Translate this page into:
Simultaneous adsorption of tetracycline, amoxicillin, and ciprofloxacin by pistachio shell powder coated with zinc oxide nanoparticles
⁎Corresponding author. tariq.almusawi@iu.edu.jo (Tariq J. Al-Musawi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Pistachio shell powder coated with zinc oxide nanoparticles (CPS): A new adsorbent. CPS was evaluated for removal of three antibiotics in the ternary adsorption system. The kinetic, thermodynamic, and isotherm processes, and their models were studied. Excellent adsorptive performance was revealed for CPS. CPS is an efficacious adsorbent for the eradication of pharmaceutical wastewater.
Abstract
A new adsorbent formed from pistachio shell powder that was coated with ZnO nanoparticles (CPS) was examined in terms of simultaneous adsorption of tetracycline (TEC), amoxicillin (AMO), and ciprofloxacin (CIP) from an aqueous solution. Initially, the characterization properties of a CPS-like surface morphology, functional groups, and structure were obtained using advanced analysis of TEM, SEM, XRD, EDS, and FT-IR. Post coating with ZnO nanoparticles, several surface and structural characteristics relating to the adsorption ability of the pistachio shell were significantly improved. The correlation of the kinetic data by a pseudo second-order model was successful for three antibiotics. High compatibility resulted between the TEC and CIP isotherm data and the Fruendlich model. However, the Langmuir model produced a better fit to the AMO isotherm curves. In addition, its spontaneous and exothermic nature was the main feature for the adsorption process of the three antibiotics onto CPS. Through the results, the chemical adsorption has been governed by the AMO, CIP, and TEC reaction onto the homogeneous and heterogeneous sites of CPS surfaces. The CPS exhibited a highest adsorption capacity for AMO (132.240 mg/g), then for TEC (98.717 mg/g), and CIP (92.450 mg/g). These results place CPS one among the highly efficient adsorbents that can be used to eradicate wastewater containing antibiotics.
Keywords
Adsorption
Antibiotics
ZnO
Simultaneous
Competitive
Characterization
1 Introduction
At the present time, pollution of water resources by pharmaceutical wastes is one of the greatest and serious environmental challenges facing communities, especially in places where there is reliance on these sources for drinking purposes (Gasser et al., 2008; Oh et al., 2017). Excessive pharmaceutical compounds in environmental matrices are classified as hazardous materials that can damage the natural ecosystem by changing the status of the equilibrium (Kalhori et al., 2018; Ji et al., 2014). These chemicals can enter the aquatic bodies including surface and ground water through various anthropogenic activities, such as, waste streams from hospitals and pharmaceutical and veterinary industries, from their manufacture to disposal (BIO Intelligence Service, 2013). Antibiotics are a part of pharmaceutical compounds, which are consumed in great quantities, as they are highly effective in treating a wide spectrum of bacterial diseases in humans, livestock, poultry, and fish (Soori et al., 2016; Huang et al., 2017; Hassani et al., 2014; Legnoverde et al., 2014). Among the various types of antibiotics; tetracycline, amoxicillin, and ciprofloxacin are widely used in the world as efficacious therapeutic agents in the treatment of a variety of bacterial diseases (Al-Musawi et al., 2019; Mohammed and Kareem, 2019; Samarghandi et al., 2015). The discharge of pharmaceutical wastewater loaded with antibiotics can cause lethal health risks to non-target humans and biota, related to their chronic or acute exposure, as they contain toxic and carcinogenic elements. Besides, they may cause fetal abnormalities (Shi et al., 2019; Bondarczuk and Piotrowska-Seget, 2019; Pouretedal and Sadegh, 2014). From another side, various studies have reported that the disinfection of by-product chemicals, which are considered as carcinogenetic agents, were detected in the final treating stages due to the chlorination of wastewater containing antibiotics. Therefore, there is an urgent need to reduce the antibiotic compounds to the permissible limits prior to being discharged into water bodies (Balcioglu and Otker, 2004). Without a doubt, antibiotics are characterized by their stability in the environment and their long degradation time. Moreover, they are not completely removed using conventional treatment methods (Xian et al., 2010; Davis et al., 2003). With regard to the last point of characterization, an efficacious and advanced technology has to be applied to treat wastewater containing antibiotics. One of the most interesting treatment methods is the adsorption process (Al-Musawi et al., 2018; Zou et al., 2019).
The adsorption treatment method is an efficient, simple, and universal advanced treatment technology. This treatment method employs using natural or synthesized material (known as sorbent, adsorbent, or biosorbent) for the abatement of pollutant molecules (known as adsorbates) from contaminated solutions (Zou et al., 2019; Ahalya et al., 2003; Al-Musawi et al., 2018). For the adsorption process to be integrated in terms of removal efficiency and economic feasibility, the adsorbent should be chosen with great care (Gisi et al., 2016; Ghorai et al., 2014). In this context, the adsorbents should be tested for their adsorption performance toward numerous contaminants before being applied. Additionally, these tests should take into consideration the availability and cost-effectiveness of the tested adsorbents (Cheng et al., 2019; Wang et al., 2018). In recent times, agricultural waste, in its original or modified form, has attracted a great deal of attention by the scientific workers, to be utilized as adsorbents (Kamar et al., 2017; Sulaymon et al., 2014; Hossain et al., 2012). This is because of their low cost, availability in large quantities, and also they are found to be efficient for removal of several organic and inorganic matters in the adsorption systems (Khodadadi et al., 2019; Holan and Volesky, 1995). To further improve the adsorption capacity of agricultural wastes and to enhance their practical performance, some surface modifications have been suggested. For example, different particles of nano size have been successfully used for coating the surfaces of several adsorbents. The result of this modification was the formation of a more effective adsorbent (Mohammed et al., 2018; Dhiman and Sharma, 2018).
Zinc oxide nanoparticles were examined and studied by several adsorption studies and they successfully passed the tests and were selected as adsorbents and catalysts in the treatment systems (Bazrafshan et al., 2019; Zhang et al., 2016). ZnO is easy to manufacture, cheap, has an adjustable morphology, is eco-friendly, and it is not affected by any change in the environmental conditions (Li et al., 2017). In addition, the isoelectric point of ZnO is high and equal to 9.5, which improves the positive charge of its surface (Bazrafshan et al., 2019; Joshi, 2018; Wang et al., 2016). Of late, ZnO nanoparticles have been used to coat various dry adsorbents such as mesoporous silica (Jeong et al., 2014), zeolite (Wang et al., 2016), cross-linked chitosan/polyvinyl alcohol microspheres (Abdelwahab and Ghoneim, 2018), 13X zeolite, activated carbon (Changsuphan et al., 2012), and SiO2 nanoparticles (Zhang et al., 2016), with good results being demonstrated.
Agriculture waste, such as a pistachio shell (PS), provides an effective surface for coating with ZnO nanoparticles, which is the goal of this research. Moreover, ZnO nanoparticles are promising agents in the adsorption systems. Pistachio shells, similar to other agricultural materials, are comprised of a number of functional groups having a negative charge, such as, polysaccharides and carbohydrates. These groups have a high tendency to bond with the positive charge of the ZnO particles (Piness, 2010; Hashemian and Shayegan, 2014). To date, using pistachio shell powder coated with ZnO nanoparticles (CPS) as an adsorbent agent for the remediation of pharmaceutical wastewater has not been reported. Moreover, it is important, in the adsorption studies, to investigate the mechanism of competition removal of several antibiotics in the adsorption treatment process. This is mainly because of the analysis of wastewater samples taken from the effluents from hospitals and drugs factories, which showed that various antibiotics can be present simultaneously in this wastewater (Lien et al., 2016; Binh et al., 2018). Therefore, the present research aims to prepare and characterize the CPS. Later, the adsorption performance of the CPS toward simultaneous removal of tetracycline (TEC), amoxicillin (AMO), and ciprofloxacin (CIP) antibiotics is studied in detail, where the effect of some environmental parameters on the adsorption behavior are taken into consideration.
2 Materials and methods
2.1 Chemicals
Powdered TEC hydrochloride (chemical formula: C22H24O8N2.HCl, molecular weight: 480.9 g/mol, and purity: 98%), AMO (chemical formula: C16H19N3O5S, molecular weight: 365.4 g/mol, and purity: 97.5%), and CIP hydrochloride (chemical formula: C17H19ClFN3O3, molecular weight: 367.8 g/mol, and purity: 99%) were obtained from a general company in the drugs industry (Samarra, Iraq) without further purification (original manufacturer: Merck, Germany). Fig. 1 shows the chemical structures of these three antibiotics. The ternary stock solution of TEC, AMO, and CIP of 500 mg/L each, was prepared by dissolving an appropriate quantity of powdered antibiotics in 1 L of deionized water. To get a homogenous solution, the stock solution was vigorously stirred for 30 min, thereafter, the working solutions were prepared by dilution. On account of the instability in the laboratory conditions, the stock and diluted solutions were prepared at the time of the experimental study. The pH value of the solutions was controlled during the experiments by addition of the buffer solution dropwise.
