Translate this page into:
Research on the regulatory mechanism of pore structure in soil matrix prepared by fluidized bed calcination of coal gangue
⁎Corresponding author at: Yulin University, No.51, Chongwen Road, Yulin, Shaanxi Province 719000, PR China. chenxiaodong@yulinu.edu.cn (Xiaodong Chen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
As a bulk industrial solid waste, coal gangue has caused serious pollution to the environment, and its application in the field of soil amendments has been widely studied, but the mechanism of pore structure regulation of coal gangue as soil substrate has not been reported in the literature. In this paper, the physical and chemical properties of coal gangue are analyzed, and its particle size, element content, and occurrence state are defined. The regulation mechanism of pores and pores in preparing matrix soil with coal gangue is revealed, and an efficient and energy-saving fluidizing activation technology is proposed to prepare active matrix soil. The results show that matrix soil with optimal pore structure can be obtained by fluidized bed calcination at 700 °C for 15 min, with a porosity of 55.0 %, volumetric water content of 28.7 %, and gas phase rate of 24.8 %. The technology also removes carbon, which is prone to natural fires, and fixes sulfur, which is good for plant growth. The formation and regulation mechanism of pores structure of soil matrix prepared by fluidized calcination of coal gangue is as follows: The oxidation reaction of carbon and the decomposition of minerals in coal gangue will form pore structures. Adjusting the reaction temperature and time, controlling the rate of carbon oxidation reaction, and the rate of mineral decomposition can achieve the goal of regulating the pore structure. Large pores with a pore size greater than 0.03 mm are formed by the oxidation of carbon and the decomposition of kaolinite. The pores with a diameter of 0.0001–0.03 mm are formed by the overflow of carbon dioxide gas, which is generated by the oxidation reaction of carbon embedded in coal gangue particles and the decomposition of calcium carbonate. The results of this study will provide technical and theoretical support for promoting the comprehensive utilization of coal gangue and improving environmental protection.
Keywords
Regulation mechanism
Coal gangue
Fluidized bed
Matrix soil
Pore structure
1 Introduction
Coal gangue is the solid waste produced during the mining of coal resources, accounting for about 10–20 % of the output of raw coal. According to statistics, the accumulated amount of coal gangue in China has reached more than 7 billion tons(Zhang and Ling, 2020; Wang et al., 2019; Chang et al., 2022). A considerable amount of coal gangue will bring many harms. For example, seizing land resources, the leaching water of coal gangue will pollute soil and groundwater, and the gas discharged by the spontaneous combustion of coal gangue will pollute the atmospheric environment. Coal gangue mountains will have geological hazards and so on(Wang and Niu, 2023; Wei and Sun, 2016; Li and Wang, 2019; Bi et al., 2021). However, coal gangue is also a resource for redevelopment and utilization. The utilization of coal gangue is now mainly used for power generation(Yang, 2020; Yan et al., 2016), subgrade materials(Liu et al.,2021), eco-friendly unburned bricks(Wang et al., 2021), production of building materials(Xu et al., 2017; Yang et al., 2023), underground backfilling (Li et al., 2020; Song et al., 2022), etc. From which the recovery of mineral products, the production of chemical products, road construction, and the application in agriculture are still less(Jia and Wu, 2019). At present, most coal enterprises in central and western China are located in the transition zone between the desert and Loess Plateau, and there are problems such as drought, water shortage, and desertification of land, which seriously affect the development of agriculture and forestry(Zhao et al., 2015). Coal gangue comes from the natural environment, so it has a high suitability for the environment. If it is used to make active matrix soil to repair mine land or desertification land, it can achieve waste treatment and in-situ utilization, and it can also obtain rich economic and environmental benefits.
The main application methods of coal gangue in agriculture are directly used as the filler of composite soil for land reclamation and improvement, and preparing silicon fertilizer(Zhang et al., 2023; Li et al., 2018; Ren et al., 2021). However, because coal gangue contains sulfur, aromatics, heterocyclic compounds, and a certain amount of harmful heavy metals, the direct application of coal gangue will cause pollution to water, air, and soil. Due to the dense structure of coal gangue, its ability to retain moisture and fertilizer is poor. Directly using it as soil may lead to soil erosion, soil compaction, and decreased soil thermal conductivity (Su, 2021; Kang and Louis, 2015). For most dry land crops, the suitable soil (hereinafter referred to as suitable soil) three-phase ratio is a solid phase ratio of about 50 %, volume water content of 25 % to 30 %, and gas phase ratio of 15 % to 25 %. If the gas phase ratio is too low, it will hinder soil aeration and inhibit plant roots and aerobic microbial activity. According to the characteristics and effects of different pores, the total porosity is about 50 %, the water-holding pores of 0.0001 mm-0.03 mm correspond to the soil's volume water content of 25–30 %, and the ventilation pores of > 0.03 mm correspond to the soil's gas phase of 15–25 %. Water-holding pores are designed to absorb the water required by plant growth and provide space for nutrients required by plants. Ventilation pores are mainly used for ventilation and drainage and provide space for plant root growth(Wang et al., 2021; Lv et al., 2022). The pore structure and channels of untreated coal gangue obviously cannot meet the requirements of water and fertilizer retention for plant growth. Therefore, coal gangue can not be directly used, and activation treatment is needed to increase the porosity of coal gangue and regulate the pore to meet the soil index requirements. So it is necessary to regulate the pore structure of coal gangue to make it meet the requirements of soil structure. Now some scholars have found that the temperature of coal gangue activation has a significant effect on the phase structure of coal gangue, and these studies make it possible to regulate the pore structure.
