Translate this page into:
Study of new cellulosic dressing with enhanced antibacterial performance grafted with a biopolymer of chitosan and myrrh polysaccharide extract
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The treatment of chronic wounds represents a major interest for public health both medically and economically. Hence the need for a modern wound dressing that actively promotes the physiological process specific to healing. In this perspective we have studied the development of a new dressing able to offer a serious contribution to the dilemma of the various chronic wounds. A dressing grafted with two natural polysaccharides known for their multiple biological effects, chitosan and a carbohydrate polymer extracted from Commiphora myrrha (CMP). We began by studying the grafting of the two natural biopolymers onto cellulose dressings, via a polyacrylic acid as a crosslinking agent. An optimization study, revealed the different grafting parameters, the polymer concentration as well as the heat-setting time and temperature. After, different characterization techniques were carried out in order to evaluate the effectiveness of our grafting. The swelling test revealed a hydrophilicity enhancement which increased with the degree of grafting, a desired property for effective dressings. Infrared characterization as well as thermogravimetric analysis (TGA and DTA) confirmed the binding mode and the permanence of our grafting. XRD and mechanical characterization showed no change in the crystallinity or in the original mechanical properties of the functionalized dressings. Morphological SEM study, confirmed the presence of our grafting as well as its mode of distribution. Finally, a bacteriological study conducted, showed a clear improvement of the antimicrobial behavior of cellulosic wound dressings functionalized by our combined natural biopolymers.
Keywords
Commiphora myrrha
Polymer extraction
Chitosan
Grafted dressing
Characterization
Antibacterial activity
1 Introduction
Chronic wounds are a public health problem whose prevalence increases with age, the patient's state of health (diabetes) and sedentary lifestyle (Fonder et al., 2008). The infection risk of chronic wounds increases with the healing delay, so it is necessary to provide regular care to prevent or control the infection of these wounds up to their complete healing (Margolis et al. 2002). In case of infection, critical colonization or localized infection may lead, in the absence of appropriate treatment, to the need for surgery to prevent systemic infection or even death of the patient (Armstrong, 2017).
Indeed, in the presence of chronic wound and especially in the case of infected wounds, the application of a simple dressing is not enough. A new range of so-called modern dressings can improve healing and prevent or treat the infection (Aditya et al., 2014). The combination of dressings with an active ingredient allow to obtain medical devices that permit both to maintain an adequate environment for healing wounds and provide added value whose purpose is to perform antibacterial action, healing or analgesic. A major interest has been shown by several researchers for the development of new interactive dressings or with more features, the bioactives. Among the interactive dressings we find, the hydrogels (Chan and Leong, 2008; Kim, et al., 2012), mostly composed of water (between 77 and 90%), are formed by gelling various hydrophilic substances (carboxymethylcellulose, calcium alginate, pectin, polyvinylpyrrolidone, polypropylene glycol, xanthan gum, etc.) (Boateng, et al., 2008). They contribute to the debridement of dry, fibrinous and necrotic wounds, help in the healing of non-exudative wounds and the softening of necrosis plaques. They often require a secondary dressing, such as a film, to maintain sufficient moisture at the wound.
The interfaces, also called tulles (Zahedi et al., 2010), are fatty dressings consisting of a mesh coated with non-adhering fatty substance such as petroleum jelly or silicone. They are inexpensive, not absorbent, their removal is sometimes painful and hemorrhagic with a risk of tearing buds. They are generally not recommended. Their combination with hydrocolloids called lipo-colloids (Fletcher, 2005) makes it possible to limit these disadvantages and to promote their use for the protection of superficial wounds in the epidermis phase. The charcoal dressings (Kerihuel, 2013), are composed of vegetable charcoal, which is the predominantly active element, they are not very absorbent. They limit bacterial growth and retain odors. These bacteriostatic properties can be enhanced by their silver ion impregnation which have a broad-spectrum anti-bacterial activity. They are indicated for fibrinous, infected, smelly wounds such as wounds in cancer patients or diabetics. Silver dressings (Dimikakos, et al., 2009; Michaels, et al., 2009), which are sometimes used alone but most often associated with charcoal, hydrofibres, hyaluronic acid or alginate. Silver is also present in these dressings in the form of silver sulfadiazine (Lansdown, 2004). They are indicated in wounds infected or at risk of infection and for a short period (a few weeks). Recently more effective dressings were developed and studied, the bioactive dressings. The purpose of bioactive dressings is to directly promote skin repair and provide the necessary properties for rapid healing. Different bioactive polymers are investigated to functionalize several natural and synthetic dressings. Among the most efficient polymers were chitosan and its derivatives aiming to improve its biological properties (Huang, et al., 2019: Ong, et al., 2008; Yang and Lin, 2004). We find also alginate polymers (Chiu et al., 2008) in addition to various extracted biological molecules and polysaccharides from various natural plants such as aloe vera (Salah et al., 2017; Salah et al., 2019), curcumin (Xu et al., 2018), cyclodextrins (El Ghoul et al., 2017; Salah et al., 2016; Ammar et al., 2015; El-Ghoul et al., 2014), hematoxylin (Alminderej and El-Ghoul, 2019), etc. Furthermore, different functional dressings have been elaborated via the electrospinning technique which providing nanomaterials based on biological polymers and other by the complexing of polymers with different biological metals (Chen et al., 2017; Sharma et al., 2014). Lately, polymer-based composite films loaded with various natural or glass nanoparticles have been studied. These nanomaterials have shown an interesting hemostatic effect with a good tissue regeneration (Francis et al., 2016).
