Translate this page into:
Resveratrol-loaded copolymer nanoparticles with anti-neurological impairment, antioxidant and anti-inflammatory activities against cerebral ischemia–reperfusion injury
⁎Corresponding author. gushuo007@hainmc.edu.cn (Shuo Gu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
During cerebral ischemia/reperfusion (I/R) injury, oxidative stress and inflammation are major contributors to disability. Finding compounds that reduce oxidative stress, inflammation and strengthen the antioxidant defense system properties is one of the vital areas of research. Therefore, the present research aimed to evaluate the effects of resveratrol loaded in copolymer nanoparticles (RES.CP.NPs) on cerebral I/R injury in rats. After preparing and characterization RES.CP.NPs, these platforms were administered to cerebral I/R injured rats at a dose of 20 mg/kg after 2 h occlusion and the neurological disability scores were measured 24 h later. The content of malondialdehyde (MDA), glutathione (GSH), and catalase (CAT) activity as well as the expression levels of TNF-α, IL-10 and NF-κB-p65 in rat brain homogenates were measured. The RES.CP.NPs synthesized in the present study had a spherical structure with a zeta potential of −38 mV and a stable RES release for 72 h. Administration of RES.CP.NPs to I/R rats resulted in significant improvement in neurological disability score, decreased MDA levels, increased GSH content, increased CAT activity, decreased TNF-α expression, increased IL-10 expression, and decreased NF-κB-p65 expression (P < 0.0001). RES.CP.NPs can have neuroprotective effects in cerebral I/R conditions as a result of their strong antioxidant and anti-inflammatory activities.
Keywords
Resveratrol
Inflammation
Oxidative stress
Cytokine
Ischemia
Reperfusion
1 Introduction
According to the World Health Organization (WHO), stroke has been reported as the second leading cause of death in the world over the past decade (Schweizer et al., 2013). Blockage of cerebral blood flow occurs during Ischemia, which leads to the cessation of oxygen supply and nutrients to brain tissue cells in the ischemic region, activating a series of complex cellular destructive pathways that eventually lead to neuronal death and nerve tissue damage (Fagan et al., 2004; Maddahi and Edvinsson, 2010). Decreased blood flow to the brain, on the one hand, reduces the production of adenosine triphosphate (ATP) due to dysfunction of mitochondria, and on the other hand, by damaging this organ, it produces oxygen free radicals and peroxides the cell membrane lipids (Valko et al., 2007; Mohammadi et al., 2013). Overproduction of reactive oxygen species (ROS) during cerebral I/R is an important cause of nerve damage and the antioxidant defense system plays an important role in preventing this damage (Niizuma et al., 2009; Wang et al., 2010; Sharma et al., 2014). During brain ischemia, the overproduction of these free radicals occurs in the ischemic brain for a variety of reasons. The findings of various studies show that after cerebral I/R, the antioxidant defense system of this tissue and the scavenging of ROS is weakened (Lai et al., 2000). Accordingly, the scavenging of free radicals as well as strengthening of antioxidant defense system of brain tissue during cerebral I/R can reduce this type of damage.
Resveratrol (RES) is a polyphenol compound with extensive pharmacological properties due to its anti-inflammatory and antioxidant properties (Walle, 2011). This compound is abundant in grapes and red wine (Frémont, 2000). The neuroprotective effects of this compound have been reported in many studies and it has been suggested that RES pretreatment may lead to a reduction in cerebral I/R lesions (Wang et al., 2002; Yousuf et al., 2009). The mechanisms of neuroprotective effects of this antioxidant compound are attributed to reducing mitochondrial dysfunction, preventing neuronal death (Yousuf et al., 2009), inhibiting matrix metalloproteinase 9 (MMP-9) levels (Gao et al., 2006), increasing nitric oxide (NO) production (Tsai et al., 2007), activating adenosine monophosphate-activated protein kinase (AMPK) (Pineda-Ramírez et al., 2018), and upregulating Bcl-2 expression (Li et al., 2012).
There have been many advances in the development of natural product-loaded nanocarriers over time, and this has led to improved cures for many diseases (Zhang and Huang, 2012; Zorkina et al., 2020). These nanoformulations have improved loading, bioavailability, blood–brain barrier (BBB) passage, and drug uptake compared to non-NP counterparts (Santos et al., 2019). Also, the degradation of polyphenols such as RES in the liver reduces its plasma concentration (Walle, 2011) and the development of nanocarriers is a good option to protect it (Santos et al., 2019). Various nanoformulations such as liposomes, niosomes, dendrimers, NPs, nanoemulsion, metallic organic framework and gold NPs have been used for RES drug delivery to improve bioavailability and cellular absorption (Chopra et al., 2022). However, there are few studies on the application of CP.NPs for RES loading.
Therefore, the present research aimed to investigate the effects of the synthesized RES.CP.NPs on cerebral I/R injury in rats.
2 Materials and methods
2.1 Resveratrol loaded in copolymer nanoparticles preparation
All chemical were purchased from Sigma Company (USA), otherwise stated. In the present study, precipitation was used to prepare RES.CP.NPs. To prepare stock, 100 mg/L resveratrol (RES, Hongda Biotechnology Co., Ltd. (Linxia, China)) was mixed with 100 mg/L poly (Lactide-co-Glycolide) (PLGA) (Sigma, Cat#P2191, USA) in 60 mL acetone. In the next step, the solution was poured into 100 mL emulsifier solution containing 1 g/L tetradecyltrimethylammonium bromide (TTAB) and centrifuged for 30 min at 16000g. The solution was shaken on a magnetic stirrer plate (400 rpm) for 8 h and centrifuged again at 12000g for 30 min. Then, pellet NPs resuspended in deionized water and glucose as cryoprotectant was added and stored at −80 °C for further physiochemical evaluations.
