Translate this page into:
Study of acetylated EGCG synthesis by enzymatic transesterification in organic media
⁎Corresponding author. zhusong@jiangnan.edu.cn (Song Zhu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
(-)-Epigallocatechin-3-O-gallate (EGCG), the most abundant polyphenolic compound in catechins, exerts excellent physiological effects including antioxidant. However, with its high hydrophilicity and poor lipophilicity, the application of EGCG in oil products is limited. In this study, EGCG acetylated derivatives were prepared by transesterification of EGCG with vinyl ester in acetonitrile/isopropanol (1:1 v/v). Lipase Lipozyme RM IM was found to be the optimum catalyst at concentration of 12 U/g EGCG, with a molar ratio of 1:5 of EGCG to vinyl acetate as the substrates. And 83.2% conversion was obtained after 10 h reaction at 50 °C. Based on the ping-pong model, the kinetic equation was constructed to determine the reaction kinetic parameters. The analysis of the initial rate and progress curve indicated that the transesterification of EGCG and vinyl acetate was kinetically regulated. Two major acetylated derivatives were identified as 5″-O-acetyl-EGCG and 3″, 5″-di-O-acetyl-EGCG by LC-MS/MS and NMR. Their enhanced lipophilicity was confirmed by transmittance test and octanol–water partition coefficient. The antioxidant activity of di-acetylated EGCG was superior to mono-acetylated EGCG and EGCG, but slightly lower than tert-butyl hydroquinone (TBHQ) as determined by peroxide values (POV) and Rancimat test. Acetylated EGCG might be used as a potent antioxidant for controlling oxidation of oil.
Keywords
(-)-Epigallocatechin-3-O-gallate
Acetylation
Lipase
Antioxidant activity
Kinetics
1 Introduction
EGCG accounts for about 50–80% of tea polyphenols (Nagle et al. 2006), has excellent physiological effects including antioxidation (Han, 2014; Vignes et al., 2006), anti-tumor (Du et al., 2012; Johnson et al. 2010) and anti-inflammatory activities (Zhang et al. 2016; Zhong et al. 2012). EGCG has eight phenolic hydroxyl groups, that is highly hydrophilic, hence, its application in oil-based food industry is limited. It has been reported that EGCG derivatives with a modified molecular structure retain its activity and applicability, while its stability, lipophilicity, and bioavailability were significantly improved (Rautio et al., 2008; Sang et al. 2006). Present EGCG modification methods primarily include methylation (Kirita et al., 2010), acylation (Mori et al., 2008) and glycosylation (Dyer et al., 2016) on the phenolic hydroxyl group.
Direct esterification and transesterification are the primary reactions for the enzymatic acylation of polyphenols (Chebil et al. 2006). Transesterification of polyphenols via lipase-mediated catalysis with vinyl ester as acyl donor has been reported previously (Enaud et al. 2004; Mellou et al., 2005). The effects of enzymatic acylation are associated with the properties of the enzyme, substrate properties, and the acyl donor. Milivojević et al. (2017) found that the rate of transesterification between hydroxyl compounds and vinyl esters was 20–100 times higher than that of esterification between hydroxyl compounds and alkyl ester. This is because the vinyl alcohol generated in the process of transesterification can be isomerized into acetaldehyde with low boiling point, which is easy to remove, thus promoting the reaction equilibrium to move towards the direction of ester formation. However, several lipases (Such as from Candida rugosa and Geotrichum candidun) have been recently reported to be inactivated in the presence of acetaldehyde (Sun et al. 2016). When alkyl esters and fatty acids are used as acyl donors, molecular sieves are often used to adsorb and remove the generated ethanol or water to maintain the low water activity required for lipase catalysis. At the same time, the removal of the reaction products also causes the reaction equilibrium moves to the direction of ester formation (Jaiswal and Rathod, 2018). However, limited studies have compared the conversion of EGCG by esterification and transesterification reactions to the best of our knowledge.
The ping-pong bi-bi kinetic mechanism and sequential double substrate reaction mechanism have been proposed to explain lipase-catalyzed reactions. The first product is released before the second substrate binds to the enzyme in the ping-pong bi-bi kinetic mechanism. According to fatty acid esterification, some studies have explained the ordered bi-bi mechanism with a dead-end complex of the substrate in non-aqueous media. Yadav and Lathi (2003) reported that lipase-mediated synthesis of butyl isobutyrate with n-butanol followed the ping-pong bi-bi mechanism with substrate inhibition by n-butanol. While, lipase-mediated synthesis of perlauric acid and citronellol laurate followed an ordered bi-bi mechanism (Yadav and Lathi, 2004). Devi et al. (2017) studied the kinetics of lipase catalyzed transesterification of ethyl caprate and butyric acid. The reaction rate was described in terms of Michaelis-Menten equation with a ping-pong bi-bi mechanism and competitive inhibition by both substrates. Nevertheless, the kinetic model of EGCG and vinyl acetate is still unknown.
In the present study, we aimed to establish a synthesis route for EGCG via enzymatic acylation by comparing the effects of esterification and transesterification of EGCG conversion. The influence of several synthesis parameters, including enzyme screening, reaction temperature, substrates concentration and molar ratio of substrates on the EGCG conversion and the initial reaction rate were studied. The kinetics studies were conducted with EGCG-vinyl acetate transesterification by Lipozyme RM IM. The molecular structures of acetylated EGCG were determined by NMR and LC-MS/MS, and the antioxidant activity of EGCG derivatives were evaluated in edible oils.
2 Materials and methods
2.1 Materials
The lipases: Novozym 435, Lipozyme TL IM, Lipozyme RM IM were supplied by Novozymes (Bagsvaerd, Denmark). EGCG (98.7%) was provided by Ningbo HEP Biotech Ltd. (Ningbo, Zhejiang, China). Vinyl acetate, acetonitrile, ethyl acetate, ethyl propionate, ethyl butyrate, vinyl butyrate, and other analytical reagents were purchased from Sinopharm Group Chemical Reagent Ltd. (Shanghai, China).
2.2 Determination of lipase activity
The lipase activity was assayed as described by Li et al. (2015a,b). One unit (U) of lipase activity was defined as the amount of enzyme which liberated 1 µmol p-nitrophenol per min under assay conditions. The specific activities of Novozym 435, Lipozyme RM IM, and Lipozyme TL IM were determined to be 2.52, 0.60, and 0.75 U/mg, respectively.
