Translate this page into:
Review on applications of carbon nanomaterials for simultaneous electrochemical sensing of environmental contaminant dihydroxybenzene isomers
⁎Corresponding author. filik@istanbul.edu.tr (Hayati Filik)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Carbonaceous nanostructures stand out as an excellent electrode material to enhance the electrocatalytic, electroconductivity, and long-term stability of the electrochemical sensor in recent years. This review article focus on the important advancement in developing carbonaceous nanomaterials-based electrochemical sensors for simultaneous electrochemical sensing (binary and ternary mixtures) of environmental contaminants dihydroxybenzene isomers. The fabrication of electrochemical sensors such as graphene/carbon nanotubes hybrid composite, graphene/ carbon nanotubes supported nanomaterials, mesoporous carbon, carbon nanofiber, carbon nano-fragment and biochar modified electrode was presented coupled with suitable applications. This review discussed the selective reports on the application of dihydroxybenzene sensors during the period from 2015 to 2020.
Keywords
Carbon nanomaterials
Nanotubes
Graphene
Dihydroxybenzene
Hydroquinone
Catechol
Resorcinol
Isomers
Pollutants
1 Introduction
Hydroquinone (HQ), catechol (CC) and resorcinol (RC) are three isomers of dihydroxybenzene (DHB). The above-mentioned compounds are important chemicals in industrial production and traces of these toxic compounds are widely distributed in our ecosystem, in particular water sources (Suresh et al., 2012). HQ, CC and RC are important chemical materials in diverse formulated products (such as dyes, medications, cosmetics, pesticides, and so on) (DeSelms, 2008; Svobodová et al., 2003). The chemical structures of these DHBs are shown in Scheme 1. In environmental water samples, the three DHB isomers usually co-exist as pollutants. The DHB isomers can be the source of serious health risks if discharged into the environment (Bieniek, 1996; Marcheterre et al., 1988). Huge levels of DHB isomers could be prompting some illnesses such as kidney damage, tachycardia, cancer and even death. Hence, synchronous detection of DHB isomers with similar chemical characteristics is also a sensible interest in analytical chemistry.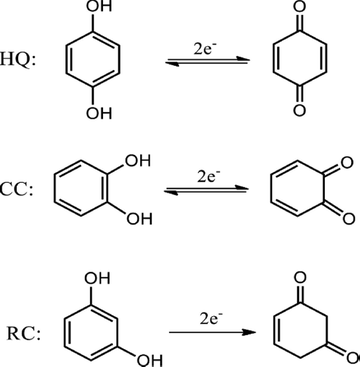
The chemical structure and the possible electrooxidation mechanism of HQ, CC and RC (Meng et al. 2017; Patel et al. 2020).
The gold standard methods such as chromatographic (Gimeno et al., 2015; Htet et al., 2016; Kanamori et al., 2015), chemiluminescence (Han et al., 2016; Yang et al., 2017a), capillary electrophoresis (Xie et al., 2006; Yang et al., 2001), phosphorescence (Wang et al., 2013), fluorescence (Patil et al., 2017) or spectrophotometric (Khoshneviszadeh et al., 2015) based-methods are assigned as expensive, laborious and time-consuming techniques, which illustrate the necessity for new approaches for on-site application (Batchelor-McAuley et al., 2012; Chaplin, 2018; Girault, 2004). The unmodified or bare working electrodes have a poor electrochemical oxidation/reduction current response of these mentioned-above DHB compounds, it is challenging to simultaneously identify the two or three DHB isomers applying unmodified electrodes. Consequently, it is always necessary to form a new effective working electrode by decorating some nano-sized substances. Carbon-based nano-sized materials have been esteemed potent supporting materials for various potential implementations such as sensors, catalysis, and energy devices due to their remarkable characteristics. Over the past years, graphene oxide (GO), graphene (Gr) and reduced graphene oxide (RGO) have drawn much attention due to its privileged features (Li et al., 2017a; Li et al., 2017b; Qiu et al., 2016; Smith et al., 2019; Tarcan et al., 2020; Yu et al., 2020) The outstanding physical, chemical features, such as broad surface area, ideal electrical conductivity, ease of surface modification and exceptional bio-conformity have exhibited great capabilities in the implementations of graphene-based sensors, including electrochemical sensing platforms for analysis of electroactive species. Renovation of the physical architecture of pristine-graphene (p-Gr) can almost feasibly by the reduction of GO (Zhu et al., 2010). To remove the oxygen-containing functional groups, GO can be reduced by chemical, electrochemical, thermal and multistep reduction process (Li et al., 2017a; Li et al., 2017b; Smith et al., 2019; Tarcan et al., 2020; Yu et al., 2020). Hereby, GO can be fundamentally reduced to form RGO. Nevertheless, the quantity of leftover functional oxygen-containing groups seriously affected the electrical capability of RGO which depends on the functional oxygen-containing groups that remaining after the decrease procedure. The synthesized RGO has the highest electrical, thermal and mechanical features (Li et al., 2017a; Li et al., 2017b; Smith et al., 2019; Tarcan et al., 2020; Yu et al., 2020). Although the electrical conductivity of GO is not as great as Gr, it is also considered as an appropriate candidate for sensing application (Li et al., 2008). Consequently, a broad variety of graphene-based nano-sized materials have been ameliorated, for instance, RGO, GO, N-doped Gr, and flower-like Gr. GO and RGO is known to have superior characteristics for several graphene-based electrochemical sensor implementations (Chen and Chatterjee, 2013; Chen et al., 2010; Compton and Nguyen, 2010; Mahmoudi et al., 2019).
On the other hand, carbon nanotubes (CNTs) (Multi-walled carbon nanotubes MWCNTs or single-wall carbon nanotubes SWCNTs) have been largely utilized in electrochemical sensor implementations because of their unique porous architecture, the largest surface area, long term chemical stability, elevated electron transfer rates, and robust mechanic features (Ibrahim, 2013; Janas, 2018). However, electrode adaptation using pristine CNTs surfaces some adversities such as insolubility and activity. To remove these drawbacks, the chemical or electrochemical functionalization of CNTs (f-CNTs) can improve the electrochemical feature and solubility of pristine CNTs due to the presence of carbonyl and hydroxyl functional groups in the sidewalls of functioned-CNTs. Latest researches have shown that the CNT-based electrochemical sensors offered poor back-ground peak current, reproducible sensor activity and convenient physical features, which make them adequate efficient for actual implementations and serial fabrication (Chen and Chatterjee, 2013; Rahman et al., 2019).
Conducting polymers (CPs) are electrically conductive matters formed of organic polymers; their significant benefit is their processability. Material specifications of CPs are similar to those of some metals and inorganic semiconductor materials while maintaining polymer specifications such as construction, flexibility and ease of processing, frequently correlated with regular functional polymers (Nambiar and Yeow, 2011). The organisation of the extraordinary attributes of carbonaceous substance (CNTs, GO, RGO and so on) with CPs builds of these nanomaterials exceptional multifunctional systems with outstanding potential in diverse employments such as sensors, photovoltaic cells, photodiodes, supercapacitors and so on. Due to these characteristics, the investigative efforts in CPs have enlarged plentiful traction to compose several classes of CPs since its investigation four decades ago. CPs are regularly classified into different cases based on the nature of electric charges (e.g., delocalized Pi electrons (π electrons), ions, or conductive nanomaterials) trustworthy for conduction. By changing the electrochemical parameters, the layer thickness, permeability and charge transfer properties can be managed. Electropolymerization, among deposition techniques, is a mild approach to immobilize polymers to generate a proper substrate for sensors. CPs contain alternative single and double bonds in their polymer chain, resulting in the production of delocalized electrons which serve as charge transporters (Das and Prusty, 2012; Nambiar and Yeow, 2011; Yang et al., 2017b). To promote sensitivity and selectivity of the electrochemical sensors, redox mediators are dispersed, added as dopants or chemically conjugated into the polymer pattern (Das and Prusty, 2012).
There has been an increasing number of reports on the development and application of carbon nanomaterials (CNMs) in electrochemical sensors because CNMs-based electrochemical sensors generally have higher sensitivities, lower limit detection, and faster electron transfer kinetics than traditional detection electrodes. Patel et al. (2020) and Wang and Hu (2016) reported an effort to mainly highlight the recent progress and focused on the electrochemical sensors based on CNTs and GR for sensing of environmental pollutants such as heavy metals, organophosphorus pesticides, some small organic pollutants, antibiotics and pathogens, etc. Electrochemical sensors remain an attractive option for the fast and sensitive determination of DHB pollutants. In this review article, we evaluate the state-of-art of Gr/CNTs hybrids composite, Gr/CNTs supported nanomaterials, mesoporous carbon, carbon nanofiber and carbon nano-fragment sensors developed for the simultaneous analysis of DHB isomers. Furthermore, we have orderly evaluated nanomaterial-based design approaches and the state-of-art of nanomaterial-based sensing platforms. Following that, we indicated the sensing performance of the described sensors and sample utilisation.
