Translate this page into:
Studies on hydrolysis/alcoholysis/ammonolysis mechanisms of ethylene terephthalate dimer using DFT method
⁎Corresponding authors. jbhuang@gzmu.edu.cn (Jinbao Huang), Dongcw126@gzmu.edu.cn (Changwen Dong)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
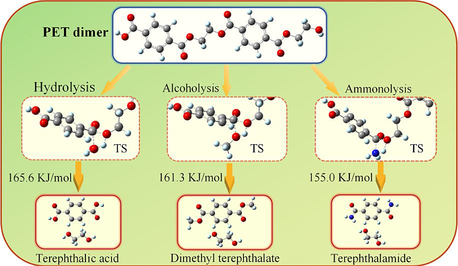
Hydrolysis/alcoholysis/ammonolysis are considered as viable routes for efficient degradation and recycling of polyethylene terephthalate waste plastic. The possible hydrolysis/alcoholysis/ammonolysis pathways for the degradation of ethylene terephthalate dimer were investigated by the density functional theory (DFT) method M06/cc-pVDZ. The geometric structure optimization and frequency calculation of various intermediates, transition states and products involved in the reaction were carried out to obtain thermodynamic and kinetic parameter values. The results show that the reaction energy barrier of the cracking process of ethylene terephthalate dimer can be reduced under the conditions of hydrolysis/alcoholysis/ammonolysis, which makes the reaction easier. Additionally, at 298 K and 1 atm, the total rate constants of hydrolysis/alcoholysis/ammonolysis are 1.51 × 10-43 cm3 molecular-1s−1, 1.86 × 10-41 cm3 molecular-1s−1, and 2.82 × 10-40 cm3 molecular-1s−1, respectively. The hydrolysis products of ethylene terephthalate dimer are mainly terephthalic acid and ethylene glycol; the alcoholysis products of ethylene terephthalate dimer are dimethyl terephthalate and ethylene glycol; and ammonolysis products mainly include terephthalamide and ethylene glycol. Furthermore, this study shows that the Gibbs free energy change of hydrolysis/alcoholysis/ammonolysis is negatively correlated with temperature and an increase in temperature enhance the spontaneity of the reaction.
Keywords
Ethylene terephthalate dimer
Density functional theory
Hydrolysis
Alcoholysis
Ammonolysis
Reaction mechanism
1 Introduction
Polyethylene terephthalate (PET) is one of the most common and significant thermoplastics. Due to its excellent physical and chemical properties, such as being lightweight, having good heat resistance and dimensional stability, and being resistant to chemicals, among others, it is widely used in various industries (Das et al., 2021; Jankaukaite et al., 2016; Samak et al., 2020). With the mass production and application of PET plastic, the amount of PET plastic waste has increased sharply, which has not only seriously polluted the environment but also caused a waste of resources (Al-Sabagh et al., 2016; Raheem et al., 2019; Sojobi et al., 2016). Due to its enduring stability, a substantial portion of utilized PET requires several centuries to naturally degrade (Wei et al., 2017). Furthermore, the degradation process can lead to the fracturing of PET into particles and even microplastics. These microplastics accumulate in soil and even flow into rivers, posing threats to the health and safety of both aquatic and terrestrial life (Patidar et al., 2023a, 2023b; Perumpully et al., 2023). In the context of carbon neutrality, the degradation and recycling of PET plastic waste have become important issues around the world (Lee et al., 2022). On the one hand, the chemical conversion of PET waste plastic has a high atom economy, which can greatly reduce the impact of plastic waste on the environment. On the other hand, it also extends the service life of PET plastics. Therefore, effective recycling of valuable chemical raw materials from PET waste plastic is of great significance in contributing to a circular economy and reducing the environmental impact of plastic waste. Many conversion processes have been developed to recycle waste PET. Pyrolysis is a method of converting high-molecular-weight polymers into recyclable minor molecular compounds under anaerobic conditions. For instance, PET can decompose into 4-(methoxycarbonyl) benzoic acid and benzoic acid by pyrolysis, while caprolactam is the main pyrolysis product of nylon 6. These monomers can then be purified and reused in the production of new PET or nylon polymers. (Kumartasli et al., 2020; Wang et al., 2023). In recent years, pyrolysis has been considered one of the most effective methods to deal with PET waste plastic, which has important practical significance for reducing energy pressure and environmental pollution (Ayodeji and Oni, 2019; Dhahak et al., 2020; Xue et al., 2017). However, the process of polymer pyrolysis into small molecule compounds requires higher temperatures, and the complex product components result in a high cost and difficulty of recycling waste plastic raw materials. An appropriate change in the degradation conditions can effectively control the composition and distribution of the products and reduce energy consumption in the process of degradation (Barth et al., 2015; Songip et al., 1994). Water (H2O) is the most important and non-toxic solvent in nature, which comes from a wide range of sources and does not even need to be removed from the products (Sako et al., 1997). Methanol (CH3OH) is an important organic chemical material and plays an important role in the decomposition reaction medium (Genta et al., 2007). Ammonia (NH3) is a reducing gas, and the presence of ammonia can promote the breaking of acyl-oxygen bonds in the ester bonds of polymer backbones to form chemical intermediates with terminal hydroxyl groups (Yang, 2012). Therefore, hydrolysis/alcoholysis/ammonolysis have been considered as one of the most effective feasible proposals to deal with PET waste plastic.
At present, there have been many macroscopic experimental studies on PET hydrolysis/alcoholysis/ammonolysis (Abedsoltan et al., 2023; Du et al., 2020). Ügdüler et al. (2020) have demonstrated the efficient recycling of multilayer and colored PET plastic waste through a two-step aqueous alkaline hydrolysis process. They successfully converted PET waste into monomers such as ethylene glycol and terephthalic acid, achieving yields of up to 95 % under mild conditions (≤80 °C, atmospheric pressure). Čolnik et al. (2021) performed hydrolysis experiments on PET waste using sub- and supercritical water in a batch reactor. The results show that a hydrolysis temperature of 300 ℃ can obtain a high yield and purity of terephthalic acid. Ambrose-Dempster et al. (2023) have significantly improved the degradation efficiency of PET through a mechanoenzymatic hydrolysis approach. Pereira et al. (2023) used post-consumer PET hydrolyzed in pure water and at neutral PH over a wide range of temperatures (190–400 °C) and pressures (1–35 MPa) to produce terephthalic acid in a short reaction time. Yao et al. (2021) discovered the reaction mechanism of the PET alcoholysis collaborative catalytic system and obtained the action mechanism of PET at different interfaces of zinc oxide (ZnO) and the first-order reaction kinetics of PET alcoholysis. The experimental results showed that ZnO/SBA-15 has high catalytic activity. Yang et al. (2012) investigated the ammonolysis of PET catalyzed by metal function ionic liquid and showed that the ammonolysis of PET could generate the corresponding terephthalamide and ethylene glycol. Xie et al. (2024) effectively converted PET into terephthalonitrile with a high yield of 32.5 % at 550 °C using urea-assisted in-situ catalytic pyrolysis. They also elucidated the pivotal role of active ammonia in PET depolymerization and found that the ammonolysis of PET was more likely to form amide rather than aromatic ammonium salt. A recent study by Liang et al. (2024) has made significant strides in this area by developing a catalyst-free ammonolysis process for the conversion of PET waste into terephthalamide with a one-step reaction process at 40–120 ℃. This approach can simplify the recycling process by eliminating the need for catalysts and solvents, yielding more than 95 mol% and more than 95 % purity of terephthalamide.
Compared with the experimental research, the theoretical study by quantum chemistry methods can interpret the PET hydrolysis/alcoholysis/ammonolysis reaction processes and the formation and evolution mechanisms of the product in more detail. A better understanding of the mechanisms underlying PET hydrolysis/alcoholysis/ammonolysis at the molecular level is very important for optimizing these methods. Hence, it is necessary to further study its mechanism in order to efficiently converting PET into high-value chemical raw materials.
PET plastic is a polymer with the ester bond formed by the condensation of terephthalic acid and ethylene glycol (Zimmermann et al., 2020). The microscopic mechanisms of polymers degradation are usually studied by various model compounds (Huang et al., 2022), and ethylene terephthalate dimer contains all the chemical characters appearing in PET, thus, ethylene terephthalate dimer was selected as a PET model compound in order to study the hydrolysis/alcoholysis/ammonolysis mechanisms of PET in this work. We carried out thermodynamic calculations using density functional theoretical methods, and analysis of the possible reaction paths in hydrolysis/alcoholysis/ammonolysis of ethylene terephthalate dimer. Finally, we calculated the effect of temperature on the main pathways of hydrolysis/alcoholysis/ammonolysis. These studies of the thehydrolysis/alcoholysis/ammonolysis mechanisms of ethylene terephthalate dimer using quantum chemical method are intended to be an important guide for energy conversion and recycling technology for PET waste.
