Translate this page into:
RP-HPLC-UV validation method for levofloxacin hemihydrate estimation in the nano polymeric ocular preparation
⁎Corresponding author. ronnymartien@ugm.ac.id (Ronny Martien),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The specific and accurate reversed-phase HPLC-UV method has been validated to determine levofloxacin hemihydrate (LEVH) level. The separation was conducted at C 18 analytical column by administering mobile phase acetonitrile, methanol, and phosphate buffer (pH 3) with the ratio of 17:3:80. The flow rate of the mobile phase was 1 mL/min with a UV detector at 295 nm wavelength. Analytical methods validation evaluated includes specificity, linearity, accuracy, precision, LOD, LOQ, and robustness. The implementation of the analytical method was employed to determine LEVH level in ocular polymeric nanoparticles preparations. The test was specific for LEVH with the retention time of 7.66 min. Linearity was obtained from the concentration range of 4.8 µg/mL to 29.04 µg/mL. All method validation criteria are within the acceptable range. The developed method can be applied for LEVH polymeric nano-formulation analysis.
Keywords
Levofloxacin hemihydrate
Validation
RP-HPLC
Polymeric nanoparticle
1 Introduction
Bacterial keratitis is an infectious disease causing vision loss or blindness (AlMahmoud et al., 2019; Farahani et al., 2017). Based on the microorganisms' culture and sensitivity test, the disease can be overcome by prescribing proper antibiotics. Levofloxacin (Fig. 1), a third-generation fluoroquinolone antibiotics, can treat bacterial keratitis by inhibiting DNA-gyrase and topoisomerase IV (Blondeau, 2004; Kasetsuwan et al., 2011; Kowalski et al., 2010; Wong et al., 2012). Levofloxacin is available in 5 mg/mL eyedrops solution, however, due to the anatomic and physiological constrictions of the eye, topical administration of the drops results on low bioavailability (Addo et al., 2016; Ameeduzzafar et al., 2018; Cholkar et al., 2013; Kaskoos, 2014; Patel et al., 2012). This bioavailability issue can be addressed by applying nanoparticles to increase permeability across biological membranes, increase drug availability in the cul-de-sac and extend residence time, and sustain the release of the drug (Ghafoorianfar et al., 2020; Weng et al., 2017).
Chemical structure of levofloxacin.
The entrapment efficiency percent of nanoparticles (% E.E.) is an essential parameter to be determined in formulation. Specific and accurate measurement of the value of % E.E. is essential to ensure just right amount of levofloxacin to be delivered by the preparation. Some options have been introduced to determine levofloxacin in the preparations and biological fluids matrix, including HPLC-UV (Dafale et al., 2015; Helmy, 2013), HPLC LC-MS (Fang et al., 2010; Xu et al., 2011), HPLC-PDA (Locatelli et al., 2015), U.V. spectroscopy, HPLC-fluorescent spectroscopy, 1H NMR spectroscopy, UPLC Vis spectroscopy, and capillary electrophoresis (Czyrski, 2017).
When HPLC system is opted, mobile phase to be employed for levofloxacin analysis is a combination of water or aqueous buffers and organic solvents. Triethanolamine (TEA) may be added in the mobile phase to improve peak shape (Czyrski and Szałek, 2016; Locatelli et al., 2015; Watabe et al., 2010); Other than that, sodium or potassium phosphate buffer in the 10-30 mM concentration range can be used (Helmy, 2013; Locatelli et al., 2015). Acetonitrile in the range of 14–43% in isocratic elution is the most frequently used organic solvent for HPLC separation (Czyrski and Szałek, 2016; Locatelli et al., 2015; Sousa et al., 2012; Xu et al., 2011).
The determination of % E.E. of nanoparticles for levofloxacin has been performed using a U.V. spectrophotometer (Gevariya et al., 2011; Gupta et al., 2011; López-López et al., 2019, p.). HPLC-UV with acetic acid and acetonitrile as mobile phases (Ameeduzzafar et al., 2020), and HPLC-UV with H20 (pH3) and methanol as mobile phase (Siafaka et al., 2019).
