Translate this page into:
Robust synthesis of mono-dispersed spherical silica nanoparticle from rice husk for high definition latent fingermark development
⁎Corresponding author. nikf@usm.my (Nik Fakhuruddin Nik Hassan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Silica is one of the most functional metalloid oxides with a widespread application as semiconductor, fillers, silicone and ceramic primarily due to its structural versatility. In this work, a robust step-wise thermochemical treatment was successfully formulated for the controlled fabrication of high-purity mono-dispersed spherical silica nanoparticle from rice husk. The silica nanoparticle with the desired morphology was formed in two stages; thermal-assisted seed particle formation followed by particle growth through acidification of the solvent modified sodium silicate solution. The obtained powder was characterised, and the effectiveness of the powder for latent fingermark development across varied donors and surfaces was tested at the introductory level. The formed spherical particles were in the range of 200 to 400 nm, as confirmed by FESEM and HRTEM analysis. Minimising the degree of silica nanoparticle agglomeration notably affected their selectivity to fingermark residue. There was a striking improvement in the selectivity of the silica nanoparticle to fingermark residue. The improvement was attributed to the strong interaction between the silica nanoparticle and the lipid components in the fingermark residue, as compared to the commercial white powder that works solely based on mechanical adherence. Additionally, the size and morphology of the fabricated silica nanoparticle were optimised to enhance the clarity of the developed fingermark. Findings of this study could improve quality of fingermarks obtained in a crime scene due to considerably lower background interference without compromising the effectiveness of fingermark development.
Keywords
Silica nanoparticle
Rice husk
Fingerprint
Powder dusting
Sol-gel
1 Introduction
Fingermarks remain one of the best means of individual identification during crime scene investigations (Fish et al., 2014). Latent fingermarks encountered at the crime scene require development using physical or chemical agents to enable recovery and perform ridge comparison analysis (Dhall et al., 2013). Five primary techniques; powder dusting, chemical treatment, chemical fuming, small particle reagents and nanoparticle-based reagents are among the techniques, which could develop latent fingermark (Becue et al., 2012; Chen et al., 2009; Fieldhouse, 2015; Jelly et al., 2009; Kabklang et al., 2009). Each method possesses unique advantages whereby its application is dependent on the circumstances of the fingermark recovery such as surface bearing the fingermark, age of fingermark and humidity level (Mink et al., 2013).
Powder dusting technique is an in-situ fingermark development technique that has proven to be the most versatile due to the ease of application and effectiveness on a large number of surfaces (Arshad et al., 2015). The effectiveness of the powder dusting technique principally depends on the characteristics of the powder particles. Certain physiognomies contribute to the superiority of any fingermark powders such as spherical shaped, mono-dispersed, small-sized particles that possesses an affinity for fingermark residue (Sodhi and Kaur, 2001). Nevertheless, powders made from a sustainable source using a low-cost method and reduced toxicity are some of the other qualities valuable in improving the aesthetics of the powder.
The effectiveness evaluation of the fingerprint dusting powders encompasses two different aspects, clarity (the discernibility of the ridges due to selective particle adhesion on ridge residues) (Hicklin et al., 2013) and contrast (clear visibility of the developed ridges against the background) (Becue et al., 2011). Hence, there are four levels of fingerprint dusting powder’s effectiveness, from ideal to the least ideal powder based on the clarity and contrast of fingermark developed.
These are the subjective evaluation of fingerprint dusting powder hierarchy used in this research based on the fingermarks developed from ideal powder to the least ideal.
-
Powder with both high clarity and contrast are considered ideal.
-
Powder with high clarity but lower contrast, yet still enough to provide sufficient contrast for evaluation.
-
Powder with high contrast and low clarity, sufficient to provide ridge details.
-
Powder with low clarity and low contrast are considered the least ideal.
Years of fingermark related research proposed numerous powder fabrication techniques that focused on optimising fingerprint dusting powder to satiate the characteristics of the ideal powder. The first evolution began with the transition from carbon powder to iron oxide-based black powder due to superior selectivity (Sodhi and Kaur, 2001). Subsequently, powders with varied colours such as white and grey containing titanium dioxide and aluminium oxide, respectively, were commercialised for the development of fingermarks on dark surfaces (Friesen, 2015). Nonetheless, the use of luminescent powders increased fingermark recovery on challenging surfaces such as multi-coloured and luminescent background (Errington et al., 2016). Luminescent powders provided increased contrast as compared to the conventional black or white powder; however, concentrated particle interaction with the substrate bearing the fingermarks compromised the clarity of the developed fingermark (Dilag et al., 2011). Besides, the luminescent powders also suffer from another drawback in the form of increased toxicity levels (Becue et al., 2011). The utilisation of many reagents and powders with varied colour, fluorescence, selectivity along with sustainable production methods alleviated the current issues with fingerprint dusting powder.
Advances in nanomaterial fabrication tailored to its end application have contributed to remarkable progress in the fingermark development research, primarily improving the sensitivity of development technique. Smaller sized nanoparticles enable the detection of aged latent marks with minimal residue through multi-metal and single metal deposition techniques (Becue et al., 2012; Moret and Bécue, 2015), while the use of quantum dots resulted in increased contrast in bloody fingermark visualisation (Becue et al., 2009). Nevertheless, issue such as stringent application processes and tuning the luminescent property of the particle while maintaining the small-sized remains as a challenge (Dilag et al., 2011).
A mixture of zinc oxide and silica have been studied previously for their fingermark developing ability due to their intense photoluminescence property (Arshad et al., 2015). Zinc oxide and silica used in these studies were synthesised from synthetic precursors and were crystalline, as indicated by XRD analysis. SEM analysis of the powder showed large clusters of highly agglomerated particles. Findings indicated that the powders exhibited high performance when used to develop fresh sebaceous fingermarks with excellent clarity.
Dye-doped silica nanoparticles have been produced from synthetic precursors to yield crystalline and amorphous silica nanoparticles using non-polar and polar solvent extraction. These micro (1–22 µm) and nanoparticles (100 to 400 nm) of silica was doped with several dyes and applied to latent fingermarks. Results revealed a concentrated distribution of the powder along the fingermark ridges, more particularly with the crystalline powder (Theaker et al., 2008). A study using commercial silica gel powder revealed that silica elicited an excellent contrast on the multi-coloured surface compared to conventional white powder (Singh et al., 2013). On the other hand, a fluorescent variant of silica nanoparticles synthesised using polyvinylpyrrolidone coating for a similar purpose have been introduced as well (Kim et al., 2016).
Silica is a mineral found abundantly in various natural resources such as coconut husks, rice husks and bamboo leaves of which, silica content was highest in rice husks (Adam et al., 2011; Kow et al., 2014; Sivasubramanian and Kurcharlapati, 2015). Rice is the staple food in most Asian countries, and their mass production leaves behind the semi-biodegradable husks in large amounts, millions of tonnes (Luduena et al., 2011). Traditionally, the husks are burnt to ashes, utilised as hay for farm animals and, there are even cutleries made of rice husks (Mor et al., 2017). Rice husks are rich in lignin, cellulose, hemicellulose and silica from which many advanced materials can be extracted (Abu et al., 2016). Previous reports of nano-silica, nano-cellulose and nanocarbon extraction from rice husks have established the plausibility of advance material synthesis from agricultural waste for use in various sectors (Jembere and Fanta, 2017; Luduena et al., 2011; Rajan et al., 2018).
Our previous work highlights the synthesis of nanocarbon and fluorescent silica nanoparticle from rice husk for latent fingermark development. Nanocarbon extracted by acid leaching of the rice husks, followed by thermal treatment in hot air oven developed latent fingermarks with appreciable clarity and contrast (Rajan et al., 2018). On the other hand, the curcumin doped silica nanoparticle powder provided enhanced contrast as compared to the white particles (Rajan et al., 2019).