2.2 Characterization of resveratrol loaded in copolymer nanoparticles (RES.CP.NPs)
The diameter, size and zeta potential of the RES.CP.NPs were determined using transmission electron microscopy (TEM, JOEL TEM machine) and dynamic light scattering (DLS, Model Nano ZS90, Malvern instruments, UK) analyses.
2.3 RES loading and release
Dialysis technique was used to determine the amount of RES.CP.NP loading and the absorption intensity at 306 nm were measured using a UV–Vis device (JascoV-570). The following formula was used to determine the encapsulation efficiency (EE):
The release of RES from NPs was measured by dynamic dialysis method in phosphate buffer solution (PBS) by using the following formula:
2.4 Animals and induction of cerebral I/R injury
Forty-five adult male Wistar rats (270–300 g) were purchased and kept at a standard temperature of 25 ± 2 °C in 12 h of light and dark cycle. Animals had free access to water and food, and the treatment of animals was in accordance with accepted standard guidelines for the care and use of animals in scientific research and animal ethical guidelines approved by Hainan Medical University, Haikou, Hannan, China.
To induce transient focal cerebral ischemia in animals we used a routine conventional method. Rats were initially anesthetized with 2.5 % isoflurane. After fixing the animal on the operating table, a sagittal incision was performed in the neck middle and the connective tissue and muscles were removed to allow easy access to the common carotid arteries. In ischemia groups, a temporary incision was made in the external carotid branch by temporarily closing the common carotid artery on the right side, and at the same time as closing the lower part, the nylon thread (No. 0–3), was entered the internal artery and from there gently into the skull and side. The Willis ring was guided to the beginning of the middle artery of the brain. By passing the tip of the thread through the beginning of the middle artery of the brain, there was little resistance in guiding (Mohammadi and Dehghani, 2014). After making sure it was in place, the nylon thread was fixed in place for 90 min. For reperfusion, the suture was gently removed from the artery and the wound was sutured by closing the external artery.
2.5 Animals grouping
The rats were divided as follows:
Group 1 (Control): The healthy rats without any treatment.
Group 2 (Sham): In this group, surgery was performed but no cerebral artery occlusion was performed without any treatments.
Group 3 (Cerebral I/R): transient focal cerebral ischemia was done in the rats without any treatment.
Group 4: Cerebral I/R rat’s tail injected with CP.NPs.
Group 5: Cerebral I/R rat’s tail injected with 20 mg/kg RES after 2-h occlusion.
Group 6: Cerebral I/R rat’s tail injected with 20 mg/kg RES.CP.NPs after 2-h occlusion.
2.6 Neurological score
24 h after ischemic induction, neurological disorders were blindly assessed using a 5-point test (Mohammadi et al., 2012). In this test, each animal was given a score of 1–5 as follows:
A score of 1 was given to animals that did not show any movement disorders.
A score of 2 was given to animals that flexed the opposite side of the ischemic hemisphere when hanging (Flexion).
A score 3 was given to an animal that spun toward the opposite of the ischemic hemisphere at the beginning of the movement on a flat surface.
Animals that lost the standing reflex were given a score of 4.
Finally, the animals that did not have any spontaneous movement were given a score of 5.
2.7 Biochemical parameters
2.7.1 Malondialdehyde (MDA)
At the end of the experiment, the animals were first anesthetized and then killed, and their brains were quickly removed. The tissue was then homogenized by a homogenizer (Polytron) in a ratio of 10 % (W/V) in PBS at pH 7.4 and stored in the freezer. The Placer method was used to measure the concentration of MDA (Placer et al., 1966). In this method, the reaction of thiobarbituric acid reactive substances (TBARS) with MDA to form a colored complex TBA-MDA was quantified spectrophotometrically at 532 nm.
2.7.2 Glutathione (GSH)
Tietz's method was used to measure brain GSH (Tietze, 1969). A suitable concentration of the homogenized sample was mixed with 10 μL of sulfosalicylic acid 5 % and centrifuged for 10 min at 4° C at 2000 rpm. Then, 100 μL of the supernatant was removed and 0.3 M was added to 810 μL of disodium phosphate. Then the reaction was started by adding 90 μL of dithiobis-nitrobenzoic acid (DTNB) reagent: 0.04 % solution in 0.1 % sodium citrate. Absorption at 412 nm was read over 5 min. Using 1 mg/mL GSH solution, the standard GSH curve was plotted and the GSH concentration was calculated in µM/mL. The standard GSH solution was prepared at concentrations of 200–25 μM.
2.7.3 Catalase (CAT)
Catalase (CAT) activity was determined by Genet et al. (2002) with slight modification. The final assay mixture in a final total volume of 1 mL contained 50 mM sodium phosphate buffer pH 7, 10 mM hydrogen peroxide and 20 μL of enzymatic extract. The change in light absorption was measured at 240 nm of the final assay mixture for 1 min at 25 °C compared to the blank solution. One unit of enzyme is the amount of enzyme that converts 1 µmol of hydrogen peroxide per minute to 1 mg of protein.
2.7.4 TNF-α and IL-10
The expression levels of TNF-α and IL-10 were analyzed by enzyme-linked immunosorbent assay (ELISA) using relevant kits (Invitrogen™, USA) based on the manufacturers' protocols.
2.8 Enzyme-linked immunosorbent assay (ELISA) assay
First, the brain homogenate protein content (described in Section 2.7.1) was determined using the BCA kit (Thermo, Pierce, USA) and then 60 μg were used for Enzyme-Linked immunosorbent assay (ELISA). NF-κB-p65 ELIZA kit (Mybiosource, Cat# MBS2505513, San Diego, USA) was used based on manufacturing instructions.