This study validated the analytical method to determine levofloxacin hemihydrate (LEVH) concentration using HPLC-UV with acetonitrile, methanol, and phosphate buffer (pH 3) as mobile phase. The method was then applied to measure % E.E. of LEVH ocular nanoparticles. This study reported selectivity, linearity, accuracy, precision, LODs, LOQ, and robustness as guided by the International Harmonization Conference (ICH).
2 Experimental procedure
2.1 Instrumentation
A Hitachi L-2000 HPLC with an L2130 pump and an L-2420 UV–Vis detector was used for chromatographic separation. A manual injector system with a 20 μL injector loop and a 50 μL syringe (Hamilton, Microliter 705LT) was applied. A Luna Phenomenex® C18 (250 × 4.6 mm; 5 µm) was used as column, and the D-2000 Elite software controlled the HPLC apparatus and data collecting. Analytical balance, vortex mixer (MaxiMixTM), pH meter (Hanna), a 0.45-µm PTFE membrane filter (Phenomenex®), nylon syringe filter 0.22 µm, glassware (Pyrex), micropipette (Biologix, USA), and ultrasonic bath were used for sample preparations.
2.2 Materials
Analytical grades of levofloxacin hemihydrate (LEVH) and ciprofloxacin (CPR) (Sigma-Aldrich, Buchs, Switzerland) were obtained from P.T. Pharos Indonesia Tbk (Indonesia). Other materials used in this study were potassium dihydrogen phosphate, sodium hydroxide, orthophosphoric acid, acetic acid, hydrochloric acid analytical grade, acetonitrile (ACN), and methanol (MeOH) HPLC grade (Merck, Darmstadt, Germany), Aquabidest (Ikapharmindo, Jakarta, Indonesia) and purified water (Onemed, Surabaya, Indonesia). As excipient materials of the nanoparticles, chitosan medium molecular weight and pectin (Sigma-Aldrich, Buchs, Switzerland) were used.
2.3 Buffer preparation
Three grams of potassium dihydrogen phosphate was dissolved with distilled water in a 1000 mL volumetric flask. The pH was then adjusted with 180 µL of phosphoric acid to pH 3.0, and volume was adjusted to 1 L with water. The solution was filtered using a 0.45 µm membrane filter (Phenomenex®) and ultrasonic-degassed for 15 min.
3 Methods
3.1 Chromatographic conditions
Isocratic elution was implemented in the study. Acetonitrile, methanol, and phosphate buffer pH 3.0 (17:3:80 v/v/v) were used as mobile phase, flowing at 1 mL/min through a Luna Phenomenex® C18 (250 4.6 mm; 5 m) column. Per run, a volume of 20 μL of the sample was injected and disclosed with a U.V. detector set at 295 nm.
3.2 Preparation of standard solution and calibration curve
As internal standard, 10 mg of LEVH and CPR were placed into a 10.0 mL volumetric flask, dissolved with sufficient acetic acid, and diluted with mobile phase to attain a concentration of 1 mg/mL. The standard solution was diluted with the mobile phase to obtain LEVH concentration of 4.84; 9.68; 14.52; 19.36; 24.20; and 29.04 µg/mL. Each concentration of standard LEVH solution contains an internal standard as much as 20 µg/mL. Calibration was done by curve-fitting concentration to LEVH to CPR area ratio.
3.3 Analysis method validation
3.3.1 System suitability test
System suitability test was conducted by injecting six replications of the standard solution before the analysis. The observed parameters were relative standard deviation (%RSD) for peak area and retention time, tailing factor, resolution, and number of theoretical plates.
3.3.2 Specificity
Method’s specificity was tested by comparing levofloxacin chromatogram and ciprofloxacin with the chromatogram of the nanoparticles matrix. The matrix was used to observe the effect of excipients on the retention time of levofloxacin and ciprofloxacin analytes.
3.3.3 Linearity
Linearity test was conducted by assessing the ratio of each area of 4.84; 9.68; 14.52; 19.36; 24.20; and 29.04 µg/mL LEVH and 20 µg/mL CPR to the common area.
3.3.4 Accuracy
Determination of the recovery value in this study was performed at three different concentrations: 4.8 µg/mL, 14.5 µg/mL, and 29.04 µg/mL.
3.3.5 Precision
Precision is determined by the fulfilment of coefficient of variation (RSD) value to 2% of intra- and inter-day examinations.
