Translate this page into:
Review of different CdS/TiO2 and WO3/ g-C3N4 composite based photocatalyst for hydrogen production
⁎Corresponding author. zainashfaq147@gmail.com (Zain Ashfaq)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
The production of hydrogen from water is one of the best sustainable energy resources to fulfill the increasing energy deficiency of the world. As most of the available energy resources are harmful in one way or another as nuclear waste is dangerous for human life and burning fossil fuels caused the production of carbon, nitrogen, and Sulphur oxides which increases global warming as well as air pollution. The earth is almost 70% consist of water so that there is need to use this water in efficient way to produce hydrogen as it is more sustainable energy resource and environment friendly. This is an emerging field for researchers and they trying to find the best photocatalyst for hydrogen production. The motivation of this study is to provide improved mechanism for hydrogen production because the developing countries like Pakistan faced serious energy crisis and available energy resources in these countries are the fossil fuels mostly which are not environmentally friendly creating chaos of global warming. The background of this study shows that researcher used doped, composite, and many other different techniques to enhance its efficiency. For the last decade, researchers mainly focused on the doped photocatalyst. In recent decade researcher shifted to composite based photocatalysts. The hypothetical question arises in this study weather these composite useful to improve efficiency of hydrogen production by using photocatalyst method? This review paper especially focuses on different composite photocatalysts for hydrogen production. The composites of Cadmium Sulfite/Titanium dioxide (CdS/TiO2) and Tungsten trioxide/graphite Carbon nitride (WO3/ g-C3N4) have been the main area of interest in this review due to their immense use in research to produce hydrogen. This study will also help to understand the mechanism of the production of hydrogen through water splitting and organic compounds. The study also elaborates on the zigzag (Z) and step (S) schemes used for the photocatalytic activity of composites. Present work showed that composite photocatalyst are better and increased efficiency of hydrogen production because active sites of surface increased. This review will be helpful for new apprentices in this field to understand the basic mechanism, helpful for industrialists and experts to decide which composite photocatalyst is best according to their interests.
Keywords
Photocatalyst activity
Semiconductor photocatalyst
Hydrogen production
Water splitting
Cadmium Sulfide (CdS) and Tungsten trioxide (WO3)
Titanium dioxide (TiO2) and Graphite Carbon Nitride (gC3N4)
1 Introduction
Immense use of fossil fuels causes worse environmental issues. So, it's now need for the world to find sustainable energy resources. With the passage of time researchers shift the environmentally friendly energy resources method in which solar energy. But one of the best sustainable energy resources is hydrogen production through water splitting by using the photocatalytic method which is environment friendly and cost-effective method. The technoeconomic analysis showed that 1 kg hydrogen production through photocatalyst cost almost 9$ and electrolysis method cost 18$ (Hermesmann and Müller, 2022, Montoya et al., 2017, Chu et al., 2017). Mostly photocatalysts based on semiconductors are reported by researchers due to their cleanliness and simplicity. This phenomenon was explored by Fujishima and Honda (Anpo et al., 1997). Many synthesized photocatalysts are used to obtain stability and higher efficiency, including nitrides of carbon (Huang et al., 2021), polymers (Ajma et al., 2022), carbides (Soni et al., 2022), metal oxides (Nunes et al., 2021), sulfides (Sudhaik et al., 2022), organic complex metals (Younis et al., 2020), and many others (Nguyen et al., 2015, Hu et al., 2015, Janaky et al., 2012, Wang et al., 2009). The semiconductor material lies between the conductor and the insulator. They have a band gap of up to 1ev usually. When a photon target on semiconductor molecule the electrons of molecule absorb the energy and jumps from valence to the conduction band. During this mechanism electron at the conduction band used for reduction and hole charge carriers produced at the valence band used for oxidation reaction to increase the efficiency of this photocatalytic process recombination of electron and hole must be prevented as much as possible.
Titanium dioxide (TiO2) is a frequently reported photocatalysts semiconductor used for the production of hydrogen because TiO2 has several advantages inert biologically and chemically, simple production and use, cost-effective, environmentally friendly, and stable but its disadvantage is activated in the UV region, not the ultraviolet region (Akpan and Hameed, 2009).
There are thousands of articles published in the last 20 years on TiO2-based photocatalysts as shown in Fig. 1.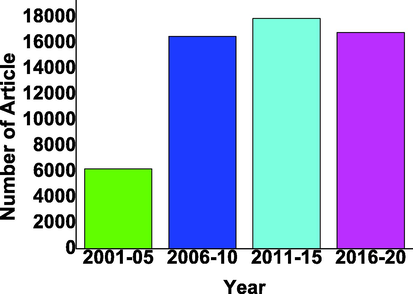
TiO2 based photocatalyst reported in last two decades with the gap of 5 years from 2001 to 2020 from google scholar (Scholar, 2001–2020).
Another mostly used semiconductor photocatalyst for hydrogen production recently tungsten trioxide (WO3) attracts the researchers due to its high stability, sharp band edges, non-toxicity, long life, low cost, easy access and activation in the visible region at a wavelength of 470 nm. But, WO3 has a few limitations as well. Firstly, WO3 has low specific area. Secondly, a less visible light response of more than 470 nm wavelength. Thirdly due to the low conduction band many reactions do not occur one of them oxygen reduction for a single electron and fourthly it has fast electro-hole pair recombination rate (Liao et al., 2021).
There are thousands of articles published in the last 20 years on WO3-based photocatalysts as shown in Fig. 2.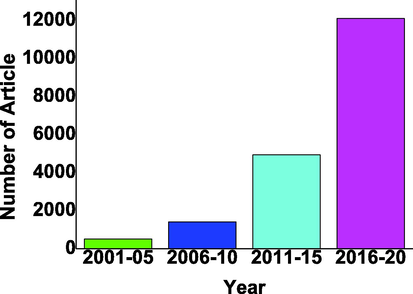
WO3 based photocatalyst reported in last two decades with the gap of 5 years from 2001 to 2020 from google scholar (Scholar, 2001-2020c).
Another interesting photocatalyst semiconductor used for hydrogen production is cadmium sulfite (CdS) due to its great response in the visible region, narrow bandgap, and good conduction band for protons reduction.
CdS has some shortcomings occur such as CdS is alterable, instable and cannot be useable for the long terms and Its efficiency decreased with passage of time (Cheng et al., 2018).
There are thousands of articles published in the last 20 years on CdS-based photocatalysts as shown in Fig. 3.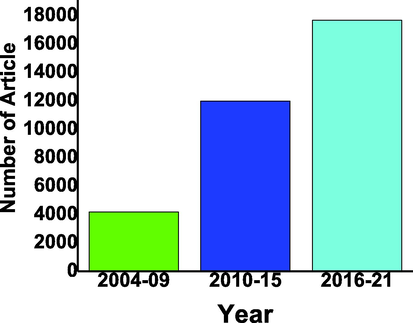
CdS based photocatalyst reported in last two decades in with the gap of 5 years from 2004 to 2021 from google scholar (Scholar, 2001-2020a).
Graphite Carbon nitride (g C3N4) based photocatalyst for hydrogen production gain the attraction of researchers due to their high chemical stability, easy availability as g C3N4 is the most earthbound elements.
Despite all the interesting features, it has a few drawbacks like lacking solar light absorption, fast electron-hole pair recombination, and less surface area (Nasir et al., 2019a).
There are thousands of articles published in the last 20 years on g C3N4-based photocatalyst as shown in Fig. 4.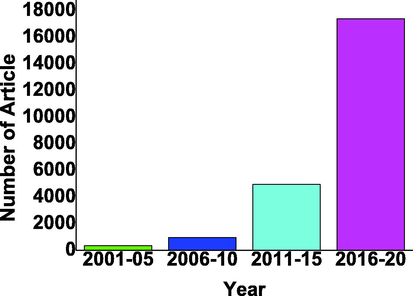
g C3N4 based photocatalyst reported in last two decades with the gap of 5 years from 2001 to 2020 from google scholar (Scholar, 2001-2020b).
Immense of research articles published by the researcher on TiO2, CdS, g C3N4, and WO3-based photocatalysts in the last two decades (Akpan and Hameed, 2009, Cheng et al., 2018, Munawar et al., 2022, Dutta et al., 2021, Liao et al., 2021, Luo et al., 2017, Chen et al., 2020, Liu et al., 2021, Yang et al., 2021, Wen et al., 2017). Recently in the last seven years, researchers shifted their interest towards the composite of this semiconductor photocatalyst for the production of hydrogen to deal with the drawback of the above-discussed semiconductor-based photocatalyst and comparison shown in Table 1.
Photocatalyst semiconductor
Band Gap
Advantages
Limitation
CDs
2.42 eV
Activation in visible
Narrow band gapUnstable
Not useable for long term(Cheng et al., 2018)
TiO2
3.2 eV
Stable
Simple ProductionNo activation in the visible range
(Akpan and Hameed, 2009)
WO3
2.5 eV
Stable
Activation in visibleLow conduction band
Fast Recombination(Liao et al., 2021)
g C3N4
2.7 eV
Stable
Easily availableLow surface area
Fast Recombination(Nasir et al., 2019b)
The main purpose of this study is to focus especially on the discussion of different WO3 composites, CdS composites, TiO2 composites, and g C3N4 composites for hydrogen production. Because the research gap found that no review article has been published on photocatalyst based composite for hydrogen production and there is need for research to focus on two-dimension (2D) composites for further study in this domain. Then after the study of different composite provide the reader with a detailed comparison of different composites. This study will also focus on the crone and prone of different composite. In the end, this study will give the future aspects, challenges, and limitations in using the different composite photocatalysts for the production of hydrogen. This study also studies the brief mechanism of water splitting and organic compounds for the production of hydrogen. Further, Schemes used by composites for photocatalytic are also discussed briefly. This study will help the new researcher to understand the mechanism and it will also help the expert to decide which composite is best for further study.
2 Composites-based photocatalysts for hydrogen (H2) evolution
Hydrogen production from water splitting by using photocatalyst semiconductor is cost-effective, sustainable and pollution-free energy resource. Even many researchers tried to take the best outcomes but few restrictions like nonstable, less activation in the visible region, and fast recombination of electron-hole pair. For solving this issue composite (Elmacı et al., 2019, Elmaci et al., 2015, Elmacı et al., 2016, Elmacı et al., 2020) of two catalysts help in modifying the valence and conduction band by enhancing reduction and oxidation and is also helpful in activation in the visible region. The different composites are reported by researchers some of them illustrated below discussion (Weng and Xu, 2015, Li et al., 2020, An and Jimmy, 2011). There are thousands of articles published in this era on composite for hydrogen production (Ghouri et al., 2021, Mao et al., 2022, Guan et al., 2004, Lee, 2005) as shown in Fig. 5.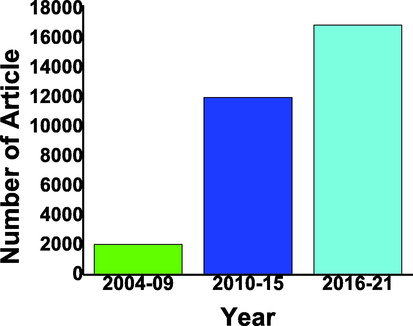
Publication in last two decades composites based photocatalysts hydrogen evolution with the gap of 5 years from 2004 to 2021 from google scholar (Scholar, 2001-2022a).
