Translate this page into:
Statistical analysis of the physico-chemical characteristics of industrial effluents: Case of evaporating basins used as final storage for effluent
⁎Corresponding author. adam.abdeljalil@gmail.com (Abdeljalil Adam),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
Evaporating basins are common storage for industrial effluent in most renewable power plants. Various types of research on the environmental consequences and issues of this experience have suggested this method of disposal to prevent industrial effluents withdrawal into the ocean, and dams and prevent water contamination.
Nevertheless, this approach may have significant worldwide environmental implications. Focusing on dedicated research methods for a power plant in the south of Morocco that employed evaporating basins for storing its effluent, we evaluated the development of the effluent's Physico-chemical characteristics over a timeframe of one year.
The evaporation basins undergo thorough assessment to evaluate the effects of industrial effluents, leading to the observation of a significant increase in the physicochemical characteristics of the discharged effluents, mainly EC, BOD5, Na, and Cl2. This rise in water contamination can be attributed to the aforementioned factors. Statistical analysis, specifically correlation and regression, was employed to examine the coefficients of the primary physicochemical properties. The objective was to determine the effluent characteristics that exhibited strong associations and close relationships.The primary goal of this study is to highlight the drawbacks associated with storing wastewater in evaporation ponds, a common practice among many industrial companies. It is crucial to consider that these ponds attract a diverse range of bird and animal species, and any contamination of the water can pose significant environmental and ecological risks in the event of an accidental leakage or spill, potentially harming wildlife. To mitigate these potential issues, it is recommended to explore alternatives such as industrial effluent reuse and recycling, which can help minimize the environmental and ecological impacts.
Keywords
Evaporating basins
Industrial effluents
Correlation and regression analysis
Physical-chemical parameters
1 Introduction
Resource extraction deterioration and permanent resource shortages, especially with regard to groundwater, remain key problems in the twenty-first decade.
Earthen ponds with a liner where the concentration spontaneously increases due to direct sunlight are known as evaporating basins. As when the surface water evaporated out from pools, which are regularly collected and discharged off-site, the chemicals in the concentrates crystallize into the brine (Abdeljalil et al., 2022).
Water from saline treatments has long been removed using evaporating basins. There are several benefits to disposing of waste seawater in evaporating basins (Pontius et al., 1996).
They assert that evaporating basins are less complicated to construct, a need less upkeep, and user supervision than mechanical equipment (Salzman et al., 2001).
The necessity for big areas of land whenever the evaporating rate has dropped or the disposing rates are very high, as well as the demand for these huge parcels of land when these conditions exist, seem to be some negatives of using evaporating basins (Amoatey et al., 2021; Abdeljalil et al., 2022).
Industrial effluents can be properly disposed of in evaporating basins, particularly in hot regions, and inexpensive territories. It is possible to seal evaporating basins in order to minimize the likelihood of polluting the groundwater (Ahmed et al., 2000; Adam et al., 2023).
Effluents are concentrated in evaporating basins for this reason. As a result, they attempt to reduce the amount of effluent by evaporation, which might lead to the formation of salt (Adam et al., 2023).
(Adam et al., 2023) studied the possibility for groundwater infiltration, management and surveillance frequencies, re-use, and other factors were taken into account while creating a grading system to evaluate disposal basins (Sustainable water management and climate change, 2009).
Industrial effluents can be treated by injecting them into large ponds where they gradually evaporate under the sun's direct rays (Dehghani and Taleb Beydokhti, 2018). They are a widely utilized method of managing salty water in various nations throughout the world (Al-Ismaili and Jayasuriya, 2016).
According to study (Curiel-Esparza et al., 2014), industrial effluent may be handled by being dumped into sizable basins with the proper risk strategy approach, where it will be subjected to intense sunlight and eventually evaporate.
Evaporating basins may raise a number of ecological and environmental issues despite their many advantages (Dehghani and Taleb Beydokhti, 2018). For instance, any effluent spill from the evaporating basins might be extremely harmful to the ecology (soil, surface water, and groundwater). Evaporating basins also attract wildlife since they are open water surfaces, resulting in a rise in the deaths of some wildlife if the wastewater they collect seems to be of poor quality and exceeds the permitted levels (Martínez-Gallardo et al., 2022).
In order to protect water resources and preserve the environment, it is advisable for industries utilizing evaporation ponds to explore sustainable alternatives for wastewater management instead of relying on them as a final disposal method. By investigating the potential of recycling wastewater, the industry can move away from the practice of dumping it into evaporation ponds.
This research focused on examining the effluent from a Moroccan power plant to shed light on the drawbacks associated with industries that currently dispose of their effluent in evaporation ponds. Additionally, a comprehensive physico-chemical assessment was conducted to identify the presence of water contaminants. Statistical analysis was employed to establish correlations between the physicochemical parameters and to identify key factors influencing the effluent's characteristics.
