Recent advances on sustainable adsorbents for the remediation of noxious pollutants from water and wastewater: A critical review
⁎Corresponding author at: Department of Environmental Health Engineering, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran. hdehghani@tums.ac.ir (Mohammad Hadi Dehghani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This review article evaluates and compares the efficiency of various sustainable adsorbents for the removal of noxious pollutants from water environments. This study discusses the various types of adsorbents concerning their performance and suitability. Adsorbent types include natural-based, carbon-based, waste-based, biomass-based, biopolymers-based, and nanocomposites-based adsorbents, as well as metallic organic frameworks, aerogels, networking crystalline solids, zeolite nanoparticles, and dendrimers were reviewed. Characterisation, modification, fabrication, adsorption capacities under different conditions, isotherm models, and kinetics of noxious pollutants were also reviewed. Adsorption capacities for different pH values, adsorbent doses, adsorbent concentrations, temperature, and the size of particles have been described. One of the basic needs in water and wastewater adsorbents is their formulation and optimisation, using columnar operations, and more importantly, they can be produced quickly and should be cost-effective. Various studies reported a broad range of values for the thermodynamic parameters for noxious pollutants, suggesting the adsorbent's nature as an essential factor affecting the thermodynamics of noxious pollutant sorption. The regeneration and reusability potential of other adsorbents have also been discussed, along with molecular modelling, simulation, knowledge gaps, and future perspectives of noxious pollutants from the water environments.
Keywords
Noxious pollutants
Water and wastewater
Kinetics and isotherms
Nanotechnology
Sustainable adsorbents
Molecular modelling
1 Introduction
The world faces water shortage as one of the current threats due to increasing population, climate change, and the continuous demand for its regular use in agriculture and industry (Dehghani et al., 2010; Krishna et al., 2017). This excess use has led to water pollution, one of the significant environmental factors currently threatening humankind. Furthermore, the leakage of toxic pollutants into rivers, seas, and oceans causes water pollution, reducing water quality and ultimately affecting human health directly or indirectly through drinking or agricultural usage (Manes, 1998, Cooney, 1998, Dąbrowski, 2001; Mohammadi et al., 2017)(Dehghani et al., 2008).
When the water is polluted with toxic pollutants, including pesticides, fungicides, and herbicides, it causes chronic effects on human health, such as immunotoxicity, cancers, congenital disabilities, and neurological toxicity (Mohammed et al., 2011, Hussain et al., 2021, Dehghani and Fadaei, 2012). Also, heavy metals and toxic minerals are an example of pollutants that causes abdominal pain, dehydration, cardiomyopathy, the nervous system, liver and kidney damage, and DNA damage (Krishna et al., 2017, Karri et al., 2021). Various recent water treatment technologies, including Ion exchange (IE), Electrodialysis (ED), Reverse osmosis (RO), Adsorption, coagulation/ flocculation, and flotation, have been developed and practiced over time (Younas et al., 2021). Although these technologies are effective, they cause disadvantages, including partial removal of specific ions, the restoration of membranes, and high operational costs (Rubalcaba et al., 2007, Choudhary et al., 2020).
Among the mentioned technologies, adsorption is the most used because it is efficient and low-cost (Gupta et al., 2016). Organic and inorganic adsorbents can be used, including activated carbon (AC) and other carbon-based adsorbents such as biochar, polymer materials, zeolite, bio-fuels, and farming waste (Liu et al., 2019, Jha et al., 2023). In addition, nanotechnology has been used to resolve environmental problems, including removing water pollutants. Nanostructured adsorbents have a high surface area, resulting in a faster adsorption rate and high efficiency in water and wastewater treatment (Sadegh et al., 2016).
Many nano adsorbents such as metal oxide-based nanoparticles (NPs), including the oxides of iron, magnesium, zinc, titanium, plant nanocomposites (NCs), carbon nanotubes (CNTs), and graphite, are used to remove pollutants (Santhosh et al., 2016, Wang et al., 2012, Rai, 2022). Such activated carbon has many limitations, including low regeneration capacity that can be toxic to many living organisms, and its use and reuse are relatively expensive (Aichour and Zaghouane-Boudiaf, 2020). Therefore, to minimize the costs and produce environmentally-friendly natural adsorbents with higher efficiency (Kyzas and Kostoglou, 2014), green adsorbents are highly effective with low costs and bio-degradable, bio-compatible, and renewable nature (Tofan et al., 2016). Li et al. (2016) showed that green adsorbent has been more effective in removing heavy metals from water than commercial AC.
Bio-adsorbents selectively aid in heavy metals removal, ions, and dyes from polluted water (Abdel-Ghani et al., 2007). The dead biomass (such as bark, sawdust, peat), natural fibres (cotton and flax, plants), and other organic substances (polysaccharides or biopolymers such as alginate, cellulose, starch, and chitin and their derivative products like cyclodextrins and chitosan) are in this category (Zhao and Zhou, 2016).
Biosorbents also include algae, bacteria, fungi, and yeasts, which can potentially remove water pollutants depending on the biosorption process that happens through binding materials derived from various biomasses (Vahabisani and An, 2021). In a study, Kuppusamy and Yun (Vijayaraghavan and Yun, 2008) showed that bacterial cell wall composition, including functional groups of peptidoglycan, teichoic acids, phospholipids, lip polysaccharides, and proteins, are involved in elemental biosorption. Furthermore, fungal cell wall composition consists of polysaccharides, chitin, proteins, lipids, and pigments with different functional groups that can remove the binding of toxic metal ions (Wang and Chen, 2009).
The uses of green adsorbents for removing noxious pollutants from water environments have shown great interest due to their low cost, abundance, and eco-friendly properties (Osagie et al., 2021, Othmani et al., 2021b). The large quantity of waste generated from different sources has been dramatically increased, encouraging their use for environmental applications (Del Sole et al., 2021, Khan et al., 2022). The effectiveness of the adsorption in water and wastewater treatment has encouraged using green and cheap adsorbents prepared from wastes, including agricultural materials, to remove noxious pollutants from industrial and aqueous environments (Mustapha et al., 2020). A lot of methods have been used for the preparation, modification, and characterization of green adsorbents like chemical and physical modifications (Adewuyi, 2020), chemical pretreatment (Bensah et al., 2011), oxidation (Zhang et al., 2020), nanoparticles grafting (Yanat and Schroën, 2021), grafting of carboxyl groups, amines or amides on green materials (de Quadros Melo et al., 2016).
2 Adsorbents modification
Chemical pretreatment is among the widely used methods for modifying vegetable materials for better performance and high adsorption capacity uptake. In this context, many works have been done to modify the surface characteristics of vegetable materials with NaHCO3, HCl, HNO3, CaCl2, H2SO4, H2O2, CaO, Na2CO3, NaOH, formaldehyde, acetic acid, citric acid, methanol, and EDTA. According to the results, masking or removing the functional moieties and exposing more binding sites can cause changes in surface characteristics. Therefore, chemically modifying these materials has affected the hydrophobicity, elasticity, capacity for water uptake, ion exchange and adsorption capacity, thermal resistance, and microbiological attack resistance (Othmani et al., 2021a). Other studies have discussed the methods used to modify vegetable materials by coating, electrodeposition, irradiation, and hydrothermal reactions (Liew et al., 2020). During the modification of the vegetable materials, a special focus must be given to the acid-base characteristics of the adsorbent before and after modification to identify the main difference and to have an idea about the main interactions that may occur during this process (Gui et al., 2019).
Also, attention must be given to ascertaining the association between the acid-base characteristics of natural materials and the adsorption capacities. Recently many researchers have focused on the chemical modification of vegetable materials by metal oxides like ZnO and TiO2 by in situ hydrolysis (Hu et al., 2010), decomposing bacterial cellulose infiltrated with zinc acetate (Qingfeng et al., 2011), hydrothermal method (Perelshtein et al., 2009), ultrasound irradiation (Li et al., 2015), and precipitation method in the presence and absence of alternating current (Seffen). Othmani et al. (2022) showed that modifying the raw material decreased the number of carboxylic and phenolic groups and increased the number of lactonic groups. The fractal mathematical model shows that the increase in the number of places on the material surfaces is confirmed by reducing the τc equilibrium time and the adsorbed values. Table 1 shows some methods used to modify vegetable materials and their use to remove emergent pollutants. Fig. 1 shows the main methods used for the adsorbent modification.
| Adsorbent | Modification | Synthesis method | Applications |
|---|---|---|---|
| Cellulose | TiO2 | In situ hydrolysis | Biosorption of Pb2+ ions |
| Bacterial Cellulose (BC) | ZnO | Decomposing bacterial cellulose infiltrated with zinc acetate | Photocatalytic degradation of Methyl orange |
| Wood | TiO2 | Hydrothermal method | Photocatalytic degradation of pollutants |
| Cotton fiber | ZnO | Ultrasound irradiation | Breakdown of bacteria |
| L. cylindrica | (1%, 2%, and 4% ZnO) | Precipitation method in the presence and absence of alternating current | Biosorption of methylene blue, industrial wastewater, and phenol |
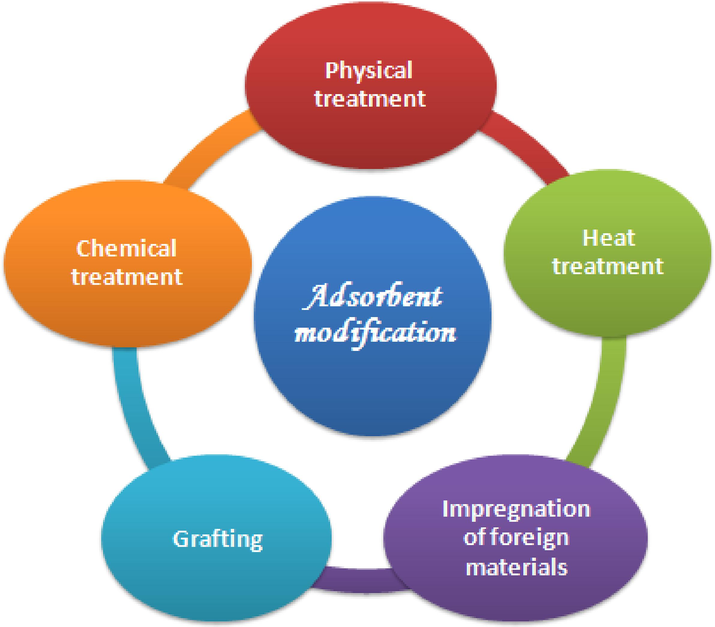
- The main methods used for the adsorbent modification.
3 Adsorbents fabrication
During these last few years, great attention has been paid to fabricating various adsorbents, like nanoporous adsorbents, which showed a good ability for water and wastewater decontaminating (Rashed, 2013). During these last years, great attention has been paid to fabricating various adsorbents like nanoporous adsorbents, which showed a good ability for wastewater decontamination. Tran et al. (2017) have discussed the performance of the synthesized nanoporous adsorbent based on the removal of aromatic sulfur compounds. They found that adsorptive desulfurization has shown a good ability to fabricate nanoporous adsorbents with low equipment investment, easy operation, and high energy efficiency.
Also, carbonaceous adsorbents present performing adsorbents for noxious pollutants removal. Li et al. (2012) have studied the modification of carbonaceous adsorbents with phosphoric acid through HTC at low-temperature hydrothermal method. They noticed an enhancement of many oxygen-containing groups and pore channels, significantly enhancing the adsorbent capacity uptake. Ahmad et al. (2019) evaluated the efficiency of the synthesized molecularly imprinted magnetite nanomaterials with iron oxide core and silica shell (Fe3O4/SiO2) to remove heavy metals. Their high adsorption capacity and easy separation ability confirmed that these adsorbents present performing strategies for remediation technology, especially for contaminated heavy metals.
4 Adsorbent characterisation
Suitable adsorbents must have a large surface area, available polar sites, and reproducibility in the degree of activation (Kose, 2010). The adsorbent's structure, morphology, composition, functions, and adsorption capacity significantly identify the adsorbent properties (Anbia and Amirmahmoodi, 2016). Scanning electron miscopy (SEM) determines the morphology of the adsorbent's surface and basic physical properties (Othmani et al., 2019). Fourier transform infrared spectroscopy (FTIR) identifies functional groups on the adsorbent material (Rytwo et al., 2015). X-photoelectron spectroscopy (XPS) spectroscopy can be used to determine the initial composition of the adsorbent (Rahdar et al., 2019a). Nuclear Magnetic Resonance (NMR), High-Performance Liquid Chromatography (HPLC), and Extended X-ray absorption fine structure are used to identify texture and can also determine the possible route of removal of pollutants from water and wastewater (Godejohann et al., 2011). Instrumental Neutron Activation Analysis (INAA), Inductively Coupled Plasma Mass Spectrometry (ICP – MS), and ICP Atomic Emission Spectroscopy (ICP-AES) are used to determine trace element content in coal (Chajduk and Polkowska-Motrenko, 2017). The N2 - BET equation can be used for the porosity of the adsorbent while removing the tested contaminant. Fig. 2 shows essential properties useful for identifying adsorbent performance for environmental applications. The adsorbent characterization is critical to understanding and identifying the different retention phenomena (adsorbent-adsorbate). Therefore, many mathematical models have been used for this utility (Othmani et al., 2019).
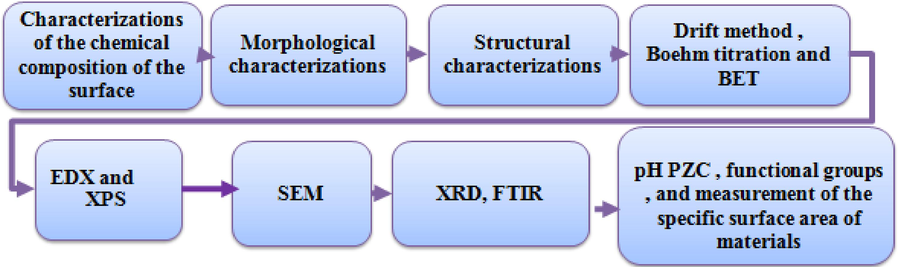
- Adsorbents characterisations.
5 Adsorption mechanisms
The term “sorption”, which is both a physical and chemical process, together with the terms “sorbent”, “sorbate”, and “sorptive”, is used to further describe both adsorption and absorption due to a synchronous event or trouble in distinctive them. In adsorption science, the essential idea is called adsorption isotherm (Seffen). The latter characterizes the harmonious connection at a consistent temperature between the amount of the material adsorbed and the tension or focus in the mass liquid stage (Ahmadi and Igwegbe, 2018).
Adsorption is a surface peculiarity described by grouping synthetic animal categories (adsorbate), considering its fume point and the pore of a strong adsorbent. This surface abundance happens every day when the appealing energy of a substance with a strong surface (i.e., the glue work) is more prominent than the firm energy of the actual substance (Krishnapriya and Kandaswamy, 2010; Gupta et al., 2016). The adsorptive take-up is enhanced if the strong material has a high surface region. If the adsorption happens by London-van der Waals forces of the strong and adsorbate, it is called actual adsorption. On the other hand, if the powers supporting the adsorption process are identified with compound-holding powers, then the adsorption is defined as chemisorption (Ahmadi and Igwegbe, 2018; Gupta et al., 2016; Osagie et al., 2021).
Nonetheless, the qualification between actual adsorption and chemisorption is not sharp in every case. For instance, the adsorption of polar fumes onto polar solids might fall under one or the other order, contingent upon the adsorption energy. From a thermodynamic perspective, when substances gather from a weakened fume point or when arranged onto a strong surface, it means that there will be a decrease in freedom of particle movement in this manner to misfortune in framework entropy (Osagie et al., 2021, Ahmadi and Igwegbe, 2018).
In explaining the interfacial layer concerning trial proof, it comprises two locales: the gas part, which exists in the power field of the strong surface, and its layer, which has the strong. Notably, the term 'adsorption' is an interaction that indicates the collection of particles in the interfacial layer, while desorption, then again, can be characterized by the opposite cycle. One eminent peculiarity frequently happens during one or the other adsorption or desorption (Ahmadi and Igwegbe, 2018). It is called hysteresis and is said to happen when a considerable deviation between the adsorption and desorption bends from each other. When the above occurs, the isotherm produces what is called a hysteresis circle, whose shape varies starting with one adsorption framework and then onto the next. Hysteresis circles in such a manner happen for the most part with mesoporous solids, which are the centre of the alleged fine buildup. Adsorbate can be defined as the material in the adsorbed state, while adsorptive refers to the one in the fume point before being adsorbed (Krishnapriya and Kandaswamy, 2010; Tran et al., 2017). Moreover, the infiltration, which takes place by the adsorbate particles and then straight to the strong mass stage, is characterized as 'assimilation’ (Krishnapriya and Kandaswamy, 2010; Tran et al., 2017). Fig. 3 shows basic terminologies used in the adsorption process to remove noxious pollutants from the environment.
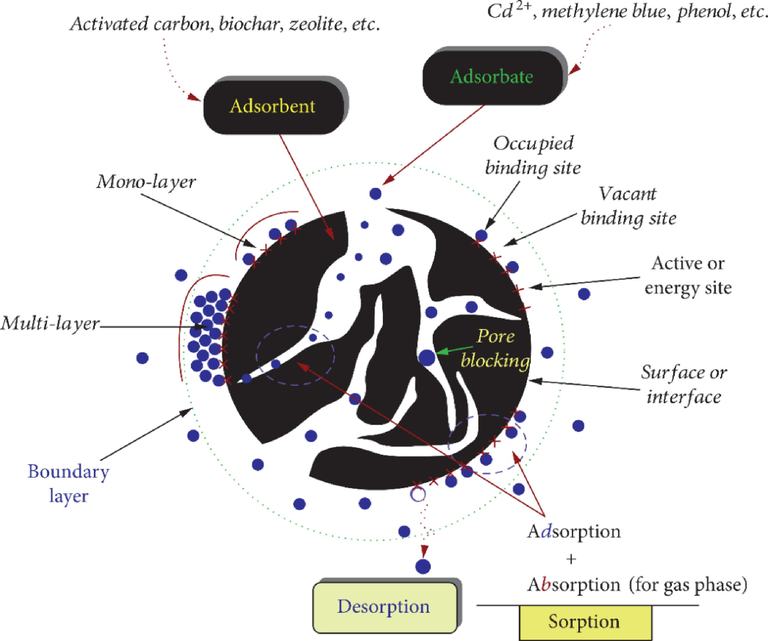
- Basic terminologies used in the adsorption process (Tran et al., 2017).
The main advantages and disadvantages of adsorption processes are; cost effectiveness / low-cost, wide pH range, easy operation or simple design (advantages) and waste products and weak selectivity (disadvantages) (Sukmana et al., 2021; Osagie et al., 2021).