2.3 Enzymatic acylation procedure
The acetylation reaction was carried out in accordance with the modification method described by Zhu et al. (2014). EGCG (100 mm), vinyl acetate (adjusted to different molar ratios), a suitable amount of lipase, and 10 mL acetonitrile and isopropanol (v/v, 1:1) as reaction medium were added to a closed reaction bottle. The reaction bottle was placed in a RET heating magnetic stirrer (IKA, Staufen, Germany) and the reaction was carried out at different temperatures. Samples were collected at intervals for high-performance liquid chromatography (HPLC) analysis. Finally, the reaction was terminated and the immobilized enzyme was filtered off. Acetylated EGCG derivatives were obtained by vacuum drying. The experimental design for optimization of reaction conditions was shown in Table 1.
Effect of factors
Conditions of reaction
Lipase concentration (U/g EGCG)
Temperature (℃)
Molar ratio of EGCG to vinyl acetate
Reaction time(h)
Effect of lipase concentration
6, 9, 12, 15, 18
50
1:5
10
Effect of temperature
12
30, 40, 50, 60, 70
1:5
10
Effect of molar ratio
12
50
1:1, 1:2, 1:3, 1:4, 1:5, 1:6, 1:7
10
Effect of reaction time
12
50
1:5
2, 4, 6, 8, 10, 12, 14, 16
2.4 HPLC analytical procedure
The reaction was monitored by an Agilent 1100 series HPLC system (Agilent Technologies Inc., Santa Clara, CA, USA) equipped with a Hypersil ODS C18 column (4.6 mm × 150 mm, 5 μm). The UV detector was set at 280 nm. The temperature of that column was 30 °C. The flow rate was 1.0 mL min−1, and the injection volume was 10 μL. The mobile phase consisted of 0.05% phosphoric acid aqueous (A) and acetonitrile (B). The gradient elution process was as follows: 0–9 min, 25% of B; 9–20 min, 25–50% of B; 20–26 min, 50% of B.
Conversion of EGCG was calculated using the following equation:
Where A0 is the concentration of EGCG in the initial reaction solution; A1 is the residual concentration of EGCG in solution.
2.5 Determination of the initial rate of the reaction
A 0.5 mL aliquot was drawn from the aforementioned reaction system every 0.5 h and then filtered using a 0.45 μm microporous membrane. EGCG concentration was determined by HPLC. Because the EGCG acylation product was a mixture, the number of moles of the resulting product cannot be accurately calculated. Therefore, the initial rate of reaction was defined as the number of moles of EGCG consumed per gram of lipase catalyzed per unit time. The initial reaction rate was calculated as follows:
where v is the initial rate of the reaction, expressed as mmol/g·min; C0 is the EGCG concentration before the reaction, expressed as mmol/L; Ct is the EGCG concentration at time t, expressed as mmol/L; t is reaction time, expressed as min; m is weight of lipase, expressed as g.
2.6 Preparative HPLC purification procedure
The reaction mixture was centrifuged at 4000 rpm for 10 min by an TD6 low speed centrifuge (Herexi Instrument Equipment Ltd., Changsha, Hunan, China). The supernatant containing most of the reaction products was concentrated. Subsequently, the EGCG derivatives were separated and purified using a Waters 2545 series preparatory HPLC (Waters, Milford, MA, USA) equipped with a Waters XBridge Prep C18 column (250 mm × 19 mm i.d., 10 μm). The injection volume was 500 μL, and the flow rate was 10 mL min−1. The mobile phase consisted of 0.1% formic acid aqueous (A) and acetonitrile (B). The gradient elution process was as follows: 0–10 min, 25% of B; 10–20 min, 25–50% of B; 20–26 min, 50% of B. The effluent was monitored at 280 nm. The collected samples were concentrated using a KA-RV10 rotary evaporator (IKA Works GuangZhou, Guangzhou, Guangdong, China), and the excess organic solvent was removed. Finally, the processed samples were freeze-dried using freeze dryer (SCIENTZ-10 N, Ningbo, Zhejiang, China) to obtain the target products.
2.7 Determination of the structure of the reaction products
Mass spectroscopic analysis was performed on a Waters SYNAPT quadrupole time of flight mass spectrometry (Q-TOF MS) instrument (Waters, Milford, MA, USA). The effluent entered the electrospray ionization source in the negative mode. The operating conditions were as follows: capillary voltage of 3.5 kV, cone hole voltage of 45 V, ion source temperature of 110 °C, desolvation gas was set to 500 L/h at a temperature of 400 °C, the cone gas was set to 50 L/h, mass range of 200–2000 m/z. All the acquisition and analysis of data were controlled by Waters MassLynx v 4.1 software.
The 1H and 13C spectra were obtained on a Bruker Avance III 400 MHz NMR spectrometer (Bruker, Karlsruhe, Germany). The purified compounds were obtained using deuterated methanol (CD3OD) as solvent, and the 13C chemical shifts being measured in relation to tetramethylsilane (TMS) (δ = 0).
2.8 Comparison of liposolubility of acetylated EGCG
10 mg of acetylated EGCG, unmodified EGCG, butylated hydroxytoluene (BHT) and TBHQ were weighed and dissolved in 10 mL of chloroform, respectively. The transmittance of the solution at 800 nm was measured by UV-2550 UV–Vis spectrophotometer (Shimadzu, Kyoto, Japan). The lipophilicity of the identified EGCG derivatives was determined as octanol/water partition coefficient (logP) by a shake flask method (Zhu et al. 2014).
2.9 Antioxidant activity of acetylated EGCG in different oils
POV were measured according to AOCS official methods. The antioxidant activity of the EGCG acetylated derivatives and EGCG was compared to TBHQ and BHT. The calculated quantities of each (200 mg/kg) were added to 40 g of oil in conical flask and held at 60 ± 2 °C in the dark. POV were measured every 3 days during 21 days storage period.
