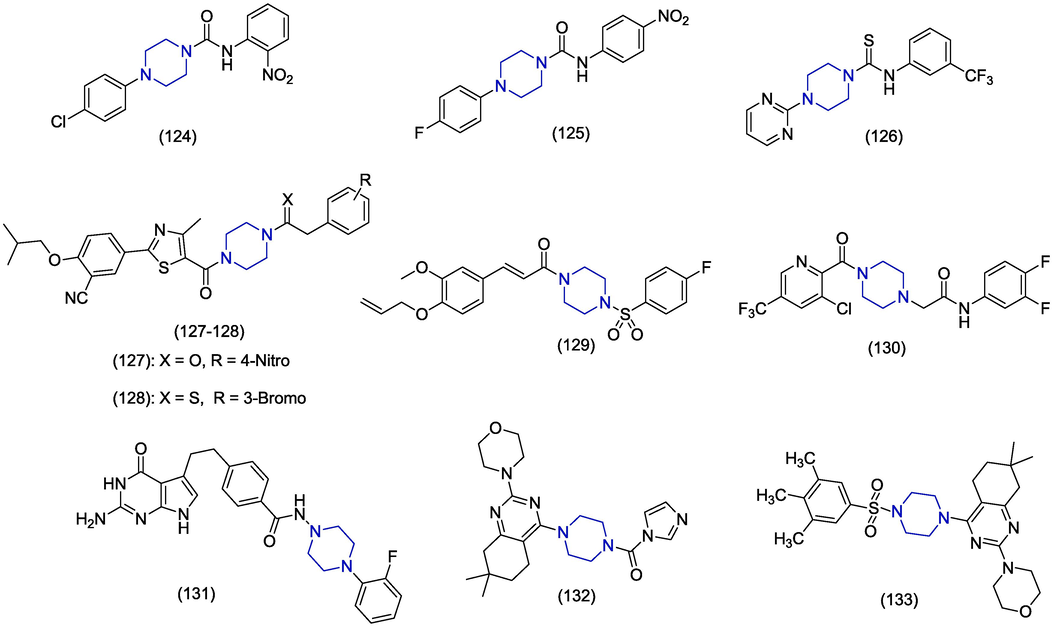
Tobacco mosaic virus (TMV) infects all tobacco species and many other plants worldwide and causes considerable loss of crops (R. Liu et al., 2013; W. Zhang et al., 2017). In this review, we identified piperazine containing favourable compounds having anti-TMV activities. In 2020, Nagalakshmamma et al. synthesized novel pyrimidine and piperazine containing derivatives by introducing urea/thiourea moieties and evaluated their inhibitory activity against TMV. Derivatives 124, 125 and 126 (Fig. 17) have exhibited high inclination towards TMV-coat proteins, TMV-helicase and were found as the most potent antiviral compounds versus TMV at 5.0 mg/mL concentration. The in vitro inhibition rates of 124, 125 and 126 were 68.3 ± 1.8%, 67.1 ± 2.2% and 65.1 ± 1.7% and found comparable to the standard drug ningnanmycin (72.3 ± 2.1%). In molecular docking studies, derivative 124 showed MM/GBSA score of −56.063 kcal/mol and presented hydrogen bonding interaction of its amino group and oxygen with Met245 and Ile275 of TMV-helicase enzyme (Nagalakshmamma et al., 2020). Febuxostat is non-purine analogue and is effective in the treatment of hyperuricemia and gout (Bruce, 2006). In 2013, Reddy et al. attached urea and thiourea analogues of piperazine with febuxostat and also evaluated them against TMV and other microbes. Derivatives 127 and 128 have shown significant in vivo anti-TMV activities with the inactivation rate of 85.72 ± 0.33% and 85.93 ± 0.54%, respectively as compared to standard drug ninganamycin (88.45 ± 0.68%). These derivatives contain 4-nitrophenyl and 3-bromophenyl attached with piperazine through amide and thioamide groups, respectively (Reddy et al., 2013). Yuan et al. synthesized ferulic acids containing piperazine moiety as potent inhibitor of mosaic viruses. The compound 129 exhibited outstanding antiviral activity against tobacco mosaic virus (TMV) and cucumber mosaic virus (CMV). The experiment was performed on the upper leaves of Nicotiana tobaccum and showed remarkable antiviral activity with EC50 values of 189.0 µM and 401.7 µM against TMV and CMV, respectively compared to the reference drugs ningnanmycin (387.0, 519.3 µM) and ribavirin (542.1, 721.5 µM) (Yuan et al., 2022). The in silico studies are mentioned in Table 1. A number of trifluoromethylpyridine piperazine derivatives were prepared and tested for their anti-CMV activities, with compound 130 showing the most impressive anti-CMV activities; specifically, the EC50 of compound 130 for curative, protective, and inactivation activities against CMV viruses was found to be 169.1, 95.0, and 18.1 µM, respectively, which were better than those of the commercial product ningnanmycin (Zhang et al., 2023).(Fig. 17)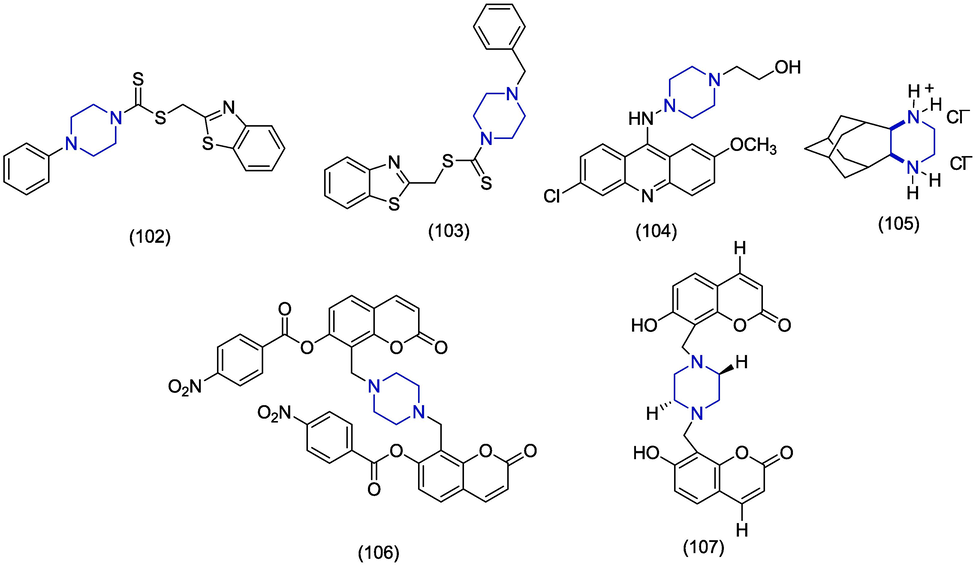
Piperazine-based anti-RSV and anti-BVDV active agents.
Piperazine-based human anti-human norovirus and anti-human adenovirus agents.
