Translate this page into:
Solidified floating organic drop microextraction in tandem with syringe membrane miro-solid phase extraction for sequential detection of thallium (III) and thallium (I) by graphite furnace atomic absorption spectrometry
⁎Corresponding author. chenshizhong62@163.com (Shizhong Chen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the present study, a novel method based on solidified floating organic drop microextraction (SFODME) combined with syringe membrane micro-solid phase extraction (SMMSPE) was proposed for the sequential separation and enrichment of Tl(III) and Tl(I) followed by graphite furnace atomic absorption spectrometry detection. In SFODME, Tl(III) can react with 1-(2-Pyridylazo)-2-naphthol at pH 8.0 to form the complexes which can be extracted into an organic drop, while Tl(I) was remained in the solution. In SMMSPE, flexible TiO2@SiO2 nanofiber membrane was used as the sorbent for the enrichment of Tl(I) in the sample solution after the separation of Tl(III). This method did not require tedious pre-oxidation/pre-reduction operation and time-consuming centrifugation/filtration steps, which may cause sample contamination and analysis errors. Main parameters influencing the separation and enrichment of the target species were studied. Under the selected conditions, the detection limits for this method were 1.7 and 2.6 ng/L for Tl(III) and Tl(I) with relative standard deviations of 6.1 % and 5.2 %, respectively. This method was successfully used for the determination of the target species in environmental water samples and two certified reference materials. The determined values were in good agreement with the certified values.
Keywords
Solidified floating organic drop microextraction
Syringe membrane micro-solid phase extraction
Thallium speciation
Graphite furnace atomic absorption spectrometry
Water samples
1 Introduction
Speciation analysis of a given element is one of the greatest challenges in analytical sciences. Thallium (Tl) is a rare and nonessential element which exhibits more toxicity than Pb, Cd and Hg for humans (Rodríguez-Mercado et al., 2013, Wang et al., 2020). Exposure to Tl, even at low levels, can result in various health problems such as headache, paralysis, alopecia, vomiting, diarrhea, cardiovascular diseases and kidney/liver failure (Hutapea et al., 2021, Soltani et al., 2021). Moreover, bioavailability and toxicity of Tl strongly depends on its chemical forms (Firouzabadi et al., 2017). In the environment, Tl occurs usually in two species of Tl(I) and Tl(III) (Biadun et al., 2016). It was reported that the toxicity of Tl(III) is approximately several thousand times higher than that of Tl(I) (Chen et al., 2018). The estimated lethal dose for adults is 8.0–12 µg g−1 (Das et al., 2007). The maximum amount of Tl in drinking water is 2.0 μg L−1 recommended by the United States Environmental Protection Agency (Lopez-Garcia et al., 2021). Foods and occupational exposure are main source in daily Tl intake. Consequently, there is an increasing demand to develop reliable and inexpensive methods for the determination of Tl species in environmental water samples.
In general, it is not an easy task to determine different species of Tl in real samples owing to its very low contents, and each species only involves a fraction of its total concentration (Escudero et al., 2013). Besides, the matrices from samples may affect the determination of the target species. To solve these problems, various sample preparation methods were developed for elemental species, including ion exchange, flow injection, solid phase extraction, phase transformation, dispersive solid phase extraction, dispersive magnetic solid phase extraction, dispersive liquid–liquid microextraction, dispersive micro-solid phase extraction and mixed-micelle cloud point extraction (Chen et al., 2017, Dadfarnia et al., 2007, Firouzabadi et al., 2017, Gil et al., 2009, Islam et al., 2016, Lin et al., 1999, Liu et al., 2018, Meeravali et al., 2008, Mohammadi et al., 2016, Rezabeyk et al., 2018). However, it is worth noting that the non-chromatographic procedures for Tl species are based on the detection of Tl(III)/T(I), and total Tl after pre-oxidation/pre-reduction reactions. The concentration of Tl(III)/T(I) is from the difference between total Tl and Tl(I)/T(III). Apparently, the redox speciation methods may cause some problems. Firstly, it is very difficult to completely convert one target species into another at its trace/ultra-trace levels. Secondly, the addition of concentrated oxidation/reduction agents such as bromine, Ce(SO4)2 and hydroxylamine hydrochloride may lead to sample contamination. Moreover, the use of bromine as an oxidation agent may pose potential health risks and environmental pollution. Thirdly, the redox step for speciation conversion usually involves the time-consuming and complicated operations (Liu et al., 2020). Thus, the development of non-redox methods for the direct and sequential detection of elemental species is a very interesting work.
Nowadays, modern trends in sample preparation are towards simplified, miniaturized and green techniques (Jagirani et al., 2020, Jalili et al., 2022, Lu et al., 2022, Noormohammadi et al., 2022, Zou et al., 2018). Syringes as facile, easy portable and low-cost devices have been used widely in various microextraction techniques (Chen et al., 2022, Li et al., 2020, Pourshamsi et al., 2021). Solidified floating organic drop microextraction (SFODME) is based on that with a syringe, organic solvent is injected the surface of stirred sample solution to form a floating drop for the extraction of analytes. Due to its simple and efficient merits, SFODME has gained significant popularity in analytical sciences (Mortada et al., 2022, Tavakoli et al., 2021, Vinas et al., 2015). Besides, the solid/micro-solid phase extraction based on the syringes as extraction devices has attracted considerable attention (Chen et al., 2020, Li et al., 2020, Li et al., 2021, Zhou et al., 2021). It is worth noting that the sorbents play a key role in the solid/micro-solid phase extraction processes. Over the last two decades, nanomaterials have been known to be fascinating materials in the separation, enrichment and degradation of organic/inorganic pollutants (Ahmad et al., 2019, Ahmad et al., 2021, He et al., 2022, Li et al., 2022a, Li et al., 2022b, Li et al., 2022c, Liu et al., 2021, Wang et al., 2022, Xu et al., 2018, Zou et al., 2019). More recently, the membrane materials consisting of inorganic/organic substances have become potential candidates as the sorbents (Ahmad et al., 2022a, Ahmad et al., 2022b, Ahmad et al., 2022c, Ahmad et al., 2021, Nisah et al., 2022, Srirachat et al., 2021). Due to its merit of high extraction efficiency, TiO2@SiO2 nanofiber membrane (TSNFM) was used for the separation and enrichment of analytes in syringe membrane micro-solid phase extraction (SMMSPE) (Yan et al., 2022). To our knowledge, however, there is no report on combining SFODME with SMMSPE based on TSNFM for elemental species in literatures.