2 Material & method
2.1 Overview of the research region
To collect a sampling of effluent from two evaporating basins during 2021/ 2022, a renewable solar power plant in the south of Morocco that uses an indoor water treatment facility was selected to perform the experiences. Fig. 1 depicts the precise location of the study area as presented in the map.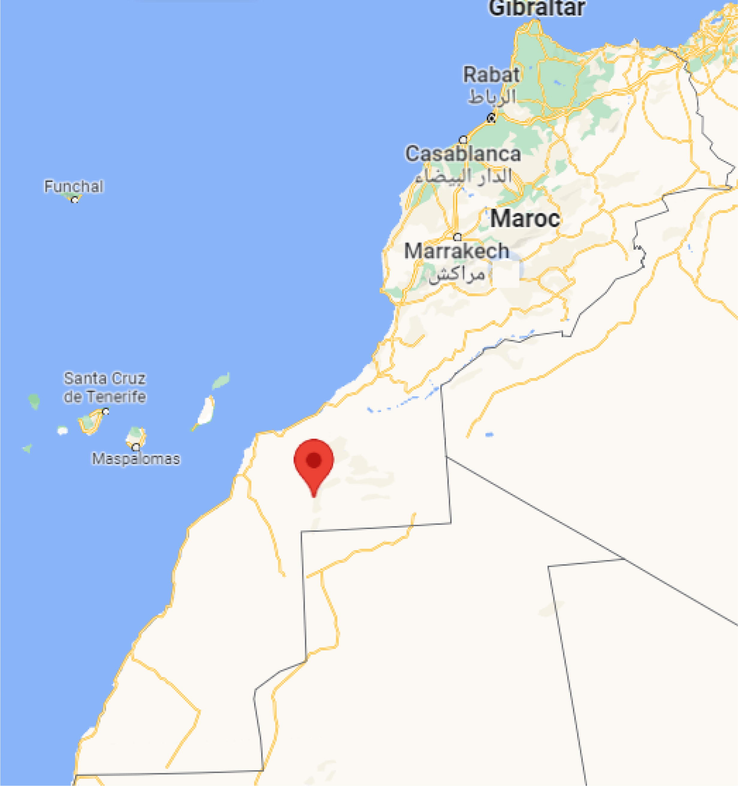
Location map of the study area.
The evaporation ponds constructed using geomembrane materials have dimensions of 165 m in length, 85 m in width, and 2 m in depth.
2.1.1 Meteorological pattern
The Moroccan southern region is known for its arid climate and continental characteristics. Both the south-western Ocean and desert air currents have an impact, although the former predominates (Web, 2020).
-
Annual rainfall:
Limited precipitation is a distinctive feature of the region. About 187 mm of rainfalls on average each year.
The climate variability dispersion of precipitation pattern has a highly irregular pattern. The area has both hot seasons, with just a seasonal rainfall of 65 mm, together with warmer seasons, with average annual precipitation as high as 293 mm.
There are just a few days with hail in the area each year on average, and there are only a few days with thunderstorms. The project location and its immediate environs seldom get snow.
-
The region’s temperatures:
The region maintains a global average temperature of 26.8 °C. The warmest months of the year, July and August, experience average high temperatures reaching 40.0 °C. In contrast, January exhibits the coolest median high temperature, recorded at 15.0 °C. The wintertime ranges in temperature from 10 °C up to 26.0 °C. The extent of summertime temperatures is 26.0 °C up to 41.2 °C. Just a handful of days during the seasons, the temperatures are lower than zero degrees.
This region had temperatures as high as 42.5 °C and as low as 09.0 °C, all in 2021 (Fig. 2.).
-
Humidity:
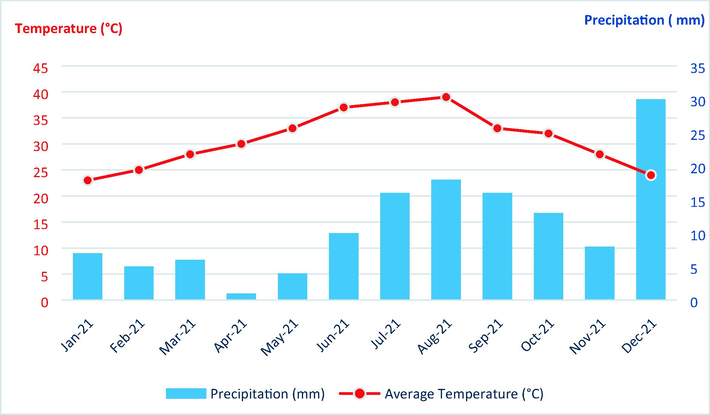
- Umbro rainfall and heat graph in the south region of Morocco (Web, 2020).
The levels of the average humidity decreased when the average temperature increases. Relative humidity varies by month, from 41% in July to 63% in December, according to data gathered in the area between 2000 and 2018.
The maximum level of evaporation exceeds 1.8 m/year, is considered the greatest in Morocco, in this region, a peak in July and the highest concentration of evaporation from May to September (which accounts for 50% of the total evaporation).
2.2 Sampling’s effluent analysis
The sampling was gathered between April 2022 and November 2021 using a certified automated tester, the SIGMA type 940P.
We used conventional techniques at the Laboratory of chemical and environmental to do the Physicochemical study of the wastewater.
All the sampling was delivered in several unique bottles since the effluent from the evaporating basin included oil residues (type glass bottle). This sampling was analyzed on the same day they were collected from the power plant.
The Common Techniques for the Examination of Effluent provides a complete description of standard processes for wastewater analysis. (Silva et al., 2022).