The chemical structure of TEC (a); AMO (b) CIP, and (c) antibiotics (Ghadim et al., 2013; Sharma et al., 2009).
2.2 Synthesizing of the CPS
In the present study, the target adsorbent of CPS was prepared in the laboratory according to the following basic methodology. Initially, a certain quantity of pistachio shells was collected from the flour mills. Then, it was subjected to the following steps: washing with deionized water, oven drying at 105 °C overnight, grinding, and sieving. The quantity of retained powder on mesh No. 200 was collected and kept in an appropriate container. On the other hand, 300 g of ZnO nanoparticles were purchased from Xi’an Lyphar Biotec (China). The obtained ZnO nanoparticles were dispersed thoroughly in acetone under a sonolysis process for 30 min. Following this, the powdered pistachio shells were added to the ZnO nanoparticles solution at a ratio of 5:1 (g/g) and vigorously stirred until a homogenous mixture was obtained. Next, the liquid was decanted and the resulting precipitate of CPS was filtered, and placed in an oven at 60 °C until complete dryness. Finally, the dried CPS was stored in flasks with stoppers to be used in the required experiments.
2.3 Characterizations
An X-ray diffractometer (XRD, Philips-Magix Pro MPD, Netherlands) was used to obtain the XRD patterns of the crystalline structure of CPS. These patterns were collected in a range of 2θ, from 5° to 60°, with a step width of 0.02° and scan rate of 1° per second. Scanning electron microscopy (SEM) images were taken using the SEM device (TESCAN-Vega3, Czech Republic). These images helped to detect the changes in the surface morphology that occurred due to adsorbent synthesizing or due to the adsorption process. The elemental composition of CPS was obtained using EDS analysis technique. Furthermore, to identify the surface morphology and size of the ZnO nanoparticles, a Philips-CM30 scanning transmission electron microscopy (TEM, Netherlands) was used for taking a TEM image. A Shimadzu FTIR spectroscopy instrument (Japan) was used to detect the functional groups existing on the CPS and their significance in the removal process. Notably, these analyses were carried out within the wave number region of 500–4000 cm−1, as all the well-known groups of agricultural adsorbents fell within this range. The specific surface area of PS and CPS was determined using Brunauer–Emmett–Teller (BET) analysis. For this test, a CPS sample of 120 mg was transferred to the BET holder tube (inner diameter = 0.7 cm) and degassed at temperature of 150 °C for 4 h, under vacuum, before analysis. Moreover, the pH of the zero point of charge (pHzpc) of CPS was determined according to the method described in the article by Mohseni-Bandpi et al., (2016). This test, in fact, was essential to identify the type of CPS surface charge, with respect to the pH of the solution.
2.4 Adsorption experiments
The adsorption experiments in this study were performed in a batch-ternary system of three antibiotics. The experiments in this study were conducted using varying factors: pH (3–9); adsorbent dose (0.02–1 g/100 mL antibiotic solution); contact time (0–120 min); initial antibiotic concentration (30–70 mg/L), and temperature (25–35 °C). For this purpose, several flasks of 500 mL were filled with 100 mL solution containing 100 mg/L of a mixture of TEC; AMO, and CIP antibiotics. The antibiotic solutions in each flask were mixed with CPS of dose = 0.1 g/100 mL (expect in the adsorbent dose experiment) and shaken for an equilibrium time, as determined from a contact time experiment, and at a constant shaking speed of 150 rpm. The optimized value of each parameter, in which the highest adsorption capacity was recorded, was used in the subsequent experiment. At different time intervals during the adsorption experiments, three replicates of samples, of 3 mL each, were taken out from each flask and analyzed for the remaining antibiotic concentrations. The initial and remaining TEC; AMO, and CIP concentrations were determined according to the calibration data that were obtained by using UV/VIS spectrophotometers, with an absorbency reading of peak wavelengths at 360; 272, and 276 nm, respectively. It is worth noting that the adsorption data of the effects of temperature, contact time at different initial concentrations values, and adsorbent dose, were utilized in the thermodynamic, kinetic, and isotherm studies, respectively. The ability of the CPS to remove the antibiotic pollutants from aqueous solutions was judged according to the values of the removal efficiency (
) and adsorption capacity (
, mg/g) (Noroozi et al., 2018; Mohseni-Bandpi et al., 2016), which are calculated by the following two formulae:
3 Results and discussion
3.1 Characterization of ZnO, PS, and CPS
A high resolution TEM image (Fig. 2) showed that the ZnO nanoparticles have an irregular shape, but most of them are roughly spherical with an average size of <100 nm. Thus, these particles are within the nano-size range. Also, the agglomeration of these nanoparticles is low, as their dispersion appears to be in a mono-dispersed manner. This is a positive adsorptive characteristic for ZnO nanoparticles, as the mono-dispersed particles offer a maximum benefit from the surface area that is allocated for adsorption of the pollutant molecules, rather than multi-dispersed particles (Bazrafshan et al., 2019; Chen et al., 2011). Therefore, ZnO can be a promising material for coating of adsorbents with low adsorption capacity.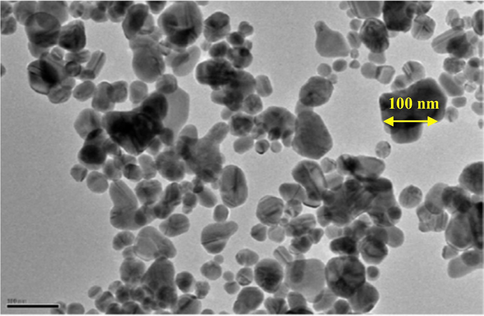
TEM image of ZnO nanoparticles.
The typical SEM micrographs of PS and CPS before and after simultaneous adsorption of TEC, AMO, and CIP molecules are presented in Fig. 3. Clearly, the morphological characteristics of PS (Fig. 3a) are completely changed after coating with ZnO nanoparticles as seen in Fig. 3b. The SEM of the CPS indicates that the surface of this adsorbent is coarse; as well as it consists of several non-uniform and separated aggregates. In addition, there are many big ravines and long grooves in the outer wall of CPS. In fact, these morphological properties of the CPS surface represent a positive point, as they provide a high surface area and active sites for sorbing the adsorbate molecules. In this manner, the specific surface area of PS has been determined to be 0.97 m2/g, which significantly increases to 4.24 m2/g, after coating with ZnO nanoparticles. On comparing Fig. 3b and the SEM image of CPS after adsorption (Fig. 3c), it can be seen that the morphological properties of CPS are significantly altered during the simultaneous antibiotic adsorption. Noticeably, the surface of CPS becomes smoother, and several pre-separated aggregates are coalesced. This situation appeared as a result of adsorption of three antibiotics on active sites of the CPS.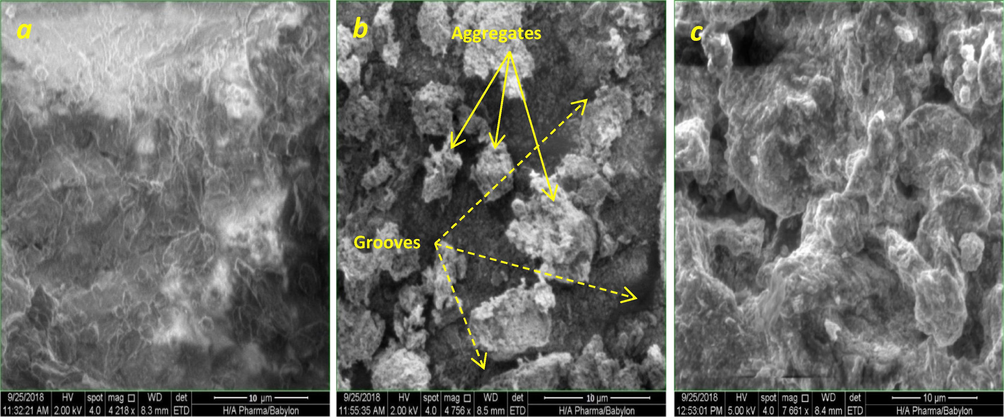
SEM micrographs of PS (a) and CPS before (b), and after (c) simultaneous adsorption of TEC; AMO, and CIP molecules.