Currently, porous materials prepared from coal gangue cover all ranges of porosities, that is, macro-, meso-, and microporous materials(Li et al., 2023). Microporous(<2nm) and mesoporous(2–50 nm) materials are used for fine chemical and environmental applications, such as adsorption, membrane, catalysis, separation, energy storage, and bioengineering applications(Miao et al.,2022). The macroporous (>50 nm) materials include cement-based porous materials, porous bricks, porous geopolymers, porous glass–ceramics, porous ceramics(0.05–0.3 mm), aerogels, Highly porous glasses(4–400 nm), and other composite porous materials, which can be used as fire-resistant materials, building materials, catalyst carriers, optical instrument dehydrators, and solid–liquid separation membranes. The core indicators of these macroporous materials are specific surface area, compressive strength, and adsorption capacity. The soil matrix macroporous materials require water-holding pores of 0.0001 mm −0.03 mm and ventilation pores larger than 0.03 mm, and appropriate pore distribution is required. In general, there is much research on the comprehensive utilization and application of coal gangue in the field of soil improvement, but there is no literature report on the regulation mechanism of the pore structure of coal gangue as a soil matrix. In addition, The commonly used calcination equipment is the rotary kiln, which has some insurmountable drawbacks such as high energy consumption, poor temperature regulation, product “sintering”, incomplete activation, and limited output of a single unit (Huang and Xu, 2010). Therefore, to promote the technological progress of coal gangue as a soil conditioner, it is necessary to study the new technology and its theory in this field.
In this paper, the coal gangue in the southern margin of the Mu Us Desert of China was used as raw material, the physical and chemical characteristics of the coal gangue were studied and analyzed, and the particle size characteristics, mineral composition, element content and occurrence state of the coal gangue were determined. The regulation mechanism of pore channels and pores of coal gangue was revealed, and a new technology for preparing active matrix soil by regulating the pore structure of coal gangue with high efficiency and energy-saving fluidized activation was put forward. At the same time, a kind of matrix soil is obtained, which can be suitable for the soil structure required by the growth of most dry land crops, meet the needs of ventilation, water retention, and fertilizer retention for plant planting, and provide new technical support for the subsequent deployment of coal gangue based composite ecological soil for soil improvement in desert areas and restoration of coal and oil mines to improve the ecological environment.
2 Materials and methods
2.1 Materials
Coal gangue produced in Dahaize Coal Mine, Yulin City, Shaanxi Province, China, was used as raw material in all experiments in this paper, as shown in Fig. 1a. The raw material of coal gangue was a large block. Before the experiment, the coal gangue was crushed and screened with 100–200 mesh particles (Fig. 1b) for the fluidized bed calcination experiment.
Coal gangue raw materials, (a) block coal gangue, (b). 100–200 mesh coal gangue.
2.2 Experimental facility
The experiment was carried out with a small fluidized calcining device, as shown in Fig. 2. The device consists of a reactor, a heating furnace, a gas cylinder, and a gas path. The reactor adopts a quartz fluidized bed reactor. The device is used to carry out fluidized calcining of coal gangue at different times and temperatures to regulate pore size and pore distribution and optimize calcining conditions.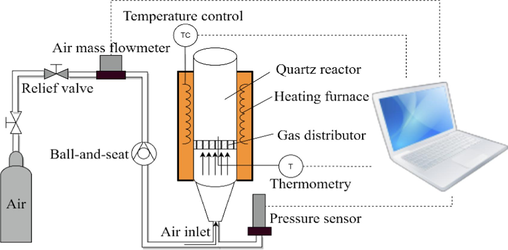
Small fluidized bed experimental equipment.
2.3 Analyze and test instruments
In this paper, a thermal field emission scanning electron microscope (SEM, JSM-7001F, Nippon Electronics Corporation) was used to observe the micromorphologies of different reaction products in the process of pore regulation of the sample. X-ray diffractometer (XRD, X'Pert PRO MPD, Panaco Analytical Instruments, Netherlands) Cu Kα radiation scanning range of 2θ = 5–90°, phase analysis of the sample. A powder tablet preparation method was used, the samples were pressed into tablets with boric acid, and then the content of elements and chemical components in the samples was determined by X-ray fluorescence spectrometer (XRF, AXIOS max model, Panaco Analytical Instruments, Netherlands). The sample was analyzed by TG-DTA6300, Seiko Co., LTD., to characterize the oxidation weight loss heat absorption, and release of the sample under an oxygen atmosphere. A carbon and sulfur analyzer (CS-2800G, Steel Yanak Testing Technology Co., LTD.) was used to determine the content of carbon and sulfur in the sample. Tungsten particle was used as the flux in the test, and all samples were tested three times, the measurement error was less than 0.1 %, and the average value was taken at last. The mercury intrusion method is used to determine the pore size distribution and porosity of the sample with a high-performance automatic mercury injection instrument (AutoPore Iv 9510, McMuritik Instruments, USA) with a test pressure of up to 60,000 Psia and a test range of 5 nm to 800 µm.
2.4 Preparation of active matrix soil
After crushing the lumpy coal gangue, 100–200 mesh particles are screened for a fluidized calcination experiment. Firstly, the small fluidized bed reactor is preheated to the required temperature(such as 500 °C, 800 °C, etc), the 40 g screened coal gangue particles are added to the quartz fluidized bed reactor (28 mm), and the fluidized calcination reaction of coal gangue is carried out under atmospheric pressure by injecting air at 3.0 L/min. After the reaction is completed, the air path is closed, the quartz fluidized bed reactor is removed, and the reaction product is removed after cooling to room temperature.