The measurements for the physicochemical analysis are highlighted below (Table 1):
Parameter
Analysis Device
Temperature (T°)
CDC641T Analyzer
Potential of hydrogen (pH)
HQ2200 Analyzer
Total Nitrogen (TN)
Nitrogen 250,494 Analyzer
Total suspended solids (TSS)
TSS analyzer HQ0490
COD “Chemical Oxygen demand”
COD Z7000 Analyzer
BOD5 “Biochemical Oxygen Demand”
BOD5 Z7100 Analyzer
Zinc (Zn)
Zinc EZ1040 analyzer
Sodium (Na)
Na 9240 analyzer
Adsorbable Organically bound halogens (AOX)
AOX analyzer
Oil and grease (OAG)
INFRACAL 2 ATR-SP
Chlorine (Cl2)
XPLORER analyzer (TE Instrument)
Electrical conductivity (EC)
EC HQ2200 Analyzer
Iron (Fe)
Fe EZ2308 Analyzer
Total Phosphorus (P)
EZ7801 Total Phosphorus, 0.025–––5 mg/L
3 Effluent parameters analysis using statistical tools
3.1 Statistical regression and correlation
When evaluating effluent conformity in the evaporating basin, regression and correlation examination is helpful. This statistical evaluation was conducted using Minitab software.The correlation coefficients (r) between physicochemical parameters in industrial effluents were computed for this study using the Pearson correlation method. The most common correlation types employed in the main statistical analysis are Pearson and Spearman. The most common method for determining how closely two variables are connected linearly is Pearson correlation. The following equation is used to compute the Pearson coefficient (r) correlation: (Javadzadeh et al., 2020):
In this equation, r is the Pearson correlation coefficient, N represents the number of data points, x represents the values of a variable and y represents the values of the other variable.
∑x and ∑y are the sum of all × and y scores respectively, ∑x2 and ∑y2 are the sum of × and y squared scores, where ∑xy is the sum of the products of × and y paired scores.
This least-squares regression method in statistical data is used to predict how reliant factors will change over time. Amongst datasets, there must be one that perfectly matches the trend being taken into account, according to the least-squares approach. Regression analysis may be used to see the association between the dependent variable and several independent ones (Amaral and Ferreira, 2005). The following regression equation was created using the parameter estimates and the proposed model for this relationship (Stoichev et al., 2020):
In this equation, y is the dependent, x is the independent, a is the coefficient of linear correlation and b is the constant at the origin.
The aim of this statistical study is to assess relationships between the physico-chemical properties of effluent. This study uses a bivariate correlation research to evaluate the strength of the link between two factors as well as the direction of the connection (Javadzadeh et al., 2020).
The coefficient (r) of −1 or + 1 indicates the largest negative and positive link for two variables; furthermore, when the obtained value is close to 1, the linear connection level for the variables is extremely strong. And when the correlation coefficient value decreases toward zero, the linear association among those variables will diminish in significance (Kumar et al., 2006).
Through using p-value, the linear correlation's strength was therefore evaluated. Consequently, the correlation in this instance is strong when the p-value is lower than 0.05 or lower than 0.01. Nevertheless, the linear connection is not strong if the p-value is above 0.05. At 0.01 and 0.05 values, the relevance is assessed (second tailed assessment) (Shroff et al., 2015).
4 Results & discussion
4.1 Evolution of the physico-chemical characteristics of the effluents over time
Table 2 presents the outcomes regarding Physicochemical examination of the effluent for a year.
Parameter
Unit
Limit value (Moroccan limit-values-of-discharges, 2017)
Apr-21
Jun-21
Aug-21
Oct-
21
Dec-21
Feb-22
Apr-22
T°
°C
30
14.8
24
27
22
21
20
17.8
pH
PH unit
5.5–9.5
8.19
8.58
8.8
8.7
8.85
8.9
8.22
TN
mg/l
40
8.4
9.2
8.2
10.3
11.5
11.6
9.6
TSS
mg/l
100
12
23
41
36
18
11
19
DCO
mg O2/l
500
48
112
241
56
87
123
145
DBO5
mg O2/l
100
20
36
145
178
153
98
126
Zn
mg/l
05
0.15
0.25
0.36
0.18
0.75
0.63
0.28
Na
mg/l
09 (for irrigation)
358
456
1025
985
456
526
415
AOX
ug/l
5000
170
210
325
165
254
326
415
OAG
mg/l
30
5
6
4
3
5
8
3
Cl2
mg/l
0.05
0.08
0.1
0.1
0.09
0.07
0.1
0.1
EC
mS/cm
2.7
4.5
15.6
21
23
13.5
12.8
23
Fe
mg/l
5
0.1
0.13
0.21
0.32
0.25
0.28
0.18
P
mg/l
15
0.2
0.3
1.2
2.3
0.9
1.8
2.1
Four effluent parameters (EC, BOD5, Na, and Cl2) surpass the limit maximum values established by Moroccan regulations for effluents discharged into surface resources, according to Table 2 (Moroccan limit-values-of-discharges, 2017):
The average EC value of 16.2 ms/cm is greater than the maximum limit value which is 2.7 ms/cm.
The average BOD5 value of 108 mg O2/l is more than the maximum fixed limit which is 100 mg O2/l.
The average of Na value of 603 mg/l is pretty high.
The median chlorine value of 0.09 mg/l is more than the limit value of 0.05 mg/l.
The bacteriological investigation demonstrates that there are no microorganisms in this effluent.
The following Figs. 3–6 illustrate the yearly variations in the Physicochemical properties of non-compliant effluent as compared to limit criteria.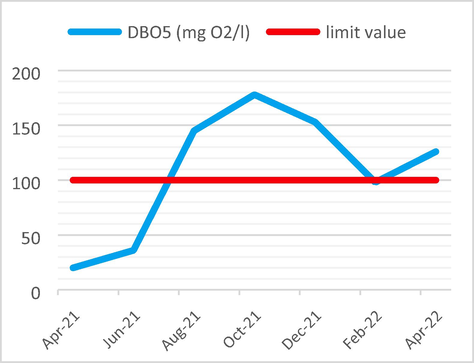
DBO5 Analysis results vs the limit value.