According to the FT-IR analysis of the raw PS sample (Fig. 4a), the presence of fats in this agricultural material is generally detected by bands of aliphatic C—H, stretching in the range of 3000–2800 cm−1 and C⚌O bonding in the range of 1745–1725 cm−1. Although, carbohydrates have defining bands in the range of 1400–800 cm−1, due to bonds between the glucose polymer chains (Stuart, 2004). In fact, the pistachio FT-IR spectrum consists of four functional groups of importance in the present study (see Fig. 4a). The broad band detected at 3414 cm−1 is assigned to the O—H bond of alcohol. The band detected at 2929.09 cm−1 is attributed to the appearance of a C—H bond stretching functional group. The two bands at 1500.62 and 1384 cm-1are related to bonds between polymer chains. Finally, the band at 894.97 cm−1 is assigned to vibrations of benzene and its derivatives (Piness, 2010). These groups offer effective sites for adhering ZnO nanoparticles. Fig. 4b is the result of the FT-IR analysis of CPS. Generally, the metal oxides demonstrate absorption bands in the FT-IR spectrum curve below 1000 cm−1 (Kumar and Rani, 2013). In this context, the band at 563.21 cm−1 corresponds to inorganic ZnO stretching (Handore et al., 2014). Compared to the spectrum of PS (Fig. 4a), there are obvious shifts in the CPS FT-IR bands (Fig. 4b) to new values: 3410.15, 2912.51, 1427.32, 1373.32, and 898.83 cm−1, and this can be attributed to interactions between raw PS and the coating nanoparticles of zinc oxide nanoparticles. Also, the appearance of bands at 1735.93 cm−1 belongs to the ν(C⚌O) vibration in the carbonyl group or carboxylic bonds. The occurrence of this group is probably from the reactive carbon-containing plasma groups, which are used during the synthesizing processes of ZnO nanoparticles (Rana et al., 2012). The band at 1604.77 cm−1 is attributed to the presence of a C⚌O ring or a C⚌C stretching of aromatic groups. The absorption of the two bands detected at 1330.88 and 1045.02 cm−1 correspond to the stretching vibration of the C—H and C—O groups of alcohol, respectively (Rana et al., 2012). Fig. 4c represents the FT-IR spectrum for CPS after the adsorption process of three pharmaceutical products. On comparing between the FT-IT spectrum for CPS before (Fig. 4b) and after (Fig. 4c) the adsorption process, it is obvious that the intensity of the band at 3410.15 in Fig. 4b is slightly increased. This is due to the overlapping of the —OH and —NH2 stretching vibrations by the pollutant molecules. In addition, bands observed at 1604.77, 1427.32, 1373.32, 1330.88, 1246.02, 898.83, and 555.5 cm−1 are shifted to new locations, which suggest the involvement of these functional groups in the sorbing of pollutant molecules. Weak bands at 1111 cm−1 are assigned to O-H stretching and deformation, which indicate the adsorption of water or a hydroxyl group on the metal surface of CPS (Rana et al., 2012). Moreover, absorption bands near 555.5 and 644.28 cm−1 normally correspond to ZnO stretching vibrations (Kumar and Rani, 2013). It can be seen that the band corresponding to ZnO (<1000 cm−1) was still exist in the FT-IR spectrum of the CPS after adsorption. This finding suggests that the ZnO nanoparticles are still attached onto PS surface during the adsorption process.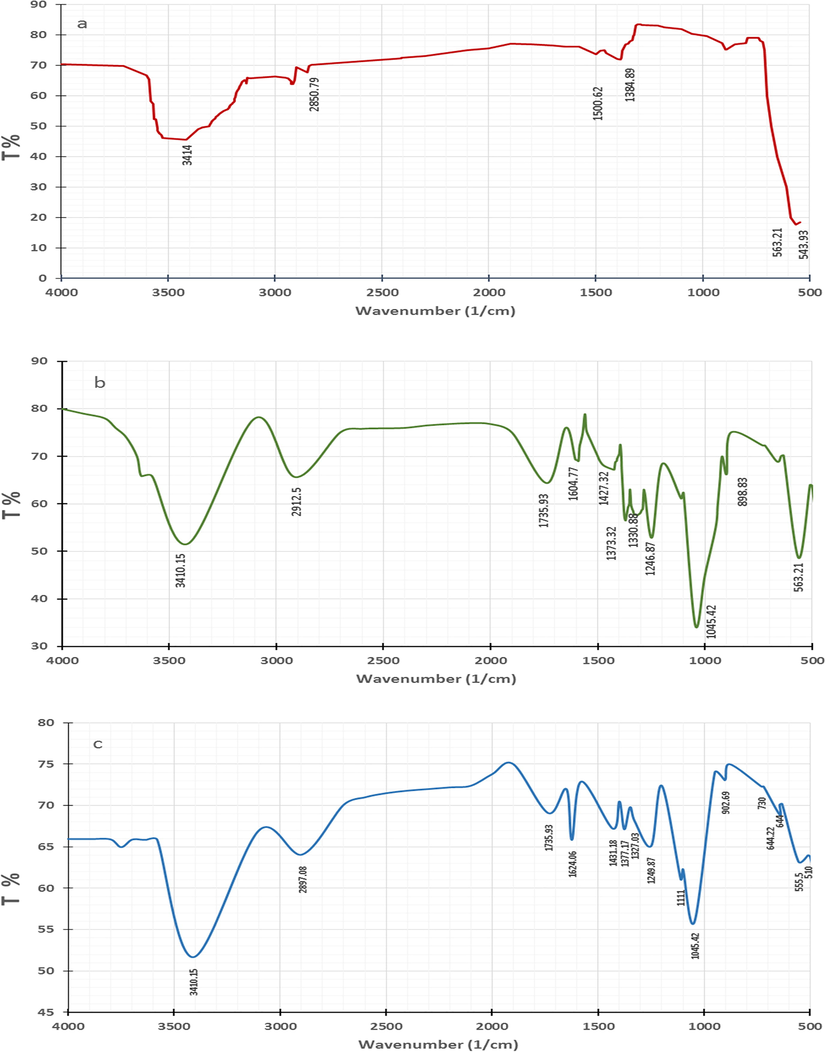
FT-IR analysis of PS (a), and CPS before (b), and after (c) simultaneous adsorption of TEC; AMO, and CIP molecules.
The results of XRD analysis of PS and CPS in the range of 5°–60° are presented in Fig. 5. It can be seen that the dominant material in the crystallographic structure of PS (Fig. 5a) is silica, as SiO2 compounds present naturally in PS. It is worth mentioning that similar to many agricultural materials, PS in its internal structure, is naturally rich in minerals such as zinc and magnesium (Ghaseminasab et al., 2015), so these elements appear in small traces in the PS XRD pattern. In the XRD spectra of CPS (Fig. 5b), additional peaks are observed after PS is coated with ZnO nanoparticles. The sharp peaks observed in the range of 30°–40° are associated with zinc of high quantity, confirming its existence as a PS coating layer (Bazrafshan et al., 2019).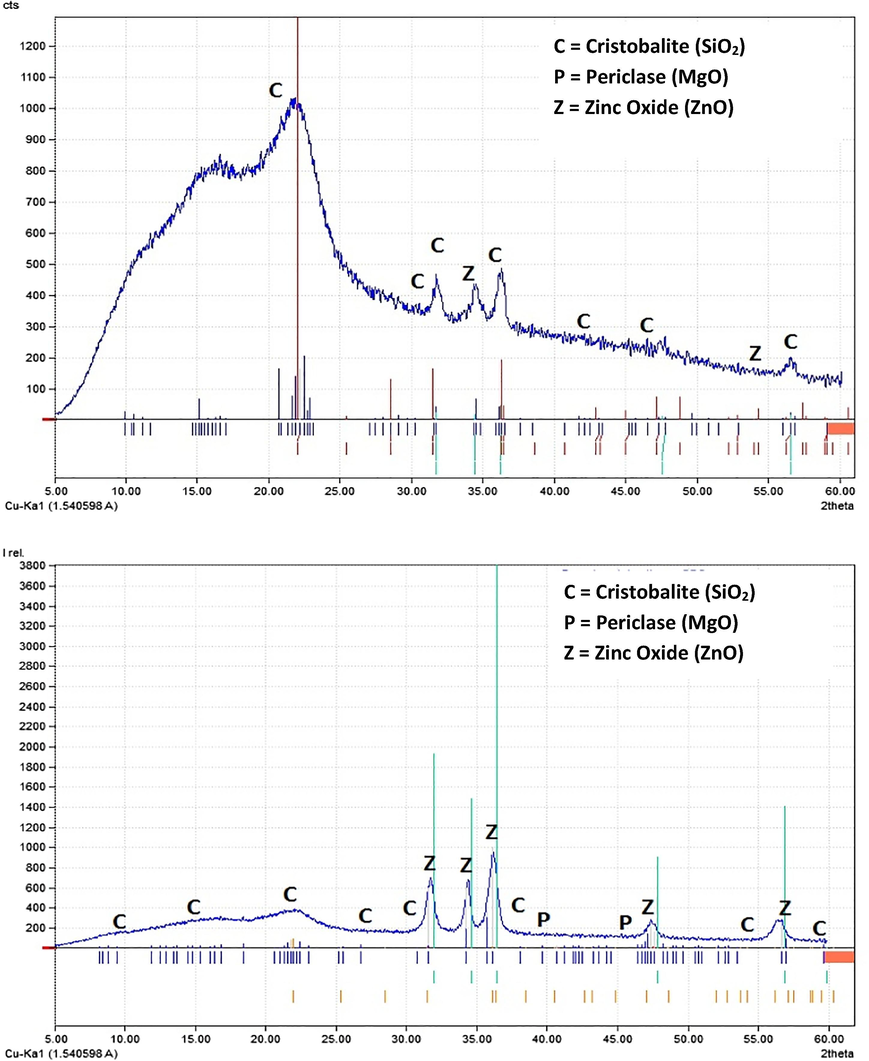
XRD spectra of PS (a) and CPS (b).
