Translate this page into:
Starburst pamam dendrimers: Synthetic approaches, surface modifications, and biomedical applications
⁎Corresponding author at: Department of Pharmaceutics, Dadasaheb Balpande College of Pharmacy, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, MS 440037, India. nmmahajan78@gmail.com (Nilesh Mahajan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Dendrimers are having novel three dimensional, synthetic hyperbranched, nano-polymeric structure. Among all of the dendrimers, Poly-amidoamine (PAMAM) dendrimer are used enormously applying materials in supramolecular chemistry. This review described the structure, characteristic, synthesis, toxicity, and surface modification of PAMAM dendrimer. Various strategies in supramolecular chemistry of PAMAM for synthesizing it at commercial and laboratory scales along with their limitations and applications has also discussed. When compared to other nano polymers, the characteristics of supramolecular PAMAM dendrimers in nanopolymer science has shown significant achievement in transporting drugs for molecular targeted therapy, particularly in host–guest reaction. It also finds its applications in gene transfer devices and imaging of biological systems with minimum cytotoxicity. From that viewpoint, this review has elaborated the structural and safety aspect of PAMAM for targeted drug delivery with pharmaceuticals in addition to the biomedical application.
Keywords
PAMAM
Supramolecular dendrimer
Synthesis
Biomedical application
Nanotherapeutics
Cytotoxicity
1 Introduction
From the current research of polymeric chemistry and technology, significant work had already been completed for developing the therapeutically effective dosage form and transportation of adequate medicament to target site (Al-Jamal et al., 2005; Tupally et al., 2015). Synthetic biodegradable polymeric macromolecules and nano biopolymers have enlightened the field of pharmaceutical and biomedical applications of novel drug delivery systems (Tomalia et al., 1985). In that perspective, dendritic polymers provide an essential scientific breakthrough in the structural designing of different bioactive materials to enhance and exposed their specific functions (Bosman et al., 1999; Tomalia et al., 1985).
In 1978, Vogtle and co-workers reported to synthesize and propose the dendritic structure of polypropylene amine through repetitive monomer addition and activation of branched molecules shown in Scheme 1. The above synthesized dendritic structure initially named “cascade molecule” (Tomalia and Fréchet, 2002). However, this concurrent synthetic investigation Tomalia and associates at Dow chemical’s near the beginning of 1980, synthesized hyperbranched monodisperse poly(amidoamine) PAMAM molecules (Tomalia et al., 1986). George Newkom’s parallelly synthesized the analogous macromolecules called arborols, which means trees in Latin, but ‘dendrimer’ is the best established one (Newkome et al., 1986).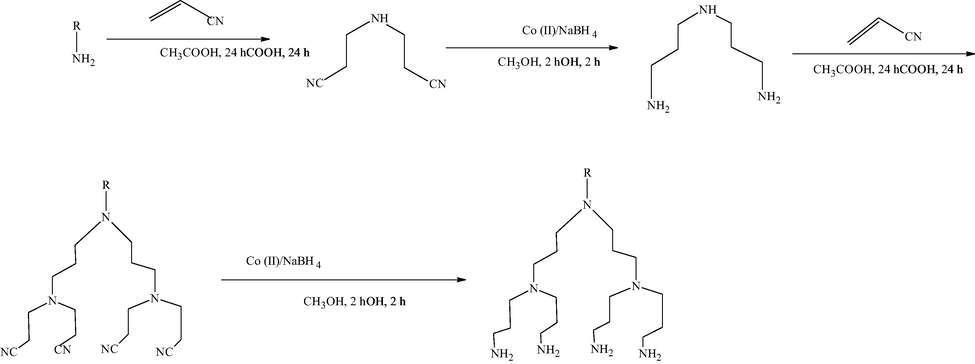
Synthesis of cascade molecule by Vogtle and co-workers (Tomalia and Fréchet, 2002).
Dendrimers are well repeating branched unit monodispersed spherical macromolecule characterized with a multifunctional core with high density and precise functional groups attached to a surface (Alper, 1991; Newkome et al., 1986). Table 1 represents different types of dendrimers with its structural specificity and applications. Compared to conventional linear, branched, and hyperbranched polymers of the same molecular weight, dendrimers have attracted wide attention due to unique functional properties confined within a small three-dimensional space (Caminade et al., 2005).
Sr. No.
Types of dendrimers
Synthesis/First reported by
Structural specificity
Applications
Structure
References
1
Polyamidoamine dendrimers (PAMAM)
Divergent method/Tomalia et al.
Spheroidal or ellipsoidal with the presence of several peripheral functional groups and internal cavities
Diagnostic agent. Targeting drug delivery and gene therapy.
Naylor, 1989; Negar and Rouhollah, 2013; Pagé and Roy, 1997; Tomalia et al.,1985
2
Chiral dendrimers
Convergent method/Diederich et al.
Peripheral primary amines groups and tertiary propylene amines as a core. Chirality based on the constitutionally different but chemically analogous branches.
Enantioselective catalysis and molecular recognition process.
Ritźen and Frejd, 1999; Smith and Diederich, 1998
3
Liquid crystalline dendrimers
Divergent or convergent method /Virgil percec et al.
Mesogenic liquid crystalline monomers or thermotropic liquid crystalline phases which are rod-like (calamitic) or disk-like (Discotic) molecules.
Use for specific physical properties.
Cheng et al., 2015a, 2015b; Guillon and Deschenaux, 2002; Percec et al., 1992; Smith and Diederich, 1998
4
Polypropylene ether imine dendrimers (PPI)
Divergent method/Newkome et al.
Amino butane core with primary amines as functional end groups.
Antimicrobial and solubility enhancement agent.
Gillies et al., 2005; Inoue, 2000; Kaur et al., 2016; Newkome et al., 1986
5
Tecto dendrimers
Divergent method/Tam et al.
Fluorescein core (thermotropic agent) and folate as a functionally active peripheral moiety.
Used for Multidrug delivery, gene therapy, and environmental remediation.
Schilrreff et al., 2012; Tam and Lu, 1995; Welch and Welch, 2009
6
Peptide dendrimers
Convergent method/Sadler et al.
Amino acid as peripheral and interior unit.
Used for the diagnostic purpose, vaccine delivery, protein replacement, Chemotherapeutic agent and gene therapy.
Ammonium, 1995; Backbone, 1990; Sadler and Tam, 2002
7
Carbosilane dendrimers
Divergent and convergent method/Bermejo et al.
Two different cores 1,3,5-(HO)3C6H3 and Si(C3H5)4 having carboxylate and sulfonate as an end functional groups.
Potential application in catalysis, host–guest chemistry, novel polymer topology.
Bermejo et al., 2007; Kesharwani et al., 2014
8
Glycodendrimers
Divergent and convergent method/Benjamin Davis et al.
Glycodendrimers include sugar moieties such as mannose, glucose, galactose, and disaccharide.
In biotechnology and material science.
Benjamin, 2002; Roy and Baek, 2002;
9
Triazine dendrimers
Convergent method (cycloaddition reaction or triazene substitution)/Astruc et al.
The core is containing triazine trichloride and other triazine based compounds.
Used in the synthesis of simazine herbicide also applied in non-viral gene delivery.
Astruc et al., 2012; Steffensen et al., 2006
10
Hybrid dendrimer
A Divergent method (Diels- Alder cycloaddition) by Roovers et al.
These are hybrids of dendritic and linear polymers.
Composed of elastic carbosilane core and rigid aromatic shell.As drug carriers.
Kesharwani et al., 2014; Roovers and Comanita, 1999
11
PAMAMOS (Poly amidoamine Organosilicon Dendrimers)
Divergent method/Peter Dvornic et al.
PAMAM as an interior and hydrophobic organosilicon as a peripheral moiety.
Use for drug conjugate and nanocarrier.
Dvornic, 2006; Tomalia, 1993; Torchilin, 2006
12
Multiple antigen peptide dendrimers
Convergent and Divergent method/Tam et al.
Dendron like molecular configuration based on a polylysine framework.
Improved efficiency of antibiotics and antimicrobial molecules.
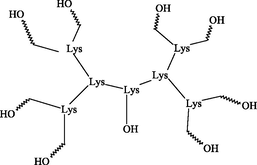
Joshi et al., 2013; Tam and Lu, 1995
13
Frechet-type dendrimers
Convergent method/Hawker and Frechet et al.
Structural framework based polybenzyl ether with Carboxylic acid group act as a functionally active peripheral moiety.
Pharmaceutically active compound, imaging agent, radioligand and targetting molecule.
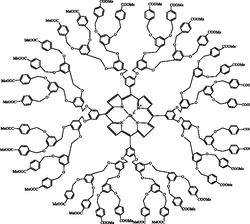
Hawker and Fréchet, 1990; Ihre et al., 2002
According to Tomalia, dendritic structures categorized into four sub-classes that are random hyperbranched polymer, dendrigraft polymers, dendrons, and dendrimers (Tomalia et al., 1990, 1987). Among all the dendrimers poly(amidoamine) PAMAM dendrimer is used enormously as applying materials in supramolecular chemistry (Naylor,1989). PAMAM provides the perfect structure for the development of active drug carriers (Jansen et al., 1994), gene transfer devices, host–guest interactions (Hu et al., 2012), and imaging biological systems with minimum cytotoxicity (Hsu et al., 2017; Roberts et al., 1996). From the above aspects, we have discussed synthesis, structure, cytotoxicity, characteristic, surface modification of PAMAM dendrimers, and their possible biomedical, as well as pharmaceutical applications in the field of nanotechnology.
2 Structural chemistry
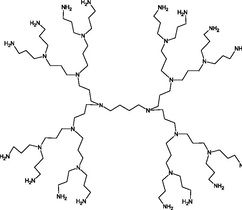
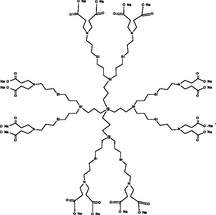
In 1984, Tomalia and co-workers developed, characterized, reported, and commercialized the first absolute PAMAM dendrimers family. These molecules start from an ammonia (NH3) or ethylenediamine (C2H8N2) core and bind amine or amide groups on the periphery (Esfand and Tomalia, 2001). From the central core, ‘wedges’ or ‘dendrons’ radiates toward the periphery, resulting in homo-structural layers (branching unit) (Nanjwade et al., 2009; Santos et al., 2019). Each layer of concentric branching units considered as one generation (Menjoge et al., 2010). Commercially PAMAM dendrimers with terminal carboxylic groups are called half generation (0.5, 1.5, 2.5…9.5), and the amino group are called a full generation (1, 2, 3…10). Fig. 1 represents the structure of PAMAM dendrimer with the generation’s number and the peripheral end groups (Twyman et al., 2002). Table 2 gives information about the diameter of PAMAM, which linearly increased according to generation and peripheral functional groups.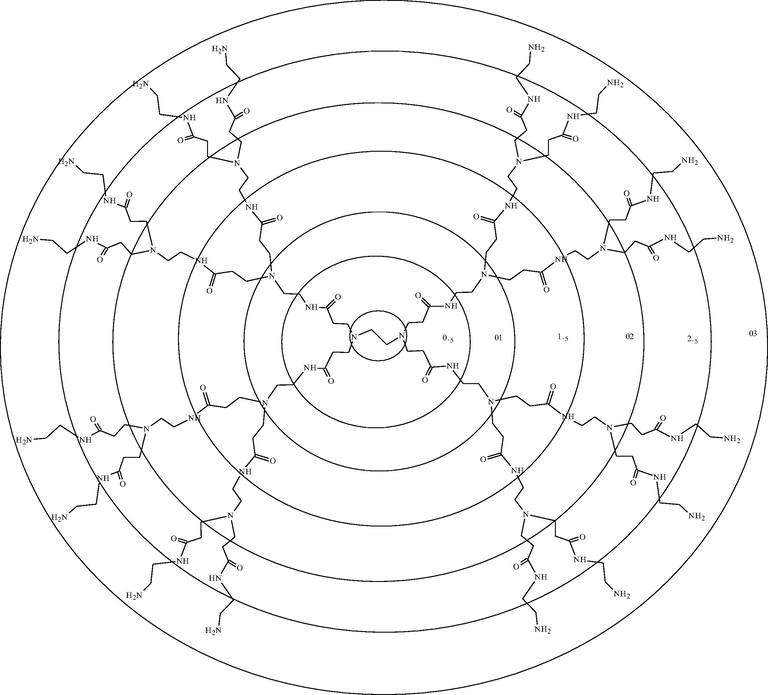
Structure of PAMAM dendrimer with the generation’s number and the peripheral end groups.
Generation
Molecular formula
Hydro dynamic diameter
Ammonia core
Ethylenediamine (EDA) core
Molecular mass
No of end groups
Molecular mass
No of end groups
0
C24H52N10O4
1.5 nm
359
3
516
4
1
C64H132N26O12
2.2 nm
1043
6
1428
8
2
C144H292N58O28
2.9 nm
2411
12
3252
16
3
C304H612N122O60
3.6 nm
5147
24
6900
32
4
C624H1252N250O124
4.5 nm
10,619
48
14,196
64
5
C1264H2532N506O252
5.4 nm
21,563
96
28,788
128
6
C2544H5092N 1110O508
6.7 nm
43,451
192
57,972
256
7
C5104H10212N2042O1020
8.1 nm
87,227
384
116,340
512
8
C10222H20448N409002044
9.8
174,779
768
233,076
1024
9
C20462H40928N818604092
11.4
349,883
1536
466,548
2048
10
C40942H81888N1637808188
∼13.0
700,091
3072
933,492
4096
“Starburst dendrimer” is a trade name for the subclass of PAMAM starlike, three-dimensional, highly ordered oligomeric compounds (Vlasov, 2006), which has been commercially manufactured by Dendritech TM (U.S.A.) since 1990 (Abbasi et al., 2014). PAMAM dendrimer becomes compactly crowded towards the periphery of the higher generation (Sadekar and Ghandehari, 2012). The steric crowding of branches increases after PAMAM G7, which leads in the formation of defective structure (De Gennes dense packing effect). After the tenth generation, the synthetic yield has reduced (Bugno et al., 2015), and dendrimers cannot grow because of insufficient space. This effect is known as ‘Starburst effect’ (Gupta and Nayak, 2015; Maiti and Goddard, 2006). After this step, the speed of reaction drops suddenly, and further reaction with the end group cannot occur (Peterson et al., 2001; Scott et al., 2005).
The molecular weight of the dendrimer can be predicted mathematically using Eq. (1) (Jackson et al., 1998; Walter and Malkoch, 2012).
Mc: The molecular weight of the core
Mm: Molecular weight of the branched monomer
Mt: Molecular weight of terminal groups
nc: Core multiplicity
nm: Branch juncture multiplicity
G: Generation number.
The number of dendrimer terminal groups increases with geometric progression (Eq. (2)) (Jackson et al., 1998; Walter and Malkoch, 2012).
This important contribution of Tomalia in well-defined structure holds great promise in nanotechnological approaches.
3 Properties of pamam dendrimers
Dendrimers are well distinguished from all other traditional linear polymers through considerably enhanced physicochemical properties. Dendrimers also have immense potential in the field of novel and targeted drug delivery (Nishikawa et al., 1996). The physical and chemical properties of PAMAM with immense potential in the field of drug delivery are shown in Fig. 2. Due to the controlled fundamental properties, including monodispersity, size and shape, peripheral charge, flexibility or rigidity, structural design, and fundamental composition, PAMAM proved itself best over the other nanocarriers (Thiagarajan et al., 2013). Researchers provide sufficient information for predicting and elucidating some techniques for characterization of PAMAM dendrimer molecule which are described below.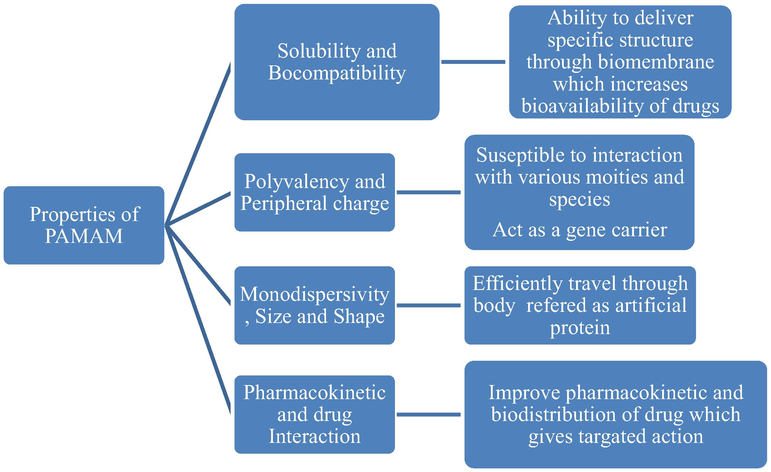
Physical and chemical properties of PAMAM with immense potential in the field of drug delivery.
3.1 Monodispersity, size, and shape
Monodispersity means a well distinct molecular arrangement without variations, which shows a significant role in predicting the pharmacokinetic behaviour of the molecule (Kaminskas et al., 2011). PAMAM is the class of dendritic polymers having a well distinct molecular arrangement, and reproducible nanoscale size through their controlled synthesis and purification process (Zhong et al., 2011). The Monodispersity of PAMAM confirmed by various analytical techniques includes mass spectroscopy, size exclusion chromatography, gel electrophoresis, and transmission electron microscopy (TEM) (Kaminskas et al., 2011; Zhong et al., 2011). Mass spectroscopic data explicitly defined monodispersity of lower generation PAMAM (G1 - G5) synthesized by the divergent method. Subsequent generation leads to polydispersity because of the partial elimination of ethylenediamine in every forwarded generation. The polydispersity index is less than 1.08 for G5–G10 PAMAM dendrimer, which shows that even particle size distribution in every step of generation(Lin et al., 2015; Maiti et al., 2005).
