Regenerative potential of platelet derived growth factor in nicotine induced intervertebral disc degenerative model – In vivo study
⁎Corresponding author. cjjhr@126.com (Jianjun Chang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
-
Platelet derived growth factor (PDGF) was isolated from human blood.
-
Intervertebral disc (IVD) degenerative model induced by Nicotine in BALB/C mice.
-
oxidative stress markers were accessed through Lipid peroxidation (LPO), Myeloperoxidase (MPO) and Nitric oxide (NO) estimation.
-
Endochondral bone formation confirmed by histology, Alizarin and Alcian blue staining, Immunohistochemistry (IHC) and qPCR.
Abstract
Intervertebral disc (IVD) degeneration is an age-related disease affecting the elderly population worldwide. The disease is contributed by degeneration of the cartilage tissue in the vertebral column. The current study explores the possibility of exploiting platelet derived growth factor (PDGF), a growth factor secreted by platelets, as a therapeutic agent against the IVD degeneration. BALB/c mice were exposed to nicotine at higher concentrations to induce IVD degeneration. Three different concentrations of PDGF (1 ng/mL, 2 ng/mL, 3 ng/mL) were used in the study. Oxidative stress parameters were assessed through Lipid peroxidation (LPO), Myeloperoxidase (MPO) and Nitric oxide (NO). Histopathology was done for assessing the extent of degeneration, collagen II, mucin, and calcium deposition by Haematoxylin and Eosin staining, immunohistochemistry, Alcian blue and Alizarin red staining respectively. Gene expression studies were carried out by quantitative real-time Polymerase chain reaction (RT-PCR) for Aggrecan, alkaline phosphatase, RUNX2, Collagen I alpha and osteocalcin. IVD degeneration was prominent and PDGF treatment restored the tissue as evidenced by H&E staining. Oxidative stress was induced by the nicotine treatment and all the biomarkers of oxidative stress were restored back to normal by PDGF in a dose dependent manner. Biomarkers of endochondral bone formation (aggrecan, alkaline phosphatase, RUNX2, Collagen I alpha and osteocalcin) was observed to be normalized by exposing to PDGF in mRNA level though RT-PCR. Mucin, calcium contents were also brought back to normal by PDGF treatment. In conclusion, current work strongly supports that PDGF could be used as a therapeutic agent for the treatment of IVD degeneration.
Keywords
Nicotine
Platelet derived growth factor
Intervertebral disc
Nucleus pulposus
Calcium deposition
Cartilage regeneration
1 Introduction
It is generally recognized that intervertebral disc (IVD) degeneration contributes to back, neck, and radiating discomfort in the aging population. It triggers low back pain which is most common musculoskeletal disorders globally and is a major healthcare burden among aged population. (Cheung et al., 2009; Hong et al., 2013; Takatalo et al., 2011; Vos et al., 2012). During the degeneration of the IVD there is a reduction in the number of cells in the disc and the inner, gelatinous nucleus pulposus (NP) is a salient feature of the IVD degeneration. The NP is made of chondrocyte-like cells (Trout et al., 1982; Zhao et al., 2007) with a jelly like consistency due to the accumulation of water enclosed by proteoglycans. (An et al., 2006) Inflammatory mediators such as TNFα, IL-1, IL-6, and IL-17 are found to be active in aggravating the progression of the disease by degrading the extracellular matrix. Smoking is one of the contributors of IVD degeneration, especially, nicotine has been observed to be causing IVD degeneration (Oates et al., 1988; Uematsu et al., 2001) via vasoconstriction, arteriosclerotic, carboxyhemoglobin synthesis, affecting blood flow, and impeded fibrinolytic activity.(Akmal et al., 2004).
| Sample | Kit | Concentration |
|---|---|---|
| Isolated PDGF | Elabs - Human PDGF-AB (platelet derived growth factor ab) Elisa kit (E-EL-H1576) |
293.57 pg/mL (1x104 dilution) |
Table showed the concentration of isolated PDGF at 10000-fold dilution estimated by kit.
Growth factor prolotherapy is considered as one of the vital method providing long term cure in management of IVD degeneration preventing surgical intervention in early stage (Kennon et al., 2018).Platelet derived growth factor (PDGF) is composed of two different polypeptide chains originating from platelets, bone matrix and osteosarcoma cells. As blood coagulates, it is triggered and released by platelets that have broken down. The ability to produce and release PDGF also exists in macrophages, endothelial cells, and epithelial cells. PDGF prevents apoptosis of annulus fibrosus cells, increases collagen-3 matrix production and maintains the disc architecture, and promotes biomechanical property which has drawn interest in intervertebral disc regeneration.(Paglia et al., 2016) Sub endothelial basement membrane, or collagen also induced the secretion of PDGF (Schecter et al., 2000; Weiss et al., 1979) and they act by binding to PDGF receptors (PDGFR). PDGF has been shown to promote collagen degradation possibly through production of collagenase and therefore a chronic exposure could result in a decline in the bone mass whereas acute exposure could be beneficial since through its mitogenic property. It also acts as chemoattractant by increasing anabolic process and decreasing catabolism through reduction in apoptosis specifically in IVD.(Romaniyanto et al., 2020).
It is well-known that nicotine, a key addictive substance found in tobacco products, may contribute to a number of pathophysiologies, including IVD degeneration possibly through vasoconstriction, arteriosclerotic, carboxy hemoglobin production, changes in blood flow, and impaired fibrinolytic activity. The earlier study on nicotine-induced osteoarthritis and to mimic chronic accumulation of nicotine-induced toxicity in human umbilical vein endothelial cells (HUVECs), higher doses of nicotine was used to establish mouse model.(Montgomery et al., 2012) In the current study, we evaluated the therapeutic potential of PDGF in alleviating the IVD degeneration and studied the osteogenic and chondrogenic potency behind the ameliorative potential of PDGF. We generated IVD degeneration model in rodent by exposing to high-doses of nicotine followed by administering three different concentrations of PDGF to the rodent model. After the exposure antioxidant activity, immunohistochemistry, histopathological analysis, calcium and mucin deposition, quantitative real-time PCR were done to evaluate the mechanism of action of PDGF.
