Translate this page into:
Silica-based chelating resin bearing dual 8-Hydroxyquinoline moieties and its applications for solid phase extraction of trace metals from seawater prior to their analysis by ICP-MS
⁎Corresponding author. asuhaimi@taibahu.edu.sa (Awadh O. AlSuhaimi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Solid phase extraction (SPE) using chelating resins has been established as a convenient technique for samples pretreatment prior to trace metal analysis from complex matrices. Oxine chelating agents (e.g., 8-Hydroxyquinoline (8-HQ)) are popular moieties in the synthesis of chelating resins, due to their characteristic coordination chemistry. So far most of the reported silica-oxine chelators encompasses a single oxine molecule per spacer arm. In this work, two 8-HQ ligands have been covalently attached onto silica surface throughout a single linkage. The synthesized resin characterized with FTIR, elemental analysis and SEM. The main parameters affecting SPE procedures, such as pH, and sorption kinetics, investigated using batch experiments. The capacity exchange of the produced resin under optimized conditions was 0.219 and 0.161 mmol g−1 for Cu(II) and Mn(II) respectively. The resin packed into 10 ml standard cartridges and used with a typical SPE manifold for matrix removal prior to an ICP-MS analysis of transition metals (i.e., Cu, Cd, Ni, Pb, Zn, and Co) in seawater certified reference material samples and real samples from high saline seawater near the discharge zone of Yanbu desalination plant. The obtained results confirm the usefulness of the method.
Keywords
SPE
Chelating reins
Dual moieties
8-HQ
Trace metals
Seawater
Desalination
1 Introduction
Over the last decades, the accumulative development in many technologies have enabled the design and manufacture of highly sensitive analytical instrumentation to meet the increased requirements for trace and ultra-trace elemental analysis in many fields, such as the water and semiconductor industries (Namiesnik, 2002). Unfortunately, the direct analysis of samples with a complex matrix composition, using “dilute and shoot” approach is still not viable. Nevertheless, sample preparation steps are essential, mainly to convert a sample matrix to one that is easily handled by analytical apparatus and/or to improve the method’s sensitivity (Namiesnik, 2002; Primel et al., 2010).
In recent years, there has been an increased shift toward the application of green analytical practices. Therefore, many conventional sample preparation methods, such as liquid-liquid extraction (LLE), precipitation, co-precipitation, and evaporation have been widely replaced by solid-phase extraction (SPE). SPE is currently the most popular sample preparation technique for metal ions and organic analytes (Primel et al., 2010) and used in 22 official methods (Bendicho et al., 2011; Domini et al., 2005). The techniques offer many genetic advantages, such as simplicity, high enrichment factor, and reusability for multiple sorption/desorption cycles, reduced consumption of and minimized exposure to solvents, rapid processing, and ability for integration with different instruments in either online or offline modes (Fritz, 1999).
Various sorbents materials, such as activated carbon (Jiang et al., 2008), silica gel (Tokalıoglu et al., 2004), ion exchange resins (Sharma and Pant, 2009; Tokalıoglu et al., 2006), nanomaterials (Zhai et al., 2006; Cui et al., 2007), and chelating resins (Bag et al., 2000; Zougagh et al., 2005; Jerez Veguería et al., 2013), have been applied to extract trace metals from various matrices. However, chelating resins are well recognized as efficient SPE materials for selective matrix removal and improved preconcentrating capability (Garg et al., 1999; Gong, 2002; Chang et al., 2001). They are superior in selectivity when compared with solvent extraction and other sorbents, such as ion exchange, due to their intrinsic triple function of ion exchange, chelate formation, and physical adsorption (Chang et al., 2001; Wang et al., 2014; Tokalıoglu et al., 2009; Mashhadizadeh et al., 2008; Baraka et al., 2007).
In the most straightforward synthetic method for chelating resins, a chelating reagent, with favorite functionality (i.e., chelating center), is attached (immobilized) onto solid supports. Oxines and their derivatives are among the initial chelating reagents to be successfully utilized in the synthesis of chelating resins, due to their chelating chemistry characteristics and preference for transition and heavy metal cations over alkaline earth cations (Crompton, 2003). Their immobilization on natural polymers (e.g., cellulose) (Zih-Perenyi et al., 1998; Gurnani et al., 2003), organic polymers (e.g., polystyrenes) (Kan, 1996; Wen, 2002) and inorganic polymers (e.g., silica) (Dierssen and Landing, 2001; Shahata, 2016) are popular; however, the use of other materials, such as magnetic multi-walled carbon nanotubes (MMWCNT) (Madannejad et al., 2016) and bentonite clay (Wang et al., 2012), also reported. Among these materials, silica and porous glass supports are favorable, due to their high surface area, reactivity, mechanical rigidity, and straightforwardness of functionalization (Crompton, 2003).