The size of PAMAM dendrimers for G0 to G10 ranges from 10 Å to 130 Å. Lower generations possess open, highly planar, elliptical, asymmetric shapes as compared to a higher generation. The spheroidal structure of PAMAM dendrimers contains the ability to incorporate drug molecules (Lin et al., 2005; Malik et al., 2000), due to the presence of peripheral protonated primary amino groups at a physiological pH (Lin et al., 2005; Percec et al., 1998). G3, G4, and G5 PAMAM dendrimers with ammonia core have structural similarity with insulin (30 Å), cytochrome C (40 Å), and hemoglobin (55 Å), respectively. Because of its biostructural similarity, PAMAM efficiently travels throughout the body and called ‘artificial protein’ (Lee and Larson, 2008; Liu et al., 2009). Earlier researchers reviewed various scattering techniques together with small-angle X-ray diffraction, small-angle neutron scattering, and laser light scattering for characterization of PAMAM dendrimers, suggesting two types of microscopy, including transmission electrons microscopy (TEM) and atomic force microscopy (AFM) for imaging hydrodynamic radius, the structure of complete dendrimer, and radius of gyration in solution (Caminade et al., 2005). Monodispersity and size concern its pharmacokinetic behaviour in a biological system for pharmaceutical application.
3.2 Polyvalency and peripheral charge
Polyvalency determined the number of reactive sites present on the surface of the dendrimer. PAMAM consists of several peripheral moieties with positive, negative, or neutral charges, which makes them more susceptible to interact with various materials and species (Cakara et al., 2003; Pryor et al., 2014). Caminade et al. have proposed electron paramagnetic resonance technique (EPR) to identify the interaction between end groups and the substitution efficiency of PAMAM. They also suggested the electrophoresis technique and differential scanning calorimetry (DSC) for the assessment of PAMAM surface groups (Caminade et al., 2005; Pryor et al., 2014). PAMAM dendrimers with a positive surface charge can be applied as a gene carrier because it forms complex with negatively charged DNA (Nomani et al., 2010). Maiti et al. also demonstrated the significance of peripheral charge on PAMAM. He explained that how peripheral charge plays a vital role to synthesize PAMAM encapsulated nanoparticles (Maiti and Messina, 2008; Nomani et al., 2010; Pryor et al., 2014).
The peripheral positive charge of polycationic PAMAM dendrimers interacts with the negatively charged biological membrane; showing smooth intracellular delivery. Among these advantages, polycationic PAMAM dendrimers shows some toxicities (Nomani et al., 2010; Pryor et al., 2014). Nomani et al. synthesized self-assembled antisense/PAMAM dendrimer nanoparticles. He observed that cytotoxicity of nanoparticles depends on generation as well as the charge ratio of PAMAM. He also noticed in-vitro cytotoxicity of Polyfect® (G6 PAMAM) and Superfect® (G6 PAMAM), which can be overcomed by surface modification (Nomani et al., 2010; Sadekar et al., 2013). In that concern, polyvalency of PAMAM has a potential role in intracellular targeted drug delivery by surface modification.
Sadekar and Ghandehari studied the effect of peripheral charge and PAMAM generation in oral delivery. They observed the effect of PAMAM dendrimer's surface charge on cytotoxicity, transepithelial transport, and cellular uptake. In their study, they used Caco-2 cell lines to transport dendrimers by paracellular and transcellular mediated routes (Ernandez and Pedro, 2014; Sadekar and Ghandehari, 2012). After the study, they concluded that the surface-modified PAMAM dendrimers give reduced toxicity with enhanced transepithelial transport.
3.3 Solubility and biocompatibility
As compared to linear polymer, PAMAM dendrimer generally showed higher solubility and miscibility due to the presence of many branching ends. The solubility of PAMAM dendrimers is robustly dependent on various components such as peripheral group, number of generations and nature of terminal functional units (Cheng et al., 2007; Kayser et al., 2016). Generally, PAMAM are soluble, and therefore it acts as solubilizers for low solubility drugs by ionic interaction, hydrogen bonding, and hydrophobic interactions.
PAMAM dendrimers with hydrophilic surface groups showed solubility in polar solvents, while hydrophobic surface groups showed solubility in nonpolar solvents. High degree hydration of PAMAM dendrimers forms gel-like structures due to repulsive interaction between positive functional groups (Wang et al., 2017). As a result, PAMAM dendrimers with hydrophilic surface groups produced large hydrodynamic radius as compared to that in a dry condition. PAMAM dendrimers can also solubilize water-insoluble drugs. Consequently, these drugs easily transport through the biomembrane and increase its bioavailability (Dib et al., 2019; Negar et al., 2014).
PAMAM dendrimer should accomplish a variety of requirements for potential drug carriers such as non-toxicity, non-immunogenic, ability to cross biological barriers, stay in blood circulation long enough, and ability to deliver specific structure (Agashe et al., 2006; Markowicz-Piasecka et al., 2014).
Piasecka et al. examined concentration and generation dependent biocompatibility and adverse effect of PAMAM towards the endothelial monolayers. They also examined the impact of PAMAM on clot formation without disturbing the thrombin activity (Markowicz-Piasecka et al., 2014). On that background, Duncan et al. designated PAMAM dendrimers as ‘safe’ molecules and the chemistry of PAMAM used in specific applications like biomedical and pharmaceutical applications (Duncan and Izzo, 2005; Fuchs et al., 2004). Therefore, PAMAM dendrimers are capable of enhancing solubility with related biodistribution, biocompatibility and efficacy of several drugs.
3.4 Pharmacokinetic and drug interaction
Pharmacokinetic properties and their interference with biological components of PAMAM depend on its physicochemical properties (Jevprasesphant et al., 2003a, 2003b). The design of PAMAM dendrimers has modified to improve the pharmacokinetics and biodistribution of drugs with the aim of targeted action. Surface modified PAMAM dendrimers reported for giving improved pharmacokinetic profiles than the basic one. Amine terminated G3, and G4 dendrimers showed very rapid initial clearance and the high initial volume of distribution (Jevprasesphant et al., 2003a, 2003b). In that concern, researchers studied the effect of surface charge on the excretion profile of non-biodegradable G5 PAMAM dendrimer. The result showed that neutral charge dendrimer was excreted via urine and feces twice over seven days as compared to polycationic dendrimer (Nigavekar et al., 2004). Nigavekar and his coworkers compared PAMAM G5 dendrimer with surface modified acetylated PAMAM dendrimer for biodistribution. They used B16 melanoma cell line and DU145 human prostate cancer cell line. They observed that tissue deposition of polycationic PAMAM is better as compared to the neutral surface charge (Nigavekar et al., 2004). From that observation, they conclude that neutralization of surface charge of PAMAM dendrimers is required to utilize it as a systemic biomedical delivery device. Drugs interact with the PAMAM dendrimer molecule and encapsulate into the interior cavities by electrostatic interactions or by conjugation with the end groups.
Sadekar et al. compared the effect of the multiple branched PAMAM dendrimers and linear N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers on tumor-bearing mice. They identified the effect of molecular weight and hydrodynamic size of PAMAM and HPMA on biodistribution using compartmental pharmacokinetic analysis. They observed that hydroxyl-terminated PAMAM dendrimers have higher tumor extravasations rate than linear HPMA copolymers. Therefore they conclude that after circulation, hydroxyl-terminated PAMAM dendrimers have a greater affinity towards the tumor with a smaller amount of clearance than linear HPMA copolymer (Sadekar et al., 2013). From the above discussed prospective, PAMAM dendrimer-drug interaction shows significant importance to design and optimize the PAMAM-based drug delivery system. Molecular PAMAM properties and characteristics are central to releasing their potential.
4 Synthesis of pamam
PAMAM dendrimers are effectively used in various biomedical applications. For that purpose, it must be readily available in the required quantity with consistent quality. Unlike conventional polymers, dendrimers are synthesized via iterative reaction to maintain controlled branched structures and minimum polydispersity (Labieniec-Watala and Watala, 2015; Svenson and Tomalia, 2005). The synthesis of this supramolecular structure is complicated with low yield because the purification processes are difficult. Besides, the design and synthesis of PAMAM dendrimers require the appropriate handling of energy parameters, called “the architecture of the molecule,” to target the supramolecule having different structural and physicochemical properties (Hernández Ramírez et al., 2020).
4.1 Conventional synthesis
Dendrimers were usually synthesized using a series of iterative and activation steps. Since PAMAM having perfectly branched structure, the synthesis requires to apply the robust organic reactions that can efficiently proceed even at a macromolecular level (Alper, 1991; Caminade et al., 2005). Scheme 2 provides two main synthetic strategies, divergent and convergent approach to accomplish dendrimers (Zhong et al., 2011).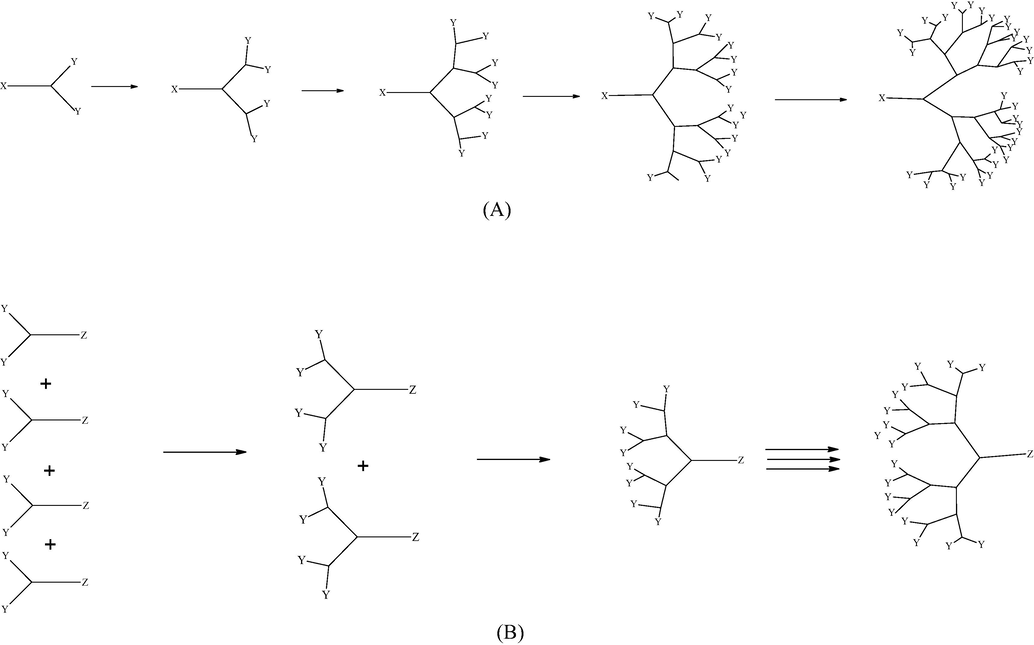
Diagramatic illustration of divergent (A) and convergent (B) synthetic approach for dendrimers.
4.1.1 Solution synthesis
In conventional solution synthesis of PAMAM, generally, two schemes were used that are divergent synthesis and convergent synthesis. However, the divergent scheme is superior for controlled branched PAMAM structure and predominantly employed for solid-phase synthesis of PAMAM dendrimers (Ihre et al., 2003).
4.1.1.1 Divergent approach
PAMAM dendrimers were synthesized by the divergent approach in a stepwise manner, starting from multifunctional core molecule ethylenediamine (EDA) as a precursor and building up the molecule towards periphery using michael addition and amidation reactions (Tomalia et al., 1987). Half generation of PAMAM dendrimer synthesized by the Michael addition, where as amidation reaction involved two reactions steps (i) coupling reaction for the activation of carboxylic group (ii) amidation step generatting amine-terminated full generation PAMAM dendrimers. The above two steps continue for multiple times to get optimized generations of PAMAM (Ihre et al., 2003) given in Scheme 3. After reaching the desired generation, the functionally active end groups are available for further post-functionalization. The divergent growth approach is also called the inside-outside approach (Labieniec-Watala and Watala, 2015).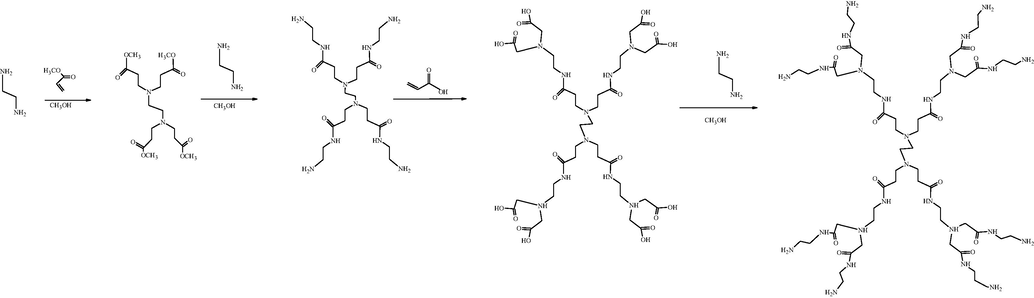
Divergent approach for synthesizing PAMAM dendrimer (Labieniec-Watala and Watala, 2015).
Structural imperfection is a significant concern in the growth of PAMAM dendrimer beyond G4 that unavoidably occurs from imperfect Michael addition reactions, retro-Michael reactions, intramolecular cyclization, dimerization during amidation and steric hindrance. It is difficult to separate the defective dendrimers from intact dendrimers because of their similar chemical compositions and physicochemical properties (Miller, 1990; Grayson and Fréchet, 2001). Fig. 3 provides brief information about structural defects occured in the synthesis of PAMAM dendrimer.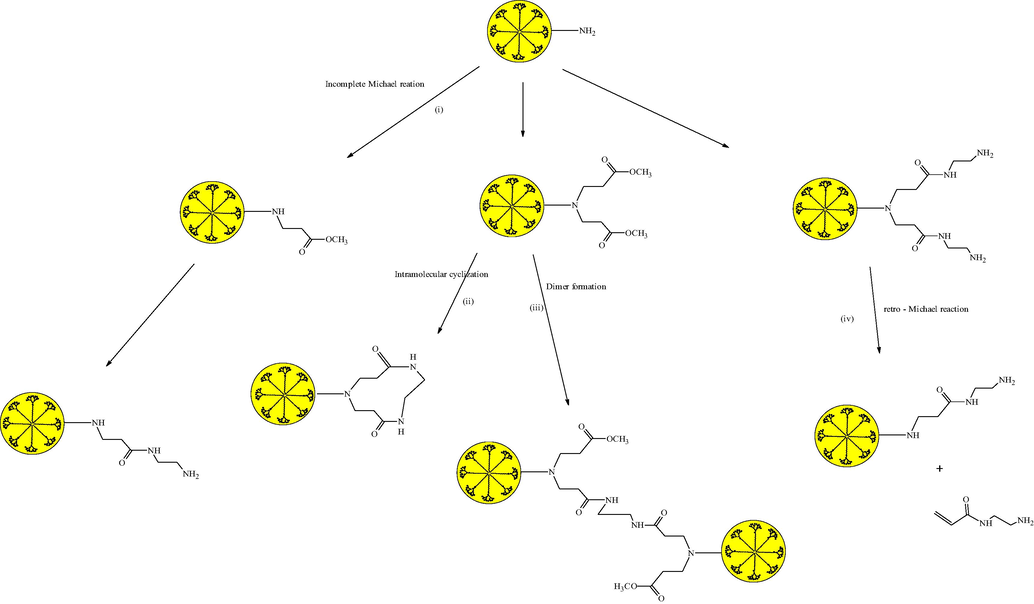
Structural defects occurred by the different side reaction in the synthesis of PAMAM dendrimer (Grayson and Fréchet, 2001; Miller, 1990).
In incomplete Michael addition reaction, nitrogen substitute one of the amine hydrogens of EDA (Michael donor), and another one fails to react. This missing moiety remains as such, and synthesis proceeds using such incomplete form of dendrimer. Sometimes intramolecular cyclization occurs in the amidation step between two branches with the same EDA molecule that stop the further expansion of PAMAM dendrimer (Gupta and Nayak, 2015). This type of reaction can only occur in the synthesis of full generation dendrimer. There is a chance of ester hydrolysis, which also generates defects in dendrimer. Beginning from the first generation, sometimes there are chances of retro Michael reactions in which arm degrades back to free –NH2 group. An excess of EDA avoids the side reactions in the amidation step. Peterson et al. suggested the most uncomplicated and easy Capillary zone electrophoresis (CZE) technique for the evaluation of different generations and analysis of homogeneity of a single generation. They also gives valuable information about “structural errors” in generations of dendrimer by electropherogram, which allows assessing the presence of side products in synthesized dendrimers (Peterson et al., 2001). Therefore it can be observed that the synthesis of PAMAM by divergent approach is at present accomplished with the well-known reaction in a controlled manner, where efficient protection or deprotection and coupling reactions are of primary importance.