2 Materials and method
2.1 Experimental design and IVD degeneration induction with nicotine
Male BALB/c mice aged 8 weeks (n = 6 per group) were chosen for this study. Male rats were chosen due to less behavioural variability in response to stressors when compared to female counterparts. The experiments performed based on guidelines provided by ethical committee (Ethical No. SXBH-2022–034). Except control group, the mice from all other groups were received a subcutaneous injection of 1.5 mg/kg Nicotine (Lo et al., 2021a) twice daily for 30 days. According to the prior study (Lo et al., 2021a), IVD degeneration was induced and treated with 100 µl of 1 ng/mL, 2 ng/mL and 3 ng/mL of PDGF with subcutaneous injection into skin over the spine, after one month of nicotine induction. Mice without any treatment kept as control PDGF treatment was done once in a week totally for 4 weeks for treatment group (n = 6/group). The group was separated as follows,
Group 1 – Control/ Untreated group.
Group 2 – Induction Group.
Group 3 – PDGF (1 ng/ml).
Group 4 – PDGF (2 ng/ml).
Group 5 – PDGF (3 ng/ml).
Finally, animals were euthanized and all euthanization was performed under isoflurane (2.5–5 % inhalation) (Hofgaard et al., 2012). IVD’s were harvested in each group of mice for histopathology, alcian blue, alizarin red and immunohistochemistry studies.
2.2 Isolation of PDGF
Clinically expired human blood was collected from blood bank (Ethical No. SXBH-2022–034). The collected blood was centrifuged at 100 x g for 20 min at room temperature. Then, the plasma layer was transferred to the new tube and centrifuged at 800 x g for 20 min to pellet the platelet. Then, the platelet was washed twice with the following solution - Tris-Hydrochloride (17 mM, pH 7.5) – nine volume, 0.15 M NaCl, 0.1 % glucose and acid citrate dextrose buffer - one volume. The washed platelets were centrifuged to concentrate. For isolation of PDGF(Tsutsui, 2020), the pellet was mixed with Na3PO4 (0.01 M) and NaCl (0.08 M, pH 7.4) and the suspension kept at 100℃ for 10 min and centrifuged. Supernatant was collected and subjected to precipitation. The precipitation was performed four times with 1 M NaCl overnight. Supernatant fluids were collected by centrifugation at 7500 x g for 30 min and dialyzed against 20 vol of 0.08 M NaCl and 0.01 M Na3PO4 (pH 7.4). The PDGF-AB collected was subjected to Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) with silver nitrate staining according to the Morrissey method (Morrissey, 1981). Purity and relative molecular weight were assessed using SDS-PAGE and estimated by human PDGF-AB (platelet derived growth factor ab) Elisa kit (ELABScience, USA).
2.3 Oxidative stress parameters
The intervertebral tissue sections were separately homogenised with liquid nitrogen and prepared with PBS buffer pH: 7.0 to produce the homogenates, which were then subjected to cold centrifugation (12,000g; 15 min at 4 °C). Following centrifugation, the tissue supernatant was used for further biochemical evaluations.
Malondialdehyde (MDA) generated during lipid peroxidation was used to determine LPO levels.(Ohkawa et al., 1979) In brief, the IVD collected from all the groups were homogenized using LN2 and ice cold KCl buffer used to collect the homogenate. Then, to the 100 µl of homogenate, 200 µl of 8.1 % SDS, 1.5 ml of 20 % Acetic acid and 1.5 ml of TBARS and 700 µl water was added. Then, the content was mixed and kept for boiling for 60 min. The content was cooled to room temperature and read at 532 nm. Reactive nitrogen compounds were detected with the Griess reagent as end products include nitrates and nitrites. (Yaraee et al., 2011) Briefly, the IVD from all the groups were homogenized using LN2. The homogenate was collected and centrifuged at 2000 rpm for 10 min. The supernatant was used for the analysis. 100 µl of supernatant was taken in a Eppendorf and 50 µl of Griess A (1 % sulfanilamide in 5 % phosphoric acid) and 50 µl of Griess B (0.1 % NED) Solution was added and incubated in dark for 5 – 10 min. Then, the OD value was taken at 540 nm using UV spectrophotometer and expressed in µM/mL. Intervertebral tissue (5 % w/v) was homogenised in 0.5 % hexadecyltrimethylammonium bromide (HTAB, Sigma-Aldrich, Co., St. Louis, MO, USA) with 50 mM potassium phosphate buffer (pH:6) with O-dianisidine and diluted H2O2 for quantification of myeloperoxidase (MPO). The homogenate was freeze-thawed three times and subjected to ten seconds of sonication, then centrifuged at 14,000 g for 45 min at 4 °C. The MPO was estimated from the supernatant by spectrophotometric analysis at 450 nm with 30sec intervals and measured as U/mg of tissue (Kim et al., 2012).
2.4 Histology analysis
Intervertebral discs were removed and collected after sacrifice, fixed in 10 % formalin, acid-decalcified, and processed according to standard histological procedures. The paraffin embedded tissue was cut into thin sections (5 mm) and subjected to Hematoxylin and Eosin staining as per standard protocol.