2.10 Antioxidant activity evaluated by Rancimat test
Inhibition of lipid peroxidation by acetylated EGCG in sunflower oil was evaluated using Rancimat test. The mearurement was carried out on a Rancimat apparatus 743 (Metrohm AG, Herisau, Switzerland) according to the method by Oueslati et al. (2020). Antioxidant (0.02%, w/w) was added to each sunflower oil. The air flow rate was at 20 L/h, and the temperature was at 120 °C. TBHQ, BHT and EGCG were used as comparative compounds. The induction periods (IP) was determined according to the time when the curve reached the inflection point and the protection factors (PF) were calculated according to the following formula: PF = IP of antioxidant / IP of control.
2.11 Statistical analysis
All data used were analysed by Origin 9.0 software (OriginLab Corp., Northampton, MA, USA). Experimental results were obtained in triplicate, and the data was represented as mean ± SD. Significant differences were evaluated using Statistix 9.
3 Results and discussion
3.1 The pathway for EGCG enzymatic acylation
Different acyl donors, such as ethyl acetate, vinyl acetate, ethyl propionate, ethyl butyrate, and vinyl esters, were used to investigate the effect on EGCG acylation when lipase and acetonitrile/isopropanol (v/v = 1:1) were used as the catalyst and solvent, respectively. Fig. 1 shows EGCG conversion using several acyl donors with EGCG. Upon using vinyl acetate and vinyl butyrate as acyl donors, EGCG displayed relatively high conversion rate (35.3% and 29.8%, respectively). In comparison, EGCG displayed an extremely low conversion rate of 4.3%, 3.3%, and 1.8% upon using ethyl acetate, ethyl propionate, and ethyl butyrate, respectively.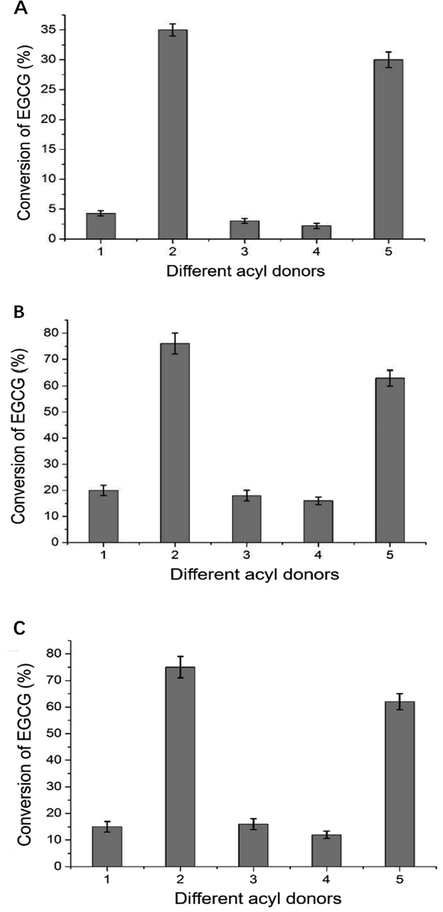
The conversion of EGCG with different acyl donors and lipase. (A) Novozym 435; (B) Lipozyme RM IM; (C) Lipozyme TL IM (1:Ethyl acetate; 2:Vinyl acetate; 3:Ethyl propionate; 4:Ethyl butyrate; 5:Vinyl butyrate).
Moreover, the conversion of EGCG by Lipozyme RM IM and Lipozyme TL IM as catalysts was generally higher than that using Novozym 435 (Fig. 1). However, the overall trend was that the conversion of transesterification between vinyl ester and EGCG was significantly higher than that of the esterification between alkyl ester and EGCG. According to the results, it was concluded that the transesterification reaction of vinyl ester as the acyl donor increased the EGCG acylation conversion.
3.2 Reaction conditions optimization
The concentrations of lipase, as a catalyst for transesterification, directly affects the progress of the reaction. Achieving high esterification rates with low lipase concentration is important. Lipozyme RM IM was selected as the catalyst to assess EGCG acylation. The effects of varying lipase concentrations on the conversion of EGCG in acetonitrile/isopropanol (1:1 v/v) system are shown in Fig. 2A. While lipase concentrations were increased from 6 U/g EGCG to 12 U/g EGCG, the EGCG conversion rate increased almost linearly, indicating saturation of the lipase with the substrate. This phenomenon was consistent with other lipase transesterification reactions (Celiz and Daz, 2011). The conversion rate tends to increase with an increase in lipase concentration, probably because a high lipase concentration increases the chance of contact with the substrate and increases the reaction rate in a certain range of lipase concentration. However, with further increase in lipase concentration, the effect of viscosity on the conversion system was intensified, thereby the effective intermolecular collision of the enzyme was decreased. Thus, no further improvements were observed in the conversion rate. Considering the economic efficiency and yield, an lipase concentration of 12 U/g EGCG was selected.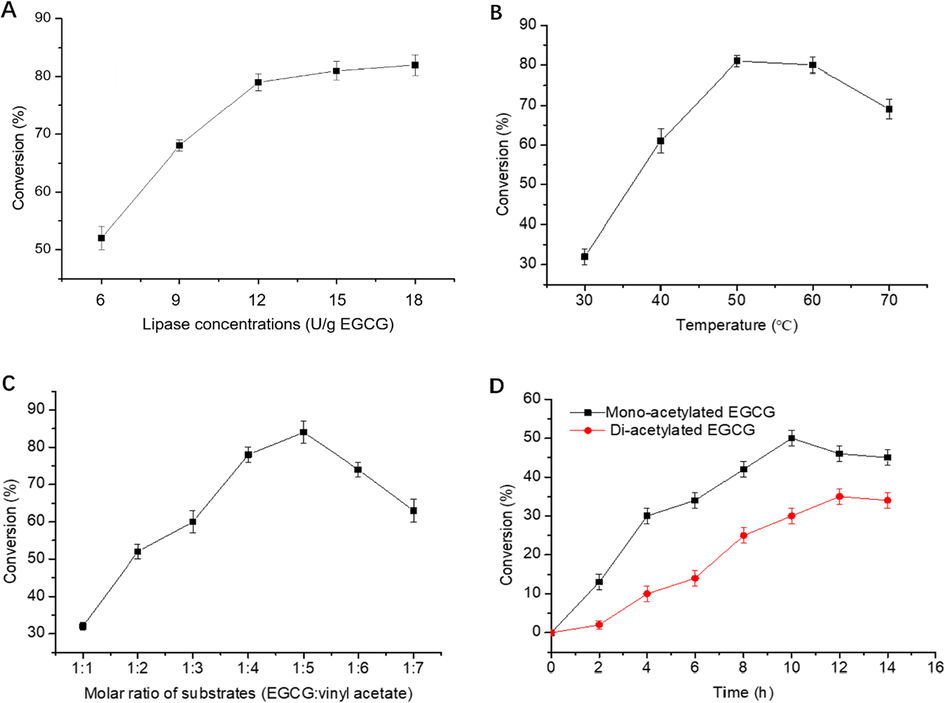
The effect of lipase concentration (A), reaction temperature (B), molar ratio of EGCG to vinyl acetate (C) and reaction time (D) on the conversion of EGCG.