4.1.1.2 Convergent synthesis
Besides divergent synthesis, Hawker and Fréchet in 1990 introduced a convergent growth scheme for the synthesis of PAMAM dendrimer (Hawker and Fréchet, 1990). In the convergent approach, the synthesis of PAMAM dendrimer forwarded from the surface towards the core by “one to one’ coupling of monomers (Gupta and Nayak, 2015). This method relies on the construction of perfectly branched dendrons (dendrimer wedges), coupled to a core moiety after activation of their focal point (Grayson and Fréchet, 2001). They first synthesized dendrons from G1 to G3 via iterative amide linkage and amine-deprotection. The obtained amine-containing dendrons were then attached to the core, as shown in Scheme 4 (Brouwer et al., 2001; Lee et al., 2006).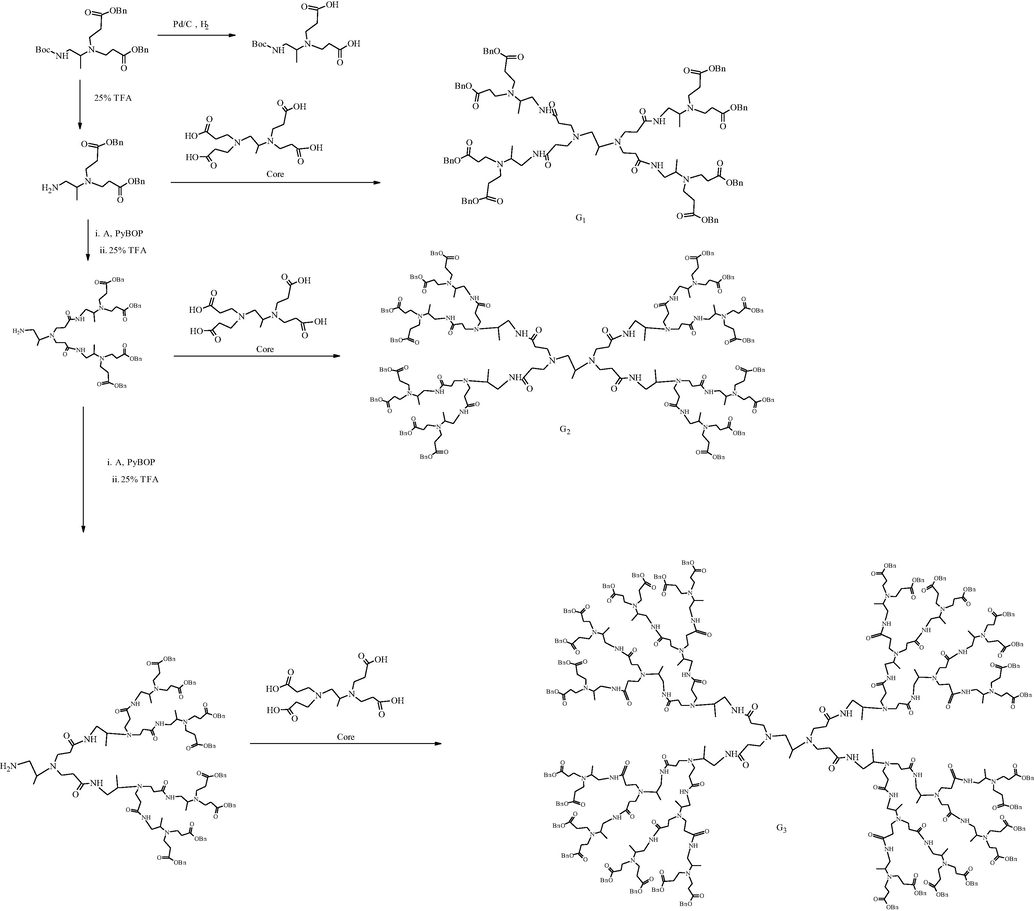
The synthesis of PAMAM dendrimers via the convergent growth approach using peptide synthesis chemistry (Huang et al., 2013).
PAMAM dendrimers synthesis via the convergent growth scheme presented a significant challenge for solution-phase synthesis because the use of an excess of reagent is not feasible. However, this approach overcomes the impurity and structural imperfection issues of divergent synthesis (Pittelkow and Christensen, 2005). By this method more consistent and symmetric dendrimers can be produced, although it has some limitations due to steric hindrance between dendrons which are going to attach the core of high generation dendrimers. Therefore by using the convergent approach, eight or fewer generation dendrimers can be obtained (Pittelkow and Christensen, 2005; Wells et al., 1998).
This approach is more accessible to synthesized heterogeneous or asymmetric dendrimers by incorporating several active sites in one dendrimer molecule. Pittelkow et al. report a convergent approach to synthesize a series of unsymmetrical internally branched PAMAM dendrimers using a highly effective protection group in combination with a very efficient peptide coupling reagent (Pittelkow and Christensen, 2005). The purity of dendrimers were confirmed by using the purity of dendrimers by SEC (scanning electron microscope), NMR (nuclear magnetic resonance), and MALDI-MS (mass spectroscopy) (Huang et al., 2013).
4.1.2 Solid phase synthesis
Other than solution synthesis, in 1988, Tam and his co-workers first reported the solid-phase synthesis approach for the synthesis of PAMAM dendrimer (Tam and Lu, 1995; Tam and Merrifield, 2009). They synthesized polylysine dendrimers by divergent approach through a conventional solid-phase peptide route (Tam and Lu, 1995). Bradley et al. synthesized PAMAM dendrimers conveniently and efficiently on the solid support by using Tomalia iterative growth for solution synthesis,viz., michael addition and amidation (Egner et al., 1995; Guillier et al., 2000; Wells et al., 1998). Scheme 5 represents the synthetic scheme for PAMAM dendrimers using peptide synthesis chemistry via the iterative growth approach. They achieved the growth of PAMAM dendrimers up to G4 with good homogeneity, characterized by 1H and 13C NMR, and electrospray mass spectrometry. They also reported that these solid-phase dendrimers employed for the bead loading amplification tool as well as for the effective synthesis of dendrimer conjugates (Egner et al., 1995). By solid-phase synthesis approach, peptides and small drug molecules can be attached to the dendrimer due to their multivalency (Grayson and Fréchet, 2001; Swali et al., 1997).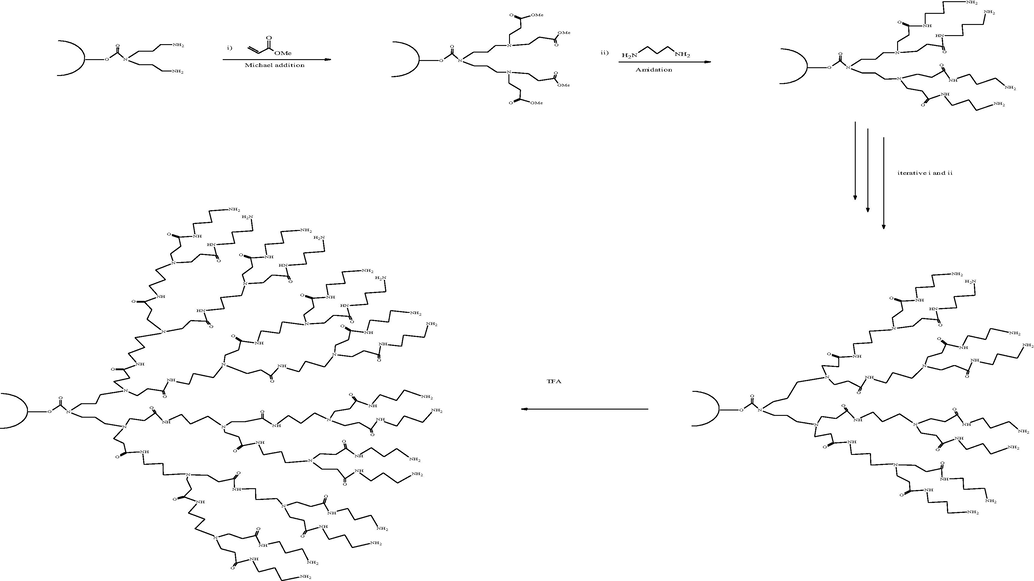
Solid-phase synthetic approach of PAMAM dendrimers using peptide synthesis chemistry via iterative Michael addition and amidation (Swali et al., 1997; Huck et al., 1998).
Huang et al. developed a concise solid-phase synthesis of inverse PAMAM dendrimers by using peptide synthesis chemistry. In this synthetic scheme, the PAMAM dendrimers designed using the peptide synthesis approach with amide linkage in the reverse direction. Therefore dendrimers synthesized by that approach were called inverse PAMAM dendrimers. He prepared dendron by incorporating 1,3-propylene diamine moiety as a core instead of ethylenediamine and carboxylic acid and amine as a peripheral end moiety (Huang et al., 2013). In contrast to the conventional PAMAM synthesis, the synthetic peptide approach appreciably reduces the formation of side products, showing structural defect that begins from Michael addition and amidation. This approach also shortened the reaction steps with greater yield as compared to the conventional synthetic approach (Kawaguchi et al., 1995). Huang et al. ensured that the inverse PAMAM dendrimers were similar in size to the PAMAM dendrimers prepared by the conventional approach with reduction in the costs and time associated with prepared dendrons, particularly higher-generation dendrons (Huang et al., 2013). Despite all these valuable advantages, solid-phase dendrimer synthesis has not commonly used because the production of PAMAM in the large quantity is still a challenging task (Huck et al., 1998; Kawaguchi et al., 1995).
4.2 Other approaches
4.2.1 Hybrid convergent-divergent synthesis
In 1994, Kawaguchi et al. reported a hybrid convergent-divergent synthesis method also called “double exponential growth” synthesis by combining the advantages of divergent and convergent synthesis with the additional advantage that, the hyper core grows along with the peripheral monodendrons (Pyzowski et al., 2003). Double exponential dendrimer growth is the fastest convergent approach for the formation of monodendrons via bi-directional synthesis as exposed in Fig. 4. This approach was more efficient to prepare higher PAMAM generation by reducing the synthesis and refining step. This approach has the facility to alter the dendrons and synthesize the dendrimers with structural diversity (Lyu et al., 2019; Pyzowski et al., 2003). Besides all these advantages, a double exponential dendrimer growth approach has some disadvantages, which are requirement of a pair of orthogonal protecting groups (rather than one) and formation of the restricted generation before steric hindrance (Grayson and Fréchet, 2001; Pyzowski et al., 2003).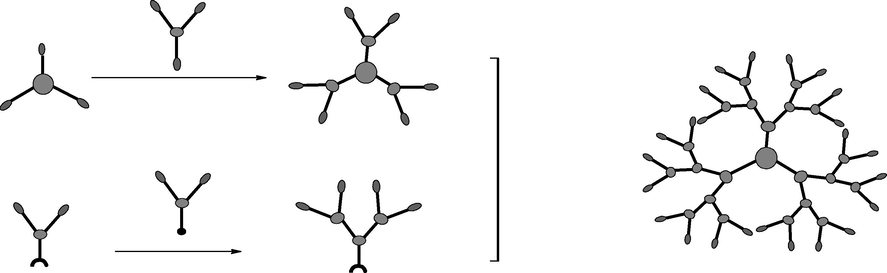
Combined divergent/convergent synthesis (Pyzowski et al., 2003; Huang et al., 2012).
4.2.2 Lego chemistry
Several approaches have implemented to reduce the time as well as the cost for simplified PAMAM synthesis. Pyzowski et al. described the lego chemistry approach for the synthesis of PAMAM dendrimers (Pyzowski et al., 2003). This approach was new, simple, and straightforward employed for the synthesis of phosphorus dendrimers by using two-branched monomers and multi functionalized cores. By using this approach, each generation obtained in a single quantitative step with 48 to 250 surface end groups along with nitrogen and water as by-products (Lyu et al., 2019; Menjoge et al., 2010). In this G4 PAMAM dendrimer is obtained only in four steps with alternative phosphines, and hydrazines end groups. This method has the advantage of utilizing the least volume of solvent and simplified purification procedure. However, two reactions should be simultaneously run for synthesizing G1 is the only disadvantage of this synthetic approach (Huang et al., 2012).
4.2.3 Click synthesis approach
With the asset of good production yield, high efficiency, and minimum reaction time with mild reaction conditions, ‘Click chemistry’ has come forward as an acceptable approach for synthesizing different dendrimers. In click chemistry, Sun et al. successfully implemented divergent, convergent, and double exponential growth approaches (Sun et al., 2015). Dendrimers synthesized by click chemistry approach give a link between dendritic architectures and nanomaterials. Among the various click reaction, formation of 1,2,3-triazole ring by copper (Cu)-catalyzed cycloaddition reaction of alkyne and azide moiety is of great importance (Geraldo et al., 2011; Sun et al., 2015).
Huang et al. synthesized G5 PAMAM dendrimer by peripheral conjugation of three azido moiety, viz., methotrexate (MTX), folic acid, and fluorescein having cyclo-octene ligands through copper-free click chemistry. They prepared mono-, di-, trifunctional PAMAM dendrimer conjugates via mixing of various azido modified functionally active moiety and therapeutic drug MTX. They concluded that copper-free click reactions give excellent yield and in vitro cytotoxicity study of folic acid facile conjugated G5 PAMAM dendrimer with MTX gives efficient binding to KB cells (Huang et al., 2012).
Lee and co-workers synthesized azide moiety containing PAMAM dendrons and azidopropylamine as a core by the divergent method. They suggested these dendrons are useful for the synthesis of uniform and controlled structure of PAMAM containing 1, 2, 3- triazole rings as linkage. Attachment of these triazole rings with different alkynes is based on Cu-catalyzed cycloaddition reaction between an alkyne and an azide. Scheme 6 gives a synthetic pathway for the synthesis of symmetrical as well as asymmetrical PAMAM dendrimers using click chemistry (Lee and Larson, 2008).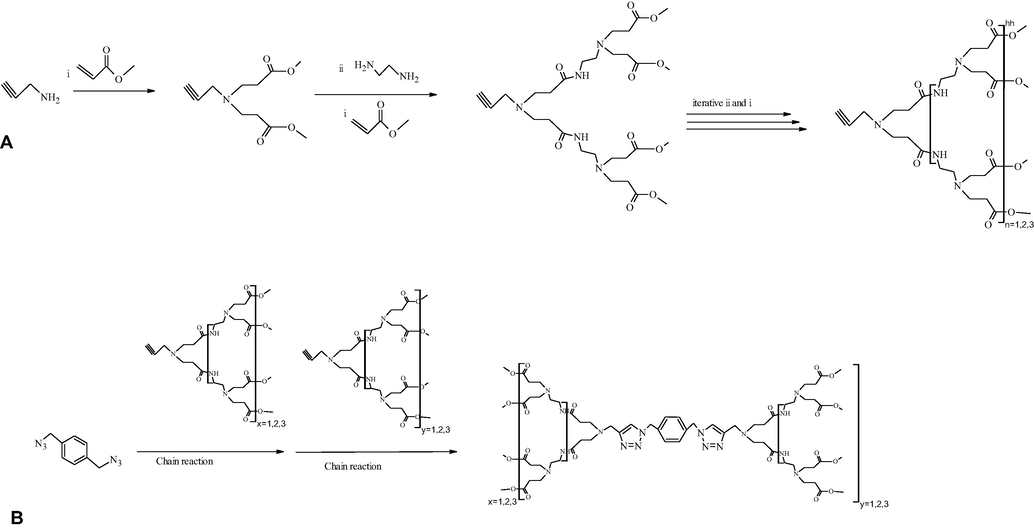
(A) Divergent synthesis of PAMAM dendrons bearing the alkyne functionality. (B) Convergent synthesis of symmetrical and asymmetrical PAMAM dendrimers using click chemistry (Pittelkow and Christensen, 2005; Sun et al., 2015).
4.2.4 Supramolecular synthesis
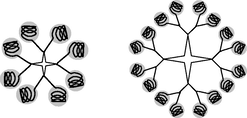
In a supramolecular synthetic approach, dendrimers synthesized by the use of three strategies. The first strategy includes synthesizing dendrons with a core unit having the capability to recognize itself. This step helps for the spontaneous formation of a dendrimer (Lyu et al., 2019; Sun et al., 2015). In the second strategy, add generation non-covalently to the peripheral groups. Finally, the higher generation of PAMAM dendrimer formed by using the self-assembly of monofunctionalised dendrons. Step by step reaction of supramolecular synthesis are as given in Scheme 7. Large noncovalent supramolecular dendrimer synthesized by this method gives a guarantee for structural accuracy. The above approach reduces the steps from the conventional multistep approach (Chen et al., 2004; Geraldo et al., 2011). Assembly of monofunctionalized dendrons into large supramolecular dendrimers is the collective effect of multiple non-covalent interactions (Al-Jamal et al., 2005). Zimmerman et al. reported the synthesis of a fourth-generation disk-shape supramolecular dendritic structure from small dendritic building blocks by a hydrogen bond (Zeng and Zimmerman, 1997; Zimmerman, 1997). Percec and his colleagues reported the synthesis of monodendrons, which are self-assembled in rod-like, cylindrical, and spherical supramolecular dendrimers molecules. Thermotropic hexagonal columnar lattice and thermotropic cubic liquid crystal lattice self-organized and formed cylindrical and spherical supramolecular dendrimers, respectively (Percec et al., 1998). Geraldo et al formulated and evaluated a gel containing supramolecular complexes of CdSe by the supramolecular chemistry of PAMAM. Geraldo first synthesized a new quantum dots (QDs)/PAMAM dendrimers containing water-soluble 3-mercaptopropionic acid. Finally, this supramolecular PAMAM was conjugated with folic acid and produced a new QDs/PAMAM-folate derivative. This QDs/PAMAM-folate complex showed an efficient and selective marker for gastric cancer cells (Geraldo et al., 2011).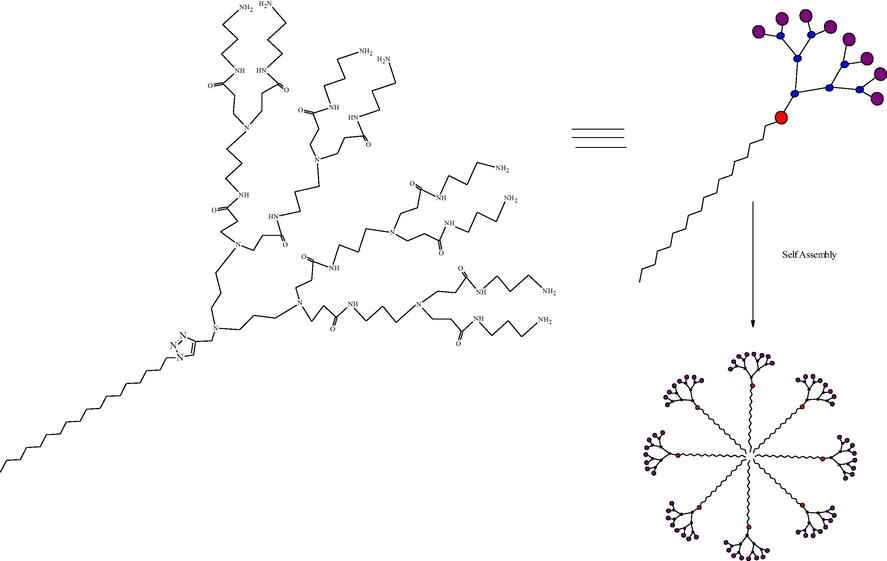
Supramolecular dendrimers constructed via self-assembly of long hydrophobic alkyl chains and small hydrophilic PAMAM dendrons (Sun et al., 2015; Zeng and Zimmerman, 1997).