2 Electrochemical behavior of DHBs
In water samples, the three DHB isomers usually co-exist as pollutants. Due to three isomers have similar stereochemical structure and contiguous redox potentials on the conventional electrode, the simultaneous determination of them is nearly impossible. To overcome these shortcomings, various nanomaterials modified electrodes have been used to the simultaneous determination of them. Usually, experiments with cyclic voltammetry (CV) can be utilized to study the kinetics of the electrocatalytic reactions of the DHBs on modified electrodes and also to determine the active surface area of the nanocomposite modified electrodes. Generally, CV investigations are performed for the modified electrodes in the presence of a redox probe such as [Fe(CN)6]3−/4− at different scan rates (0.01–0.5 V/s). The peak currents (anodic and the cathodic) can be plotted against either the scan rate or the square root of the scan rate. If the plot displays a linear dependence of peak current with scan rate (Ip-v) then it usually means it is a surface-controlled process. If the plot displays a linear dependence of the anodic and the cathodic peak currents with the square root of the scan rate (Ip-v1/2) then it usually means a diffusion-controlled process. pH effect study can be useful in providing substantial knowledge about the electrooxidation mechanisms of the redox reactions occurring on the surface of modified electrodes. Usually, an increase in the pH of the analyte solution would result in shifting of the peak potential towards lower potential, which indicates the involvement of the protons during the electrode reactions. Based on previous studies, the electrochemical behavior of RC was correctly different from that confirmed by the other two isomers (HQ and CC) and no reverse current signal has appeared. The two-electron and two-proton oxidation mechanism do not help in generating a stable quinone with RC due to its meta structure. This decision can be described as the position of the two hydroxyl functional groups on the HQ, CC and RC benzene rings are different, and so the charge density population is different (Scheme 1). Besides, HQ and CC have a reversible quinolinic form, and a cathodic reduction peak appears in their CVs. Recent studies by Fotouhi et al. (2018); Kokulnathan et al. (2017); Meng et al. (2017); Yang et al. (2019) reported that all three DHB isomers underwent two-electron and two-proton processes as their electrooxidation mechanisms (Scheme 1). However, other researchers such as Edris et al. (2019); Nady et al. (2017); Sabbaghi and Noroozifar (2019); Tohidinia et al. (2018) have reported contrasting electrooxidation mechanism (Scheme 2). For RC, the resonance between the hydroxyl group and the π electrons is compromised due to its meta-structure which impedes its oxidation and normally requires overpotential to undergo oxidation. The RC undergoes keto-enol tautomerism reaction mechanism, which would lead to polymerization involving the hydroxyl groups of the RC.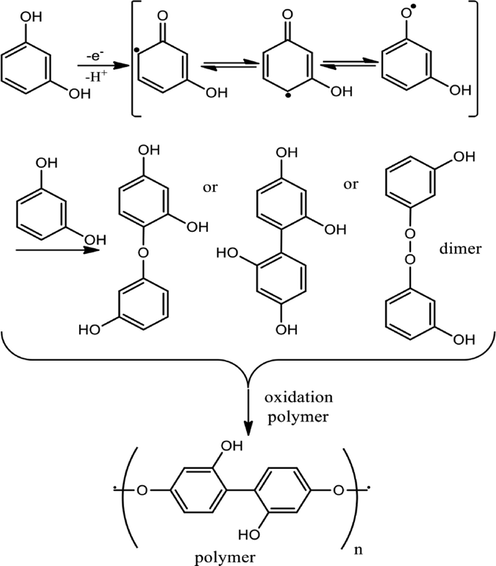
Proposed keto-enol tautomerism redox mechanism for electrooxidation of RC (Tohidinia et al. 2018; Patel et al. 2020).
3 Representative applications
3.1 Graphene/carbon nanotube hybrid nanocomposite
The synthesis/fabrication of graphene and carbon nanotube hybrid structure (GNHSs) generally can be categorized into four different approaches, including solution processing/casting, layer-by-layer deposition, vacuum filtration and chemical vapour deposition (CVD) (Fan et al., 2010; Ong et al., 2019; Wang and Hu, 2016; Zhu et al., 2012). The development of graphene and graphene-based structure is currently in a fast line with enormous research achievement on a daily basis. GNHSs is one of the promising graphene derivatives and material scientists have devoted tremendous effort in realizing their potential applications in areas such as energy storage, supercapacitor and building blocks composite (Fan et al., 2010). Experimental studies have shown that nicely aligned hybrid structures between graphene and carbon nanotube are achievable. Their highly tailorable structures endow them with highly tunable mechanical properties. GNHS is one of the promising graphene derivates. The structure of the GNHS composite was highly porous, with carbon nanotubes sandwiched between graphene sheets that functioned as spacers and provided diffusion paths for smooth and rapid ion conduction. The surfaces of GO sheets are highly negatively charged, apparently as a result of the existence of the carboxylic and hydroxyl groups on these sheets. Yang and Weikun (2015) have prepared the hybrid MWCNTs in graphene oxide-cetyl trimethylammonium bromide (CTAB) composites modified GCE for the determination of trace DHB isomers by differential pulse voltammetry (DPV). Although CNT is a neutral, CTAB/CNTs is negatively and positively charged, respectively. For this reason, there could be a tight connection between each surfactant and CNTs. In cases of CTAB/GNHS, they are also negatively and positively charged, respectively. However, the size of these hybrids decreased. From this, they have a good connection between CNT with each surfactant and GO. Graphene oxide nanoribbons (GONRs) are thin strips of Gr. The composition and physical characteristics of GONRs differ significantly depending on the construction technique (Jalalvand and Goicoechea, 2017). The MWCNTs coupled with RGONR based sensing platform was prepared by Yang et al. (2019) for simultaneous detection of HQ, CC and RC by DPV with excellent performance. In this study, the reduced graphene oxide nanoribbon (RGONR) composite was synthesized via hydrothermal method. Chen et al. (2019b) have also demonstrated that three-dimensional (3D) CNT-graphene decorated with gold nanoparticles (AuNPs) was modified onto GCE (AuNPs/CNTs/Gr/GCE) surface through casting and subsequent electrodeposition method. The resultant electrode was employed as an electrochemical interface for simultaneous determination of DHB isomers of HQ, CC and RC. According to the reported results (Table 1), the CTAB cationic surfactant functionalized MWCNTs/GO nanocomposite (Yang and Weikun, 2015) exhibited a very wide linear range and low detection limit compared to other reported hybrid composites such as MWCNTs coupled with RGONR (Yang et al., 2019) and 3D CNT-Gr decorated with AuNPs (Chen et al., 2019b). Abbreviations: 3D: Three-dimensional, AuNPs: Gold nanoparticles, B-Gr: Boron-doped graphene, CDs: Carbon dots, CFs: Carbon nanofibers, Chit: Chitosan, CL: Carbon cloth, CNCs: Carbon nanocages, CN-F: Carbon nano-fragment, CNNS: Graphitic carbon nitride nanosheets, CS: Core-shell, CTAB: Cetyl trimethylammonium bromide, ERGO: Electrochemically reduced graphene oxide, f-MWCNT: Functionalized MWCNTs, GCE: Glassy carbon electrode, GO: Graphene oxide, Gr: graphene, Gr-CNS: Graphene-like carbon nanosheets, HMCCSs: Hollow Mo2C/carbon spheres, LDR/LOD: Linear detection range/detection limit, MOF: Metal-organic framework, MPC: Mesoporous carbon, MWCNTs: Multi-walled carbon nanotubes, NF: Nanoflower, NMCS: N-doped mesoporous carbon spheres, NR: Nanorods, Nr: Not reported, PDA: poly(dopamine), PDDA: poly(diallyl-dimethylammonium chloride, PEBT: poly(Eriochrome black T), PEDOT: poly(3, 4-ethylenedioxythiophene), pMel: poly(melamine), P-RGO: Porous RGO, p-Trp: poly-tryptophan, RGO: Reduced graphene oxide, RGONR: Reduced graphene oxide nanoribbon, WBC: White myoga ginger-derived biochar.
