Translate this page into:
Review on biologically active natural insecticides from Malaysian tropical plants against Aedes aegypti and Aedes albopictus
⁎Corresponding authors. vijay.kotra@qiu.edu.my (Vijay Kotra), allan.mathews@qiu.edu.my (Allan Mathews)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Dengue fever is the most common mosquito-borne viral disease in the world, caused by the dengue viruses (DENV 1–4) and it was transmitted in Malaysia by two main Aedes mosquito species: Aedes aegypti and Aedes albopictus. The overuse of synthetic chemical insecticides in managing these vectors leads to an increase in insecticide resistance, which has occurred in most arthropod species, including Aedes mosquitoes. Bio-insecticides have been suggested as a new potential alternative method which can replace synthetic chemical insecticides to overcome the vector issues. Analytical data were used to compare with Malaysian plants that have larvicidal ability; and those plants were Areca catechu, Azolla pinnata, Lantana camara, Mukia maderaspatana, and Leucas aspera. The most prevalent chemical components found in all five plants were fatty acids (oleic acid, palmitic acid, tetradecanoic acid), fatty acid methyl esters (hexadecanoic acid methyl ester, 9-octadecenoic acid (Z)-methyl ester), and flavonoids (catechin). These chemical compounds have been patented for pesticide formulations due to their biodegradable qualities and capacity to increase pesticide efficiency. Furthermore, the key advantages of these chemical compounds to combat vector difficulties are their enzyme inhibitory characteristics, biochemical alterations, and structural deformation of mosquito larvae. According to the findings of this study, these five plants have the potential to be used as bio-insecticides.
Keywords
Biological activity
Chemical constituents
Areca catechu L
Aedes
Fatty acids
Flavonoids
1 Introduction
Infections caused by transmission of pathogens by arthropods are called vector-borne diseases. Ticks, lice, sand flies, black flies, mosquitoes and others related insects are the few examples of arthropods that can spread vector-borne diseases (Kamaraj, Rahuman, Mahapatra, Bagavan, & Elango, 2010; Wilson et al., 2020). According to Emran, Sherin, Thein, and Aung (2018), mosquito-borne diseases cause morbidity. Dengue fever is a mosquito-borne disease caused by dengue viruses. It can be transmitted by two main species of Aedes mosquito in Malaysia; those are Aedes aegypti and Aedes albopictus mosquitoes through blood feeding from one individual to another individual. Currently, there are 64,078 reported dengue cases in Malaysia compare to 25,794 the same period of time in the year of 2021 (WHO, 2022). According to (WHO, 2022), a cumulative amount of 50 death were reported in 2022 and where else there were only 19 reported in the year of 2021. The resurgence or re-emergence of mosquito-borne arboviruses gives concerns to the importance of public health because it can lead to disease outbreaks which occurs globally (Huang, Higgs, & Vanlandingham, 2019). It also has been reported that the clinical characteristics of dengue were similar to that of the coronavirus diseases (COVID-19) (Wu et al., 2020).(See Table 1).
Apparatus model
Chemical compound
Reference
Shimadzu GC4CMPF with FID detector, JMS DX-300 (JEOL Co., Akishima,Japan)
- lauric acid,
- myristic acid,
- pentadecanoic acid
- palmitic acid
- oleic acid
- stearic acid
- nonadecanoic acid(Chareonviriyaphap et al., 2013)
Shimadzu (Japan) QP2010 GC–MS
- Hexadecanoic acid methyl ester
− 9-Octadecenoic acid methyl ester (E)-
− 9,12-Octadecadienoic acid methyl ester (E,E)-
-Acetic acid
−4-Hydroxy-2-methylacetophenone
-Pentadecanoic acid, 14-methyl, methyl ester
- Pantolactone(Elumalai, Hemalatha, & Kaleena, 2017)
According to (Shafique et al., 2019), the common present-day methods which can be used to control these disease vectors can be classified into three types of measures: environmental, chemical and biological. The environmental measures includes few actions such as wearing clothes with long sleeves, applying bed nets and covering the substances that can stack water easily such as tires and jars. Application of mosquito coils, mosquito repellent cream or lotions, temephos and some chemical insecticidal spray are the chemical measures for vector controls. The utilization of microbe species like Bacillus thuringiensis israelensis, guppy fishes and sterile insect technique are the main measures of biological vector control methods(Hamed, El-Sherbini, Abdeltawab, & Parasitology, 2022). Vaccination is also one of a strategy for vector control problem but it has been reported that the effectiveness of vaccine towards dengue diseases was low (Redoni et al., 2020). The Ministry of Health Malaysia utilized certain methods in order to control the diseases vector problem in Malaysia. For instance, fogging at dengue-suspected household regions with certain chemicals and tentative inspection at some mosquito breeding sites are the methods for vector controls in Malaysia (Bharathithasan et al., 2022). Besides that, educational talks or campaign related to mosquito vector and dengue also has been carried out. These talks and campaign makes the society aware about the risks of dengue and the necessary actions to prevent the spread of the disease.
The classes of insecticides most frequently applied for vector controls are pyrethroids. This is due to some of their properties such as high levels of toxicity towards target vectors and low possibility to harm mammalian. In the last three decades, pyrethroids were used widely because insecticides still played the major roles for the controls of mosquito vector, Ae. aegypti within endemic regions (Smith, Kasai, & Scott, 2018). However, development of insecticide resistances within target vectors, harmful effects towards non-target organisms and environmental contaminations were the few main ecological problems caused by the application of synthetic chemical insecticides (Manorenjitha Malar et al., 2017). According to (Prasannath & Review, 2016), bio-insecticides consist of some benefits which can be used for vector control. For example, bio-insecticides have their own specific mode of action that can target the related vectors so that it does not harm others non-target organisms. Bio-insecticides can also reduce the amount of bio-accumulate substances within the soils due to its biodegradable properties. The environmental pollution rate can be decreased with its biodegradable properties. Besides that, there is very low chance for these target vectors to develop resistances towards certain insecticides. Hence, bio-insecticides were recommended as an alternative of chemical insecticides which can be used for vector controls.
2 Dengue
Dengue is a well-known transmissible vector-borne disease and dengue viruses are the major factors for this disease. Dengue viruses consist of different serotypes and there are four major types that can be classified as DENV-1, DENV-2, DENV-3 and DENV-4 (Khetarpal & Khanna, 2016). According to (Teoh et al., 2012), the dengue virus contains single-stranded RNA which has the lengths of 11 kb. The RNA is the components which located within the nucleocaspid core and covered by core proteins. The lipid bilayer membrane attached with certain constituents like 180 replications of membrane proteins and 180 replications of envelope proteins. The membrane of lipid bilayer plays their role to enclose the nucleocaspid core. The envelope protein of DENV plays its role as main antigen. These viral envelope protein forms dimeric features and aligned themselves to the lipid membrane of virus. There are some structural and non-structural proteins can be translated from single-stranded RNA of dengue virus. Envelope, capsid and pre-membrane are the structural protein of dengue virus. The non-structural proteins of dengue virus include NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5 (Khetarpal & Khanna, 2016).
According to (Guzman et al., 2016), the dengue virus can be transmitted female Aedes mosquitoes bite while humans are the main hosts for dengue infections. Normally, the female mosquito will be infected during the blood feeding process. During incubation periods, the virus infects the mosquitoes’ midgut cells and followed by virus replication within the tissues. After 8 to 10 days, the mosquito salivary gland is also infected and it became infective for dengue viruses’ transmission to the host. Dengue can be classified on several categories: dengue fever, dengue haemorrhagic fever and dengue shock syndrome (Oishi, Saito, Mapua, Natividad, & chemotherapy, 2007). Although there are several therapies and drugs are promoted for treatment of dengue, but the effectiveness is very low to cure the dengue diseases (Subenthiran et al., 2013). Children who are infected with dengue diseases may have undifferentiated fever but adults may have the possibility to experience these several symptoms such as headache, fever, discomfort and musculoskeletal pain (A. Guzman & Istúriz, 2010), There are no effective drugs or therapy to fully cure the dengue diseases (Subenthiran et al., 2013; Zaheer et al., 2022).
3 Aedes mosquito
Mosquitoes are in the member of Culicidae family of the order (Diptera) and suborder (Nematocera) (S. Singh, Mann, & leprology, 2013). The mosquito diversity can cause by different factors such as mating areas, different resting or reproduction sites, source of nectar and bloods (Diallo et al., 2012). According to (Rueda, 2007), mosquito species have their own life cycles or called as metamorphosis. Their life cycle comprises of eggs, larvae, pupae and adults. The egg-laying sites of mosquitoes usually located at certain areas which contained stagnant water. Female mosquitoes play the main role in laying eggs and incubation for larvae formations. These hatched larvae can depend on several food sources like organic substances and microbes to obtain nutrients for growth. The larvae move into the next phase called pupae after 7 days and emerges into adult phase (Faithpraise et al., 2014). The common characteristics of all mosquito are the coverage of external skeleton or exoskeleton over their body parts. Besides that, the type of symmetry for mosquito species is bilateral. A normal mosquito body has 3 elementary sections which includes head, thorax and abdomen. A single proboscis, a couple of maxillary palpi and compound eyes are the main components of mosquito head. The thorax of mosquito comprised with 3 segments: mesothorax, prothorax and methathorax. These segments of mosquito thorax are attached with wings and legs. Normally, a mosquito abdomen consisted of 10 segments and some segments are used for other functions like reproduction (Rueda, 2007).