Lyu et al. recently prepared supramolecular dendrimers by using small amphiphilic dendrimers, which contain long hydrophobic alkyl chains and small hydrophilic PAMAM dendrons via click chemistry. These amphiphilic dendrimers form stable, controlled, uniformly spherical nanoscale supramolecular dendrimers with different length of hydrophobic chain. The amphiphilic dendrimers functionalized with several copies of a biologically active molecule or imaging agent (Lyu et al., 2019). These self-assembling supramolecular dendrimers applied for drug delivery and bioimaging, respectively. In polycationic supramolecular PAMAM dendrimers, peripheral amine groups can interact with negatively charged nucleic acids and form nanoscale dendriplexes in non-viral gene therapy (Lyu et al., 2019;Mignani et al., 2014). Sun and Yang et al. reviewed the future outcomes of siRNA therapeutics in the field of non-viral gene therapy. They analyzed supramolecular dendrimer models for siRNA targeted delivery (Sun et al., 2015).
5 Purification of PAMAM dendrimers
The purification of PAMAM dendrimers is an essential step in laboratory synthesis because each generation batch has several trailing generation defects, which can affect the PAMAM properties. However, by removing many of these defects, the purification process improves the structural uniformity of PAMAM (Mullen et al., 2012).
Through purification, PAMAM has been prepared for non-viral gene delivery agents, host–guest interaction, and modification with functional moieties such as therapeutic molecules or imaging agents. The pharmacodynamic and biodistribution of PAMAM is size-dependent property. The structural defect possess different water solubilizing capability which likely to influence the biodistribution. So that it is essential to reduce the variability in the relative structural defect, which will improve the pharmacodynamic and biodistribution properties. After purification, it is essential to perform a characterization of PAMAM (Mullen et al., 2012; Rundel et al., 2007).
Tomalia et al. already recommended a purification of PAMAM by azeotropic distillation with a 9:1 mixture of toluene and methanol. In the beginning, the PAMAM dendrimer was dissolved in the above mixture, removing all volatile byproducts on a rotary evaporator. This procedure repeated until the evaporation of EDA. After that, the toluene residues from the PAMAM was removed by similar azeotropic distillation with pure methanol. During this step, the water bath temperature of the rotary evaporator is not more than 30 °C to avoid retro Michael reaction (Esfand and Tomalia, 2002). Mullen et al. used a membrane dialysis procedure for purification of generation 5 PAMAM dendrimer. They demonstrated its effectiveness and limitations using a lot of biomedical grade dendrimers. For purification study, he used 10,000 molecular weight cut off dialysis tubing and washed with nanopure water before use (Mullen et al., 2012).
6 Cytoxicity and surface modification
Cytotoxicity of PAMAM dendrimer mainly attributed due to the interaction of cationic peripheral groups with negatively charged biomembrane. PAMAM causes hemolytic toxicity and cytotoxicity by damaging cellular membrane (Jones et al., 2012; Kesharwani et al., 2011) mentioned in Table 3. Cationic G7 PAMAM dendrimer interacts with lipid bilayers of host cells and open with 15–40 nm diameter, which disturbs the flow of electrolyte and causes cell death (Cancino et al., 2013; El-Sayed et al., 2003). Fig. 5 represents the mechanism of interaction of polycationic PAMAM dendrimer with the biological membrane. Anionic PAMAM dendrimers were 10-folds more tolerated and less toxic than cationic ones. Hemotoxity is an additional undesired type of cytotoxicity of cationic charge PAMAM (Otto and De Villiers, 2018).
Sr. No.
Dendrimer
Cell line
Level of cytotoxicity
IC50
Reference
1
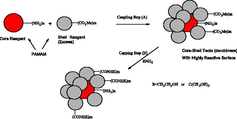
PAMAM G5 core-PAMAM G2.5 shell Tecto dendrimer
HaCaT((Human epidermal Keratinocytes)
CaCo2(Colon adenocarcinoma)
SK-Mel-28Up to 50 μM Nontoxic
Up to 50 μM Nontoxic
IC50 = 7.5 μMSchilrreff et al., 2012
2
PAMAM generation 1.5–3.5
PAMAM generation 1–2
PAMAM generation 3–4B16F10(Murine malenoma cells)
IC50 > 155 μM
IC50 > 614 μM
35 μMKesharwani et al., 2011; Lin et al., 2005
3
PAMAM dendrimers
G4 PEG2
G4PEG4CaCo2(Colon adenocarcinoma)
PEG2/PEG4
IC50 = 120/790 μM
Jevprasesphant et al., 2003a, 2003b; Nigavekar et al., 2004
4
PAMAM dendrimers
Generation4(G4)
Generation 5(G5)
Generation 6(G6)
HaCaT
(Human epidermal Keratinocytes)
SW 480(Primary adenocarcinoma of colon)G4/G5/G6
IC50 = 3.21/1.07/1.02 μM
IC50 = 16.35/1.89/1.30 μM
Fischer et al., 2003
5
PAMAM- Pyrrolidone dendrimer
N2a(Mouse Neuroblastoma)
IC50 > 200 μM
Nontoxic
King Heiden et al., 2007
6
Acylation of PAMAM dendrimer G1-G4
Caco-2 cell monolayer
Non toxic
Kolhatkar et al., 2007; Sweet et al., 2009
7
Lauroyl and PEGylated PAMAM dendrimers
Caco-2 cell
Seven fold reduction in cytotoxicity as compared to unmodified PAMAM
Jevprasesphant et al., 2003a, 2003b
8
PAMAM dendrimers
Generation4(G4)
Generation 5(G5)
Generation 6(G6)
PLHC1
(Fish hepatoma cell line)
RTG-2(rainbow trout gonad tissue)G4/G5/G6
IC50 = 2.08/12.93/0.68 μM
IC50 = 0.56/6.07/0.27 μM
Naha and Byrne, 2013
9
PAMAM- Pyrrolidone dendrimer
B14(Chinese Hamster fibroblast)
mHippoE18(mouse embryonic hippocampal cell)
BRL-3A(Rat liver derived cells)Non Toxic
Janaszewska et al., 2017, 2013
10
Carboxymethyl chetosan/Poly(amidoamine)
(CMCht/PAMAM) DendrimerU87MG
(Glioblastoma) GBM
HTERT/E6/E7(Human immortalized astrocytes)Non-Toxic
Pojo et al., 2013
11
Amino terminated PAMAM dendrimer G3 and G4
H4IIE(Rat hepatoma)
G3/G4(μg/mL)
IC50 =12.96/38.3Hernando et al., 2012
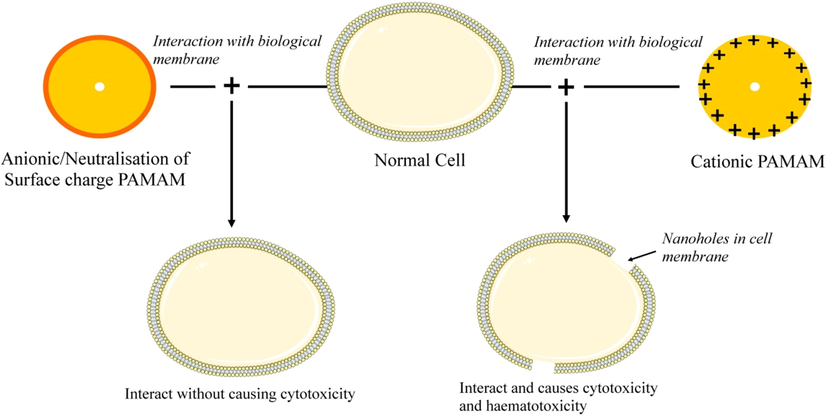
Mechanism of interaction of polycationic PAMAM dendrimers with the cellular membrane.
Pryor et al. evaluated the cytotoxicity of various full and half generation PAMAM with surface modification by cationic, anionic, and neutral terminal groups. For the above study, they used zebrafish models (Pryor et al., 2014), and observed neutral or anionic dendrimers which are not showing significant lethal effect, related cardiac impact, and pericardial edema. The authors also observed that the cytotoxicity of PAMAM was dependent on the number of terminal groups, generation, and size of molecules. However, in cationic dendrimers, they observed a contradictory effect that lower generations of polycationic PAMAM dendrimers were more toxic than a higher generation (Jain et al., 2010a, 2010b; Pryor et al., 2014). However, Cancino et al. used Caco-2 model and reported exactly the opposite statement that decreases in cell viability with a higher generation of PAMAM (Cancino et al., 2013; Woller and Cloninger, 2001).
Cancino et al. focused on the effects of single-walled carbon nanotube (SWCNT), PAMAM, and SWCNT-PAMAM nanomaterials on C2C12 murine cell line. They observed SWCNT–PAMAM causes DNA damage in C2C12 murine cell. Finally, the authors concluded that the toxicity of nanomaterial was firmly due to peripheral charge (Mukherjee and Byrne, 2013). Thiagarajan et al. evaluated PAMAM for oral drug delivery. They studied the effect of surface modification and the size of PAMAM on the epithelial barrier of the gut in CD-1 mice. They concluded that polycationic PAMAM caused more toxicity, whereas anionic surface-modified PAMAM was ten times more tolerable in oral doses. G7 cationic amine or hydroxyl-terminated dendrimers showed severe signs of toxicity include hemobilia and splenomegaly with dose 30 mg/kg. Whereas anionic G 6.5 carboxyl-, amine-, or hydroxy-terminated dendrimers tolerated dose up to 500 mg/kg with no sign of toxicity (Thiagarajan et al., 2013). From the above perception, researchers pointed out that peripheral positive charge of PAMAM is responsible for its cytotoxicity, which limits their biomedical application (Cancino et al., 2013; Woller and Cloninger, 2001). Therefore modification of cationic surface groups is one of the ways which reduces the toxicity, improve biocompatibility and circulation time of PAMAM dendrimers in the body (Fischer et al., 2003; Janaszewska et al., 2013). However, this surface modification has done by neutralizing charge through PEGylation (polyethylene glycol conjugation), acetylation, folate, and peptide conjugation or by neutralizing the negative charge (King Heiden et al., 2007; Kolhatkar et al., 2007; Sweet et al., 2009). Fig. 6 gives a diagrammatic representation of attachment of other molecules to dendrimer surface groups, which are the most outstanding solution to reduce cytotoxicity. Fig. 7 shows various strategies for modification of the PAMAM dendrimers to improve its physicochemical properties for targeted drug release.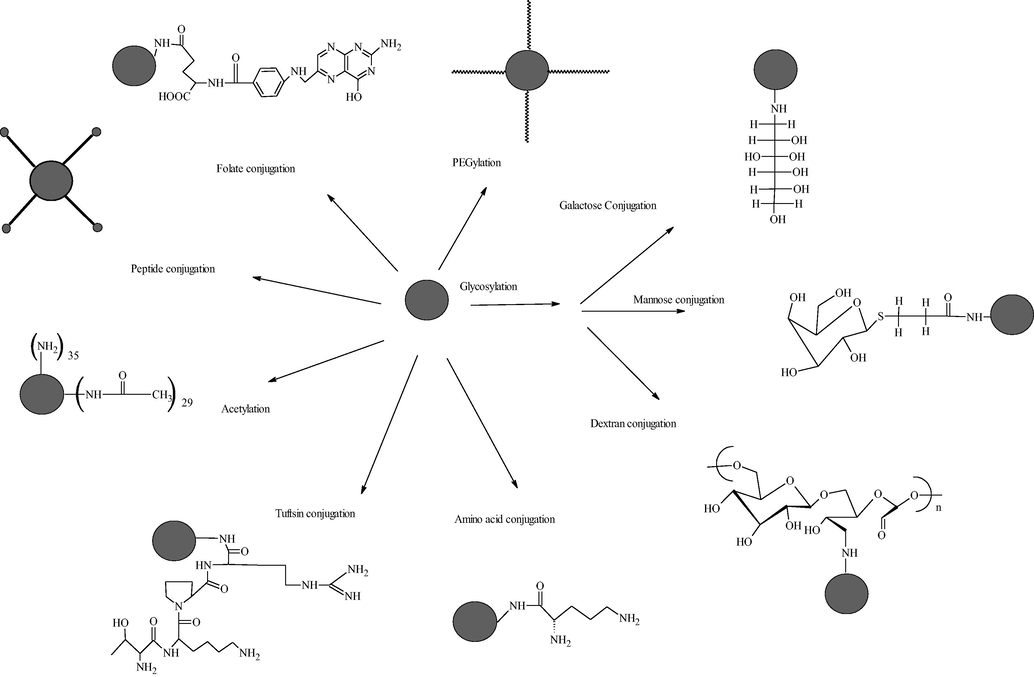
Modification of surface groups of PAMAM to improve biocompatibility and circulation time of PAMAM dendrimers in the body via neutralization of charge.
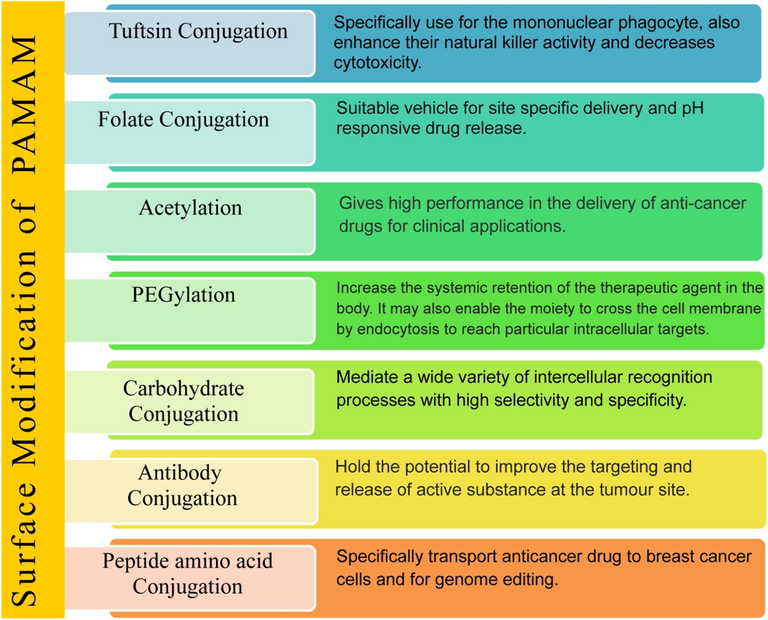
Various strategies for modification of the PAMAM dendrimers to improve its physicochemical properties for targeted drug release.
Kolhatkar et al. pointed out acetylation for decreasing cytotoxicity of PAMAM. The author observed that cytotoxicity of PAMAM could be reduced 10-fold with retaining the permeability of the cell by surface modification with acetyl moiety (Kolhatkar et al., 2007). They also observed that acetylation also increases the water solubility of PAMAM, which is essential for biomedical use. In order to improve the biocompatibility of PAMAM researchers modified G5 PAMAM dendrimers by conjugating them with polyethylene glycol (PEG) of two different molecular weights (Bertero et al., 2014; Ladd et al., 2017). They observed dendrimers conjugated with PEG of higher molecular weight were less cytotoxic as compared to conjugation with lower molecular weight PEG. This study provided a novel idea to decrease the cytotoxicity of dendrimer by PEGylation. Cytotoxicity reduction of dendrimer depends on molecular weight and chain length of PEG (Ladd et al., 2017). Kolhe et al. reported enhanced Ibuprofen loading in G4 and G3 PAMAM by facile conjugation with PEG. PEGylated G4 and G3 PAMAM dendrimers enhanced drug loading, drug release, and drug solubility with improved biodistribution and biocompatibility (Kolhe et al., 2003).
