Translate this page into:
Removal of some heavy metals and fungicides from aqueous solutions using nano-hydroxyapatite, nano-bentonite and nanocomposite
⁎Corresponding author. Tel.: +201061402725. elnagar.doaa@yahoo.com (Doaa A. El-Nagar) elnagar.doaa@sweri-eg.com (Doaa A. El-Nagar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A batch adsorption experiments were carried out to study the role of nanoparticles and nanocomposite on removal of some heavy metals and fungicides from aqueous solution. Nano-Hydroxyapatite (n-HAP), Nano-Bentonite (n-Bo) and Bentonite-hydroxyapatite nanocomposite (B-HAP NC) evaluated for the removal of some heavy metals and fungicides. The nanoparticles and nanocomposite were characterized by TEM, SEM and AFM, X-ray powder diffraction (XRD) and BET surface area. The batch adsorption was done using nanoparticles with Pb2+ and Ni2+ as example of heavy metals with concentrations up to 25 mgL−1. Also, the adsorption experiment was conducted using nano-particles (n-HAP, n-Bo and B-HAP NC) with fungicides Stop Feng and Eurozole with concentrations 20 to 200 μg L−1. Langmuir and Freundlich isotherm equations were employed to study the adsorption. The adsorption kinetics were conducted metal ion (Pb2+ and Ni2+) with residence time. The results indicated the maximum adsorption capacity of Ni+2 was occurred on (n-HAP). While that maximum adsorption capacity of Pb2+ was occurred on (B-HAP NC). The rate of Ni+2 removal was found to be very rapid during the initial 60 min. The adsorption of Pb+2 by the n-HAP and (B-HAP NC) was a slow increase with time, it did not bring any remarkable effect. Also, the efficiency of adsorbent compounds used to remove the residue of fungicides Stop Feng and Eurozole shown the highest removal rates obtained with used nano-hydroxyapatite followed by bentonite-hydroxyapatitenanocomposite and nano-bentonite, respectively. The current results are very useful in the treatment of wastewater and the removal of heavy metals and fungicides, consequently making them suitable for agricultural purposes.
Keywords
Heavy metals
Fungicides
Waste water
Nano-bentonite
Nano-hydroxyapatite
Nanocomposite
1 Introduction
The presence of high levels of heavy metals in the environment may cause long-term health risks to humans and ecosystems. Adsorption is an effective physicochemical purification and separation technique used in water and wastewater treatments. It is considered as the preferred method for removal, recovery and recycling of toxic heavy metals (Muharrem and Olcay, 2017).
Treatment of metal bearing industrial wastewater is of height ended interest (Pakshirajan and Swaminathan, 2009; Acheampong et al., 2013). This is mainly because heavy metals are toxic even at a very low concentration to human, animal andplant species.
The pollution of environmental is considered one of the important serious predicaments of the world nowadays (Helal and Abo-Seoud, 2014). The chemical pesticides and fungicides were used for non-agricultural and agricultural purposes have resulted in presence residues of them in water, air, plant and soil caused harmful effects to the environment and human also, bioaccumulation of them were taking a place in the food chain (Azmi et al., 2009; Diez, 2010). Fungicides and their metabolic product of some fungicides are used to control a broad range of fungal diseases in different crops (vegetables, fruits, medicinal herbs and ornamentals) in agriculture (Raheem et al., 2017).
Recently nanotechnology come to the fore. Nanomaterials differ from bulk materials in their large surface area, chemical stability and resistance to external influences, Nanoparticle size stability depends on the adsorption energy and density of total surface area, low densities of nanoparticle lead to large stable nanoparticles over time (He et al., 2014). These nanomaterials have the ability to effectively separate heavy metal ions and fungicides from the environment source by the adsorption them on its surface. Nanoparticles are highly suitable for removing metal, organic/inorganic contaminants from water (Jiang et al., 2012; Mohmood et al., 2013; Sai Bhargav and Prabha, 2013; Pandipriya et al., 2014).
Hydroxyapatite being an inexpensive but efficient adsorbent, is extensively used for decontaminating wastewater and soils polluted by heavy metals (Hashimoto et al., 2009; Slobodan, 2009; Wang et al., 2009; Chen et al., 2010; Yuan et al., 2010), pointed to the nano-hydroxyapatite is an ideal material for the disposal of contaminants because of its high adsorption capacity for heavy metals.
Nano-bentonite used as an alternative adsorbent to remove heavy metal and pesticides, bentonite is cost cheap and abundant in nature. The main constituents of nano-bentonite are montmorillonite minerals. Montmorillonite has a 3-layered structure causes the formation of negative charges on the surface its. This part is active site which can be used as an adsorbent to remove heavy metal (Mahfoud et al., 2014). The heavy metals adsorption by nano-bentonite is 99.03% for Cd and 99.18% for Hg (Makmur and Profita, 2018). It shows good adsorption removal for both metal ions Pb+2 and Cd+2 (Hendet al., 2016).
Nano-clays like nano-bentonite having the maximum removal capacity was used efficiently for the removal of pesticides (Shabeer et al., 2015). Therefore, the levels of heavy metals and fungicides residuals that exist in waste water and water used in agricultural and industrial purposes should to be reduced in allowable concentrations.
The aim of this study is the removal of heavy metals and fungicides from the wastewater. Consequently, making them suitable for agricultural purposes by fast, non-expensive and safe materials as nanoparticles.
2 Materials and methods
Bentonitic clay (from north Egypt), CaCO3, H3PO4 and PEG 6000 (Poly Ethylene Glycol). All chemicals are manufactured by Sigma Company (Indi) and using without any purification.
2.1 Synthesis
2.1.1 Synthesis of hydroxyapatite nanoparticles
Simple and rapid precipitation method using to the synthesis of hydroxyapatite nanoparticles in which, 1 M of CaCO3 solved in 50 ml doubled deionized distill water add to 0.6 M of H3PO4. Mixture subjected to ultrasonic irradiation under specific condition (70% amplitude, 0.5 cycles for 2 h) until white precipitate obtain.
2.1.2 Synthesis of bentonite nano sheet structure
First of all, before the synthesis of Bentonite nano sheets structure purification has been done (Sameh et al., 2017). Purified bentonite synthesis by simple Sono-chemical method by exfoliation-adsorption with PEG as a stabilizer and separator of Bentonite sheets. In typical synthesis, 0.1 g of purified bentonite added to 50 ml doubled deionized water then add 0.1 ml of PEG 6000 and subjected mixture into direct sonication using sonicated prop (Hielscher, up 400s) under condition of plus time 10 Sec., rest time 1 Sec., temperature not accessed at 60 °C, total time of sonication 2 h, 70% amplitude and 0.5 cycles. The precipitate was oven dry at 50 °C for 24 h.