Therefore, in the present study, we investigated the optimised fabrication of mono-dispersed spherical silica nanoparticles from rice husk for the development of latent fingermarks with increased sensitivity and selectivity. Also, we elucidated the underlying factors influencing controlled silica nanoparticle precipitation from sodium silicate sol in this work.
2 Materials and methods
This section illustrates the optimisation silica nanoparticle synthesis and characterisation method for latent fingermark development.
2.1 Materials
The chemicals used for this investigation consisted of hydrochloric acid (HCl), sodium hydroxide (NaOH) pellets, acetone, acetic acid and phenolphthalein. These chemicals were procured from Merck Millipore, Darmstadt, Germany and used without further purification. Meanwhile, the rice husk was obtained from a local rice mill in Peringat, Kelantan. Finally, deionised (DO) water was employed throughout this work.
2.2 Synthesis and characterisation of silica nanoparticle
2.2.1 Synthesis of silica nanoparticle from rice husk
Rice husk procured directly from the mill in its original form, was washed thoroughly to remove soil sediments and other extraneous materials. The rice husk was then dried in the oven and finely blended using counter-top blender. Next, 5.0 g of the rice husk powder was added to 100 ml of 1 M HCl and stirred homogeneously on a heated hotplate (80 °C). The filtrate was thoroughly rinsed using DO, dried in the oven at 90 °C and further treated on the hotplate at 80 °C until complete carbonisation was achieved. The black rice husk ash was calcined in the furnace at 700 °C for 5 h producing pure white ash.
Then, 0.5 g of pure white ash was dissolved in 100 ml NaOH (1 M), the mixture heated at 80 °C under constant stirring for an hour then aged in an oven overnight at 90 °C. Aged sodium silicate solution was reconstituted to original volume with DO and added with varying amount of acetone in separate experiments (10 to 40 ml). Afterwards, the solution was adjusted to pH 7 using sulphuric acid (6.0 M) or acetic acid (5.0 M) at a steady flow rate of 1 ml/min. Colloidal silica nanoparticle formed was spun at high speed (5000 rpm) for 10 min to form white pellets. The pellet was rinsed thrice with warm DO water to remove all excess ionic impurities and freeze-dried to obtain loose white silica nanoparticle powder.
The optimal fingermark developing powder was prepared by ageing the sodium silicate solution prepared using rice husk ash in the oven at 80 °C overnight. The volume reconstituted aged sodium silicate solution (100 ml) was subsequently precipitated under vigorous stirring using 5 M acetic acid after adding 40 ml of acetone.
2.2.2 Electron microscopic analysis
Two and three-dimensional images of the particles and their aggregation state as well as the surface morphology were visualised using field emission scanning (FESEM) (Quanta FEG 450) and high-resolution transmission electron microscopy (HRTEM) (FEI Tecnai G2 HRTEM) both acquired from the FEI Czech Republic. The images were captured at low voltage (5.00 kV) at ∼ 9–10 mm working distance. HRTEM samples were prepared by sonication in ethanol to ensure mono-dispersal of the particles, which was then transferred onto a carbon-coated TEM grid (300 mesh copper, formvar-carbon, Ted Pella Inc., Redding, CA) before viewing under 200.00 kV acceleration voltage.
2.2.3 Spectroscopic analysis
The determination of other properties such as the elemental composition and molecular bonding profile was performed using Fourier Transform Infrared (FTIR) and Energy Dispersive X-Ray Spectroscopic (EDX) techniques. Both techniques exploited different wavelengths of the electromagnetic radiation to study the interaction of the particle with these radiations. Infrared analysis in the range of 4000 to 400 cm−1 was employed on samples embedded in a transparent potassium bromide pellet using Tensor 27 FTIR by Bruker Corporations, UK. Alternatively, the characteristic elemental x-ray emission was recorded using EDS Xmax 50 mm2, Oxford, United Kingdom unit adjunct to the FESEM.
2.2.4 X-Ray diffraction (XRD)
Additionally, the structure of the particles was studied using D8 Advance XRD, Bruker Corp. UK. to confirm amorphousness. The analysis was conducted using monochromatic Cu Kα radiation source (λ = 1.5406 Å) in the step scan mode with a 2θ angle ranging from 5° to 90° with a step of 0.04. The scanning rate was 1 s/step at an operating voltage of 40 kV with a filament current of 40 mA.
2.2.5 Surface area and porosity analysis
Nitrogen adsorption–desorption isotherms were measured with an Autosorb 1 isothermal nitrogen sorption analyser (Quantachrome Instruments, Florida, USA) using a continuous adsorption procedure. Specific surface area calculation was based on the isotherms by using the BET equation in a relative pressure range where the isotherm behaves linearly. Whereas, adsorption branch of the isotherm was used to derive the pore size distribution using the Barrett-Joyner-Halenda (BJH) method. The volume of the nitrogen adsorption at the relative pressure of P/Po = 0.994 corresponded to the total pore volumes.
2.2.6 Development of latent fingermark
A pilot study was conducted to establish a working theory for the latent fingermark developing efficacy of the fabricated silica nanoparticle powder. The efficacy of the powder was evaluated in two phases utilising a range of fingermark donors and surfaces.
2.2.6.1 First phase
For the first part of the pilot study, un-groomed natural fingermarks from random donors were employed. Natural uncharged fingermarks were collected from five donors and aged for an hour before development. Three female donors and two males donors aged between 23 and 30 years old participated in the study. Donors were randomly selected, comprising all three types of fingermark donors, weak, medium and robust residue depositors. Donors washed their hands using tap water, dried hands using clean towels and resumed regular activity for an hour. Then the fingermarks were collected on the surfaces using assisted fingermark deposition (researcher holds the thumb and place it on the surface) to ensure consistent deposition pressure.
The multiple donor study was conducted on one selected clean surface (black glass) to minimise interference from other contaminants. Each donor was asked to deposit three fingermarks on the surface provided without recharging in between depositions. The fingermarks were split into two vertical halves; one half-developed using the synthesised powder and the other half with SIRCHIE white powder.
2.2.6.2 Second phase
The second phase of the pilot study was conducted on several surfaces with fingermarks collected from a single donor. Groomed fresh fingermarks (controlled variable) with consistent residue depositions (triplicates) were deposited on six non-porous surfaces and developed after one hour to evaluate the effectiveness of the powder (responding variable) across different surfaces. Groomed fingermarks were obtained an hour after the donors washed their hands, resumed regular activity and then charged their residue by lightly touching forehead or nose area before deposition. One set of fingermarks were developed using the silica nanoparticle powder, while the other set developed with the SIRCHIE white powder.
2.2.7 Fingermark development and grading
Regular powder dusting technique was employed for the development of the fingermarks using SIRCHIE squirrel hairbrush. The developed marks were photographed using a Canon Cybershot SX60 camera in the macro focus mode, displayed on a computer screen and graded. Fingermarks were evaluated using a holistic scale developed by the Centre for Applied Science and Technology, as outlined in Table 1 (Almog and Cantu, 2014).
Grade
Detail Visualized
0
No evidence of a fingermark
1
Some evidence of a fingermark
2
Less than 1/3 clear ridge detail
3
Between 1/3 and 2/3 clear ridge detail
4
Over 2/3 clear ridge detail
3 Results and discussion
The primary aim of this research was to synthesise silica nanoparticle with the specific morphology, which can be applied for high definition latent fingermarks. Ideal fingermark powders are mono-dispersed small-sized spherical particles that were successfully synthesised from rice husk with the aid of thermochemical treatments carried out systematically. The yield of the silica nanoparticle was in the range of 11 to 14.5%. Visual comparison of the fingermarks developed using the synthesised powder exhibited superior clarity as compared to the SIRCHIE white powder owing to selective adherence of particle to the fingermark residue. Nonetheless, statistical analysis of the fingermark grades revealed no significant difference between both powder’s performance.