2 Computational methods
2.1 Electronic structure calculations
All density functional theory (DFT) calculations were performed using Gaussian 16 program (Frisch et al., 2016). Previous studies have shown that the M06 meta function performs exceptionally well in databases involving main-group thermochemistry, kinetics, and noncovalent interactions (Zhao et al., 2008). Therefore the geometries of reactions, intermediates, transition states, and products were optimized using the density functional M06 method using the cc-pVDZ base set. The above methods have been successfully applied to the mechanistic study of pyrolysis of organic compounds (Maftei et al., 2018; Wang et al., 2023a, 2023b). Although this study employed conventional hybrid DFT method (M06) for geometry optimization and calculation of thermodynamic and kinetic parameters, it is important to note that the latest research shows newer range-separated DFT methods (such as CAM-B3LYP and wB97X) give more accurate calculations of HOMO-LUMO gaps compared to conventional hybrid functionals (Senan et al., 2023). The frequency was calculated at the same calculation level to obtain the thermodynamic parameter values at the standard conditions (298.15 K, 1 atm), and the zero vibration energy (ZPE) correction is considered for the thermodynamic quantity. The vibration frequencies of the reactants, intermediates, and products are all positive, and all transition states have one and only one imaginary frequency, which is verified by a GaussView program or calculation of intrinsic reaction coordinates (IRC) to ensure the correct transition states for the products and reactants. The enthalpy energy difference between the transition state (ETS) and the reactant (ER) under 298 K and 1 atm is taken as the potential barrier of the reaction (ΔE), and the enthalpy energy difference between the product (EP) and the reactant (ER) is taken as the enthalpy change of the reaction. We assumed that all the decomposition pathways are in the ground state (single state).
2.2 Kinetic computations
Canonical transition state theory (TST) (Bao and Truhlar, 2017) and the Eckart correction was used to calculate the rate constant from equation (1) by the kinetic program KiSThelP (Canneaux et al., 2014):
In equation (2), σrot,R and σrot,TS are the rotational symmetry numbers of reactants and transition states. Additionally, zero point energies were scaled by a factor of 0.984 to reduce systematical error and account for anharmonic effect (Alecu, 2010).
3 Results and discussion
3.1 Hydrolysis mechanisms of PET dimer
PET hydrolysis studies have shown that PET hydrolysis is mainly carried out through the synergistic reaction mechanism of Cacyl-O bond breaking in the main chain (Wang et al., 2011; Han et al., 2018; Ügdüler et al., 2020; Pereira et al., 2023). In addition, H2O can dissociate weakly into H+ and OH-. Based on the study by Xu et al. (2020), the influence of H+ produced by the ionization of H2O is fairly limited, so we ignored the ionization of water during the calculation. Therefore, this paper mainly considers the concerted reaction mechanisms through 4-membered cyclic transition states for the Cacyl-O bond breaking in the main chain of PET dimer, and we design three possible reaction pathways, as shown in Scheme 1 (Path (1)). The optimized structures of the transition states are shown in Fig. 1, and the reaction energy barriers are shown in Fig. 2.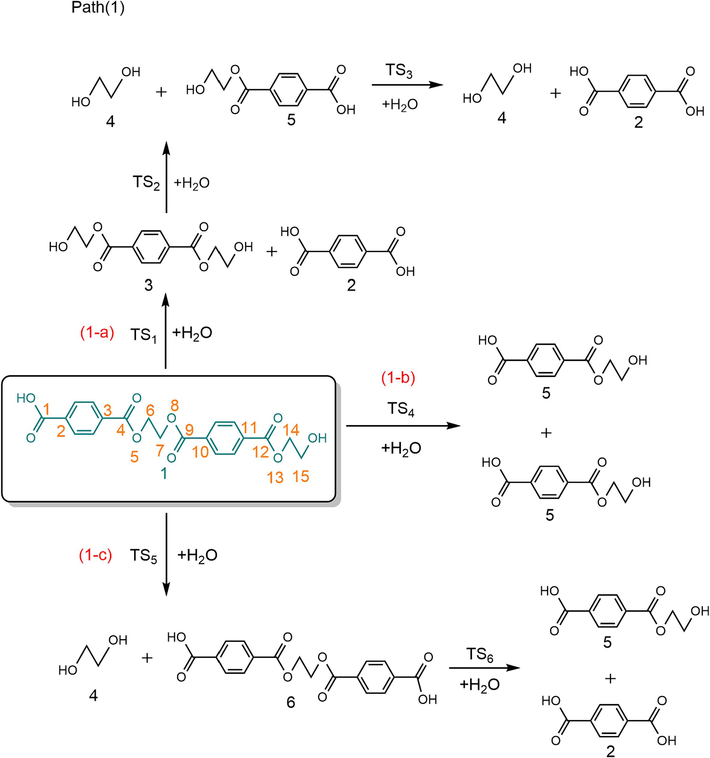
Possible reaction paths for PET dimer hydrolysis.
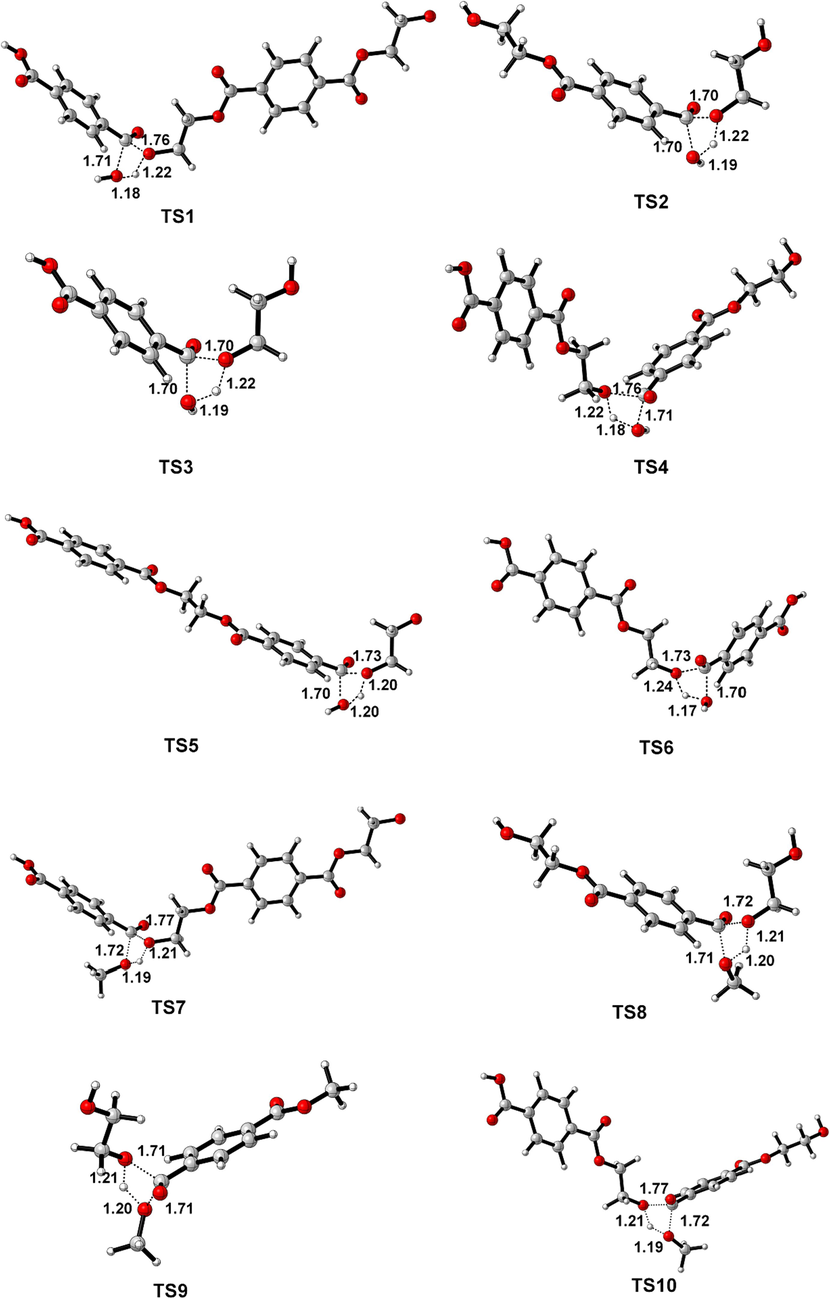
Geometrically optimized structures of all transition state. The crucial distance (Å) are marked.