The effect of temperature on the enzymatic acetylation of EGCG was assessed under different reaction temperatures, including 30 °C, 40 °C, 50 °C, 60 °C and 70 °C (Fig. 2B). The EGCG conversion rate displayed an increasing pattern with an increase in temperature from 30 °C to 50 °C and peaked at 50 °C with the conversion of 83.2%. However, it reduced to 78.5% with a further increase in temperature up to 60 °C. The increasing temperature has a considerable effect on lipase stability and activation (Tomke and Rathod, 2016). A low reaction temperature is not conducive to the enzymatic reaction and mass transfer between the reactants. With an increase in reaction temperature, the mass transfer speed of the reaction system increased, and lipase activity was gradually optimized. However, an excessively high temperature tends to deactivate lipase, thereby decrease the EGCG conversion rate (Yadav and Dhoot, 2009).
The effect of molar ratio of EGCG to vinyl acetate on the conversion of EGCG was investigated. The conversion of EGCG reached its peak of 83.6% at a 1:5 substrate ratio after 10 h, and conversion rate is descended to 73.9% at 1:6 (Fig. 2C), probably because excess of vinyl acetate prevented itself from entering the active site of lipases to act as acyl receptors. Electron-donating combined with steric hindrance effects accounted for the overall esterification reaction rate. Similar effects have also been reported (Sun et al., 2009). In the case of reaction efficiency, the ideal substrate ratio for the higher conversion of EGCG was 1:5 (EGCG/vinyl acetate, w/w).
Fig. 2D shows that at a certain temperature (50 °C), the conversion of EGCG increased linearly with time within 0–10 h. Thereafter, the EGCG conversion rate decreased gradually. Analysis by HPLC showed that acetylated EGCG derivatives was a mixture of mono- and di-acetylated EGCG. In the initial stage of the reaction, the main products were mono-acetylated EGCG, while the di-acetylated EGCG was less. With the reaction proceeded, mono- and di-acetylated EGCG were both increased. After 10 h of reaction, the total conversion rate of EGCG reached the highest, with the content of mono-acetylated EGCG and di-acetylated EGCG were 51.1% and 30.2%, respectively.
3.3 Initial reaction rate
The effects of different lipase concentrations on the initial reaction rate of acetylated EGCG were measured within 1 h of the reaction. Fig. 3A clearly shows that the initial reaction rate increased linearly with an increase in lipase concentrations between 3 U/g EGCG and 18 U/g EGCG. It apparently indicated that the internal diffusion effect of immobilized enzyme had a limited effect on the transesterification process and the reaction was intrinsically kinetically dominated. A similar reaction pattern has been observed for lipase-catalyzed esterification reactions (Gogoi et al. 2008). Thus, the effect of immobilized lipase diffusion on initial reaction rate can be ignored in the establishment of reaction kinetics model (Romero et al. 2007).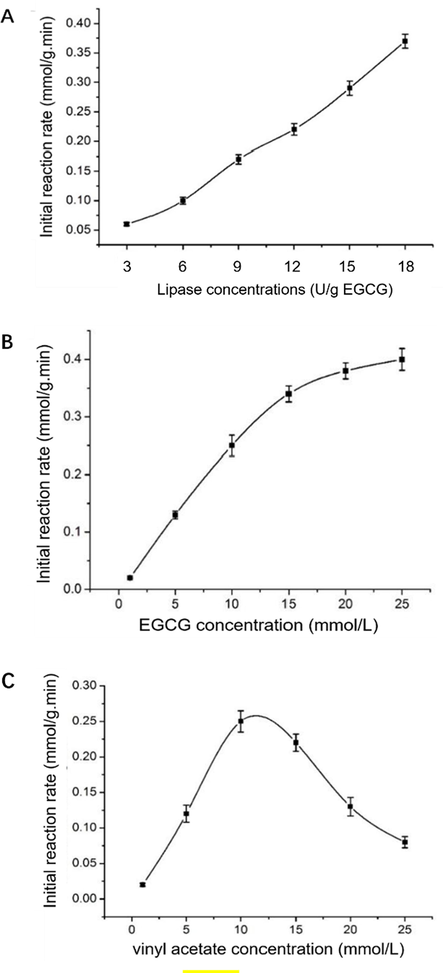
The effects of lipase concentration (A), EGCG concentration (B) and vinyl acetate concentration (C) on the initial reaction rate.
The effect of varying EGCG concentrations on the initial velocity was measured at an initial reaction time of 1 h. Fig. 3B shows that the initial reaction velocity continuously increased with an increase in EGCG concentration and peaked at the critical EGCG concentration. When the concentration of EGCG was above 15 mmol/L, the slope of the curve slightly decreased, and the increasing initial reaction velocity decreased gradually, probably owing to the saturation of EGCG bounding to the active site of the immobilized lipase. Simultaneously, it was deduced from the curve that excess EGCG failed to cause substrate inhibition within the concentration range of EGCG used herein.
Analogously, the effect on the initial the reaction rate was determined at vinyl acetate concentrations from 1 mmol/L to 25 mmol/L. The initial reaction velocity displayed a perspicuous increasing–decreasing trend with the increasing in vinyl acetate concentration (Fig. 3C). Vinyl acetate concentration peaked (0.25 mmol/g·min) at 11 mmol/L, indicating that the active site of the immobilized lipase was completely saturated with the substrate. The initial reaction rate rapidly decreased and substrate inhibition occurred when the vinyl acetate concentration was over 11 mmol/L. Therefore, in order to avoid substrate inhibition caused by excessive vinyl acetate, its concentration should be selected within 11 mmol/L in subsequent kinetic model studies.
