Translate this page into:
Stigma and petals of Crocus sativus L.: Review and comparison of phytochemistry and pharmacology
⁎Corresponding authors. peixjin@163.com (Jin Pei), microelements@sina.com (Hongyan Ma)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Crocus sativus L. (C. sativus), known as “plant gold ”, has numerous functions in traditional Chinese medicine, including promoting blood circulation, removing blood stasis, cooling blood, detoxifying, relieving depression, and calming the nerves. Its stigma, the main medicinal part, performs extremely low yield and high price, thus the scarce resources, while its petals, the by-product, are usually discarded or employed as fertilizer or feed, resulting in huge waste, as the petals have been proved to contain various chemical components covering terpenoids, flavonoids, and glycosides, which exhibits pharmacological activities of analgesia, anti-inflammatory, cardiovascular protection, liver protection, and antidepressant. This paper aims to compare the material basis of the pharmacological similarities or differences between stigmas and petals, clarify their research status, and evaluate the potential application value of petals. As a by-product of a precious traditional herbal medicine, the petals of C. sativus have been elucidated in previous studies. This review explores the chemical constituents and pharmacological effect of stigma and petals of C. sativus, confirming their similar material bases, and the application prospect of petals.
Keywords
Adjuvant therapy
Antidepressant
By-products
Crocus sativus L.
Flavonoids
- Abbreviations
-
Full name
- Aβ
-
Amyloid-beta
- AMPK
-
Adenosine 5′-monophosphate (AMP)-activated protein kinase
- AD
-
Alzheimer disease
- BDNF
-
Brain-derived neurotrophic factor
- BDI
-
Beck Depression inventory
- BAI
-
Beck Anxiety Inventory
- Crocus sativus L.
-
C. sativus
- CREB
-
cAMP response element binding
- CRC
-
Colorectal cancer
- CK-MB
-
Cardiac creatine kinase
- DASS
-
Depression Anxiety Stress Scale
- FST
-
Forced swimming test
- GHQ
-
General Health Questionnaire
- GSH
-
Glutathione
- GM
-
Gentamicin sulfate
- HDRS
-
Hamilton Depression Rating Scale
- HARS
-
Hamilton Anxiety Rating Scale
- HFRDIS
-
Hot Flash-Related Daily Interference Scale
- HCY
-
Homocysteine
- ICP-MS
-
Inductively coupled plasma mass spectrometry
- IDS-SR
-
Revised Symptomatology self-rated Version
- LDH
-
Lactate dehydrogenase
- L-NAME
-
NG-nitro-L-arginine methyl ester
- LSEQ
-
Leeds Sleep Evaluation Questionnaire
- MADRS
-
Montgomery-Asberg Depression Rating Scale
- MDQ
-
Mood Disorder Questionnaire
- MDA
-
Malondialdehyde
- MCP-1
-
Monocyte chemoattractant protein 1
- NF-κB
-
Nuclear factor-k-gene binding
- NOS
-
Nitric oxide synthase
- ox-LDL
-
Low-density cholesterol
- PSQI
-
Pittsburgh Sleep Quality Index
- POM
-
Primary outcome measure
- PANAS
-
Positive and Negative Affect Schedule
- POMS
-
Profile of Mood States
- qRT-PCR
-
quantitative Reverse Transcription-Polymerase Chain Reaction
- RCADS
-
Revised Child Anxiety and Depression Scale
- RONS
-
Reactive Oxygen-nitrogen Species
- ROS
-
Reactive oxygen species
- SWLS
-
Satisfaction with life scale
- STAI
-
State-Trait Anxiety Inventory
- SOD
-
Superoxide dismutase
- SIRT1
-
Sirtuin 1
- VGF
-
Nerve growth factor inducible
Abbreviations
1 Introduction
Crocus sativus L. (C. sativus), a bulbous herb of the genus Crocus of the family Iridaceae, native to Iran, Spain, India and other countries, was introduced to China through Tibet (Basker and Negbi 2008), and first recorded in Compendium of Materia Medica. It has been employed as a natural traditional Chinese medicine for thousands of years. The historical records of Iranian traditional medicine take that C. sativus relieves headaches and toothache, and promotes diuresis, restoration, cosmetology, aphrodisiac, detoxification, antihypertensive, and blood circulation (Liu 2007, Shariatifar et al., 2014), while traditional Chinese medicine books record that C. sativus has the function of promoting blood circulation, removing blood stasis, cooling blood, detoxifying, relieving depression, and calming the nerves (Commission 2020). In addition, it is adopted as a high-grade dye owing to the carotenoids contained, while its unique aroma as a spice (Fabre 2003). There exist many studies on the pharmacological effect and chemical constituents of C. sativus, validating its role in preventing cardiovascular diseases such as ischemia reperfusion injury (Farjah et al., 2017), hyperlipidemia (Tajaddini et al., 2021), and atherosclerosis, and determining its main active components of terpenoids and flavonoids (Hatziagapiou and Lambrou 2018). Lambrianidou et al reviewed the anti-cancer effect of C. sativus, with crocin, crocetin, picrocrocin, and safranal serving as the main active component (Lambrianidou et al., 2020). In reality, only the stigma at the top of the 3 pistils in each C. sativus is rationally employed (Salehi et al., 2022). This part is completely harvested by hand, and only about 1 g of dry stigma can be obtained from about 90 flowers, which explains its extremely low yield (Serrano-Díaz et al., 2014). The petals, however, are usually used as fertilizer, fodder, or even discarded, leading to a huge waste.
The chemical constituents and pharmacological effect of the non-medicinal parts of C. sativus have caught the eyes of researchers, thus the growing reports on the petals of C. sativus. The chemical composition of petals is complex, including flavonoids and their glycosides, terpenoids and their glycosides, alkaloids, and organic acids, among which terpenoids and their glycoside are the main components (Chen and Yang 2018). Lu Zhuo et al. (Lv et al., 2021)explored the amino acids and trace elements in petals using an amino acid analyzer, inductively coupled plasma mass spectrometry (ICP-MS), Kjeldahl nitrogen analyzer, etc., revealing the 6 essential amino acids (threonine, valine, isoleucine, leucine, phenylalanine, and lysine) required by the human body, and 6 trace elements (chromium, iron, cobalt, copper, molybdenum and zinc). It also proved petals’ role in antioxidation (Huang et al., 2013, Zhao and Qu 2013), cardiovascular system and nervous system protection (Khazdair et al., 2015), liver and kidney protection (Azizi et al., 2019), antifungal and cytotoxic effects, etc. (Chen and Yang, 2018).
Studies concerning C. sativus mostly focus on stigmas, with very few on petals. The review discusses the chemical constituents and pharmacological activities of petals with previous literature as reference, in the hope of providing theoretical support for better utilization, and giving an insight into the development and rational application of C. sativus.
2 Botany
C. sativus is native to southern Europe and was introduced to China through Tibet, where it is commonly cultivated throughout China, including Beijing, Shandong, Zhejiang, and Sichuan provinces. The plant is a perennial herb, and the 3 cm in diameter, the oblate-spherical build is covered in a membranous layer that is yellowish brown in cover. It contains 9–15 basal leaves that are banded, grayish-green, 15–20 cm long, and 2–3 mm wide, and have inverted margins, 4–5 membranous sheath-like leaves are enclosed within the base of the plexus. The flower’s stalk is quite short and does not stick out above the surface. The ovary is narrowly spindle-shaped, and the stigma is orange-red, somewhat flattened, wedge-shaped at the apex, and shallowly serrated. Flowers begin to bloom in late October and open during the day before closing at night.
3 Chemistry and bioactivity of stigma and petals compounds
C. sativus is a mixture of various chemical constituents, including primary metabolites (like polysaccharides, proteins, fats, and vitamins), compounds of different classes of secondary metabolites (like carotenoids, monoterpenoids, flavonoids, and anthocyanins) (Masi et al., 2016), fat-soluble pigments represented by zeaxanthin, phytoene, phytoene, β-carotene, etc., volatile components represented by C. sativus aldehyde and isophorone, and flavonoids represented by kaempferol and its glycosides (Mykhailenko et al., 2019, Abu-Izneid et al., 2022). Metabolites with pharmacological activity are classified into four categories. The first refers to water-soluble crocus acid; the second crocin, the glycoside of crocetin, which is the colored component of C. sativus and its main component, as well as an uncommon water-soluble carotenoid (dicarboxylic acid polyene monosaccharide ester); the third picrocrocin, which is largely responsible for the bitterness of C. sativus; while the fourth safranal, which is a volatile oil that largely explains the characteristic odor and aroma of C. sativus. (Wang et al., 2015).
Despite the differences in the chemical components of petals and stigma, they share plentiful chemical components. The stigma contains more terpenoids, and among the 50 kinds of terpenoids collected, 36 observed in stigma cannot be found in petals, including 7 kinds of tetraterpenoids. Besides, crocin, a common component, is the only component in petals that has been reported (Wang et al., 2019). On the other hand, petals contain more flavonoids, which belong to chemical components with a higher proportion of common components in stigma and petals. Among the 43 flavonoids collected, 22 are chemical constituents isolated from petals, while 13 are common constituents of stigma and petals. The paper confirmed the chemical constituents of stigmas and petals, including 50 terpenoids, 43 flavonoids, 7 alkaloids, 5 phenylpropanoids, as well as 16 amino acids, 11 organic acids, and 2 steroids, most of which are conjugated with one or more glycosyl moieties to form glycoside esters, and the conjugated glycosyl structure is shown in Fig. 1A.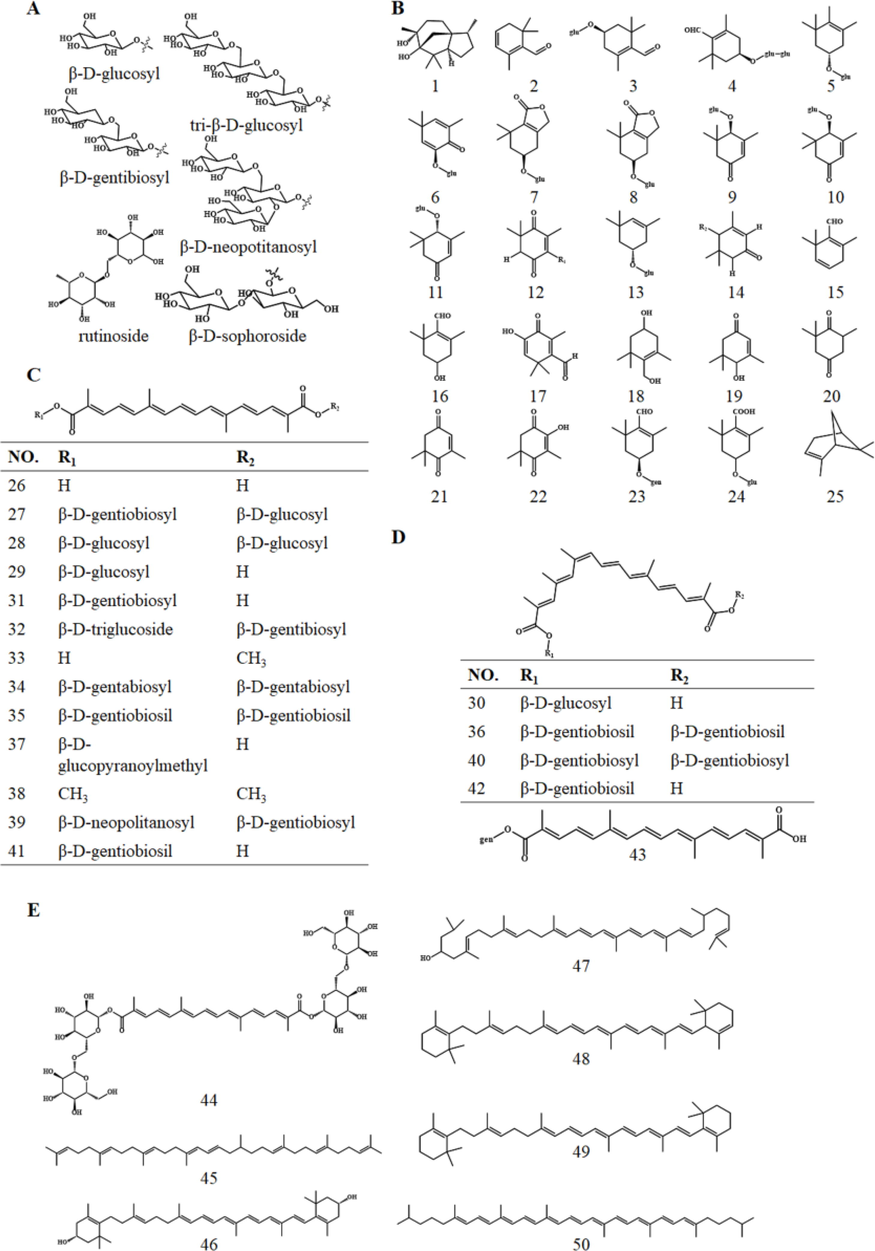
Conjugated glycosyl structure (A) and Chemical Structures of terpenoids (B: sesquiterpenoids and monoterpenoids; C: trans-diterpenoid; D: cis-diterpenoid; E: tetraterpenoids).
3.1 Terpenoids
Terpenoids act as the most characteristic and important phytochemicals of C. sativus, and the highest concentrations reported in the stigma of C. sativus are water-soluble crocetin and its glycoside esters conjugated with one or more glycosyls (crocin, crocetin-β-D-glucosyl ester, Dimethylcrocetin, etc.). Table 1 lists the 50 terpenoids and their glycosides in the stigma and petals of C. sativus, including 1 sesquiterpene, 24 monoterpenoids, 18 diterpenoids, and 7 tetraterpenoids.
Species
NO.