In spite of, the many efforts and investigations of research developed for an efficient bioactive dressing, there still is a shortage and deserves more efforts, especially that the expectations are great to reach a solution in the level of importance of this vital and important humanitarian field.
In the current paper, we proposed the development of a new functional dressing grafted by two extracted natural polymers, well known by their multiple biological effects. We have synthesized a new crosslinked polymer-based chitosan/polysaccharide myrrh extract. Chitosan is produced by the chemical or enzymatic deacetylation of chitin (the most abundant natural polysaccharide after cellulose). The latter is present in the exoskeleton of many invertebrates (crustaceans, mollusks, insects) and in the cell membrane of green algae, fungi and yeasts (Alves et al., 2008; Francesko et al., 2011). Numerous chitosan studies have shown its antichololesterolemic, bacteriostatic, fungistatic and hemostatic activities (Barbosa et al., 2010; Kim et al., 2008). Its biocompatibility and affinity for many proteins supports its interest in tissue engineering (Senel and McClure, 2004). These multiple biological properties explain why chitosan has been evaluated in various forms (beads, films, sponges, tubes, hydrogels, fibers, membranes) in the context of various biomedical and pharmaceutical applications (Jayakumar et al., 2011; Mangiapia, et al., 2007). The second biological polymer investigated was extracted from the Arabian myrrh resin obtained from Commiphora myrrha (so called myrrh). Myrrh is a natural compound secreted by shrubs of genus Commiphora of the Burseraceae. It is composed of alcohol-soluble resins, volatile oils (essential oils) and water-soluble Ammar, 2015gum (Hanus et al., 2005; Tucker, 1986). The water-soluble gum in myrrh is about 30–60%. It is composed mainly of acidic polysaccharide with D-galactose, D-glucuronic acid and L-arabinose in a ratio of 4:3:1, with approximately 18–20% proteins (Hough, 1952). Myrrh, is a commonly-used herb in Saudi society. It is very well reported to possess a wide variety of medicinal properties and has been investigated in traditional medicines to treat various diseases including ulcerative colitis, skin infections, fever, dysmenorrhea, amenorrhea, tumors, and in burn treatment (Langhorst et al., 2013; Pećanac et al., 2013; Shen et al., 2012). The present research study aiming to perform the optimal conditions of grafting able to bring an efficient chemical functionalization of a cellulosic dressing via a crosslinked polymer of chitosan and CMP extract. The combination of the different biological properties of the two grafted natural polymers will allow us a bioactive dressing, effective in the treatment of serious and chronic wounds.
Firstly, the structure and the different macromolecular properties of the polysaccharide extracted from the myrrh resin was confirmed by SEC analysis and then by infrared spectroscopy study, results were compared to the literature (Lee et al., 2017).
Next, we studied the grafting of the Commiphora myrrha extracted polysaccharide (CMP) and the alginate polymer via a polyesterification reaction through a PAA crosslinking agent. We varied the grafting parameters such as temperature and heat setting time as well as the concentration of polyPAA-Ag-CMP. All these parameters showed a clear influence on the grafting rate of cellulosic dressings. Then, we carried out infrared and thermogravimetric characterizations (TGA and DTA) in order to evaluate the functionalization process and the efficiency of the selected parameters. The effectiveness of the hydrophilicity was then assessed on various dressings treated with different grafting rates. In addition, an evaluation of the mechanical properties of the untreated and functionalized samples was carried out. Scanning electron microscopy was used, allowing morphology and grafting mode via our new bioactive combined polymer.
Finally, we performed a biological and bacteriological study of functionalized dressings. This study revealed the effectiveness of our functionalization, an efficient substitution for a new generation of bioactive dressings with multiple desired functionalities.
2 Experimental
2.1 Chemicals and materials
Myrrh resin was procured from a commercial market in Buraydah, Saudi Arabia.
Citric acid (CTR), sodium dihydrogen hypophosphite (SHP catalyst) and chitosan (CS) were provided by Sigma Aldrich. The CS used is of low molecular weight (190 kDa, viscosity: 20–300 cps, soluble in 1% v/v acetic acid) with a degree of deacetylation of 75 to 85%. The cellulose dressings materials were textile woven of 24 yarns/cm with 44 g/m2 surface weight. They were a gift from SOTUPA company (TUNISIA) intended for research. functionalization was performed by padding, squeezing and then a heat-setting step in an oven. The initial impregnation bath contains CTR (80 gL−1), SHP (20 gL−1), chitosan (40 gL−1) and CMP (40 gL−1) in purified water. An alkaline post-treatment (using 2 gL−1 of ammonium hydrogen phosphate solution, Aldrich chemicals) was applied, in order to eliminate the excess of the unfixed polymer and to improve the surface properties of the grafted samples.
2.2 Extraction of Commiphora myrrha polysaccharide (CMP)
The myrrh resin was first washed and dried before being ground in a mortar. The resulting fine powder was then used for extraction. The myrrh powder was treated with water (1:10) at 90 °C for 90 min. After cooling to room temperature, the extract was filtered. The extract solution was then evaporated and the solid CMP extract recovered.