5.1 Kinetics and equilibrium isotherms
The following models are used to predict the kinetics of the adsorption process: Pseudo-First Order, Pseudo-Second Order model, Brouers-Sotolongo, etc. Langmuir, Freundlich, Temkin, Dubinin-Radushkevich, and Sips are used to predict the isotherms of the reactions adsorption isotherm. The best models for isothermal and kinetic are reported: two Langmuir isotherm models and quasi-quadratic kinetic models. The Langmuir isotherm can show that a monolayer's adsorption happens at specific homogeneous sites and that no further adsorption occurs when a contaminant occupies the adsorbent at a specific site. Therefore, the adsorbent is saturated when put in contact with the adsorbate (Krishnapriya and Kandaswamy, 2010; Gupta et al., 2016,). A brief description of equilibrium isotherms and kinetic modelling of hazardous contaminants has been provided in Table 2.
| Adsorbents | Contaminants | Best-fit kinetic | R2 | Best-fit isotherm | R2 | Ref |
|---|---|---|---|---|---|---|
| Sesame leaves and stems as a kind of useless agricultural waste | cadmium | PSO | 0.9929 | Langmuir | 0.993 | (Swain et al., 2013) |
| Palm Shell Powder (PSP) | Methylene Blue | PSO | 0.9966 | Freundlich | 0.9449 | (Rigueto et al., 2021) |
| Palm Shell Powder (PSP) | Rhodamine 6G | PSO | 0.9537 | Langmuir | 0.9813 | (Rigueto et al., 2021) |
| arborvitae leaves | Pb(II) | – | – | Langmuir | 0.974 | (Liu et al., 2018) |
| arborvitae leaves | Cu(II) | – | – | Langmuir | 0.994 | (Liu et al., 2018) |
| arborvitae leaves | Co(II) | – | – | Langmuir | 0.997 | (Liu et al., 2018) |
| agroforestry waste mixtures | Cu | PSO | 0.9976 | Langmuir | 0.9786 | (Fatima et al., 2021) |
| agroforestry waste mixtures | Ni | PSO | 0.9962 | Langmuir | 0.9412 | (Fatima et al., 2021) |
| agroforestry waste mixtures | Mn | PSO | 0.9964 | Langmuir | 0.9415 | (Fatima et al., 2021) |
| agroforestry waste mixtures | Cr | PSO | 0.9763 | Langmuir | 0.9579 | (Fatima et al., 2021) |
| agroforestry waste mixtures | Zn | PSO | 0.9946 | Langmuir | 0.9385 | (Fatima et al., 2021) |
| modified bentonite | phenol | PSO | 0.999 | Freundlich | 0.997 | (Rawajfih and Nsour, 2006) |
| modified bentonite | aniline | PSO | 0.999 | Langmuir | 0.99 | (Ahmadi and Igwegbe, 2018) |
| modified saxaul ash | arsenic (V) | PSO | 0.991 | Langmuir | 0.949 | (Rahdar et al., 2019b) |
| modified barley husk (MBH) | phenol | PSO | 0.997 | D–R model | 0.971 | (BALARAK et al., 2020) |
| biocompatible nanocomposite | malachite green | PSO | 0.984 | Langmuir | 0.970 | (Raval et al., 2016) |
| nutraceutical industrial fenugreek seed spent | malachite green | PSO | 0.94 | – | – | (Gümüş, 2022) |
| cellulose acetate/graphene oxide (CA-GO) nanocomposite, | Ni2+ | PSO | 0.982 | Langmuir | 0.997 | (Aldalbahi et al., 2020) |
| red clay | Brilliant Green | PSO | 0.999 | Redlich–Peterson | 0.999 | (Rehman et al., 2013) |
| Walnut shell powder | methylene blue dye | PSO | 0.999 | Langmuir | 0.961 | (Miyah et al., 2018) |
| distilled washed Bambusa Tulda (DBT), | Brilliant green | PSO | 0.999 | Langmuir | 0.930 | (Laskar and Kumar, 2019) |
| Na2CO3-treated Bambusa Tulda (NCBT) | Brilliant green | PSO | 0.999 | Langmuir | 0.920 | (Laskar and Kumar, 2019) |
| Hydrochloric acid-treated Bambusa Tulda (HABT) | Brilliant green | PSO | 0.999 | Langmuir | 0.986 | (Laskar and Kumar, 2019) |
| composite snail shell–rice husk | Brilliant green | PSO | 1 | Langmuir | 0.995 | (Popoola et al., 2018) |
5.2 Thermodynamics studies
Three important parameters in thermodynamic modelling include ΔG°, ΔH°, and ΔS° (Laskar and Kumar, 2019). When the amount of Gibbs free energy is negative, it indicates spontaneity. Also, when the value of ΔS is positive, it indicates a tendency to pollutants and an accidental increase in adsorption. A negative ΔH value means being exothermic in the adsorption process. The results show that the adsorption of pollutants is less than 80 kJ/mol, so the adsorption mechanism is physical. The effect of temperature on the adsorption process shows that at low temperatures, the adsorption is exothermic (Sreelatha and Padmaja, 2008). Table 3 summarizes the research on thermodynamic modelling of noxious pollutants.
| Adsorbents | Temp. (K) | Contaminants | Enthalpy (ΔH°) (kJ/mol) | Entropy (ΔS°) J/Kmol | Free energy (ΔG°) (kJ/mol) | Ref |
|---|---|---|---|---|---|---|
| Palm Shell Powder (PSP) | 333 | Rhodamine 6G | 43.1270 | 0.1481 | −6.1906 | (Rigueto et al., 2021) |
| Palm Shell Powder (PSP)300 | 333 | Methylene Blue | 9.4526 | 0.0532 | −8.2590 | (Rigueto et al., 2021) |
| modified saxaul ash | 323 | arsenic (V) | 14.05 | 20.6 | 7.50 | (Rahdar et al., 2019b) |
| modified barley husk (MBH) | 293 | phenol | 23.88 | 0.087 | −1.431 | (BALARAK et al., 2020) |
| biocompatible nanocomposite | 293 | malachite green | −28.824 | −0.086 | −3.256 | (Raval et al., 2016) |
| nutraceutical industrial fenugreek seed spent | 323 | malachite green | 4.28 | 23.27 | −2.23 | (Taqui et al., 2021) |
| cellulose acetate/graphene oxide (CA-GO)nanocomposite | 293 | Ni2+ | −65 × 103 | 0.28 | −4.236 | (Aldalbahi et al., 2020) |
| red clay | 45 | Brilliant Green | 5.18 | 126.45 | 11.63 | (Rehman et al., 2013) |
| Walnut shell powder | 303 | methylene blue | −4.169 | −9.118 | −1.307 | (Miyah et al., 2018) |
| distilled washed Bambusa Tulda (DBT), | 288 | Brilliant Green | 3.819 | 17 | −1.482 | (Laskar and Kumar, 2019) |
| NCBT | 288 | Brilliant Green | 7.181 | 25 | −0.5576 | (Laskar and Kumar, 2019) |
| HBT | 288 | Brilliant Green | 4.146 | 18 | −1.329 | (Laskar and Kumar, 2019) |
| composite snail shell–rice husk | 323 | Brilliant Green | +61.189 | +159.404 | +9.702 | (Popoola et al., 2018) |
6 Types and efficiency of new-generation adsorbents
Adsorption is commonly used to eliminate diverse pollutants from water, wastewater, and other aqueous streams. Adsorption is an effective and efficient process for separation and purification, flexibility and simplicity of design while playing an essential role in removing different pollutants from water and wastewater. The most widely used method, activated carbon, has a highly porous nature and high adsorption capacity (Bansal and Goyal, 2005). However, the development of suitable adsorbents for various purposes has ensured the availability of various adsorbents, including natural adsorbents, alumina & alumina-based adsorbents, calcium-based adsorbents, clay & bentonite, pumice, zeolite, among others. This study discusses the various types of adsorbents concerning their performance and suitability.
6.1 Natural-based adsorbents
Agricultural by-products, including shells, bone, wood, and peat processed into activated carbon, are low-cost and unconventional adsorbents (Ahmedna et al., 2000, Dastgheib and Rockstraw, 2001). Biomass such as Aspergillus Tereus (Saifuddin M and Kumaran, 2005), Pseudomonas sp (Salah Azab and Peterson, 1989), and coconut shells (Amuda and Ibrahim, 2006) are essential adsorbents for removing pollution. The adsorption capacity of natural adsorbents changes according to the type of pollutant. Based on the general average, the heavy metal removal percentage is around 87.6 to 92.2%. Therefore, the adsorption capacity of heavy metals with natural attractions is high (corn cob > paddy husk > peanut skin > human hair > wheat bran > bagasse) (Amuda and Ibrahim, 2006). Gupta et al. (2009) reported efficiently removing the material's Cr (VI). They showed that at optimum conditions (pH 6, C0: 100 mg/L, contact time 90 min, and dosage: 3.5 g/L), removal was found to be 91%.
6.2 Alumina and alumina-based adsorbents
Alumina is a potentially efficient adsorbent material for fluoride removal because it shows a high adsorption capacity (Ayoob et al., 2008). However, it has not limited its utilization as an adsorbent material. Kumar et al. (2014) reported the utilization of alumina and aluminium-based adsorbents as water treatment sorbents. These include aluminium oxides, hydroxides, and ox hydroxides. Among all the derivatives of aluminium metal, aluminium oxides have received increased attention in water treatment processes in recent years. Their abundance and ability to influence the environmental behaviour of many contaminants, including divalent metals such as Pb2+, Co2+, and Ni2+ in soils and sediments, is a result of their reactivity further sets them apart (Kumar et al., 2014, Scheidegger et al., 1997). Removal of excess fluoride ions from municipal waters via transition alumina is an excellent example of illustrating alumina adsorption processes in water (Neidel et al., 2006). Jeong et al. (2007) evaluated the use of Aluminium oxide (Al2O3) for As(V) removal in water. Results of this study showed that over 95% of As (V) adsorption was shown at lower pH (less than7) and contact time of 20–60 min.
6.3 Calcium-based adsorbents
Wang et al. (2018) showed that calcium adsorbents have an excellent ability to remove dye with the removal dyes (97% and 91% Congo red and Titan yellow, respectively). Ramesh et al. (2017) also reported the adsorption of indigo carmine dye onto calcium hydroxide. Calcium hydroxide is effective at pH 12 (50 min) and follows a Langmuir-type isotherm to remove the dye. Dai et al. (2018) utilized calcium-rich biochar (CRB), and they reported high adsorption capacities of 12,502 and 20317 mg/g for cationic malachite green and anionic Congo red, respectively.
6.4 Clay and nano-clay minerals
Clay mineral types vary, including kaolin, ball clay, fire clay, Smectite and montmorillonite, Chlorite, and bentonite. The structural geometry of clay minerals is shown in Fig. 4. The use of clay minerals for the adsorption of organic and inorganic pollutants like-pesticides, heavy metals, dyes, antibiotics, humic acid, and other chemicals have been investigated by researchers (El Ass, 2018, Kennedy et al., 2018, Awasthi et al., 2019). The advantages of using clay minerals as suitable adsorbents for removing environmental pollutants such as heavy metals include high adsorption, stability, high ion exchange capacity and large specific surface area (Awasthi et al., 2019). By integrating nanomaterials and producing clay nanocomposites, the effectiveness of clay minerals in removing water pollutants can be increased (Hernández-Hernández et al., 2016, Annan et al., 2018, Das et al., 2018a).
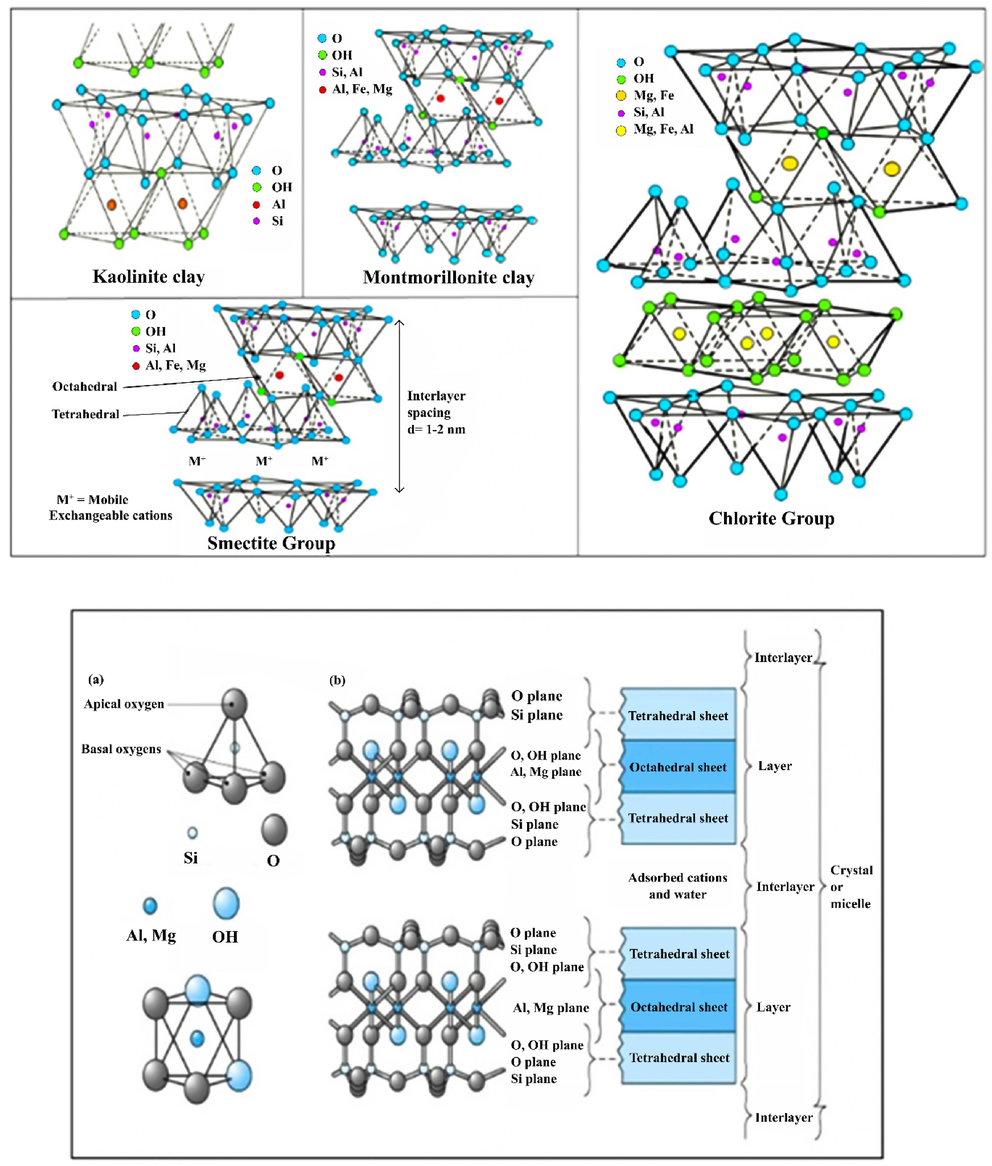
- Schematic of common clay minerals (Awasthi et al., 2019).
Gupta et al. (2009) report that the net negative charge on the structure of clay minerals increases its adsorption capacity. This negative charge in the clay attracts positively charged species. Also, Gupta et al. (2009) indicated that the qmax of Fuller’s earth was 220 and 120 mg/g for basic and acid blue, respectively (Yanat and Schroën, 2021). Espantaleon et al. (2003) showed that qmax for acid yellow 194 (24.9 mg/g), acid blue 349 (92.7 mg/g), and acid red 423 (29.1 mg/g) on natural bentonite. Clays remain very high potential candidates for green adsorbent types with their high adsorption capacity in aqueous media.
6.5 Pumice
Pumice stone as a natural stone is consists mainly of SiO2 and is formed when volcanic gas from the nucleus of viscous magma bubbles up. The significant proportion of silica in the pumice stone is the negative charge on the pumice surface, prompting it to adsorb heavy metal easily. Its application for industrial purposes, including textile and detergents, has been reported chiefly because of the many metals, organics, and dyes they can adsorb and its high adsorption capacity while maintaining its stance as a low-cost adsorbent (Liu et al., 2014). Modifying pumice has become a mainstay for its enhancement as an adsorbent, with findings documented in favour of modified pumice species. In the research by Asgari et al. (2012), pumice clay modified with surfactant presented a qmax of 41 mg/g, and removal of 96% fluoride (dose 0.5 g/L, pH 6, C0 = 10 mg/L, contact time = 30 min). For example, SEM micrographs of the pure pumice adsorbent and Co-supported pumice are shown in Fig. 5 (a-e).
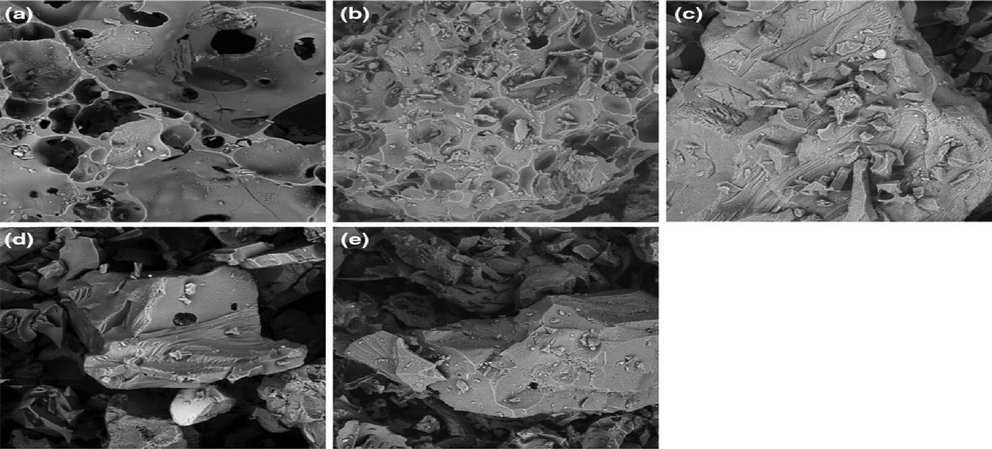
- SEM images of pure pumice (a), Co-supported pumice (b), 2-anisidine-adsorbed Co-supported pumice (c), 3-anisidine-adsorbed Co-supported pumice (d), 4-anisidine-adsorbed Co-supported pumice (e), (Bardakci et al., 2013).
6.6 Zeolites
Zeolites are naturally occurring, and synthetic microporous crystalline hydrated aluminosilicates characterized by high surface areas and cage-like three-dimensional networks of tetrahedral TO4 units (T is Si4+ and Al3+) linked by the sharing of all oxygen atoms. Partial substitution of Si4+ by Al3+ leads to excess negative charges, which are compensated for by extra framework alkali and earth alkaline cations. These cations reside in cavities and channels within the aluminosilicate structure along with the water molecules, and the water molecules can be removed or replaced by other sorbates (Tarasevich, 1999). Bowman (Bowman, 2003) studied the applications of surfactant-modified zeolites as a permeable barrier for the removal of environmental contaminants from water using a natural Clinoptilolite (74% purity) modified with hexadecyltrimethylammonium chloride (HDTMA-Cl).
The use of Surfactant Modified Zeolite as a sorbent was evaluated in a permeable barrier at a laboratory scale to remove chromate from polluted groundwater. Tests reveal that the SMZ permeable barrier effectively removed chromate from groundwater. Chromate was fully retained by the barrier, with no detectable concentrations. There has been researched on the use of cationic surfactant-modified zeolite to remove Phosphate, PO4+, and the results showed that surfactant-modified zeolite using cetylpyridinium chloride could remove phosphate ranging from 50% to 90% depending on variables such as the concentration of cetylpyridinium chloride on the preparation of surfactant modified zeolite (Widiastuti et al., 2008).
Another study of using cationic surfactant zeolite to remove arsenate, AsO43-, was reported by Macedo-Miranda and Olguin (Macedo-Miranda and Olguin, 2007). They demonstrated that modifying the adsorption properties of natural zeolite with lanthanum, hexadecyltrimethylammonium, and iron compounds improves arsenic adsorption capacities. The findings indicated that arsenic retention is affected by the priority of the zeolite material, the type of chemical species of arsenic, the pH, and the properties of the modified natural zeolites. Gebremedhin-Haile et al. (2003) investigated mercury sorption from aqueous solutions in the presence and absence of heavy metals (Cu2+, Ni2+, Zn2+) onto modified zeolite with cysteamine hydrochloride or cysteamine dihydrochloride in acidic pH. It was discovered that the elimination of mercury from an aqueous solution (without the heavy metals) ranges between 80% and 90%. Because copper, nickel, and zinc compete for the exchange sites in the zeolite network, reducing mercury by modified pretreatment zeolite from mixed metal solution is lower, around 42 %.
Ćurković et al. (1997) used a modified clinoptilolite treated with NaCl to remove Pb2+ and Cd2+ from water. They discovered 90% and 70% clearance efficiencies, respectively. Altare et al. (Altare et al., 2007) investigated the removal of volatile organic compounds (VOCs) such as benzene, toluene, ethyl benzene, and p, m-, and o-xylene (BTEX) from oil and gas-field wastewaters using surfactant-modified zeolite (SMZ). The cationic surfactant used was hexadecyltrimethylammonium (HDTMA). This study suggests that surfactant-modified zeolite is an effective sorptive medium for removing volatile organics from oil-and-gas-field wastewaters.
6.7 Carbon-based adsorbents
Thanks to a set of characteristics like the high surface, the high porosity, and the distinct chemical and physical properties of adsorbents based on carbon like activated carbon, carbon nanotube, graphene, and fullerene present the most performing adsorbents for noxious pollutant removal (Kabiri et al., 2014; Zhao et al. 2018; Cukierman et al., 2019; Mashkoor and Nasar, 2020). These carbonaceous materials (Fig. 6) have highly porous internal structures obtained from the pyrolysis and chemical treatment of sources such as wood, coal, nutshells, and other organic materials (Sweetman et al., 2017; Zhao et al. 2018; Mashkoor and Nasar, 2020; Reynel-Ávila et al., 2021).