3 CdS and TiO2 composite
The bandgap energy of CdS 2.42 eV is smaller and makes it worthy to use under visible light for the decomposition of water through the photocatalyst process. But it has a fast recombination rate of electron-hole pair which make it less efficient for photocatalyst (Husseina et al., 2022). On the contrary, TiO2 has good electron-hole pair recombination which makes it more efficient for photocatalyst but it has a band gap energy of 3.42 eV which make it disreputable for visible region (Doubi et al., 2022). The researcher started to work on CdS/TiO2 composite to overcome the photocatalytic barrier of TiO2 and CdS for the evolution of hydrogen. The composite improved the recombination rate and, in some cases, work in near-visible regions successfully from the range of 420–550 nm. In different sizes (nano bulk, nano, quantum dot) (Doubi et al., 2022) and with different preparation methods (hydrothermal, precipitation, sol–gel) CdS/TiO2 composites photocatalysts reported by the researcher for the production of hydrogen by different substances (air, water, hydrogen sulfide) (Wang et al., 2022).
In the last decade's lot of publications reported using composite Cds/TiO2 for hydrogen evolution (Jiang et al., 2017, Lian et al., 2015, Khatamian et al., 2014, Jang et al., 2007b) as shown in Fig. 6.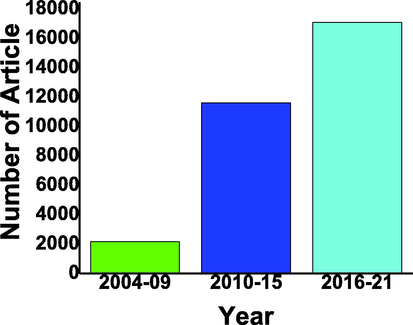
CdS, TiO2 composite based photocatalyst reported in last two decades with the gap of 5 years from 2004 to 2021 from google scholar (Scholar, 2022b).
3.1 CdS and TiO2 nanocomposite treated with TiCl4
The first CdS and TiO2 composite was reported by won-wook so et al in 2003 at the nano level with a treated film of TiCl4. won-wook so et al prepared nano-size CdS and TiO2 solution mixed by using the mole ratio. The further hydrothermal method was used for 12 h at 120–240-degree Celsius (C) temperature. In this way composite of CdS and TiO2 was prepared and TiCl4 was annealed for 30 min at 400C temperature to obtain the crystalline phase of the composite. By using XRD, SEM, and UV techniques nano composite characteristics and structure were studied (So et al., 2004). Finally, the effect of composite photocatalyst check on the photoreactor as shown in Fig. 7.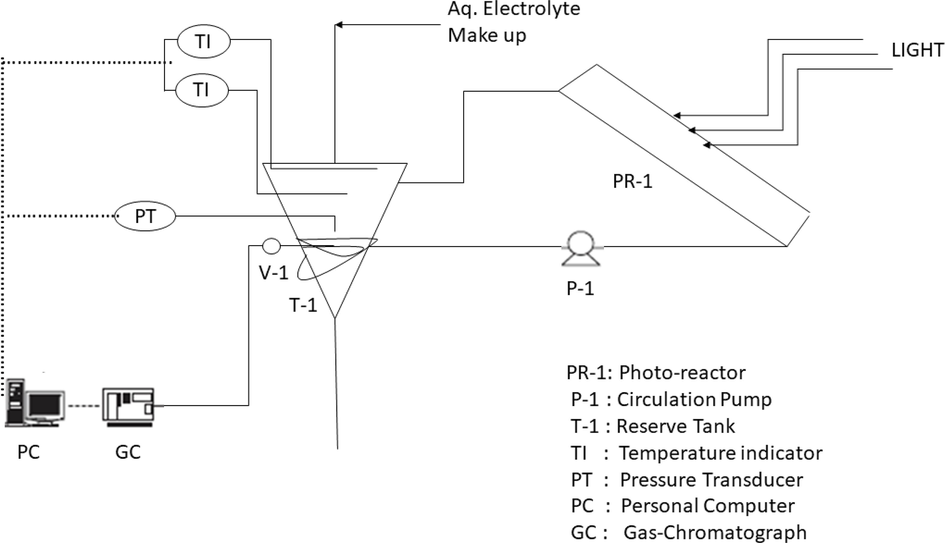
Photoreactor used to study consist of photo reactor, circulation pump, reserve tank, temperature indicator, pressure transduce, personal computer and gas chromatograph (So et al., 2004).
The structure and characteristics of the composite treated with TiCl4 illustrates by studying the obtained results from XRD and SEM shown in Fig. 8.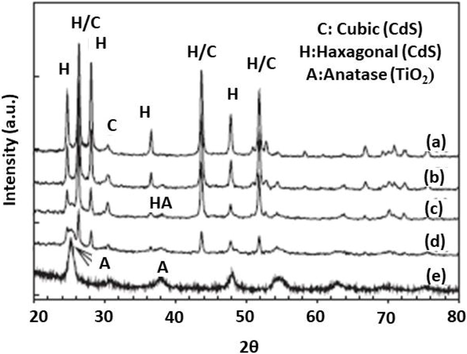
XRD at mole ratio TiCl4 with composite of CdS/TiO2 at(a)0 (b)0.2 (c) 0.5 (d) 0.8 (e) 1 here A represents anatase of TiO2, H hexagonal of CdS and C cubic of CdS (So et al., 2004).
On the base of TiCl4 treated TiO2/CdS composite production of hydrogen with the time shown in Fig. 9.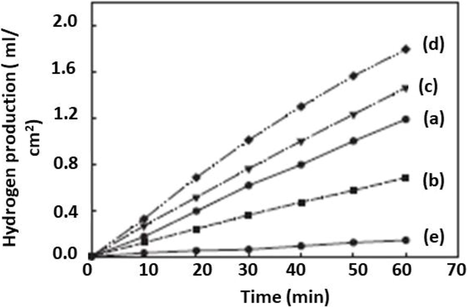
The figure showed rate of hydrogen (H2) production in ml/cm2 in time (min) for CdS/TiO2 composite particulate prepared with TiCl4 treatment at a mole ratio of (a)0 (b)0.2 (c) 0.5 (d) 0.8 (e) 1 (So et al., 2004).
The result showed that the production of hydrogen increased with time as photocurrent increased, photocatalytic activity enhanced, and optimum production obtain at a mole ratio of 0.8.
3.2 CdS and TiO2 nano bulk composite (NBC)
Although there are many limitations in the production of hydrogen but high production of H2 and the removal of hydrogen sulfite (H2S) observed by using the composite photocatalyst CdS (bulk)/TiO2 in the presence of water and 0.1 M Na2S + 0.02 M Na2SO3. The hydrogen is produced from H2S when H2S dissolve in alkaline water solution where H2S reacts with hydroxyl ion (OH–). Then water forms and by reduction or addition of electron with water molecules it produced hydrogen. In this process hydrogen separated from sulfide. The reaction of this process is shown below. In this process, water is also involved and contributes to the production of hydrogen (Jang et al., 2007a, Jang et al., 2006).
During this process production of hydrogen with the passage of time and the dissolution of H2S in different electrolytes are shown in Fig. 10.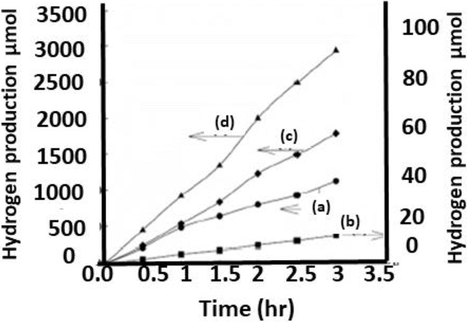
The rate of hydrogen production in time (hr) when H2S mixed with (a) Na2S (b) H2O (c) 0.1 M NaOH (d) 1 M NaOH(Jang et al., 2006).
The hydrogen (H2) production model for CdS bulk with nano sized TiO2 composite shown in Fig. 11 from H2S and Fig. 12 from 0.1 M Na2S + 0.02 M Na2SO3 (Jang et al., 2006, Jang et al., 2007a).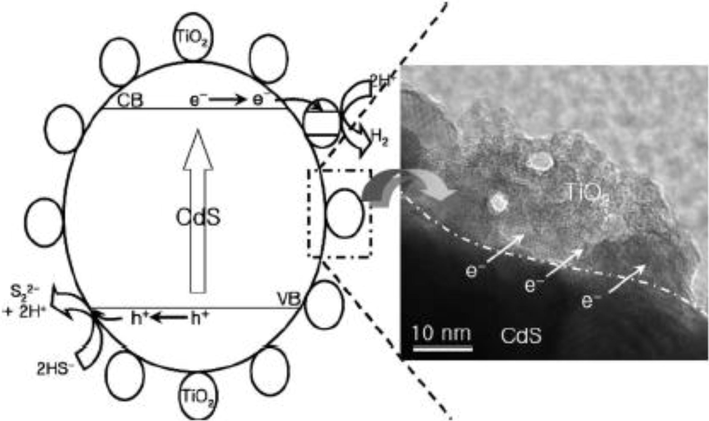
Hydrogen production mechanism for CdS bulk with nano sized TiO2 composite from H2S (Jang et al., 2006).
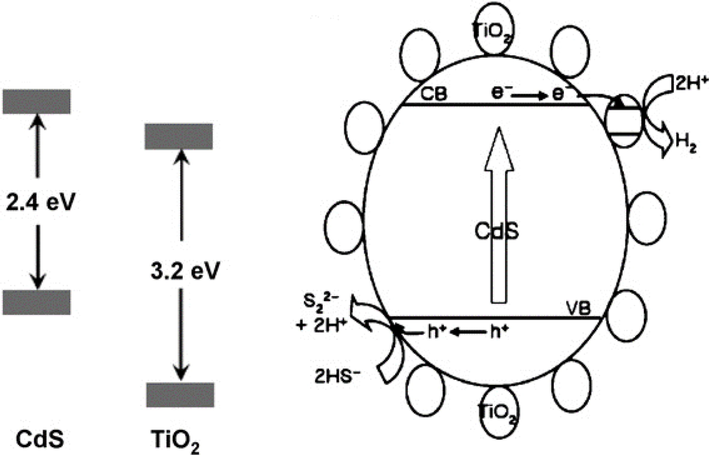
Hydrogen (H2) production model for CdS bulk with nano-sized TiO2 composite from H2S in presence of 0.1 M Na2S + 0.02 M Na2SO3 (Jang et al., 2007a).