Piperazine-based anti-tobacco mosaic virus, anti-cucumber mosaic virus, and anti-avian paramyxovirus agents.
Piperazine-based anti-SARS-CoV-2 compounds.
Piperazine-based anti-Nipah virus and anti-ZIKV agents.
Studies on piperazine-based derivatives that target the TMV have found a good structure–activity relationship (SAR). Ferulic acid with a piperazine moiety attached (1 2 9) demonstrated the strongest anti-TMV activity among the various groups, with an 89.9% inactivation rate. Other derivatives, such as urea and thiourea analogues (124–128), also showed excellent anti–TMV activity, with inactivation effect rates ranging from 65 to 85 percent.
3.14 Anti-avian paramyxovirus activity of piperazine derivatives
Economically significance of avian paramyxoviruses has been reported around the world because huge mortality and morbidity rate is associated with them (Gogoi et al., 2017). Huge economic losses are reported from all over the world due to the devastating outbreaks of viruses particularly affecting the poultry industry and impose serious threats to chicken (Munir et al., 2012; Pedersen et al., 2013; Al-ilbadi, 2019). In 2018, Balaraman et al. synthesized novel benzamide derivatives by replacing the glutamic acid part of pemetrexed with different amines. The biological activity was evaluated against paramyxovirus (PMV) by plaque reduction assay. The piperazine containing derivative 131 has exhibited moderate plaque reduction (47%) at the minimal test concentration of 1 mM as compared to ribavirin (60% at 0.3 mM) (Balaraman et al., 2018). In 2017, Selvakumar et al. synthesized piperazine and morpholine derived compounds with N-aroyl/benzyl substitutions and screened their anti-viral activities against avian paramyxovirus (APMV-1). The compound 132 having carbonyl functionality appeared as the most potent derivative against avian paramyxovirus (APMV-1) (28% inhibition) as compared to reference drug ribavirin (32% inhibition) in plaque reduction assay (Selvakumar et al., 2017). In another research paper in 2018, Selvakumar et al. carried out the synthesis and anti-viral activity of piperazine and morpholine substituted derivatives against avian paramyxo virus (APMV-1). Among all the compounds piperazine substituted derivative 133 demonstrated higher antiviral activity (84% inhibition) in plaque reduction assay as compared to the reference drug ribavirin (32% inhibition) (Selvakumar et al., 2018).
The investigations focused on piperazine-based derivatives that specifically target the avian paramyxovirus, and these studies demonstrate a noteworthy relationship between the compound's structure and its activity. Among the various groups attached to the piperazine moiety, tetrahydroquinazoline (1 3 3) displayed the most potent anti-avian paramyxovirus activity, with inactivation rate of 84%. Furthermore,piperazine containing benzamide (1 3 1), demonstrated anti-avain paramyxovirus activity, with inactivation effect rate of 47%.(Fig. 17)
3.15 Anti-SARS-CoV-2 activity of piperazine derivatives
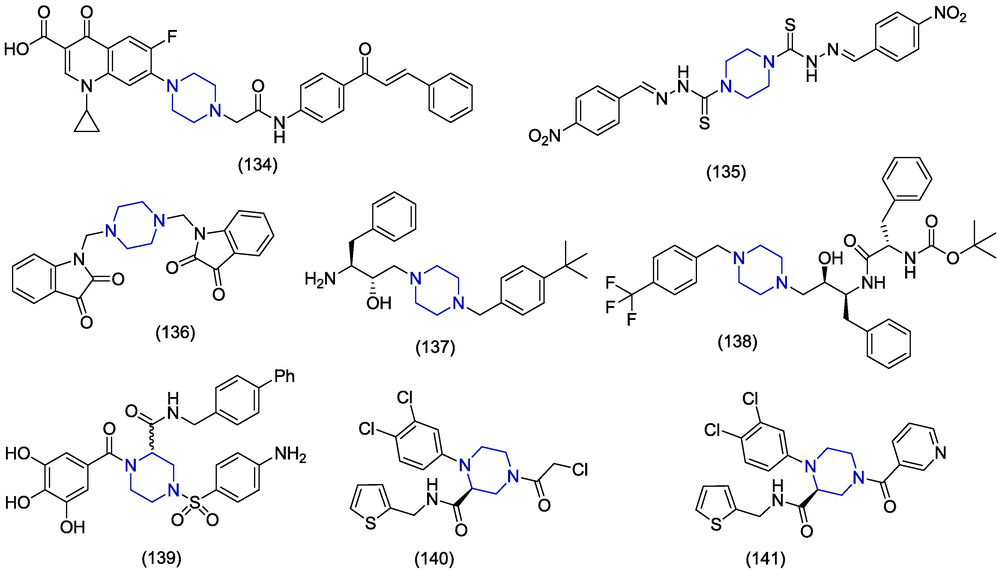
The ongoing COVID-19 pandemic has resulted in a global panic because of its continual evolution and recurring spikes. This serious malignancy is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Rafiq et al., 2023b). Alaaeldin et al. investigated the in vitro antiviral activity of 7-(4-(N-substituted-carbamoyl-methyl)piperazin-1-yl)-chalcone 134 supported by molecular docking against SARS-CoV-2 main protease. The experiment was carried out using Vero cells which have an IC50 value of 0.6 ± 0.05 μM, EC50 value of 0.00393 µM, and an energy score of −8.9 kcal/mol, while ciprofloxacin was used as a reference drug displaying an IC50 value of 5.13 ± 0.19 µM and an energy score of −7.0 kcal/mol (Alaaeldin et al., 2022). Omar et al. synthesized some novel piperazine compounds and evaluated them for their best antiviral activity against the SARS-CoV-2 protease enzyme through a virtual screening approach. Among these derivatives, the piperazine hybrids 135 and 136 showed the best docking scores towards the SARS-CoV-2 protease enzyme, i.e., −6.87 kcal/mol and −6.81 kcal/mol, respectively. The piperazine hybrid 135 showed hydrogen bond interactions with Thr26, Gln189, Asn142, and Gly143, while the compound 136 showed hydrogen bond interactions with His163 and hydrophobic interactions with His41 and Thr25 (Omar et al., 2021). Kumar et al. synthesized a novel piperazine-based compound 137 and tested it in vitro and in silico against the SARS-CoV-2 non-structural protein NSP15. The molecular docking simulations revealed the antiviral behavior of compound 137 via MM/GBSA analysis (ΔG-Bind), glide energy and potential energy values of −55.62 kcal/mol, 43.96 kcal/mol, and −84.37 kcal/mol, respectively. The viral entry assay showed that (2S,3S)-3-amino-1-(4-(4-(tert-butyl)benzyl)piperazin-1-yl)-4-phenylbutan-2-ol 137 demonstrated good inhibitory activity with an IC50 value of 4.97 µM when compared to the reference drug ivermectin which had an IC50 value of 7.28 µM. The spread assay indicated that compound 137 showed an IC50 value of 8.46 µM compared to the ivermectin IC50 value, i.e., 5.53 µM (Kumar et al., 2021). Antiviral activity of hydroxyethylamine analogs was investigated by Gupta et al. against SARS-CoV-2 main protease (3CLpro) using computational and in vitro approaches. The piperazine-based compound 138 was synthesized, and its antiviral behavior was checked using the MM/GBSA score of −52.14 kcal/mol using an in vitro spread assay performed on Vero E6 cells that exhibited an IC50 value of 12.44 µM against SARS-CoV-2 3CLpro (Gupta et al., 2021). Gao et al. identified piperazine-based polyphenols as potent inhibitors of SARS-CoV-2 RdRp. The compound 139 demonstrated the best binding energy of −7.196 kcal/mol