Translate this page into:
Solvent effectiveness factor: A new correlation to study the influence of solvent, temperature, and stirring rate on synthesis yield of Ionic Liquids
⁎Corresponding author. amirengr@gmail.com (Amir Shafeeq)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The main objective of this study is to investigate the effect of solvent, temperature, and stirring on synthesis yield of Ionic Liquids (ILs). A representative Ionic Liquid [BMIM][BF4] is prepared using seven different solvents and their influence on percentage yield is explained using a new correlation; solvent effectiveness factor (SEF). The SEF of solvent is governed by nature of solvent, operating temperature and stirring rate. Acetone has the highest SEF 1.275 and affords maximum IL yield 88.11% while toluene has the least SEF 0.674 and produces minimum IL 63.21%. When synthesis temperature is increased from 50 to 80 °C, synthesis yield is substantially increased from 76.7 to 93.08% because rise in temperature increases the SEF from 1.234 to 1.333, mainly due to the decrease in solvent viscosity. Similarly, when stirring rate is increased from 80 to 200 RPM, IL yield slightly increased from 87.37 to 91.35% due to addition of mechanical energy in the reaction mixture which in turn reduced solvent viscosity and increased SEF from 1.278 to 1.297.
Keywords
Ionic Liquids
Synthesis
Solvent effectiveness factor
Viscosity
Boiling point
1 Introduction
Over that past two decades, the field of Ionic Liquids (ILs) has advanced enormously. ILs have remarkable properties such as negligible vapor pressure and high thermal stability. These properties have made them interesting solvents among academia and industry (Rogers and Seddon, 2003). Further, ILs have successfully been employed in separation processes (Pereiro et al., 2012; Zhang et al., 2015; Ge et al., 2008), chemical reactions (Ratti, 2014), removal of metal ions, cellulose dissolution (Keskin et al., 2007), electroplating (Abbott et al., 2013) and numerous other applications (Plechkova and Seddon, 2008). The growing trend regarding the applications of ILs indicates that they will gradually replace conventional organic solvents.
Due to the increasing interest in ILs, many researchers have introduced new techniques for IL synthesis. Microwave irradiated synthesis technique has gain popularity among researchers because ILs can be prepared in very short time (Varma and Namboodiri, 2001). However, this method affords colored and low purity ILs. Further, this synthesis route cannot be scaled up for large production of ILs. Therefore, solvent aided synthesis is still the widely adapted technique by researchers for preparation of high purity ILs (Gordon et al., 2006).
Solvent aided synthesis method essentially employs a specific solvent. The main objective of using a solvent is to safeguard the reactants from the effects of air and moisture (Gordon et al., 2006). A number of researchers have prepared ILs using different solvents such as toluene (Lucas et al., 2000), acetonitrile (Min et al., 2006), and acetone (Dharaskar et al., 2016). Further, synthesis reaction is carried out in a suitable temperature range depending upon the reactants. Moreover, a random stirring rate is also applied to facilitate efficient synthesis of ILs.
Although, solvent does not take part in chemical reaction yet it significantly facilitates it. Therefore, selection of a particular solvent may either increase or decrease the percentage synthesis yield of an IL. However, no study is available explaining, how a solvent may affect the synthesis yield of IL. Moreover, previous studies are also unable to justify the solvent selection for IL synthesis on technical grounds. Similarly, the effect of temperature on percentage yield has also not been investigated. The only insight available, suggests that IL yield increases with rise in temperature and synthesis reaction must not be conducted beyond 80 °C because it may impart color impurities to ILs (Gordon et al., 2006). However, there is no research account available which illustrates the relationship between temperature and percentage yield of IL. Likewise, all the studies reporting IL synthesis via solvent aided method have employed a random stirring rate, and the possible effect of stirring rate on IL yield was not considered at all.
To properly, scale up the process for IL synthesis, all the above mentioned parameters must be optimized and controlled. Optimization of these process variables will not only increase the IL yield but also make the process energy efficient. A typical IL, 1-butyl-3-methylimidazolium tetrafluoroborate, has been synthesized using seven different solvents. The effect of solvent nature, temperature and stirring rate on synthesis yield of ILs, was explained in terms of a new correlation; solvent effectiveness factor (SEF).