2.1.3 Synthesis of Bentonite-hydroxyapatite nanocomposite (B-HAP NC)
Equivalent weight (1:1) mixed between Bentonite sheets nanostructure and hydroxy apatite nanoparticles (5 g) added to 500 ml doubled deionized water and subjected to direct sonication at the condition of plus time 30 Sec., rest time 2 Sec., temperature not accessed at 60 °C, total time of sonication 4 h, 80% amplitude and 0.8 cycles for 2 h. The precipitate was oven dry at 50 °C for 24 h. Finally, precipitate is centrifuged at 500 rpm for 20 min and oven dry the final precipitate at 50 °C for 24 h.
2.2 Characterization
The characterization goal of Bentonite-hydroxyapatite nanocomposite not only determines physical and chemical properties, but also influence these properties to its removal of heavy metals. Characterized classified into three classes namely texture, identification and index class. Identification class carried out to prove the successes of synthesis method without any contamination or undesired chemicals from synthesis process. Identification class was characterized by XRD (D8 Discovery –Bruker Company) under measurement condition 40 KV and 40 mA (1600 W) at speed scan 0.01 and 2 theta range from 5° to 100°. Texture class carried out to confirm 2D and 3D shape and size by Transmission Electronic Microscope (TEM) (model EM-2100, High-Resolution) at magnification 20X and voltage 200 kV. Scanning Electronic Microscope (SEM) carried out by Jol 2000. AFM images carried out by AFM instrument 5600 LS Agilent Technology Company. Sample preparation before measurement on AFM was done by adding sample in the form of powder in water and sonication for 45 min suspension added on mica slide, at condition of measurement (size 100 X 100 nm, speed 0.4 in./sec, I Gain = 0.4 and P Gain = 18 using contact mode). Index class carried out by determining the BET surface area and pore size by surface area and pore size analyzer model of Nova Touch LX2 manufacture by Quantachrome Company. Degaussing was done to remove moisture content and dust at 50 °C under vacuum (10 °C/min for 3 h then soaking for 1 h). Characterization carried out for hydroxy apatite nanoparticles and Bentonite – hydroxy apatite nanocomposite.
2.3 Adsorption experiments
2.3.1 Effect of initial metal concentration
The stock solutions of the Pb2+ and Ni2+ were (1000 mg/l). Subsequent dilutions of (5, 10, 15, 20 and 25 mg/l), were prepared by diluting the stock solution with distilled water. Performing experiments in flasks containing 25 ml of Pb2+ or Ni2+ of different concentration plus 0.1 g of Nano-Hydroxyapatite (n-HAP), Nano-Bentonite (n-Bo) and Bentonite-hydroxyapatite nanocomposite (B-HAP NC) with three replicates. The pH value was adjusted to 7 andthe temperature of the adsorption experiments 20 0CThe mixture was shaken in a rotary shaker at 50 rpm for one hour followed by filtration. The filtrate containing the residual concentration of Pb+2 or Ni+2 was saved for analysis.
2.3.2 Effect of equilibration time
The effect of equilibration time on each metals adsorption were studied in different time intervals ranging from 30, 60, 90, 120 and 180 min. To determine the contact time was studied using 0.1 g of Nano-Hydroxyapatite (n-HAP), Nano-Bentonite (n-Bo) and Bentonite -hydroxyapatite nanocomposite (B-HAP NC) with three different sizes and 25 ml of Pb2+ or Ni2+ solution of concentration 20 mg/l. The pH value was adjusted to 7 and the temperature of the adsorption experiments 20 °C. The mixture was shaken in a rotary shaker at 50 rpm at the same time intervals followed by filtration. The filtrate contained the residual concentration of Pb+2 or Ni+2 were stored for analysis.
Soluble (Pb+2 and Ni+2) were determination using Inductively Coupled Plasma Spectrometry, ICP (Ultima 2 JY Plasma).
According to (Vijayaraghavan et al., 2006; Vieira and Volesky, 2003) the equilibrium adsorption capacity (mg/g) of the heavy metals were calculated by:
Eq. (1) Where: Ce and C0(mg/l) are the concentrations of the metals in the initial solution and the equilibrium solution after the experiment, respectively. V(l) is the solution volume. m: adsorbent mass (g).
Heavy metal adsorption models and kinetic models:
Langmuir and Freundlich isotherm models using software IsoFit (Matotte, 2007).
Freundlich equation
Eq. (2) Where, KF and n are adsorption capacity and affinity, respectively.
Langmuir equation
Eq. (3) Where, Ce (mg/l) is the supernatant concentration at the equilibrium state of the system, qm (mg/g) and KL are the Langmuir constants, representing the maximum adsorption capacity and the energy constant respectively.
Pseudo second-order model
Eq. (4) where K2 is the rate constant of pseudo second- order adsorption rate constant (g/mg/min). The values of k2, R2 and qe were calculated from the plots of t/qt versus t. The regression R2 > 0.9 (Das and mondal, 2011).
2.3.3 Fungicides and adsorbent
The adsorbent compounds such as nano-hydroxyapatite, nano-bentonite and bentonite-hydroxyapatite nanocomposite were used at 0.1 g for each for removing fungicides residues from water.
Fungicides Stop Feng 70% and Eurozole 25% were used with different concentrations at 20 to 200 μg L−1 and added to 1 L aqueous solution. The four solutions were used in this experiment. First solution, added to it each adsorbent compound in each concentration and shaken for 90 min. The second solution, was added each adsorbent compound in each concentration of each fungicide without shaken. Third solution, with the same concentrations of fungicides and without adsorbents compounds were used and shaken for 90 min. The fourth solution was used as of fungicides only without shaken.
Extraction of fungicides: Each solution was extracted with dichloromethane (3 × 60 ml) and transferred into a separatory funnel and added saline solution (25 ml, 10%) into the samples to improve the efficiency of partition and prevent formation of the emulsion. After that, added anhydrous Na2SO4 to get rid of moisture in extractions. Each extraction was evaporated by a rotary evaporator to dryness. The obtained residue was re-dissolved in 1 ml hexane and analyzed
Analysis of fungicides: Samples were analyzed with a gas chromatography system model 7890B coupled with mass spectrometry model 5977A (GC/MS) instrument and an autosampler (Agilent, Little Falls, DE). The system was equipped with a splitting/splitless injection inlet, and electronic pressure control (EPC). The system was controlled by MSD ChemStation software and data analysis was done by MassHunter GC/MS acquisition software. Extracts and recovery samples (2 μl) were injected in the GC/MS system in splitless mode. An HP-5MS capillary column (30 m X 0.53 mm i.d. 0.25 µm film thickness) was used to separate the components. Helium was used as a carrier gas. Separation conditions were according to the AOAC (2007) as the following: initial column temperature set at 80 °C for 6 min., increased to 215 °C at 15 °C/min (hold for 1 min), then to 230 °C at 5 °C/min and finally to 290 °C at 5 °C/min (hold for 2 min). The carrier gas was at a constant flow rate of 1.1 ml/min. Fungicides were identified by their full mass spectra scans and retention time using the total ion current as a monitor to give a Total Ion Chromatogram (TIC). The use of the full scan mode allowed the contrast of the spectrum of the compounds with the EI-MS library.