than remdesivir (-6.5 kcal/mol) to RdRp of SARS-CoV-2. The results revealed the confirmation of in silico analysis, i.e., the compound 139 demonstrated an IC50 value of 9.2 ± 1.1 µM. It 139 blocked the active site by chelating with Mg+2 thereby inhibiting the replication function of RdRp (Gao et al., 2022a). Gao et al. discovered nonpeptidic piperazine derivatives as SARS-CoV-2 main protease inhibitors. Among 30 synthesized compounds, compound 140 showed the significant enzymatic inhibitory activity of Mpro i.e., IC50 = 0.18 µM and possessed the best antiviral activity against SARS-CoV-2 with an EC50 value of 2.64 µM similar to that of remdesivir (EC50 = 2.27 µM). The crystallographic studies revealed the covalent binding of the compound with the active site of Mpro (Gao et al., 2022b). Gao et al. discovered 1,2,4-trisubstituted piperazine derivatives as SARS-CoV-2 main protease inhibitors. Among synthesized compounds, compound 141 displayed the significant enzymatic inhibitory activity of Mpro i.e., IC50 = 0.4 µM and demonstrated the excellent antiviral activity against SARS-CoV-2 with an EC50 value of 1.1 µM. The study revealed that the compound 141 showed non-covalent, non-peptidic interactions with low cytotoxicity and high target specificity (Gao et al., 2022c).
Among piperazine-based anti-SARS-CoV-2 derivatives, chalcone (1 3 4) demonstrated the best anti-SARS-CoV-2 activity as compared to the other groups attached with the piperazine-moiety, with EC50 value 0.0039 µM and IC50 value 0.6 µM. On the other hand, 1,2,4-trisubstituted piperazine derivatives (1 4 1) exhibited the remarkable anti-SARS-CoV-2 activity with EC50 value of 1.1 µM and IC50 value of 0.4 µM. Other molecules with excellent anti-SARS-CoV-2 activity (IC50) include phenylbutan-2-ol (1 3 7), hydroxyethylamine analogs (1 3 8), piperazine-based polyphenols (1 3 9), and nonpeptidic piperazine derivatives (1 4 0) in the range of 0.18 µM to 12.44 µM.(Fig. 18)
3.16 Anti-Nipah virus and anti-Zika virus activity of piperazine derivatives
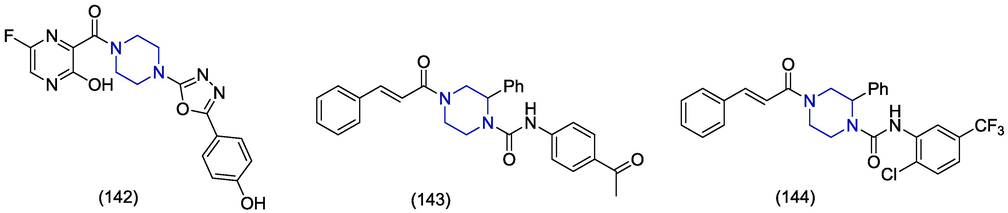
Lipin et al. unraveled some piperazine-based derivatives of favipiravir for the treatment of Nipah virus through in silico approach. The compound 142 was observed to be the most potent compound against Nipah virus with docking score of −8.909 kcal/mol and glide energy value of −55.229 kcal/mol. The hydrogen bond residues were seen with Gln530, Ala532, and Ile580 and showed hydrophobic interactions with Val507, Pro488, Ala532, Cys240, Tyr581, Ala558, Ile580, Pro590, Ile588, Ile217, and Cys216. The predicted IC50 value (pIC50) was found out to be 4.86 µM, endorsing the docking studies, and predicted using a web server. (Lipin et al., 2021). In the context of the structure–activity relationship described by Lipin et al. (2021), piperazine-based derivatives of furans with phenyl groups that have electron-donating substituents (1 4 2) showed a stronger affinity to bind with the Nipah virus target than the compounds with electron-withdrawing substituents on the phenyl ring attached to the furan ring that is directly bonded to the piperazine. del Rosario García-Lozano et al. (2023) reported piperazine-derived compounds as inhibitors against Zika virus. Two lead compounds, 143 and 144, with promising broad-spectrum activity against ZIKV (IC50 6.6 μM and 1.9 μM respectively) were identified with a good security profile. Regarding structure activity relationship, piperazine ring possessing chalcone moiety (143, 144) exhibited better activity against ZIKV than the piperazine-based indole derivatives which are found to be inactive.(Fig. 19)
4 Conclusion
The piperazine ring system defines the major class of N-heterocyclic compounds including several blockbuster drugs as discussed above. This review reflects the contribution of piperazine towards the development of compounds as antiviral agents. Piperazine served as a linker, substituent and as the main pharmacophore in new antiviral agents. Piperazine is present as the central scaffold in some new anti-HIV agents which presented prominent activity. The attachment of piperazine with some privileged scaffolds like indole and tetrahydroisoquinoline also produced compounds with exceptional activities. Piperazine has been used as the suitable linker in the preparation of symmetric compounds which have shown notable antiviral activities. The incorporation of phenyl piperazine also produced potent antivirals especially when the aromatic ring is attached with suitable substituents. The benzyl piperazine scaffold also served an important role in the activity of some derivatives. The methyl piperazine has also been incorporated as the substituent in some derivatives but these derivatives were found inactive or showed moderate activities. Conversion of piperazine nitrogen into guanidine group increased the toxicity of resultant derivatives. In some derivatives, piperazine ring has been directly attached with an additional heterocyclic ring where moderate antiviral activity was achieved. In this manuscript, some potent antiviral compounds having piperazine core are summarized. Hopefully, the present report will be useful in anticipating the medicinal significance of piperazine hybrids which have the potential to fight against viral infections, including epidemics and pandemics. On these grounds, further therapeutic investigations on this motif are highly recommended.
Acknowledgements
The authors acknowledge the support from Government College University Faisalabad and Higher Education Commission of Pakistan.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- Antioxidant and antimicrobial studies of novel N′-(substituted-2-chloroquinolin-3-yl)methylidene-4-hydroxy-2H-1,2-benzothiazine-3-carbohydrazides 1, 1-dioxides. Med. Chem. Res.. 2012;21:2340-2348.