2.9 Statistical analysis
Results were presented as Mean ± SEM. One-way analysis of variance (ANOVA) and Tukey post hoc test were used to compare the obtained data between different groups. Also, non-parametric comparison method and Mann-Whitney U statistical test at a level of probability P < 0.05 were used to compare the data of neurological disorders.
3 Results
3.1 Characterization of RES.CP.NPs
The results of TEM showed that the prepared RES.CP.NPs had a spherical shape and a size of about 30 nm, which is in the appropriate range (Fig. 1a). Zeta potential was also determined using DLS analysis and the results showed that RES.CP.NPs had a zeta potential of about −38 mV (Fig. 1b). The examination of RES.CP.NPs with DLS showed a range size between 30 and 100 nm (Fig. 1c). Therefore, according to the results of zeta potential, it can be said that the prepared nanoformulation loading with RES has a good colloidal stability potential.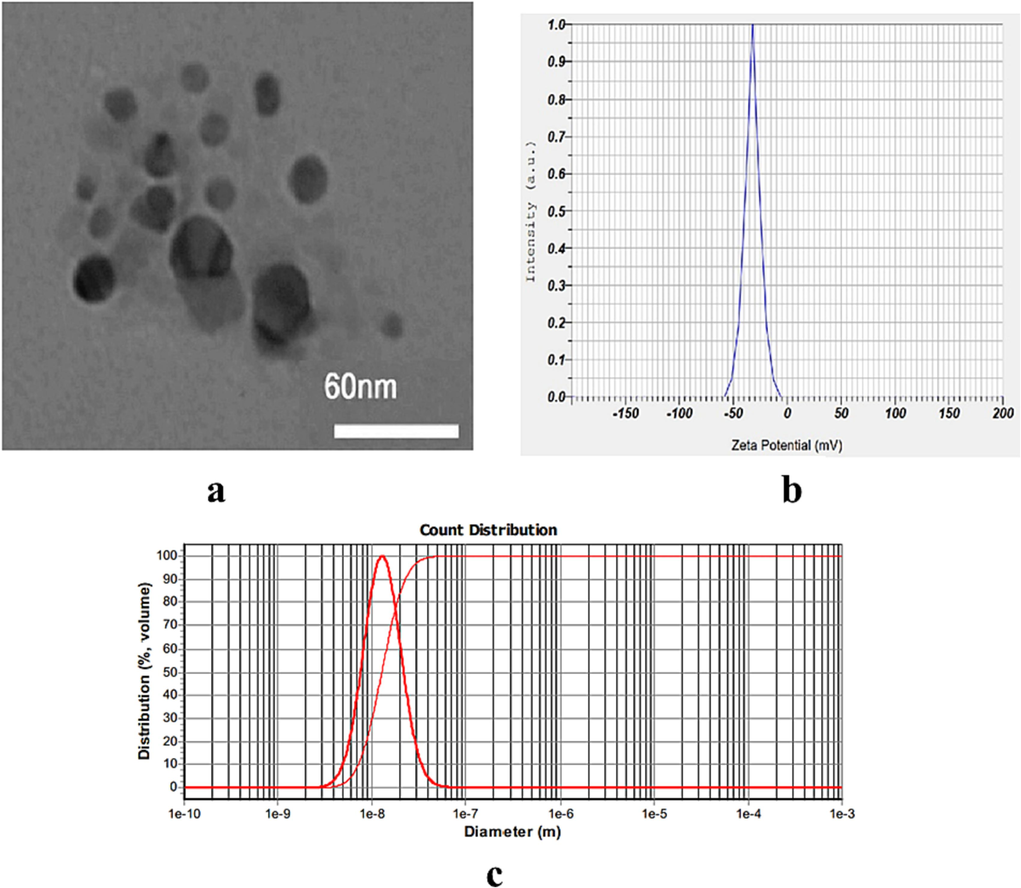
The characterization of resveratrol loaded in copolymer nanoparticles (RES.CP.NPs) morphology by TEM (a), Zeta potential (b) and particle size (c) .
3.2 RES loading and release
RES loading and EE values in the nanoformulated system were calculated and the results showed that RES.CP.NPs have a loading value of 59.5 % and an EE% of 93.6 % for RES. The RES concentration inside these NPs was then estimated to be about 3.13 μg/mg. The time-dependent drug release curves for RES and RES.CP.NPs are shown in Fig. 2. The slow release of RES from the nanoformulation was observed over a period of 72 h, indicating its stable release, which makes it a suitable system for drug delivery.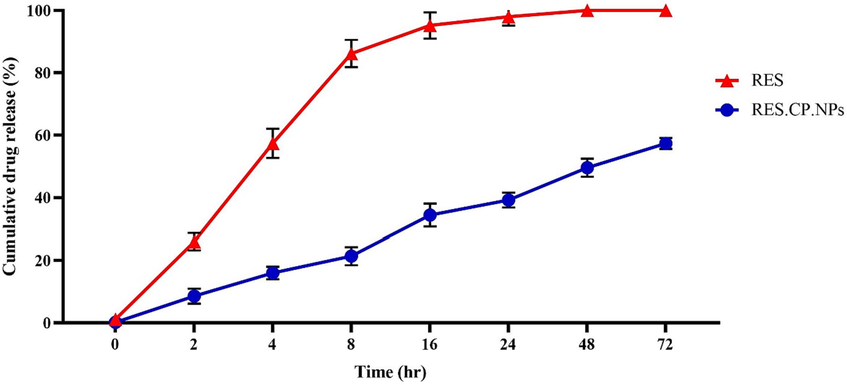
The time and drug release curves for RES and RES.CP.NPs at pH = 7.4.