Over the last 40 years or so, various approaches to synthesizing chelating resins by chemically attaching of oxine moieties onto silica materials have been described (Hill, 1973; Jezorek and Freiser, 1979; Goswami et al., 2003; Lan and Yang, 1994; Wang et al., 2006). However, the diazo coupling onto aminated silica, following a method developed originally by Hill in 1974 for the immobilization of enzymes, is a conventional, preferable chemical transformation route for synthesizing these chelating resins (Hill, 1973; Jezorek and Freiser, 1979; Willie et al., 1998; AlSuhaimi and McCready, 2012; Lofthouse et al., 1999; Rao and Gladis, 2001).
Most of the reported immobilization methods for the chemical modification of silica with oxines, however enable the attachment of a single chelating moiety per spacer arm; thus, the obtained capacity is usually similar. In this work, the conventional Hill’s functionalization procedure based on amination and diazotization coupling (Hill, 1973) was improved for the first time to synthesize silica-based chelating resins encompassing two oxine moieties per aminopropyl spacer arm. The proposed resin is expected to demonstrate an enhanced capacity exchange over the classical ones in which a single moiety is usually attached per linkage arm.
2 Materials and methods
2.1 Chemical reagents
All reagents used in the immobilization or analytical process were of analytical grade. Solid support silica gel (mesh size 70–230, surface area 300 m3/g) and sodium nitrite (NaNO2) were purchased from Lobal Cheme (Gujarat, India), silylation reagent 3-aminopropyltriethoxysilane was obtained from Acros Organics (Geel, Belgium), hydrochloric acid, anhydrous toluene and ethanol from Scharlau (Barcelona, Spain), and super purity nitric acid from Chem-Lab NV (Zedelgem, Belgium). Element stock solutions (1 mg/ml; Acros Organics) were used in the preparation of standard solutions of the studied metals. Ammonium acetate, aqueous ammonium oxide solution, and acetic acid from Fisher Scientific (Waltham, MA, USA) were used to adjust the pH of solutions. The solutions passed through column packed with Chelex-100 (BioRad, California, USA) for purification. High-purity water was used throughout.
All glassware was thoroughly cleaned with Fairy® liquid detergent and soaked overnight in 10% HNO3. Immediately before use, all acid-soaked glasses were rinsed with high-purity water.
2.2 Instrumentation and measurements
Elemental analysis was performed with a CHNSO 2400 instrument, Perkin Elmer (Waltham, MA, USA) and FT IR spectra were obtained with a Fourier transform infrared instrument, Shimadzu 8300-FTIR (Kyoto, Japan Japan). Surface morphology study carried out using a scanning electron microscope (SEM ssx 550, Shimadzu, Kyoto, Japan). An inductively coupled plasma mass spectrometer, ICP-MS 7500 series, Agilent Technologies (Santa Clara, California, United States) used for metal ion determination; instrument parameters were those of the manufacturer. pH measurements performed using a Basis pH meter, Denver Instrument Company (Bohemia, NY, USA).
2.3 Preparation of chelating resin
The chelating resin referred to as dioxin-silica chelating resin and symbolized in this work as Si(—HQ)2 was prepared as follows: 20 g of silica gel (denoted as Si—OH) was gently heated at 80 °C in a mixture of 5 ml of 2 M hydrogen peroxide solution, 5 mL of 2 M ammonium oxide solution, and 50 ml of water for about 30 min. The silica gel was filtered and washed with distilled water over a Büchner funnel until the filtrate attained pH 7, and then dried in an oven at 100 °C for three hours. The dried beads were silanized with 2.5 ml of 2% 3-aminopropyltriethoxysilane solution prepared using 50 mL of anhydrous toluene for four hours at room temperature. The aminated silica beads (identified as Si—NH2) were then filtered and washed with toluene and alcohol before being dried at 70 °C in an oven overnight to ensure complete curing. The cured silica gel beads were left to cool at room temperature and then reacted with 3,5-dinitrobenzoyl chloride dissolved in acetonitrile and 2.5 mL triethylamine. The resultant product was filtered, washed, and dried under vacuum before being treated with 5% sodium dithionite in 20 mL of water for 24 h. After that, the resultant product was filtered, washed, and dried, then treated with 2% NaNO2 in 1 M acetic acid at 0–5 °C for 30 min. This reaction resulted in the formation of diazonium salt–silica gel, which was quickly filtered; washed with three 10 mL portions of cold, distilled water; and added to 10 ml of a 2% solution of 8-HQ in cold ethanol. The appearance of a deep red color indicated the formation of the azo-coupled 8-HQ. This coupling reaction was carried out for 30 min, after which the silica gel-immobilized 8-HQ was filtered and washed sequentially with ethanol, 0.1 M HCl, and water. This material was then allowed to dry over a Büchner funnel for two hours and stored in a desiccator for subsequent use.