The mechanics of producing highly pure silica ash from rice husk using thermochemical treatment has been described in detail in our previous work. Structural characterisation of the by-products after each treatment was discussed. Rice husk was thoroughly washed and leached with HCl to remove trace metal impurities. The leachate was ashed in the furnace at 700 °C for 5 h to produce silica ash. Silica produced after this treatment was coarse amorphous powder and particles as revealed by FESEM was highly agglomerated silica in the dimensions of 1–5 nm (Rajan et al., 2020).
Alkaline solubilisation and acidic re-precipitation of the silica ash produced silica nanoparticle. The pure silica ash obtained from calcination was dissolved in an alkaline medium to form sodium silicate solution, following equation (1) for the reaction.
Sodium silicate formation
According to previous research, the silica ash dissolution in NaOH was achieved through heated stirring for four hours (Zaky et al., 2008). Nevertheless, it was observed in this study that maximum dissolution could be achieved in a shorter duration (an hour) due to the high purity of the ash utilised.
3.1 Effects of various precipitation parameters on the morphology of the silica nanoparticle
The composition of the sodium silicate sol at the time of precipitation, the strength of the acid and its concentration play a crucial role in determining the properties of the precipitated silica. The various effects of the components such as heat, pH and sol composition on the morphology of the precipitated silica are discussed in the following sections.
3.2 Effect of heating method
The dissociation of the sodium silicate compound into sodium ion and silanol group takes place in an aqueous medium. In the presence of water, the silanol undergoes hydrolysis and forms siloxane bond, which in turn aggregates to form silica particles, following equations (2) and (3) (Milea and Bogatu, 2011).
Dissociation in water
Water condensation/hydrolysis
It was observed that the conversion of silanol into silica occurs at a much slower rate at the room temperature. Formation of sponge-like material was evident in sodium silicate solution kept at room temperature after a month. Even so, the process can be accelerated by supplying heat or reducing the pH of the sodium silicate solution. Previously, the concept of forming seed particle in order to serve as the growth site for the controlled growth of gold nanoparticle was successful in synthesising spherical small sized gold nanoparticle with homogeneous size distribution (Kimling et al., 2006). Similarly, in this study, the formation of silica seed particle was prompted by exposing the sodium silicate solution to uniform heat (convection). Although direct heating and pH reduction produce accelerated seed particle formation as compared to uniform heating in a hot air oven, the silica particle nucleation was localised in some part of the solution directly exposed to heat or pH reduction (Fig. 1). Hence, a considerable size diversity was observed in the silica particle size after the growth step. Uniformly sized seed particle formation is imperative in ensuring the consistent particle growth at the subsequent precipitation step.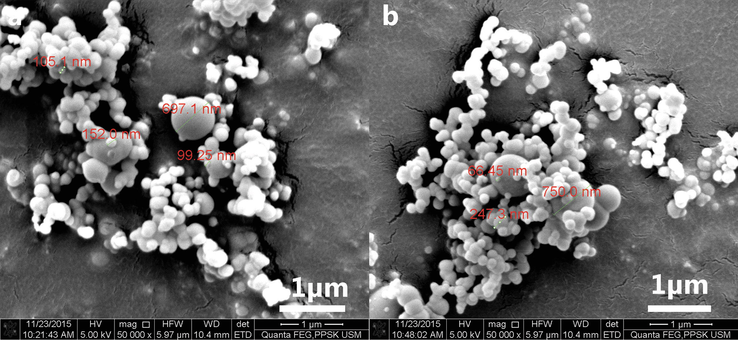
FESEM image of silica particle resulting from acidic precipitation of sodium silicate using. a 10 M sulphuric acid. b 5 M acetic acid (direct heating on hot plate). Images revealed spherical silica particle with a wide range of size distribution (10 nm – 700 nm).
Separating the nucleation and the growth step offered more significant control over the size of the silica particles formed later. Nucleation of silica particles using pH reduction leads to the cross-linking of the particle in a tightly interlinked manner forming a sol–gel rather than mono-dispersed particles as desired. Thus, a two-step approach by initiating nucleation using uniform heating and followed by pH reduction using acid was adopted in this study to facilitate the formation of mono-dispersed silica particles.
3.3 Effect of acid strength
Acid has been reported to act as the catalyst in accelerating the hydrolysis process by facilitating the further decomposition of sodium silicate into silicic acid (Kumar, 2003; Milea and Bogatu, 2011). As the pH of the aged sodium silicate solution drops during the acid titration process, the concentration of silicic acid reaches a saturation point, and spontaneous condensation polymerisation of silica occurs. This point can be perceived as a sudden change of the solution into colloidal sol. The titration must be performed under vigorous stirring to distribute the acid throughout the solution. Continuous addition of acidic solution into the sodium silicate solution after reaching the saturation point led to the growth of the silica particles, which eventually sunk to the bottom of the sol. The titration was discontinued after the point of neutralisation to avoid the formation of clustered and massive silica particles.
Use of mineral acids, as well as organic acids to reduce the pH of the sodium silicate solution, has been widely explored in previous studies (Hessien et al., 2009; Noushad et al., 2012). Therefore, both mineral and organic acids were tested in this phase to study the effect of acid strength on the morphology of silica nanoparticles formed. Mineral acids are inorganic compounds, whereas organic acids are essentially composed of carbon and hydrogen. The mineral acids dissociate entirely into their ionic counterparts in a water solution, thereby affecting pH at a faster rate compared to the organic acid. Organic acid only partially dissociates into ionic counterpart in a water solution, with a lower concentration of hydrogen ions (Pirrung, 2017). During the acid titration process, the following reactions (equations (4) and (5)) occur depending on the type of acid used.
Mineral acid titration
Organic acid titration
Even though the sodium silicate solution was aged before neutralisation, the process still promoted polymerisation, which led to the formation of sol-el rather than desired mono-dispersed particles regardless of the strength of acid employed. Neutralisation using mineral (sulphuric acid 6 M) and organic acid (10 M acetic acid) resulted in the formation of rhombohedral blocks made up of tightly interlinked nanostructured silica (Fig. 2).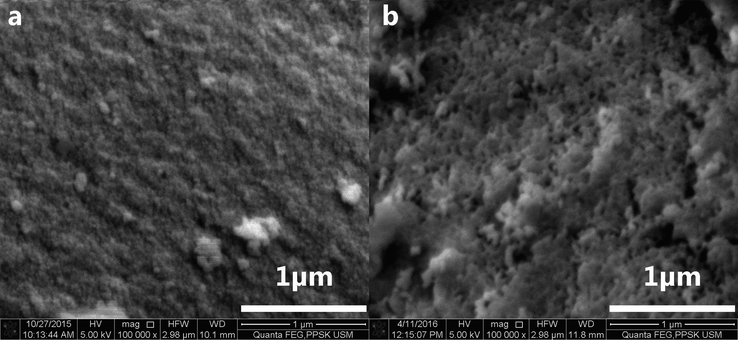
FESEM images of silica nanoparticles resulting from acidic precipitation of sodium silicate solution using. a 6 M sulphuric acid. b 10 M acetic acid.
3.4 Effect of solvent addition
The rate of hydrolysis, condensation and polymerisation during acid titration governs the particle growth and degree of aggregation (Keefer, 1990). High order polymerisation of minuscule particles leads to the formation of translucent sol–gel. On the other hand, particle growth with low aggregation order led to the formation of opaque colloidal sol. Increasing the rate of hydrolysis while retarding polymerisation favoured the formation of a colloidal sol, which was preferred to synthesise mono-dispersed particles. As such, the following section elaborates the mechanics of the neutralisation. Additionally, changes made to the system using solvents to shift the chemical equilibrium to achieve a stable, unaggregated particle growth is elaborated as well.