3.3 Neurological score
Induction of cerebral ischemic reperfusion injury led to increased neurological disability score (NDS) in rats. Although RES mitigated the NDS, RES.CP.NPs significantly further reduced that compared with I/R rats (Fig. 3).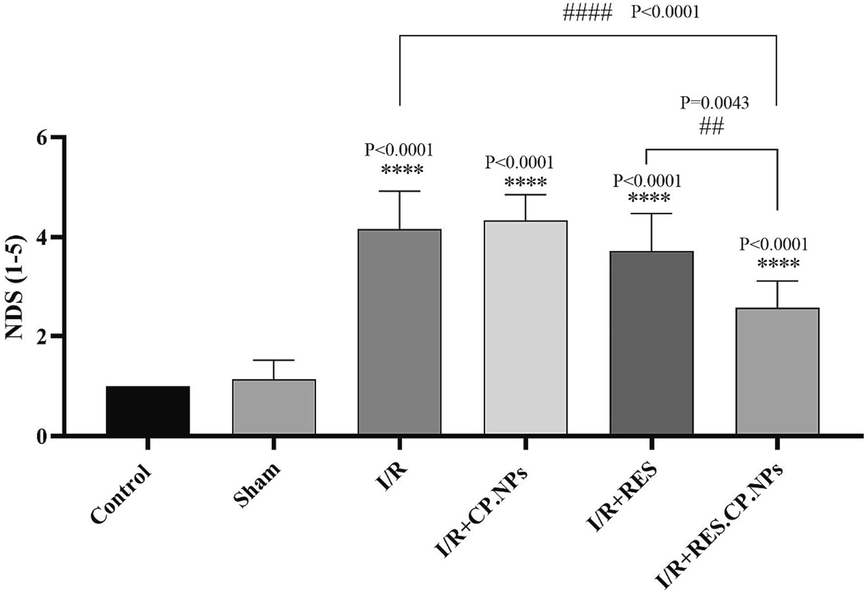
The effect of resveratrol (RES) and resveratrol loaded in copolymer nanoparticles (RES.CP.NPs) on the neurological scores in rats affected by induction of cerebral ischemic reperfusion (I/R) injury (n = 8). **** Indicates a significant difference at the probability level P < 0.0001 compared control; ## shows significant differences at a probability level of P < 0.01 between I/R + RES and I/R + RES.CP.NPs groups; #### shows significant differences at a probability level of P < 0.0001 between I/R + RES.CP.NPs and I/R groups.
3.4 Malondialdehyde (MDA), glutathione (GSH) and catalase (CAT)
Induction of cerebral I/R injury resulted in a significant increase in MDA (Fig. 4a), decreased GSH content (Fig. 4b) and CAT (Fig. 4c) enzyme activity compared to healthy control rats (P < 0.0001). Although RES resulted in a slight decrease in brain MDA content and a slight increase in CAT activity, RES.CP.NPs resulted in a significant decrease in MDA content and brain CAT activity compared to I/R rats. Also, brain GSH content in I/R rats receiving RES and RES.CP.NPs showed a significant increase compared to I/R rats, and this increase was stronger in rats receiving RES.CP.NPs (Fig. 4b). Thus, application of RES.CP.NPs resulted in a decrease in the cerebral lipid peroxidation, while led to an increase in the GSH content and brain CAT activity under cerebral I/R injury.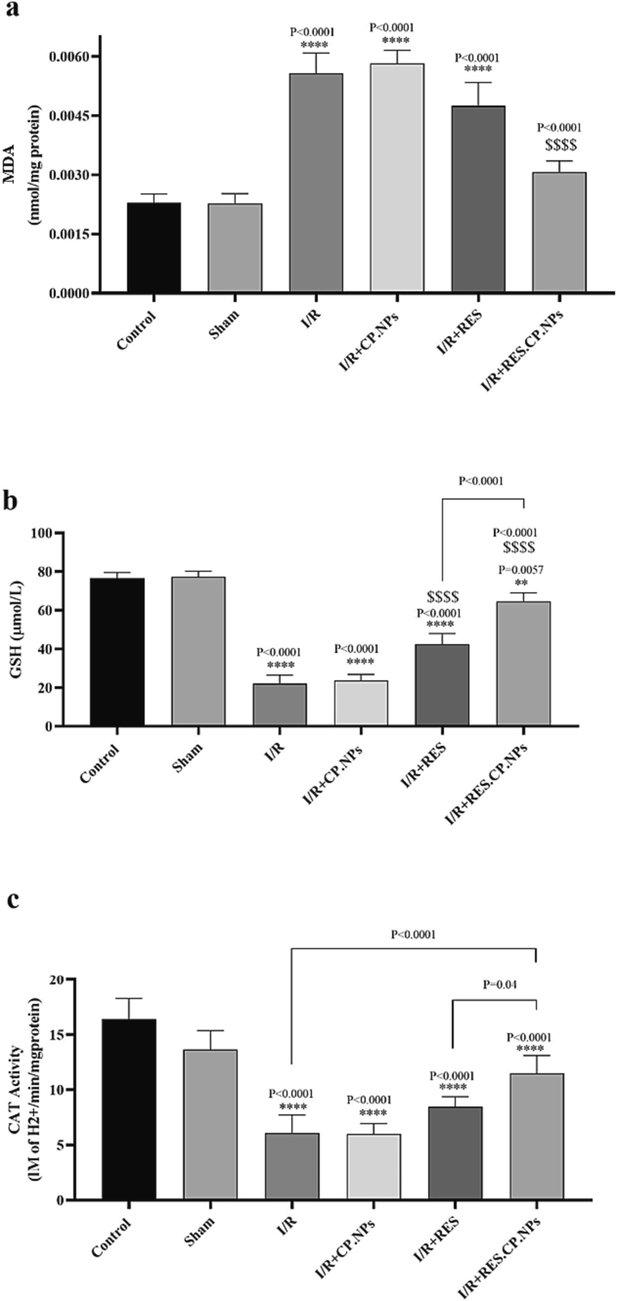
Effects of resveratrol (RES) and resveratrol loaded in copolymer nanoparticles (RES.CP.NPs) on brain malondialdehyde (MDA, a), glutathione (GSH) content (b) and catalase (CAT) activity (c) in rats under cerebral I/R injury conditions (n = 5). **** and $$$$ Indicates significant difference in the means at the probability level P < 0.0001 compared with healthy and I/R rats, respectively.