Eq. (5) Where, Co and Ce (mg/l) represent initial and equilibrium concentrations of fungicides.
3 Results
3.1 Characterization
Identification class results using XRD chart show the finger print XRD pattern for hydroxy apatite nanoparticles (COD9014313) with hexagonal lattice and bentonite sheets nanostructure (COD9002779 for montmorillonite and COD9000122 for dickite) according to ICCD data base without any contamination from other chemicals during synthesis steps. XRD chart of Bentonite-hydroxyapatite nanocomposite shows the characteristic peaks of both hydroxyapatite nanoparticles and bentonite sheets nanostructure which indicated the success of synthesis without any undesired. Fig. 1.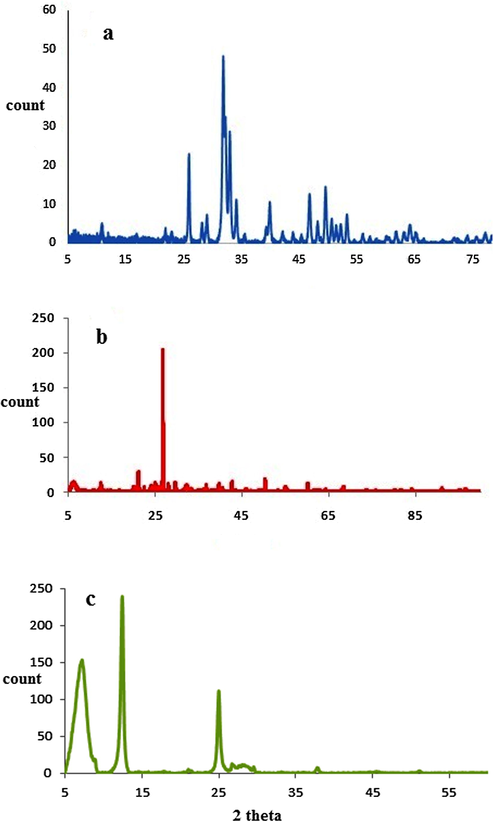
(a) XRD pattern of nano-hydroxyapatite (b) bentonite-hydroxyapatite nanocomposite and (c) nano-bentonite.
TEM and SEM show the sheet structure of Bentonite with thickness of sheet about 10 nm size and semi-spherical shape of hydroxyapatite with 45 nm size. Hydroxyapatite-bentonite nanocomposite TEM and SEM illustrated combination of subspherical hydroxyapatite nanoparticles and bentonite nanosheet Fig. 2. AFM images show the good distribution of hydroxyapatite nanoparticles on the surface of Bentonite sheets nanostructure with humongous in size and shape Fig. 3. AFM images show also penetrate part of hydroxyapatite nanoparticles inside Bentonite sheets nanostructure which conformed good quality of Bentonite-hydroxy apatite nanocomposite. AFM images of bentonite nanosheets and hydroxyapatite nanoparticles illustrated the good grain size distribution and deagglomeration of subspherical and sheets of them Figs. 3 and 4.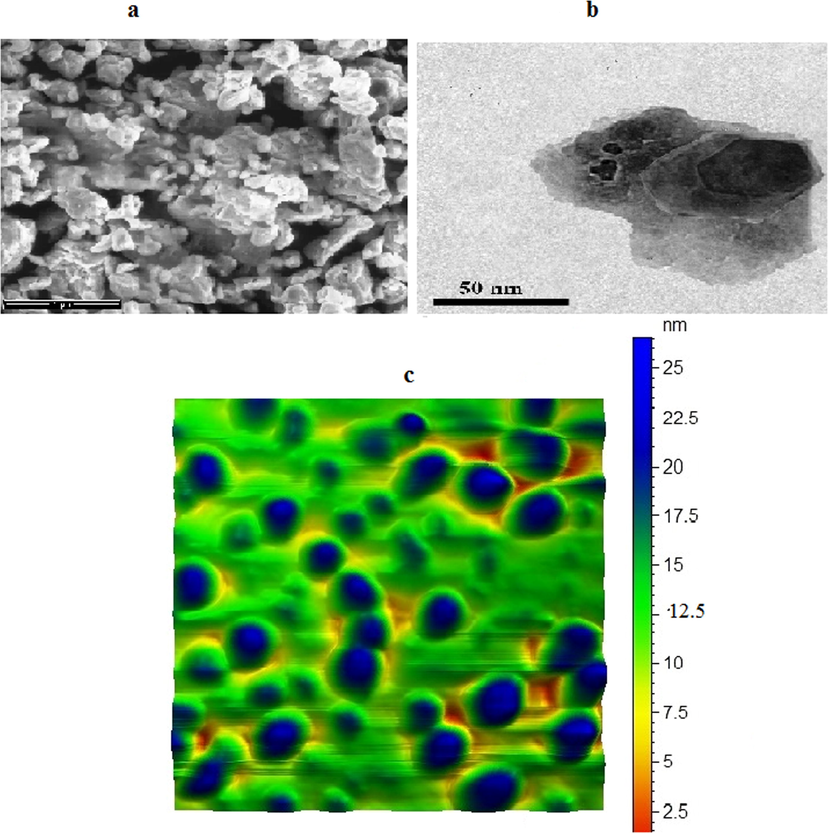
(a) SEM (b) TEM (c) AFM of bentonite-hydroxyapatite nanocomposite.
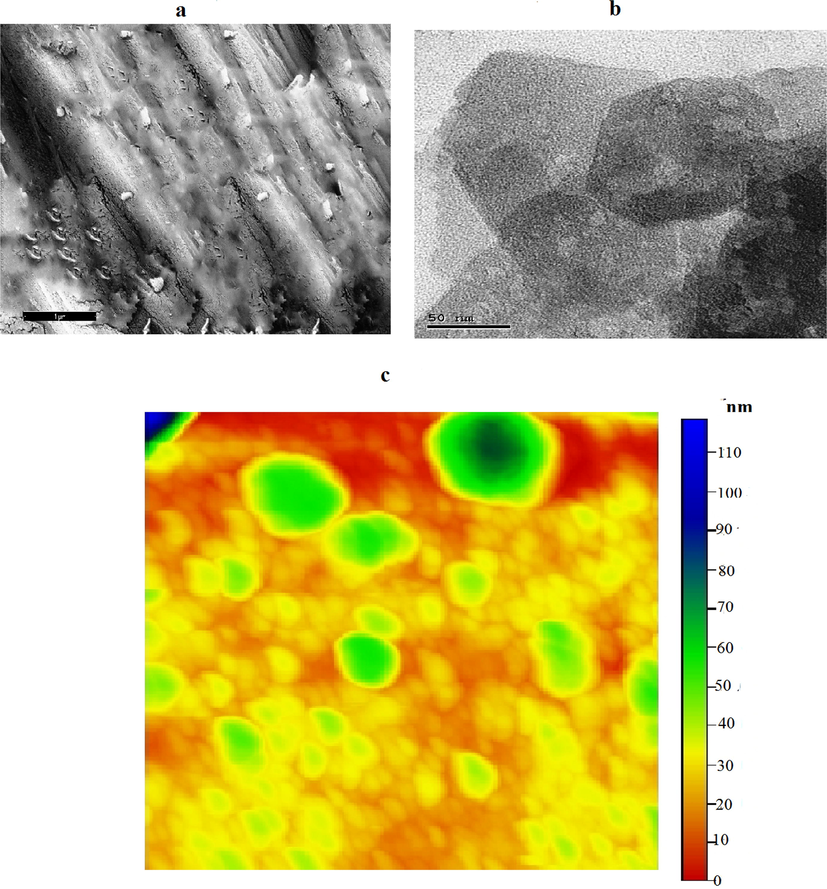
(a) SEM (b) TEM (c) AFM of of nano-bentonite.