2 Materials and methods
2.1 Materials
1-methylimidazole (CAS No. 616-47-7, reagent plus 99%, Sigma Aldrich), 1-bromobutane (CAS No. 109-65-9, reagent plus 99%, Sigma Aldrich), sodium tetrafluoroborate (CAS No. 13755-29-8, 98%, Sigma Aldrich), acetone (CAS No. 67-64-1, 99.9%, Merck Millipore), methanol (CAS No. 67-56-1, anhydrous 99.8%, Sigma Aldrich), ethanol (CAS No. 64-17-5, analytical standard, Riedel-de-Haen), n-hexane (CAS No. 110-54-3, anhydrous 95%, BDH), cyclohexane (CAS No. 110-82-7, analytical standard, Sigma Aldrich), benzene (CAS No. 71-43-2, reagent plus 99%, Riedel-de-Haen), toluene (CAS No. 108-88-3, analytical standard, Sigma), ethyl acetate (CAS No. 141-78-6, anhydrous 99.8%, Sigma Aldrich) and dichloromethane (CAS No. 75-09-2, anhydrous 99.8%, Sigma Aldrich).
2.2 Methods
13.72 g (0.10 mol) of 1-bromobutane and 9.03 g (0.11 mol) of 1-methylimidazole were weighed using digital balance with accuracy of 0.0001 g. The weighed reactants and solvent were added in a three neck round bottomed flask, fitted with a reflux condenser. The mixture was continuously heated at 60 °C and stirred at 100 RPM using PCSIR Rota mantle. Nitrogen gas was circulated through the reaction mixture and the reaction was carried out for 38–46 h until the formation of two distinct layers. The bottom layer was decanted and washed thrice with 20 ml of ethyl acetate to obtain quaternary salt. The residual solvent from quaternary salt was evaporated using vacuum distillation. This quaternary salt was further reacted with 10.98 g (0.10 mol) of NaBF4 in a simple flask. The mixture was continuously heated at 45 °C and stirred at 100 RPM for 6 h. The reaction mixture was filtered to remove NaBr precipitate. The filtrate was vacuum distilled at 70 °C to afford final product. Seven different solvents were used in similar fashion and their corresponding IL yields were determined. The reactions involved in synthesis of [BMIM][BF4] are illustrated in Fig. 1.![Synthesis of [BMIM][BF4].](/content/184/2020/13/2/img/10.1016_j.arabjc.2019.04.005-fig1.png)
Synthesis of [BMIM][BF4].
IR spectra of IL were recorded on Agilent Technologies ATR spectrophotometer. The 1H NMR spectra of IL were recorded in CDCl3 on a Bruker DRX-400 Avance Spectrometer. IR (ATR): 2963 w, 2874 w, 1572 w, 1466 w, 1169 m, 1050 s, 847 w, 756 w cm−1. 1H NMR (400 MHz, CDCl3): δ 9.16 (s, 1H), 7.29 (s, 1H), 7.24 (s, 1H), 4.17 (t, J = 6.0 Hz, 2H), 3.97 (s, 3H), 1.88–1.81 (m, 2H), 1.38–1.32 (m, 2H), 0.93 (t, J = 7.6 Hz, 3H).
2.3 SEF calculations
The solvent effectiveness factor for different solvents was calculated using Eq. (1).
Ts = Boiling point of solvent used
Tw = Boiling point of water
ηs = Viscosity of solvent at synthesis temperature
ηw = Viscosity of water at STP
The effect of temperature on IL yield was investigated from 50 to 80 °C and using acetone as solvent. The effect of stirring rate on IL yield was investigated by stirring 50 ml of acetone at 30 °C for 1 h. Further, solvent was stirred in completely insulated conditions from 80 to 200 RPM. The observed rise in solvent temperature is related to its corresponding temperature and viscosity at 60 °C as shown in Table 1.
Stirring rate (RPM)
Ti (°C)
ηi (cp)
Tf (°C)
ηf (cp)
80
60
0.224
60.8
0.222
100
60
0.224
61.4
0.220
120
60
0.224
61.7
0.219
140
60
0.224
62.3
0.217
160
60
0.224
62.9
0.214
180
60
0.224
63.2
0.213
200
60
0.224
63.6
0.211
2.4 IL yield calculations
IL yields are calculated using Eq.2.
3 Results and discussion
3.1 Effect of solvent on IL yield
In IL synthesis, solvent safeguards the reaction mixture from the effects of air and moisture to prevent side reactions. Further, as the reaction progresses in the absence of solvent, the viscosity of reaction mixture increases due to the formation of viscous quaternary salt. Moreover, flow characteristic of the reaction mixture can be disturbed because of the increased viscosity and inhibit reaction propagation. Therefore, solvent also serves as a viscosity reducing agent for proper propagation of reaction. Further, it also facilitates the uniform distribution of heat energy in the reaction mixture as well as discards the excess heat from reaction mixture by exchanging it with external coolant. Therefore, selection of a proper solvent is essential for high synthesis yield of IL. A solvent for IL synthesis can be selected based on its physical properties. Since IL synthesis involves both heating and mechanical agitation, and hence boiling point and viscosity become significant parameters for the solvent selection.