2.4 Extraction study of the resin
2.4.1 Batch method
The most significant parameters affecting resin performance; pH, loading capacity, and kinetic of sorption were evaluated using the static batch method as described in the literature (Willie et al., 1998; AlSuhaimi and McCready, 2012). Briefly, a suspension of 50 mg of the dry chelated resin in 10 mL of metal solutions containing 3 mg/L of the investigated metals was mechanically stirred at room temperature. After extraction, the solid phase was separated by centrifugation at 3500 rpm for five minutes. The residual metal concentration of the supernatant was measured versus the original concentration using ICP-MS. All experiments were performed in triplicate.
2.4.2 Solid phase extraction manifold and procedure
A 12-way standard SPE manifold (Ato Science, China) was used for the solid-phase extraction process. The SPE cartridges (bond straight barrel; Agilent Technologies) were packed with 200–300 mg of the washed resin to make discs 5–10 mm in height between two porous Teflon filters. The manifold was operated with a vacuum pump (AP-9950; Ato Science, China). For the SPE process, the SPE cartridges were conditioned with a buffer solution passing at a flow rate of 0.5 mL/min, and then the samples were loaded at 0.5 ml/min. The cartridges were flushed with water to remove any trapped metals and matrices, and then the chelated metals were eluted with 5 ml of 2 M nitric acid at 2 ml/min. The eluted metals were collected into 10 ml PTFE sample tubes and analyzed using ICP-MS. This procedure was followed for all standards and the real samples.
2.4.3 Collection and analysis of sea water samples
Seawater samples were collected from different sampling sites within the harbor near Yanbu desalination plants from surface water (top 1 m depth) in 1 L acid cleaned polyethylene bottles. The samples were filtered in site, transported into ice boxes to the laboratory and processed using SPE manifold as detailed in Section 2.4.2.
3 Results and discussion
3.1 Resin synthesis and characterization
The chemical transformation developed to synthesize Si—(HQ)2 resin is shown schematically in Fig. 1. Briefly, in the first step, 3-aminopropyltriethoxysilane was used to aminate the silica gel surface flowing the typical silylation reaction in dry toluene. The second step in the sequence used to couple 3,5-dinitrobenzoyl chloride to the aminated silica to attach two nitro groups. The nitro groups were reduced into amino groups in the third step, to allow the subsequent diazotization coupling of two oxine (HQ) moieties on a single spacer arm. The completion of synthesis can be recognized visually by the appearance of prominent deep red color of the final product.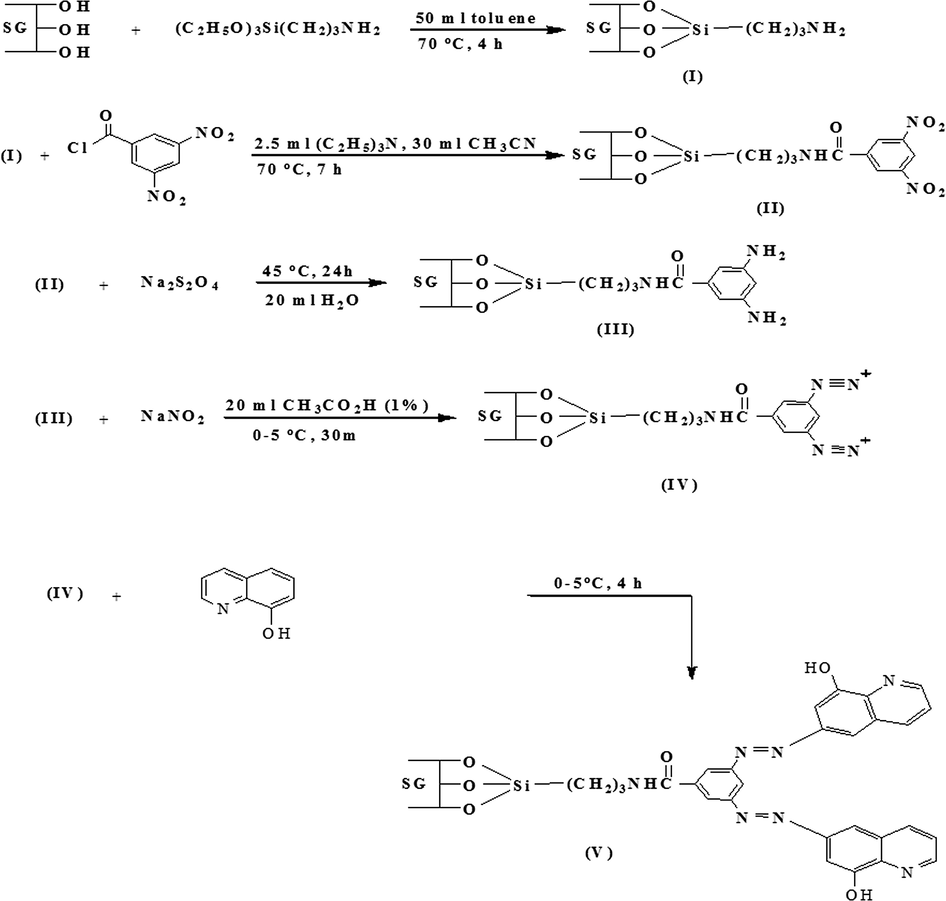
Reaction scheme for chelating resin synthesis.
