Translate this page into:
Silanization of functionalized PET fabric to improve PET-nitrile rubber (NBR) adhesion; effects of functionalization type and silane concentration
⁎Corresponding author. mjamshidi@iust.ac.ir (Masoud Jamshidi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Surface modification could increase affinity of PET fibers to polymer matrix by changing the interfacial physical interactions to covalent bonds. In this research, surface modification of a superfine polyethylene terephthalate (PET) fabric was performed by bis(triethoxysilylpropyl)tetrasulfide (TESPT). The surface grafting was performed at different silane concentrations (i.e. 1, 5 and 10X). Before silanization, the fabrics were functionalized using photochemical method (i.e. irradiation of UV light in presence of glutaric acid peroxide (GAP)) and also hydrolysis by Sodium hydroxide (NaOH). Due to functionalization, carboxyl and hydroxyl groups were formed on the PET surface that made it ready to condense with hydrolyzed silane molecules. The fabrics were characterized using FTIR, TGA, FE-SEM, XPS analysis for evaluation of grafting performances. XPS analysis confirmed the presence of new peaks of Si and S after surface modification with TESPT. Comparing the results of TESPT-grafted functionalized PETs, it was found that the silane grafting content on PET surface is dependent not only to the silane concentration but also to the functionalization method. Finally, the influences of silane grafting on the functionalized PET fabrics to NBR adhesion were evaluated using H-pull and T-peel tests. The results showed that silane grafted-carboxylated PET fabric illustrated more adhesion to NBR than silane grafted-hydroxylated PET (i.e. 33 and 12% improvement in the pullout strength and T-peel adhesion, respectively).
Keywords
Surface modification
Adhesion
PET Fabric
Nitrile rubber (NBR)
TESPT
1 Introduction
Surface modification of reinforcing materials (i.e. fibers or particles) to improve their adhesion to polymeric materials has been a topic in the composites science and technology (Cruz & Fangueiro, 2016; Ghamarpoor & Jamshidi, 2022a, 2022c; Kockmann et al., 2018). Today, the surface modification of fabrics as reinforcement of rubbery composites is a well-known method to enhance their adhesion to rubber matrix and strengthen their interfacial transition zone (Biuk Afshari et al., 2022; Dharmasiri et al., 2022; Fang et al., 2022; Wu et al., 2022). In fact, proper adhesion at reinforcement-substrate interface facilitates stress transfer from matrix to reinforcement and prevents crack creation and propagation at interfacial zone (i.e. fracture) (Jose et al., 2022; Yang et al., 2019). Nitrile rubber (NBR) is a fuel resistant rubber that is used in production of oil and fuel resistant O-rings, packings, sealants, hoses and tanks (Ghamarpoor & Ebrahimabadi, 2019; Ghamarpoor & Jamshidi, 2022b, 2023; Weatherhead, 2012). Polyethylene terephthalate (PET) fibers/fabrics are of the most important reinforcements used in NBR-based composites. Since the surface of polyesters is naturally inactive, they have poor adhesion to the NBR compound (Jincheng et al., 2005). However, due to huge applications of PET fabrics in rubbery composites, improving their adhesion to rubbers by surface modification is an interesting subject from the scientific and industrial points of view.
Many efforts have been performed to increase the adhesion of PET fibers/fabrics to rubbers, so far. In some researches, physical/mechanical treatments were used to improve PET-rubber adhesion by enhancing the mechanical interlocking (Koc et al., 2008; HUDEC Krump et al., 2006; H Krump et al., 2005). However, chemical treatment of PET fibers has been the most interested strategy for increasing PET-rubber interfacial bonding, during the last decades. For instance, coating of PET surface by resorcinol–formaldehyde-latex (RFL) as a coupling agent have been a traditional method in tire industry for many years (Jamshidi et al., 2005; Kondo et al., 2008). Nonetheless, due to the environmental problems caused by resorcinol and formaldehyde, several studies have been conducted in recent years to replace RFL. Dierkes et al (Dierkes et al., 2019) used plasma treatment of PET fabric in presence of a sulfur-containing precursor to develop adhesion to rubber in absence of RFL. They found that the material was not successful as well as RFL for improving PET to rubber adhesion. Zhang et al (Zhang et al., 2020) introduced ethylene glycol diglycidyl ether (EGDE) and diethylenetriamine (DETA) as replacement of the toxic resorcinol and formaldehyde in RFL dipping system. They assessed T-peel adhesion of the modified PET fabric to rubber compared to RFL-coated PET and found that the new system has capability of replacing RFL. Cazan et al (Cazan et al., 2017) functionalized PET surface by non-ionic polyethylene glycol and sodium dodecyl sulphate. It was illustrated that the PET-rubber interface properties improved by the functionalization step. Gholshaei et al (Golshaei & Güven, 2017) modified surface of PET by graft copolymerization of N-isopropylacrylamide as a thermo-sensitive layer. Prior to surface modification, the PET surface was functionalized by H2O2 to prepare COOH groups on the surface which were later reacted with allylamine to introduce vinyl end groups at the surface. The vinyl groups that created on the PET surface could be co-vulcanized by rubber unsaturated bonds (—C⚌C—) to create strong PET-rubber adhesion.
However, the other weakness of RFL adhesive (i.e. in one-bath method) is its weak interfacial interactions (i.e. physical bonds) to inert PET surface. On this basis, many efforts have been performed to find binding agents with ability of providing chemical bond to PET fabric. Liu et al (Liu et al., 2013) grafted isocyanate based compound on PET fiber after surface functionalization via NaOH and corona discharge treatments. They showed that rubber to PET adhesion improved due to created strong chemical bonds between rubber-MDI-functionalized PET. Razavizadeh et al (Razavizadeh & Jamshidi, 2016a, 2016b) functionalized PET surface by UV irradiation in presence of glutaric acid peroxide (GAP) to prepare carboxyl groups on the surface. Thereafter, the functionalized PET were grafted by methylenediphenyl diisocyanate (MDI) via chemical reaction to surface carboxyl groups It was illustrated that the chemical modification caused considerable improvement in the adhesion between PET fabric and NBR. In another study, they illustrated that MDI could incorporate in sulfur vulcanization of NBR as a co-vulcanizing agent (Razavizadeh & Jamshidi, 2017).
Nowadays, using silanes as functional coupling agents to increase interfacial interactions between different reinforcing materials (i.e. nanoparticles and fibers) and rubbers is a well-known subject (Ghamarpoor et al., 2023b; Ghamarpoor et al., 2023c; Riaz et al., 2019). For example, Riaz et al. (Riaz et al., 2021) applied TiO2 nanoparticles modified with (3-Glycidoxypropyl)trimethoxysilane (GPTS) and 1,2-Bis(triethoxysilyl)ethane (BTSE) in textiles. However, there are few studies on silanization of PET fabric to improve its interfacial bonding to rubbers. Kondo et al (Kondo et al., 2008) functionalized inert surface of PET by electron beam (EB) irradiation in presence of acrylate based silane. They found that the silanization of PET improved the mechanical properties of NR-PET composite via development of chemical strong bonds between them. In another study, bis-[3-(triethoxysilyl)propyl] tetrasulfide (TESPT) silane was used to modify functionalized PET surface that completely different results were obtained. The results showed that the adhesion of silanized PET to rubber extremely reached to 70% of the RFL coated PET to rubber adhesion (Dierkes et al., 2019).
Based on the literature review, many binding agents have been applied on PET fiber to improve its interfacial interactions to rubber matrix, hitherto. However, to enhance the performance of binding agent and creating strong bonding, it is necessary to increase surface functionality of the PET fibers. There are few researches that assessed functionalization of PET fibers and its effects on the PET-rubber adhesion.