According to (Farajollahi & Price, 2013), Ae. aegypti is the primary vector for the dengue disease. These types of mosquito have the preference to bite the bottom parts of host body. The common feeding time of Ae. aegypti is daytime but within the shadows. Besides that, the feeding characteristic of Ae. aegypti mosquito is mainly on anthropophilic where human is chosen as their nutrient resources. However, it can also feed on other animals when they are obtainable. Aedes aegypti can be scared easily by surrounding during feeding times. The mosquitoes stop feeding and move away after interrupted by something but they will come back within short periods. This action may cause Ae. aegypti to search others hosts for blood feeding which give rise to increase of disease transmission rates (Farajollahi & Price, 2013). According to (Rezza, 2012), Ae. albopictus, the Asian tiger mosquito are the Aedes mosquito species which commonly known as low effective diseases vectors compared to Ae. aegypti (Fig. 1.). Low anthropophilic properties and low ability to adapt within urban household environments are the main reasons for Ae. albopictus to become a low efficiency vector. Nonetheless, Ae. albopictus can tolerate benign climate compared to Ae. aegypti. Thus, some of the disease outbreaks can be caused by Ae. albopictus but not Ae. aegypti. Aedes albopictus has its own dorsal shape which is single longitudinal stripe. Stored water substances which can be found at forest, rural and urban environment are the main preferences for female Ae. albopictus to lay their eggs (Das and Subaharan, 2017).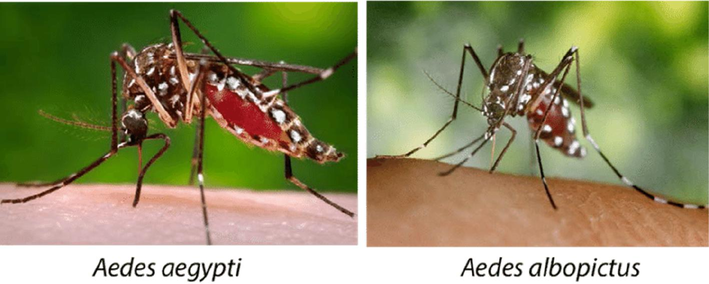
Aedes aegypti and Ae. albopictus mosquitoes (Okafor, Health, & Care, 2016).
4 Chemical insecticidal compound
Potential chemical compound from plant extract act as insecticidal agent. Plant naturally consist of bioactive compound which able to act as insecticides. Most of the compounds found on plants are to fight against pathogens and herbivores and they act as defence system of plants. Scientist has found the chemical compound as more valuable and able to utilize as main compound on insecticides. There are thousands of compounds from plants are potential to be used as insecticides but only fistful of bio-insecticide is considered by industries into their products.
4.1 Pyrethroids
According to (Davies et al., 2007), pyrethroids are the well-known neurotoxic insecticides. Pyrethrin is the organic compounds which it can be obtained from a flower species, Chrysanthemum cinerafolis. This type of dried flowers was applied as insecticides to prevent the biting of insects because of its insecticidal abilities in ancient China. The usages of pyrethroids became more general within some countries such as Persia and Europe. In the mid of 19th century, the development and marketing of various pyrethroid related insecticides were done and these insecticidal products were manufactured by using Chrysanthemum flower or pyrethrum. There are two types of pyrethrin compounds which can be extracted from a flower species, Chrysanthemum cinerafolis (Kshirsagar & Phal, 2022). These two pyrethrin compounds are chrysanthemic acid (pyrethrin I) and pyrethric acid (pyrethrin II). The pyrethroids are the substitute compound of insecticidal esters of both pyrethrin that produced naturally. There are 3 natural variation which derived from alcohol moiety of pyrethrin and it give rise to different version of pyrethroids such as jasmolin I, jasmolin II, cinerin I, cinerin II, pyrethrin I and pyrethrin II. Pyrethroids can be categorized into 2 classes which are class I and class II. Allethrin and permethrin are the examples of class I pyrethroids because they consisted of cyclopropane carboxylic ester for the basic structure which required for pyrethroids formations. Class II pyrethroids are slightly different with class I because they contain cyano group which can lead to choreoathetosis and salivation. Cypermethrin and deltamethrin are the examples of class II pyrethroids (Gajendiran & Abraham, 2018).
According to (Soderlund, 2012), it has been reported that several agencies like academic research laboratories, governments and agrochemical industries have conducted plenty of researches and studies to prove the wide ranges of pyrethroid structures. Furthermore, most of the researchers also proved the usages of pyrethroids to different field types such as medical, agriculture and control of pests. However, the stability of natural pyrethroid insecticides can be reduced when it exposed under air and light. This factor became the weakness of natural pyrethroids because the effect of pyrethroid insecticide cannot be fully expressed to control the pest problems and protects the crops. Thus, synthetic insecticides were developed from the original structure of natural pyrethroids can boost up the stability at light and air conditions. Modified pyrethroids also can retain its own functions such as reduction the toxicity towards mammalian and high rate of insecticidal effects. Permethrin and deltamethrin are the two examples of synthetic pyrethroid insecticides. These two synthetic pyrethroids insecticides upgraded the overall functions of natural pyrethroid insecticides such as insecticidal activity and photostability. The pyrethroid insecticides attaches to the open sodium channels and alter the transition of channel gating. This situation can cause the extension of periods for the opening of sodium channels. The insects can have several symptoms such as paralysis, conduction blockage of nerve and even death can be happened due to the action of pyrethroids which alters the sodium channel gating (Soderlund, 2012); (Du, Nomura, Zhorov, & Dong, 2016).
4.2 Dichlorodiphenyltrichloroethane (DDT)
Dichlorodiphenyltrichloroethane (DDT) is a lipid soluble types of organophosphate insecticides and the version of this insecticide is older compared to other versions. The scientific name of DDT is called as 1, 1, 1-Trichloro-2,2-bis(p-chlorophenyl) ethane. India is the major manufacturing country which produces high quantities of DDT insecticides while DDT has been applied by some of the countries like Africa to solve the vector control problems (Du et al., 2016). According to (Harada, Takeda, Kojima, & Tomiyama, 2016), it has been reported that the neurotoxicity properties which can be expressed by DDT insecticides are avoiding the activation of potassium gates and extends the opening periods of sodium ion channels. In addition, some of the neuronal ATPase is aimed by DDT insecticides through nervous system to alter the flow rate of elements like calcium, potassium and sodium. The calcium ions are the main element which can help in neurotransmitter release processes but DDT insecticides can restrict the transfer of calcium ions. These merged actions can enhance the sustainable for nerve depolarization, strengthen the transmitter release and give rise to excitation of central nervous system such as tremors and hyperexcitability.
According to (Davies et al., 2007), the characteristic of DDT was discovered by a chemist from Sweden called Paul Muller and he make an important contribution for pest controls. There are several factors to prove DDT can be the suitable insecticides. For example, the production of DDT insecticides can be produced with large or limitless amounts. The production costs of DDT insecticides were relatively low and it also can maintain its toxicity for certain periods. Although, the toxicity is harmful towards insects relatively it is safe to human kind. After the establishment of DDT insecticides, other related types of insecticides which contain chlorinated hydrocarbon were also created and sold. The effect of DDT insecticides can mainly influence on peripheral nervous systems. The exposure of insecticides can cause the neuron to trigger several symptoms which can be happened to insect body such as body quivering, muscle twitching and even appendages and joints tremors. The increase of neurotransmitter release can cause the rising of miniature post synaptic dispersion. These actions can affect the neuromuscular junctions due to depolarization. The depletion of neurotransmitter can lead to inhibition of neurotransmitter joints as the depolarization process keeps on (Perry, Yamamoto, Ishaaya, & Perry, 1998); (Davies et al., 2007).
5 Resistance
Insecticide resistance has been a serious threat especially among mosquitoes. Synthetic insecticides are applied by most of the countries in order to solve the problem of mosquito vector and also decrease the rate of vector diseases transmission. Still, the usages of synthetic insecticides for vector controls gives rise to certain problems such as formation of insecticide resistance within the vectors while affecting non-target organism. Accoding to (Smith et al., 2018), the two well-known pyrethroid insecticides resistance which can be found within 2 main species of Aedes mosquito (Aedes aegypti and Aedes albopictus) are cytochrome P450 monooxygenases detoxification and knockdown resistance (kdr). 450 monooxygenases detoxification and knockdown resistance (kdr). It has been reported that the A. aegypti mosquitoes develop single and combined mutations at voltage-gated sodium channels.