In the above models of CdS generated photoelectrons collected by active sites of TiO2 which provide more time for electron-hole pair recombination increase and make the photocatalyst process more efficient.
3.3 Platinum in platinized CdS/TiO2 composite photocatalysts
Platinization of photocatalyst composite CdS/TiO2 has been done by using different means like wet impregnation (WI), photo deposition (PD), and chemical reduction (CR) for the production of hydrogen in the visible region. For this purpose, platinum (Pt) in a metallic state is planted on the composite. On the other hand, the photocatalyst of WI and PD consists of electron-deficient Pt. In WI Pt-Ti is formed to fill this deficiency, in this way the electron-hole pairs recombination required more time due to two electrons deficient photocatalysts and makes hydrogen production more efficient from water.
The Pt becomes much more important because of extra higher and lower states created due to WI, PD, and CR processes which make the CdS/TiO2 composite photocatalysts more efficient. The activity of this model, operation of composite and production of hydrogen with time by using PD, WI, and CR in platinum is shown in Fig. 13 (Jang et al., 2008). These results showed that WI method is more efficient in the production of hydrogen.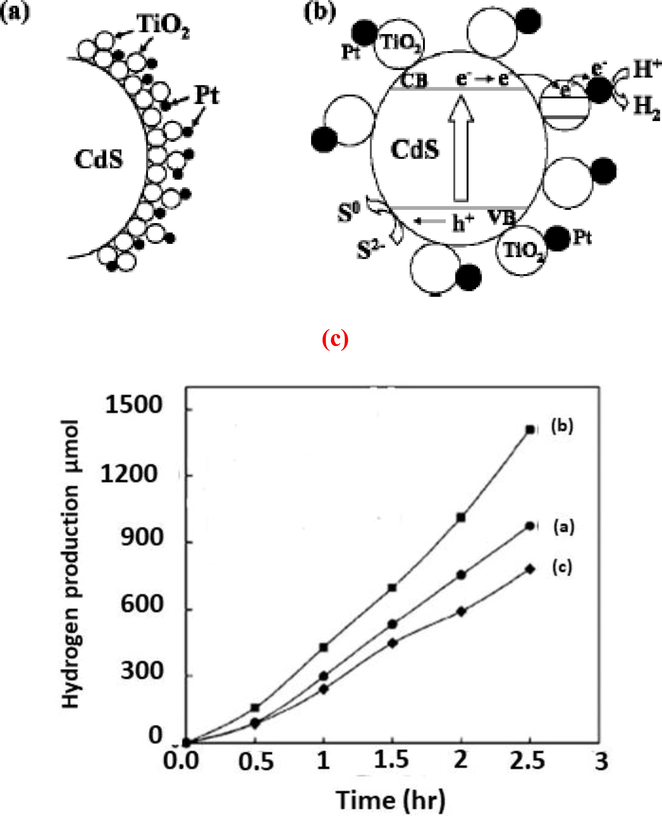
(a) Operational mechanism of composite with CdS/TiO2 in the presence of catalyst Pt (b) Activity model of CdS/TiO2 composite in the presence of catalyst Pt (c) Rate of hydrogen production in time (hr) (Jang et al., 2008).
3.4 CdS / TiO2 (Nanotubes) composite photocatalyst for hydrogen production
Nanotubes (NTs) of titanate is used for the systemization of CdS/TiO2 composite with changing diameters by a simple method of ion change and sulfurization process followed at moderate temperature. Using this composite as a photocatalyst increased the production of hydrogen (H2). In this process, almost 43.4% yield was obtained under the visible region around the wavelength of 525 nm. High efficiency might be obtained due to these reasons (1) The effects of synergetic among TiO2 NTs and nanoparticles (NP) of CdS (2) Another major role of homogeneous distribution of CdS particle and quantum effect.
In this photocatalytic hydrogen production process following reactions occur
The production of hydrogen was recorded by using a composite of CdS (NP)/ TiO2 (NTs) with time efficiency like for the first NT CdSNT-1, for the second used after 4 h denoted as CdSNT-2, physical mixture and neat powder of CdS. The average hydrogen (H2) evolution during the whole process is shown in Fig. 14.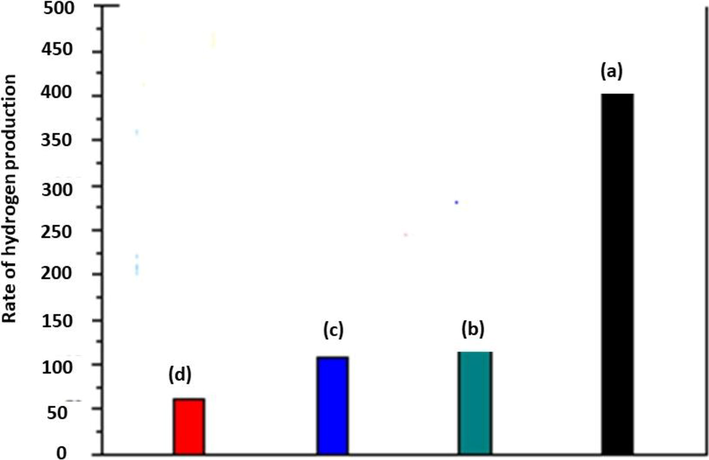
The average hydrogen production by using (a) CdSNT-1 (b) CdSNT-2 (c) Physical mixture 20% CdS + 80% TiO2 (d) neat powder of CdS (Li et al., 2010).
3.5 Elucidating the factors affecting hydrogen production activity using a CdS/TiO2
The photocatalytic efficiency of the CdS/TiO2 composite was observed by factors of hydrogen overvoltage, transfer of an electron, substrate adsorption, and cocatalyst effect. In CdS/TiO2 composite transfer of electron play an effective role in the recombination rate of electron-hole pair. It could be more effective when a co-catalyst like Pt is used and increased the reducing and oxidizing capacity of generated electron-hole pair which makes the photocatalysis process more efficient. For this purpose, it is important to choose a catalyst carefully that matched the required output and is designed as well. The design used for elucidating factors is shown in Fig. 15 (Nagakawa and Nagata, 2021).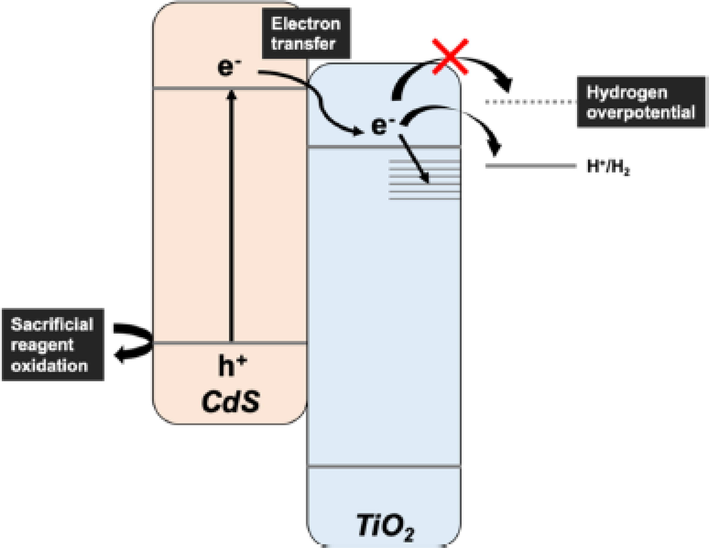
Hydrogen production mechanism CdS/ TiO2 by using reducing agent (Nagakawa and Nagata, 2021).
The above discussion shows that CdS/TiO2 composite photocatalyst is important for the production of hydrogen. The CdS/TiO2 have been reported by using various methods, materials, and shapes, under different experimental conditions, and the efficiency of hydrogen production is shown in Table 2.
Material
Experimental condition
Production rate of hydrogen
Ref
CdS/TiO2 nanocomposite with TiCl4
Variation in use of catalyst from mole ratio 0,0.2,0.5,0.8 and 1.
1.6 mol/cm2
(Guan et al., 2004)
TiO2 nanotubes Pt/CdS
Catalyst with aqueous solution 300 W (W) Xe lamp wavelength > 400 nm from water
402umolh−1
(Li et al., 2010)
Pt/TiO2 / CdS zeolite Y
Catalyst 50 g aqueous solution 200 W Xe lamp 320 < wavelength < 570 nm from water
12 umolh−1
(White and Dutta, 2011)
CdS/TiO2 nano bulk
Catalyst 0.1 g Aqueous solution 500 W Hg lamp wavelength > 420 nm from H2S
980umolh−1
(Jang et al., 2006)
TiO2 / CdS
Catalyst 0.08 g Aqueous solution 500 W Hg lamp wavelength > 420 nm
72% after 6 h
(Huo et al., 2011)
TiO2 nanotubes/ CdS
Catalyst 25 mg aqueous solution 200 W Halogen lamp 420 < wavelength < 800 nm
91.6% after 2 h
(Zhou et al., 2011)
TiO2 / CdS/ cross-linked chitosan
Catalyst 50 mg with aqueous solution 300 W Xe lamp wavelength > 400 nm
37.4% after 5 h (visible)
(Zhu et al., 2013)
Pt/TiO2 / CdS
aqueous solution 400 W Xe lamp monochromatic (470 nm) radiation
0.21 μmol min−1
(Strataki et al., 2010)
TiO2 / CdS graphene
Catalyst 50 m × 75 mm film 8 W LED lamp
33% after 2.5 h
(Park et al., 2013)
Nanowires TiO2 / CdS film
4-tert-butyl pyridine (0.125 M) in 3-meth- oxy propio nitrile,and 1-vinyl-3-methyl imidazolium iodide (0.70 M),LiI(0.1 M),I2 (40 mM), 300 W Xe lamp
16.4 % after 1 h
(Lee et al., 2009)
TiO2 / CdS/Pt
Reducing agent MeOH with effecting factor overvoltage and electron transfer
459.5 umolh−1
(Nagakawa and Nagata, 2021)
Ti3CN@TiO2 / CdS
nanoflower morphology from water splitting
3393.4 μmolg-1h−1
(Li et al., 2022)
4 WO3/g C3N4 composite photocatalyst for hydrogen production
Graphitic Carbon Nitride (g C3N4) polymeric semiconductor recently has gained the attention of researchers because of its great efficiency in photocatalysis activity, g C3N4 polymer inorganic coupling with Z scheme nanocomposite of semiconductor behaves as an efficient photocatalyst in the irradiation of visible light (Ge et al., 2012, Su et al., 2022, Tian et al., 2015, Lang et al., 2015).