In this work, an attempt was made to combine SFODME with SMMSPE for the direct and sequential separation and preconcentration of T(III) and Tl(I) without any pre-redox reaction prior to graphite furnace atomic absorption spectrometry (GFAAS) detection. In SFODME, 1-(2-Pyridylazo)-2-naphthol (PAN) selectively reacts Tl(III) to form Tl(III)-PAN complexes, which can be extracted into 1-undecanol, while Tl(I) was remained in the solution. In the next step, the original solution after SFODME was used for the enrichment of Tl(I) by SMMSPE based on TSNFM as the sorbent. Finally, the extract from SFODME and the eluate from SMMSPE were utilized for GFAAS detection of Tl(III) and Tl(I), respectively. This procedure was successfully utilized for the determination of trace/ultra-trace Tl species in environmental water samples.
2 Materials and methods
2.1 Apparatus
Tl was determined by a Z-2000 GFAAS (Hitachi, Japan). A hollow cathode lamp for Tl was utilized as the light source with a wavelength of 276.8 nm. The working parameters of GFAAS are summarized as follows: sampling volume, 20 µL; argon flow rate, 250 mL min−1; temperature program (ramp time and hold time): drying, 100 °C (15 s, 30 s); ashing, 800 °C (15 s, 20 s); atomization, 2000 °C (0 s, 4.0 s); cleaning, 2500 °C (1.0 s, 3.0 s). In this work, 1.2 % Pd(NO3)2 was employed the chemical modifier. The pH of solutions was adjusted by a pH meter supplied with a combined electrode (Thermo Fisher Scientific, USA). Ultrapure water was obtained from a Milli-Q device (Millipore Corporation, USA). A 79–1 magnetic stirrer with temperature control (Yitong Co. ltd., Jiangsu, China) was used for promoting the extraction of analytes.
2.2 Reagents
Standard solutions (1.0 mg mL−1) of Tl(I) and Tl(III) were prepared by dissolving appropriate amounts of TlNO3 and Tl(NO3)3·3H2O (Sinopharm Chemical Reagent Company, Shanghai, China) in 1.0 % (v/v) HNO3 solution, respectively. Working solutions were prepared by diluting the original standard solution before use. PAN solution was prepared by dissolving proper amount of PAN in 1-undecanol (Sinopharm Chemical Reagent Company, Shanghai, China). Unless otherwise stated, all other reagents used in this work were of high purity available but at least of analytical grade (Sinopharm Chemical Reagent Company, Shanghai, China). Certified reference materials of tea leaves (GBW 10016) and cabbage (GBW 10014) were obtained from the Institute of Geophysical and Geochemical Prospecting (Langfang, China). TSNFM was synthesized and characterized in our laboratory (Yan et al., 2022). The polypropylene and glass vessels were soaked in 2.0 mol/L HNO3 solutions for at least one night. The vessels were rinsed with 0.1 mol/L HNO3 solution and subsequently with deionized water prior to their use.
2.3 Sample pretreatment
Water samples were collected from local districts (Wuhan, China), including well water, tap water, river water and wastewater. Then, the sample solutions were filtered with a 0.45 μm membrane filter to remove the possible suspended materials. The obtained samples were placed in a refrigerator at 4 °C for future analysis. The certified reference materials of tea leaves and cabbage (0.5000 g) were treated for the analysis according to the literature (Chen et al., 2014). All blanks were prepared exactly as the samples in this work.
2.4 Extraction procedure
2.4.1 Solidified floating organic drop microextraction
A 20 mL of sample or standard solution containing Tl(III) and Tl(I) was put into a beaker with a stirrer bar. The sample solution was adjusted to pH 8.0 with the buffer solution containing acetic acid/acetate, and then spiked with 50 μL of 3.5 mmol/L PAN in 1-dodecanol. The resulting solution was magnetically stirred for 15 min at 35 °C. During this step, Tl(III) can react with PAN to form the hydrophobic complexes, which were extracted into 1-undecanol, while Tl(I) was remained in the solution. After the extraction, the sample vial was transferred into an ice bath. The organic drop was solidified after 4.0 min. The solidified drop was put into a conical vial where it melted rapidly at room temperature. The extract was diluted to 100 μL with ethanol. Finally, 20 μL of the extract was used for the detection of Tl(III) by GFAAS. The extraction recovery (ER) was calculated by the following formula: ER(%) = [(Co-C)/Co]x100, where Co and C are the initial and final concentration of the species, respectively.
2.4.2 Syringe membrane micro-solid phase extraction
SMMSPE system used in this work was described in the literature (Yan et al., 2022). Briefly, TSNFM was cut into a disk shape with 2.0 cm of diameter as the sorbent, which was placed into a reusable syringe filter. Then, the filter was connected to the tip of a syringe, and conditioned with 5.0 mL of 1.0 mol/L HNO3 and 5.0 mL of ultrapure water by pushing and pulling the plunger, respectively. To enrich Tl(I), 20 mL of the sample solution after SFODME of Tl(III) was adjusted to pH 6.0, and then pulled and pushed for eight cycles in 8.0 min with the syringe. Tl(I) adsorbed on TSNFM was eluted with 0.3 mL of 1.0 mol/L HNO3 solution for four cycles within 2.0 min. Finally, 20 μL of the eluate was injected into GFAAS for determining Tl(I).