5.1 Knockdown resistance
Voltage-gated sodium channels are the integrated membrane protein which located at entire cell membrane. The main roles of this sodium channels are launching of neuron activity potentials and distributes them across the body parts. Domain I, domain II, domain III and domain IV are the four similar replicate domains of pore forming α-subunits. The α-subunit are the main components of voltage gated sodium channels. There are six portions of trans- membrane (S1–S6) and linked by certain loops from both intracellular and extracellular. The formation of voltage sensing domain structures is done by S1 to S6 segments. S5 and S6 help to build the central structures of pore. The activation of sodium channels can lead to depolarization of membranes. It can be done by the passing of sodium ions within the cells due to the opening of sodium channels. These actions also can give rise to changes of membrane potential and become depolarized. The stages of action potential can also be increased rapidly by initiation of sodium channels. Fast inactivation is a process which a deactivated component blocks the channel pore after the milliseconds of channel opening. This process can cause the action potentials to be terminated. Sodium channels are the favour sites for most of the insecticides due to its role in alteration of action potentials (Du et al., 2016).
According to (Bass et al., 2007), knockdown resistance (kdr) is a type of common insecticide resistances which it related to the sodium channel of target pests. Basically, the sodium channels of target vectors are the suitable sites for the distributions of synthetic insecticides like DDT and pyrethroids which caused insect mortality. However, the main principle of this insecticide resistance is the para-type sodium channel occurred some certain point mutations within insect body systems. These mutations can reduce the effectiveness of insecticides towards target insects. The alteration of sodium channels can lead to low capacity of insecticides towards insect who developed resistance compared to non– resistance insects. There are related studies showed that pyrethroids and DDT insecticides are the main insecticide type which leads to kdr mutation. Besides that, it also reported that the combination of both DDT and pyrethroids insecticides can give rise to cross resistances and caused insecticide resistances. (Davies et al., 2007); (Kushwah et al., 2015).
5.2 Behaviour resistance
Physiological resistances and behavioural avoidance are the two main common characterizations of insect responses towards insecticides (Chareonviriyaphap et al., 1997); (Chareonviriyaphap et al., 2013). Physiological resistances are a type of insecticide resistance which certain populations of insects manage to live at certain environments and expose themselves towards insecticides which its concentration levels of insecticides normally can lead to mortality of insects (Carrasco et al., 2019; Chareonviriyaphap et al., 2013; Roberts, Andre, & hygiene, 1994). There are still having some possibilities for the combination of more than one physiological resistances. For examples, the detoxification through enhancement of activity for certain enzymes (mediated P450 mono-oxygenase or glutathione S-transferases) and the modification of target site nerve receptors are the mechanisms which can work together for physiological resistances (Ranson et al., 2011). Behavioural avoidance or behavioural resistance is the resistance which insects can avoid the exposure towards insecticides by its own altered behaviour. The insects which had developed behavioural resistances can detect the insecticides within the environments and move to another site. This is due to its properties which have the stimulus for the detection of environmental changes (Gatton et al., 2013); (Panini, Manicardi, Moores, & Mazzoni, 2016). The changes of mosquito behaviour can be categorized into two types: direct contact excitation and non-contact spatial repellence. Direct contact excitation is the ability of some insecticides-threated mosquitoes to escape from areas which covered by insecticides. The mosquito behaviour which can avoid the insecticide-threated areas before they have any contact with insecticides is called non-contact spatial repellence (Roberts, Chareonviriyaphap, Harlan, & Hshieh, 1997); (Chareonviriyaphap et al., 2013); (Amelia-Yap, Chen, Sofian-Azirun, Low, & vectors, 2018).
There are four major phases of resistances which can be categorized from behavioural resistances: qualitative, quantitative, tolerance and exploitation. The first phase of behavioural resistance is called qualitative behavioural resistances whereby mosquitoes prevent any exposure towards insecticides. The mosquitoes can avoid any insecticide exposures by altering their activities times to prevent any clashing with insecticide applied periods. The changing of feeding locations can be done by mosquitoes to reduce the insecticide exposure rates. Quantitative behavioural resistances are the second phase of behavioural resistances whereby mosquitoes may have some reactions to get away from area where insecticide can found easily. Thus, the exposure time towards insecticides also can be reduced (Carrasco et al., 2019). Temperature regulations are also one of the quantitative behavioural resistances for mosquitoes to lower the reactions of insecticides within target sites. (Maliszewska & Tęgowska, 2017); (Carrasco et al., 2019). The third phase of resistances is behavioural tolerance and this resistance is suitable to mosquitoes that cannot escape from exposure or decrease the rate of insecticidal effects. For instances, mosquitoes can alter the production and distribution of their eggs. Besides that, the increases of nutrient qualities of bloods and decrease the usage of energies can be occurred due to this resistance. Reorganization of egg distribution and production also can be caused by this resistance (Cutler, 2013); (Carrasco et al., 2019). Behavioural exploitation is the last phase of resistance which the mosquitoes have physiological resistance and they can utilize these properties to detection the surrounding insecticides.
5.3 Reduce cuticle penetration resistance
The most of the insects synthesized certain amounts of apical extracellular matrix around their body parts such as tracheal, epidermis, epithelial at foregut and hindgut. These extracellular matrixes are usually called as cuticle which only produced at certain periods such as replacement of new cuticle during metamorphosis and moulting process. Embryogenesis is the biological process which can also leads to the formation of insect cuticles (Moussian & biology, 2010). According to (Vincent, Wegst, & development, 2004), the cuticles can provide some basic functions like locomotion, waterproof properties and give stable shapes to insects. Temporarily food reserved sites and obstacles to harmful diseases or parasite are also play as a parts of cuticle functions. It also consisted of several mechanical related functions such as high flexibility, regulation of diffusion and wear resistances. Chitins are the main constituents for the basic structural formations of cuticles. These arranged crystallized chitin nano–fibres embedded within the matrix of protein. There are others relevant substances like lipids, polyphenols and waters also included in cuticle productions.
According to (Strycharz et al., 2013), reduced cuticle penetration resistances are a well-known insecticides resistance mechanism which can be produced within insect body systems. The scattering levels of insecticides within the insect’s hemolymph became low as the penetration rate of insecticides through the cuticle decreased. The slow-going distribution rate of insecticides in hemolymph of insects can also leads to time extension for the detoxification of insecticides. Hence, it can reduce the insecticide accumulation at the targeted regions of insect body system. There are certain relevant studies showed that Ae. albopictus had developed resistances towards pyrethroid insecticides within Kuala Lumpur, Malaysia and these insecticide resistances developments were caused by reduced cuticle penetration resistances. The main factor that gives rise to cuticle penetration resistances was due to the over-expression of relevant cuticular protein genes in Ae. albopictus mosquitoes (Ishak et al., 2016). The modifications of cuticle are the elementary requirement to develop cuticle penetration resistance. The increases of cuticle thickness and the alteration of cuticle constituents are the two major parameters which involved in cuticle modifications. Among these 2 parameters, the cuticle thickness is more correlated with insecticide resistances but there are some studies showed the linkage between the reduction of xenobiotic penetration and constituents of cuticle (Dang, Doggett, Veera Singham, Lee, & vectors, 2017);(Balabanidou, Grigoraki, & Vontas, 2018). These types of insecticide resistance can defend against many different types of insecticides but the rates of resistance towards insecticides are very low. These cuticle penetration resistances can work with other types of insecticide resistance to boost up the resistance effects (Oppenoorth & Welling, 1976); (Scott, 1990); (Strycharz et al., 2013); (Kasai et al., 2014); (Panini et al., 2016).
6 Bio-insecticide
According to (Rattan, 2010), different types of defence mechanisms were created through the evolution of plants in order to protect themselves from their natural enemies. Besides plants, insects were also evolved so that they can defeat the protection mechanism of plants. Plants usually consisted of constituents called allele chemicals and these compounds can provide some defence properties towards their predators or microbes. These allele chemicals compounds also benefit plants to defend against most vertebrates due to the high similarity of neuronal signalling pathways among the animal kingdoms. The combinations of secondary metabolites may provide longer blockage effects towards herbivores and pests compared to single compounds. Besides that, its physical characteristic also can give more lasting protections to plants. It has been reported that different types of plants contain several groups of phytochemical compounds like steroids, terpenoids, phenolics, alkaloids and essential oils showed its insecticides effects against pests (A Shaalan & V Canyon, 2015). In search of bio insecticide, essential oils produced from aromatic plants have also researched extensively as alternatives to conventional insecticides (Zoubiri & Baaliouamer, 2014).