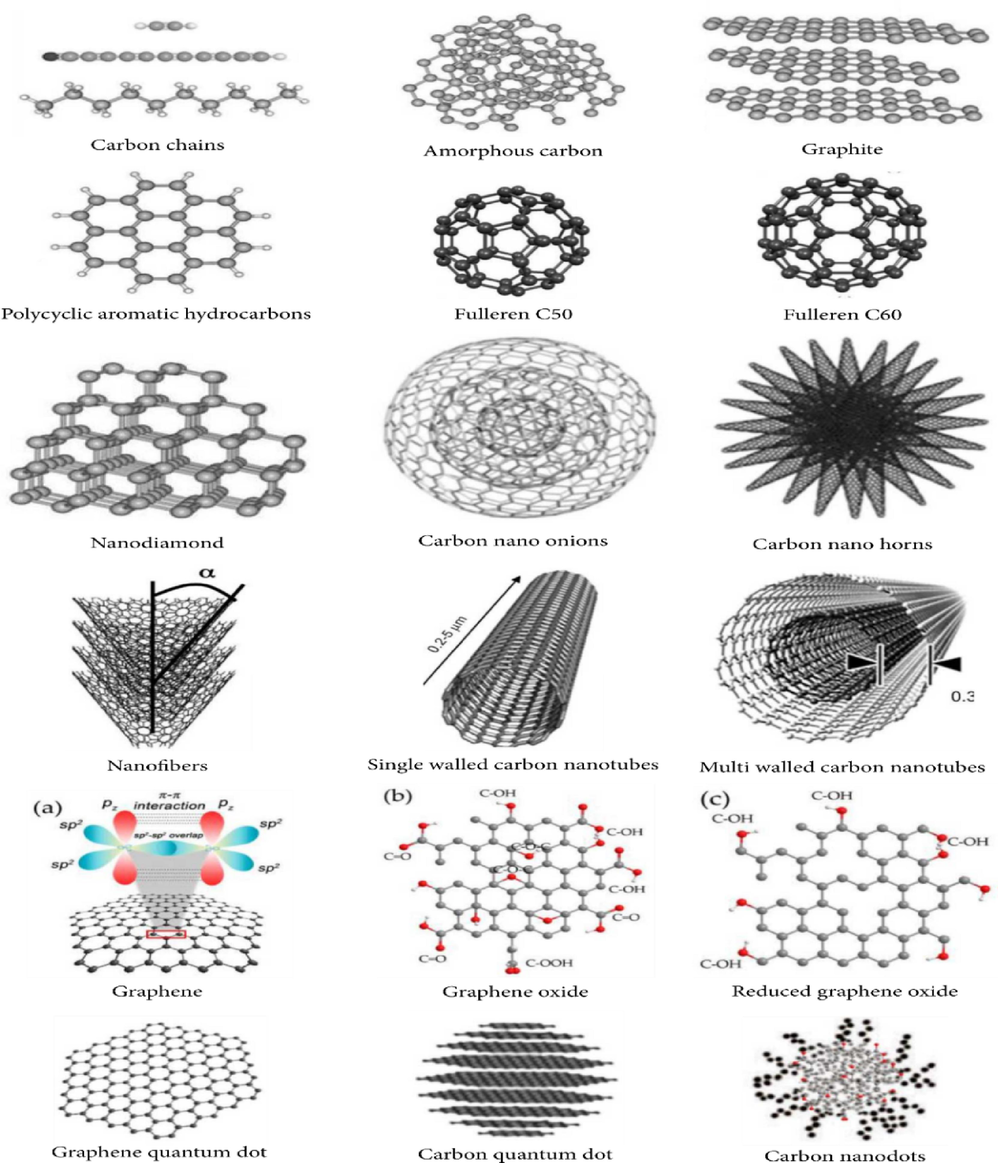
- Description of the structure forms of carbon materials (Reynel-Ávila et al., 2021).
6.7.1 Activated carbon
Activated carbon is available in the form of powder, granules, and fibers (Nazal, 2020). They are commonly used for water treatment (Varma, 2019). Powdered activated carbon with an average diameter of 15 to 25 µm has a particle size of fewer than 100 µm (Sanchez, 2011). Granular activated carbons are particles with a size greater than 1 mm, a small pore diameter, a large inner surface, and a relatively small outer surface. Therefore, diffusion phenomena inside the pores are essential in adsorption. The influence of the activated carbon's physical characteristics on adsorption can definitively affect the processing speed. If the speed of the fluid phase is high enough, then the overall adsorption rate is limited by the internal diffusion step (in the pores and surface). This phenomenon explains the influence of particle size on the dynamics of adsorption (Li et al., 2012; Varma, 2019).
A change in the particle size of the carbon (by grinding, for example) will increase the effective kinetics of adsorption: access to the core of the carbon is faster since the distance to be covered for the molecules is shorter (Lawtae and Tangsathitkulchai, 2021). However, grinding did not affect the specific surface of the carbon; the adsorption capacity remained the same (Mestre et al., 2016). The absorption capacity depends partly on the match between the pores' size and the adsorbed molecules' size (Othmani et al., 2017). Activated carbon is a generally microporous adsorbent; it will be more effective for molecules with dimensions smaller than the micropores than for larger organic molecules (Koonaphapdeelert et al., 2018). Possible interactions between different contaminants and activated carbon are shown in Fig. 7.
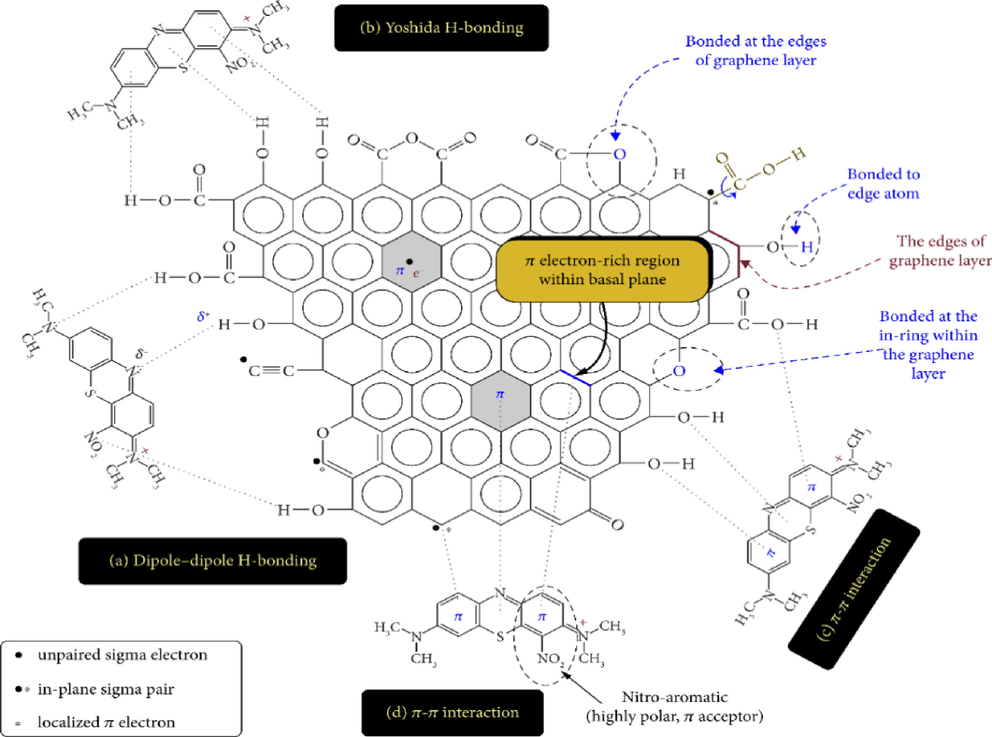
- Interactions between different contaminants and activated carbon (Sharma et al., 2022).
6.7.2 Carbon nanotubes
The sole physicochemical, mechanical, and electrical properties of carbon nanotube adsorbents allow them to be used in many applications, primarily as adsorbents, and have been widely used for environmental applications due to their similarities to activated carbon (Das et al., 2014). The latter have bonding structures of the carbon backbone and the relative ease of chemical modification. One of the main advantages of using carbon nanotubes is their ability is design specific functionalization or modification processes depending on their application and the type of pollutant tested (Aslam et al., 2021).
Therefore, they have similar adsorption mechanisms to activated carbon during adsorption (De Luca et al., 2021). Multi-wall carbon nanotubes (MWCNTs) and Single-wall carbon nanotubes (SWCNTs) are the main groups of carbon nanotubes (Saifuddin et al., 2013). Fig. 8 shows possible adsorption sites for interacting pollutants with carbon nanotubes. Carbon nanotubes have been successfully used for emergent pollutant removal. However, some precautions concerning their safety depending on their types, physicochemical properties, lengths, shapes, and charges must be considered (Das et al., 2018b; Mashkoor and Nasar, 2020).
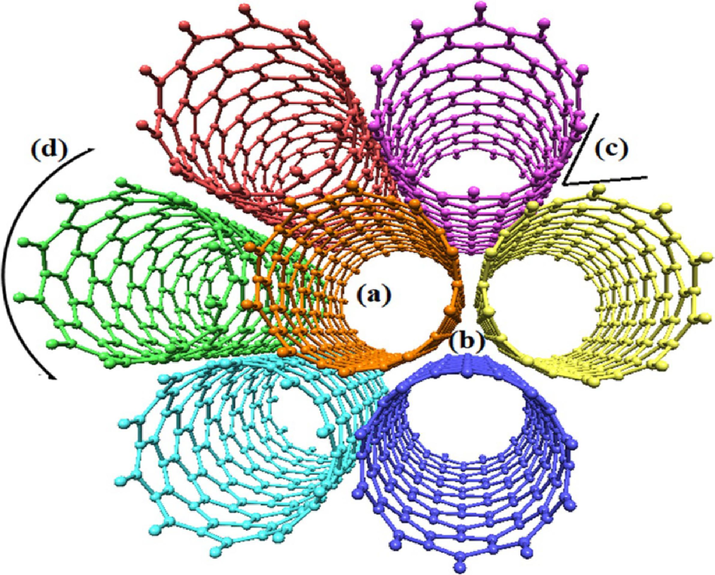
- Possible adsorption sites for the interaction of contaminants with carbon nanotubes: (a) internal sites, (b) interstitial channels, (c) external grooves, and (d) exposed surface sites. Due to the hollow structure of carbon nanotubes, the internal sites have the potential to adsorb contaminants. The interstitial channels that are available between the individual nanotubes are easily accessible for the adsorbate molecules. External grooves on the periphery of the nanotube bundles and the exposed surface sites also provide the sites to adsorb pollutants in water (Mashkoor and Nasar, 2020).
6.7.3 Graphene/graphene oxide
According to the literature, Graphene oxide (GO) presents excellent hydrophilicity, high surface area, abundant functional groups, and strong π interactions that allow them to be used in thin films for emergent pollutant removal and environmental applications (Ma et al., 2017). Recently, graphene and graphene oxide have been used to remove many pollutants, such as heavy metals, dyes, phenols, metals, organic and inorganic pollutants, etc. (Velusamy et al., 2021). Huang et al. (2021), have investigated the Pb2+ ions' adsorption from water on pristine graphene nanosheets and thermally modified graphene nanosheets. The results showed that the adsorption of Pb2+ ions was better by heat treatment than pristine graphene nanosheets. Gandhi et al. (2016) have highlighted the efficacy of using graphene and graphene oxide for the adsorption of inorganic (cationic and anionic) contaminants. In this context, many works were discussed, like the work done by Zhao et al. (2011), who have prepared graphene oxide nanosheets to adsorb Co2+ and Cd2+ from water. The qmax reached 106.3 mg/g for Cd2+ and 68.2 mg/g for Co2+, respectively. The results indicated that the prepared reduced graphene oxide-Fe (0)-Fe3O4 adsorbed Cr(VI) (31.2 mg/g), Hg2+(22 mg/g), Pb2+ (19.2 mg/g), and Cd2+ (1.91 mg/g).
6.7.4 Biochar
High carbon-containing materials, be they rich in lignin or cellulose, have been processed to biochar through various processes, including (i) pyrolysis under an inert atmosphere of nitrogen gas, (ii) temperature-programmed drying of carbonaceous material between 473 and 573 K, (iii) charring of moist biomass in a pressure vessel under high temperature and pressure, and (iv) biomass degradation at high temperature under gaseous atmosphere like O2, H2O vapour, and CO2. Customized pyrolysis is the most trusted route for cost and energy efficiency (Krishnapriya and Kandaswamy, 2010; Li et al., 2021). Biochar requires bio-based renewable fodder and is completely distinguishable from producing activated carbon, which rather requires activation through chemicals. However, various commissions define the precursor for biochar, as few consider high-content ashy substances as precursors. Furthermore, there are a few limits to the plant live derivative only: shell, oilseed residue, fruit, sawdust, seed, vegetable, bark, wood chip, etc.(Isaeva et al., 2021; Li et al., 2021). More focus on biochar these days is based on the fact that their applicability has increased several folds to remove organic and inorganic pollutants, including organic dyes, heavy metal ions, phenols, antibiotics, pesticides, etc., (Peiris et al., 2017; Conte et al., 2021; Liang et al., 2021; Qiu et al., 2022) from water environments (Fig. 9).
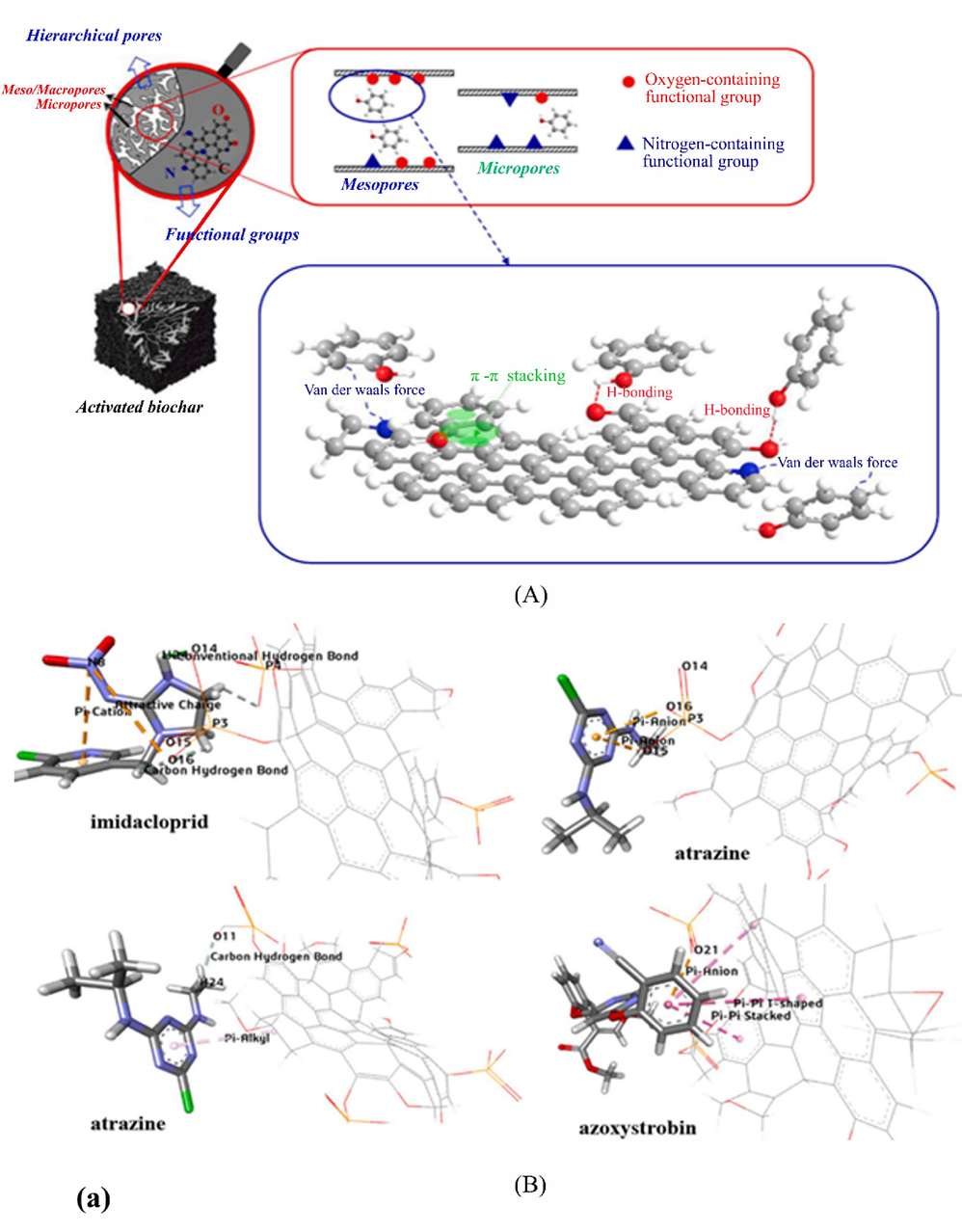
- Adsorption mechanism of some pollutants on biochar (A) adsorption mechanism of phenol (Qiu et al., 2022), (B) Important molecular non-bonding interactions that played a major role in the removal of imidacloprid, atrazine and azoxystrobin (Qiu et al., 2022), (C) Cr(VI) removal mechanisms (a) on the surface of biochar (b) on the surface of modified biochar (Liang et al., 2021), (D) Adsorption mechanisms of sulfonamides and tetracyclines on biochar surfaces (Peiris et al., 2017).
- Adsorption mechanism of some pollutants on biochar (A) adsorption mechanism of phenol (Qiu et al., 2022), (B) Important molecular non-bonding interactions that played a major role in the removal of imidacloprid, atrazine and azoxystrobin (Qiu et al., 2022), (C) Cr(VI) removal mechanisms (a) on the surface of biochar (b) on the surface of modified biochar (Liang et al., 2021), (D) Adsorption mechanisms of sulfonamides and tetracyclines on biochar surfaces (Peiris et al., 2017).
There is no bar in producing biochar from waste or fresh bio-based materials, as it has been evident in the literature that biochar has been obtained from fresh woody biomass and refuse biomasses like agriculture wastes, industrial wastes, and organics processed for environmental remedy (Shyam et al., 2022). Biochar obtained from sewage, agriculture waste, and industrial wastes are found to be rich in carbonates and oxides of transition metals besides the elements of groups 1 and 2, which tends to remove the deadly pollutants both by ion exchange and adsorption (Abdeljaoued et al., 2020, Nzediegwu et al., 2021). This mineral-rich biochar is multifunctional besides acting as a green adsorbent and has applicability in many other uses like catalysis, soil enrichment, nutrient enrichment, etc. Mechanisms of the interactions of biochar with organic pollutants and inorganic pollutants as below (Ahmad et al., 2014; Ahmad et al., 2019): (A) Mechanisms of the interactions of biochar with organic pollutants. I. electrostatic interaction, II. electrostatic attraction, and III. electrostatic attraction between biochar and organic pollutants, (B) Mechanisms of biochar interactions with inorganic contaminants. I. ion exchange, II. electrostatic attraction, III. precipitation, and IV. electrostatic attraction of metal.
6.8 Waste-based adsorbents
The necessity of producing cheap or low-cost adsorbents to reduce the costs of wastewater treatment and the increasing production of large quantities of waste around the world led to an urgent upsurge a technology to recycle the wastes and use them in the treatment and purification of water and wastewater (Hossain et al., 2020). Recycling waste conserves energy and preserves the greenery of the environment. Sources of waste used as waste adsorbents include municipal waste, agricultural and industrial wastes, and biomass and natural clay sources (Hossain et al., 2020). Exploiting waste adsorbents has many environmental benefits, including soil amendment supplements, adsorbents regeneration, biofuel regeneration, and cementitious material (Zwain et al., 2014). Furthermore, these waste adsorbent materials can be implemented directly or used after modifying with different chemical, thermal and hydrothermal treatments (Sun et al., 2015).