3.3.6 Limit of detection (LOD) and limit of quantification (LOQ)
LOD and LOQ were calculated by the signal-to-noise ratio of three and ten times respectively.
3.3.7 Robustness
Rigidity test was performed by diversifying the maximal wavelength (294 and 295 nm) and the speed of flow time (0.9 and 1 mL/min).
3.4 Nanoparticles preparation
0.1% w/v Levofloxacin nanoparticle containing chitosan (0.1% w/v) and pectin (0.02 w/v) was prepared by mixing 500 µL of chitosan solution and 500 µL of LEVH into a microtube, homogenized using a vortex mixer for 60 s at 1250 rpm. Then, 500 µL of pectin solution was added and vortex-mixing was continued for 60 s.
3.5 %E.E. Determination
%E.E. of LEVH in nano polymeric formulation was determined by indirect method. The dispersion were centrifuged for 50 min at a speed of 15000 rpm. Twenty microliters of supernatant were taken, CPR standard solution was added and then diluted using mobile phase ad 1 mL. A total of 20 µL of sample were injected into the column, and then %E.E. was calculated using Eq. (1).
4 Results
4.1 System suitability test
Measured parameters fulfilled the criteria of acceptance. The RSD of retention time and area < 2%, tailing factor < 2, N (number of theoretical plates) > 2000, and Rs (resolution) > 2 as presented in Table 1.
Parameters
LEVH (n = 6)
CPR (n = 6)
Average
RSD (%)
Average
RSD (%)
Retention Time (minutes)
7.66 ± 0.01
0.11
8.44 ± 0.01
0.15
Area
563189.33 ± 6301.42
0,81
879621.00 ± 9945.98
0.69
Tailling Factor
1.18 ± 0.01
1.02
1.21 ± 0.02
1.22
Resolution
2.51 ± 0.02
0.5
2.51 ± 0.02
0.5
Number of Theoretical Plates
10748.17 ± 166.83
1.19
10963.50 ± 160.81
1.23
4.2 Specificity
LEVH peaks (Rt = 7.66 min) and ciprofloxacin peaks (Rt = 8.43 min) were well separated with a value of Rs > 2. The results of the nanoparticle matrix chromatogram presented that no peaks occurred at 7.66 and 8.43 min (Fig. 2.). The result indicates that the HPLC assay possesses good selectivity for levofloxacin assay administering the standard internal method.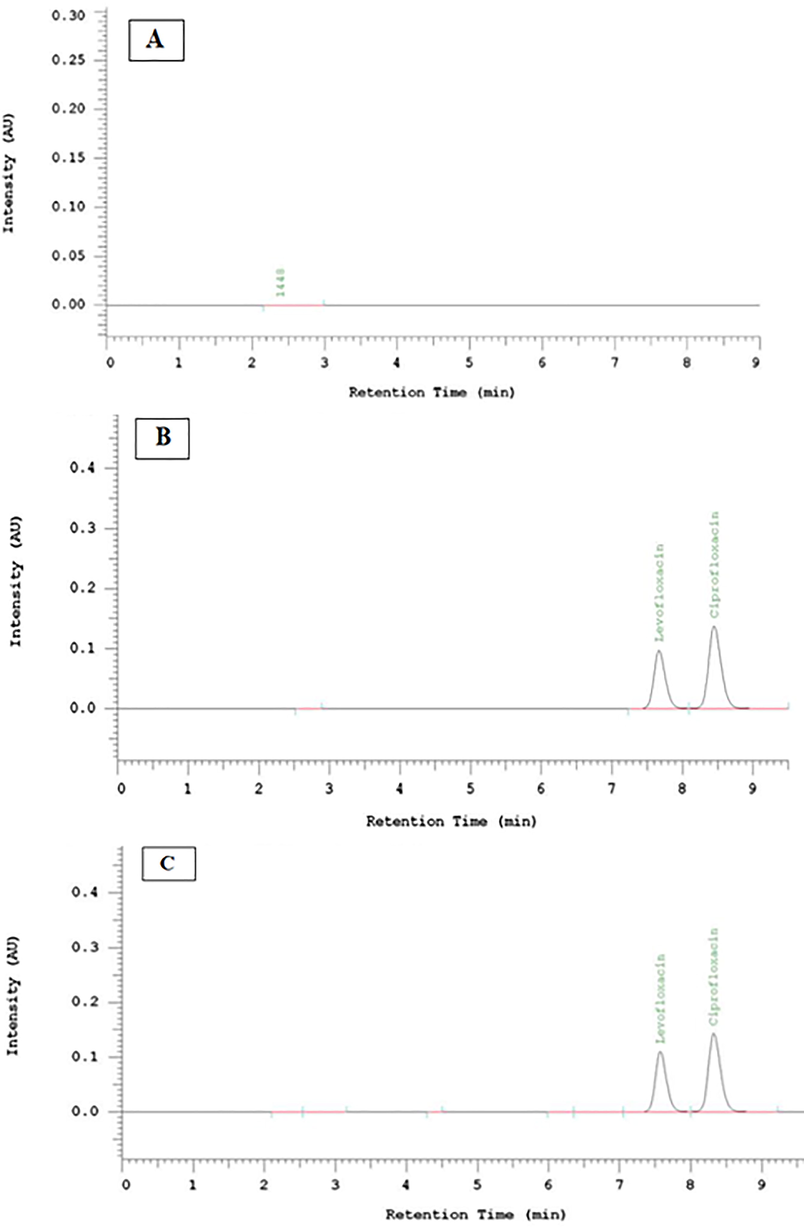