The elemental composition of CPS prior and post adsorption reactions with three antibiotics was obtained by capturing the EDS spectrums (Fig. 6). Clearly, the elements which appeared in the EDS spectrum of CPS before the adsorption are Mg, Si, C, Ca, and F. In addition, the mass ratio of Zn and O elements was high, which highlighted the successful interaction and shell of PS with ZnO nanoparticles. In addition, the Zn is also detected in the EDS spectrum of CPS after reaction. This confirms the stability of ZnO nanoparticles onto the CPS surface after adsorption process.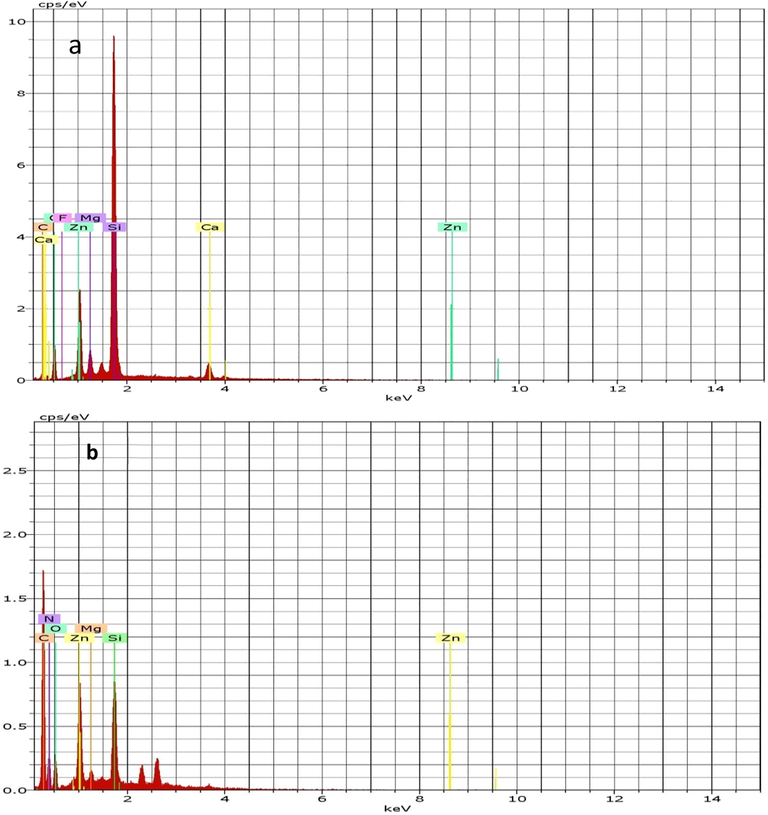
EDS spectra of CPS before (a) and after (b) adsorption process.
3.2 Adsorption mechanism
3.2.1 Effect of pH
In general, the removal efficiency of any pollutant in the adsorption system is dependable on the surface characteristics of the used adsorbent and speciation of the adsorbate, at specific pH values. In this case, the analysis of pHpzc is very important, to indicate the pH value at which the surface of the adsorbent is of neutral charge. In other words, the surface charge of the adsorbent is negative when the pH values are higher than pHpzc and vice versa (Sun et al., 2016). On the other hand, at different pH values the adsorbate molecules may have different surface charges, which greatly affect their tendency toward adsorbent particles. The variation in the removal percent of TEC, AMO, and CIP as a function of pH and contact time are displayed in Fig. 7. Clearly, the amount of each antibiotic adsorbed onto the CPS in the ternary system increases, showing a pH increase from 3 to 5, and subsequently it decreases with the increase of pH. Such adsorption behavior can be explained according to the electrostatic interactions between antibiotic molecules and surface of the CPS. For example, the TEC molecule has several ionizable functional groups like dimethyl ammonium, tricarbonyl amide, and phenol diketone groups. These active groups undergo protonation–deprotonation reactions, as a result they may be formation of a species of H2TEC+ at pH < 3.3. While, the zwitterion species of H2TEC0 are formed in case the pH of the solution ranges from 3.3 to 7.7. At pH > 7.7, anion species of HTEC−/TEC2− are dominant in the aqueous solution (Hsu et al., 2018). According to the analysis, the pHpzc of the CPS was determined to be 6.5. Also, it is important to note that the surface of the ZnO nanoparticle typically comprises of hydroxyl (OH) groups of neutral charge. The charge of this group may vary according to the pH value (Qu and Morais, 1999; Qu and Morais, 2001). At pH > pHpzc, the H+ ions leave the particle surface owing to a negative charge of ZnO with partially bonded oxygen atoms, that is, ZnO−. Conversely, at pH < pHpzc, ions of H+ are transferred to the particle surface and combined with the OH– groups leading to a positive charge of the ZnO surface due to formation of ZnOH2+ groups. Under these circumstances, the net surface charges of the ZnO nanoparticles and CPS at pH = 5 are positive, thus electrostatic attraction may take place among them and the negatively charged tricarbonylamide groups of TEC, which results in high TEC removal efficiency. When the pH is below 5, the electrostatic repulsion between the positively charged ZnO nanoparticles and cationic moieties of TEC causes a decrease in the TEC adsorption efficiency. Similar results were obtained in the case of AMO adsorption onto CPS. For CIP adsorption, it is known that the molecules of this antibiotic are positively charged at aqueous solutions of pH lower than 4 where about 99% of the CIP molecules exist as CIP+ (cationic), while at a pH higher than 10, over 95% of CIP molecules are present in a CIP- form (anionic). At the pH = 7, the CIP0 (zwitterion) is dominant within the system (Wu et al., 2010). It is observed that CIP adsorption is favored wherever the dissimilarity of adsorbents and CIP molecules charges occur. This is mainly due to the fact that when the adsorbate molecules and adsorbent surface have dissimilar charges, electrostatic attraction forces tend to exist, which can benefit the adsorption process. Therefore, a chemisorption reaction may occur between the CIP molecules and CPS active sites at pH = 5.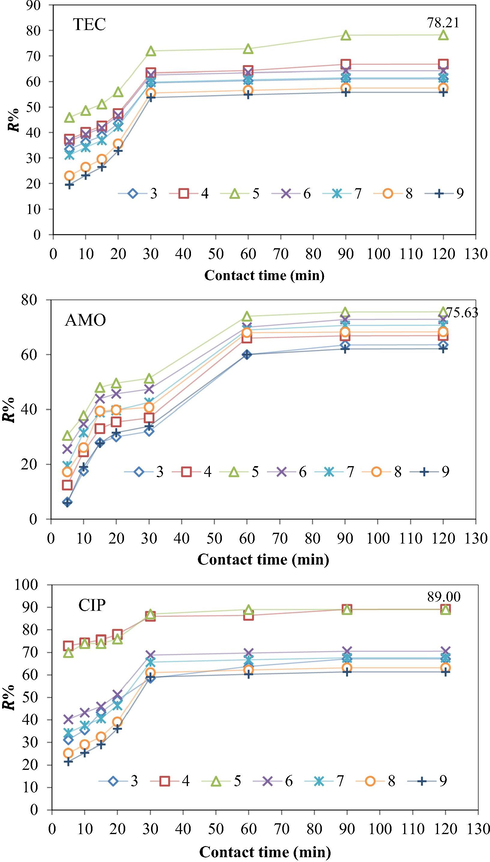
Effect of pH on the TEC, AMO, and CIP adsorption efficiency onto CPS in the ternary system (initial antibiotic concentration = 60 mg/L, temperature = 25 ± 2 °C, agitation time from 0 to 120 min, agitation speed = 150 rpm, adsorbent dose = 0.1 g/100 mL).
3.2.2 Effect of contact time
In addition to the effects of the pH solution, the results plotted in Fig. 7 also show the simultaneous effect of an increase in the contact time. The removal efficiencies of three antibiotics presented in the ternary system increase with prolonged contact time and then they reach plateau adsorption within the first 30 min for TEC and CIP, and 60 min for AMO. For all the results recorded during the first 30 min of contact time, the removal rate of TEC, AMO, and CIP has been fast, and this can be attributed to the abundance of uncovered adsorption sites on the used adsorbent that are not occupied by contaminant molecules. In fact, the rapid pollutant removal by the applied adsorbent is considered as a positive property. Therefore, such adsorbents are good agents in the adsorption treatment processes. Subsequently, as the contact time moves from 60 to 120 min, the removal rate will slow down for all pH values. This phenomenon can be accounted as being due to the continuous decrease in both the adsorbent reaction sites and the concentration of non-adsorbed or non-reacted molecules of TEC, AMO, and CIP, until that moment, thus the removal percentage of antibiotic molecules increases slowly. The highest removal of TEC, AMO, and CIP efficiencies is reached at pH = 5 and a contact time of 120 min can be ranked as: CIP > TEC > AMO. The obtained results are compared with other similar researches (Mohammed et al., 2019; Abdelkareem et al., 2019) and are found to be alike.