3.4 Kinetic model
The initial reaction rate increased at the range of low concentrations of vinyl acetate and decreased at the range of high concentrations (>11 mmol/L). Furthermore, there is no substrate inhibition in the range of EGCG concentration (1–25 mmol/L). It indicated that the reaction of EGCG and vinyl acetate is a two-substrate bimodal reaction (Aljawish et al., 2014; Ledford et al., 2017). This reaction mechanism was in accordance with the double-substrate ping-pong mechanism. Therein, the reaction process was not formed the ternary complex but the binary complex (Yu et al. 2008). Based on the ping-pong mechanism, vinyl acetate (A) initially forms an EA binary complex with lipase (E) as an acyl donor, and EA bind to EGCG (B) to form the ternary complex E.Ac.BP, which then releases vinyl alcohol P to form the intermediate E.Ac .B. Finally, E.Ac.B is converted to EQ and releases the desired product Q, while simultaneously releasing the lipase E.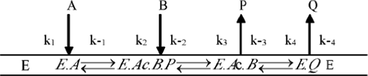
Consistent with the requirements of the kinetic study, the assumptions were as follows: (i) lipase concentration is lesser than the substrate concentration; thus, complex formation cannot cause a significant change in substrate concentration; (ii) at the beginning of the reaction, no reversible product formation occurs; (iii) during the reaction, lipase concentration remains stable.
Transesterification is consistent with the Michaelis-Menten equation:
Where v is the initial velocity of the reaction; Kma and Kmb are the kinetic constants of EGCG and vinyl acetate, respectively; [A] and [B] are the EGCG and vinyl acetate concentrations, respectively.
The kinetic constants of the enzyme represent enzyme affinity to the substrate whose value is the substrate concentration at which the reaction rate reaches half of Vmax. The transformation of equation (1) into Lineweaver-Burk double-reciprocal equation can be described as follows (Li et al., 2015a,b):
According to equation (2), upon maintaining a constant vinyl acetate concentration and changing the EGCG concentration, a series of straight lines are obtained with 1/v as the abscissa and 1/v max as the intercept and Kma/Vmax as the slope, from which the kinetic parameters of the reaction can be calculated.
Upon maintaining a constant EGCG concentration and varying the vinyl acetate concentration, the relationship curve between the initial reaction rate and vinyl acetate concentration was obtained (Fig. 4A). The increasing concentration of EGCG and vinyl acetate, increased the initial velocity of the reaction, confirming that the hypothetical substrate is not inhibited. Taking the reciprocal of the initial velocity of the reaction as the ordinate, the reciprocal of vinyl acetate concentration is the double-reciprocal fitting curve of the abscissa, and a series of substantially parallel straight lines are obtained (Fig. 4B). Specific parameters are shown in Table 2.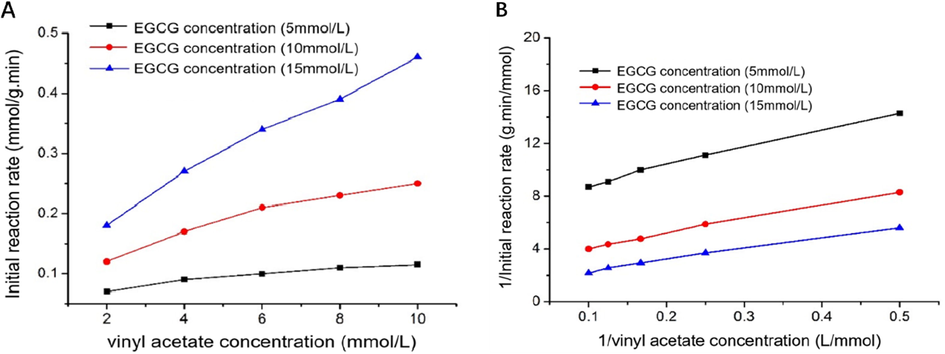
Effect of vinyl acetate concentration and EGCG concentration on the initial reaction rate (A); Double-reciprocal fit plot of the initial reaction rate with vinyl acetate concentration (B).
Concentration of EGCG (mmol/L)
Slope
Intercept
R2
5
13.78
4.02
0.993
10
10.70
2.01
0.996
15
8.33
1.49
0.993