3.1.1 FTIR spectrum
Infrared absorption used to characterize the resins. The FT-IR spectra of Si—NH2, and Si—(HQ)2 are shown in Fig. 2. Strong adsorption peak at about 1093.1 cm−1 was observed in both samples, confirming the presence of Si—O—Si stretching vibration of silanol groups. The existing of aromatic CH2 groups in Si—(HQ)2 were confirmed by —C—H scissoring vibration at 1384.53 cm−1, whereas the distinguished —O—H bending vibrations could be detected at 3600–3300 cm−1 in both spectra. However, these strengthened peaks mainly from silica support. The common characteristics of Si—NH2 and Si—(HQ)2 due to N—H bond is recognizable around 1636.12 cm−1. The week peak at 2400 cm−1 observed Si—(HQ)2 spectrum corresponded to C—N stretching. Because the mass of resin attached to the silica surface compared with the mass of silica beads is very minute, the intensities of all peaks in the IR spectrum are quite low.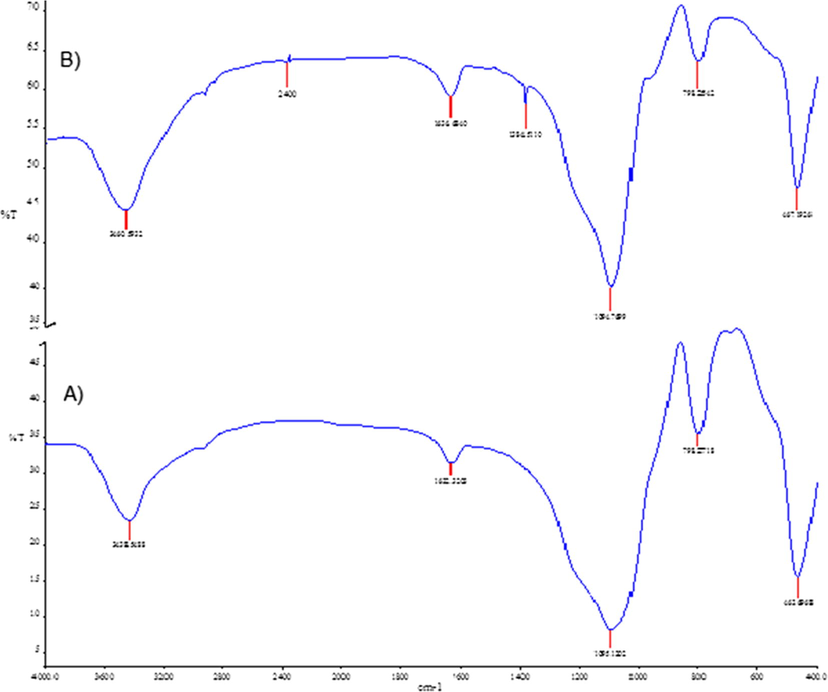
FTIR spectrum of (A) aminated silica Si—NH2, and (B) chelating resin Si—(HQ)2.
3.1.2 CHN
The CHN elemental analysis is a convenient mean to determine ratios of carbon, hydrogen and nitrogen in organic matrices. It has been used confirmed the success of the immobilization by comparing the C/N ratio. As shown in Table 1, the ratio calculated (estimated) from the formula weight was about 3.4, and that obtained from the elemental analysis was found to be 3.31. The insignificant variation in ratio is expected, because the yield of such reactions cannot reach 100% and ethoxy groups in 3-aminotriethoxysilane are not included in the calculated formula weight, as the number of the hydrolyzed or remainder groups are not definite. However, these comparable values confirm the attainment of chelator immobilization. The capacity exchange results (Section 3.2) support these findings.
Calculated (F. Wt)
Obtained (C, N analysis)
Ratio C/N
C
66.66
19.43
3.40
N
2.68
0. 81
3.31
3.1.3 SEM
Scanning electron microscope (SEM) which utilizes a focused beam of electrons to produces images of a sample is a reliable and convenient tool to study resin surface topography and composition (Saxena and Meena, 2014; Cheira, 2015).
The micrographs (Fig. 3) clearly reveals that the plain silica had a smooth and even surface, while the modified silica (chelating resin) shows relatively dense microstructures and rough surface. This confirms the chemical attachment of ligand onto the silica surface. Clearly, the substrate surface altered because of functionalization with 8-hydroxyquinoline moieties.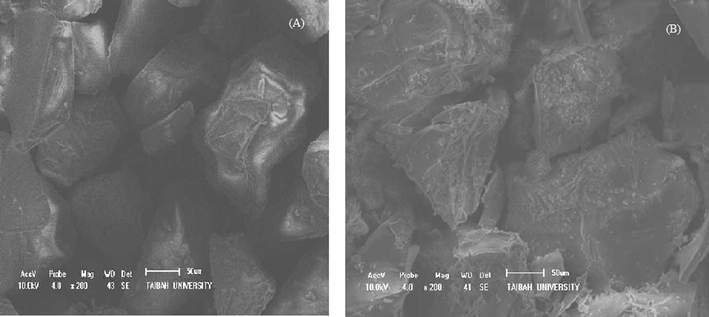
SEM images of (A) plain silica, and (B) chelating resin Si—(HQ)2.