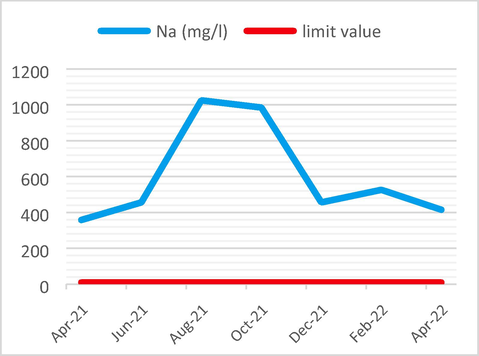
Sodium Analysis results vs the limit value.
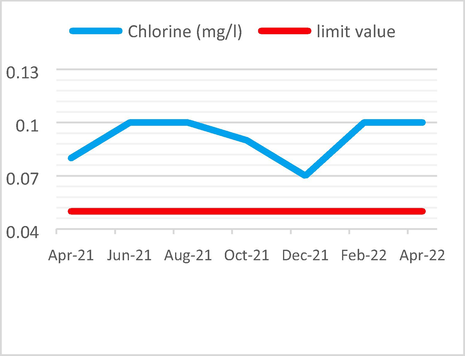
Chlorine Analysis results vs the limit value.
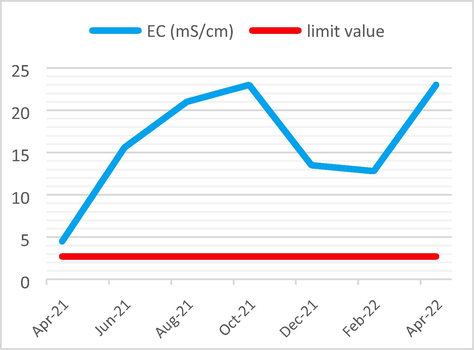
EC Analysis results vs the limit value.
4.2 Correlation results
The correlation data for the entire year (winter and summer) are summarized in Table 3. *Correlation is significant at the 0.05 level (2-tailed). **Correlation is significant at the 0.01 level (2-tailed).
T°
pH
TN
TSS
DCO
DBO5
Zn
Na
AOX
OAG
Cl2
EC
Fe
P
T°
1.000
pH
0.670
1.000
TN
−0.102
0.562
1.000
TSS
0.774*
0.300
−0.381
1.000
DCO
0.639
0.268
−0.325
0.478
1.000
DBO5
0.399
0.505
0.399
0.569
0.248
1.000
Zn
0.142
0.688
0.755*
−0.306
0.152
0.327
1.000
Na
0.698
0.484
−0.176
0.913**
0.414
0.648
−0.166
1.000
AOX
0.064
0.003
0.105
−0.070
0.693
0.272
0.342
−0.082
1.000
OAG
−0.039
0.400
0.399
−0.588
−0.066
−0.493
0.502
−0.400
−0.031
1.000
Cl2
0.380
0.002
−0.263
0.275
0.594
−0.066
−0.298
0.270
0.489
0.088
1.000
EC
0.546
0.183
0.007
0.710*
0.471
0.726*
−0.131
0.617
0.424
0.598
0.504
1.000
Fe
0.303
0.715*
0.698
0.312
−0.011
0.826*
0.445
0.563
0.072
−0.035
−0.017
0.477
1.000
P
0.057
0.202
0.414
0.275
0.135
0.746*
0.068
0.443
0.444
−0.340
0.349
0.724*
0.761*
1.000
4.2.1 Results interpretation
The data analysis data described in Table 3 demonstrates that effluent temperature has a positive association with TSS, DCO, and Na; whereas, the linear correlation between T° and TN, Zn, AOX, OAG, and P is low.
The temperature has an impact on the liberation of TSS and Na in effluent is significant, although its linear correlation with TN, Zn, AOX, and P is minimal. This might be understood by the evaporating impacts on effluent, that raise the concentration of water and saline.
pH shows a strong link with TN, DBO5, Zn, and Fe, but perhaps a weak correlation with chlorine and AOX.
The impact of pH demonstrated that the release of Fe, DBO5, TN, and Zn rises when wastewater conditions are alkaline.
TN has a strong positive correlation with Zn and Fe, and has a very weak correlation with EC, AOX, Na and Cl2.
TN is an indispensable component for all living organisms. However, an overabundance of nitrogen in the effluent can result in low amounts of dissolved oxygen and severely impact several plant and animal species.
TSS demonstrates a weak association with AOX, Cl2, and P. TSS, in fact, has a high positive correlation with Na, EC, DBO5, and a negative correlation with OAG.
The oxidation of some heavy metals with ambient oxygen can also lead to an increase in total suspended solids in the evaporating basins.
COD has a strong linear association with AOX and Cl2, whereas its linear correlation with Fe and OAG is minor.
High COD concentrations might be understood by the oxidation resilience of organic matter into evaporating basins in addition to the accumulation of sodium (Lee et al., 2016; Sukmawati et al., 2021).
BOD5 shows a positive linear relationship with Na, EC, P, and Fe, and a negative linear relationship with OAG; however, its linear correlation with AOX and Cl2 is relatively weak.