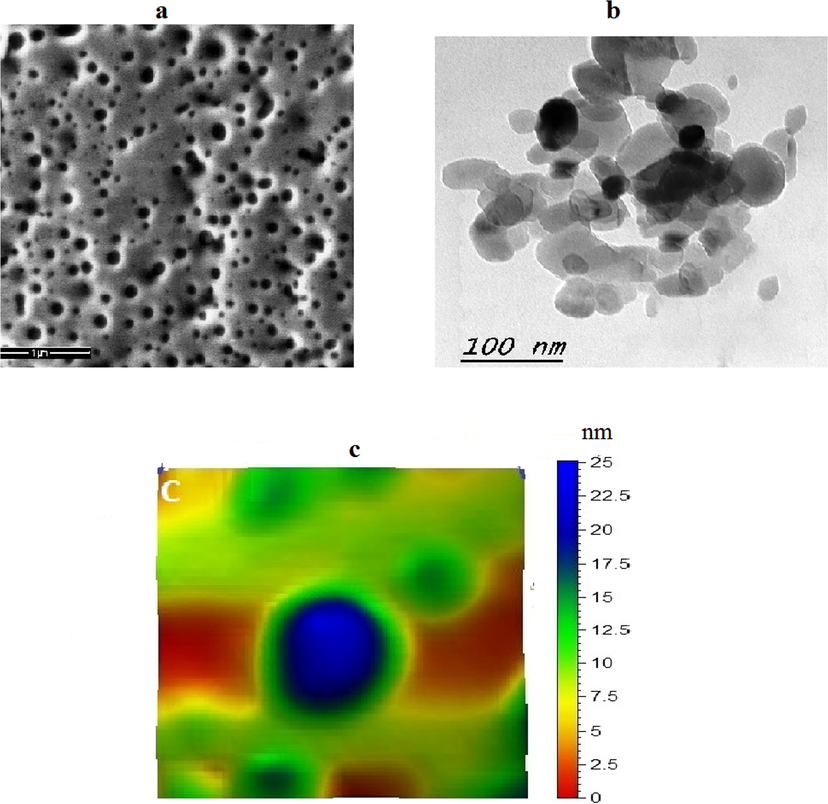
(a) SEM (b) TEM (c) AFM of of nano-hydroxyapatite.
BET surface area of bentonite nanosheet, hydroxyapatite nanoparticles and bentonite-hydroxyapatite nanocomposite are 245.6, 24.5 and 204.2 m2 g−1, respectively. According to IUPAC isotherm is IV type with mesoporosityas a show in Fig. 5.The decreased of a BET surface area of nanocomposite due to attached the hydroxyapatite nanoparticles on bentonite nanosheet. Total pore size is 12.3, 1.1 and 10.2 nm for bentonite nanosheet, hydroxyapatite nanoparticles and and bentonite-hydroxyapatite nanocomposite, respectively. The decreased of the total pore size of nanocomposite respect to bentonite nanosheet due to blocking of hydroxyapatite nanoparticles for most of the macro and mesopores.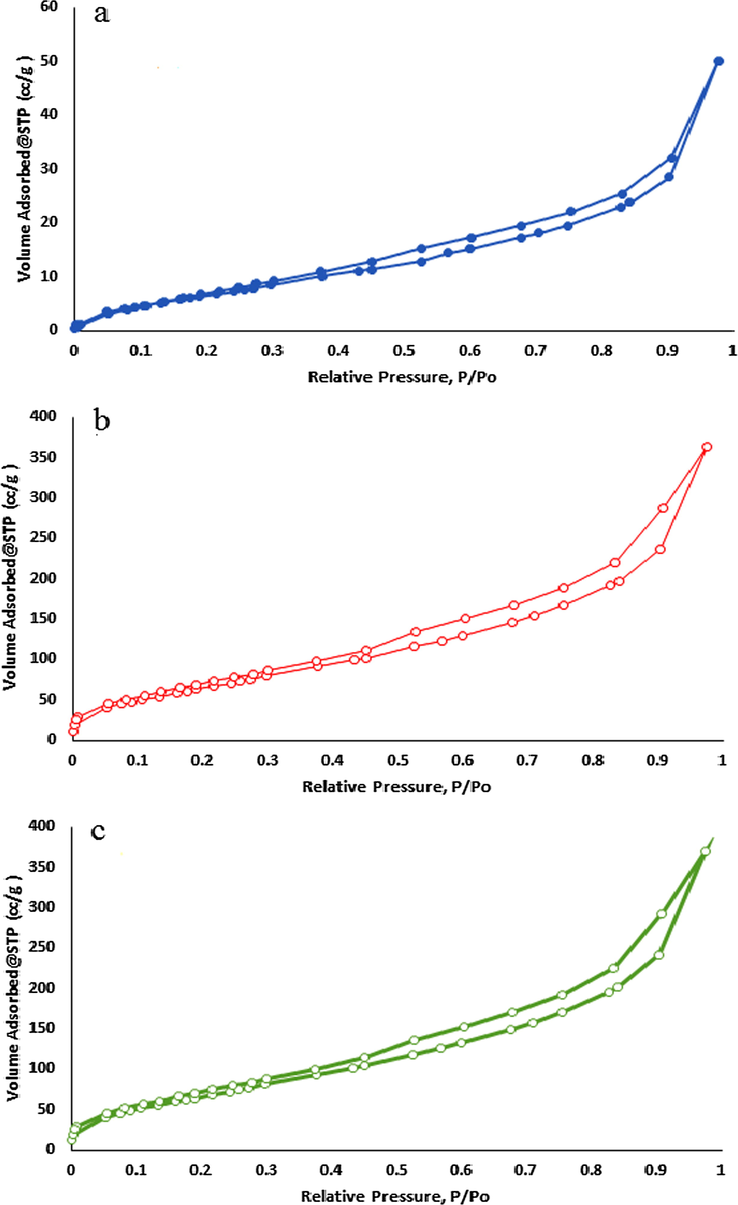
Isotherm of (a) of nano-hydroxyapatite, (b) bentonite-hydroxyapatite nanocomposite and (c) nano-bentonite.