WO3 also gains attention due to its narrow band gap activated in visible light but WO3 is not suitable for single electron O2 reduction in the conduction band due to low photooxidation ability. The researcher tried their best to overcome these limits of WO3 such as deposition of noble metal, controlling of size, and many other coupling phenomena but still no satisfactory result was obtained. g C3N4 also gains attain due to its activation in the visible region and favorable in the reduction as conduction band minimum −1.12 ev which makes it to best for the production of hydrogen(Chen et al., 2014b). But is a limitation in photocatalytic efficiency in that its electron-hole pair of recombination is too fast. Many strategies adopted to overcome this limitation such as controlling size and coupling with some other semiconductors were most favorable (Cheng et al., 2017, Han et al., 2018, Kim et al., 2022, Bi and Xu, 2011).
g C3N4 and WO3 due to narrow semiconductors driven by visible light within the solar spectrum. In this regard composites of WO3/g C3N4 became the center of interest due to their high efficiency in photocatalytic reaction for the production of hydrogen. Photocatalyst Composites of WO3/g C3N4 has been investigated for the production of hydrogen by using different techniques of preparation, different shape or size (nanocomposite, nanotubes, nanosheets, bulk, and others), different dimensions (1D,2D,3D) and different scheme (Z and S) (Chen et al., 2014b, Fu et al., 2019a, Cui et al., 2017, Kim et al., 2022, Zhou et al., 2022).
In this domain of composite of WO3/g C3N4 photocatalyst for hydrogen production there are hundreds of researches article published as shown in Fig. 16.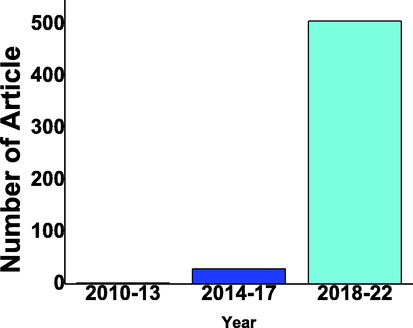
No of Publication on WO3/g C3N4 composite photocatalyst for hydrogen production with the gap of 4 years from 2010 to 2022 from google scholar (Scholar, 2023).
4.1 Composite WO3/g C3N4 with a different mass content of WO3
The phase structure, the separation efficiency of electron-hole pair, optical adsorption, and absorption are key factors involved in the photocatalytic activity of any substance. The composite of WO3/g C3N4 formed by using a calcination process with variation in mass % of WO3 (6.1 %,9.7 %, and 16.6 %) with a specific value of g C3N4. The photocatalytic activity efficiency was observed by the degradation of methyl blue. The photocatalyst properties by using different techniques. WO3 and g C3N4 not altered their structure crystal phase after hybridization as shown in XRD pattern Fig. 17 (Huang et al., 2013a).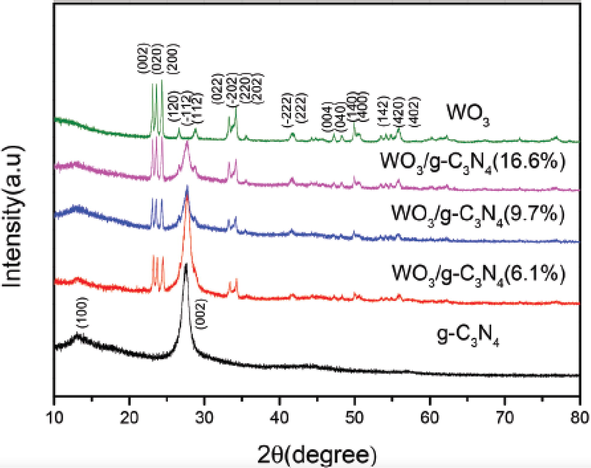
XRD pattern of WO3,g-C3N4 and composite of WO3/g-C3N4 with variation in percentage of WO3 6.1%, 9.7% and 16.6% (Zhang et al., 2012).
The comparison between pure WO3, g-C3N4, and their composite showed that the composite is more efficient for photocatalytic activity as compared to pure one. Further analysis shows that 16.6% WO3 quantity has produced more sites for photocatalytic reactivity. Because the surface increased with an increase in % of WO3 quantity which provides a favorable position for the diffusion of products and adsorption of reactants. Its increased efficiency of photocatalytic reactivity (Kang et al., 2012, Dong et al., 2013, Sun et al., 2012, Tersoff, 1984).
The above discussion has showed that visible range absorption and adsorption enhancement key point for an increase in photocatalytic activity. But there is another important effect involved in the efficiency of photocatalytic activity which is charge separation. The separation of electrons and holes depends on the band edge position of two semiconductors, as band structure is accountable for the efficient separation and generation of charge carriers. The proper direction of the band structure is useful in the creation of interface depletion of charge carriers (Alferov, 1998, Yan et al., 2010c).
The possible mechanism involved in this process has shown in Fig. 18. The bottom of the valence band of WO3 has 3.43 eV and the conduction band 0.75 eV energy. The g-C3N4 has the valence band (VB) bottom 1.57 eV and conduction band (CB) −1.13 eV energy. The band gap of WO3 has 3.43-0.75 = 2.68 eV and g-C3N4 1.57- (-1.13) = 2.70 eV energy. This shows that both semiconductor irradiation under visible light creates electron-hole pairs. Its shows that the potential of the g-C3N4 CB edge is less than the WO3, which means the g-C3N4 electrons in the excitation state directly insert into the WO3 CB. In the same way, the VB of g-C3N4 is less than the WO3. So, holes are transfer from WO3 to g-C3N4. The distribution of electrons is on one side of the junction and holes on another side of the junction increased the separation time of the electron and hole pair which has increased the photocatalytic activity of the composite. This is clear that the composite of g-C3N4/WO3 has a higher efficiency as compared to the pure g-C3N4 and WO3 (Chakraborty et al., 2012, Cui et al., 2017).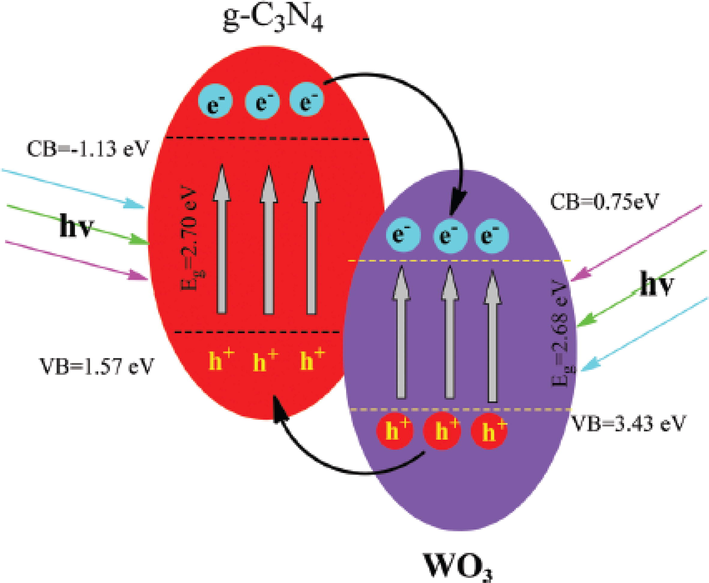
Photocatalytic mechanism of g-C3N4/WO3 composites under irradiation of visible light (Xing et al., 2019).
The degradation efficiency of the substance is calculated by using the formula
Here Ao and A are corresponding absorption values of concentration of a substance at initial Co and concentration after some time (t) C (Xing et al., 2019).
g-C3N4/WO3 composite has a greater value of substance degradation as compared to the pure g-C3N4 and WO3 due to its higher efficiency in photocatalytic activity and Composite g-C3N4/WO3 enhanced due to the enhanced absorption and electron-hole pair separation(Cui et al., 2017).
The p hotocatalytic activity of g-C3N4/WO3 composite increased as the WO3 % content increased until 25% after that the photocatalytic activity decreased so maximum photocatalytic activity achieved in g-C3N4/WO3 composite as 25% content of WO3 as shown in Fig. 19 (Han et al., 2018).
Shows degradation activity under light irradiation with different mass % content of WO3 at 0, 0.05, 0.15,0.25,0.4,0.5 in g-C3N4/WO3 composite and without light (Yan et al., 2010b).
Change in different properties like surface area and band gap energy with different mass content % shown in Table 3 (Cui et al., 2017).
WO3 % in g-C3N4/WO3
Surface area
(m2/g)
Energy
(eV)
5%
68.1
2.57
15%
84.6
2.62
25%
100.6
2.73
35%
75
2.54
40%
72.7
2.52
50%
70.5
2.4
In the same way when methanol was used and activity of hydrogen production rate was observed every half hour of WO3/g-C3N4 composite prepared under the Z-Scheme. It provides the maximum hydrogen production of 224.4 umol/h with 25% WO3 content as shown in Fig. 20 (Cui et al., 2017).
Hydrogen production rate for WO3, g-C3N4 and WO3/g-C3N4 composite with time irradiation (Xing et al., 2019).
4.2 Two-dimensional (2D) composite of g-C3N4/WO3 photocatalytic activity for hydrogen production
The 2D g-C3N4/WO3 composite was prepared by using the cost-effective and simple method of hydrothermal. The production of hydrogen was observed under the irradiation of visible light. During the process of the hydrothermal composite formed by using nanoparticles (NP) of WO3 attached uniformly with g-C3N4 nanosheets (NS). This was observed in orthorhombic shape NP grain size 5 nm-80 nm of WO3 attached uniformly with NS of g-C3N4. The synthesis process of the composite is shown in Fig. 21 (Han et al., 2018, Yan et al., 2010a).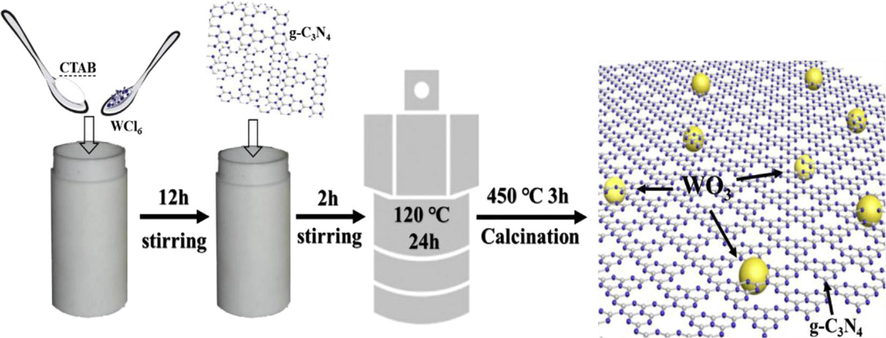
Schematic of preparation of 2D g-C3N4/WO3 composite by using hydrothermal method stirring for 12 h of WO3,g-C3N4 for 2 h and calcination at 450 °C for 3 h (Ge et al., 2013).