Principle of Le Chateliar argues that when a constraint is applied to a system in equilibrium, a shift occurs to accommodate the effect of the constraint (Daintith, 2008). In the conventional sodium silicate neutralisation process, the equilibrium is such that it favours a higher rate of polymerisation to rate of hydrolysis. It was found that varying the strength of acid utilised for neutralisation failed to serve as the best constraint in facilitating controlled growth and polymerisation of the silica particles. Thus, an external constraint, solvent, was applied to the system to shift the reaction equilibrium. The addition of solvents changed the acid-base ionisation equilibrium, subsequently affecting the equilibrium of the neutralisation reaction. The influence of the solvent’s dielectric constant over the acid-base equilibria altered the basicity of the sodium silicate solution (Leffler and Grunwald, 1963).
In a solution with water as the primary solvent, sodium silicate undergoes the strongest dissociation due to water being the solvent with the highest polarity. However, the addition of solvents with lower polarity acted as a buffer to reduce the strength of sodium silicate dissociations as well as retard rate of polymerisation leading to more controlled growth (Reichardt and Welton, 2011). Two conditions favoured colloid formation; use of concentrated sodium silicate solution or inclusion of solvent in the sodium silicate solution before neutralisation. Low concentration of water in concentrated sodium silicate solution causes acid-base ionic dissociation to occur at a reduced capacity promoting precipitation of amorphous silica. Additionally, since there were no water molecules to buffer the growth of the particles, micro-sized particles were formed. Previously, Noushad et al. (2012) have reported the production of silica nanoparticles with a high order of agglomeration and low surface area by titrating organic acid into sodium silicate solution spiked with a certain amount of solvent. Similarly, in this study, when solvents were added, the acid-base dissociation was reduced without reducing the buffering capacity of the water and solvent molecule, forming nano-sized mono-dispersed amorphous silica, explained in the next section.
Solvent addition before precipitation favoured the morphological transformation from crystalline silica formation to amorphous silica. In this study, three solvents (acetone, 1-butanol and ethanol) of increasing polarity were studied for their effect on the growth of mono-dispersed silica nanoparticle. Due to the partial miscibility of 1-butanol, the precipitated silica was too dense although particles were amorphous. Silica precipitated using acetone and ethanol formed a stable colloidal solution; hence the effect of the solvent volume on the morphology of silica precipitated was further investigated.
The effect of acetone and ethanol addition in varied amount is shown in Fig. 3. Under lower magnification, the particles from acetone addition morphed from large heavily agglomerated clusters into the smaller cluster of spherical particles before achieving mono-dispersity. Further probing into the structure of these clusters revealed flaky mesh which turned into nanoflower structure and then spherical particles. On the other hand, particles precipitated with ethanol exhibited large nanoflower structured clusters with the 10 ml solvent addition and progressed into spherical particles with the size smaller than 100 nm embedded in a mesh. Mono-dispersity of the silica particles could not be achieved using ethanol addition. Silica nanoparticle synthesised by adding 40 ml of acetone exhibited mono-dispersity as well as a smooth spherical shaped particle.
FESEM images of silica nanoparticle resulting from the precipitation of sodium silicate solution (SS) and solvent (Ace: Acetone; Eth: Ethanol) mixtures at varied ratios† using 10 M acetic acid. †a SS: Ace (1.0:0.0). b SS: Ace (0.9:0.1). c SS: Ace (0.8:0.2). d SS: Ace (0.77:0.23). e SS: Ace (0.71:0.29). f SS: Eth (0.9:0.1). g SS: Eth (0.8:0.2). h SS: Eth (0.77:0.23).
3.5 Effect of acid concentration
Two varied concentrations of acetic acid and as well as sulfuric acid has been employed in this study. The morphology of the silica particles precipitated with these acidic concentrations are illustrated in Fig. 4. Although silica precipitated with sulfuric acid obtained spherical shapes with the addition of 40 ml of acetone, the particle surface was highly porous and possessed a wide range of particle size distribution (20 nm to 400 nm) (Fig. 4a). Likewise, using 10 M acetic acid sped up the neutralisation process. However, particle size distribution remained wide and also produced distorted spherically shaped particles (Fig. 4b). On the contrary, the use of 5 M acetic acid at a steady feed rate of 1 ml/minute enabled the production spherical shaped silica nanoparticle with narrower particle size distribution (Fig. 4c).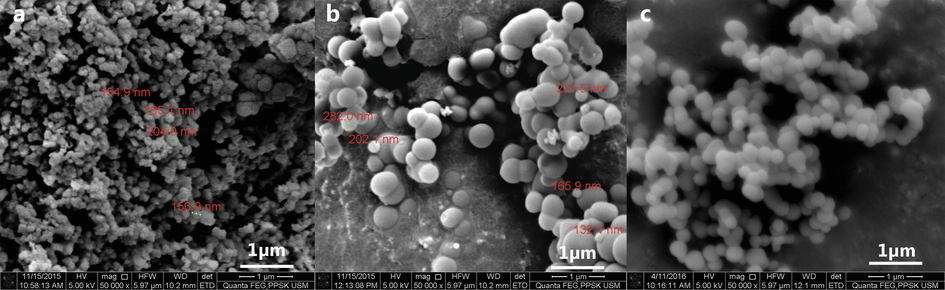
FESEM image of spherical silica nanoparticles resulting from acidic precipitation of sodium silicate solution (100 ml) and acetone (40 ml) mixture using. a 6 M sulphuric acid. b 10 M acetic acid. c 5 M acetic acid.
Concentration can be defined as the strength of a substance per unit volume. Increasing concentration of the solution is directly proportional to the increase in the chances of a more significant number of molecular collisions between reacting reagents, hence plays a vital role in the rate of the reaction (Lind, 2015). Hydrolysis, a naturally slow reaction can be accelerated with the presence of a catalyst, and in this case acid solution. Hence, it is apparent that the higher acid concentration must attribute to a higher hydrolysis rate. The effect of the acid solution is not only to promote hydrolysis but also to stimulate the polymerisation rate, resulting in larger particle sizes (Park et al., 2002).
3.6 Morphological and elemental analysis of silica nanoparticle
Structural and morphological characterisations of the silica nanoparticle have been performed on the final product, produced using the optimised parameters. The FESEM analysis revealed mono-dispersed spherical silica with a smooth surface, HRTEM analysis showed slightly porous particles (Fig. 5). Particle size analysis was conducted using Image J software on the FESEM micrographs, and a corresponding histogram (Fig. 6) was generated by the IBM SPSS software revealing the mean particle size to be 272 nm. Three peaks were detected in the EDX analysis analogous to carbon (8.72%), oxygen (31.66%), silicon (59.62%) excluding peak corresponding to gold (atomic percentages). Silicon and oxygen have a 2 to 1 atomic ratio confirming the formation of silica. Carbon and gold peaks were the interference peaks generated from the carbon tape and gold sputtering used to prepare the sample.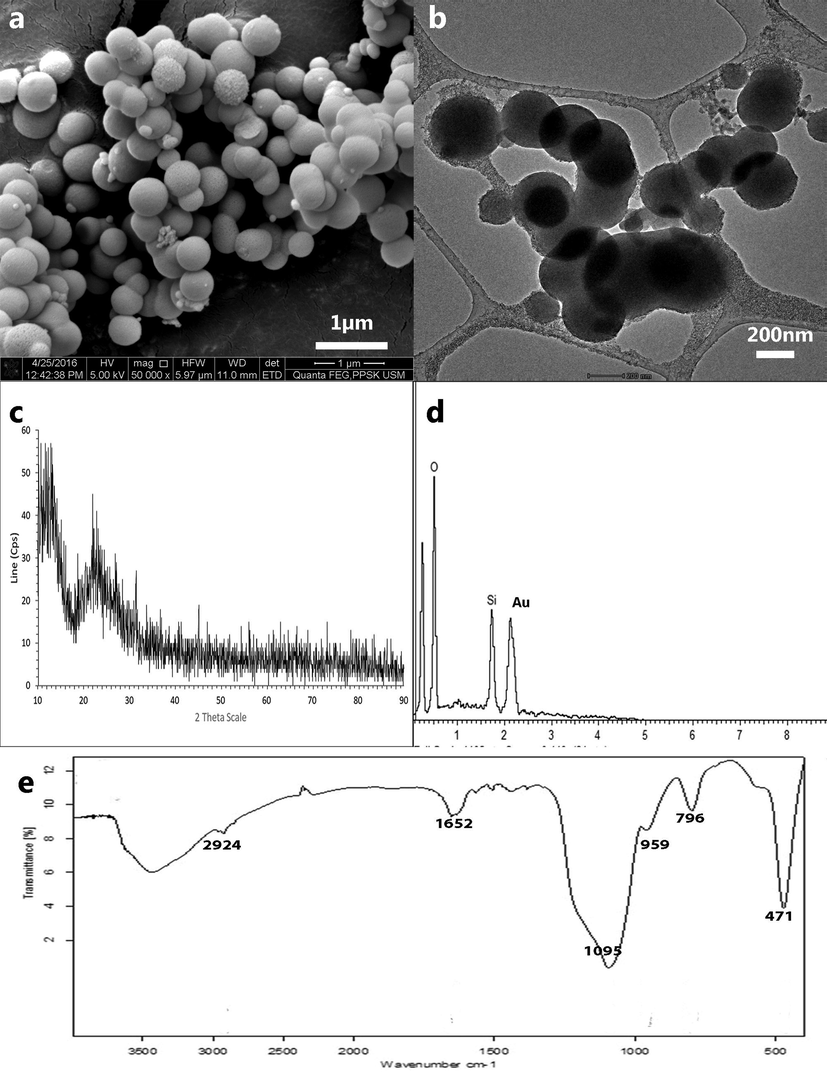
Microscopic image of silica nanoparticle synthesised using final parameters† of precipitation viewed under. a FESEM. b HRTEM. Images revealed spherical porous particles with a smooth surface and homogenously dispersed. †Sodium silicate solution and acetone mixture (0.71:0.29) precipitated with 5 M acetic acid.