2.3 Steric exclusion chromatography (SEC)
The determination of the molar mass of the extracted polysaccharide as well as the various macromolecular quantities were carried out in steric exclusion chromatography (SEC) coupled to a multi-angle light scattering detector and a differential refractometer. The analysis was performed in DMF. The reported molecular weights are expressed as equivalent poly (ethylene oxide). The results are exploited using the OmniSEC software.
2.4 Grafting of polymer-based Ag/CMP onto cotton dressings
The general method of dressing’s functionalization by the polyPAA-Ag-CMP is based on the method of padding, squeezing, drying and thermofixation. Firstly, the textile samples were washed on soxhlet by three cycles with isopropanol and then rinsed by three cycles with distilled water to remove impurities. After drying at 90 °C for 15 min, the samples were weighed (mi: initial mass) before being functionalized with polyPAA-Ag-CMP. The impregnating solution was then prepared and the samples are impregnated in the solution at a rate of 1 ml/cm2 and then were expressed in the padder in order to eliminate the excess solution. Then, the samples were dried at 90 °C for 15 min in a finishing oven (Minithermo®). After they were subjected to the curing temperature where the cross-linking reaction is carried out between the PAA and the other protagonists; the textile, the alginate polymer and the extracted CMP. Finally, the samples were washed successively, with a 1% acetic acid solution and ultrapure water (20 min for each) under ultrasound. This washing step was necessary to eliminate unreacted reagents. The samples were then dried at 90 °C for 15 min and weighed (mf: final mass) in order to calculate the grafting rate (%-wt):
2.5 Swelling assessment
A gravimetric method via the difference of weighing before and after impregnation was applied during the swelling test on virgin and grafted samples. First, the samples were dried and weighed (mi). Then they are impregnated for 48 h in ultra-pure water. Different times of impregnations were selected. Finally, the samples were wiped and we completed the corresponding weight (mf). The following formula allowed us the rate of swelling.
2.6 Thermogravimetric analysis (TGA and DTA measurements)
The thermal properties of virgin and functionalized dressings were evaluated via a TA Instruments device. The parameters applied for the measurements were a heating rate of 10 °C min−1 and a temperature variation range of 25–600 °C under controlled atmosphere. The different resulting curves reported the percentage of the weight loss of the heated samples as a function of temperature increases.
2.7 Infrared spectroscopy analysis (ATR-FTIR)
Infrared spectroscopy analysis was realized using a FT-IR spectrometer (Agilent Technologies, Cary 600 Series FTIR Spectrometer PIKE Technologies GladiATR, from Qassim University, Saudi Arabia) via attenuated total reflection (ATR) accessory. A range of 4000–400 cm−1, and a resolution of 2 cm−1, were fixed for recording different spectra
2.8 Mechanical characterization
Mechanical properties of virgin and functionalized dressings were evaluated according to standard NF EN ISO 13 934-1, via a traction machine (Lhomargy 2/M). The jaws of this unit are equipped with a constant pressure clamping system. The gap between jaws was set at 200 mm. The pulling speed, 100 mm/min, was kept constant during the elongation of the samples. A force sensor of 100 N was applied.
Wet and dry samples were tested. Ten different measurements were taken for each sample.
2.9 Scanning electron microscopy (SEM)
A carbon coating was sprayed onto the textile dressings to make them conductive. SEM micrographs were recorded using a scanning electron microscope (FEI Quanta SEM) operating at a voltage of 5 kV. The morphology of untreated and functionalized surfaces has been compared.
2.10 Antibacterial efficiency
The disc method was considered for the evaluation of the antibacterial activity of the different virgin and treated samples. A method based on the agar diffusion technique. Before being sterilized, the virgin and functionalized dressings were cut in the form of discs (11 mm in diameter). Four different bacteria were tested, two Gram positive; Micrococcus luteus (NCIMB 8166) and Staphylococcus aureus (ATCC 25923) and two Gram-negative ones: Pseudomonas aeruginosa (ATCC 33787) and Escherichia coli (ATCC 35218). These bacteria were cultured in two successive stages, the first in a nutrient broth and the second on nutrient agar (Mueller-Hinton) for 24 h at 37 °C for each step. A bacterial load (106 CFU/ml) was suspended in physiological saline solution. Then 1 ml of each bacterial suspension was spread on Mueller-Hinton agar and incubated at 37 °C. Thirty minutes later, the samples were deposited on the prepared MH agar. After 24 h of incubation at 37 °C, we evaluate the antibacterial activity by measuring the diameter of inhibition which corresponds to the clear zone around the assessed samples.
3 Results and discussions
3.1 Extraction and properties of the polysaccharide from Commiphora myrrha
Compared to previous researches, the infrared characterization applied on the polymer extracted from myrrh resin (Table 1), confirmed the chemical structure of the extracted polysaccharide composed of D-galactose/D-glucuronic acid/L-arabinose (Fig. 1). Table 1 presents an overview of the assignments of the different FTIR bands of the polymer extracted from myrrh. the results confirmed the structure of the polysaccharide obtained.
