Translate this page into:
Silica-supported Schiff-based palladium nanocatalyzed cross-coupling reactions and its applications in the synthesis of antiplatelet and fungicidal
⁎Corresponding author. sunxianke666@163.com (Xianke Sun)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The readily available silica gel was converted into an amino functionalized silica by refluxing with 3-aminopropylthiethoxy silane. The amino functionalized silica was transformed into the respective Schiff-based ligand by the incorporation of [2,2′-bithiophene]-5,5′-dicarbaldehyde. The Pd(0) nanocatalyst on the silica gel support was developed by further processing the Schiff-based ligand with ammonium tetrachloropalladate and hydrazine. The Schiff-based Pd(0) nanocatalyst was extensively characterized using a range of spectroscopic techniques, including Raman, SEM, EDX, TEM, XRD, ICP, and XPS. Raman spectroscopy was used to confirm that the organic moieties have been successfully incorporated, and XPS, XRD, and EDX analyses supported the existence of palladium species on the silica surface. According to the TEM analysis, Pd-nanocomplex with a size distribution of 5.3 ± 0.6 nm was found to be evenly distributed on the silica surface. Palladium was incorporated into the silica surface at a rate of 1.9 w%, according to ICP and EDX analysis. The Mizoroki-Heck and Suzuki-Miyaura coupling reactions were successfully catalyzed by the Schiff-based Pd(0) catalyst using a diverse range of aryl halides, olefines, and organoboronic acids. High yields were achieved in each example in the case for the coupling products. Despite being recovered from the reaction medium and used multiple times, the catalyst still maintained the majority of its catalytic activity. Furthermore, this Schiff-based Pd(0) catalyst was used to produce biologically active molecules of Ozagrel, an antiplatelet agent and fungicidal Boscalid with high yield.
Keywords
Silica gel
Bithiophene-5,5′-dicarboxaldehyde
Palladium
Schiff-base
Heck reaction
Suzuki reaction
1 Introduction
The use of cross-coupling reactions that are catalyzed by transition metals is one of the greatest effective and efficient tools for the synthesis of natural products, industrial applications, functional materials, fine chemicals, pharmaceuticals, material science, and agrochemicals (Zhao et al., 2021; Castillo and Buchwald, 2016). Since of their relatively mild reaction conditions, the Suzuki-Miyaura (Suzuki) and Mizoroki-Heck (Heck) coupling reactions are considered as the most flexible and broadly used functional group tolerance reactions for the construction of C–C bonds (Fihri et al., 2011; Han, 2013; Sarkar et al., 2015; Ashraf et al., 2023). The most efficient Mizoroki-Heck and Suzuki-Miyaura reactions employed phosphine-based auxiliary ligands and homogeneous Pd-complex catalysts in organic solvents (Beletskaya et al., 2019; Beletskaya and Cheprakov, 2000; Chinchilla and Nájera, 2011). However, these catalysts suffer from inherent toxicity, high cost, the need for post-processing to extract ligands, thermal sensitivity, limited catalyst recycling (Shende et al., 2022), and expensive downstream metal isolation (Schnoor et al., 2019). Moreover, the use of hazardous solvents, complex synthesis procedures, air sensitivity during handling, and protracted chromatographic purification for product purity have posed significant challenges in these reactions. These setbacks and failures highlight the need for alternative approaches to overcome the drawbacks associated with homogeneous Pd catalysts and optimize the efficiency, cost-effectiveness, and sustainability of Mizoroki-Heck and Suzuki-Miyaura reactions. Therefore, homogeneous Pd catalysts are not suitable for industrial use (Das and Linert, 2016; Torborg and Beller, 2009). Traditional homogeneous catalysts suffer from a number of drawbacks, such as weak metal bonding, inconsistency, functional group intolerance, and efficiency. Sometimes they need extremely high operating temperatures in addition to harsh operating conditions. The challenging problem of “greening up” the reaction method in modern organic synthesis has recently gained prominence. In this regard, numerous cross-coupling reactions have been conducted under favorable conditions, including quick reaction times, environmentally friendly solvents, and room temperature. The addition of external bases, ligands, additives, or promoters, on the other hand, reduces the ability of cross-coupling reactions to green up.
A fascinating solution to these issues involves immobilizing metal catalysts on solid supports, which combines the advantages of homogeneous metal catalysts and solid supports (Fatahi et al., 2021; Hong et al., 2020; Ahadi et al., 2019; Algarou et al., 2020). Due to their high surface area and excellent reactivity, which significantly improve the interaction between the reactant and the metal's catalytic active sites, metal nanoparticles have attracted a lot of attention in both the academic and industrial worlds (Liu and Corma, 2018). Due to its outstanding catalytic activity, wide substrate range, and simplicity of handling, heterogeneous metal catalysis has recently gained a great deal of attention (Varma and Sustain, 2016). For long-term sustainable practices and economic viability, heterogeneous metal-based transformation reactions are viewed as a safer and more ecologically responsible approach (Ganesh and Ramakrishna, 2020). Heterogeneous catalysts based on transition metals (Au, Pt, Cu, Pd, and their alloys) enable efficient cross-coupling with diverse substrates, leading to enhanced catalytic performance and productivity (Herget et al., 2017). Nanomaterials in the small size range exhibit augmented surface area and atoms, leading to enhanced metal coordination ability and ligand binding capacity, making them promising candidates for diverse applications (Deng et al., 2016; Garcia et al., 2010).
Heterogeneous metal catalysts have numerous uses in industry, electronics, and pharmaceutical chemistry as a result, and they have shown promise as alternatives to traditional homogeneous metal complexes (Elias et al., 2017; Song et al., 2012; Thomas et al., 2014; Nasrollahzadeh et al., 2019). The use of heterogeneous metal catalyzed Suzuki and Heck reactions in particular has increased significantly over the past ten years. It is necessary to address the problems of their poor selectivity, the necessity of harsh process conditions, the draining of metal catalyst from the solid support, and the emergence of side products (Molnár, 2011). The controversy over whether the catalyst in the heterogeneous pathway or the metal species leached in the homogeneous pathway is actually in charge of the catalysis process needs to be given more consideration. To overcome these drawbacks of the homogenous transition metals catalysts, it is still challenging to find effective pseudo-homogeneous nanocatalysts with better performance (Nasrollahzadeh et al., 2019; Yang et al., 2013). In order to prevent metal agglomeration and maintain their coherent distribution, researchers are looking for effective solid supported heterogeneous catalysts that increase surface area while delivering superior performance, provide a significant number of active catalytic centers, and increase surface area (Li et al., 2013; Li et al., 2012; Sun et al., 2013). The introduction of a new catalyst that is more effective, environmentally friendly, simple to use, and reliable has received a lot of attention in recent years. This catalyst is made by placing metal catalyst on a solid support. For the progression of stabilized palladium-based nanocatalysts, for instance, carbon-based materials (Kamal et al., 2012; Shekarizadeh and Azadi, 2020), cellulose (Sarkar et al., 2017; Sarkar et al., 2015), polymers (Sato et al., 2015), silica (Sarkar et al., 2015), magnetic Fe3O4 (Sutar et al., 2023; Hafizi et al., 2022), MOF (Luo et al., 2019; Rostamnia et al., 2016), carbon nanotubes (Luo et al., 2021), Pd-NHCs complex in MIL-101 (Cr) (Niknam et al., 2021), composite materials (Tubio et al., 2023), and fiber (Sultana et al., 2016) were investigated. They are however constrained by issues like high cost, the use of hazardous solvents during the preparation of the catalyst, and high chemical consumption.
This study describes a Schiff-based palladium bithiophene-5,5′-dicarboxaldehyde derivative catalyst supported on silica gel that effectively promoted the Heck and Suzuki reaction in mild reaction conditions. The catalyst exhibited a high efficacy in producing up to 95% of the desired cross-coupling products and retained its catalytic activity even after being recycled up to seven times. Additionally, this catalyst supported by silica gel was used to produce biologically active molecules Boscalid and Ozagrel which are known as a potent fungicides and antiplatelet agent respectively.