2.5 Immunohistological analysis
IHC staining of fixed tissue sections was carried out (Lo et al., 2021a). Xylene was used to remove the paraffin wax from the unstained slice, and ethanol in progressively lower quantities was used to rehydrate the tissue sections. We utilised 5 % BSA buffer to prevent non-specific binding. Tissue sections were treated with the Collagen II Monoclonal antibody (6B3) (Invitrogen, USA) and incubated overnight at 4 °C. After rinsed with 1X TBST thrice, these sections were treated with stabilized peroxidase conjugated Goat anti – mouse (H + L) secondary antibody (Invitrogen, USA) which contains 0.3 % H2O2, was employed to suppress endogenous peroxidase activity and the peroxidase activity was seen using 3,3 - diaminobenzidine (SRL, India). Slides were counterstained with hematoxylin and then given one more water rinse and observed under microscope and photographed.
2.6 Alcian blue and alizarin red
Alcian blue staining was performed to analyse the mucilage presence in IVD in all groups.(Lo et al.,2021) and Calcium deposition was observed using Dahl method. For this 1 % Alizarin Red S was used for staining (5 min incubation) and fast green used as a counter stain. After washing the slides visualised under microscope and photographed. (Rutges et al., 2010) were also used to further stain the sections in order to observe the expression of mucin and calcium mineralization.
2.7 Real-time polymerase chain reaction (QPCR)
The total RNA was extracted from intervertebral disc region of mice by RNA isolation kit (Promega, USA) and Real-Time Polymerase Chain Reaction (qPCR) was performed as per manufacturer instruction. The Qiagen PCR system (USA) was used to carry out qPCR assay and the relative gene expression was evaluated with calibration samples in each experiment. The used primers are given below:
| Primer | Forward Sequence | Reverse Sequence |
|---|---|---|
| Aggrecan | CCGCTACGACGCCATCTG | CCCCCACTCCAAAGAAGTTT |
| ALP | CTTCATAAGCAGGCGGGGG | TTAATTGACGTTCCGATCCTGC |
| RUNX2 | TGCTCACTCCGTTTTGTTTTGT | ACCATACCCAGTCCCTGTTTT |
| Col 1α | CTGGCAAAGACGGACTCAAC | AGCTGAAGTCATAACCGCCA |
| BGLAP | TGGCTTAAAGACCGCCTACA | AGAGAGAGAGGACAGGGAGG |
| β- actin | TCTGTGTGGATCGGTGGCT | GTAAAACGCAGCTCAGTAACAGTC |
2.8 Statistical analysis
All data were subjected to statistical analysis using Graphpad Prism version 8.0. All experiments were triplicated and expressed as Mean ± SD. Data were analysed through One Way Analysis of Variance (ANOVA) and the comparison between the groups were analysed by Tukey’s multiple comparison test. The P value is < 0.001 considered as significant.
3 Results
PDGF has been isolated and confirmed by SDS-PAGE, shown in Fig. 1 and quantified using Elisa kit given in Table 1We developed an in vivo animal model to evaluate the regenerative bioactivities influence of PDGF against IVD degeneration and the overall experimental design is shown in Fig. 2.
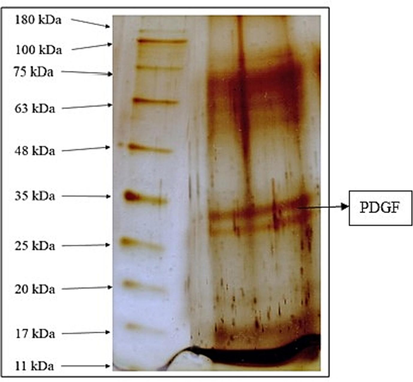
- Qualitative and quantitative analysis of PDGF Fig. 1 denotes the qualitative and quantitative analysis of isolated PDGF. Gel image shows the qualitative confirmation of PDGF by SDS PAGE - silver staining method. PDGF bands was observed in 33 KDa.
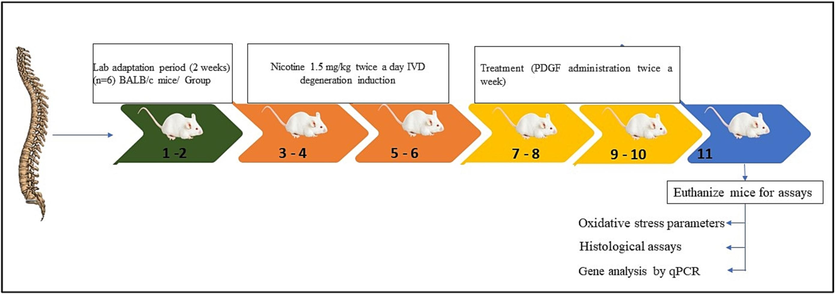
- Time line of experiment and group separation Fig. 2 showed the time line of experimental design. The chart indicates the induction and treatment schedule.
3.1 Oxidative stress markers: NO, LPO and MPO
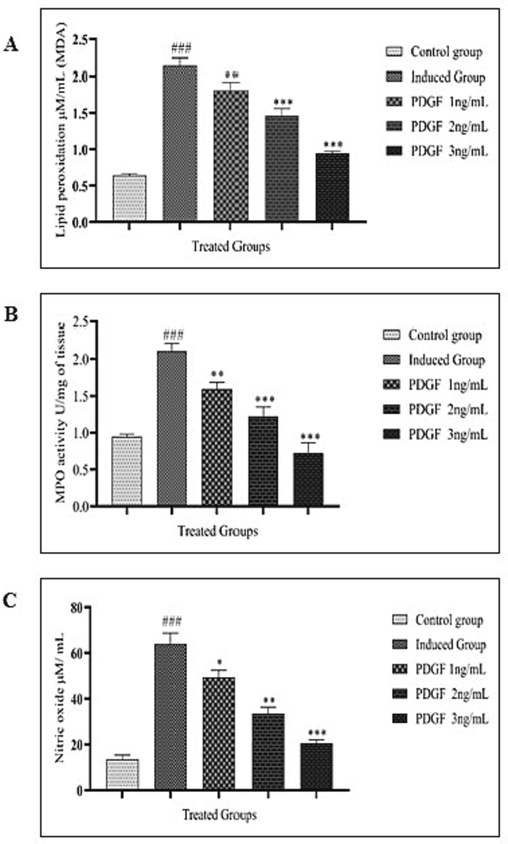
The lipid peroxidation level was elevated in the nicotine-induced intervertebral disc degenerated group. With the treatment of PDGF there was a dose-dependent decline in lipid peroxidation (Fig. 3A). Complementing to the result, there was a dose-dependent reduction in the MPO (Fig. 3B) was also observed. The same trend was also observable in the nitric oxide levels. (Fig. 3C).