- [CrossRef] [Google Scholar]
- Synthesis and antioxidant studies of novel N-substitutedbenzyl/phenyl-2-(3,4-dimethyl-5,5-dioxidopyrazolo[4,3-c][1,2]benzothiazin-2(4H)-yl) acetamides. Med. Chem. Res.. 2013;22:794-805.
- [CrossRef] [Google Scholar]
- Synthesis of novel pyrazolobenzothiazine 5, 5-dioxide derivatives as potent anti-HIV-1 agents. Med. Chem. Res.. 2014;23:1309-1319.
- [CrossRef] [Google Scholar]
- Design, synthesis, in silico study and anticancer potential of novel N-4-piperazinyl-ciprofloxacin-aniline hybrids. Pak. J. Pharm. Sci.. 2019;32(5):2215-2222.
- [Google Scholar]
- In vitro inhibition and molecular docking of a new chalcone against SARS-CoV2 main protease. Fundam. Clin. Pharmacol.. 2022;36(1):160-170.
- [CrossRef] [Google Scholar]
- Anti-adenovirus activity, antioxidant potential, and phenolic content of black tea (Camellia sinensis Kuntze) extract. J. Complement. Integr. Med.. 2016;13(4):357-363.
- [CrossRef] [Google Scholar]
- Synthesis and structure–activity relationship studies of HIV-1 virion infectivity factor (vif) inhibitors that block viral replication. ChemMedChem. 2012;7(7):1217-1229.
- [CrossRef] [Google Scholar]
- Interactions of paramyxovirus: A review. Al-Qadisiyah J. Vet. Med. Sci.. 2019;18(1):105-112.
- [Google Scholar]
- Nucleozin targets cytoplasmic trafficking of viral ribonucleoprotein-Rab11 complexes in influenza A virus infection. J. Virol.. 2013;87(8):4694-4703.
- [CrossRef] [Google Scholar]
- Novel spirothiazamenthane inhibitors of the influenza A M2 proton channel. Eur. J. Med. Chem.. 2016;120:64-73.
- [CrossRef] [Google Scholar]
- Design, synthesis, and biological evaluation of 1-(thiophen-2-yl)-9H-pyrido [3,4-b] indole derivatives as anti-HIV-1 agents. Chem. Biol. Drug Des.. 2015;85(6):722-728.
- [CrossRef] [Google Scholar]
- A study of cytotoxicity of novel chlorokojic acid derivatives with their antimicrobial and antiviral activities. Eur. J. Med. Chem.. 2010;45(9):4089-4095.
- [CrossRef] [Google Scholar]
- Synthesis and antiviral study of novel 4-(2-(6-amino-4-oxo-4,5-dihydro-1H-pyrrolo[2,3-d]pyrimidin-3-yl)ethyl) benzamide derivatives. Med. Chem. Res.. 2018;27(11–12):2538-2546.
- [CrossRef] [Google Scholar]
- Bassetto, M., Leyssen, P., Neyts, J., Yerukhimovich, M. M., Frick, D. N., 2017. Courtney-Smith, M.; Brancale, A., In silico identification, design and synthesis of novel piperazine-based antiviral agents targeting the hepatitis C virus helicase. European Journal of Medicinal Chemistry 125, 1115-1131. https://doi.org/10.1016/j.ejmech.2016.10.043.
- Targeting influenza a virus RNA promoter. Chem. Biol. Drug Des.. 2015;86(4):663-673.
- [CrossRef] [Google Scholar]
- Piperazine derivatives with central pharmacological activity used as therapeutic tools. Fundam. Clin. Pharmacol.. 2019;33(1):13-24.
- [CrossRef] [Google Scholar]
- Treatment for emerging viruses: convalescent plasma and COVID-19. Transfus. Apher. Sci.. 2020;59(3):102790
- [CrossRef] [Google Scholar]
- Febuxostat: a selective xanthine oxidase inhibitor for the treatment of hyperuricemia and gout. Ann. Pharmacother.. 2006;40(12):2187-2194.
- [CrossRef] [Google Scholar]
- In silico structure-based design and synthesis of novel anti-RSV compounds. Antiviral Res.. 2015;122:46-50.
- [CrossRef] [Google Scholar]
- Innovation and trends in the development and approval of antiviral medicines: 1987–2017 and beyond. Antiviral Res.. 2018;155:76-88.
- [CrossRef] [Google Scholar]
- design, synthesis, and anti-HIV evaluation of novel triazine derivatives targeting the entrance channel of the NNRTI binding pocket. Chem. Biol. Drug Des.. 2015;86(1):122-128.
- [CrossRef] [Google Scholar]
- Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med.. 2011;365(6):493-505.
- [CrossRef] [Google Scholar]
- Heterocyclic pharmacochemistry of new rhinovirus antiviral agents: a combined computational and experimental study. Eur. J. Med. Chem.. 2017;140:528-541.
- [CrossRef] [Google Scholar]
- Cytomegalovirus infection in pregnancy. Birth Defects Res.. 2017;109(5):336-346.
- [CrossRef] [Google Scholar]
- Antivirals and antiviral strategies. Nat. Rev. Microbiol.. 2004;2(9):704-720.
- [CrossRef] [Google Scholar]
- Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev.. 2016;29(3):695-747.
- [CrossRef] [Google Scholar]
- Challenges and recent progress in drug discovery for tropical diseases. Nature. 2018;559(7715):498-506.
- [CrossRef] [Google Scholar]
- The future of antivirals: broad-spectrum inhibitors. Curr. Opin. Infect. Dis.. 2015;28(6):596-602.
- [CrossRef] [Google Scholar]
- del Rosario García-Lozano, M., Dragoni, F., Gallego, P., Mazzotta, S., López-Gómez, A., Boccuto, A. Martínez-Cortés, C., Rodríguez-Martínez, A., Pérez-Sáanchez, H., Vega-Pérez, J. M., Del campo, J. A., Vicenti, I., Vega-Holm, M., Iglesias-Guerra, F., 2023. Piperazine-derived small molecules as potential Flaviviridae NS3 protease inhibitors. In vitro antiviral activity evaluation against Zika and Dengue viruses. Bioorganic Chemistry 133, 106408. https://doi.org/10.1016/j.bioorg.2023.106408.
- Design, synthesis, and biological activity of novel 1, 4-disubstituted piperidine/piperazine derivatives as CCR5 antagonist-based HIV-1 entry inhibitors. Bioorg. Med. Chem. Lett.. 2012;22(9):3284-3286.
- [CrossRef] [Google Scholar]
- Adenoviruses in immunocompromised hosts. Clin. Microbiol. Rev.. 2008;21(4):704-715.