2 Experimental
2.1 General
Ammonium tetrachloropalladate(II) [(NH4)2PdCl4] was acquired from Aldrich Chemical company. The proton NMR (500 MHz) and carbon 13 NMR (125 MHz) spectra were recorded using a BRUKER-500 spectrometer. Chemical shifts were referenced to tetramethylsilane (TMS) at 0.00 ppm for both proton and carbon 13 NMR, with deuterated chloroform (77.0 ppm) present as the solvent. The ICP-AES analysis was performed using Shimadzu ICPS-8100 equipment. Raman spectra were recorded using a LabRAM HR Evolution spectrometer, HORIBA, while XPS (X-ray photoelectron spectroscopy) measurements were performed using the Kratos AXIS ULTRA spectrometer (X-ray Microprobe PHI Quantera II) and an Al Ka1486.58 eV monochromatic X-ray source at 300 W (20 mA, 15 kV). For imaging purposes, the FE-SEM images were captured using JSM7800F, and TEM images with HT-7700 from Hi-Tech Instruments. Additionally, column chromatography was carried out using Wakogel C-200 silica gel.
2.2 Modification of silica gel surface with 3-aminopropyl-trimethoxysilane (3-APTS)
The 3-aminopropyl-functionalized silica 1 was obtained by refluxing of 6 g silica gel (60–100 mesh, heated at 130 °C for 6 h) and 4.2 g of 3-aminopropyl-trimethoxysilane in dry toluene (80 ml) for 24 h under nitrogen atmosphere. After being filtered through a glass filtration system, the solid product was repeatedly cleaned with absolute ethanol before being dried under vacuum for 6 h at 80 °C. The resulted amino functionalyzed silica 1 was characterized by Raman spectroscopy analysis. The weight gain analysis revealed that the silica gel 1 had an amino group concentration of 0.56 mmol/g.
2.3 Modification of 3-APTS group into a Schiff base chelating ligand 2
A 150 ml flask was charged with 10 g (5.6 mmol) of amino functionalyzed 1, 2.723 g [2,2′-bithiophene]-5,5′-dicarbaldehyde (2.8 mmol) and 50 ml of toluene. Following that, the resulting mixture was refluxed for 24 h in a nitrogen atmosphere. The resulting light yellow silica gel solution showed that the appropriate Schiff base had formed on the silica gel surface (a bi-dentate ligand). Bi-dentate ligand 2 was obtained as a light yellow solid after the silica gel-supported Schiff base bidented ligand was filtered off, cleaned with methanol, and dried for 4 h at 80 °C. The elemental analysis and weight gain analysis showed that 0.25 mmol/g of thiophene based bi-dentate chelating group was incorporated onto the silica gel supported 2.
2.4 Preparation of silica gel supported Schiff base palladium nanocatalyst 3
A stirred suspension of the thiophene-based bi-dented ligand 2 (3 g in 20 ml of methanol) was slowly transferred to an aqueous solution of (NH4)2PdCl4 (0.25 g in 20 ml of water). The pale yellow ligand 2 quickly changed into the light brown Pd(II) complex. The light brown solid turned dark brown when 0.5 ml of hydrazine hydrate (NH2NH2·H2O) was introduced to the suspension and the mixture was continued to stir for an additional hour. The Schiff base Pd(II) complex was reduced into Pd(0) in presence of NH2NH2·H2O (Khan et al., 2014). The Schiff base Pd(0) catalyst 3 was obtained after filtration and cleaning with acetone, water, and methanol. The dark brown silica gel supported Schiff base catalyst 3 was dried at 50 °C for four hours before being packed under nitrogen atmosphere. The XPS and EDX analysis of 3 suggested that 4.9 wt% of palladium was incorporated onto the Schiff base catalyst 3.
2.5 Cross-coupling reaction
2.5.1 Mizoroki-Heck reaction
A screwcap 10 ml glass-vial was filled with 10 mg of 3 (0.0173 mg of Pd, 0.0173 mol%), aryl halide (1 mol equiv.), olefine (1.5 mol equiv.), triethylamine (Et3N) (3.2 mmol mol equiv.), and 2 ml of dimethylformamide (DMF). The glass-vial was heated to 125 °C for 7 h, and the reaction progress and forming of the coupling product was constantly monitored using GC–MS. Once the reaction was completed, the reaction vial was bring-down at room temperature, diluted with ethyl acetate, and centrifuged to separate the solid catalyst 3. The organic phase was rinsed with saturated brine solution, dried over MgSO4, and the solvents were extracted by a rota-evaporator. Hexane and ethyl acetate (4:1) were used as the eluent during the short silica gel column chromatography process to purify the crude product.
2.5.2 Preparation of intermediate compound of ozagrel butyl ester 4z
The initial intermediate compound 4y was synthesized according to the Heck reaction. The second intermediate compound 4z was prepare by the mixing of 1 mmol of 4y and 2 mmol of carbonyldiimidazole in an anhydrous N-methylpyrrolidone (NMP) (15 ml) at 160 °C for 3 h. Following the complete conversion of 4y to 4z, the reaction mixture was diluted with ethyl acetate (EtOAc, 20 ml) and cooled to 25 °C. The organic layer was rinsed with brine (3 × 20 ml), dried over MgSO4, and evaporated using a rota-evaporator. The resulting ozagrel butyl ester 4z was purified using column chromatography in presence of CHCl3/MeOH (25/1) as an elution.
2.5.3 Preparation of ozagrel 5
(Khan et al., 2014) Ozagrel butyl ester 4z (300 mg), 0.80 M NaOH (2 ml), and MeOH (7 ml) were combined in a 20 ml round bottomed flask and the reaction mixture was stirred at room temperature for 3 h. The reaction mixture was then evaporated at a lower pressure, and the leftover material was then acidified with 1 M HCl (5 ml) solution. The residue was extracted with dichloromethane and evaporated under reduced pressure. The obtained solid ozagrel, was washed with diethyl ether to yield pure ozagrel with a 91% yield.
2.5.4 Suzuki-Miyaura reaction
10 mg (0.0173 mol% of Pd) of silica gel-supported Schiff base 3 was added into a 10 ml screw-cap glass vial along with 1 mmol of an aryl halide, 1.25 mol equiv. of an organoboronic acid, 3 mol equiv. of K2CO3, and 4 ml of aqueous ethanol (1/1). The mixture was warmed to 90 °C for 5 h while GC–MS analysis was used to continuously track the formation of biaryl. Once there was no longer an aryl halide peak present in the GC–MS spectrum, the reaction mixture was cool to room temperature before being diluted with water and ethyl acetate. After separating the ethyl acetate layer, it was dried and evaporated under low pressure. The resulting crude biaryl was purified using silica gel column chromatography with a hexane/ethyl acetate eluent (5:1 ratio).
2.5.5 Synthesis of Boscalid 7
According to the general Suzuki reaction procedure, the intermediate compound 6w was produced in 82% yield. The bioactive molecules Boscalid had been generated by reacting 2-amino-4-chlorobiphenyl 6w (406 mg, 2 mmol) and 2-chloronicotinoyl chloride (2 mmol) for 1 h at room temperature in the presence of 4 mol equiv. of Et3N in 6 ml of CH2Cl2. The reaction mixture was transferred into a separating funnel and diluted with water and dichloromethane after the reaction was completed. The dichloromethane layer was concentrated under reduced pressure and dried over MgSO4. The resulting crude Boscalid was purified using silica gel column chromatography to yield a colorless solid with an 86% yield.