Bio-insecticides are the insecticides which they utilize most of the biological material such as isolated components from animal, plants, microorganisms and even certain natural environments as their main ingredients for its productions. (Chowański, Kudlewska, Marciniak, & Rosiński, 2014). According to (Dinesh, Kumari, Kumar, & Das, 2014), there are various common and significant issues happened due to the unsystematic usages of chemical pesticides. For example, target insects produced genetic resistances towards certain insecticides so that these insecticides are not effectives to insects. The adverse health risk of handling toxic substances and high amount of cost were also the problems created from chemical pesticides. It has been reported that bio-insecticides had been suggested as an alternative method for chemical insecticides to overcome the vector control problems. Bio-insecticides consisted of few benefits which make it as a new strategy for vector controls. It can protect the non-target organisms from hazard effects because it contains low amounts or no toxic substances. The amount of costs for usage of bio-insecticides is lower than chemical insecticides. The bio-insecticides also can avoid users to get harm because its low toxicity can enhance the protection towards users. It also can decrease the rate of bio-accumulation so that the residues within the environment also can be reduced. Besides that, the concentrations of bio-insecticides required to eradicate the target vectors are very low (Ohia & Ana, 2015). The effective levels of plant-based bio-insecticides are varies based on different types of factors such as the chosen plant segments, plant species and species of target. Furthermore, the plant-based bio-insecticides also can produce different effectiveness of insecticidal effects which depends on extraction techniques and solvent polarity.
6.1 Larvicidal activities in Malaysia
(Shivakumar, Srinivasan, Natarajan, & Research, 2013) reported that plants are the resources which can help to overcome the vector problems because they have some bioactive constituents which are harmless to environments. The bioactive constituents of plants consisted of several unique modes of action which can be applied against targeted pests or insects. Traditionally, human communities overcame the problems related to insects or pests by using plant-based products for many centuries. Normally, most of the plants can synthesize several compounds called secondary metabolites and these compounds plays important roles in defence mechanism to fight against insects. These secondary metabolites also contain various types of bioactive chemical which can be applied for others functions. For instances, there are several applications like insecticides, repellents, growth inhibitors, moulting hormones, antifeedants, oviposition deterrents, attractants, juvenile hormone mimics and anti-moulting hormones. Besides that, plant-based pesticides are low toxicity, low rate of resistance developments are the properties of plant-based pesticides due to its high biodegradable rate and its new structures. It has been reported that nine essential oils and seventeen methanol extracts derived from several plants within Malaysia were determined for their larvicidal activity against Ae. aegypti. Most of the plants which found in Malaysia showed effective results which they give high mortality rates to larvae of Ae. aegypti. Malaysia cinnamon species, Cinnamomum microphyllum (Ridl) and Cinnamomum impressicostatum (Kosterm), pear mangosteen, Garcinia griffithii (T. Anders), turmeric, Curcuma domestica (Valeton) and button mangosteen, Garcinia praniana (King) are the few main examples which have significant mortality rates towards Ae. aegypti. (Jantan, Ping, Visuvalingam, & Ahmad, 2003). Azolla pinnata, commonly known as mosquito weed and it can be found around Malaysia. There were some current researches showed that it can yield high effectiveness of larvicidal activities to both Ae. aegypti and Ae. albopictus mosquito species (Ravi et al., 2018).
6.2 Areca catechu (L.)
Areca catechu is a type of palm tree which can be broadly cultivated and noticeable at various countries within Asia like Thailand, India, Malaysia, Taiwan and other relevant countries. Areca catechu plant is commonly known as betel nut tree or betel palm. The height of 20 m is the maximum height for an A. catechu plant grows. The general diameters of its trunks are located within the ranges which approximately between 20 m and 30 cm. The leaves of A. catechu plant have its own features which called as pinnate shapes and it can be used to differentiate with others plants. The normal length of A. catechu leaves can be 1.5 m to 2.0 m (Jaiswal, Kumar, Singh, & Singh, 2011). According to (Binoj, Raj, Sreenivasan, & Thusnavis, 2016), the flowers of A. catechu plant are typically grown in cluster forms and each spathe of flower can produce about 30 to 50 nuts. Areca catechu nuts usually have their own diameters which can grow up to the ranges of 5 cm to 10 cm. The external parts of nut shells were covered by certain amount of fibres which approximately 2.5 g to 2.75 g. Raw, ripe and mature are the 3 main maturity stages of an A. catechu nut. These stages can be determined easily by its colours and features. The nut and husk of a raw A. catechu nut are soft and the nuts are in green colour. The yellow A. catechu nut and juicy husk showed its ripe conditions. A fully matured nut has brownish colour appearance with shaggy fibres (Yusriah, Sapuan, Zainudin, & Mariatti, 2014).
(Amudhan et al., 2012), reported that there are several main compounds of phytochemicals which incorporated within an A. catechu nut such as polyphenol, alkaloids, fatty acids and others related mineral constituents. The isolation of phytochemicals constituents from A. catechu nut differs based on the nut maturity stages (Sumalatha, Ishwara Bhat, & Environment, 2019). Flavonoids such as catechin, epicatechin and leucocyanidin are the major constituents of polyphenols in an A. catechu nut. The fatty acid of A. catechu nut can be categorized into major and minor compounds. The few examples of major fatty acid within the nuts are myristic acid, lauric acid, linoleic acid, oleic acid, hexadecanoic acid and palmitic acid. Stearic acid and decanoic acid are the compounds which play as minor fatty acid groups in the nuts. A. catechu nut also consisted different types of oligomeric procyanidins. The major alkaloids which can be found in A. catechu nut are guvacine, guvacoline, arecaidine and arecoline (Amudhan et al., 2012). Several types of minerals such as calcium, iron, phosphorus and vitamins also can be extracted from A. catechu nuts (Sumalatha et al., 2019).
The betel nut can be processed by various ways such as dried, baked, roasted, cured and boiled. Betel quid is a type of quid which included several components such as betel leaves, tobacco leaves, quench lime mushes and betel nuts. The proper methods to consume betel quid are combine these components and chew it. The staining of teeth and tongue can be happened for the peoples who chew betel quid. The practices of chewing betel quid are mostly occurred within South Asia and even at certain rural areas in India (Hossain, Anwar, Akhtar, & Numan, 2015). Areca catechu nut was used for treatment purposes to certain diseases such as hypoglycemic, regulation of blood pressure and anti-diabetic due to its pharmacological functions (Patil, Rakesh, Dhabale, & Burade, 2009). According to (Dhanraj, Veerakumari, & Ashwini, 2018), it has been reported that A. catechu nut extracts also possessed some anthelmintic activities which it can eradicate Cotylophoron cotylophorum parasites in livestock with high efficiency. The rate of antioxidant activities of A. catechu nut extracts can be enhanced by using extraction techniques with methanol or methanol–water (Hannan, Karan, & Chatterjee, 2012). The dried A. catechu nut is commonly used in these extractions (Fig. 2). Nevertheless, A. catechu has shown very effective response against larvae with just a minimal concentration of 200 mg/l (Bharathithasan et al., 2022).
Dried Areca catechu nut (Murugakoothan et al., 2014).
6.3 Azolla pinnata
Azolla pinnata is a type of free-floating aquatic fern plants which can be found in most of the tropical and subtropical in the world, especially at stagnant water areas or mild freshwater ecosystem (Shamna, Peethambaran, Jalaludeen, Leo, & Muhammad, 2013); Fig. 3). This type of plant species belongs to the family called Azollaceae (E. V. Kumar, Avinash, Bakshi, Kiran, & Narender, 2019). There are several common names for A. pinnata such as mosquito fern, water fern, mosquito weeds and water velvet. Basically, the fern frond of A. pinnata is the main body parts of plants. Polygonal or triangular are the common shape of A. pinnata fern fron d. The stems are the essential part of A. pinnata fern frond and they grow at the surfaces of aquatic habitats. The adventitious roots and alternate leaves also grow by the side of stems at regular intervals. These fronds usually float individually or mats forms at the water surfaces (Israa et al., 2012) (Wilson et al., 2020). The formation of thick mats can also avoid the egg laying processes by adult mosquito which can decrease the rate of mosquito larvae development (AhbiRami et al., 2014); (Ravi et al., 2018).
Fresh Azolla pinnata plants (Husna Zulkrnin et al., 2018).