This relationship might be read as the BOD5 result being underestimated if the effluent is nutrient-deficient. If this nutrient level is excessively high, however, nitrification occurs and the BOD is exaggerated.
The presence of non-biodegradable or minimally biodegradable organic substances requires the bacteria to adapt for an extended period of time before they can degrade the organic molecules.
Zn shows a positive linear relationship with Fe, OAG, whereas its linear relationship with EC, Na and P is very weak.
The presence of Zn in effluent may produce hydrogen, which interacts exponentially with oxygen. In higher quantities, zinc salts result in the turbidity and Fe release in effluent. Additionally, zinc may provide a bitter odor to effluent in the evaporation basins.
Na has a moderate negative linear association with AOX and chlorine and a positive linear correlation with EC, Fe, and P.
This demonstrates that freshwater and saltwater interact in the evaporation basins.
AOX has also a positive linear correlation with chlorine, EC and P. however AOX, shows a very weak linear association with OAG and Fe.
In addition, the concentration of organic halogens, which are substances containing chlorine, phosphorus or salt, in a sample of effluent may be determined by the presence of AOX. Increases are frequently connected to water pollution.
OAG have a positive linear relationship with EC and a negative linear relationship with P; and a very weak linear relationship with Cl2 and Fe.
OAG can produce unattractive deposits and interfere with organic organisms in surface evaporating basins, and also could be explained by a decreases of dissolved oxygen and phosphorus.
Cl2 shows a positive linear association with EC and P. however Cl2 has a very weak linear association with the iron Fe.
The occurrence of Cl2 residue in effluent may be a sign that enough chlorine has originally injected to the effluent to make the microorganisms inactive.
In wastewater, the amount of spontaneous residual chlorine is associated with the lack of pathogens and the presence of a high EC.
The drop of irons in the evaporation basin could be attributed to the existence of a chlorine-based oxidant (Amoatey et al., 2021).
EC shows a positive linear correlation with Fe and P. The excessive value of EC, that is proportional to a rise in Fe and P, can be interpreted by the breakdown of organic matter in the effluent of the evaporating basins; EC in this case is elevated while a great deal of organic contamination.
The statistical study indicated the main significant linear associations between effluent variables:
The TSS showed a strong positive linear correlation with Na (r = 0.913, p < 0.05) and EC(r = 0.710, p < 0.05). an increase in TSS might be interpreted by the Na accumulation into evaporating basin.
The effluent’ temperature showed a strong negative linear association with TSS (r = 0.774, p < 0.05).
The DBO5 showed a strong positive linear correlation with EC (r = 0.726, p < 0.05), Fe (r = 0.826, p < 0.05) and P (r = 0.746, p < 0.05). Because of this, the high EC/BOD5 ratios indicated that the evaporating basin is now expanding with the non-biodegradable portion of total organic pollutants.
The TN showed a strong positive linear relationship with Zn (r = 0.755, p < 0.05) and Fe (r = 0.698, p < 0.05).
The pH showed the most significant positive linear correlations with Zn (r = 0.688, p < 0.01) and Fe (r = 0.715, p < 0.01).
The P showed a significant positive linear correlation with Fe (r = 0.761, p < 0.05) and EC (r = 0.724, p < 0.05).
Table 4 reveals the appropriate statistical outcomes of the potential linear association between effluent properties and the climate of the region. These results indicate that the ambient temperature of the region shows a negative linear relationship along with pH, TN, Zn, oil and grease, and Fe; and a positive linear relationship with Temperature, TSS, and chlorine; in addition, this temperature shows a very weak linear association with DCO, BOD5, AOX, and P.
T°
pH
TN
TSS
DCO
DBO5
Zn
Na
AOX
OAG
Chlorine
EC
Fe
P
Ambiant temperature
Humidity
Precipitation
Ambiant temperature
0.435
−0.305
-0.754
0.633
0.262
−0.170
-0.803
0.401
−0.192
−0.429
0.535
0.417
−0.358
−0.112
1
Humidity
0.555
0.244
−0.478
0.624
0.843
0.326
0.028
0.624
0.376
−0.251
0.264
0.306
0.031
0.049
0.230
1
Precipitation
0.588
0.666
0.271
0.393
0.232
0.539
0.599
0.327
−0.065
−0.087
−0.461
0.162
0.357
−0.124
−0.225
0.337
1
The area's relative humidity shows a favorable linear connection with Temperature and TSS, otherwise, it shows a negative linear correlation with TN and a very weak linear correlation with Zn, Fe and P.
The region precipitation shows a positive linear association with Temperature, pH, DBO5, Zn, and a negative linear association with Cl2, whereas it shows a very weak linear association with AOX, OAG.
The significant linear association has revealed for humidity with DCO (r = 0.843, p < 0.05), for ambient temperature with Zn (r = -0.803, p < 0.05), and for ambient temperature with TN (r = 0.754, p < 0.05).
4.3 Regression analysis outcomes
Employing a statistical tool, the regression model for the effluent characteristics was undertaken.
Table 5 presents the regression model’s last square focused on effluent variables with statistically strong correlations.