The mean values of the slope of the three fitting parameters are acquired from equation (2) = 10.93. Since the intercept of the fitted line is , to intercept the mapping, it can be concluded that = 19.24, =0.16. Upon solving simultaneous equations, Vmax = 6.25 mmol/g.min, Kma = 68.31 mmol/L, Kmb = 120.25 mmol/L, ultimately, the kinetic equation of lipase Lipozyme RM IM catalyzed transesterification kinetic equation of EGCG and vinyl acetate can be expressed as follows:
3.5 Identification and structure elucidation of EGCG derivatives
EGCG derivatives were purified by prep-HPLC and the fractions 1, 2 and 3 were identified by MS and NMR. The molecular ion peak of fraction 1 was 456.8, which is consistent with [M−H]- of EGCG (Fig. 5A). Therefore, fraction 1 was EGCG without reaction. The molecular ion peak of fraction 2 was 498.8 (Fig. 5B), and one hydroxyl hydrogen was replaced by acetyl group (molecular weight: 42). The fragment ion peaks located at m/z 168.9 and 210.9 corresponding to [M−289]- and [M−289−42]-, respectively. Derived from cleavage of the (-)-epigallocatechin portion (molecular weight: 289). Similarly, the molecular ion peak of fraction 3 is 540.8 (Fig. 5C), with two hydroxyl hydrogen substituted by acetyl groups. The fragment ion peaks m/z 498.8 and 456.8 derived from the molecular ion peak that losses one or two mass of 42, representing the ions of [M−42]- and [M−2 × 42]-, respectively. On the base of the presence of the molecular ion and fragments in the mass spectrum, fraction 2 and 3 were confirmed to be mono-acetylated EGCG and di-acetylated EGCG, respectively.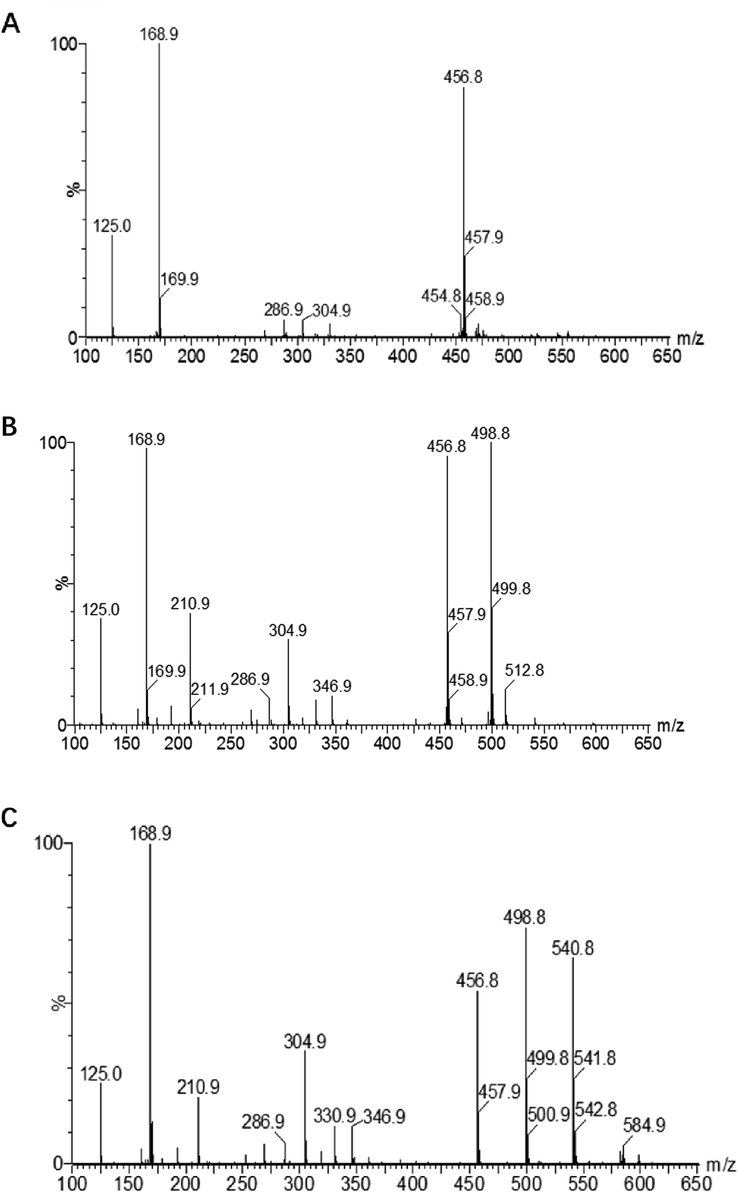
Mass spectra of fraction 1(A) , fraction 2 (B) and fraction 3(C).
1H NMR (400 MHz, CD3OD) spectral data of fraction 2: δ 6.98 (s, 2H, H-2′’, H-6′’), 6.56 (s, 2H, H-2′, H-6′), 6.02 (s, 2H, H-6, H-8), 5.56 (s, 1H, H-3), 5.02 (s, 1H, H-2), 3.10 (m, 2H), 2.06 (s, 3H). 13C NMR (120 MHz, CD3OD) spectral data of fraction 2: δ 173.46 (COO-5′’), 167.56 (COO), 157.73 (C-7), 157.67 (C-9), 156.10 (C-5), 146.55 (C-3′’, C-5′’), 145.15 (C-3′, C-5′), 139.70 (C-4′’), 133.66 (C-4′), 130.70 (C-1′), 121.37 (C-1′’), 110.17 (C-2′’, C-6′’), 106.79 (C-2′, C-6′), 99.34 (C-10), 96.44 (C-8), 95.79 (C-6), 78.38 (C-2), 69.82 (C-3), 26.72 (C-4), 20.40 (–CH3).
1H NMR (400 MHz, CD3OD) spectral data of fraction 3: δ 6.90 (s, 2H, H-2′’, H-6′’), 6.51(s, 2H, H-2′, H-6′), 5.96 (s, 2H, H-6, H-8), 5.50 (s, 1H, H-3), 4.94 (s, 1H, H-2), 3.02 (m, 2H), 2.02 (6H, –2CH3); 13C NMR (120 MHz, CD3OD) spectral data of fraction 3: δ 173.50 (COO-5′’), 170.92 (COO-3′’), 167.62 (COO), 157.76 (C-7), 157.70 (C-9), 156.18 (C-5), 146.64 (C-3′’, C-5′’) , 146.22 (C-3′, C-5′), 139.78 (C-4′’), 133.80 (C-4′), 130.76 (C-1′), 121.42 (C-1′’), 110.22 (C-2′’, C-6′’), 106.82 (C-2′, C-6′), 99.40 (C-10), 96.42 (C-8), 95.82 (C-6), 78.52 (C-2), 69.98 (C-3), 26.82 (C-4), 20.50 (–2CH3).
By comparing the chemical shifts of EGCG and EGCG acylation products, the chemical shifts at C-3″ and C-5″ decreased significantly, confirmed that hydroxyl groups at C-3″ and C-5″ were replaced by acetyl groups. The two acetylated derivatives were identified as 5″-O-acetyl-EGCG and 3″, 5″-di-O-acetyl-EGCG by LC-MS/MS and NMR. The reaction scheme was shown in Fig. 6.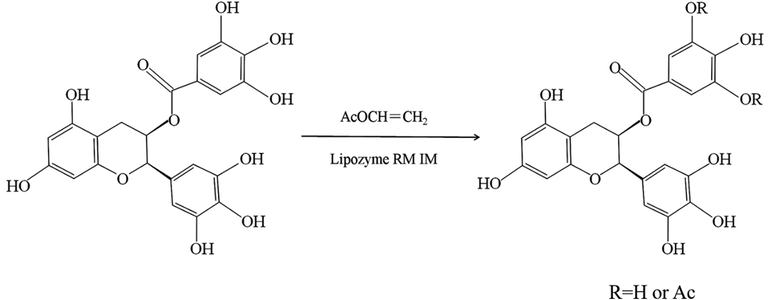
The reaction scheme of EGCG acetylated derivatives by lipase-catalyzed transesterifications.