Modified electrode
Detection Technique - pH
Water Sample
HQ LDR/LOD (µM)
CC LDR/LOD (µM)
RC LDR/LOD (µM)
Reference
CTAB-GO/MWCNTs/GCE
CV-7.0
Tap
0.1–200/0.03
0.1–400/0.01
1–100/0.2
Yang and Weikun (2015)
MWCNTs@RGONR/GCE
DPV-7.0
Tap, River
15–921/3.89
15–1101/1.73
15–1301/5.77
Yang et al. (2019)
3D CNT-Gr/AuNPs/GCE
DPV-5.7
Tap, River
0.0–80/0.80
0.0–80/0.95
0.0–80/0.10
Chen et al. (2019b)
Au@PdNF/RGO/GCE
DPV-7.0
Tap, River, Lake
1.6–100/0.5
2.5–100/0.8
2.0–100/0.7
Chen et al. (2017)
Au@Pd/RGO/GCE
DPV-8.0
Tap Lake
0.01–400/0.01
0.1–400/0.1
–
Wang et al. (2018)
P-RGO/GCE
DPV-7.0
Tap
5–90/0.08
5–120/0.18
5–90/0.62
Zhang et al. (2015a)
ZnO/Gr-Ta
CV-7.0
Nr
0–70/0.1
0–80/0.2
0–700/1.0
Ge et al. (2015)
ZnONR/CL/GCE
DPV-7.0
Nr
2.0–30/0.57
2.0–45/0.81
2.0–385/7.2
Meng et al. (2017)
NiO/MWCNT/GCE
DPV-6.0
Nr
7.4–56/0.039
7.4–56/0.015
–
Goulart and Mascaro (2016)
NiO/MWCNT/GCE
DPV-7.0
Tap
10–500/2.5
10–400/2.5
–
Zhao et al. (2018)
Co3O4/CS/GCE
DPV-7.0
River
0.8–127/0.03
0.6–116.4/ 0.03
–
Zhou et al. (2019)
p-Trp/Gr/GCE
DPV-7.0
Tap
5–300/0.221
5–100/0.086
–
Jiang et al. (2015)
MWCNTs/PDDA/Gr/GCE
DPV-7.0
Tap, Seawater
0.5–400/0.02
0.5–400/0.018
–
Song et al. (2015)
CuNP/pMeL/ERGO/GCE
DPV-7.0
Tap and Lake
2.0–566/0.21
2.0–181/0.15
–
Dorraji and Jalali (2015)
MWCNTs/PDA/AuNPs/ Chit/GCE
DPV-7.0
Tap, Lake
0.1–10/0.035
0.1–10/0.047
–
Wang et al. (2016)
PEDOT/Gr/GCE
DPV-7.0
–
5.0–250/0.06
2.00–400/0.08
1.0–250/0.16
Tian et al. (2017)
HMCCSs/GCE
DPV −7.0
River,Tap, Vegetable
0.30–1000/ 0.12
2.00–2000/0.19
–
Ren et al. (2019)
NMCS-Gr/GCE
DPV-6.0
Tap
0.5–400/0.15
1–300/0.3
3–200/1
Peng et al. (2018)
PEBT/AuNPs/ERGO/GCE
DPV-6.0
Tap
0.52–31.7/0.015
0.44–31.2/0.008
–
Mohammed Modawe Alshik Edris et al. (2019)
Chit/f-MWCNTs/GCE
DPV-7.0
Pond, Tap, Drinking
0.09–171/0.027
0.09–155/ 0.029
0.3–175/ 0.11
Kokulnathan et al. (2017)
Chit/MWCNTs/TiO2/GCE
DPV −7.0
River, Tap
0.4–276/0.06
0.4–159/ 0.07
3.0–657/0.52
Fotouhi et al. (2018)
CDs/MWCNTs/GCE
DPV-5.4
Tap
0.1–200/0.03
0.1–200/0.03
–
Gu et al. (2018)
CNCs-RGO/GCE
DPV-4.0
Tap
1–300/0.87
1–400/0.4
–
Huang et al. (2015)
Gr-CNS/GCE
DPV-7.0
Tap
0.1–30/0.02
0.5–50/0.05
–
Jiang et al. (2017)
B-Gr/GCE
DPV-5.6
Tap
5–100/0.3
1–75/0.2
–
Zhang et al. (2015c)
CNNS-CNT/GCE
DPV-7.0
Tap
1–200/0.13
1–250/0.09
–
Zhang et al. (2015b)
N,S-MPC/GCE
DPV-4.5
Tap
1–110/0.056
1–110/0.209
–
Xu et al. (2016)
Fe/MPC/GCE
AMP-7.0
Tap-Lake
0.1–120/0.014
1–120/0.033
–
Huang et al. (2018)
UiO-66/MPC-3/GCE
DPV-6.0
Tap-Lake
0.5–100/0.056
0.4–100/0.072
30–400/3.51
Deng et al. (2017)
Cu-MOF-Gr/GCE
DPV-7.0
Tap water
1.0–100/0.59
1.0–100/0.33
–
Li et al. (2018)
MOF@Pt@MOF-RGO/GCE
DPV-7.0
Lake River
0.05–200/0.015
0.1–160/0.032
0.4–300/0.133
Ye et al. (2019)
CFs-Sm2O3/GCE
DPV-5.5
Tap-Lake
1–500/ 0.09
1–500/ 0.07
–
He et al. (2018)
CuS-CN-F/GCE
DPV-5.0
Tap
3–200/0.293
7–150/0.259
–
Alshahrani et al. (2019)
CN-F/GCE
DPV-5.9
River
6–200/0.25
2–200/0.1
–
Alshahrani et al. (2018)
CN-F/GCE
DPV-6.0
River
10–120/0.5
10–120/0.8
10–120/0.4
Liu et al. (2016)
WBC/Au-850–15/GCE
DPV-6.0
Tap
0.008–7.0/ 0.002
0.01–7.0/0.004
–
Wang et al. (2020)
A summary of the recent nanocomposite-based simultaneous electrochemical determination of DHB isomers has been collated in Table 1.
3.2 Carbon material-supported bimetallic composites
Bimetallic nanoparticles, composed of two distinct metal elements are a new class of nanomaterials. On the other hand, they exhibited different properties than their bulk bimetallic materials; on the other hand, they exhibit unique properties that differ from those of the corresponding pure monometallic counterparts and their physical mixing. Bimetallic materials have presented exceptional potential in chemical catalytic utilisation owing to their unusual microstructures and improved catalytic efficiency. The properties of bimetallic nanoparticles are also dependent on their sizes. Bimetallic (Au@Pd) nanoparticles are the most ordinarily applied reactant material. Au@Pd nanoparticles serve as valuable signal amplifiers can be anchored to the surface of RGO sheets to extra magnify the catalytic oxidation peak currents (Mejía-Rosales et al., 2007; Tadayon et al., 2016). Chen et al. (2017) and Wang et al. (2018) demonstrated the synthesis of Au@Pd nanoflower (PdNF)/RGO (Au@PdNF/RGO) and Au@Pd/RGO, respectively for simultaneous determination of DHB isomers. The developed Au@PdNF/RGO modified glassy carbon electrode (GCE) combined the unique conductivity of RGO with the superior catalytic ability of the Au@Pd. In this work, the Au@PdNFs/RGO nanomaterials were deposited onto the GCE using CV. The combination of excellent electrocatalytic of Au@Pd and tremendous conductivity of RGO showed excellent performance. The modified electrodes have exhibited high sensitivity for the detection of three target isomers with good activity in comparison to AuNPs/RGO-GCE and RGO/GCE. The detection limits (LODs) were 0.5 µM, 0.8 µM and 0.7 µM, respectively. Wang et al. (2018) also reported that the Au@Pd nanoplates were loaded onto RGO to give an Au@Pd/RGO nanohybrid to obtain Au@Pd/RGO/GCE. The detection limit was 0.01 µM for HQ and 0.1 µM for CC. It was found that the Au@Pd/RGO modified electrode showed high electrochemical activity than the electrodeposited Au@PdNF/RGO modified GCE. Consequently, the Au@Pd/RGO composite electrode has a large surface area which creates high catalytic activity; therefore, this electrode has a wide linear range (0.01–400 for HQ and 0.1–400 for CC) and detection limit for simultaneous detection of HQ and CC.
3.3 Carbon material-supported metal oxides
Metal oxide nanoparticles (MeONPs: ZnO, MnO2, NiO and so on) have excited much sensation in recent decades owing to their huge surface region, selectivity, tunable morphologies, and remarkable catalytic activity (Narayan et al., 2019; Ong et al., 2017; Ong et al., 2019) MeONPs are one of extraordinary one-dimensional (1D) nanotextures (such as nanowires, nanorods, nanotubes, etc.). The MeONPs showed a microstructure with a homogeneous dispersion of graphene in the matrix (Ding et al., 2005). Single-layer MeO nanosheets have gained important research attention in the areas of biosensing and biomedicine. Mesoporous MeO nanosheets exhibit a large surface area, intense and broad optical absorption, strong oxidation ability, exceptional catalytic activity, and potent mechanical characters (Chen et al., 2019a). All these properties make MeO nanosheets useful candidates for electrochemical sensors. Several techniques have been proposed for the synthesis nanometal oxides such as direct current (DC) arc plasma jet; chemical vapour deposition (CVD), sol–gel synthesis, microwave synthesis, atomic layer deposition, chemical bath deposition (CBD) and so on. In this regard, Zhang et al. (2015a) revealed that ZnO nanorods (ZnONR) as electrodes inhibit aggregation of RGO leaves and support form a porous building. In a neutral aqueous solution, the positively charged ZnO nanospheres and negatively charged GO can be easily combined by the strong electrostatic synergy. After direct electrochemical reduction of GO onto GCE, ZnO quickly discarded by HCl to reach the porous RGO (P-RGO)/GCE. On the other hand, the current response of P-RGO/GCE was approximately two-fold compared with that of electrochemically reduced graphene oxide (ERGO) ERGO-GCE. Fig. 1 shows the preparation of P-RGO/GCE sensor for simultaneous detection of three DHB isomers. Ge et al. (2015) detailed the synthesis of few-layered Gr nanosheets on a Ta thin sheet by a direct current (DC) arc plasma jet CVD protocol. Sequentially, the ZnO/Gr-Ta sensing electrode was collected. The resulting electrode exhibited a unique conductivity and electron-transfer rate than the Gr/GCE. The simultaneous oxidation of three isomers was realized in PBS (pH 7.0). ZnONRs with an average diameter of 50 nm were uniformly anchored on the surface of carbon cloth directly by a simple hydrothermal method. The ZnONRs growing in situ along the specific direction of (0 0 2) have single-crystalline features and a columnar structure. Based on the ZnONR/carbon cloth (ZnO NR/CL) composite, free-standing electrodes were fabricated for the simultaneous determination of DHB isomers (Meng et al., 2017). NiO is emerging electrode element for energy storage utilisation owing to its thermal resistance, high chemical durability, high theoretical distinct ability, low cost, typically plentiful and environment-friendliness. In this context, NiO-based composite electrodes have also been employed for simultaneous detection of bisphenol A (BPA), HQ and CC (Goulart and Mascaro, 2016). This work reported the electrochemical determination of BPA, HQ and CC using GCE modified with MWCNT/NiOPs. Goulart and Mascaro (2016) and Zhao et al. (2018) have also prepared and reported the NiO/MWCNTs modified GCE (NiO/MWCNTs/GCE). Goulart and Mascaro (2016) demonstrated that NiONPs were installed on MWCNTs surface to create a nanocomposite structure employing simple chemical bath deposition (CBD) technique. Atomic layer deposition (ALD) has emerged as an extensive and robust vapour-phase deposition procedure. Zhao et al. (2018) have prepared the NiO/MWCNTs hybrid electrode through ALD method. ALD has been widely considered as an encouraging technology for surface modification and preparation of nanostructured materials. NiONPs with a size of ∼ 4.9 nm prepared by ALD method and the obtained NiONPs were highly dispersed on the MWCNTs (Zhao et al., 2018). Zhou et al. (2019) developed a cost-effective Co3O4 carbon core–shell nanocomposite (Co3O4/CS) by exploiting glucose as the carbon source using a hydrothermal method. This kind of nanocomposite can greatly reduce the fabrication cost for potential commercialization, however, the researchers did not perform any interference studies with resorcinol or other phenolic compounds generally found in polluted aquifers. From the Table 1, the reported Co3O4/CS composite electrode (Zhou et al., 2019) presented well-sensing efficiency, including a wide linear scale than those achieved at P-RGO/GCE (Zhang et al., 2015a), ZnO/Gr/Ta (Ge et al., 2015) ZnONR/Carbon (Meng et al., 2017) and NiO/CNTs/GCEs (Goulart and Mascaro, 2016; Zhao et al., 2018). Lower LODs were collected at Co3O4/CS/GCE than those obtained at (Zhang et al., 2015a), (Ge et al., 2015), (Meng et al., 2017), (Goulart and Mascaro, 2016) and (Zhao et al., 2018). As a result, the high sensitivity originates from a synergistic effect of Co3O4 and superior conductivity of the carbon layer and its beneficial structure characteristics (high surface area and porous structure).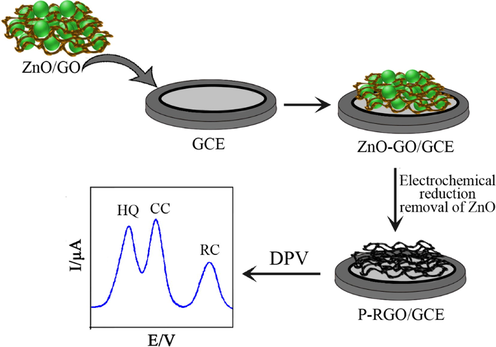
Schematic representation of P-RGO/GCE sensor for simultaneous detection of three DHB isomers (Zhang et al., 2015a).