3.2 Resin exchange capacity
The exchange capacity of a resin is an important factor in determining the mass of the resin needed to achieve quantitative extraction/preconcentration of the elements from a given sample solution. The sorption capacity of Si—(HQ)2 resin was determined for Cu and Mn individually in batch approach using 50 mg of dry chelating resin and 10 mL of 50 mg/L solution of these ions prepared in ammonium acetate solution (0.1 M, pH 6). The difference in concentration (before/after) used to calculate the capacity using Eq. (1) (Willie et al., 1998; AlSuhaimi and McCready, 2012).
Resin
Cu
Mn
Refs.
8-HQ immobilized onto silica monoliths
–
0.136
Lofthouse et al. (1999)
8-HQ immobilized onto controlled pore glass
0.106
0.098
AlSuhaimi and McCready (2012)
8-HQ immobilized onto controlled pore glass
–
0.086
Al-Jibouri et al. (2013)
8-HQ immobilized onto porous silica gel
0.060
–
Nelms et al. (1995)
8-HQ immobilized onto porous silica gel
0.185
–
Sturgeon et al. (1981)
8-HQ immobilized onto porous silica gel
0.219
0.161
Current work
3.3 Effect of pH on the retention of the elements
The pH of medium is an important variable in achieving quantitative extraction of elements, as it affects the extent of complexation, which in turn determines the percentage of metal retained by the resin. Commonly, the relative amount of metal taken up by resin increases as a function of the pH of solution. In this study, the influence of solution pH investigated using 0.05 M ammonium acetate solution in pH range from 2 to 8 (AlSuhaimi and McCready,2012; Lofthouse et al., 1999). The recovered percentage of the selected metals plotted vis. pH presented in Fig. 4. The general view of this figure agrees with the above observation. Noticeably, all the metal retained by the resins quantitatively in the 5–6 pH range. The retention ratios ranged from 80% for Ni and 100% for Cu and Zn. The percentages for the other metals were 94%, 87%, and 87% for Cd, Pb, and Co, respectively. The data indicated that the best operating pH under the seawater matrix for these elements was around 5.5, which also provided the highest recoveries. However, the sorption of major matrix ions, such as Ba, Ca, and Mg, on the SPE cartridge at this pH might encountered. Accordingly, it is considered that the optimum pH for the SPE of these metals was 5.0.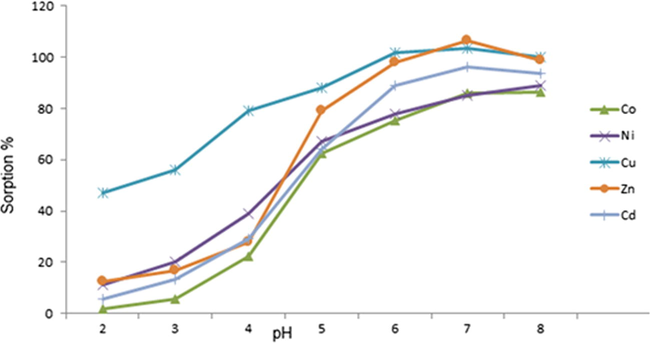
Effect of pH on the retention of elements.
Remarkably, these results cannot be compared with the results of Willie et al. (1998), who reported poor recovery results at pH 5.3 using 8-hydroxyquinoline immobilized onto silicone tubing. However, it is in consistent with finding stated by Zih-Perenyi et al. (1998), for Cd, Co, Ni, and Pb ions (near 100% at pH 5) using 8-hydroxyquinoline-5-sulphonic acid cellulose (sulphoxine cellulose) chelating resin.