Compound
Molecular formula
Part of plant
Reference
Sesquiterpenoids
1
Cedrol
C15H26O
petals
(Wang et al., 2012)
Monoterpenoids
2
Safranal
C10H14O
stigmas
(Tarantilis and Polissiou 1997, Yu et al., 2008, Li et al., 2017)
3
Picrocrocin
C16H26O7
stigmas
petals(Rios et al., 1996, Zhou et al., 2011, Montoro et al., 2012, Christodoulou et al., 2015)
4
(4R)-4-hydroxy-2,6,6-trimethylcyclohex-1-enecarbaldehyde 4-O-[β-D-glucopyranosyl (1 → 3)-β-D-glucopyranoside
C22H36O12
stigmas
(Straubinger 1997)
5
(4R)-4-hydroxy-2,6,6-trimethylcyclohex-1-enecarboxylicacid O-β-D-glucopyranoside
C16H26O8
stigmas
(Straubinger 1997)
6
6-hydroxy-3-(hydroxymethyl)-2,4,4-trimethylcyclohexa-2,5-dienone 6-O-β-D-glucopyranoside
C16H23O8
stigmas
(Straubinger et al., 1998, Li et al., 2004)
7
(5S)-5-hydroxy-7,7-dimethyl-4,5,6,7-tetrahydro-3Hisobenzofuran-1-one O-β-D-glucopyranoside
C16H24O8
stigmas
(Straubinger 1997, Straubinger et al., 1998)
8
(5S)-5-hydroxy-7,7-dimethyl-4,5,6,7-tetrahydro-3Hisobenzo-Furanone 5-O-β-D-gentibioside
C22H35O13
stigmas
(Carmona et al., 2006)
9
(4S)-4-hydroxymethyl-3,5,5-trimethyl-cyclohex-2-en-1-one 4-O-β-D-gentibioside
C22H36O12
stigmas
(Carmona et al., 2006)
10
(4R)-4-hydroxy-3,5,5-trimethylcyclohex-2-enone 4-O-β-D-glucopyranoside
C15H24O7
stigmas
(Straubinger 1997, Straubinger et al., 1998)
11
(4S)-4-hydroxy-3,5,5-trimethylcyclohex-2-enone 4-O-β-D-glucopyranoside
C15H24O7
stigmas
(Straubinger 1997)
12
(2Z)-3-methylpent-2-enedioic acid1-[1-(2,4,4-trimethyl-3,6-dioxocyclohexenyloxy)-O-β-D-glucopyranosid-6-yl] ester
C21H28O11
stigmas
(Straubinger 1997)
13
(1R)- 3,5,5-trimethylcyclohex-3-enol O-β-D-glucopyranoside
C15H26O6
stigmas
(Straubinger 1997)
14
(4S,3′R)-4-Hydroxy-4-(3′-hydroxy-1′-butenyl)-3,5,5-trimethyl-2-cyclohexen-1-one 3′-O-β-D-glucopyranoside
C19H30O8
stigmas
(Straubinger 1997)
15
2,6,6-trimethyl-1,4-cyclohexadiene-1-carboxaldehyde
C10H14O
stigmas
(Christodoulou et al., 2015)
16
2,6,6-trimethyl-4-hydroxy-1-cyclohexen-1-carboxaldehyde
C10H16O2
stigmas
(Zarghami and Heinz 1971)
17
2,4,4-trimethyl-3-formyl-6-hydroxy-2,5-cylohexadien-1-one
C10H12O3
stigmas
(Zarghami and Heinz 1971)
18
4-hydroxymethyl-3,5,5-trimethylcyclohex-3-enol
C10H18O2
stigmas
(Li and Wu 2002)
19
3,5,5-trimethyl-4-hydroxy-1-cyclohexanon-2-ene
C9H14O2
stigmas
(Zarghami and Heinz 1971)
20
3,5,5-trimethyl-1,4-cyclohexadione
C9H14O2
stigmas
(Zarghami and Heinz 1971)
21
3,5,5-trimethyl-1,4-cyclohexadion-2-ene
C9H12O2
stigmas
(Zarghami and Heinz 1971)
22
3,5,5-trimethyl-2-hydroxy-1,4-cyclohexadion-2-ene
C9H12O3
stigmas
(Zarghami and Heinz 1971)
23
(4R)-4-Hydroxy-2,6,6-trimethylcyclohex-1-enecarbaldehyde-O-β-D-gentiobioside
C26H34O11
stigmas
(Li and Wu 2002, Tung and Shoyama 2013)
24
4-Hydroxy-2,6,6-trimethylcyclohex-1-enecarboxylic acid-O-β-D-glucopyranoside
C16H26O8
stigmas
(Straubinger 1997)
25
α-Pinene
C10H16
stigmas
(Mounira 2014)
Diterpenoids
26
Crocetin
C20H24O4
stigmas
petals(Montoro et al., 2012, Christodoulou et al., 2015, Garcia-Rodriguez et al., 2016)
27
crocetin-(β-D-gentiobiosyl)-(β-D-glucosyl)-ester
C38H54O19
stigmas
petals(Pfander and Wittwer 1975, Pfister et al., 1996, Carmona et al., 2006, Montoro et al., 2012)
28
crocetin di-(β-D-glucosyl) ester
C32H44O14
stigmas
petals(Pfander and Wittwer 1975, Pfister et al., 1996, Rios et al., 1996, Montoro et al., 2012)
29
trans-crocetin (β-D-glucosyl) ester
C26H34O9
stigmas
petals(Montoro et al., 2012, García-Rodríguez et al., 2017)
30
cis-crocetin (β-D-glucosyl) ester
C26H34O9
stigmas
petals(Montoro et al., 2012, García-Rodríguez et al., 2017)
31
crocetin-mono-(β-D-gentiobiosyl)-ester
C32H44O14
stigmas
(Pfander and Wittwer 1975, Pfister et al., 1996)
32
crocetin (β-D-triglucoside)-(β-D-gentibiosyl) ester
C50H74O29
stigmas
(Carmona et al., 2006, Zhou et al., 2011)
33
Methyl-crocetin
C21H26O4
stigmas
(Christodoulou et al., 2015)
34
crocetin di(β-D-gentabiosyl) ester
C44H64O24
petals
(Montoro et al., 2012)
35
trans-crocetin di-(β-D-gentiobiosil) ester
C44H64O24
stigmas
petals(Tarantilis et al., 1995, Carmona et al., 2006, Montoro et al., 2012)
36
cis-crocetin di-(β-D-gentiobiosil) ester
C44H64O24
stigmas
petals(Tarantilis et al., 1995, Carmona et al., 2006, Montoro et al., 2012)
37
crocetin β-D-glucopyranoylmethyl ester
C27H36O9
stigmas
(Moraga et al., 2009, Rubio-Moraga et al., 2009, Zhou et al., 2011)
38
Dimethylcrocetin
C22H28O4
stigmas
(Zhou et al., 2011, García-Rodríguez et al., 2017)
39
crocetin (β-D-neopolitanosyl)-(β-D-gentiobiosyl) ester
C50H74O28
stigmas
(Carmona et al., 2006, Carmona et al., 2006)
40
cis-crocetin di-(β-D-gentiobiosyl) ester
C44H64O24
stigmas
petals(Tarantilis et al., 1995, Carmona et al., 2006, Carmona et al., 2006)
41
trans-crocetin (β-D-gentiobiosil) ester
C32H44O14
stigmas
petals(Tarantilis et al., 1995, Carmona et al., 2006, Carmona et al., 2006)
42
cis-crocetin (β-D-gentiobiosil) ester
C32H44O14
stigmas
petals(Tarantilis et al., 1995, Carmona et al., 2006, Carmona et al., 2006)
43
crocetin-1-al 1-O-β-D-gentiobiosyl ester
C31H44O13
stigmas
(Pfander and Schurtenberger 1982, Carmona et al., 2006)
Tetraterpenoids
44
Crocin
C44H64O24
stigmas
petals(Termentzi and Kokkalou 2008, Georgiadou et al., 2012, Christodoulou et al., 2015, Shahi et al., 2016, Wang et al., 2019)
45
Phytoene
C40H64
stigmas
(Wang et al., 2014, Andrade et al., 2016)
46
Zeaxanthin
C40H56O2
stigmas
(Pfander and Schurtenberger 1982, Andrade et al., 2016)
47
Lycopene
C40H56
stigmas
(Wang et al., 2014, Andrade et al., 2016)
48
α-carotene
C40H56
stigmas
(Pfander and Schurtenberger 1982, Pitsikas et al., 2008, Andrade et al., 2016)
49
β-Carotene
C40H56
stigmas
(Pfander and Schurtenberger 1982, Andrade et al., 2016)
50
Tetrahydrolycopene
C40H60
stigmas
(Pfander and Schurtenberger 1982, Andrade et al., 2016)
3.1.1 Sesquiterpenoids and monoterpenoids
One sesquiterpene (1) and 24 monoterpenoids (2–25) were identified from the stigma and petals (Fig. 1 B) represented by safranal (2) and picrocrocin (3), which explain the aroma and bitterness of C. sativus, respectively, while picrocrocin is the precursor of safranal (Li and Wu 2002, Li et al., 2004, Mounira et al., 2015). Cedrol (1), as sesquiterpene alcohol isolated from petal species, is the only sesquiterpene compound that exists only in petals. Among the 24 monoterpenoids, 23 can be found in stigma, and picrocrocin (3) is the only common component of stigma and petals (Table 1).
3.1.2 Diterpenoids
Diterpenoids, one of the most important active components in C. sativus, refer to esters formed by the carboxyl group of crocetin (26) and the hydroxyl group of glucose, gentiobiose, and triglucose. A total of 18 compounds are summarized (Table 1). It’s reported that there are 4 kinds of crocetin compounds containing cis and trans, and the difference between trans (Fig. 1C) and cis (Fig. 1D) lies in the configuration of C-13 on crocetin (26). Tung and Shoyama isolated a special crocetin (26) ester compound, trans-crocetin di- (β-D-gentiobiosil) ester (35), from the stigma of C. sativus in 2012(Tung and Shoyama 2013). Among the 16 kinds of diterpenoids, 7 are commonly found in stigma and petal, and 8 are isolated and identified from the stigma, leaving crocetin di (β-D-gentabiosyl) ester (34) the only one isolated from petals.
3.1.3 Tetraterpenoids
The various fat-soluble carotenoids that have been isolated and identified from the stigma and petals mainly include the following 7 compounds (Fig. 1E), crocin (44), phytoene (45), zeaxanthin (46), lycopene (47), α-carotene (48), β-carotene (49), and tetrahydrolycopene (50). The tetraterpenoids that mainly come from the stigma are represented by crocin (44), which is one of the main active components in the stigma (Maggi et al., 2022), and the only tetraterpenoid shared by stigma and petals (Table 1). Zeaxanthin (46) refers to the precursor compound of crocin (44), picrocrocin (3), and safranal (2) (Pfander and Schurtenberger 1982, Wang et al., 2014, Several 2016).
3.2 Flavonoids
As the second most abundant bioactive in C. sativus, flavonoids perform anti-inflammatory, antioxidant, antibacterial, and cancer chemopreventive activities. They mainly consist of flavonoids and their glycosides, flavonols, and their glycosides (Fig. 2), among which kaempferol and its glycosides abound (Fig. 2A) (Nørbæk et al., 2002, Li et al., 2004). Though distributed in both stigma and petals, the most abundant species are observed in petals (Yu et al., 2008, Moraga et al., 2009, Montoro et al., 2012). Among the 48 compounds (51–93) collected (Table 2), 24 are chemical constituents identified from petals, while 15 are common constituents.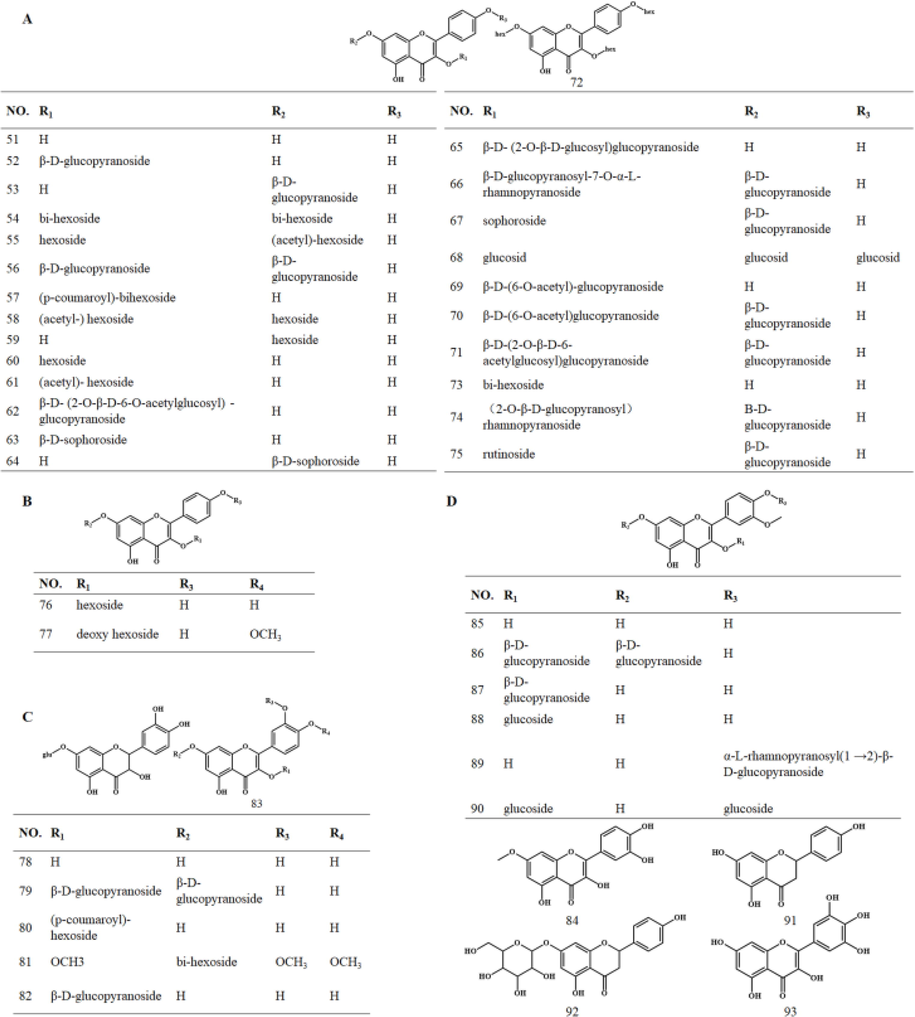
Chemical structures of flavonoids and their glycosides (A: Kaempferol and its glycosides; B: Dihydrokaempferol and its glycosides; C: Quercetin and its glycosides; D: Isorhamnetin and its glycosides).
Species
NO.