-
Fig. 3 A Lipid peroxidation estimation Fig. 3 B MPO estimation Fig. 3C Nitric oxide estimation Fig. 3A showed the LPO estimation in untreated, induced and treated groups. LPO was very high in induced group compared to control with very high significance (#) and decreased in treated groups in dose dependent manner compared to induced group (*). One way ANOVA was performed. ### - P < 0.0001, ** - P < 0.001, *** - P < 0.05. Fig. 3B showed the MPO estimation in control, induced and treated groups. MPO was very high in induced group compared to control with high significance. Gradual decrease of MPO was observed in treated groups in dose dependent manner compared to induced group. One way ANOVA was performed. # - indicates the comparison between control and induced group. * - indicates the comparison between induced and treated group. ### - P < 0.0001, ** - P < 0.001, *** -P < 0.0001 Fig. 3C showed the estimation of Nitric oxide level in control, induced and treated groups. NO level was high in induced group and gradually decreased in treated groups as dose dependent manner. One way ANOVA was performed. # - indicates the comparison between control and induced group. * - indicates the comparison between induced and treated group. ### - P < 0.0001, * - P < 0.05, ** - P < 0.001, *** -P < 0.0001.
3.2 Histological screening
3.2.1 Hematoxylin and eosin staining
H&E staining confirmed the degenerative changes which occurred in the tissue whereas there was a regenerative signature observable in the PDGF treated animal groups in a dose dependent manner. (Fig. 4).
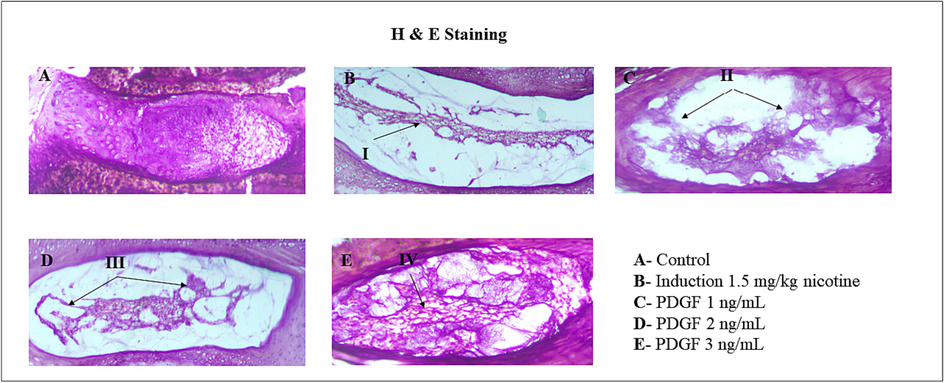
- Histopathological analysis of IVD disc Fig. 4 showed the histopathological analysis of IVD disc in BALB/c mice. I – showed degeneration of NP disc matrix and less cartilaginous structure. II – indicates less NP cells and recovering cartilaginous structure. III – indicates regeneration of NP disc matrix and cartilaginous structure. IV – indicates regenerated disc matrix and cartilaginous structure was observed in high dose.
3.2.2 Immunohistochemistry
Immunohistochemistry was done to evaluate the level of type II collagen. Similar to other experiments, in the animal group where the intervertebral disc degeneration was induced, a reduced level of type II collagen was observed. On contrary, there was a dose-dependent increase in the type II collagen levels upon treatment with PDGF. (Fig. 5).
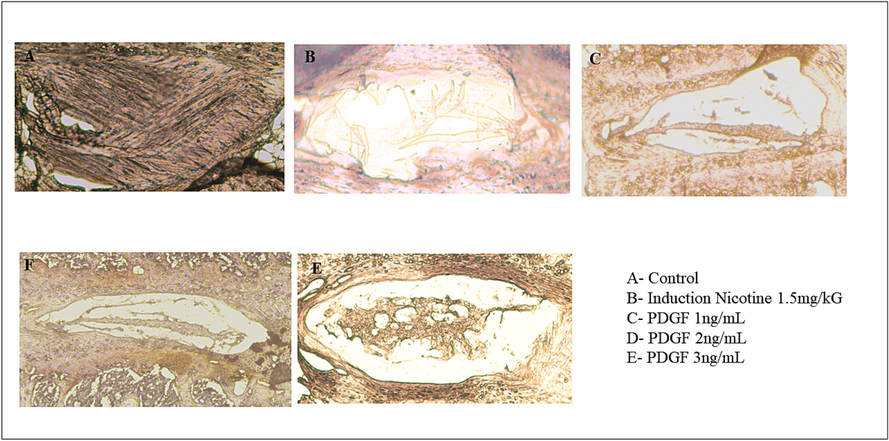
- Immunohistochemistry analysis Fig. 5 showed the immunohistochemistry staining of Collagen II of IVD disc. There was loss of NP cells in induction group (5B), and the increased regeneration of NP cells was observed in dose dependent manner (5C, 5D and 5E).