FTIR bands (cm-1)
Affectation
Conclusion
875–2920
C–H stretch vibration
Polysaccharide s pyranose
1028
C–C stretch vibration
Polysaccharide s pyranose
1084
C–O stretch vibration
Polysaccharide s pyranose
1235
C–O–C stretch vibration (cyclic ether)
Polysaccharide s pyranose
1032–1120
C–OH stretch vibration
First and second alcohol in polysaccharide
1350–1430
CH2 deformation
Aliphatic CH2 in galactose
1734
C=O stretch vibration
COOH D-glucuronic acid
3000–3600
OH stretch vibration
First and second alcohol in polysaccharide
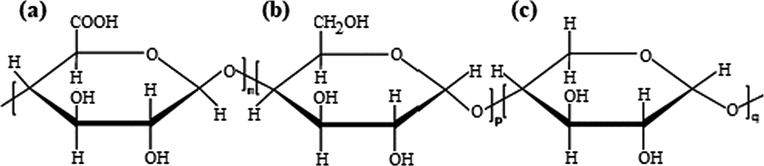
Chemical structures of polysaccharide extracted from Commiphora myrrha (a) D-glucuronic acid, (b) D-galactose and (c) g L-arabinose.
In addition, Analysis by steric exclusion chromatography performed on the extracted polysaccharide revealed the following macromolecular characteristics (Table 2).
Extract
Mn (g/mol)
Mw (g/mol)
Ð (Mw/Mn)
[ƞ] (mL/g)
CMP polymer
121000
236000
195
295
The polydispersity index result for the extracted polysaccharide was 1.95. This value is considered very acceptable, especially since it is a natural extract; The obtained polymer has satisfactory homogeneity.
3.2 Grafting of the polymer-based chitosan/CMP on cotton dressings
3.2.1 Influence of the curing time on the grafting rate
The evolution of the grafting rate was recorded over a time interval varying from 5 to 25 min (5, 10, 15, 20, 25). The curing temperature was set at 140 °C. and the polymer concentration at 30 g L−1.
From the results illustrated in Fig. 2, we noted that the grafting rate of polyPAA-CS-CMP increased gradually in the first 20 min of heat setting before reaching a plateau corresponding to the maximum conversion of the reagents reacting with the dressing during the treatment process. The minimum time of reactivity necessary to allow good grafting rate of the PolyPAA-CS-CMP polymer is 10 min. Besides, the results showed that the PAA alone revealed a grafting rate that increased with curing time. For each polymer applied in the impregnation solution results showed the same phenomenon, a grafting which increased further with the curing time. These findings have confirmed the hypothesis of the polyesterification reaction of the PAA, with the cellulose dressing material and with the two grafted biopolymers, chitosan, and CMP extract (Fig. 3).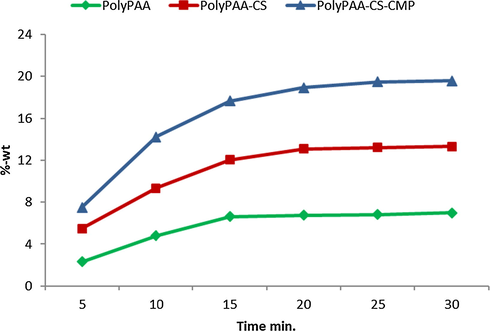
Grafting rate according to curing time, temperature of treatment 140 °C.
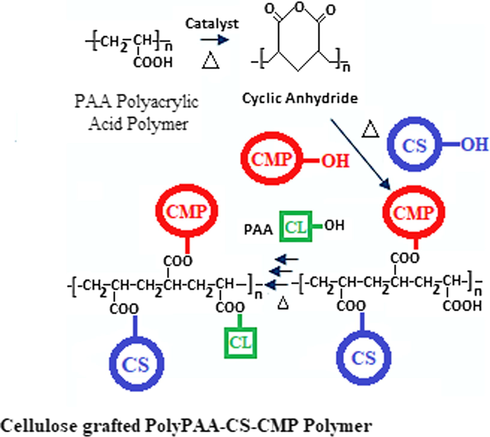
Grafting polyesterification reactions of the chitosan and the Myrrh polysaccharide extract onto the cellulosic dressing via the polyacrylic acid as a crosslinking polymer.
3.2.2 Influence of the curing temperature on the grafting rate
As indicated in the literature, the choice of the field of study of this parameter is considered important. Indeed, a low temperature may be insufficient to cause the polyesterification reaction (formation of the cyclic anhydride) between the polysaccharide, the cellulose and the polycarboxylic acid. A high temperature is disadvised since it is capable of hydrolyzing the already formed polymer network and degrading the dressing, hence the need to select the appropriate temperature allowing a sufficient degree of grafting without altering the properties of the dressing or the grafted biopolymers. Therefore, a range of varied temperatures from 120 to 160 was investigated. Results in Fig. 4, showed a progressive increase of the grafting rate of different polymers according to the temperature. The PAA was able to be grafted alone, and the important increase of the grafting rate noticed with the adding of the alginate and after the CMP extract confirmed the formation of the reticulated polymer grafted to the dressing via the PAA as a crosslinking agent.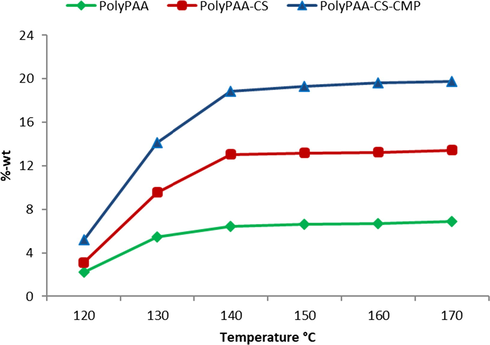
Grafting rate according to curing temperature, time of treatment 20 min.