3 Results and discussion
3.1 Selection of SFODME variables
3.1.1 Effect of pH
In SFODME, the selective isolation of Tl(III) and Tl(I) is related to the pH values of sample solution as it directly affects the formation of hydrophobic Tl-PAN complexes, which are extracted into the organic drop. For this reason, the influence of pH values on the recovery of the target species was examined within the range of 1.0–9.0. It can be seen from in Fig. 1a that at pH 7.0–9.0, Tl(III) can was quantitatively extracted into the organic drop containing PAN, while Tl(I) remained in the solution. The reason for the above facts is that at the selected pH values, Tl(III) can chelate with PAN to produce the hydrophobicity complexes, which are extracted by 1-undecanol. However, PAN cannot react with Tl(I) at the same pH range, leading to its poor extraction percentages. Thus, pH 8.0 was utilized for the enrichment of the interesting species.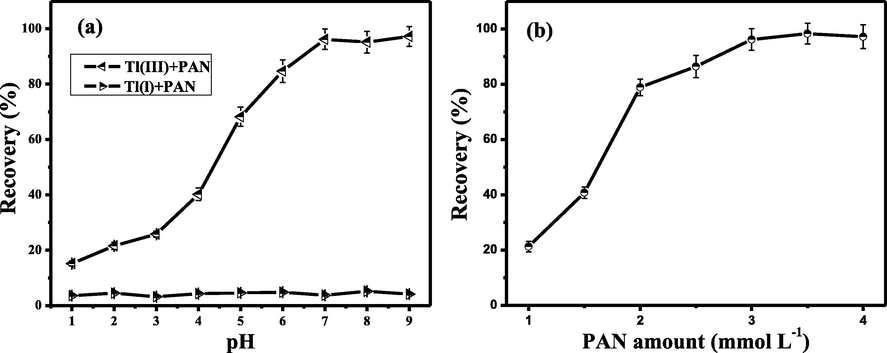
Effect of pH (a) and PAN concentration (b) on recovery of Tl(III) in SFODME. Conditions: (a) Tl(III) and Tl(I), 2.0 ng mL−1; PAN concentration, 3.5 mmol/L; 1-undecanol solvent, 50 µL; extraction time, 15 min; sample volume, 20 mL. (b) Tl(III), 2.0 ng mL−1; pH, 8.0; 1-undecanol solvent, 50 µL; extraction time, 15 min; sample volume, 20 mL.
3.1.2 Effect of PAN concentration
PAN concentration is another key factor influencing the formation of Tl(III)-PAN complexes. Therefore, the effect of PAN dosage on the extraction efficiency of Tl(III) was investigated with its concentration varying from 1.0 to 4.0 mmol/L. As shown in Fig. 1b, the extraction percentages of Tl(III) increased with increasing PAN concentration up to 3.0 mmol/L. The quantitative extraction of Tl(III) occurred between 3.0 and 4.0 mmol/L PAN concentration. It is worth noting that in this work, the chelating reagents are in excess amount, which favors the formation of PAN-Tl(III). Based on that PAN may react with other coexisting ions, a PAN amount of 3.5 mmol/L was selected in further work.
3.1.3 Type and volume of extraction solvent
In order to obtain quantitative extraction efficiency, the extraction solvents used for SFODME generally have the following merits of suitable melting point, low water solubility, density below water and less volatility. Hence, 1-dodecanol, 1-undecanol, 1-chlorooctadecane and 1, 2-dichoroethane were used for the extraction of Tl(III)-PAN complexes. The results in Fig. 2a show that 1-undecanol provided the best extraction percentages for the interesting species. In addition, 1-undecanol was easily solidified in ice bath and transferred from the solution. Thus, 1-undecanol was used as the extraction solvent in this study.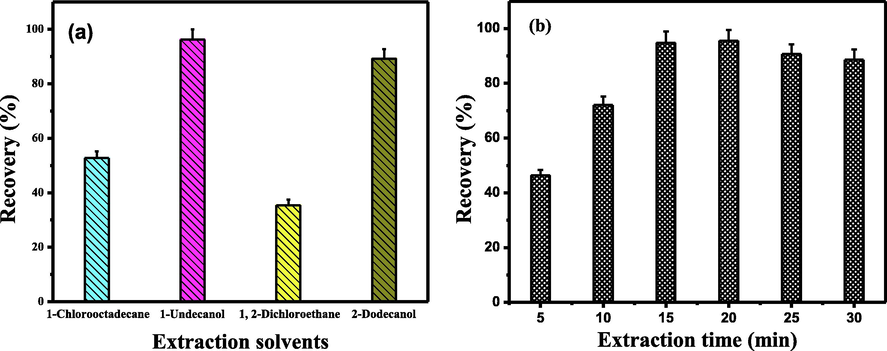
Effect of extraction solvent type (a) and extraction time (b) on recovery of Tl(III) in SFODME. Conditions: (a) Tl(III), 2.0 ng mL−1; pH, 8.0; PAN concentration, 3.5 mmol/L; solvent volume, 50 µL; extraction time, 15 min; sample volume, 20 mL. (b) Tl(III), 2.0 ng mL−1; pH, 8.0; PAN concentration, 3.5 mmol/L; solvent volume, 50 µL; sample volume, 20 mL.
The extraction solvent volume is also one of the essential parameters, which influence the extraction of the target species for SFODME. The large volume of extraction solvent can increase the surface area of the drop to result in the increase of the extraction efficiency, but the enrichment factor may decrease owing to the dilution effect. Therefore, the effect of 1-undecanol volume on the extraction efficiency of Tl(III) was studied using different solvent volumes changing from 20 to 60 µL. The experimental results indicated that the extraction percentage of Tl(III) increased with the increase of 1-undecanol volumes up to 50 µL, and then remained constant. Therefore, 50 µL of 1-undecanol was employed in the following experiments.