The Schematic shows that WO3 dissolved hexadecyl trimethyl ammonium bromide (CTAB) and stirred for 12 h, g-C3N4 also stirred for 12 h, and then both mixed and stirred for 24 h at a temperature of 240 °C. After this calcination of the composite was done almost for 3 h at a temperature of 450 °C. in this way 2D composite of WO3/g-C3N4 was prepared(Wang et al., 2016).
Then 2D g-C3N4/WO3 was used for the hydrogen production and the whole experiment was conducted in the evacuation and closed gas circulation system. The water circulation is used to keep the temperature of the system at room temperature. The photocatalytic powder of 100 mg is suspended in aqueous triethanolamine (TEOA). The co-catalyst of Pt was directly deposited on the photocatalyst and irradiation started after 15 min vacuum. The concentration of H2 was collected after every hour and analyzed in a chromatograph (Huang et al., 2013a, Yi et al., 2017).
The morphology of the composite showed that it has a layered structure which caused to increase in the surface area as an increase in the surface area increased the active sites for photocatalytic activity. In the same way, its enhanced absorption of visible light generates more electron-hole pairs. The 2D structure of the composite is also helpful in enhancing the separation of electron hole-pair. The co-catalyst of Pt was further used to enhance the photocatalytic activity of the composite for the hydrogen evolution from the water. As the Pt has high metallic characteristics the electrons in CB of g-C3N4 combine with H+ and produced H2 and holes in WO3 VB oxidized the molecule of TEOA. In this process, Z-Scheme is used for hydrogen production. Z- Scheme slows down the process of recombination of an electron from CB to combine with a hole from the VB. As the recombination rate increased the photocatalytic activity of the composite increased in the production of hydrogen. In this way, Z-Scheme plays an important role in increasing the efficiency of photocatalyst composite. The mechanism is shown in Fig. 22 (Xu et al., 2018, Guan et al., 2017, Nie et al., 2018).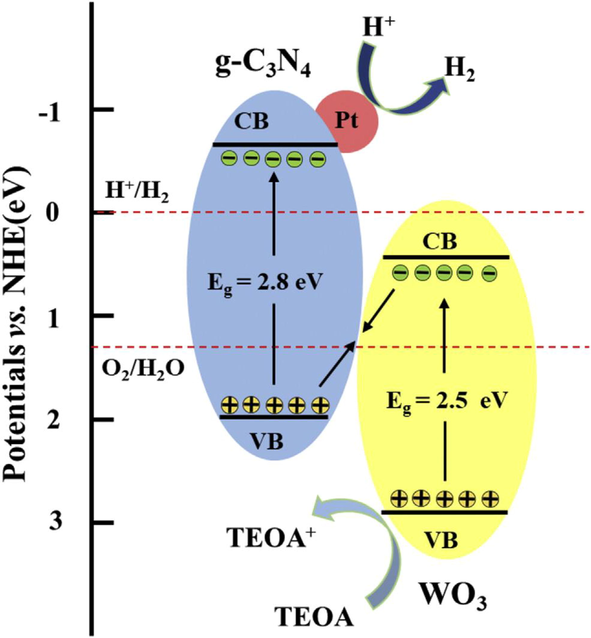
Mechanism of 2D g-C3N4/WO3 composite for hydrogen production (Fu et al., 2019b).
The highest generation rate of H2 was observed at 1853 mmol/h by using a composite of 2D g-C3N4/WO3 which was almost 6.5 times as compared to g-C3N4 NS. The WO3 synergistic effect and nano architectures effect of the g-C3N4 plays a vital role in the performance of their 2D composite in the production of hydrogen. The Z-Scheme not only increased the separation time of the electron-hole it also increased the surface area which creates more active sites. All these factors make a more efficient 2D composite of WO3/g-C3N4 (Liu et al., 2016).
4.3 2D/2D composite of WO3/g-C3N4 for production of hydrogen
To improve the photocatalytic activity of WO3/g-C3N4 composite 2D structure of both WO3 and g-C3N4 used for preparation and step (S) scheme used. The 2D/2D design of surface–surface contact increased the surface area which developed more active sites and increased photocatalytic activity efficiency. For the fabrication of 2D/2D structure, the ultrathin nanosheet of WO3 and g-C3N4 sized ranged from 2.5 nm to 3.5 nm used. WO3 /g-C3N4 composites were prepared by using self-assembly electrostatic nanosheets of WO3 and g-C3N4 as shown in Fig. 23 (Zhang et al., 2020).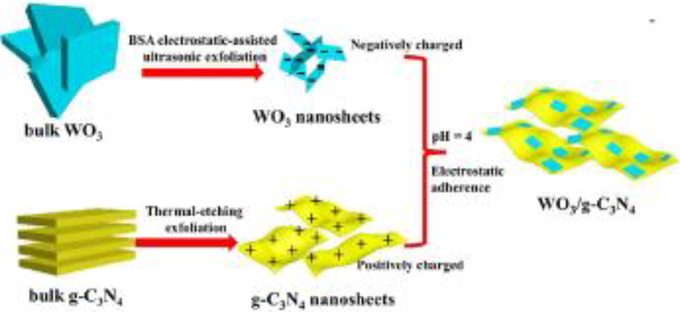
Schematic of 2D/2D WO3 /g-C3N4 composites preparation (Zhang et al., 2020).
Then this composite was used for the production of hydrogen with a co-catalyst of Pt. the mechanism used for the hydrogen production was S Scheme for this composite. The whole mechanism is shown in Fig. 24 (Zhang et al., 2020).
Schematic of mechanism of WO3 and g-C3N4 (a) before contact (b) after contact and (c) light irradiation by using S scheme charge transfer (Byskov et al., 2000a).
In the S-Scheme WO3 was used as oxidation due to a lower Fermi level and g-C3N4 reduction to a larger fermi level but when both are in close contact fermi level became the same. As WO3 accepts electrons from the g-C3N4 an electric field induces at the interface. The band edge of WO3 moves downward due to the acceptance of electrons and g-C3N4 upward due to the transfer of electrons. So, when the light falls on composite electron transfer from both VB to CB. In this way, coulomb interaction between charges, edge bending, and internal field increased the recombination of some electron-hole pairs but at the same time increased the separation of some electron-hole pairs. The inactive electron holes are eliminated by using S-Scheme but it holds the active electron-hole pair. This process provides the 2D/2D WO3/g-C3N4 composite strong force for photocatalytic activity in the splitting of water for the production of hydrogen (Liu et al., 2017, Yang et al., 2018).
The above discussion shows that S scheme 2D/2D WO3/g-C3N4 composite is more efficient as compared to pure WO3 and g-C3N4 for the production of hydrogen. The mechanism involved in this process is interesting as it eliminates useless electron-hole pairs in photocatalytic activity and holds useful electron-hole from VB and CB (Xu et al., 2017b).
4.4 WO3/g-C3N4 composite performance enhanced by using nickel sulfide (NiS) for hydrogen production
Already being discussed composite is better than pure or doped semiconductor for photocatalytic efficiency. But the composite efficiency also increased by using different co-catalysts. The NiS directly involved in the composite of WO3/g-C3N4 increased its efficiency 8.9 times of pure g-C3N4 previously it was reported 6.5 times. The in-situ growth of NiS on the WO3 modifies the composite of WO3/g-C3N4 because it enhances surface area which provides more active sites for the production of hydrogen by using photocatalytic activity (Zhang et al., 2020).
The sample was prepared through the hydrothermal method and NiS plays the role of catalyst in this process which increased active sites for photocatalytic reaction in VB and CB. The synthesis procedure is shown in Fig. 25 (Zhang et al., 2020).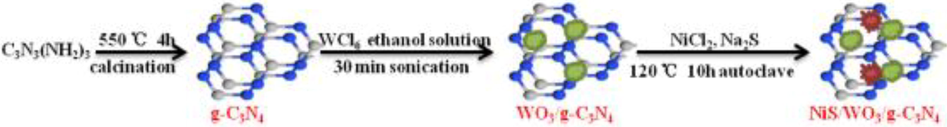
Schematic of NiS-assisted WO3/g-C3N4 composite for 4 h at 550 °C temperature, 30 min sonication and autoclaves for 10 h 120 °C temperature (Zhang et al., 2020).
The mechanism involved in this process shows when light irradiation on the sample the electron-hole pair is generated in the WO3/g-C3N4 composite. The NiS behave as a cocatalyst which accelerates the charge carrier in the g-C3N4 CB which drives the production of hydrogen increased and at the same time, holes in VB of WO3 oxidized strongly with the TEOA. NiS also promotes an electron from the oxidation of TEOA which increased active sites for the production of hydrogen. In this way, the production rate of hydrogen was enhanced significantly by involving NiS in composite and boosted its photocatalytic efficiency. The mechanism is shown in Fig. 26 (Zhang et al., 2020, Chen et al., 2014a, Byskov et al., 2000b, Liu et al., 2017, Yang et al., 2018, Xu et al., 2017c, Xu et al., 2017a).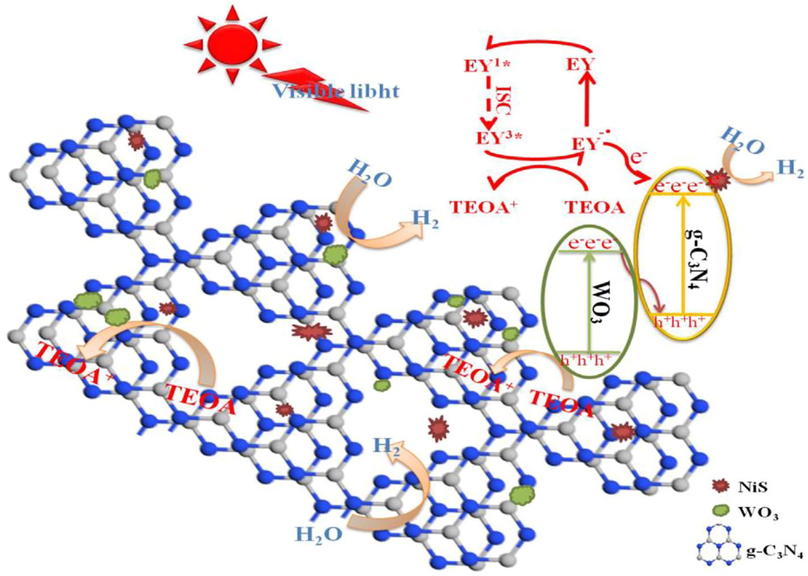
Schematic mechanism of WO3/g-C3N4 composite assisted with NiS for the hydrogen production (Zhang et al., 2020).