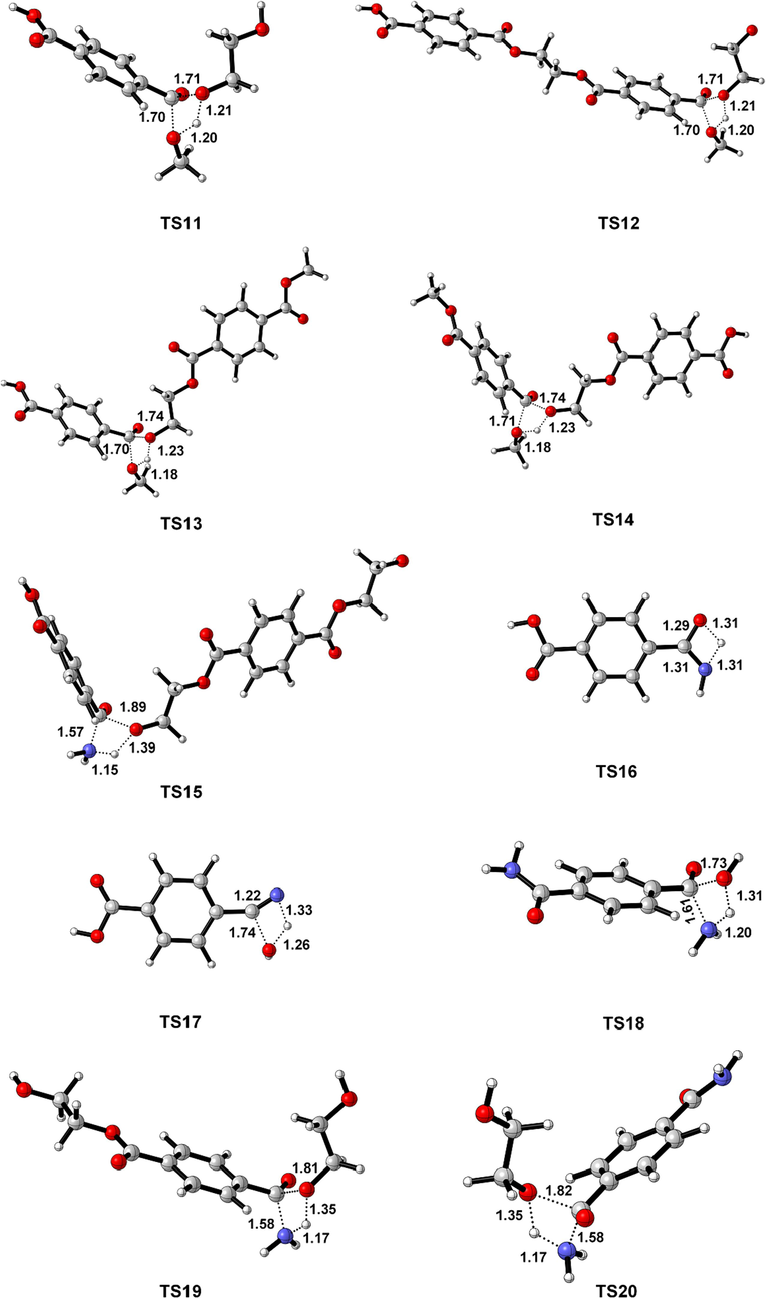
Geometrically optimized structures of all transition state. The crucial distance (Å) are marked.
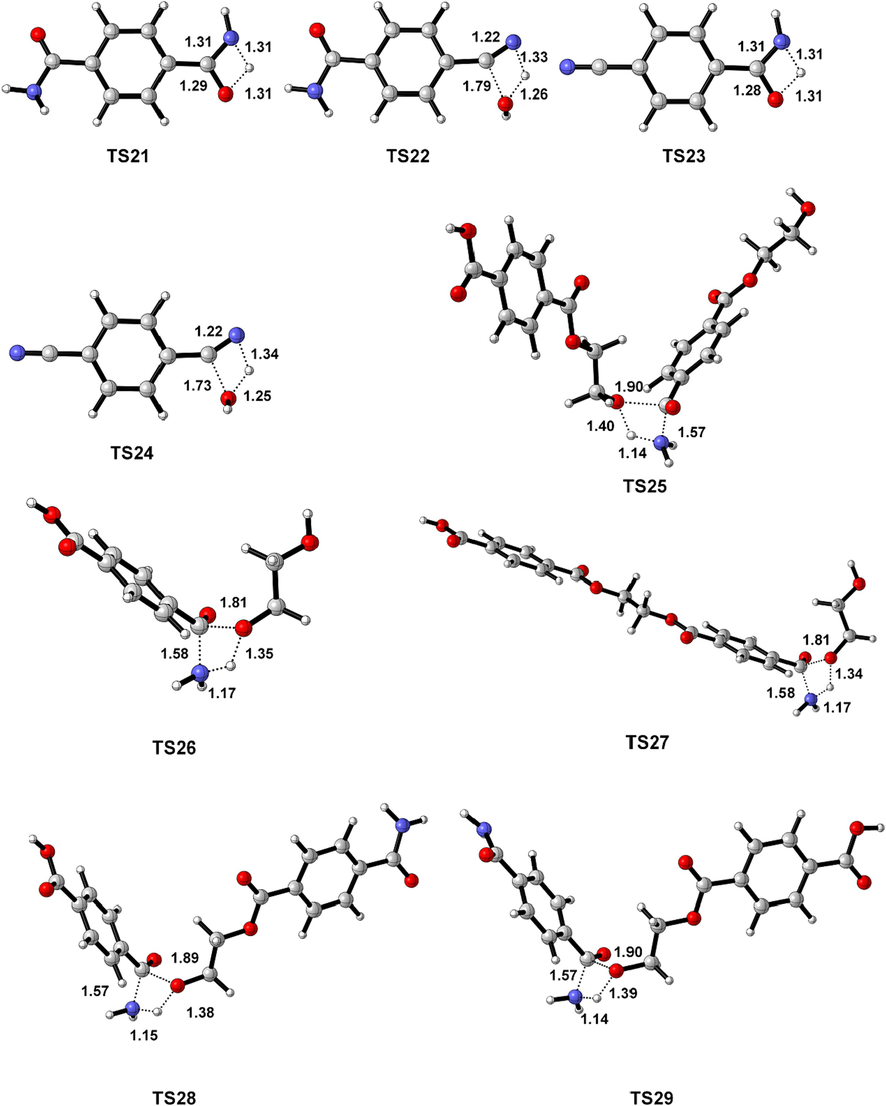
Geometrically optimized structures of all transition state. The crucial distance (Å) are marked.
Reaction energy barriers for the hydrolysis of PET dimer.