-
Regression graphs:
| Y | X | r | R2 | a | B | Y = ax + b |
|---|---|---|---|---|---|---|
| TSS | T° | 0.774 | 0.598 | 2.23 | –23.98 | TSS = 2.23 T° –23.98 |
| TSS | Na | 0.913 | 0.834 | 0.0377 | 0.1181 | TSS = 0.0377Na + 0.1181 |
| TSS | EC | 0.710 | 0.503 | 1.217 | 3.139 | TSS = 1.217 EC + 3.139 |
| BOD5 | EC | 0.726 | 0.527 | 6.484 | 2.952 | BOD5 = 6.484 EC + 2.952 |
| BOD5 | Fe | 0.826 | 0.682 | 623.68 | –22.974 | BOD5 = 623.68 Fe − 22.974 |
| BOD5 | P | 0.746 | 0.556 | 53.205 | 41.114 | BOD5 = 53.205P + 41.114 |
| TN | Zn | 0.755 | 0.570 | 4.490 | 8.160 | TN = 4.490 Zn + 8.160 |
| TN | Fe | 0.698 | 0.487 | 12.026 | 7.303 | TN = 12.026 Fe + 7.303 |
| pH | Zn | 0.688 | 0.473 | 0.873 | 8.281 | pH = 0.873 Zn + 8.281 |
| pH | Fe | 0.715 | 0.511 | 2.634 | 8.052 | pH = 2.634 Fe + 8.052 |
| P | Fe | 0.761 | 0.579 | 8.052 | −0.433 | P = 8.052 Fe − 0.433 |
| P | EC | 0.724 | 0.524 | 0.090 | −0.210 | P = 0.090 EC − 0.210 |
| DCO | AOX | 0.693 | 0.480 | 0.987 | 151.88 | DCO = 0.987 AOX + 151.88 |
The Figs. 7–19 illustrate linear relationships between effluent Physico-chemical variables: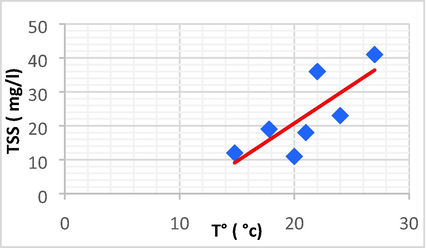
Linear plot between TSS and T.
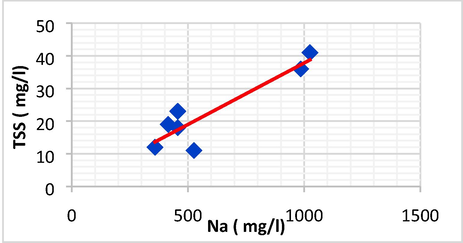
Linear plot between Na and TSS.
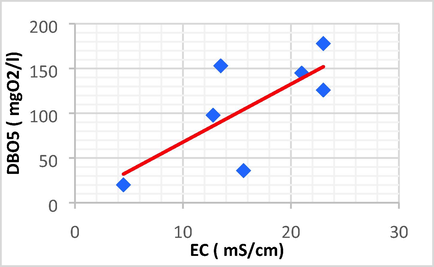
Linear plot between DBO5 and EC.
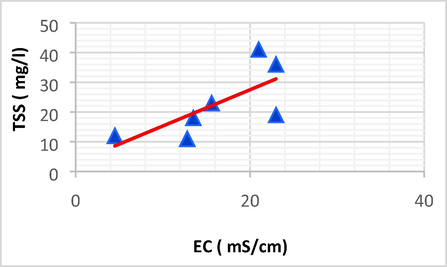
Linear plot between TSS and EC.
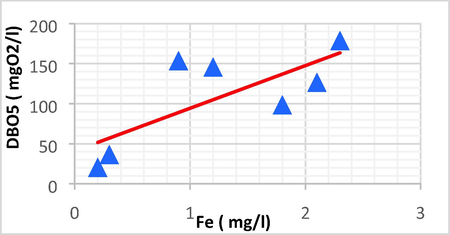
Linear plot between DBO5 and Fe.
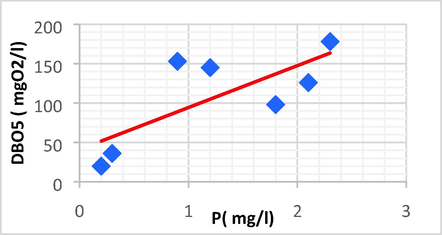
Linear plot between DBO5 and P.
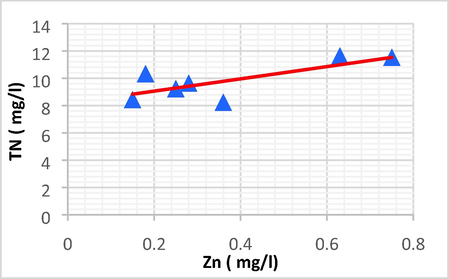
Linear plot between TN and Zn.
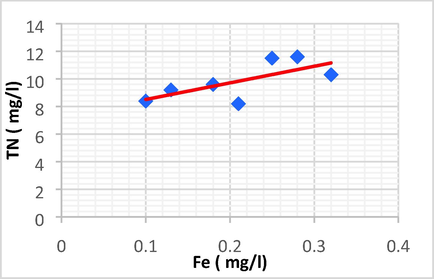
Linear plot between TN and Fe.
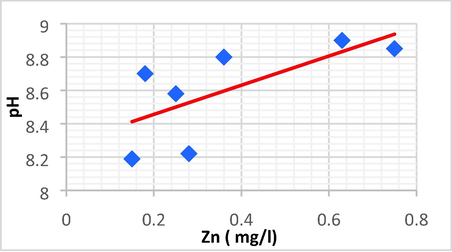
Linear plot between pH and Zn.