3.6 Lipophilicity of EGCG derivatives
When the sample was dissolved in a low polarity solvent (such as chloroform), the spectrophotometer was used to determine the transmittance of the solution (or suspension). The higher the transmittance, the stronger the lipophilicity of the sample. The transmittance of EGCG group was 17% and that of acetylated EGCG group was 85%. It showed that the liposolubility of acetylated EGCG was obviously better than that of EGCG, and that of BHT, TBHQ group was over 90%. Therefore, the liposolubility of acetylated EGCG was significantly higher than that of EGCG, slightly lower than that of BHT and TBHQ.
LogP can also evaluated the lipophilicity of acetylated EGCG derivatives. The higher LogP value indicated that high lipophilic properties. As expected, the LogP of EGCG derivatives was higher than that of EGCG. The LogP of EGCG, mono- and di-EGCG were 1.89 ± 0.24, 2.46 ± 0.28 and 3.57 ± 0.38, respectively. With the increase of the number of hydroxyl substituents, The LogP increased gradually, the polarity became weaker and the lipophilicity increased.
3.7 Antioxidant activity of acetylated EGCG in different oils
The antioxidant properties of EGCG, EGCG derivatives and TBHQ in homogeneous oil were evaluated using soybean oil and corn oil without exogenous antioxidants. Hydroperoxides, the primary oxidation products of oils, were measured and represented as POV. The higher the POV, the more serious oxidation of oil. According to soybean oil in Fig. 7A, the POV of the blank sample increased rapidly after 21 days of storage, and reached the maximum value (49.32 mmol/kg). The POV of EGCG, mono-acetylated EGCG, di-acetylated EGCG and TBHQ were 32.01, 21.52, 16.67 and 11.14 mmol/kg, respectively. Acetylated EGCG also showed strong antioxidant activity in corn oil Fig. 7B. The antioxidant activity of acetylated EGCG were higher than that of EGCG, and slightly lower than TBHQ.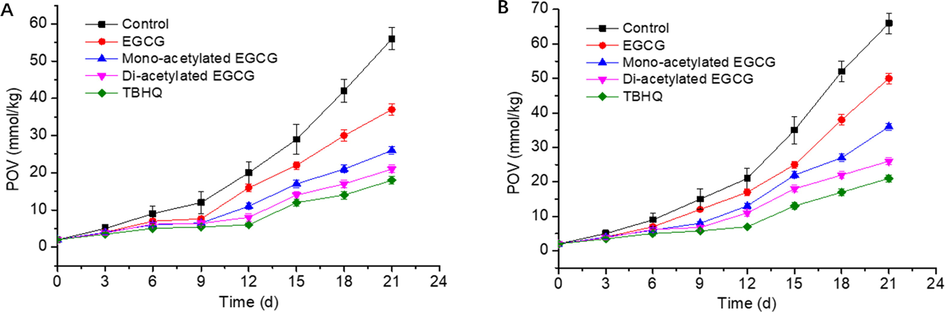
The effect of various antioxidants on POV of soybean oil (A), Corn oil (B).
The efficacy of EGCG derivatives as antioxidants in comparison with EGCG and TBHQ was also evaluated using the Rancimat test. The PF value of all antioxidants were over 1.0, which indicated an antioxidant activity against accelerated oxidation (Table 3). TBHQ had the highest PF value, followed by di-acetylated EGCG, then the slightly less, mono-acetylated EGCG, and followed by EGCG with the lowest PF value. Averagely, the IP of EGCG derivatives were 150 min higher than that of EGCG. The results of Rancimat test was consistent with the POV analysis. Note: Significant differences (P < 0.05) between antioxidants are marked as a, b, c, d and e. Significant differences (P < 0.05) between oils are marked as A, B, C and D.
antioxidants
Soybean oil
Corn oil
Palm oil
Lard
IP (min)
PF
IP (min)
PF
IP (min)
PF
IP (min)
PF
Control
185 ± 2.09 aB
1.00
205 ± 3.28 aD
1.00
210 ± 1.21 aC
1.00
132 ± 3.29 aA
1.00
EGCG
241 ± 4.20 bB
1.30
284 ± 1.53 bD
1.39
268 ± 3.76 bC
1.28
208 ± 2.88 bA
1.58
Di-acetylated EGCG
287 ± 3.22 cA
1.55
398 ± 2.97 dB
1.94
506 ± 1.89 dD
2.41
427 ± 1.09 dC
3.23
Mono-acetylated EGCG
245 ± 1.09 bA
1.32
301 ± 2.30 cB
1.47
421 ± 3.24cD
2.00
365 ± 4.77 cC
2.77
TBHQ
334 ± 1.87 dA
1.81
451 ± 4.11 eB
2.20
568 ± 0.96 eD
2.70
475 ± 3.62 eC
3.60
It was reported that the antioxidant activity of phenolic compounds was ascribed to the position and number of hydroxyl groups (Bodoira et al. 2017). The antioxidant activity of acetylated EGCG should be lower than that of EGCG because some hydroxyl groups were substituted by acetyl groups. However, because of the improvement of acetylated EGCG solubility in oil, the effective threshold of antioxidant concentration can be reached.
4 Conclusion
This study systematically assessed EGCG acetylation using EGCG and vinyl acetate, including the screening of various acyl donors and optimization of reaction conditions such as lipase concentration, molar ratio of substrates, and temperature. The reaction kinetic was agreed with Ping-Pong mechanism, and the constants values of the kinetic model were obtained. The two major acetylated derivatives were identified as 5″-O-acetyl-EGCG and 3″, 5″-di-O-acetyl-EGCG by LC-MS/MS and NMR. Their enhanced lipophilicity was confirmed by transmittance test and octanol–water partition coefficient. The antioxidant activity of di-acetylated EGCG was superior to mono-acetylated EGCG and EGCG as determined by POV in oil as well as by the Rancimat test, but slightly lower than TBHQ. Acetylated EGCG is shown to be a potent antioxidant derived for controlling oxidation of oil.
Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (No. 31871794, 21676122), the Innovative Project of State Key Laboratory of Food Science and Technology (SKLF-ZZB-201809, SKLF-ZZB-202010), and Fundamental Research Funds for the Central Universities (JUSRP21905).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Laccase-catalysed oxidation of ferulic acid and ethyl ferulate in aqueous medium: A green procedure for the synthesis of new compounds. Food Chemistry. 2014;145:1046-1054.