According to (Pabby et al., 2003) and (Abd Kadir et al., 2018), Anabaena azollae is a type of cyanobacteria which have symbiotic relationships with A. pinnata. This cyanobacterium typically hosts by them at the space which located at the leaf centre. The main role of A. azollae cyanobacteria includes productions of high level of nitrogen within A. pinnata plant tissues by absorbing nitrogen gas (N2) from atmosphere. This makes A. pinnata become a suitable plant which can be used as green manure by various countries (Abd Kadir et al., 2018). It has been reported that A. pinnata contained 21–23 % of high crude protein level which cosidered as a good protein resource. There are various vitamins (Vitamin B-12 and A) and minerals (potassium, calcium, iron, magnesium) have been discovered within A. pinnata. Azolla pinnata also has been utilized as feed supplements for different livestock such as chicks, goats and cattle. The feeding of A. pinnata as feed supplements to livestock led to positive results such as increase of egg laying rates, milk and meat productions (Mathur, Sharma, & Choudhary, 2013); (G. Kumar & Chander, 2017). There are studies reported the high effectiveness of A. pinnata in wastewater treatments and removals of several metals such as cadmium, copper, lead and manganese (Akinbile et al., 2019). There are studies related to A. pinnata showed that the plant extracts caused high mortality rates against 2 main Aedes mosquito larvae species (Ae. aegypti and Ae. albopictus). According to Ravi et al. (2018), the methanol solvent extracts of A. pinnata using soxhlet extraction were analyzed by GC–MS analysis. The GC–MS chromatogram displayed 44 peaks which showed that 44 phytochemical compounds were presented within A. pinnata extracts. The mass spectra consisted of 44 phytochemical compounds were compared with mass spectra within NIST 08 library but there were only 35 compounds were characterized and identified in Table 2. These studies proved the A. pinnata contained potent larvicidal activities against Aedes mosquito larvae (Ravi et al., 2018).
Compound
Retention time
Area %
Activity
1- Methyldecylamine
3.623
0.347
Insecticidal activity
1-Heptadecene
26.824
1.270
Insecticidal and antibacterial activity
1-Nonadecene
34.437
1.946
Insecticidal and fungizide activity
Neophytadiene
35.926
27.853
Larvicidal, insecticidal and antimicrobial activity
3,7,11,15- Tetramethyl-2- hexadecen-1-ol
37.446
9.061
Insecticidal, anti-parasitic, nematicide and antimicrobial activity
Phytol
45.188
10.043
Insecticidal, fungicide, miticide activity
6.4 Lantana camara
The Lantana is the plant genus which is belonging to family called Verbenaceae. Genus of Lantana is indigenous from America, especially from the tropical and subtropical regions. There are also certain taxa which are native to tropical Africa and other Asia countries. The genus Lantana contains one species from Ethiopia and six species from South America as reported by Linnaeus in 1753. This genus also included about 150 species of shrubs or herbs. The cultivated flower species of genus Lantana can be found around 50 countries (Munir, 1996); (Negi et al., 2019). Lantana camara is the one of the plant species derived from genus Lantana and it commonly known as red sage. Lantana camara plant is mostly used as an ornamental plant for gardening purposes (Munir, 1996); (Shah, Alharby, & Hakeem, 2020).
The wide of the L. camara leaves are within 3 cm to 6 cm and the ranges of its length are about 3 cm to 8 cm. The shape of L. camara leaves consisted of several characteristics such as wrinkled leaves surfaces, acute, ovate and both sides of leaves contain scabrid. The maximum width of L. camara plant can reach up to 2.5 m and its height can grow up to the range of 1 m to 3 m. L. camara plant consisted some rough hairs which coated their stems and leaves. The flower of L. camara plant is small and appeared as cluster forms. Orange is the most common colour of L. camara flower (Fig. 4). The alteration of flower colour may occur from white to red in different shades and it depends on their ages. The strong scents of L. camara plant are similar to blackcurrants (Verma & Therapeutics, 2018). Callosobruchus chinensis (L.) is the harmful pest which can penetrate and destroy the entire grain kernel. This type of pests can spoil the grains which can lead to loss of total grain productions (S. J. J. o. E. Singh & Studies, 2017). It has been reported that L. camara plant can give high effectiveness of antiovipositional and insecticidal activities towards C. chinensis (L.) (Saxena, Dixit, & Harshan, 1992); (Shah et al., 2020). The pharmacological studies about L. camara also have been proved that it has the powerful anti-inflammatory and antinociceptive properties (Silva et al., 2015). Some studies also showed that L. camara plant extracts give strong larvicidal effects to Ae. aegypti, Anopheles stephensi and Culex quinquefasciatus larvae (Hemalatha et al., 2015).
Lantana camara plant (Dubey & Padhy, 2013).
6.5 Mukia maderaspatana
Mukia maderaspatana is a plant that belongs to Cucurbitaceae family (A. J. A. J. o. C. Petrus, 2012). Normally, this type of plant can be found in some abandoned fields or unwanted areas. It distributed nearly all over India but it also found in other countries such as Africa, New Zealand, Taiwan, Malaysia, China and Australia (Petrus et al., 2011). (A. J. A. J. o. C. Petrus, 2012), reported that the growing patterns of M. maderaspatana are usually climbing or prostrate. This type of plant contains some tendrils, scattering with certain amount of stiff hairs and mostly branched. The colour of M. maderaspatana flowers are pale yellow and their flower size are small. The M. maderaspatana flowers have categorized into two parts: male and female. Both of flowers are slightly different in appearances. Female flowers usually joint directly to base without peduncle and borne singly while male flowers are attached with a short peduncle.
The length and width of M. maderaspatana plants are generally within the range of 3 cm to 11 cm. Their characteristics of leaves included dark green colour, acute apices, irregularly serrated edge and consist of 3 to 5 lobes per leaf. The length of hairy petioles can reach about 0.6 cm to 2.5 cm. The pale green is the fruit colour of M. maderaspatana plants and reddish colour are the maturation stages of fruits (A. J. A. J. o. C. Petrus, 2012) Fig. 5). It has been reported that the M. maderaspatana plants contain several types of polyphenols which consist of strong antioxidant properties such as saponarin, carotenoids, vitamin E, vitamin C. These polyphenols can against the oxidative stresses and reduce the development of chronic diseases (Petrus et al., 2011). Mukia maderaspatana plant also contains larvicidal properties towards larvae of Ae. aegypti and gave efficient mortality results (Ramamurthy & Krishnaveni, 2014). Some studies also reported that M. maderaspatana plants consisted of high antimicrobial activity against causative agent of tuberculosis, Mycobacterium tuberculosis. Mukia maderaspatana extracts showed the lowest minimum inhibitory concentration against M. tuberculosis compared with others experimental plants (Nirmal et al., 2020).
Mukia maderaspatana plant (Petrus et al., 2011).
6.6 Leucas aspera
Leucas aspera is a type of herbaceous plants which belongs to family called Lamiaceae (Labiatae). Normally, the distribution of these plants includes some temperate or tropical regions of Asia countries. It can be found at various areas such as roadsides, uncultivated land, farmhouse and crop fields located at high land (Prajapati, Patel, Modi, & Shah, 2010); (Srinivasan et al., 2011); (Islam, Ohno, Suenaga, & Kato-Noguchi, 2014). According to (Saritha, Rajesh, Manjulatha, Setty, & Yenugu, 2015) and (E. V. Kumar et al., 2019), the lance head-liked shape, tiny petiole and coated pubescents are the main characteristics of L. aspera leaves (Fig. 6). The height of L. aspera plants can grow within the ranges of 15 cm to 60 cm. The stems and branches are upright, thick and quadrangular shapes. The L. aspera fruits look like a brown colour nut which have diameter of 2.5 mm. The flowers of L. aspera are white in colour and it also comprised with terminal forms of whorls and tiny sessile.
Leucas aspera plants (Suganya, Karthi, & Shivakumar, 2014).
It has been reported that L. aspera plant extracts consisted of antimicrobial activities towards microorganisms such as Escherichia coli. The small pores formation on bacterial membrane is due to the antimicrobial actions of small molecules and protein content within L. aspera plant extracts. These formations of small pores caused the cellular contents leaked out from its membrane (Saritha et al., 2015). The antioxidant properties of L. aspera plant extracts also have been proved by some studies. The root parts of L. aspera plant showed the potent antioxidant activities compared to others plant parts and its activities almost reached the level of vitamin E. Thus, it can become the new potential antioxidant resources (Santhanam Selvaraj, Ponraj, Rameshkumar, & et al., 2021) The larvicidal activities of L. aspera plant extracts against Ae. aegypti, A. stephensi and Cx. quinquefasciatus has been proved by some studies. The results also showed mortality rate on the larvae of these mosquito species (Elumalai et al., 2017).
7 Lethal concentration
Toxicology is the major components in pharmacology which manage with the negative impacts of phytochemical compounds towards living organisms before it is applied as drugs or chemical in clinical uses. Most of the studies also focused on toxicity analysis because it can help to determine the safety level of the targeted plants and their related products. The toxicity analysis is significant since there have been reports of toxicity effects from long-term ingestion of various plant herbs. (Subha & Geetha, 2017). This toxicity analysis can make sure the safety of chemical which can be uses as drugs or pesticides prior to apply in clinical or industry fields. It can also determine the mechanism of toxic effects which may be found from other studies. The toxicity test has been applied for quantifications of chemical toxicology in ecotoxicology (Arome, Chinedu, & BioSciences, 2013).