3 Results and discussions
3.1 Phase and composition analysis of coal gangue
The physical and chemical characteristics of coal gangue are discussed in this paper, and the mineral composition, element content, and occurrence state of coal gangue are clarified. The microscopic morphology of pulverized coal gangue presents dense and irregular particles, as shown in Fig. 3. Table 1 is the analysis of the main elements of coal gangue. It can be observed in the table that coal gangue is mainly composed of Si, Al, Ca, Fe, S, K, Ti, Na, Mg, and other elements, among which Si has a high content, accounting for 24.686 %. The second element is Al, accounting for 10.601 %. The contents of S, K, and Ca required for plant growth in coal gangue are 2.689 %, 2.134 %, and 6.06 %, which are significantly higher than those in soil, which are 0.085 %, 1.36 % and 1.37 % respectively(Huang and Xu, 2010). Fig. 4 displays the phase analysis of coal gangue. It can be seen that the main phases of coal gangue are quartz, kaolinite, and carbon, with dense structures and few pores, which are not conducive to water and fertilizer retention.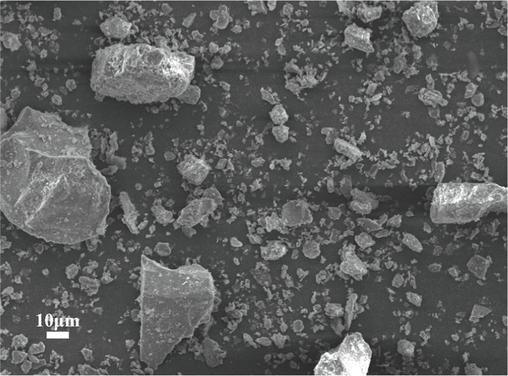
SEM diagram of coal gangue.
Serial number
Element
Content(wt%)
1
O
47.549
2
Si
24.686
3
Al
10.601
4
Ca
6.06
5
Fe
3.176
6
S
2.689
7
K
2.134
8
Ti
1.297
9
Na
0.811
10
Mg
0.770
11
Mn
0.075
12
Dy
0.053
13
P
0.037
14
Cr
0.018
15
Sr
0.016
16
Zr
0.008
17
Cu
0.007
18
Rb
0.007
19
Zn
0.006
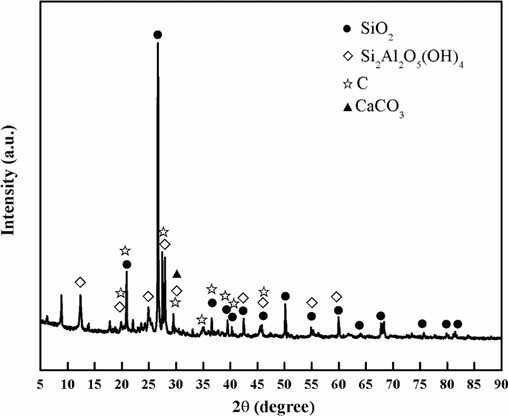
Phase analysis of coal gangue.
The temperature of coal gangue activation has an obvious influence on the phase structure of coal gangue(Gong et al., 2020; Luo et al., 2022; Meng et al., 2013; Jiao et al., 2022). To determine the basic conditions for fluidized calcination of coal gangue, thermogravimetric analysis of coal gangue under air atmosphere was carried out, and the results are shown in Fig. 5.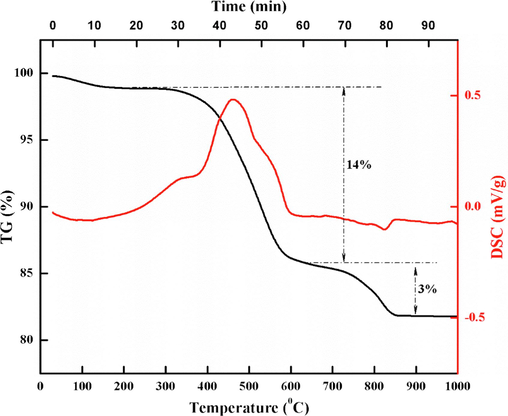
Thermogravimetric analysis of coal gangue in air atmosphere.
Coal gangue has two weight loss stages with the increase of temperature in the air atmosphere. The first weight-loss stage is 330–600 °C, the weight-loss rate is 14 %, and there is a large heat release peak. This stage is mainly the oxidation of carbon and part of organic matter in coal gangue, which releases a lot of heat. This weight loss stage is completed in about 30 min. The second weight-loss stage is 700–850 °C and completed within 15 min, corresponding to 3 % weight loss, accompanied by a small heat absorption peak. This stage is mainly the decomposition of kaolinite in coal gangue, resulting in the precipitation and volatilizing of structural water, and requires heat absorption to provide energy for the decomposition reaction, which is consistent with previous literature reports(Zhang et al., 2019; Platov et al., 2021). According to the reaction characteristics of coal gangue at different temperatures, the weight loss rate of coal gangue is the fastest at about 500 °C, and the weight loss is complete at 850 °C. Therefore, it can be preliminarily determined that the temperature of calcination of coal gangue in an air atmosphere to control pore activation is 500–850 °C, and the calcination time is 15 min-30 min.
3.2 Influence of calcination temperature on pore structure
According to the results of the thermal analysis of coal gangue, calcination conditions were further optimized, and a small fluidized calcination device was used to calcinate coal gangue at 500 °C, 600 °C, 700 °C, 750 °C, and 800 °C for 30 min, respectively, to study the effect of temperature on the pore structure of coal gangue after calcination.