Agricultural residues such as citrus fruit peel, black gram husk, rice husk, wood sawdust, bagasse, and egg-shells (Reza and Ahmaruzzaman, 2015) are converted into a gelatinous substance or biochar/activated carbon engineered for wastewater treatment (Hossain et al., 2020). For example, rice husk has been chemically activated with K2CO3 and further modified by urea, forming urea-modified activated carbons to uptake nitrate from aqueous effluents (Satayeva et al., 2018). Previous studies (Aybar et al., 2016) have reported that leaf adsorbents are the most efficient biosensors for removing metal ions. Another type of agricultural residue is modified eucalyptus sorbent and birch sawdust, which was efficient for adsorbing inorganic species, accounting for 45.37% Fe, 21.99% Al, 15.37% Si, and 4.88% Cu from the contaminated waters (Chen et al., 2018). Also, alfalfa biomass is transformed into chemically activated biochar using NaOH to achieve the qmax of 302.37 mg/g to remove tetracycline from wastewater (Jang and Kan, 2019). Table 4 summarizes the adsorption capacities of some adsorbents based on agricultural residue. The biosorption of the examined heavy metals onto raw leaf biosorbents at different experimental conditions followed, in most cases, the Langmuir isotherm. The maximum monolayer adsorption capacity obtained from the Langmuir isotherm for the studied heavy metals ranged from 3.9 to 300 mg/g.
| Adsorbents | Contaminants | Maximum capacity; mg/g | Ref. |
|---|---|---|---|
| Guava leaves (Psidiumguajava) | Cd2+ | ∼ 12(exp.a) | (Kee et al., 2015) |
| Fig leaves | 103.09(exp.) | (Rahul et al., 2015) | |
| Sesame waste (leaf and stem parts) | 84.74 (Langmuir)22.88 (column studies) |
(Swain et al., 2013) | |
| Ficusreligiosa leaves | 27.14 (Langmuir) | (de Oliveira Farias et al., 2015) | |
| Tobacco leaves | Cu2+ | ∼ 10.66 (exp.)17.182 (Langmuir) |
(Goudarztalejerdi et al., 2015) |
|
TectonagrandisL.f. leaves |
∼ 10 mg/g (exp.)15.43 (Langmuir) |
(Pi et al., 2016) | |
| Ricinus communis leaves | 127.27(exp.) | (Varghese and Das, 2015) | |
| Melaleuca diosmifolia leaves | Cr6+ | 49.38 (exp.)62.5 (Langmuir) |
(An et al., 2019) |
| Rubber leaves | 21.45(exp.) | (An et al., 2014) | |
| Colocasiaesculenta leaves | 43.61(exp) | (Rajeswari et al., 2016) | |
| Ficusauriculata leaves | 6.80 (Langmuir) | (Jagtap et al., 2011) | |
| Castor tree (Ricinuscommunis L.) leaves | Hg2+ | 37.2(exp.) | (Xu et al., 2005) |
| Bamboo leaves | 27.11(exp.) | (Sayed and Jardine, 2015) | |
| Tomato (Lycopersicum esculent) leaves | Ni2+ | 58.82 (Langmuir) | (Shen et al., 2017) |
| SyzygiumcuminiL Leaves | Pb2+ | 32.47 mg/g (Langmuir) | (Christensen and Rorrer, 2009) |
| Phoenix tree leaves | 71.0 (Langmuir) | (Hubbe et al., 2013) | |
| Black tea leaves | 19.7(exp.) | (Aksu and Kutsal, 1990) | |
| Ficusreligiosa Leaves | 37.45 (Langmuir) | (Tam et al., 2002) | |
| Solanummelongena Leaves | 71.42 (Langmuir) | (Zhang et al., 2013) | |
| Neem (Azadirachta indica) Leaves | 300 (Langmuir) | (Christensen and Rorrer, 2009) | |
| Bael (Aegle marmelos) Leaves | 104(exp.) | (Nwosu et al., 2018) | |
| Cinnamomumcamphora Leaves | 75.82(exp.) | (Sharma et al., 2018) | |
| Diceriocaryumeriocarpum Leaves | 41.9 (Langmuir) | (Ighalo et al., 2021) | |
| Brazilian Orchid Tree (Pata-de-vaca) leaves | Cu2+ Cd2+ |
0.238 mmol/g (exp.) 0.113 mmol/g (exp.) |
(Reverberi et al., 2017) |
| Cabbage leaves | Pb2+ Cu2+ Cd2+ |
6.081 mg/g (exp.) 5.493 mg/g (exp.) 4.843 mg/g (exp.) |
(Tijani et al., 2019) |
| Oak leaves (in a mixture of fern bark and rice husks) | Cu2+ Ni2+ Mn2+ Zn2+ Cr6+ |
32.52 (Langmuir)3.90 (Langmuir)4.68 (Langmuir)6.82 (Langmuir)23.80 (Langmuir) |
(Fatima et al., 2021) |
| Arborvitae leaves | Pb2+ Cu2+ Co2+ |
35.84 (Langmuir)7.94 (Langmuir)6.78 (Langmuir) |
(Liu et al., 2018) |
| Mistletoe leaves | Pb2+ Cd2+ |
68.53 (Langmuir)50.07 (Langmuir) |
(Pardo et al., 2021) |
| Teak (Tectona grandis) leaves | Ni2+ Co2+ |
18.112 (Langmuir)27.215 (Langmuir) |
(Laabd et al., 2022) |
| Citrus documana | Reactive red 2 (dye) |
0.608 mg/g | (Phan et al., 2009) |
| Citrus medica | 0.580 mg/g | ||
| Citrus aurantifolia | 0.566 mg/g | ||
| Orange peel (Citrus sinensis L.) | Remazol brilliantBlue (dye) |
11.62 mg/g (20 °C), 10.70 mg/g (30 °C), 8.61 mg/g (40 °C), 6.39 mg/g (50 °C), 5.54 mg/g (60 °C). |
(Shan et al., 2020) |
| Mosambi peel | Erichromeblack T (dye) |
90% (Initial dye concentration 50 mg/L, adsorbent dose 4 g/L) |
(Yu et al., 2021) |
| Palm nutshell carbon | Dark green PLS (dye) |
0.84 mg/g | (Navarathna et al., 2020) |
| Cashew nutshell carbon | 1 mg/g | ||
| Broomstick carbon | 0.63 mg/g | ||
| Coconut shell char | Rhodamine-B (dye) |
41.67 mg/g | (Bazargan et al., 2021) |
| Coir pith char | Coomassie brilliant (dye) |
31.84 mg/g | (Solis et al., 2020) |
| Palm shell-activated carbon | Reactive red 3 BS (dye) | 7 mg/g | (Omran and Abdel-Salam, 2021) |
| Palm shell powder | Methylene blue | 121.5 mg/g | (Rigueto et al., 2021) |
| Rhodamine 6G | 105 mg/g | ||
| Rice husk carbon | Congo red | 10 to 99% (Initial dye concentration 25 ppm & adsorbent dose 0.08 g/L) |
(Rusch et al., 2020) |
| Sugarcane bagasse | Reactive orange | 3. 48 mg/g | (Li et al., 2020) |
| Sugarcane bagasse (ZnCl2 treated) | 2.83 mg/g | ||
| Sugarcane bagasse (H3PO4 treated) | 1.8 mg/g | ||
| Sugarcane bagasse fly ash | Remazol Black B Remazol brilliant blue R Remazol Brilliant red |
16.42 mg/g 32.468 mg/g 18.282 mg/g |
(Smiri et al., 2020) |
| Sugarcane bagasse | Basic blue 3 Reactive orange 16 |
37.59 mg/g 34.48 mg/g |
(Miao et al., 2021) |
| Sugarcane dust | Basic violet 1 Basic violet 10 Basic green 4 |
50.4 mg/g 13.9 mg/g 20.6 mg/g |
(Elella et al., 2021) |
| Rice hull | Basic blue 3 Reactive orange 16 |
14.68 mg/g 6.24 mg/g |
(Sowinska and Urbanczyk-Lipkowska, 2014) |
| Saw dust | Ethylene blue | 87.7 mg/g(Natural saw dust) . 188.7 mg/g(Treated saw dust) . |
(Ahmadi and Igwegbe, 2018) |
| Beechwood sawdust | Direct orange 26 Acid green 20 Aid orange 7 |
2.78 mg/g 7.81 mg/g 5.06 mg/g |
(Laskar and Kumar, 2019) |
6.9 Industrial wastes
Industrial wastes are considered the most crucial problem worldwide due to their increasing environmental load and mutagenicity (Iqbal et al., 2019). Therefore, industrial waste management has become a significant active research area that attracts several disciplines, such as chemical, environmental and civil ones (Soliman and Moustafa, 2020). The use of industrial waste as waste adsorbents is also one of the high availability/efficiencies and green/eco-friendly alternative approaches for removing anionic pollutants. Generally, these industrial wastes are generated as by-products (Gil et al., 2018, Yin et al., 2020b).
For instance, by-products such as fly ash (Wang et al., 2016), red mud (Wang et al., 2008), tailings (Zeng, et al., 2004), and blast furnace slag (Yasipourtehrani, et al., 2019) have been used for the manufacture of ceramic site adsorbents to remove phosphorus from the wastewater (Zeng et al., 2004, Yasipourtehrani et al., 2019). In addition, the production of the ceramic site with other waste was also performed to use the elements contained in the waste to enhance its adsorption capacity. For example, in the Shandong province, China's river water has been polluted by industrial wastes that become sediment on the riverside bed. Therefore, the riverside sediment has been added to the fly ash and red mud to manipulate the ceramic site (Yin et al., 2020a).
Similarly, the wastewater treatment plant produces many extremely cheap liquid waste by-products known as sewage sludge. Further, the sludge is carbonized under a 10 mL/min nitrogen flow at 600 °C for 1 h, followed by physical activation with CO2 to convert it to activate carbon used extensively in metal adsorption (Rio et al., 2005). Furthermore, studies have also shown that low-cost adsorbents obtained from fertilizers and steel industries' wastes were used to remove the anionic dyes (ethyl orange, acid blue 113, and metanil yellow) (Jain et al., 2003) because of their toxic nature (Sundarrajan et al., 2000). The study reveals physical, exothermic adsorption of 198, 219, and 211 mg/g of ethyl orange, acid blue 113, and metanil yellow, respectively, and conforms to the Langmuir model with first-order kinetic data (Jain et al., 2003).
Notably, the adsorption mechanisms of these adsorbents are largely dependent on the contact time between the adsorbate and adsorbents, and this varies between the adsorbents due to the changes in the adsorbent’s chemical structure, surface area, and availability of active surface sites, adsorption binding constants and the difference in the ionic size of the metal ions (Sundarrajan et al., 2000). Therefore, combining these industrial waste adsorbents is considered the most efficient alternative for removing environmental waste. Industrial wastes are considered the most crucial problem worldwide due to their increasing environmental load, genotoxicity, and mutagenicity (Senberber et al., 2017). Therefore, industrial waste management has become an important active research area attracting several disciplines, such as chemical, environmental, and civil (Sundarrajan et al., 2000). The heavy metal inside the riverside sediments created metal hydroxyl on the surface of the adsorbent, causing a high phosphorus adsorption capacity from the water (Yin et al., 2020b). Biopolymers-based adsorbents are ingredients produced naturally by live organisms and have been studied to be sustainable and eco-friendly (Mok et al., 2020). Nanocomposite films and biopolymer hydrogels have been employed as operational bio-sorbents in removing inorganic and organic pollutants, dye, toxic agents, and heavy metals from wastewater (Mok et al., 2020; Yin et al., 2020b). They play critical roles in environmental applications, such as proton-conducting membranes in anti-desertification, electrochemical devices, and bio-natural sealants to prevent specific leakage (Mok et al., 2020). On the other hand, expensive and high-energy commercial adsorbents used to remove undesirable agents from wastewater can be replaced with inexpensive adsorbents based on biopolymers (Shikuku et al., 2020). Biopolymers can additionally be utilized as a filter in wastewater treatment and as native flocculants and coagulants (Ferral-Pérez et al., 2016).
Biopolymers are obtained from eukaryotes or prokaryotes cells, such as plants or animals. Currently, the decrease in oil reserves and global warming related to synthetic polymers has a detrimental impact. The glycolipids, lip polysaccharides, polysaccharides, polyhydroxyalkanoates, or proteins are biopolymers that are well suited for environmental applications (Petrila et al., 2021). Among all biopolymers, chitosan is the most abundant biopolymer, followed by cellulose (Rudhziah et al., 2015). Other biopolymers such as tannin (Bacelo et al., 2016), starch (Yusof and Kadir, 2016), lignin (Ge et al., 2016), pectin (Sharma et al., 2016), polycaprolactone (Vila et al., 2011), guar gum (Kee et al., 2015), inulin (Rahul et al., 2015), alginate (Swain et al., 2013), agar (de Oliveira Farias et al., 2015), polyhydroxyalkanoates (Goudarztalejerdi et al., 2015), and xanthan gum (Pi et al., 2016), were implemented for widespread environmental functions such as dye-sensitized solar cell, removal of ion and fluoride, desalination, and water treatment. Biopolymers-based absorbents could be activated by linked chemical groups that can interact with pollutants (Varghese and Das, 2015). Bio-composites are highly valuable in which at least one of the matrix or enhancers a component consists of a biopolymer (An et al., 2019). The usage of biopolymer-based adsorbents is limited to removing heavy metals and dyes. It encompasses a wide range of pollutants, including phosphates (An et al., 2014), nitrates (Rajeswari et al., 2016), fluorides (Jagtap et al., 2011), hydrocarbons (Xu et al., 2005) per chlorates (Sayed and Jardine, 2015), and pesticides (Sayed and Jardine, 2015).
6.10 Biomass adsorbents
Adsorbents have gained much attention in the environmental field as a potential method for removing organic pollutants (Shen et al., 2017). Biosorption is a subgroup of adsorption that describes the physicochemical adsorption and ion exchange on an organism's cellular (Christensen and Rorrer, 2009). Fig. 10 (a) shows classification of biosorption mechanisms. This phenomenon occurs in all living or dead cells because it is unrelated to metabolism. Instead, it happens through the binding to materials derived from various biomasses. Using inactive or dead cells to remove pollutants could be favourable because harmful pollutants may not affect such cells, keeping them relatively easy to handle. On the other hand, dead cells do not require any further treatment or nutrition and can be directly implemented/recycled (Christensen and Rorrer, 2009). Fig. 10 (b) shows different biosorbent materials and their functional groups involved in biosorption.
![Classification of biosorption Mechanisms (a) (Redha, (2020)), different biosorbent materials and their functional groups involved in biosorption (b)[Gouda and Taha (2023)], schematic representation of the biomass chemotactic biosorption process (c).](/content/184/2023/16/12/img/10.1016_j.arabjc.2023.105303-fig11.png)
- Classification of biosorption Mechanisms (a) (Redha, (2020)), different biosorbent materials and their functional groups involved in biosorption (b)[Gouda and Taha (2023)], schematic representation of the biomass chemotactic biosorption process (c).
Hubbe et al. (2013) showed that algal biomass as a biosorption matrix for absorbing the dissolved fractions of petroleum pollutants proved to be a promising environmentally beneficial. In this context, Aksu and Kutsal (1990) found that Chlorella vulgar is biomass can adsorb contaminants, including heavy metal ions, to the same level, if not greater than, living cells. Furthermore, Tam et al. (2002) explored the elimination of tributyltin by adsorption on dead microalgal cells. Previous researchers clarified that several types of biomass, including bacteria, fungi, algae, and plant cuticles, are considered to eliminate various pollutants, including heavy metals, dyes, pesticides, and other pollutants (Tam et al., 2002). Fig. 10 (c) shows biomass chemotactic biosorption process. Using biomass to remove organic contaminants from water could be cost-effective, dependable, and efficient (Zhang et al., 2013).
6.11 Biopolymers-based adsorbents
Biopolymers-based adsorbents can remove multiple heavy metals, dyes, and toxic pollutants. Chitosan-derived biopolymer was prepared by grafting a 4-hydroxy-3-methoxy-5-[(4-methyl piperazine-1-yl) methyl] benzaldehyde (L) with chitosan (Krishnapriya and Kandaswamy, 2010). Various methods have been implemented for the chemical modification of biopolymers. The current phase of research and the mechanisms for the uptake of hazardous substances (dyes and heavy metals) using nanochitosan and nanocellulose were extensively investigated. Functional cellulose and chitosan nano-fibre mattresses packed in small columns can be used to remediate small quantities of Pb(II) and Cd(II) in water and wastewater (Krishnapriya and Kandaswamy, 2010). Chitosan is well indicated, especially for its dye and heavy metals adsorption in water and wastewater. Absorbents derived or modified from chitosan are interesting for film-forming capacity, biocompatibility, hydrophilicity, reactivity, and non-toxicity wastewater (Krishnapriya and Kandaswamy, 2010).
Oysters' exoskeleton is one of the main sources of chitin, and it is predicted that the annual production of chitin and chitosan equals cellulose. Chitosan is produced by the chitin deacetylation using NaOH hydrolysis that breaks the β-(1–4) glycosidic bonds of D-glucosamine and N-acetyl-D-glucosamine (Krishnapriya and Kandaswamy, 2010). Chitosan and chitin are the main compounds that are used in the process of the adsorption of wastewater pollutants. In this process, hydroxyl and amino groups are involved in possible interactions between chitin and chitosan with contaminants (pesticides, dyes, phenols, metals, ions, drugs, herbicides, etc.) (Krishnapriya and Kandaswamy, 2010). A primary amine group is the main adsorption site of chitosan, which is protonated to form NH3+ in acidic solutions (Krishnapriya and Kandaswamy, 2010). Physical and structural parameters such as surface, particle size, particle type, crystallinity, porosity, and water content are various factors that can play a role in chitosan uptake. The crystallinity is quite high for both chitosan and chitin (Krishnapriya and Kandaswamy, 2010).
Commercial chitosan is a semi-crystalline polymer, and its crystallinity is a function of the degree of acetylation. In addition, crystallized chitosan is more effective in absorbing anionic (Krishnapriya and Kandaswamy, 2010; Sharma et al., 2018). Chitosan exists in various forms, such as gels, powders, shells, and particles. Usually, chitosan beads are selected for adsorption because of their scaly nature, while the polymer forms are unsuitable for adsorption due to their lack of porosity and low surface area (Krishnapriya and Kandaswamy, 2010; Nwosu et al., 2018). In addition, the molecular weight of chitosan also has an essential role in the adsorption of various pollutants from wastewater because it affects the viscosity and solubility in the solution. Furthermore, increased adsorption of chitosan also depends on the higher degree of acetylation of N that increases the number of amino groups in the polymer, which, together with the increased molecular weight, causes the ball or chain formation of the polymer in the aqueous environment (Krishnapriya and Kandaswamy, 2010; Nwosu et al., 2018, Sharma et al., 2018).
6.12 Nanocomposites
Nanocomposites comprise homogeneous multi-functional matter crafted by combining two or many chemical and physical substances. The important aspect of this creation is that one of the constituent materials involved in composition should be of the order of nano-size in terms of its dimension. If any component involved in composite formation is magnetically active, the composition formed is known as magnetic nanocomposite (Pardo et al., 2021). There is always an edge in creating magnetic nanocomposites, as they have already stolen attention because of their high applicability and multifunctional characteristics (Reverberi et al., 2017, Tijani et al., 2019). These composites can attain the form of colloid, film, fiber, membrane, or powder (Lau et al., 2020, Jun et al., 2020). Their multi-functionality can further be enhanced by incorporating certain key functional groups as per their required applications like drug/fertilizer delivery, sensors, wastewater treatment (catalytic degradation, adsorption, magnetic separation, etc.), and bio-separation (Krishnapriya and Kandaswamy, 2010; Reverberi et al., 2017, Tijani et al., 2019).
Water treatment in terms of desalinating and purifying water to enhance its quality to fit in the drinking standards has employed a lot of adsorbents in nano dimensions, including nanoparticle metal oxides, graphene oxide, and nano-fiber nanotube-based carbon, and nano-sized polymers (Narayana et al., 2022, Mehmood et al., 2021, Ruthiraan et al., 2019). The possession of bi-dimensional porous carbon sheets in graphene oxide has made it a good candidate for the synthesis of water-repelling nanocomposites to be recovered and recycled easily post their use as adsorbents in water treatment (Laabd et al., 2022).
Polyaniline, a low-cost and green polymer with abundant functional groups available for linage (imine and amine), has been processed with graphene oxide to develop nanocomposite for adsorption processes (Reverberi et al., 2017; Tijani et al., 2019). Magnetic nanocomposites, which tend to produce external magnetic field lines, can easily remove small-sized pollutants when used as an adsorbent. However, it is not suitable for bigger molecules as their aggregate as they cannot diffuse through the composite's small pores (Krishnapriya and Kandaswamy, 2010; Reverberi et al., 2017;). Such problems have been addressed by functionalizing the magnetic composite's surface with a polymeric shell, which does not allow large pollutants to aggregate and thus enhances the adsorption (Reverberi et al., 2017; Tijani et al., 2019). The polymer shell is preferably produced from a bio-extract to make the nanocomposite adsorbent greener and safer and remove both anionic and cationic pollutants. Such nanocomposites behave as multifunctional materials because of their porous, colloidal, water-repelling, and magnetic characteristics, making them promising green adsorbents for water management (Reverberi et al., 2017; Tijani et al., 2019).
6.13 Metal-organic framework (MOF)
Covalently bonded organ metallic frameworks with particular reference to MOF nanocomposites have a set of featured characteristics, including better porosity, functionalized morphology, and high surface area. They are mixed homogenously with magnetically active nanoparticles to produce magnetic nanocomposites for catalyzing a wide range of applications, including slow-releasing fertilizers/drugs, catalysis, and adsorption (Phan et al., 2009, Shan et al., 2020). Scientists have been developing magnetically active metal–organic framework nanocomposite recyclable adsorbents that possess semi-conduction properties and are thus applicable for various applications, including impurity removal from food, water, and hydrocarbon fuels. The only limitation of such framework nanocomposites is the instability in water due to the blocking of active sites by water molecules, hindering the adsorption of pollutants (Yu et al., 2021).
Ferric chloride was coupled with a linker in terephthalic acid to produce the Fe-metal organic framework, which was further impregnated over hybrid biochar-Fe3O4 to produce magnetic recyclable multifunctional nanocomposite acting as both adsorbents as well as photo-catalyst for the removal and degradation of rhodamine B, a carcinogenic dye (Navarathna et al., 2020). Such nanocomposites can find a place in broad industrial employ because they operate as adsorbents and catalytic degradants of contaminant material (Navarathna et al., 2020; Yu et al., 2021).
Initially, MOF nanocomposite adsorbents were considered applicable to those pollutants present in the gas phase only as there was a stability issue of such adsorbents in liquids. However, recent studies have addressed such limitations by synthesizing stable composite MOFs even in liquid solutions (Bazargan et al., 2021). Also, the MOF has been coupled with bio-based adsorbents like activated carbon to form greener and safer nanocomposites that can remove cationic and anionic impurities simultaneously and hence can be used at a large scale for the treatment of aquatic pollution (Solis et al., 2020). MOF coupled with Fe3O4-based eggshell membrane to craft a magnetically active green composite to adsorb both dye molecules and metallic ions from wastewater (Pardo et al., 2021). The main characteristics of such metal framework bio-composites are their cost-efficacy and recyclability after use (Bazargan et al., 2021). The adsorption mechanisms of some pollutants onto the MOFs are shown in Fig. 11(a-d).