Chromatograms of nanoparticle matrix (A), levofloxacin and ciprofloxacin (B), and levofloxacin-unloaded nanoparticle (C).
4.3 Linearity
The obtained linear regression equation is Y = 0.1097x + 0.0439, with the correlation coefficient of 0.9998 (Table 2), indicating qualified linearity parameter. The levofloxacin standard curve is displayed in Fig. 3.
LEVH concentration (µg/mL
Area Ratio
4.84
0.578
9.68
1.126
14.52
1.625
19.36
2.150
24.20
2.676
29.04
3.261
Correlation Coefficient (r)
0.9998
Slope
0.1097
intercept
0.0439
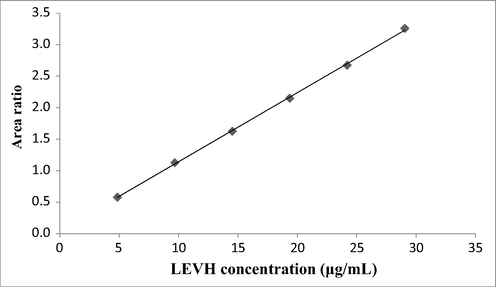
Standard curve of Levofloxacin.
4.4 Accuracy
The value of % recovery generated as shown in Table 3 fulfills the requirements of % recovery in the range of 80–110%.
Theoretical concentration (µg/mL)
Measured concentration (µg/mL ± SD) (n = 3)
% Recovery
4.8
4.88 ± 0.02
100.92 ± 0.33
14.5
14.53 ± 0.01
100.05 ± 0.70
29.04
29.35 ± 0.04
101.09 ± 0.12
4.5 Precision
The results of the intermediate precision intra- and inter-day testings showed the required precision (RSD < 2) (Table 4).
Day
Actual LEVH concentration (µg/mL)
Measured concentration (µg/mL) (n = 3)
%R.S.D
1
4.8
4.88 ± 0.02
0.32
14.5
14.53 ± 0.08
0.70
29.04
29.88 ± 0.15
0.51
2
4.8
4.86 ± 0.04
0.75
14.5
14.42 ± 0.03
0.22
29.04
29.35 ± 0.04
0.12
3
4.8
4.98 ± 0.01
0.11
14.5
14.36 ± 0.08
0.54
29.04
29.56 ± 0.15
0.50
4.6 Limit of detection (LOD) and limit of quantification (LOQ)
The LOD and LOQ values obtained in the method analysis were 0.66686 µg/mL and 2.22286 µg/mL, respectively, based on the standard curve equation.
4.7 Robustness
The results showed that the RSD value is <2 (Table 5), indicating fulfilment of robustness criteria.