3.4 Carbon material-supported conducting polymers
Composites of carbon and CPs can be formed via chemical synthesis, electrochemical deposition on used electrodes, or by electrochemical co-deposition (Kim et al., 2015; Li et al., 2019). Gr or CNTs conductive polymer composite exhibited a synergetic effect, leading to the augmentation of the electronic and mechanical characteristics of the constituent components (Singh, 2017; Tiwari et al., 2017; Yang et al., 2017b; Yusoff et al., 2019). The electrochemical capability of the poly-tryptophan-functionalized Gr (p-Trp-Gr) toward oxidation of both analytes was evaluated by Jiang et al. (2015). Trp has an indole conjugate structure and it helps the dispersion of Gr through π–π interactions. They reported that the addition of Trp on Gr could accelerate the electron transfer rate of two isomers in 0.1 M PBS at pH 7.0 and gives good LOD ranges of 0.221 and 0.086 μM for HQ and CC, respectively. Song et al. (2015) and Dorraji and Jalali (2015) have been reported the poly(diallyl-dimethylammonium chloride) (PDDA)/MWCNTs/Gr-PDDA/MWCNTs/Gr, and poly(melamine)/ERGO (pMel/ERGO), respectively for simultaneous detection of DHBs. In the first method (Song et al., 2015), PDDA (an ordinary and water-soluble cationic polyelectrolyte) was employed as the covalent cross-linking agent to bind MWCNTs and GR. In this approach, the positively charged colloid of PDDA can be easily coated on the negatively charged Gr or MWCNTs surfaces by electrostatic interaction. The resulting MWCNTs-PDDA-Gr has a high electroactivity toward the HQ and CC oxidation. Dorraji and Jalali (2015) displayed that poly-melamine film-ERGO-CuNPs electrode showed excellent performance for the simultaneous measurement of two DHB species and the current responses were recorded by DPV and the LODs were 0.21 µM for HQ and 0.15 µM for CC. Wang et al. (2016), Tian et al. (2017) and Ren et al. (2019) have reported polymer- based as electrode platforms i.e. poly(dopamine) (PDA)/Chit/MWCNTs/AuNPs, one-dimensional (1D) Poly(3, 4-ethylenedioxythiophene)-Gr (PEDOT-Gr) and hollow Mo2C/carbon spheres, respectively for detection of DHB isomers simultaneously. The AuNPs/MWCNTs with needle-like structural of AuNPs possessed large surface area and excellent active sites, which lead to the enhancement of catalytic performances toward the electrochemical oxidation of two species Wang et al. (2016). Fig. 2 shows the fabrication procedure of MWCNTs/PDA/AuNPs/Chit modified electrode. The sensor-based on PEDOT/Gr composite showed good stability and acceptable sensitivity (Tian et al., 2017). Ren et al. (2019) reported that hollow Mo2C/carbon spheres (HMCCSs) had a good electrochemical catalytic activity for DHB isomers. In this study, hollow molybdenum-dopamine spheres (HMPDSs) were synthesized easily by one-step method at room temperature and thermally annealed to form hollow Mo2C/C spheres. The obtained electrode was marked as the HMCCSs/GCE. In 2018, Peng et al. (2018) have also synthesized hollow N-doped mesoporous carbon spheres (NMCS) decorated Gr (NMCS-Gr). In this regard, polydopamine microspheres were synthesized on the surface of SiO2 nanoparticles to design SiO2@PDA hybrids in the presence of non-ionic surfactant polyol (F127). After that, the NMCS-Gr hybrid materials were prepared through high-temperature carbonization method and the obtained NMCS-Gr was used as electrode material for determination of DHBs. Due to excellent catalytic properties of AuNPs, Edris et al. (2019) incorporated AuNPs into poly(Eriochrome black T) (PEBT) for simultaneous detection of HQ, CC and RC. In this study, the bare GCE modified with electrochemically reduced graphene oxide-PEBT and AuNPs. According to the Table 1, it is clear that the PDDA/MWCNTs/Gr electrode (Song et al., 2015) was superior in some cases when compared to other reported electrode materials (Dorraji and Jalali, 2015; Edris et al., 2019; Peng et al., 2018; Ren et al., 2019; Tian et al., 2017; Wang et al., 2016). The good electrocatalytic response of DHB isomers at the MWCNTs-PDDA-Gr/GCE can be contributed to the following reasons. Firstly, Gr has great electronic conductivity, and its large surface area may improve the attraction of analytes toward the electrode, therefore enhancing the current response. Secondly, the hydroxyl and carboxyl groups in MWCNTs can interact with the hydroxyl groups in DHB isomers, which increases the separation of oxidation peaks of the DHBs. Thirdly, PDDA is not only a conducting polymer but also a strong ionic polymer. These results also suggest that the PDDA/MWCNTs/Gr modified electrode displays excellent selectivity for the determination of DHB isomers without mutual interference.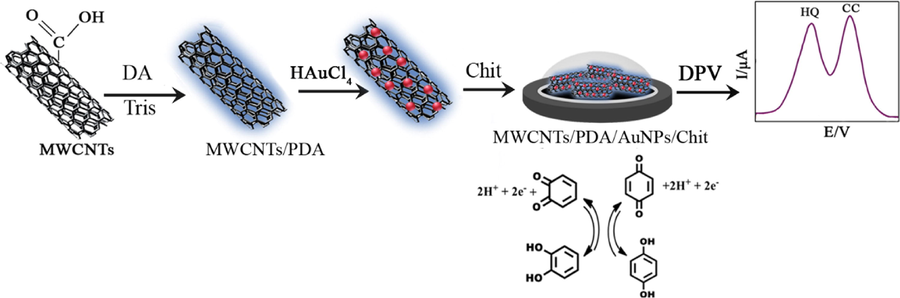
Schematic representation of MWCNTs/PDA/AuNPs/Chit sensor for simultaneous detection of two isomers (Wang et al., 2016).
Chitosan (Chit) is a linear polysaccharide produced by the deacetylation of chitin, a naturally occurring polymer (Younes and Rinaudo, 2015). The effect of the degree of deacetylation on properties such as solubility and antimicrobial activity have been studied in several articles. Chitosan is widely used in a range of diverse fields, including waste management, medicine, food and agriculture. Despite its huge availability, the utilization of chitin has been restricted by its intractability and insolubility. The fact that chitin is as an effective material for sutures essentially because of its biocompatibility, biodegradability and non-toxicity together with its antimicrobial activity and low immunogenicity, points to wide potential for future development. Chitosan has been utilized in a broad of electrochemical sensing implementations (Divya and Jisha, 2018; Szymańska-Chargot et al., 2019; Younes and Rinaudo, 2015). However, pure chitosan films have poor tensile strength and elasticity. The assembling of the carbon composites with Chit exhibits a steady dispersion and could be strongly used as an electroactive film (Rizeq et al., 2019; Suginta et al., 2013). In this regard, the GCE was modified with MWCNTs dispersed in Chit solution. A combination of Chit with MWCNTs has been used to improve the current response of HQ, CC and RC (Kokulnathan et al., 2017). TiO2 nanoparticles as alternative electrode materials have gained more interest because of low cost, high abundance, biocompatibility, and friendly. Due to desirable surface properties of TiO2 nanoparticles, they have been widely used in sensor technology, coating, and photocatalyst applications. In this context, the TiO2 nanoparticles and MWCNTs in Chit matrix were well-suited for simultaneous detection of HQ, CC and RC (Fotouhi et al., 2018). Remarkably, the DHB oxidation ability of Chit/f-MWCNTs was higher than Chit/MWCNTs/TiO2. The Chit/f-MWCNTs electrode provided an evidently larger current response with a sensitivity augmentation of 2.2-fold compared to the Chit/f-MWCNTs/TiO2 electrode to decrease the detection limit from 0.06 µM (Chit/MWCNTs/TiO2) to 0.027 µM (Chit/f-MWCNTs).