In this work, for the first time, a superfine PET fabric was functionalized using photochemical and chemical methods to find out which one created more effective groups on the surface. The photochemical method (i.e. carboxylation) was performed oxidation of PET surface in presence of glutaric acid peroxide/H2O2 under UV irradiation to prepare COOH groups. The chemical functionalization (i.e. hydrolysis) was performed by NaOH solution to prepare OH groups on the PET surface. Thereafter, the functionalized PET were surface modified using bis-[3-(triethoxysilyl)propyl] tetrasulfide (TESPT) at different concentrations (i.e. 1, 5 and 10 times to stoichiometric silane concentration). The pristine, functionalized and silanied PET fabrics were carefully characterized to determine the success of functionalization and silanization processes. XPS results confirmed functionalization and surface modification. They were applied to nitrile rubber (NBR). The composites were evaluated for thermomechanical properties (using DSC and DMTA analysis) and PET-NBR adhesion (Pullout and peel tests).
2 Experimental
2.1 Materials
Polyethylene terephthalate (PET) fine fabric prepared by HEJAB Co (Iran) was used in this study. The structural parameters of PET fabric are presented in Table 1. To remove oil and pollutants, the fabric was immersed in a 1 wt% solution of non-ionic detergent in distilled water at temperature of 50 °C and then dried at 60 °C for 15 min.
Fabric type
Mesh size
(µm)2
Warp and weft density
(number/10 cm)Thickness
(mm)linear mass density of fiber
(Denier)
PET
100
38
0.25
50
Acrylonitrile-butadiene rubber (NBR) with acrylonitrile content of 33% and specific density of 1.31 (g/cm3) were supplied from LG company. Bis-(triethoxysilylpropyl) tetrasulfide (TESPT) supplied from Evonik Company was used as coupling agent. Glutaric anhydride, hydrogen peroxide, acetone, ethanol, Sodium hydroxide (NaOH) and acetic acid were supplied from Merck Company.
Table 2 shows the used NBR compound in this study. The rubber ingredients were mixed by a laboratory two-roll mill and then mixing followed by a Bunbury according to ASTM D-3182 standard. The prepared compound was processed on a two roll mill to prepare rubber sheets with thickness of 2 mm.
Ingredients
phr
NBR
100
Carbon black (N660)
65
Calcium carbonate
35
DOP oil
10
Zinc oxide
5
Stearic acid
1
Sulfur
1.2
CBS
2
TMTD
0.6
2.2 Functionalization of PET fabric
2.2.1 Carboxylation by GAP/UV irradiation
Glutaric acid peroxide (GAP) was prepared by reaction of glutaric anhydride with hydrogen peroxide at 0 °C. For surface carboxylation, the PET fabric was immersed in a water/acetone solution containing 5 wt% of GAP and irradiated by a UV lamp (400 W, ULTRAMED400, OSRAM Co., Germany) for 90 min. The water and acetone solution was placed in an ice bath to prevent temperature rising and solvent evaporation. The carboxylated fabric was washed with deionized water and dried for 15 min at 60 °C (see Scheme 1a and b).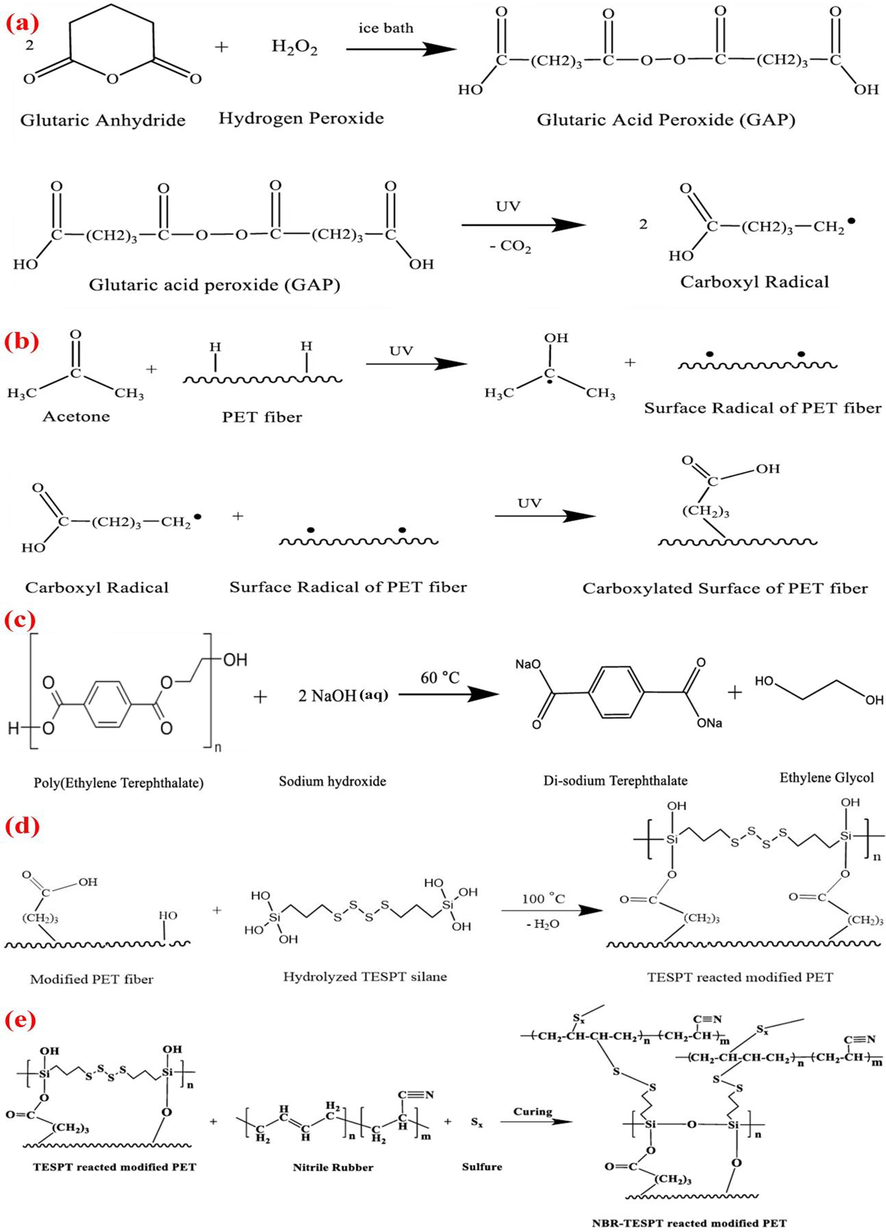
(a) The reaction of carboxylic free radical production under UV radiation, (b) carboxylation of PET in presence of GAP and under UV irradiation, (c) hydrolysis of PET structure in the presence of sodium hydroxide solution, (d) The reaction between PET fibers and TESPT, (e) Mechanism of co-vulcanization of NBR rubber and silanized PET fibers.
2.2.2 Hydroxylation by NaOH
To prepare OH groups on the PET surface, the fabric was exposed to NaOH solution (10 wt%) for 60 min at temperature of 60 °C. The hydroxylated fabric was then washed with deionized water and dried for 15 min at 60 °C (see Scheme 1-c).