Compound
Molecular formula
Part of plant
Reference
Flavonoids
51
Kaempferol
C15H10O6
stigmas
petals(Termentzi and Kokkalou 2008, Montoro et al., 2012, Ahrazem et al., 2015)
52
kaempferol 3-O-β-D-glucopyranoside
C21H20O11
stigmas
petals(Li et al., 2004, Montoro et al., 2012, Tung and Shoyama 2013, Liu et al., 2021)
53
kaempferol 7-O-β-D-glucopyranoside
C21H20O11
stigmas
(Montoro et al., 2012, Liu et al., 2021)
petals
54
kaempferol 7-O-bihexoside-3-O hexoside
C33H40O21
petals
(Termentzi and Kokkalou 2008, Montoro et al., 2012)
55
kaempferol 3-O-hexoside, 7-O-(acetyl)-hexoside
C29H33O17
petals
(Termentzi and Kokkalou 2008, Montoro et al., 2012)
56
kaempferol-3,7-di-O-β-D-glucopyranoside
C27H30O16
petals
(Li et al., 2004, Termentzi and Kokkalou 2008, Montoro et al., 2012)
57
kaempferol 3-O- (p-coumaroyl)-bihexoside
C36H36O18
petals
(Termentzi and Kokkalou 2008, Montoro et al., 2012)
58
kaempferol 3-O-(acetyl-) hexoside-7-O-hexoside
C29H33O17
petals
(Termentzi and Kokkalou 2008, Montoro et al., 2012)
59
kaempferol 7-O-hexoside
C21H20O11
petals
(Termentzi and Kokkalou 2008)
60
kaempferol 3-O-hexoside
C21H20O11
petals
(Termentzi and Kokkalou 2008)
61
kaempferol 3-O-(acetyl)- hexoside
C23H23O12
petals
(Termentzi and Kokkalou 2008, Montoro et al., 2012)
62
keampferol-3-O-β-D-(2-O-β-D-6-O-acetylglucosyl)-glucopyranoside
C29H32O17
stigmas
petals(Li et al., 2004, Montoro et al., 2012)
63
kaempferol-3-O-β-D-sophoroside
C27H30O16
stigmas
petals(Moraga et al., 2009, Moraga et al., 2009, Tarantilis 2016)
64
kaempferol 7-O-β-D-sophoroside
C27H30O16
stigmas
(Tung and Shoyama 2013, Ahrazem et al., 2015, García-Rodríguez et al., 2017)
65
kaempferol-3-O-β-D- (2-O-β-D-glucosyl)glucopyranoside
C27H30O16
petals
(Li et al., 2004, Montoro et al., 2012)
66
kaempferol-3-(O-β-D-glucopyranosyl-7-O-α-L-rhamnopyranoside)-7-O-β-D-glucopyranoside
C33H40O20
petals
(Harborne and Williams 1984)
67
kaempferol 3-O-sophoroside-7-O-β-D-glucopyranoside
C33H40O21
stigmas
(Ahrazem et al., 2015, Christodoulou et al., 2015)
68
kaempferol-3,7,4′-triglucosid
C33H40O21
stigmas
(Ahrazem et al., 2015)
69
kaempferol 3-O-β-D-(6-O-acetyl)-glucopyranoside
C23H22O12
stigmas
(Slimestad et al., 1995, Li et al., 2004)
70
kaempferol-3-O-β-D-(6-O-acetyl)glucopyranoside-7-O-β-D-
glucopyranosideC29H33O17
stigmas
(Veit et al., 1995, Li et al., 2004)
71
kaempferol-3-O-β-D-(2-O-β-D-6-acetylglucosyl)glucopyranoside-7-O-β-D-glucopyranoside
C35H43O22
stigmas
petals(Han et al., 2001, Li et al., 2004, Montoro et al., 2008)
72
kaempferol tetrahexoside
C39H50O26
stigmas
(Carmona et al., 2007, Moraga et al., 2009, Moraga et al., 2009)
73
kaempferol 3-O-bihexoside
C27H30O16
stigmas
petals(Carmona et al., 2007, Termentzi and Kokkalou 2008, Montoro et al., 2012)
74
Kaempferol-3-O-α-L-(2-O-β-D-glucopyranosyl)rhamnopyranoside-7-O-β-D-glucopyranoside
C33H40O20
stigmas
petals(Harborne and Williams 1984, Li et al., 2004)
75
kaempferol 3-O-rutinoside-7-O-β-D-glucopyranoside
C33H40O20
petals
(Harborne and Williams 1984)
76
Dihydrokaempferol 3-O hexoside
C21H22O11
petals
(Baba et al., 2015, Baba et al., 2015)
77
4′ -Methyl ether dihydrokaempferol 3-O-deoxyhexoside
C22H24O10
petals
(Termentzi and Kokkalou 2008)
78
Quercetin
C15H10O7
stigmas
petals(Harborne and Williams 1984, Bate‐Smith 2008, Gismondi 2012)
79
quercetin-3,7-di-O-β-D-glucopyranoside
C27H30O17
petals
(Termentzi and Kokkalou 2008, Montoro et al., 2012)
80
quercetin 3-O-(p-coumaroyl)-hexoside
C30H26O14
petals
(Termentzi and Kokkalou 2008, Montoro et al., 2012)
81
3,3′,4′ - trimethyl ether quercetin 7-O-bihexoside
C30H36O17
petals
(Termentzi and Kokkalou 2008, Montoro et al., 2012)
82
quercetin-3-O-β-D-glucopyranoside
C21H20O12
petals
(Montoro et al., 2008, Montoro et al., 2012)
83
Dihydroquercetin 7-glucoside
C21H22O12
stigmas
petals(Montoro et al., 2008, Baba et al., 2015, Baba et al., 2015)
84
Rhamnetin
C16H12O7
petals
(Termentzi and Kokkalou 2008, Montoro et al., 2012)
85
Isoramnetin
C16H12O7
petals
(Montoro et al., 2012)
86
isorhamnetin-3,7-di-O-β-D-glucopyranoside
C28H32O17
petals
(Montoro et al., 2012)
87
isorhamnetin-3-O-β-D-glucopyranoside
C22H22O12
stigmas
petals(Montoro et al., 2008, Baba et al., 2015, Baba et al., 2015)
88
isorhamnetin-3-O-glucoside
C22H22O12
stigmas
petals(Li and Wu 2002, Montoro et al., 2012)
89
isorhamnetin-4′-O-α-L-rhamnopyranosyl(1 → 2)-β-D-glucopyranoside
C28H32O16
stigmas
(Song and Xu 1991)
90
isorhamnetin-3,4′-diglucoside
C28H32O17
stigmas
petals(Li and Wu 2002, Montoro et al., 2008)
91
Naringenin
C15H12O5
petals
(Termentzi and Kokkalou 2008)
92
Narinrenin 7-O-hexoside
C21H22O10
petals
(Montoro et al., 2012)
93
Myricetin
C15H10O8
stigmas
(Gismondi 2012)
3.3 Alkaloids
Alkaloid compounds are also shared by stigma and petals (Fig. 3A). Li et al. isolated and identified seven alkaloids (94–100) from both parts (Li and Wu 2002), among which 5-methyl uracil (94), pyridin-3-ylmethanol (95), and uracil (96) are only observed in stigma, while the other 4 (97–100) are shared (Table 3). As a result, no alkaloid is unique to petals.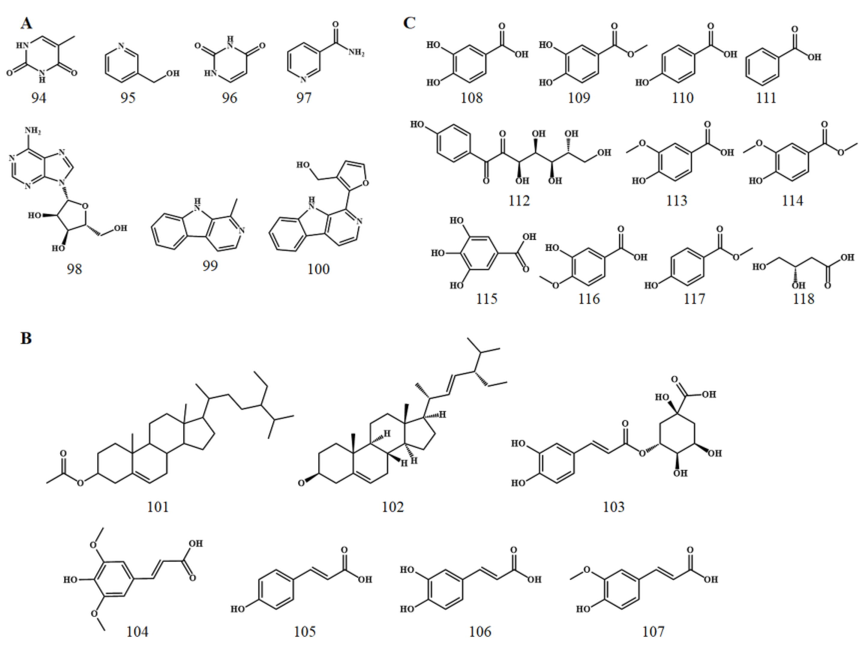
Chemical structures of alkaloids (A), steroids and phenylpropanoids (B), and organic acids (C).
Species
NO.
Compound
Molecular formula
Part of plant
Reference
Alkaloids
94
5-methyluracil
C5H6N2O2
stigmas
(Li and Wu 2002)
95
pyridin-3-ylmethanol
C6H7NO
stigmas
(Li and Wu 2002)
96
Uracil
C4H4N2O2
stigmas
(Li and Wu 2002)
97
Nicotinamide
C6H6N2O
stigmas
petals(Li and Wu 2002, Li et al., 2004)
98
Adenosine
C10H13N5O4
stigmas
petals(Li et al., 2004, Termentzi and Kokkalou 2008)
99
Harman
C12H10N2
stigmas
petals(Li and Wu 2002, Li et al., 2004)
100
Tribulusterine
C16H12N2O2
stigmas
petals(Li and Wu 2002, Li et al., 2004, Termentzi and Kokkalou 2008)
Sitosterol
101
β-Sitosterol
C30H52O
stigmas
(Zheng et al., 2011, Feizy and Reyhani 2016)
102
Stigmasterol
C29H50O
stigmas
(Zheng et al., 2011, Feizy and Reyhani 2016)
Phenylpropanoids
103
Chlorogenic acid
C16H18O9
stigmas
(Gismondi 2012)
104
Sinapic acid
C11H12O5
petals
(Termentzi and Kokkalou 2008, Montoro et al., 2012)
105
p-coumaric acid
C9H8O3
stigmas
(Li et al., 2004)
106
Caffeic acid
C9H8O4
stigmas
(Gismondi 2012, Loizzo et al., 2016)
107
Ferulic acid
C10H10O4
stigmas
(Loizzo et al., 2016)
Organic acid
108
Protocatechuic acid
C7H6O4
stigmas
petals(Li et al., 2004)
109
Protocatechuic acid methyl ester
C8H8O4
stigmas
petals(Li and Wu 2002, Li et al., 2004)
110
4-hydroxybenzoic acid
C7H6O3
stigmas
petals(Li et al., 2004)
111
benzoic acid
C7H6O2
stigmas
(Li and Wu 2002)
112
1-O-(4-hydroxybenzoyl)-β -D-glucose
C13H16O8
stigmas
(Li and Wu 2002)
113
vanillic acid
C8H8O4
stigmas
petals(Li et al., 2004)
114
methylvanillate
C9H10O4
stigmas
(Li et al., 2004)
115
gallic acid
C7H6O5
stigmas
(Karimi et al., 2010, Gismondi 2012)
116
3-Hydroxy-4-methoxybenzoic acid
C8H8O4
petals
(Li et al., 2004)
117
methylparaben
C8H8O3
stigmas
petals(Li and Wu 2002, Li et al., 2004)
118
(S)-3,4-dihydroxybutyric acid
C4H8O4
petals
(Mounira 2014)
Amino acid
119
Asparagine
C4H7NO4
stigmas
petals(Lv et al., 2021)
120
Threonine
C4H9NO3
stigmas
petals(Lv et al., 2021)
121
Serine
C3H7NO3
stigmas
petals(Lv et al., 2021)
122
Glutamicacid
C5H9NO4
stigmas
petals(Lv et al., 2021)
123
Glycine
C2H5NO2
stigmas
petals(Lv et al., 2021)
124
Alanine
C3H7NO2
stigmas
petals(Lv et al., 2021)
125
Valine
C5H11NO2
stigmas
petals(Lv et al., 2021)
126
Methionine
C5H11NO2S
stigmas
petals(Lv et al., 2021)
127
Isoleucine
C6H13NO2
stigmas
petals(Lv et al., 2021)
128
Leucine
C6H13NO2
stigmas
petals(Lv et al., 2021)
129
Tyrosine
C9H11NO3
stigmas
petals(Lv et al., 2021)
130
Phenylalanine
C9H11NO2
stigmas
petals(Lv et al., 2021)
131
Lysine
C6H14N2O2
stigmas
petals(Lv et al., 2021)
132
Histidine
C6H9N3O2
stigmas
petals(Lv et al., 2021)
133
Arginine
C6H14N4O2
stigmas
petals(Lv et al., 2021)
134
Proline
C5H9NO2
stigmas
petals(Lv et al., 2021)
3.4 Steroids and phenylpropanoids
There exist fewer types of steroids in stigma and petals. Specifically, only two phytosterols, β-sitosterol (1 0 1) and stigmasterol (1 0 2), were isolated and identified from stigma (Table 3), while no steroids in petals were mentioned.
Phenylpropanoids refer to compounds in which a phenyl group is attached to three carbons. A total of four phenylpropionic acid compounds were isolated from stigma and petals, namely chlorogenic acid (1 0 3), sinapic acid (1 0 4), p-coumaric acid (1 0 5), caffeic acid (1 0 6), and ferulic acid (1 0 7). Among them, the first is an ester formed by the condensation of caffeic acid (1 0 6) and quinic acid (Fig. 3B). Such compounds are mainly found in stigma, and only sinapic acid is obtained from petals, leaving no common chemical composition.
3.5 Others
Except for the above-mentioned material bases, the stigma and petals also include essential minerals such as magnesium, iron, manganese, copper, calcium, and zinc, high levels of amino acids (119–134) including aspartic acid (1 1 9), alanine (1 2 4), and proline (1 3 4), certain organic acids (108–118) (Fig. 3C), and protein. Accordingly, the protein content of C. sativus is as high as about 20% (Mykhailenko et al., 2019).
The stigma and petals are rich in amino acids, and 16 amino acids are isolated and identified, all of which are shared (Table 3). The hydrogen on the benzene ring of organic acids is mainly replaced by –OH, –COOH, –OCH3, and other groups, such as protocatechuic acid (1 0 8), benzoic acid (1 1 1), methylparaben (1 1 4), gallic acid (1 1 5), etc (Song et al., 2020). In some organic acid molecules, –OH, –COOH, and glycosyl are further condensed into glycosides or esters (Liu et al., 2022), such as protocatechuic acid methyl ester, and 1-O-(4-hydroxybenzoyl)-β-D-glucose. Such compounds are commonly found in stigma and petals (Mykhailenko et al., 2019). Of the 11 kinds of organic acids collected, 5 are common components, 4, that is, benzoic acid (1 1 1), 1-O-(4-hydroxybenzoyl)-β-D-glucose (1 1 2), methylvanillate (1 1 4), and Gallic acid (1 1 5), are only observed in stigma, while the remaining 2, 3-Hydroxy-4-methoxybenzoic acid (1 1 6) and (S)-3,4-Dihydroxybutyric acid (1 1 7), are isolated and identified from petals.
4 Pharmacological activity
Given their shared chemical composition, the stigma and petals have similar pharmacological effects. In traditional Chinese medicine, stigma is frequently used to treat amenorrhea, postpartum stasis, depression, palpitations, and madness. Pharmacological studies clarified safranal (2), picrocrocin (3), crocetin (26), and crocin (44) as the primary material basis for pharmacological activity and confirmed the anti-psychiatric, neurodegeneration, anti-atherosclerosis, and anti-tumor effects of stigma (Abu-Izneid et al., 2022) (Fig. 4).Studies involving petals demonstrated the pharmacological activities of petal extracts in the nervous system, cardiovascular system, and antitumor role, which calls attention to their application as a potential drug source for illnesses such as neurodegenerative diseases, ischemia–reperfusion injury, and cancer (Khazdair et al., 2015).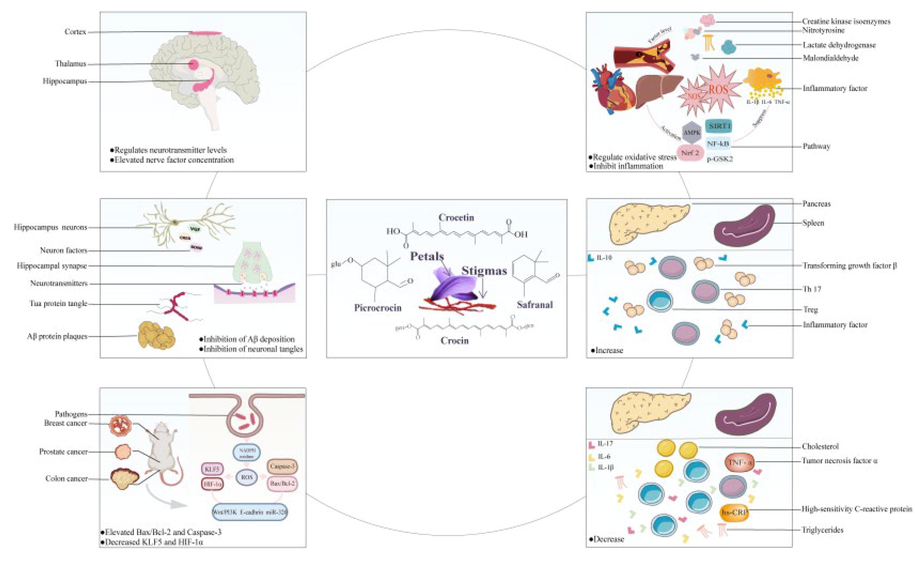
Pharmacological activity of stigma and petals and their active ingredients.
4.1 Mental illness
C. sativus stigma reduces depression symptoms by regulating neurotransmitter levels and promoting neuroplasticity. For example, using the isolated depressed rat model, acute intraperitoneal injection of 50 mg/kg and oral administration of 200 mg/kg of stigma aqueous extract significantly increased the swimming time and climbing times and their preference for sucrose in comparison to the control group, confirming the antidepressant effects of stigma extract (Orio et al., 2020). The C. sativus stigma ethanol extract (0.2–0.8 g/kg) and its constituents, safranal (2) (0.15–0.5 mg/kg) and crocin (44) (50–600 mg/kg) increased swimming time in depressed mice, during the forced swim test (FST) of the depression mice model, confirming that stigma extract and its components had an antidepressant effect by activating serotonergic, noradrenergic and dopaminergic systems. These results indicate that stigma plays an anti-depression role by regulating neurotransmitter levels (Hosseinzadeh et al., 2004). Neuroplasticity is the ability of the nervous system to adapt to internal and external stimuli and adapt to future stimuli. The neuroplasticity function of the hippocampus and prefrontal cortex, which are involved in emotion regulation, is impaired in depressed patients, and this is reflected in the decline of neurotrophic factors and other growth factors (Jones 2016). Roustazade et al. found that both doses of stigma reduced anxiety in rats in the stress group when they orally administered low-dose (30 mg/kg) and high-dose (60 mg/kg) stigma aqueous extracts to rats, respectively. Its mechanism is related to the expression of brain-derived neurotrophic factor (BDNF) and tumor necrosis factor-α (TNF-α) genes (Roustazade et al., 2022). Stigma aqueous extracts (40 and 80 mg/kg/ day) were intraperitoneally injected into depressed rats for 21 days. According to a Western blot analysis of the levels of the proteins cAMP response element binding (CREB), nerve growth factor inducible (VGF), and BDNF in the rats cerebellum, 80 mg/kg/day can significantly increase the expression of CREB protein in the rats cerebellum (Asrari et al., 2018). Vahdati et al. studied crocin (44), an active ingredient of C. sativus, and found that the immotility time of rats was shortened on the 21st day following intraperitoneal injection of crocin (44) (12.5, 25 and 50 mg/kg). According to results from Western blotting and quantitative reverse transcription-polymerase chain reaction (qRT-PCR), crocin (44) increased VGF levels in a dose-dependent manner at all does. At doses of 25 and 50 mg/kg, CREB and BDNF levels were significantly increased in a dose-dependent manner. At a dose of 12.5 mg/kg, BDNF transcription level significantly increased. The results show that crocin (44), which has an antidepressant effect by enhancing neuroplasticity, can increase the concentration of CREB, BDNF and VGF neurofactors in the hippocampus (Vahdati Hassani et al., 2014).