3.1.1 Effect of solvent’s boiling point
To analyze the effect of solvent’s boiling point (BP) on IL yield, different solvents and their corresponding synthesis yields are plotted in Fig. 2. Solvent S1 with the lowest BP 56 °C, afforded maximum IL yield 88.11%. Solvent S7with the highest BP 111°Cproduced the least amount of IL 63.21%. It suggests that the synthesis yield generally decreases with elevation in solvent’s BP. This is because when a high BP solvent is used for IL synthesis, the heat supplied to the reaction mixture is largely consumed by the solvent itself and is not distributed among the reactants. Therefore, IL yield is reduced because the energy needed for reactants to cross the energy barrier is lost in getting the solvent’s temperature close to its BP. On the contrary, low BP solvents consume less energy to reach their BP and hence energy supplied to the reaction mixture is utilized by reactants to cross energy barrier to form products.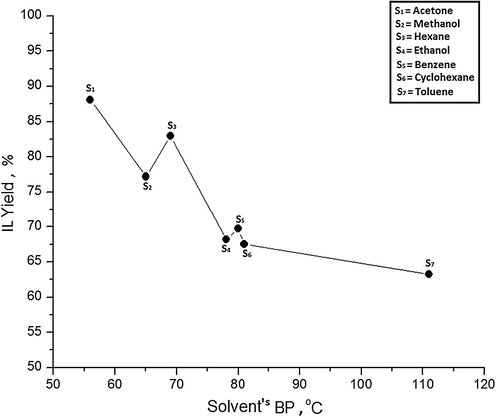
Effect of solvent's boiling point on IL yield.
However, it is worth noticing that solvent S3with higher BP 69 °C has afforded better yield of82.97% compared to solvent S2 with BP 65 °C and producing only 77.20% IL. Similarly, slight deviation has also been observed with solvents S4 and S5. Since, a regular trend of IL yield cannot be predicted based on BP of a solvent. Therefore, BP appears to be a defective criterion for solvent selection for IL synthesis.
3.1.2 Effect of solvent’s viscosity
To better rationalize the solvent’s control over synthesis yield of IL, viscosity of a solvent may prove an effective parameter. Therefore, viscosities of different solvents at 60 °C are plotted against their IL yields. Solvent S1 with the lowest viscosity 0.224 cp gives maximum IL yield 88.11% and solvent S7 with viscosity 0.374 cp has produced minimum IL of 77.20%. Generally, IL yield decreases when viscosity of solvent increases which can be observed from Fig. 3. It indicates that less viscous solvents are more efficient in IL synthesis because they can easily circulate through the reaction mixture and able to uniformly distribute heat energy to the reactants. Further, enhanced flow ability of solvent also increases the number of effective collisions among reacting species and elevating the chances of product formation.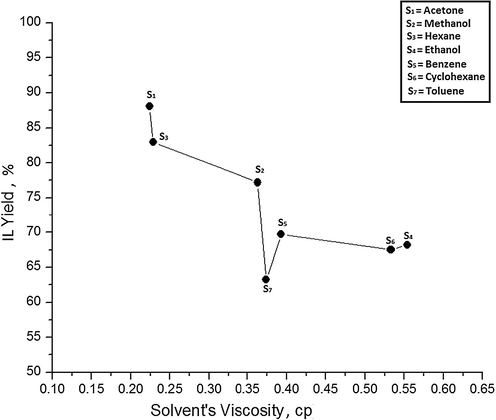
Effect of solvent's viscosity on IL yield at 60 °C.
However, solvent S7 has deviated from the regular trend and afforded the lowest yield despite the fact it has lower viscosity compared to S4, S5, and S6. The relationship between solvent viscosity and percentage yield is more uncertain because with the slightest variation in viscosity, IL yield alters prominently as from solvent S1 to S3 and from S4 to S7. These results suggest that viscosity cannot be adapted as a reliable standard of solvent selection for IL synthesis.