2.3 Silanization of PET fabric
The silanization process includes hydrolysis and condensation steps (see Scheme 1-d). In the hydrolysis step, silanol groups (-Si-OH) are formed by hydrolysis of alkoxy groups of silane molecules. In condensation step, silanol groups react to oxygen-containing surface functional groups of PET fabrics (i.e. OH or COOH) via heating. In this research, water and ethanol were firstly combined at a ratio of 5:95. The pH of the solution was adjusted to 3–4 using acetic acid and the TESPT was added dropwise to the solution. The silane solutions were mixed for 60 min to complete the hydrolysis process. The functionalized fabrics were then immersed in the silane solutions at room temperature for 60 min. The fabrics were then exposed to the open air for 10 min and finally subjected to 100 °C for 30 min in an oven for condensation of TESPT molecules with surface functional groups of PET to form siloxane structure at the surface.
2.4 Calculations
The calculations were performed based on Mrkoci method to determine the amount of desired silane (Parent et al., 2003). Considering this fact that one TESPT molecule is needed to react each PET surface functional group (due to hindrance of attached TESPT molecules), the following stoichiometric equation was used to determine needed TESPT content:
Which
and
are the weight of TESPT and PET fabric, respectively,
is the molecular mass of silane and
is the Avogadro number. X is a coefficient for stoichiometric concentration of the TESPT that is needed to react to the surface functional groups. The used X coefficients were 1, 5, and 10 in this study.
is the number of hydroxyl/carboxyl groups relative to the weight of PET fabric and was calculated from the following equation (Huang et al., 2014; Sneh & George, 1995):
Which
is the weight of water required for hydrolysis of TESPT,
is the molecular mass of water, h and n are the hydrolysis ratio and the number of silane alkoxy groups, respectively (see Table 3).
Type of silane
h
(Hydrolysis ratio)n
(Number of alkoxy groups)
TESPT
9
6
The calculated weight percentages of TESPT needed for hydrolysis step is presented in Table 4.
Components
Soluble components of
TESPT silane (wt.%)
Coupling agent
11
Deionized water
19.8
Ethanol
69.2
2.5 Adhesion tests
H-pull test of PET fibers from NBR rubber was performed according to ASTM-D4776 standard at ambient temperature with a pulling speed of 120 mm/min. T-peel adhesion of PET fabric to nitrile rubber was evaluated according to ASTM-D413 standard at ambient temperature with a separation speed of 50 mm/min.
2.6 Characterization
Thermogravimetric analysis (TGA) was performed with METTLER-TOLEDO analyzer. TGA was performed on fabrics by heating them under a continuous air flow at 10 °C/min from 50 °C to 600 °C. Attenuated total reflectance infrared spectroscopy (ATR-FTIR) was carried out on the samples by Bruker EQUINOX 55 spectrometer. The surface of the treated and untreated fabrics was studied using a TESCAN-Mira III Scanning Electron Microscope- Field Emission (FE-SEM) and energy-dispersive X-ray spectroscopy (EDX). To study chemical reactions between rubber chains and different silanes (at 5 phr), differential scanning calorimetry (DSC) was performed using METTLER-TOLEDO analyzer at a heating rate of 10 °C/min on rubber compounds contained 5 phr of each TESPT. The chemical characteristics of the surface modification of PET by grafting TESPT, carboxylation and hydroxylation were investigated in X-ray photoelectron spectroscopy (XPS, 8025-BesTec twin anode XR3E2, Bwstec Company, Germany).
2.7 Sample codes
The codes of prepared samples in this study are listed in Table 5.
Sample code
Functionalization type
TESPT
NaOH
GAP/UV
1X
5X
10X
PET (Pristine PET)
–
–
–
–
–
PET-Ha
√
–
–
–
–
PET-Cb
–
√
–
–
–
PET-H-T1
√
–
√
–
–
PET-H-T5
√
–
–
√
–
PET-H-T10
√
–
–
–
√
PET-C-T1
–
√
√
–
–
PET-C-T5
–
√
–
√
–
PET-C-T10
–
√
–
–
√
3 Results and discussions
3.1 Characteristics of PET fabrics
Fig. 1 shows TGA and DTG results for hydroxylated PET fabrics grafted by TESPT at different concentrations. The weight losses at 120–350, 350–450 and 450–600 °C were corresponded to decomposition of surface OH groups, PET backbone and crystalline phases of PET, respectively (Andideh et al., 2021; X. Liu et al., 2022). It is clearly seen that hydroxylation of PET surface caused increase in the weight loss at the temperature ranges of 120–350 and 450–600 °C but declined it at 350–450 °C. On this basis, the creation of OH groups on PET surface through hydroxylation process was confirmed. Furthermore, it is obviously seen that the hydroxylation decreased thermal stability of the PET fabric due to damages that occurred by hydrolysis process.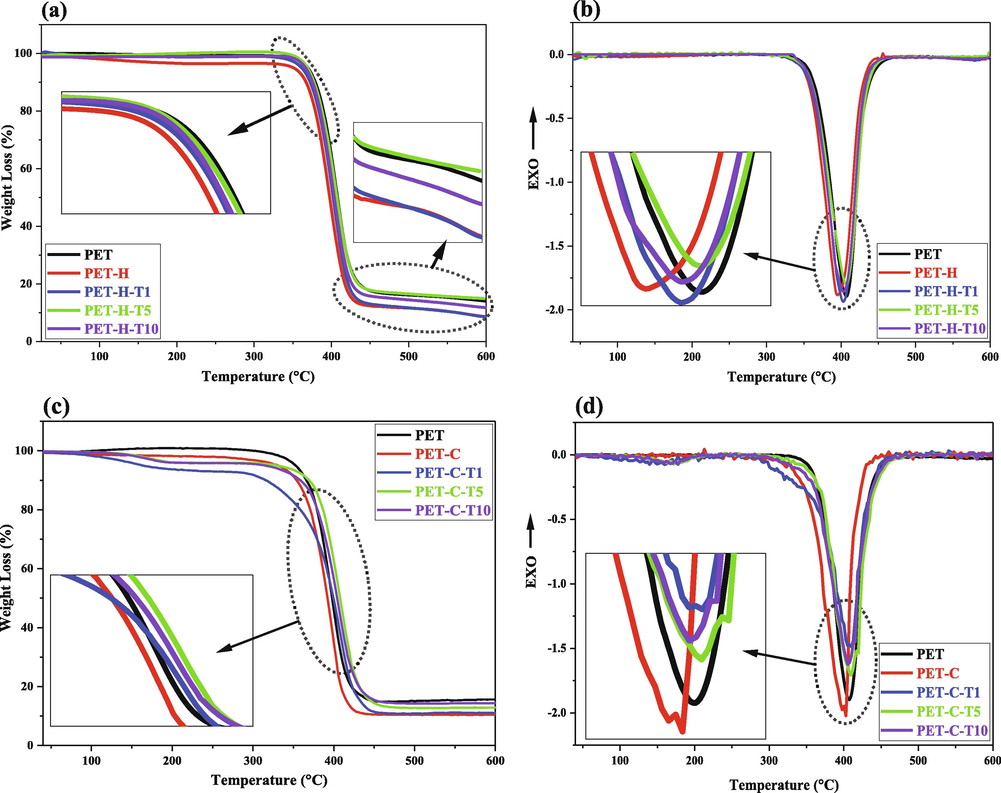
TGA and DTG analysis of the TESPT grafted; (a, b) hydroxylated PET and (c, d) carboxylated PET.