3.1.4 Extraction time and temperature
In the liquid microextraction, the mass transfer of the target species between two phases is a time-dependent process. To improve extraction efficiency, it is necessary to select a suitable exposure time to guarantee the complete equilibrium of the target species between two phases. Thus, the effect of extraction time on the recoveries was examined with the extraction time ranging from 5.0 to 30 min. As shown in Fig. 2b, the recoveries of the target species increased with the increase of the extraction time up to 15 min. The quantitative recoveries were obtained in the range of 15–20 min. Hence, an extraction of 15 min was used for further experiments.
In addition, the mass transfer of the target species between two phases is related to the temperature. Thus, the effect of extraction temperature on the recoveries of the interesting species was explored in the range of 20–50 °C. The results showed that the target species was quantitatively extracted into the drop in the range of 30–40 °C. However, the extraction efficiency slightly decreased with further increasing the temperature due to the increase in solubility of organic phase at higher temperature. Therefore, the temperature of 35 °C was used in the following extraction steps.
3.1.5 Effect of sample volume
Sample solution volume may affect the extraction efficiency in the given times. To examine the effect of sample volume on the extraction percentages, some experiments were performed in the range of 5.0–40 mL. The results showed that the quantitative recoveries were achieved with the sample volumes changing from 5.0 to 20 mL. However, the recovery of the analytes decreased by increasing the sample volumes above 20 mL because the convection is not sufficient in the aqueous phase at a fixed stirring rate to result in less extraction efficiency. Based on that the organic drop was diluted to 100 μL with ethanol, an enrichment factor of 200-fold for Tl(III) was achieved. Thus, the sample volume of 20 mL was used in this work.
3.2 Optimization of SMMSPE conditions
3.2.1 Effect of pH
Among various factors of SMMSPE, pH value of sample solution is one of the most key parameters due to its strong effect on the surface charges of sorbent and existing forms of the target species. Therefore, the effect of pH on the adsorption percentages of Tl(I) was investigated by varying the pH values in the range 2.0–8.0. As shown in Fig. 3a, the recoveries of Tl(I) increased with increasing pH values up to 6.0, and then keep constant at pH 6.0–7.0. The extraction mechanism of Tl(I) may be explained by the surface charges of the sorbent (Vu et al., 2012). At an acidic condition (at pH 2.0–5.0), the surface of FTOSNFM is electropositive (TiOH2+), which repels Tl(I) cations. With the further increase of pH (>5.0), the surface of FTOSNFM becomes electronegative (TiO-), which favors the adsorption of Tl(I). At pH values higher than 7.0, precipitation of Tl(I) may occur to result in the decrease of the extraction efficiency. Thus, a pH of 6.0 was utilized as the optimum value for the subsequent experiments.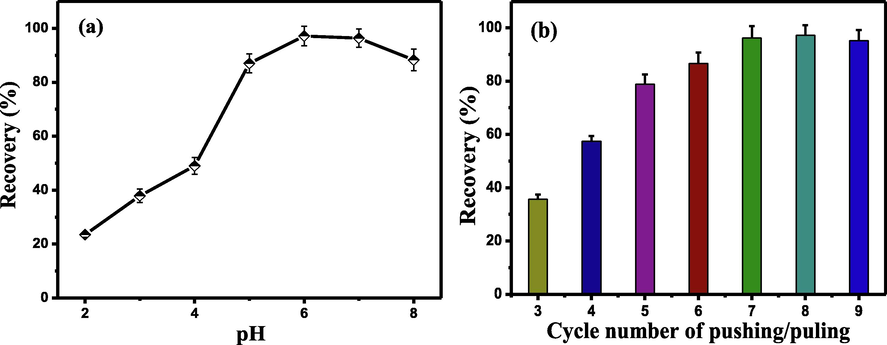
Effect of pH (a) and cycle number of pushing and pulling (b) on recovery of Tl(I) in SMMSPE. Conditions: (a) Tl(I), 2.0 ng mL−1; cycles of pushing and pulling, 8; eluent concentration, 1.0 mol/L; sample volume, 20 mL. (b) Tl(I), 2.0 ng mL−1; pH, 6.0; eluent concentration, 1.0 mol/L; sample volume, 20 mL.
3.2.2 Effect of pushing and pulling cycles
The adsorption of Tl(I) is an equilibrium-based extraction process in SMMSPE. The pushing and pulling cycles greatly affects the preconcentration/separation and elution of Tl(I). Thus, the effect of the number for pushing and pulling cycles was examined in the range of 3–9 cycles. The results in Fig. 3b demonstrate that the extraction percentages increased from 3 to 7 cycles, and then maintained constant. Hence, 8 cycles of pushing and pulling with 8.0 min were adopted in the following studies.