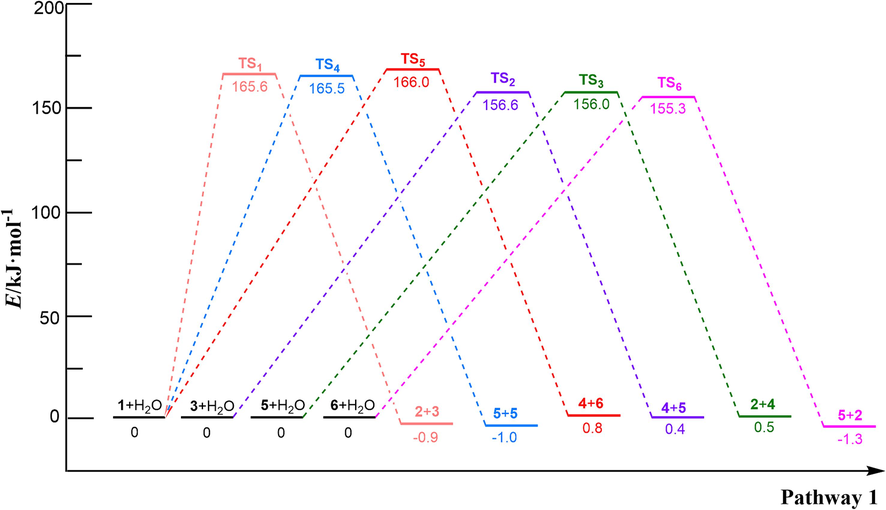
In the reaction pathway (1-a), PET dimer 1 reacts with H2O through a 4-membered cyclic transition state (TS1) to form terephthalic acid 2 and bis (2-hydroxyethyl) benzene-1,4-dicarboxylate 3. As shown in Fig. 1, the length of the O–H bond in H2O molecules was 0.960 Å, and the length of the Cacyl-O bond (C(4)-O(5)) in PET dimer was 1.347 Å; nevertheless, the length of the O–H bond in transition state TS1 increased to 1.183 Å, and the length of the Cacyl-O bond (C(4)-O(5)) is increased to 1.759 Å. In this hydrolysis reaction mechanism, –OH in H2O would attack the C(4) atom in the Cacyl-O bond, causing the cleavage of the C(4)-O(5) bond. At that time, –OH binds to the carbon atom C(4) of the carbonyl group to form terephthalic acid, while the remaining H atom binds to O(5) to form a hydroxyl terminal compound. This process requires overcoming a barrier height of 165.6 kJ/mol and emitting a lower heat of 5.2 kJ/mol. Bis (2-hydroxyethyl) benzene-1,4-dicarboxylate 3 can hydrolyzes to ethylene glycol 4 and ethylene terephthalate 5 via TS2, with an energy barrier of 156.6 kJ/mol. Next, the ethylene terephthalate 5 is further degraded into the main PET products, i.e., terephthalic acid 2 and ethylene glycol, 4 in the presence of H2O via TS3. In the reaction pathway (1-b), PET dimer 1 can also be hydrolyzed into ethylene terephthalate 5 via the 4-membered cyclic transition state (TS4), with an energy barrier of 165.5 kJ/mol. In reaction pathway (1-c), PET dimer 1 decomposes into ethylene glycol 4 and 1,2-bis-(4-carboxy-benzoyloxy)-ethane 6 in the presence of H2O via TS5, with an energy barrier of 166.0 kJ/mol. Compound 6 can be further hydrolyzed via TS6 leading to the formation of terephthalic acid 2 and ethylene terephthalate 5, with a slightly lower energy barrier (155.3 kJ/mol).
In the hydrolysis of PET dimer, the energy barriers for hydrolysis at the positions in the middle and terminal of the main chain are essentially the same, which are 165.6, 165.5, and 166.0 kJ/mol, respectively. This result showed that the hydrolysis reaction of PET may be caused by the random cracking of the Cacyl-O bond in the main chain, resulting in the formation of main products such as terephthalic acid 2 and ethylene glycol 4, which is consistent with the results of related PET hydrolysis experiments (Wang et al., 2011; Han et al., 2018; Ügdüler et al., 2020; Pereira et al., 2023).
3.2 Alcoholysis mechanisms of PET dimer
Based on related alcoholysis experiments (Lorenzetti et al., 2006; Dimitrov et al., 2013; Shojaei et al., 2020), the alcoholysis reaction of PET dimer may involve three distinct routes, as illustrated in Scheme 2. The optimized structures of transition states are shown in Fig. 1 and reaction energy barriers are shown in Fig. 3. In the reaction path (2-a), PET 1 and CH3OH pass through the 4-centered transition state (TS7) to form mono-methyl terephthalate 7 and bis (2-hydroxyethyl) benzene-1,4-dicarboxylate 3, which need to overcome a barrier height of 161.3 kJ/mol with an exothermic of −6.6 kJ/mol. As shown in Fig. 1, the O–H bond length of CH3OH molecule was optimized to 0.964 Å, and the length of the Cacyl-O bond (C(4)-O(5)) in PET dimer was 1.347 Å; whereas the length of the O–H bond becomes 1.189 Å, and the length of the acyl oxygen bond (C(4)-O(5)) becomes 1.771 Å in the transition state TS7. In this alcoholysis reaction mechanism, –OCH3 in CH3OH would attack the C(4) atom in the carbonly group, causing the breakage of the C(4)-O(5) bond. At the same time, the remaining H atom in CH3OH could combine with the O(5) atom, and then Bis (2-hydroxyethyl) benzene-1,4-dicarboxylate 3 and mono-methyl terephthalate 7 were obtained. Bis (2-hydroxyethyl) benzene-1,4-dicarboxylate 3 is further alcoholized to generate ethylene glycol 4 and diethylene glycol terephthalate 8 via TS8, with a barrier height of 147.2 kJ/mol. Next, diethylene glycol terephthalate 8 can decompose into ethylene glycol and dimethyl terephthalate 9 with the participation of CH3OH via TS9, which are the main products of PET alcoholysis. The required activation energy is 147.0 kJ/mol. In the reaction path (2-b), PET dimer is able to form diethylene glycol terephthalate 8 and ethylene terephthalate 5 with the participation of CH3OH via TS10, and ethylene terephthalate 5 is further alcoholized and decomposed into mono-methyl terephthalate 7 and ethylene glycol 4 via TS11. The energy barriers of the above processes are 161.0 kJ/mol and 146.7 kJ/mol, respectively. In the reaction path (2-c), PET dimer 1 and CH3OH can be degraded into ethylene glycol 4 and compound 10 via TS12, with a reaction energy barrier of 145.5 kJ/mol. Compound 10 is further alcoholized by TS13 to form mono-methyl terephthalate 7 and diethylene glycol terephthalate 8 with an energy barrier of 145.8 kJ/mol. In addition to this, compound 10 can also be alcoholized to generate dimethyl terephthalate and ethylene glycol by TS14, with an energy barrier of 145.8 kJ/mol, which is identical to that of TS13.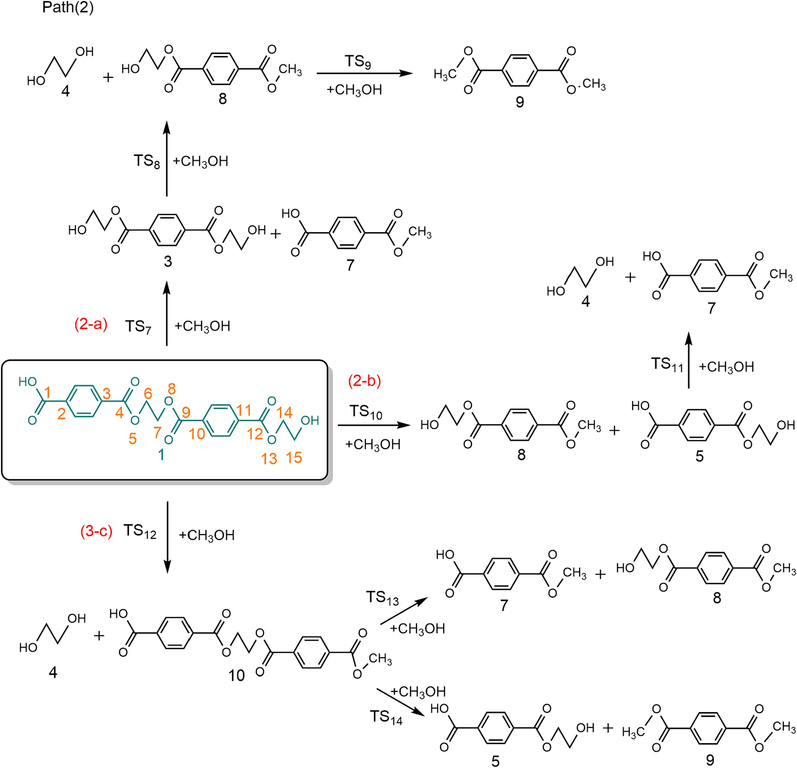
Possible reaction paths for ethylene terephthalate dimer alcoholysis.
Possible reaction paths for ammonolysis of PET dimer.
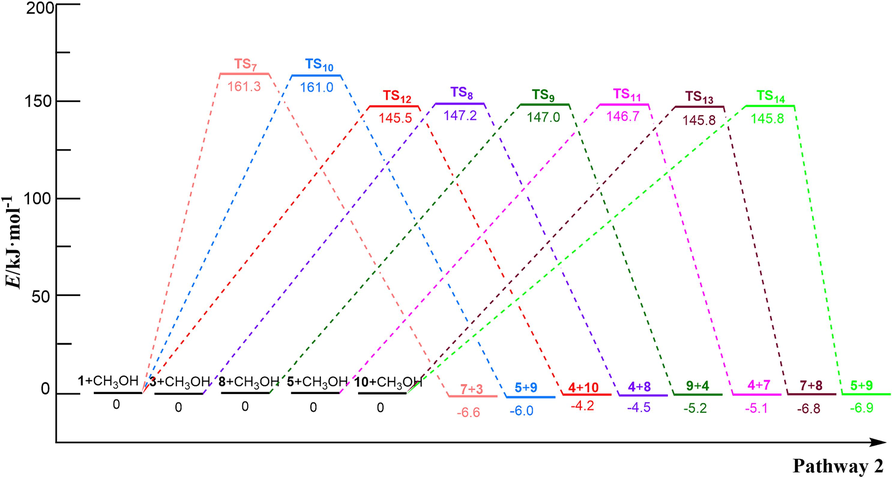
The energy barrier for PET dimer alcoholysis reaction
In the alcoholysis of PET dimer, the reaction energy barriers for the breakage of the middle or terminal Cacyl-O bonds in the main chain is 145.5–161.3 kJ/mol. This indicates that the alcoholysis process of PET dimer may be multiple alcoholysis of the ester bond at any position of the main chain, leading to the formation of the main products (such as dimethyl terephthalate 9 and ethylene glycol 4), which is basically in accordance with the results of the related PET alcoholysis experiments (Lorenzetti et al., 2006; Dimitrov et al., 2013; Shojaei et al., 2020).