After silanization of PET by TESPT, the hydroxyl content decreased at the temperature range of 120–350 °C due to the consumption of OH groups in the condensation process with silane molecules. This also confirmed successful grafting of TESPT on the hydroxylated PET fabrics. By increment in the TESPT concentration, weight loss in the grafted samples at temperature ranges of 350–450 and 450–600 °C was observed that related to the attachment of TESPT molecules and formation of inorganic silanol (Si-OH) layer at the surface. Based on the results, silanization at stoichiometric concentration (i.e. PET-H-T1 sample) was selected as the best TESPT grafting condition for the hydroxylated PET fabrics (see Table 6).
Sample code
Weight loss (%)
120–350 (˚C)
350–450 (˚C)
450–600 (˚C)
PET
1.35
84.4
1.35
PET-H
2.6
82.73
3.68
PET-H-T1
1.75
84.23
4.48
PET-H-T5
0.1
81.45
2.88
PET-H-T10
0.41
81.95
3.93
The decrement in the OH content of the silanized samples at concentrations of 5 and 10X (i.e. PET-H-T5 and PET-H-T10) was attributed to the self-assembly of silane molecules in the solution phase due to the homo-polymerization. In fact, the attached silane molecules inhibited more reactions by overlapping free OH groups of PET surface. Fig. 1-b shows that the melting point of PET had negligible changes due to the hydroxylation process.
Despite of much more OH creation through carboxylation process (GAP/UV irradiation) on PET fabric, however, they did not illustrate more TESPT grafting contents compared to the hydroxylated fabrics except at stoichiometric concentration of silane (see Fig. 1-c and Table 7). However, silanized sample at concentration of 5X (i.e. 5 times to stoichiometric concentration) was selected as the best TESPT grafted-carboxyalted PET sample due to more consumption in the created OH groups and higher weight loss related to silane degradation. The higher OH content in the PET-C-T1 sample was attributed to the less attachment of hydrolyzed silane molecules on the PET surface due to low silane concentration that increase free OH groups on the PET surface. This limits more TESPT grafting and also formation of Si-O-Si network during condensation step. Fig. 1d shows that carboxylation of PET under GAP/UV irradiation caused decrement in the fibers strength that it was compensated after silanization.
Sample code
Weight loss (%)
120–270 (˚C)
270–370 (˚C)
370–450 (˚C)
450–600 (˚C)
PET
0.44
4.54
77.11
3.66
PET-C
0.95
17.22
69.91
0.1
PET-C-T1
4.7
17.52
63.84
0.36
PET-C-T5
3.42
6.03
75.33
1.66
PET-C-T10
3.47
9.54
70.83
0.94
Fig. 2 shows ATR-FTIR analysis of the TESPT grafted PET fabrics. The results show that after functionalization the intensity of peak related to OH group increased in both hydroxylated and carboxylated samples. The peak at 1720 cm−1 in all samples was corresponded to the stretching vibration of C⚌O bond. It is clearly seen that the peak intensified after hydrolysis (i.e. PET-H sample) compared to the pristine PET. This was attributed to hydrolysis of ester (i.e. —COO) groups in the polymer backbone that makes broken chains with new —OH or —COOH groups at the tails. This increases the carbonyl (—C⚌O) group content in hydroxylated PET fabrics. The same trend was also observed after carboxylation (i.e. PET-C sample) that was related to creation of —COOH groups on the surface of PET fabric during reaction to GAP under UV irradiation (Ghamarpoor et al., 2023a).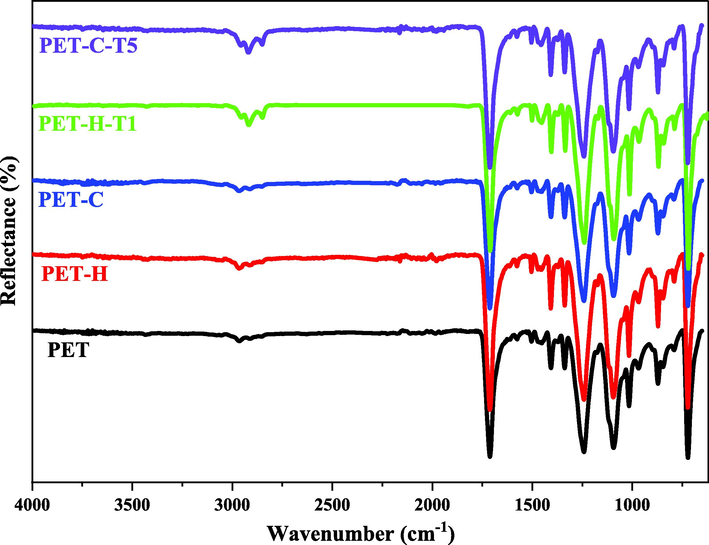
ATR-FTIR Spectrum of the TESPT grafted PET fabrics.
The presence of intensified stretch peaks in the silanized samples at 2800–3000 cm−1 were attributed to stretching vibration of CH and CH2 groups that confirmed successful TESPT grafting on the PET surface.
A wide scan of the pristine, hydroxylated, carboxylated and silane-modified PET fabric is shown in Fig. 3. The quantitative XPS data were given in Table 8. Fig. 3a shows the intact PET fabric with two peaks of C1s and O1s. Fig. 3-b shows the hydroxylated PET fabric with NaOH, which increased the amount of O1s and decreased the amount of C1s. Fig. 3c shows the hydroxylated PET fabric modified with TESPT, after surface modification, new peaks of Si2s, Si2p and S1p appeared, also O1s was consumed in the reaction and C1s increased. Fig. 3d shows the carboxylated PET fabric with GAP, which was strengthened after modification of C1s and O1s elements. In Fig. 3e, the new elements Si2s, Si2p and S1p were created as a result of modification of the carboxylated fabric with TESPT.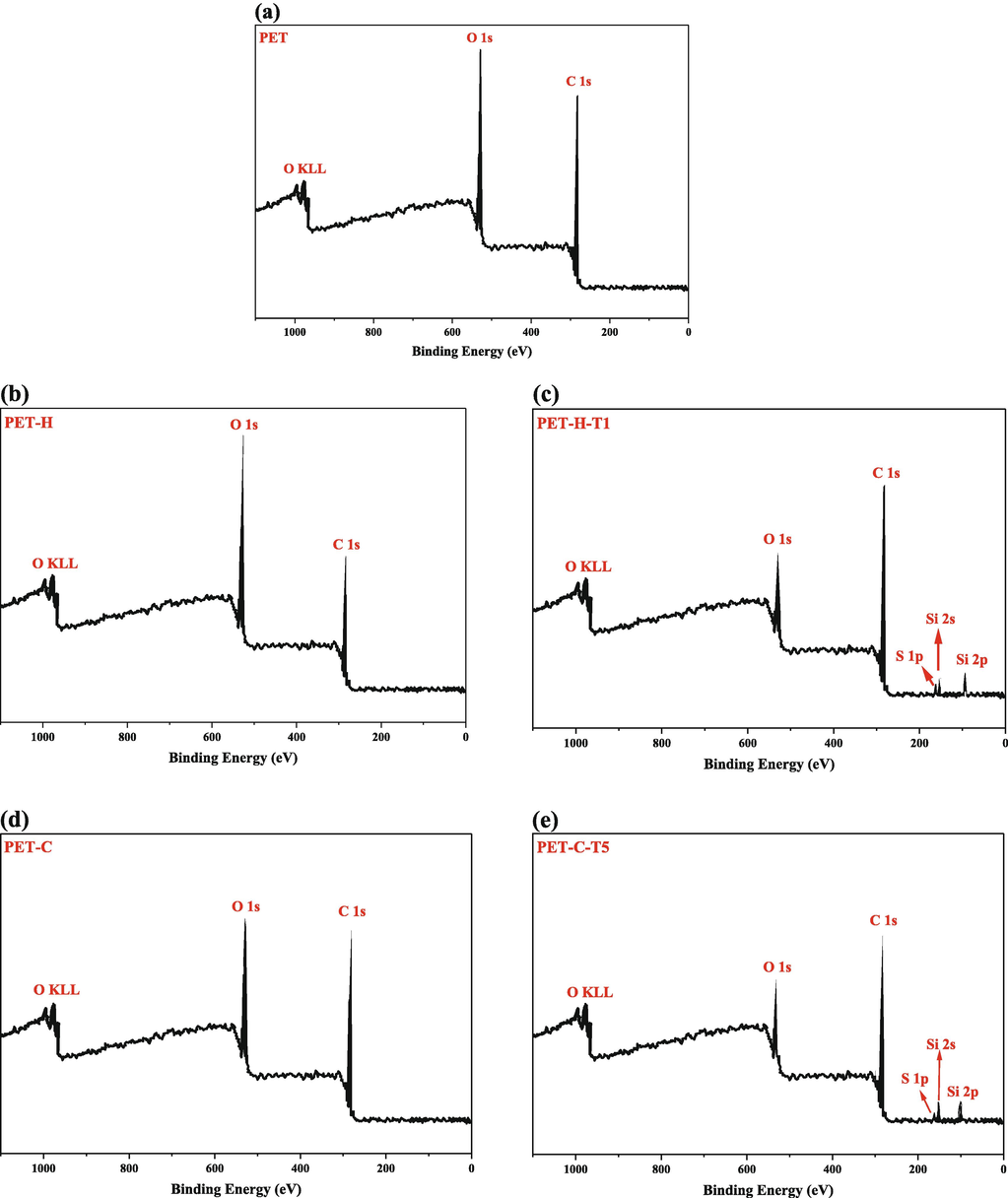
XPS spectra of PET fabric before and after treatments; (a) PET; (b) PET-H; (c) PET-H-T1, (d) PET-C and (e) PET-C-T5.