3.2.3 Influence of the polymer concentration on the grafting rate
The results in Fig. 5, showed that the grafting rate of the treated dressings evolved in an almost linear evolution as a function of the concentration. The importance of linearity lies in the possibility of predicting the grafting rate for a given concentration.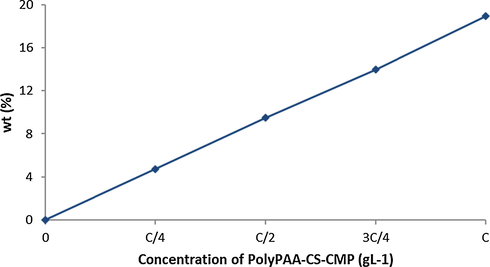
Grafting rate according to the concentration of polyPAA-CS-CMP, Curing 20 min at 140 °C.
3.3 Swelling study
Swelling is an important property that is directly related to the absorbance capacity of dressings. Besides, Retention and Absorptive Capacity are considered essential parameters for assessing the quality of absorbent dressings. The importance of this parameter lies in its ability to limit the spread of harmful exudates released around the wound. Untreated and functionalized dressings were evaluated.
Results in Fig. 6, showed a significant increase in the swelling capacity following the grafting of alginate. This increase in absorbance performance was shown to be higher after the addition of the CMP polymer. The significant improvement in the absorption properties of the functionalized dressings was certainly due to the hydrophilic character of the polyacid (PAA) and especially of the two natural grafted biopolymers, the alginate and the extracted CMP.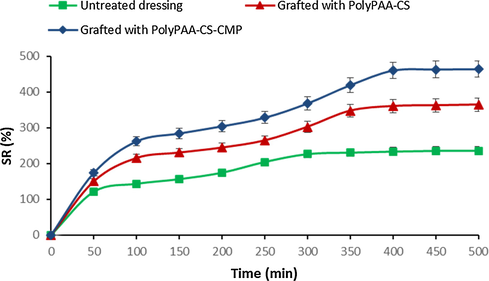
Swelling behavior of untreated and grafted dressings with polyPAA-CS and polyPAA-CS-CMP.
3.4 Thermogravimetric analysis (TGA and DTA)
The first loss of weight percentage detected at about 100 °C was attributed to moisture presented in the samples. Evaporation of water from the virgin and grafted cellulosic fibers was characterized by a weight loss of 4% and 6% respectively. This difference in weight loss can be explained by the more hydrophilic character of dressings after functionalization.
The degradation of the virgin cellulose fibers began at 310 °C, and continued to 410 °C, with a weight loss of 85% (Fig. 7A). The treated sample presented two areas a first weight loss varying from 180 °C to 310 °C referring to the grafted polymer, and a second from 310 °C to 410 °C mentioning the untreated sample. This decrease in degradation temperature is caused by the treatment of cellulose fibers with polyPAA-CS-CMP. Therefore, the virgin sample exhibited a single degradation temperature for the cellulose dressing material. On the thermogram of the treated sample we noticed the appearance of a new degradation temperature (temperature of 220 °C) reference of the grafted polymer. The change in thermal behavior was attributed to the presence of the grafted polymer, which has proved the success of a permanent functionalization.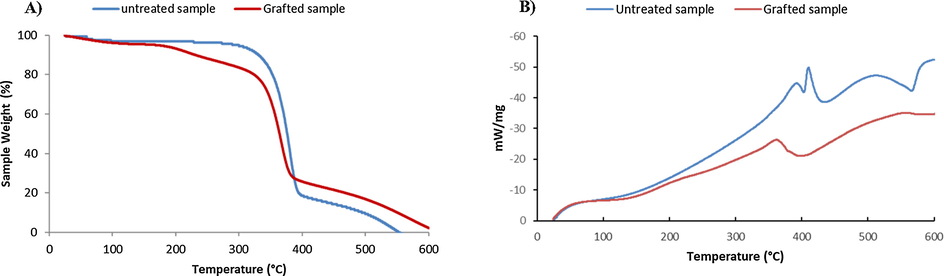
TGA (A) and DTA (B) thermograms of virgin and grafted cellulosic dressing with PolyPAA-CS-CMP.
The dressings treated with the CS/CMP polymer had a relatively greater residual weight after degradation, from 410 °C to 600 °C under a purge of nitrogen, hence a more improved thermal stability. The increase in the amount of residues during pyrolysis, with treated dressing, was explained by the presence of chemically grafted polymers.
In addition, DTA analysis results of untreated and grafted samples (Fig. 7B), were confirmed the functionalization via the appearance of a new exothermic peak (around 220 °C) due to the degradation of the grafted polymer.
3.5 Infrared spectroscopy analysis (ATR-FTIR)
Infrared analysis via ATR mode was performed on polysaccharide myrrh extract, virgin and treated dressings. The chemical grafting, in particular the polyesterification reaction, could be confirmed by the appearance of the new bands on the grafted samples proving the reaction.