3.4 Kinetics of metal sorption
The kinetics of metal sorption is one of the important parameters for the quantitative retention of analytes in SPE studies. Therefore, the sorption of metal ions as a function of time was studied using the batch method. The resins were shaken (using an electrical shaker) with 50 ml of a metal ion solution containing 5 mg/L for different time intervals (2, 5, 10, 15, 30, and 60 min). The concentration of metal ions loaded onto the matrix and present in the supernatant solution was quantified by ICP-MS after appropriate dilution and acidification. The profile of the sorption of the investigated metal ions as a function of time is shown in Fig. 5. It is usually observed that the adsorption of metal ions increased as a function of shaking time (Wang et al., 2014). An equilibration time of 10 min was found to be sufficient for Co, Ni, Pb, and Zn to reach 50% of the maximum sorption on the chelating resin, while Cu, Cd, and Pb attained 78, 42, and 39% of the maximum, respectively, during this time. An equilibration time of 15 min was effective for maximum sorption of Cu, Co, and Zn, whereas 20 min equilibration was enough to attain 90% of the maximum sorption of Pb, Cd, and Ni.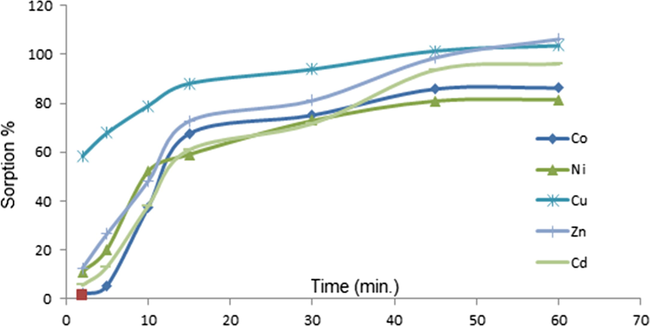
Kinetics of metal sorption.
3.5 Accuracy of the method
The chelating resin was packed in the standard cartridges and used for sample pretreatment with regular SPE manifold. This simple approach has allowed simultaneous processing for multi samples and is ideal for field work. The method accuracy was examined using seawater reference materials (CRM), CASS-5 and NASS-6. Ammonium acetate solution (2 M) was used to adjust the pH (buffer) of the standards and the real sample immediately before their passage through the cartridge. The calibration standards were prepared in aqueous solution of 1% nitric acid, covering the dynamic range shown in Table 3, and used to calculate the concentration of targeted metals in the real samples (seawater reference materials and real samples). Table 4 shows the concentration (recovery values) of the analyzed metals in reference materials together with the certified values. Apart from the Ni ion (recovery 83%) in reference sample NASS-6, all the analyzed metals were quantified with agreeable results.
Calibration parameters
Metal ions (μg/l)
Co
Cu
Cd
Ni
Zn
Concentration range (μg/L)
Blank-5
Blank-25
Blank-2
Blank-5
Blank-30
% RSD at 1 ng/ml (n = 4)
1.53
1.35
1.11
1.75
1.21
Correlation coefficient, R2
0.997
0.997
0.998
0.991
0.995
Sensitivity, CPS ratio/(μg/L)
0.83
0. 86
1.42
1.01
0.71
LOD (μg/L)
0.01
0.03
0.007
0.04
0.05
CASS-5
NASS-6
Found
Certified
Found
Certified
Co
0.097 ± 0.05
0.095 ± 0.021
0.018 ± 0.003
0.015
(102%)
(120%)
Cu
0.41 ± 0.03
0.380 ± 0.028
0.243 ± 0.06
0.248 ± 0.025
(108%)
(98%)
Ni
0.324 ± 0.021
0.330 ± 0.023
0.298 ± 0.074
0.301 ± 0.025
(98.2%)
(83.3%)
Zn
0.708 ± 0.061
0.719 ± 0. 068
0.262 ± 0.031
0.257 ± 0.020
(98.5%)
(102%)
Cd
0.0209 ± 0.015
0.0215 ± 0.001
0.0313 ± 0.003
0.0311 ± 0.001
(97.2%)
(100.2%)
3.6 Resin stability and reusability
To evaluate resin stability its sorption capacity was determined after treated with acidic and alkaline solutions of different concentrations. It was found that the sorption capacity for chelating resin treated with nitric acid (2 M HNO3) was comparable with the value for the untreated one; the maximum variation is below 3%. However, upon suspension in more concentrated acid (4 M HNO3), a light red color was observed in the solution, which could be ascribed to the cleavage of the organic moieties together with the linkage arm or due the silica dissolution in the strong acidic medium. The resin sorption capacity was reduced by around 12% after such treatment. Similarly, when the material was placed in an alkaline solution with pH above 10, an orange color was observed in the solution, which can be attributed to the effect of alkaline solutions on silica. To test reusability, the resin exchange capacity for the metal ions was determined after 50 loading and elution cycles without a noticeable capacity change; therefore, it can be reused many times. In addition, the sorption capacity of resin did not show a significant change upon stored in desiccators for more than one year.