- [CrossRef] [Google Scholar]
- Discovery of dihydroxyindole-2-carboxylic acid derivatives as dual allosteric HIV-1 Integrase and Reverse Transcriptase associated Ribonuclease H inhibitors. Antiviral Res.. 2020;174:104671
- [CrossRef] [Google Scholar]
- Rational modifications, synthesis and biological evaluation of new potential antivirals for RSV designed to target the M2–1 protein. Bioorg. Med. Chem.. 2020;28(8):115401
- [CrossRef] [Google Scholar]
- Gao, S., Sylvester, K., Song, L., Tobias, Claff, lanlan, Jing., Wooson, M., WeiBh, R. H., Cheng, Yusen., Schakel.,L.,Petry., Gutschow, M., Schiedel, A. C., Strater, N., Kang, Dongwei., Shujing, Xu., Toth, K., Tavis, J., Tollefson, A. E., Muller, C. E., Liu, X., Zhan, P., 2022c. Discovery and crystallographic studies of trisubstituted piperazine derivatives as non-covalent SARS-CoV-2 main protease inhibitors with high target specificity and low toxicity. Journal of Medicinal Chemistry 65 (19), 13343-13364. https://doi.org/10.1021/acs.jmedchem.2c01146.
- Identification of polyphenol derivatives as novel SARS-CoV-2 and DENV non-nucleoside RdRp inhibitors. Molecules. 2022;28(1):160.
- [CrossRef] [Google Scholar]
- Discovery and crystallographic studies of nonpeptidic piperazine derivatives as covalent SARS-CoV-2 main protease inhibitors. J. Med. Chem.. 2022;65(24):16902-16917.
- [CrossRef] [Google Scholar]
- Design, synthesis and anti-HBV activity evaluation of new substituted imidazo [4,5-b] pyridines. Bioorg. Chem.. 2020;98:103580
- [CrossRef] [Google Scholar]
- Current treatment guidelines of chronic hepatitis B: the role of nucleos(t)ide analogues and peginterferon. Best Pract. Res. Clin. Gastroenterol.. 2017;31(3):299-309.
- [CrossRef] [Google Scholar]
- A new antiviral scaffold for human norovirus identified with computer-aided approaches on the viral polymerase. Sci. Rep.. 2019;9(1):1-20.
- [CrossRef] [Google Scholar]
- Avian paramyxovirus: a brief review. Transbound. Emerg. Dis.. 2017;64(1):53-67.
- [CrossRef] [Google Scholar]
- Single-cell virology: on-chip investigation of viral infection dynamics. Cell Rep.. 2017;21(6):1692-1704.
- [CrossRef] [Google Scholar]
- Gupta, Y., Kumar, S., Zak, S. E., Jones, K. A., Upadhyay, C., Sharma, N., Azizi, S. A., Kathayat, R. S., Poonam., Herbert, A. S., Durvasula, R., Dickinson, B. C., Dye, J. M., Rathi, B., Kempaiah, P., 2021. Antiviral evaluation of hydroxyethylamine analogs: Inhibitors of SARS-CoV-2 main protease (3CLpro), a virtual screening and simulation approach. Bioorganic Med. Chem. 47, 116393. https://doi.org/10.1016/j.bmc.2021.116393.
- Synthesis and antiviral evaluation of novel N-6 substituted adenosine analogues. Tetrahedron Lett.. 2017;58(3):190-193.
- [CrossRef] [Google Scholar]
- Identification of novel non-nucleoside vinyl-stilbene analogs as potent norovirus replication inhibitors with a potential host-targeting mechanism. Eur. J. Med. Chem.. 2019;184:111733
- [CrossRef] [Google Scholar]
- chlorcyclizine inhibits viral fusion of hepatitis c virus entry by directly targeting HCV envelope glycoprotein 1. Cell Chem. Biol.. 2020;27(7):780-792.
- [CrossRef] [Google Scholar]
- Design, synthesis, and biological evaluation of novel 2-methylpiperazine derivatives as potent CCR5 antagonists. Bioorg. Med. Chem.. 2015;23(5):1157-1168.
- [CrossRef] [Google Scholar]
- Exploiting the tolerant region I of the non-nucleoside reverse transcriptase inhibitor (NNRTI) binding pocket: discovery of potent diarylpyrimidine-typed HIV-1 NNRTIs against wild-type and E138K mutant virus with significantly improved water solubility and favorable safety profiles. J. Med. Chem.. 2019;62(4):2083-2098.
- [CrossRef] [Google Scholar]
- Activity of bis (7-hydroxycoumarin) Mannich bases against bovine viral diarrhoea virus. Antiviral Res.. 2016;134:153-160.
- [CrossRef] [Google Scholar]
- Safety, efficacy and pharmacodynamics of vesatolimod (GS-9620) in virally suppressed patients with chronic hepatitis B. J. Hepatol.. 2018;68(3):431-440.
- [CrossRef] [Google Scholar]
- Jiang, X., Huang, B., Rumrill, S., Pople, D., Zalloum, W. A., Kang, D., Zhao, F., Ji, X., Gao, Z., Hu, L., Wang, Z., Xie, M., De Clercq, E., Ruiz, F. X., Arnold, E., Pannecouuque, Liu, X., Zhan, P., 2023. Discovery of diarylpyrimidine derivatives bearing piperazine sulfonyl as potent HIV-1 nonnucleoside reverse transcriptase inhibitors. Communications Chemistry 6 (1), 83. https://doi.org/10.1038/s42004-023-00888-4.
- New strategies against drug resistance to herpes simplex virus. Int. J. Oral Sci.. 2016;8(1):1-6.
- [CrossRef] [Google Scholar]
- 2-((4-arylpiperazin-1-yl) methyl)benzonitrile derivatives as orally available inhibitors of HCV with a novel mechanism of action. J. Med. Chem.. 2020;63(11):5972-5989.
- [CrossRef] [Google Scholar]
- Synthesis and antiviral activity of a series of novel N-phenylbenzamide and N-phenylacetophenone compounds as anti-HCV and anti-EV71 agents. Acta Pharm. Sin. B. 2015;5(3):201-209.
- [CrossRef] [Google Scholar]
- Design and synthesis of a novel series of non-nucleoside HIV-1 inhibitors bearing pyrimidine and N-substituted aromatic piperazine. Bioorg. Med. Chem. Lett.. 2018;28(22):3491-3495.
- [CrossRef] [Google Scholar]
- Improving the positional adaptability: structure-based design of biphenyl-substituted diaryltriazines as novel non-nucleoside HIV-1 reverse transcriptase inhibitors. Acta Pharm. Sin. B. 2020;10(2):344-357.
- [CrossRef] [Google Scholar]
- Benzimidazole ring system as a privileged template for anticancer agents. Pharm. Chem. J.. 2018;51:1068-1077.
- [CrossRef] [Google Scholar]
- Anti-HIV activity of new pyrazolobenzothiazine 5, 5-dioxide-based acetohydrazides. Med. Chem. Res.. 2015;24:3671-3680.