Fig. 8, illustrated the spectra for CMP extract and the two virgin and grafted dressings. The spectrum of the extracted polymer from myrrh confirmed the structure of the obtained polysaccharide as explained above. Characteristic bands of cellulosic material were shown in the two spectra of untreated and grafted samples, as reported in literature (Lumbreras et al., 2019). The different bands relating to the grafted polymers appeared well on the spectrum of the functionalized dressing. Two bands of deformation vibrations at 900 and 1400 cm −1 corresponding to the amide functions resulting from the reaction of chitosan with PAA. A new band centered at 1718 cm−1 clearly appeared with grafting, which corresponded to the ester group of the PolyPAA-CS-CMP polymer grafted onto the treated dressings. a wider band, closed at 3290 cm−1, appeared on the spectrum of the functionalized sample relative to the hydroxyl functions of the grafted polymers and the amine functions of chitosan. Various research studies reported the functionalization of cellulosic materials by polysaccharides, via other polycarboxylic acids, had shown the effectiveness of grafting process by the formation of ester connecting groups (Salah et al., 2016; Alonso et al. 2009). The analysis of the different spectra revealed the covalent chemical grafting via a polyesterification reaction (between PAA and the other protagonists the cellulosic dressing, chitosan, and CMP polymer) and also a polyamidification reaction (between chitosan and PAA). The analysis thus confirmed the grafting of the two natural biopolymers onto the treated dressings via the PAA as crosslinking polymer.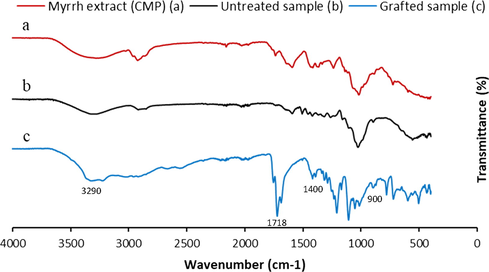
FT-IR spectra analysis of CMP extract (a), untreated cellulosic dressing (b) and grafted cellulosic dressing with the PolyPAA-CS-CMP (c).
3.6 Mechanical characterization
The mechanical behavior of the modified dressing samples was checked after the finishing treatment through the breaking strength test. The study of mechanical properties (Fig. 9) has shown that functionalization does not affect the breaking strength of modified materials. It has improved slightly with high grafting rates. This can be explained by the gradual recovery of surfaces and the crystalline restructuring due to the thermal conditions of grafting.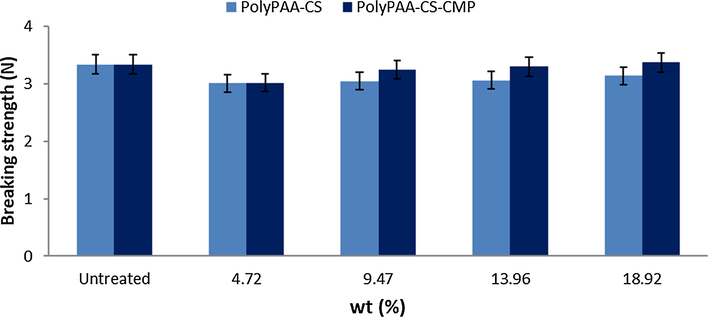
Breaking strength function of the grafting rate of cellulosic dressings with PolyPAA-CS-CMP.
3.7 Scanning electron microscopy (SEM)
The comparison of the different SEM images (Fig. 10) showed a clear difference in the morphology of the surfaces before and after grafting. On virgin samples, a rather regular surface is observed, presenting in its texture a very fine granulosity. On the micrographs of the grafted samples we noticed a change in the morphology of the surface. The grafting of the alginate and CMP biopolymers is perfectly visible on the fibers. The surface is much less smooth, and less regular. The empty spaces observed between the virgin fibers are filled by the presence of the polymer. We can also point out the absence of degradation in the treated fibers; The grafting treatment respected the original properties of the cellulosic fibers.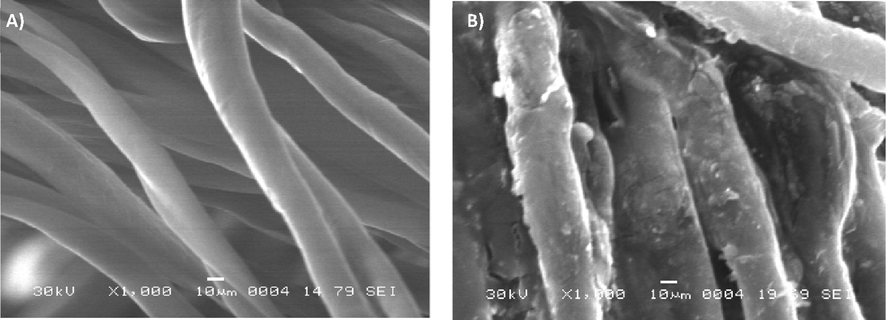
SEM images of virgin (A) and polyPAA-CS-CMP grafted cellulose dressing (B).
Finally, we noted that the grafting occurred at the fiber scale, not the threads. Therefore, the treatment has preserved the porosity of the functionalized dressings, which is a necessary property for the proper functioning of a dressing during the wound healing process.
3.8 Antibacterial efficiency
Two different gram-positive pathogenic bacteria (Micrococcus luteus, Ml and Staphylococcus aureus, Sa) and two gram-negative bacteria (Pseudomonas aeruginosa, Pa and Escherichia coli, Ec) were selected for bacteriological evaluation of virgin dressings and those functionalized by the polyPAA-CS-CMP. According to the agar diffusion technique described above, we have determined the diameter of the zone of inhibition which appeared around the tested samples.