3.3 Ammonolysis mechanism of ethylene terephthalate dimer
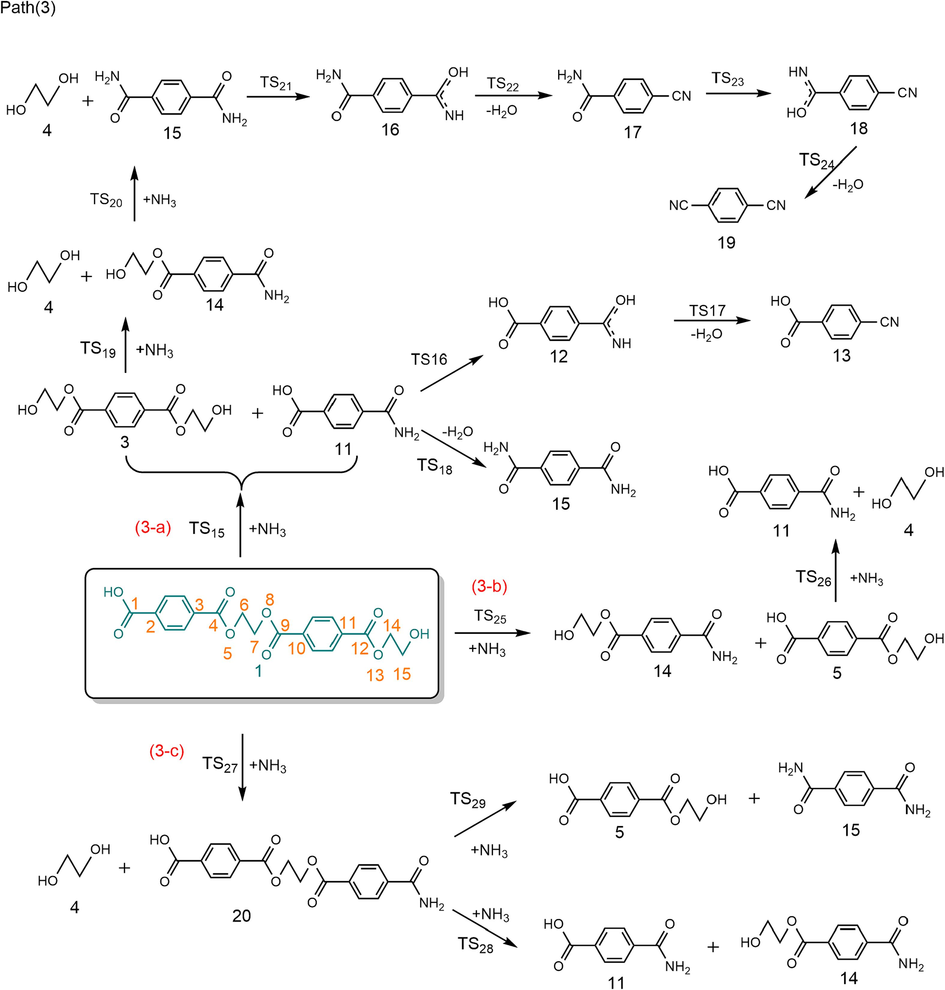
According to the experimental results of PET ammonolysis under an ammonia atmosphere (Spychaj et al., 2001; Shukla and Harad, 2006; Xie et al., 2024), three possible reaction paths for the ammonolysis of PET dimer were designed as shown in Scheme 3. Fig. 1 shows the optimal structure of the transition states; Fig. 4 and Fig. 5 describe a schematic diagram for the reaction energy barriers of PET dimer ammonolysis. In the reaction pathway (3-a), PET dimer 1 ammonolysis to terephthalic acid monoamide 11 and bis (2-hydroxyethyl) benzene-1,4-dicarboxylate 3 via TS15. Before the reaction, the N–H bond length of the NH3 molecule was optimized to be 1.022 Å, and the length of the Cacyl-O bond (C(4)-O(5)) in the PET dimer was 1.347 Å. In the transition state, the N–H bond length of TS15 becomes 1.146 Å, and the acyl oxygen bond (C(4)-O(5)) bond length becomes 1.894 Å. In this reaction mechanism, –NH2 in NH3 would attack the C(4) atom in the Cacyl-O bond to form a terephthalic acid monoamide, while the remaining H atom combines with O(5) to form a bis (2-hydroxyethyl) benzene-1,4-dicarboxylate, and the C(4)-O(5) bond would be broken. This process required overcoming a barrier of 148.3 kJ/mol, which is in correct agreement with the value obtained by Xie et al. (2024) at the B3P86/6–31++G(d,p) level of theory (151.5 kJ/mol). Terephthalic acid monoamide 11 undergoes H transfers (TS16) in the amino group to form compound 12 by overcoming an energy barrier of 165.7 kJ/mol and endothermic by 59.5 kJ/mol. And then, –OH and the H (on the N atom) of compound 12 dehydration through β-elimination (TS17) yield H2O and 4-cyanobenzoic acid 13, with reaction energy barriers of 305.6 kJ/mol and endothermicity of 70.4 kJ/mol. Note that the barrier heights given by Xie et al. (2024) are 174.5 and 310.9 kJ/mol, in agreement with our values. Further, a ammonolysis reaction may occur in terephthalic acid monoamide 11 to form terephthalamide 15 and H2O by TS18 (170.4 kJ/mol). In comparison, reactions for dehydration (β-elimination) and H transfers have high energy barriers as well as being heat absorbing, which are not advantageous. This signifies that PET dimer readily generates terephthalamide 15 through the ammonolysis reaction. Xie et al. (2024) reached the same conclusion through theoretical calculation. This result is also consistent with the experimental observation of Liang et al. (2024). Bis (2-hydroxyethyl) benzene-1,4-dicarboxylate 3 is degraded to ethylene glycol 4 and compound 14 with the participation of NH3 through TS19, with a reaction energy barrier of 154.6 kJ/mol. Compound 14 was further ammolyzed to form ethylene glycol and terephthalamide 15 by TS20, with an energy barrier of 154.7 kJ/mol. Terephthalamide 15 isomerizes to compound 16 through H transfers (TS21) in amino group by overcoming an energy barrier of 165.2 kJ/mol and endothermic by 60.6 kJ/mol. Subsequently, compound 16 dehydration (TS22) to form 4-Cyanobenzamide 17 and H2O, the energy barriers is 306.2 kJ/mol. It can be seen from the optimized structures of TS21 and TS22 that the H atom from the amide group transfers from the N atom to the O atom to form an OH group, and then dehydration can occur between the OH group and the H atom on the N atom to form 4-cyanobenzamide 17. For TS20, Xie et al. (2024) computed energy barriers equal to 164.0 kJ/mol, in good agreement with our values. However, for TS21 and TS22, large differences are observed (17.6 and 13.9 kJ/mol). In the same way, 4-cyanobenzamide 17 can undergo H transfer (TS23) and dehydration reaction (TS24), leading to the formation of H2O and 1,4-diisocyanobenzene 19 with energy barriers of 166.9 kJ/mol and 307.3 kJ/mol, respectively. As expected,dehydration remains the most difficult process, requiring an energy barrier of more than 300 kJ/mol. This indicated that terephthalamide 15 is hard to convert to 1,4-diisocyanobenzene 19, which is consistent with the previous study (Xie et al., 2024). In the reaction pathway (3-b), PET dimer 1 is decomposed into compound 14 and ethylene terephthalate 5 with the participation of NH3 via TS25, which required overcoming a barrier of 148.2 kJ/mol. Ethylene terephthalate 5 was further ammoniated into ethylene glycol 4 and terephthalic acid monoamide 11 via TS26, with an energy barrier of 154.3 kJ/mol. In the reaction pathway (3-c), compounds 1 and NH3 were degraded to ethylene glycol 4 and compound 20 via TS27, with an energy barrier of 155.0 kJ/mol. Compound 20 can be ammoniated by TS28 to form terephthalic acid monoamide 11 and compound 14. An additional possible pathway for compound 20 is TS29, leading to terephthalamide 15 and ethylene terephthalate 5. The reaction energy barriers for TS28 and TS29 are 145.8 kJ/mol and 150.0 kJ/mol, respectively. These intermediates (5, 11, and 14) can easily react by ammonolysis to form terephthalamide 15 further.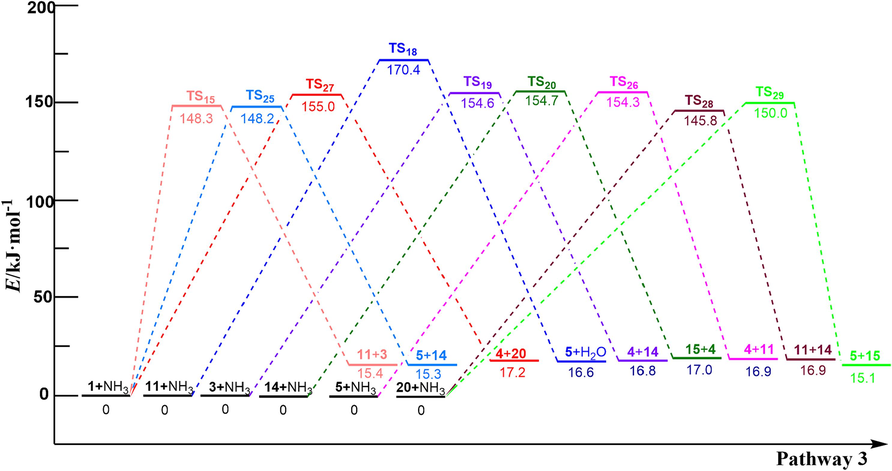
Ammonolysis reaction energy barriers of PET dimer.