In this process maximum hydrogen production rate of 2929.1 umol/gh was observed and it involved two factors one surface area increased and the second time of separation electron-hole pair increased (Zhang et al., 2020).
All the above discussion shows composites of Cds/TiO2 and WO3/g-C3N4 increased the efficiency of hydrogen production through photocatalytic activity. The researchers are using these composites for hydrogen production from water splitting and air. The basic principle of hydrogen production through water splitting and organic compound by using schemes like Z and S used involved in the composite is briefly discussed below discussion.
5 Hydrogen production as renewable energy resource using photocatalyst
The production of hydrogen becomes of huge interest to the researcher as it needs an hour to find renewable energy resource which must be environmentally friendly and the method of photocatalyst best compare to another method as it is low cost and involve visible region irradiation. Hydrogen fuel is the future of the world. First the production of hydrogen from water splitting important topic of discussion for the researcher (Di et al., 2019, Xu et al., 2020a, Fajrina and Tahir, 2019, Konn et al., 2015).
5.1 Hydrogen production by water splitting
The basic concept in the production of hydrogen through water splitting involves a semiconductor. When a semiconductor material dissolves in water and light fall on it then an electron is generated in the CB which react with the positive ion of hydrogen and produce hydrogen on the contrary hole in VB react with the negative hydroxyl ion of water and form oxygen. This is the basic photocatalytic concept of water splitting and the production of hydrogen. The whole process involved in hydrogen production is shown in Fig. 27 (Takanabe, 2017).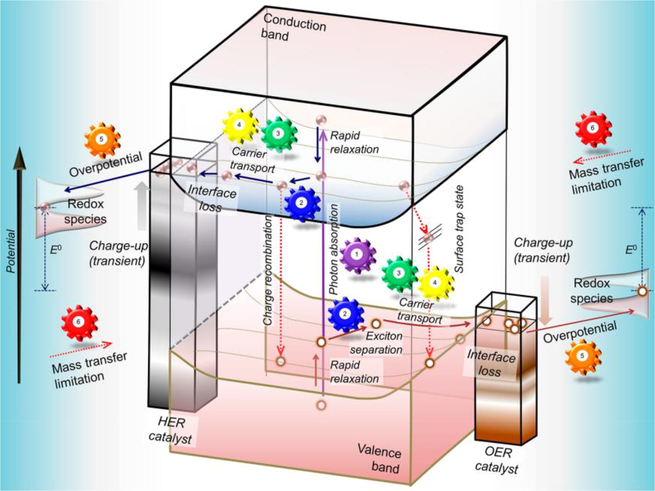
Mechanism of water splitting shows first mass transfer then light fall on it and oxidation reduction occur (Takanabe, 2017).
The main parameters involved in which efficiency of hydrogen production through water splitting are photon absorption, carrier diffusion, catalytic efficiency, mass transfer, carrier transport, and excitation separation. As more photon is absorbed the more electron-hole more generated which increased photocatalytic activity. In the same way, when there are more active sites, the carrier diffuses and excites the water molecule and split to produce hydrogen. The separation of charge carriers also plays an important role as electron-hole pairs separate for a long time they will reduce and oxidize water molecules at a large scale.in this way large quantity of hydrogen is produced. The parameter involved in this process is shown in the schematic of Fig. 28 (Takanabe, 2017).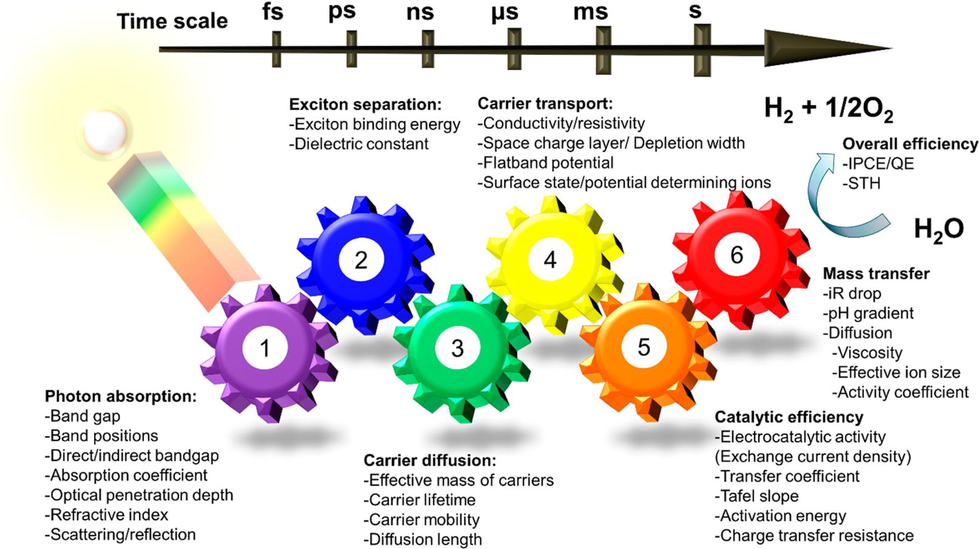
Parameters affect the production of hydrogen through water splitting involves Carrier transport, Excitation separation, Photon absorption, Carrier diffusion, Catalytic efficiency and Mass transfer (Takanabe, 2017).
5.2 Hydrogen production by using organic compound
Another famous and used method for hydrogen production use of the organic compound as organic compound combined with VB holes efficiently and used as sacrificial agents for the production of hydrogen. The organic compound uses a different mechanism as compared to water splitting. There are two main steps involved in this mechanism:
-
Direct capture of hole.
-
Indirectly as free radicals of hydroxide attacked.
The using of an organic compound like methanol for hydrogen production involves the following reaction takes place.
The electron from CB is involved in the reduction of proton for the production of hydrogen given by photo-oxidation of methanol. The whole mechanism is shown in schematic Fig. 29 (Chiarello and Selli, 2014).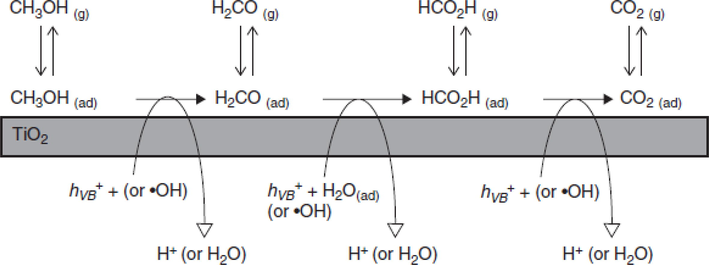
Schematic for Hydrogen Production through Organic Compound (Chiarello and Selli, 2014).
The production rate of hydrogen in this process depends upon the optical, and electrical characteristics and surface area of an organic compound.
6 Schemes involved in photocatalytic activity of composites for hydrogen production
There are two main schemes involved in the production of hydrogen through photocatalytic activity. First is Z Scheme and other S Schemes.
6.1 Z Scheme
The Z Scheme idea was first discussed by A.J. Bard for photocatalysts. In Z Scheme observed in composites of two semiconductors. One act as oxidized and the second as a reduced agent the electron-hole pair generated when light fall on it. Then photogenerated electron from CB reacts with the acceptor and creates a donor (Bard, 1979, Low et al., 2017, Madhusudan et al., 2021).
And photogenerated holes in VB react with the donor and form the acceptor.
In this way, the electron-hole pair reacted with the substance in the presence of light. This is the simplest concept to understand the Z Scheme. The Schematic of the direct Z Scheme shown in Fig. 30 used in composite used for serval purposes and one of the important purposes for the production of hydrogen (Yuan et al., 2017, Liu et al., 2018).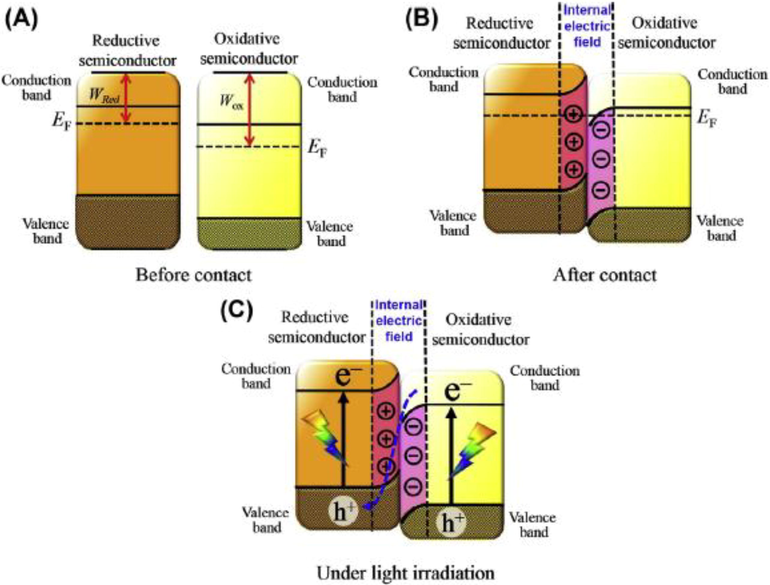
Schematic of Z Scheme (A) Before Contact no change in fermi energy(B) After Contact change in fermi energy (C) Under light Irradiation creates band edge (Low et al., 2020).
6.2 S Scheme
The Z Scheme has several limitations (1) confined to the phase of the solution (2) Side reactions involved (3) pH sensitivity and (4) light shielding.