PAMAM dendrimers have a great interest in non-viral gene delivery because of the presence of primary amine groups on their periphery, which can bind to DNA and promote the cellular uptake of genes (Eichman et al., 2000; Qi et al., 2009). However, cationic PAMAM dendrimers are associated with their cytotoxicity, hemolysis, and liver toxicity. Cationic PAMAM dendrimers interact with the negatively charged cell surfaces, which limit theirs in vitro and in vivo applications (Faraji and Wipf, 2009). Qi et al. conjugated G5 and G6 PAMAM dendrimer to PEG at three different molar ratios of 4%, 8%, and 15% to improve its characteristics as gene delivery carriers. They observed 8% PEG conjugated PAMAM dendrimers are the most efficient muscular gene expression with decreased in-vivo and in-vitro cytotoxicity (Maciejewski, 1982; Qi et al., 2009).
Bhadra et al. developed and explored the PEGylated and non-PEGylated G4 PAMAM dendrimers for delivery of the anti-cancer drug, 5-fluorouracil. The results showed that PEGylated PAMAM dendrimers increased drug loading capacity, reduce drug release rate, and hemolytic toxicity. Hence, they conclude that, PEGylated dendrimers can be used as a nanoparticulate depot system for drug administration (Maciejewski, 1982; Bhadra et al., 2005). From the above data it can be concluded that, PAMAM dendrimers are promising structure for drug and gene delivery but, its cytotoxicity and hematotoxicity is a basic problem. However, dimishing the cationic charge on surface reduces the therapeutic activity i.e., ability to create complex with nuclic acid. Therefore, partial modification with perfect molecule is applied to get balance between reduced toxicity and retained activity.
7 Application of PAMAM
PAMAM dendrimer has several applications in pharmaceutical as well as the biomedical field. PAMAM dendrimer can improve biological properties such as bioavailability, solubility, and specific target selectivity by linking or entrapping bioactive compounds. Fig. 8 shows the potential applications of PAMAM dendrimers. The summary of the applications of PAMAM dendrimers in both biomedical and diagnostic purpose is tabulated in Table 4.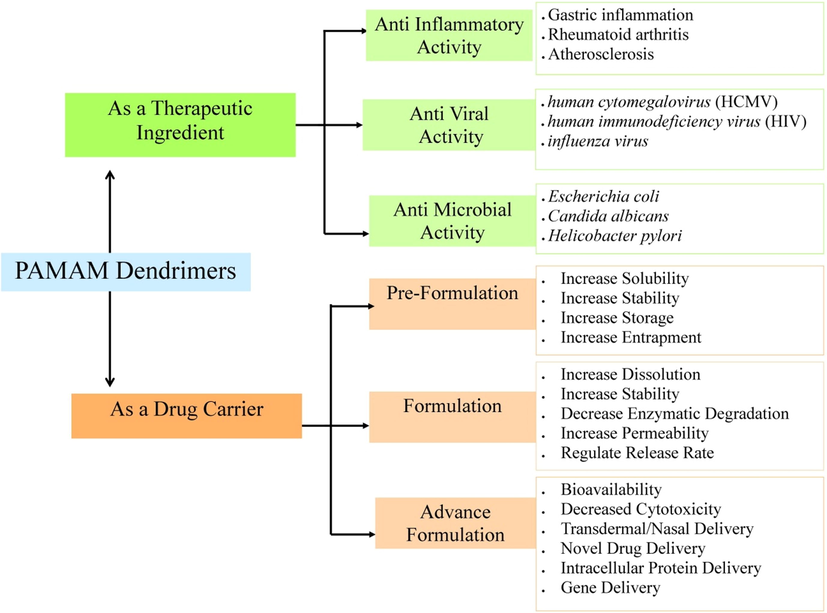
Schematic representation of the potent application of PAMAM dendrimer as a therapeutic agent and as a drug carrier.
Applications
Dendrimers
Drugs
Outcome
References
Enhance solubility
PAMAM G1-G3
Clotrimazole and Ketoconazole
Improve antifungal activity by increasing drug solubility in water
Matsubara et al., 2010
Folic acid (FA)-PAMAM conjugate
Flavonoid 3,4-difluorobenzylidene diferuloylmethane
Targeted FA-PAMAM-CDF showed high anticancer activities on HeLa and SKOV3 cell line with increasing solubility
Longmire et al., 2008; Zong et al., 2012
Hybrid PAMAM dendrimer G4
Paclitaxel
Lipid-PAMAM hybrid nanosystem proved to be a promising approach for delivering hydrophobic drugs
Parsian et al., 2016; Tajarobi et al., 2001
G0 and G3 PAMAM functionalized with lauryl chain
Naproxen
The lauroyl chain conjugated to lower PAMAM generation terminal groups (G0) rather than high PAMAM generations terminal groups for solubility of Naproxen, which gives optimal drug delivery.
De Araújo et al., 2018; Najlah et al., 2017; Sadekar et al., 2013
Targeted action
Half generations magnetic PAMAM dendrimers
Gemcitabin
An effective system for targeted gemcitabine delivery with great therapeutic potential at the tumor site and to minimize the side effects in normal tissue
Parsian et al., 2016
Folic acid (FA)-modified generation 5 (G5) PAMAM dendrimers through a pH-sensitive cis-aconityl linkage
doxorubicin (DOX)
The developed G5 FA-DOX conjugates used as a promising nanodevice for targeted cancer chemotherapy
Cheng et al., 2015a, 2015b, 2011
Trastuzumab (TZ) grafted PAMAM dendrimer
Docetaxel
The developed TZ-G4 PAMAM dendrimer use to improve the delivery of Docetaxel to HER2-positive breast cancer cells.
Kulhari et al., 2016
Transdermal delivery
4.0 G PAMAM dendrimers with amino and hydroxyl terminal and 4.5 G PAMAM dendrimers
Indomethacin
In vivo, pharmacokinetic and pharmacodynamic studies in Wistar rats show significant indomethacin concentration in blood.
Chauhan, 2018
5.0 G PAMAM dendrimers
Ketoprofen
3–4 times permeation rate increase due to complex as compared to plain ketoprofen
Chauhan, 2018; Chauhan et al., 2003
Lower generation PAMAM
5- Fluorouracil
PAMAM dendrimer enhanced the rate of penetration of 5-fluorouracil by increasing partitioning in the skin.
Venuganti and Perumal, 2008
Sustain drug delivery
PAMAM with carboxyl or hydroxyl active peripheral group
Pilocarpine
Increase the retention time of pilocarpine within the eyes.
Vandamme and Brobeck, 2005
Enhance Bioavailability
G2, G2.5, and G3 PAMAM dendrimers
Heparin and Enoxaparin
Enoxaparin –PAMAM complex is effective in preventing deep vein thrombosis with increasing bioavailability up to 40% without any adverse effect on mucociliary transport rate.
Bai and Thomas, 2007
Inhanced Cellular Uptake
Carboxyl-terminated PAMAM G3.5
Carboplatin
Prepared for nano-based drug delivery system with good cytocompatibility
Cheng et al., 2015a, 2015b
PAMAM
Sialic acid
They are potentially inhibiting the infection caused by H3N2 (influenza virus) by increasing the delivery of sialic acid and reduced toxicity.
Schilrreff et al., 2012
MRI contrast agents (contrast or imaging agent
PAMAM G4
(1B4M-Gd)64
MRI agent for a kidney in Nude mice
Guo et al., 2011
PAMAM-Cystamine G 6.0
(1B4M-Gd) 256
MRI agent for breast cancer in mice
Wang et al., 2012
PAMAM-Cystamine G 8.0
(1B4M-Gd)1024
MRI agent for sentinel (mammary) lymph nodes in mice
Kojima et al., 2011
Immunotherapy (antibody conjugate)
PAMAM dendrimers (5.0 G)
60bca and J591
Carrier
Longmire et al., 2008; Oddone et al., 2016
Gene transfection
PAMAM dendrimers and PAMAM-Arginine dendrimers
DNA
Lysine functionalized PAMAM dendrimers as a novel vaccine delivery vector for a schistosomiasis japonica DNA vaccine
Bahadoran et al., 2017
4.0 G PAMAM
siRNA
Effective intracellular accumulation of siRNA with co-delivery of the drug was obtained with a developed system
Bahadoran et al., 2017; Dutta et al., 2010; Heegaard et al., 2010
Therapeutic Activity
PAMAM G0 and G1
Conjugates with carbon nanodots and Tetracyclin
Conjugated PAMAM G0 and G1 dendrimers with carbon nanodots with drug observed synergic antibacterial action.
Khandare et al., 2012; Mukherjee et al., 2010a, 2010b
PAMAM
BRI-2923 and BRI- 6195
Inhibit the replication of HIV by interfering virus adsorption.
Winnicka et al., 2011
PAMAM G3-G5
Covalently bound to DC-SIGN receptor and interfering with virus adsorption.
Nishikawa et al., 1996
PAMAM G7
Potentially able to inhibit the growth gram-negative and positive bacteria
Landers et al., 2002; Matsubara et al., 2010
7.1 Drug delivery agent
Dendrimers have excellent host–guest properties (Faraji and Wipf, 2009). By these unique characteristics, PAMAM has applied in the targeted delivery of drugs by physical interaction or covalently bonding. Drug molecules can be encapsulated in the interior space of dendrimers or attached to the peripheral group, which is shown in Fig. 9 (Maciejewski, 1982; Peng et al., 2008).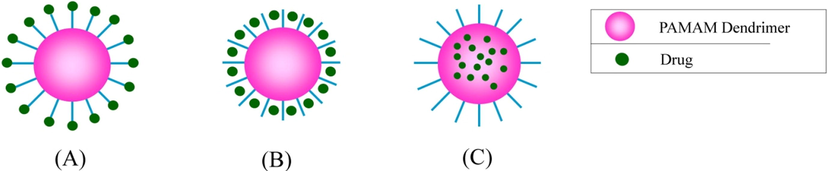
Schematic representation of entrapment of drug in PAMAM dendrimer. (A) Covalent binding (B) Electrostatic interaction (C) Encapsulation.
The core of dendrimers is hydrophobic, while the peripheral part is hydrophilic, which is analogous to micelles like structure and able to bind ionic surfactant molecules via hydrophobic and ionic interaction (Hu et al., 2012). This structural property makes it a potential carrier in targeted drug delivery (Choudhary et al., 2017; Hsu et al., 2017). Hydrophobic drug molecules formed inclusion complexes with internal tertiary amine and peripheral amine groups of PAMAM (Choudhary et al., 2017; Jain et al., 2010a, 2010b). The peripheral hydrophilic groups help for drug solubilization. The unique properties of PAMAM dendrimers allow them to administer by different routes such as oral, injectable, transdermal, ocular, nasal, colon, rectal, and pulmonary delivery systems (Mehta et al., 2019; Mintzer and Grinstaff, 2011). Nanjwade et al. reported PAMAM are excellent carriers for drug delivery due to multiple branching structures which terminated with cationic amine (—NH2), neutral hydroxyl (—OH), or anionic carboxylic acid (—COOH) surface groups which conjugated with biological agents and to control the release rate of the drug in the body (Nanjwade et al., 2009; Peng et al., 2008).
Jansen and co-workers reported the concept of “dendritic box” for trapping small molecules of rose Bengal and p-nitrobenzoic acid. They observed that the solubilization of drugs depends on various factors, including generation, size, concentration, pH, focal point, and terminal active groups (Jansen et al., 1994). The drug entrapment efficiency of PAMAM increases with generation as well as the concentration of PAMAM (Jansen et al., 1994; Malik et al., 2000; Padilla De Jesús et al., 2002). Chauhan et al. reported the encapsulation efficiency of indomethacin in G4 and G4.5 PAMAM dendrimers through electrostatic interaction. They also observed the encapsulation of ibuprofen in G4 PAMAM dendrimers and conclude that solubility of Ibuprofen molecules was increased by encapsulation in PAMAM dendrimer (Chauhan et al., 2003). PAMAM also transports the bioactive compounds by a covalent bond (dendrimer prodrug), ionic interaction, or adsorption in the internal space of PAMAM (Dias et al., 2020).
Twyman and Astruc et al. reported the drug release from arborol type dendrimers by calorimetric experiments. They compared four antibacterial and antifungal compounds for study (Astruc and Chardac, 2001; Twyman et al., 2002). Najlah et al. explored the potential of PAMAM in oral drug delivery. They concluded that the oral drug delivery potential of PAMAM increases with branching and surface amine groups, which immobilize drugs, enzymes, antibody, or other bioactive agents. In this study, the authors studied trans-epithelial permeability of Naproxen-PAMAM conjugate. They observed PAMAM-Naproxen conjugate enhance the oral bioavailability and serve as the most potent candidate for controlled release (Najlah et al., 2017).
Mignani et al. introduced the concept of “dendrimer space” to provide medicinal chemists with a new approach by identifying original dendrimer based drug delivery systems. According to their observations, dendrimers space shapes the boundaries of a new drug cluster that included in a considerable volume of chemical space. This concept fully integrated into the drug discovery process (Mignani et al., 2014).
Wiwattanapatapee et al. reported in vitro cell uptake of polycationic and polyanionic PAMAM in an account of its size, charge, and concentration (Wiwattanapatapee et al., 2000). The result of Wiwattanapatapee experiment showed transport of I-labelled dendrimers across the intestinal membrane was charge-dependent, and tissue uptake of cationic PAMAM was higher than anionic(Malik et al., 2000; Wiwattanapatapee et al., 2000). Dendrimers have also investigated for oral delivery of the anticancer drug. Zong et al. reported that facile conjugated folic acid G5 PAMAM improved the target release of MXT by 2500–170,000 fold due to specific binding with folate receptors (Cheng et al., 2011; Zong et al., 2012).
The highly impermeable and lipophilic stratum corneum layer limited the transdermal drug delivery (Chauhan, 2018). To this end, dendrimers found to be the effective and efficient carrier of drugs to improve drug penetration across the skin by increasing drug solubility and drug partitioning. Among various surface modifications in PAMAM, PAMAM with amino groups shows a higher perfusion rate than neutral and carboxyl terminated PAMAM dendrimers (Chauhan, 2018; Chauhan et al., 2003). Jain et al investigated G4 PAMAM for transdermal delivery of Indomethacin. They performed in vivo pharmacokinetic and pharmacodynamic studies with Wistar rats. They observed a significant increase in the concentration of Indomethacin in blood with PAMAM dendrimers as compared to pure drug suspension (Chauhan et al., 2004). Venuganti et al. found that cationic lower generation PAMAM dendrimers increased penetration of 5-flurouracil by increasing partitioning into the skin (Venuganti and Perumal, 2008).
Intra-ocular delivery of bioactive suffers from poor bioavailability problems due to the elimination of the drug by nasolacrimal duct mediated drainage of fluid (Wang et al., 2009). Dendrimers have explored to overcome the above limitations by increasing the retention time of the drug in eyes. Vandamme and Brobeck reported the PAMAM dendrimers with carboxyl or hydroxyl active peripheral groups increases the retention time of Pilocarpine within the eyes (Vandamme and Brobeck, 2005). Dendrimers have also evaluated for pulmonary delivery of heparin and enoxaparin by Bai et al. In this study, they assessed G2, G2.5, and G3 PAMAM dendrimer for pulmonary absorption of Enoxaparin and observed that the resulting drug dendrimer complex is effective in preventing deep vein thrombosis. They also observed that cationic charged G2 and G3 PAMAM dendrimer increases the bioavailability of Enoxaparin about 40% without any adverse effect on mucociliary transport rate (Bai et al., 2007).
Dendrimers also used as a passive as well as active targeting delivery of medicament due to the presence of active surface groups (Kolhe et al., 2003). In cancer therapy, drug targeting is essential to maximize the therapeutic potential at the tumor site with minimizing side effects in normal tissues, which can be achieved by entrapment of drugs into the PAMAM or facile conjugation of drugs with the terminal active group of PAMAM (Cheng et al., 2015a, 2015b; Parsian et al., 2016). Patri et al. compared the efficacy of covalently attached MTX G5 PAMAM dendrimer complex with noncovalent methotrexate G5 PAMAM dendrimer. They concluded that covalently attached methotrexate conjugated dendrimer was more stable compared to noncovalent MTX dendrimer complex (Patri et al., 2005).
Cytosolic protein delivery shows a significant important role in protein therapies. Cheng et al. reported that cationic polymer like PAMAM encompasses great characteristic features in gene delivery. However, cytosolic protein delivery was limited because of cationic polymers can not prepare stable complexes with proteins (Cheng et al., 2020). Liu et al. reported that Boronic acid rich dendrimers can strengthen the binding affinity of cationic polymer with protein with high efficiency for cytosolic delivery of native proteins (Liu et al., 2019b, 2019a). They also demonstrated that supramolecular chemistry used to describe the reversible connection of the cationic PAMAAM. He also ensured complex stability and minimal toxicity by shielding the cationic charge with anionic polymer or lipid PEG (Lv et al., 2019; Cheng et al., 2020). Araujo reviewed that PAMAM dendrimers activate the immune response and, therefore, positively charged dendrimers employed as vaccine carriers, due to their ability to increase cytokine production. He reported that PAMAM was not immunogenic by itself. However, when conjugation with protein carrier, (albumin, Interleukin-3) is induced the formation of antibody and specified antigenic effect (De Araújo et al., 2018).