Lethal concentration (LC) is one of the toxicity analyses which it can quantify the threshold level of chemical toxicity in various conditions of exposures. The lethal concentration 50 (LC50) means the concentration of certain chemicals within the treated media which can kill half of the test populations (Zhao, Newman, & Journal, 2004). Lethal concentration 95 (LC95) is the chemical concentration which can give mortality to 95 % of test populations. Lethal concentration has also been used for some larvicidal studies of target plants. For example, both LC50 and LC95 have been used as the toxicity analysis to determine the larvicidal activities of A. pinnata plant extracts towards Ae. aegypti and Ae. albopictus. These lethal concentrations can detect the effectiveness of targeted plant extracts by their lethal concentrations which can cause mortality to Aedes mosquito larvae (Ravi et al., 2018).
8 Conclusion
The conclusion of this study has shown bio-insecticides are found in various plants which act against major dengue vectors. These chemical compounds also included in several patented inventions for various types of pesticide formulations. Besides that, these are some studies showed the related larvicidal mechanisms of fatty acids, fatty acid methyl esters and flavonoids which can cause mortality against different mosquito larvae species. The finding from this study showed that the five plants could be potential bio-insecticides for overcome the dengue vectors, Ae. aegypti and Ae. albopictus. The plants consisted of some potential chemical compounds which can cause larvicidal effects against mosquito larvae. Further studies would be required for experimental studies about larvicidal activities against Aedes mosquito larvae. The gas chromatography-mass spectrometry (GC–MS) analysis of these plants with multiple extraction methods could be also done for further studies in order to discover more bioactive compounds from these plants.
Acknowledgement
We would like to thank the Malaysia Ministry of Higher Education (MoHE), Fundamental Research Grant Scheme (FRGS), FRGS/1/2020/WAB02/QUEST/03/1, Quest International University staffs, research students who had contributed to this work and the management who provided facilities.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Comparative phytotoxicity of Azolla pinnata and Lemna minor in treated palm oil mill effluent. Inter. J. Eng. Technol.. 2018;7(4):2499-2505.
- [Google Scholar]
- Larvicidal efficacy of different plant parts of railway creeper, Ipomoea cairica extract against dengue vector mosquitoes, Aedes albopictus (Diptera: Culicidae) and Aedes aegypti (Diptera: Culicidae) J. Insect Sci.. 2014;14(1)
- [Google Scholar]
- Assessing the efficacy of Azolla pinnata in four different wastewater treatment for agricultural re-use: a case history. Sust. Water Resour. Manage.. 2019;5(3):1009-1015.
- [Google Scholar]
- Amelia-Yap, Z. H., Chen, C. D., Sofian-Azirun, M., Low, V. L. J. P., & vectors. (2018). Pyrethroid resistance in the dengue vector Aedes aegypti in Southeast Asia: present situation and prospects for management. 11(1), 1-17.
- Amudhan, M.S., Begum, V.H., Hebbar, K., 2012. A review on phytochemical and pharmacological potential of Areca catechu L. seed. Inter. J. Pharmaceut. Sci. Res.
- Arome, D., Chinedu, E. J. J. o. P., & BioSciences. (2013). The importance of toxicity testing. 4, 146-148.
- Balabanidou, V., Grigoraki, L., & Vontas, J. J. C. o. i. i. s. (2018). Insect cuticle: a critical determinant of insecticide resistance. 27, 68-74.
- Bass, C., Nikou, D., Donnelly, M. J., Williamson, M. S., Ranson, H., Ball, A., Field, L. M. J. M. J. (2007). Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. 6(1), 1-14.
- Bharathithasan, M., Ravindran, D. R., Rajendran, D., Sugathan, S., Yahaya, Z. S., Said, A. R., Ishak, I. H. (2022). Areca catechu Linn as a potential insecticide for mosquitoes. Paper presented at the AIP Conference Proceedings.
- Binoj, J., Raj, R. E., Sreenivasan, V., & Thusnavis, G. R. J. J. o. B. E. (2016). Morphological, physical, mechanical, chemical and thermal characterization of sustainable Indian areca fruit husk fibers (Areca catechu L.) as potential alternate for hazardous synthetic fibers. 13(1), 156-165.
- Carrasco, D., Lefèvre, T., Moiroux, N., Pennetier, C., Chandre, F., & Cohuet, A. J. C. o. i. i. s. (2019). Behavioural adaptations of mosquito vectors to insecticide control. 34, 48-54.
- Chareonviriyaphap, T., Roberts, D., Andre, R. G., Harlan, H., Manguin, S., & Bangs, M. J. J. o. t. A. M. C. A.-M. N. (1997). Pesticide avoidance behavior in Anopheles albimanus, a malaria vector in the Americas. 13(2), 171-183.
- Chareonviriyaphap, T., Bangs, M. J., Suwonkerd, W., Kongmee, M., Corbel, V., Ngoen-Klan, R. J. P., & vectors. (2013). Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. 6(1), 1-28.
- Chowański, S., Kudlewska, M., Marciniak, P., & Rosiński, G. J. P. J. o. E. S. (2014). Synthetic Insecticides--is There an Alternative? , 23(2).
- Cutler, G. C. J. D.-r. (2013). Insects, insecticides and hormesis: evidence and considerations for study. 11(2), dose-response. 12-008. Cutler.
- Dang, K., Doggett, S. L., Veera Singham, G., Lee, C.-Y. J. P., & vectors. (2017). Insecticide resistance and resistance mechanisms in bed bugs, Cimex spp.(Hemiptera: Cimicidae). 10(1), 1-31.
- Plant-based Essential Oils in the Management of Dengue Vector, Aedesaegypti sp. Inter. J. Life Sci. 2017
- [Google Scholar]
- Davies, T., Field, L., Usherwood, P., & Williamson, M. J. I. l. (2007). DDT, pyrethrins, pyrethroids and insect sodium channels. 59(3), 151-162.
- Dhanraj, K. M., Veerakumari, L., & Ashwini, S. J. D. R. (2018). Anthelmintic efficacy of ethanol extract of Areca catechu on the carbohydrate metabolism of Cotylophoron cotylophorum.
- Diallo, D., Sall, A. A., Buenemann, M., Chen, R., Faye, O., Diagne, C. T., Watts, D. J. P. n. t. d. (2012). Landscape ecology of sylvatic chikungunya virus and mosquito vectors in southeastern Senegal. 6(6), e1649.
- Dinesh, D. S., Kumari, S., Kumar, V., & Das, P. J. J. o. v. b. d. (2014). The potentiality of botanicals and their products as an alternative to chemical insecticides to sandflies (Diptera: Psychodidae): A review. 51(1), 1.
- Du, Y., Nomura, Y., Zhorov, B. S., & Dong, K. J. J. o. B. C. (2016). Evidence for dual binding sites for 1, 1, 1-trichloro-2, 2-bis (p-chlorophenyl) ethane (DDT) in insect sodium channels. 291(9), 4638-4648.
- Dubey, D., & Padhy, R. N. J. J. o. H. M. (2013). Antibacterial activity of Lantana camara L. against multidrug resistant pathogens from ICU patients of a teaching hospital. 3(2), 65-75.
- Elumalai, D., Hemalatha, P., & Kaleena, P. J. J. o. t. S. s. o. a. s. (2017). Larvicidal activity and GC–MS analysis of Leucas aspera against Aedes aegypti Anopheles stephensi and Culex quinquefasciatus. 16(4), 306-313.
- Emran, A., Sherin, A., Thein, T. T., & Aung, T. S. J. J. o. V. B. D. (2018). Circulation of all dengue virus serotypes during dengue outbreak in Sandakan, Sabah, Malaysia (2016). 55(2), 168.
- Faithpraise, F., Idung, J., Usibe, B., Chatwin, C., Young, R., Birch, P. J. I. J. o. I. R. i. S. E., & Technology. (2014). Natural control of the mosquito population via Odonataand Toxorhynchites. 3(5), 12898-12911.
- Farajollahi, A., & Price, D. C. J. J. o. t. A. M. C. A. (2013). A rapid identification guide for larvae of the most common North American container-inhabiting Aedes species of medical importance. 29(3), 203-221.
- Gajendiran, A., & Abraham, J. J. F. i. B. (2018). An overview of pyrethroid insecticides. 13(2), 79-90.
- Gatton, M. L., Chitnis, N., Churcher, T., Donnelly, M. J., Ghani, A. C., Godfray, H. C. J., . . . Ranson, H. J. E. i. j. o. o. e. (2013). The importance of mosquito behavioural adaptations to malaria control in Africa. 67(4), 1218-1230.
- Guzman, A., & Istúriz, R. E. J. I. j. o. a. a. (2010). Update on the global spread of dengue. 36, S40-S42.