3.2.3 Choice of elution parameters
It can be seen from in Fig. 3a that under the acidic conditions, the adsorption percentages of Tl(I) were relatively low (pH < 3.0). Thus, it is possible to use an acid as the eluent for desorbing Tl(I) on FTSNFM. In this work, the different concentration of HNO3 on the recoveries of Tl(I) was examined within the range of 0.2–1.2 mol/L. The results in Fig. 4a show that Tl(I) can be quantitatively desorbed in the concentration range with 0.8–1.2 mol/L HNO3. Hence, 1.0 mol/L HNO3 was utilized as the eluent. In addition, the effect of eluent volume was also investigated by keeping the concentration of 1.0 mol/L HNO3 solution. The experimental results indicated that the quantitative recoveries of Tl(I) were achieved with 0.3 mL of 1.0 mol/L HNO3. Therefore, 0.3 mL was used as the eluent volume in this work.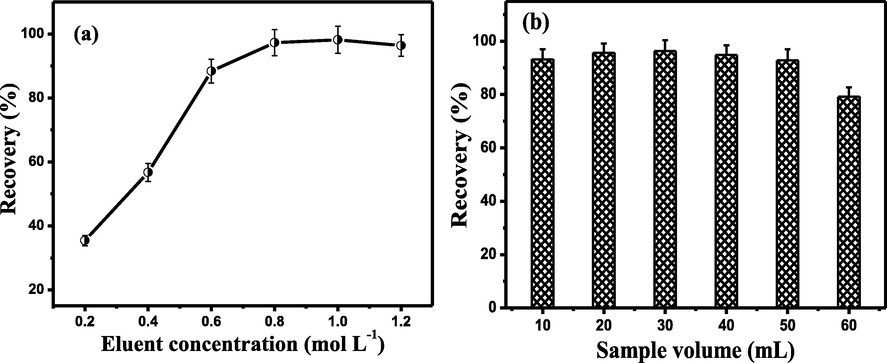
Effect of eluent concentration (a) and sample volume (b) on recovery of Tl(I) in SMMSPE. Conditions: (a) Tl(I), 2.0 ng mL−1; pH, 6.0; cycles of pushing and pulling, 8; eluent concentration, 1.0 mol/L; sample volume, 20 mL. (b) Tl(I), 2.0 ng mL−1; pH, 6.0; cycles of pushing and pulling, 8; eluent concentration, 1.0 mol/L.
3.2.4 Effect of sample volume
The effect of sample solution volume on the recoveries of Tl(I) was investigated in the range of 10–60 mL. It can be seen from Fig. 4b that the quantitative recoveries of Tl(I) (>90 %) were obtained with sample volume varying from 10 to 50 mL. Based on using 0.3 mL eluent, a enrichment factor of 167-fold for Tl(I) was achieved. In this work, 20 mL of sample solution was used for the preconcentration and separation of Tl(I) in term of the real analysis needs.
3.3 Effect of diverse ions
To evaluate the application potential of this method in real sample analysis, the effect of commonly coexisting ions in environmental water samples was examined under the selected conditions. The tolerance limit of interference ions is defined as the largest amount making the recovery of target species varying from 90 to 110 %. The selected interfering ions, such as K+, Na+, Ca2+, Mg2+, Al3+, Fe3+, Zn2+, Pb2+, Cd2+, Cu2+, Cl− and NO3−, were added into the model solutions, which were analyzed by the proposed method. It can be seen from Table 1 that the above common coexisting ions did not interfere with the extraction and determination of the target species in the range of their tested amounts. Thus, the developed procedure can be utilized for the analysis of the real samples.
Interfering ion
Concentration (mg/L)
Na+, K+
8000
Ca2+, Mg2+
5000
Cu2+, Zn2+, Pb2+, Cd2+
100
Fe3+, Al3+
50
NO3–, Cl-
10,000
3.4 Analytical performance
The analytical merit of the proposed method was studied under the optimum conditions. Detection limits (DLs), defined as the concentration of analytes corresponding to three times the standard deviation for 11 replicate determination of blank solution, were found to be 1.7 and 2.6 ng/L for Tl(III) and Tl(I), respectively. Relative standard deviations (RSDs), achieved by analyzing the standard solutions containing 1.0 ng mL−1 of the analytes in sequence for nine times, were 6.1 % and 5.2 % for Tl(III) and Tl(I), respectively. The linear range of calibration curves were found to be in the concentration range of 0.05–40 ng mL−1 for Tl(III) and Tl(I) with the correlation coefficients better than 0.9971. In addition, Table 2 provided the comparison of the analytical merits of this method with those reported in the literatures (Afzali et al., 2013, Asadpour et al., 2016, Escudero et al., 2015, Gil et al., 2009, Javedani-Asleh et al., 2016, Krishna et al., 2020, Lopez-Garcia et al., 2021, Pacheco et al., 2009). As shown in Table 2, the proposed method possesses low DLs and higher enrichment factors. RSDs are similar to those of other methods. Besides, the combination of SFODME and SMMSPE avoided the time-saving and complicated pre-oxidation/pre-reduction and centrifugation/filtration steps, which may cause the risk of sample contamination and analysis errors. Moreover, this method can provide DLs and RSDs of both Tl(III) and Tl(I). The majority of the non-chromatographic methods reported in the literatures only offered one of them owing to the requirement of speciation conversion. USAE-SFO, ultrasound-assisted emulsification based on solidification of floating organic drop microextraction; SPE, solid phase extraction; Cell-PME, cellular phase microextraction; FI-SPE, flow injection solid phase extraction; DLLME, dispersive liquid–liquid microextraction; CPE, cloud point extraction; DMSPE, dispersive micro-solid phase microextraction; ETAAS, electrothermal atomic absorption spectrometry; EF, Enrichment factor; LR, linear range; DL, detection limit; RSD, relative standard deviation.