Fig. 11, showed the different diameters detected around the samples after 24 h of incubation in the MH prepared agar. No inhibition zone was detected around the untreated samples and this with the four different bacteria tested. The results revealed a significant inhibition zone around the samples grafted with the different bacteria strains. We noted that the diameters of the inhibition zones are greater in the case of gram-positive bacteria, Sa and Ml. The grafting of polyPAA-CS-CMP conferred significant antibacterial activity to the treated dressings. The applied grafting method and the different selected parameters of functionalization have preserved the combined bacteriological effect of both chitosan and the polysaccharide extracted from myrrh.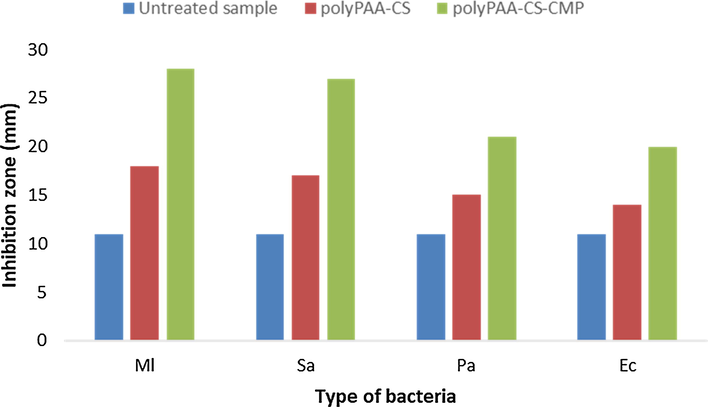
Antibacterial effect of grafted cellulosic disks on different bacteria via the evaluation of the inhibition area around bacteria (diameter of disk samples = 11 mm).
4 Conclusion
The present study aimed to provide an insight on the physicochemical, morphological, and bacteriological characteristics of new bioactive dressings functionalized by two natural polymers known by their biological properties. These two biopolymers studied were chitosan and a polysaccharide extracted from Commiphora myrrha resin. We started by extracting the polysaccharide via a simple method using water as a solvent. The FTIR and ESC characterizations of the extracted polymer have been compared to previous studies, and have confirmed its physicochemical properties and its polysaccharide structure with D-galactose/D-glucuronic acid/L-arabinose.
Then we performed a grafting study of these two natural biopolymers on cellulose based dressings using polyacrylic acid as crosslinking agent. This study allowed us to determine the influence of the different parameters studied such as the time, the curing temperature and the polymer concentration. The optimization of the grafting reaction allowed the selection of different grafting parameters. The study of the variation of these parameters showed the increase of the degree of grafting with the time and temperature of thermofixation. A linear increase in the grafting rate according to the polymer concentration has been considered as a very interesting result in that we could predict the degree of grafting by fixing the initial concentration of the polymer in the impregnating bath. Characterizations via both spectroscopic FTIR using ATR mode and thermogravimetric TGA/DTA analysis have well confirmed the permanence of the grafting which happened via a polyesterification and polyamidification reactions with the PAA as crosslinking agent and an intermediate for the binding of grafted polymers to the treated dressings. The swelling test has indeed shown a significant increase in the hydrophilicity of grafted dressings which is a required property for an effective dressing for the absorption of wound exudates. Evaluation of the mechanical properties of functionalized dressings showed that with the optimized grafting conditions there was no modification of the original mechanical characteristics of the dressings. Finally, the study of the morphology of the grafted surfaces, by SEM, revealed the mode of distribution of the polymer on the fibers, a uniform grafting around the fibers which respected the porosity of the functionalized surfaces.
The bacteriological study performed via the agar diffusion method, revealed a significant antibacterial activity of graft dressings against the four pathogenic bacteria tested. The literature is full of research and pharmacological applications, enumerating the various therapeutic effects of chitosan and the extracted polysaccharide from myrrh. The successful functionalization of the dressings by these two natural biopolymers, will offer an effective bioactive medical device with an efficient potential in the treatment of various acute and chronic wounds.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aditya, S., Mark, S.G., Nancy, L.T., 2014. Wound dressings and comparative effectiveness data Adv wound care (New Rochelle), 3 (8) (2014) 511–529.
- Alminderej, F.M., El-Ghoul, Y., 2019. Synthesis and study of a new biopolymer‐based chitosan/hematoxylin grafted to cotton wound dressings, J. Appl. Polym. Sci. 136 (2019) 47625.
- Cross-linking chitosan into UV-irradiated cellulose fibers for the preparation of antimicrobial-finished textiles. Carbohydr. Polym.. 2009;77(2009):536-543.
- [Google Scholar]
- Alves, A.N.M., Mano, J.F., 2008. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 43 (2008) 401–14.
- Alves, A.N.M., Mano, J.F., 2008. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 43 (2008) 401–14.
- Finishing of polypropylene fibers with cyclodextrins and polyacrylic acid as a crosslinking agent. Textile Research Journal. 2015;85(2):171-179.
- [Google Scholar]
- Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med.. 2017;376:2367-2375.
- [CrossRef] [Google Scholar]
- Barbosa, J.N., Amaral, I.F., Aguas, A.P., Barbosa, M.A., 2010. Evaluation of the effect of the degree of acetylation on the inflammatory response to 3D porous chitosan scaffolds J Biomed Mater Res A. 93 (2010) 20–28.
- Wound healing dressings and drug delivery systems: a review. J. Pharm. Sci.. 2008;97:2892-2923.
- [Google Scholar]
- Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur. Spine. J.. 2008;17:467-479.