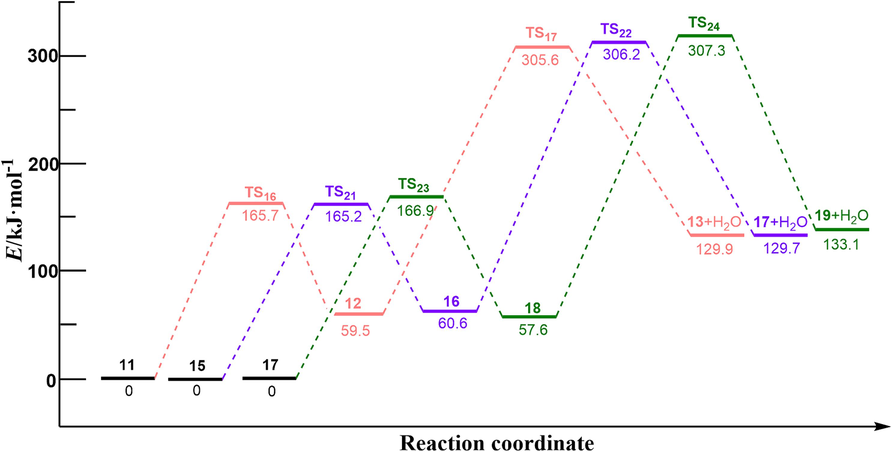
H transfer and dehydration energy barrier in Scheme 3.
In the ammonolysis of PET dimer, the reaction energy barriers of ammonolysis reactions at the Cacyl-O bond in the middle or terminal position of the main chain were 148.3–155.0 kJ/mol. This indicates that the ammonolysis of PET may be caused by multiple degradations of the Cacyl-O bond at any position of the main chain, resulting in the formation of main products such as terephthalamide 15 and ethylene glycol 4, which is basically the same as the results of relevant PET ammonolysis experiments (Spychaj et al., 2001; Shukla and Harad, 2006; Xie et al., 2024).
By comparing the computational results of the hydrolysis/alcoholysis/ammonolysis of the PET dimer to the pyrolysis, it can be seen that the reaction energy barriers of the hydrolysis/alcoholysis/ammonolysis of the PET dimer are lower than the thermal decomposition (the energy barriers of concerted reaction through the six-membered ring and four-membered ring transition states and free radical reaction are 184.0, 267.0, and 345.0 kJ/mol, respectively.) (Huang et al., 2022). As can be observed, hydrolysis/alcoholysis/ammonolysis can reduce the energy barriers of the main reaction steps of the degradation of PET dimer to a certain extent, making the reaction easier to proceed. Besides, calculation data show that the reaction energy barriers for the cleavage of PTE dimer in NH3 atmosphere are the smallest, followed by cracking in CH3OH medium and H2O medium.
3.4 Effect of different temperature on hydrolysis/alcoholysis/ammonolysis mechanisms of PET dimer
3.4.1 Effect of different temperature on hydrolysis/alcoholysis/ammonolysis thermodynamics of PET dimer
This work considers the influence of temperature on the thermodynamics of ethylene terephthalate dimer hydrolysis/alcoholysis/ammonolysis mechanism. To study a reaction from the perspective of thermodynamics, it is only necessary to consider the beginning and end states of the reaction without considering its intermediate process. We calculated the reaction free energy (ΔG) and enthalpy change (ΔH) parameters of the PET dimer degradation process under different temperature conditions (298, 400, 500, 600, 700, 800, and 900 K) to estimate the feasibility of the reaction. The free energy change of the reaction ΔG is an important thermodynamic parameter used to determine the direction of spontaneous reactions and the reactant conversion to products when the reaction reaches equilibrium. When free energy change ΔG is less than zero, the reaction can be spontaneous, and the smaller the reaction ΔG, the greater the conversion of reactant when the reaction reaches equilibrium. From the perspective of reaction energy, ΔH greater than 0 indicates an endothermic reaction, whereas an exothermic reaction (Huang et al., 2015). Table.1 shows the enthalpy change (ΔH), free energy change (ΔG) and entropy change (ΔS) of the degradation process of PET dimer during hydrolysis/alcoholysis/ammonolysis at different temperatures.
T(K)
Hydrolysis/alcoholysis/ammonolysis(Path (1)/ Path (2)/ Path (3))
ΔH/
ΔG/
ΔS/
ΔH/
ΔG/
ΔS/
ΔH/
ΔG/
ΔS/
298
0.0
−15.8
53.1
−16.4
−20.5
13.8
49.4
31.0
61.7
400
−10.5
−28.7
45.5
−16.4
−21.9
13.8
48.6
24.9
59.3
500
−12.6
–33.1
41.0
−16.4
–23.3
13.8
48.4
19.0
58.8
600
−14.1
−37.0
38.0
−16.4
−24.7
13.8
48.4
13.1
58.8
700
−15.3
−40.7
36.3
−16.3
−26.0
13.9
48.6
7.2
59.1
800
−16.1
−44.3
35.3
−16.2
−27.4
14.0
48.9
1.3
59.5
900
−16.8
−47.8
34.4
−16.2
−28.8
14.0
49.1
−4.6
59.7
As can be seen clearly from the data in Table 1, in the hydrolysis of PET dimer ΔG and ΔH are negative above 298 K, and both ΔG and ΔH decrease with increasing temperature. Therefore, it is speculated that the PET hydrolysis reaction is more spontaneous above 298 K and increasing the temperature can increase the spontaneity of PET hydrolysis. In the PET dimer alcoholysis reaction,the temperature dependence of ΔH is limited, which means that the change in ΔH with increasing temperature is small. ΔH and ΔG are always less than zero, and ΔG decrease with increasing temperature, similar to hydrolysis. As a result, it is speculated that PET hydrolysis/alcoholysis could be a type of spontaneous exothermic reaction. In the process of PET dimer ammonolysis, the values of ΔH are positive at all reaction temperatures, indicating an endothermic nature of these chemical reactions so it is necessary to intake thermal energy from outside to push the processes of PET ammonolysis. The value of ΔG goes negative at about 900 K. Therefore, it is speculated that PET ammonolysis can be spontaneous at 900 K or higher. In addition, temperature also has little effect on ΔH in the ammonolysis process of PET dimer. ΔS is always positive in hydrolysis/alcoholysis/ammonolysis, but the entropy effect is more favorable to PET ammonolysis. The revelation of this result may help chemical scientists optimize the chemical recovery process of PET by developing a more rational reaction temperature, and applying it in practical industrial. For example, increasing the reaction temperature to about 900 K during PET ammonolysis can tilting the chemical balance toward the product. This will benefit to reduced the required of catalytic, increased the yield, thus the production cost can be reduced and the efficiency improved. However, it is also necessary to pay attention to the increase in equipment costs and possible side effects caused by rising temperatures.