7.2 Diagnostic agent
As compared to low molecular weight substances, high molecular weight substances mainly used as contrast agents. These agent obtained an X-ray image with high resolution and easily detect disease lesions or organs (Roberts et al., 1990). From that perception, unique and distinct properties of PAMAM dendrimers have become an exciting class of polymer to develope it as a diagnostic agent (Roberts et al., 1996, 1990). Biocompatible PAMAM dendrimer has efficiently used in radiotherapy, as imaging agent or tracer, X-ray or magnetic resonance imaging (MRI) contrast agent, and radionucleotide to detect cancerous cells. Dade International Inc. (U.S.A.) introduced a new method for cardiac test by the use of blood proteins. These blood proteins binds to immunoglobulins with the help of PAMAM dendrimers (Roberts et al., 1990; Tajarobi et al., 2001). Oddone et al. reported the cellular uptake by using the fluorescein isothiocyanate (FITC) tagged PAMAM dendrimers (Oddone et al., 2016).
Guo et al. demonstrated a novel concept for enhancement of X-ray attenuation property in potential computed tomography (CT) and angiography. They combined gold and iodine within single dendrimer based nanodevice (Guo et al., 2011). G5 PAMAM dendrimers was used as a template for gold particles entrapment (AuNPs), after that it was conjugated with diatrizoic acid via 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) coupling reaction (Guo et al., 2011). He observed that the above nano-complex encompasses high efficacy as a contrast agent in dynamic computerized tomography (CT) imaging and angiography.
Wang et al. also synthesized gold (Au) entrapped PAMAM nanoparticles with distinct sizes. They evaluated the effect of size on CT imaging of passively targeted tissues (Wang et al., 2012). They observed that, contrast agent with small sizes was a natural aspect for CT imaging of tumor by using liver endocytosis. These nanoparticles were also useful in tumor imaging because of the enhanced permeability and retention time (Guo et al., 2011; Wang et al., 2012).
In addition to the imaging agent, PAMAM-gadolinium (Gd) chelate used for MRI and as a contrast agent. However, their applications restrict due to their rapid clearance from the blood (Yang and Chuang, 2012). Several complexes of Gd with high molecular weight compounds identified to overcome this problem. Dendrimers based diethylenetriaminepentacetate (DTPA) complexes potentially improved the limitations of previously used MRI contrast agent and improve the visualization of vascular structure (Tomalia et al., 2007). Kojima et al. evaluated PEGylated PAMAM (G4 and G5)-Gd chelates for pharmacokinetic and paramagnetic properties. They observed that PEGylated Gd-PAMAM dendrimers showed negligible liver and kidney cell uptake with less plasma clearance and prolonged retention time (Kojima et al., 2011).
The generation and size of PAMAM also play a significant role in altering the pharmacokinetics of contrast agents by altering blood retention time, tissue perfusion rate, and excretion rate. The circulation half-life of the contrast agent was increased by 2–10 times with increasing generation. G7, G8, and G2 PAMAM identified as to be the best diagnostic agent for imaging intratumoral, lymphangiography, and renal imaging, respectively (Barrett et al., 2017). However, compared to hydrophilic PAMAM, hydrophobic PAMAM shows a better contrast effect in liver imaging. Surface conjugating PAMAM with antibodies and ligands such as folic acid used for the targeted delivery of contrast agents (Longmire et al., 2008).
In molecular biology, PAMAM efficiently works as a molecular probe by immobilizing the sensor unit on the branching ends. Surface modified PAMAM intended to apply as a molecular probe for avidin, DNA, amino alcohols, and pH (Barrett et al., 2017; Longmire et al., 2008).
7.3 Gene therapy
Gene therapy is a new technology for DNA transfection by expressing target protein or small interfering RNA to silent the target gene, which is represented in Fig. 10. It provides a new platform to eradicate genetic disease (Kesharwani et al., 2015). Viruses were an attractive gene delivery vectors for gene therapy. However, due to the lack of specificity, a low transfection rate, immunogenicity, toxicity, poor cell uptake, oncogenicity, and susceptibility to nuclease degradation limits the use of viruses for gene therapy. Therefore the non-viral vectors like cationic polymers and lipids are safer and recommend more flexibility for gene transfection agents. In this context, dendrimers are acting as efficient non-viral-mediated vectors for gene delivery system (Li et al., 2018). Peripheral amino functional groups of PAMAM dendrimer can effectively bind to the phosphate group of nucleic acids in physiological pH. Terminal amino groups of PAMAM leads to continuous formation of transfection complexes with nucleic acid (Heegaard et al., 2010; Wu et al., 2005).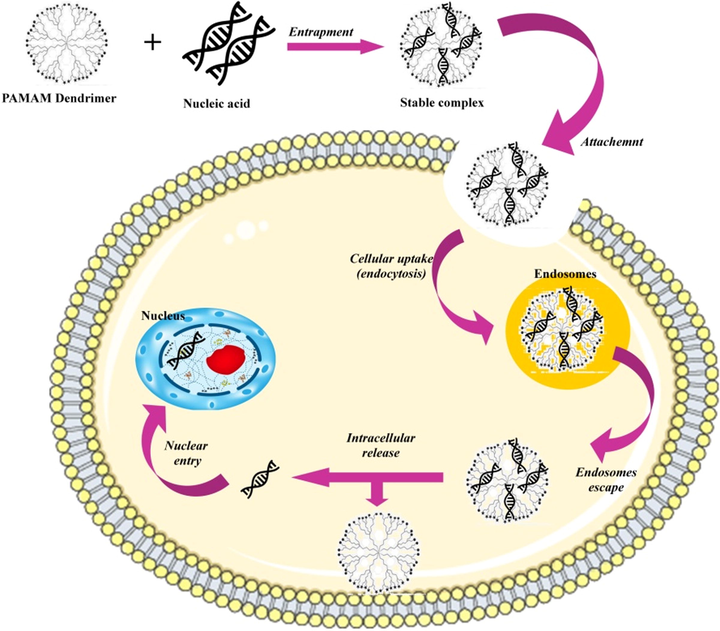
Mechanism of efficient non-viral mediated gene delivery with emergence use of PAMAM dendrimers (Kesharwani et al., 2015).
A transfection reagent “Superfect TM” is a trademark name for activated dendrimers which carry a high quantity of genetic material than viruses. The transfection efficiency of PAMAM may not only due to the well-defined structure but depend on low pKa of amines (3.9 and 6.9). The low pKa value of the PAMAM dendrimer produces it to buffer for pH change in the endosomal compartment (Abedi-Gaballu et al., 2018; Shcharbin et al., 2009). Haensler and Szoka published the report of dendrimer mediated transfection using luciferase and beta-galactosidase containing the plasmid. This plasmid dendrimer complex showed high efficiency towards the DNA transfection into different mammalian cell lines. They also found that the transfection efficiency of PAMAM dendrimer depended on both dendrimers–DNA ratio and diameter of the dendrimers. They also observed that the transfection rate has reached a stable level in G8 PAMAM dendrimers. They used different strategies to improve the properties of PAMAM for non-viral mediated gene delivery agents (Haensler and Francisco, 1993).
Nussbaumer et al. use group II chaperonin thermosome (THS) nanoparticle hallow protein for entrapment of macromolecules. They conjugated PAMAM to THS for entrapment of siRNA and observed THS-PAMAM protects SiRNA from degradation by RNase A and traffics Kinesin family member-11 (KIF 11). This molecule showed a good efficiency for gene delivery, carrying KIF11 and glyceraldehydes-3-phosphate dehydrogenase (GAPDH)-siRNA to cancer cells with significantly inhibit the proliferation of that cell (Nussbaumer et al., 2016). Wang et al. developed a lysine (lys) modified PAMAM dendrimers as a novel vaccine delivery vector for a Schistosomiasis japonica DNA vaccine. They investigated its ability to enhance a protective effect against Schistosoma japonicum infection. It was observed that when PAMAM-lys combined with DNA vaccine, it exhibited a higher efficacy as compared to the free DNA with reduced infection by 45–50% (Wang et al., 2014).
Dutta et al. explored the potential of dendrimers based siRNA against E7 and E6 cervical cancer. They optimized dendrimer generation by nitrogen to phosphate ratio using dendrimer- fluorescein isothiocyanate oligo complexes (Dutta et al., 2010). The in-vitro study showed that these formulations were able to inhibit target genes against E6 and E7 cervical cancer with the decrease side effect of drugs, overcome drug resistance and increase the survival rate of individual infected by cervical cancer (Dutta et al., 2010; Wu et al., 2013). Bahadoran developed a new method for delivering the H5-DNA vaccine against avian influenza. He designed a complex system with an H5-green fluorescent protein gene cloned into the expression vector pBud (pBud-H5-GFP), then conjugated to transactivator transcription (TAT) and PAMAM (PAMAM-TAT) (Bahadoran et al., 2017). He observed that the above system demonstrated low toxicity with good skin permeation. The cellular uptake of the above complex system was also better than free dendrimer (Bahadoran et al., 2017). Recently Cheng et al. also observed the boronic acid-rich dendrimer intracellular delivery of Cas9 protein into various cell lines with high efficiency in CRISPRCas9 genome editing (Liu et al., 2019b). In the current study Pishavar et al. used modified PAMAM (G4 and G5) dendrimers for effective trial gene therapy in colon adenocarcinoma. They used alkyl-carboxylate, PEG and cholesteryl chloroformate for enhancement of cellular transfection efficiency while reducing cytotoxicity. Gene delivery efficiency of the above novel vectors was evaluated by a green fluorescent protein reporter gene in the colon cancer cell, in-vitro and in-vivo. After a complete evaluation, they represented that cholesterol-grafted PAMAM dendrimer with alkyl-PEG surface modifier was the best gene delivery vector in-vitro as well as in-vivo (Pishawar et al., 2018). Lee et al. also concluded that PAMAM with histidine and KRTR surface modification could be used as a potential gene vector with prolonged gene therapy with the enhancement of gene expression (Lee et al., 2019).
7.4 Therapeutic activity
In recent times, dendrimer has significant attention in the invention of drugs by their therapeutic value against prion and infectious diseases like viral, parasitic infection, Alzheimer's disease, HIV, Herpes simplex virus (Tyssen et al., 2010) shown in Fig. 8. Dendrimers act as a therapeutic agent in viral infection by preventing the formation of amyloid fibrils and disaggregate previously formed fibrils and prevent viral adhesion and replication (Mhlwatika and Aderibigbe, 2018). Polycationic PAMAM dendrimers (generation greater than 3) react with bacterial membranes and disturb their integrity, while the lower generation did not show antimicrobial activity (Khandare et al., 2012). PAMAM also improves the cell uptake of antibiotics into the bacterium and, as a result showing synergistic effects (Khandare et al., 2012; Mukherjee et al., 2010b). Modified PAMAM dendrimers have shown to be efficacious in tissue repair also have the potential for neutralizing toxins and removing drugs and metal overdose from the body. Ngu-Schwemlein et al. conjugated PAMAM G0 and PAMAM G1 dendrimers with carbon nanodots to increase their aminated cationic concentration and evaluated their antimicrobial activity. The author additionally assessed the above conjugate with tetracycline and cilistin, observed synergic antibacterial action (Ngu-Schwemlein et al., 2016).
Dendrimers with certain functional groups on their periphery show anti-retroviral activity by breaking key contact of the virus to target cell. It includes three potential binding sites, i.e., CD4 receptor, glycosphingolipids (GSLs), and dendritic cell-specific ICAM-3-grabbing on integrin (DC-SIGN). Hence dendrimers act at several stages of viral infection for prevention of viral genome replication in the host cells and act as an antiretroviral. The phenyl dicarboxylic acid BRI-2923 and naphthyl disulfonic acid BRI-6195 are fourth-generation PAMAM dendrimer built from ammonia and ethylenediamine core respectively. BRI-2923 is fully capped with 24 naphthyl disulfonic acids group while BRI-6195 capped with 32 phenyl dicarboxylic acids group (Tyssen et al., 2010). BRI-2923 and BRI- 6195 dendrimers inhibit the replication of HIV by interfering with virus adsorption. A gp120 binding assay and virus adsorption assay indicated that the PAMAM has an effect on docking of HIV to the host cells also inhibit binding of TAT protein to trans-acting responsive element (TAR) RNA of HIV (Mhlwatika and Aderibigbe, 2018; Mukherjee et al., 2010b). Araujo and Santos et al. measured the binding affinity of PAMAM to the DC-SIGN receptor and observed that PAMAM covalently bound to it. DC-SIGN act as a receptor with a high affinity for mannose glycans moieties, which are present on the surface of the virus (De Araújo et al., 2018). HIV possesses a high density of Mannose-α1 and 2-mannose motif on gp 120. Thus PAMAM encoded with carbohydrate focused on mannose and its derivatives suggested that an assembly of mannose with aryl group at the peripheral region is beneficial for DC-SIGN binding. This modified PAMAM intensively block the interaction between gp120 and DCs at 10 μM, whereas a natural polymer mannose- mannan only partially inhibited the interaction (Mukherjee et al., 2010b; Wang et al., 2009, 2014). Hong Zhao studied the binding of PAMAM dendrimer to TAT peptide using microgravimetric quartz crystal microbalance (QCM) analysis (Zhao et al., 2004). They observed 3–5 generation PAMAM dendrimers easily prevented the binding of TAT protein to TAR RNA. They showed that PAMAM dendrimers could form complexes with TAR RNA and disrupt the interaction of TAT protein with TAR RNA (Mukherjee et al., 2010a; Zhao et al., 2004).
PAMAM –G7 potentially able to inhibit the growth gram-negative and positive bacteria, and their cytotoxicity was dependent on the exposure time and the concentration (Rastegar et al., 2017). Rastegar et al synthesized PAMAM G7 and evaluated for antibacterial properties against gram-positive and gram-negative bacteria. The dendrimer exhibited a good response against both bacteria. According to authors, the antibacterial property of dendrimer is due to disruption of the bacterial membrane by terminal amine groups, which interact with the cytoplasmic membrane, resulting in its disruption and disintegration (Winnicka et al., 2011; Rastegar et al., 2017).
Winnicka et al. reported the PAMAM dendrimer to improve the antifungal activity of clotrimazole by increasing drug solubility in water. Besides, authors also formulated hydrogel of polyacrylic acid containing clotrimazole and ketoconazole, PAMAM–G2, and PAMAM–G3, which was demonstrated 16 times more potent against Candida with induced bioadhesive feature as compared to free drug. PAMAM dendrimer G1-G3 also improve amphotericin B water solubility and sustained release action (in-vitro) with higher generation (Winnicka et al., 2011).
Influenza commonly treated with antiviral drugs such as Oseltamivir, Amantadine, and Rimantadine, which have developed resistance due to losing its ability to bind and inhibit the function of the virus nucleic acid proteins (Landers et al., 2002; Naha and Byrne, 2013). Landers et al. conjugated sialic acid-based PAMAM dendrimers for the inhibition of hemagglutinin adhesion of influenza subtype A (H3N2 and H2N2). Results showed that the dendrimers were potentially inhibiting the infection caused by H3N2 by increasing the delivery of sialic acid and reduced toxicity (Landers et al., 2002). PAMAM-G4 conjugated to glutathione was assessed for Parkinson’s disease. Glutathione (GSH) is a tripeptide that is an important antioxidant and antiapoptotic in the brain. PAMAM–GSH was able to lower intracellular reactive oxygen species and to reduce the level of cleaved caspase-3 at low concentration (Pojo et al., 2013). Recently Gao et al. reported the use of G4 PAMAM to remineralize human teeth to treat dentine hypersensitivity. They demonstrated that PAMAM created precipitates on the surface and within the dentinal tubules with a strong ability to resist acid and showed great potential in the treatment of dentine hypersensitivity (Gao et al., 2017).
8 Conclusion
PAMAM dendrimers are hyperbranched organic molecules with distinguished characteristics from all other traditional linear polymers like size, shape, monodispersity, surface charge and peripheral functional group. For achieving these unique characteristics optimally, it must be synthesized with consistent quality and purity. Various approaches for synthesizing PAMAM dendrimers have been available, including divergent, convergent along with click chemistry, lego synthesis and hybrid convergent-divergent synthesis. However, preparation of high generation and defect-free PAMAM dendrimers on a large scale remains challenging because the purification processes are complicated and require the appropriate handling of energy parameters.
Due to its physicochemical properties and bio structural similarities, it determined its immense potential in the field pharmaceuticals as well as the bio-medicals. PAMAM dendrimer can improve biological properties such as bioavailability, solubility and specific target selectivity by linking or entrapping bioactive compounds. The peripheral positive charge of PAMAM is responsible for its cytotoxicity which can be overcome by neutralizing charge through PEGylation, acetylation, folate, and peptide conjugation. The structure of PAMAM dendrimers with a wide scope of applications will provides several opportunities for commercialization in the future.
Declaration of Competing Interest
The research did not receive any scientific grants from funding agencies in the public, commercial, or not for profit sectors.
References
- Dendrimers: synthesis, applications, and properties. Nanoscale Res. Lett.. 2014;9:1-10.
- [Google Scholar]
- PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today. 2018;12:177-190.
- [CrossRef] [Google Scholar]
- Investigations on the toxicological profile of functionalized fifth-generation poly(propylene imine) dendrimer. J. Pharm. Pharmacol.. 2006;58:1491-1498.
- [CrossRef] [Google Scholar]
- Supramolecular structures from dendrons and dendrimers. Adv. Drug Deliv. Rev.. 2005;57:2238-2270.
- [CrossRef] [Google Scholar]
- Rising chemical “stars” could play many roles. Science. 1991;251(5001):1562-1564.
- [CrossRef] [Google Scholar]
- Ammonium, C., 1995. F-1; 578, 578–581.
- Dendritic catalysts and dendrimers in catalysis. Chem. Rev.. 2001;101:2991-3023.