3.5 TNF-α and IL-10
Overexpression of the inflammatory cytokine TNF-α and downregulation of the cytokine IL-10 with induction of I/R damage were observed in rats, indicating a state of inflammation in their brain. However, RES.CP.NPs decreased TNF-α expression (Fig. 5a) and increased IL-10 (Fig. 5b) expression in I/R rats, which may indicate the anti-inflammatory effects of this compound. Comparison of the anti-inflammatory effects of RES and RES.CP.NPs in cerebral I/R indicated stronger anti-inflammatory effects of RES.CP.NPs.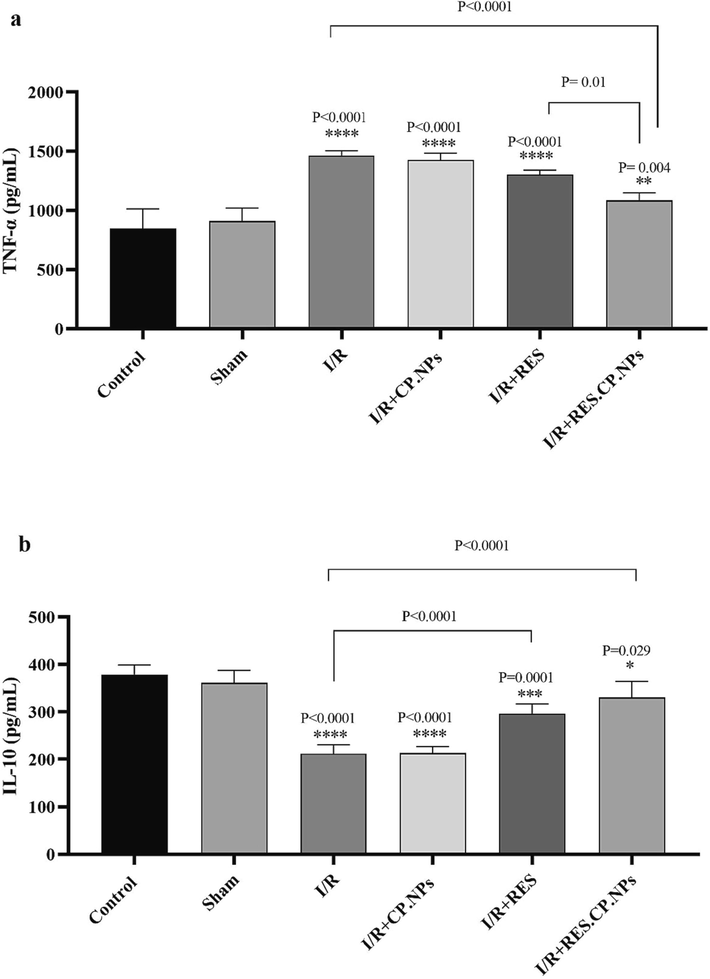
Effects of resveratrol (RES) and resveratrol loaded in copolymer particles (RES.CP.NPs) on Tumor necrosis factor α (TNF-α) (a) and interleukin 10 (IL-10) (b) in the brain of rats under cerebral I/R injury (n = 5). The symbol *, **, *** and **** indicates significant difference at probability levels of P < 0.05, P < 0.01, P < 0.001 and P < 0.0001, respectively, compared with healthy rats.
3.6 NF-κB-p65
Up-regulation of the NF-κB-p65 protein was observed at the time of induction of cerebral I/R injury in rats, which may indicate their inflammatory conditions in the brain. Nevertheless, RES.CP.NPs significantly decreased the regulation of this protein compared to I/R rats (P = 0.0006, Fig. 6). Therefore, it can be stated that one of the anti-inflammatory mechanisms of RES.CP.NPs are mediated by inhibition of NF-κB-p65 protein expression.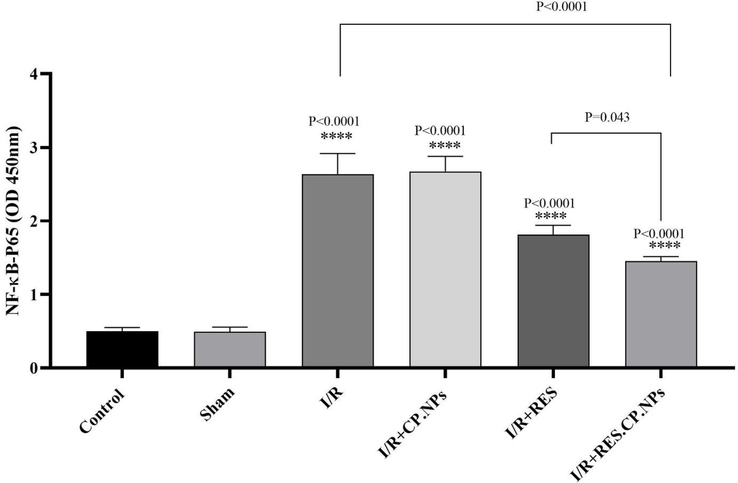
The effects of resveratrol (RES) and resveratrol loaded in copolymer nanoparticles (RES.CP.NPs) on the expression of NF-κB-p65 in rats in cerebral I/R injury (n = 5). **** shows significant differences at a probability level of P < 0.0001 compared with healthy rats. # shows significant differences at a probability level of P < 0.05 between I/R + RES and I/R + RES.CP.NPs groups; #### shows significant differences at a probability level of P < 0.0001 between I/R + RES.CP.NPs and I/R groups.