3.4.2 Effect of different temperature on hydrolysis/alcoholysis/ammonolysis kinetics of PET dimer
The conventional TST was used to obtain the rate constants of the reactions of PET hydrolysis/alcoholysis/ammonolysis in the KiSThelP program. The rate constants were computed using frequencies, energy barriers, and the geometries of the transition states, and the Eckart correction was applied. The rate constants at 298 K for the initial reactions of PET hydrolysis/alcoholysis/ammonolysis are given in Table 2. Additionally, the branching ratios (R) of the initial reactions of hydrolysis/alcoholysis/ammonolysis at 298 K are also listed in Table 2. The branching ratios are calculated by dividing the reaction rate constants for each initial reaction by the total reaction rate constant (R = k/ktot), and the hydrolysis, alcoholysis, and ammonolysis are calculated separately.
Paths
k298K
R
ktot
PET dimer + H2O → IM3 + IM2
4.85E-44
0.321
ktot_hydrolysis = 1.51E-43
PET dimer + H2O → 2IM5
9.09E-44
0.602
PET dimer + H2O → IM4 + IM6
1.15E-44
0.077
PET dimer + CH3OH → IM3 + IM7
1.73E-43
0.009
ktot_alcoholysis = 1.86E-41
PET dimer + CH3OH → IM8 + IM5
4.63E-44
0.002
PET dimer + CH3OH → IM4 + IM10
1.84E-41
0.988
PET dimer + NH3 → IM3 + IM11
1.14E-40
0.403
ktot_ammonolysis = 2.82E-40
PET dimer + NH3 → IM14 + IM5
1.50E-40
0.534
PET dimer + NH3 → IM4 + IM20
1.77E-41
0.063
To assess the relative importance of the different conditions for the degradation of PET dimer at different temperatures, the total rate constants for the hydrolysis/alcoholysis/ammonolysis pathways (ktot_hydrolysis, ktot_alcoholysis, and ktot_ammonolysis) were calculated over a range of temperatures, from 298 to 498 K in 20 K increments, as shown in Fig. 6. The results show that the total rate constants of hydrolysis/alcoholysis/ammonolysis increases with increasing temperature in the rage of 298–498 K. Total rate constants of the ammonolysis reactions of PET are generally one to two orders of magnitude higher than hydrolysis and alcoholysis at all temperatures. This indicates that ammonolysis is more likely to occur kinetically than hydrolysis and alcoholysis. The kinetic findings are also consistent with the above reaction mechanisms.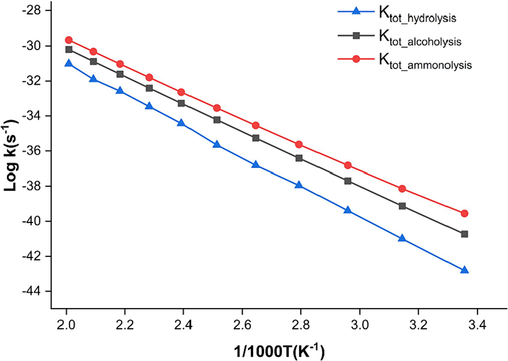
Calculated rate constants for the initial reaction of PET hydrolysis/alcoholysis/ammonolysis between 298 and 498 K and 1 atm.
This study employs DFT methods to calculate thermo-kinetic parameters to explain the degradation mechanism of PET plastic waste. However, the DFT methods are an approximate approach for solving the electronic structure of multi-electron system. This could potentially result in reduced precision of the computed outcomes and the introduction of errors, thereby affecting the comprehensive comprehension and forecasting of chemical reactions. Additionally, it is not pragmatic to calculate the thermo-kinetic parameters for PET polymers due to the high cost of DFT computation. The PET model compound (PET dimer) was used to loosely explain the degradation mechanism of PET polymers. Despite the fact that achieving quantitative correctness in macromolecular systems is still a challenge, the qualitative insights provided by our theoretical calculations are reliable. Moreover, our study offers a valuable theoretical framework that can guide future experimental and computational research in the field of PET recycling.
4 Conclusions
In this study, the elementary steps associated with hydrolysis/alcoholysis/ammonolysis pathways for the common and significant thermoplastics PET, were successfully probed via DFT method M06/cc-pVDZ, analyzed their thermodynamic and kinetic feasibility. Mechanism study shows that the main products of hydrolysis of ethylene terephthalate dimer are terephthalic acid and ethylene glycol; the main products of alcoholysis are dimethyl terephthalate and ethylene glycol; and the main products of ammonolysis are terephthalamide and ethylene glycol. The analysis about thermodynamics and kenitics reveals that the energy barrier for the degradation process of PET dimer can be reduced under hydrolysis/alcoholysis/ammonolysis conditions compared to pure pyrolysis, facilitating the reaction. And a rise in reaction temperatures can boost the spontaneity of the reaction and the reaction rate constants during the hydrolysis/alcohololysis/ammonolysis of PET. This study can serve as a theoretical model and guide for experimental and industrial applications in the filed of PET recycling.
Despite the theoretical potential of hydrolysis/alcohololysis/ammonolysis, hydrolysis/alcohololysis/ammonolysis usually requires catalysts and high reaction pressures to increase the reaction rate, so there are still some challenges in practical applications. Future research needs to concentrate on screening catalysts, optimizing reaction conditions, improving product recovery rates, and reducing overall processing costs.
CRediT authorship contribution statement
Jinbao Huang: Data curation, Writing – original draft. Weifeng Xu: Visualization, Investigation. Yang Long: Visualization, Investigation. Yan Zhu: Writing – review & editing. Song Chen: Writing – review & editing. Wenjing Duan: Conceptualization, Methodology. Jiankai Ou: Conceptualization, Methodology. Hong Wang: Formal analysis. Changwen Dong: Formal analysis. Shuang Tian: Supervision.
Acknowledgments
This research was supported by Research Fund of Guizhou Minzu University, China [No. GZMUZK[2023]YB07, GZMUZK[2023]YB05], Science and Technology Funds of Guizhou province, China [No. ZK(2021)278], and Construction Project of Characteristic Key Laboratory in Guizhou Colleges and Universities, China [No. KY(2021)003].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A focused review on recycling and hydrolysis techniques of polyethylene terephthahate. Polym. Eng. Sci.. 2023;63:2651-2674.
- [CrossRef] [Google Scholar]
- Computational thermochemistry: scale factor databases and scale factors for vibration frequencies obtained from electronic model chemistries. J. Chem. Theory Comput.. 2010;6:2872-2887.
- [CrossRef] [Google Scholar]
- Al-Sabagh, A.M., Yehia, F.Z., Eshaq, G., Rabie, A.M., EIMetwally, A.E., 2016. Greener routes for recycling of polyethylene terephthalate. Egypt. J. Petrol. 25, 53-64. https://doi.org/10.1016/j.ejpe.2015.03.001.
- Mechanoenzymatic reactions for the hydrolysis of PET. RSC Adv. 2023;13:9954.
- [CrossRef] [Google Scholar]
- Thermal pyrolysis production of liquid fuel from a mixture of polyethylene terephthalate and polystyrene. Heat Transfer-Asian Re.. 2019;48:1648-1662.
- [CrossRef] [Google Scholar]
- Variational transition state theory: theoretical framework and recent developments. Chem. Soc. Rev.. 2017;46:7548-7596.
- [CrossRef] [Google Scholar]
- Effect of hydrolysis products on the enzymatic degradation of polyethylene terephthalate nanoparticles by a polyester hydrolase from Thermobifida fusca. Biochem. Eng. J.. 2015;93:222-228.
- [CrossRef] [Google Scholar]
- KiSThelP: a program to predict thermodynamic properties and rate constants from quantum chemistry results. J. Comput. Chem.. 2014;35:82-93.
- [CrossRef] [Google Scholar]
- Sub-and supercritical water for chemical recycling of polyethylene terephthalate waste. Chem. Eng. Sci.. 2021;233:116389
- [CrossRef] [Google Scholar]
- Plastic recycling of polyethylene terephthalate (PET) and polyhydroxybutyrate (PHB)-A comprehensive review. Mate. Circ. Econ.. 2021;3:9.
- [CrossRef] [Google Scholar]
- Real time monitoring of slow pyrolysis of polyethylene terephthalate (PET) by different mass spectrometric techniques. Waste Manage.. 2020;106:226-239.
- [CrossRef] [Google Scholar]
- Analysis of recycled PET bottles products by pyrolysis-gas chromatography. Polym. Degrad. Stabil.. 2013;98:972-979.