The fluid calcination of coal gangue must first maintain the material in the fluid state during the calcination process, and pressure is one of the important parameters to reflect the fluid state. The pressure change curve of coal gangue during fluidized calcination at different temperatures is shown in Fig. 6(A). The coal gangue is calcined at 500 °C, 600 °C, and 700 °C for 30 min respectively.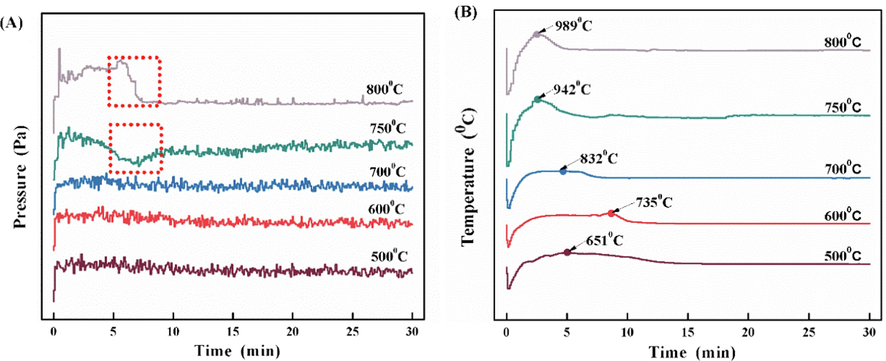
(A)Pressure change curve of coal gangue calcined at different temperatures for 30 min;(B) Temperature change curve of material during fluidized bed calcination of coal gangue for 30 min.
The pressure in the fluidized bed reactor fluctuates within a relatively stable range with the calcination time, indicating that the fluidization effect is good, and the samples produced by calcination have distinct particles without sintering (see Fig. 7a,b,c). However, at temperatures of The samples after calcination showed different degrees of caking (see Fig. 7d,e), indicating that.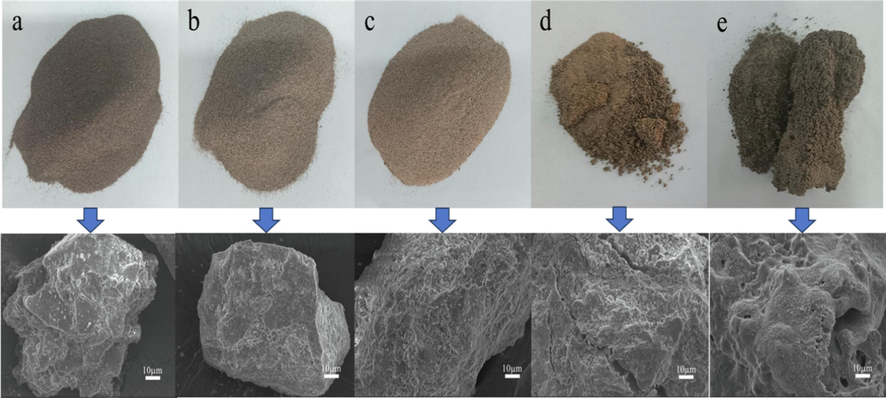
Sample and SEM morphology of coal gangue calcined at different temperatures for 30 min by fluidization(a. 500 °C;b. 600 °C;c. 700 °C;d. 750 °C;e. 800 °C).
the samples were sintered, resulting in fluidization gas drift and a short time drop in pressure. Coal gangue has two weight loss stages with the increase of temperature in the air atmosphere. The temperature change curve of the calcination process is further analyzed as shown in Fig. 6(B). With the extension of calcination time, the calcination temperature of all the samples showed a tendency of first dropping sharply, then rising, and then dropping to a stable state. This is due to the heat absorption process of coal gangue raw material added at room temperature in the quartz reactor, resulting in a sharp decrease in the temperature in the reactor. Then, as the carbon in the coal gangue began to oxidize and release heat, the temperature in the reactor increased, and a high-temperature zone was formed for a certain period. The temperature drops to stable after the carbon is completely oxidized. The high-temperature zone formed by the oxidation of carbon in coal gangue is gradually shortened with the increase of the set temperature, which is caused by the different oxidation rates of carbon in coal gangue at different temperatures. Because the higher the temperature is, the higher the oxidation rate is, and the shorter the oxidation time is, the shorter the duration of the high-temperature region is as the temperature increases. Fig. 7 also shows that the samples calcined at 750 °C and 800 °C for 30 min. All have different degrees of sintering, and the temperature change curves in Fig. 6(B) have sharp vertices, and the highest temperatures are 942 °C and 989 °C respectively. This shows that the carbon oxidation rate of coal gangue is accelerated, resulting in the high temperature of coal gangue particles in a short time, and sintering will be caused when the temperature in the material bed is greater than or equal to 942 °C. Therefore, sintering should be avoided when fluidized calcined coal gangue.