3.2 Effect of initial metal ion concentration
The effect of initial metal concentration (Pb+2 and Ni+2) on adsorption on to Nano-Hydroxyapatite (n-HAP), Nano-Bentonite (n-Bo) and Bentonite-hydroxyapatite nanocomposite (B-HAP NC) and adsorption models. The increase in adsorption capacity of Nano-Hydroxyapatite (n-HAP), Nano-Bentonite (n-Bo) and Bentonite-hydroxy apatite nanocomposite (B-HAP NC) were corresponded with increasing in the concentrations of Pb+2 and Ni+2 (up to 25 mg/l), Where the energetically less active sites are active with increasing metal concentration in solution. The maximum adsorption capacity of Pb+2 by these materials were 5.83, 2.05 and 5.99 mg/g, respectively. The maximum adsorption capacity of Ni+2 were 3.72, 1.26 and 1.01 mg/g, respectively. The results indicated that maximum adsorption capacity of Ni+2 was occurred on nano-hydroxyapatite followed by nano-bentonite. On other hand, the maximum adsorption capacity of Pb+2 was occurred on Bentonite-hydroxyapatite nanocomposite (B-HAP NC) followed by nano-hydroxyapatite. The removal percentage of Pb+2 was more than Ni+2 indicated that the ability of Pb+2 to bind with nanoparticles more than Ni+2. The experimental data (adsorption isotherm) obtained from the batch studies of heavy metals adsorption on nanoparticles were modeled using two adsorption models such as Freundlich and Langmuir models (Table 1).
Adsorption model
Nanoparticles
Parameters
Pb+2
Ni+2
Freundlich
Nano-Hydroxyapatite (n-HAP)
Kf
344.64
100.31
1/n
1.07
1.25
R2
0.987
0.994
Nano-Bentonite (n-Bo)
Kf
86.06
1/n
0.80
R2
0.997
Bentonite-hydroxy apatite nanocomposite (B-HAP NC)
Kf
610.44
34.90
1/n
1.20
1.20
R2
0.967
0.971
Langmuir
Nano-Bentonite (n-Bo)
Kl
5.134
qm
186.75
R2
0.898
The results show the adsorption parameters of heavy metals (Pb+2 and Ni+2) on different nanomateriles indicated that maximum adsorption of Ni+2 was occurred on Nano- hydroxyapatite Freundlich adsorption models, while maximum adsorption of Pb+2 was occurred on Bentonite-hydroxyapatite nanocomposite (B-HAP NC)Freundlich adsorption models. This indicates multilayer adsorption on the surface. Adsorption parameters of Pb+2 on nano-bentonite used Langmuir adsorption models where the maximum adsorption exist when a saturated monolayer of Pb+2 is existent on the adsorbent surface. The nano-hydroxyapatite has more ability for retention of Ni+2 and the freundlich isotherm parameter (Kf) was 100.31. The freundlich isotherm parameter (Kf) was 610.44 for Pb+2 by Bentonite-hydroxy apatite nanocomposite (B-HAP NC), indicated that Pb+2 is more binding on n-HAP and (B-HAP NC) than Ni+2. The more ability of n-HAP and (B-HAP NC) to retain the Pb2+ than Ni2+ reflected in the high value of the freundlich isotherm parameter (Kf).
3.3 Effect of contact time
The Effect of equilibrium time on the adsorption of Pb and Ni on Nano-Hydroxyapatite (n-HAP), Nano-Bentonite (n-Bo) and Bentonite-hydroxyapatite nanocomposite (B-HAP NC).
The adsorption of Ni+2 is found to be very rapid during the initial 60 min. No significant change in Ni+2 ion adsorption is observed by all nanomaterials after about 60 min and for 60 min for Pb+2 by Nano-bontente. The results of adsorption studies, carried out as a function of contact time, for pb+2 on n-HAP and (B-HAP NC). It suggested that the adsorption of Pb+2 by the n-HAP and (B-HAP NC) were slow increase with time, it did not bring any remarkable effect, and so a contact time of 60 min was chosen for further experiment.
Table 2 and Figs. 6 and 7 illustrates the kinetic parameters of Pb+2 and Ni+2 adsorption on (n-HAP), (n-Bo) and (B-HAP NC) according to kinetic model.
Kinetics model
Nanoparticles
Parameters
Pb+2
Ni+2
Pseudo second-order rate equation
Nano-Hydroxyapatite (n-HAP)
qe
4.92
2.28
K2
0.121
0.053
R2
0.99
0.99
Nano-Bentonite (n-Bo)
qe
1.25
0.79
K2
0.041
0.193
R2
0.98
0.99
Bentonite-hydroxy apatite nanocomposite (B-HAP NC)
qe
4.96
0.79
K2
0.101
0.043
R2
1.00
0.99
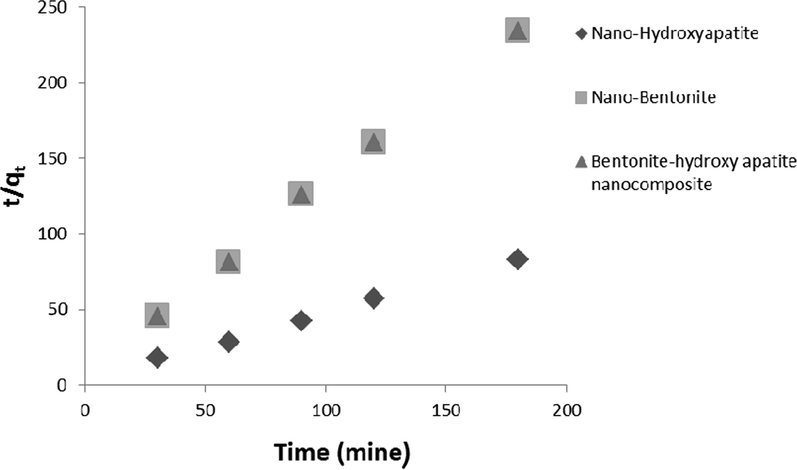
Pseudo-second order rate plot for the Ni2+ adsorption on nano-hydroxyapatite, nano-bentonite and bentonite-hydroxyapatite nanocomposite.