3.5 Carbon material-supported other forms of carbon-based nanomaterials
Other carbon nanomaterials that are emerging recently, like carbon dots (CDs), carbon nanohorns (CNHs), graphene-like carbon nanosheets (Gr-CNS), carbon nanocages (CNCs) boron-doped graphene (B-Gr) and graphitic carbon nitride nanosheets (CNNS-CNTs), are the great promise for future developments. Carbon dots, as a new class of “zero-dimensional” carbon materials, have recently attracted much attention for a variety of purposes and applications, especially for their potential applications in fluorescence, biosensors and imaging. Carbon dots are an emerging family of nanosystems displaying a range of fascinating properties (Mahmoudi et al., 2020; Rani et al., 2020). The amphiphilic nature of carbon dots with a hydrophobic graphitic core could effectively interact with the CNTs surface, whereas hydrophilic oxygenated functionalization on the CD surface provided excellent water dispersibility (Mandani et al., 2017). CDs are prepared by fragmentation of large carbon materials by using acid oxidation, arc discharge, laser ablation, ultrasonic/electrochemical exfoliation, or hydrothermal/solvothermal exfoliation methods. On the other hand, in the bottom-up approaches, molecular precursor materials are carbonized via hydrothermal, microwave, and thermal pyrolysis methods (Boakye-Yiadom et al., 2019; Lu et al., 2016). Gu et al. (2018) synthesized a stable MWCNTs/CDs catalyst through a simple one-pot hydrothermal method and used as a modifier for the determination of DHB isomers. The MWCNTs/CDs modified electrode exhibited good electrocatalytic action towards the quantification of HQ and CC. The detection limit was 0.03 µM for both compounds. Fig. 3 shows the fabrication procedure of MWCNTs/CDs modified electrode. Carbon nanocages (CNCs), nanosize cage-type mesoporous carbon material with a regular framework as well as nanographene shell, have aroused considerable interest in recent years (Wang et al., 2010). As a promising candidate material, CNCs are of importance in a wide range of applications including rechargeable batteries, hydrogen production and storage, catalysis, sensing, and drug and gene delivery owing to their unique geometrical structure, excellent physical and electrical properties (Zhang et al., 2010). Up to now, the most common methods to prepare CNCs are conventional hard-templating, sacrificial templating, soft templating, and template-free method. In this regard, Huang et al. (2015) have investigated the synthesis of porous nanostructure of graphitized carbon nanocages (CNCs) reduced GO (CNCs-RGO) via one-pot in situ solvothermal strategy. Due to the addition of CNCs to RGO, the surface of the modified electrode becomes more porous structure, larger surface areas and more excellent electrical conductivity. The experimental results indicated that the CNCs-RGO porous structures via interactive effect displayed the highest film-forming ability comparing with the individual modifier, which enhanced the long-term stability of the constructed electrode. The LODs for HQ and CC were 0.87 and 0.4 μM, respectively. A novel approach was reported by Jiang et al. (2017), that has produced a Gr-like carbon nanosheet (Gr-CNS). The Gr-CNS have higher electrosorption capacity (38.62 μmol/L) compared with other carbon materials. In that study, glucose was used as carbon precursors, NH4Cl as blowing agents. Co(NO3)2 employed as a graphitization catalyst. The obtained Gr-CNS texture modified on the GCE surface. For DPV detection of HQ and CC, the theoretical LOD was 0.02 and 0.05 µM, respectively.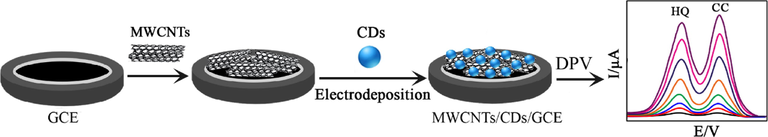
Schematic representation of MWCNTs/CDs sensor for simultaneous determination of HQ and CC (Gu et al., 2018).
Boron strengthens the crystal structure making cross‐links between molecules and thus increasing the mechanical stability of the final product. The addition of boron impurities into pure Gr or CNTs increases the activation region on its surface, enhances its catalytic ability, accelerates redox reactions (Agnoli and Favaro, 2016). A simple and highly sensitive electrochemical sensor based on a boron-doped Gr composite modified GCE was developed for the simultaneous detection of HQ and CC by Zhang et al. (2015c). This study indicated that the as-prepared B-Gr (which contains 1.4 percent of B) shown high electrocatalytic activity towards the oxidation of two isomers. The obtained detection limit was 0.3 μM for HQ and 0.2 μM for CC. In another example, Zhang et al. (2015b) synthesized strongly coupled 3D graphitic carbon nitride nanosheets (CNNS-CNT) nanocomposite by in situ self-assembly of 2D CNNS and 1D CNT-COOH by π-π stacking and electrostatic interactions with the solvothermal method. The designed electrode (CNNS-CNTs) also displayed good reproducibility, stability and good anti-interference features. Among composites (Gu et al., 2018; Huang et al., 2015; Jiang et al., 2017; Zhang et al., 2015b; Zhang et al., 2015c), Gr-like carbon nanosheet (Gr-CNS) (Jiang et al., 2017) exhibited excellent electrocatalytic performance for the detection of DHB isomers. The linear response range was 0.1–30 µM for HQ and 0.5–50 µM for CC. The detection limit was 0.02 µM and 0.05 µM, respectively. Comparing with other works (Gu et al., 2018; Huang et al., 2015; Zhang et al., 2015b; Zhang et al., 2015c), the Gr-CNSs modified electrode displays low detection limit and broad linear range. Gr-CNSs not only have a relatively large accessible surface area to accommodate more ions but also have a high graphitization degree to accelerate ion diffusion. The high sensitivity of the Gr-CNS electrode can be explained by the porous texture of the nanostructured platform. The Gr-CNSs based sensors displayed excellent results in terms of sensitivity for the considered analytes and allowed the sensitive electrochemical measurement of these isomers in water samples.
3.6 Mesoporous carbon, carbon nanofiber, carbon nano-fragment and biochar nanocomposites
Different mesoporous/ordered mesoporous (MP/OMP) carbon-based nano-sized materials have been fabricated to raise the electrochemical characteristics and electrocatalytic capabilities of target analytes by a combination of MPC with highly conductive and mechanically durable nanomaterials. MPC or OMPC nanomaterials are a flexible nanotexture generating interdependent channels for the effective penetration of electroactive substances in electrochemical systems. However, the direct implementation of single component MOFs in the electrochemical system is restricted owing to their poor conductivities, low mechanical durabilities, and ordinary electrocatalytic potentials (Ndamanisha and Guo, 2012). MOFs are constructed from transition or rare earth metal ions as nodes and multidentate organic ligands containing O- or N-donors as linkers. The nitrogen and sulfur dual-doped MPC (N,S-MPC) based electrochemical sensor was prepared and used for simultaneous quantification of the two target isomers (HQ and CC) in real water samples by Xu et al. (2016). Another research team synthesized (Huang et al., 2018) an MPC composite doped with an iron species (Fe/MPC) by carbonizing a mixture of Zeolite imidazole framework-8 (ZIF-8) in the presence of Fe(II). After carbonization in the nitrogen atmosphere, both ZIF-8 and Fe/ZIF-8 were converted to PC and Fe/PC. However, this work also has some limitation. For example, the synthetic process of porous carbons derived from ZIF-8 is more complicated than other materials (Gr, CNTs, and MPC), and the morphology is affected by carbonization temperature and carbonization environment (nitrogen or air). The marked Fe-ZIF-8 electrode was used for the determination of HQ and CC. The two analytes were detected by amperometry (AMP).
A metal–organic framework (MOF) is formed of organic molecules and metal ions, coordinated in such a way to form one-, two-, or three-dimensional rigid porous textures with high chemical stability and larger surface areas. Due to their distinctive characteristic features, they are very useful in the field of chemistry, nanotechnology, material science, and drug delivery systems (DDSs). Deng et al. (2017) have reported a Zr-based MOF (marked as UiO-66)/MPC nanomaterial for the simultaneous multiple detections of three DHB compounds. The authors reported that the incorporation of MPC greatly improved the stability and electrocatalysis of the UiO-66. The UiO-66/MPC nanocomposite was synthesized using a conventional hydrothermal protocol. The UiO-66/MPC electrode showed a wide linear response. Li et al. (2018) reported copper-based metal–organic frameworks graphene nanocomposites having good electronic conductivity and stability for electrochemical detection of CC and HQ, In this work, the Cu-MOF-Gr has been synthesized using a hydrothermal method and characterized by SEM and XRD. A novel type of sandwich MOF was successfully synthesized on reduced graphene oxide (denoted as MOF@Pt@MOF−RGO) by an in situ synthesis method. The obtained MOF@Pt@MOF-RGO possesses excellent electrochemical properties for the determination of three DHB isomers (Ye et al., 2019).