There is extensive clinical research on the study of C. sativus stigma to treat depression. In a randomized, double-blind, placebo-controlled trial, Lopresti et al. gave stigma extract (14 mg bid) or placebo to 80 adolescents with mild to moderate depression. The average internalization of the 17% total score on the Revised Child Anxiety and Depression Scale (RCADS) was 33% lower in the treatment group than in the placebo group. Stigma extract showed mild to moderate improvement of depressive symptoms in adolescents (Lopresti et al., 2018). In a meta-analysis of 12 studies on stigma's role in the treatment of depression, Dai et al. found no appreciable distinction between the effects of stigma and antidepressants (Dai et al., 2020). In a randomized, double-blind, placebo-controlled trial for major depression, 123 patients with major depressive disorder were randomly assigned to receive placebo, low-dose curcumin extract (250 mg b.i.d.), high-dose curcumin extract (500 mg b.i.d.), or combined low-dose curcumin extract plus stigma (15 mg b.i.d.). After 12 weeks, the combination therapy had a significantly greater improvement effect than the placebo, but there was no difference between the treatment groups receiving additional active drugs, and the combination therapy can effectively reduce the depressive and anxiolytic symptoms in patients with major depressive disorder (Lopresti and Drummond 2017). The combination of stigma and other antidepressants can improve severe depression. Many meta-analyses have concluded that C. sativus helps improve depressive symptoms with minimal side effects (Lu et al., 2021, Musazadeh et al., 2022, Zhang et al., 2022). Table 4 displays the clinical studies on anti-depression and anxiolytic effects of C. sativus stigma in recent 5 years. In conclusion, one of the hotspots in clinical research is the use of C. sativus to alleviate and treat mental illness, which may open up a new field for C. sativus to prevent negative emotions or as an adjuvant therapy for depression.
NO.
Administration
group (dose)
Control group
Subjects
Participate in case/Complete case
Indicators
Conclusion
Reference
1
Stigma capsule
(30 mg/d)Placebo
Overweight women with mild to moderate depression.
73/52
BDI-II
WeightStigma capsules as a safe over-the-counter supplement, it may help reduce the symptoms of depression in patients who experience mild or moderate depression and are overweight.
(Akhondzadeh et al., 2020)
2
Stigma capsule
(30 mg/d)Citalopram
(40 mg/d)Major depressive disorder accompanied by anxious distress
66/60
HDRS
HARSStigma as a potential efficacious and tolerable treatment for major depressive disorder with anxious distress.
(Ghajar et al., 2017)
3
Stigma alcohol extract
(30 mg/d)Placebo
Type 2 diabetes patients with mild to moderate depression and anxiety
54/54
HDRS
HARS
PSQI
SLSThe results indicate the beneficial effect of Stigma on the mild to moderate comorbid depression-anxiety in type 2 diabetic patients.
(Milajerdi et al., 2018)
4
Stigma tablet
(28 mg/d、22 mg/d)Placebo
Healthy adults
128/128
POM
PANAS
DASS
PSQIStigma increased mood, reduced anxiety and managed stress without side effects, offering a natural alternative to standard treatments.
(Kell et al., 2017)
5
Stigma tablet
(14 mg bid)Placebo
Adults with persistent depression who are taking medication antidepressants
160/139
MADRS
The experimental results are insufficient to support the clinical benefit of stigma as an adjunctive treatment for adults with persistent depressive symptoms after antidepressant treatment, and further research is needed to clarify.
(Lopresti et al., 2019)
6
Stigma extract tablet
(14 mg bid)Placebo
Youth with mild to moderate anxiety and depression
80/68
RCADS
Mild to moderate symptoms of depression and anxiety improved in adolescent patients after 8 weeks of treatment with stigma extract tablets.
(Lopresti et al., 2018)
7
Combined low-dose curcumin extract plus stigma
(15 mg bid)Placebo
Major depression
123/123
IDS-SR
STAICurcumin/ stigma combination therapy is effective in reducing depression and anxiety symptoms in patients with major depressive disorder.
(Lopresti and Drummond 2017)
8
Combined Rhodiola tablet (154 mg) plus stigma extract tablet (15 mg)
/
Mild-moderate depression
45/45
HDRS
As a primary care study, rhodiola and stigma testing may be useful in managing mild to moderate depression and improving symptoms of depression and anxiety.
(Bangratz et al., 2018)
9
Stigma (15 mg capsule) or fluoxetine (20 mg capsule)
/
Mild to moderate postpartum depression
29/29
HDRS
Preliminary studies show that stigma is a safe alternative medication for improving depressive symptoms of postpartum depression.
(Kashani et al., 2017)
10
Luoxetine (20 mg/day)and stigma (30 mg /day)
Placebo
Major depression
40/40
HCY levels
BDIStigma has beneficial effects on depression and homocysteine level in patients with major depression.
(Jelodar et al., 2018)
11
Crocin tablet
(30 mg)Placebo
Depression in subjects with metabolic syndrome
34/33
BDI
Crocin reduced depressive symptoms in subjects and this effect was independent of its effect on the serum pro-oxidant/anti-oxidant balance.
(Jelodar et al., 2018)
12
Stigma tablet
(15 mg/Bid)Placebo
New mothers with mild to moderate postpartum depression
60/60
BDI-II
When administered to nursing mothers for mild postpartum depression, stigma had a more significant effect on depressive symptoms than placebo.
(Tabeshpour et al., 2017)
13
Stigma capsule
(30 mg/d)Placebo
Major depressive disorder associated with post-menopausal hot flashes
60/56
HFRDIS
HDRSStigma is a safe and effective treatment in improving hot flashes and depressive symptoms in post-menopausal healthy women, and with fewer adverse effects, may offer a non-hormonal and alternative herbal medicine option in treatment of women with hot flashes.
(Kashani et al., 2018)
14
Stigma capsule
(60 mg/d)Sertraline
(100 mg/d)older people with major depressive
50/50
HDRS
Both stigma and sertraline have the potential to significantly decrease symptoms of depression.
(Ahmadpanah et al., 2019)
15
Crocin tablet
(30 mg/d)Placebo
Major depression
40/40
BDI
GHQ
MDQCrocin can be used to treat patients with major depression.
(Talaei et al., 2015)
16
Stigma capsule
(30 mg/d)Placebo
Healthy adults
73/56
POMS
Stigma extract appears to improve subclinical depressive symptoms in healthy individuals and may contribute to increased resilience against the development of stress-related psychiatric disorders.
(Jackson et al., 2020)
17
Stigma extract
(15.5 mg/d)Placebo
Mild to moderate sleep disorder associated with anxiety
66/66
LSEQ
PSQIThe results suggest that a saffron extract could be a natural and safe nutritional strategy to improve sleep duration and quality.
(Pachikian et al., 2021)
18
Stigma capsule
(100 mg/d)Placebo
Adult patients with anxiety and depression
60/54
BDI
BAIStigma appears to have a significant impact in the treatment of anxiety and depression disorder. Side effects were rare.
(Mazidi et al., 2016)
4.2 Alzheimer disease
Alzheimer disease (AD) can be treat with C. sativus stigma extract and its active ingredients by improving amyloid β-protein (Aβ) and neurofibrillary tangles (Finley and Gao 2017, Ghosh et al., 2020, Koulakiotis et al., 2020). For example, stigma aqueous alcohol extract showed anti-AD effects in 5XFAD mice by upregulating synaptic proteins and attenuating Aβ pathologically related neuroinflammation (Batarseh et al., 2017). Crocetin (26) and crocin (44) are terpenoid active components in C. sativus with antioxidant activity. After one month of oral crocetin (26) (10 mg/kg/day) treatment for wild-type male C57BL/6AD mice, Wani et al. found that there was a significant 45% decrease in total Aβ levels in the mouse brains. It was found that in the crocetin (26) (25 μM) treatment group, the expression of STK11/LKB1 and CAMKK2/ CAMKβ kinase was increased in N9 microglia and primary neuronal cells, and the red fluorescence of fluorescently labeled Aβ42-Hilyte Fluor 555 was significantly decreased. These results indicated that the increase in Aβ clearance rate was promoted by the activation of autophagy. To prevent and treat AD, crocetin (26) induces autophagy, promoting the clearance of Aβ (Wani et al., 2021). Hadipour et al. found that intraperitoneal injection of crocin (44) (30 mg/kg) into AD rat model preserved the number of viable cells in hippocampal pyramidal neurons and granule cells in DG area in CA3 of AD rats and reversed the reduction of axons, spines and dendritic dendritic structures (Hadipour et al., 2021). Reducing neuronal apoptosis appears to improves synaptic loss and neuronal death in AD rats, which in turn improve their capacity for learning and memory (Lin et al., 2019). Stigma extract and its active components improve protein plaque accumulation and Aβ plaque clearance (Wani et al., 2021), showing a promising future for the treatment of AD (Wang et al., 2019).
Clinical research has demonstrated that C. sativus is effective in treating AD. In a randomized, double-blind, controlled trial, 54 patients with mild to moderate AD were given either 30 mg/day of C. sativus stigma or 10 mg/day of donepezil. After 22 weeks, the stigma capsule group experienced fewer adverse events than the control group and similar effects to donepezil in mild-to-moderate AD. There is preliminary evidence that C. sativus extract can help patients with mild to moderate AD (Akhondzadeh et al., 2010). In a double-blind, parallel study, 68 patients with moderate-to-severe AD were divided two group and given either memantine (20 mg/day) or C. sativus extract (30 mg/day) for a 12-months period. Comparable effectiveness to memantine was seen in the C. sativus capsule group (Farokhnia et al., 2014). In another randomized, placebo-controlled trial, 46 mild-to-moderate patients were given a daily 30mgC. sativus capsule or a placebo. After 16 weeks, C. sativus produced cognitive effects that were superior to placebo while having no negative side. The results suggest that C. sativus is, at least temporarily, a safe and effective treatment for mild to moderate AD (Akhondzadeh et al., 2010). C. sativus has a therapeutic effect on mild cognitive impairment and cognitive decline in AD patients and frequently has a synergistic effect with other nutritional supplements, suggesting that C. sativus can be used as a natural source of medicine for the prevention and treatment of AD (Tsolaki et al., 2016, Cicero et al., 2017).
4.3 Cardiovascular diseases
Similar to AD, the incidence of cardiovascular disease is age-related, manifested as ischemia–reperfusion injury and atherosclerosis. Reactive Oxygen-nitrogen Species (RONS) are a by-product of cellular metabolic redox reactions which must be controlled in order to reduce tissue damage and treating cardiovascular diseases (Fig. 5) (Speer and McKune 2021). In C. sativus extract, safranal (2), crocetin (26), and crocin (44) demonstrate the positive effects of oxidative stress and have potential to prevent age-related diseases (Su et al., 2021).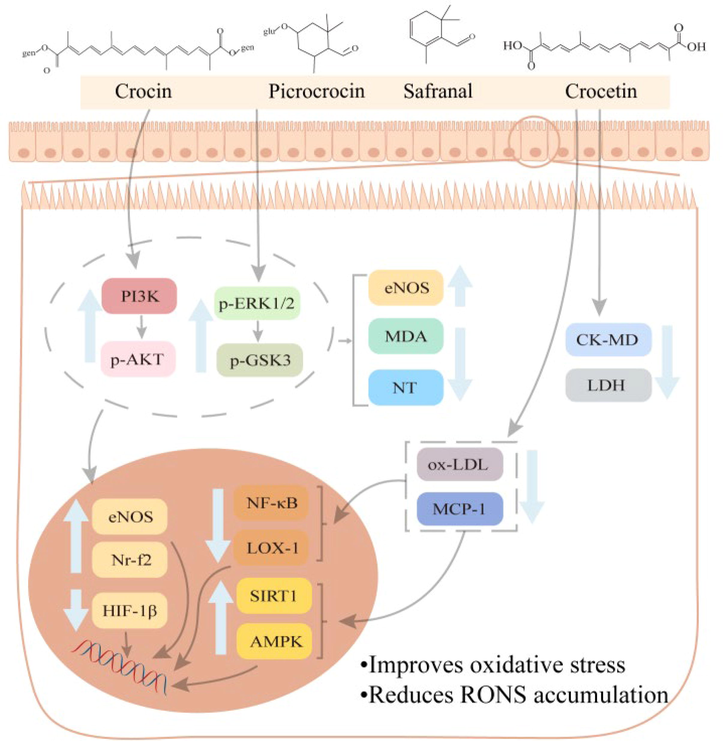
Anti-cardiovascular mechanism of Crocus sativus L. and its active components.
C. sativus plays a protective role in ischemia–reperfusion injury by regulating oxidative stress. For example, C. sativus aqueous extract (60 mg/kg/d) can decrease the size of myocardial infarctions, lower levels of malondialdehyde (MDA) and nitrotyrosine (NT), and increase levels of eNOS, p-Akt, P-ERK1/2 and P-GSK3 in Wild Type and ApoE mice with myocardial ischemia–reperfusion injury models. The Akt/eNOS/ERK1/2/GSK3-β and Nrf2 pathways are activated by stigma aqueous extracts to provide antioxidant protection against myocardial ischemia–reperfusion injury (Efentakis et al., 2017). Arrhythmia is brought on by excessive ROS production and an excess of calcium in the early stages of reperfusion. After intragastric administration of safranal (2) (0.025 mL/kg/d), the activities of creatine kinase (CK-MB), lactate dehydrogenase (LDH) and the oxidative stress factor MDA in serum decreased, while the activity of superoxide dismutase (SOD) increased in the rat model of myocardial ischemia injury. Its mechanism of action may involve inhibiting ROS accumulation, myocardial contractility, and calcium influx, according to speculations (Xue et al., 2020). In another study, administration of an ethanol extract of stigma significantly increased antioxidant enzyme content and decreased ROS concentration in rats with hepatic ischemia–reperfusion injury. By regulating protein oxidation, the ethanol extract of stigma can lessen ischemia and perfusion injury, which aids in the development of new therapeutic strategies for diseases caused by ROS (Pan et al., 2013).
C. sativus stigma extract and its active components can improve atherosclerosis by regulating ROS levels and improving inflammation (Rahman et al., 2016, Li et al., 2018). For example, gavage stigma aqueous extract (60, 90 mg/kg/day) for 4 weeks improved aortic stenosis and triglyceride levels and significantly decreased the proinflammatory factor il-6 in the dosing group in a high-fat diet apolipoprotein E (ApoE) mouse model. Antiatherogenic effects of C. sativus were dose-dependent and probably resulted from improvements in inflammatory mechanisms (Christodoulou et al., 2018). One of the pharmacologically active components of C. sativus, crocin (44), can regulate eNOS levels and is beneficial for atherosclerosis (Hatziagapiou and Lambrou 2018). Crocin (44) (100 mg/kg/day) was gavaged to atherosclerotic apolipoprotein E knockout (ApoE-/-) mice. In the mice, eNOS expression significantly increased after 16 weeks, while HIF −1α expression significantly reduced. Crocin (44) may reduce atherosclerosis in ApoE-/- mice by modifying eNOS and HIF-1α expression (Makaritsis et al., 2022). The levels of low-density cholesterol (ox-LDL) and monocyte chemoattractant protein 1 (MCP-1) were lower in crocin(44) (30 mg/d) group than in the placebo group after 8 weeks of treatment in a clinical trial. Crocin (44) may have beneficial effects in atherosclerotic patients by modulating atherogenic genes (increasing SIRT1 and AMPK gene expression and decreasing LOX1 and NF-κB expression (Abedimanesh et al., 2020). In another randomized, double-blind, placebo-controlled clinical trial, 63 atherosclerotic patients were randomly assigned to receive C. sativus stigma capsule (100 mg/day) or placebo, the results showed significantly greater Macnew scores (<0.001), physical domains (=0.025), and social domains (<0.001) after receiving the stigma capsule (Ahmadikhatir et al., 2022). C. sativus, which is a potential treatment strategy for coronary atherosclerosis, can effectively improve the quality of life of patients with atherosclerosis.