3 Results and discussion
3.1 Ligand and catalyst characterization
The silica gel supported Schiff base palladium catalyst 3 was constructed by the surface modification of readily available silica gel. Scheme 1 depicts the steps involved in the synthesis of a Schiff base Pd(0) catalyst. An oven dried silica gel was treated with (3-aminopropyl)triethoxysilane (APTES) in the refluxing toluene to afford the amino functionalized silica gel 1 where 0.56 mmol/g of amino functionality is contained. To introduce thiophene bi-dented chelating ligand onto the amino functionalized 1, the [2,2′-bithiophene]-5,5′-dicarbaldehyde was heated with 1 yield the corresponding Schiff base bi-dented ligand 2. Then, we subjected 2 with an aqueous medium of ammoniumtetrachloropalladate at room temperature to produce a Schiff-based Pd(II) complex supported by silica gel. The resulting lite yellow Schiff-based Pd(II) complex was then addressed with an aqueous medium of NH2NH2·H2O to afford the dark brown solid powder of the corresponding silica gel supported Schiff base Pd(0) catalyst 3. The Schiff base catalyst 3 was characterized using various spectroscopic techniques, and the XPS and based on EDX analyses, the palladium content was 4.9 wt%.(See Scheme 2).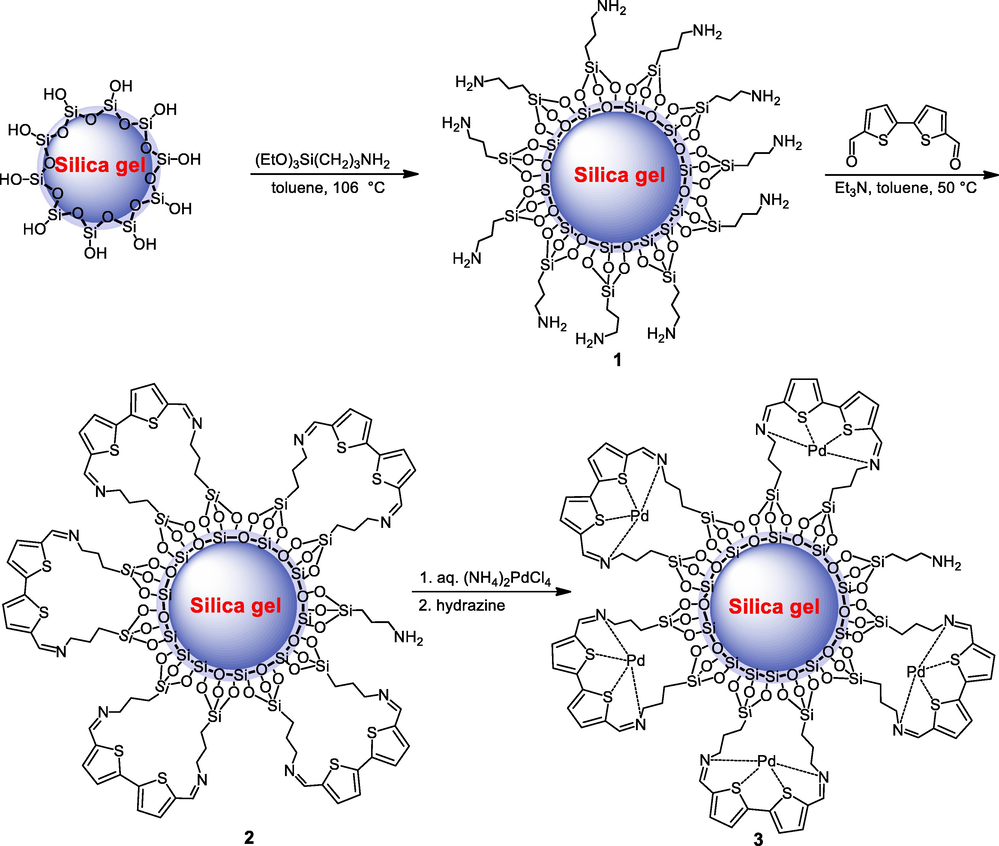
Synthesis of silica gel supported Schiff base Pd(0) 3 catalyst.
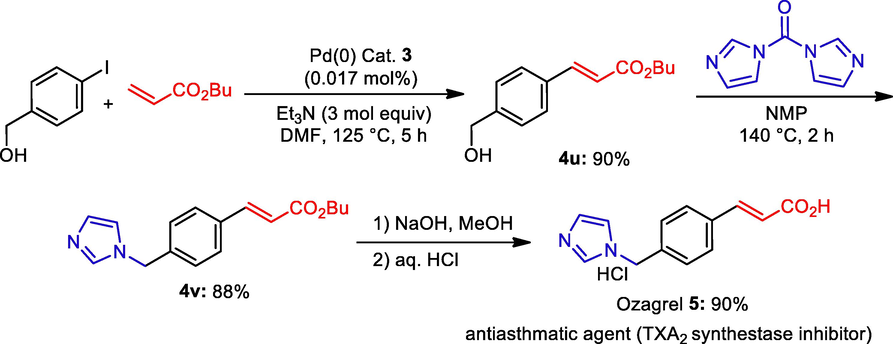
Synthesis of Ozagrel hydrochloride.
Peaks in the Raman spectra of 1 at 2904 cm−1, 2932 cm−1, 2988 cm−1, and 3056 cm−1 were associated with alkane carbon chain of sp3 C–H stretching (Fig. 1a). The N–H stretching distinct peaks were observed at 3318 cm−1 and 3381 cm−1, indicating the presence of the NH2 group in the sample. While, after the integration of thiophene chelating ligand onto 2 the Raman peaks were observed at 2902 cm−1, 2967 cm−1, and 3007 cm−1 and the N–H stretching peak was not observed owing to the formation of Schiff-base (Fig. 1b). Furthermore, the N = C stretching peaks at 3199 cm−1 and 3251 cm−1 were found due to the formation of an imine bond onto the 2. Furthermore, after complexation of palladium with the N and S atoms, the Raman peaks were observed in the direction of the lower wave number for both aliphatic sp3 C–H at 2849 cm−1, 2888 cm−1, 3014 cm−1, 3028 cm−1 and the C = N stretching band were observed at 3147 cm−1, 3184 cm−1, 3201 cm−1, 3223 cm−1 due to the pushing of none-pair electrons on nitrogen and sulfur atoms toward the vacant d-orbital of palladium (Fig. 1c).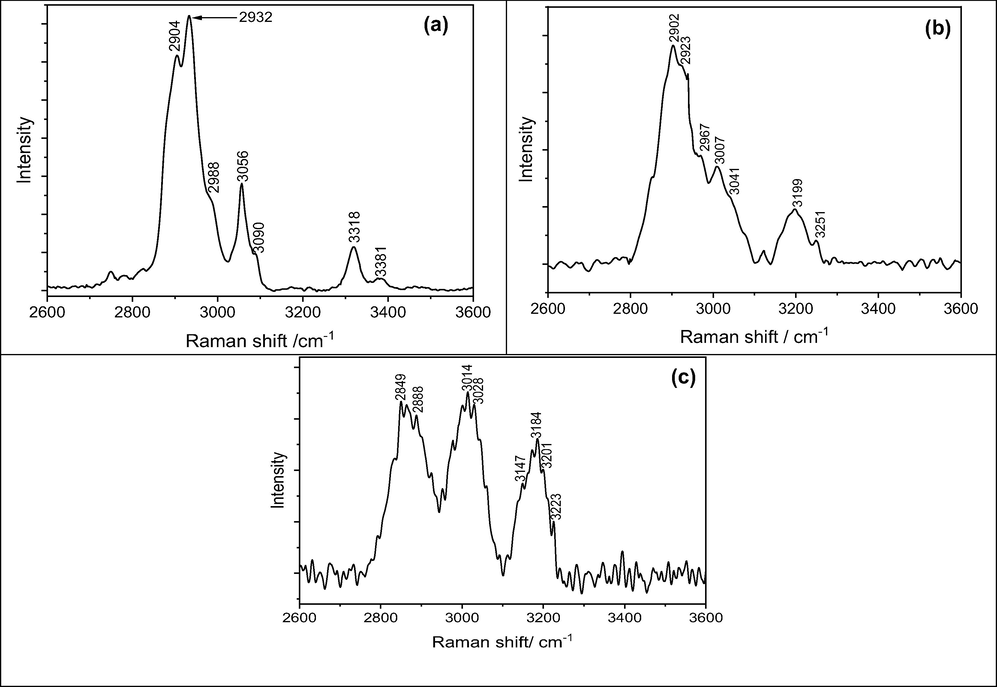
(a) Raman spectra of 1, (b) Raman spectra of 2, and (c) Raman spectra of 3.