4 Discussion
The results of the present study showed that RES.CP.NPs could have neuroprotective effects in cerebral I/R conditions due to their antioxidant and anti-inflammatory effects, and these effects were stronger than free RES.
In the present study, the EE value of RES.CP.NPs was calculated as 93.6 %, which indicates a high EE and is suitable for drug delivery. Pandita et al., used solid lipid NPs (SLN) for RES drug delivery and the results indicated 88.9 % EE (Pandita et al., 2014). While, the EE value of RES loaded in lipid NPs was reported to be 70 % by Neves et al., (2013). Therefore, the CP.NPs used in the present research had a higher EE value than the aforementioned studies, which indicates the appropriateness of their design.
The first treatment strategies for stroke are to remove the blockage caused by the clot. After the blockage is removed, blood flow is restored to the ischemic region, although it appears to reduce the severity of ischemic damage by delivering cellular metabolites and removing excess waste, extensive cellular destructive pathways and signals are activated in this area, leading to reperfusion damage (Posada-Duque et al., 2014). In the present study, induction of cerebral I/R resulted in high oxidative stress in the ischemic region, in parallel with which these changes led to widespread neuromotor disorders in animals. One of the destructive pathways that plays a key role in reperfusion injuries after ischemia is the production of oxygen-free radicals (Bozkurt et al., 2014). Increased production of free radicals occurs in the ischemic hemisphere for a variety of reasons. The results of studies have shown that after cerebral ischemia, the brain's antioxidant defense system is weakened and the scavenging of oxygen-free radicals produced in the reperfusion phase is impaired (Deng et al., 2000). Antioxidant enzymes are among the most important enzymes that their activities/contents are reduced following cerebral I/R (Sinha et al., 2001). In the present study, an increase in MDA content was observed after induction of cerebral I/R damage in rats, which indicates damage to the membrane of neurons. In fact, lipids in brain tissue are more exposed to peroxidation compared to other tissues due to their high content of unsaturated fatty acids as well as high oxygen consumption and low activity of antioxidant enzymes (Faruk et al., 2010; Kakkar et al., 2013). Finally, the combination of these radicals with nitrogen-free radicals creates a very dangerous compound in the ischemic region (peroxynitrite), which is the most important cause of tissue necrosis and stimulation of programmed cell death (Sharipov and Kotsiuruba, 2014). The findings of the present study showed that the use of RES and RES.CP.NPs could improve neuromotor disorders and also reduce the MDA of brain tissue, indicating the neuroprotective effects of these compounds, which is consistent with the findings of other studies (Li et al., 2011; Aktaş et al., 2019). Nevertheless, the comparison of the neuroprotective effects of RES and RES.CP.NPs indicated the stronger effects of RES.CP.NPs compared to RES, which can attribute to the slow and sustained release of RES, increase its bioavailability and cross the BBB. The slow and sustained release of RES from other NPs including chitosan NPs (Yao et al., 2006), functionalized mesoporous silica NPs (Chaudhary et al., 2019) and Bovine serum albumin-based NPs (Fonseca et al., 2017) have been reported, which is in line with the current research findings.
Some clinical studies have shown that an increased risk of stroke is associated with lower levels of GSH in the brain and that GSH levels decrease following an increase in oxidative stress factors in ischemic brain tissue (Demirkaya et al., 2001; Cojocaru et al., 2013). GSH neutralizes and eliminates free radicals and their destructive effects by reducing free radicals by thiols, and has protective effects against cerebrovascular endothelial dysfunction and cell death (Takahashi et al., 2010). The results of the present study showed that RES and RES.CP.NPs have the ability to increase GSH content in the brains of ischemic rats, indicating their neuroprotective effects. CAT is one of the most important enzymes in the body's defense system against oxygen-free radicals, which reduces their destructive effects by converting hydrogen peroxide to water and oxygen. According to some studies, CAT prevents ROS destructive effects by removing hydrogen peroxide (Sepasi Tehrani and Moosavi-Movahedi, 2018). In the present study, a decrease in CAT enzyme activity weakened the antioxidant system of the brain following I/R conditions, which could lead to the destruction of brain tissue and increased brain damage. However, RES and RES.CP.NPs increased CAT activity, indicating the potentiating effects of these compounds on the brain's antioxidant defense system under cerebral I/R injury.