Pore distribution is the key to the preparation of active matrix soil. The pore distribution of samples calcined for 30 min at different temperatures was analyzed by mercury injection method, as shown in Fig. 8. The total porosity of coal gangue samples calcined at different temperatures did not change much, and all were above 50 %, which met the requirement of about 50 % of the total porosity of suitable soil. However, in meeting the index requirements of suitable soil, only the sample calcined at 700 °C for 30 min met the index requirements, with a gas phase ratio of 26.8 % and a volume moisture content of 31.5 %. The pore structure is also closest to the pore range of suitable soil, and the fluidization state is better. It shows that 700 °C is the appropriate temperature to control the fluid pore structure of coal gangue.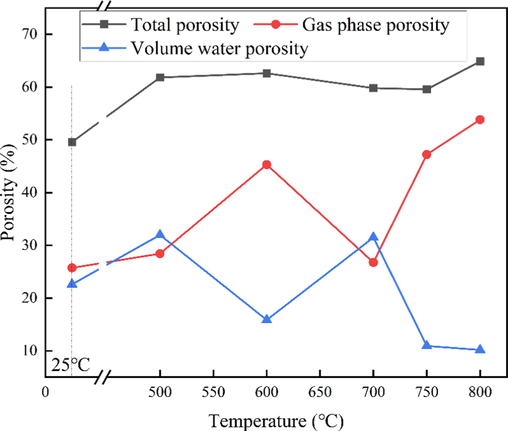
Pore distribution of coal gangue calcined at different temperatures for 30 min.
3.3 Effect of calcination time on pore structure
Based on optimizing the calcination temperature mentioned above, the calcination conditions were further optimized. The coal gangue was fluidized calcined at 700 °C for 15 min, 30 min, 45 min, 60 min, 75 min, and 90 min respectively, and the effect of calcination time on pore structure was studied. The pressure change curve of coal gangue in the process of fluidized calcination at 700 °C is shown in Fig. 9(A). After the sample was added, the pressure in the fluidized bed increased, and then the pressure fluctuated within a relatively stable range without sudden changes, which indicates that the coal gangue did not appear sintering phenomenon at different times of calcination at 700 °C, and the material in the reactor was in a good fluidization state.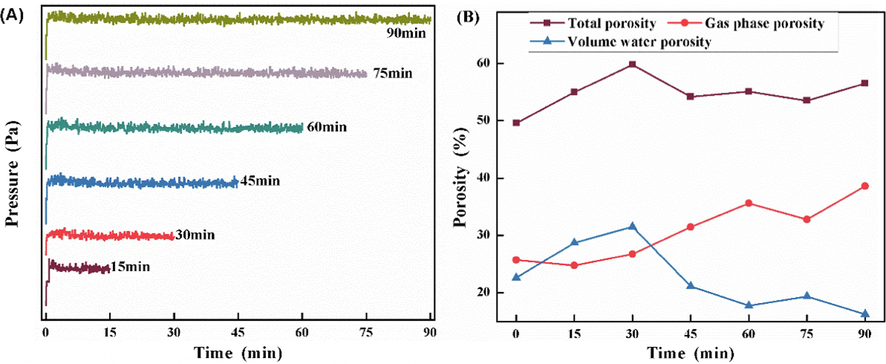
(A)Pressure change curve of coal gangue in the process of fluidized calcination at 700 °C;(B) Change curve of total porosity, gas phase, and solvent water content of coal gangue calcined at 700 °C with time.
The pore analysis results of samples calcined at 700 °C at different times are given in Fig. 9(B). With the extension of calcination time, the total porosity of coal gangue rose first and then decreased to a stable state, the gas phase continued to increase up to 38.6 %, and the volume water content showed a trend of first increasing and then decreasing. The total porosity of the samples calcined at 700 °C for different times is about 50 %, and the total porosity reaches the maximum of 59.8 % when calcined for 30 min. The porosity of coal gangue samples calcined at 700 °C at different times meets the total porosity requirement of suitable soil. The gas phase ratio is an index to evaluate ventilation pores, which is for soil ventilation, and drainage, and conducive to plant root growth. The gas phase ratio of suitable soil is usually 15–25 %, and cannot be less than 8–15 %. Depending on the test results in Fig. 9(B), the gas phase ratio of samples calcined for 15 min is 24.8 %, and that of samples calcined for 30 min is 26.8 %, which is in line with the gas phase ratio range of suitable soil. The volume of water content is an index to evaluate the volume of water content pores, which is extremely important for the preservation of plant growth water and water-soluble nutrients. In Fig. 9(B), the sample calcined for 30 min has a volume moisture content of 31.5 %, reaching the maximum volume moisture content at 700 °C, which is slightly greater than the volume moisture content range of suitable soil, while the sample calcined for 15 min has a gas phase ratio of 24.8 %, which is in line with the volume moisture content range of suitable soil. Can provide suitable water and water-soluble nutrient elements storage space. In summary, the active matrix soil prepared by fluidized calcination at 700 °C for 15 min has a good pore structure, which is suitable for preparing the soil, improving desertification, and providing a good soil environment for plant growth.
3.4 Changes of elements before and after calcination of coal gangue
Excessive heavy metal seriously endangers land security and has a serious impact on the application of coal gangue in agriculture and forestry. Coal gangue contains heavy metals such as Cr, Cu, and Zn. The content of heavy metal elements before and after the calcination of coal gangue according to the standard HJ780 test method is given in Table 2. The content of three heavy metals in the coal gangue before and after calcination is not much different, indicating that calcination has little effect on the content of heavy metals in the sample, and the content of three heavy metals in the coal gangue after calcination meets the GB15618-2018 standard for soil pollution risk control of agricultural land. Therefore, this kind of coal gangue can be used to prepare active matrix soil after adjusting pore structure by fluidized calcination.