3.1.3 Solvent’s effectiveness factor (SEF)
Although there seems to be a generalized correlation between IL yield and either of the solvent properties, i.e. BP and viscosity, the solvent choice for IL synthesis cannot be done based on those properties. Therefore, a new correlation solvent effectiveness factor (SEF) has been developed by incorporating both properties of solvent because these reciprocate each other. The SEF of different solvents has been calculated using Eq. (1). It is worth noticing that SEF values are temperature dependent despite the fact, it is a dimensionless value because viscosity of a solvent alter with variation in temperature. To analyze the relationship between SEF and IL yield, a graphical representation is shown in Fig. 4. It can be noticed that the lowest synthesis yield 63.21% has been obtained with S7 because it has the lowest SEF value 0.6738. IL yield has increased smoothly with rise in SEF as manifested by all solvents especially by S4 and S6 because a minor increase in SEF from 0.744 to 0.749has increased an agreeable yield from 67.5% to 68.1%. Solvent S1 has afforded maximum IL yield 88.11% because it has the highest SEF 1.275. The observed results indicate that, IL yield is directly proportional to SEF. Further, IL yield at a particular temperature can also be predicted for a specific solvent based on its SEF.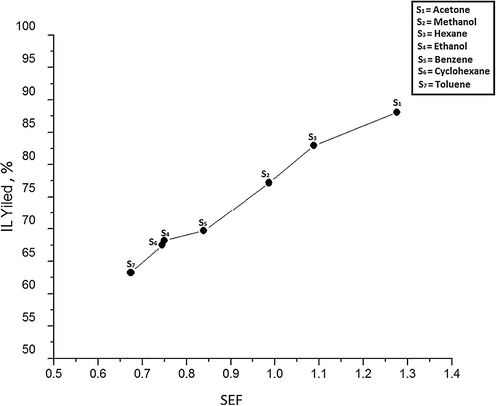
Effect of SEF on IL Yield at 60 °C and 100 RPM.
3.2 Effect of temperature on IL yield
Percentage yield of ILs cannot be enhanced in solvent aided method, without precisely controlling the temperature of reaction mixture. Although, both reactions involved in IL synthesis are exothermic yet energy is required to initiate these chemical reactions. However, to precisely control the reaction temperature, heat energy is continuously supplied to sustain the reaction rate followed by removal of excess heat using reflux conditions. A number of reports regarding IL synthesis suggest that IL yield increases at higher temperatures (Wasserscheid and Welton, 2008; Dzyuba and Bartsch, 2001). “Since, quaternization reaction is carried out at relatively higher temperature compared to anion exchange and requires very precise temperature control to obtain high IL yields. Further, it is also very complex and time consuming reaction (38–46 h) compared to anion exchange which is a mere anion replacement in the IL structure”. Therefore, effect of temperature on IL yield is investigated considering the quaternization reaction, only. The dependence of IL yield on temperature in terms of SEF has been graphical represented in Fig. 5.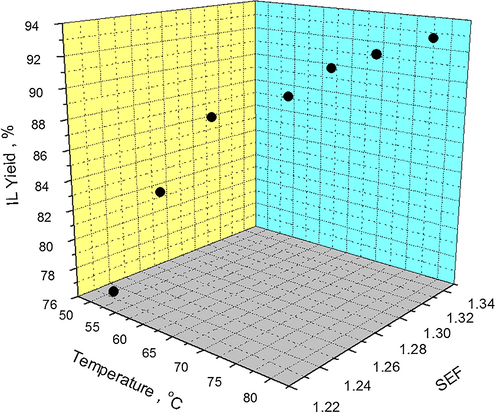
Effect of temperature on IL yield as a function of SEF at 100 RPM, solvent-acetone.
At 50 °C, the SEF of Acetone is 1.234 and the afforded IL yield is 76.73%. When reaction temperature is increased to 55 °C, SEF increases to 1.250 and IL yield is enhanced to 83.79%. Further, Conducting IL synthesis at 60 °C raises SEF to 1.275 and the observed IL yield becomes 88.11. Similarly, a general trend of increasing IL yield can be noticed with rise in temperature and SEF. %. It is important to note that when temperature increases, SEF also increases mainly due to the decrease in solvent’s viscosity.
However, when temperature is further increased from 60 °C to 80 °C, SEF has also increased accordingly from 1.275 to 1.333 but IL yield has only increased from 88.11 to 93.08. The reduced growth in IL yield can be explained in terms of reactants concentration and threshold temperature. Although, SEF increases accordingly when synthesis temperature is increased above 60 °C, but the lower concentration of reactants reduces the frequency of product formation. Therefore, increasing the temperature above 60 °C does not increase IL yield agreeably with SEF values because SEF can only enhance IL yield when there is a sufficient amount of reactants left in the reaction mixture. Moreover, when higher temperatures are employed for IL synthesis, the reaction mixture moves closer to the threshold temperature where side reactions start. These reactions do not allow IL yield to increase substantially. Therefore, the effect of temperature in terms of SEF over IL yield becomes less significant.