Method
EF
LR (ng mL−1)
DL (ng L-1)
RSD (%)
Reference
Tl(I)
Tl(III)
Tl(I)
Tl(III)
USAE-SFO-GFAAS
200
0.2–10
–
30
–
3.3
Afzali et al. (2013)
SPE-GFAAS
100
0.6–2.5
87
–
6.4
–
Asadpour et al. (2016)
Cell-PME- GFAAS
50
–
–
8.3
–
5.1
Escudero et al. (2015)
FI-SPE-ETAAS
20
–
9.0
–
3.9
–
Gil et al. (2009)
DLLME-ETAAS
125
0.1–2.0
–
30
–
4.1
Javedani-Asleh et al. (2016)
CPE-GFAAS
25
0.5–25
15
15
2.0–10
2.0–10
Krishna et al. (2020)
DMSPE-ETAAS
185
0.05–1.0
10
–
5.0
–
Lopez-Garcia et al. (2021)
SPE-ETAAS
40
–
–
3.0
–
3.4
Pacheco et al. (2009)
SFODME-SMMSPE-GFAAS
167/200
0.05–40
1.7
2.6
6.1
5.2
This work
3.5 Sample analysis
The proposed method was used for the sequential detection of Tl(III) and Tl(I) in the environmental water samples, including tap water, well water, river water and wastewater. The obtained results are tabulated in Table 3. In addition, the accuracy of this method was also validated by analyzing the certified reference materials of tea leaves (GBW 10016) and cabbage (GBW 10014) (Table 4). It can be seen from Table 4 that the determined results were in good agreement with certified values. Student’s t-test was performed and no statistically significant differences were observed at 95 % confidence level. Since there is no available aqueous certified reference materials for Tl species, the reliability of the analytical results was further checked by determining the recoveries of the analytes in the spiked samples with the known Tl(III) and Tl(I) concentrations. As shown in Table 3 and 4, there is a good agreement between the added and determined amounts of the target species with the recovery range of 90.0 % − 110 %.
Sample
Added (ng mL−1)
Found a (ng mL−1)
Recovery (%)
Tl(I)
Tl(III)
Tl(I)
Tl(III)
Tl(I)
Tl(III)
Tap water
0
0
0.38 ± 0.02
Ndb
–
–
0.2
0.2
0.57 ± 0.03
0.18 ± 0.01
95.0
90.0
1.0
1.0
1.31 ± 0.09
1.09 ± 0.07
93.0
109
Well water
0
0
0.52 ± 0.03
0.07 ± 0.005
–
–
0.2
0.2
0.70 ± 0.05
0.28 ± 0.02
90.0
105
1.0
1.0
1.48 ± 0.11
1.05 ± 0.06
96.0
98.0
River water
0
0
0.19 ± 0.01
Ndb
–
–
0.2
0.2
0.41 ± 0.02
0.21 ± 0.01
110
105
1.0
1.0
1.16 ± 0.07
0.94 ± 0.05
97.0
94.0
Wastewater
0
0
3.65 ± 0.18
0.93 ± 0.06
–
–
0.5
0.5
4.13 ± 0.19
1.47 ± 0.12
96.0
108
5.0
5.0
8.57 ± 0.46
5.62 ± 0.37
98.4
93.8
Sample
Added (ng g -1)
Found (ng g -1)a
Certified (ng g -1)
Recovery (%)
Tl(I)
Tl(III)
Tl(I)
Tl(III)
Tl(I)
Tl(III)
Tea leaves
0
0
45.3 ± 2.8
3.1 ± 0.2
50b
–
–
5.0
5.0
49.8 ± 2.1
7.9 ± 0.4
–
90.0
96.0
20
20
65.0 ± 3.1
21.9 ± 1.3
–
98.5
94.0
Cabbage
0
0
5.17 ± 0.32
0.96 ± 0.07
6.3b
–
–
2.0
2.0
7.21 ± 0.48
2.82 ± 0.13
–
102
93.0
10
10
14.9 ± 1.0
10.1 ± 0.4
–
97.3
91.4
4 Conclusions
In the present study, a novel method of combining SFODME with SMMSPE was developed for the sequential separation and preconcentration of Tl(III) and Tl(I) followed by GFAAS detection. Compared with conventional redox speciation strategies, this method did not require time-consuming pre-oxidation/pre-reduction operation steps, which may lead to incomplete conversion of target species at trace/ultra-trace levels, sample contamination by concentrated oxidation/reduction agents and interferences of the ions from redox processes. Moreover, this method only needs some facile devices such as septum-lined cap, stir bar, magnetic stirrer and microsyringes. Therefore, the proposed method has several advantages of simple operations, low cost, high efficiency and direct speciation determination without calculation by difference. Besides, this method exhibited the merits of high preconcentration factor, low detection limits and good precision. As a conclusion, the proposed method may become an ideal and potential sample preparation technique for elemental species at trace/ultra-trace levels in real-word samples.
Acknowledgments
This work was financially supported by the Key Research and Development Project of Hubei Province (No.2020BBB068), Central Committee Guides Local Science and Technology Development Special Project of Hubei Province (No. 2019ZYYD059) and Nature Science Foundation of Hubei Province (No.2020CFB400).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ultrasound-assisted emulsification/microextraction based on solidification of trace amounts of thallium prior to graphite furnace atomic absorption spectrometry determination. Toxic. Environ. Chem.. 2013;95(7):1080-1089.
- [Google Scholar]
- Bioinspired 2D carbon sheets decorated with MnFe2O4 nanoparticles for preconcentration of inorganic arsenic, and its determination by ICP-OES. Microchim. Acta. 2019;186(9):649.
- [CrossRef] [Google Scholar]
- Preconcentration and determination of trace Hg(II) using ultrasound-assisted dispersive solid phase microextraction. RSC Adv.. 2021;12(1):53-61.
- [Google Scholar]
- Selective Extraction of trace arsenite ions using a highly porous aluminum oxide membrane with ordered nanopores. ACS omega. 2022;7(3):3044-3051.
- [Google Scholar]
- Cellulose nanofibers@ZrO2 membrane for the separation of Hg(II) from aqueous media. J. Phys. Chem. Solids. 2022;168:110812
- [CrossRef] [Google Scholar]
- Enrichment of trace Hg(II) ions from food and water samples after solid phase extraction combined with ICP-OES determination. Microchem. J.. 2022;175:107179
- [CrossRef] [Google Scholar]
- Ultra-thin graphene oxide membrane deposited on highly porous anodized aluminum oxide surface for heavy metal ions preconcentration. J. Hazard. Mater.. 2021;415:125661
- [CrossRef] [Google Scholar]
- On-line preconcentration of ultra-trace thallium(I) in water samples with titanium dioxide nanoparticles and determination by graphite furnace atomic absorption spectrometry. Arab. J. Chem.. 2016;9:S1833-S1839.