4.4 Anti-diabetic
The active components in C. sativus stigma extract lower blood sugar by reducing insulin resistance, are anti-inflammatory, and anti-oxidation, and can help with hypertension, hyperlipidemia, and other complications associated with diabetes (Jiang and Zhu 2019, Lingli and Wenfang 2022). In rats with streptozotocin-induced diabetes, Samarghandian et al. administrated stigma aqueous extract (20, 40, and 80 mg/kg),they found that the rats gained weight and had lower serum levels of TNF-α blood glucose, cholesterol, and triglyceride. The results indicated that stigma extract could lower blood glucose and hyperlipidemia risks and that it could help treat chemoinduced diabetes mellitus and its complications (Samarghandian et al., 2014). Jiang et al. administered stigma aqueous extract (100 mg/kg) intravenously to diabetic mice by streptozotocin. Water intake, fasting blood glucose, and the area under the stigma aqueous extract curve were all significantly lower than in the homologous diabetes group. Total cholesterol was also lower, but high-density lipoprotein cholesterol and insulin were both significantly higher (Jiang et al., 2018). In streptozotocin induced diabetic mice, C. sativus aqueous extract can reduce blood glucose and blood lipid levels, suggesting that C. sativus may be useful in the treatment of diabetes. In another study, C57BL/6 mice with streptozocin-induced autoimmune diabetes received an oral administration of an aqueous alcohol extract of stigma (500 mg/kg) for 3 weeks. The incidence of hypoglycemia and proinflammatory interleukin-17 (il-17) production were decreased, and the production of the anti-inflammatory factors molecules IL-10 and transforming growth factor-β (TGF-β) was increased in pancreatic cell populations. It has been provided that C. sativus stigma extract has a hypoglycemic effect and can be used to treat diabetes (Faridi et al., 2019). Crocin (44), picrocrocin (3), crocetin (26), safranal (2), and other compounds that are the primary anti-diabetic active ingredients in C. sativus, of which crocin (44) is the representative (Delkhosh-Kasmaie et al., 2018, Yaribeygi et al., 2019, Sepahi et al., 2022). In diabetes rats induced by nicotinamide and streptozotocin, the levels of aspartate aminotransferase (AST), superoxide dismutase (SOD), glutathione (GSH), creatinine, cholesterol and triglyceride were measured in kidney and blood. After diabetes is induced, treatment with 50 mg/kg crocin(44) helps to restore blood glucose, reduce blood lipid, cholesterol and other parameters, and alleviate some complications of diabetes (Margaritis et al., 2020).
C. sativus has been shown to be effective in treating type 2 diabetes by recent clinical evidence. In a triple-blind trial, 54 patients with type 2 diabetes were randomly assigned to receive either C. sativus stigma extract capsules (15 mg/kg) or placebo. After 8 weeks, the stigma group's fasting blood glucose levels were significantly lower than those of the placebo group. Aqueous alcohol extract of C. sativus improves glycemic control in T2D patients by reducing serum levels of LDL and HDL, total cholesterol, and triglycerides (TG), as well as glycosylated hemoglobin (HbA1c) (Milajerdi et al., 2018). In another clinical trial, the mean differences in fasting blood glucose (FPG), cholesterol, LDL C, and LDL/HDL ratio were significantly lower in 64 patients with type 2 diabetes who were randomized to receive C. sativus capsules (15 mg/day) or placebo after 3 months (Moravej Aleali et al., 2019). There is no lack of clinical use of C. sativus as a supplement in the treatment of type 2 diabetes. For example, in a clinical trial of C. sativus as a supplement, 60 obese patients with type 2 diabetes received C. sativus (100 mg/d), training (resistance + aerobic), and placebo. Patients with type 2 diabetes mellitus who lost weight after training experienced significant reductions in insulin, high activity cytokine (TNF-α), C-reactive protein (hs-CRP) and inflammatory factors such as IL-6, IL-1β and IL-10 (Hooshmand Moghadam et al., 2022). Supplemental C. sativus has an adjuvant effect on diabetes and a strong anti-inflammatory effect.
4.5 Anticancer effect
It’s been proved that safranal (2), picrocrocin (3), crocetin (26), and crocin (44) in stigma exhibit obvious anti-tumour effect, particularly the therapeutic effect on breast cancer, colon cancer, gastric cancer, uterine cancer, lung cancer, cervical cancer and other cancers (Liu and Mao 2014, Bhandari 2015, Colapietro et al., 2019). The possible anticancer mechanisms of C. sativus include promoting cancer cell apoptosis, inhibiting cell proliferation, migration, and invasion to play preventive and therapeutic roles (Fig. 6) (Hu et al., 2014, Naeimi et al., 2019). Considering antitumor activity and toxicity, crocin (44) has become one of the most promising anticancer drugs among the active components of C. sativus (Veisi et al., 2020).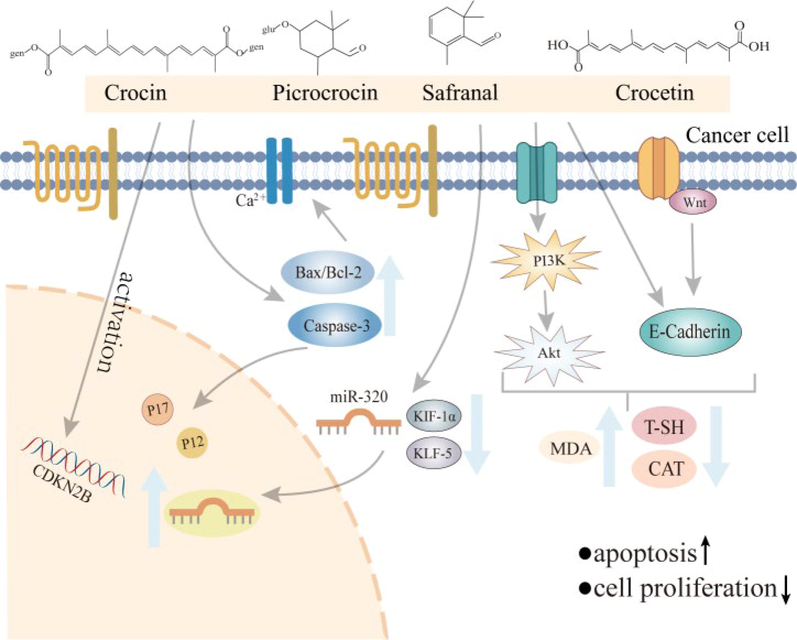
Anticancer mechanism of Crocus sativus L. and its active components.
Research in vitro and in vivo studies have shown that the active components in C. sativus have anti-cancer effects by promoting apoptosis and inhibiting cell proliferation, migration, and invasion. Oral administration of C. sativus stigma extract (200 mg/kg) extended the lives of mice with endoperitoneal metastases of Sarcoma-180 (S-180), Ehrlich AScites Carcinoma (EAC), and Dalton's ASCITES (DLA), by 111.0%, 83.5%, and 112.5%, respectively. Hematological and biochemical parameters in toxicology studies were within normal ranges, indicating the potential value of C. sativus as an anticancer agent (Nair et al., 1991). Bax/Bcl-2 and caspase-3 levels in stigma aqueous extract-treated mice were significantly higher than those in other controls in the 4 T1 cell line-induced breast cancer experiment in female mice, indicating that stigma extract may mediate cancer cell apoptosis by rasing Bax/Bcl-2 and caspase-3 levels in breast cancer mice (Ahmadabadi et al., 2021). At different concentrations (100, 200, 400, 600, 800, 1600 and 3200 μg/mL), stigma aqueous extract was applied to human prostate cancer cells (PC3). The 1600 μg/mL group manifested severe granulation, a reduction in cell volume, and partial cell death after 72 h. Inhibited human prostate cells by stigma aqueous extract was dose-dependent (Ahmadnia et al., 2020). In colorectal cancer (CRC) rat experiments, long-term treatment with crocin (44) (400 mg/kg) improved the survival rate of female colon cancer rats without causeing significant toxic effects (García-Olmo et al., 1999). Amerizadeh et al. discussed the effect of crocin (44) (200 ppm in drinking water) on a mouse model of colon cancer (Amerizadeh et al., 2018). The results reveal that total thiol (T-SH) and catalase (CAT) levels were decreased, and MDA activity was increased, while the size and number of colon tumors were smaller and fewer in treated mice than in the control group. By using flow cytometry, the inhibition of crocin (44) on the proliferation of HCT116 cells was identified, and the expression levels of several genes were evaluated. Both the release of the P-STAT3/STAT3 ratio and cytokine were decreased. Crocin (44) has antitumor activity in CRC and may play a role in reducing inflammation in mucosal ulcers and highly dysplastic crypts. Crocin (44) inhibits the growth and invasion of CRC cells by regulating the Wnt pathway and E-cadherin, as well as colonic inflammation (Wang et al., 2020). Crocetin (26) can induce apoptosis in gastric cancer cells (He et al., 2014, Naeimi et al., 2019). The mechanism of action of crocetin (26) on gastric cancer cells demonstrated that crocetin (2 or 3 μm) inhibited the migration, invasion and epithelial-mesenchymal transition of gastric cancer cells, and the expression of KLF5 and HIF-1α decreased, while Mir-320 expression increased. Crocetin (26) prevents gastric cancer cells from migrating, invading, and undergoing the epithelial-mesenchymal transition, which is mediated by Wnt/PI3K, Mir-320 / KLF5 / HIF-1α (Khodir et al., 2019, Zhou et al., 2019). Liu et al. found that C. sativus extract and its active components protect against cancer organ damage by inhibiting oxidative stress (Liu et al., 2022), and activating the Wnt/PI3K and miR-320 / KLF5 / HIF-1α signalling pathways (Amerizadeh et al., 2018). The cell inhibition of cancer cells is more active than that of non-cancer cells, which may be regarded as a successful anticancer agent with broad clinical implications for cancer treatment (Hire et al., 2017).
4.6 Other activities
In addition to the pharmacology listed above, C. sativus also have antiasthmatic, memory-enhancing and diuretic activities. In a clinical trial, 80 patients with mild to severe allergic asthma were randomized to receive either C. sativus capsules or a placebo. When compared to the placebo group, the C. sativus group's clinical sympotoms and asthma severity were significantly reduced (Zilaee et al., 2019). Many studies have shown that C. sativus has the effect of enhancing memory. It has been demonstrated that morphine-induced memory deficits in mice can be avoided by using C. sativus aqueous extracts (Naghibi et al., 2012). C. sativus extract can improve learning and memory impairment brought on by AD and cerebral ischemia–reperfusion injury (Ghadrdoost et al., 2011). According to Shariatifar N et al., gaving rats oral doses of C. sativus aqueous extracts (60 mg/kg, 120 mg/kg, 240 mg/kg) produced diuretic effects comparable to those of the commonly prescribed diuretic hydrochlorothiazide (10 mg/kg B.W., i.p.) (Shariatifar et al., 2014). Additionally, more research is required to determine the mechanisms of action, possibly other side effects, and interactions with other medications.
5 Safety and/or toxicity of Crocus sativus L.
Although C. sativus and its active ingredients are widely used in food and medicine, more research is required to determine the safety and toxicity of the medicinal herbs. The stigma and petals of C. sativus had different toxicological effects. In a subacute toxicity test, the stigma (1.2–3.6 g/kg) and the petal ethanol extract (0.16–0.48 g/kg) both coused anemia and pulmonary and hepatocytosis in rats after intraperitoneal administration, respectively. The LD50 values of stigma and petals were 1.6 and 6.0 g/kg, respectively, indicating that the toxicity of petals was lesser than that of stigma (Mohajeri et al., 2007). There are a variety of toxicological literature reports on the stigma of C. sativus. The LD50 value of stigma aqueous extract to CCD-18LU human normal lung cells was 50–400 mg/mL in the toxicity study, and there was no cytotoxicity (Abdullaev et al., 2003). The mice showed symptoms of nausea, vomiting, diarrhea and bleeding after receiving an intraperitoneal injection of stigma aqueous extract at the dose of 1.2–2 g/kg (Schmidt et al., 2007). In another study, oral administration of stigma aqueous extract (4 g/kg/d) to mice did not result in any negative side effects (Melnyk et al., 2010). Bahmani et al. invesrigated the toxicity of stigma aqueous extract on lactating mice and neonatal mice, and found that the LD50 value of lactating mice after oral administration (500, 1000 or 2000 mg/ kg/ day) was 4120 ± 556 mg/kg, did not show any toxic effects on the liver, but at high doses (2000 mg/kg/d) neonatal mouse kidneys showed morphological changes (Bahmani et al., 2014). In the subacute toxicity test of stigma ethanol extract in rats, intraperitoneal injection of 0.35, 0.70, and 1.05 g/kg/d increased the damage of liver and kidney tissue in a dose-dependent manner (Mohajeri et al., 2007). Compared with ethanol extract, aqueous extract may have lower toxicity. It's possible that oral administration damages tissue less than intraperitoneal administration.
Safranal (2) has been reported to have more toxic effects than other active ingredients in C. sativus (Hosseinzadeh et al., 2010). Rat mortality was decreased when C. sativus aqueous extract (25–100 mg /kg, IP) and safranal(2) (1.2 mL /kg, IP) were combined as opposed to when safranal(2) was used alone, and no deaths were observed when C. sativus aqueous extract was used at a dosage of 10 mg/kg. Safranal (2) can be made less toxic when when combined with aqueous extract of C. sativus (Ziaee et al., 2014). According to Hosseinzadeh et al., crocin (44) had a low toxicity for acute intraperitoneal administration and was nearly non-toxic for acute oral administration. Safranal (2) was given intraperitoneally, resulting in LD50 values for male mice of 1.48 mL/kg, female mice of 1.88 mL/kg, and rats of 1.50 mL/kg, all of which were<5.0 mg/kg. Oral administration resulted in LD50 values of 21.42 mL/kg in male mice, 11.42 mL/kg in female mice, and 5.53 mL/kg in rats, all of which were higher than 5.0 mg/kg (Hosseinzadeh et al., 2013). However, the results of acute and subacute trials using intraperitoneal and oral doses of 3 g/kg crocin (44) showed no mortality and no negative effects on organs in experimental rats and mice (Hosseinzadeh et al., 2010). In the studier by Moallem et al., pregnant mice were given intraperitoneal doses of crocin (44) (200 mg/kg or 600 mg/kg) or safranal (2) (0.075 mL/kg or 0.225 mL/kg), which led to skeletal malformation and growth retardation in newborn mice. Additionally, the severity of embryo malformation induced by the two is similar (Moallem et al., 2016). This result suggests that pregnant women should use C. sativus-related products with caution because C. sativus has a strong blood-activating effect. Other blood-activating and anticoagulant drugs, such as Chuanxiong rhizoma, Salvia miltiorrhiza, enteric-coated aspirin, warfarin, heparin, etc., should generally be avoided during the period of C. sativus, (Qiu et al., 2021), and pregnant women should be cautious to avoid bleeding risks. To sum up, studies on the long-term toxicity of C. sativus in vivo as well as studies to establish an effective dose are still lacking.
6 Potential medicinal source possibilities for petals
It was believed that the stigma and petals of C. sativus had comparable pharmacological activities on the basis of common material basis. Petal methanol extract on rat cardiomyocytes showed negligble inotropic and chronotropic intrinsic activity, but significant intrinsic activity on smooth muscle. Kaempferol (51) and crocin (44) were isolated and purified from petals and demonstrated selective negative inotropic activity (Zeka et al., 2020). Another study using angiotensin II and NG-nitro-L-arginine methyl ester (L-NAME, a NOS inhibitor) on anesthetized rats confirmed the significant attenuation of cardiovascular responses caused by AII and L-NAME in the petal pretreatment group, including the antihypertension effect and application prospect of hydroalcoholic extract petal in cardiovascular diseases (Mohebbati et al., 2021).