Variation condition
Flow rate 0.9 mL/min
Flow rate 1.0 mL/min
Measured concentration
% recovery
R.S.D
Measured concentration
% recovery
R.S.D
Wavelength 294 nm
4.8
4.88 ± 0.02
0.32
4.8
4.88 ± 0.02
0.32
Wavelength 295 nm
14.5
14.53 ± 0.08
0.70
14.5
14.53 ± 0.08
0.70
5 Discussion
5.1 Chromatographic conditions
Reverse-phase HPLC method is used to separate LEVH. Commonly used in reverse phase HPLC is C18 bonded to the silica surface as the stationary phase due to the chemical interaction between chlorosilane and silanol groups. LEVH and CPR are polar, therefore the elution power of the mobile phase must be decreased to increase interaction between the analyte and the stationary phase. Combining solvents ACN (proton acceptor) with methanol (proton donors) increases selectivity. Interactions between the analyte and stationary phase are generally hydrophobic, and secondary interactions with silanol residues can cause tailings in compounds with amine groups so that pH adjustment is carried out to prevent the ionization of silanol residue. LEVH is base for its amine group on the piperazine ring. The interaction between positively ionized amine base groups (−NH2+−) and negatively ionized silanol residues (SiO–) causes peak tailing thus reduces resolution. This adverse secondary interaction can be avoided by keeping the silanol residue in the unionized form. At pH > 8, the silica as a solid support of the stationary phase can be dissolved, while at pH < 2, siloxane linkages can be hydrolyzed in the stationary phase. Consequently, a generally safe and effective mobile phase may be performed at pH 3 with a suitable buffer, one of which is a phosphate buffer (a combination of H3PO4 and KH2PO4) (Nemutlu et al., 2007; Sousa et al., 2012).
UV spectrophotometer detector was used. The levofloxacin compound has chromophore and auxochrome groups to be detected with this detector. Ciprofloxacin was used as an internal standard because it has physical and chemical properties similar to LEVH. Internal standard procedure was used to precisely and sensitively assess levofloxacin level. The developed method can provide good separation with<10 min of retention time so that analytical methods may be used to determine levofloxacin level in pharmaceutical formulations.
5.2 Implementation of the analytical method in polymeric nanoparticle formulas
The % E.E.value id the quantification of the amount of drug absorbed in the nanoparticle preparation. The test was carried out by indirect method. The % E.E. is 21.34 ± 1.53% with unloaded nanoparticles and LEVH chromatograms showed similar retention time. Furthermore, no peaks presented at the retention time of LEVH and CPR in the chromatogram of the nanoparticle matrix. This result indicates that the developed method could specificity determine LEVH levels in polymeric nanoparticles preparations.
6 Conclusions
The development and validation methods of HPLC for analyzing LEVH using acetonitrile: methanol: phosphate buffer pH 3 (17:3:80) is specific, accurate, precise, and robust. The system can be implemented to analyze LEVH in polymeric nanoparticles.
Acknowledgements
The author would like to express his gratitude to the Ministry of Education and Culture of the Republic of Indonesia (2021), which has provided funding for this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anatomy of the Eye and Common Diseases Affecting the Eye. In: Addo R.T., ed. Ocular Drug Delivery: Advances, Challenges and Applications. Cham: Springer International Publishing; 2016. p. :11-25.
- [Google Scholar]
- Management of infective corneal ulcers in a high-income developing country. Medicine (Baltimore). 2019;98:e18243.
- [CrossRef] [Google Scholar]
- Formulation and optimization of levofloxacin loaded chitosan nanoparticle for ocular delivery: In-vitro characterization, ocular tolerance and antibacterial activity. Int. J. Biol. Macromol.. 2018;108:650-659.
- [Google Scholar]
- Improvement of Ocular Efficacy of Levofloxacin by Bioadhesive Chitosan Coated PLGA Nanoparticles: Box-behnken Design, In-vitro Characterization, Antibacterial Evaluation and Scintigraphy Study. Iran J Pharm Res.. 2020;19:292-311.
- [CrossRef] [Google Scholar]
- Fluoroquinolones: mechanism of action, classification, and development of resistance. Surv. Ophthalmol.. 2004;49:S73-S78.
- [CrossRef] [Google Scholar]
- Novel Strategies for Anterior Segment Ocular Drug Delivery. J. Ocul. Pharmacol. Ther.. 2013;29:106-123.
- [CrossRef] [Google Scholar]
- Analytical Methods for Determining Third and Fourth Generation Fluoroquinolones: A Review. Chromatographia. 2017;80:181.