3.7 Method application for seawater samples
The SPE procedure using the synthesized chelating resin was applied for the cleaning up of seawater samples prior to the analysis of transition metal ions by ICPMS. This part of the study also aimed to evaluate the effect of desalination activities on seawater within the harbor near Yanbu desalination plant, Red Sea Saudi Arabia. The map in Fig. 6 shows location of samples. The samples were contained high levels of salts (i.e., salinity 41.10–43.50 mg/l) and elevated amounts of organic components originate from petrochemical industry plants and seaport due to the heavy traffic of ships and oil tankers nearby. The concentration of the investigated metal ions is represented in Table 5. Obviously, the levels of all ions of concern within the desalination plant zones; S1 to S3, are relatively higher than the level detected in reference site; S4. The discharge site, S1 has the highest level in comparison to uptake and the mid sites; S2 and S3. Fig. 7 demonstrates a graphical comparison for the level of the investigated ions in each sampling site. This finding reflects the potential influence of dilution factor in seawater. However, a sufficient predilution for brine prior to its discharge (Falkenberg and Styan, 2015) and regular investigation for the trace levels of heavy metals, as well as other chemicals in seawater around desalination plants and prohibition of fishing in these areas are recommended. ■(Sample number). The symbols S1, S2 and S3 assigned for discharge, uptake and mid sites, and S4 referred to reference site outside plant zone.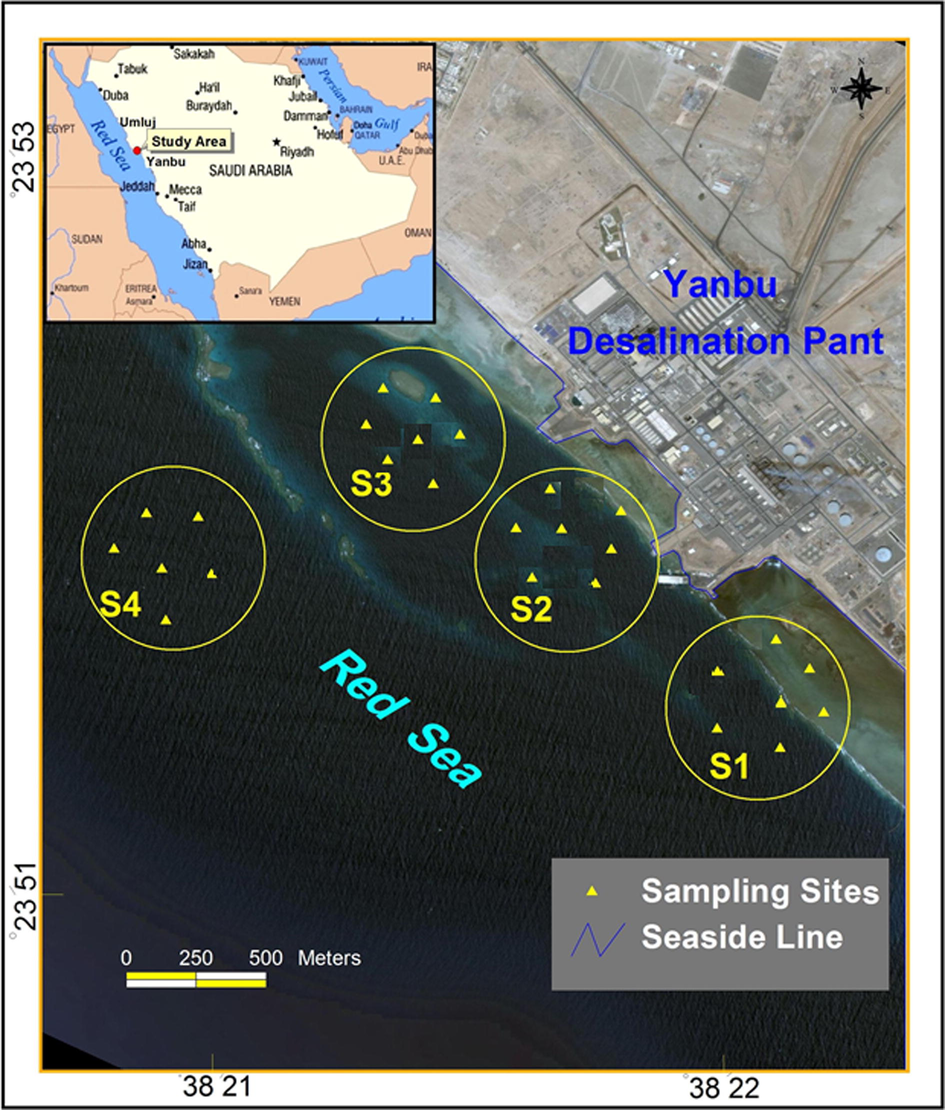
Sampling sites near yanbu desalination plant; S1 is the discharge site, S2 intermediate zone, S3 is the uptake site for plant and S4 is control site outside plant zone.
Sampling sites
Elements
Co
Cu
Ni
Zn
Cd
S1■ (7)
0.34
19.91
1.19
28.42
0.094
S2 (7)
0.25
17.84
1.00
15.53
0.087
S3 (7)
0.19
17.61
0.84
17.312
0.067
S4 (6)
0.13
13.92
0.76
12.03
0.052
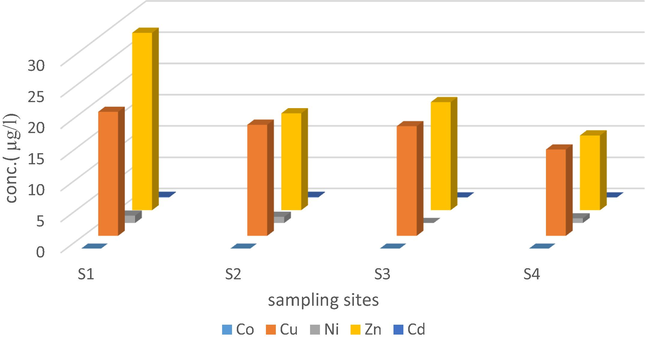
Comparison of metals concentration in the sampling sites in the Desalination Plant harbor, Yanbu.