3.2.3 Thermodynamic study
The antibiotic removal efficiency was examined as a function of temperature from 25 °C to 35 °C and the results are depicted in Fig. 8. This figure indicates that the increase in temperature had minimal impact on the adsorption ability of CPS for the three antibiotics. However, the removal efficiencies of three antibiotics were slightly decreased with the increase in the temperature. Indeed, the increase in the temperature will decrease the viscosity of the aqueous solution bearing the pollutant molecules which in turn permits the increase of diffusion rate of the adsorbate across the bulk (external) and pore (internal) boundaries of the adsorbent particles (Mohammed et al., 2019).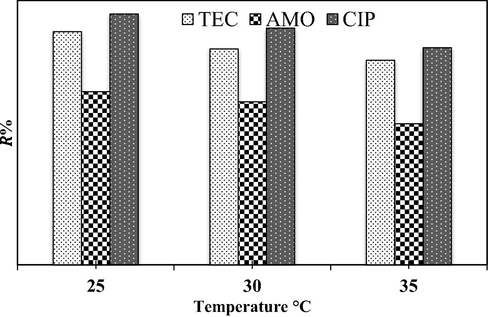
Effect of temperature on the TEC, AMO, and CIP adsorption efficiency in the ternary system (initial antibiotic concentration = 60 mg/L, CPS dose = 0.1 g/100 mL, agitation time = 120 min, agitation speed = 150 rpm, pH = 5).
To investigate the thermodynamic analysis of CPS for simultaneous adsorption of TEC; AMO, and CIP, the thermodynamic parameters of Gibbs free energy change (ΔG°, kJ/mol); surface adsorption of entropy change (ΔS°, kJ/mol K), and enthalpy change (ΔH°, kJ/mol) were calculated using the following equations (Khodadadi et al., 2019):
In the present study, the thermodynamic analysis was conducted based on the obtained data of Fig. 7, and the results of this analysis are tabulated in Table 1. It can be evidenced that the adsorption reactions of the TEC, AMO, and CIP adsorption onto CPS was spontaneous, as negative values of
were determined for all the temperatures. Although, the negative values of
and
demonstrated that the nature of the adsorption was exothermic, and there was a decrease in randomness occurring during the reaction of TEC; AMO, and CIP with the CPS particles.
Adsorbate
T (°C)
qe (mg/g)
Thermodynamic parameters
(kJ/mol)
(kJ/mole)
(kJ/mole K)
AMO
25
47.570
−3380.260
−30.347
−90.498
30
46.192
−2927.734
35
43.191
−2475.250
TEC
25
55.744
−5861.166
−86.033
−269.030
30
53.383
−4516.001
35
51.863
−3170.836
CIP
25
58.150
−5824.924
−38.415
−109.362
30
56.212
−5278.114
35
53.541
−4731.304
3.2.4 Effect of antibiotic concentration and kinetic study
The effect of the initial TEC, AMO, and CIP concentrations varied from 30 to 60 mg/L and their removal percentage by CPS was further tested. These experiments were conducted under environmental conditions: pH = 5; CPS dose = 0.1 g/100 mL; contact time = 0 to 120 min; agitation speed = 150 rpm, and temperature = 25 ± 2 °C. The evolution curves of the removal efficiencies depicted in Fig. 9 (a to c), demonstrated that the removal efficiencies of TEC, AMO, and CIP increased remarkably with an increase in the initial concentration. This was due to the high concentration gradients that formed at higher adsorbate concentrations (Gupta et al., 2017). The same explanation above was mentioned in the Arami et al. (2005) study.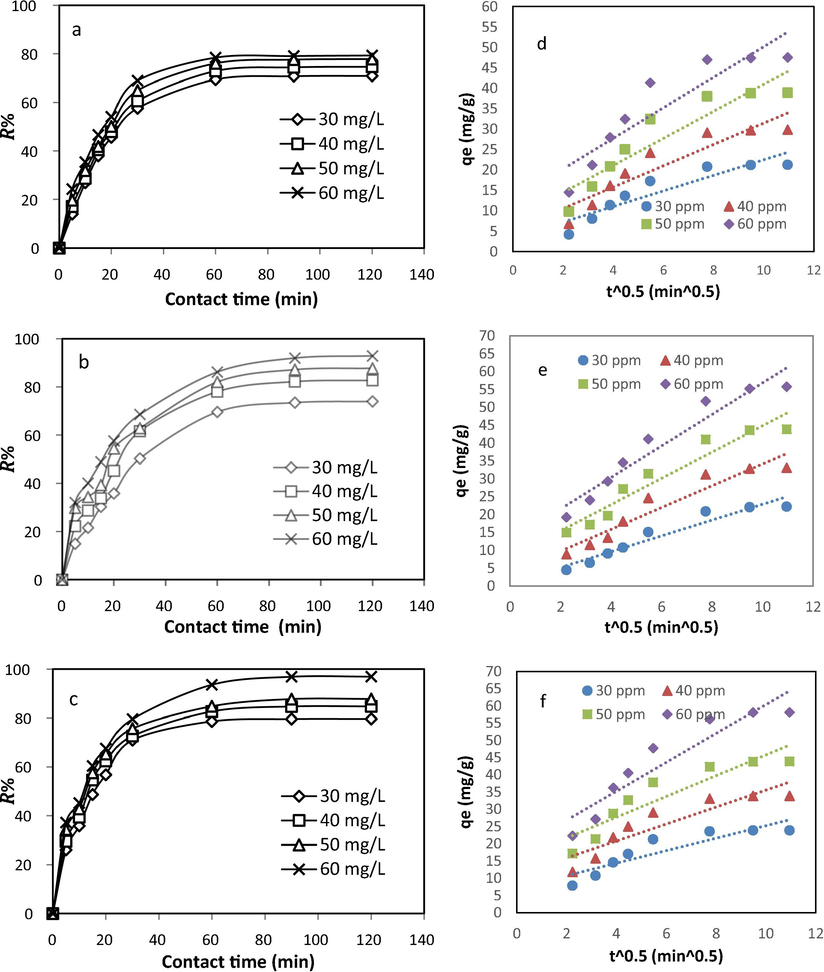
Effect of initial TEC (a), AMO (b), and CIP (c) concentrations on their adsorption efficiency in the ternary system, and results of the application of intra-particle diffusion model of TEC (d), AMO (e), and CIP (f) adsorption data onto CPS.
The adsorption kinetics study is a very important stage for any practical application of adsorption treatment systems (Brouers and Al-Musawi, 2018). This is due to the obtained kinetic results that provide valuable insights into the reaction types and pathways, and the mechanism of pollutant removal. As a result, the design, operation, and control of the treatment process depend largely on the results of the kinetic study. In this manner, there is a necessity that highlighted the need for the experimental kinetic data to be properly modeled and fitted with the relevant kinetic models, in order to have an accurate design of the treatment system (Brouers and Al-Musawi, 2018). In this study, the two kinetic models of pseudo-first-order (Eq. (6)) and pseudo-second-order (Eq. (7)) were used to model the kinetic data of TEC; AMO, and CIP adsorption onto CPS. These models are widely applied in literature for modeling of the adsorption data of inorganic and organic pollutant removal from water solutions. The parameters of each model with their corresponding coefficient of regression values are determined using nonlinear fitting algorithms using a MATLAB program, the results are tabulated in Table 2. Based on the R2 values, one can infer that the pseudo-second-order model was best-fitted equation for TEC; AMO, and CIP adsorption kinetic onto CPS. In addition for the pseudo-second-order model, a small deviation between the experimental and calculated uptake values, was determined. This finding indicates that the adsorption process of the TEC, AMO, and CIP, using CPS is chemical in nature (Mohammed et al., 2019; Aljeboree et al., 2017). Furthermore, in order to account for the contribution of intra-particle diffusion mechanism in all the kinetics of TEC, AMO, and CIP adsorption processes, the data were evaluated with the intra-particle diffusion model (Eq. (8)). This model is often used to determine if intra-particle diffusion is the dominant rate-limiting step or there is another process like film, surface, or pore diffusion that can control the kinetics of the process (Boparai et al., 2011; Ozcan et al., 2007). According to Eq. (8), in case the linear trend linefit of qt versus t0.5 point passes through or is close to the origin point (i.e., the parameter
), then the intra-particle diffusion is the sole rate-limiting step in the adsorption kinetic. Therefore, the kinetic data of TEC, AMO, and CIP adsorption onto CPS at different initial concentrations is plotted according to the intra-particle diffusion equation (Eq. (8)) and the results are presented in Fig. 9 (d to f). These figures show that the plots of the kinetic data, according to the intra-particle diffusion equation, are not fitted to the linear equations. In addition, all the calculated
values are higher than zero. This finding indicates that the adsorption process of TEC, AMO, and CIP onto CPS is controlled by more than one mechanism of adsorption and intra-particle diffusion is not the dominant mechanism in the adsorption process.