- [Google Scholar]
- Direct speciation analysis of thallium based on solid phase extraction and specific retention of a Tl(III) complex on alumina coated with sodium dodecyl sulfate. Microchim. Acta. 2016;183(1):177-183.
- [Google Scholar]
- Speciation analysis of thallium in tobaccos using liquid chromatography inductively coupled plasma mass spectrometry. Microchem. J.. 2018;141:104-109.
- [Google Scholar]
- Solid phase extraction with titanium dioxide nanofibers combined with dispersive liquid-liquid microextraction for speciation of thallium prior to electrothermal vaporization ICP-MS. Microchim. Acta. 2017;184(8):2797-2803.
- [Google Scholar]
- Dual in-syringe microextraction with electrothermal vaporization (etv) inductively coupled plasma-mass spectrometry (ICP-MS) for determination of rare earth elements (REEs) in food. Anal. Lett.. 2022;55(10):1623-1636.
- [Google Scholar]
- Comparison and application of two microextractions based on syringe membrane filter. J. Sep. Sci.. 2020;43(2):462-469.
- [Google Scholar]
- Speciation of chromium and its distribution in tea leaves and tea infusion using titanium dioxide nanotubes packed microcolumn coupled with inductively coupled plasma mass spectrometry. Food Chem.. 2014;150:254-259.
- [Google Scholar]
- Speciation and determination of thallium by on-line microcolumn separation/preconcentration by flow injection-flame atomic absorption spectrometry using immobilized oxine as sorbent. J. Hazard. Mater.. 2007;148(1–2):446-452.
- [Google Scholar]
- Sensitive determination of thallium species in drinking and natural water by ionic liquid-assisted ion-pairing liquid-liquid microextraction and inductively coupled plasma mass spectrometry. J. Hazard. Mater.. 2013;244:380-386.
- [Google Scholar]
- An eco-friendly cellular phase microextraction technique based on the use of green microalgal cells for trace thallium species determination in natural water samples. Anal. Meth.. 2015;7(18):7480-7487.
- [Google Scholar]
- Preconcentration and speciation of thallium by ferrofluid based dispersive solid phase extraction and flame atomic absorption spectrometry. Microchem. J.. 2017;130:428-435.
- [Google Scholar]
- Speciation analysis of thallium using electrothermal AAS following on-line pre-concentration in a microcolumn filled with multiwalled carbon nanotubes. Microchim. Acta. 2009;167(3–4):187-193.
- [Google Scholar]
- Dielectric barrier discharge plasma for nanomaterials: Fabrication, modification and analytical applications. TRAC-Trends Anal. Chem.. 2022;156:116715
- [CrossRef] [Google Scholar]
- Adsorption of thallium from wastewater using disparate nano-based materials: a systematic review. Arab. J. Chem.. 2021;14(10):103382
- [CrossRef] [Google Scholar]
- A graphene oxide decorated with triethylenetetramine-modified magnetite for separation of chromium species prior to their sequential speciation and determination via FAAS. Microchim. Acta. 2016;183(1):289-296.
- [Google Scholar]
- A review: Recent advances in solid phase microextraction of toxic pollutants using nanotechnology scenario. Microchem. J.. 2020;159:105436
- [CrossRef] [Google Scholar]
- Microextraction techniques for sampling and determination of polychlorinated biphenyls: a comprehensive review. Microchem. J.. 2022;179:107442
- [CrossRef] [Google Scholar]
- Determination of total thallium in water and spinach samples by ligandless microextraction using ion pair-based dispersive liquid-liquid microextraction followed by electrothermal atomic absorption spectrometry. Spectrosc. Lett.. 2016;49(6):420-425.
- [Google Scholar]
- A new sequential and simultaneous speciation analysis of thallium in coal effluents by graphite furnace atomic absorption spectrometry after a novel ligandless mixed micelle cloud point extraction. Int. J. Environ. Anal. Chem.. 2020;100(10):1079-1093.
- [Google Scholar]
- Li, L., Xu, K., Huang, Z., Xu, X., Iqbal, J., Zhao, L., Du., Y. 2021. Rapid determination of trace Cu2+ by an in-syringe membrane SPE and membrane solid-phase spectral technique. Anal. Meth. 13(39), 4691-4698.
- Two-dimensional material-based electrochemical sensors/biosensors for food safety and biomolecular detection. Biosensors-Basel. 2022;12(5):314.
- [CrossRef] [Google Scholar]
- Sb-doped WO3 based QCM humidity sensor with self-recovery ability for real-time monitoring of respiration and wound. Sensor. Actuat. B-Chem.. 2022;361:131691
- [CrossRef] [Google Scholar]
- The combination of two-dimensional nanomaterials with metal oxide nanoparticles for gas sensors: a review. Nanomaterials. 2022;12(6):982.
- [CrossRef] [Google Scholar]
- Rapid detection of sulfamethoxazole in plasma and food samples with in-syringe membrane SPE coupled with solid-phase fluorescence spectrometry. Food Chem.. 2020;320:126612
- [CrossRef] [Google Scholar]
- Thallium speciation in river waters with Chelex-100 resin. Anal. Chim. Acta. 1999;395(3):301-307.
- [Google Scholar]
- Effective extraction of Cr(VI) from hazardous gypsum sludge via controlling the phase transformation and chromium species. Environ. Sci. Technol.. 2018;52(22):13336-13342.