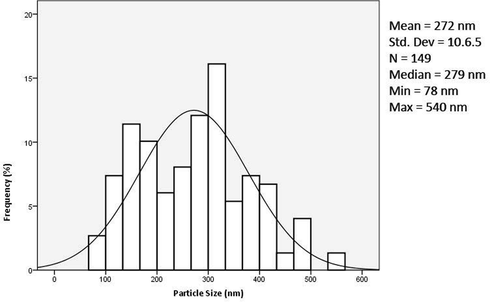
Particle size distribution of silica nanoparticles.
3.7 Structural and chemical bond analysis of silica nanoparticle
The X-ray diffraction patterns displayed a broad peak from 18° to 28° centred at approximately 22.5° corresponding to amorphous silica (Mor et al., 2017). The FTIR spectrum of the synthesised silica nanoparticle shows fundamental absorption of SiO2 within the range of 400 to 1100 cm−1. The spectral peaks corresponded to asymmetric and symmetric stretching/bending of the Si-O bond. Moreover, the peak at 1652 cm−1 implied the presence of water molecules, indicating that the sample was not thoroughly dried.
3.8 Surface area and pore size analysis
Nitrogen adsorption–desorption isotherms and the pore volume progression with a pore size of the silica nanoparticle is displayed in Fig. 7. The type IV isotherms with an H3 hysteresis loop was indicative of a mesoporous structure. Pore size distribution, according to the BJH method, was estimated to be in the range of 0.80 to 58.00 nm with an average pore width of 4.19 nm. The BET (outer surface area) and BJH (total surface area including pores) surface areas were found to be 162.00 m2/g and 238.60 m2/g, which further supports that the silica nanoparticle synthesised was mesoporous (Lu and Hsieh, 2012). The reported surface area values for commercial and rice husk derived silica are 326.90 m2/g and 120–288 m2/g, respectively. The lower surface area of the silica nanoparticle synthesised in this study could presumably be due to the larger particle size as compared to the previous reports. Nevertheless, the total pore volume of 0.169 cm3/g was consistent with the commercial and rice husk silica, which fell between the range of 0.195–0.220 cm3/g (Lu and Hsieh, 2012).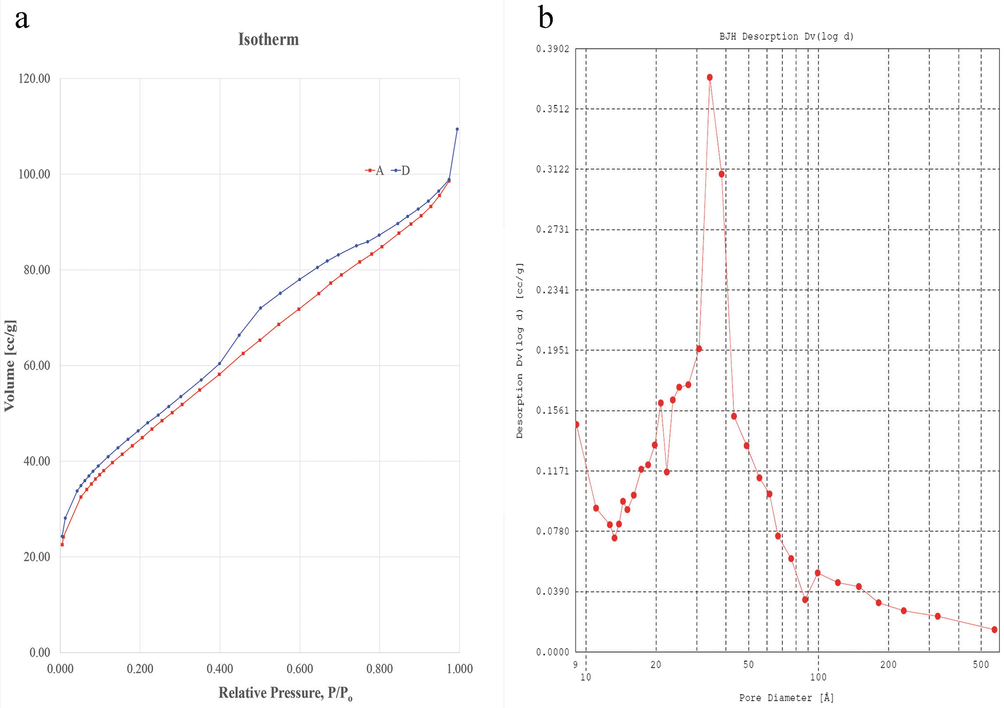
Surface area and pore size analysis of silica nanoparticle. a Nitrogen adsorption–desorption isotherm. b Pore size distribution curve indicating mesoporous structure with an average pore diameter of 4.19 nm.