3.3 Effect of stirring on IL yield
Besides the solvent and reaction temperature, stirring rate may also affect the IL yield. Stirring enables the solvent to uniformly distribute the heat energy to the entire reaction mixture. Further, it also increases the number of effective collisions among the reactants, increasing the probability of product formation. Since, stirring adds mechanical energy to the reaction mixture which may either appear as rise in temperature or decrease in viscosity of solvent.
The results of Table 1indicate that a higher stirring rate further reduces the viscosity of solvent and SEF increases. The effect of stirring rate on IL yield in terms of SEF is shown in Fig. 6. A slight and gradual increase in IL yields can be observed when stirring rates are increased from 80 to 200 RPM. At first when stirring rate is 80 RPM the corresponding SEF is 1.278 and IL yield is 87.37%. When stirring rate is increased to 200 RPM the corresponding SEF becomes 1.297 and a synthesis yield of 91.3% has been obtained. It has been observed that IL yields have not increased significantly, even at very high stirring rates. This indicates that high stirring rates do not add substantial mechanical energy to the reaction mixture to increases the reaction temperature. Therefore, SEF do not increase significantly, thus giving rise to a minor increase in IL yield.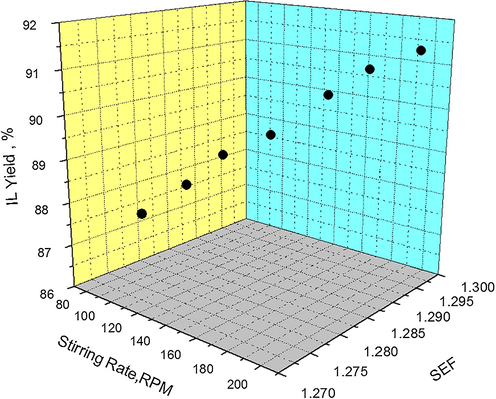
Effect of Stirring Rate on IL Yield as a function of SEF at 60 °C, solvent-acetone.
4 Conclusion
The study has revealed that SEF can be used to optimize the IL synthesis process. Further, IL yields may also be predicted using SEF when synthesis conditions are known. SEF is mainly affected by the nature of solvent and synthesis temperature. Since, agitation does not alter SEF substantially therefore, any moderate stirring rate can be employed for IL synthesis. Finally, it can be concluded that solvents with higher SEF values ought to be used for higher IL yields.
Acknowledgement
The authors of this manuscript acknowledge the cooperation of Higher Education Commission of Pakistan and HEJ Research Centre, Karachi.
References
- Synthesis, characterization and application of 1-butyl-3-methylimidazolium tetrafluoroborate for extractive desulfurization of liquid fuel. Arabian J. Chem.. 2016;9(4):578-587.
- [Google Scholar]
- Efficient synthesis of 1-alkyl (aralkyl)-3-methyl (ethyl) imidazolium halides: precursors for room-temperature ionic liquids. J. Heterocycl. Chem.. 2001;38(1):265-268.
- [Google Scholar]
- Selection of ionic liquids as entrainers for separation of (water+ethanol) J. Chem. Thermodyn.. 2008;40(8):1248-1252.
- [Google Scholar]
- Gordon, C.M., Davis, Jr., James H., M., Charles, 2006. Gordon, Claus Hilgers, and Peter Wasserscheid. Ionic Liquids in Synthesis, pp. 7.
- A review of ionic liquids towards supercritical fluid applications. J. Supercrit. Fluids. 2007;43(1):150-180.
- [Google Scholar]
- Expedient synthesis of symmetric aryl ketones and of ambient-temperature molten salts of imidazole. Synthesis. 2000;2000(09):1253-1258.
- [Google Scholar]
- Synthesis and properties of ionic liquids: imidazolium tetrafluoroborates with unsaturated side chains. Bull. Korean Chem. Soc.. 2006;27(6):847-852.
- [Google Scholar]
- Ionic liquids in separations of azeotropic systems – a review. J. Chem. Thermodyn.. 2012;46:2-28.
- [Google Scholar]
- Applications of ionic liquids in the chemical industry. Chem. Soc. Rev.. 2008;37(1):123-150.
- [Google Scholar]
- Ionic liquids: synthesis and applications in catalysis. Adv. Chem.. 2014;2014:1-16.
- [Google Scholar]
- An expeditious solvent-free route to ionic liquids using microwaves. Chem. Commun.. 2001;7:643-644.
- [Google Scholar]
- Ionic Liquids in Synthesis. John Wiley & Sons; 2008.
- Selective separation of toluene/n-heptane by supported ionic liquid membranes with [Bmim][BF4] Chem. Eng. Technol.. 2015;38(2):355-361.
- [Google Scholar]