- [CrossRef] [Google Scholar]
- A novel piperazine derivative that targets hepatitis B surface antigen effectively inhibits tenofovir resistant hepatitis B virus. Sci. Rep.. 2021;11(1):1-13.
- [CrossRef] [Google Scholar]
- A novel compound active against SARS-CoV-2 targeting Uridylate-specific endoribonuclease (NendoU/NSP15): In silico and in vitro investigations. RSC Med. Chem.. 2021;12(10):1757-1764.
- [CrossRef] [Google Scholar]
- Clinical features and treatment of adenovirus infections. Rev. Med. Virol.. 2008;18(6):357-374.
- [CrossRef] [Google Scholar]
- Li, D. K., Chung, R. T., 2019. Overview of direct-acting antiviral drugs and drug resistance of hepatitis C virus. In Hepatitis C Virus Protocols 1911, 3-32. https://doi.org/10.1007/978-1-4939-8976-8_1.
- Amide-containing α-hydroxytropolones as inhibitors of hepatitis B virus replication. Antiviral Res.. 2020;177:104777
- [CrossRef] [Google Scholar]
- Piperazine-substituted derivatives of favipiravir for Nipah virus inhibition: What do in silico studies unravel? SN Appl. Sci.. 2021;3:1-18.
- [CrossRef] [Google Scholar]
- Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov. Today Technol.. 2004;1(4):337-341.
- [CrossRef] [Google Scholar]
- Antiretroviral-drug resistance among patients recently infected with HIV. N. Engl. J. Med.. 2002;347(6):385-394.
- [CrossRef] [Google Scholar]
- Humans have antibodies against a plant virus: evidence from tobacco mosaic virus. PLoS One. 2013;8(4):e60621.
- [Google Scholar]
- Design, synthesis and biological evaluation of novel piperazine derivatives as CCR5 antagonists. PLoS One. 2013;8(1):e53636.
- [Google Scholar]
- 9-Aminoacridine-based agents impair the bovine viral diarrhea virus (BVDV) replication targeting the RNA-dependent RNA polymerase (RdRp) Bioorg. Med. Chem.. 2018;26(4):855-868.
- [CrossRef] [Google Scholar]
- Current progress in antiviral strategies. Trends Pharmacol. Sci.. 2014;35(2):86-102.
- [CrossRef] [Google Scholar]
- Lucero, Y., Vidal, R., O'Ryan G, M., 2018. Norovirus vaccines under development. Vaccine 36 (36), 5435-5441. https://doi.org/10.1016/j.vaccine.2017.06.043.
- Rethinking the old antiviral drug moroxydine: Discovery of novel analogues as anti-hepatitis C virus (HCV) agents. Bioorg. Med. Chem. Lett.. 2015;25(22):5372-5376.
- [CrossRef] [Google Scholar]
- Discovery of a novel 9-position modified second-generation anti-HCV candidate via bioconversion and semi-synthesis of FR901459. Bioorg. Med. Chem. Lett.. 2020;30(18):127423
- [CrossRef] [Google Scholar]
- Mamidala, E., Davella, R., Gurrapu, S., Shivakrishna, P., 2020. In silico identification of clinically approved medicines against the main protease of SARS-CoV-2, causative agent of COVID-19. arXiv preprint arXiv:2004.12055. https://doi.org/10.48550/arXiv.2004.12055.
- A potential long-acting bictegravir loaded nano-drug delivery system for HIV-1 infection: A proof-of-concept study. Antiviral Res.. 2019;167:83-88.
- [CrossRef] [Google Scholar]
- Design, synthesis, and evaluation of WC5 analogues as inhibitors of human cytomegalovirus immediate-early 2 protein, a promising target for anti-HCMV treatment. ChemMedChem. 2013;8(8):1403-1414.
- [CrossRef] [Google Scholar]
- Optimization of piperazine-derived ureas privileged structures for effective antiadenovirus agents. Eur. J. Med. Chem.. 2020;185:111840
- [CrossRef] [Google Scholar]
- Piperazine-based CCR5 antagonists as HIV-1 inhibitors. III: synthesis, antiviral and pharmacokinetic profiles of symmetrical heteroaryl carboxamides. Bioorg. Med. Chem. Lett.. 2003;13(3):567-571.
- [CrossRef] [Google Scholar]
- Future landscape of hepatitis C research–basic, translational and clinical perspectives. J. Hepatol.. 2016;65(1):S143-S155.
- [CrossRef] [Google Scholar]
- Concentration-targeted phase I trials of atevirdine mesylate in patients with HIV infection: dosage requirements and pharmacokinetic studies. Antiviral Res.. 2000;45(1):47-58.
- [CrossRef] [Google Scholar]
- Newcastle disease virus in pakistan: genetic characterization and implication in molecular diagnosis. Indian J. Virol.. 2012;23(3):368-373.
- [CrossRef] [Google Scholar]
- Design, synthesis, anti-tobacco mosaic viral and molecule docking simulations of urea/thiourea derivatives of 2-(piperazine-1-yl)-pyrimidine and 1-(4-fluoro/4-chloro phenyl)-piperazine and 1-(4-chlorophenyl)-piperazine–A study. Bioorg. Chem.. 2020;102:104084
- [CrossRef] [Google Scholar]
- Studies on the anti-hepatitis C virus activity of newly synthesized tropolone derivatives: identification of NS3 helicase inhibitors that specifically inhibit subgenomic HCV replication. Bioorg. Med. Chem.. 2010;18(14):5129-5136.
- [CrossRef] [Google Scholar]
- Antioxidant, antimicrobial and antiproliferative activities of peel and pulp extracts of red and white varieties of Ipomoea batatas (L) Lam. Trop. J. Pharm. Res.. 2017;16(9):2221-2229.
- [CrossRef] [Google Scholar]
- Biochemical activity of RAGs is impeded by Dolutegravir, an HIV integrase inhibitor. Cell Death Discovery. 2020;6(1):50.
- [CrossRef] [Google Scholar]
- Genetic susceptibility to human norovirus infection: an update. Viruses. 2019;11(3):226.
- [CrossRef] [Google Scholar]
- Respiratory syncytial virus seasonality: a global overview. J Infect Dis. 2018;217(9):1356-1364.
- [CrossRef] [Google Scholar]
- Novel piperazine based compounds as potential inhibitors for SARS-CoV-2 Protease Enzyme : Synthesis and molecular docking study. J. Mol. Struct.. 2021;1245:131020
- [CrossRef] [Google Scholar]
- The fight against the influenza A virus H1N1: synthesis, molecular modeling, and biological evaluation of benzofurazan derivatives as viral RNA polymerase inhibitors. ChemMedChem. 2014;9(1):129-150.
- [CrossRef] [Google Scholar]
- Avian paramyxovirus serotype 1 (newcastle disease virus), avian influenza virus, and Salmonella spp. in mute swans (cygnus olor) in the great lakes region and atlantic coast of the united states. Avian Dis.. 2013;58(1):129-136.