The compositing of material both of conductive carbon nanofibers (CFs) and inorganic materials can afford new platform in the electroanalytical and electrocatalytic area. In the composite material, the CFs work as the conductive substrate and electron collector. CFs with straight and porous/mesoporous textures can be fabricated by electrospinning and chemical vapour deposition technique with subsequent chemical treatment. Besides, CF surfaces are easy to modify with various nano-sized materials to enlarge the implementations of CF-based nanomaterials in different fields (Wang et al., 2019). Based on this concept, He et al. (2018) prepared a voltammetric sensing platform based on Sm2O3-carbon nanofibers. which were dispersed in DMF and the as-prepared Sm(OH)3 solution of was added and the mixture was poured into an autoclave and calcined. Fig. 4 shows the fabrication procedure of Sm2O3-carbon nanofiber electrodes. The prepared electrode displayed two pairs of independent well-defined quasi-reversible redox peaks in the CV curve and two well-distinguished anodic peaks in DPV curve. The calculated LODs were 0.09 µM and 0.07 µM for HQ and CC, respectively. According to the results, the Sm2O3-carbon nanofibers based sensors displayed excellent results in terms of sensitivity for the considered isomers.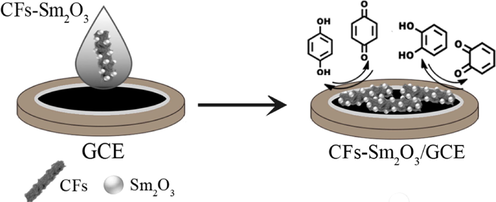
Schematic representation of CFs-Sm2O3 electrode for simultaneous determination of HQ and CC (He et al., 2018).
Carbon nano-fragment (CN-F) reveal unique nanostructured fragments with high surface area, abundant functional groups, excellent structural stability and high electrical conductivity and electrocatalytic oxidation efficiency. The CN-Fs derived from spent graphite exhibit various applications after converting into another form. CN-F as a new electrode material demonstrates a perfect fragment perspective in nano size, abundant functional groups, and effective electrocatalytic oxidation efficiency (Baptista et al., 2015). Alshahrani et al. (2019) have prepared the CN-F modifier using the graphite cycled in lithium-ion batteries as the raw material through a controllable refluxing process. In this work, the CuS decorated CN-Fs (CuS@CN-F) were prepared by a controllable refluxing method. The CuS/CN-F used to modify GCE (CuS-CN-F/GCE) for simultaneous determination of HQ and CC. The calculated LODs were 0.293 μM and 0.259 μM, respectively. Besides, Alshahrani et al. (2018) developed CuO/CN-Fs based electrode for the detection of two isomers. The study indicated that the preparation of the metal-based nanomaterials-anchored CN-F may be an effective method to improve the performance of the modified electrode. The two analyte responses (HQ and CC) linearly increased with the concentration in the range of 6.0–200 and 2.0–200 μM, respectively. The performances of the CuS-CN-F/GCE were better and competitive with previously reported values of metal-doped carbon materials (CuO/CN-Fs) modified electrode. The electrochemical characteristics and simultaneous detection of three target isomers were examined using a thermally reduced CN-Fs modified GCE by Liu et al. (2016). The modified electrode was also employed to the simultaneous detection of HQ, CC and RC. The linear ranges of three DHB isomers ranged from 10 to 120 µM. Particularly, the sensing properties of detection limit and linear range of MOF@Pt@MOF-RGO/GCE composites (Ye et al., 2019) are the best compared with other reported works (Alshahrani et al., 2019, 2018; Deng et al., 2017; He et al., 2018; Huang et al., 2018; Li et al., 2018; Liu et al., 2016; Xu et al., 2016). The sensing performance of the sandwiched MOF@Pt@MOF-RGO/GCE could be further improved by optimizing the synthesis conditions, such as reactant ratio, reaction temperature and reaction time and so on. The resultant nano-sandwiched electrode (MOF@Pt@MOF-RGO) can behave as a promising electrode for simultaneous and sensitive determination of DHB isomers without interference with each other.
Biochar (BC) is a carbon-rich material (namely biomass-derived Charcoal), which is largely synthesized from the pyrolysis of biomass under oxygen-limited conditions. BC has attracted enormous attention and research interest, due to its exceptional characteristics such as great surface area, highly porous texture, and excellent chemical stability. Over the last few years, BC has been widely employed in various areas including soil rehabilitation (Kong et al., 2018; Yadav et al., 2016), remove heavy metal ions, electrochemical implementation, and adsorbing CO2 (Cheng et al., 2017; Li et al., 2016). In brief, the preparation and application of BC are exciting subjects because of its extraordinary physical and mechanical characteristics. In this field, Wang et al. (2020) have prepared and characterized an AuNPs decorated the seedling of white myoga ginger-derived biochar (WBC/Au). The prepared electrode was marked as WBC/Au-850–15/GCE. Further, the BC based-electrode was used for the simultaneous detection of HQ and CC.
4 Conclusions
To date, various modified electrodes have been fabricated for the detection of HQ, CC and RC simultaneously and the binary (HQ and CC) and ternary (HQ, CC and RC) mixtures of DHB isomers were determined with satisfactory results. Researchers have been working on electrode modification by using different carbon-based nanomaterials to accomplish those challenges and raise their potential separation, the limit of detection. selectivity and sensitivity. The analytical performance and the rapid analyses of the electrochemical sensors towards DHB isomers are mostly dependent on the small size, the large surface area of the electrodes and the type of modifier materials. The reported sensors have been usually applied to the simultaneous determination of DHB isomers in the environmental water samples. Convenient sensors for simultaneous monitoring is increasingly needed in environmental monitoring. Although, the number of electrochemical sensors for these isomers has greatly increased in the last five years. However, the new designs are usually applied to the same isomers, so that there are several sensors for the most common, HQ, CC and RC, and very few or none for other important phenols. Despite the number of published studies, there is a lack of information regarding the application in real industrial wastewaters. Future work should emphasize the estimation of the cost of fabrication and the application of nanomaterial-based sensors in real industrial or domestic wastewater systems.
Acknowledgements
The authors gratefully acknowledge the financial support from Istanbul University-Cerrahpaşa Research grant.
Declaration of Competing Interest
The author declare that there is no conflict of interest.
References
- Doping graphene with boron: A review of synthesis methods, physicochemical characterization, and emerging applications. J. Mater. Chem. A. 2016;4:5002-5025.
- [CrossRef] [Google Scholar]
- Copper oxide and carbon nano-fragments modified glassy carbon electrode as selective electrochemical sensor for simultaneous determination of catechol and hydroquinone in real-life water samples. J. Electroanal. Chem.. 2018;815:68-75.
- [CrossRef] [Google Scholar]
- 3D-flower-like copper sulfide nanoflake-decorated carbon nanofragments-modified glassy carbon electrodes for simultaneous electrocatalytic sensing of co-existing hydroquinone and catechol. Sensors (Basel).. 2019;19:2289.
- [CrossRef] [Google Scholar]
- Recent developments in carbon nanomaterial sensors. Chem. Soc. Rev.. 2015;44:4433-4453.
- [CrossRef] [Google Scholar]
- Simultaneous determination of phenol, cresol, xylenol isomers and naphthols in urine by capillary gas chromatography. J. Chromatogr. B Biomed. Appl.. 1996;682:167-172.
- [CrossRef] [Google Scholar]
- Carbon dots: Applications in bioimaging and theranostics. Int. J. Pharm.. 2019;564:308-317.
- [CrossRef] [Google Scholar]
- Advantages, disadvantages, and future challenges of the use of electrochemical technologies for water and wastewater treatment. In: Martínez-Huitle, C. A., Rodrigo, M. A., Scialdone, O. (Eds.), Electrochemical Water and Wastewater Treatment. Butterworth-Heinemann, pp. 451–494.
- Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev.. 2013;42:5425-5438.
- [CrossRef] [Google Scholar]
- Graphene-based materials in electrochemistry. Chem. Soc. Rev.. 2010;39:3157-3180.
- [CrossRef] [Google Scholar]
- Recent advances in functionalized MnO2 nanosheets for biosensing and biomedicine applications. Nanoscale Horizons. 2019;4:434-444.
- [CrossRef] [Google Scholar]
- Simultaneous determination of dihydroxybenzene isomers using glass carbon electrode modified with 3D CNT-graphene decorated with Au nanoparticles. Int. J. Electrochem. Sci. 2019;14:7037-7046.
- [Google Scholar]
- Ultrasensitive and simultaneous detection of hydroquinone, catechol and resorcinol based on the electrochemical co-reduction prepared Au-Pd nanoflower/reduced graphene oxide nanocomposite. Electrochim. Acta. 2017;231:677-685.
- [CrossRef] [Google Scholar]
- Recent developments of post-modification of biochar for electrochemical energy storage. Bioresour. Technol.. 2017;246:224-233.
- [CrossRef] [Google Scholar]
- Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small. 2010;6:711-723.
- [CrossRef] [Google Scholar]
- Review on conducting polymers and their applications. Polym. Plast. Technol. Eng.. 2012;51:1487-1500.
- [CrossRef] [Google Scholar]
- Simultaneous and sensitive electrochemical detection of dihydroxybenzene isomers with UiO-66 metal-organic framework/mesoporous carbon. Talanta. 2017;174:527-538.