Serial number
Element
Before calcination(mg/Kg)
After calcination(mg/Kg)
Risk screening value
(GB15618-2018) (mg/Kg)
1
Cr
180
110
250
2
Cu
70
80
150
3
Zn
60
80
200
3.5 Mechanism of pore formation in fluidized calcination of coal gangue
The pore formation mechanism of fluidized calcination of coal gangue was revealed in this paper. Fig. 10 shows the phase analysis of coal gangue and its calcination at different temperatures for 15 min. The main phases of coal gangue are quartz, kaolinite, and carbon, and a small amount of calcium carbonate. After calcination at 500 °C, the diffraction peak of carbon disappears first (reaction equation (1)). Coal gangue raw materials contain more kaolinite (Si2Al2O5(OH)4), but the diffraction peak of kaolinite can no longer be observed in the sample after calcination at 500 °C for 15 min. This shows that at 500 °C, the structure of kaolinite has been destroyed, kaolinite gradually out of the structural water at high temperature, to metakaolinite structure transformation (reaction equation (2)), metakaolinite further decomposed into amorphous SiO2 and Al2O3 (reaction equation (3)). In the process of coal gangue, kaolinite and other minerals dissolving structural water and forming amorphous substances will form pore structure. In Fig. 11, the total porosity and gas phase ratio increase simultaneously, indicating that a certain amount of porosity greater than 0.3 mm is formed by calcination oxidation of carbon at high temperatures and decomposition of kaolinite.
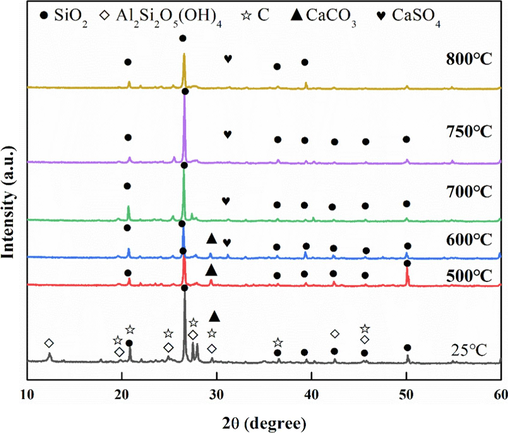
Phase analysis of coal gangue calcined at different temperatures for 15 min.
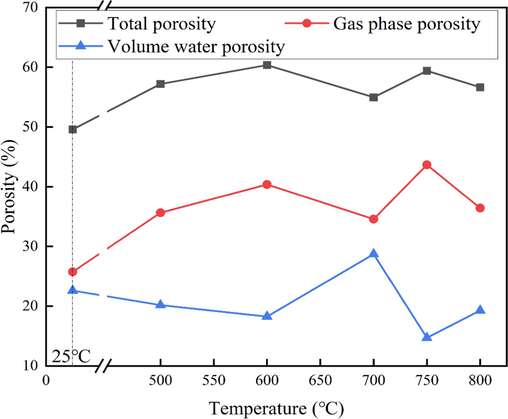
Pore distribution of coal gangue calcined at different temperatures for 15 min.
Samples calcined at 500 °C and 600 °C still have the diffraction peak of CaCO3. When the temperature increases to 700 °C, the diffraction peak of CaCO3 disappears, indicating the decomposition of CaCO3 (reaction equation (4)).
As can shown in Table 3, the carbon content of coal gangue is 9.39 %, and the remaining 1.37 % after calcination at 500 °C for 15 min. As the temperature rises, the carbon content gradually decreases, and when the temperature rises to 700 °C, the carbon has been fully oxidized and dropped to 0. As seen in Fig. 11, after calcination at 700 °C, the total porosity and gas phase ratio simultaneously decreased, while the volumetric water content increased. This shows that carbon oxidation embedded in coal gangue particles and decomposition of CaCO3 produce CO2 gas escape to form 0.0001–0.03 mm pore. It also demonstrates that the orderly and controlled oxidation of carbon in coal gangue and the decomposition of minerals will form pore structures. The key to pore structure regulation is to regulate the carbon oxidation rate and mineral decomposition rate, and the key to carbon oxidation rate and mineral decomposition rate is temperature, too low temperature can not completely oxidize carbon and completely decompose minerals, on the contrary, it will cause the rapid oxidation of carbon, and instantly release a lot of heat resulting in sintering between particles, which is not conducive to the formation of pore structure.
Temperature(°C)
C(wt%)
S(wt%)
25
9.49
3.95
500
1.37
0.43
600
0.81
0.93
700
0.00
2.47
750
0.00
1.77
800
0.00
1.78
According to the changes of sulfur content at different temperatures in Table 3, it is found that the sulfur content of coal gangue is 3.95 %, and the sulfur content only remains 0.43 % after calcination at 500 °C for 15 min, indicating that about 89 % of sulfur at this temperature oxidized to form SOx during the fluidized calcination process, which is also conducive to the formation of pore structure. With the increase of temperature, at 700 °C, the sulfur content in the calcined sample is 2.47 %, and the sulfur is fixed to the greatest extent, accounting for about 63 % of the sulfur content in the coal gangue raw materials. Depending on the phase analysis of the samples calcined at different temperatures in Fig. 12, it can be found that neither the original coal gangue nor the samples calcined at 500 °C have the diffraction peak of CaSO4, but when the temperature reaches 600 °C, the diffraction peak of CaSO4 appears, indicating that after the temperature rises, the combination of S element and Ca element forms CaSO4. CaSO4 is produced in the highest amount at 700 °C, and the S element is fixed to the greatest extent. Under this condition, the release of SOx with flue gas is reduced and the pressure of exhaust gas desulfurization is decreased. Downstream calcination at 700 °C can not only completely oxidize carbon to form a fine pore structure, but also fix S element to the greatest extent, providing nutrient elements for plant growth.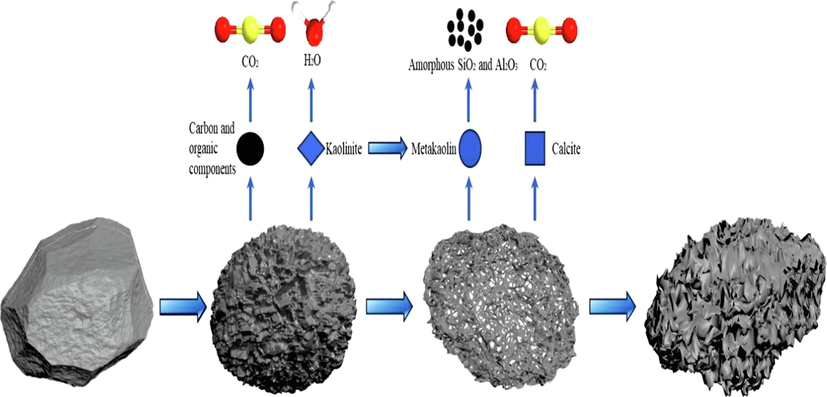
Schematic diagram of the formation mechanism of pore structure in coal gangue.