- [CrossRef] [Google Scholar]
- The evolution of seasonal influenza viruses. Nat. Rev. Microbiol.. 2018;16(1):47.
- [CrossRef] [Google Scholar]
- Recent synthetic approaches towards thienothiophenes: a potential template for biologically active compounds. Mol. Divers.. 2023;1–29
- [CrossRef] [Google Scholar]
- A comprehensive update of various attempts by medicinal chemists to combat COVID-19 through natural products. Molecules. 2023;28(12):4860.
- [CrossRef] [Google Scholar]
- Recent advancements in oxadiazole-based anticancer agents. Trop. J. Pharm. Res.. 2017;16(3):723-733.
- [CrossRef] [Google Scholar]
- Piperazine derivatives for therapeutic use: a patent review (2010-present) Expert Opin. Ther. Pat.. 2016;26(7):777-797.
- [CrossRef] [Google Scholar]
- Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol. Hepatol.. 2018;3(6):383-403.
- [CrossRef] [Google Scholar]
- Herpesvirus: an underestimated virus. Folia Microbiol.. 2017;62(2):151-156.
- [CrossRef] [Google Scholar]
- Reddy, K., Chenna, R., Rasheed, S., Subba Rao, D., Adam, S., Venkata Rami Reddy, Y., Raju, C. N., 2013. New urea and thiourea derivatives of piperazine doped with febuxostat: synthesis and evaluation of anti-TMV and antimicrobial activities. The Scientific World Journal 2013. https://doi.org/10.1155/2013/682603.
- 3-((R)-4-(((R)-6-(2-bromo-4-fluorophenyl)-5-(ethoxycarbonyl)-2-(thiazol-2-yl)-3,6-dihydropyrimidin-4-yl)methyl)morpholin-2-yl)propanoic acid (HEC72702), a novel hepatitis b virus capsid inhibitor based on clinical candidate GLS4. J. Med. Chem.. 2018;61(3):1355-1374.
- [CrossRef] [Google Scholar]
- Riitho, V., Strong, R., Larska, M., Graham, S. P., Steinbach, F., 2020. Bovine pestivirus heterogeneity and its potential impact on vaccination and diagnosis. Viruses 12 (10), 1134.references in formatted form.docx.
- Anti-HIV-1 activity of a tripodal receptor that recognizes mannose oligomers. Eur. J. Med. Chem.. 2015;106:132-143.
- [CrossRef] [Google Scholar]
- Novel quinolyl-thienyl chalcones and their 2-pyrazoline derivatives with diverse substitution pattern as antileishmanial agents against Leishmania major. Med. Chem. Res.. 2012;21:1322-1333.
- [CrossRef] [Google Scholar]
- Preclinical pharmacological development of chlorcyclizine derivatives for the treatment of hepatitis C virus infection. J. Infect. Dis.. 2018;217(11):1761-1769.
- [CrossRef] [Google Scholar]
- 2-Aminobenzoxazole ligands of the hepatitis C virus internal ribosome entry site. Bioorg. Med. Chem. Lett.. 2014;24(15):3521-3525.
- [CrossRef] [Google Scholar]
- Sánchez-Céspedes, J., Martínez-Aguado, P., Vega-Holm, M., Serna-Gallego, A., Candela, J. I., Marrugal-Lorenzo, J. A., Pachón, J. n., Iglesias-Guerra, F., Vega-Pérez, J. M., 2016. New 4-acyl-1-phenylaminocarbonyl-2-phenylpiperazine derivatives as potential inhibitors of adenovirus infection. synthesis, biological evaluation, and structure–activity relationships. Journal of Medicinal Chemistry 59 (11), 5432-5448. https://doi.org/10.1021/acs.jmedchem.6b00300.
- Inhibition of adenovirus replication by a trisubstituted piperazin-2-one derivative. Antiviral Res.. 2014;108:65-73.
- [CrossRef] [Google Scholar]
- Selvakumar, B., Vaidyanathan, S., Subbiah, M., Elango, K. P., 2017. Synthesis and antiviral activity of 4-(7, 7-dimethyl-4-[4-{N-aroyl/benzyl}1-piperazinyl]-5,6,7,8-tetrahydroquinazolin-2-yl) morpholine derivatives. Arkivoc 2017 (4), 353-364.http://dx.doi.org/10.24820/ark.5550190.p010.037.
- Synthesis and antiviral study of 4-(7,7-dimethyl-4-(piperazin-1-yl)-5,6,7,8-tetrahydroquinazolin-2-yl) morpholine derivatives. Med. Chem. Res.. 2018;27(2):512-519.
- [CrossRef] [Google Scholar]
- Discovery of a novel 5-carbonyl-1H-imidazole-4-carboxamide class of inhibitors of the HIV-1 integrase–LEDGF/p75 interaction. Bioorg. Med. Chem.. 2013;21(19):5963-5972.
- [CrossRef] [Google Scholar]
- HIV-1 protease and reverse transcriptase mutations for drug resistance surveillance. AIDS (London, England). 2007;21(2):215.
- [CrossRef] [Google Scholar]
- Molecular design, synthesis and biological evaluation of cage compound-based inhibitors of hepatitis C virus p7 ion channels. Eur. J. Med. Chem.. 2018;158:214-235.
- [CrossRef] [Google Scholar]
- Coxsackievirus B escapes the infected cell in ejected mitophagosomes. J. Virol.. 2017;91(24):e01347-e10417.
- [CrossRef] [Google Scholar]
- Synthesis and anti-HIV properties of new carbamate prodrugs of AZT. Chem. Biol. Drug Des.. 2012;80(6):947-952.
- [CrossRef] [Google Scholar]
- Manassantin B shows antiviral activity against coxsackievirus B3 infection by activation of the STING/TBK-1/IRF3 signalling pathway. Sci. Rep.. 2019;9(1):1-10.
- [CrossRef] [Google Scholar]
- Synthesis, anti-HIV and antitubercular activities of lamivudine prodrugs. Eur. J. Med. Chem.. 2005;40(12):1373-1376.
- [CrossRef] [Google Scholar]
- Current perspectives in transfusion-transmitted infectious diseases: emerging and re-emerging infections. ISBT Sci. Ser.. 2014;9(1):30-36.
- [CrossRef] [Google Scholar]
- Design, synthesis, and mechanism study of benzenesulfonamide-containing phenylalanine derivatives as novel HIV-1 capsid inhibitors with improved antiviral activities. J. Med. Chem.. 2020;63(9):4790-4810.
- [CrossRef] [Google Scholar]
- Studies of anti-HIV transcription inhibitor quinolones: identification of potent N1-vinyl derivatives. ChemMedChem. 2010;5(11):1880-1892.
- [CrossRef] [Google Scholar]
- Structural investigation of the naphthyridone scaffold: identification of a 1,6-naphthyridone derivative with potent and selective Anti-HIV activity. ChemMedChem. 2011;6(7):1249-1257.