- [Google Scholar]
- Different Pathways for Cr(III) oxidation: implications for Cr(VI) reoccurrence in reduced chromite ore processing residue. Environ. Sci. Technol.. 2020;54(19):11971-11979.
- [Google Scholar]
- Ultrasensitive exhaled breath sensors based on anti-resonant hollow core fiber with in situ grown ZnO-Bi2O3 nanosheets. Adv. Mater. Int.. 2021;8(6):2001978.
- [CrossRef] [Google Scholar]
- Dispersive micro-solid phase extraction with a magnetic nanocomposite followed by electrothermal atomic absorption measurement for the speciation of thallium. Talanta. 2021;228:122206
- [CrossRef] [Google Scholar]
- Recent application of deep eutectic solvents as green solvent in dispersive liquid-liquid microextraction of trace level chemical contaminants in food and water. Crit. Rev. Anal. Chem.. 2022;52(3):504-518.
- [Google Scholar]
- Ultra-trace speciation analysis of thallium in environmental water samples by inductively coupled plasma mass spectrometry after a novel sequential mixed-micelle cloud point extraction. J. Anal. At. Spectrom.. 2008;23(4):555-560.
- [Google Scholar]
- Speciation of Tl(III) and Tl(I) in hair samples by dispersive liquid-liquid microextraction based on solidification of floating organic droplet prior to flame atomic absorption spectrometry determination. Arab. J. Chem.. 2016;9:S1510-S1515.
- [Google Scholar]
- Microextraction of metal ions based on solidification of a floating drop: basics and recent updates. Trends Environ. Anal. Chem.. 2022;34:e00163.
- [Google Scholar]
- Nisah, K., Rahmi, Ramli, M., Iqhrammullah, M., Mitaphonna, R., Hartadi, B.S., Abdulmadjid, S.N., Sani, N.D.M., Idroes, R., Safitri, E., 2022. Controlling the diffusion of micro-volume Pb solution on hydrophobic polyurethane membrane for quantitative analysis using laser-induced breakdown spectroscopy (LIBS). Arab. J. Chem. 15(6), 103812. https://doi.org/10.1016/j.arabjc.2022.103812.
- Determination of aromatic amines in environmental water samples by deep eutectic solvent-based dispersive liquid-liquid microextraction followed by HPLC-UV. Arab. J. Chem.. 2022;15(6):103783
- [CrossRef] [Google Scholar]
- L-Tyrosine immobilized on multiwalled carbon nanotubes: A new substrate for thallium separation and speciation using stabilized temperature platform furnace-electrothermal atomic absorption spectrometry. Anal. Chim. Acta. 2009;656(1–2):36-41.
- [Google Scholar]
- A comprehensive review on application of the syringe in liquid- and solid-phase microextraction methods. J. Iran. Chem. Soc.. 2021;18(2):245-264.
- [Google Scholar]
- Speciation analysis of Tl(I) and Tl(III) after magnetic solid phase extraction using a magnetite nanoparticle composite modified with aminodibenzo-18-crown-6 functionalized MIL-101(Cr) Microchim. Acta. 2018;185(8):365.
- [CrossRef] [Google Scholar]
- Novel bimodal micro-mesoporous Ni50Co50-LDH/UiO-66-NH2 nanocomposite for Tl(I) adsorption. Arab. J. Chem.. 2021;14(4):103058
- [CrossRef] [Google Scholar]
- Selective separation of trace nickel(II) and gold(I) ions via hollow fiber supported liquid membrane enhanced by synergistic extractants D2EHPA/TBP. Arab. J. Chem.. 2021;14(12):103427
- [CrossRef] [Google Scholar]
- Ultrasound-assisted dispersive liquid-liquid microextraction (DLLME) based on solidification of floating organic drop using a deep eutectic solvent for simultaneous preconcentration and determination of nickel and cobalt in food and water samples. Anal. Lett.. 2021;54(18):2863-2873.
- [Google Scholar]
- Recent achievements in solidified floating organic drop microextraction. TRAC-Trends Anal. Chem.. 2015;68:48-77.
- [Google Scholar]
- Adsorption of Cu(II) from aqueous solution by anatase mesoporous TiO2 nanofibers prepared via electrospinning. J. colloid. Interf. Sci.. 2012;367:429-435.
- [Google Scholar]
- Mo-modified band structure and enhanced photocatalytic properties of tin oxide quantum dots for visible-light driven degradation of antibiotic contaminants. J. Enviro. Chem. Eng.. 2022;10(1):107091
- [CrossRef] [Google Scholar]
- Temporal sedimentary record of thallium pollution in an urban lake: an emerging thallium pollution source from copper metallurgy. Chemosphere. 2020;242:125172
- [CrossRef] [Google Scholar]
- Nanomaterials in speciation analysis of mercury, arsenic, selenium, and chromium by analytical atomic/molecular spectrometry. Appl. Spectrosc. Rev.. 2018;53(2–4):333-348.
- [Google Scholar]
- Syringe membrane micro-solid-phase extraction (SPE) with flexible titanium(IV) oxide@silica nanofiber membrane for the speciation of Te(IV) and Te(VI) with graphite furnace atomic absorption spectrometry (GFAAS) Anal. Lett. doi. 2022;10(1080/00032719):2022.
- [Google Scholar]
- Development of a syringe membrane-based microextraction method based on metal-organic framework mixed-matrix membranes for preconcentration/extraction of polycyclic aromatic hydrocarbons in tea infusion. Food Chem.. 2021;361:130105
- [CrossRef] [Google Scholar]
- Recent trends in atomic fluorescence spectrometry towards miniaturized instrumentation-a review. Anal. Chim. Acta. 2018;1019:25-37.
- [Google Scholar]
- Nanomaterials for photochemical vapor generation-analytical atomic spectrometry. TRAC-Trends Anal. Chem.. 2019;114:242-250.
- [Google Scholar]