To prevent all these limitations researchers introduced a new S Scheme. The S Scheme is involved in a composite of two semiconductors. Two generalized semiconductors are OP with lower work functions as compared to RP. The following S Scheme steps are involved for their photocatalytic activity. (1) When semiconductors are closed in contact RP electrons diffuse in OP, which creates a depletion layer of electrons on the interface of RP and OP. Thus, RP charged positively and OP negatively. The internal electric field induced in RP and OP accelerates the electrons to RP from OP. (2) When two semiconductors RP and OP come in close contact they are aligned at the same level. As the fermi level of OP aligned upward and RP downward. So, bending in the band urges the recombination of CB electrons of OP with VB holes of RP. (3) The electron-hole pair recombined at the interface due to the Coulomb force. On the whole useless electron-hole pair is eliminated by recombination and useable powerful electron-hole pair holds for photocatalytic activity. The whole mechanism involved in this process is shown in the Fig. 31 (Yang et al., 2018).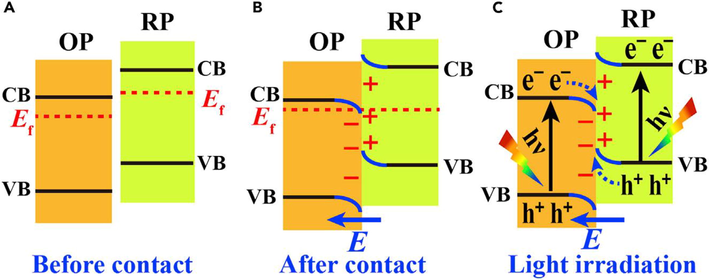
Schematic of S scheme for two general semiconductor OP and RP (A) Before contact (B) After Contact (C) Under light Irradiation (Xu et al., 2020b).
In the S Scheme, there are three main factors involved which act as driven forces band bending, electric force, and Coulombic attraction. This is a useful scheme in the production of hydrogen by using composites for Photocatalytic activity.
7 Conclusion
The production of hydrogen through the photocatalytic activity of composites becomes a great area of interest through water splitting and using organic compounds. As hydrogen production is one of the best fuels to fulfill the energy requirement in the future. This review elaborates on the different CdS/TiO2 and g-C3N4/WO3 composites under different conditions for the photocatalytic activity used for the production of hydrogen. This showed the efficiency of hydrogen production is limited due to different characteristics of semiconductors like fast recombination rate and instability. Therefore, researchers focused to develop a new design for these composites to increase their photocatalytic efficiency. These limitations are reduced by using the co-catalyst, different schemes S and Z, and 2D structure of composite material. Among Schemes, S is best to increase the efficiency of the photocatalytic activity of composites. The 2D structure composites are more efficient in photocatalytic activity and produced a large rate of hydrogen as 2D structure increased active sites for the electron-hole pair which increased the efficiency of photocatalytic activity of composite. Hence, among all 2D composites are best for photocatalytic activity. Moreover, the production of hydrogen through photocatalytic activity and its use as fuel to fight with energy crisis are the top priority of the world as it is environmentally friendly and cost-effective.
8 Future prospective
The future prospective of these composites’ that researchers will study 2D structure of composites with the assistance of the co-catalysts by using the S Scheme and this work will be laid the foundation for hydrogen production as fuel on an industrial scale.
Acknowledgments
The authors express their gratitude to the Deanship of Scientific Research at King Khalid University for funding this work through the Small Research Group Project under grant number RGP.2-122-43. Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R55), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. This study is supported via funding from Prince Sattam bin Abdulaziz University project number (PSAU/2023/R/1444).
Conflict of Interest
It is certified that there is no conflict of interest regarding the material discussed in this manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Recent advancement in conjugated polymers based photocatalytic technology for air pollutants abatement: Cases of CO2, NOx, and VOCs. Chemosphere. 2022;136358
- [Google Scholar]
- Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater.. 2009;170:520-529.
- [Google Scholar]
- The history and future of semiconductor heterostructures. Semiconductors. 1998;32:1-14.
- [Google Scholar]
- Photocatalytic reduction of CO2 with H2O on titanium oxides anchored within micropores of zeolites: effects of the structure of the active sites and the addition of Pt. J. Phys. Chem. B. 1997;101:2632-2636.
- [Google Scholar]
- Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors. J. Photochem.. 1979;10:59-75.
- [Google Scholar]
- Improved photocatalytic activity of WO3 through clustered Fe2O3 for organic degradation in the presence of H2O2. Langmuir. 2011;27:9359-9366.
- [Google Scholar]
- Edge termination of MoS 2 and CoMoS catalyst particles. Catal. Lett.. 2000;64:95-99.
- [Google Scholar]
- Preparation and characterization of WO 3/Bi 3 O 4 Cl nanocomposite and its photocatalytic behavior under visible light irradiation. React. Kinet. Mech. Catal.. 2012;106:83-98.
- [Google Scholar]
- Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod.. 2020;268:121725
- [Google Scholar]
- Study on the separation mechanisms of photogenerated electrons and holes for composite photocatalysts g-C3N4-WO3. Appl. Catal. B. 2014;150:564-573.
- [Google Scholar]
- In-situ reduction synthesis of nano-sized Cu2O particles modifying g-C3N4 for enhanced photocatalytic hydrogen production. Appl. Catal. B. 2014;152:335-341.
- [Google Scholar]
- WO3/g-C3N4 composites: one-pot preparation and enhanced photocatalytic H2 production under visible-light irradiation. Nanotechnology. 2017;28:164002
- [Google Scholar]
- Chiarello, G. & Selli, E. 2014. Photocatalytic production of hydrogen. Advances in hydrogen production, storage and distribution. Elsevier.
- Facile preparation of Z-scheme WO3/g-C3N4 composite photocatalyst with enhanced photocatalytic performance under visible light. Appl. Surf. Sci.. 2017;391:202-210.
- [Google Scholar]
- Review on metal sulphide-based Z-scheme photocatalysts. ChemCatChem. 2019;11:1394-1411.
- [Google Scholar]
- In situ construction of g-C3N4/g-C3N4 metal-free heterojunction for enhanced visible-light photocatalysis. ACS Appl. Mater. Interfaces. 2013;5:11392-11401.
- [Google Scholar]
- The high impact of solution flow rate on optical properties of TiO2 thin layers for optoelectronic applications. Cryst. Res. Technol. 20222200129
- [Google Scholar]
- An overview on WO3 based photocatalyst for environmental remediation. J. Environ. Chem. Eng.. 2021;9:105018
- [Google Scholar]
- Water oxidation catalysis by using nano-manganese ferrite supported 1D-(tunnelled), 2D-(layered) and 3D-(spinel) manganese oxides. J. Mater. Chem. A. 2016;4:8812-8821.
- [Google Scholar]
- MnO2 nanowires anchored on mesoporous graphitic carbon nitride (MnO2@ mpg-C3N4) as a highly efficient electrocatalyst for the oxygen evolution reaction. Int. J. Hydrogen Energy. 2019;44:17995-18006.
- [Google Scholar]
- Water oxidation catalysis by birnessite@ iron oxide core–shell nanocomposites. Inorg. Chem.. 2015;54:2734-2741.
- [Google Scholar]
- Enhanced water oxidation performances of birnessite and magnetic birnessite nanocomposites by transition metal ion doping. Sustain. Energy Fuels. 2020;4:3157-3166.
- [Google Scholar]
- A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy. 2019;44:540-577.
- [Google Scholar]
- Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B. 2019;243:556-565.
- [Google Scholar]
- Synthesis and efficient visible light photocatalytic hydrogen evolution of polymeric g-C3N4 coupled with CdS quantum dots. J. Phys. Chem. C. 2012;116:13708-13714.
- [Google Scholar]
- Synthesis and characterization of composite visible light active photocatalysts MoS2–g-C3N4 with enhanced hydrogen evolution activity. Int. J. Hydrogen Energy. 2013;38:6960-6969.
- [Google Scholar]
- Improved photocatalytic H2 evolution over composites based on niobium pentoxide, metal sulfides and graphene. Mater. Sci. Semicond. Process.. 2021;122:105492
- [Google Scholar]
- Photocatalytic H2 evolution under visible light irradiation on CdS/ETS-4 composite. Chem. Phys. Lett.. 2004;385:319-322.
- [Google Scholar]
- Electrostatic-driven exfoliation and hybridization of 2D nanomaterials. Adv. Mater.. 2017;29:1700326
- [Google Scholar]
- WO3/g-C3N4 two-dimensional composites for visible-light driven photocatalytic hydrogen production. Int. J. Hydrogen Energy. 2018;43:4845-4855.
- [Google Scholar]
- Green, turquoise, blue, or grey? environmentally friendly hydrogen production in transforming energy systems. Prog. Energy Combust. Sci.. 2022;90:100996
- [Google Scholar]
- A versatile strategy for shish-kebab-like multi-heterostructured chalcogenides and enhanced photocatalytic hydrogen evolution. J. Am. Chem. Soc.. 2015;137:11004-11010.
- [Google Scholar]
- Strategies to enhance photocatalytic activity of graphite carbon nitride-based photocatalysts. Mater. Des.. 2021;210:110040
- [Google Scholar]
- Visible-light-induced WO 3/gC 3 N 4 composites with enhanced photocatalytic activity. Dalton Trans.. 2013;42:8606-8616.
- [Google Scholar]
- Highly active and stable CdS–TiO2 visible photocatalyst prepared by in situ sulfurization under supercritical conditions. Appl. Catal. B. 2011;106:69-75.
- [Google Scholar]
- Energy band gaps and optical absorption properties of the CdZns and CdZns: PEO thin films prepared by chemical bath deposition. Chalcogenide Lett.. 2022;19:329-335.
- [Google Scholar]
- Bringing conjugated polymers and oxide nanoarchitectures into intimate contact: Light-induced electrodeposition of polypyrrole and polyaniline on nanoporous WO3 or TiO2 nanotube array. J. Phys. Chem. C. 2012;116:19145-19155.
- [Google Scholar]
- Fabrication of CdS/TiO2 nano-bulk composite photocatalysts for hydrogen production from aqueous H2S solution under visible light. Chem. Phys. Lett.. 2006;425:278-282.
- [Google Scholar]
- Optimization of CdS/TiO2 nano-bulk composite photocatalysts for hydrogen production from Na2S/Na2SO3 aqueous electrolyte solution under visible light (λ≥ 420 nm) J. Photochem. Photobiol. A Chem.. 2007;188:112-119.
- [Google Scholar]
- Simultaneous hydrogen production and decomposition of H2S dissolved in alkaline water over CdS–TiO2 composite photocatalysts under visible light irradiation. Int. J. Hydrogen Energy. 2007;32:4786-4791.
- [Google Scholar]
- Location and state of Pt in platinized CdS/TiO2 photocatalysts for hydrogen production from water under visible light. J. Phys. Chem. C. 2008;112:17200-17205.
- [Google Scholar]
- Carbon nitride coupled with CdS-TiO2 nanodots as 2D/0D ternary composite with enhanced photocatalytic H2 evolution: a novel efficient three-level electron transfer process. Appl. Catal. B. 2017;210:194-204.
- [Google Scholar]
- Organic-inorganic composite of g-C3N4–SrTiO3: Rh photocatalyst for improved H2 evolution under visible light irradiation. Int. J. Hydrogen Energy. 2012;37:11602-11610.