- [Google Scholar]
- Recent advances in electrospun nanofibers for wound healing. Nanomedicine. 2017;11(12):1335-1352.
- [Google Scholar]
- Development of two alginate-based wound dressings. J. Mater. Sci.. 2008;6(19):2503-2513.
- [Google Scholar]
- Infected venous leg ulcers: management with silver-releasing foam dressing. Wounds. 2009;1(21):4-8.
- [Google Scholar]
- El-Ghoul, Y., Ammar, C., El-Achari, A., 2014. New polymer based modified cyclodextrins grafted to textile fibers; characterization and application to cotton wound dressings IJARTEX 2 (2) (2014) 11–21.
- Synthesis and study of drug delivery system obtained via β-cyclodextrin functionalization of viscose/polyester dressings. Ind. Tex. J.. 2017;47(4):489-504.
- [Google Scholar]
- Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J. Am. Acad. Dermatol.. 2008;58(2):185-206.
- [Google Scholar]
- Chitosan and derivatives for wound healing and tissue engineering. Adv. Biochem. Eng. Biotechnol.. 2011;125:1-27.
- [Google Scholar]
- Novel P(3HB) composite films containing bioactive glass nanoparticles for wound healing. Appl. Roy. I. Polym. Inter.. 2016;65(6):661-674.
- [Google Scholar]
- Hanus, L.O., Rezanka, T., Dembitsky, V.M., 2005. Myrrh-Commophora chemistry. Biomed Pap. 149 (2005) 3–28.
- Huang, J., Cheng, Y. Wu, Y., Shi, X., Du, Y., Deng, H., 2019. Chitosan/tannic acid bilayers layer-by-layer deposited cellulose nanofibrous mats for antibacterial application. Int. J. Biol. Macromol. 139 (2019) 191–198.
- Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv.. 2011;29:322-337.
- [Google Scholar]
- Kerihuel, J.C., 2013. Effect of activated charcoal dressings on healing outcomes of chronic wounds, J. Wound Care 19 (5) (2013) 208–215.
- Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv.. 2008;26:1-21.
- [Google Scholar]
- Modulation of cell adhesion of heparin-based hydrogel by efficient physisorption of adhesive proteins. Macromol. Res.. 2012;20(3):271-276.
- [Google Scholar]
- Randomised clinical trial: an herbal preparation of myrrh, chamomile and coffee charcoal compared with mesalazine in maintaining remission in ulcerative colitis – a double-blind, double-dummy study Aliment. Pharmacol. Ther.. 2013;38:490-500.
- [Google Scholar]
- Lansdown, A.B.G., 2004. A review of the use of silver in wound care: facts and fallacies. Br. J. Nurs. 13 (6) (2004) 6-–9.
- Dyeing properties and deodorizing/antibacterial performance of cotton/silk/wool fabrics dyed with myrrh (Commiphora myrrha) extract Tex. Res. J.. 2017;87(8):973-983.
- [Google Scholar]
- Poly (methacrylic acid)-modified medical cotton gauzes with antimicrobial and drug delivery properties for their use as wound dressings. Carbohydr. Polym.. 2019;205:203-210.
- [Google Scholar]
- Physicochemical and structural properties of hydrogels formed by chitosan, in the presence and absence of poly(vinylpyrrolidone) and sodium decylsulfate. Phys. Chem. Chem. Phys.. 2007;9:6150-6158.
- [Google Scholar]
- Venous leg ulcer: incidence and prevalence in the elderly. J. Am. Acad. Dermatol.. 2002;46(3):381-386.
- [Google Scholar]
- A prospective randomized controlled trial and economic modelling of antimicrobial silver dressings versus non-adherent control dressings for venous leg ulcers: the vulcan trial. Health. Technol. Assess.. 2009;13(56):1-114.
- [Google Scholar]
- Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials. 2008;29(32):4323-4332.
- [Google Scholar]
- Effect of the deacetylation degree on the antibacterial and antibiofilm activity of acemannan from Aloe vera. Ind. Crops Prod.. 2017;103:13-18.
- [Google Scholar]
- Bacteriological effects of functionalized cotton dressings. Tex Inst. J.. 2016;107(2):171-181.
- [Google Scholar]
- Development, characterization, and biological assessment of biocompatible cellulosic wound dressing grafted Aloe vera bioactive polysaccharide. Cellulose. 2019;26(8):4957-4970.
- [Google Scholar]
- Potential applications of chitosan in veterinary medicine. Adv. Drug Deliv. Rev.. 2004;56:1467-1480.
- [Google Scholar]
- Sharma, R., Singh, H., Joshi, M., Sharma, A., Garg, T., Goyal, A.K., Rath, G., 2014. Recent advances in polymeric electrospun nanofibers for drug delivery. Crit. Rev. Ther. Drug Carr. Syst., 31(3) (2014) 187–2017.
- The genus Commiphora: a review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol.. 2012;142:319-330.
- [Google Scholar]
- Tucker, A.O., 2005. Frankincense and myrrh. Econ. Bot. 40 (1986) 425–433.
- Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int. J. Biol. Macromol.. 2018;117:102-107.
- [Google Scholar]
- Properties of chitosan containing PP-g-AA-g-NIPAAm bigraft nonwoven fabric for wound dressing. J. Membr. Sci.. 2004;243(2):1-7.
- [Google Scholar]
- A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Poly. Adv. Techn.. 2010;2(21):77-95.
- [Google Scholar]