3.3 Alizarin red stain
Calcium deposition was evaluated from alizarin red staining in nucleus pulposus disc (Fig. 6). Calcium level was much reduced in the induced group compared to the control whereas low dose of PDGF (1 ng/mL) there was a slight improvement in the calcium deposition. However, in the high dose of PDGF treatment there was a pronounced improvement in the calcium deposition. In the middle concentration (2 ng/mL) there was a moderate amount of calcium deposition.
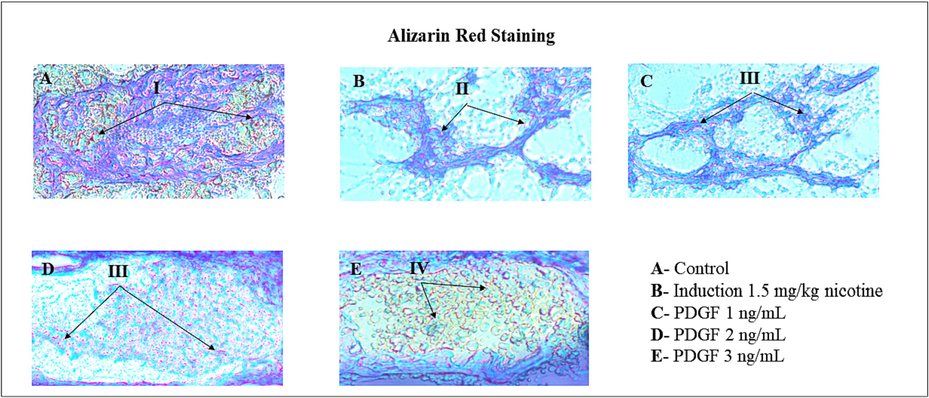
- Calcium deposition by Alizarin red staining method Fig. 6 showed the images of Calcium mineralization in IVD disc by Dahl’s Method. I – indicates reddish orange color shows the Calcium deposition. II – indicates reduced NP disc matrix and cartilaginous structure and also low level Calcium accumulation. III – indicates regeneration NP disc matrix, cartilaginous structure and increased level of Calcium deposition as dose dependent manner. IV- indicates the progressive regeneration of disc matrix with high level of Calcium deposition.
3.4 Alcian blue staining
Alcian blue selectively stains the mucin in the tissue sections (Fig. 7). The tissue degeneration was observed in the intervertebral disc of animals treated with nicotine indicating the low level of mucin. However, there was a marked elevation in the mucin upon treatment with PDGF in a dose-dependent manner. In the 1 ng/mL PDGF treated group increased mucin level compared to induced group is observable by increased blue color. The intensity of mucin further increased in 2 ng/mL PDGF treated group and in the 3 ng/mL PDGF treated group the mucin level is as equal to control group indicative of a near to complete restoration of mucin.
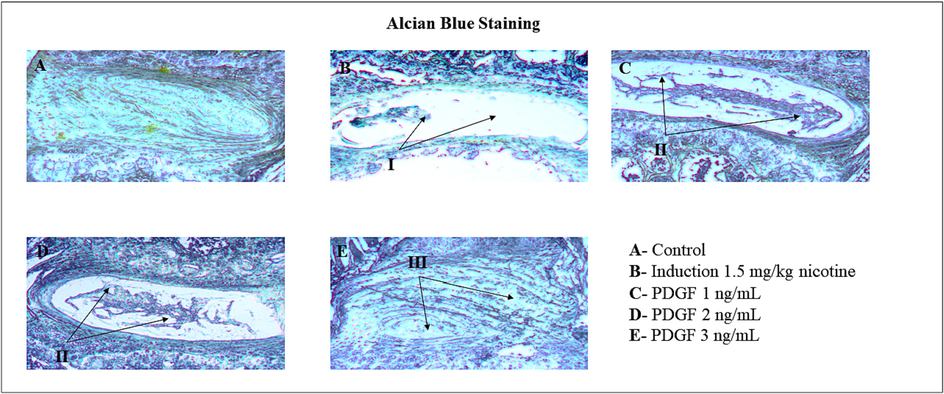
- Alcian blue staining of mucilage tissues of IVD disc Fig. 7 showed the Alcian blue staining of mucilaginous tissues of IVD disc. I – indicates degeneration of NP disc matrix and less cartilaginous structure. II and III – indicates regeneration of NP disc matrix and cartilaginous structure.
3.5 Gene expression analysis by quantitative real-time PCR
The expression level of certain genes involved in intervertebral disc regeneration was evaluated by quantitative real-time PCR shown in Fig. 8. The expression levels of aggrecan, alkaline phosphatase, RUNX2, type 1 collagen alpha, and osteocalcin was much reduced in intervertebral disc degenerated group. However, the expression levels of all the genes were restored while treated with PDGF in a dose-dependent manner.
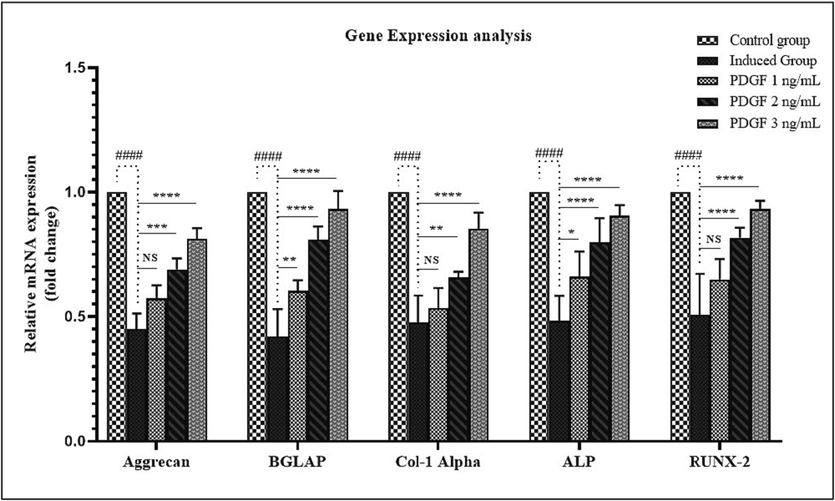
- Gene expression analysis Fig. 8 showed the Gene expression analysis of IVD disc in BALB/c mice. Aggrecan, Osteocalcin (BGLAP), Collagen I α, Alkaline Phosphatase (ALP) and RUNX-2 gene expression were analysed using qPCR normalization with β actin gene. Figure showed the reduced gene expression levels in induced group, whereas the progressive upregulation was observed in treated groups in dose dependent manner compared to control group. # - indicates the comparison between control and induced group. * - indicates the comparison between induced and treated group. NS – Non significant, * - P < 0.05, **- P < 0.001, ***-P < 0.0001.