- [CrossRef] [Google Scholar]
- ZnO nanodispersion as pseudohomogeneous catalyst for alcoholysis of polyethylene terephthalate. Chem. Eng. Sci.. 2020;220:115642
- [CrossRef] [Google Scholar]
- Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Petersson, G.A., Nakatsuji, H., Li, X., Caricato, M., Marenich, A.V., Bloino, J., Janesko, B.G., Gomperts, R., Mennucci, B., Hratchian, H.P., Ortiz, J.V., Izmaylov, A.F., Sonnenberg, J.L., Williams-Young, D., Ding, F., Lipparini, F., Egidi, F., Goings, J., Peng, B., Petrone, A., Henderson, T., Ranasinghe, D., Zakrzewski, V.G., Gao, J.,Rega, N., Zheng, G., Liang, W., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Throssell, K., Montgomery, J.A., Jr., Peralta, J.E., Ogliaro, F., Bearpark, M.J., Heyd, J.J., Brothers, E.N., Kudin, K.N., Staroverov, V.N., Keith, T.A., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A.P., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Millam, J.M., Klene, M., Adamo, C., Cammi, R., Ochterski, J.W., Martin, R.L., Morokuma, K., Farkas, O., Foresman, J.B., and Fox, D.J., 2016. Gaussian 16, Revision A.03. Gaussian Inc, Wallingford CT.
- Supercritical methanol for polyethylene terephthalate depolymerization: Observation using simulator. Waste Manage.. 2007;27:1167-1177.
- [CrossRef] [Google Scholar]
- Han, M., 2018. Depolymerization of PET bottle via methanolysis and hydrolysis, in: S. Thomas, K. Kanny, M.G. Thomas, A. Rane, V.K. Abitha (Eds.). Recycling of Polyethylene Terephthalate Bottles, Plastics Design Library, pp. 85-108. https://doi.org/10.1016/C2016-0-01084-7.
- A computational study on thermal decomposition mechanism of β-1 linkage lignin dimer. Comput. Theor. Chem.. 2015;1054:80-87.
- [CrossRef] [Google Scholar]
- Insight into the thermal degradation mechanisms of polyethylene terephthalate dimer compound. Chemosphere. 2022;291:133112
- [CrossRef] [Google Scholar]
- Recycled polyethylene terephthalate waste for different application solutions. Environ. Res. Eng. Manage.. 2016;72:5-7.
- [CrossRef] [Google Scholar]
- Kumartasli, S., Avinc, O., 2020. Important step in sustainability: polyethylene terephthalate recycling and the recent developments. Sustain. Tex. Appar. Ind., Turkey, pp. 1-19. https://doi.org/10.1007/978-3-030-38013-7_1.
- A unified view on catalytic conversion of biomass and waste plastics. Nat. Rev. Chem.. 2022;6:635-652.
- [CrossRef] [Google Scholar]
- Valorization of polyethylene terephthalate wastes to terephthalamide via catalyst-free ammonolysis. J. Ind. Eng. Chem.. 2024;132:578-587.
- [CrossRef] [Google Scholar]
- Chemical recovery of useful chemicals from polyester (PET) waste for resource conservation: a survey of state of the art. J. Polym. and Environ.. 2006;14:89-101.
- [CrossRef] [Google Scholar]
- Trends in bond dissociation energies of brominated flame retardants from density functional theory. Struct. Chem.. 2018;29:921-927.
- [CrossRef] [Google Scholar]
- Microplastics as heavy metal vectors in the freshwater environmental: distribution, variations, sources and health risk. Phys. Chem. Earth. 2023;131:103448
- [CrossRef] [Google Scholar]
- Microplastic contamination in water and sediments of Mahanadi River, India: A assessment of ecological risk along rural-urban area. J. Environ. Manage.. 2023;348:119363
- [CrossRef] [Google Scholar]
- An inclusive trend study of evaluation and scientometric analysis of microplastics. Phys. Chem. Earth.. 2023;132:103455.
- [CrossRef] [Google Scholar]
- Neutral hydrolysis of post-consumer polyethylene terephthalate waste in different phases. ACS Sustainable Chem. Eng.. 2023;11:7203-7209.
- [CrossRef] [Google Scholar]
- Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: A review. J. Clean. Prod.. 2019;225:1052-1064.
- [CrossRef] [Google Scholar]
- Depolymerization of polyethylene terephthalate to monomers with supercritical methanol. J. Chem. Eng. Jpn.. 1997;30:342-346.
- [CrossRef] [Google Scholar]
- Recent advances in biocatalysts engineering for polyethylene terephthalate plastic waste green recycling. Environ. Int.. 2020;145:106144
- [CrossRef] [Google Scholar]
- Synthesis, structure charaterization, DFT calculations, and computational anticancer activity investigations of 1-phenyl ethaol derivatives. J. Mol. Struct.. 2023;1294:136323.
- [CrossRef] [Google Scholar]
- Chemical recycling of PET: a stepping-stone toward sustainability. Polym. Advan. Technol.. 2020;31:2912-2938.
- [CrossRef] [Google Scholar]
- Aminolysis of polyethylene terephthalate waste. Polym. Degrad. Stabil.. 2006;91:1850-1854.
- [CrossRef] [Google Scholar]
- Recycling of polyethylene terephthalate (PET) plastic bottle wastes in bituminous asphaltic concrete. Cogent Eng.. 2016;3:1133480.
- [CrossRef] [Google Scholar]
- Kinetic studies for catalytic cracking of heavy oil from waste plastics over REY zeolite. Energy Fuels. 1994;8:131-135.
- [CrossRef] [Google Scholar]
- Aminolysis and aminoglycolysis of waste poly (ethylene terephthalate) J. Mater. Cycles Waste. 2001;3:24-31.
- [CrossRef] [Google Scholar]
- Towards closed-loop recycling of multilayer and coloured PET plastic waste by alkaline hydrolysis. Green Chem.. 2020;22:5376.
- [CrossRef] [Google Scholar]
- Wang, G.Y., Zhang, Z.H., Xu, D., Xing, B., Zhu, L.j., Wang, S.R., 2023. Insight into pyrolysis mechanism of plastic waste with C-O/C-N bonds in the backbone. Sci. Total Environ. 897, 165359. https://doi.org/10.1016/j.scitotenv.2023.165359.
- A mechanistic and kinetic investigation on the oxidative thermal decomposition of decabromodiphenyl ether. Environ. Pollut.. 2023;333:121991.
- [CrossRef] [Google Scholar]
- Theoretical study on pyrolysis mechanism of decabromodiphenyl ether (BDE-209) using DFT method. Chemosphere. 2023;310:136904.
- [CrossRef] [Google Scholar]
- Depolymerization of polyethylene terephthalate in sub- and supercritical water. J. Chem. Eng. Chinese U.. 2011;25:904-910.
- [Google Scholar]
- Biocatalysis as a green route for recycling the recalcitrant plastic polyethylene terephthalate. Microb. Biotechnol.. 2017;10:1302-1307.
- [CrossRef] [Google Scholar]
- Valorization of waste PET: understanding the role of active ammonia in facilitating PET depolymerization and aromatic nitrile formation. J. Clean. Prod.. 2024;434:140204
- [CrossRef] [Google Scholar]
- Directing the simultaneous conversion of hemicellulose and cellulose in raw biomass to lactic acid. ACS Sustain. Chem. Eng.. 2020;8:4244-4255.
- [CrossRef] [Google Scholar]
- Effect of catalyst contact mode and gas atmosphere during catalytic pyrolysis of waste plastics. Energy Convers. Man.. 2017;142:441-451.
- [CrossRef] [Google Scholar]
- PET glycolysis catalyzed by zinc task special ionic liquids. J. Mater. Sci. Eng.. 2012;30:747-751.
- [CrossRef] [Google Scholar]
- Yang, W., 2012. Study on halogen-free synergistic flame retardant and mechanism of polyester composites. PhD dissertation.
- Yao, H.Y., 2021. Study on construction of synergistic catalysis system and reaction mechanism for PET catalytic alcoholysis. Beijing: University of Chinese Academy of Sciences (Institute of Process Engineering, Chinese Academy of Sciences). https://doi.org/10.27550/d.cnki.ghgys.2021.000039.
- Density functionals with broad applicability in chemistry. Acc. Chem. Res.. 2008;41:157-167.
- [CrossRef] [Google Scholar]
- Biocatalytic recycling of polyethylene terephthalate plastic. Phil. T. R. Soc. A. 2020;378:20190273.
- [CrossRef] [Google Scholar]