3.9 Fingermark development efficiency
3.9.1 Multiple donor study
The synthesised silica nanoparticle powder was validated in a preliminary assay for the development of latent fingermarks. It was expected that a right fingermark development agent would produce fingermark ridges with high clarity and contrast. Referring to the results of the Phase 1 study, it was evident that the silica nanoparticle powder was efficient in developing latent fingermarks. The fingermarks developed using silica nanoparticle powder, and SIRCHIE white powder is presented in Fig. 8. In general, the visual comparison of the latent fingermarks developed in Phase 1 reflected that the SIRCHIE white powder exhibited better contrast owing to the higher number of particle adhesion. Nonetheless, the clarity of the fingermarks developed using silica nanoparticle powder was found to be enhanced due to highly selective adhesion of the particle to fingermark ridge residue. Furthermore, the forensic significance of the fingermark lies in obtaining precise ridge details, whereby over-powdering could lead to the obstruction of ridge details. As discussed earlier, the effects of smaller particle size and minimal agglomeration of the silica nanoparticle powder resulted in a notable improvement in the quality of the fingermarks developed. Independent t-test (Table 2) revealed no significant difference between the performance of silica nanoparticle powder and SIRCHIE white powder in developing latent fingermarks.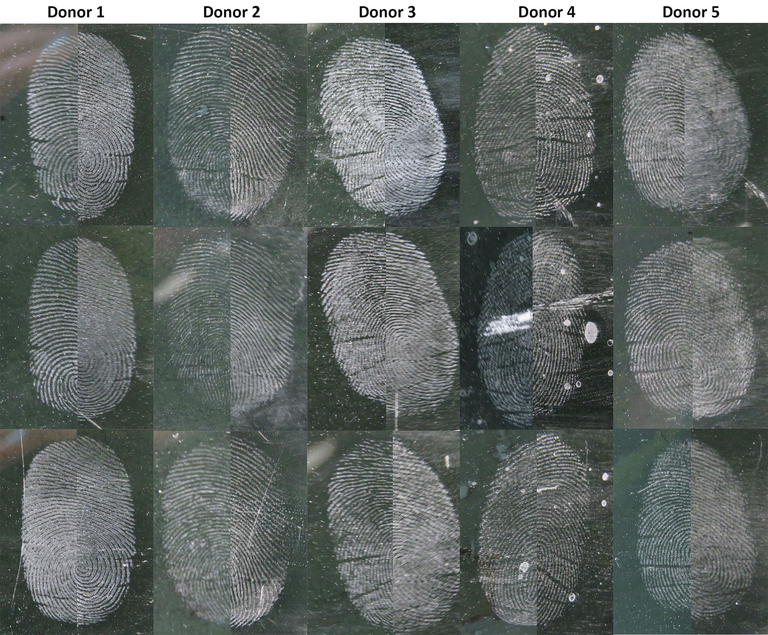
Natural latent fingermarks obtained from multiple donors developed using silica nanoparticle (left) and SIRCHIE white powder (right) on the black glass surface.
Sample
t /Z
p-value
Multiple donor study, Mean ± SD
Silica nanoparticle powder (n = 15)
3.8 ± 1.01
0.497a
0.623
White powder (n = 15)
3.6 ± 1.18
Multiple surface study, Median (IQR)
Silica nanoparticle powder (n = 15)
4.0 (1.0)
−0.237b
0.864
White powder (n = 15)
4.0 (1.0)
3.9.2 Multiple surface study
Since the synthesised powder exhibited the excellent potential to develop latent fingermarks from several donors, the second part of the powder testing on fingermarks was performed on several non-porous surfaces. Fingermarks are often recovered on various types of surfaces and thus establishing the versatility of the fingerprint powder across surfaces is a critical area that needs focus. The variance in the development efficiency is due to the nature of the surface itself, such as the texture, smoothness, porosity, the behaviour of surface under extreme temperature, which will eventually influence the quality of the developed fingermark. Fig. 9 represents latent fingermarks developed on six non-porous surfaces using both powders. There was no significant difference between the performance of both powders indicated by Mann-Whitney U test (Median (IQR) = 4.0 (1.0) for NS and WP) in developing latent fingermarks, U = 156.0 and p = 0.864.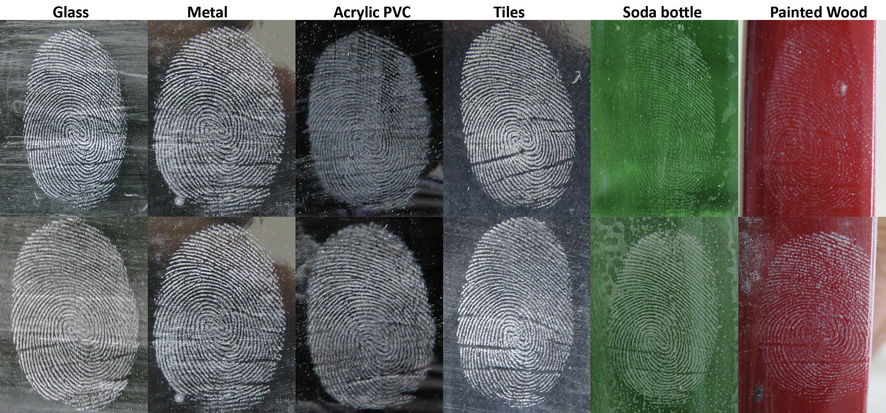
Groomed latent fingermarks from the same donor developed using silica nanoparticle (top) and SIRCHIE white powder (bottom) on multiple non-porous surfaces.
3.9.3 Efficiency of silica nanoparticle powder compared to other nano-powders
Fingermarks have served as the leading means of individual identification in the course of forensic investigations and as such copious literature have been dedicated to improving the existing physical and chemical techniques of fingermark visualisation. Fingerprint dusting technique remains the foremost used technique to treat fixed surfaces in the crime scene owing to the ease of applicability and cost-effectiveness.
Little has changed in the principle of powder dusting technique over the years, however many new formulations that provide enhanced contrast and sensitivity have been proposed. Primarily, titanium oxide powder has been extensively researched as the potential white powder in fingermark development (Choi et al., 2007; Wade, 2002). There are many white powder products available in the market from different companies. Although the main component is titanium oxide, the composition and the surface properties vary between products. A new formulation of white fingerprint dusting powder has not been widely researched as much as the investigation of the extent of the titanium oxide powder effectiveness.
One of the research undertaken to compare the fingermarks developed using WP from two different companies reported a significant difference between the composition and the effectiveness of the powders. Although the surface composition of the powders varied, the morphology (∼300 nm) and particle aggregation were noted to be similar, the selectivity of the powder corresponded to the surface composition (Reynolds et al., 2008).
Their subsequent research also confirmed these findings that aluminosilicates coating used as an anti-caking agent in the powder formulations plays a vital role in the differences background staining (Jones et al., 2010). In comparison, the silica nanoparticle powder does not require any coating or addition of other chemicals to prevent powder caking. Titanium dioxide powder has also been reported to exhibit over powdering along the ridges, leading to diminished ridge detail clarity (De Alcaraz-Fossoul et al., 2013), which is avoided by using the silica nanoparticle powder.
One of the research employing dyed titanium dioxide powders for the development of latent fingermarks on various surface noted that commercial powder formulation, as well as the proposed powder, performed very poorly of the glossed wood surface (Choi et al., 2007). Findings of this research indicate that silica nanoparticle was able to develop fingermark on painted/glossed wood surface with high clarity and contrast, revealing secondary level fingermark details.
The research focused on exploiting silica nanoparticle as the fingerprint dusting powder, or wet powder suspension have only gained interest in the latter part of a recent decade. The use of silica nanoparticle synthesised from synthetic precursors (18 nm) doped with nano-phosphors has been investigated previously for the development of fingermarks on smooth non-porous surfaces (Saif et al., 2015). Results revealed the superiority of the nano-phosphor doped silica nanoparticle in producing strong photoluminescence; however, over-powdering and indistinct ridge border demarcation were observed in the developed fingermarks.
The amorphous nature of the fingermark powder is a vital component needed to produce fingermarks with a well-defined border (Singh et al., 2013). Similar to the proposed silica nanoparticle powder excellent fingermark developing capability was exhibited by highly agglomerated amorphous zinc oxide and silica nanoparticle hybrid powder on fresh fingermarks as demonstrated in this research (Arshad et al., 2015). In comparison, the application of finely ground crystalline silica nanoparticle powder did not achieve superior results in comparison to un-agglomerated amorphous spherical silica nanoparticle (Theaker et al., 2008).
The nanoparticle is much smaller than most of the particles currently used in fingerprint detection, which are in the order 1–10 μm in size. Nevertheless, it is vital to understand the distinction between nanoparticles and nanostructured particles. On the one hand, nanoparticles are distinct and non-aggregated nano-sized particles. On the other hand, nanostructured particles, which may exist up to microns in diameter, often exist as aggregates of nano-sized primary particles (Choi et al., 2008). Silica nanoparticle proposed in this research falls under nanoparticle category while the commercial powder formulation is a nanostructured particle.