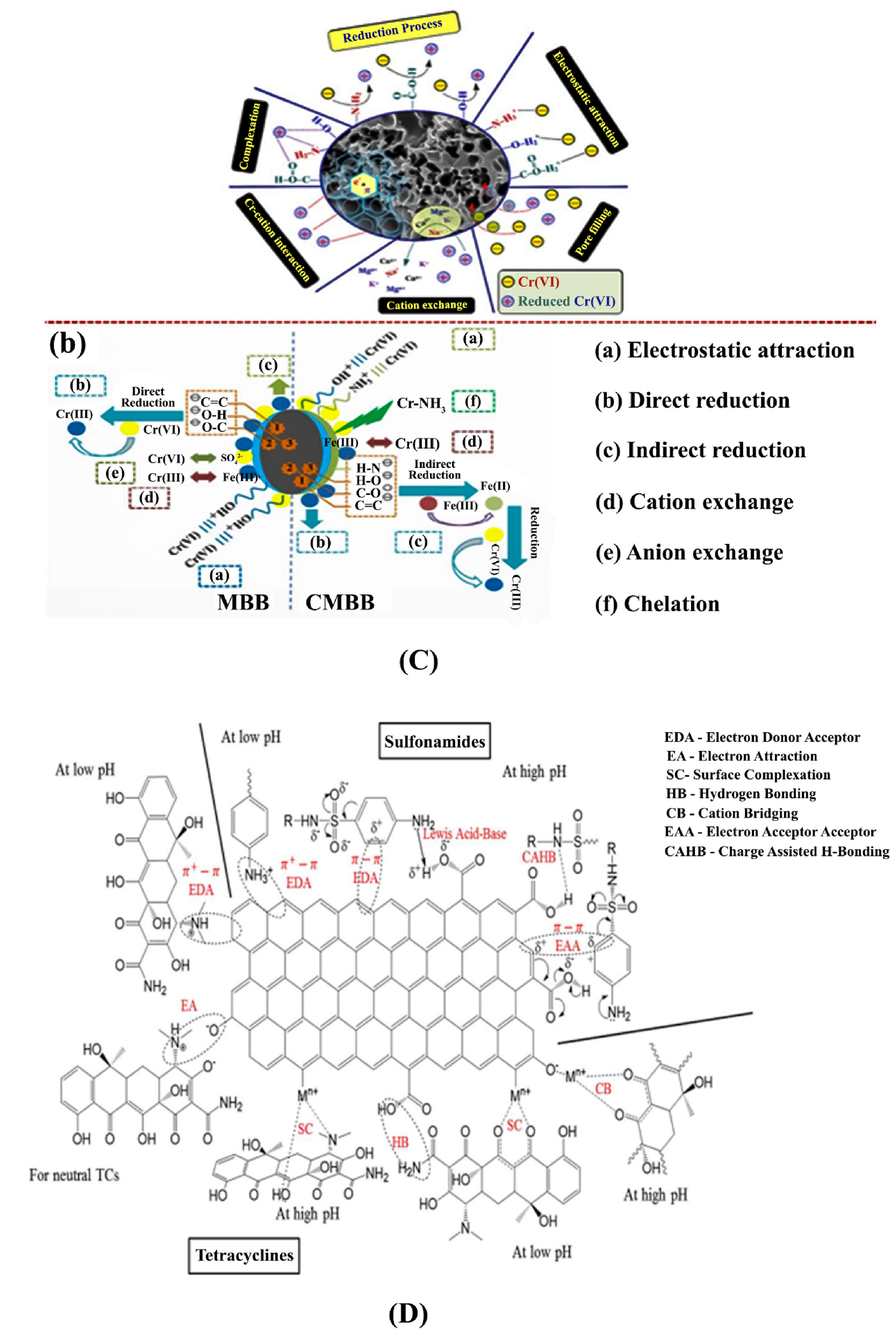
- Potential mechanisms of dyes removal by Zr-MOFs-PUF membrane in Rhodamine B (RB)/ methylene blue (MB)/ congo red (CR) ternary system (a), Adsorption mechanisms of some pharmaceuticals and personal care products (PPCPs) on MIL-101(Cr)/CS composite bead ((benzoic acid (BEN), ibuprofen (IBU) and ketoprofen (KET)) (b), Adsorption mechanisms of 2,4-dichloro phenoxy acetic acid (2,4-D) onto the UiO-66-NMe3+, (c) Adsorption mechanisms of Hg(II) onto the Cys-UiO-66 (d) (Jeong et al., 2022).
6.14 Aerogels
Aerogels are a class of adsorbents (Fig. 12) whose popularity has recently increased tremendously, considering their cost-effectiveness and efficiency. However, they need not be misjudged as hydrogels, considering their bulk network is either vacuum or filled with air, not liquid (Rusch et al., 2020; Memetova et al., 2022). Though acquainted with better surface characteristics of high porosity and good surface area, aerogels still do not have high adsorption capabilities as they lack such functional groups (Memetova et al., 2022). However, this limitation can be overcome by modifying the aerogel with nano-cellulose fibril to produce nanocomposite. This composite is soluble in water and possesses a range of actively involving functional groups for adsorption and to enhance wet strength (Rusch et al., 2020; Memetova et al., 2022).
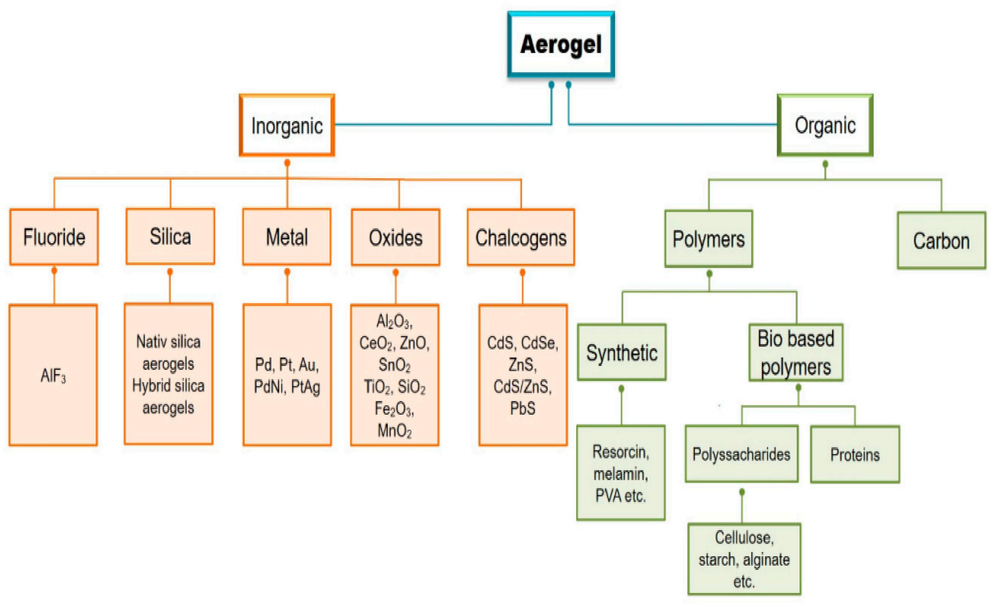
- Classification of aerogels according to the materials used (Rusch et al., 2020).
Adding poly-ethylen-1-amine enhances adsorption capacity due to the incorporation of functional group amine having an essential nature and also providing mechanical stability to the aerogels. As a result, they can remove the heavy metal ions through adsorption, including high adsorption efficiency for Cu2+ and Pb2+ ions from wastewater (Krishnapriya and Kandaswamy, 2010). The capacity to remove heavy metal ion toxins was further enhanced by co-polymerization by poly-maleic acid co-methacrylic acid, which has shown high absorptivity of more than 95% for Ni2+, Zn2+, Cd2+, and Pb2+ ions of their initial concentration (Krishnapriya and Kandaswamy, 2010). For the application of the dye removal, nanocomposite aerogels produced on compositing with poly-ethylenimine/gelatin/TiO2 have been developed for the adsorption of both negative and positive (acidic and basic) dyes (Omran and Abdel-Salam, 2021). These gelatin-based multifunctional nanocomposites behave as both photo-catalysts for degradation dye molecules and adsorbents for heavy metal ion removal, besides acting as a separator for water/oil emulsion and mixture (Krishnapriya and Kandaswamy, 2010).
Biological macromolecules obtained from sea lobster, be it chitin or chitosan, have been composited with aerogels to receive beads that possess a unique character of being stable even after the pollutant adsorption and are highly customizable as they hold many sites for adsorption (Rigueto et al., 2021). Bio-hybrid aerogels have been produced by compositing nano-cellulose assembled by a crosslinking agent with chitosan to produce adsorbents to remove dye molecules and heavy metal ions. The recyclability of such bio-hybrid aerogels is so high that their adsorption capacity for either dye molecules or heavy metal ions remains almost unchanged even after five cycles (Krishnapriya and Kandaswamy, 2010; Rusch et al., 2020). The aerogel has broad applicability in treating wastewater through adsorption because of its easy formation process, eco-friendly nature, and recyclability. Recently, specialists' attention has been focused on the performance of cellulose-based aerogel (CEBA), chitosan-based aerogel (CHBA), graphene oxide-based aerogel (GOBA), and silica-based aerogel (SBA), to remove water and hazardous wastewater contaminants (Rusch et al., 2020, Li et al., 2020, Rigueto et al., 2021).
6.15 Silica nanoparticles
Networking crystalline solids like silica with porous structures have been tested for adsorption for a long time. Still, the drawback of low adsorption capabilities has been enhanced from time to time by modifications in surface characteristics. One such advanced technique involves the preparation of a hybrid adsorbent by compositing SiO2 with carbonaceous material to enhance surface area (Krishnapriya and Kandaswamy, 2010; Smiri et al., 2020, Miao et al., 2021, Elella et al., 2021). Such compositing has been done by synthesizing nanoparticles of Silica-C shell to be used as adsorbent for wastewater treatment. These nanoparticles were synthesized under hydrothermal conditions, coated with a thin covering of SiO2 gel, and then carbonized. The carbon weight percentage of these silica-C nanoparticles was found to be very high (80–85%), which is also responsible for its high surface area of around 595 m2/g (Krishnapriya and Kandaswamy, 2010; Smiri et al., 2020).
Furthermore, due to the presence of carbon and silica, both mesopores and micropores in the adsorbent enable the removal of many pollutants from wastewater. Most of the materials which are in the nanoscale range have been converted to nanocomposites upon shelling into SiO2 with better characteristics like less toxicity, more chemical and thermal stability, and improved adsorption capacity. Magnetically active materials like Fe3O4 nanoparticles have been composited with SiO2 for wastewater treatment by adsorbing dye molecules (Krishnapriya and Kandaswamy, 2010; Miao et al., 2021). Such nanocomposites have better dye adsorption capacity due to the strong coulombic force of attraction between the nanocomposite and the dye molecule. Furthermore, upon analyzing the adsorption activity of these magnetic nanoparticles with and without compositing to SiO2 for the adsorption of humic acid from water, those with SiO2 are excellent adsorbents (Krishnapriya and Kandaswamy, 2010; Elella et al., 2021).
Further modifications were sometimes carried out to make these silica nanoparticle adsorbents more efficient, benign, and green for aquatic pollution treatment. This has been achieved by incorporating more active sites for adsorption by employing a cheap agricultural source like cellulose derivative to modify silica nanoparticle adsorbents to enhance their efficiency for dye removal (Smiri et al., 2020, Miao et al., 2021, Elella et al., 2021). Furthermore, during modification, adding various basic functional groups, like amine, imine, etc., to these silica nanoparticle adsorbents increases their adsorption capacity for removing acidic dyes (Miao et al., 2021, Elella et al., 2021). Due to their hydrophilic nature, these silica nanoparticle adsorbents have been regenerated and recycled with consistent adsorption capacities even after the 5th cycle and have also been used to remove group II element ions from the water environment (Smiri et al., 2020, Miao et al., 2021).
6.16 Zeolite nanoparticles
Zeolites are natural and have formed long back in the form of rocks or sediments beneath the earth or deep inside the sea due to high pressure and temperature. Chemically, they are aluminosilicate in association with a few of the light metal ions for balancing the charge of the Al/Si framework. They are associated with good surface area and highly porous materials like honeycombs (Krishnapriya and Kandaswamy, 2010). Their applications have been immense for a long time, including the adsorption of impurities from wastewater. Usually, naturally occurring zeolites are no different from many clays and mica minerals as all of them are aluminosilicates and crystalline. However, due to the common surface area, they stick with the drawback of low adsorption capabilities. This has led researchers to synthesize zeolite nanoparticles in laboratories and even modifications in natural zeolites with desired Si/Al ratio to have a better surface area and increase the applicability for adsorption of a wide range of pollutants (Krishnapriya and Kandaswamy, 2010).
However, the chemical synthesis of zeolites is a high-energy consuming and high-cost process with low output in pollutant removal. Hence, the researchers have switched to the greener synthesis of zeolite nanoparticles using low-cost wastes like coal fly ash, steel slag, oil palm ash, etc., to help in energy and waste management and the production of greener adsorbents and catalysts (Krishnapriya and Kandaswamy, 2010).
Furthermore, the compositing of zeolite nanoparticles with carbonaceous agricultural and other bio-wastes has produced zeolite-carbon nanocomposites with better surface area and porosity for wide application in wastewater treatment (Krishnapriya and Kandaswamy, 2010). Alumina was composited with the ash obtained from Palm oil shells and treated with NaOH, followed by heating at high temperature in a reactor under an inert atmosphere of N2to produce a carbon composite of zeolite (Erinoite) utilized for the uptake of Doxycycline from wastewater (Krishnapriya and Kandaswamy, 2010). Burnt palm oil shells were treated with kaolin under high temperatures and pressure in a pressure vessel to produce carbon-zeolite nanocomposite with a high surface area to remove methylene blue dye (Krishnapriya and Kandaswamy, 2010).
6.17 Dendrimers
Polymer-based nanocomposite adsorbents have been highly recommended for wastewater treatment due to their high adsorption capacities. Bio-based macromolecules like cellulose, chitin, and chitosan, which contain long-chain polymers of carbonaceous materials, fall under the category of dendrimers. These adsorbents have been used to recover dye molecules and transition metal ions from aquatic ecosystems (Bansal and Goyal, 2005, Sowinska and Urbanczyk-Lipkowska, 2014).
The most common biopolymers used are chitosan or chitin obtained from lobster shells, and the cationic polymer has the limitations of adsorption of only anionic dyes. Most researchers have tried to irradiate this limitation by compositing chitosan with various materials of the capacity of adsorption of cationic dyes, thus making the nanocomposite green adsorbent fit for the uptake of both kinds of dyes and increasing its range for multiple pollutant removal. For example, a clay sepiolite was composited with chitosan by cross-linking to produce beads for removing reactive orange 16 (anionic) and methylene blue (cationic) dyes through adsorption (Krishnapriya and Kandaswamy, 2010). Because of their highly carbonaceous nature, these biopolymers have been activated to enhance porosity, surface area, and adsorption capacity. Sodium hydroxide was used to activate biopolymer chitosan to produce mesoporous carbon green adsorbent to remove methylene blue dye from wastewater (Bansal and Goyal, 2005, Sowinska and Urbanczyk-Lipkowska, 2014). Many dendrimers can be classified according to their structural characteristics as layer-block (LB), segment-block (SEB), and surface-block (SUB) dendrimers (Fig. 13) (Sowinska and Urbanczyk-Lipkowska, 2014).
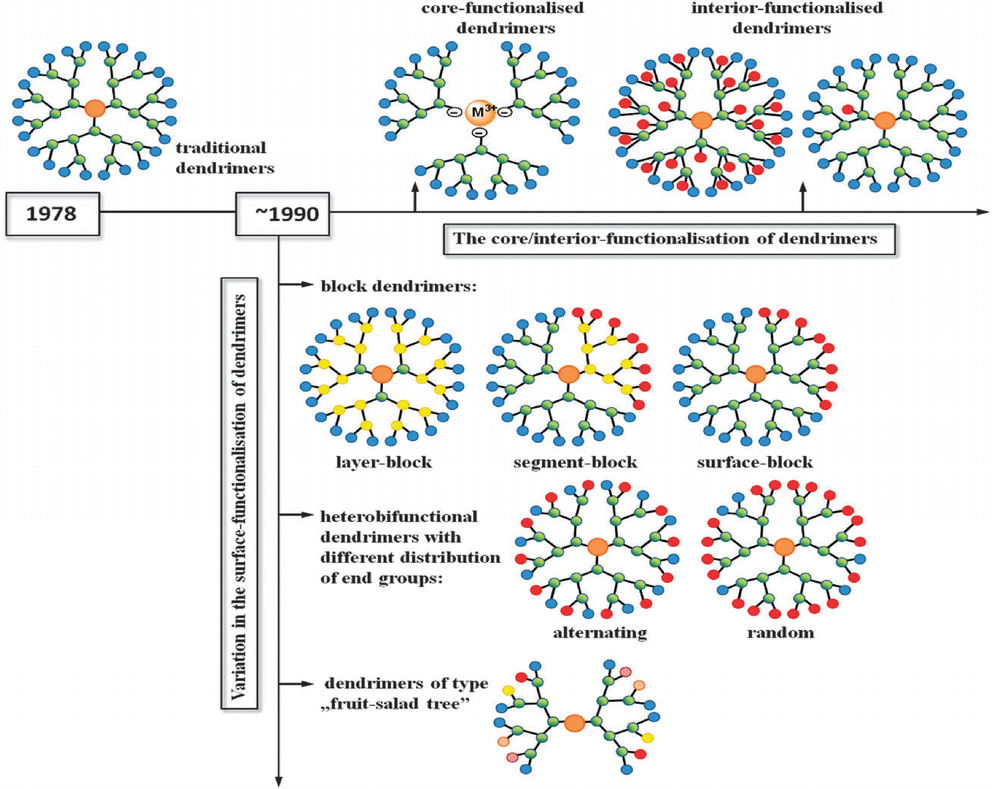
- Structural characteristics of dendrimer (Sowinska and Urbanczyk-Lipkowska, 2014).
6.18 Green synthesis of nano-adsorbents
This session will address various green syntheses of different nano-absorbents applied for adsorption, their importance, benefits, and shortcomings. Nano-adsorbents, referred to as metal-based adsorbents, are largely employed for the treatment of water and wastewater because of some associated benefits such as small size, large surface area, adsorption potential, good morphological structure, availability, and non-toxicity (Krishnapriya and Kandaswamy, 2010; Nwosu et al., 2018, Sharma et al., 2018). Examples of these nano-absorbents include titanium, zinc, Aluminum, graphene, tungsten, and copper (Krishnapriya and Kandaswamy, 2010). Other synthesis methods like sol–gel, precipitation, solvothermal, pulsed laser ablation, mechanical alloying/milling, flame spray pyrolysis, and gas condensation have been reported to synthesize different types of nano-absorbents through chemical or physical methods (Nwosu et al., 2018, Ighalo et al., 2021).
These methods have been revealed with different shortcomings, like harmful by-products and the formation of agglomerates, among others. Green synthesis can either be synthesized through plant extract or microorganisms. Different parts of the plant can be used for nano-absorbents, such as leaves, roots, flowers, and fruits, while microorganisms involve fungi, bacteria, and algae (Krishnapriya and Kandaswamy, 2010; Ighalo et al., 2021). There is a need for green synthesis to avoid dangerous by-products or unwanted compounds, which are always the aftermath of chemical synthesis techniques. Therefore, Green synthesis involves building sustainability liability and eco-friendly synthesis techniques by applying natural resources and ideal systems. Among the available green techniques of synthesis for nano-absorbents, utilization of plant extract is a relatively simple and easy method for large-scale production of nano-absorbents compared to bacterial and/or fungal synthesis known as biogenic nano-adsorbents (Nwosu et al., 2018, Sharma et al., 2018, Ighalo et al., 2021).
Green synthesis methods based on biological precursors depend on reaction parameters such as temperature, pressure, solvent, and pH conditions (neutral, acidic, or basic) (Krishnapriya and Kandaswamy, 2010). The synthesis of metal-based nano-metal-based plant biodiversity has been widely considered due to the availability of effective phytochemicals in various plant extracts, especially in leaves, such as terpenoids, amides, phenol, ketones, ascorbic acids, aldehydes, carboxylic acids, and flavones (Reverberi et al., 2017). Nano-adsorbents have proven to be suitable absorbents via their unique properties, like many active sites, and easy separation. The high surface-to-mass ratio of nano-absorbents increases adsorption efficiency, making it easy to adsorb and degrade the pollutants in water and air (Reverberi et al., 2017, Tijani et al., 2019).