Adsorbate
C0 (mg/L)
qe exp. (mg/g)
Pseudo-first order
Pseudo-second order
Intra-particle diffusion
k1 (min−1)
qe cal. (mg/g)
R2
k2 (g/mg min)
qe cal. (mg/g)
R2
C
R2
AMO
30
22.19
0.0564
27.93
0.9855
0.00117
27.01
0.9905
2.2097
0.77
0.9313
40
33.09
0.0561
40.11
0.9874
0.00109
38.33
0.9898
3.0495
3.67
0.9147
50
43.87
0.0541
47.05
0.9775
0.00108
45.59
0.9931
3.6767
8.07
0.9275
60
55.74
0.0487
50.05
0.9854
0.00102
55.00
0.9992
4.41
12.74
0.9356
TEC
30
21.26
0.0716
27.77
0.9966
0.00208
24.91
0.9899
1.9049
3.448
0.8472
40
29.85
0.0662
35.02
0.9983
0.00163
31.39
0.9990
2.6106
5.3569
0.8557
50
37.9
0.0666
45.18
0.9484
0.00138
39.99
0.9937
3.3125
7.8523
0.853
60
48.35
0.068
45.18
0.9878
0.00148
48.01
0.9906
3.7397
12.786
0.8306
CIP
30
23.89
0.085
31.00
0.9909
0.00330
26.10
0.9966
1.8126
7.1177
0.8114
40
33.889
0.0818
40.08
0.9654
0.00250
36.16
0.9992
2.4593
10.96
0.8255
50
43.88
0.0793
53.11
0.9462
0.00220
47.09
0.9981
3.0106
15.66
0.8355
60
58.14
0.0809
80.92
0.9427
0.00140
64.51
0.9981
4.1911
18.458
0.8767
3.2.4.1 Effects of CPS dose and isotherm study
From an economic perspective, the determination of an optimum adsorbent dose is one of the crucial factors that must be found in adsorption process studies. Therefore, in the present study the effect of the adsorbent dose is studied within the range of CPS content of 0.02–0.1 g in 100 mL of 60 mg/L antibiotic solution (Fig. 10). It can be seen that with an increase in the CPS dose from 0.02 to 0.06 g/100 mL, the adsorption efficiency of TEC, AMO, and CIP proportionately increases. The reason behind this behavior with the rise in the adsorbent amount in the aqueous solution is the increase in the surface area or active sites for reaction of the pollutant molecules. In case of an increase in the adsorbent dose above 0.06 g/100 mL, the removal efficiencies are not altered greatly. The agglomeration of some CPS magnetic nanoparticles at high doses in the solution is the factor behind the decrease in the percentage of removal rate, due to the aggregation of the available active groups of the used adsorbent (Bazrafshan et al., 2019). Moreover, the recorded sequence of the removal efficiencies in the pH study is not altered here and is still at the rank of CIP > TEC > AMO for all the studied values of the adsorbent dose.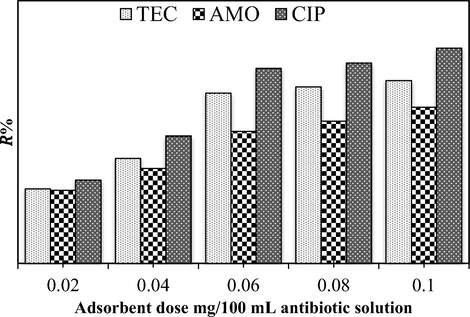
Effect of CPS dose on the TEC, AMO, and CIP adsorption efficiency in the ternary system (initial antibiotic concentration = 60 mg/L, temperature = 25 ± 2 °C, contact time from 0 to 120 min, agitation speed = 150 rpm, and pH = 5).
Langmuir (Eq. (9)) and Freundlich (Eq. (10)) isotherm models were used to simulate the experimental equilibrium data. In recent times, these two models have been widely used in published articles with regard to modeling of the experimental isotherm data of different adsorbents (Khodadadi et al., 2019; Martins et al., 2015; Sulaymon et al., 2014).
The models constants and correlation coefficients were calculated using non-linear regression; the results are presented in Table 3. Besides, Fig. 11 displayed the plot of the obtained theoretical and experimental data. It is remarkable that the adsorption data of both TEC and CIP on CPS have adjusted well to the Freundlich model, in which the adequate correlation coefficient values were determined. On the other hand, the Langmuir isotherm model was found to be the better model to describe AMO adsorption onto CPS. These findings were enlightening a complex mechanism of simultaneous antibiotic adsorption onto the adsorbent’s surface, wherein the CIP surfaces comprise of different adsorption sites; therefore, each antibiotic reacted differently from the other with the CIP. The TEC and CIP molecules reacted on a multi-layer and heterogeneous active sites of the surface of CPS, whereas, the adsorption of AMO molecules were characterized by a monolayer adsorption type that occurred on a homogeneous CPS surface. The maximum adsorption uptakes, based on Langmuir model analysis, were high, and found to be 92.45, 98.717, and 132.240 mg/g for TEC, CIP, and AMO, respectively. In addition, the results of the best-fit model, represented by n values of TEC and CIP adsorption and the RL value of AMO, determined using Eq. (11), indicated that the adsorption process using CIP adsorbent was favored. As the n values were determined to be <1, a chemisorption process was involved for the three antibiotic adsorptions onto CIP (Aljeboree et al., 2017; Kumar et al., 2010). These results were quite consistent with our findings in the kinetic study. In addition, Table 4 lists the maximum uptakes for TEC, CIP, and AMO adsorption using current adsorbent and other studied materials that taken under investigation in previous works. Based on the results presented in this table, the CPS exhibited high performance toward elimination of antibiotic molecules in the adsorption treatment process when compared with other adsorbens.
Model
Parameter
TEC
CIP
AMO
Langmuir
qmax (mg/g)
92.450
98.717
132.24
KL (L/mg)
0.146
0.149
0.103
RL
0.102
0.1
0.139
R2
0.995
0.989
0.997
Freundlich
kf (mg/g)
33.382
33.176
26.66
1/n
4.233
3.848
2.56
R2
0.997
0.995
0.989
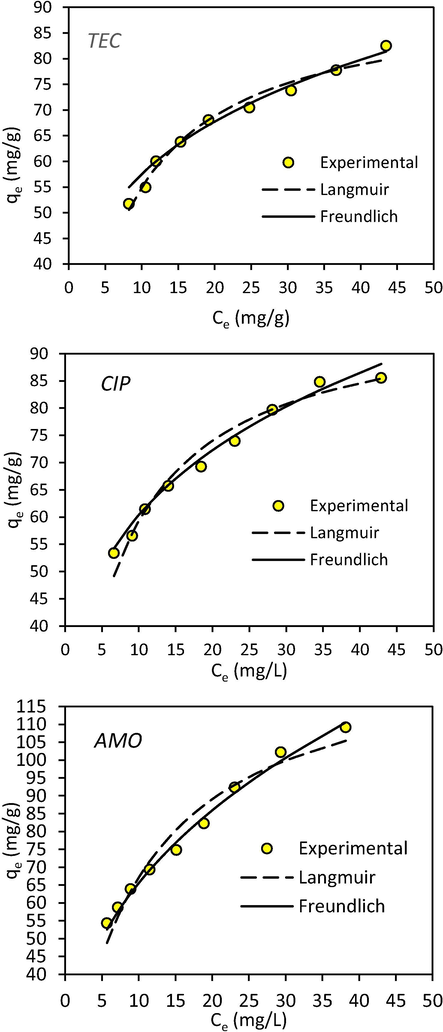
A graph showing the experimental and theoretical isotherm values of TEC, CIP, and AMO onto CPS.