Fig. 2a displays a SEM image of an amino functionalized silica gel support 1. The SEM image revealed that spherical shape of the silica gel with unsmooth surface due to the incorporation of the amino moieties onto the surface of silica gel (Fig. 2a). The SEM micrograph of silica gel supported Schiff base 2 revealed a spherical morphology with a rougher surface than 1. (Fig. 2b). Interestingly, after introduction of palladium species onto the Schiff base ligand 2, the SEM image of silica gel supported Schiff base Pd(0) catalyst 3 showed more unsmooth surface compare to 1 and 2. The Pd(0) 3 catalyst showed more unsmooth surface due to the complexation and aggregation between the ligand and palladium species (Fig. 2c). The evident changes in surface morphology not only demonstrate the successful incorporation of organic moieties but also indicate the formation of metal complexes, confirming the desired chemical transformations during the catalyst preparation.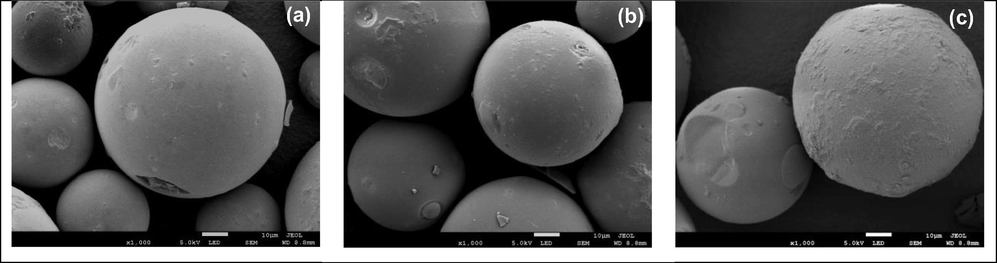
(a) SEM image of 1, (b) SEM image of 2, and (c) SEM image of 3.
The EDX image of silica gel supported amino functionalized 1 showed the contained of 37.5 wt% of oxygen, 32.5 wt% of silicon, 17.9 wt% of carbon and 2.3 wt% of nitrogen (Fig. 3a). The EDX image of silica gel supported Schiff base thiophene ligand showed 33.5 wt% of oxygen, 35.2 wt% of silicon, 14.0 wt% of carbon, 1.3 wt% of nitrogen and 0.9 wt% of sulfur (Fig. 3b). However, the peak for palladium was visible on the EDX spectra after the incorporation of the palladium species with the thiophene ligand, and it was estimated that the Schiff base Pd(0) catalyst 3 contained 1.9 wt% palladium (Fig. 3c).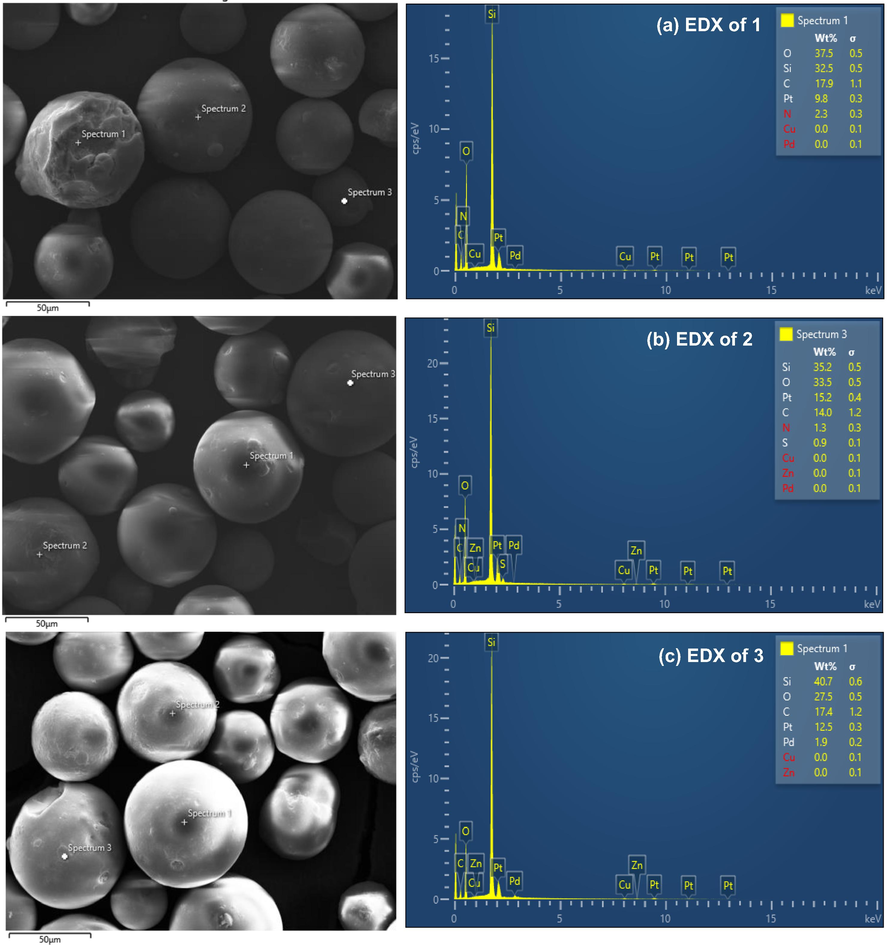
SEM-EDX images of 1–3.
Fig. 4a shows a TEM image of fresh silica gel supported Schiff base Pd(0) catalyst 3. The TEM analysis of 3 demonstrates that the palladium complexes were distributed evenly across the surface of the silica gel. The metal-chelating ligand complex formation was revealed by the magnified TEM image of Pd(0) catalyst 3. More than 225 Pd(0) complexes were analyzed to determine the average diameter of the palladium species (Fig. 5), and the average diameter of the particle was found to be 5.3 ± 0.6 nm. Surprisingly, the fresh TEM micrograph was similar to the third recycled one, and the Suzuki reaction did not cause the palladium nanocomplexes to aggregate (Fig. 4b).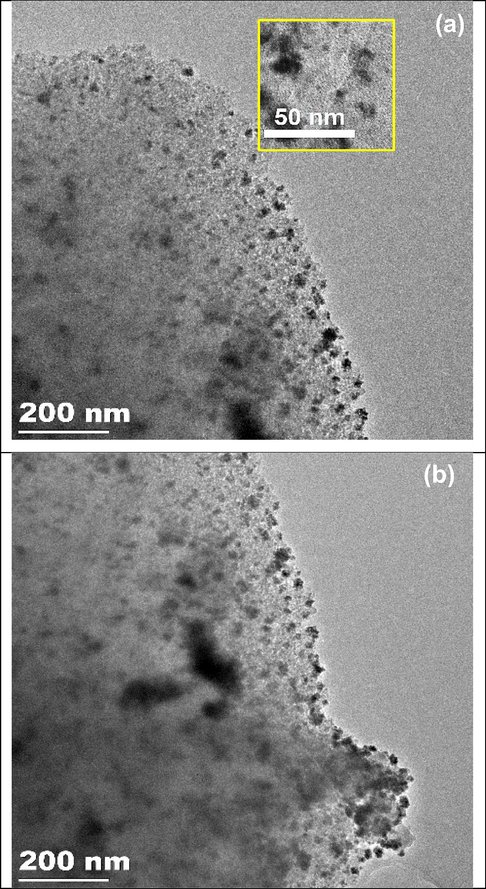
(a) TEM image of silica gel supported Schiff based Pd(0) complex 3, (b) TEM image of 3rd recycled of 3.
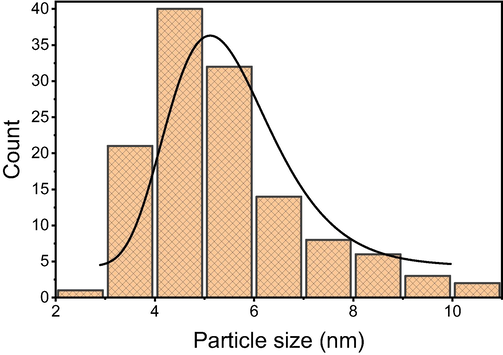
Size distribution of palladium complex in Schiff base Pd(0) catalyst 3.