The proposed silica nanoparticle powder retained the homogenous dispersal of the spherical silica nanoparticle as observed in suspension form. Hence, both dry powder and wet powder formulation exhibited the same level of increased sensitivity regardless of the application technique. Reproducibility of the synthesis method was extremely accurate, leading to constant physical and chemical properties of the silica nanoparticle synthesised and in turn, maintains the quality of the fingermark developed. In comparison to the previous reports whereby homogenous dispersal of the silica nanoparticle was only observed in suspension form, the proposed silica nanoparticle powder retains this characteristic in the dry powder form (Theaker et al., 2008), which is the primary method of fingermark development in the crime scene.
As was explained above, maintaining mono-dispersed morphology in the dry powder form is imperative to improve the clarity of fingermark ridges. Mono-dispersed dry powder particles give a significant impact in improving the recovery rate of the fingermark from the crime scene with enough details for personal identification. Successful use of silica nanoparticle and the superiority exhibited by these particles have already been previously established. Nonetheless, the proposed silica nanoparticle synthesised from rice husk gives the added benefit of cost-saving, environmentally friendly product as well as reduced health hazard with prolonged exposure. This research was a novel attempt at utilising rice husk silica as fingermark developing powder, incorporating the environmentally friendly technique into forensic science.
Our previous work on producing a fluorescent variant of silica nanoparticle for the development of latent fingermarks illustrates dye doping ability of the silica nanoparticle powder from rice husk. The natural dye pigment, significantly curcumin exhibited stable and strong fluorescence over a long period (Rajan et al., 2019). However, the silica nanoparticle used for this study was not optimised for latent fingermark development with the particles exhibited slight agglomeration. Hence, some reduction of clarity was observed on the fingermark developed. In the current work, the silica nanoparticle morphology has been optimised for a better clarity and outcome latent fingermark development.
The commercial powder (SIRCHIE), which is being used by the Royal Malaysian Police primarily contains titanium dioxide. Titanium dioxide is a compound classified as a substance that causes long-lasting harmful effects on aquatic life and carcinogenic effects. Studies were carried out to determine the effect of titanium dioxide on human and preliminary findings acknowledged the carcinogenic property of this compound (Sha et al., 2011; Shah et al., 2017). On the other hand, silica was classified as a substance that causes acute toxicity through inhalation or ingestion (UN, 2017). However, some findings suggested that the toxicity effect of silica was enhanced in the crystalline form and there was no conclusive proof that confirms the toxicity of amorphous silica (Merget et al., 2002; Plumlee and Ziegler, 2007). Another study indicated that silica is an essential mineral for bone growth and prevents osteoporosis (Price et al., 2013). Although amorphous silica may cause toxicity by inhalation, titanium dioxide was suggested to have long-lasting adverse effects. The inhalation of powder particle can be avoided by using suitable personal protective equipment.
4 Conclusion
Spherical mono-dispersed silica nanoparticle can be synthesised from rice husk by using a step-wise thermochemical treatment. A simple, rapid and practical approach was presented in this chapter for visualising latent fingermarks on various non-porous and semi-porous surfaces. The silica nanoparticle powder was found to produce clear and sharp images of latent fingermarks on most surfaces with minimal background staining, revealing excellent ridge characteristics which are useful for identification purposes in crime investigation. Excellent visibility of the minutiae or ridge detail was observed using the synthesised silica nanoparticle powder.
Silica nanoparticle powder is advantageous over the commercial white powder because of the simple synthesis technique using agricultural waste as well as the low toxicity of the powder. Silica nanoparticle from rice husk presented superiority over the commercial white powder due to the minimally agglomerated spherical particles that spread evenly over the ridges. The silica nanoparticle also displayed excellent selectivity to the ridges in comparison to its commercial counterpart, white powder.
Besides, rice husk is abundant and of the sustainable source, hence, utilising this agricultural waste helps with the cleaner disposal of rice husk. This method is also not energy intensive and does not require costly precursors or equipment. Hence, silica nanoparticle is user-friendly not only in terms of application but also through the cost of manufacturing and purchase. The method may easily be scaled-up for mass production in industrial manufacturing.
Future studies focusing on the specific structural changes of the silica particles formed under different neutralisation study may be undertaken for an in-depth analysis of the changes occurring silica morphology. Additionally, the effectiveness and robustness of the formulated powder can be established in a more significant manner by using diverse fingermark samples obtained from various conditions.
5 Declarations
Ethics Approval and consent to participate.
The human ethical clearance was approved by Human Research Ethics Committee, Universiti Sains Malaysia (reference number: USM/JEPeM/280.5(1.3)).
6 Availability of data and material
Please contact author for data requests.
7 Author’s contributions
All authors contributed to the design of the study. Revathi Rajan performed the experiments. All authors analysed the data, discussed and wrote the manuscript.
8 Author’s Information
Not applicable.
9 Research involving human participants
The human ethical clearance was approved by the Human Research Ethics Committee, Universiti Sains Malaysia (reference number: USM/JEPeM/280.5(1.3)).
Funding
Financial support for this research by the Universiti Sains Malaysia (USM) Research University Grant 1001/PPSK/812125.
Acknowledgements
This work was financially supported by the Universiti Sains Malaysia (USM) Research Grant 1001/PPSK/812125.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Production of high purity amorphous silica from rice husk. Procedia Chem.. 2016;19:189-195.
- [Google Scholar]
- A simple template-free sol – gel synthesis of spherical nanosilica from agricultural biomass. J. Sol.-Gel Sci. Technol.. 2011;59:580-583.
- [Google Scholar]
- Guidelines for the Assessment of Fingermark Detection Techniques International Fingerprint Research Group. J. Forensic Identif.. 2014;64:174-200.
- [Google Scholar]
- Development of latent fingermarks on various surfaces using ZnO-SiO2. J. Forensic Sci.. 2015;60:1182-1187.
- [CrossRef] [Google Scholar]
- Use of quantum dots in aqueous solution to detect blood fingermarks on non-porous surfaces. Forensic Sci. Int.. 2009;191:36-41.
- [CrossRef] [Google Scholar]
- Detection of fingermarks by colloidal gold (MMD/SMD) – beyond the pH 3 limit. Forensic Sci. Int.. 2012;219:39-49.
- [CrossRef] [Google Scholar]
- Infrared spectroscopic imaging of latent fingerprints and associated forensic evidence. Analyst. 2009;134:1902-1904.
- [CrossRef] [Google Scholar]
- An evaluation of nanostructured zinc oxide as a fluorescent powder for fingerprint detection. J. Mater. Sci.. 2008;43:732-737.
- [Google Scholar]
- Choi, M.J., Smoother, T., Martin, A. a., McDonagh, A.M., Maynard, P.J., Lennard, C., Roux, C., 2007. Fluorescent TiO2 powders prepared using a new perylene diimide dye: Applications in latent fingermark detection. Forensic Sci. Int. 173, 154–160. https://doi.org/10.1016/j.forsciint.2006.09.014
- A dictionary of chemistry (Seventh ed.). New York: Oxford University Press; 2008.
- De Alcaraz-Fossoul, J., Mestres Patris, C., Balaciart Muntaner, A., Barrot Feixat, C., Gen?? Badia, M., 2013. Determination of latent fingerprint degradation patterns - A real fieldwork study. Int. J. Leg. Med. 127, 857–870. https://doi.org/10.1007/s00414-012-0797-0
- A novel method for the development of latent fingerprints recovered from arson simulation. Egypt. J. Forensic Sci.. 2013;3:99-103.
- [CrossRef] [Google Scholar]
- Dilag, J., J. Kobus, H., V. Ellis, A., 2011. Nanotechnology as a new tool for fingermark detection: A review. Curr. Nanosci. 7, 153–159. https://doi.org/10.2174/157341311794653596
- Micronised Egyptian blue pigment: A novel near-infrared luminescent fingerprint dusting powder. Dye. Pigment.. 2016;132:310-315.