- [Google Scholar]
- Chia (Salvia hispanica L.) oil stability: Study of the effect of natural antioxidants. LWT. 2017;75:107-113.
- [Google Scholar]
- Biocatalytic preparation of alkyl esters of citrus flavanone glucoside prunin in organic media. Process Biochemistry. 2011;46(1):94-100.
- [Google Scholar]
- Lipase catalyzed transesterification of ethyl butyrate synthesis in n-hexane— a kinetic study. J Food Sci Technol. 2017;54(9):2871-2877.
- [Google Scholar]
- Du, G., Zhang, Z., Wen, X., Yu, C., Calway, T., Yuan, C., Wang, C., 2012. Epigallocatechin gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 11, 1679-1691. https://doi.org/10.3390/nu4111679.
- An in vitro evaluation of epigallocatechin gallate (eGCG) as a biocompatible inhibitor of ricin toxin. Biochimica et Biophysica Acta (BBA) - General Subjects. 2016;1860(7):1541-1550.
- [Google Scholar]
- Enzymatic synthesis of new aromatic esters of phloridzin. Journal of Molecular Catalysis B: Enzymatic. 2004;27(1):1-6.
- [Google Scholar]
- Porcine pancreas lipase catalyzed synthesis of lauryl laurate in organic solvent media: A kinetic study. Indian J. Biochem. Bio.. 2008;45:192-197.
- [CrossRef] [Google Scholar]
- Protective effects of EGCG through Inhibition of NADPH oxidase expression in endothelial cells. Food Sci Biotechnol. 2014;23(5):1611-1614.
- [Google Scholar]
- Acoustic cavitation promoted lipase catalysed synthesis of isobutyl propionate in solvent free system: Optimization and kinetic studies. Ultrasonics Sonochemistry. 2018;40:727-735.
- [Google Scholar]
- Green tea polyphenols for prostate cancer chemoprevention: A translational perspective. Phytomedicine. 2010;17(1):3-13.
- [Google Scholar]
- Cloning of a Novel O -Methyltransferase from Camellia sinensis and Synthesis of O -Methylated EGCG and Evaluation of their Bioactivity. J. Agric. Food Chem.. 2010;58(12):7196-7201.
- [Google Scholar]
- A dual substrate kinetic model for cytochrome P450BM3-F87G catalysis: simultaneous binding of long chain aldehydes and 4-fluorophenol. Biotechnol Lett. 2017;39(2):311-321.
- [Google Scholar]
- Efficient mono-acylation of fructose by lipase-catalyzed esterification in ionic liquid co-solvents. Carbohydrate Research. 2015;416:51-58.
- [Google Scholar]
- Highly efficient and regioselective synthesis of dihydromyricetin esters by immobilized lipase. Journal of Biotechnology. 2015;199:31-37.
- [Google Scholar]
- Highly efficient enzymatic acetylation of flavonoids: Development of solvent-free process and kinetic evaluation. Biochemical Engineering Journal. 2017;128:106-115.
- [Google Scholar]
- Biocatalytic preparation of acylated derivatives of flavonoid glycosides enhances their antioxidant and antimicrobial activity. Journal of Biotechnology. 2005;116(3):295-304.
- [Google Scholar]
- Enhanced anti-influenza A virus activity of (-)-epigallocatechin-3-gallate fatty acid monoester derivatives: Effect of alkyl chain length. Bioorg. Med. Chem. Lett.. 2008;18:4249-4252.
- [CrossRef] [Google Scholar]
- Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry. 2006;67(17):1849-1855.
- [Google Scholar]
- Assessment of conventional and microwave heating effects on the variation of the bioactive compounds of Chétoui VOO using HPLC-DAD-ESI-TOF-MS. Arabian Journal of Chemistry. 2020;13(1):954-965.
- [Google Scholar]
- Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7(3):255-270.
- [Google Scholar]
- A kinetic study of isoamyl acetate synthesis by immobilized lipase-catalyzed acetylation in n-hexane. Journal of Biotechnology. 2007;127(2):269-277.
- [Google Scholar]
- Bioavailability and stability issues in understanding the cancer preventive effects of tea polyphenols. J. Sci. Food Agric.. 2006;86(14):2256-2265.
- [Google Scholar]
- Improved enantioselective esterification of dl-menthol catalyzed by immobilized TL 100L lipase. Journal of Molecular Catalysis B: Enzymatic. 2016;133:S271-S276.
- [Google Scholar]
- Solvent-free enzymatic synthesis of feruloylated diacylglycerols and kinetic study. Journal of Molecular Catalysis B: Enzymatic. 2009;57(1-4):104-108.
- [Google Scholar]
- Enzyme as biocatalyst for synthesis of octyl ethanoate using acoustic cavitation: Optimization and kinetic study. Biocatalysis and Agricultural Biotechnology. 2016;7:145-153.
- [Google Scholar]
- Anxiolytic properties of green tea polyphenol (−)-epigallocatechin gallate (EGCG) Brain Research. 2006;1110(1):102-115.
- [Google Scholar]
- Immobilized lipase-catalysed synthesis of cinnamyl laurate in non-aqueous media. Journal of Molecular Catalysis B: Enzymatic. 2009;57(1-4):34-39.
- [Google Scholar]
- Kinetics and mechanism of synthesis of butyl isobutyrate over immobilised lipases. Biochemical Engineering Journal. 2003;16(3):245-252.
- [Google Scholar]
- Synthesis of citronellol laurate in organic media catalyzed by immobilized lipases: kinetic studies. J. Mol. Catal. B-Enzym.. 2004;27:113-.
- [CrossRef] [Google Scholar]
- Study of glucose ester synthesis by immobilized lipase from Candida sp. Catal. Commun.. 2008;9:1369-1374.
- [CrossRef] [Google Scholar]
- Epigallocatechin-3-gallate (EGCG) inhibits imiquimod-induced psoriasis-like inflammation of BALB/c mice. BMC Complem. Altern. M.. 2016;16:334-339.
- [CrossRef] [Google Scholar]
- Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem.. 2012;134:742-748.
- [CrossRef] [Google Scholar]
- Lipase-catalyzed synthesis of acetylated EGCG and antioxidant properties of the acetylated derivatives. Food Res. Int.. 2014;56:279-286.
- [CrossRef] [Google Scholar]