Fig. 6 displayed a classic XRD pattern of a Schiff base Pd(0) catalyst 3 supported by silica gel. The five characteristic peaks at 2θ = 39.14° (1 1 1), 45.35° (2 0 0), 66.26° (2 2 0), 79.86° (3 1 1) and 83.45° (2 2 2) were observed (Zhang et al., 2017). The face-centered cubic structure of palladium was thought to be reflected by these attribute reflections. The silica gel-supported Schiff base ligand 2, which is represented by the broad peak at 2θ of 18–27 represent an amorphous structure.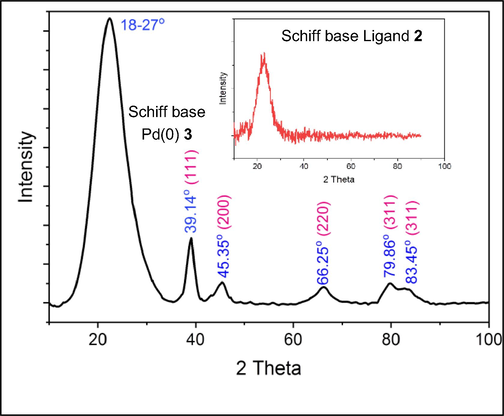
XRD spectra of Schiff base ligand 2 and Pd(0) catalyst 3.
The full scan XPS of silica gel supported Schiff base Pd(0) 3 catalyst and Schiff base ligand 2 were shown in Fig. 7. The Schiff base ligand 2 showed binding energy peaks at O 1 s 532.5, N 1 s 399.2, C 1 s 285.1, S 2p 153.25, Si 2p 102.9 however the Schiff base Pd(0) catalyst 3 showed binding energy at O 1 s 532.5, N 1 s 399.6, C 1 s 285.1, S 2p 153.7, Si 2p 103.7. The Schiff base Pd(0) 3 catalyst exhibited additional peaks corresponding to palladium species that were not detected in Schiff base ligand 2, providing confirmation of successful coordination between the palladium species and the ligand. The donation of electrons towards the palladium species resulted in a slight shift in the binding energies of the N and S atoms.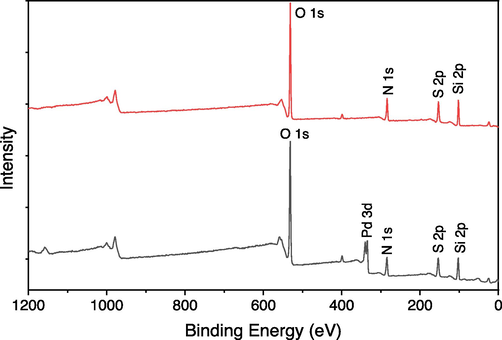
Full scan XPS images of Schiff base 2 (red line) and Pd(0) catalyst 3 (black line).
In the narrow scan profile (Fig. 8), two distinct signals were observed at 335.75 eV and 340.73 eV in the Schiff base Pd(0) 3 complex supported by silica gel. Based on their respective energies, these signals were identified as the Pd(0) species Pd3d5/2 and Pd3d3/2, respectively (Jampilek, 2019).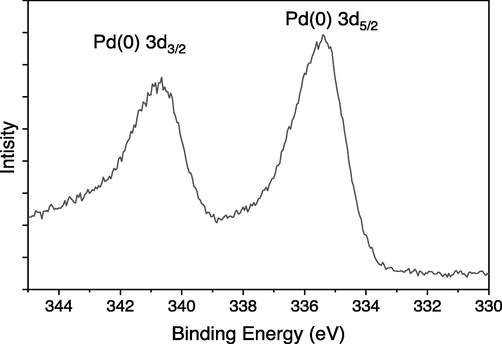
Narrow scan XPS image of Schiff base Pd(0) catalyst 3.
3.2 Useful catalytic applications
Following successful characterization, the catalytic practical application of the Schiff base Pd(0) catalyst 3 catalyst supported by silica gel to the Heck reaction was examined. The Schiff base Pd(0) catalyst 3 was used to construct C–C bonds from olefins and organic halides, resulting in excellent cross-coupling products. The preliminary reaction was conducted under a variety of conditions in order to determine the standard reaction condition for the formation of C–C bond through the Heck reaction. As a result, we chose the first reaction, using butyl acrylate and 4-iodotoluene as starting materials, and kept the catalytic dose fixed (10 mg, 0.017 mol%), the reaction temperature constant (125 °C), and the reaction time constant (7 h). The solvents and bases were then put to use in a variety of ways. It was discovered that all reaction conditions allowed for the successful production of the desired cross-coupling product 4a (Table 1). When the reaction took place in DMF with N,N-diisopropylethylamine (DIPEA) as a base, the product 4a was afforded with an 81% yield (Table 1, entry 1). However, the yield was slightly increased when triethyl amine was used instead of DIPEA (entry 2). Interestingly when inorganic base i.e Na2CO3 was and used the formation of 4a decreased to 64% (entry 3). The combination of DMA solvent and DIPEA base was not improved the yield of 4a (entry 4) however, the combination of DMA and Et3N provided showed the better result (entry 5). The other solvents i.e. NMP and DMSO with Et3N afforded only 78% and 68% yield of 4a respectively (entries 6 & 7). Remarkably, under identical reaction conditions, the presence of n-decane led to a surprising outcome, yielding an impressive 95% of the desired product. Furthermore, we reduced the loading of the Schiff base Pd(0) 3 catalyst to 0.0085 mol% while the cross-coupling reaction continued and provided a 70% yield of 4a (entry 9). As a result, suitable reaction conditions were determined to be n-decane as the solvent, Et3N as the base, and 0.017 mol% Schiff base Pd(0) 3 catalyst at 125 °C for 7 h (entry 8). [a] 4-Methyliodobenzene (1 mmol), butyl acrylate (1.5 mmol), Schiff base Pd(0) 3 catalyst (0.017 mol%), base (3.2 mmol), and 2 ml of solvent were used in the reaction. [b]Yields from GC. [c] Isolated yield. [d]Schiff base Pd(0) catalyst 3 at 0.0085 mol% was used.
Entry
Solvent
Base
Yield (%)[b]
1
DMF
DIPEA
81
2
DMF
Et3N
88(83)[c]
3
DMF:H2O
Na2CO3
64
4
DMA
DIPEA
76
5
DMA
Et3N
85(80)[c]
6
NMP
Et3N
78
7
DMSO
Et3N
68
8
n-decane
Et3N
95(91)[c]
9d
n-decane
Et3N
70(66)[c]
Following a screening of the Heck reaction, we increased the use of the Schiff base Pd(0) catalyst 3 for a wide range of substituted iodo and bromo arenes with alkynes (Table 2). Methyl acrylate, an olefine, smoothly interacted with 3-iodotoluene, 4-iodoanisole, and iodobenzene to produce the corresponding cross-coupling products 4b-d with 90%, 88%, and 90% yields, respectively. The reactions between aryl bromides and olefins to form C–C bonds have a huge market because arene bromides are cheap materials that are used in both industrial and pharmaceutical applications. We further investigated the range of the Heck reaction of electronically deficient aryl bromides with a wide variety of olefins after observing the excellent catalytic performance of 3 towards the aryl iodides. When the reactions were carried out using same derivatives having bromine as a halogen atom provided 81%, 80% and 82% yield of the respective products 4b-d. Aryl bromides were found to have lower catalytic activity than aryl iodides because, during the catalytic cycle, they added to the metal catalyst's active site oxidatively more slowly than aryl iodides. N-isopropyl acrylamide and butyl acrylate are two other olefins were also readily subjected to the cross-coupling reaction with 4-iodoacetophenone, iodobenzene, 4-nitro, 4-methyl, and 4-methoxy iodobenzene to produce the correlating coupling products 4e-j with 82–94% yield. The bromide derivatives of those aryl halides were produced in a range of 75–85% yield with the reaction proceeding without any problems. The catalytic activity of aryl halides with electron-pushing groups was found to be lower than that of those with electron-withdrawing groups. It is worthwhile to mention that the sterically hindered, bulky, and much less responsive styrene could be converted into the respective stilbene derivatives 4 k and 4 l in up to 85% yield by the Heck reaction with 4-CN and 4-Me aryl iodide respectively. Bromo derivatives were also produced the respective products 4 k and 4 l with 79% and 76%, respectively. [a] Aryl halide (1 mmol), olefin (1.5 mol equiv), 0.017 mol% of Pd(0) cat. 3, Et3N (3.2 mmol), and 2 ml of n-decane at 125 °C were used in the reaction.