- [CrossRef] [Google Scholar]
- Piperazine-based CCR5 antagonists as HIV-1 inhibitors. II. Discovery of 1-[(2,4-Dimethyl-3-pyridinyl) carbonyl]-4-methyl-4-[3(S)methyl-4-[1(S)-[4-(trifluoro-methyl)phenyl]ethyl]-1-piperazinyl]-piperidine N1-Oxide (Sch-350634), an orally bioavailable, potent CCR5 antagonist. J. Med. Chem.. 2001;44(21):3343-3346.
- [CrossRef] [Google Scholar]
- Synthesis and the biological evaluation of 2-benzenesulfonylalkyl-5-substituted-sulfanyl-[1, 3, 4]-oxadiazoles as potential anti-hepatitis B virus agents. Antiviral Res.. 2006;71(1):7-14.
- [CrossRef] [Google Scholar]
- New carbocyclic N6-substituted adenine and pyrimidine nucleoside analogues with a bicyclo [2.2.1] heptane fragment as sugar moiety; synthesis, antiviral, anticancer activity and X-ray crystallography. Bioorg. Med. Chem.. 2015;23(19):6346-6354.
- [CrossRef] [Google Scholar]
- Recent advances and developments of in vitro evaluation of heterocyclic moieties on cancer cell lines. Chem. Rec.. 2019;19(2–3):362-393.
- [CrossRef] [Google Scholar]
- Discovery and optimization of novel small-molecule HIV-1 entry inhibitors using field-based virtual screening and bioisosteric replacement. Bioorg. Med. Chem. Lett.. 2014;24(23):5439-5445.
- [CrossRef] [Google Scholar]
- Identification of a new benzimidazole derivative as an antiviral against hepatitis C virus. J. Virol.. 2016;90(19):8422-8434.
- [CrossRef] [Google Scholar]
- Synthesis, evaluation and structure–activity relationships of triazine dimers as novel antiviral agents. Bioorg. Med. Chem. Lett.. 2012;22(23):7174-7178.
- [CrossRef] [Google Scholar]
- The evolution of pleconaril: modified O-alkyl linker analogs have biological activity towards coxsackievirus B3 nancy. Molecules. 2020;25(6):1345.
- [CrossRef] [Google Scholar]
- An insight into the therapeutic potential of piperazine-based anticancer agents. Turk. J. Chem.. 2019;43(1):1-23.
- [CrossRef] [Google Scholar]
- Optimization and SAR research at the piperazine and phenyl rings of JNJ4796 as new anti-influenza A virus agents, part 1. Eur. J. Med. Chem.. 2021;222:113591
- [CrossRef] [Google Scholar]
- Phenylpropenamide derivatives: anti-hepatitis B virus activity of the Z isomer, SAR and the search for novel analogs. Bioorg. Med. Chem. Lett.. 2011;21(15):4642-4647.
- [CrossRef] [Google Scholar]
- Pharmacophore-based design, synthesis, and biological evaluation of novel chloro-pyridazine piperazines as human rhinovirus (HRV-3) inhibitors. Bioorg. Med. Chem. Lett.. 2011;21(3):1057-1059.
- [CrossRef] [Google Scholar]
- Inhibitors of human immunodeficiency virus type 1 (HIV-1) attachment. 5. An evolution from indole to azaindoles leading to the discovery of 1-(4-benzoylpiperazin-1-yl)-2-(4,7-dimethoxy-1H-pyrrolo [2,3-c] pyridin-3-yl)ethane-1,2-dione (BMS-488043), a drug candidate that demonstrates antiviral activity in HIV-1-infected subjects. J. Med. Chem.. 2009;52(23):7778-7787.
- [CrossRef] [Google Scholar]
- 2,5-diketopiperazine derivatives as potential anti-influenza (H5N2) agents: synthesis, biological evaluation, and molecular docking study. Molecules. 2022;27(13):4200.
- [CrossRef] [Google Scholar]
- Discovery and mechanistic investigation of piperazinone phenylalanine derivatives with terminal indole or benzene ring as novel HIV-1 capsid modulators. Molecules. 2022;27(23):8415.
- [CrossRef] [Google Scholar]
- Benzimidazole derivative, BM601, a novel inhibitor of hepatitis B virus and HBsAg secretion. Antiviral Res.. 2014;107:6-15.
- [CrossRef] [Google Scholar]
- Inhibitors of HIV-1 attachment. part 7: indole-7-carboxamides as potent and orally bioavailable antiviral agents. Bioorg. Med. Chem. Lett.. 2013;23(1):198-202.
- [CrossRef] [Google Scholar]
- Hepatitis C infection: A systemic disease. Clin. Liver Dis.. 2017;21(3):449-453.
- [CrossRef] [Google Scholar]
- Yuan, T., Wang, Z., Liu, D., Zeng, H., Liang, J., Hu, D., Gan, x., 2022. Ferulic acid derivatives with piperazine moiety as potential antiviral agents. Pest Management Science 78(4), 1749-1758. https://doi.org/10.1002/ps.6794.
- Identification of a small-molecule inhibitor of HIV-1 assembly that targets the phosphatidylinositol (4,5)-bisphosphate binding site of the HIV-1 matrix protein. ChemMedChem. 2013;8(3):426-432.
- [CrossRef] [Google Scholar]
- Design, synthesis and evaluation of 2, 2-dimethyl-1, 3-dioxolane derivatives as human rhinovirus 3C protease inhibitors. Bioorg. Med. Chem. Lett.. 2017;27(17):4061-4065.
- [CrossRef] [Google Scholar]
- Binding interactions between enantiomeric α-aminophosphonate derivatives and tobacco mosaic virus coat protein. Int. J. Biol. Macromol.. 2017;94:603-610.
- [Google Scholar]
- Trifluoromethylpyridine piperazine derivatives: synthesis and anti-plant virus activity. Pest Manag. Sci.. 2023;79(7):2571-2580.
- [CrossRef] [Google Scholar]
- Novel substituted heteroaromatic piperazine and piperidine derivatives as inhibitors of human enterovirus 71 and coxsackievirus a16. Molecules. 2013;18(5):5059-5071.
- [CrossRef] [Google Scholar]
- Design, synthesis and in vitro anti-influenza A virus evaluation of novel quinazoline derivatives containing S-acetamide and NH-acetamide moieties at C-4. Eur. J. Med. Chem.. 2020;206:112706
- [CrossRef] [Google Scholar]
- Discovery of novel N-aryl piperazine CXCR4 antagonists. Bioorg. Med. Chem. Lett.. 2015;25(21):4950-4955.
- [CrossRef] [Google Scholar]
- Synthesis, biological evaluation and molecular docking study of N-(2-methoxyphenyl)-6-((4-nitrophenyl)sulfonyl)benzamide derivatives as potent HIV-1 Vif antagonists. Eur. J. Med. Chem.. 2017;129:310-324.
- [CrossRef] [Google Scholar]