- [CrossRef] [Google Scholar]
- An HPLC method for levofloxacin determination and its application in biomedical analysis. J Anal Chem.. 2016;71:840-843.
- [CrossRef] [Google Scholar]
- Development and validation of microbial bioassay for quantification of Levofloxacin in pharmaceutical preparations. J. Pharm. Anal.. 2015;5:18-26.
- [CrossRef] [Google Scholar]
- Simultaneous determination of isoniazid, rifampicin, levofloxacin in mouse tissues and plasma by high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. B. 2010;878:2286-2291.
- [CrossRef] [Google Scholar]
- Formulation and characterization of levofloxacin-loaded biodegradable nanoparticles. Asian J. Pharm.. 2011;5:114.
- [CrossRef] [Google Scholar]
- Efficiency of nanoparticles for treatment of ocular infections: Systematic literature review. J. Drug Deliv. Sci. Technol.. 2020;57:101765.
- [CrossRef] [Google Scholar]
- Biodegradable levofloxacin nanoparticles for sustained ocular drug delivery. J. Drug Target.. 2011;19:409-417.
- [CrossRef] [Google Scholar]
- Simultaneous quantification of linezolid, tinidazole, norfloxacin, moxifloxacin, levofloxacin, and gatifloxacin in human plasma for therapeutic drug monitoring and pharmacokinetic studies in human volunteers. Ther. Drug Monit.. 2013;35:770-777.
- [CrossRef] [Google Scholar]
- The efficacy and safety of 0.5% Levofloxacin versus fortified Cefazolin and Amikacin ophthalmic solution for the treatment of suspected and culture-proven cases of infectious bacterial keratitis: a comparative study. Asian Biomed.. 2011;5:77-83.
- [CrossRef] [Google Scholar]
- Investigation of moxifloxacin loaded chitosan–dextran nanoparticles for topical instillation into eye: In-vitro and ex-vivo evaluation. Int. J. Pharm. Investig.. 2014;4:164-173.
- [Google Scholar]
- Topical levofloxacin 1.5% overcomes in vitro resistance in rabbit keratitis models. Acta Ophthalmol. (Copenh.). 2010;88:e120-e125.
- [CrossRef] [Google Scholar]
- Determination of ciprofloxacin and levofloxacin in human sputum collected from cystic fibrosis patients using microextraction by packed sorbent-high performance liquid chromatography photodiode array detector. J. Chromatogr. A. 2015;1419:58-66.
- [CrossRef] [Google Scholar]
- Optimized Preparation of Levofloxacin Loaded Polymeric Nanoparticles. Pharmaceutics. 2019;11:1-13.
- [Google Scholar]
- Simultaneous Separation and Determination of Seven Quinolones Using HPLC: Analysis of Levofloxacin and Moxifloxacin in Plasma and Amniotic Fluid. Chromatographia. 2007;66:15-24.
- [CrossRef] [Google Scholar]
- Novel Ophthalmic Drug Delivery Approach: An Ophthalmic Inserts. Res. J. Pharm. Technol.. 2012;5:729-735.
- [Google Scholar]
- Design and characterization of nanocarriers loaded with Levofloxacin for enhanced antimicrobial activity; physicochemical properties, in vitro release and oral acute toxicity. Braz. J. Pharm. Sci.. 2019;55:e18295.
- [CrossRef] [Google Scholar]
- Analytical methods for determination of new fluoroquinolones in biological matrices and pharmaceutical formulations by liquid chromatography: a review. Anal. Bioanal. Chem.. 2012;403:93-129.
- [CrossRef] [Google Scholar]
- Simultaneous measurement of pazufloxacin, ciprofloxacin, and levofloxacin in human serum by high-performance liquid chromatography with fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci.. 2010;878:1555-1561.
- [CrossRef] [Google Scholar]
- Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin. B. 2017;7:281-291.
- [CrossRef] [Google Scholar]
- New Treatments for Bacterial Keratitis. J. Ophthalmol.. 2012;2012:e831502.
- [CrossRef] [Google Scholar]
- Microwave-assisted extraction and in situ clean-up for the determination of fluoroquinolone antibiotics in chicken breast muscle by LC-MS/MS. J. Sep. Sci.. 2011;34:142-149.
- [CrossRef] [Google Scholar]