In the present study, it was observed that in I/R rats, the inflammatory cytokine TNF-α was upregulated and the anti-inflammatory cytokine IL-10 was downregulated. However, RES and RES.CP.NPs were able to largely prevent the decrease in IL-10 expression and the increase in TNF-α. This indicates that RES and especially RES.CP.NPs have anti-inflammatory effects and one of the neuroprotective mechanisms of these compounds is their anti-inflammatory effects. The anti-inflammatory effects of RES have been reported in other studies (Orsu et al., 2013; Prabhakar, 2013; Fang et al., 2015), which is consistent with current research findings. The results of studies have shown that inflammation due to cerebral I/R injury leads to neuronal death (Sieber et al., 2013). Therefore, finding compounds with anti-inflammatory effects can be a good treatment option in I/R conditions and prevent neuronal death. One of the key proteins in the inflammatory pathway is NF-κB, which enters the nucleus and activates the important inflammatory cytokine genes such as TNF-α (Maddahi and Edvinsson, 2010; Yasuda et al., 2011). Upregulation of both NF-κB and TNF-α in cerebral I/R conditions was observed in the present study, which is consistent with the findings of other studies (Wang et al., 2017; Chen et al., 2018). However, RES.CP.NPs significantly prevented the expression of these two proteins involved in the inflammatory response, indicating the anti-inflammatory effects of this compound. Increased expression of these proteins has been shown to be associated with severe impairment of BBB function and cerebral edema (Maddahi et al., 2011). Thus, RES.CP.NPs have shown neuroprotective effects in the present study by down-regulating NF-κB and TNF-α and thus preventing these serious brain injuries. Clinical research is recommended to evaluate the effects of RES.CP.NPs in cerebral I/R patients.
From the point of view of safety, CP.NPs have been proposed as one of the best forms of NPs for drug delivery, and so far, there is no report on the toxicity effects of these NPs. Therefore, CP.NPs have a high potential in drug delivery, and in future studies, the development of drugs-loaded CP.NPs, which are designed in the present study, are recommended for drug delivery of various types of drugs or other natural products. Nevertheless, the current research has limitations such as the study on an animal model and requires studies on human samples for recommendations in clinical conditions. Also, it is recommended for future studies to study the ability of this nanoformulation to cross the BBB and its pharmacokinetics in the brain in the cerebral I/R damage or other brain diseases including Parkinson's disease or Alzheimer's disease.
5 Conclusion
The RES.CP.NPS developed in the present research had suitable physicochemical properties that make it suitable for pharmacological applications. Also, this nanoformulation, when administered to cerebral I/R rats, showed stronger protective effects than free RES and led to the improvement of neurological disorders, reduction of brain lipid peroxidation, improvement of the antioxidant defense capacity of neurons, and reduction of inflammatory cytokine levels. In general, it was concluded that RES.CP.NPs aveh stronger neuroprotective effects in cerebral I/R injury than free RES. These effects are mediated by the strong antioxidant and anti-inflammatory effects of this compound.
Funding
This study was supported by Hainan Province Clinical Medical Center (NO. QWYH202175, 2021 Hainan Natural Science Foundation High Level Talent Project (NO. 821RC1133), and Finance Science and Technology Project of Hainan Province (NO. ZDYF2020225).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Protective effects of resveratrol on hepatic ischemia reperfusion injury in streptozotocin-induced diabetic rats. Mol. Cell. Biochem.. 2019;460(1):217-224.
- [Google Scholar]
- Syringaldehyde exerts neuroprotective effect on cerebral ischemia injury in rats through anti-oxidative and anti-apoptotic properties. Neural Regen. Res.. 2014;9(21):1884-1890.
- [Google Scholar]
- Encapsulation and controlled release of resveratrol within functionalized mesoporous silica nanoparticles for prostate cancer therapy. Front. Bioeng. Biotechnol.. 2019;7
- [Google Scholar]
- Plumbagin inhibits neuronal apoptosis, intimal hyperplasia and also suppresses TNF-α/NF-κB pathway induced inflammation and matrix metalloproteinase-2/9 expression in rat cerebral ischemia. Saudi J. Biol. Sci.. 2018;25(6):1033-1039.
- [Google Scholar]
- Emerging trends in the delivery of resveratrol by nanostructures: applications of nanotechnology in life sciences. J. Nanomater.. 2022;2022:1-17.
- [Google Scholar]
- Evaluation of oxidative stress in patients with acute ischemic stroke. Roman. J. Internal Med. = Revue roumaine de medecine interne. 2013;51(2):97-106.
- [Google Scholar]
- Malondialdehyde, glutathione peroxidase and superoxide dismutase in peripheral blood erythrocytes of patients with acute cerebral ischemia. Eur. J. Neurol.. 2001;8(1):43-51.
- [Google Scholar]
- Antagonistic effect of 3,6-dimethamidodibenzopyriodonium gluconate on lipid peroxidation in cerebral cortical neuronal cultures and rat brains during focal cerebral ischemia reperfusion. Acta Pharmacol. Sin.. 2000;21(5):460-462.
- [Google Scholar]
- Targets for vascular protection after acute ischemic stroke. Stroke. 2004;35(9):2220-2225.
- [Google Scholar]
- Resveratrol alleviates nerve injury after cerebral ischemia and reperfusion in mice by inhibiting inflammation and apoptosis. Int. J. Clin. Exp. Med.. 2015;8(3):3219-3226.
- [Google Scholar]
- Neuroprotective effects of postconditioning on lipid peroxidation and apoptosis after focal cerebral ischemia/reperfusion injury in rats. Turk. Neurosurg.. 2010;20(1)
- [Google Scholar]
- Bovine serum albumin-based nanoparticles containing resveratrol: Characterization and antioxidant activity. J. Drug Delivery Sci. Technol.. 2017;39:147-155.
- [Google Scholar]
- Resveratrol reduces the elevated level of MMP-9 induced by cerebral ischemia–reperfusion in mice. Life Sci.. 2006;78(22):2564-2570.
- [Google Scholar]
- Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: Effect of vanadate and fenugreek (Trigonella foenum graecum) Mol. Cell. Biochem.. 2002;236(1):7-12.
- [Google Scholar]
- Curcumin loaded solid lipid nanoparticles: An efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur. J. Pharm. Biopharm.. 2013;85(3,Part A):339-345.