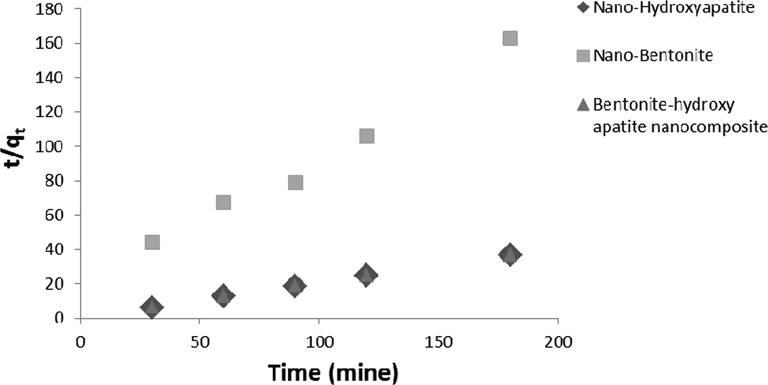
Pseudo-second order rate plot for the Pb2+ adsorption on nano-hydroxyapatite, nano-bentonite and bentonite-hydroxyapatite nanocomposite.
The adsorption kinetics study describes metal ion uptake rate controls with a residence time of adsorption at solid and solution interface. The results of the kinetics study were tested with four kinetics models; pseudo second-order rate equation, Elovich rate equation, Intra-particle diffusion rate equation and Lagergen pseudo first-order equation. Kinetic studies reveal the pseudo second-order model as best describing the process of lead and nickel adsorption on (n-HAP), (n-Bo) and (B-HAP NC). The regression R2 > 0.9, for both Ni+2 and Pb+2 meaning that this model provided the best fit for the adsorption data than other kinetic models. K2 and R2 values were greater for Pb than Ni. These results suggest that (n-HAP), (n-Bo) and (B-HAP NC) can be applied as an adsorbent for lead and nickel ion removal from waste water.
3.4 Removing of fungicides residues
Efficiency of adsorbent compounds used to removal residue of fungicides were showing the highest removal rates obtained with used nano-hydroxyapatite followed by Bentonite-hydroxyapatite nanocomposite and nano-bentonite, respectively. The increasing in the efficiency of removing was obtained at low concentrations of each fungicide, while at high concentration the efficiency was decreasing. The shaken may play role in removing residue of fungicide. The obtained data was recorded as chart for each fungicide Fig. 8 was showed the removal residue of Stop Feng at different concentrations. nano-hydroxyapatite was shown increasing removal of Stop Feng from 68.9% to 91.5% with shaken but without shaken from 20.1% to 29.7%. The lowest removal was obtained with nano-bentonite from 42.7% to 56.2% with shaken but without shaken from 14.5% to 16.3%. The data belongs Eurozole was shown in Fig. 9 and showed that the adsorbent compounds less efficiency in removal Eurozole comparing with removal Stop Feng. The same data was obtained the most efficiency of adsorbent compounds was nano-hydroxyapatite followed by Bentonite-hydroxyapatite nanocomposite and nano-bentonite, respectively. nano-hydroxyapatite was ranged from 50% to 70.4% with a shaken, while 14.3% to20.5 % without shaken followed by Bentonite-hydroxyapatite nanocomposite with a range from 36.8 to 55% with shaken and 12.7 to 15.4 without shaken. Finally, the use of fungicides with adsorbent compounds and shaking for 90 mins were giving the best removal of theses fungicides.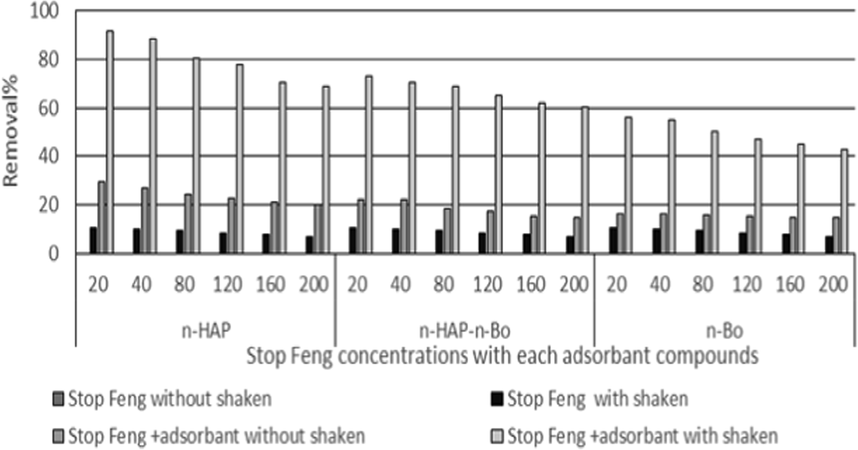
Using nano-hydroxyapatite, nano-bentonite and Bentonite-hydroxyapatite nanocomposite to removal Stop Feng residue.
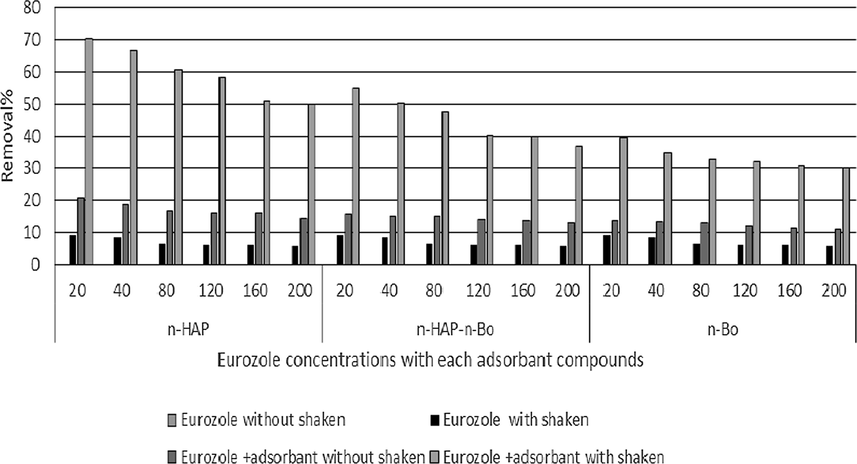
Using nano-hydroxyapatite, nano-bentonite and Bentonite-hydroxyapatite nanocomposite to removal Eurozole residue.