- [Google Scholar]
- Visible-light response photocatalytic water splitting over CdS/TiO2 and CdS–TiO2/metalosilicate composites. Int. J. Energy Res.. 2014;38:1712-1726.
- [Google Scholar]
- Photo-assisted electrolysis of urea using Ni-modified WO3/g-C3N4 as a bifunctional catalyst. Int. J. Hydrogen Energy. 2022;47:5797-5806.
- [Google Scholar]
- The production of methane, hydrogen, and organic compounds in ultramafic-hosted hydrothermal vents of the Mid-Atlantic Ridge. Astrobiology. 2015;15:381-399.
- [Google Scholar]
- Fabrication of the heterostructured CsTaWO6/Au/g-C3N4 hybrid photocatalyst with enhanced performance of photocatalytic hydrogen production from water. Appl. Surf. Sci.. 2015;358:252-260.
- [Google Scholar]
- Photocatalytic water splitting under visible light with particulate semiconductor catalysts. Catal. Surv. Asia. 2005;9:217-227.
- [Google Scholar]
- Growth of CdS nanorod-coated TiO2 nanowires on conductive glass for photovoltaic applications. Cryst. Growth Des.. 2009;9:4519-4523.
- [Google Scholar]
- Nanoflower-like Ti3CN@ TiO2/CdS heterojunction photocatalyst for efficient photocatalytic water splitting. Int. J. Hydrogen Energy. 2022;47:19580-19589.
- [Google Scholar]
- TiO2 nanotubes incorporated with CdS for photocatalytic hydrogen production from splitting water under visible light irradiation. Int. J. Hydrogen Energy. 2010;35:7073-7079.
- [Google Scholar]
- Macro-/nanoporous Al-doped ZnO/cellulose composites based on tunable cellulose fiber sizes for enhancing photocatalytic properties. Carbohydr. Polym.. 2020;250:116873
- [Google Scholar]
- C60-decorated CdS/TiO2 mesoporous architectures with enhanced photostability and photocatalytic activity for H2 evolution. ACS Appl. Mater. Interfaces. 2015;7:4533-4540.
- [Google Scholar]
- Strategies to improve WO3-based photocatalysts for wastewater treatment: a review. J. Mater. Sci.. 2021;56:14416-14447.
- [Google Scholar]
- Charge transmission channel construction between a MOF and rGO by means of Co–Mo–S modification. Cat. Sci. Technol.. 2017;7:4478-4488.
- [Google Scholar]
- Promoting photogenerated holes utilization in pore-rich WO3 ultrathin nanosheets for efficient oxygen-evolving photoanode. Adv. Energy Mater.. 2016;6:1600437
- [Google Scholar]
- 0D (MoS2)/2D (g-C3N4) heterojunctions in Z-scheme for enhanced photocatalytic and electrochemical hydrogen evolution. Appl. Catal. B. 2018;228:64-74.
- [Google Scholar]
- Low, J., Yu, J. & Jiang, C. 2020. Design and fabrication of direct Z-scheme photocatalysts. Interface Science and Technology. Elsevier.
- Present perspectives of advanced characterization techniques in TiO2-based photocatalysts. ACS Appl. Mater. Interfaces. 2017;9:23265-23286.
- [Google Scholar]
- Construction of highly efficient Z-scheme ZnxCd1-xS/Au@ g-C3N4 ternary heterojunction composite for visible-light-driven photocatalytic reduction of CO2 to solar fuel. Appl. Catal. B. 2021;282:119600
- [Google Scholar]
- S-scheme heterojunction of CuBi2O4 supported Na doped P25 for enhanced photocatalytic H2 evolution. Int. J. Hydrogen Energy. 2022;47:8214-8223.
- [Google Scholar]
- Enhanced photocatalytic, antibacterial, and electrochemical properties of CdO-based nanostructures by transition metals co-doping. Adv. Powder Technol.. 2022;33:103451
- [Google Scholar]
- Elucidating the factors affecting hydrogen production activity using a CdS/TiO2 type-II composite photocatalyst. ACS Omega. 2021;6:4395-4400.
- [Google Scholar]
- Recent development in graphitic carbon nitride based photocatalysis for hydrogen generation. Appl. Catal B. 2019;257:117855
- [Google Scholar]
- Efficient photocatalytic H2 evolution: controlled dewetting–dealloying to fabricate site-selective high-activity nanoporous Au particles on highly ordered TiO2 nanotube arrays. Adv. Mater.. 2015;27:3208-3215.
- [Google Scholar]
- Self-assembled hierarchical direct Z-scheme g-C3N4/ZnO microspheres with enhanced photocatalytic CO2 reduction performance. Appl. Surf. Sci.. 2018;441:12-22.
- [Google Scholar]
- Metal oxide-based photocatalytic paper: A green alternative for environmental remediation. Catalysts. 2021;11:504
- [Google Scholar]
- Preparation of novel CdS-graphene/TiO2 composites with high photocatalytic activity for methylene blue dye under visible light. Bull. Mater. Sci.. 2013;36:869-876.
- [Google Scholar]
- Photo-production of hydrogen over the CdS–TiO2 nano-composite particulate films treated with TiCl4. Int. J. Hydrogen Energy. 2004;29:229-234.
- [Google Scholar]
- Emerging architecture titanium carbide (Ti3C2Tx) MXene based photocatalyst toward degradation of hazardous pollutants: Recent progress and perspectives. Chemosphere. 2022;133541
- [Google Scholar]
- Visible-light photocatalytic hydrogen production from ethanol–water mixtures using a Pt–CdS–TiO2 photocatalyst. Catal. Today. 2010;151:53-57.
- [Google Scholar]
- Structural distortion of g-C3N4 induced by N-defects for enhanced photocatalytic hydrogen evolution. Catalysts. 2022;12:1496.
- [Google Scholar]
- Copper sulfides based photocatalysts for degradation of environmental pollution hazards: A review on the recent catalyst design concepts and future perspectives. Surfaces and Interfaces 2022:102182.
- [Google Scholar]
- Fabrication of composite photocatalyst gC 3 N 4–ZnO and enhancement of photocatalytic activity under visible light. Dalton Trans.. 2012;41:6756-6763.
- [Google Scholar]
- Photocatalytic water splitting: quantitative approaches toward photocatalyst by design. ACS Catal.. 2017;7:8006-8022.
- [Google Scholar]
- Theory of semiconductor heterojunctions: The role of quantum dipoles. Phys. Rev. B. 1984;30:4874
- [Google Scholar]
- Mixed-calcination synthesis of CdWO4/g-C3N4 heterojunction with enhanced visible-light-driven photocatalytic activity. Appl. Surf. Sci.. 2015;358:343-349.
- [Google Scholar]
- A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater.. 2009;8:76-80.
- [Google Scholar]
- Synchronous surface hydroxylation and porous modification of g-C3N4 for enhanced photocatalytic H2 evolution efficiency. Int. J. Hydrogen Energy. 2016;41:3888-3895.
- [Google Scholar]
- Construction of Bi-assisted modified CdS/TiO2 nanotube arrays with ternary S-scheme heterojunction for photocatalytic wastewater treatment and hydrogen production. Fuel. 2022;322:124163
- [Google Scholar]
- What if the electrical conductivity of graphene is significantly deteriorated for the graphene–semiconductor composite-based photocatalysis? ACS Appl. Mater. Interfaces. 2015;7:27948-27958.
- [Google Scholar]
- Assembly of nanoparticles in zeolite Y for the photocatalytic generation of hydrogen from water. J. Phys. Chem. C. 2011;115:2938-2947.
- [Google Scholar]
- Preparation of WO 3/gC 3 N 4 composites with enhanced photocatalytic hydrogen production performance. Appl. Phys. A. 2019;125:1-7.
- [Google Scholar]
- Making co-condensed amorphous carbon/g-C3N4 composites with improved visible-light photocatalytic H2-production performance using Pt as cocatalyst. Carbon. 2017;118:241-249.
- [Google Scholar]
- Enhanced visible-light photocatalytic H 2-generation activity of carbon/gC 3 N 4 nanocomposites prepared by two-step thermal treatment. Dalton Trans.. 2017;46:10611-10619.
- [Google Scholar]
- Constructing 2D/2D Fe2O3/g-C3N4 direct Z-scheme photocatalysts with enhanced H2 generation performance. Solar Rrl. 2018;2:1800006
- [Google Scholar]
- Organic–inorganic composite photocatalyst of gC 3 N 4 and TaON with improved visible light photocatalytic activities. Dalton Trans.. 2010;39:1488-1491.
- [Google Scholar]
- Compounds, Synthesis, formation mechanism and illuminated sensing properties of 3D WO3 nanowall. J. Alloy. Compd.. 2010;495:88-92.
- [Google Scholar]
- Ni-Mo-S nanoparticles modified graphitic C3N4 for efficient hydrogen evolution. Appl. Surf. Sci.. 2018;427:587-597.
- [Google Scholar]
- Recent advances in CdS-based photocatalysts for CO2 photocatalytic conversion. Chem. Eng. J.. 2021;418:129344
- [Google Scholar]
- Noble-metal-free cobalt phosphide modified carbon nitride: an efficient photocatalyst for hydrogen generation. Appl Catal B. 2017;200:477-483.
- [Google Scholar]
- Metal-organic framework as a photocatalyst: Progress in modulation strategies and environmental/energy applications. Prog. Energy Combust. Sci.. 2020;81:100870
- [Google Scholar]
- Noble-metal-free janus-like structures by cation exchange for Z-Scheme photocatalytic water splitting under broadband light irradiation. Angew. Chem.. 2017;129:4270-4274.
- [Google Scholar]
- Performance of WO3/g-C3N4 heterojunction composite boosting with NiS for photocatalytic hydrogen evolution. Appl. Surf. Sci.. 2020;499:143862
- [Google Scholar]
- A facile band alignment of polymeric carbon nitride semiconductors to construct isotype heterojunctions. Angew. Chem.. 2012;124:10292-10296.
- [Google Scholar]
- Assembly, characterization, and photocatalytic activities of TiO2 nanotubes/CdS quantum dots nanocomposites. J. Nanopart. Res.. 2011;13:6661-6672.
- [Google Scholar]
- 3D g-C3N4/WO3/biochar/Cu2+-doped carbon spheres composites: Synthesis and visible-light-driven photocatalytic hydrogen production. Mater. Today Commun.. 2022;30:103084
- [Google Scholar]
- CdS nanocrystals/TiO2/crosslinked chitosan composite: Facile preparation, characterization and adsorption-photocatalytic properties. Appl. Surf. Sci.. 2013;273:661-669.
- [Google Scholar]