- [CrossRef] [Google Scholar]
- UV-active phenol ester compounds. Washington, DC: Enigen Science Publishing; 2008.
- Direct simultaneous determination of dihydroxybenzene isomers at C-nanotube-modified electrodes by derivative voltammetry. J. Electroanal. Chem.. 2005;575:275-280.
- [CrossRef] [Google Scholar]
- Chitosan nanoparticles preparation and applications. Environ. Chem. Lett.. 2018;16:101-112.
- [CrossRef] [Google Scholar]
- A nanocomposite of poly(melamine) and electrochemically reduced graphene oxide decorated with Cu nanoparticles: Application to simultaneous determination of hydroquinone and catechol. J. Electrochem. Soc.. 2015;162:B237-B244.
- [CrossRef] [Google Scholar]
- A three-dimensional carbon nanotube/graphene sandwich and its application as electrode in supercapacitors. Adv. Mater.. 2010;22:3723-3728.
- [CrossRef] [Google Scholar]
- Electrochemical sensor based on nanocomposite of multi-walled carbonnanotubes / TiO2 nanoparticles in chitosan matrix for simultaneous and separate determination of dihydroxybenzene isomers. J. Electrochem. Soc.. 2018;165:B202-B211.
- [CrossRef] [Google Scholar]
- Synthesis of a ZnO nanorod/CVD graphene composite for simultaneous sensing of dihydroxybenzene isomers. Carbon N. Y.. 2015;95:1-9.
- [CrossRef] [Google Scholar]
- HPLC–UV method for the identification and screening of hydroquinone, ethers of hydroquinone and corticosteroids possibly used as skin-whitening agents in illicit cosmetic products. J. Chromatogr. Sci.. 2015;54:343-352.
- [CrossRef] [Google Scholar]
- Analytical and Physical Electrochemistry, Analytical and Physical Electrochemistry. New York: EPFL Press; 2004. http://doi.org/10.1201/9781439807842
- GC electrode modified with carbon nanotubes and NiO for the simultaneous determination of bisphenol A, hydroquinone and catechol. Electrochim. Acta. 2016;196:48-55.
- [CrossRef] [Google Scholar]
- Simultaneous determination of hydroquinone, and catechol using a multi-walled carbon nanotube/GC electrode modified by electrodeposition of carbon nanodots. J. Nano Res.. 2018;54:42-53.
- [CrossRef] [Google Scholar]
- Silver nanoparticle induced chemiluminescence of the hexacyanoferrate-fluorescein system, and its application to the determination of catechol. Microchim. Acta. 2016;183:917-921.
- [CrossRef] [Google Scholar]
- A carbon nanofibers-Sm2O3 nanocomposite: A novel electrochemical platform for simultaneously detecting two isomers of dihydroxybenzene. Anal. Methods. 2018;10:1852-1862.
- [CrossRef] [Google Scholar]
- Chemical analysis of hydroquinone and retinoic acid contents in facial whitening creams. Asian J. Pharm. Sci.. 2016;11:89-90.
- [CrossRef] [Google Scholar]
- Amperometric determination of hydroquinone and catechol using a glassy carbon electrode modified with a porous carbon material doped with an iron species. Microchim. Acta. 2018;185:37.
- [CrossRef] [Google Scholar]
- One-pot hydrothermal synthesis carbon nanocages-reduced graphene oxide composites for simultaneous electrochemical detection of catechol and hydroquinone. Sensors Actuators, B Chem.. 2015;212:165-173.
- [CrossRef] [Google Scholar]
- Carbon nanotubes-properties and applications: a review. Carbon Lett.. 2013;14:131-144.
- [CrossRef] [Google Scholar]
- Applications of electrochemical data analysis by multivariate curve resolution-alternating least squares. TrAC - Trends Anal. Chem.. 2017;88:134-166.
- [CrossRef] [Google Scholar]
- Towards monochiral carbon nanotubes: A review of progress in the sorting of single-walled carbon nanotubes. Mater. Chem. Front.. 2018;2:36-63.
- [CrossRef] [Google Scholar]
- Graphene-like carbon nanosheets as a new electrode material for electrochemical determination of hydroquinone and catechol. Talanta. 2017;164:300-306.
- [CrossRef] [Google Scholar]
- A novel sensitive electrochemical sensor for the simultaneous determination of hydroquinone and catechol using tryptophan-functionalized graphene. Anal. Lett.. 2015;48:1426-1436.
- [CrossRef] [Google Scholar]
- Development of analytical method for catechol compounds in mouse urine using hydrophilic interaction liquid chromatography with fluorescence detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci.. 2015;985:142-148.
- [CrossRef] [Google Scholar]
- UV spectrophotometric determination and validation of hydroquinone in liposome. Iran. J. Pharm. Res.. 2015;14:473-478.
- [Google Scholar]
- Fabrication of various conducting polymers using graphene oxide as a chemical oxidant. Chem. Mater.. 2015;27:6238-6248.
- [CrossRef] [Google Scholar]
- Chitosan stabilized multi-walled carbon nanotubes for electrochemical determination of dihydroxybenzene isomers. J. Electrochem. Soc.. 2017;164:H958-H966.
- [CrossRef] [Google Scholar]
- Biochar accelerates PAHs biodegradation in petroleum-polluted soil by biostimulation strategy. J. Hazard. Mater.. 2018;343:276-284.
- [CrossRef] [Google Scholar]
- Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol.. 2008;3:101-105.
- [CrossRef] [Google Scholar]
- An electrochemical sensor based on copper-based metal-organic frameworks-graphene composites for determination of dihydroxybenzene isomers in water. Talanta. 2018;181:80-86.
- [CrossRef] [Google Scholar]
- Ag/N-doped reduced graphene oxide incorporated with molecularly imprinted polymer: An advanced electrochemical sensing platform for salbutamol determination. Biosens. Bioelectron.. 2017;90:210-216.
- [CrossRef] [Google Scholar]
- Modifying reduced graphene oxide by conducting polymer through a hydrothermal polymerization method and its application as energy storage electrodes. Nanoscale Res. Lett.. 2019;14:226.
- [CrossRef] [Google Scholar]
- Graphene and related two-dimensional materials: Structure-property relationships for electronics and optoelectronics. Appl. Phys. Rev.. 2017;4:021306
- [CrossRef] [Google Scholar]
- Biochar as a renewable source for high-performance CO2 sorbent. Carbon N. Y.. 2016;107:344-351.
- [CrossRef] [Google Scholar]
- Electrochemical behavior and simultaneous determination of catechol, resorcinol, and hydroquinone using thermally reduced carbon nano-fragment modified glassy carbon electrode. Anal. Methods. 2016;8:605-613.
- [CrossRef] [Google Scholar]
- Multifarious roles of carbon quantum dots in heterogeneous photocatalysis. J. Energy Chem.. 2016;25:927-935.
- [CrossRef] [Google Scholar]
- Distinguishing characteristics and usability of graphene oxide based on different sources of graphite feedstock. J. Colloid Interface Sci.. 2019;542:429-440.
- [CrossRef] [Google Scholar]
- Improving membrane bioreactor performance through the synergistic effect of silver-decorated graphene oxide in composite membranes. J. Water Process Eng.. 2020;34:101169
- [CrossRef] [Google Scholar]
- Carbon dots as nanodispersants for multiwalled carbon nanotubes: reduced cytotoxicity and metal nanoparticle functionalization. Langmuir. 2017;33:7622-7632.
- [CrossRef] [Google Scholar]
- Environmental photochemistry of herbicides. Rev. Environ. Contam. Toxicol.. 1988;103:61-126.
- [CrossRef] [Google Scholar]
- On the structure of Au/Pd bimetallic nanoparticles. J. Phys. Chem. C. 2007;111:1256-1260.
- [CrossRef] [Google Scholar]
- Simultaneous detection of dihydroxybenzene isomers with ZnO nanorod/carbon cloth electrodes. ACS Appl. Mater. Interfaces. 2017;9:12453-12460.
- [CrossRef] [Google Scholar]
- Voltammetric determination of hydroquinone, catechol, and resorcinol by using a glassy carbon electrode modified with electrochemically reduced graphene oxide-poly(Eriochrome black T) and gold nanoparticles. Microchim. Acta. 2019;186:261.
- [CrossRef] [Google Scholar]
- Electrochemical oxidation behavior of some hazardous phenolic compounds in acidic solution. Egypt. J. Pet.. 2017;26:669-678.
- [CrossRef] [Google Scholar]
- Conductive polymer-based sensors for biomedical applications. Biosens. Bioelectron.. 2011;26:1825-1832.
- [CrossRef] [Google Scholar]
- Metal nanoparticles as green catalysts. Materials (Basel).. 2019;12:3602.
- [CrossRef] [Google Scholar]
- Ordered mesoporous carbon for electrochemical sensing: A review. Anal. Chim. Acta. 2012;747:19-28.
- [CrossRef] [Google Scholar]
- Integrated adsorption-solar photocatalytic membrane reactor for degradation of hazardous Congo red using Fe-doped ZnO and Fe-doped ZnO/rGO nanocomposites. Environ. Sci. Pollut. Res.. 2019;26:33856-33869.
- [CrossRef] [Google Scholar]
- Solar photocatalytic and surface enhancement of ZnO/rGO nanocomposite: Degradation of perfluorooctanoic acid and dye. Process Saf. Environ. Prot.. 2017;112:298-307.