4 Conclusion
The modified chemical functionalization procedure adapted in this study enabled the attachment of two oxine moieties onto single aminopropyltriethoxy spacer arm grafted onto silica surfaces. FTIR and elemental analysis confirmed the success of resin synthesis. The capacity exchange test showed that the produced resin has higher sorption capability compared with the reported values for most similar resins. In addition, the resin exhibited excellent performance as an SPE material for sample preparation of trace metals in seawater reference materials prior to ICP–MS quantification. The accuracy of the method and its application to seawater sample proved that the method is appropriate for matrix removal in the analysis of difficult environmental samples such as high saline water. The results of metals analysis in seawater samples within the desalination plant zone have demonstrated that desalination industry might alter the levels of trace/metals in seawater, especially nearby brine discharge sites. Therefore, it is advised that sludge disposal policy may need to consider the occurrence of trace metals in brine and their levels should be included in the routine monitoring programs.
Acknowledgment
The authors are grateful for the Scientific Research Deanship, Taibah University for the generous financial support, grant No. 766/432.
References
- Sci. J. Chem.. 2013;1(4):38-49.
- Global NEST J.. 2012;14(1):55-65.
- Spectrochim. Acta. B. 2000;55(7):1101-1108.
- React. Funct. Polym.. 2007;67(7):585-600.
- Green sample preparation methods. In: de la Guardia M., Garrigues S., eds. Challenges in Green Analytical Chemistry. London: RSC; 2011. p. :63-75.
- [Google Scholar]
- Anal. Chim. Acta. 2001;450(1–2):231-238.
- J. Environ. Chem. Eng.. 2015;3:642-652.
- Preconcentration Techniques for Natural and Treated Waters: High Sensitivity Determination of Organic and Organometallic Compounds, Cations and Anions. London: Spon Press; 2003. p. :410-421.
- Microchem. J.. 2007;86(1):23-28.
- Mar. Chem.. 2001;73(3–4):173-192.
- Sample Preparation for Chromatographic Analysis of Environmental Samples. In: Nollet L.M.L., ed. Chromatographic Analysis of the Environment. NY: CRC; 2005. p. :31-39.
- [Google Scholar]
- Desalination. 2015;368:3-9.
- Analytical Solid-Phase Extraction. New York: Wiley-VCH; 1999. p. :11-24.
- Microchem. J.. 1999;61(2):94-114.
- Talanta. 2002;57:89-95.
- Talanta. 2003;60(6):1141-1154.
- Anal. Chim. Acta. 2003;485(2):221-232.
- J. Chromatogr. A. 1973;76(2):455-458.
- Microchem. J.. 2013;106:121-128.
- Anal. Chem.. 1979;51(3):366-373.
- Microchim. Acta. 2008;161(1–2):137-142.
- React. Funct. Polym.. 1996;31(3):207-218.
- Anal. Chim. Acta. 1994;287(1–2):101-109.
- J. Anal. Atom. Spectrom.. 1999;4:1839-1842.
- Int. J Environ. Anal. Chem.. 2016;96(6):595-607.
- Spectrochim. Acta B. 2008;63(8):885-888.
- Crit. Rev. Anal. Chem.. 2002;32(4):271-300.
- J. Anal. Atom. Spectrum.. 1995;10:929-933.
- Int. J. Environ. Anal. Chem.. 2010;90(14–15):1048-1062.
- Rev. Anal. Chem.. 2001;20(2):145-159.
- RSC Adv.. 2014;2014(4):20216-20225.
- J. Arab. Chem.. 2016;9(6):755-763.
- Hazard. Mater.. 2009;163(1):295-301.
- Anal. Chem.. 1981;53(14):2337-2340.
- J. Braz. Chem. Soc.. 2006;17(1):98-106.
- Anal. Chim. Acta. 2004;511:255-260.
- J. Hazard. Mater.. 2009;169(1–3):593-598.
- J. Environ. Anal. Toxicol.. 2012;2(7)
- [CrossRef]
- Chin. J. Anal. Chem.. 2006;34(4):459-462.
- Talanta. 2014;128:337-344.
- Talanta. 2002;56(4):681-687.
- Talanta. 1998;47(2):439-445.
- Microchim. Acta.. 2006;154:253-259.
- Talanta. 1998;47(3):673-679.
- Anal. Bioanal. Chem.. 2005;381:1103-1113.