Several nanoparticles have been reported to be synthesized via the green technique and are used as a nano-absorbent to remove pollutants such as inorganic, organic, and even microbes. Fatima et al. (2021) reported the green synthesis of ZnO-CdWO4 applied for the adsorption of organic dye. 5 mg/g of Congo red was observed to be adsorbed, successfully applying the green synthesized nanocomposites. Furthermore, Liu et al. (2018)reported tannin-hexamethylenediamine synthesis as an adsorbent through a green synthesis approach. It was revealed that there was an efficient removal of chromium (Cr (VI)) of 283.29 mg/g at 30 °C. It was further revealed that polyethylene glycol diacrylate-3-sulfopropyl methacrylate potassium could be synthesized via the green synthesis approach, and its adsorption capacity was reported to be 263.158, 227.27, 117.647, 102.041, and 99.010 mg/g for Pb2+, Ag+, Zn2+, Ni2+, and Cu2+ respectively. The different plants used for the green synthesis of different types of iron nanoparticles are presented in Table 5.
| Plant | Size (nm) | Plant’s part |
|---|---|---|
| Camellia sinensis (Green Tea) | 10–100 | leaves |
| Citrulluslanata (water melon) | < 20 | rind |
| Musa acuminate (Green banana) | 100–200 | Peel extract |
| Kappaphycusalvarezii | 147 | seaweed |
| Plantain peel | < 50 | peel |
| Mimosa pudica | 6 | root |
7 Molecular modelling and simulation
In previous sections, experimental investigations have been presented on the adsorption of noxious pollutants using green, sustainable adsorbents. The present section presents adsorptions’ computational and molecular simulation aspects using green, sustainable adsorbents. Most adsorbents derived from green synthesis are computationally modelled by activated carbon. Furthermore, in most investigations, the activated carbon is modelled using graphene sheets or similar systems. It should be noted that the molecular modelling of adsorbent materials remains a challenge in the computational community (Li et al., 2014, Abdel-Aziz et al., 2021). Nevertheless, some investigations successfully presented reliable results based on molecular modelling of activated carbons. An exploration of the literature shows that several computational approaches have been used for the molecular simulation of the adsorption of noxious pollutants using activated carbons. Most investigations are performed using density functional theory (DFT), grand canonical Monte Carlo simulations (GCMC), and molecular dynamics simulations. At the same time, very few other computational approaches have also been used (Abdel-Aziz et al., 2021, Cam et al., 2013).
Several authors have used DFT to model the adsorption onto activated carbon. The difference between the investigations usually lies in the functional and the modelling of the activated carbon. Some investigations based on DFT have only calculated DFT-based descriptors of the adsorbates to provide insights into the adsorption processes (Abdel-Aziz et al., 2021). This approach is independent of adsorbents; thus, this will not be insightful regarding the specific case of the adsorption onto activated carbon. This work only considers the investigations explicitly modelled activated carbon in their molecular simulations.
Fig. 14 shows the adsorption of three phenol molecules onto truncated graphene used as activated carbon. Cam et al. (2013) investigated the adsorption of phenol onto activated carbon at the PBE/DZP level of theory. The activated carbon has been modelled using the pristine molecule and pristine functionalized with the following functional groups: OH, CHO, or COOH. The authors have shown that the phenol cannot be adsorbed by pristine-activated carbon through weak van der Waals interactions. The results show that the pristine functionalized with the COOH group has the highest adsorption energy. This result highlights that pristine functionalized with the COOH group could efficiently adsorb phenol from wastewater (Cam et al., 2013, Abdel-Aziz et al., 2021).
- Example of the adsorption of three phenol molecules onto the surface as truncated graphene used as activated carbon.
Li et al. (2014) reported ethyl mercaptan adsorption onto four different types of activated carbon (AC). The AC is modelled by four six-membered rings of carbon atoms functionalized with four different functional groups. It comes out that the activated carbon functionalized with the COOCO functional group has the highest adsorption energy. The adsorption of sulfamethoxazole, sulfadiazine, and sulfamethazine onto activated carbon has been investigated using static DFT calculations (Serna-Carrizales et al., 2021). The activated carbon was modelled using pristine, ketone, and pyran. The investigation has been performed at the B3LYP/6–31 + G(d,p) level of theory, while implicit solvation has been performed using the IEF-PCM (integral equation formalism – polarizable continuum model). The results show that adsorption through hydrogen bonding has the highest adsorption energy, followed by adsorption occurring through π—π interactions (Serna-Carrizales et al., 2021). In addition, Jan et al. (2021) have modelled the adsorption of azo dye onto activated carbon using DFT. The activated carbon surface was built based on their experimental XRD and FTIR results. The modelled surface is based on a graphitic structure and contains the following functional groups: O–H, –CH2 or − CH3, −C = C, −C = O, −SO3, and − NO2. The authors have explored different adsorption sites. The results show that the highest adsorption energy is −27.1 kcal/mol, highlighting the adsorption capacity of the modelled activated carbon.
Adsorption of acetone, toluene, and methanol onto activated carbon has been performed by Zhou et al. (2019) using DFT. The activated carbon was modelled as a monolayer graphene slab in an 8x8 carbon ring unit cell. The calculations have been performed using the PBE functional associated with the DNP basis set. The calculated adsorption energies indicate that the adsorption is more favourable for toluene, followed by methanol and acetone. Besides, Liu et al. (2019) have examined phenol uptake using activated carbon as an adsorbent and employing the DFT method.
The calculations have been performed at the PW91/DNP level of theory using the COSMO solvation model. The activated carbon was modelled using a monolayer of graphene and graphene substituted with functional groups containing nitrogen atoms: amine, pyridine, and pyrrole. Different positions of the adsorption have been examined by the authors (Liu et al., 2019). It comes out that graphene doped with one nitrogen atom has the highest adsorption energy (89.3 kJ/mol). In addition to static density functional theory, molecular dynamics simulations have been used to investigate the adsorption of noxious pollutant s onto activated carbon. Prosenjak et al., (2010) have performed molecular dynamics simulations to explore the phenol uptake by activated carbon. The calculations have been performed in GROMACS using the OPLSA-AA force field. The liquid phase was simulated using the TIP4P water molecules. The authors showed that the adsorption rate varies with the pressure and the percentage of removed carbon atoms on the activated carbon surface.
Furthermore, the adsorption of benzene, phenol, and paracetamol onto the virtual porous carbon models of activated carbons has been performed by Terzyk and coworkers in 2011 using classical molecular dynamics. Simulations have been performed using the OPLSA-AA force field at 298.15 K on 84 systems. The liquid phase was modelled using the TIP4P water model and Lennard-Jones potential. The authors show that the adsorption decreases from benzene to paracetamol and depends on the collision diameter of molecules (Terzyk et al., 2011). In addition, molecular dynamics simulations have been used to understand the adsorption of crystal violet dye onto activated carbon (Depci et al., 2016). Molecular dynamics simulations have been performed using the Amber force field and software in the solvent phase. The authors stated that the molecular dynamics simulations have shown that the crystal violet molecules move around the activated carbon after adsorption (Depci et al., 2016). A certified material, BAM-P109 standard activated carbon, has been used to adsorb the n-perfluorohexane pollutant using molecular dynamics simulations (Herdes et al., 2016). Recently, molecular dynamics simulations have been used for the adsorption of benzene on activated carbon by Li et al. (2020) at room temperature. In addition, adsorptions of N2 and CH4 onto activated carbon using molecular dynamics have also been reported by Chen et al. (2020).
Usually, molecular dynamics simulations are associated with Monte Carlo simulations. A few authors have also applied this approach to understanding the adsorption of some noxious pollutants using activated carbons. For example, grand canonical Monte Carlo (GCMC) simulations have been performed to study the adsorption of ibuprofen onto activated carbon by Bahamon et al., (2017). The authors have identified the most favourable type of activated carbon for removing ibuprofen from water. It has been found that GCMC simulation can reproduce the macroscopic adsorption of pharmaceutical molecules. In addition, GCMC simulations have been used to remove some pharmaceutical pollutants by Bahamon and Vega (2017a) and Bahamon et al. (2017). The following pharmaceutical pollutants have been used in the study: ibuprofen, diclofenac, naproxen, paracetamol, and amoxicillin. The activated carbon has been modelled using polyaromatic units of nanoporous carbon, including defects and polar-oxygenated sites. The results show that the adsorption follows the following trend: paracetamol > diclofenac, naproxen > ibuprofen > amoxicillin (Bahamon and Vega, 2017b). Recently, GCMC simulations have been used to study the adsorption of volatile organic compounds onto activated carbons (An et al., 2014). GCMC has also been used to adsorb H2S, CO2, and CH4 onto activated carbon (Gonçalves et al., 2018).
An exploration of the literature shows that various molecular simulation approaches have been used to study the adsorption of noxious pollutants using activated carbons as adsorbents. Various models of activated carbons have been proposed depending on the computational method. Despite the effort and the attention devoted to the molecular study of the adsorption with activated carbon, the accurate and universal modelling of the activated carbon material remains challenging for researchers. Therefore, future works concentrating on the reliable modelling of activated carbon surfaces would greatly interest the remediation of noxious pollutants using molecular simulations.
8 Desorption/regeneration
Desorption/Regeneration has benefited pollutant remediation and economic recovery for adsorption (Indah et al., 2018). Desorption is the release of ions or molecules from the solid phase into the liquid (Thompson and Goyne, 2012). It is regarded as the key process involved in the recycling and reusing of sorbents (solid substances) used during adsorption. Desorption is the opposite of adsorption (when a molecule or ion, called adsorbate, present in a gaseous or liquid bulk, sticks on a solid surface called adsorbent) (Luosujarvi et al., 2008).
Desorption involves the release of one substance from another, either from the surface or through the surface, while adsorption is the accumulation of chemicals at the solid–liquid interface. Both desorption and adsorption are surface processes (the surface of the adsorbent is involved, and adsorbate does not diffuse into the structure of the adsorbent (Kulkarni and Kaware, 2014). Desorption and regeneration of the adsorbents in metal-recovery processes from water and wastewater are extremely important. The control of metal-loaded and used adsorbents after the adsorption process helps to give useful information for the economic design of an overall operation. In most cases, when the adsorption process is over, the adsorbent is to be discarded as waste, which constituent severe environmental problems. Interestingly, adsorbents can be regenerated and reused in the same process line (Kulkarni and Kaware, 2014, Indah et al., 2018).
The regeneration of an adsorbent involves removing the adsorbed substance(s) from its surface and restoring its initial adsorptive properties as far as possible. The primary purpose of the regeneration is to recover the original adsorbent capacity by removing the adsorbed contaminants (Shah et al., 2013). In industrial practice, this is either associated with recovering valuable materials adsorbed on the adsorbent or using the same adsorbent many times to remove toxic substances. It is important to note that regeneration is a crucial factor in improving the economy of the adsorption process. Therefore, it is relevant to assess the potential of the adsorbent for commercial applications (Shah et al., 2013, Kulkarni and Kaware, 2014).
Understanding methods involved in desorption /regeneration is relevant to the optimum performance and efficiency of the process. Some authors say desorption may occur either through thermal treatment or suitable desorbing agents (Kulkarni and Kaware, 2014). Similarly, it is worth noting that technical and economic considerations play an essential role in the final choice. Some studies opined that desorption mechanisms include thermal desorption, change of chemical conditions, stripping, and partial reduction (Lata et al., 2015). Other researchers also reported various recovery and regeneration of adsorbents. The methods include solvent washing, chemical, electrochemical and thermal methods used effectively to regenerate adsorbent (Kulkarni and Kaware, 2014). Table 6 shows the desorption of various adsorbents to remove noxious pollutants efficiently from aqueous solutions.
| Contaminants | Adsorbents | Adsorption conditions | Desorption solution | Desorption efficiency (%) | No. of adsorption recycles | Removal efficiency /adsorption capacity (first–last) | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | Adsorbent dose (g/L) | pH | Temperature (℃) | Contact time (min) | |||||||
| Cu2+ | Activated carbon | 0.2 M solution | 100.0 g | 2–11 | 333 K | 30 | 6 M HCl solution | 13.3% loss | 10 | Very fast in first 10 min. slower in the second stage, from 10 min to 30 min | (Kim et al., 2001) |
| chromium (VI) | Raw groundnut husk powder | 25 mg/L ofmetal ion [Cr(VI) | 2.0 g/L | NA | 105 °C1 | 60 | 0.1 M HCl and 0.1 M H2SO4 | 76.1%, | 3 | There was a gradual decrease in Cr(VI) and Pb(II) removal efficiencies with an increasing number of cycles | (Taşar and Özer, 2020) |
| Pb(II) | Raw groundnut husk powder | 25 mg/L of metal ion Pb(II)] in 100 mL. | 2.0 g/L | NA | 105 °C | 60 | 0.1 M HCl and 0.1 M H2SO4 | 82.1% | 3 | There was a gradual decrease in Cr(VI) and Pb(II) removal efficiencies with an increasing number of cycles | (Taşar and Özer, 2020) |
| indium ions | phosphorylated sawdust | Indium(III ion | 1.0 mg/mL | NA | 60 | 0.5 M HCl, | 97% | 4 | 85% in the 4th cycle | (Taşar and Özer, 2020) | |
| iron | Natural pumice | iron ions | 15 mg/L | 5 | 20–25 | 60 | 0.1 M HCl | 90% | 3 | Slight decrease | (Indah et al., 2018) |
9 Knowledge gaps, future perspective, and conclusion
Researchers categorized various water and wastewater treatment methods primarily in other chemicals, physical, and biological groups. The chemical approach includes precipitation, oxidation, solvent extraction, electrochemical, dissolved air flotation, coagulation, hydrolysis, oxidation, flocculation, neutralization, and ion exchange. In contrast, the physical approach involves adsorption, filtration, skimming, distillation, oil and grease, steam stripping, oil/water, sedimentation, separation, membrane, and technologies. The biological treatment includes nitrogen removal, activated sludge, bio-augmentation, sequencing batch reactors, anaerobic processes, extended is ration, rotating biological contactors, and tracking filters.
Researchers use waste as a raw material to produce alternative sorbents to save natural resources and increase cost-effectiveness and efficiency. In addition, it provides an alternative and sustainable approach to waste assessment and management. Implementing justifiable water and wastewater management methods to reduce pollution and recuperate environmental health is urgent. This has been observed that much of the waste is still a valuable resource with untapped economic value.
In this perspective, the change from a linear economy (meaning the fabrication, use, and disposal of waste) to the concept of a circular economy, which means recycling materials and returning them to the market or industry, has been welcomed worldwide. The circular economy concept emphasizes the zero-waste appeal and the rip-off of renewable resources. Thus, in the case of adsorbent production, research has been carried out on using renewable, inexpensive, and abundant raw materials such as waste. Nevertheless, on the other hand, converting these wastes to adsorbents can be regarded as a “win-win” appeal to protecting the environment.
According to the literature, the origin of substitution in benzene rings, molecular size, pKa, and solubility in water are among the optimal adsorption properties. Also, critical factors include adsorption potential such as temperature, ionic strength, adsorbate initial concentration, pH, compounds' competition in the matrix, the method used, adsorbent dose, agitation speed, and contact time. Despite all these assumptions, it is still impossible to foresee the achievement of an adsorbent for the targeted adsorption of particular substances (dye, toxic elements, organic and inorganic adsorbate, heavy metals, etc.). Therefore, various factors involved in the adsorption process must be examined and tested individually. Notwithstanding much research on wastewater adsorbents, multi-component studies are lacking. Given several contaminants in real water matrices, producing adsorbents with an extended affinity and high selectivity is essential.
Various studies have shown that the adsorption properties are improved by modification and activation. However, the disadvantages of such processing steps have not been studied. For instance, activation versus deactivation should be given more attention, especially regarding economic and environmental impacts. “Low-cost” is one of the essential factors in classifying alternative adsorbents; however, critical discussions about cost-effectiveness and related economic evaluation are rare in the review articles. As a result, it is hard to foresee if the fabrication and utilization of such attractions is a lucrative approach. In addition, these reported studies are limited to the adsorbent production costs and do not explore the utilization of the adsorbent in a real application. Conversely, applied studies lack cost analysis.
Further, efforts should be made toward accurate economic and market analysis at the same time as the application of the targeted adsorbent. The performance of adsorbents is difficult to compare due to experimental incompatibility; for instance, adsorption capacities for different pH values, adsorbent dose, adsorbent concentration, particle size, and temperature have been reported. Correspondingly, adsorbents are produced under various heat treatment temperatures, atmospheres, and residence times. Despite the various challenges that currently exist in wastewater adsorbents, according to the studies by specialized scientists, in the future, it can be expected that an improvement may occur in this area. One of the basic needs of wastewater adsorbents is their formulation, optimization, and columnar operations, and more importantly, they can be produced easily and should be cost-effective.
Currently, the remediation of noxious pollutants from water and wastewater aims for many researchers worldwide. Although numerous techniques were employed for wastewater treatment, making them sustainable, efficient, feasible, low-cost, and bio-degradable, the scale-up process for production, locally generated, selective, eco-friendly, and reusable, is still a global concern. The cost of the adsorbent depends on the need for modification, lifetime, activation, availability of raw materials, and reutilization. This review article reported the efficacy of novel green adsorbents for removing noxious pollutants from water and wastewater.
Different studies reported a broad range of values for the adsorption thermodynamic parameters (ΔG°, ΔH°, ΔS°) of noxious pollutants onto different adsorbents. According to the studies, the chemical nature of the adsorbent and adsorbate and the textural properties of the adsorbent were essential factors in estimating the thermodynamic adsorption parameters. The regeneration and reusability potential of various adsorbents for noxious pollutants has also been discussed, along with molecular modelling and simulation of noxious pollutants removal from the water and wastewater.
CRediT authorship contribution statement
Mohammad Hadi Dehghani: Conceptualization, Supervision, Writing – review & editing. Shabnam Ahmadi: Conceptualization, Supervision. Soumya Ghosh: Writing – original draft. Amina Othmani: Writing – original draft. Christian Osagie: Writing – original draft. Maryam Meskini: Writing – original draft. Samar Sami AlKafaas: Writing – original draft. Alhadji Malloum: Writing – original draft. Waheed Ahmad Khanday: Investigation, Visualization. Ajala Oluwaseun Jacob: Investigation, Visualization. Ömür Gökkuş: Investigation, Visualization. Andrew Oroke: Investigation, Visualization. Obialor Martins Chineme: Investigation, Visualization. Rama Rao Karri: Writing – review & editing. Eder C. Lima: Writing – review & editing.
Acknowledgement
This research has been supported by the Tehran University of Medical Sciences.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- DFT and experimental study on adsorption of dyes on activated carbon prepared from apple leaves. Carbon Lett.. 2021;31:863-878.
- [Google Scholar]
- Removal of lead from aqueous solution using low cost abundantly available adsorbents. Int. J. Environ. Sci. Technol.. 2007;4:67-73.
- [Google Scholar]
- Bibliometric analysis of the evolution of biochar research trends and scientific production. Clean Techn. Environ. Policy. 2020;22:1967-1997.
- [Google Scholar]
- Chemically modified biosorbents and their role in the removal of emerging pharmaceutical waste in the water system. Water. 2020;12:1551.
- [Google Scholar]
- Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere. 2014;99:19-33.
- [Google Scholar]
- Synthesis and characterization of molecularly imprinted magnetite nanomaterials as a novel adsorbent for the removal of heavy metals from aqueous solution. J. Mater. Res. Technol.. 2019;8:4239-4252.
- [Google Scholar]
- Adsorptive removal of phenol and aniline by modified bentonite: adsorption isotherm and kinetics study. Appl. Water Sci.. 2018;8:1-8.
- [Google Scholar]
- Production of granular activated carbons from select agricultural by-products and evaluation of their physical, chemical and adsorption properties. Bioresour. Technol.. 2000;71:113-123.
- [Google Scholar]
- Single and competitive adsorption studies of two cationic dyes from aqueous mediums onto cellulose-based modified citrus peels/calcium alginate composite. Int. J. Biol. Macromol.. 2020;154:1227-1236.
- [Google Scholar]
- A comparative study for biosorption characteristics of heavy metal ions with C. vulgaris. Environ. Technol.. 1990;11:979-987.
- [Google Scholar]
- Development of green and sustainable cellulose acetate/graphene oxide nanocomposite films as efficient adsorbents for wastewater treatment. Polymers. 2020;12:2501.
- [Google Scholar]
- Regeneration and long-term stability of surfactant-modified zeolite for removal of volatile organic compounds from produced water. Microporous Mesoporous Mater.. 2007;105:305-316.
- [Google Scholar]
- Industrial wastewater treatment using natural material as adsorbent. Afr. J. Biotechnol.. 2006;5
- [Google Scholar]
- Performance evaluation of activated carbon with different pore sizes and functional groups for VOC adsorption by molecular simulation. Chemosphere. 2019;227:9-16.
- [Google Scholar]
- Preparation and characterization of polymeric ligand exchanger based on chitosan hydrogel for selective removal of phosphate. React. Funct. Polym.. 2014;85:45-53.