- Dengue infection. Nature reviews Disease primers. Inter. J. Antimicrobial Agents. 2016;2(1)
- [Google Scholar]
- Hamed, A. M., El-Sherbini, M. S., Abdeltawab, M. S. J. E. A. J. o. B. S., E. Medical Entomology, & Parasitology. (2022). Eco-friendly mosquito-control strategies: Advantages and disadvantages. 14(1), 17-31.
- Hannan, A., Karan, S., & Chatterjee, T. K. J. I. J. P. P. S. (2012). A comparative study of in-vitro antioxidant activity of different extracts of Areca seed collected from Areca catechu plant grown in Assam. 4(2), 420-427.
- Harada, T., Takeda, M., Kojima, S., & Tomiyama, N. J. T. R. (2016). Toxicity and carcinogenicity of dichlorodiphenyltrichloroethane (DDT). 32(1), 21-33.
- Larvicidal activity of Lantana camara aculeata against three important mosquito species. J. Entomol. Zoology Stud.. 2015;3(1):174-181.
- [Google Scholar]
- Hossain, M. F., Anwar, M., Akhtar, S., & Numan, S. M. J. I. J. C. M. P. H. (2015). Adverse effects on health posed by consumption of Areca nut (Areca catechu L., family: Palmaceae). 2(4), 357-360.
- Huang, Y.-J. S., Higgs, S., & Vanlandingham, D. L. J. C. o. i. v. (2019). Emergence and re-emergence of mosquito-borne arboviruses. 34, 104-109.
- Husna Zulkrnin, N. S., Rozhan, N. N., Zulkfili, N. A., Nik Yusoff, N. R., Rasat, M. S. M., Abdullah, N. H., . . . Mohd Amin, M. F. J. J. o. p. r. (2018). Larvicidal effectiveness of Azolla pinnata against Aedes aegypti (Diptera: Culicidae) with its effects on larval morphology and visualization of behavioural response. 2018.
- Ishak, I. H., Riveron, J. M., Ibrahim, S. S., Stott, R., Longbottom, J., Irving, H., & Wondji, C. S. J. S. r. (2016). The Cytochrome P450 gene CYP6P12 confers pyrethroid resistance in kdr-free Malaysian populations of the dengue vector Aedes albopictus. 6(1), 1-13.
- Islam, A. M., Ohno, O., Suenaga, K., & Kato-Noguchi, H. J. J. o. p. p. (2014). Two novel phytotoxic substances from Leucas aspera. 171(11), 877-883.
- Israa, A. A.-B., Siti, R., Fatihah, S., Nurina, A., Mushrifah, I. J. I. P. o. C., Biological, & Engineering, E. (2012). Preliminary test of hydrocarbon exposure on Azolla pinnata in phytoremediation process. 33, 244-247.
- Jaiswal, P., Kumar, P., Singh, V., & Singh, D. J. R. J. o. M. P. (2011). Areca catechu L.: A valuable herbal medicine against different. 5(2), 145-152.
- Jantan, I., Ping, W. O., Visuvalingam, S. D., & Ahmad, N. W. J. P. B. (2003). Larvicidal activity of the essential oils and methanol extracts of Malaysian plants on Aedes aegypti. 41(4), 234-236.
- Kamaraj, C., Rahuman, A. A., Mahapatra, A., Bagavan, A., & Elango, G. J. P. r. (2010). Insecticidal and larvicidal activities of medicinal plant extracts against mosquitoes. 107, 1337-1349.
- Kasai, S., Komagata, O., Itokawa, K., Shono, T., Ng, L. C., Kobayashi, M., & Tomita, T. J. P. n. t. d. (2014). Mechanisms of pyrethroid resistance in the dengue mosquito vector, Aedes aegypti: target site insensitivity, penetration, and metabolism. 8(6), e2948.
- Khetarpal, N., & Khanna, I. J. J. o. i. r. (2016). Dengue fever: causes, complications, and vaccine strategies. 2016.
- Kshirsagar, R., & Phal, L. W. D. (2022). Novel method for screening Mosquitocidals against Aedes aegypti.
- Kumar, G., & Chander, H. J. A. J. A. B. S. (2017). Study on the Potential of Azolla pinnata as livestock Feed Supplement for climate Change adaptation and Mitigation. 5(2), 65-68.
- Kumar, E. V., Avinash, N., Bakshi, V., Kiran, G., & Narender, B. J. I. T. S. (2019). A review on Leucas aspera for phytopharmacological studies. 2, 3-7.
- Kushwah, R., Mallick, P., Ravikumar, H., Dev, V., Kapoor, N., Adak, T., & Singh, O. J. J. o. v. b. d. (2015). Status of DDT and pyrethroid resistance in Indian Aedes albopictus and absence of knockdown resistance (kdr) mutation. 52(1), 95.
- Maliszewska, J., & Tęgowska, E. J. I. J. o. P. M. (2017). A comparison of the effectiveness of insecticides in constant and variable thermal conditions. 63(4), 331-340.
- Toxicity of white flesh Citrus grandis Osbeck fruit peel extracts against Aedes aegypti (Linnaeus) larvae and its effect on non-target organisms. Inter. J. Mosquito Res. 2017
- [Google Scholar]
- Mathur, G., Sharma, R., & Choudhary, P. J. S. J. A. L. M. O. S. O. K. V. (2013). Use of azolla (Azolla pinnata) as cattle feed. 73.
- Moussian, B. J. I. b., & biology, m. (2010). Recent advances in understanding mechanisms of insect cuticle differentiation. 40(5), 363-375.
- Munir, A. A. J. J. o. t. A. B. G. (1996). A taxonomic review of Lantana camara L. and L. montevidensis (Spreng.) Briq.(Verbenaceae) in Australia. 1-27.
- Murugakoothan, P., Ananth, S., Vivek, P., Arumanayagam, T., 2014. Natural dye extracts of areca catechu nut as dye sensitizer for titanium dioxide based dye sensitized solar cells. J. Nano Electronic Phys.
- Negi, G., Sharma, S., Vishvakarma, S. C., Samant, S. S., Maikhuri, R. K., Prasad, R. C., & Palni, L. J. T. B. R. (2019). Ecology and use of Lantana camara in India. 85(2), 109-130.
- Nirmal, C. R., Ebenezer, R. S., Kannan, P., Balasubramanian, M., Thirunavukkarasu, I., Mondal, R., & Dusthackeer, A. J. E. J. o. I. M. (2020). Anti-tuberculosis activity of bio-active compounds from Lantana camara L., Euphorbia hirta L., Mukia maderaspatana (L.) M. Roem, and Abutilon indicum (L.). 35, 101105.
- Ohia, C., & Ana, G. J. J. B. A. H. (2015). Bio-insecticides: the one-health response to mosquito-borne diseases of public health importance. 5, 22-26.
- Oishi, K., Saito, M., Mapua, C. A., Natividad, F. F. J. J. o. i., & chemotherapy. (2007). Dengue illness: clinical features and pathogenesis. 13(3), 125-133.
- Okafor, I. I. J. D., Health, E. i., & Care. (2016). Zika virus: The emerging global health challenge. 13(6).
- Oppenoorth, F., & Welling, W. (1976). Biochemistry and physiology of resistance. In Insecticide biochemistry and physiology (pp. 507-551): Springer.
- Azolla-Anabaena symbiosis-from traditional agriculture to biotechnology. Indian J. Biotechnol. 2003
- [Google Scholar]
- Panini, M., Manicardi, G. C., Moores, G., & Mazzoni, E. J. I. S. J. (2016). An overview of the main pathways of metabolic resistance in insects. 13(1), 326-335.
- Patil, P. R., Rakesh, S. U., Dhabale, P., & Burade, K. J. J. o. P. R. (2009). Pharmacological activities of Areca catechu Linn.-a review. 2(4), 683-687.
- Perry, A., Yamamoto, I., Ishaaya, I., & Perry, R. (1998). Retrospects and Prospects. In: Springer-Verlag, Berlin.
- Antioxidative constitution of Mukia maderaspatana (Linn.) M. Roem. leaves. J. Asian J. Chem. 2011
- [Google Scholar]
- Petrus, A. J. A. J. o. C. (2012). Mukia maderaspatana (L.) M. Roemer-A Review of Its Global Distribution, Phytochemical Profile and Antioxidant Capacity. 24(6).
- Prajapati, M., Patel, J., Modi, K., & Shah, M. J. P. r. (2010). Leucas aspera: A review. 4(7), 85.
- Prasannath, K. J. I. J. o. A. R., & Review. (2016). Botanical insecticides-special reference to horticultural insect pest management: a review. 1(5), 14-18.
- Ramamurthy, V., & Krishnaveni, S. J. J. E. Z. S. (2014). Larvicidal efficacy of leaf extracts of Heliotropium Indicum and Mukia maderaspatana against the dengue fever mosquito vector Aedes aegypti. 2, 40-45.