- [CrossRef] [Google Scholar]
- Click dendrimers and triazole-related aspects: Catalysts, mechanism, synthesis, and functions. A bridge between dendritic architectures and nanomaterials. Acc. Chem. Res.. 2012;45:630-640.
- [CrossRef] [Google Scholar]
- Backbone, P.L., 1990. X (x * 425–430).
- Induction of a robust immune response against avian influenza virus following transdermal inoculation with H5-DNA vaccine formulated in modified dendrimer-based delivery system in mouse model. Int. J. Nanomed.. 2017;12:8573-8585.
- [CrossRef] [Google Scholar]
- Dendrimer as a carrier for pulmonary delivery of enoxaparin, a low – molecular weight heparin. J. Pharm. Sci.. 2007;96:2090-2106.
- [CrossRef] [Google Scholar]
- Benjamin, D., 2002. Synthesis and use of glycodendrimer reagents related application data.
- Water-soluble carbosilane dendrimers: Synthesis biocompatibility and complexation with oligonucleotides; evaluation for medical applications. Chem. – A Eur. J.. 2007;13:483-495.
- [CrossRef] [Google Scholar]
- Surface functionalisation regulates polyamidoamine dendrimer toxicity on blood-brain barrier cells and the modulation of key inflammatory receptors on microglia. Nanotoxicology. 2014;8:158-168.
- [CrossRef] [Google Scholar]
- Pegylated lysine based copolymeric dendritic micelles for solubilization and delivery of artemether. J. Pharm. Pharm. Sci.. 2005;8:467-482.
- [Google Scholar]
- About dendrimers: structure, physical properties, and applications. Chem. Rev.. 1999;99:1665-1688.
- [CrossRef] [Google Scholar]
- Convergent synthesis and diversity of amino acid based dendrimers. Eur. J. Org. Chem.. 2001;25:1903-1915.
- [CrossRef] [Google Scholar]
- Recent advances in targeted drug delivery approaches using dendritic polymers. Biomater. Sci.. 2015;3:1025-1034.
- [CrossRef] [Google Scholar]
- Microscopic protonation equilibria of poly(amidoamine) dendrimers from macroscopic titrations. Macromolecules. 2003;36:4201-4207.
- [CrossRef] [Google Scholar]
- Characterization of dendrimers. Adv. Drug Deliv. Rev.. 2005;57:2130-2146.
- [CrossRef] [Google Scholar]
- In vitro nanotoxicity of single-walled carbon nanotube-dendrimer nanocomplexes against murine myoblast cells. Toxicol. Lett.. 2013;219:18-25.
- [CrossRef] [Google Scholar]
- Solubility enhancement of indomethacin with poly(amidoamine) dendrimers and targeting to inflammatory regions of arthritic rats. J. Drug Target.. 2004;12:575-583.
- [CrossRef] [Google Scholar]
- Dendrimer-mediated transdermal delivery: Enhanced bioavailability of indomethacin. J. Control. Release. 2003;90:335-343.
- [CrossRef] [Google Scholar]
- Cytotoxicity, hemolysis, and acute in vivo toxicity of dendrimers based on melamine, candidate vehicles for drug delivery. J. Am. Chem. Soc.. 2004;126:10044-10048.
- [CrossRef] [Google Scholar]
- Construction and evaluation of PAMAM-DOX conjugates with superior tumor recognition and intracellular acid-triggered drug release properties. Colloids Surf. B Biointerfaces. 2015;136:37-45.
- [CrossRef] [Google Scholar]
- PH- and redox-responsive self-assembly of amphiphilic hyperbranched poly(amido amine)s for controlled doxorubicin delivery. Biomater. Sci.. 2015;3:597-607.
- [CrossRef] [Google Scholar]
- Polyamidoamine (PAMAM) dendrimers as biocompatible carriers of quinolone antimicrobials: An in vitro study. Eur. J. Med. Chem.. 2007;42:1032-1038.
- [CrossRef] [Google Scholar]
- A coordinative dendrimer achieves excellent efficiency in cytosolic protein and peptide delivery. Angew. Chemie – Int. Ed.. 2020;59:4711-4719.
- [CrossRef] [Google Scholar]
- Design of biocompatible dendrimers for cancer diagnosis and therapy: Current status and future perspectives. Chem. Soc. Rev.. 2011;40:2673-2703.
- [CrossRef] [Google Scholar]
- Impact of dendrimers on solubility of hydrophobic drug molecules. Front. Pharmacol.. 2017;8:1-23.
- [CrossRef] [Google Scholar]
- New advances in general biomedical applications of PAMAM dendrimers. Molecules. 2018;23:1-27.
- [CrossRef] [Google Scholar]
- Dendrimers in the context of nanomedicine. Int. J. Pharm.. 2020;573:118814.
- [CrossRef] [Google Scholar]
- Formation of dendrimer-guest complexes as a strategy to increase the solubility of a phenazine N,N′-dioxide derivative with antitumor activity. Heliyon. 2019;5
- [CrossRef] [Google Scholar]
- Best practices for purification and characterization of PAMAM dendrimer. Macromolecules. 2012;45:5316-5320.
- [CrossRef] [Google Scholar]
- Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev.. 2005;57:2215-2237.
- [CrossRef] [Google Scholar]
- Dendrosome-based delivery of siRNA against E6 and E7 oncogenes in cervical cancer. Nanomed. Nanotechnol. Biol. Med.. 2010;6:463-470.
- [CrossRef] [Google Scholar]
- PAMAMOS: the first commercial silicon-containing dendrimers and their applications PETAR. J. Polym. Sci.. 2006;44:2755-2772.
- [CrossRef] [Google Scholar]
- Solid phase chemistry: direct monitoring by matrix-assisted laser desorption/ionization time of flight mass spectrometry. A tool for combinatorial chemistry. J. Org. Chem.. 1995;60:2652-2653.
- [CrossRef] [Google Scholar]
- The use of PAMAM dendrimers in the efficient transfer of genetic material into cells. Pharm. Sci. Technol. Today. 2000;3:232-245.
- [CrossRef] [Google Scholar]
- Transport mechanism(s) of poly (amidoamine) dendrimers across Caco-2 cell monolayers. Int. J. Pharm.. 2003;265:151-157.
- [CrossRef] [Google Scholar]
- Evaluation of electrostatic binding of PAMAM dendrimers and charged phthalocyanines by fluorescence correlation spectroscopy hysical. Chem. Chem. Phys. 2014:1-10.
- [CrossRef] [Google Scholar]
- Laboratory synthesis of poly(amidoamine) (PAMAM) dendrimers. Dendrimers Other Dendritic Polym.. 2002;1:587-604.
- [CrossRef] [Google Scholar]
- Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today. 2001;6:427-436.
- [CrossRef] [Google Scholar]
- Nanoparticles in cellular drug delivery. Bioorg. Med. Chem.. 2009;17:2950-2962.
- [CrossRef] [Google Scholar]
- Fischer, D., Li, Y., Ahlemeyer, B., Krieglstein, J., Kissel, T., 2003. Fischer-2003-In vitro cytotoxicit.pdf 24, pp. 1121–1131. https://doi.org/10.1016/S0142-9612(02)00445-3.
- A surface-modified dendrimer set for potential application as drug delivery vehicles: synthesis, in vitro toxicity, and intracellular localization. Chem. – A Eur. J.. 2004;10:1167-1192.
- [CrossRef] [Google Scholar]
- Effect and stability of poly(amido amine)-induced biomineralization on dentinal tubule occlusion. Materials (Basel). 2017;10:1-15.
- [CrossRef] [Google Scholar]
- Supramolecular complexes of quantum dots and a polyamidoamine (PAMAM)-folate derivative for molecular imaging of cancer cells. Anal. Bioanal. Chem.. 2011;400:483-492.
- [CrossRef] [Google Scholar]
- Biological evaluation of polyester dendrimer: Poly(ethylene oxide) “bow-tie” hybrids with tunable molecular weight and architecture. Mol. Pharm.. 2005;2:129-138.
- [CrossRef] [Google Scholar]
- Convergent dendrons and dendrimers: From synthesis to applications. Chem. Rev.. 2001;101:3819-3867.
- [CrossRef] [Google Scholar]
- Linkers and cleavage strategies in solid-phase organic synthesis and combinatorial chemistry. Chem. Rev.. 2000;100:2091-2157.
- [CrossRef] [Google Scholar]
- Liquid-crystalline dendrimers. Curr. Opin. Solid State Mater. Sci.. 2002;6:515-525.
- [CrossRef] [Google Scholar]
- Enhanced X-ray attenuation property of dendrimer-entrapped gold nanoparticles complexed with diatrizoic acid. J. Mater. Chem.. 2011;21:5120-5127.
- [CrossRef] [Google Scholar]
- Dendrimers: A review on synthetic approaches. J. Appl. Pharm. Sci.. 2015;5:117-122.
- [CrossRef] [Google Scholar]
- Haensler, J., Francisco, S., 1993. Haensler-1993-polyamidoamine cascaade polymers mediate efficient transfection of cells in culture, pp. 372–379.
- Preparation of polymers with controlled molecular architecture. a new convergent approach to dendritic macromolecules. J. Am. Chem. Soc.. 1990;112:7638-7647.
- [CrossRef] [Google Scholar]
- Dendrimers for vaccine and immunostimulatory uses. A review. Bioconjug. Chem.. 2010;21:405-418.
- [CrossRef] [Google Scholar]
- Synthesis of PAMAM dendrimers with porphyrin core and functionalized periphery as templates of metal composite materials and their toxicity evaluation. Arab. J. Chem.. 2020;13:27-36.
- [CrossRef] [Google Scholar]
- In vitro dose-response effects of poly(amidoamine) dendrimers [amino-terminated and surface-modified with N-(2-hydroxydodecyl) groups] and quantitative determination by a liquid chromatography-hybrid quadrupole/time-of-flight mass spectrometry based metho. Anal. Bioanal. Chem.. 2012;404:2749-2763.
- [CrossRef] [Google Scholar]
- Dendrimer-based nanocarriers: a versatile platform for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol.. 2017;9:1-21.
- [CrossRef] [Google Scholar]
- NMR insights into dendrimer-based host-guest systems. Chem. Rev.. 2012;112:3856-3891.
- [CrossRef] [Google Scholar]
- Concise solid-phase synthesis of inverse poly(amidoamine) dendrons using AB2 building blocks. Chem. Commun.. 2013;49:5784-5786.
- [CrossRef] [Google Scholar]
- The facile synthesis of multifunctional PAMAM dendrimer conjugates through copper-free click chemistry. Bioorg. Med. Chem. Lett.. 2012;22:3152-3156.
- [CrossRef] [Google Scholar]
- Convergent and divergent noncovalent synthesis of metallodendrimers. J. Am. Chem. Soc.. 1998;120:6240-6246.
- [CrossRef] [Google Scholar]
- Ihre, H., Jesu, O.L.P. De, Fre, J.M.J., 2003. JACS 2001 123 5908 (Frechet) Fast and Convenient Divergent Synthesis of Aliphatic Ester Dendrimers by Anhydride Coupling.pdf, pp. 5908–5917.
- Polyester dendritic systems for drug delivery applications: Design, synthesis, and characterization. Bioconjug. Chem.. 2002;13:443-452.
- [CrossRef] [Google Scholar]
- Functional dendrimers, hyperbranched and star polymers. Prog. Polym. Sci.. 2000;25:453-571.
- [CrossRef] [Google Scholar]
- Visualization of dendrimer molecules by transmission electron microscopy (TEM): Staining methods and cryo-TEM of vitrified solutions. Macromolecules. 1998;31:6259-6265.
- [CrossRef] [Google Scholar]
- Poly propyl ether imine (PETIM) dendrimer: A novel non-toxic dendrimer for sustained drug delivery. Eur. J. Med. Chem.. 2010;45:4997-5005.
- [CrossRef] [Google Scholar]
- Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm.. 2010;394:122-142.
- [CrossRef] [Google Scholar]
- Modified PAMAM dendrimer with 4-carbomethoxypyrrolidone surface groups reveals negligible toxicity against three rodent cell-lines. Nanomed. Nanotechnol. Biol. Med.. 2013;9:461-464.
- [CrossRef] [Google Scholar]
- Modified PAMAM dendrimer with 4-carbomethoxypyrrolidone surface groups-its uptake, efflux, and location in a cell. Colloids Surf. B Biointerfaces. 2017;159:211-216.
- [CrossRef] [Google Scholar]
- Encapsulation of guest molecules into a dendritic box. Science (80-.). 1994;266:1226-1229.
- [CrossRef] [Google Scholar]
- Engineering of dendrimer surfaces to enhance transepithelial transport and reduce cytotoxicity. Pharm. Res.. 2003;20:1543-1550.
- [CrossRef] [Google Scholar]
- The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int. J. Pharm.. 2003;252:263-266.
- [CrossRef] [Google Scholar]
- Cationic PAMAM dendrimers aggressively initiate blood clot formation. ACS Nano. 2012;6:9900-9910.
- [CrossRef] [Google Scholar]
- Multiple antigenic peptide (MAP): A synthetic peptide dendrimer for diagnostic, antiviral and vaccine strategies for emerging and re-emerging viral diseases. Indian J. Virol.. 2013;24:312-320.
- [CrossRef] [Google Scholar]
- Poly(amidoamine) dendrimers increase antifungal activity of clotrimazole. Biol. Pharm. Bull.. 2011;34(7):1129-1133.
- [CrossRef] [Google Scholar]
- Dendrimer pharmacokinetics: The effect of size, structure and surface characteristics on ADME properties. Nanomedicine. 2011;6:1063-1084.
- [CrossRef] [Google Scholar]
- A review on comparative study of PPI and PAMAM dendrimers. J. Nanopart. Res.. 2016;18
- [CrossRef] [Google Scholar]
- Double exponential dendrimer growth. J. Am. Chem. Soc.. 1995;117:2159-2165.
- [CrossRef] [Google Scholar]
- The impact of nanobiotechnology on the development of new drug delivery systems. Curr. Pharm. Biotechnol.. 2016;6:3-5.
- [CrossRef] [Google Scholar]
- PAMAM dendrimers as promising nanocarriers for RNAi therapeutics. Mater. Today. 2015;18:565-572.
- [CrossRef] [Google Scholar]
- Evaluation of dendrimer safety and efficacy through cell line studies. Curr. Drug Targets. 2011;12:1478-1497.
- [CrossRef] [Google Scholar]
- Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci.. 2014;39:268-307.
- [CrossRef] [Google Scholar]
- Multifunctional dendritic polymers in nanomedicine: Opportunities and challenges. Chem. Soc. Rev.. 2012;41:2824-2848.
- [CrossRef] [Google Scholar]
- Developmental toxicity of low generation PAMAM dendrimers in zebrafish. Toxicol. Appl. Pharmacol.. 2007;225:70-79.
- [CrossRef] [Google Scholar]
- Dendrimer-based MRI contrast agents: The effects of PEGylation on relaxivity and pharmacokinetics. Nanomed. Nanotechnol. Biol. Med.. 2011;7:1001-1008.
- [CrossRef] [Google Scholar]
- Surface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeability. Bioconjug. Chem.. 2007;18:2054-2060.
- [CrossRef] [Google Scholar]
- Drug complexation, in vitro release and cellular entry of dendrimers and hyperbranched polymers. Int. J. Pharm.. 2003;259:143-160.
- [CrossRef] [Google Scholar]
- Trastuzumab-grafted PAMAM dendrimers for the selective delivery of anticancer drugs to HER2-positive breast cancer. Sci. Rep.. 2016;6:1-13.
- [CrossRef] [Google Scholar]
- PAMAM dendrimers: Destined for success or doomed to fail? Plain and modified PAMAM dendrimers in the context of biomedical applications. J. Pharm. Sci.. 2015;104:2-14.
- [CrossRef] [Google Scholar]
- Design and synthesis of dendrimers with facile surface group functionalization, and an evaluation of their bactericidal efficacy. Molecules. 2017;22
- [CrossRef] [Google Scholar]
- Prevention of influenza pneumonitis by sialic acid-conjugated dendritic polymers. J. Infect. Dis.. 2002;186:1222-1230.
- [CrossRef] [Google Scholar]
- Coarse-grained molecular dynamics studies of the concentration and size dependence of fifth- and seventh-generation PAMAM dendrimers on pore formation in DMPC bilayer. J. Phys. Chem. B. 2008;112:7778-7784.
- [CrossRef] [Google Scholar]
- Convergent synthesis of PAMAM dendrimers using click chemistry of azide-functionalized PAMAM dendrons. Tetrahedron. 2006;62:9193-9200.
- [CrossRef] [Google Scholar]
- Gene Delivery by PAMAM dendrimer conjugated with the nuclear localization signal peptide derived from influenza B virus nucleoprotein. Macromol. Res.. 2019;27:360-368.
- [CrossRef] [Google Scholar]
- Poly(amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int. J. Pharm.. 2018;546:215-225.
- [CrossRef] [Google Scholar]
- Effect of poly(amidoamine) dendrimers on the structure and activity of immune molecules. Biochim. Biophys. Acta – Gen. Subj.. 2015;1850:419-425.
- [CrossRef] [Google Scholar]
- Dynamics and thermodynamics of water in PAMAM dendrimers at subnanosecond time scales. J. Phys. Chem. B. 2005;109:8663-8672.
- [CrossRef] [Google Scholar]
- Natural polyphenols augment cytosolic protein delivery by a functional polymer. Chem. Mater.. 2019;31:1956-1965.