4 Discussion
It is generally recognized that intervertebral disc (IVD) degeneration contributes to back, neck, and radiating discomfort in the aging population. (Ding et al., 2013) In the current study we evaluated the therapeutic potential of PDGF in alleviating IVD degeneration. IVD degenerative condition in BALB/c mice was created by applying increasing doses of nicotine in which the ihNPs population was drastically decreased. First, we evaluated the oxidative stress in the degenerative regions of the disc since oxidative stress is a major contributor of the condition. ROS alters matrix proteins present in the IVD to result in oxidative damage of the extracellular matrix which further weakens the mechanical function of IVD. (Feng et al., 2017). ROS mediates the IVD degeneration through inflammatory signaling, induction of autophagy, mitophagy, DNA methylation, manifestation of advanced glycation end products etc. (Cao et al., 2022) In our experiment it was observed that PDGF showed a dose-dependent decline in the oxidative stress as indicated by the markers LPO, MPO and NO. Therefore, it is clear from the experiments that the treatment with PDGF could effectively clears the ROS to result in amelioration of IVD degeneration.
IVD is composed of a specialized cartilaginous tissue composed of collagens I and II. (Eyre & Muir, 1976). Analysis of type II collagen by immunohistochemistry revealed a drastic decline in the IVD degenerated tissue while the increasing doses of PDGF gradually improved the collagen type II in such tissues. ROS has been shown to degrade type II collagen in articular cartilage.(Watari et al., 2011) Similarly, Tanaka et al., demonstrated that ROS diminishes the biosynthesis of collagen in the human dermal fibroblasts. (Tanaka et al., 1993) Therefore, it is apparent that ROS not only degrades the type II collagen but also blocks its synthesis. Our data further sheds light on the possible therapeutic potential of PDGF by improving the type II collagen content as shown by the immunohistochemistry. In addition, H&E staining also strengthens the therapeutic efficacy of PDGF against the IVD degeneration. A network of collagen type II and elastin fibers surrounds aggrecan, the main proteoglycan found in the nucleous pulposus.(Adams & Roughley, 2006) Decreased aggrecan and collagen type II degradation in the nucleus pulposus, altered IVD cell morphologies, and greater levels of inflammatory cytokines were associated with IVD degeneration. (Risbud & Shapiro, 2014; Roughley, 2004) In earlier research, PDGF increased the proliferation of IVD cells (Pratsinis & Kletsas, 2007; Thompson et al., 1973) Similarly in our study, aggrecan expression decreased in mRNA level of IVD group which is increased on treatment with PDGF in dose dependent manner.
Mucins are one of the components of cartilages which are vulnerable to ROS attack on terminal sugars and protein residues. (Brownlee et al., 2007) It is reasonable to hypothesize that during the IVD degeneration through nicotine, elevated ROS is likely to degrade mucins. The hypothesis was evaluated by staining with alcian blue which revealed a decreased amount of mucin in IVD degenerated tissues strengthening the hypothesis. PDGF could effectively restore the mucin content in a dose-dependent manner.
Mineralization of bone is an essential process for the strength of bones and cartilages during which calcium phosphate nanocrystals are filled into the bone matrix. During IVD degeneration there was a loss of calcium as observed through alizarin red staining which could be possibly through the adverse effects of reactive oxygen species. On the other hand, treatment with PDGF increased the concentration of calcium mineralization in a dose-dependent manner. To evaluate the mechanism of remineralization we evaluated the expression of certain genes. Calcification process is a complex one during which calcium apatite crystals are accumulated into the bone matrix to provide strength to the bone by the enzyme alkaline phosphatase (ALP). (Christoffersen & Landis, 1991; Miller & Demarzo, 1988; Roach, 1999) The enzyme ALP derives and mobilizes free phosphate ions from organic and inorganic phosphate sources. (Anderson et al., 2004; Kamekura et al., 2006) These free monophosphate ions in the presence of calcium ions synthesizes hydroxy apatite crystals. Therefore, evaluating the dynamics of ALP expression by quantitative real-time PCR revealed a dose-dependent increase which parallels to the mineralization.
Osteocalcin is another factor involved in calcium mineralization through aiding in hydroxyapatite crystal formation. Osteocalcin is an extracellular factor secreted into the microenvironment and subsequent conformational changes allows it to orient its Gla residues to bind with calcium ions in hydroxyapatite.(Price et al., 1976; Zoch et al., 2016) In our experiment quantification of osteocalcin revealed that in the induced group a stronger suppression whereas treatment with PDGF resulted in an upregulation of osteocalcin in a dose dependent manner which is in line with dose-dependent calcium accumulation. Indirect evidences also support our result that PDGF enhances mineralization through restoration of the activity of osteocalcin. (Vordemvenne et al., 2011).