Based on the above analysis, pore regulation of coal gangue in the calcination process is mainly closely related to the following four reactions. Combined with the pore distribution of coal gangue in calcination for 15 min at different temperatures, reactions (1), (2), and (3) occur at 500 and 600 °C, and some large pores (>0.03 mm) will be formed. Increasing gas phase and total porosity. There are some small pore structures in coal gangue raw materials, but with the oxidation of carbon in the components, the small pores disappear, resulting in a decrease in volume water content during fluidized calcination at 500 and 600 °C. Reactions (3) and (4) occur when calcination is carried out at 700 °C, causing part of the macropores to transform into smaller pores, resulting in a decrease in the gas phase ratio and an increase in the available water porosity of the sample. When the temperature increases by 750 °C and 800 °C, the sample sintering is caused by flying temperature, and the small pores of the sample are reduced, mostly large pores, that is, compared with the pore structure at 700 °C, the gas phase ratio increases, but the volume water content decreases.
In summary, according to the formation process of coal gangue pores, the formation mechanism diagram of coal gangue pores is drawn, as shown in Fig. 12. Mechanism of pore formation in fluidized calcination of coal gangue: At a certain temperature, orderly and controllable oxidation of carbon in coal gangue and decomposition of CaCO3 minerals form pore structure, and control the oxidation rate of carbon and decomposition rate of minerals can achieve the purpose of regulating pore structure.
4 Conclusion
Turn waste into treasure, reduce the environmental pollution of coal gangue, the physical and chemical characteristics of coal gangue in the Daze mining area of Yulin City are studied and analyzed, and the particle size characteristics, mineral composition, element content and occurrence state of coal gangue are defined. The calcination conditions of high efficiency and energy-saving fluidized activated coal gangue to prepare active matrix soil were studied by using a small fluidized calcination device. The pore formation mechanism and control mechanism of coal gangue were revealed. The main conclusions are as follows:
The optimal pore structure can be obtained by fluidized calcination of coal gangue at 700 °C for 15 min. The total porosity is 55.0 %, the volumetric water content is 28.7 %, and the gas phase ratio is 24.8 %. The pore distribution of both is very similar to the pore structure of ‘suitable soil’.
The formation and regulation mechanism of pores structure of soil matrix prepared by fluidized calcination of coal gangue are as follows: The oxidation reaction of carbon and the decomposition of minerals in coal gangue will form pore structures. Adjusting the reaction temperature and time, controlling the rate of carbon oxidation reaction, and the rate of mineral decomposition can achieve the goal of regulating the pore structure. Large pores with a pore size greater than 0.03 mm are formed by the oxidation of carbon and the decomposition of kaolinite. The pores with a diameter of 0.0001–0.03 mm are formed by the overflow of carbon dioxide gas, which is generated by the oxidation reaction of carbon embedded in coal gangue particles and the decomposition of calcium carbonate.
Coal gangue at 700 °C down flow calcination can completely oxidize carbon to obtain the appropriate volume water content and can maximize the fixed sulfur element, accounting for about 63 % of the total sulfur content in coal gangue raw materials. After carbon removal by calcination, spontaneous combustion of coal gangue can be prevented and sulfur element fixed to provide soil fertility. Realize the utilization of solid waste resources and help improve the ecological environment. Moreover, Coal gangue has a natural advantage as a soil matrix due to its homology with local soil. The active soil matrix obtained from coal gangue calcination has a pore structure and some elements such as S, Ca, P, and Fe for plant growth. To become a soil suitable for plant growth. It is necessary to add organic and inorganic fertilizers.
Fluidized calcination of coal gangue, when the temperature in the reactor bed exceeds 942 °C, sintering will occur, resulting in a sudden drop in pressure during the fluidized process of coal gangue, greatly affecting the fluidization state, and then affecting the pore structure of the calcined sample.
CRediT authorship contribution statement
Meiju Zhang: Supervision, Validation, Writing – original draft. Zhan Qu: Data curation, Investigation, Writing – original draft. Mihui Xie: Data curation, Investigation. Xiaodong Chen: Funding acquisition, Supervision, Validation, Writing – original draft, Writing – review & editing. Yonglin Yang: Investigation, Writing – original draft, Funding acquisition. Yuxiao Bai: Investigation, Writing – original draft.