- [CrossRef] [Google Scholar]
- An Investigation into the Effects of Force Applied During Deposition on Latent Fingermarks and Inked Fingerprints Using a Variable Force Fingerprint Sampler. J. Forensic Sci.. 2015;60:422-427.
- [Google Scholar]
- Fish, J.T., Miller, L.S., Braswell, M.C., Wallace, E.W., 2014. Chapter 4 – Fingerprints and Palmprints. Crime Scene Investig. 85–110. https://doi.org/10.1016/B978-1-4557-7540-8.00004-6
- Forensic chemistry: The revelation of latent fingerprints. J. Chem. Educ.. 2015;92:497-504.
- [CrossRef] [Google Scholar]
- Controlling the synthesis conditions for silica nanosphere from semi-burned rice straw. Mater. Sci. Eng. B. 2009;162:14-21.
- [Google Scholar]
- Assessing the clarity of friction ridge impressions. Forensic Sci. Int.. 2013;226:106-117.
- [CrossRef] [Google Scholar]
- The detection of latent fingermarks on porous surfaces using amino acid sensitive reagents: A review. Anal. Chim. Acta. 2009;652:128-142.
- [Google Scholar]
- Studies on the synthesis of silica powder from rice husk ash as reinforcement filler in rubber tire tread part: Replacement of commercial precipitated silica. Int. J. Mater. Sci. Appl.. 2017;6:37-44.
- [CrossRef] [Google Scholar]
- Nano-scale composition of commercial white powders for development of latent fingerprints on adhesives. Sci. Justice. 2010;50:150-155.
- [CrossRef] [Google Scholar]
- Latent fingerprint detection by various formulae of SPR. J. Sci. Res. Chula. Univ.. 2009;34:59-64.
- [Google Scholar]
- Structure and Growth of Silica Condensation Polymers. In: Zeigler J.M., Fearon F.W.G., eds. Silicon-Based Polymer Science. Washington: American Chemical Society; 1990. p. :227-240.
- [CrossRef] [Google Scholar]
- Rapid imaging of latent fingerprints using biocompatible fluorescent silica nanoparticles. Langmuir. 2016;32:8077-8083.
- [CrossRef] [Google Scholar]
- Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B. 2006;110:15700-15707.
- [CrossRef] [Google Scholar]
- Kow, K.-W., Yusoff, R., Aziz, a. R.A., Abdullah, E.C., 2014. From bamboo leaf to aerogel: Preparation of water glass as a precursor. J. Non. Cryst. Solids 386, 76–84.
- A Textbook of Inorganic Chemistry (Ninth ed.). New Delhi: New Age International (P) Limited Publishers; 2003.
- Rates and Equilibria of Organic Reactions: As Treated by Statistical, Thermodynamic and Extrathermodynamic Methods. Wiley, New York, NY: Dover Books on Chemistry; 1963.
- Effect of synthesis duration and HCl acid concentration on the formation of hydrothermally synthesised TiO 2 nanoparticles. Cape Penisula University of Technology; 2015.
- Highly pure amorphous silica nano-disks from rice straw. Powder Technol.. 2012;225:149-155.
- [Google Scholar]
- Nanocellulose from rice husk following alkaline treatment to remove silica. BioResources. 2011;6:1440-1453.
- [Google Scholar]
- Health hazards due to the inhalation of amorphous silica. Arch. Toxicol.. 2002;75:625-634.
- [CrossRef] [Google Scholar]
- The influence of parameters in silica sol-gel process. Bull. Transilv. Univ. Brasov Eng. Sci.. 2011;4:59-66.
- [Google Scholar]
- Determination of efficacy of fingermark enhancement reagents; the use of propyl chloroformate for the derivatisation of fingerprint amino acids extracted from paper. Sci. Justice. 2013;53:301-308.
- [CrossRef] [Google Scholar]
- Nanosilica extraction from processed agricultural residue using green technology. J. Clean. Prod.. 2017;143:1284-1290.
- [CrossRef] [Google Scholar]
- Single-Metal Deposition for Fingermark Detection—A Simpler and More Efficient Protocol. J. Forensic Identif.. 2015;65:118-137.
- [Google Scholar]
- A simple method of obtaining spherical nanosilica from rice husk. Int. J. Adv. Sci. Inf. Technol.. 2012;2:28-30.
- [Google Scholar]
- Preparation of silica nanoparticles: Determination of the optimal synthesis conditions for small and uniform particles. Colloids Surf. A Physicochem. Eng. Asp.. 2002;197:7-17.
- [CrossRef] [Google Scholar]
- Appendix 8 - Acidities of Organic Functional Groups. In: Handbook of Synthetic Organic Chemistry (Second Edition). Academic Press; 2017. p. :259-260.
- [Google Scholar]
- 9.07 - The Medical Geochemistry of Dusts, Soils, and Other Earth Materials. In: Holland H.D., Turekian K.K., eds. Treatise on Geochemistry. Oxford: Pergamon; 2007. p. :1-61. https://doi.org/https://doi.org/10.1016/B0-08-043751-6/09050-2
- [Google Scholar]
- Silicon: A review of its potential role in the prevention and treatment of postmenopausal osteoporosis. Int. J. Endocrinol.. 2013;2013:1-6.
- [Google Scholar]
- Fluorescent variant of silica nanoparticle powder synthesised from rice husk for latent fingerprint development. Egypt. J. Forensic Sci.. 2019;9:1-9.
- [Google Scholar]
- Nanocarbon powder for latent fingermark development : a green chemistry approach. Egypt. J. Forensic Sci.. 2018;8:1-10.
- [Google Scholar]
- Morphological and Compositional Changes Exhibited by Rice Husk When Subjected to Synergistic Thermochemical Treatments. Malaysian J. Med. Heal. Sci.. 2020;16:1-7.
- [Google Scholar]
- Solvents and Solvent Effects in Organic Chemistry. Hoboken, NJ, USA: Wiley; 2011.
- Nano-scale analysis of titanium dioxide fingerprint-development powders. J. Phys. Conf. Ser.. 2008;126:1-4.
- [CrossRef] [Google Scholar]
- Saif, M., Shebl, M., Nabeel, A.I., Shokry, R., Hafez, H., Mbarek, A., Damak, K., Maalej, R., 2015. Novel Non-toxic and Red Luminescent sensor based on Eu3+:Y2Ti2O7/SiO2 Nano-powder for Latent Fingerprint detection, Sensors Actuators B. Elsevier B.V. https://doi.org/10.1016/j.snb.2015.05.040
- Cytotoxicity of titanium dioxide nanoparticles differs in four liver cells from human and rat. Compos. Part B Eng.. 2011;42:2136-2144.
- [CrossRef] [Google Scholar]
- Hazardous effects of titanium dioxide nanoparticles in ecosystem. Bioinorg. Chem. Appl.. 2017;2017:1-12.
- [CrossRef] [Google Scholar]
- Visualisation of latent fingerprints using silica gel G : A new technique. Egypt. J. Forensic Sci.. 2013;3:20-25.
- [Google Scholar]
- Synthesis and characterisation of silica nano particles from coconut shell. Int. J. Pharma Bio Sci.. 2015;6:530-536.
- [Google Scholar]
- Powder method for detecting latent fingerprints: a review. Forensic Sci. Int.. 2001;120:172-176.
- [Google Scholar]
- Doped hydrophobic silica nano- and micro-particles as novel agents for developing latent fingerprints. Forensic Sci. Int.. 2008;174:26-34.
- [CrossRef] [Google Scholar]
- UN, E., 2017. Globally harmonised system of classification and labelling of chemicals (GHS), Seventh Ed. ed. United Nations Publications, Herndon, VA, USA.
- Development of latent prints with titanium dioxide (TiO2) J. Forensic Identif.. 2002;52:551-559.
- [Google Scholar]
- Preparation of silica nanoparticles from semi-burned rice straw ash. Powder Technol.. 2008;185:31-35.
- [Google Scholar]