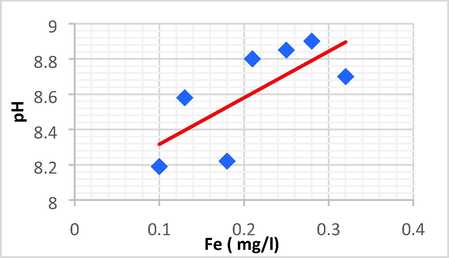
Linear plot between pH and Fe.
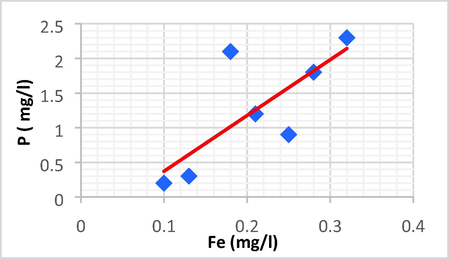
Linear plot between P and Fe.
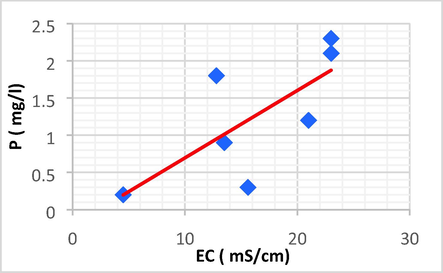
Linear plot between P and EC.
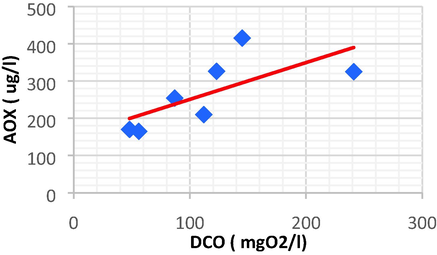
Linear plot between AOX and DCO.
The plots demonstrated that TSS has a direct linear and positive connection with EC and Na, but a negative association with T°. Using linear regression, the regression coefficients (R) and (R2) for these relationships were determined.
The graph reveals that Na and EC are dependent on TSS, with an increase in TSS leading to an increase in EC and Na.
Also, BOD5 with EC,P and Fe have a direct linear and positive connection; hence, EC,P and Fe are dependent on BOD5, with an increase in BOD5 leading to an increase in EC,P and Fe.
TN has a positive direct linear relationship with Zn and Fe, with an increase in TN leading to a rise in Zn and Fe.
pH with Zn and Fe and have the same positive direct linear relationship, a simple increase of pH resulting in another increase of Zn and Fe.
P has a positive direct linear relationship with EC and Fe, with a rise in P leading to an increase in EC and Fe.
DCO and AOX and have a positive direct linear relationship, with an increase of DCO resulting in another increase of AOX.
Ordinarily, a regression analysis is conducted primarily for one of two reasons: to anticipate the significance of the effluent physicochemical dependent parameters for those for whom some information about the response variable is available, or to estimate the impact of some explanatory physicochemical parameters on the dependent ones, hence have complete understanding of the wastewater pollutants.
4.4 Analysis of results
The outcomes of evaluating Physico-Chemical Characteristics of the effluent released in evaporating basins indicate that evaporation concept shows a negative impact on wastewater, as demonstrated by a huge rise in different effluent properties (EC, Na, TSS..etc). Such impact may have adverse environmental and ecological consequences.
The large impact of the temperature on the generation of Na, Fe, and TSS in effluent can be understood by evaporation factors that increase the salinity and saturation of the effluent.
The oxidation of Fe ions by atmospheric oxygen can also result in elevated TSS and Na levels in the evaporating basins.
AOX may be used to measure the quantity of organic halogens, which are compounds comprising chlorine, phosphorus, or salt, in an effluent test. Typically, increases are associated with water contamination.
Microbial degradation in the effluent of evaporating basins might underlie the high EC level, which would be associated to a rise in several physicochemical variables; EC is increased in case of a plenty of organic contaminants.
Hydrogen may be produced through the concentration of Zn in the effluent, which reacts significantly with oxygen. In wastewater, zinc ions in high concentrations cause turbulence and the liberation of iron. In consequence, zinc may provide an unpleasant odor to the effluent in the evaporation basins.
The occurrence of peroxidation reliability of organic substances, especially in the effluent pit, the appearance of Na might contribute to the maximum DBO5 and AOX interpretations. Since some particles are limited or not biodegradable, pathogens could be required to acclimate for a prolonged period before it can alter the organic matter.
The presence of Cl2 residual in the effluent may indicate that sufficient chlorine was initially added to render the bacteria dormant.
The quantity of unintentional residual Cl2 in the effluent is related to the absence of microorganisms and the occurrence of a significant EC.
In surface evaporating basins, oil and grease can generate unsightly deposits and interfere with living life, which could be described by a reduction in oxygen concentration and phosphorus.
Total Nitrogen is essential for all living creatures. Nevertheless, an excess of nitrogen in the wastewater can lead to low levels of dissolved oxygen and have devastating effects on a variety of species of plants and animals.
The linear correlation between regional climate and effluent features imply that warm and hot weather has an effect on effluent characteristics.
Based on these findings, we advocate reusing the effluent recovered in the evaporating basins by applying a sustainable approach like the solar still techniques, especially in arid and hot regions.
5 Conclusions
The evaluation of the Physico-chemical characteristics of the discharged effluent into the evaporation pond reveals the negative effects of evaporation on this industrial wastewater. As a result, evaporation causes a notable increase in various Physico-chemical parameters such as EC, Na, Cl2, BOD5, and others. This rise can be attributed to water contamination caused by external factors like soil, wildlife, and organic waste from poultry.