Adsorbent
(mg/g)
Reference
TEC
The red soil
12.00
Wang et al., (2010)
HCl-modified zeolite
20.40
Zou et al., (2012)
Bamboo charcoal
23.50
Liao et al., (2013)
Hydroxyapatite/clay
76.02
Ersan et al., (2015)
Nanosheet-layered double hydroxide
98.04
Soori et al., (2016)
CPS
98.71
This study
CIP
Corylus avellana (hazelnut) activated carbon
73.64
Balarak et al., (2016)
ZnO nanoparticles
8.30
Dhiman and Sharma (2018)
Groundnut (Arachis hypogaea) shell powder
8.86
Dhiman and Sharma (2018)
MgO nanoparticles
3.46
Khoshnamvand et al., (2017)
Magnetic activated carbon/chitosan
90.00
Danalıoğlu et al., (2017)
CPS
92.45
This study
AMO
Almond shell
2.50
Homem et al., (2010)
MNPs-PAC
142.85
Kakavandi et al., (2013)
Chitosan beads
8.71
Adriano et al., (2005)
Olive stone activated carbon
57.00
Limousy et al., (2016)
Magnetically modified grapheme nano-platelets
14.10
Kerkez-Kuyumcu et al., (2016)
CPS
132.24
This study
4 Conclusions
The present study has investigated the adsorptive performance of the CPS toward TEC, AMO, and CIP adsorption in the ternary system. The CPS adsorbent was characterized by advanced means of TEM, SEM, XRD, and FT-IR. The results of the characterization study demonstrated that the coating process of the pistachio shell by ZnO nanoparticles had positive effects on the prepared adsorbent, in terms of practical utilization and adsorption ability. In addition, the parameters of the adsorption process were optimized with the variation in values of pH, contact time, initial antibiotic concentration, temperature, and CPS dose. From the optimized data of the effect study, the isotherm, kinetic, and thermodynamic studies were accomplished. The isotherm data of AMO adsorption could be predicted using the Langmuir model, while the TEC and CIP isotherm data followed the Fruendlich model. Thus, the isotherm study showed that the surface of CPS comprised of both heterogeneous and homogeneous adsorption sites that were ready for reaction with antibiotic molecules. The thermodynamic parameters highlighted that the nature of the simultaneous adsorption process was spontaneous and exothermic. The CPS maximum adsorption capacity based on the Langmuir model results for the three antibiotics could be ranked as: AMO > CIP > TEC. The kinetic data of the three antibiotics are adequately modeled by the pseudo-second-order model, indicating that the nature of the kinetic adsorption is chemical. In addition, the kinetic study showed that there was more than one process that controlled the kinetic rate. The present study shows CPS to be an effective adsorbent, which can be used for the sequestration of antibiotics in the adsorption treatment systems. In the future studies, it is recommended to identify the magnetic force of CPS and the effect of agitation speed on the adsorption performance of CPS.
Acknowledgments
The researchers appreciate the scientific cooperation between the University of Baghdad (Iraq) and the IsraUniversity (Jordan). Also, a special thanks to GlobalEdico (India) for their valuable efforts to edit and improve the English language of this research.
References
- Photocatalytic activity of ZnO coated magnetic crosslinked chitosan/polyvinyl alcohol microspheres. Mater. Sci. Eng., B. 2018;228:7-17.
- [Google Scholar]
- Adsorption of amoxicillin on chitosan beads: Kinetics, equilibrium and validation of finite bath models. Biochem. Eng. J.. 2005;27(2):132-137.
- [Google Scholar]
- Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arabian J. Chem.. 2017;10:S3381-S3393.
- [Google Scholar]
- What can the use of well-defined statistical functions of pollutants sorption kinetics teach us? A case study of cyanide sorption onto LTA zeolite nanoparticles. Environ. Technol. Innov.. 2018;10:46-54.
- [Google Scholar]
- Optimization the effects of physicochemical parameters on the degradation of cephalexin in sono-fenton reactor by using box-behnken response surface methodology. Catal. Lett.. 2019;149(5):1186-1196.
- [Google Scholar]
- Removal of dyes from colored textile wastewater by orange peel adsorbent: Equilibrium and kinetic studies. J Colloid Interface Sci.. 2005;288:371-376.
- [Google Scholar]
- Adsorption kinetics and equilibrium of ciprofloxacin from aqueous solutions using corylus avellana (Hazelnut) activated carbon. Brit. J. Pharm. Res.. 2016;13(3):1-14.
- [Google Scholar]
- Pre-Treatment of Antibiotic Formulation Wastewater by O3, O3/H2O2, and O3/UV Processes. Turk. J. Eng. Environ. Sci.. 2004;28(5):325-332.
- [Google Scholar]
- Photocatalytic degradation of catechol using ZnO nanoparticles as catalyst: Optimizing the experimental parameters using the Box-Behnken statistical methodology and kinetic studies. Microchem. J.. 2019;147:643-653.
- [Google Scholar]
- Thai P.K., Antibiotics in the aquatic environment of Vietnam: Sources, concentrations, risk and control strategy. Chemosphere. 2018;197:438-450.
- [Google Scholar]
- Study on the environmental risks of medicinal products, Final Report prepared for Executive Agency for. Health Consum. 2013
- [Google Scholar]
- Microbial diversity and antibiotic resistance in a final effluent-receiving lake. Sci. Total Environ.. 2019;650:2951-2961.
- [Google Scholar]
- H.K. Boparai M. Joseph D.M. O’Carroll Kinetics and thermodynamics of cadmium ion removal by adsorption onto nanozerovalent iron particles, J. Hazard. Mater. 2011, 186: (1): 458-465.
- Brouers-Sotolongo fractal kinetics versus fractional derivative kinetics: a new strategy to analyze the pollutants sorption kinetics in porous materials. Hazard. Mat.. 2018;350:162-168.
- [Google Scholar]
- Removal of benzene by ZnO nanoparticles coated on porous adsorbents in presence of ozone and UV. Chem. Eng. J.. 2012;181–182:215-221.
- [Google Scholar]
- Removal of methyl orange from aqueous solution using bentonite-supported nanoscale zero-valent iron. J. Colloid Interface Sci.. 2011;363:601-607.
- [Google Scholar]
- Highly efficient removal of ceftiofur sodium using a superior hydroxyl group functionalized ionic liquid-modified polymer. Sci. Total Environ.. 2019;662:324-331.
- [Google Scholar]
- Efficient removal of antibiotics by a novel magnetic adsorbent: Magnetic activated carbon/chitosan (MACC) nanocomposite. J. Mol. Liq.. 2017;240:589-596.
- [Google Scholar]
- A review of the biochemisty of heavy metals biosorption by brown algae. Water Res.. 2003;37:4311-4330.
- [Google Scholar]
- Batch adsorption studies on the removal of ciprofloxacin hydrochloride from aqueous solution using ZnO nanoparticles and groundnut (Arachis hypogaea) shell powder: a comparison. Indian Chem. Eng. 2018:1-10.
- [Google Scholar]
- Synthesis of hydroxyapatite/clay and hydroxyapatite/pumice composites for tetracycline removal from aqueous solutions. Process Saf. Environ. Prot.. 2015;96:22-32.
- [Google Scholar]
- Humic acid adsorption onto Mg/Fe layered double hydroxide, Colloids and Surfaces A: Physicochem. Eng. Aspects. 2008;331:195-201.
- [Google Scholar]
- Adsorption properties of tetracycline onto graphene oxide: equilibrium. Kinetic Thermodyn. Stud.. 2013;8(11):1-9.
- [Google Scholar]
- A review on pistachio: Its composition and benefits regarding the prevention or treatment of diseases. JOHE. 2015;4(1):57-69.
- [Google Scholar]
- Rapid adsorptive removal of toxic Pb2+, ion from aqueous solution using recyclable, biodegradable nanocomposite derived from templated partially hydrolyzed xanthan gum and nanosilica. BioresourTechnol.. 2014;170(5):578-582.
- [Google Scholar]
- Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater.Technol.. 2016;9:10-40.
- [Google Scholar]
- Application of response surface methdology to optimize the adsorption performance of a magnetic graphene oxide nanocomposite adsorbent for removal of methadone from the environment. J. Colloid Interface Sci.. 2017;497:193-200.
- [Google Scholar]
- Novel green route of synthesis of ZnO nanoparticles by using natural biodegradable polymer and its application as a catalyst for oxidation of aldehydes. J. Macromol. Sci., Part A: Pure Appl. Chem.. 2014;51:941-947.
- [Google Scholar]
- A comparative study of cellulose agricultural wastes (almond shell, pistachio shell, walnut shell, tea waste and orange peel) for adsorption of violet B dye from aqueous solutions. Orient. J. Chem.. 2014;30(4):2091-2098.
- [Google Scholar]
- Performance of iron-zero (nZVI) nanno particles in removal of cephalexin from synthetic wastewater. J Water Wastewater. 2014;25:85-92.
- [Google Scholar]
- Accumulation of cadmium, lead and nickel by fungal and wood biosorbents. Appl. Biochem. Biotechnol.. 1995;53:133-142.
- [Google Scholar]
- Amoxicillin removal from aqueous matrices by sorption with almond shell ashes. Int. J. Environ. Anal. Chem.. 2010;90(14–15):1063-1084.
- [Google Scholar]
- Adsorption and desorption of copper(II) ions onto garden grass. J. Bioresour. Technol.. 2012;121:386-395.