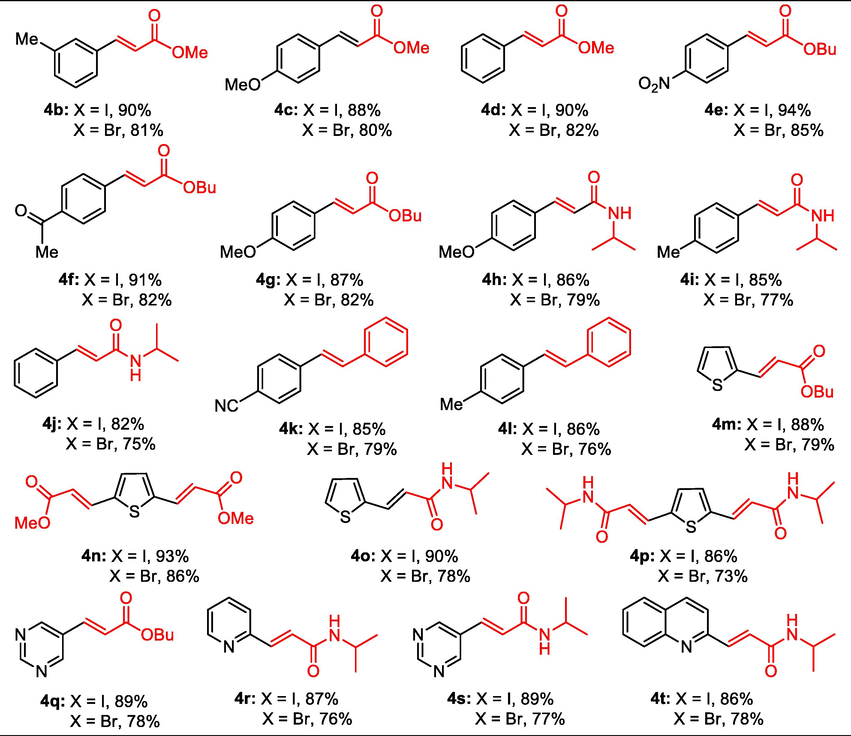
More than 85% of all chemical compounds with physiological activity contain heterocycles. In the field of medicine, heterocyclic compounds have become more popular because they are pharmacologically and physiologically active (Ji et al., 2015). Heterocyclic molecules make up a large portion of the molecules found in many biological compounds connected to living things, including vitamins, hormones, and antibiotics (Panchal et al., 2020). Heterocyclic compounds with nitrogen atoms in their structures comprise the largest group of chemical compounds and are widely utilized in medicinal chemistry due to their prevalence in biologically active complexes and natural products (Ocampo et al., 2019; Wahab et al., 2018). In medicine, many of these compounds have been found to have biological activities and are used as drugs to treat various diseases. For example, the anti-cancer drug paclitaxel contains an indole ring in its structure, and the anti-inflammatory drug naproxen contains a heterocyclic ring with a nitrogen atom. In addition, a significant portion of therapeutically effective compounds and FDA-approved pharmaceuticals are heterocyclic compounds containing sulfur. Scientific research has demonstrated that these nitrogen-containing heterocyclic compounds have a diverse range of biological effects, including anti-diabetic, anti-cancer, antiviral, anti-inflammatory, antimicrobial, anti-hypertensive, anti-malaria, anti-Alzheimer's, and antifungal properties. Heterocyclic compounds that contain sulfur are utilized extensively in chemical research (Feng et al., 2016; Schutte and Teranishi, 1974; Tomishima et al., 2013). These compounds are also present in numerous natural products and pharmaceuticals.
Therefore, we have further investigated to derivatize the heterocyclic aryl halides using a variety of olefins. Product 4 m was obtained in an 88% yield by combining 2-iodothiophene with butyl acrylate under identical reaction conditions. The yield was obtained 79% when 2-bromothiophene was used. Interestingly, the coupling reactions involving 2,5-dioodo and 2,5-dibromothiophenes with methyl acrylate proceeded smoothly, giving rise to the formation of disubstituted thiophene derivative 4n. Impressively, these reactions exhibited remarkable yields of 93% and 86% for the respective products. Furthermore, the application of the Heck reaction to N-isopropylacrylamide in conjunction with the mono and 2,5-diiodosubstituted thiophenes proved to be successful, leading to the generation of cross-coupling products 4o and 4p. Notably, these products were obtained with noteworthy yields of 90% and 86%, respectively. These findings highlight the efficient and effective nature of these reactions, underscoring their potential significance in synthetic organic chemistry. Similarly, mono and 2,5-dibromosubstituted thiophene was also provided 4o and 4p 78% and 73% yield respectively. Further, the nitrogen contained heterocyclic compounds were also investigated. In contrast to 5-bromopyrimidine, which provided 78% yield, 5-iodopyrimidine allowed to treat with butyl acrylate smoothly forwarded the coupling reaction to provide the pyrimidine derivative 4q with 89% yield. The coupling reaction with N-isopropylacrylamide was also smoothly carried out on the 2-iodopyridine, 5-iodopyrimidine, and 2-iodoquinoline to produce the respective combining products 4r, 4 s, and 4 t with 87%, 89%, and 86% yield, respectively. The bromo substituted of these n-heterocyclic aromatic halides were also efficiently forwarded the reaction with 76%, 77% and 78% yield respectively.
Then we focused the further utility of the Schiff base Pd(0) catalyst 3 for the synthesis of biological active molecule. Ozagrel hydrochloride has been shown to significantly reduce mortality rates, serum alanine aminotransferase levels, hepatic centrilobular necrosis, hemorrhaging, DNA fragmentation, and plasma 2,3-dinor thromboxane B2 levels caused by APAP injection. It also inhibits the expression of jun oncogene, FBJ osteosarcoma oncogene (fos), C/EBP homologous protein, and other cell death-related mRNAs induced by N-acetyl-p-aminopheno (APAP) in the liver. Although ozagrel hydrochloride does not affect CYP2E1 activity, which results in N-acetyl-p-benzoquinone imine (NAPQI) production, it has been found to significantly reduce cell damage caused by NAPQI in RLC-16 (Bodaghifard, 2019). Due to the high demand and excellent biological activity we began with synthesis of ozagrel. The first intermediate compound (E)-butyl 3-(4-(hydroxymethyl)phenyl)acrylate was synthesized by the utilization of Schiff base Pd(0) catalyst 3. Under the same circumstances as those listed in Table 2, the coupling reaction between 4 and iodophenylmethanol and butyl acrylate was conducted, and the intermediate compound (E)-butyl 3-(4-(hydroxymethyl)phenyl)acrylate 4u was produced with a 90% yield. The incorporation of an imidazole moiety into 4u after treating it with carbonyldiimidazole (CDI) in N-methylpyrrolidone (NMP) for two hours at 140 °C led to the production of 4v with an 88% yield. Following the hydrolysis of the ester group in 4v in the presence of a basic methanol solvent and acidification with hydrochloric acid, ozagrel hydrochloride 5 was produced as a white colour solid with 90% yield.
Suzuki-Miyaura C–C chemical bond formation reactions were used to further investigate the catalytic activity of Schiff base Pd(0) 3. The Suzuki coupling reaction between 4-bromotoluene and phenylboronic acid was used as an ideal reaction to determine the optimum reaction conditions. Table 3 displays the results of the cross-coupling reaction using different bases and solvents. The initial reaction, which took place for four hours in clear water at 80 °C with potassium carbonate acting as a base, used a Schiff base Pd(0) 3 catalyst at a concentration of 0.017 mol% (10 mg). The coupling reaction resulted in the production of 56% of the desired product 6a (Table 3, entry 1). When this reaction was conducted in the same reaction conditions in an aqueous solution of isopropanol, the corresponding product 6a was produced with a 73% yield (entry 2). However, a 86% yield of the product 6a was obtained when the coupling reaction was conducted for four hours while using aqueous ethanol (entry 3). Surprisingly, 96% of the desired product was achieved after six hours prolonging time (entry 4). When bases were changed, it was discovered that the yield of the corresponding product was essentially unaffected (entries 5–8). Additionally, when this reaction was carried out with the phase transfer catalyst tetrabutylammonium bromide (TBAB) (0.5 mol%) the desired biphenyl 6a was generated with an 85% yield (entry 9). [a] The reaction was conducted using 1 mmol of 4-bromotoluene and 1.2 mmol of phenylboronic acid in the presence of 2 mmol of base and 0.017 mol% (10 mg) of Pd(0) 3 catalyst. The reaction mixture was heated to 90 °C and maintained at this temperature for 5 h.