Samples
Peak
Binding Energy (eV)
Atomic concentration (%)
PET
O1s
532.18
55.32
C1s
284.62
44.68
PET-H
O1s
531.59
69.13
C1s
284.1
30.87
PET-H-T1
O1s
530.09
32.12
C1s
285.6
47.35
S1p
164.3
7.29
Si2p
99.79
13.24
PET-C
O1s
533.12
56.78
C1s
285.6
43.22
PET-C-T5
O1s
534.79
33.41
C1s
287.29
48.31
S1p
161.11
4.18
Si2p
100.27
14.1
The FE-SEM images of TESPT grafted PET fabrics are shown in Fig. 4. It is seen that hydroxylation made grooves on the PET surface (see Fig. 4a & b). The PET surfaces were covered by silane layer (see Fig. 4c). The EDS results confirmed presence of Si and S elements that were related to condensed silane molecules (see Table 9). The rough surface of silane layer could improve the mechanical bonding (i.e. interlocking) of modified PET to NBR matrix. The healing effect of silane layer was likely observed in the carboxylated PETs (see Fig. 4d & e). The results of EDS analysis of the samples are listed in Table 9. As can be seen, carboxylated PET fabric showed more TESPT grafting. The results were in good correlation to the TGA results.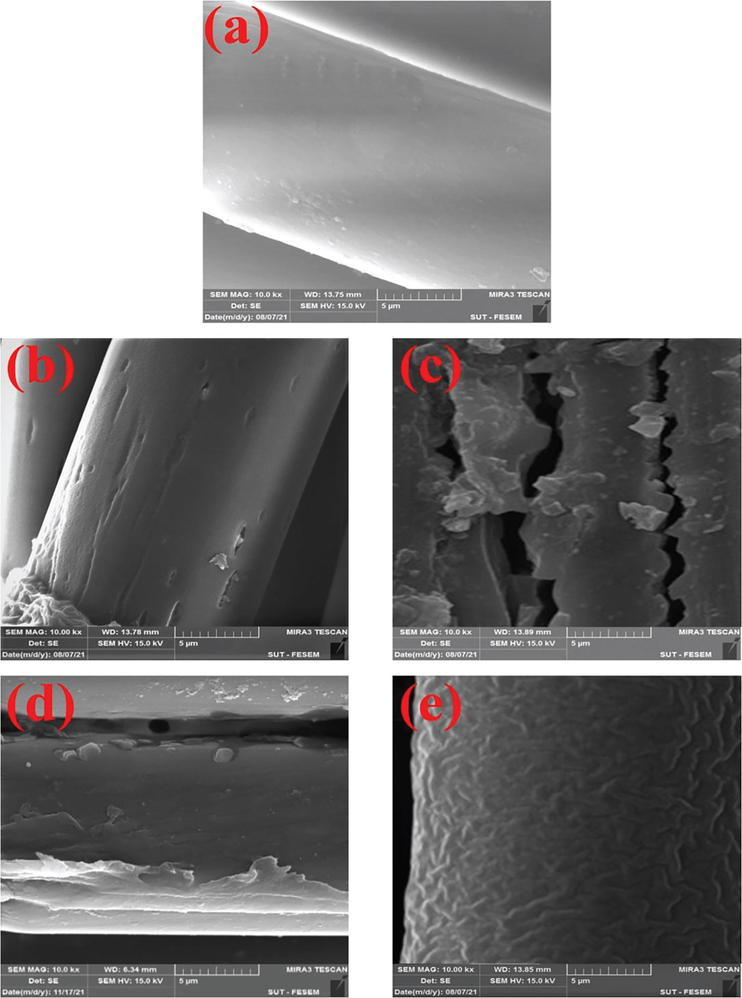
FE-SEM images of the TESPT grafted PET fabrics; (a) pristine PET, (b) PET-H, (c) PET-H-T1, (d) PET-C, (e) PET-C-T5.
Element (wt.%)
SampleC
O
Si
S
PET
74.23 ± 0.19a
25.77 ± 0.19
–
–
PET-H
71.1 ± 0.2
27.39 ± 2
–
–
PET-H-T1
70.21 ± 0.19
29.72 ± 0.19
0.04 ± 0.02
0.03 ± 0.02
PET-C
71.3 ± 0.2
27.7 ± 0.2
–
–
PET-C-T5
75.9 ± 0.19
23.85 ± 0.19
0.08 ± 0.02
0.17 ± 0.02
According to the results of TESPT grafted PETs, it was found that the silane grafting content on PET surface is dependent on not only to the silane type but also the functionalization method. In fact, depending on the silane chemical structure, different functionalization method could be suitable for higher silane grafting.
Fig. 5 shows the tensile test results of carboxylated, hydroxylated and silanized PET yarns. Based on the obtained results, the silanization process of carboxylated and hydroxylated PET fabric with TESPT has increased elongation at break and reduced breaking force. In fact, the toughness and the ability to change the shape of the fibers against the applied stress have increased and the resistance of the fibers against tearing and stretching has increased.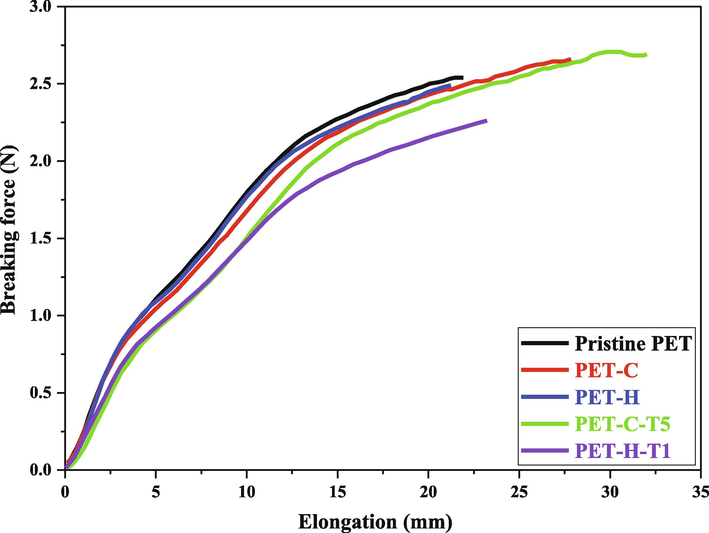
Comparing the tensile strength results of PET yarns before and after the silanization process.