The C. sativus petals have antidepressant effects, according to in vivo tests. Karim et al. found that gavage administration of an aqueous and ethanol extract (30 mg/kg) from C. sativus petals significantly reduced immobilization time and had antidepressant effects in depressed mice (Karimi et al., 2001). In a randomized, double-blind, placebo-controlled clinical study, 40 depressed patients were given petals supplement (30 mg/ day). After 6 weeks, the petals group significantly outperformed the placebo group on the Hamilton Depression Rating Scale (HDRS) and the subjects' depressive mood (Akhondzadeh et al., 2005). In a randomized, double-blind, controlled trial, 40 moderately depressed patients received either petals capsules (15 mg bid) or fluoxetine (10 mg bid). HDRS significantly reduced by 25% in both groups after 8 weeks. The antidepressant effect of C. sativus petals was similar to fluoxetine, and the side effects were not significantly different. C. sativus petals may be used as a potential source of antidepressants (Akhondzadeh Basti et al., 2007). Another double-blind randomized clinical trial involved 40 patients with mild to moderate depression who were randomized to receive either C. sativus petals capsules (30 mg/day) or placebo. After 6 weeks, the HDRS results in the petals capsule group were significantly better than those in the placebo group, and there were no side effects, indicating that petals are very effective in the treatment of mild to moderate depression (Moshiri et al., 2006). C. sativus petals may be used as an adjuvant therapy for mild to moderate depression.
Petal performance surpasses stigma in the areas of antibacterial, liver, and kidney protection in addition to the previously mentioned cardiovascular system and nervous system activities. With a 13–22 mm diameter inhibitory zone, the 1000 mg/mL methanol extract of petals has antibacterial activity against Staphylococcus aureus, Bacillus cereus, Salmonella typhi, Escherichia coli, and Shigella dysenteriae (Asgarpanah et al., 2013). Rats were intraperitoneally injected daily with low-dose (40 mg/kg) and high-dose (80 mg/kg) petal extracts to test for gentamicin sulphate-induced nephrotoxicity. The serum blood urea nitrogen (BUN) and creatinine levels of rats were decreased at the dose of 40 mg/kg. The damaging effects of gentamicin sulfate (GM) on the kidney can be lessened by an ethanol extract of petals (Omidi and Totrabi 2016). In the acetaminophen-induced liver and kidney injury experiment, rats pretreated with low-dose (10 mg/kg) of hydroalcoholic extract of petals showed mild necrosis in the hepatic lobule area, but rats pretreated with high dose (20 mg/kg) showed only modest hepatocyte degeneration (Omidi et al., 2014). Increased serum creatinine and uric acid are signs acute nephrotoxicity from acetaminophen, which were significantly reduced by a 20 mg/kg dose of C. sativus hydroalcoholic extract (Omidi et al., 2015). As a result, liver and kidney damage brought on by gentamicin sulfate and acetaminophen can be made up for by the hydroalcoholic extract of petals.
Despite the evidence that stigma and petals both have similar pharmacological effects, stigma has been the subject of more extensive and in-depth pharmacological research than petals. This paper summarized and analyzed the connection between the common and different main active ingredients of stigma and petals and their pharmacological effects based on the study of the chemical constituents of petals and stigma, as shown in Table 5. Antidepressant Reducing ROS accumulation, inhibiting myocardial contractility and reducing calcium influx Anti-diabetic Anti-diabetic Antidepressant Inhibit the aggregation of Aβ monomers into oligomers or fibers, and degrade the formed Aβ oligomers or fibers Inhibit pancreatic lipase and improve hyperlipidemia Gastric cancer, hepatocellular carcinoma, cervical cancer Antidepressant Induces autophagy in N9 microglia and primary neuronal cells, promotes increased Aβ clearance and improves protein plaque accumulation Anti-atherosclerosis Restore some parameters after diabetes induction and relieve some complications of diabetes Breast cancer, colon cancer Inhibits the inflammatory response and apoptosis of renal tissue in rats with diabetic nephropathy, and has a protective effect on renal function and histopathological damage Anti-atherosclerosis Anti-inflammatory and analgesic, hypoglycemic, anti-osteoporosis, anti-fertility. Immune regulation, disease prevention, protective effect on damaged tissue Antibacterial Breast cancer, lung cancer Treating Rheumatoid Arthritis Anti-fibrosis Antibacterial Breast cancer Note: + indicates that the site contains the compound; - Indicates that the site does not contain the compound.
Compounds
Petals
Stigmas
Effect
Reference
Safranal (2)
–
+
(Wang et al., 2015, Tabeshpour et al., 2017, Xue et al., 2020)
Picrocrocin (3)
+
+
(Roshanravan and Ghaffari 2022)
Crocetin (26)
+
+
(Zhu 2019, Yu et al., 2020) (Chen et al., 2015, Wang et al., 2015, Mohan et al., 2021, Zang et al., 2021)
Crocin(44)
+
+
(Li et al., 2018, Xiao et al., 2019, Margaritis et al., 2020, Farahi et al., 2021, Wani et al., 2021, Bakshi et al., 2022)
Kaempferol(51)
+
+
(Kong 2014, Lei et al., 2017, Ming 2018, Tang et al., 2018, Li et al., 2020)
Quercetin(78)
+
+
(Wang et al., 2018, Ezzati et al., 2020, Chen et al., 2021, Jiang et al., 2021)
7 Remaining problems and trends
As a by-product, there are some limitations in the exploitation and utilization of petal. By comparing the metabolites in the stigma and petals, Zhou et al. discovered that the composition of the metabolites in the stigma and petals was essentially the same. The petals contained flavonoids, alkaloids, coumarins, and other medicinal components, which were of great development value to medicine and food (Zhou et al., 2022). The remaining petals must be dried quickly once the stigma has been collected to prevent spoiling. For the purpose of controlling the quality of fresh petals, some rapid drying techniques, such as infrared drying and microwave drying, can be applied (Zhao et al., 2019, Qiu et al., 2022). In addition, although the petals are valuable for medicine and foods and have potential utility in multiple areas such as the natural spice, cosmetic, health drink, and natural health product industries, it lacks in necessary safety measures, and further studies of the mechanism of action and toxicological properties of petals are also required, especially research to establish an effective dose and its long-term toxicity in vivo.
8 Conclusion
Modern chemical and pharmacological studies have clarified the relationship between complex chemical composition and clinical application of C. sativus. Terpenoids, flavonoids and their derivatives are the main material basis for pharmacological effects in C. sativus(Liu et al., 2020), accounting for 69.39% of the total compounds. The stigma and petals contain a large number of chemical compounds that are chemically similar and make up 33.57% of the total compounds. Flavonoids and their derivatives account 31.25% of all flavonoids and their derivatives, making them the class with the most similar chemicals. The material basis overlap rate is extremely high. Besides, flavonoids with good antioxidant activity are proved to be the most abundant compounds in the stigma and petals(Hatziagapiou and Lambrou 2018), and kaempferol glycosides are abundant in dried petals with a concentration of up to 126 mg/g DW and crocin concentration of 6.4 mg/g DW (Zeka et al., 2020), which indicates the role of petals as a source of phytochemicals and their application prospect in adjuvant treatment of various diseases, especially depression. Therefore, we believe it's necessary to review it, which will help to provide ideas for the development and utilization of petals.
9 Authors’contributions
Hongyan Ma and Jin Pei conceived and designed all the review. Xue Li, Jin Xie and Hong Fan wrote and edited the review. Jin Tan and Yang Bao completed data analysis and graphing. Funeng Geng and Dingkun Zhang collected documents and literatures.
Acknowledgements
We are grateful for the support of the Sichuan Science and Technology Program (grant NO. 2020YFN0082) and the help of Chengdu University of Traditional Chinese Medicine and Good Doctor Pharmaceutical Group Co., Ltd., Chengdu, Sichuan.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Use of in vitro assays to assess the potential antigenotoxic and cytotoxic effects of saffron (Crocus sativus L.) Toxicol. In Vitro. 2003;17:731-736.
- [CrossRef] [Google Scholar]
- Effects of crocin and saffron aqueous extract on gene expression of SIRT1, AMPK, LOX1, NF-κB, and MCP-1 in patients with coronary artery disease: a randomized placebo-controlled clinical trial. Phytother. Res.. 2020;34:1114-1122.
- [CrossRef] [Google Scholar]
- Nutritional and health beneficial properties of saffron (Crocus sativus L): a comprehensive review. Crit. Rev. Food. Sci. Nutr.. 2022;62:2683-2706.
- [CrossRef] [Google Scholar]
- Treatment-induced tumor cell apoptosis following high-intensity interval training and saffron aqueous extract in mice with breast cancer. Physiol. Int. 2021
- [CrossRef] [Google Scholar]
- Saffron (Crocus sativus L.) supplements improve quality of life and appetite in atherosclerosis patients: a randomized clinical trial. J. Res. Med. Sci.. 2022;27:30.
- [CrossRef] [Google Scholar]
- Cytotoxic effect of saffron stigma aqueous extract on human prostate cancer and mouse fibroblast cell lines. Urol. J.. 2020;18:633-638.
- [CrossRef] [Google Scholar]
- Crocus Sativus L. (saffron) versus sertraline on symptoms of depression among older people with major depressive disorders-a double-blind, randomized intervention study. Psychiatry Res.. 2019;282:112613
- [CrossRef] [Google Scholar]
- Saffron: its phytochemistry, developmental processes, and biotechnological prospects. J. Agric. Food Chem.. 2015;63:8751-8764.
- [CrossRef] [Google Scholar]
- Comparison of petal of Crocus sativus L. and fluoxetine in the treatment of depressed outpatients: a pilot double-blind randomized trial. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31:439-442.
- [CrossRef] [Google Scholar]
- Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized and placebo-controlled trial. Phytother. Res.. 2005;19:148-151.
- [CrossRef] [Google Scholar]
- Saffron in the treatment of patients with mild to moderate Alzheimer's disease: a 16-week, randomized and placebo-controlled trial. J. Clin. Pharm. Ther.. 2010;35:581-588.
- [CrossRef] [Google Scholar]
- A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer's disease. Psychopharmacology. 2010;207:637-643.
- [CrossRef] [Google Scholar]
- A placebo controlled randomized clinical trial of Crocus sativus L. (saffron) on depression and food craving among overweight women with mild to moderate depression. J. Clin. Pharm. Ther.. 2020;45:134-143.
- [CrossRef] [Google Scholar]
- Crocin synergistically enhances the antiproliferative activity of 5-flurouracil through Wnt/PI3K pathway in a mouse model of colitis-associated colorectal cancer. J. Cell Biochem.. 2018;119:10250-10261.
- [CrossRef] [Google Scholar]
- Andrade, P., L. Catarino, M. Cheesman, et al., 2016. Herbal Medicine in Depression: Traditional Medicine to Innovative Drug Delivery.
- In-vitro evaluation of Crocus Sativus L. petals and stamens as natural antibacterial agents against food-borne bacterial strains. Iran. J. Pharmaceut. Sci.. 2013;9:69-82.
- [Google Scholar]
- Antidepressant effects of aqueous extract of saffron and its effects on CREB, P-CREB, BDNF, and VGF proteins in rat cerebellum. J. Pharmacopuncture. 2018;21:35-40.
- [CrossRef] [Google Scholar]
- Investigating the effect of Crocus sativus L. petal hydroalcoholic extract on inflammatory and enzymatic indices resulting from alcohol use in kidney and liver of male rats. J. Inflamm. Res.. 2019;12:269-283.
- [CrossRef] [Google Scholar]
- Phytochemical analysis and antioxidant activity of different tissue types of Crocus sativus and oxidative stress alleviating potential of saffron extract in plants, bacteria, and yeast. S. Afr. J. Bot.. 2015;99:80-87.
- [CrossRef] [Google Scholar]
- Comprehensive transcriptome analysis of Crocus sativus for discovery and expression of genes involved in apocarotenoid biosynthesis. BMC Genomics. 2015;16:698.
- [CrossRef] [Google Scholar]
- Nephrotoxicity and hepatotoxicity evaluation of Crocus sativus stigmas in neonates of nursing mice. J. Nephropathol.. 2014;3:81-85.
- [CrossRef] [Google Scholar]
- Crocin inhibits angiogenesis and metastasis in colon cancer via TNF-α/NF-kB/VEGF pathways. Cells. 2022;11
- [CrossRef] [Google Scholar]
- A preliminary assessment of a combination of rhodiola and saffron in the management of mild-moderate depression. Neuropsychiatr. Dis. Treat.. 2018;14:1821-1829.
- [CrossRef] [Google Scholar]
- Crocus sativus extract tightens the blood-brain barrier, reduces amyloid β load and related toxicity in 5XFAD mice. ACS Chem. Neurosci.. 2017;8:1756-1766.
- [CrossRef] [Google Scholar]
- The phenolic constituents of plants and their taxonomic significance. J. Linn. Soc. Lond. Bot.. 2008;60:325-356.
- [CrossRef] [Google Scholar]
- Crocus sativus L. (saffron) for cancer chemoprevention: a mini review. J. Tradit. Complement. Med.. 2015;5:81-87.
- [CrossRef] [Google Scholar]
- Generation of saffron volatiles by thermal carotenoid degradation. J. Agric. Food Chem.. 2006;54:6825-6834.
- [CrossRef] [Google Scholar]
- Crocetin esters, picrocrocin and its related compounds present in Crocus sativus stigmas and Gardenia jasminoides fruits. tentative identification of seven new compounds by LC-ESI-MS. J. Agric. Food Chem.. 2006;54:973-979.
- [CrossRef] [Google Scholar]
- Identification of the flavonoid fraction in saffron spice by LC/DAD/MS/MS: comparative study of samples from different geographical origins. Food Chem.. 2007;100:445-450.
- [CrossRef] [Google Scholar]
- Chen, N. and B. Yang, 2018. Research progress of chemical components and pharmacological effects of non-medicinal parts of Crocus sativus. China Journal of Chinese Materia Medica 43, 2884-2891. https://doi.org/10.19540/j.cnki.cjcmm.20180425.002.
- Inhibitory and protective effects of quercetin on hepatic fibrosis induced by carbon tetrachloride in rats. Chinese J. Immunol.. 2021;37:46-50.
- [Google Scholar]
- Crocetin downregulates the proinflammatory cytokines in methylcholanthrene-induced rodent tumor model and inhibits COX-2 expression in cervical cancer cells. Biomed. Res. Int.. 2015;2015:829513
- [CrossRef] [Google Scholar]
- Research progress of chemical components and pharmacological effects of non-medicinal parts of Crocus sativus. Zhongguo Zhong Yao Za Zhi. 2018;43:2884-2891.
- [CrossRef] [Google Scholar]
- Saffron: a natural product with potential pharmaceutical applications. J. Pharm. Pharmacol.. 2015;67
- [CrossRef] [Google Scholar]
- Saffron: a natural product with potential pharmaceutical applications. J. Pharm. Pharmacol.. 2015;67:1634-1649.
- [CrossRef] [Google Scholar]
- Crocus sativus L. aqueous extract reduces atherogenesis, increases atherosclerotic plaque stability and improves glucose control in diabetic atherosclerotic animals. Atherosclerosis. 2018;268:207-214.
- [CrossRef] [Google Scholar]
- Short-term impact of a combined nutraceutical on cognitive function, perceived stress and depression in young elderly with cognitive impairment: a pilot, double-blind, randomized clinical trial. J. Prev. Alzheimers Dis.. 2017;4:12-15.
- [CrossRef] [Google Scholar]
- Crocetin and Crocin from Saffron in cancer chemotherapy and chemoprevention. Anticancer Agents Med. Chem.. 2019;19:38-47.
- [CrossRef] [Google Scholar]
- Commission, C. P., 2020. Pharmacopoeia of the People's Republic of China. Beijing, People's Medical Publishing House
- Safety and efficacy of Saffron (Crocus sativus L.) for treating mild to moderate depression: a systematic review and meta-analysis. J. Nerv. Ment. Dis.. 2020;208:269-276.
- [CrossRef] [Google Scholar]
- The effects of safranal, a constitute of saffron, and metformin on spatial learning and memory impairments in type-1 diabetic rats: behavioral and hippocampal histopathological and biochemical evaluations. Biomed. Pharmacother.. 2018;107:203-211.
- [CrossRef] [Google Scholar]
- Saffron (Crocus sativus) intake provides nutritional preconditioning against myocardial ischemia-reperfusion injury in Wild Type and ApoE((-/-)) mice: Involvement of Nrf2 activation. Nutr. Metab. Cardiovasc. Dis.. 2017;27:919-929.