Osteogenic cells migrating into the adjacent regions chondro-osseous junction produce type I collagen. (Sandberg & Vuorio, 1987) Therefore, we evaluated the expression levels of type I collagen alpha by quantitative real-time PCR which revealed a strong downregulation during the nicotine mediated degeneration of IVD and upon treatment with PDGF, concentration dependent increase was observed confirming the induction of rebuilding of cartilage. Similar results were also observed in RUNX-2 expression, which is a transcription factor for genes that regulates osteoblast differentiation and chondrocyte hypertrophy, which plays vital role in endochondral bone formation. (Jonason et al., 2009; Liao et al., 2019; Sato et al., 2008). Thus confirming osteogenic and chondrogenic ability of PDGF in restoration of IVD degeneration. It may induce differentiation of mesenchymal stem cells to osteocytes and chondrocytes through the bone morphogenetic protein signalling pathway (Ghodadra & Singh, 2008). Most of the growth factor have been administered depending on the stage of degeneration but research has been constrained to in vitro and in vivo studies. Therefore, safe clinical trial needs to be performed for human application which may provide painless and non-invasive treatment with alleviation of IVD degeneration
5 Conclusion
Overall, the current work highlights the importance of cartilage regeneration by PDGF in a dose dependent manner with a maximum dose of 3 ng/mL. IVD was degenerated using nicotine exposure and elevated reactive oxygen species was observed in the degenerated tissue. Upon treatment with PDGF, there was a dose-dependent decline in the ROS level. Staining of tissue sections revealed that PDGF treatment restored the calcium and mucin levels. Type I collagen and type II collagen were restored upon treatment with PDGF. Gene expression studies provided endochondral bone formation markers such as Aggrecan, Col 1α, RUNX-2, ALP and osteocalcin were downregulated in the IVD degenerated tissues whereas they are brought back to normal in the PDGF treated groups. Thus, proliferating chondrocytes give rise to hypertrophic cells during the process of endochondral bone formation which then deposit a mineralized matrix to form calcified cartilage prior to replacement by bone. Overall, the study reveals the therapeutic potential of PDGF for the treatment of IVD degeneration.
Ethical statement
Research experiments conducted in this article with humans and animals were approved by the Ethical Committee and responsible authorities of Shanxi Bethune Hospital (No. SXBH-2022–034), following all guidelines, regulations, legal and ethical standards as required for experimental objects.
Author’s contribution
All authors are equally contributed.
Funding source
This work is funded by Shanxi provincial Natural Science Foundation 201901D111411.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adams, M. A., & Roughley, P. J. (2006). What is intervertebral disc degeneration, and what causes it? In Spine (Vol. 31, Issue 18). https://doi.org/10.1097/01.brs.0000231761.73859.2c.
- Effect of nicotine on spinal disc cells: a cellular mechanism for disc degeneration. Spine. 2004;29(5):568-575.
- [CrossRef] [Google Scholar]
- Intervertebral disc degeneration: biological and biomechanical factors. Journal of Orthopaedic Science : Official Journal of the Japanese Orthopaedic Association. 2006;11(5):541-552.
- [CrossRef] [Google Scholar]
- Impaired Calcification Around Matrix Vesicles of Growth Plate and Bone in Alkaline Phosphatase-Deficient Mice. Am. J. Pathol.. 2004;164(3)
- [CrossRef] [Google Scholar]
- Action of reactive oxygen species on colonic mucus secretions. Free Radic. Biol. Med.. 2007;43(5)
- [CrossRef] [Google Scholar]
- Cao, G., Yang, S., Cao, J., Tan, Z., Wu, L., Dong, F., Ding, W., & Zhang, F. (2022). The Role of Oxidative Stress in Intervertebral Disc Degeneration. In Oxidative Medicine and Cellular Longevity (Vol. 2022). https://doi.org/10.1155/2022/2166817.
- Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine. 2009;34(9):934-940.
- [CrossRef] [Google Scholar]
- A contribution with review to the description of mineralization of bone and other calcified tissues in vivo. Anat. Rec.. 1991;230(4)
- [CrossRef] [Google Scholar]
- Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem. J. 1976;157(1)
- [CrossRef] [Google Scholar]
- Feng, C., Yang, M., Lan, M., Liu, C., Zhang, Y., Huang, B., Liu, H., & Zhou, Y. (2017). ROS: Crucial Intermediators in the Pathogenesis of Intervertebral Disc Degeneration. In Oxidative Medicine and Cellular Longevity (Vol. 2017). https://doi.org/10.1155/2017/5601593.
- Recombinant human bone morphogenetic protein-2 in the treatment of bone fractures. Biologics: Targets and Therapy. 2008;2(3):345-354.
- [CrossRef] [Google Scholar]
- A Novel Mouse Model for Multiple Myeloma (MOPC315.BM) That Allows Noninvasive Spatiotemporal Detection of Osteolytic Disease. PLoS One. 2012;7(12):e51892.
- [Google Scholar]
- Costs associated with treatment of chronic low back pain: An analysis of the UK general practice research database. Spine. 2013;38(1):75-82.