3.2 Assessing co-vulcanization of silane with NBR
DSC analysis was performed to study possible reactions of silane coupling agents with NBR. For this purpose, the silane was added at 5 phr to the rubber compound and the samples were analyzed. The results are presented in Fig. 6 and Table 10. In the temperature range of 100–200 °C, exothermic peaks were observed for all samples which were related to the vulcanization of the rubber compound. By adding TESPT silane to the rubber compound, the enthalpy of vulcanization increased that confirmed co-curing of silane with NBR via sulfur. This was attributed to presence of tetra sulfide group in TESPT molecule that separates during heating and involves in vulcanization process. The curing temperature of NBR showed negligible changes by addition of silane.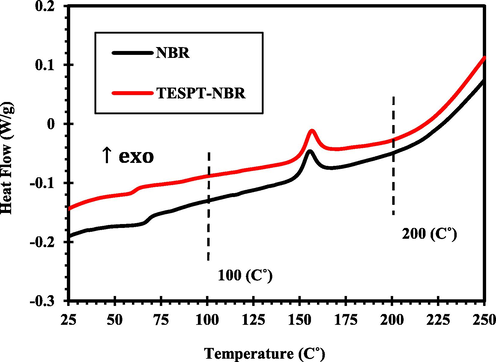
Comparison of DSC results of NBR rubber compounds containing TESPT.
Sample
Tonset (C˚)
Tpeak (C˚)
Tend (C˚)
NBR
149.30
155.49
162.26
2.26
TESPT-NBR
15.21
156.50
163.72
2.9
3.3 Adhesion tests
3.3.1 H-Pull test results
Fig. 7 shows the pullout adhesion between PET yarns and NBR that calculated based on pullout test results. The results showed a slight decrement in the pullout adhesion of the hydroxylated/carboxylated PET yarns to nitrile rubber. This was attributed to the damages that occurred during functionalization step (i.e. hydroxylation and carboxylation) (see Fig. 4-b & d) that could decline the strength of PET fibers/yarns and cause their rupture during pulling out from rubber matrix. However, silane grafting compensated the declined pullout adhesion of both hydroxylated and carboxylated yarns to the NBR.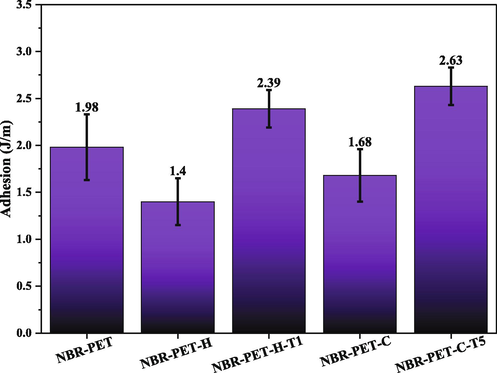
PET-NBR pullout adhesion for hydroxylated PET and carboxylated PET.
Fig. 8a shows the separation surface of PET yarn before surface modification of rubber. The pieces separated from the rubber can be seen on the surface of the witness fibers after the test of pulling out the thread from the rubber. On the other hand, due to the relatively low polarity of the control fibers, the rubber has been able to slightly penetrate inside the thread. Fig. 8b shows the surface of yarn treated with GAP after the rubber pull-out test. It is seen that the surface of the yarn is almost smooth and without pieces of rubber that was attributed to the increase in the polarity of the PET surface after carboxylation process and the decrease in the compatibility of the carboxylated yarn with the amphiphilic NBR matrix [4]. However, in the fibrillated areas, due to the mechanical contact of rubber with these surfaces, very small pieces of rubber were observed. Fig. 8d shows the sample of thread hydroxylated with sodium hydroxide, which probably decreased due to the increase of polarity of the fiber surface. In scattered areas of fibers, large pieces of rubber can be seen that are separated and left on the surface. Fig. 8c and e show the surface of fiber modified with TESPT, which completely changed its surface after pulling out from the rubber, which indicate its proper adhesion to the rubber. Table 11 shows the comparison of the previous research with the current research.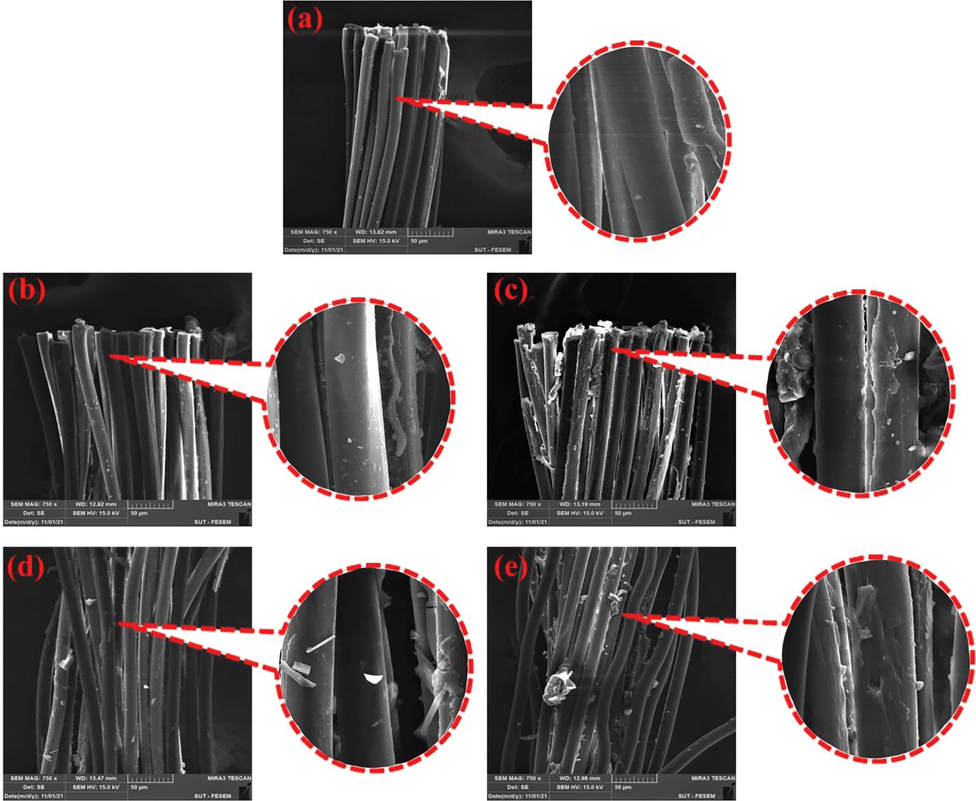
FE-SEM images of the surface of PET yarns after pullout adhesion test; (a) pristine PET, (b) hydroxylated PET, (c) hydroxylated/TESPT grafted PET, (d) carboxylated PET and (e) carboxylated/ TESPT grafted PET.