- [CrossRef] [Google Scholar]
- A review on anti-cancer properties of Quercetin in breast cancer. Life Sci.. 2020;248:117463
- [CrossRef] [Google Scholar]
- Use of Ancient texts in modern therapeutic research. Rev. Hist. Pharm. (Paris). 2003;51:239-250.
- [Google Scholar]
- Crocin and Metformin suppress metastatic breast cancer progression via VEGF and MMP9 downregulations: in vitro and in vivo studies. Mol. Cell Biochem.. 2021;476:3341-3351.
- [CrossRef] [Google Scholar]
- Beneficial effects of hydroalcoholic extract of Saffron in alleviating experimental autoimmune diabetes in C57bl/6 mice. Iran. J. Allerg. Asthma Immunol.. 2019;18:38-47.
- [Google Scholar]
- Protective effect of Crocus sativus L. (Saffron) extract on spinal cord ischemia-reperfusion injury in rats. Iran. J. Basic Med. Sci.. 2017;20:334-337.
- [CrossRef] [Google Scholar]
- Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer's disease: a double-blind randomized clinical trial. Hum. Psychopharmacol.. 2014;29:351-359.
- [CrossRef] [Google Scholar]
- Gas chromatographic determination of phytosterols and fatty acids profile in Saffron petals. Canad. Chem. Trans.. 2016;4:389-397.
- [CrossRef] [Google Scholar]
- A perspective on Crocus sativus L. (Saffron) constituent Crocin: a potent water-soluble antioxidant and potential therapy for Alzheimer's disease. J. Agric. Food Chem.. 2017;65
- [CrossRef] [Google Scholar]
- Effects of long-term treatment of colon adenocarcinoma with crocin, a carotenoid from saffron (Crocus sativus L.): an experimental study in the rat. Nutr. Cancer. 1999;35:120-126.
- [CrossRef] [Google Scholar]
- Comparative evaluation of an ISO 3632 method and an HPLC-DAD method for Safranal quantity determination in Saffron. Food Chem.. 2016;221
- [CrossRef] [Google Scholar]
- Comparative evaluation of an ISO 3632 method and an HPLC-DAD method for safranal quantity determination in saffron. Food Chem.. 2017;221:838-843.
- [CrossRef] [Google Scholar]
- Effects of the active constituents of Crocus Sativus L., crocins, in an animal model of obsessive-compulsive disorder. Neurosci. Lett.. 2012;528:27-30.
- [CrossRef] [Google Scholar]
- Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur. J. Pharmacol.. 2011;667:222-229.
- [CrossRef] [Google Scholar]
- Crocus sativus L. versus Citalopram in the treatment of major depressive disorder with anxious distress: a double-blind, controlled clinical trial. Pharmacopsychiatry. 2017;50:152-160.
- [CrossRef] [Google Scholar]
- Alzheimer's disease-related dysregulation of mRNA translation causes key pathological features with ageing. Transl. Psychiatry. 2020;10:192.
- [CrossRef] [Google Scholar]
- Biochemical, antioxidant and antineoplastic properties of Italian Saffron (Crocus sativus L.) Am. J. Plant Sci.. 2012;03:1573-1580.
- [CrossRef] [Google Scholar]
- Crocin attenuates the granular cells damages on the dentate gyrus and pyramidal neurons in the CA3 regions of the hippocampus and frontal cortex in the rat model of Alzheimer's disease. J. Chem. Neuroanat.. 2021;113:101837
- [CrossRef] [Google Scholar]
- Flavonol glycosides from the stems of Trigonella foenum-graecum. Phytochemistry. 2001;58:577-580.
- [CrossRef] [Google Scholar]
- 6-hydroxyflavones and other flavonoids of Crocus. Zeitschrift für Naturforschung C. 1984;39:18-23.
- [CrossRef] [Google Scholar]
- The protective role of Crocus Sativus L. (Saffron) against ischemia- reperfusion injury, hyperlipidemia and atherosclerosis: nature opposing cardiovascular diseases. Curr. Cardiol. Rev.. 2018;14:272-289.
- [CrossRef] [Google Scholar]
- Crocetin induces apoptosis of BGC-823 human gastric cancer cells. Mol. Med. Rep.. 2014;9:521-526.
- [CrossRef] [Google Scholar]
- Antiproliferative activity of crocin involves targeting of microtubules in breast cancer cells. Journal. 2017;7:44984.
- [CrossRef] [Google Scholar]
- The effects of saffron (Crocus sativus L.) in conjunction with concurrent training on body composition, glycaemic status, and inflammatory markers in obese men with type 2 diabetes mellitus: a randomized double-blind clinical trial. Br. J. Clin. Pharmacol.. 2022;88:3256-3271.
- [CrossRef] [Google Scholar]
- Hosseinzadeh, H., V. Shariaty, A. Sameni, et al., 2010. Acute and sub-acute toxicity of crocin, a constituent of Crocus sativus L. (saffron), in mice and rats. Pharmacologyonline.
- Antidepressant effect of Crocus sativus L. stigma extracts and their constituents, crocin and safranal, in mice. Acta Hortic.. 2004;650:435-445.
- [CrossRef] [Google Scholar]
- Acute and subacute toxicity of safranal, a constituent of saffron, in mice and rats. Iran. J. Pharm. Res.. 2013;12:93-99.
- [Google Scholar]
- Hu, J., D. Yao, J. Zhang, et al., 2014. Research Progress of Crocus sativus L. Antitumor Role. Journal of Anhui Agricultural Sciences. 42, 699-701+703. https://doi.org/10.13989/j.cnki.0517-6611.2014.03.102.
- Huang, Y., Y. Liu, W. Wu, et al., 2013. Bioactivity-guided fractionation of petals of Crocus sativusand their antioxidant activityinvestigation. Natural Product Research and Development. 25, 1489-1493+1567. https://doi.org/10.16333/j.1001-6880.2013.11.004.
- Effects of Saffron extract supplementation on mood, well-being, and response to a psychosocial stressor in healthy adults: a randomized, double-blind, parallel group, clinical trial. Front. Nutr.. 2020;7:606124
- [CrossRef] [Google Scholar]
- Saffron improved depression and reduced homocysteine level in patients with major depression: a randomized, double-blind study. Avicenna J. Phytomed.. 2018;8:43-50.
- [Google Scholar]
- Effect of saffron aqueous extract on the level of blood glucose in experimental diabetes mice. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2018;34:173-176.
- [CrossRef] [Google Scholar]
- Research progress of quercetin in treatment of rheumatoid arthritis. Chin. J. Exp. Tradit. Med. Formulae. 2021;27:243-250.
- [CrossRef] [Google Scholar]
- Research progress on antidiabetic active components and mechanism of Crocus Sativa. Zhejiang J. Trad. Chinese Med.. 2019;54:697-699.
- [CrossRef] [Google Scholar]
- Jones, K., 2016. Review of neuroplasticity and depression: evidence for the neurotrophic or neuroplasticity theory of depression pathophysiology and systematic review of the neurophysiological implications of long-term antidepressant treatment.
- Study of antidepressant effect of aqueous and ethanol extract of Crocus sativus in mice. Iran. J. Basic Med. Sci.. 2001;4:11-15.
- [Google Scholar]
- Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules. 2010;15:6244-6256.
- [CrossRef] [Google Scholar]
- Comparison of Saffron versus Fluoxetine in treatment of mild to moderate postpartum depression: a double-blind, randomized clinical trial. Pharmacopsychiatry. 2017;50:64-68.
- [CrossRef] [Google Scholar]
- Efficacy of Crocus sativus (saffron) in treatment of major depressive disorder associated with post-menopausal hot flashes: a double-blind, randomized, placebo-controlled trial. Arch. Gynecol. Obstet. 2018;297:717-724.
- [CrossRef] [Google Scholar]
- affron(®) a novel saffron extract (Crocus sativus L.) improves mood in healthy adults over 4 weeks in a double-blind, parallel, randomized, placebo-controlled clinical trial. Complement. Ther. Med.. 2017;33:58-64.
- [CrossRef] [Google Scholar]
- The effects of Crocus sativus (saffron) and its constituents on nervous system: a review. Avicenna J. Phytomed.. 2015;5:376-391.
- [Google Scholar]
- Targeting Nrf2/HO-1 signaling by crocin: role in attenuation of AA-induced ulcerative colitis in rats. Biomed. Pharmacother.. 2019;110:389-399.
- [CrossRef] [Google Scholar]
- Kong, X., 2014. Explore of the anti-atherosclerosis mechanism of kaempferol and study of it's pharmacokinetics 硕士, Chongqing medical university.
- Crocus-derived compounds alter the aggregation pathway of Alzheimer's Disease: associated beta amyloid protein. Sci. Rep.. 2020;10:18150.
- [CrossRef] [Google Scholar]
- Recent advances on the anticancer properties of Saffron (Crocus sativus L.) and its major constituents. Molecules. 2020;26
- [CrossRef] [Google Scholar]
- Research progress on pharmacological effects of kaempferol. Stud. Trace Elements Health. 2017;34:61-62.
- [Google Scholar]
- Antityrosinase principles and constituents of the petals of Crocus sativus. J. Nat. Prod.. 2004;67:437-440.
- [CrossRef] [Google Scholar]
- Crocin alleviates coronary atherosclerosis via inhibiting lipid synthesis and inducing M2 macrophage polarization. Int. Immunopharmacol.. 2018;55:120-127.
- [CrossRef] [Google Scholar]
- GC-MS analysis of volatile constituents from Crocus sativus L. Shanghai Med. Pharmaceut. J.. 2017;38:74-78.
- [Google Scholar]
- Constituents of the pollen of Crocus sativus L. and their tyrosinase inhibitory activity. Chem. Pharm. Bull. (Tokyo). 2002;50:1305-1309.
- [CrossRef] [Google Scholar]
- Constituents of the stigmas of Crocus sativus and their tyrosinase inhibitory activity. J. Nat. Prod.. 2002;65:1452-1456.
- [CrossRef] [Google Scholar]
- Effect and mechanism of kaepferol on proliferation and apoptosis of breast cancer cells. Chinese J. Clin. Pharmacol.. 2020;36:3679-3682.
- [CrossRef] [Google Scholar]
- Crocin improves cognitive behavior in rats with Alzheimer's disease by regulating endoplasmic reticulum stress and apoptosis. Biomed. Res. Int.. 2019;2019:9454913.
- [CrossRef] [Google Scholar]
- Characteristics and molecular mechanisms through which SGLT2 inhibitors improve metabolic diseases: a mechanism review. Life Sci.. 2022;300:120543
- [CrossRef] [Google Scholar]
- Progress in the development of pharmaceutical active ingredients and their products of Crocus sativus. Subtrop. Plant Sci.. 2020;49:506-512.
- [Google Scholar]
- The molecular mechanism of chronic stress affecting the occurrence and development of breast cancer and potential drug therapy. Transl. Oncol.. 2022;15:101281
- [CrossRef] [Google Scholar]
- Advances in pharmacological effects and tissue culture of Saffron. Pharm. Biotechnol.. 2014;21:593-596.
- [CrossRef] [Google Scholar]
- Chemical constituents from the petals of Crocus sativus L. Pharmaceut. Clin. Res.. 2021;29:327-330.
- [CrossRef] [Google Scholar]
- Effects of organic acids on the release of fruity esters in water: an insight at the molecular level. Molecules. 2022;27
- [CrossRef] [Google Scholar]
- Crocus cancellatus subsp. damascenus stigmas: chemical profile, and inhibition of α-amylase, α-glucosidase and lipase, key enzymes related to type 2 diabetes and obesity. J. Enzyme Inhib. Med. Chem.. 2016;31:212-218.
- [CrossRef] [Google Scholar]
- Efficacy of curcumin, and a saffron/curcumin combination for the treatment of major depression: a randomised, double-blind, placebo-controlled study. J. Affect. Disord.. 2017;207:188-196.
- [CrossRef] [Google Scholar]
- affron(®), a standardised extract from saffron (Crocus sativus L.) for the treatment of youth anxiety and depressive symptoms: a randomised, double-blind, placebo-controlled study. J. Affect. Disord.. 2018;232:349-357.
- [CrossRef] [Google Scholar]
- Efficacy of a standardised saffron extract (affron®) as an add-on to antidepressant medication for the treatment of persistent depressive symptoms in adults: a randomised, double-blind, placebo-controlled study. J. Psychopharmacol.. 2019;33:1415-1427.
- [CrossRef] [Google Scholar]
- Saffron (Crocus sativus L.) and health outcomes: a meta-research review of meta-analyses and an evidence mapping study. Phytomedicine. 2021;91:153699
- [CrossRef] [Google Scholar]
- Content of amino acids, trace elements and other components inpetals of Crocus sativus. Modern Chinese Med.. 2021;23:1023-1028.
- [CrossRef] [Google Scholar]
- Content of amino acids, trace elements and other components in petals of Crocus sativus. Modern Chinese Med.. 2021;23:1023-1028.
- [CrossRef] [Google Scholar]
- Saffron and retinal neurodegenerative diseases: Relevance of chemical composition. J. Anat. 2022
- [CrossRef] [Google Scholar]
- Mechanistic insights on the effect of crocin, an active ingredient of saffron, on atherosclerosis in apolipoprotein E knockout mice. Coron Artery Dis.. 2022;33:394-402.
- [CrossRef] [Google Scholar]
- Effect of crocin on antioxidant gene expression, fibrinolytic parameters, redox status and blood biochemistry in nicotinamide-streptozotocin-induced diabetic rats. J. Biol. Res. (Thessalon). 2020;27:4.
- [CrossRef] [Google Scholar]
- PTR-TOF-MS and HPLC analysis in the characterization of saffron (Crocus sativus L.) from Italy and Iran. Food Chem.. 2016;192:75-81.
- [CrossRef] [Google Scholar]
- A double-blind, randomized and placebo-controlled trial of Saffron (Crocus sativus L.) in the treatment of anxiety and depression. J. Complement. Integr. Med.. 2016;13:195-199.
- [CrossRef] [Google Scholar]
- Chemical and biological properties of the world's most expensive spice: Saffron. Food Res. Int.. 2010;43:1981-1989.
- [CrossRef] [Google Scholar]
- The effect of saffron (Crocus sativus L.) hydroalcoholic extract on metabolic control in type 2 diabetes mellitus: a triple-blinded randomized clinical trial. J. Res. Med. Sci.. 2018;23:16.
- [CrossRef] [Google Scholar]
- The effects of alcoholic extract of saffron (Crocus satious L.) on mild to moderate comorbid depression-anxiety, sleep quality, and life satisfaction in type 2 diabetes mellitus: a double-blind, randomized and placebo-controlled clinical trial. Complement. Ther. Med.. 2018;41:196-202.
- [CrossRef] [Google Scholar]
- Ming, D., 2018. Molecular mechanism of inhibitory effet of kaempferol on staphylococcus aureus biofilm formation 硕士, Jilin university.
- Evaluation of teratogenic effects of crocin and safranal, active ingredients of saffron, in mice. Toxicol. Ind. Health. 2016;32:285-291.
- [CrossRef] [Google Scholar]
- Subacute toxicity of Crocus Sativus L. (Saffron) stigma ethanolic extract in rats. Am. J. Pharmacol. Toxicol.. 2007;2:189-193.
- [CrossRef] [Google Scholar]
- Crocetin imparts antiproliferative activity via inhibiting STAT3 signaling in hepatocellular carcinoma. IUBMB Life. 2021;73:1348-1362.
- [CrossRef] [Google Scholar]
- Effects of hydroalcoholic extract of saffron petal on blood pressure and heart rate in hypertension induced by angiotensin II and L-NAME in anesthetized rats. Vet. Res. Forum. 2021;12:185-190.
- [CrossRef] [Google Scholar]
- Montoro, P., C. Tuberosob, M. Maldini, et al., 2008. Qualitative Profile and Quantitative Determination of Flavonoids from Crocus sativus L. Petals by LC-MS/MS. Natural product communications
- Radical scavenging activity and LC-MS metabolic profiling of petals, stamens, and flowers of Crocus sativus L. J. Food Sci.. 2012;77:C893-C900.
- [CrossRef] [Google Scholar]
- Cloning and characterization of a glucosyltransferase from Crocus sativus stigmas involved in flavonoid glucosylation. BMC Plant Biol.. 2009;9:109.
- [CrossRef] [Google Scholar]
- Metabolite and target transcript analyses during Crocus sativus stigma development. Phytochemistry. 2009;70:1009-1016.