- [Google Scholar]
- Removal of Hg (II) and Mn (II) from aqueous solution using nanoporous carbon impregnated with surfactants. Arab. J. Chem.. 2016;9:S319-S325.
- [Google Scholar]
- Application of clay ceramics and nanotechnology in water treatment: A review. Cogent Eng.. 2018;5:1476017.
- [Google Scholar]
- The investigation of kinetic and isotherm of fluoride adsorption onto functionalize pumice stone. J. Hazard. Mater.. 2012;217:123-132.
- [Google Scholar]
- Functionalized carbon nanotubes (Cnts) for water and wastewater treatment: Preparation to application. Sustainability. 2021;13:5717.
- [Google Scholar]
- Clay nano-adsorbent: structures, applications and mechanism for water treatment. SN Appl. Sci.. 2019;1:1-21.
- [Google Scholar]
- Performance analysis of single and double basin-inclined solar water distillation systems with and without black-fleece wick. Desalin. Water Treat.. 2016;57:17167-17181.
- [Google Scholar]
- A conceptual overview on sustainable technologies for the defluoridation of drinking water. Crit. Rev. Environ. Sci. Technol.. 2008;38:401-470.
- [Google Scholar]
- Tannin-based biosorbents for environmental applications–a review. Chem. Eng. J.. 2016;303:575-587.
- [Google Scholar]
- BAHAMON, D. & VEGA, L. F. 2017b. Pharmaceuticals removal from water effluents by adsorption in activated carbons using Monte Carlo simulations. Computer Aided Chemical Engineering. Elsevier.
- Pharmaceutical removal from water effluents by adsorption on activated carbons: a Monte Carlo simulation study. Langmuir. 2017;33:11146-11155.
- [Google Scholar]
- Computational study of ibuprofen removal from water by adsorption in realistic activated carbons. J. Colloid Interface Sci.. 2017;498:323-334.
- [Google Scholar]
- Biosorption of phenol using modified barley husk: Studies on equilibrium isotherm, kinetics, and thermodynamics of interactions. Sigma J. Eng. Nat. Sci.. 2020;38:1161-1177.
- [Google Scholar]
- BANSAL, R. C. & GOYAL, M. 2005. Activated carbon adsorption, CRC press.
- Metal–organic framework-based sorbents in analytical sample preparation. Coord. Chem. Rev.. 2021;445:214107
- [Google Scholar]
- Status and prospects for household biogas plants in Ghana–lessons, barriers, potential, and way forward. Int. J. Energy Environ.. 2011;2:887-898.
- [Google Scholar]
- Applications of surfactant-modified zeolites to environmental remediation. Microporous Mesoporous Mater.. 2003;61:43-56.
- [Google Scholar]
- Theoretical study on the adsorption of phenol on activated carbon using density functional theory. J. Mol. Model.. 2013;19:4395-4402.
- [Google Scholar]
- Application of ICP-MS, INAA and RNAA to the determination of some “difficult” elements in infant formulas. J. Radioanal. Nucl. Chem.. 2017;311:1347-1353.
- [Google Scholar]
- Effect of pore size on CH4/N2 separation using activated carbon. Chin. J. Chem. Eng.. 2020;28:1062-1068.
- [Google Scholar]
- Natural adsorbent based on sawdust for removing impurities in waste lubricants. J. Hazard. Mater.. 2018;350:38-45.
- [Google Scholar]
- CHOUDHARY, M., PETER, C., SHUKLA, S. K., GOVENDER, P. P., JOSHI, G. M. & WANG, R. 2020. Environmental issues: a challenge for wastewater treatment. Green Materials for Wastewater Treatment. Springer.
- Equilibrium partitioning behavior of naphthalene and phenanthrene with axenic microplantlets of the temperate green seaweed Acrosiphonia coalita. Chemosphere. 2009;76:1135-1142.
- [Google Scholar]
- CONTE, P., BERTANI, R., SGARBOSSA, P., BAMBINA, P., SCHMIDT, H.-P., RAGA, R., LO PAPA, G., CHILLURA MARTINO, D. F. & LO MEO, P. 2021. Recent developments in understanding biochar’s physical–chemistry. Agronomy, 11, 615.
- COONEY, D. O. 1998. Adsorption design for wastewater treatment, CRC press.
- CUKIERMAN, A. L., NUNELL, G. V. & BONELLI, P. R. 2019. Removal of emerging pollutants from water through adsorption onto carbon-based materials. Emerging and Nanomaterial Contaminants in Wastewater. Elsevier.
- Metal ion exchange by natural and modified zeolites. Water Res.. 1997;31:1379-1382.
- [Google Scholar]
- Calcium-rich biochar from crab shell: an unexpected super adsorbent for dye removal. Bioresour. Technol.. 2018;267:510-516.
- [Google Scholar]
- DAS, R., ABD HAMID, S. B., ALI, M. E., ISMAIL, A. F., ANNUAR, M. & RAMAKRISHNA, S. 2014. Multifunctional carbon nanotubes in water treatment: the present, past and future. Desalination, 354, 160-179.
- The toxic truth about carbon nanotubes in water purification: a perspective view. Nanoscale Res. Lett.. 2018;13:1-10.
- [Google Scholar]
- Clay-based nanocomposites as recyclable adsorbent toward Hg (II) capture: experimental and theoretical understanding. ACS Omega. 2018;3:6283-6292.
- [Google Scholar]
- Pecan shell activated carbon: synthesis, characterization, and application for the removal of copper from aqueous solution. Carbon. 2001;39:1849-1855.
- [Google Scholar]
- The role of carbon nanotube pretreatments in the adsorption of benzoic acid. Materials. 2021;14:2118.
- [Google Scholar]
- DE OLIVEIRA FARIAS, E. A., DOS SANTOS, M. C., DE ARAUJO DIONÍSIO, N., QUELEMES, P. V., LEITE, J. R. D. S. A., EATON, P., DA SILVA, D. A. & EIRAS, C. 2015. Layer-by-Layer films based on biopolymers extracted from red seaweeds and polyaniline for applications in electrochemical sensors of chromium VI. Materials Science and Engineering: B, 200, 9-21.
- DE QUADROS MELO, D., DE OLIVEIRA SOUSA NETO, V., DE FREITAS BARROS, F. C., RAULINO, G. S. C., VIDAL, C. B. & DO NASCIMENTO, R. F. 2016. Chemical modifications of lignocellulosic materials and their application for removal of cations and anions from aqueous solutions. Journal of Applied Polymer Science, 133.
- Assessment of medical waste management in educational hospitals of Tehran University Medical Sciences. Iranian Journal of Environmental Health Science and Engineering. 2008;5:131-136.
- [Google Scholar]
- Using sonochemical reactor for degradation of LAS from effluent of wastewater treatment plant. Desalination. 2010;250:82-86.
- [Google Scholar]
- Green aspects in molecularly imprinted polymers by biomass waste utilization. Polymers. 2021;13:2430.
- [Google Scholar]
- DEPCI, T., SARIKAYA, M., PRISBREY, K. A. & YUCEL, A. Computational Chemistry Approach to Interpret the Crystal Violet Adsorption on Golbasi Lignite Activated Carbon. IOP Conference Series: Earth and Environmental Science, 2016. IOP Publishing, 052026.
- EL ASS, K. 2018. Adsorption of cadmium and copper onto natural clay: Isotherm, kinetic and thermodynamic studies. Glob. Nest J, 20.
- Green antimicrobial adsorbent containing grafted xanthan gum/SiO2 nanocomposites for malachite green dye. Int. J. Biol. Macromol.. 2021;191:385-395.
- [Google Scholar]
- Use of activated clays in the removal of dyes and surfactants from tannery waste waters. Appl. Clay Sci.. 2003;24:105-110.
- [Google Scholar]
- Facile green synthesis of ZnO–CdWO4 nanoparticles and their potential as adsorbents to remove organic dye. Environ. Pollut.. 2021;271:116401
- [Google Scholar]
- FERRAL-PÉREZ, H., TORRES BUSTILLOS, L., MÉNDEZ, H., RODRÍGUEZ-SANTILLAN, J. & CHAIREZ, I. 2016. Sequential treatment of tequila industry vinasses by biopolymer-based coagulation/flocculation and catalytic ozonation. Ozone: Science & Engineering, 38, 279-290.
- Graphene and graphene-based composites: A rising star in water purification-a comprehensive overview. ChemistrySelect. 2016;1:4358-4385.
- [Google Scholar]
- Lignin microspheres: An effective and recyclable natural polymer-based adsorbent for lead ion removal. Mater. Des.. 2016;95:141-147.
- [Google Scholar]
- Removal of mercury ions from mixed aqueous metal solutions by natural and modified zeolitic minerals. Water Air Soil Pollut.. 2003;148:179-200.
- [Google Scholar]
- Application of industrial wastes from chemically treated aluminum saline slags as adsorbents. ACS Omega. 2018;3:18275-18284.
- [Google Scholar]
- Non-targeted analysis of wastewater treatment plant effluents by high performance liquid chromatography–time slice-solid phase extraction-nuclear magnetic resonance/time-of-flight-mass spectrometry. J. Chromatogr. A. 2011;1218:9202-9209.
- [Google Scholar]
- Prediction of the monocomponent adsorption of H2S and mixtures with CO2 and CH4 on activated carbons. Colloids Surf. A Physicochem. Eng. Asp.. 2018;559:342-350.
- [Google Scholar]
- Biosorption of heavy metals as a new alternative method for wastewater treatment: A Review. Egypt. J. Aquat. Biol. Fish.. 2023;27:135-153.
- [Google Scholar]
- Evaluation of bioremediation potential and biopolymer production of pseudomonads isolated from petroleum hydrocarbon-contaminated areas. Int. J. Environ. Sci. Technol.. 2015;12:2801-2808.
- [Google Scholar]
- Preparation and applications of electrochemical chemosensors based on carbon-nanomaterial-modified molecularly imprinted polymers. Nanoscale Adv.. 2019;1:3325-3363.
- [Google Scholar]
- Utilization of algal waste biomass-derived biochar prepared by a microwave-assisted method for aniline green adsorption. Water Air Soil Pollut.. 2022;233:1-14.
- [Google Scholar]
- GUPTA, V.K., CARROTT, P., RIBEIRO CARROTT, M. & SUHAS 2009. Low-cost adsorbents: growing approach to wastewater treatment—a review. Critical reviews in environmental science and technology, 39, 783-842.
- Study on the removal of heavy metal ions from industry waste by carbon nanotubes: effect of the surface modification: a review. Crit. Rev. Environ. Sci. Technol.. 2016;46:93-118.
- [Google Scholar]
- Predicting the adsorption of n-perfluorohexane in BAM-P109 standard activated carbon by molecular simulation using SAFT-γ Mie coarse-grained force fields. Adsorpt. Sci. Technol.. 2016;34:64-78.
- [Google Scholar]
- Polymer-clay nanocomposites and composites: structures, characteristics, and their applications in the removal of organic compounds of environmental interest. Med. Chem.. 2016;6:201-210.
- [Google Scholar]
- Waste materials for wastewater treatment and waste adsorbents for biofuel and cement supplement applications: a critical review. J. Clean. Prod.. 2020;255:120261
- [Google Scholar]
- Facile synthesis of ZnO nanoparticles based on bacterial cellulose. Mater. Sci. Eng. B. 2010;170:88-92.
- [Google Scholar]
- Effect analysis of pore wall thickness, pore size, and functional group of activated carbon on adsorption behavior based on molecular simulation. Environ. Sci. Pollut. Res.. 2021;28:59908-59924.
- [Google Scholar]
- Cellulosic substrates for removal of pollutants from aqueous systems: A Review. 3. Spilled oil and emulsified organic liquids. BioResources. 2013;8:3038-3097.
- [Google Scholar]
- Arsenic speciation and biotransformation pathways in the aquatic ecosystem: The significance of algae. J. Hazard. Mater.. 2021;403:124027
- [Google Scholar]
- CuO nanoparticles (CuO NPs) for water treatment: A review of recent advances. Environ. Nanotechnol. Monit. Manage.. 2021;15:100443
- [Google Scholar]
- Studies on desorption and regeneration of natural pumice for iron removal from aqueous solution. Water Sci. Technol.. 2018;2017:509-515.
- [Google Scholar]
- Bioassays based on higher plants as excellent dosimeters for ecotoxicity monitoring: A review. Chem. Int.. 2019;5:1-80.
- [Google Scholar]
- Modern carbon–based materials for adsorptive removal of organic and inorganic pollutants from water and wastewater. Molecules. 2021;26:6628.
- [Google Scholar]
- Defluoridation of drinking water using chitosan based mesoporous alumina. Microporous Mesoporous Mater.. 2011;142:454-463.
- [Google Scholar]
- Utilization of industrial waste products as adsorbents for the removal of dyes. J. Hazard. Mater.. 2003;101:31-42.
- [Google Scholar]
- Removal of azo dye from aqueous solution by a low-cost activated carbon prepared from coal: adsorption kinetics, isotherms study, and DFT simulation. Environ. Sci. Pollut. Res.. 2021;28:10234-10247.
- [Google Scholar]
- Engineered biochar from agricultural waste for removal of tetracycline in water. Bioresour. Technol.. 2019;284:437-447.
- [Google Scholar]
- Adsorptive removal of dyes onto cost effective biomaterials–a review. J. Environ. Treat. Tech.. 2021;9:218-223.
- [Google Scholar]
- A review on metal-organic frameworks for the removal of hazardous environmental contaminants. Sep. Purif. Technol. 2022122416
- [Google Scholar]
- Evaluation of iron oxide and aluminum oxide as potential arsenic (V) adsorbents. Chem. Eng. Process.-Process Intensif.. 2007;46:1030-1039.
- [Google Scholar]
- Biochar as sustainable alternative and green adsorbent for the remediation of noxious pollutants: A comprehensive review. Toxics. 2023;11:117.
- [Google Scholar]
- Modelling of methylene blue adsorption using peroxidase immobilized functionalized Buckypaper/polyvinyl alcohol membrane via ant colony optimization. Environ. Pollut.. 2020;259:113940
- [Google Scholar]
- Outstanding adsorption performance of graphene–carbon nanotube aerogels for continuous oil removal. Carbon. 2014;80:523-533.
- [Google Scholar]
- Wastewater—Sources, toxicity, and their consequences to human health. In: Soft Computing Techniques in Solid Waste and Wastewater Management. Elsevier; 2021.
- [Google Scholar]
- Effective remediation of phenol, 2, 4-bis (1, 1-dimethylethyl) and bis (2-ethylhexyl) phthalate in farm effluent using Guar gum–A plant based biopolymer. Chemosphere. 2015;136:111-117.
- [Google Scholar]
- Selected adsorbents for removal of contaminants from wastewater: towards engineering clay minerals. Open J. Appl. Sci.. 2018;8:355-369.
- [Google Scholar]
- KHAN, A. H., KHAN, N. A., ZUBAIR, M., AZFAR SHAIDA, M., MANZAR, M. S., ABUTALEB, A., NAUSHAD, M. & IQBAL, J. 2022. Sustainable green nanoadsorbents for remediation of pharmaceuticals from water and wastewater: A critical review. Environmental Research, 204, 112243.
- Production of granular activated carbon from waste walnut shell and its adsorption characteristics for Cu2+ ion. J. Hazard. Mater.. 2001;85:301-315.
- [Google Scholar]
- Low pressure biomethane gas adsorption by activated carbon. Energy Sustain. Dev.. 2018;43:196-202.
- [Google Scholar]
- KOSE, H. 2010. The Effects of Physical Factors on the Adsorption of Synthetic Organic Compounds by Activated Carbons and Activated Carbon Fibers.
- Environmental management: science and engineering for industry. Butterworth-Heinemann; 2017.
- A new chitosan biopolymer derivative as metal-complexing agent: synthesis, characterization, and metal (II) ion adsorption studies. Carbohydr. Res.. 2010;345:2013-2022.
- [Google Scholar]
- Regeneration and recovery in adsorption-a review. Int. J. Innov. Sci. Eng. Technol.. 2014;1:61-64.
- [Google Scholar]
- Interaction of anionic pollutants with Al-based adsorbents in aqueous media–A review. Chem. Eng. J.. 2014;241:443-456.
- [Google Scholar]
- Efficient detoxification of Cr (VI)-containing effluents by sequential adsorption and reduction using a novel cysteine-doped PANi@ faujasite composite: experimental study supported by advanced statistical physics prediction. J. Hazard. Mater.. 2022;422:126857
- [Google Scholar]
- Removal of Brilliant Green dye from water by modified Bambusa Tulda: adsorption isotherm, kinetics and thermodynamics study. Int. J. Environ. Sci. Technol.. 2019;16:1649-1662.
- [Google Scholar]
- Regeneration of adsorbents and recovery of heavy metals: a review. Int. J. Environ. Sci. Technol.. 2015;12:1461-1478.
- [Google Scholar]
- Removal of dye using peroxidase-immobilized Buckypaper/polyvinyl alcohol membrane in a multi-stage filtration column via RSM and ANFIS. Environ. Sci. Pollut. Res.. 2020;27:40121-40134.
- [Google Scholar]
- A new approach for controlling mesoporosity in activated carbon by the consecutive process of air oxidation, thermal destruction of surface functional groups, and carbon activation (the OTA Method) Molecules. 2021;26:2758.
- [Google Scholar]
- Chemical Engineering Research and Design Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem. Eng. Res. Des.. 2012;91:361-368.
- [Google Scholar]
- In situ growing directional spindle TiO2 nanocrystals on cellulose fibers for enhanced Pb2+ adsorption from water. J. Hazard. Mater.. 2015;289:140-148.
- [Google Scholar]
- Correlation of active sites to generated reactive species and degradation routes of organics in peroxymonosulfate activation by Co-loaded carbon. Environ. Sci. Tech.. 2021;55:16163-16174.
- [Google Scholar]
- A green adsorbent derived from banana peel for highly effective removal of heavy metal ions from water. RSC Adv.. 2016;6:45041-45048.
- [Google Scholar]
- Polyoxometallates trapped in a zeolitic imidazolate framework leading to high uptake and selectivity of bioactive molecules. J. Mater. Chem. A. 2014;2:2168-2173.
- [Google Scholar]
- Molecular simulation of benzene adsorption on different activated carbon under different temperatures. Microporous Mesoporous Mater.. 2020;302:110220
- [Google Scholar]
- Review of organic and inorganic pollutants removal by biochar and biochar-based composites. Biochar. 2021;3:255-281.
- [Google Scholar]
- Surface modification and functionalization by electrical discharge coating: a comprehensive review. Int. J. Extreme Manuf.. 2020;2:012004
- [Google Scholar]
- Green synthesis of tannin-hexamethylendiamine based adsorbents for efficient removal of Cr (VI) J. Hazard. Mater.. 2018;352:27-35.
- [Google Scholar]
- Enhanced dyes adsorption from wastewater via Fe3O4 nanoparticles functionalized activated carbon. J. Hazard. Mater.. 2019;373:397-407.
- [Google Scholar]
- Removal of mercury (II) and chromium (VI) from wastewater using a new and effective composite: Pumice-supported nanoscale zero-valent iron. Chem. Eng. J.. 2014;245:34-40.
- [Google Scholar]
- Desorption and ionization mechanisms in desorption atmospheric pressure photoionization. Anal. Chem.. 2008;80:7460-7466.
- [Google Scholar]
- Recent developments of graphene oxide-based membranes: A review. Membranes. 2017;7:52.
- [Google Scholar]
- Arsenic sorption by modified clinoptilolite–heulandite rich tuffs. J. Incl. Phenom. Macrocycl. Chem.. 2007;59:131-142.
- [Google Scholar]
- Activated carbon adsorption fundamentals. Encyclopedia Environ. Anal. Remed.. 1998;1:26-68.
- [Google Scholar]
- Carbon nanotube-based adsorbents for the removal of dyes from waters: a review. Environ. Chem. Lett.. 2020;18:605-629.
- [Google Scholar]
- Magnetic nanocomposites for sustainable water purification—a comprehensive review. Environ. Sci. Pollut. Res.. 2021;28:19563-19588.
- [Google Scholar]
- Reduced graphene oxide based composite aerogels for energy storage and transportation of methane. J. Clean. Prod.. 2022;379:134770
- [Google Scholar]
- Micropore size distribution of activated carbons: a key factor for a deeper understanding of the adsorption mechanism of pharmaceuticals. Boletín del Grupo Español del Carbón 2016:22-27.
- [Google Scholar]
- Feasible synthesis of hierarchical porous MgAl-borate LDHs functionalized Fe3O4@ SiO2 magnetic microspheres with excellent adsorption performance toward congo red and Cr (VI) pollutants. J. Alloy. Compd.. 2021;861:157974
- [Google Scholar]
- Adsorption of methylene blue dye from aqueous solutions onto walnut shells powder: equilibrium and kinetic studies. Surf. Interfaces. 2018;11:74-81.