Application
Functionalization Type
Ref.
Evaluation of the adhesion of nitrile rubber (NBR) to polyethylene terephthalate (PET) fabric
Treating PET fibers with ultraviolet light and creating adhesion between PET fibers and NBR rubber with isocyanate agents
(Razavizadeh & Jamshidi, 2016b)
Surface modification of PET fibers using ultraviolet rays to improve adhesion
Surface modification of PET fibers using succinic peroxide and isocyanate under UV light to increase adhesion to polyurethane rubber
(Liu et al., 2013)
Reinforcement of modified cellulose nanofibers extracted from Napier in natural rubber composite
Investigating the characteristics and application of cellulose nanofibers silanized with TESPT as reinforcement in natural rubber
(Somseemee et al., 2021)
Increasing the adhesive properties of aramid fibers by polyphenol-iron metal complexation and silane grafting
Surface modification of aramid fibers with the metal complex of iron metal polyphenol and TESPT with the aim of increasing the adhesion property to tire rubber
(Wang et al., 2021)
Functionalization and silanization of PET fabric surface to increase its adhesion to nitrile rubber
Functionalization and silanization of the surface using GAP, NaOH and TESPT
This study
3.3.2 T-peel Test results
Fig. 9 shows the PET fabric-NBR peel test results. From the comparison of the peeling strength results of the control sample with a carboxylated and hydroxylated sample, it was determined that the adhesion decreased after modification. This may be due to the increase in surface polarity due to the binding of carboxyl groups on the surface of the PET fabric, which has increased the incompatibility of the fabric with the NBR matrix and has reduced adhesion due to disruption in the rubber curing process. Adhesion strength increased in TESPT modified fabric sample compared to other samples, which is due to proper connection of modified fiber with rubber matrix.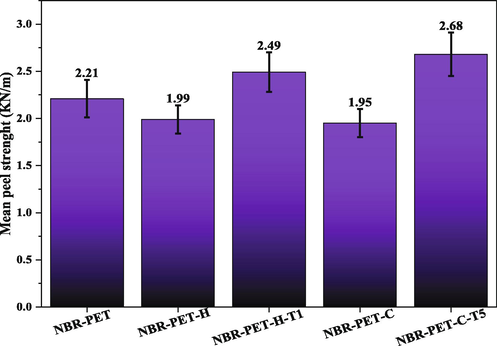
Peel strength results of PET-NBR samples.
After performing the peeling test, the surfaces of the treated fabrics were examined with FE-SEM. Fig. 10a is related to the control sample. It can be seen that in a few areas of the fabric, the rubber was able to penetrate into the fibers, which caused a mechanical conflict with the rubber matrix when the rubber was separated from the fibers. Fig. 10b and c shows the carboxylated sample with GAP under 90 min of UV light irradiation and the hydroxylated sample with NaOH after 60 min of immersion in alkaline solution. A clean and rubber-free surface indicates low compatibility of carboxylated and hydroxylated fibers with the rubber matrix and improper adhesion between fibers and rubber. Fig. 10d and e show the silane-modified samples, where a large amount of rubber is still attached to the fiber surface when removed, indicating good adhesion of PET to rubber.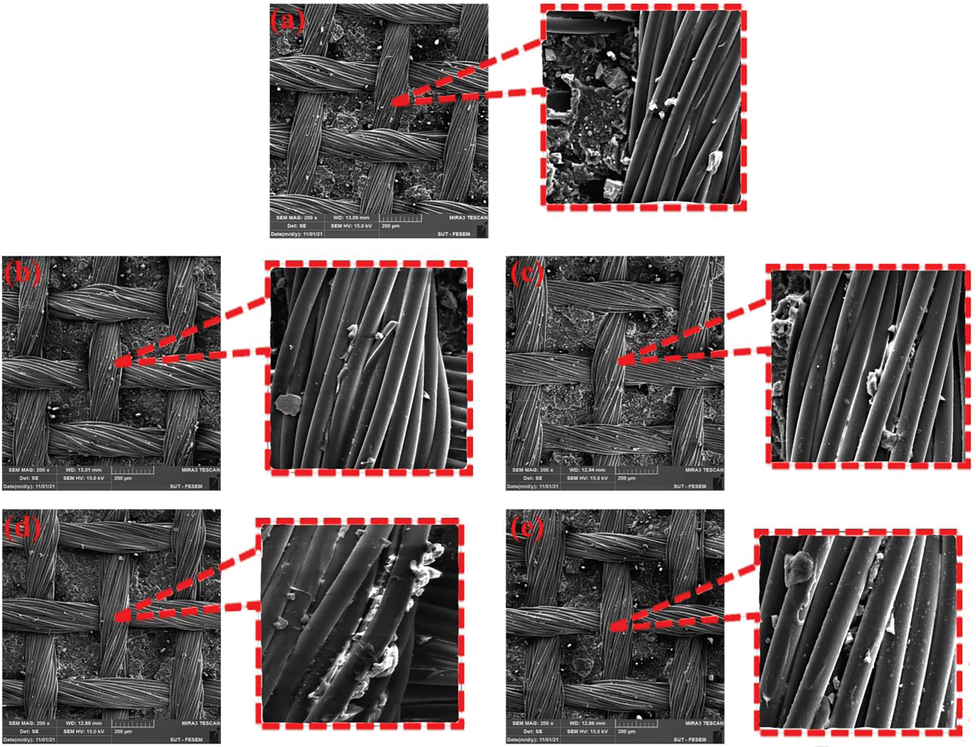
FE-SEM images of PET-NBR samples peel adhesion; (a) NBR-PET, (b) NBR-PET-C, (c) NBR-PET-H, (d) NBR-PET-C-T1, (e) NBR-PET-T-T5.
4 Conclusions
In this work, functionalized PET fabrics (i.e. by GAP/UV irradiation and hydrolysis by NaOH) were silanized by TESPT at different concentrations. The best silane concentration for TESPT was determined. The following results were concluded:
-
The hydroxylation and carboxylation of PET enhanced OH content to 2.19 and 2.83%.
-
The XPS results confirmed that the TESPT was successfully grafted on the PET surface. The highest percentage of TESPT grafting to the surface of PET fabric was observed for hydroxylated and carboxylated samples at silane concentrations of 1 and 5 times to the stoichiometric content, respectively.
-
The enthalpy of curing of NBR enhanced by 28.3% in presence of 5 phr TESPT. This ascribe that the TESPT grafted PET fabrics could incorporate in NBR vulcanization due to the presence of tetra sulfide group in the silane structure.
-
The results of H-Pull and T-peeling tests showed that the silanization of carboxylated PET fibers with TESPT silane increased the adhesion strength of PET to NBR by 33 and 12%, respectively, compared to the control sample. However, PET-NBR adhesion was lower in the case of using TESPT grafted/ hydroxylated PET fibers.
5 Availability of data
It is confirmed that all Data Availability. The raw/processed data required to reproduce these findings can be shared.
CRediT authorship contribution statement
Mohammad Sayyadian: Methodology, Investigation, Writing – original draft. Masoud Jamshidi: Supervision, Conceptualization, Methodology, Validation, Resources, Data curation. Reza Ghamarpoor: Methodology, Investigation, Data curation, Writing – original draft. Mahmoud Razavizadeh: Methodology, Validation, Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Surface modification of oxidized carbon fibers by grafting bis (triethoxysilylpropyl) tetrasulfide (TESPT) and rubber sizing agent: Application to short carbon fibers/SBR composites. Compos. A Appl. Sci. Manuf.. 2021;141:106201
- [Google Scholar]
- Improving the mechanical/anticorrosive properties of a nitrile rubber-based adhesive filled with cerium oxide nanoparticles using a two-step surface modification method. ACS Omega 2022
- [Google Scholar]
- Effect of PET functionalization in composites of rubber–PET–HDPE type. Arab. J. Chem.. 2017;10(3):300-312.