4 Discussion
Nano-materials were expected to play a crucial role in remove heavy metals and fungicides, that remove toxic and harmful substances from soil and wastewater. The maximum adsorption of Ni was occurred on nano-hydroxyapatite followed by nano-bentonite. These results may be due to the functional groups found in nano-hydroxyapatite. Where nano-hydroxyapatite contains to the O-H extend vibration of surface P-OH groups that help release the proton from the surface POH sites to the aqueous solution. Which leads to adsorbed heavy metals ions on the n-HAP surface and replaced Ca+2 ion by Ni+2 and Pb+2 through ionic exchange reaction. Similar results have been reported by (Mobasherpour et al., 2011; Vijaykiran et al., 2014). Yuan et al. (2010) investigated, the adsorption potential of n-HAP for the removal of cadmium and zinc from aqueous solutions. The maximum adsorption capacity of cadmium and zinc were 1.964 and 2.151 mmol g−1 respectively. The adsorption of Cu+2, Cd+2 and Pb+2 on nano-HAP are well fitted both using the Langmuir and Freundlich equations. The maximum adsorption of heavy metals on n-HAP follows the order Pb+2 > Cd+2 > Cu+2. The competitive adsorption of multi-mixtures of Cu+2, Cd+2 and Pb+2 on n-HAP reduced the adsorption capacity of n-HAP for individual metal ions (Chen et al., 2010). The application of n-HAP could significantly reduce water soluble Cd+2with 90% and Pb+2 with 72% (Mao et al., 2013). The removal capacity of Ni+2, Cd+2 and Pb+2 increases with the increase in the initial concentration of n-HAP. Isotherm studies indicated that the Langmuir model fits the experimental data better than Freundlich (Mobasherpour et al., 2012). The maximum adsorption capacity of heavy metals was occurred on nano-hydroxyapatite for both Pb+2 and Ni+2. These results may be due to the functional groups found in nano-hydroxyapatite (Doaa et al., 2016). Nano-bentonite increased adsorption capacity of Ni+2 this due to high cation exchange capacity, large surface area, exchangeable sites available, large active sites and mechanical stability (Pawar et al., 2016; Mahmoud et al., 2017; Erfan et al., 2019). Silica and magnesium ions in n-Bo exchanged with heavy metal ions which increased adsorption of heavy metals in surface (Makmur and Profita, 2018). Where the ability of the adsorbent mainly depends mainly on the chemical reactivity and porosity. The FTIR studies indicated that nano-bentonite forms a variety of functional groups such as hydroxyl, carbonyl, silicate, aluminum which may involve in the adsorption of heavy metals ions (Maheswari et al., 2019). The nano-bentonite adsorption showed good percentage removal for both Pb+2 and Cd+2. It was found that the percentage removal was increased by increasing the contact time till 30 min (Hend et al., 2016).
The maximum adsorption of Pb+2 was occurred on Bentonite- hydroxy apatite nanocomposite, this may be due to dual adsorption force Bentonite with hydroxy apatite, where adsorption Pb+2 depended on the presence of a large number of active sites and the accessibility its without impediment followed by nano-hydroxyapatite. Where the partial dissolution of n-HAp and precipitation leads to adsorption of Pb+2 this is the main mechanism in the adsorption Pb+2 (Mousa et al., 2016; Duyen et al., 2019; Simona et al., 2018). The application of n-HAP has converted Pb+2 from soluble to insoluble. Which would lead to more calcium lead phosphate hydroxide formation (Mao et al., 2013). From above results, the main mechanism in the adsorption of Pb+2 on n-HAp was due to the formation of a Complexes on the surface. While the adsorption of Ni+2 on n-HAp was due to ion exchange. The removal of Pb+2 more than Ni+2. This is due maximum sorption to be inversely proportional to the hydrated ionic radii (Pb+2 0.401 nm, Ni+2 0.404 nm) and hydration energy (Pb+2 1481 kJ/mol, Ni+2 2106 kJ/mol), Also the groups on n-HAP is hard Lewis bases while Pb+2 is a borderline hard Lewis acid and Ni+2 is a soft Lewis acid Which leads to greater affinity between n-HAP and Pb+2. The Pb+2 higher electronegativity than Ni+2 (Mobasherpour et al., 2012).
During the initial stage of adsorption, a large number of vacant surface sites are available for adsorption. After the laps of some time, the remaining vacant surface sites are difficult to be occupied due to repulsive forces between the solute molecules adsorbed on the solid surface and the bulk phase. Besides, the metal ions are adsorbed into the active sites that get almost saturated with metal ions during the initial stage of adsorption. Thereafter, the metal ions have to traverse farther and deeper into the sites encountering much larger resistance. This results in the slowing down of the adsorption during the later period of adsorption (Doaa et al., 2016). The decrease in the rate of adsorption of heavy metal with time may be due to aggregation of heavy metal around nanoparticles. This lead to hamper the migration of heavy metal, where the adsorption sites become full (Mousa et al., 2016).
In general, the higher molecular weight of pesticide can be removed easily in treatment than lower molecular weight pesticides (Szlachta and Adamski, 2009). In othrstudy using Graphene/Fe3O4 nanocomposite for removal of ten triazole fungicides (20 µg/mL) from water solution, the removal efficiency of fungicides used were rangedfrom 85.2% to 96.0% (Zhaokun et al., 2019).
Removal of fungicide residue from water was depended on many factors as treatment method, application time, the solute’s properties, pH of the solution, light conditions and type of adsorbents. The efficient removal of pendimethalin and atrazine were 87.8% and 71.1%, respectively, using nano-bentonite (Shabeer et al., 2015). Also, (Kaur et al., 2015) shows that the better removal efficiency of fungicides were obtained by using nano-bentonite than TiO2.
5 Conclusions
In this paper, The experiments were carried out to study the role of Nano-Hydroxyapatite (n-HAP), Nano-Bentonite (n-Bo) and Bentonite-hydroxyapatite nanocomposite (B-HAP NC) on the removal of some heavy metals and fungicides from aqueous solution. The results indicated the maximum adsorption capacity of Ni+2 was occurred on Nano-Hydroxyapatite (n-HAP). While that maximum adsorption capacity of Pb+2 was occurred on Bentonite-hydroxyapatite nanocomposite. The rate of Ni+2 removal was found to be very rapid during the initial 60 min. The adsorption of Pb+2 by the n-HAP and (B-HAP NC) was a slow increase with time, it did not bring any remarkable effect. Also, the efficiency of adsorbent compounds was used for removal residue of fungicides Stop Feng and Eurozole were showing the highest removal rates obtained with used nano-hydroxyapatite followed by Bentonite-hydroxyapatite nanocomposite and nano-bentonite, respectively.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Removal of Cu (II) by onto coconutshell in fixed-bed column systems. J. Ind. Eng. Chem.. 2013;19:841-848.
- [Google Scholar]
- Effect of pesticide residues on health and blood parameters of farm workers from rural Gadap, Karachi-Pakistan. J. Environ. Biol. Ind.. 2009;30:747-756.
- [Google Scholar]
- Adsorption of aqueous Cd Pb and Cu ions by nano-hydroxyapatite: Single- and multi-metal competitive adsorption study. Geochem. J.. 2010;44:233-239.
- [Google Scholar]
- Calcareous soils as a new adsorbent to remove lead from aqueous solution: equilibrium, kinetics and thermodynamic studies. Univ. J. Env. Res. Tech.. 2011;1(4):515-530.
- [Google Scholar]
- Biological aspects involved in the degradation of organic pollutions. J. Soil. Sci. Plant. Nutr.. 2010;10(3):244-267.