- [Google Scholar]
- Temporal and spatial variation of chemical parameter concentration in drinking water resources of Bandar-e Gaz City using geographic information system. Desalin. Water Treat.. 2017;68:170-176.
- [Google Scholar]
- MOHAMMED, A. S., KAPRI, A. & GOEL, R. 2011. Heavy metal pollution: source, impact, and remedies. Biomanagement of metal-contaminated soils. Springer.
- Adsorbents for removal of cationic dye: nanocellulose reinforced biopolymer composites. J. Polym. Res.. 2020;27:1-15.
- [Google Scholar]
- Preparation and characterization of Bio-Adsorbent from coconut husk for remazol red dye removal. Biointerface Res. Appl. Chem.. 2020;11:10006-10015.
- [Google Scholar]
- Predictive capability evaluation and optimization of Pb (II) removal by reduced graphene oxide-based inverse spinel nickel ferrite nanocomposite. Environ. Res.. 2022;204:112029
- [Google Scholar]
- Rhodamine B adsorptive removal and photocatalytic degradation on MIL-53-Fe MOF/magnetic magnetite/biochar composites. J. Inorg. Organomet. Polym Mater.. 2020;30:214-229.
- [Google Scholar]
- NAZAL, M. K. 2020. An overview of carbon-based materials for the removal of pharmaceutical active compounds. Carbon-Based Material for Environmental Protection and Remediation.
- NEIDEL, L. L., KRUMHANSL, J. L., SIEGEL, M. D. & KHANDAKER, N. R. 2006. Performance evaluation of ALCAN-AASF50-ferric coated activated alumina and granular ferric hydroxide (GFH) for arsenic removal in the presence of competitive ions in an active well: Kirtland field trial-initial studies. Sandia National Laboratories (SNL), Albuquerque, NM, and Livermore, CA ….
- Preparation and characterization of adsorbents derived from bentonite and kaolin clays. Appl. Water Sci.. 2018;8:1-10.
- [Google Scholar]
- Lead (II) adsorption on microwave-pyrolyzed biochars and hydrochars depends on feedstock type and production temperature. J. Hazard. Mater.. 2021;412:125255
- [Google Scholar]
- OMRAN, B. A. & ABDEL-SALAM, M. 2021. A new age of innovative technology for wastewater treatment using nanomaterials. Microbial Ecology of Wastewater Treatment Plants. Elsevier.
- Dyes adsorption from aqueous media through the nanotechnology: A review. J. Mater. Res. Technol.. 2021;14:2195-2218.
- [Google Scholar]
- The alternating and direct current effect on the elimination of cationic and anionic dye from aqueous solutions by electrocoagulation and coagulation flocculation. Euro-Mediterranean J. Environ. Integr.. 2017;2:1-12.
- [Google Scholar]
- Use of alternating current for colored water purification by anodic oxidation with SS/PbO2 and Pb/PbO2 electrodes. Environ. Sci. Pollut. Res.. 2019;26:25969-25984.
- [Google Scholar]
- Biochar and activated carbon derivatives of lignocellulosic fibers towards adsorptive removal of pollutants from aqueous systems: Critical study and future insight. Sep. Purif. Technol.. 2021;274:119062
- [Google Scholar]
- Removal of phenol from aqueous solution by coupling alternating current with biosorption. Environ. Sci. Pollut. Res.. 2021;28:46488-46503.
- [Google Scholar]
- Agricultural waste materials for adsorptive removal of phenols, chromium (VI) and cadmium (II) from wastewater: A review. Environ. Res.. 2022;204:111916
- [Google Scholar]
- Magnetic nanocomposite hydrogels for tissue engineering: design concepts and remote actuation strategies to control cell fate. ACS Nano. 2021;15:175-209.
- [Google Scholar]
- Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: a critical review. Bioresour. Technol.. 2017;246:150-159.
- [Google Scholar]
- Antibacterial properties of an in situ generated and simultaneously deposited nanocrystalline ZnO on fabrics. ACS Appl. Mater. Interfaces. 2009;1:361-366.
- [Google Scholar]
- Polyelectrolyte multilayers: An overview on fabrication, properties, and biomedical and environmental applications. Materials. 2021;14:4152.
- [Google Scholar]
- PHAN, A., DOONAN, C. J., URIBE-ROMO, F. J., KNOBLER, C. B., O’KEEFFE, M. & YAGHI, O. M. 2009. Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks.
- Novel and environmentally friendly oil spill dispersant based on the synergy of biopolymer xanthan gum and silica nanoparticles. ACS Sustain. Chem. Eng.. 2016;4:3095-3102.
- [Google Scholar]
- Brilliant green dye adsorption onto composite snail shell–rice husk: Adsorption isotherm, kinetic, mechanistic, and thermodynamics analysis. Environ. Qual. Manag.. 2018;28:63-78.
- [Google Scholar]
- Simulations of phenol adsorption onto activated carbon and carbon black. Adsorpt. Sci. Technol.. 2010;28:797-806.
- [Google Scholar]
- Growth of hydrophobic TiO2 on wood surface using a hydrothermal method [J] J. Mater. Sci.. 2011;46:7706-7712.
- [Google Scholar]
- Biochar for the removal of contaminants from soil and water: A review. Biochar. 2022;4:1-25.
- [Google Scholar]
- Adsorption of bovine serum albumin (BSA) by bare magnetite nanoparticles with surface oxidative impurities that prevent aggregation. Can. J. Chem.. 2019;97:577-583.
- [Google Scholar]
- Adsorption of arsenic (V) from aqueous solution using modified saxaul ash: isotherm and thermodynamic study. Appl. Water Sci.. 2019;9:1-9.
- [Google Scholar]
- Cationic inulin: A plant based natural biopolymer for algal biomass harvesting. Int. J. Biol. Macromol.. 2015;72:868-874.
- [Google Scholar]
- Novel adsorbents in remediation of hazardous environmental pollutants: Progress, selectivity, and sustainability prospects. Clean. Mater.. 2022;3:100054
- [Google Scholar]
- Adsorption studies for the removal of nitrate using chitosan/PEG and chitosan/PVA polymer composites. J. Water Process Eng.. 2016;9:123-134.
- [Google Scholar]
- Calcium hydroxide as low cost adsorbent for the effective removal of indigo carmine dye in water. J. Saudi Chem. Soc.. 2017;21:165-171.
- [Google Scholar]
- Adsorption technique for the removal of organic pollutants from water and wastewater. Org. Pollut.-Monit. Risk Treat.. 2013;7:167-194.
- [Google Scholar]
- Nanoparticles loaded biopolymer as effective adsorbent for adsorptive removal of malachite green from aqueous solution. Water Conserv. Sci. Eng.. 2016;1:69-81.
- [Google Scholar]
- Characteristics of phenol and chlorinated phenols sorption onto surfactant-modified bentonite. J. Colloid Interface Sci.. 2006;298:39-49.
- [Google Scholar]
- Removal of heavy metals from aqueous media by biosorption. Arab J. Basic Appl. Sci.. 2020;27:183-193.
- [Google Scholar]
- Adsorption of Brilliant Green dye from aqueous solution onto red clay. Chem. Eng. J.. 2013;228:54-62.
- [Google Scholar]
- New trends in the synthesis of nanoparticles by green methods. Chem. Eng. Trans.. 2017;61:667-672.
- [Google Scholar]
- REYNEL-ÁVILA, H., CAMACHO-AGUILAR, K., BONILLA-PETRICIOLET, A., MENDOZA-CASTILLO, D., GONZÁLEZ-PONCE, H. & TREJO-VALENCIA, R. 2021. Engineered Magnetic Carbon-Based Adsorbents for the Removal of Water Priority Pollutants: An Overview. Adsorption Science & Technology, 2021.
- Comparative study of waste derived adsorbents for sequestering methylene blue from aquatic environment. J. Environ. Chem. Eng.. 2015;3:395-404.
- [Google Scholar]
- Production and environmental applications of gelatin-based composite adsorbents for contaminants removal: a review. Environ. Chem. Lett.. 2021;19:2465-2486.
- [Google Scholar]
- Production and characterization of adsorbent materials from an industrial waste. Adsorption. 2005;11:793-798.
- [Google Scholar]
- Phenol wastewater remediation: advanced oxidation processes coupled to a biological treatment. Water Sci. Technol.. 2007;55:221-227.
- [Google Scholar]
- Biopolymer electrolytes based on blend of kappa-carrageenan and cellulose derivatives for potential application in dye sensitized solar cell. Electrochim. Acta. 2015;175:162-168.
- [Google Scholar]
- Control over structure and properties in nanocrystal aerogels at the nano-, micro-, and macroscale. Acc. Chem. Res.. 2020;53:2414-2424.
- [Google Scholar]
- RUTHIRAAN, M., MUBARAK, N., ABDULLAH, E., KHALID, M., NIZAMUDDIN, S., WALVEKAR, R. & KARRI, R. R. 2019. An overview of magnetic material: preparation and adsorption removal of heavy metals from wastewater. Magnetic Nanostructures: Environmental and Agricultural Applications, 131-159.
- RYTWO, G., ZAKAI, R. & WICKLEIN, B. 2015. The use of ATR-FTIR spectroscopy for quantification of adsorbed compounds. Journal of Spectroscopy, 2015.
- SADEGH, H., SHAHRYARI, G. R., MASJEDI, A., MAHMOODI, Z. & KAZEMI, M. 2016. A review on Carbon nanotubes adsorbents for the removal of pollutants from aqueous solutions.
- SAIFUDDIN M, N. & KUMARAN, P. 2005. Removal of heavy metal from industrial wastewater using chitosan coated oil palm shell charcoal. Electronic journal of Biotechnology, 8, 43-53.
- SAIFUDDIN, N., RAZIAH, A. & JUNIZAH, A. 2013. Carbon nanotubes: a review on structure and their interaction with proteins. Journal of Chemistry, 2013.
- The removal of cadmium from water by the use of biological sorbents. Water Sci. Technol.. 1989;21:1705-1706.
- [Google Scholar]
- SANCHEZ, J. 2011. Characterization of activated carbon produced from coffee residues by chemical and physical activation.
- Role of nanomaterials in water treatment applications: a review. Chem. Eng. J.. 2016;306:1116-1137.
- [Google Scholar]
- Investigation of rice husk derived activated carbon for removal of nitrate contamination from water. Sci. Total Environ.. 2018;630:1237-1245.
- [Google Scholar]
- Chitosan derivatives as important biorefinery intermediates. Quaternary tetraalkylammonium chitosan derivatives utilized in anion exchange chromatography for perchlorate removal. Int. J. Mol. Sci.. 2015;16:9064-9077.
- [Google Scholar]
- Spectroscopic evidence for the formation of mixed-cation hydroxide phases upon metal sorption on clays and aluminum oxides. J. Colloid Interface Sci.. 1997;186:118-128.
- [Google Scholar]
- SEFFEN, M. Amina Othmani, Aida Kesraoui &.
- Adsorption of Cr (III) from aqueous solution using borax sludge. Acta Chim. Slov.. 2017;64:654-660.
- [Google Scholar]
- Adsorption of sulfamethoxazole, sulfadiazine and sulfametazine in single and ternary systems on activated carbon. Experimental and DFT computations. J. Mol. Liq.. 2021;324:114740
- [Google Scholar]
- Steam regeneration of adsorbents: an experimental and technical review. Chem. Sci. Trans.. 2013;2:1078-1088.
- [Google Scholar]
- Remarkable phosphate removal and recovery from wastewater by magnetically recyclable La2O2CO3/γ-Fe2O3 nanocomposites. J. Hazard. Mater.. 2020;397:122597
- [Google Scholar]
- TiO2-GO nanocomposite for photocatalysis and environmental applications: A green synthesis approach. Vacuum. 2018;156:434-439.
- [Google Scholar]
- SHARMA, G., SHARMA, S., KUMAR, A., LAI, C. W., NAUSHAD, M., IQBAL, J. & STADLER, F. J. 2022. Activated Carbon as Superadsorbent and Sustainable Material for Diverse Applications. Adsorption Science & Technology, 2022.
- A multifunctional nanocomposite pectin thorium (IV) tungstomolybdate for heavy metal separation and photoremediation of malachite green. Desalin. Water Treat.. 2016;57:19443-19455.
- [Google Scholar]
- Immobilization of tetrabromobisphenol A by pinecone-derived biochars at solid-liquid interface: synchrotron-assisted analysis and role of inorganic fertilizer ions. Chem. Eng. J.. 2017;321:346-357.
- [Google Scholar]
- SHIKUKU, V. O., ACHIENG, G. O. & KOWENJE, C. O. 2020. Removal of dyes from wastewater by adsorption onto low-cost adsorbents. Impact of textile dyes on public health and the environment. IGI Global.
- Biomass as source for hydrochar and biochar production to recover phosphates from wastewater: A review on challenges, commercialization, and future perspectives. Chemosphere. 2022;286:131490
- [Google Scholar]
- Remove of humic acid from water using magnetite nanoparticles. Eur. J. Adv. Chem. Res.. 2020;1
- [Google Scholar]
- Industrial solid waste for heavy metals adsorption features and challenges; a review. J. Mater. Res. Technol.. 2020;9:10235-10253.
- [Google Scholar]
- Metal organic framework UiO-66 and activated carbon composite sorbent for the concurrent adsorption of cationic and anionic metals. Chemosphere. 2020;238:124656
- [Google Scholar]
- A review on adsorbent parameters for removal of dye products from industrial wastewater. Water Qual. Res. J. 2021
- [Google Scholar]
- Study of removal of cationic dyes using palm shell powder as adsorbent. J. Environ. Prot. Sci.. 2008;2:63-71.
- [Google Scholar]
- Adsorption and coagulation in wastewater treatment – Review. Prog. Agric. Eng. Sci.. 2021;17:49-68.
- [Google Scholar]
- Performance, kinetics, and equilibrium of methylene blue adsorption on biochar derived from eucalyptus saw dust modified with citric, tartaric, and acetic acids. Bioresour. Technol.. 2015;198:300-308.
- [Google Scholar]
- Overexpression of G1/S cyclins and PCNA and their relationship to tyrosine phosphorylation and dephosphorylation during tumor promotion by metanil yellow and malachite green. Toxicol. Lett.. 2000;116:119-130.
- [Google Scholar]
- Efficient removal of fluoride using new composite material of biopolymer alginate entrapped mixed metal oxide nanomaterials. Desalin. Water Treat.. 2013;51:4368-4378.
- [Google Scholar]
- Activated carbon, carbon nanotubes and graphene: materials and composites for advanced water purification. C. 2017;3:18.
- [Google Scholar]
- Removal of tributyltin (TBT) by live and dead microalgal cells. Mar. Pollut. Bull.. 2002;45:362-371.
- [Google Scholar]
- Sustainable adsorption method for the remediation of malachite green dye using nutraceutical industrial fenugreek seed spent. Biomass Convers. Biorefin. 2021:1-12.
- [Google Scholar]
- TARASEVICH, Y. I. 1999. Application of natural adsorbents and adsorption-active materials based thereon in the processes of water purification. Studies in Surface Science and Catalysis. Elsevier.
- A thermodynamic and kinetic evaluation of the adsorption of Pb (II) ions using peanut (Arachis Hypogaea) shell-based biochar from aqueous media. Pol. J. Environ. Stud.. 2020;29
- [Google Scholar]
- First molecular dynamics simulation insight into the mechanism of organics adsorption from aqueous solutions on microporous carbons. Chem. Phys. Lett.. 2011;515:102-108.
- [Google Scholar]
- Introduction to the sorption of chemical constituents in soils. Nat.Edu. Knowl.. 2012;4:7.
- [Google Scholar]
- One-step green synthesis of WO3 nanoparticles using Spondias mombin aqueous extract: effect of solution pH and calcination temperature. Appl. Phys. A. 2019;125:1-12.
- [Google Scholar]
- Natural and waste materials as green sorbents for Cd (II) removal from aqueous effluents. Environ. Eng. Manag. J. (EEMJ). 2016;15
- [Google Scholar]
- Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res.. 2017;120:88-116.
- [Google Scholar]
- Use of biomass-derived adsorbents for the removal of petroleum pollutants from water: a mini-review. Environ. Syst. Res.. 2021;10:1-10.
- [Google Scholar]
- Removal of Hg (II) ions from aqueous environment using glutaraldehyde crosslinked nanobiocomposite hydrogel modified by TETA and β-cyclodextrin: optimization, equilibrium, kinetic and ex situ studies. Ecol. Eng.. 2015;85:201-211.
- [Google Scholar]
- Biomass-derived renewable carbonaceous materials for sustainable chemical and environmental applications. ACS Sustain. Chem. Eng.. 2019;7:6458-6470.
- [Google Scholar]
- VELUSAMY, S., ROY, A., SUNDARAM, S. & KUMAR MALLICK, T. 2021. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide‐Based Adsorption Strategies for Textile Wastewater Treatment. The Chemical Record, 21, 1570-1610.
- Novel biopolymer-coated hydroxyapatite foams for removing heavy-metals from polluted water. J. Hazard. Mater.. 2011;192:71-77.
- [Google Scholar]
- Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes. Chemosphere. 2008;72:1621-1635.
- [Google Scholar]
- Biosorbents for heavy metals removal and their future. Biotechnol. Adv.. 2009;27:195-226.
- [Google Scholar]
- In situ synthesis of magnetically recyclable graphene-supported Pd@ Co core–shell nanoparticles as efficient catalysts for hydrolytic dehydrogenation of ammonia borane. J. Mater. Chem.. 2012;22:12468-12470.
- [Google Scholar]
- Comparison study of phosphorus adsorption on different waste solids: Fly ash, red mud and ferric-alum water treatment residues. J. Environ. Sci.. 2016;50:79-86.
- [Google Scholar]
- Synthesis of aminated calcium lignosulfonate and its adsorption properties for azo dyes. J. Ind. Eng. Chem.. 2018;61:321-330.
- [Google Scholar]
- The potential application of natural zeolite for greywater treatment. Desalination. 2008;218:271-280.
- [Google Scholar]
- Use of slow-release fertilizer and biopolymers for stimulating hydrocarbon biodegradation in oil-contaminated beach sediments. Mar. Pollut. Bull.. 2005;51:1101-1110.
- [Google Scholar]
- Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym.. 2021;161:104849
- [Google Scholar]
- YASIPOURTEHRANI, S., STREZOV, V., EVANS, T. & ANAWAR, H. M. 2019. Pyrometallurgical process for recycling of valuable materials and waste management: valorisation applications of blast furnace slags. Sustainable and Economic Waste Management. CRC Press.
- YIN, Y., XU, G., LI, L., XU, Y., ZHANG, Y., LIU, C. & ZHANG, Z. 2020a. Fabrication of ceramsite adsorbent from industrial wastes for the removal of phosphorus from aqueous solutions. Journal of Chemistry, 2020.
- Fabrication of ceramsite adsorbent from industrial wastes for the removal of phosphorus from aqueous solutions. J. Chem.. 2020;2020:8036961.
- [Google Scholar]
- YOUNAS, F., MUSTAFA, A., FAROOQI, Z. U. R., WANG, X., YOUNAS, S., MOHY-UD-DIN, W., ASHIR HAMEED, M., MOHSIN ABRAR, M., MAITLO, A. A. & NOREEN, S. 2021. Current and emerging adsorbent technologies for wastewater treatment: trends, limitations, and environmental implications. Water, 13, 215.
- Recent advances in metal-organic framework membranes for water treatment: a review. Sci. Total Environ.. 2021;800:149662
- [Google Scholar]
- Electrochemical characterizations and the effect of glycerol in biopolymer electrolytes based on methylcellulose-potato starch blend. Mol. Cryst. Liq. Cryst.. 2016;627:220-233.
- [Google Scholar]
- Adsorptive removal of phosphate from aqueous solutions using iron oxide tailings. Water Res.. 2004;38:1318-1326.
- [Google Scholar]
- A green biochar/iron oxide composite for methylene blue removal. J. Hazard. Mater.. 2020;384:121286
- [Google Scholar]
- Biosorption of phenanthrene by pure algae and field-collected planktons and their fractions. Chemosphere. 2013;93:61-68.
- [Google Scholar]
- Nanomaterials for treating emerging contaminants in water by adsorption and photocatalysis: Systematic review and bibliometric analysis. Sci. Total Environ.. 2018;627:1253-1263.
- [Google Scholar]
- Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ. Sci. Tech.. 2011;45:10454-10462.
- [Google Scholar]
- Synthesis and applications of pectin-based nanomaterials. Curr. Nanosci.. 2016;12:103-109.
- [Google Scholar]
- Experimental and DFT study on the adsorption of VOCs on activated carbon/metal oxides composites. Chem. Eng. J.. 2019;372:1122-1133.
- [Google Scholar]
- ZWAIN, H. M., VAKILI, M. & DAHLAN, I. 2014. Waste material adsorbents for zinc removal from wastewater: a comprehensive review. International Journal of Chemical Engineering, 2014.
- Photocatalytic oxidation of organophosphorus pesticides using zinc oxide. Res. J. Chem. Environ. 2012;16:104-109.
- [Google Scholar]