- [Google Scholar]
- Free radical scavenging activity of fullerenol on grafts after small bowel transplantation in dogs. Transpl. Proc. 2000
- [Google Scholar]
- Resveratrol attenuates brain damage in a rat model of focal cerebral ischemia via up-regulation of hippocampal Bcl-2. Brain Res.. 2012;1450:116-124.
- [Google Scholar]
- Neuroprotective effects of resveratrol on ischemic injury mediated by improving brain energy metabolism and alleviating oxidative stress in rats. Neuropharmacology. 2011;60(2):252-258.
- [Google Scholar]
- Cerebral ischemia induces microvascular pro-inflammatory cytokine expression via the MEK/ERK pathway. J. Neuroinflammation. 2010;7(1):14.
- [Google Scholar]
- The role of tumor necrosis factor-α and TNF-α receptors in cerebral arteries following cerebral ischemia in rat. J. Neuroinflammation. 2011;8(1):107.
- [Google Scholar]
- Effects of atorvastatin on the hypertension-induced oxidative stress in the rat brain. Iran. Biomed. J.. 2013;17(3):152-157.
- [Google Scholar]
- Mohammadi, M.T., Dehghani, G.A., 2014. Evaluation of cerebral blood flow autoregulation during early phase of reperfusion in rat model of transient focal ischemia.
- Contribution of nitric oxide synthase (NOS) in blood–brain barrier disruption during acute focal cerebral ischemia in normal rat. Pathophysiology. 2012;19(1):13-20.
- [Google Scholar]
- Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomed.. 2013;8:177-187.
- [Google Scholar]
- Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J. Neurochem.. 2009;109(s1):133-138.
- [Google Scholar]
- Cerebroprotective potential of resveratrol through anti-oxidant and anti-inflammatory mechanisms in rats. J. Neural Transm.. 2013;120(8):1217-1223.
- [Google Scholar]
- Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Res. Int.. 2014;62:1165-1174.
- [Google Scholar]
- Current evidence for AMPK activation involvement on resveratrol-induced neuroprotection in cerebral ischemia. Nutr. Neurosci.. 2018;21(4):229-247.
- [Google Scholar]
- Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem.. 1966;16(2):359-364.
- [Google Scholar]
- Protection after stroke: cellular effectors of neurovascular unit integrity. Front. Cell. Neurosci.. 2014;8
- [Google Scholar]
- Cerebroprotective effect of resveratrol through antioxidant and anti-inflammatory effects in diabetic rats. Naunyn Schmiedebergs Arch. Pharmacol.. 2013;386(8):705-710.
- [Google Scholar]
- Nanotechnology-based formulations for resveratrol delivery: Effects on resveratrol in vivo bioavailability and bioactivity. Colloids Surf. B Biointerfaces. 2019;180:127-140.
- [Google Scholar]
- Epigenetic mechanisms in cerebral ischemia. J. Cereb. Blood Flow Metab.. 2013;33(9):1335-1346.
- [Google Scholar]
- Induction of nitrosative stress in mitochondria of rats hearts in experimental ischemia-reperfusion of the brain and its correction by ecdysterone. Fiziolohichnyi zhurnal (Kiev, Ukraine: 1994). 2014;60(5):3-13.
- [Google Scholar]
- Solasodine protects rat brain against ischemia/reperfusion injury through its antioxidant activity. Eur. J. Pharmacol.. 2014;725:40-46.
- [Google Scholar]
- Attenuated inflammatory response in triggering receptor expressed on myeloid cells 2 (TREM2) knock-out mice following stroke. PLoS One. 2013;8(1):e52982.
- [Google Scholar]
- Effect of melatonin on ischemia reperfusion injury induced by middle cerebral artery occlusion in rats. Eur. J. Pharmacol.. 2001;428(2):185-192.
- [Google Scholar]
- Buthionine sulfoximine promotes methylglyoxal-induced apoptotic cell death and oxidative stress in endothelial cells. Biol. Pharm. Bull.. 2010;33(4):556-560.
- [Google Scholar]
- Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem.. 1969;27(3):502-522.
- [Google Scholar]
- Resveratrol neuroprotective effects during focal cerebral ischemia injury via nitric oxide mechanism in rats. J. Vasc. Surg.. 2007;46(2):346-353.
- [Google Scholar]
- Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol.. 2007;39(1):44-84.
- [Google Scholar]
- Orientin attenuates cerebral ischemia/reperfusion injury in rat model through the AQP-4 and TLR4/NF-κB/TNF-α signaling pathway. J. Stroke Cerebrovasc. Dis.. 2017;26(10):2199-2214.
- [Google Scholar]
- Shikonin protects mouse brain against cerebral ischemia/reperfusion injury through its antioxidant activity. Eur. J. Pharmacol.. 2010;643(2):211-217.
- [Google Scholar]
- Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res.. 2002;958(2):439-447.
- [Google Scholar]
- Study on the preparation of resveratrol chitosan nanoparticles with free amino groups on the surface. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chinese Mater. Med.. 2006;31(3):205-208.
- [Google Scholar]
- Temporal and sequential changes of glial cells and cytokine expression during neuronal degeneration after transient global ischemia in rats. J. Neuroinflammation. 2011;8(1):1-10.
- [Google Scholar]
- Resveratrol exerts its neuroprotective effect by modulating mitochondrial dysfunctions and associated cell death during cerebral ischemia. Brain Res.. 2009;1250:242-253.
- [Google Scholar]
- Micro- and nano-carrier mediated intra-articular drug delivery systems for the treatment of osteoarthritis. J. Nanotechnol.. 2012;2012:748909
- [Google Scholar]
- Nano carrier drug delivery systems for the treatment of neuropsychiatric disorders: advantages and limitations. Molecules. 2020;25(22)
- [Google Scholar]