- [CrossRef] [Google Scholar]
- Post-translational regulation of Runx2 in bone and cartilage. In. J. Dent. Res.. 2009;Vol. 88, Issue 8
- [CrossRef] [Google Scholar]
- Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum.. 2006;54(8)
- [CrossRef] [Google Scholar]
- Current insights on use of growth factors as therapy for Intervertebral Disc Degeneration. Biomolecular Concepts. 2018;9(1)
- [CrossRef] [Google Scholar]
- Investigating intestinal inflammation in DSS-induced model of IBD. J. Vis. Exp.. 2012;60
- [CrossRef] [Google Scholar]
- Runx2 is required for postnatal intervertebral disc tissue growth and development. J. Cell. Physiol.. 2019;234(5)
- [CrossRef] [Google Scholar]
- Platelet-Derived Biomaterials Inhibit Nicotine-Induced Intervertebral Disc Degeneration Through Regulating IGF-1/AKT/IRS-1 Signaling Axis. Cell Transplant.. 2021;30
- [CrossRef] [Google Scholar]
- Ultrastructural localization of matrix vesicles and alkaline phosphatase in the swarm rat chondrosarcoma: Their role in cartilage calcification. Bone. 1988;9(4)
- [CrossRef] [Google Scholar]
- The Effect of Timing of rhBMP-2 Injection on Intervertebral Disc Degeneration in a Rat Tail Model. Spine J.. 2012;12(9)
- [CrossRef] [Google Scholar]
- Silver stain for proteins in polyacrylamide gels: A modified procedure with enhanced uniform sensitivity. Anal. Biochem.. 1981;117(2)
- [CrossRef] [Google Scholar]
- Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addiction. N. Engl. J. Med.. 1988;319(20):1318-1330.
- [CrossRef] [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95(2)
- [CrossRef] [Google Scholar]
- PDGF-BB Delays Degeneration of the Intervertebral Discs in a Rabbit Preclinical Model. Spine. 2016;41(8)
- [CrossRef] [Google Scholar]
- PDGF, bFGF and IGF-I stimulate the proliferation of intervertebral disc cells in vitro via the activation of the ERK and Akt signaling pathways. Eur. Spine J.. 2007;16(11)
- [CrossRef] [Google Scholar]
- Price, P. A., Otsuka, A. S., Poser, J. W., Kristaponis, J., & Raman, N. (1976). Characterization of a γ carboxyglutamic acid containing protein from bone. Proceedings of the National Academy of Sciences of the United States of America, 73(5). https://doi.org/10.1073/pnas.73.5.1447.
- Role of cytokines in intervertebral disc degeneration: Pain and disc content. In Nature Reviews. Rheumatology. 2014;Vol. 10, Issue 1
- [CrossRef] [Google Scholar]
- Association of matrix acid and alkaline phosphatases with mineralization of cartilage and endochondral bone. Histochem. J.. 1999;31(1)
- [CrossRef] [Google Scholar]
- Intervertebral Disc Degeneration: Review Article. Systematic Reviews in Pharmacy. 2020;11(11):1042-1049.
- [CrossRef] [Google Scholar]
- Roughley, P. J. (2004). Biology of intervertebral disc aging and degeneration: Involvement of the extracellular matrix. In Spine (Vol. 29, Issue 23). https://doi.org/10.1097/01.brs.0000146101.53784.b1.
- Hypertrophic differentiation and calcification during intervertebral disc degeneration. Osteoarthr. Cartil.. 2010;18(11)
- [CrossRef] [Google Scholar]
- Localization of types I, II, and III collagen mRNAs in developing human skeletal tissues by in situ hybridization. J. Cell Biol.. 1987;104(4)
- [CrossRef] [Google Scholar]
- The distinct role of the runx proteins in chondrocyte differentiation and intervertebral disc degeneration: Findings in murine models and in human disease. Arthritis Rheum.. 2008;58(9)
- [CrossRef] [Google Scholar]
- Release of active tissue factor by human arterial smooth muscle cells. Circ. Res.. 2000;87(2):126-132.
- [CrossRef] [Google Scholar]
- Does lumbar disc degeneration on magnetic resonance imaging associate with low back symptom severity in young finnish adults? Spine. 2011;36(25):2180-2189.
- [CrossRef] [Google Scholar]
- The effect of reactive oxygen species on the biosynthesis of collagen and glycosaminoglycans in cultured human dermal fibroblasts. Arch. Dermatol. Res.. 1993;285(6)
- [CrossRef] [Google Scholar]
- Stimulation of mature canine intervertebrai disc by growth factors. Transplantation. 1973;16(3)
- [CrossRef] [Google Scholar]
- Ultrastructure of the human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell. 1982;14(2):359-369.
- [CrossRef] [Google Scholar]
- Dental Pulp Stem Cells: Advances to Applications. Stem Cells and Cloning : Advances and Applications. 2020;13:33.
- [CrossRef] [Google Scholar]
- Effects of nicotine on the intervertebral disc: an experimental study in rabbits. Journal of Orthopaedic Science : Official Journal of the Japanese Orthopaedic Association. 2001;6(2):177-182.
- [CrossRef] [Google Scholar]
- Cooperative effects in differentiation and proliferation between PDGF-BB and matrix derived synthetic peptides in human osteoblasts. BMC Musculoskelet. Disord.. 2011;12
- [CrossRef] [Google Scholar]
- Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-2196.
- [CrossRef] [Google Scholar]
- Evaluation of the effect of oxidative stress on articular cartilage in spontaneously osteoarthritic STR/OrtCrlj mice by measuring the biomarkers for oxidative stress and type II collagen degradation/synthesis. Exp. Ther. Med.. 2011;2(2)
- [CrossRef] [Google Scholar]
- Heterogeneity in Storage Pool Deficiency: Studies on Granule-Bound Substances in 18 Patients Including Variants Deficient in α-Granules, Platelet Factor 4, ß-Thromboglobulin, and Platelet-Derived Growth Factor. Blood. 1979;54(6):1296-1319.
- [CrossRef] [Google Scholar]
- The effect of MS14 on innate and cellular immune responses in BALB/c mice. Immunopharmacol. Immunotoxicol.. 2011;33(3)
- [CrossRef] [Google Scholar]
- The cell biology of intervertebral disc aging and degeneration. Ageing Res. Rev.. 2007;6(3):247-261.
- [CrossRef] [Google Scholar]
- Zoch, M. L., Clemens, T. L., & Riddle, R. C. (2016). New insights into the biology of osteocalcin. In Bone (Vol. 82). https://doi.org/10.1016/j.bone.2015.05.046.