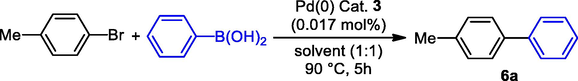
Entry
Time (h)
Base
Solvent
Yield (%)
1
4
K2CO3
H2O
56
2
4
K2CO3
i-PrOH:H2O
73
3
4
K2CO3
EtOH:H2O
86
4
6
K2CO3
EtOH:H2O
96
5
6
K2PO4
EtOH:H2O
90
6
6
NaOAc
EtOH:H2O
91
7
6
NaOH
EtOH:H2O
85
8
6
KOH
EtOH:H2O
83
9
6
K2CO3
H2O + TBAB
84
In this study, the aim was to identify the highest catalytic activity for the Suzuki-Miyaura coupling reaction using a Schiff base Pd(0) catalyst 3 with a concentration of 0.0009 mol%. The reaction was carried out using 10 mmol of 4-methyliodobenzene and 1.1 mol equivalent of phenylboronic acid for 10 h, as outlined in Table 1 entry 4. The results of the study showed that the Pd(0) catalyst 3 was highly effective, producing the desired coupling product 6a with a yield of 92%. In addition, the catalyst demonstrated a high turnover number (TON 8142) and turnover frequency (TOF 841 h−1), as indicated in Scheme 3.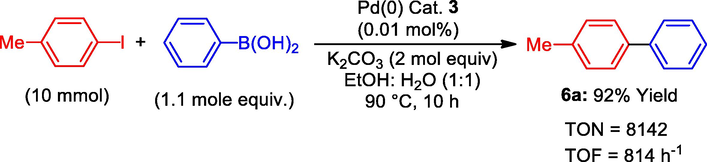
TON and TOF in the Suzuki reaction.
We concentrated on expanding the applicability of the Schiff-based Pd(0) catalyst 3 to a wide variety of aryl substrates as its catalytic performance was satisfactory. In accordance with Table 3 entry 4, the performance of Pd(0) catalyst 3 was tested at 90 °C with 0.017 mol% palladium. The catalyst was able to smoothly direct the Suzuki-Miyaura coupling reaction of a diverse range of aryl iodides and bromides, as demonstrated in Table 4. When various electron densities of aryl iodides and bromides were combined with phenylboronic acid, the resulting cross-coupling biaryl produced 6b-e up to 95% yield. In the catalytic pathway, the π-aryl Pd-complex formed at a notably faster rate than aryl bromides, leading to enhanced yields with aryl iodides. [a] The following ingredients were used in the reactions: 1 mmol of aryl halide, 1.2 mmol of arylboronic acid, 0.017 mol% of Pd(0) catalyst 3 and 4 ml of aqueous ethanol (1:1).
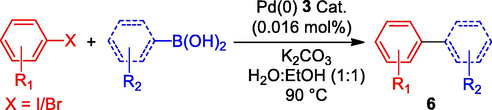
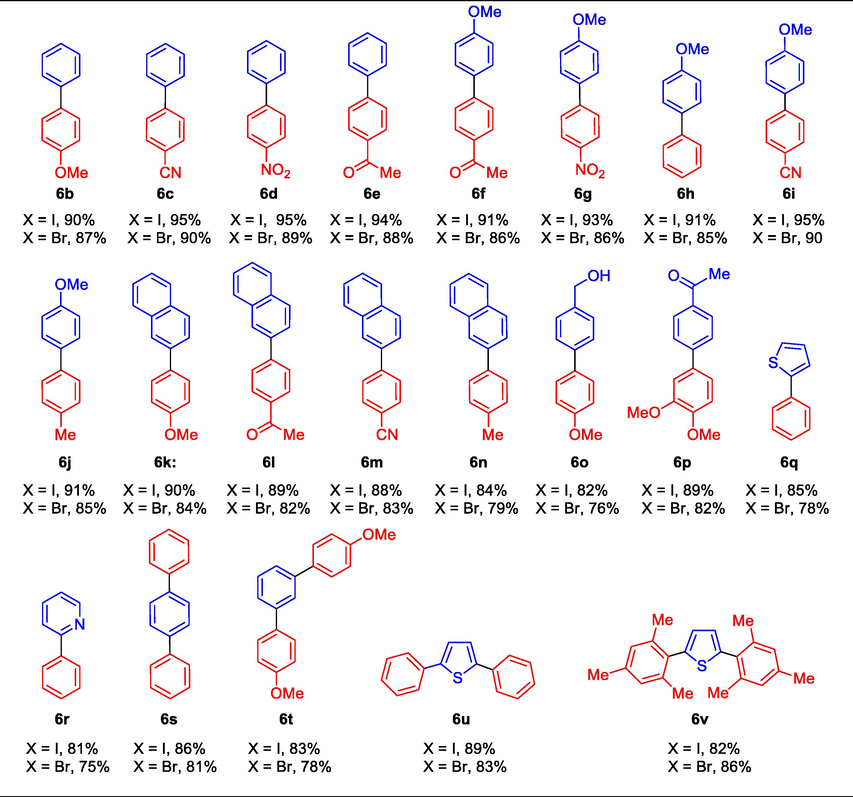
The corresponding desired coupling products were successfully produced in up to 95% yield when substituted aryl iodides and bromides were added to 4-methoxyphenyl boronic acids under the ideal reaction conditions. However, the yield of biaryl products 6f- j was lower for all aryl boromies. The fused benzene ring e.g napthylene boronicacid is relative bulky size compared to phenyl boronicacid which also underwent cross-coupling reaction with numerous aryl iodides and bromides provided the respective products 6 k-n in a range of 90–84% yield (X = I). Similarly, aryl bromides were also smoothly afforded the biaryl produce 6 k-n with 84–79% of yield (X = Br). The alcoholic substituted aryl halide e.g. 4-iodo/bromo-phenylmethanol, and 3,4-dimethoxyphenyl boronicacids were also efficiently promoted the Suzuki reaction to provide the respective products with high yield. Thiophene and pyridine, which are heterocyclic aryl halides, underwent smooth coupling reactions with phenylboronic acid to yield heterocyclic biaryls 6q and 6r with yields of 85% and 81%, respectively. Similarly, di-halogen substituted aryl halides such as 1,4-diiodo/dibromobenzene, 1,3-diiodo/dibromobenzene, and 2,5-diiodo/dibromothiophene were effectively coupled with phenylboronic acid, 4-methoxyphenylboronic acid, and mesitylboronic acid, resulting in the formation of disubstituted biaryl products 6 s-v with excellent yields.
We further turned our attention to utilize of Schiff based Pd(0) catalyst 3 for the synthesis of biological active molecule. The oxathiin fungicide family known as carboxamide, carboxin, or (carbox) anilide fungicides, includes the new pesticide Boscalid. Boscalid have been classified as “reduced risk” by the U.S. EPA. Boscalid functions by preventing fungal cells from producing ATP through mitochondrial respiration. Boscalid, a novel mode of intervention fungicide, is useful for treating pathogens resistant to certain other fungicides, such as those resistant to sterol inhibitors, dicarboximides, benzimidazoles, anilinopyrimidines, phenylamides, and strobilurins. Boscalid was first introduced to the market by BASF in 2003, and it is currently produced at a rate of more than 1000 tons annually (Wang et al., 2006). Scheme 4 illustrates the production of Boscalid 7. According to Table 4, Suzuki coupling reaction between 2 and iodoaniline and 4-chlorophenylboronic acid produced the 4-chloro-2-aminobiphenyl transitional compound 6w in 82% yield. The second step produced the desired Boscalid 7 with an 86% yield by exposing the generated amine 6w to 2-chloronicotinoyl chloride at room temperature for an hour.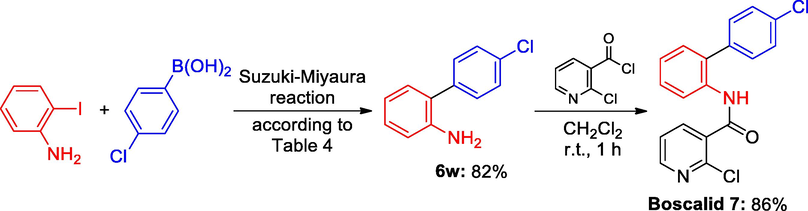
Synthesis of Boscalid via Suzuki-Miyaura reaction.