- [CrossRef] [Google Scholar]
- The effect of hydroalcoholic Saffron (Crocus sativus L.) extract on fasting plasma glucose, HbA1c, lipid profile, liver, and renal function tests in patients with type 2 diabetes mellitus: a randomized double-blind clinical trial. Phytother. Res.. 2019;33:1648-1657.
- [CrossRef] [Google Scholar]
- Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: a double-blind, randomized and placebo-controlled trial. Phytomedicine. 2006;13:607-611.
- [CrossRef] [Google Scholar]
- Phytochemical composition of Moroccan saffron accessions by headspace solid-phase-microextraction. Am. J. Essent. Oils Nat. Prod.. 2014;2:1-7.
- [Google Scholar]
- Phytochemical composition of Moroccan saffron accessions by headspace solid-phase-microextraction. Am. J. Essent. Oils Nat. Prod.. 2015;2:01-07.
- [Google Scholar]
- Saffron, as an adjunct therapy, contributes to relieve depression symptoms: an umbrella meta-analysis. Pharmacol. Res.. 2022;175:105963
- [CrossRef] [Google Scholar]
- Biologically active compounds and pharmacological activities of species of the genus Crocus: a review. Phytochemistry. 2019;162:56-89.
- [CrossRef] [Google Scholar]
- Saffron (Crocus sativus) in the treatment of gastrointestinal cancers: Current findings and potential mechanisms of action. J. Cell Biochem.. 2019;120:16330-16339.
- [CrossRef] [Google Scholar]
- Naghibi, S. M., M. Hosseini, F. Khani, et al., 2012. Effect of Aqueous Extract of Crocus sativus L. on Morphine-Induced Memory Impairment. Adv Pharmacol Sci. 2012, 494367. https://doi.org/10.1155/2012/494367.
- Antitumour activity of saffron (Crocus sativus) Cancer Lett.. 1991;57:109-114.
- [CrossRef] [Google Scholar]
- Flower pigment composition of Crocus species and cultivars used for a chemotaxonomic investigation. Biochem. Syst. Ecol.. 2002;30:763-791.
- [CrossRef] [Google Scholar]
- Hepatoprotective effect of Crocus sativus (saffron) petals extract against acetaminophen toxicity in male Wistar rats. Avicenna J. Phytomed.. 2014;4:330-336.
- [Google Scholar]
- Evaluation of protective effect of hydroalcoholic extract of saffron petals in prevention of acetaminophen-induced renal damages in rats. Vet. Sci. Dev.. 2015;5
- [Google Scholar]
- The protective role of saffron petal extracts on gentamicininduced nephrotoxicity in rats. Vet. Sci. Dev.. 2016;6
- [CrossRef] [Google Scholar]
- Antianhedonic and antidepressant effects of Affron(®), a standardized Saffron (Crocus Sativus L.) extract. Molecules. 2020;25
- [CrossRef] [Google Scholar]
- Effects of Saffron extract on sleep quality: a randomized double-blind controlled clinical trial. Nutrients. 2021;13
- [CrossRef] [Google Scholar]
- Functional proteomics reveals the protective effects of saffron ethanolic extract on hepatic ischemia-reperfusion injury. Proteomics. 2013;13:2297-2311.
- [CrossRef] [Google Scholar]
- Biosynthesis of C20-carotenoids in Crocus sativus. Phytochemistry. 1982;21:1039-1042.
- [Google Scholar]
- Carotinoid-Glykoside 2. Mitteilung Untersuchungen zur Carotinoid-Zusammensetzung im Safran. Helv. Chim. Acta. 1975;58:1608-1620.
- [Google Scholar]
- Isolation and structure elucidation of Carotenoid−Glycosyl Esters in Gardenia Fruits (Gardenia jasminoides Ellis) and Saffron (Crocus sativus Linne) J. Agric. Food Chem.. 1996;44:2612-2615.
- [Google Scholar]
- Effects of the active constituents of Crocus sativus L., crocins, in an animal model of anxiety. Phytomedicine. 2008;15:1135-1139.
- [CrossRef] [Google Scholar]
- Pharmacological and clinical application of heparin progress: an essential drug for modern medicine. Biomed. Pharmacother.. 2021;139:111561
- [CrossRef] [Google Scholar]
- Convenient use of near-infrared spectroscopy to indirectly predict the antioxidant activitiy of edible rose (Rose chinensis Jacq “Crimsin Glory” H.T.) petals during infrared drying. Food Chem.. 2022;369:130951
- [CrossRef] [Google Scholar]
- C/EBPβ in bone marrow is essential for diet induced inflammation, cholesterol balance, and atherosclerosis. Atherosclerosis. 2016;250:172-179.
- [CrossRef] [Google Scholar]
- An update review of Saffron and its active constituents. Phytother. Res.. 1996;10:189-193.
- [CrossRef] [Google Scholar]
- The therapeutic potential of Crocus sativus Linn.: a comprehensive narrative review of clinical trials. Phytother. Res.. 2022;36:98-111.
- [CrossRef] [Google Scholar]
- Therapeutic effects of saffron extract on different memory types, anxiety, and hippocampal BDNF and TNF-α gene expressions in sub-chronically stressed rats. Nutr. Neurosci.. 2022;25:192-206.
- [CrossRef] [Google Scholar]
- Cloning and characterization of a glucosyltransferase from Crocus sativus stigmas involved in flavonoid glucosylation. BMC Plant Biol.. 2009;9:109.
- [CrossRef] [Google Scholar]
- An overview on different detection methods of saffron (Crocus sativus L.) adulterants. J. Food Meas. Charact.. 2022;16:4996-5006.
- [CrossRef] [Google Scholar]
- Ameliorative effect of saffron aqueous extract on hyperglycemia, hyperlipidemia, and oxidative stress on diabetic encephalopathy in streptozotocin induced experimental diabetes mellitus. Biomed. Res. Int.. 2014;2014:920857
- [CrossRef] [Google Scholar]
- Saffron in phytotherapy: pharmacology and clinical uses. Wien Med. Wochenschr. 2007;157:315-319.
- [CrossRef] [Google Scholar]
- Effect of crocin on diabetic patients: a placebo-controlled, triple-blinded clinical trial. Clin. Nutr. ESPEN. 2022;50:255-263.
- [CrossRef] [Google Scholar]
- Flavonoid determination in the quality control of floral bioresidues from Crocus sativus L. J. Agric. Food Chem.. 2014;62:3125-3133.
- [CrossRef] [Google Scholar]
- Several, 2016. Herbal medicine in depression: Traditional medicine to innovative drug delivery.
- Main chemical compounds and pharmacological activities of stigmas and tepals of 'red gold'; saffron. Trends Food Sci. Technol.. 2016;58
- [CrossRef] [Google Scholar]
- Study on diuretic activity of saffron (stigma of Crocus sativus L.) Aqueous extract in rat. J. Adv. Pharm. Technol. Res.. 2014;5:17-20.
- [CrossRef] [Google Scholar]
- Syringetin 3-O-(6″-acetyl)-β-glucopyranoside and other flavonols from needles of norway spruce, Picea abies. Phytochemistry. 1995;40:1537-1542.
- [CrossRef] [Google Scholar]
- New progress in the pharmacology of protocatechuic acid: a compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res.. 2020;161:105109
- [CrossRef] [Google Scholar]
- Studies on the constituents of Crocus sativus III. the structural elucidation of two new glyeosides. Acta Chim. Sin. 1991:917-920.
- [Google Scholar]
- Aging under pressure: the roles of Reactive Oxygen and Nitrogen Species (RONS) production and aging skeletal muscle in endothelial function and hypertension-from biological processes to potential interventions. Antioxidants (Basel). 2021;10
- [CrossRef] [Google Scholar]
- Identification of novel glycosidic aroma precursors in Saffron (Crocus sativus L.) J. Agric. Food Chem.. 1998;46:3238-3243.
- [Google Scholar]
- Straubinger, M., 1997. Novel glycosidic constituents from saffron. Journal of agricultural and food chemistry. v. 45, pp. 1678-1681-1997 v.1645 no.1675. https://doi.org/10.1021/jf960861k.
- The beneficial effects of Saffron extract on potential oxidative stress in cardiovascular diseases. Oxid. Med. Cell Longev.. 2021;2021:6699821.
- [CrossRef] [Google Scholar]
- A double-blind, randomized, placebo-controlled trial of saffron stigma (Crocus sativus L.) in mothers suffering from mild-to-moderate postpartum depression. Phytomedicine. 2017;36:145-152.
- [CrossRef] [Google Scholar]
- Saffron improves life and sleep quality, glycaemic status, lipid profile and liver function in diabetic patients: a double-blind, placebo-controlled, randomised clinical trial. Int. J. Clin. Pract.. 2021;75:e14334.
- [Google Scholar]
- Crocin, the main active saffron constituent, as an adjunctive treatment in major depressive disorder: a randomized, double-blind, placebo-controlled, pilot clinical trial. J. Affect. Disord.. 2015;174:51-56.
- [CrossRef] [Google Scholar]
- The protective effect of kaempferol on renal function and tissue of diabetic nephropathy rats induced by high glucose. Immunol. J.. 2018;34:1041-1046.
- [CrossRef] [Google Scholar]
- Polyphenol composition and in vitro antiproliferative effect of corm, tepal and leaf from Crocus sativus L. on human colon adenocarcinoma cells (Caco-2) J. Funct. Foods. 2016;24:18-25.
- [CrossRef] [Google Scholar]
- Isolation and identification of the aroma components from Saffron (Crocus sativus) J. Agric. Food Chem. - J. AGR. FOOD CHEM.. 1997;45
- [CrossRef] [Google Scholar]
- Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV-visible photodiode-array detection-mass spectrometry. J. Chromatogr. A. 1995;699:107-118.
- [CrossRef] [Google Scholar]
- LC-DAD-MS (ESI+) analysis and antioxidant capacity of crocus sativus petal extracts. Planta Med.. 2008;74:573-581.
- [CrossRef] [Google Scholar]
- Efficacy and safety of Crocus sativus L. in patients with mild cognitive impairment: one year single-blind randomized, with parallel groups, clinical trial. J. Alzheimers Dis.. 2016;54:129-133.
- [CrossRef] [Google Scholar]
- New minor glycoside components from saffron. J. Nat. Med.. 2013;67:672-676.
- [CrossRef] [Google Scholar]
- Antidepressant effects of crocin and its effects on transcript and protein levels of CREB, BDNF, and VGF in rat hippocampus. Daru. 2014;22:16.
- [CrossRef] [Google Scholar]
- Role of crocin in several cancer cell lines: an updated review. Iran. J. Basic Med. Sci.. 2020;23:3-12.
- [CrossRef] [Google Scholar]
- Interspecific and intraspecific variation of phenolics in the genus Equisetum subgenus Equisetum. Phytochemistry. 1995;38:881-891.
- [CrossRef] [Google Scholar]
- Investigation of the neuroprotective effects of crocin via antioxidant activities in HT22 cells and in mice with Alzheimer's disease. Int. J. Mol. Med.. 2019;43:956-966.
- [CrossRef] [Google Scholar]
- Research progress on chemical constituents of Crocus sativus and their pharmacological activities. Chin. Tradit. Herb. Drug. 2014;45:3015-3028.
- [CrossRef] [Google Scholar]
- Research progress on chemical constituents of Crocus sativus and their pharmacological activities. Chin. Tradit. Herb. Drug. 2014;45:3015-3028.
- [Google Scholar]
- Wang, X., T. Ye, S. Zhou, et al., 2012. Analysis and comparison of the Volatile olis fron Crocus sativus by GC-MS. Natural Product Research and Development. 24, 1239-1241+1260. https://doi.org/10.16333/j.1001-6880.2012.09.018.
- Research progress of hypolipidemic effect to saffron. Chinese J. Clin. Pharmacol.. 2015;31:1218-1220.
- [CrossRef] [Google Scholar]
- Research progres of hypolipidemic effect to saffron. Chinese J. Clin. Pharmacol.. 2015;31:1218-1220.
- [CrossRef] [Google Scholar]
- Crocin has pharmacological effects against the pathological behavior of colon cancer cells by interacting with the STAT3 signaling pathway. Exp. Ther. Med.. 2020;19:1297-1303.
- [CrossRef] [Google Scholar]
- Determination of crocin-Ⅰ and crocin-Ⅱ in different parts of Crocus sativus by HPLC. Chinese Trad. Patent Med.. 2019;41:1102-1105.
- [Google Scholar]
- Bacteriostatic effect of Quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Prot.. 2018;81:68-78.
- [CrossRef] [Google Scholar]
- Crocetin promotes clearance of amyloid-β by inducing autophagy via the STK11/LKB1-mediated AMPK pathway. Autophagy. 2021;17:3813-3832.
- [CrossRef] [Google Scholar]
- Antidepressant activity of crocin-I is associated with amelioration of neuroinflammation and attenuates oxidative damage induced by corticosterone in mice. Physiol. Behav.. 2019;212:112699
- [CrossRef] [Google Scholar]
- Safranal, an active constituent of saffron, ameliorates myocardial ischemia via reduction of oxidative stress and regulation of Ca(2+) homeostasis. J. Pharmacol. Sci.. 2020;143:156-164.
- [CrossRef] [Google Scholar]
- Antidiabetic potential of saffron and its active constituents. J. Cell Physiol.. 2019;234:8610-8617.
- [CrossRef] [Google Scholar]
- Comparative study of composition of essential oil from stigmas and of extract from corms of Crocus sativus. Chem. Nat. Compd. - CHEM. NAT. COMPD.. 2008;44:666-667.
- [CrossRef] [Google Scholar]
- Anti-depression mechanism of Croci stigma based on network pharmacology. Acad. J. Shanghai Univ. Trad. Chinese Med.. 2020;34:70-75.
- [CrossRef] [Google Scholar]
- Crocetin suppresses angiogenesis and metastasis through inhibiting sonic hedgehog signaling pathway in gastric cancer. Biochem. Biophys. Res. Commun.. 2021;576:86-92.
- [CrossRef] [Google Scholar]
- Monoterpene aldehydes and isophorone-related compounds of saffron. Phytochemistry. 1971;10:2755-2761.
- [CrossRef] [Google Scholar]
- Activity of antioxidants from Crocus sativus L. petals: potential preventive effects towards cardiovascular system. Antioxidants (Basel). 2020;9
- [CrossRef] [Google Scholar]
- Medicinal herbs for the treatment of anxiety: a systematic review and network meta-analysis. Pharmacol. Res.. 2022;179:106204
- [CrossRef] [Google Scholar]
- Edible flowers: Review of flower processing and extraction of bioactive compounds by novel technologies. Food Res. Int.. 2019;126:108660
- [CrossRef] [Google Scholar]
- Extraction of petals of Crocus sativus and antioxidant activity investigation. Food Res. Dev.. 2013;34:29-33.
- [Google Scholar]
- Chemical constituents and bioactivities of the liposoluble fraction from different medicinal parts of Crocus sativus. Pharm. Biol.. 2011;49:756-763.
- [CrossRef] [Google Scholar]
- Comparative metabolomics analysis of stigmas and petals in Chinese Saffron (Crocus sativus) by widely targeted metabolomics. Plants (Basel). 2022;11
- [CrossRef] [Google Scholar]
- Zhou, J., G. Xie and X. Yan, 2011. Encyclopedia of Traditional Chinese Medicines - Molecular Structures, Pharmacological Activities, Natural Sources and Applications
- Crocin inhibits the migration, invasion, and epithelial-mesenchymal transition of gastric cancer cells via miR-320/KLF5/HIF-1α signaling. J. Cell Physiol.. 2019;234:17876-17885.
- [CrossRef] [Google Scholar]
- Zhu, A., 2019. Synthesis, Metabolism and Anti-Alzheimer's Disease Investigations of Crocetin Glucuronide 硕士, Zunyi Medical University
- Saffron reduced toxic effects of its constituent, safranal, in acute and subacute toxicities in rats. Jundishapur J. Nat. Pharm. Prod.. 2014;9:3-8.
- [CrossRef] [Google Scholar]
- An evaluation of the effects of saffron supplementation on the asthma clinical symptoms and asthma severity in patients with mild and moderate persistent allergic asthma: a double-blind, randomized placebo-controlled trial. Respir. Res.. 2019;20:39.
- [CrossRef] [Google Scholar]