Acknowledgments
The authors express their gratitude to the National Natural Science Foundation of China (Grant 22268046), the Science and Technology Project of Yulin City (Grant CXY-2021-106-01), the Education Department of Shaanxi Province (Grant 22JS047)
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Pyrolysis characteristics, artificial neural network modeling, and environmental impact of coal gangue and biomass by TG-FTIR. Sci. Total Environ.. 2021;751:142293
- [Google Scholar]
- Current situation of the comprehensive utilization of coal gangue in China and the related problems and recommendations. China Environ. Protection Ind.. 2022;08:13-17.
- [Google Scholar]
- GB15618-2018 Soil environmental quality risk control standard for soil contamination of agricultural land. Beijing, China Environmental Science Press, 2018.
- Thermogravimetric analysis and kinetic characteristics of coal gangue under oxygen-rich atmosphere. Therm. Energy Power Eng.. 2020;35(10):94-102.
- [Google Scholar]
- Huang, C., Xu, J., 2010. Soil Science (3rd Ed.). Beijing: China Agriculture Press, 112-116.
- Properties and comprehensive utilization status of coal gangue resource. Coal Technol.. 2019;38(11):37-40. in Chinese
- [Google Scholar]
- Research progress on activation and mechanism of coal gangue. Appl. Chem. Ind.. 2022;51 2362–2366+2372 (in Chinese)
- [Google Scholar]
- Enhanced series-parallel model for estimating the time-dependent thermal conductivity of fly ash soil mixtures. Granul. Matter. 2015;17:579-592.
- [Google Scholar]
- Fabrication and application of porous materials made from coal gangue: A review. Int. J. Appl. Ceram. Technol.. 2023;20:2099-2124.
- [Google Scholar]
- Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod.. 2019;239:117946
- [Google Scholar]
- Literature overview of Chinese research in the field of better coal utilization. J. Clean. Prod.. 2018;185:959-980.
- [Google Scholar]
- Reutilisation of coal gangue and fly ash as underground backfill materials for surface subsidence control. J. Clean. Prod.. 2020;254:120113
- [Google Scholar]
- Effect of drying–wetting cycles on the hydromechanical behavior of compacted coal gangue. Environ. Geotech. 2021:1-11.
- [Google Scholar]
- Activation and evaluation of coal gangue. Shanxi Construct.. 2022;48:100-103. in Chinese
- [Google Scholar]
- Sustainable and clean utilization of coal gangue: activation and preparation of silicon fertilizer. J. Mater. Cycles Waste Manage.. 2022;24:1579-1590.
- [Google Scholar]
- Pyrolysis and combustion behavior of coal gangue in O 2 /CO 2 and O 2 /N 2 mixtures using thermogravimetric analysis and a drop tube furnace. Energy Fuels. 2013;27:2923-2932.
- [Google Scholar]
- Review of the fabrication and application of porous materials from silicon-rich industrial solid waste. Int. J. Miner. Metall. Mater.. 2022;29(3):424-438.
- [Google Scholar]
- Using decomposition of IR spectra to analyze structural-phase transformations of kaolinite. J. Appl. Spectrosc.. 2021;87:1029-1036.
- [Google Scholar]
- Research progress of coal gangue fertilizer. China Coal. 2021;47(1):103-109. in Chinese
- [Google Scholar]
- Study on preparation technology and properties of artificial soil based on coal gangue. Shanxi University; 2021.
- Synthesis and characterization of sustainable Eco-friendly unburned bricks from slate tailings. J. Mater. Res. Technol.. 2021;14:1697-1708.
- [Google Scholar]
- Research progress of coal gangue soil amendments. China Coal. 2021;47(12):49-56. in Chinese
- [Google Scholar]
- Summary of research on coal gangue: classification, harm, and comprehensive utilization. Chem. Miner. Process. 2023:1-9.
- [Google Scholar]
- Current situation research on resource utilization of coal gangue. Chem. Eng.. 2019;35(04) 68–69+63 (in Chinese)
- [Google Scholar]
- The Situation China Faces in Comprehensive Utilization of Coal Gangue and Tactful Research. China Land Resour. Econ.. 2016;29(04) 51–53+72 (in Chinese)
- [Google Scholar]
- Utilization of coal gangue for the production of brick. J. Mater. Cycles Waste Manag.. 2017;19:1270-1278.
- [Google Scholar]
- Emission characteristics of volatile organic compounds from coal-, coal gangue-, and biomass-fired power plants in China. Atmos. Environ.. 2016;143:261-269.
- [Google Scholar]
- Current situation analysis and prospect discussion on comprehensive utilization of coal resources for power generation. J. China Coal. 2020;46(10):67-74.
- [Google Scholar]
- Reviews of research on mechanical properties and durability of coal Gangue coarse aggregate concrete. Ind. Build.. 2023;53(01):212-222.
- [Google Scholar]
- Reactivity activation of waste coal gangue and its impact on the properties of cement-based materials – A review. Constr. Build. Mater.. 2020;234:117424
- [Google Scholar]
- Research progress on resource utilization of waste coal gangue. Environ. Chem.. 2023;43(6):1-14.
- [Google Scholar]
- Decomposition of key minerals in coal gangues during combustion in O2/N2 and O2/CO2 atmospheres. Appl. Therm. Eng.. 2019;148:977-983.
- [Google Scholar]
- Research and practice of ecological restoration technology in Mu Us Sandy Land Mining area in northern Shaanxi. Shaanxi: Northwest A&F University Press 221; 2015.