- [Google Scholar]
- Adsorption of tetracycline on Fe (hydroxides): effects of pH and metal cation (Cu2+, Zn2+ and Al3+) addition in various molar ratios. R. Soc. Open Sci.. 2018;5:171941. eCollection 2018 Mar
- [CrossRef] [Google Scholar]
- Removal of antibiotics and resistance genes from swine wastewater using vertical flow constructed wetlands: Effect of hydraulic flow direction and substrate type. Chem. Eng. J.. 2017;308:692-699.
- [Google Scholar]
- ZnO shell on mesoporous silica by atomic layer deposition: Removal of organic dye in water by an adsorbent and its photocatalytic regeneration. Appl. Surf. Sci.. 2014;307:468-474.
- [Google Scholar]
- Degradation of ciprofloxacin and sulfamethoxazole by ferrousactivatedpersulfate: implications for remediation of groundwater contaminated by antibiotics. Sci Total Environ.. 2014;472:800-808.
- [Google Scholar]
- Facile photochemical synthesis of ZnO nanoparticles in aqueous solution without capping agents. Materialia. 2018;2:104-110.
- [Google Scholar]
- Magnetic Fe3O4@C nanoparticles as adsorbents for removal of amoxicillin from aqueous solution. Water Sci. Technol.. 2013;69(1):147-155.
- [Google Scholar]
- Effective reduction of metronidazole over the cryptomelane-type manganese oxide octahedral molecular sieve (K-OMS-2) catalyst: facile synthesis, experimental design and modeling, statistical analysis, and identification of by-products. Environ. Sci. Pollut. Res.. 2018;25:34164-34180.
- [Google Scholar]
- Aqueous Phase Biosorption of Pb(II), Cu(II), and Cd(II) onto Cabbage Leaves Powder. Int. J. Chem. Reactor Eng.. 2017;15(2)
- [CrossRef] [Google Scholar]
- Antibiotic amoxicillin removal from aqueous solution using magnetically modified graphene nanoplatelets. J. Ind. Eng. Chem.. 2016;36:198-205.
- [Google Scholar]
- A comparative study of using barberry stem powder and ash as adsorbents for adsorption of humic acid. Environ. Sci. Pollut. Res. 2019
- [CrossRef] [Google Scholar]
- Kinetic and isotherm studies on ciprofloxacin an adsorption using magnesium oxide nanopartices. J. Appl. Pharm. Sci.. 2017;7(11):79-83.
- [Google Scholar]
- Structural and optical characterization of ZnO nanoparticles synthesized by microemulsion route. Int. Lett. Chem., Phys. Astron.. 2013;14:26-36.
- [Google Scholar]
- Adsorption of dye from aqueous solution by cashewnut shell: studies on equilibriumisotherm, kinetics and thermodynamics of interactions. Desalination. 2010;261:52-60.
- [Google Scholar]
- Influence of pH on cephalexin adsorption onto SBA-15 mesoporous silica: Theoretical and experimental study. Appl. Surf. Sci.. 2014;300:37-42.
- [Google Scholar]
- Three-dimensional hierarchical structures of ZnO nanorods as a structure adsorbent for water treatment. J. Mater. Sci. Technol.. 2017;33:864-868.
- [Google Scholar]
- Adsorption of tetracycline and chloramphenicol in aqueous solutions by bamboo charcoal: a batch and fixed-bed column study. Chem. Eng.. 2013;228:496-505.
- [Google Scholar]
- Antibiotics in wastewater of a rural and an urban hospital before and after wastewater treatment, and the relationship with antibiotic use—A one year study from Vietnam. Int J Environ Res Public Health. 2016;13(6):588.
- [Google Scholar]
- Amoxicillin removal from aqueous solution using activated carbon prepared by chemical activation of olive stone. Environ. Sci. Pollut. Res.. 2016;24(11):9993-10004.
- [Google Scholar]
- Removal of tetracycline by NaOH-activated carbon produced from macadamianut shells: kinetic and equilibrium studies. Che. Eng. J.. 2015;260:291-299.
- [Google Scholar]
- Role of Fe3O4 magnetite nanoparticles used to coat bentonite in zinc(II) ions sequestration. Environ. Nanotechnol. Monit. Manage.. 2018;10:17-27.
- [Google Scholar]
- Adsorption of tetracycline fom wastewater by using Pistachio shell coated with ZnO nanoparticles: Equilibrium, kinetic and isotherm studies. Alexandria Engineering Journal 2019
- [Google Scholar]
- Adsorptive performance of a mixture of three nonliving algae classes for nickel remediation in synthesized wastewater. J. Environ. Health Sci. Eng. 2019
- [CrossRef] [Google Scholar]
- 2016, Improvement of zeolite adsorption capacity for cephalexin by coating with magnetic Fe3O4 nanoparticles. J. Mol. Liq.. 2016;218:615-624.
- [Google Scholar]
- Removal of cyanide using surface-modified Linde type-A zeolite nanoparticles as an efficient and ecofriendly material. Water Process Eng.. 2018;21:44-51.
- [Google Scholar]
- A comprehensive performance evaluation of heterogeneous Bi2Fe4O9/peroxymonosulfate system for sulfamethoxazole degradation. Environ. Sci. Pollut. Res. 2017
- [CrossRef] [Google Scholar]
- Adsorption kinetics and isotherms of anionic dye of reactive blue 19 from aqueous solutions onto DTMA-sepiolite. In: Lewinsky A.A., ed. Hazardous Materials and Wastewater—Treatment, Removal and Analysis. New York: Nova Science Publishers; 2007.
- [Google Scholar]
- Physical and chemical structural analysis of pistachio shells. J. Undergrad. Mater. Res.. 2010;4
- [CrossRef] [Google Scholar]
- Effective removal of amoxicillin, cephalexin, tetracycline and penicillin G from aqueous solutions using activated carbon nanoparticles prepared from vine wood. J. Water Process. Eng.. 2014;1:64-73.
- [Google Scholar]
- The pH dependence of the surface charge density in oxide-based semiconductor nanoparticles immersed in aqueous solution. IEEE Trans. Magn.. 2001;37:2654-2656.
- [Google Scholar]
- Energy levels in metal oxide semiconductor quantum dots in waterbased colloids. J. Chem. Phys.. 1999;111:8588-8594.
- [Google Scholar]
- Influence of surface modification by 2-aminothiophenol on optoelectronics properties of ZnO nanoparticles. J. Exp. Nanosci. 2012:1-15.
- [Google Scholar]
- Adsorption of cephalexin from aqueous solution using natural zeolite and zeolite coated with manganese oxide nanoparticles. J. Mo. Liq.. 2015;2015(211):431-441.
- [Google Scholar]
- Fluoroquinoloneantibacterials: a review on chemistry, microbiology and therapeutic prospects. Acta PoloniaePharmaceutica -Drug Res.. 2009;66:587-604.
- [Google Scholar]
- Occurrence, toxicity and transformation of six typical benzotriazoles in the environment: A review. Sci. Total Environ.. 2019;661:407-421.
- [Google Scholar]
- Intercalation of tetracycline in nano sheet layered double hydroxide: an insight into UV/VIS spectra analysis. J. Taiwan Ins. Chem. Eng.. 2016;67:271-285.
- [Google Scholar]
- Infrared spectroscopy: fundamentals and applications. Sussex: Wiley; 2004.
- Comparative study of removal of cadmium (II) and chromium (III) ions from aqueous solution using low-cost biosorbent. Int. J. Chem. Reac. Eng.. 2014;12(1):1-10.
- [Google Scholar]
- Characterization and ciprofloxacin adsorption properties of activated carbons prepared from biomass wastes by H3PO4 activation. Bioresour. Technol.. 2016;217:239-244.
- [CrossRef] [Google Scholar]
- Arsenic removal from water/wastewater using layered double hydroxide derived adsorbents, a critical review. RSC Adv.. 2018;8:22694-22709.
- [Google Scholar]
- An innovative zinc oxide-coated zeolite adsorbent for removal of humic acid. J. Hazard. Mater.. 2016;313:283-290.
- [Google Scholar]
- Phosphate affects the adsorption of tetracycline on two soils with different characteristics. Geoderma. 2010;156(3–4):237-242.
- [Google Scholar]
- Adsorption and intercalation of ciprofloxacin on montmorillonite. Appl. Clay Sci.. 2010;50(2):204-211.
- [CrossRef] [Google Scholar]
- Removal of nutrients and veterinary antibiotics from swine wastewater by a constructed macrophyte floating bed system. J. Environ. Manage.. 2010;91:2657-2661.
- [Google Scholar]
- Removal of Pb(II) from aqueous solutions by adsorption on magnetic bentonite. Environ. Sci. Pollut. Res.. 2019;26:1315-1322.
- [CrossRef] [Google Scholar]
- High adsorption capability and selectivity of ZnO nanoparticles for dye removal. Colloids Surf., A. 2016;509:474-483.
- [Google Scholar]
- Adsorption research of tetracycline from water by HCl-modified zeolite. Adv. Mater. Res.. 2012;573–574:43-47.
- [Google Scholar]