- Ranson, H., N’guessan, R., Lines, J., Moiroux, N., Nkuni, Z., & Corbel, V. J. T. i. p. (2011). Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? , 27(2), 91-98.
- Rattan, R. S. J. C. p. (2010). Mechanism of action of insecticidal secondary metabolites of plant origin. 29(9), 913-920.
- Ravi, R., Zulkrnin, N. S. H., Rozhan, N. N., Nik Yusoff, N. R., Mat Rasat, M. S., Ahmad, M. I., . . . Amin, M. F. M. J. P. o. (2018). Chemical composition and larvicidal activities of Azolla pinnata extracts against Aedes (Diptera: Culicidae). 13(11), e0206982.
- Redoni, M., Yacoub, S., Rivino, L., Giacobbe, D. R., Luzzati, R., & Di Bella, S. J. R. i. M. V. (2020). Dengue: Status of current and under‐development vaccines. 30(4), e2101.
- Rezza, G. J. B. p. h. (2012). Aedes albopictus and the reemergence of Dengue. 12(1), 1-3.
- Roberts, D. R., Andre, R. G. J. T. A. j. o. t. m., & hygiene. (1994). Insecticide resistance issues in vector-borne disease control. 50(6 Suppl), 21-34.
- Roberts, D. R., Chareonviriyaphap, T., Harlan, H. H., & Hshieh, P. J. J. o. t. A. M. C. A. (1997). Methods of testing and analyzing excito-repellency responses of malaria vectors to insecticides. 13(1), 13-17.
- Rueda, L. M. (2007). Global diversity of mosquitoes (Insecta: Diptera: Culicidae) in freshwater. In Freshwater animal diversity assessment (pp. 477-487): Springer.
- Santhanam Selvaraj, C. P., Ponraj, G., Rameshkumar, K. J. A. A. o. M. P. L. E. A. S. S. J., & et al. (2021). Antibacterial Activities of Medicinal Plant Leaves Extract Against Isolated Escherichia coli Bacteria from Different Water Sources. Research & Reviews: A Journal of Microbiology & Virology. 2021; 11 (3): 34–40p. 2.
- Saritha, K., Rajesh, A., Manjulatha, K., Setty, O. H., & Yenugu, S. J. F. i. m. (2015). Mechanism of antibacterial action of the alcoholic extracts of Hemidesmus indicus (L.) R. Br. ex Schult, Leucas aspera (Wild.), Plumbago zeylanica L., and Tridax procumbens (L.) R. Br. ex Schult. 6, 577.
- Saxena, R., Dixit, O., & Harshan, V. J. J. o. S. P. R. (1992). Insecticidal action of Lantana camara against Callosobruchus chinensis (Coleoptera: Bruchidae). 28(4), 279-281.
- Scott, J. G. (1990). Investigating mechanisms of insecticide resistance: methods, strategies, and pitfalls. In Pesticide resistance in arthropods (pp. 39-57): Springer.
- A Shaalan, E., & V Canyon, D. J. C. B. C. (2015). A review on mosquitocidal activity of botanical seed derivatives. 11(2), 78-90.
- Shafique, M., Lopes, S., Doum, D., Keo, V., Sokha, L., Sam, B., Liverani, M. J. P. n. t. d. (2019). Implementation of guppy fish (Poecilia reticulata), and a novel larvicide (Pyriproxyfen) product (Sumilarv 2MR) for dengue control in Cambodia: A qualitative study of acceptability, sustainability and community engagement. 13(11), e0007907.
- Shah, M., Alharby, H. F., & Hakeem, K. R. J. L. A. N. B. S. (2020). Lantana camara: a comprehensive review on phytochemistry, ethnopharmacology and essential oil composition. 9(3), 1199-1207.
- Shamna, T., Peethambaran, P., Jalaludeen, A., Leo, J., & Muhammad, A. J. A. S. R. (2013). Broiler characteristics of Japanese quails (Coturnix coturnix japonica) at different levels of diet substitution with Azolla pinnata. 7(2).
- Shivakumar, M., Srinivasan, R., Natarajan, D. J. A. J. o. P., & Research, C. (2013). Larvicidal potential of some Indian medicinal plant extracts against Aedes aegypti (L.). 6(3), 77-80.
- Silva, T., Suffredini, I., Ricci, E., Fernandes, S., Gonçalves Jr, V., Romoff, P., Bernardi, M. J. R. B. d. P. M. (2015). Antinociceptive and anti-inflammatory effects of Lantana camara L. extract in mice. 17, 224-229.
- Singh, S., Mann, B. K. J. I. j. o. d., venereology, & leprology. (2013). Insect bite reactions. 79, 151.
- Singh, S. J. J. o. E., & Studies, Z. (2017). Natural plant products-As protectant during grain storage: A review. 5(3), 1873-1885.
- Smith, L. B., Kasai, S., & Scott, J. G. J. P. m. s. (2018). Voltage‐sensitive sodium channel mutations S989P+ V1016G in Aedes aegypti confer variable resistance to pyrethroids, DDT and oxadiazines. 74(3), 737-745.
- Soderlund, D. M. J. A. o. t. (2012). Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. 86(2), 165-181.
- Srinivasan, R., Ravali, B., Suvarchala, P., Honey, A., Tejaswini, A., Neeraja, P. J. I. J. o. P., & Sciences, B. (2011). Leucas aspera-medicinal plant: a review. 2(1), 153-159.
- Strycharz, J. P., Lao, A., Li, H., Qiu, X., Lee, S. H., Sun, W., physiology. (2013). Resistance in the highly DDT-resistant 91-R strain of Drosophila melanogaster involves decreased penetration, increased metabolism, and direct excretion. 107(2), 207-217.
- Subenthiran, S., Choon, T. C., Cheong, K. C., Thayan, R., Teck, M. B., Muniandy, P. K., Medicine, A. (2013). Carica papaya leaves juice significantly accelerates the rate of increase in platelet count among patients with dengue fever and dengue haemorrhagic fever. 2013.
- Subha, D., & Geetha, N. J. J. S. I. R. (2017). Evaluation of acute toxicity of the methanolic extract of Tanacetum parthenium L. in albino wistar rats. 6(3), 113-115.
- Suganya, G., Karthi, S., & Shivakumar, M. S. J. P. r. (2014). Larvicidal potential of silver nanoparticles synthesized from Leucas aspera leaf extracts against dengue vector Aedes aegypti. 113(3), 875-880.
- Sumalatha, K., Ishwara Bhat, J. J. I. J. o. E., & Environment. (2019). Extraction, Characterization and Identification of Major Chemical Components of Areca Nut Extract at its Different Stages. 10(2), 136-146.
- Teoh, E. P., Kukkaro, P., Teo, E. W., Lim, A. P., Tan, T. T., Yip, A., Leo, Y. S. J. S. t. m. (2012). The structural basis for serotype-specific neutralization of dengue virus by a human antibody. 4(139), 139ra183-139ra183.
- Verma, S. J. J. o. D. D., & Therapeutics. (2018). Medicinal potential of lantana camara: Verbenaceae. 8(4), 62-64.
- Vincent, J. F., Wegst, U. G. J. A. s., & development. (2004). Design and mechanical properties of insect cuticle. 33(3), 187-199.
- WHO. (2022). Update on the Dengue situation in the Western Pacific Region. Retrieved from World Health Organization.
- Wilson, A. L., Courtenay, O., Kelly-Hope, L. A., Scott, T. W., Takken, W., Torr, S. J., & Lindsay, S. W. J. P. n. t. d. (2020). The importance of vector control for the control and elimination of vector-borne diseases. 14(1), e0007831.
- Wu, D., Lu, J., Liu, Q., Ma, X., He, W. J. I. C., & Epidemiology, H. (2020). To alert coinfection of COVID-19 and dengue virus in developing countries in the dengue-endemic area. 41(12), 1482-1482.
- Yusriah, L., Sapuan, S., Zainudin, E. S., & Mariatti, M. J. J. o. C. P. (2014). Characterization of physical, mechanical, thermal and morphological properties of agro-waste betel nut (Areca catechu) husk fibre. 72, 174-180.
- Zaheer, S., Tahir, M. J., Ullah, I., Ahmed, A., Saleem, S. M., Shoib, S., Surgery. (2022). Dengue outbreak in the times of COVID-19 pandemic: Common myths associated with the dengue. 81, 104535.
- Zhao, Y., Newman, M. C. J. E. T., & Journal, C. A. I. (2004). Shortcomings of the laboratory‐derived median lethal concentration for predicting mortality in field populations: Exposure duration and latent mortality. 23(9), 2147-2153.
- Zoubiri, S., & Baaliouamer, A. J. J. o. S. C. S. (2014). Potentiality of plants as source of insecticide principles. 18(6), 925-938.