- [CrossRef] [Google Scholar]
- A boronic acid–rich dendrimer with robust and unprecedented efficiency for cytosolic protein delivery and CRISPR-Cas9 gene editing. Sci. Adv.. 2019;5:1-12.
- [CrossRef] [Google Scholar]
- Poly(amidoamine)dendrimers: covalent and supramolecular synthesis. Mater. Today Chem.. 2019;13:34-48.
- [CrossRef] [Google Scholar]
- Concepts of trapping topologically by shell molecules. J. Macromol. Sci. Part A – Chem.. 1982;17:689-703.
- [CrossRef] [Google Scholar]
- Effect of solvent and pH on the structure of PAMAM dendrimers. Macromolecules. 2005;38:979-991.
- [CrossRef] [Google Scholar]
- Solvent quality changes the structure of G8 PAMAM dendrimer, a disagreement with some experimental interpretations. J. Phys. Chem. B. 2006;110:25628-25632.
- [CrossRef] [Google Scholar]
- Counterion distribution and ζ-potential in PAMAM dendrimer. Macromolecules. 2008;41:5002-5006.
- [CrossRef] [Google Scholar]
- Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J. Control. Release. 2000;65:133-148.
- [CrossRef] [Google Scholar]
- Studies towards biocompatibility of PAMAM dendrimers – Overall hemostasis potential and integrity of the human aortic endothelial barrier. Int. J. Pharm.. 2014;473:158-169.
- [CrossRef] [Google Scholar]
- Sialic acid-mimic peptides as hemagglutinin inhibitors for anti-influenza therapy. J. Med. Chem.. 2010;53:4441-4449.
- [CrossRef] [Google Scholar]
- Dendrimers for pulmonary delivery: Current perspectives and future challenges. New J. Chem.. 2019;43:8396-8409.
- [CrossRef] [Google Scholar]
- Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov. Today. 2010;15:171-185.
- [CrossRef] [Google Scholar]
- Application of dendrimers for the treatment of infectious diseases. Molecules. 2018;23
- [CrossRef] [Google Scholar]
- Dendrimer-based contrast agents for molecular imaging Michelle. Bone. 2008;8:1180-1186.
- [CrossRef] [Google Scholar]
- Dendrimer space exploration: An assessment of dendrimers/dendritic scaffolding as inhibitors of protein-protein interactions, a potential new area of pharmaceutical development. Chem. Rev.. 2014;114:1327-1342.
- [CrossRef] [Google Scholar]
- Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev.. 2011;40:173-190.
- [CrossRef] [Google Scholar]
- Polyamidoamine dendrimer nanoparticle cytotoxicity, oxidative stress, caspase activation and inflammatory response: Experimental observation and numerical simulation. Nanomed. Nanotechnol. Biol. Med.. 2013;9:202-211.
- [CrossRef] [Google Scholar]
- In vitro mammalian cytotoxicological study of PAMAM dendrimers - Towards quantitative structure activity relationships. Toxicol. Vitr.. 2010;24:169-177.
- [CrossRef] [Google Scholar]
- Mechanistic studies of in vitro cytotoxicity of poly(amidoamine) dendrimers in mammalian cells. Toxicol. Appl. Pharmacol.. 2010;248:259-268.
- [CrossRef] [Google Scholar]
- Generation of intracellular reactive oxygen species and genotoxicity effect to exposure of nanosized polyamidoamine (PAMAM) dendrimers in PLHC-1 cells in vitro. Aquat. Toxicol.. 2013;132–133:61-72.
- [CrossRef] [Google Scholar]
- In vitro evaluation of third generation PAMAM dendrimer conjugates. Molecules. 2017;22:1-12.
- [CrossRef] [Google Scholar]
- Dendrimers: Emerging polymers for drug-delivery systems. Eur. J. Pharm. Sci.. 2009;38:185-196.
- [CrossRef] [Google Scholar]
- Starburst Dendrimers: molecular shape control. J. Am. Soc.. 1989;32:2339-2341.
- [CrossRef] [Google Scholar]
- Bioapplications of poly(amidoamine) (PAMAM) dendrimers in nanomedicine. J. Nanoparticle Res.. 2014;16
- [CrossRef] [Google Scholar]
- Poly(amidoamine) (PAMAM) nanoparticles: synthesis and biomedical applications poli(amidoamin) (pamam) nanopartiküller: sentezi ve biyomedikal uygulamalari. J. Biol Chem. 2013;41:289-299.
- [Google Scholar]
- Cascade molecules:1 synthesis and characterization of a benzene[9]3-arborol. J. Am. Chem. Soc.. 1986;108:849-850.
- [CrossRef] [Google Scholar]
- Carbon nanodots as molecular scaffolds for development of antimicrobial agents. Bioorg. Med. Chem. Lett.. 2016;26:1745-1749.
- [CrossRef] [Google Scholar]
- Organ/Tumor Distrib.. 2004;21:476-483.
- Pharmacokinetic evaluation of polymeric carriers. Adv. Drug Deliv. Rev.. 1996;21:135-155.
- [CrossRef] [Google Scholar]
- Physicochemical and biological properties of self-assembled antisense/poly(amidoamine) dendrimer nanoparticles: The effect of dendrimer generation and charge ratio. Int. J. Nanomed.. 2010;5:359-369.
- [CrossRef] [Google Scholar]
- In vitro and in vivo uptake studies of PAMAM G4.5 dendrimers in breast cancer. J. Nanobiotechnol.. 2016;14:1-12.
- [CrossRef] [Google Scholar]
- Poly(amidoamine) dendrimers as a pharmaceutical excipient. are we there yet? J. Pharm. Sci.. 2018;107:75-83.
- [CrossRef] [Google Scholar]
- Polyester dendritic systems for drug delivery applications: In vitro and in vivo evaluation. Bioconjug. Chem.. 2002;13:453-461.
- [CrossRef] [Google Scholar]
- Synthesis and biological properties of mannosylated starburst poly(amidoamine) dendrimers. Bioconjug. Chem.. 1997;8:714-723.
- [CrossRef] [Google Scholar]
- Half generations magnetic PAMAM dendrimers as an effective system for targeted gemcitabine delivery. Int. J. Pharm.. 2016;515:104-113.
- [CrossRef] [Google Scholar]
- Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv. Drug Deliv. Rev.. 2005;57:2203-2214.
- [CrossRef] [Google Scholar]
- Bimetallic dendrimer-encapsulated nanoparticles as catalysts: A review of the research advances. Chem. Soc. Rev.. 2008;37:1619-1628.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of branched liquid-crystalline polyethers containing cyclotetraveratrylene-based disklike mesogens. Macromolecules. 1992;25:1164-1176.
- [CrossRef] [Google Scholar]
- Structural analysis of cylindrical and spherical supramolecular dendrimers quantifies the concept of monodendron shape control by generation number. J. Am. Chem. Soc.. 1998;120:11061-11070.
- [CrossRef] [Google Scholar]
- Synthesis and Cze analysis of pamam dendrimers with an ethylenediamine core. Proc. Est. Acad. Sci.. 2001;50:156-166.
- [Google Scholar]
- Convergent synthesis of internally branched PAMAM dendrimers. Org. Lett.. 2005;7:1295-1298.
- [CrossRef] [Google Scholar]
- Modified PAMAM vehicles for effective TRAIL gene delivery to colon adenocarcinoma: in vitro and in vivo evaluation. Artif. Cells, Nanomed. Biotechnol.. 2018;46:S503-S513.
- [CrossRef] [Google Scholar]
- In vitro evaluation of the cytotoxicity and cellular uptake of CMCht/PAMAM dendrimer nanoparticles by glioblastoma cell models. J. Nanopart. Res.. 2013;15
- [CrossRef] [Google Scholar]
- Comparative toxicological assessment of PAMAM and thiophosphoryl dendrimers using embryonic zebrafish. Int. J. Nanomed.. 2014;9:1947-1956.
- [CrossRef] [Google Scholar]
- Pyzowski, J., Caminade, A., Majoral, J., 2003. “Lego” Chemistry for the Straightforward Synthesis of Dendrimers chemistry, it is desirable that the byproducts are envi- ronmentally friendly, that only a very slight excess of reagents is used for quantitative reactions , and that no as byproducts. pp. 6043–6046.
- PEG-conjugated PAMAM dendrimers mediate efficient intramuscular gene expression. AAPS J.. 2009;11:395-405.
- [CrossRef] [Google Scholar]
- Antibacterial activity of amino- and amido- terminated poly (amidoamine)-G6 dendrimer on isolated bacteria from clinical specimens and standard strains. Med. J. Islam. Repub. Iran. 2017;31:368-376.
- [CrossRef] [Google Scholar]
- Synthesis of a chiral dendrimer based on polyfunctional amino acids. Chem. Commun.. 1999;2:207-208.
- [CrossRef] [Google Scholar]
- Using starburst dendrimers as linker molecules to radiolabel antibodies. Bioconjug. Chem.. 1990;1:305-308.
- [CrossRef] [Google Scholar]
- Preliminary biological evaluation of polyamidoamine (PAMAM) Starburst™ dendrimers. J. Biomed. Mater. Res.. 1996;30:53-65.
- [CrossRef] [Google Scholar]
- Dendrimers and dendrimer-polymer hybrids. Adv. Polym. Sci.. 1999;142:179-228.
- [CrossRef] [Google Scholar]
- Glycodendrimers: Novel glycotope isosteres unmasking sugar coding. Case study with T-antigen markers from breast cancer MUC1 glycoprotein. Rev. Mol. Biotechnol.. 2002;90:291-309.
- [CrossRef] [Google Scholar]
- Organic solvent nanofiltration for microfluidic purification of poly(amidoamine) dendrimers. J. Chromatogr. A. 2007;1162:167-174.
- [CrossRef] [Google Scholar]
- Transepithelial transport and toxicity of PAMAM dendrimers: Implications for oral drug delivery. Adv. Drug Deliv. Rev.. 2012;64:571-588.
- [CrossRef] [Google Scholar]
- Comparative pharmacokinetics of PAMAM-OH dendrimers and HPMA copolymers in ovarian tumor-bearing mice. Drug Deliv. Transl. Res.. 2013;3:260-271.
- [CrossRef] [Google Scholar]
- Peptide dendrimers: Applications and synthesis. Rev. Mol. Biotechnol.. 2002;90:195-229.
- [CrossRef] [Google Scholar]
- Dendrimers as pharmaceutical excipients: synthesis, properties, toxicity and biomedical applications. Materials 2019
- [CrossRef] [Google Scholar]
- Selective cytotoxicity of PAMAM G5 core-PAMAM G2.5 shell tecto-dendrimers on melanoma cells. Int. J. Nanomed.. 2012;7:4121-4133.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, and applications of dendrimer-encapsulated nanoparticles. J. Phys. Chem. B. 2005;109:692-704.
- [CrossRef] [Google Scholar]
- Dendrimers Based on [1,3,5]-Triazines. J. Polym. Sci.. 2006;44:3411-3433.
- [CrossRef] [Google Scholar]
- Functional dendrimers: Unique biological mimics. Chem. – A Eur. J.. 1998;4:1353-1361.
- [CrossRef] [Google Scholar]
- Supramolecular assembly models of siRNA delivery systems. Chin. J. Chem.. 2015;33:79-89.
- [CrossRef] [Google Scholar]
- Dendrimers in biomedical applications – Reflections on the field. Adv. Drug Deliv. Rev.. 2005;57:2106-2129.
- [CrossRef] [Google Scholar]
- Solid-phase dendrimer synthesis and the generation of super-high-loading resin beads for combinatorial chemistry. J. Org. Chem.. 1997;62:4902-4903.
- [CrossRef] [Google Scholar]
- Transepithelial transport of PEGylated anionic poly(amidoamine) dendrimers: Implications for oral drug delivery. J. Control. Release. 2009;138:78-85.
- [CrossRef] [Google Scholar]
- Transport of poly amidoamine dendrimers across Madin-Darby canine kidney cells. Int. J. Pharm.. 2001;215:263-267.
- [CrossRef] [Google Scholar]
- Coupling difficulty associated with interchain clustering and phase transition in solid phase peptide synthesis. J. Am. Chem. Soc.. 1995;117:12058-12063.
- [CrossRef] [Google Scholar]
- Solid phase synthesis of gastrin I. Int. J. Pept. Protein Res.. 2009;26:262-273.
- [CrossRef] [Google Scholar]
- Charge affects the oral toxicity of poly(amidoamine) dendrimers. Eur. J. Pharm. Biopharm.. 2013;84:330-334.
- [CrossRef] [Google Scholar]
- Tomalia, B.D.A., Naylor, A.M., Goddard, W.A., 1990. AngChemieIE_138_1990.pdf 29, pp. 138–175.
- Starburst™/cascade dendrimers: Fundamental building blocks for a new nanoscopic chemistry set. Aldrichim. Acta. 1993;26:91-101.
- [Google Scholar]
- A new class of polymers: Starburst-dendritic macromolecules. Polym. J.. 1985;17:117-132.
- [CrossRef] [Google Scholar]
- Dendritic macromolecules:1 Synthesis of starburst dendrimers. Macromolecules. 1986;19:2466-2468.
- [CrossRef] [Google Scholar]
- Discovery of dendrimers and dendritic polymers: A brief historical perspective. J. Polym. Sci. Part A Polym. Chem.. 2002;40:2719-2728.
- [CrossRef] [Google Scholar]
- Starburst dendrimers. 4. Covalently fixed unimolecular assemblages reiminiscent of spheroidal micelles. Macromolecules. 1987;20:1164-1167.
- [CrossRef] [Google Scholar]
- Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem. Soc. Trans.. 2007;35:61-67.
- [CrossRef] [Google Scholar]
- Dendrimers. Control. Release Syst. Adv. Nanobottles Act. Nanoparticles. 2015;48:259-285.
- [CrossRef] [Google Scholar]
- Structure activity relationship of dendrimer microbicides with dual action antiviral activity. PLoS One. 2010;5
- [CrossRef] [Google Scholar]
- Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J. Control. Release. 2005;102:23-38.
- [CrossRef] [Google Scholar]
- Effect of poly(amidoamine) (PAMAM) dendrimer on skin permeation of 5-fluorouracil. Int. J. Pharm.. 2008;361:230-238.
- [CrossRef] [Google Scholar]
- Starlike branched and hyperbranched biodegradable polymer systems as DNA carriers. Russ. J. Bioorganic Chem.. 2006;32:205-218.
- [CrossRef] [Google Scholar]
- Simplifying the synthesis of dendrimers: Accelerated approaches. Chem. Soc. Rev.. 2012;41:4593-4609.
- [CrossRef] [Google Scholar]
- Dendrimer-entrapped gold nanoparticles as potential CT contrast agents for blood pool imaging. Nanoscale Res. Lett.. 2012;7:1-8.
- [CrossRef] [Google Scholar]
- Highly water-soluble, pH sensitive and biocompatible PAMAM ‘dendrizyme’ to maintain catalytic activity in complex medium. Mater. Sci. Eng. C. 2017;78:315-323.
- [CrossRef] [Google Scholar]
- The decrease of PAMAM dendrimer-induced cytotoxicity by PEGylation via attenuation of oxidative stress. Nanotechnology. 2009;20
- [CrossRef] [Google Scholar]
- PAMAM-Lys, a novel vaccine delivery vector, enhances the protective effects of the SjC23 DNA vaccine against schistosoma japonicum infection. PLoS One. 2014;9:1-10.
- [CrossRef] [Google Scholar]
- Tecto-dendrimers: A study of covalently bound nanospheres. Macromolecules. 2009;42:7571-7578.
- [CrossRef] [Google Scholar]
- Solid-phase dendrimer synthesis. Biopolym. – Pept. Sci. Sect.. 1998;47:381-396.
- [CrossRef] [Google Scholar]
- Anionic PAMAM dendrimers rapidly cross adult rat intestine in vitro: A potential oral delivery system? Pharm. Res.. 2000;17:991-998.
- [CrossRef] [Google Scholar]
- Mannose functionalization of a sixth generation dendrimer. Biomacromolecules. 2001;2:1052-1054.
- [CrossRef] [Google Scholar]
- Dendrimers as carriers for siRNA delivery and gene silencing: A review. Sci. World J.. 2013;2013
- [CrossRef] [Google Scholar]
- Polycationic dendrimers interact with RNA molecules: Polyamine dendrimers inhibit the catalytic activity of Candida ribozymes. Chem. Commun. 2005:313-315.
- [CrossRef] [Google Scholar]
- Gd(iii) chelates for MRI contrast agents: From high relaxivity to “smart”, from blood pool to blood-brain barrier permeable. Medchemcomm. 2012;3:552-565.
- [CrossRef] [Google Scholar]
- Dendrimers in supramolecular chemistry: From molecular recognition to self-assembly. Chem. Rev.. 1997;97:1681-1712.
- [CrossRef] [Google Scholar]
- Polyamidoamine dendrimers inhibit binding of Tat peptide to TAR RNA. FEBS Lett.. 2004;563:241-245.
- [CrossRef] [Google Scholar]
- Structures and properties of PAMAM dendrimer: A multi-scale simulation study. Fluid Phase Equilib.. 2011;302:43-47.
- [CrossRef] [Google Scholar]
- Dendrimers in molecular recognition and self-assembly. Curr. Opin. Colloid Interface Sci.. 1997;2:89-99.
- [CrossRef] [Google Scholar]
- Bifunctional PAMAM dendrimer conjugates of folic acid and methotrexate with defined ratio. Biomacromolecules. 2012;13:982-991.
- [CrossRef] [Google Scholar]