The recent catalyst is compared to the previously described catalyst to determine the efficiency and practicability of the established catalyst Pd(0) catalyst 3. The Heck coupling results of iodobenzene was chosen in order to more accurately compare the timing of product formation and catalyst dosage to those of the catalysts listed in Table 5 . There are no significant drawbacks associated with the catalyst utilized in these Hack reactions to construct C–C bond, such as high speed and dosage, poor catalytic performance, high temperature, or long reaction time. Additionally, compared to previously reported methods, this new catalyst's reusability and recycling are easier and quicker.
Catalyst/Amount
Solvent/Base
Temp. (°C)
Time (h)
Yield (%)
Ref.
MNPs-TDA-Pd/20 mg
DMF/K2CO3
100
8 h
93
(Bodaghifard, 2019)
Fe3O4@SiO2-NH2-Pd/30 mg
CH3CN/H2O/NaOAc
reflux
12 h
71
(Wang et al., 2006)
TiO2@Pd NPs/1%
DMF/Et3N
140
10
93
(Nasrollahzadeh et al., 2014)
HMMS-NH2-Pd/4%
NMP/K2CO3
130
8
98
(Wang et al., 2014)
Fe3O4-NH2-Pd/5%
NMP/K2CO3
130
10
99
(Zhang et al., 2011)
SMNPs-DF-Pd/1%
Solvent free/DABCO
140
2
92
(Zolfigol et al., 2014)
SiO2@Schiff base-Pd/0.017%
n-decane/Et3N
125
7
95
This report
The utilization of the heterogeneous catalyst is of great economic and environmental importance. As a result, we concentrated our efforts on recovering and recycles Schiff-based Pd(0) catalyst 3 endorsed on silica gel (Fig. 9). We selected examples 4e and 6d from Tables 2 and Table 4, respectively, where X = I, as a model reaction for recycling of the Schiff base Pd(0) catalyst 3 in the Suzuki reaction. After the first catalytic reaction was competed, the reaction mixture was centrifuged after being diluted with ethyl acetate. Then the solvents were carefully decanted, and the remaining catalyst was further cleaned with methanol and decanted. The catalyst was then dried for 30 min in an oven set at 50 °C before being used again. It was observed that the Schiff based Pd(0) catalyst 3 could be successfully separated from the reaction mixture and reused up to seven times without suffering a significant loss in catalytic activity. The catalytic performance showed a slight deterioration due to a minor loss of Pd(0) catalyst 3 during its recovery process. Fig. 5b, displaying the TEM image of the third recycle of 3, clearly indicates that there was no accumulation of palladium catalysts during the Suzuki-Miyaura reaction. Therefore, the silica gel-supported thiophene-based Pd(0) catalyst 3 demonstrated high durability and catalytic efficiency in the coupling reactions.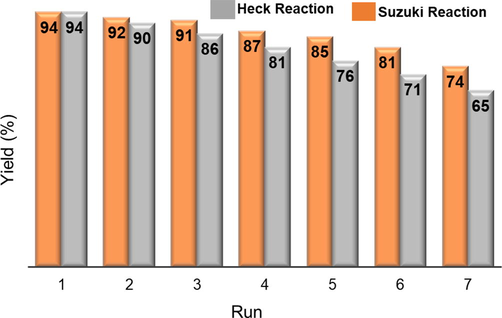
Recycling of Pd(0) catalyst 3 in the Suzuki and Heck reaction.
4 Conclusions
In conclusion, we developed an amino functionalized silica gel supported [2,2′-bithiophene]-5,5′-dicarbaldehyde Schiff-based Pd(0) nanocatalyst. Several spectroscopic techniques, including Raman, SEM, EDX, TEM, XRD, ICP, and XPS, were used to fully characterize the catalyst. The ICP and EDX analyses confirmed that the silica surface contained 1.9 w% palladium, and the TEM analysis verified the even dispersion of Pd-complexes. The measured size of the palladium complex was found to be an average of 5.3 ± 0.6 nm. Excellent coupling products were produced by using the Schiff-based Pd(0) catalyst in the Mizoroki-Heck and Suzuki-Miyaura coupling reactions with a wide range of aryl halides, olefins, and organoboronic acids. The Schiff base Pd(0) catalyst 3 could be easily taken out of the reaction mixture and reused seven times without losing any catalytic activity. Additionally, this Schiff-based Pd(0) catalyst was used to successfully synthesize the biologically active molecules of Ozagrel and Boscalid.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Catalysts. 2019;9:140.
- J. Mater. Res. Technol.. 2020;9:5858.
- Coord. Chem. Rev.. 2023;476:214928
- Coord. Chem. Rev.. 2019;385:137.
- Chem. Rev.. 2000;100:3009.
- J. Organomet. Chem.. 2019;886:57.
- Chem. Rev.. 2016;116:12564.
- Chem. Soc. Rev.. 2011;40:5084.
- Coord. Chem. Rev.. 2016;311:1-23.
- Catal. Sci. Technol.. 2016;6:4565.
- ACS Catal.. 2017;7:1462.
- J. Organomet. Chem.. 2021;936:121711
- Curr. Top. Med. Chem.. 2016;16:1200.
- Chem. Soc. Rev.. 2011;40:5181.
- Asian J. Org. Chem.. 2020;9:1341.
- ChemCatChem. 2010;2:493.
- Arab. J. Chem.. 2022;15:103914
- Chem. Soc. Rev.. 2013;42:5270.
- Adv. Mater.. 2017;29:1603823.
- ACS Appl. Nano Mater.. 2020;3:2070.
- Molecules. 2019;24:3839.
- Water Res.. 2015;87:1-9.
- Green Chem.. 2012;14:2513.
- Dalton Trans.. 2014;43:9026.].
- Catal. Commun.. 2012;17:179.
- Appl. Catal. A. 2013;459:65.
- Chem. Rev.. 2018;118:4981.
- Catal. Lett.. 2021;151:3230.
- ACS Appl. Mater. Inter.. 2019;11:32579.
- Chem. Rev.. 2011;111:2251.
- J. Mol. Catal. A Chem.. 2014;394:205.
- Coord. Chem. Rev.. 2019;397:54-75.
- Organomet. Chem.. 2021;935:121676
- Eur. J. Med. Chem.. 2019;162:435.
- Int. J. Pharm. Sci. Res.. 2020;11:4739.
- Micropor. Mesopor. Mat.. 2016;222:87.
- J. Chin. Chem. Soc.. 2015;62:33.
- New J. Chem.. 2015;39:3564.
- RSC Adv.. 2015;5:19630.
- J. Cat. 2017;350:103.
- ChemCatChem. 2015;7:2141.
- Chem. Eng. Technol.. 2019;42:2187.
- Crit. Rev. Food Sci. Nutr.. 1974;4:457.
- Appl. Organomet. Chem.. 2020;34:5775.
- Chem. Rec.. 2022;2019:19.
- J. Mater. Chem.. 2012;22:15953.
- ChemistrySelect. 2016;1:4108.
- Catal. Today. 2013;212:206.
- J. Organomet. Chem.. 2023;983:122541
- RSC Adv.. 2014;4:21688.
- BMC Gastroenterol.. 2013;13:21.
- Adv. Synth. Catal.. 2009;351:3027.
- Mater. Today Chem.. 2023;27:101355
- Chem. Eng.. 2016;4:5866.
- Mol. Divers.. 2018;22:517.
- New J. Chem.. 2014;38:1138.
- Colloids Surf. A Physicochem. Eng. Asp.. 2006;276:116.
- Green Chem.. 2013;15:3429.
- Green Chem.. 2011;13:1238.
- Appl. Surf. Sci.. 2017;396:812.
- Chem. Soc. Rev.. 2021;50:8903.
- RSC Adv.. 2014;4:40036.