- [CrossRef] [Google Scholar]
- Review—Nanocomposite-based sensors for voltammetric detection of hazardous phenolic pollutants in water. J. Electrochem. Soc.. 2020;167:037568
- [CrossRef] [Google Scholar]
- Tailor-made dicationic ionic liquid as a fluorescent sensor for detection of hydroquinone and catechol. J. Mol. Liq.. 2017;244:39-45.
- [CrossRef] [Google Scholar]
- Simultaneous determination of hydroquinone, catechol and resorcinol with high selectivity based on hollow nitrogen-doped mesoporous carbon spheres decorated graphene. J. Electrochem. Soc.. 2018;165:B212-B219.
- [CrossRef] [Google Scholar]
- An enhanced electrochemical platform based on graphene oxide and multi-walled carbon nanotubes nanocomposite for sensitive determination of Sunset Yellow and Tartrazine. Food Chem.. 2016;190:889-895.
- [CrossRef] [Google Scholar]
- An overview of the recent progress in the synthesis and applications of carbon nanotubes. J. Carbon Res.. 2019;5:3.
- [CrossRef] [Google Scholar]
- A review of carbon quantum dots and their applications in wastewater treatment. Adv. Colloid Interface Sci.. 2020;278:102124
- [CrossRef] [Google Scholar]
- Synthesis of hollow Mo2C/carbon spheres, and their application to simultaneous electrochemical detection of hydroquinone, catechol, and resorcinol. Microchim. Acta. 2019;186:306.
- [CrossRef] [Google Scholar]
- Synthesis, bioapplications, and toxicity evaluation of chitosan-based nanoparticles. Int. J. Mol. Sci.. 2019;20:5776.
- [CrossRef] [Google Scholar]
- Nanoraspberry-like copper/ reduced graphene oxide as new modifier for simultaneous determination of benzenediols isomers and nitrite. Anal. Chim. Acta. 2019;1056:16-25.
- [CrossRef] [Google Scholar]
- Composites based on conducting polymers and carbon nanotubes for supercapacitors. In: Kumar V., Kalia S., Swart H., eds. Conducting Polymer Hybrids. Springer Series on Polymer and Composite Materials. Cham: Springer; 2017.
- [CrossRef] [Google Scholar]
- Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci.. 2019;1:31-47.
- [CrossRef] [Google Scholar]
- Multiwall carbon nanotubes-poly(diallyldimethylammonium chloride)-graphene hybrid composite film for simultaneous determination of catechol and hydroquinone. Sensors Actuators, B Chem.. 2015;206:111-118.
- [CrossRef] [Google Scholar]
- Electrochemical biosensor applications of polysaccharides chitin and chitosan. Chem. Rev.. 2013;113:5458-5479.
- [CrossRef] [Google Scholar]
- Adsorption of catechol, resorcinol, hydroquinone, and their derivatives: A review. Int. J. Energy Environ. Eng.. 2012;3:32.
- [CrossRef] [Google Scholar]
- Natural phenolics in the prevention of UV-induced skin damage. A review. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub.. 2003;147:137-145.
- [CrossRef] [Google Scholar]
- Influence of chitosan addition on the mechanical and antibacterial properties of carrot cellulose nanofibre film. Cellulose. 2019;26:9613-9629.
- [CrossRef] [Google Scholar]
- Au-Pd/reduced graphene oxide composite as a new sensing layer for electrochemical determination of ascorbic acid, acetaminophen and tyrosine. Mater. Sci. Eng. C. 2016;68:805-813.
- [CrossRef] [Google Scholar]
- Synthesis of one-dimensional poly(3,4-ethylenedioxythiophene)-graphene composites for the simultaneous detection of hydroquinone, catechol, resorcinol, and nitrite. Synth. Met.. 2017;226:148-156.
- [CrossRef] [Google Scholar]
- Conductive polymer composites based on carbon nanomaterials. In: Kumar V., Kalia S., Swart H., eds. Conducting Polymer Hybrids. Springer Series on Polymer and Composite Materials. Cham: Springer; 2017.
- [CrossRef] [Google Scholar]
- Poly(quercetin)-bismuth nanowires as a new modifier for simultaneous voltammetric determination of dihydroxybenzene isomers and nitrite. RSC Adv.. 2018;8:1237-1245.
- [CrossRef] [Google Scholar]
- Room-temperature phosphorescent discrimination of catechol from resorcinol and hydroquinone based on sodium tripolyphosphate capped Mn-doped ZnS quantum dots. Anal. Chem.. 2013;85:1920-1925.
- [CrossRef] [Google Scholar]
- Simultaneous determination of hydroquinone and catechol using a glassy carbon electrode modified with Au@Pd loaded on reduced graphene oxide. Anal. Methods. 2018;10:1331-1338.
- [CrossRef] [Google Scholar]
- Gold nanoparticles decorated biochar modified electrode for the high-performance simultaneous determination of hydroquinone and catechol. Sensors Actuators, B Chem.. 2020;306:127590
- [CrossRef] [Google Scholar]
- Carbon nanocages with nanographene shell for high-rate lithium ion batteries. J. Mater. Chem.. 2010;20:9748-9753.
- [CrossRef] [Google Scholar]
- Applications of carbon nanotubes and graphene for electrochemical sensing of environmental pollutants. J. Nanosci. Nanotechnol.. 2016;16:7852-7872.
- [CrossRef] [Google Scholar]
- Selective sensing of hydroquinone and catechol based on multiwalled carbon nanotubes/polydopamine/gold nanoparticles composites. Sensors Actuators, B Chem.. 2016;223:501-508.
- [CrossRef] [Google Scholar]
- Carbon nanofiber-based functional nanomaterials for sensor applications. Nanomaterials. 2019;9:1045.
- [CrossRef] [Google Scholar]
- Simultaneous determination of positional isomers of benzenediols by capillary zone electrophoresis with square wave amperometric detection. J. Chromatogr. A. 2006;1109:317-321.
- [CrossRef] [Google Scholar]
- Nitrogen, sulfur dual-doped mesoporous carbon modified glassy carbon electrode for simultaneous determination of hydroquinone and catechol. J. Electrochem. Soc.. 2016;163:B617-B623.
- [CrossRef] [Google Scholar]
- Vacuum pyrolysed biochar for soil amendment. Resour. Effic. Technol.. 2016;2:S177-S185.
- [CrossRef] [Google Scholar]
- Determination of catechol in water based on gold nanoclusters-catalyzed chemiluminescence. J. Lumin.. 2017;187:186-192.
- [CrossRef] [Google Scholar]
- Conducting polymer composites: Material synthesis and applications in electrochemical capacitive energy storage. Mater. Chem. Front.. 2017;1:251-268.
- [CrossRef] [Google Scholar]
- An ultrasensitive electrochemical sensor based on multiwalled carbon nanotube@reduced graphene oxide nanoribbon composite for simultaneous determination of hydroquinone, catechol and resorcinol. J. Electrochem. Soc.. 2019;166:B547-B553.
- [CrossRef] [Google Scholar]
- Study of a novel cationic calix[4]arene used as selectivity modifier in capillary electrophoresis with electrochemical detection. J. Chromatogr. A. 2001;910:311-318.
- [CrossRef] [Google Scholar]
- Simultaneous determination of catechol, hydroquinone, and resorcinol on CTAB functionalized graphene oxide/multiwalled carbon nanotube modified electrode. Fullerenes. Nanotub. Carbon Nanostructures. 2015;23:410-417.
- [CrossRef] [Google Scholar]
- In situ synthesis of sandwich MOFs on reduced graphene oxide for electrochemical sensing of dihydroxybenzene isomers. Analyst. 2019;144:2120-2129.
- [CrossRef] [Google Scholar]
- Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs. 2015;13:1133-1174.
- [CrossRef] [Google Scholar]
- Progress in the functional modification of graphene/graphene oxide: a review. RSC Adv.. 2020;10:15328-15345.
- [CrossRef] [Google Scholar]
- Conductive polyelectrolyte multilayers PANI membranes synthesis for tunable filtration ranges. J. Mater. Sci.. 2019;54:12988-13005.
- [CrossRef] [Google Scholar]
- Electrochemical preparation of porous graphene and its electrochemical application in the simultaneous determination of hydroquinone, catechol, and resorcinol. Sensors Actuators, B Chem.. 2015;220:919-926.
- [CrossRef] [Google Scholar]
- Self-assembly of graphitic carbon nitride nanosheets-carbon nanotube composite for electrochemical simultaneous determination of catechol and hydroquinone. Electrochim. Acta. 2015;176:28-35.
- [CrossRef] [Google Scholar]
- Carbon nanocages grown by gold templating. Carbon N. Y.. 2010;48:424-430.
- [CrossRef] [Google Scholar]
- Boron-doped graphene as high-performance electrocatalyst for the simultaneously electrochemical determination of hydroquinone and catechol. Electrochim. Acta. 2015;156:228-234.
- [CrossRef] [Google Scholar]
- Nickel oxide/carbon nanotube nanocomposites prepared by atomic layer deposition for electrochemical sensing of hydroquinone and catechol. J. Electroanal. Chem.. 2018;808:245-251.
- [CrossRef] [Google Scholar]
- Simultaneous determination of catechol and hydroquinone using non-enzymatic Co3O4@carbon core/shell composites based sensor. J. Electrochem. Soc.. 2019;166:B1069-B1078.
- [CrossRef] [Google Scholar]
- A seamless three-dimensional carbon nanotube graphene hybrid material. Nat. Commun.. 2012;3:1-7.
- [CrossRef] [Google Scholar]
- Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater.. 2010;22:3906-3924.
- [CrossRef] [Google Scholar]