- [Google Scholar]
- Carbon reinforced carbon fibers: Using surface modification as a route to enhanced physical performance. Compos. Sci. Technol.. 2022;218:109217
- [Google Scholar]
- A novel approach of promoting adhesion of reinforcing cord to elastomers by plasma polymerization. Polymers. 2019;11(4):577.
- [Google Scholar]
- Biomimetic surface modification of UHMWPE fibers to enhance interfacial adhesion with rubber matrix via constructing polydopamine functionalization platform and then depositing zinc oxide nanoparticles. Surf. Interfaces. 2022;29:101728
- [Google Scholar]
- Optimum design of water-based drilling fluid in shale formations in Khangiran oilfields. Prog. Ind. Ecol. Int. J.. 2019;13(1):42-62.
- [Google Scholar]
- Preparation of Superhydrophobic/Superoleophilic nitrile rubber (NBR) nanocomposites contained silanized nano silica for efficient oil/water separation. Sep. Purif. Technol.. 2022;291:120854
- [Google Scholar]
- Investigating the use of titanium dioxide (TiO2) nanoparticles on the amount of protection against UV irradiation. Sci. Rep.. 2023;13(1):9793.
- [CrossRef] [Google Scholar]
- Silanizing nano SiO2 and its application in recycled nitrile rubber to prepare super oil resistant/superhydrophobic/superoleophilic oil/water separator. J. Environ. Chem. Eng.. 2022;107971
- [Google Scholar]
- Synthesis of vinyl-based silica nanoparticles by sol–gel method and their influences on network microstructure and dynamic mechanical properties of nitrile rubber nanocomposites. Sci. Rep.. 2022;12(1):1-15.
- [Google Scholar]
- Synergistic effect of microwave assisted devulcanization of waste NBR rubber and using superhydrophobic/superoleophilic silica nanoparticles on oil-water separation. Alex. Eng. J.. 2023;69:67-84.
- [Google Scholar]
- Preparation of dual-use GPTES@ ZnO photocatalyst from waste warm filter cake and evaluation of its synergic photocatalytic degradation for air-water purification. J. Environ. Manage.. 2023;342:118352
- [Google Scholar]
- Achieving outstanding mechanical/bonding performances by epoxy nanocomposite as concrete–steel rebar adhesive using silane modification of nano SiO2. Sci. Rep.. 2023;13(1):9157.
- [Google Scholar]
- Chemical modification of PET surface and subsequent graft copolymerization with poly (N-isopropylacrylamide) React. Funct. Polym.. 2017;118:26-34.
- [Google Scholar]
- Toward tuning the surface functionalization of small ceria nanoparticles. J. Chem. Phys.. 2014;140(7):074703
- [Google Scholar]
- Jamshidi, M., Afshar, T. F., & Mohammadi, N. (2005). The effects of temperature on interfacial interactions of Cord-RFL-Rubber system.
- Research on the adhesive property of polyethylene terephthalate (PET) cord and nitrile-butadiene rubber (NBR) system. J. Ind. Text.. 2005;35(2):157-172.
- [Google Scholar]
- Surface modification of wool fabric using sodium lignosulfonate and subsequent improvement in the interfacial adhesion of natural rubber latex in the wool/rubber composites. Ind. Crop. Prod.. 2022;177:114489
- [Google Scholar]
- Effect of air-jet texturing on adhesion behaviour of polyester yarns to rubber. Appl. Surf. Sci.. 2008;254(21):7049-7055.
- [Google Scholar]
- Impact of nanoparticle surface modification on the mechanical properties of polystyrene-based nanocomposites. RSC Adv.. 2018;8(20):11109-11118.
- [Google Scholar]
- Surface treatment of PET fiber by EB-irradiation-induced graft polymerization and its effect on adhesion in natural rubber matrix. Eur. Polym. J.. 2008;44(5):1567-1576.
- [Google Scholar]
- Adhesion strength study between plasma treated polyester fibres and a rubber matrix. Appl. Surf. Sci.. 2005;240(1–4):268-274.
- [Google Scholar]
- Physical–morphological and chemical changes leading to an increase in adhesion between plasma treated polyester fibres and a rubber matrix. Appl. Surf. Sci.. 2006;252(12):4264-4278.
- [Google Scholar]
- Thermal stability of oxygen-containing functional groups on activated carbon surfaces in a thermal oxidative environment. J. Chem. Eng. Jpn.. 2014;47(1):21-27.
- [Google Scholar]
- UV-assisted surface modification of PET fiber for adhesion improvement. Appl. Surf. Sci.. 2013;264:61-69.
- [Google Scholar]
- Effect of interfacial interaction between Nano-SiO2 and NBR on tribological properties of NBR water-lubricated bearings. Wear. 2022;490:204191
- [Google Scholar]
- Silica agglomeration and elastomer reinforcement: influence of surface modifications. Plast., Rubber Compos.. 2003;32(3):114-121.
- [Google Scholar]
- Adhesion of nitrile rubber (NBR) to polyethylene terephthalate (PET) fabric. Part 1: PET surface modification by methylenediphenyl di-isocyanate (MDI) Appl. Surf. Sci.. 2016;360:429-435.
- [Google Scholar]
- Adhesion of nitrile rubber to UV-assisted surface chemical modified PET fabric, part II: Interfacial characterization of MDI grafted PET. Appl. Surf. Sci.. 2016;379:114-123.
- [Google Scholar]
- Effects of methylene diphenyl diisocyanate on the physical, mechanical, and vulcanization properties of nitrile rubber. J. Appl. Polym. Sci.. 2017;134(33):45200.
- [Google Scholar]
- Modification of silica nanoparticles to develop highly durable superhydrophobic and antibacterial cotton fabrics. Cellulose. 2019;26:5159-5175.
- [Google Scholar]
- Selection and optimization of silane coupling agents to develop durable functional cotton fabrics using TiO2 nanoparticles. Fibers Polym.. 2021;22:109-122.
- [Google Scholar]
- Thermal stability of hydroxyl groups on a well-defined silica surface. J. Phys. Chem.. 1995;99(13):4639-4647.
- [Google Scholar]
- Reinforcement of surface-modified cellulose nanofibrils extracted from Napier grass stem in natural rubber composites. Ind. Crop. Prod.. 2021;171:113881
- [Google Scholar]
- Enhanced adhesion property of aramid fibers by polyphenol-metal iron complexation and silane grafting. J. Adhes.. 2021;97(4):346-360.
- [Google Scholar]
- FRP Technology: Fibre Reinforced Resin Systems. Springer Science & Business Media; 2012.
- Dopamine concentration-dependent surface modification for gaining carbon fiber composites with enhanced interfacial adhesion. Compos. Commun.. 2022;29:101047
- [Google Scholar]
- Surface modification of Poly (p-phenylene terephthalamide) fibers by polydopamine-polyethyleneimine/graphene oxide multilayer films to enhance interfacial adhesion with rubber matrix. Polym. Test.. 2019;78:105985
- [Google Scholar]
- Polyester (PET) fabrics coated with environmentally friendly adhesive and its interface structure and adhesive properties with rubber. Compos. Sci. Technol.. 2020;195:108171
- [Google Scholar]