According to the results of the correlation study, the Total Suspended Solids (TSS) and 5-Day Biological Oxygen Demand (DBO5) of the effluent collected in the evaporation basins display a significant linear relationship with most of the other effluent variables. However, the effluent demonstrates the strongest and most significant linear correlation between Sodium (Na) and TSS, as well as between DBO5 and Iron (Fe). As a result, regression models were established to analyze the significant relationships between these effluent properties, and the details are provided in Table 5.This study identified a correlation among all the physicochemical properties of industrial effluents stored in evaporating basins. Additionally, it was observed that the levels of DBO5, Sodium (Na), chlorine, and Electrical Conductivity (EC) exceed the permissible limits for effluent in the study area. To mitigate potential ecological and environmental issues, it is recommended to consider the reuse or recycling of the effluent using a sustainable technological approach such as solar still. This approach can help address the challenges associated with effluent disposal and contribute to more sustainable water management practices.
Acknowledgements
The editors and reviewers who gave constructive and useful feedback on an earlier draught of this work are to be acknowledged by the authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Feasibility and sustainability of evaporation ponds as final basins for industrial wastewater: statistical evaluation of gross parameters. Desalin. Water Treat.. 2022;257:41-54.
- [CrossRef] [Google Scholar]
- Contribution to developing a new environmental risk management methodology for industrial sites. Journal of Applied and Natural Science. 2022;14:9-16.
- [Google Scholar]
- Classification of industrial wastewater discharged into effluent pits, an approach toward a sustainable recycling: case study of a water treatment facility in Morocco. E3S Web of Conferences. 2023;364:02001.
- [CrossRef] [Google Scholar]
- The reliability of evaporation ponds as a final basin for industrial effluent: Demonstration of an environmental risk management methodology. MethodsX. 2023;10:102055
- [CrossRef] [Google Scholar]
- Sustainable solar still system coupled with renewable power for industrial wastewater recycling: A review. Mater. Today:. Proc. 2023
- [CrossRef] [Google Scholar]
- Use of evaporation ponds for brine disposal in desalination plants. Desalination. 2000;130:155-168.
- [Google Scholar]
- Seawater greenhouse in Oman: A sustainable technique for freshwater conservation and production. Renew. Sustain. Energy Rev.. 2016;54:653-664.
- [CrossRef] [Google Scholar]
- Activated sludge monitoring of a wastewater treatment plant using image analysis and partial least squares regression. Anal. Chim. Acta. 2005;544:246-253.
- [CrossRef] [Google Scholar]
- A critical review of environmental and public health impacts from the activities of evaporation ponds. Sci. Total Environ.. 2021;796:149065
- [CrossRef] [Google Scholar]
- Selecting a Sustainable Disinfection Technique for Wastewater Reuse Projects. Water. 2014;6:2732-2747.
- [CrossRef] [Google Scholar]
- Investigating the quality and quantity of effluent in wastewater treatment plants of Iran: A case study of Tehran. MethodsX. 2018;5:871-880.
- [CrossRef] [Google Scholar]
- Interaction of lake-groundwater levels using cross-correlation analysis: A case study of Lake Urmia Basin. Iran. Science of The Total Environment. 2020;729:138822
- [CrossRef] [Google Scholar]
- Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. Environ. Geol.. 2006;50:1025-1039.
- [CrossRef] [Google Scholar]
- Relationships between water quality parameters in rivers and lakes: BOD5, COD, NBOPs, and TOC. Environ. Monit. Assess. 2016:188.
- [CrossRef] [Google Scholar]
- Biorecovery of olive mill wastewater sludge from evaporation ponds. J. Environ. Manage.. 2022;319:115647
- [CrossRef] [Google Scholar]
- Moroccan limit-values-of-discharges, (2017). Available at http://www.environnement.gov.ma/fr/78-cat1/1012-valeurs-limites-des-rejets.
- Regulations governing membrane concentrate disposal. J. - Am. Water Works Assoc.. 1996;88:44-52.
- [CrossRef] [Google Scholar]
- Performance of constructed evaporation ponds for disposal of smelter waste water. Water Res.. 2001;35:2121-2128.
- [CrossRef] [Google Scholar]
- Correlation study among water quality parameters of groundwater of Valsad district of south Gujarat(India) Journal of Fundamental and Applied Sciences. 2015;7:340.
- [CrossRef] [Google Scholar]
- Chemical characterization of riverine sediments affected by wastewater treatment plant effluent discharge. Sci. Total Environ.. 2022;839:156305
- [CrossRef] [Google Scholar]
- Multiple regression analysis to assess the contamination with metals and metalloids in surface sediments (Aveiro Lagoon, Portugal) Mar. Pollut. Bull.. 2020;159:111470
- [CrossRef] [Google Scholar]
- Analysis of reduction of COD (Chemical Oxygen Demand) levels in tofu waste using activated sludge method. Moroccan Journal of Chemistry. 2021;9:339-345.
- [Google Scholar]
- Sustainable water management and climate change: the North Sea Skills Integration and New Technologies (SKINT) project. International Journal of Climate Change Strategies and Management 2009;1. https://doi.org/10.1108/ijccsm.2009.41401daf.001.
- Weather Morocco Official Web site, (2020). Available at http://www.meteomaroc.com/.