- [Google Scholar]
- Removal of some heavy metals from aqueous solution using biosorbent materials and nanoparticles. J. Adv. Agric. Reseaches 2016 21,198–224
- [Google Scholar]
- Fabrication of porous hydroxyapatite granules as an effective adsorbent for the removal of aqueous Pb(II) ions. Hindawi J. Chem.. 2019;2019:1-10.
- [Google Scholar]
- Removal of arsenic and copper from water solution using magnetic iron/bentonite nanoparticles (Fe3O4/bentonite) Silicon. 2019;11:961-971.
- [Google Scholar]
- Sorption of dissolved lead from shooting range soils using hydroxyapatite amendments synthesized from industrial byproducts as affected by varying pH conditions. J. Environ. Manage.. 2009;90:1782-1789.
- [Google Scholar]
- Fungal biodegradation of pesticide in soil and aquatic system. Rad. Res. Appl. Sci. 2014:87-94.
- [Google Scholar]
- Hend, H.G., Somia, B.A., Mohamed, A.O., Saeda, A.E., Eman, R.S., 2016. Dynamic removal of cumulative toxic heavy metals Pb(II) and Cd(II) from aqueous solutions via activated nano-sized bentonite adsorbents. Int. J. Contemporary Appl. Sci. 3, 67–82.
- Nanomaterials in analytical atomic spectrometry. Trac-Trends in Anal. Chem., roč.. 2012;39:38-59.
- [Google Scholar]
- Parametric study on degradation of fungicide carbendazim in dilute aqueous solutions using nano TiO2. J. Desalination Water Treatment. 2015;54(1):122-131.
- [Google Scholar]
- Synthesis, characterization of modified nanoadsorbents and its application in removal of Zn2+ ions from battery effluent. Environ. Chem. Ecotoxicol.. 2019;1:2-11.
- [Google Scholar]
- Application of algerian bentonite in the removal of cadmium (II) and chromium (VI) from aqueous solutions. J. Surf. Eng. Mater. Adv. Technol.. 2014;4:210-226.
- [Google Scholar]
- Adsorptive removal of Zn (II), Co(II) and their radioactive isotopes 65 Zn, 60 Co on the surface of sodium nano bentonite coated with oleyl-amine. J. Rad. Nucl. Appl.. 2017;2:87-93.
- [Google Scholar]
- Preparation nature nano-bentonite as adsorbent heavy metal Cd and Hg. J. Phys.: Conf. Series. 2018;1120:1-8.
- [Google Scholar]
- Immobilization of Pb and Cd in contaminated soil using nanocrystallite hydroxyapatite. Procedia Environ. Sci.. 2013;18:657-665.
- [Google Scholar]
- Matotte, L.S., 2007. IsoFit documentation and user’s guide. Version 1.2. State University of New York at Buffalo, Department of Civil, Structural and Environmental Engineering.
- Removal of nickel (II) from aqueous solutions by using nano-crystalline calcium hydroxyapatite. J. Saudi Chem. Soc.. 2011;15:105-112.
- [Google Scholar]
- Comparative of the removal of Pb+2, Cd+2 and Ni+2 by nano crystallite hydroxyapatite from aqueous solutions: Adsorption isotherm study. Arabian J. Chem.. 2012;5:439-446.
- [Google Scholar]
- Nanoscale materials and their use in water contaminants removal—a review. Environ. Sci. Pollut. Res.. 2013;20(3):1239-1260.
- [Google Scholar]
- Removal of lead ions using hydroxyapatite nano-material prepared from phosphogypsum waste. J. Saudi Chem. Soc.. 2016;20:357-365.
- [Google Scholar]
- An overview of adsorption technique for heavy metal removal from water/wastewater: a critical review. Int. J. Pure Appl. Sci.. 2017;3(2):10-19.
- [Google Scholar]
- Biosorption of lead, copper, and cadmium by Phanerochaetechrysosporiumin ternary metal mixtures: Statistical analysis of individual and interaction effects. Appl. Biochem. Biotechnol.. 2009;158:457-469.
- [Google Scholar]
- An insight into the selection of nano particle for removing contaminants in waste water. J. Eng. Res. Appl.. 2014;4(1):203-208.
- [Google Scholar]
- Activated bentonite as a lowcost adsorbent for the removal of Cu(II) and Pb(II) from aqueous solutions: Batch and column studies. J. Ind. Eng. Chem.. 2016;34:213-223.
- [Google Scholar]
- Raheem, S.S., Al-Dossary, M.A., AL-Saad, H.T., 2017. Determination of carbendazim fungicide and oxymatrine insecticide residues in the soils of four agriculture stations in basrah governorate by HPLC. J. Biol., Agriculture Healthcare. 7(22),10–16.
- Removal of arsenic and copper metals from contaminated water using iron (III) oxide nanoparticle. Int. J. Chem. Chem. Eng.. 2013;3:107-112.
- [Google Scholar]
- Exploitation of nano-bentonite, nano-halloysite and organically modified nano-montmorillonite as an adsorbent and coagulation aid for the removal of multi-pesticides from water: a sorption modelling approach. Water Air Soil Pollut.. 2015;226(41):1-14.
- [Google Scholar]
- Synthesis and characterization of bentonite nanocomposites from Egyptian Bentonite Clay. Int. J. Nanotechnol. Allied. Sci. 2017;1:16-26.
- [Google Scholar]
- Simona, L.I., Mikae, l.M., Regis, G., Mircea, B., Adrian, C., Daniela, P., 2018. Adsorption of Pb (II) Ions onto Hydroxyapatite Nanopowders in Aqueous Solutions. Materials, 11, 1–17.
- Comments on factors influencing the removal of divalent cations by hydroxyapatite. J. Hazard. Mater.. 2009;162:1588-1589.
- [Google Scholar]
- Effects of natural organic matter removal by integrated processes: alum coagulation and PAC-adsorption. Water Sci. Technol.. 2009;59(10):1951-1957.
- [Google Scholar]
- Biosorption of copper(II) and cobalt(II) from aqueous solutions by crab shell particles. Bioresour. Technol.. 2006;97:1411-1419.
- [Google Scholar]
- Waste water treatment by nanoceramics: Removal of lead particles. Int. J. Eng. Sci. Innovative Technol. (IJESIT).. 2014;3:324-329.
- [Google Scholar]
- Effects of low-molecular-weight organic acids on Cu(II) adsorption onto hydroxyapatite nanoparticles. J. Hazard. Mater.. 2009;162:1135-1140.
- [Google Scholar]
- Adsorption of Cd (II) and Zn (II) from aqueous solutions using magnetic hydroxyapatite nanoparticles as adsorbents. Chem. Eng. J.. 2010;162:487-494.
- [Google Scholar]
- Graphene/Fe3O4 nanocomposite for effective removal of ten triazole fungicides from water solution: Tebuconazole as an example for investigation of the adsorption mechanism by experimental and molecular docking study. J. Taiwan Inst. Chem. Eng.. 2019;95:635-642.
- [Google Scholar]







